Abstract
The intestinal mucosa faces the challenge of regulating the balance between immune tolerance towards commensal bacteria, environmental stimuli and food antigens on the one hand, and induction of efficient immune responses against invading pathogens on the other hand. This regulatory task is of critical importance to prevent inappropriate immune activation that may otherwise lead to chronic inflammation, tissue disruption and organ dysfunction. The most striking example for the efficacy of the adaptive nature of the intestinal mucosa is birth. Whereas the body surfaces are protected from environmental and microbial exposure during fetal life, bacterial colonization and contact with potent immunostimulatory substances start immediately after birth. In the present review, we summarize the current knowledge on the mechanisms underlying the transition of the intestinal mucosa during the neonatal period leading to the establishment of a stable, life-long host–microbial homeostasis. The environmental exposure and microbial colonization during the neonatal period, and also the influence of maternal milk on the immune protection of the mucosa and the role of antimicrobial peptides, are described. We further highlight the molecular mechanisms of innate immune tolerance in neonatal intestinal epithelium. Finally, we link the described immunoregulatory mechanisms to the increased susceptibility to inflammatory and infectious diseases during the neonatal period.
Keywords: Intestine, Epithelial cells, Homeostasis, Tolerance, Development, Neonates
Introduction
The mammalian mucosal surfaces such as the lung, reproductive tract, urinary tract and intestine are in direct contact with the external environment populated with bacteria, fungi, viruses and parasites. This is particularly evident in the intestine where a dense and highly diverse microbiota exists in a mutually beneficial relationship with the host. Yet, the bacterial colonization of the intestinal mucosa requires a tight epithelial barrier and functional mucosal immune system to ensure maintenance of the epithelial integrity and tissue homeostasis. In addition, the intestinal mucosa is intermittently exposed to potentially harmful pathogenic microorganisms. Thus, the establishment of a mature mucosal immune system able to restrict the microbiota to the intestinal lumen and to discriminate invading pathogens from commensal members of the microbiota is required and represents a unique regulatory challenge for the mucosal immune system.
The mammalian small intestine is composed of three tissue layers consisting of an outer smooth muscle layer, stromal tissue and an inner mucosal layer covered by a single sheet of cuboideal epithelial cells. The epithelial cell layer comprises four different cell types: enterocytes (secreting hydrolases and absorbing nutrients, ions and fluid), goblet cells (producing the mucus layer), enteroendocrine cells (secreting hormones, like serotonine, substance P and secretine), and Paneth cells (secreting antimicrobial peptides like cryptidins or defensins and enzymes like lysozyme). All four lineages derive from pluripotent continuously proliferating intestinal stem cells that are situated in a protected niche close to the bottom of intestinal crypts. The intestinal epithelium regulates the selective entry of fluids, minerals, vitamins and nutrient substrates, but also forms an active barrier separating the 10–100 trillion microorganisms of the gut microbiota from the largely sterile submucosal tissue [1].
Intestinal epithelial cells express innate immune pattern recognition receptors (PRR), such as Toll-like receptors (TLRs), Nod-like receptors (NLRs) and helicases, and thereby are able to actively respond to exposure of microbe-associated molecular patterns (MAMPs) [2, 3]. The factors that allow the host to discriminate between colonization by commensal microbiota and infection by pathogenic bacteria are still largely undefined [4], but an increasing number of studies start to shed light on the molecular and cellular mechanisms underlying the maintenance of gut homeostasis under conditions of mucosal stress, in most cases using the model of oral dextran sulphate sodium (DSS) treatment. Mucosal host–microbial homeostasis is the result of a complex cross-talk between microbiota, the epithelium and host immune cells [5–8]. Several studies using chimeric animals or mice with cell lineage-specific gene deletions showed that epithelial cells, which were originally considered a simple physical barrier, actively contribute to the regulation of immune responses in the gut [2, 9–11]. Hyporesponsiveness to the microbiota and mucosal homeostasis thus appears to be an interactive, dynamic, regulatory mechanism, rather than be caused by a non-responsive surface layer of cells. In fact, there is evidence that intestinal epithelial-specific dysfunction of innate immune signaling pathways and an impaired interaction between epithelial and submucosal cells might lead to mucosal inflammation or colitis-associated cancer [7, 12].
Several functional and structural aspects of the intestinal mucosa have been identified to contribute to homeostasis in adult mice and may also support the mucosal barrier formation during the postnatal period. Epithelial barrier integrity is enforced by intercellular tight junctions that block paracellular transcytosis and actin-rich microvilli that form a dense brush border to prevent microbial attachment and invasion [5]. Goblet cells produce mucins, heavily glycosylated interlinked protein chains that form a hydrophilic matrix overlaying the epithelial cell layer. The mucus layer physically separates the great majority of luminal microbiota from the apical epithelial surface [13, 14]. The critical importance of an intact mucus layer is illustrated by mice lacking the major mucin protein MUC2 that develop spontaneous colitis [15]. Goblet cells additionally secrete the resistin-like molecule (RELM) β and trefoil factors (TFF) that play a role in intestinal homeostasis, wound healing and the host defense against worm infection [16]. Paneth cells produce antimicrobial peptides (AMPs) to limit bacterial growth and shape the microbiota composition [17, 18]. Resident CD103− CX3CR1+ phagocytic cells generate dendrite-like protrusions that reach into the intestinal lumen and allow uptake of bacteria and sample luminal antigens [19, 20]. They control T cell activation via secretion of IL-10 and TGF-β but are also able to promote Th1 and Th17 differentiation during inflammation. Also, migratory CD103+ CCR7+ dendritic cells are conditioned by microbial and epithelial-derived factors and promote the differentiation of CD4+ Foxp3+ regulatory T cells (Treg) and the secretion of immunoglobulin A (IgA) by B cells [21]. Under inflammatory conditions, they also promote IL-17-producing helper T (TH17) cells that in turn induce infiltration by professional immune cells via secretion of IL-17 and IFN-γ [22]. Treg and Tr1-like cells regulate inflammation through IL-10- and TGF-β-dependent mechanisms [21]. RORγt+ innate lymphoid cells (ILCs), including lymphoid tissue-inducer (LTi) cells and IL-22-producing NKp46+ cells, synthesize IL-22 which induces expression of the antimicrobial protein Reg3γ. Epithelial IL-25 induced by the microbiota is able to control IL-22 production [23]. Also, lymphotoxin (LT) expression by RORγt+ cells favors epithelial cell repair and and induces epithelial secretion of CXCL1 and CXCL2 and the recruitment of neutrophils and macrophages during infection [24]. Enterocytes inhibit TH1 differentiation via soluble thymic stromal lymphopoietin (TSLP) [25] and control tumor necrosis factor (TNF)-induced epithelial apoptosis [9]. Finally, continuous signaling in epithelial cells through PRRs like TLR4, TLR5, Nlrp3, Nlrp6 and associated molecules like Myd88, NEMO, IKK1 and 2 as well as Caspase-1 has been shown to protect against colitis [11, 26–28] suggesting an active role of epithelial cells in the maintenance of host–microbial balance.
Whereas the mechanisms that ensure maintenance of immune tolerance and gut homeostasis in the adult host begin to be unraveled, the factors that facilitate the establishment of this surprisingly stable and life-long host–microbial interaction after birth and during the neonatal period are largely unknown. The healthy mammalian fetus develops in a bacteria- and microbial ligand-free environment. Upon rupture of the amniotic membranes and passage through the birth canal, the neonate organism is exposed to maternal bacteria from the vaginal tract, skin and feces and to environmental microbial ligands such as endotoxin [29–31]. During this time, the neonate intestinal epithelium has to adapt to facilitate the robust adaptation from a sterile protected site to a densely colonized surface and to establish a symbiotic interaction with the bacterial microbiota, in order to establish a stable host–microbial homeostasis [32, 33]. Alterations of the microbiota composition have been linked to several diseases such as allergies, vascular diseases, cancer and autoimmunity, as well as inflammatory bowel disease (IBD) and necrotizing enterocolitis (NEC) [34–37]. In this review, we will focus on the development of the neonatal mucosal immune system, the formation of the intestinal microbiota and the establishment of the host–microbe homeostasis during the neonatal period. Additionally, we will address infant diseases that might result from a dysregulated adaptation process during the postnatal period leading to an impaired immune homeostasis in the gut.
Development of the intestinal mucosa
During the fetal period, tissue morphogenesis and cell differentiation prepare the epithelium for absorption of colostrum and milk. The primitive gut is a pseudostratified layer of endodermal origin surrounded by mesenchymal tissue, appearing early during ontogeny [38]. Later on (around embryonic day E15 in mice), an anterior-to-posterior wave of morphogenic changes occurs and the undifferentiated group of cells converts into a single-layered epithelium with columnar cells and nascent villi [39]. The mesoderm differentiates into smooth muscle and stromal tissue. Cell proliferation, first homogenously present along the intestinal tract, becomes restricted to the intervillus regions, where the crypts begin to develop as soon as epithelial cells penetrate the underlying mesenchyme. Crypts contain a small group of proliferating stem cells, giving rise to the different cell phenotypes that migrate along the crypt–villus axis. The maturity of the epithelial tissue at birth depends on the length of the gestation period. Early crypt development occurs in species with a long gestational period including humans, but not rodents, where crypts develop only after the immediate postnatal period [40]. In some vertebrate species, such as zebrafish, crypts never appear and stem cells remain localized within the intervillus region [41]. Intercellular communication between epithelial cells, which facilitates a coordinated epithelial response upon microbial challenge [42], also represents one of the key mechanisms driving epithelial morphogenic movements, differentiation and migration. The composition of the extracellular matrix detected via cell surface receptors such as E-cadherin and integrins determines intestinal epithelial polarization characterized by the separation of the apical and basolateral plasma membrane through the expression of intercellular tight junction molecules [43, 44]. Hedgehog signaling, through Sonic hedgehog (Shh) and Indian hedgehog (Ihh), plays a role in endodermal and mesodermal patterning, crypt formation and spacing [45]. Conditional deletion of β1 integrins in the intestinal epithelium of mice results in a loss of Hedgehog expression and early postnatal lethality [46]. Forkhead box transcription factors, Homeobox genes and Parahox genes, as well as GATA/FOG transcription factors, regulate intestine-specific developmental genes during fetal development [45]. The immature primitive polarized cells lead to the formation of the four different lineages of IECs, enterocytes (90% of epithelial cells), goblet cells (5%), enteroendocrine cells (<1%) and Paneth cells (10–15 per crypt, restricted to the small intestine), that are maintained in the adult gut [47]. Nevertheless, the four cell types do not emerge synchronically, since active enteroendocrine cells are already present around E10 whereas Paneth cells in mice appear only after birth. LGR4, an orphan G-protein coupled receptor, has been shown to be required for Paneth cell differentiation [48]. Epithelial proliferation is known to be low in the intestine of suckling mice and starts to increase approximately at postnatal day P15, correlating with the adaptation of the gut to utilize solid nutrient components and the formation of the crypt–villus architecture of the intestinal epithelium [49–51]. The pool of stem cells, confined to the crypt, allows constant and rapid renewal of the adult gut epithelium. Transit-amplifying cells, the progeny of stem cells, divide approximately five times and then differentiate into specialized epithelial cells [51]. Differentiated cells of the upper crypt and villus epithelium continuously migrate towards the villus tip and are replaced by newly formed cells from the stem cell pool in the crypts, making the small intestinal epithelium an extremely dynamic surface structure. Epithelial cells differentiation and proliferation are controlled by several major signaling pathways. Wnt signaling maintains cellular proliferation in crypts and controls the development of the secretory lineage and the migration along the crypt–villus axis. Bone morphogenetic protein (BMP) signaling negatively regulates cell proliferation. K-RAS regulates cell proliferation and survival. Finally, notch signaling regulates secretory lineage development and crypt proliferation [41, 45]. Recently, an interesting study connected the age-dependent expression of the transcriptional repressor Blimp1 to the developmental adaptation of the murine intestinal epithelium during the postnatal period. Blimp1 expression is high in the embryonic gut and starts to decrease at birth in cells of the intervillus region which subsequently gives rise to developing crypts. Adult enterocytes completely lack expression of Blimp1. Intestinal epithelial-specific deletion of Blimp1 leads to enhanced postnatal lethality with disturbance of small intestinal tissue architecture, vacuolation of intervillous cells and altered differentiation, illustrating the critical importance of the switch in the global transcription profile between fetal and adult intestinal epithelial cells [52].
Also, posttranscriptional regulatory mechanisms such as microRNAs (miRs) have been implicated in the development of the intestinal epithelium. In mice, epithelial-specific ablation of Dicer1, essential cofactor in the maturation of miRs, leads to a disorganization of the epithelium, decrease in the number of goblet cells and an increase in the apoptosis rate [53, 54]. miR-145 has been shown to play a critical role in promoting the maturation of the zebrafish gut epithelium through the regulation of gata6, essential for intestinal morphogenesis [55]. Also, miR-194 regulates the expression of HNF1α, a Notch signaling activator expressed during organogenesis in the gut, which determines epithelial cell maturation and differentiation in mice [56]. Finally, miR-103 has been shown to control the expression level of proteins involved in the G1/S transition regulatory network during intestinal stem cell proliferation [57]. miRs may also play an important role for epithelial differentiation and barrier homeostasis after the immediate postnatal period [58]. For example, the epithelial di/tripeptide membrane transporter PepT1 was shown to be downregulated by miR-92b. PepT1 is expressed in differentiated IECs at the top of the villi and is involved in the transport of formyl-methionyl-leucyl-phenylalanine (fMLP), muramyl dipeptide (MDP) and l-Ala-D-Glu-meso-DAP (Tri-DAP), and thus contributes to innate immune stimulation via NOD2 [58]. miR-92b via the regulation of PepT1 thereby inhibits the inflammatory response induced by bacterial peptidoglycan fragments [58]. Also, enhanced expression of miR-29a was found in a fraction of patients with irritable bowel syndrome (IBS). The same patients exhibited increased intestinal membrane permeability associated with decreased expression of the glutamine synthetase GLUL (glutamate-ammonia ligase), a target of the miR-29.
In addition to the maturity of the intestinal epithelium, the development of the gut-associated lymphoid tissue (GALT) also correlates with the length of gestational period. Lymphomyeloid precursor cells are present during early development and disseminate to seed progenitors in early structures of Peyer’s patches and mesenteric lymph nodes [59]. In mice, the initiation of Peyer’s patches genesis starts around E15–E17 [60, 61]. The migration of mature lymphocytes begins at postnatal day P2 and fully organized Peyer’s patches with follicular DCs, germinal centers, a B cell and a T cell region are evident at P4 [62]. In contrast, mouse cryptopatches and isolated lymphoid follicles (ILF) are only formed after microbial exposure [63]. In human fetuses, Peyer’s patches outlines appear at 11 weeks gestation and functional T cells and B cells are found at 16 and 12 weeks gestation, respectively [64]. At 16 weeks gestation, fully formed Peyer’s patches are present and progressively expand. During the neonatal period, the gut immune system is structurally complete, but still undergoes significant expansion and maturation. Also, innate and adaptive immune responses of intestinal immune cells during the neonatal period are different from the adult situation [59, 65]. Neonatal CD4+ T cell are more prone than adult CD4+ T cells to differentiate into TReg cells upon stimulation [66]. In addition, B cells expand during the postnatal period and develop into plasma cells that produce large amounts of secretory (S)IgA [67]. SIgA prevents inappropriate immune activation by binding to nutritional and microbial antigens. Thus, interactions with microbial ligands and food antigens facilitate the maturation of dendritic cells, T cells and B cells during the postnatal period and drive the development of immune tolerance mechanisms to avoid an inappropriate immune response [64]. As described below, the immature neonate immune system also renders the organism more susceptible towards microbial infection [32, 65, 68].
Maternal influence on postnatal mucosal homeostasis
One unique feature of the neonatal mucosal immune system is the link to maternal immunity through breast feeding. Breast milk stimulates cellular growth and tissue repair, enhances the immunocompetence and provides significant immunoprotection [68, 69]. Early breast milk (called colostrum) contains large amounts of IgA, and also immune cells such as neutrophils, macrophages/colostral corpuscules and lymphocytes, and soluble mediators such as cytokines (interleukins [IL], interferon [IFN]-γ, and TGF-β), hormones and growth factors (insulin, insulin-growth factors [IGF], erythropoietin, colony-stimulating factor [CSF], vascular endothelium factor [VEGF], epidermal growth factor [EGF], nerve growth factor [NGF], hepatocyte growth factor [HGF]), non-specific immune factors (sphingomyelin, oligosaccharides, lactoferrin), and certain miRs [64, 68, 70]. The functional importance of breast milk for the developing intestinal mucosa is highlighted by the finding that breast feeding reduces the risk to acquire inflammatory enteric diseases, such as Crohn’s disease, coeliac disease, gastrointestinal infections, NEC and food allergies [64].
Breast milk has been shown to modulate neonatal TLR-mediated microbial recognition. For example, soluble TLR2, found in the maternal milk, may help to restrict innate immune stimulation induced by Gram-positive bacteria in the neonate gut [71]. Milk-derived growth factors contribute to the maturation of the mucosa, reinforce epithelial barrier formation and enhance the ability to selectively transport and absorb nutrients [68]. Macrophages present in colostrum and mature breast milk persist in the lumen of the neonate’s gut during the first postnatal week and are able to translocate and reach the systemic circulation [68]. Macrophages are able to secrete cytokines and growth factors that favor epithelial maturation and bind SIgA to enhance the neonate’s own immune system [72]. Maternal SIgA also restricts immune activation and microbial attachment by binding to nutritional and microbial antigens. Importantly, the spectrum of the maternal IgA reflects the geographical and temporal environment of both the mother and the child and thus provides highly specific protection. Maturation of the SIgA-producing plasma cells in the GALT and expression of the polymeric immunoglobulin receptor (pIgR), a molecule that translocates SIgA into the intestinal lumen, occur gradually during the neonatal period and are influenced by environmental conditions [73].
Lactoferrin contained in breast milk limits the pool of free iron and suppressed bacterial growth in addition to its interference with the nuclear transcription factor-κB (NF-κB) [68, 70]. Interestingly, miR-584 has recently been shown to induce expression of the lactoferrin receptor in epithelial cells during the neonatal period [57]. Furthermore, the breast milk constituent lysozyme inhibits bacterial growth by disrupting the peptidoglycan layer of the microbial cell wall [70]. Oligosaccharides have prebiotic effects, but also act as receptor analogs to inhibit attachment of commensal bacteria to the epithelial surface [74]. Maternal cytokines also influence the neonates’s immune system. IFN-γ stimulates phagocyte function and TGF-β acts as an immunosuppressor and maintains the integrity of the mucosal barrier. Significant levels of miRs have been detected in breast milk despite the low pH indicating their stability and thereby potential regulatory function of the intestinal mucosa [49, 53]. Particularly, miRs associated with T cell and B cell differentiation and regulation have been observed in breast milk [70].
In addition to their nutritional and innate immune functions, factors present in breast milk also play a role in wound healing and tissue repair. Insulin-like growth factor (IGF) 1 is induced after mucosal injury to promote cell proliferation and is present in the maternal milk. Also, epidermal growth factor (EGF) has been shown to play a role in cell proliferation, maturation and differentiation, and is protective against NEC, a devastating intestinal inflammatory disease predominantly of premature neonates. EGF downregulates pro-inflammatory cytokines such as IL-18, increases anti-inflammatory cytokines such as IL-10, and restores the intestinal barrier [75]. EGF also promotes the generation of the mucus layer by goblet cells which is formed by complex interlinked mucin glycoproteins and shields particularly the colon epithelium from direct exposure to luminal substrates [76].
Immune tolerance of IECs after birth
With rupture of the membranes and passage through the birth canal, the neonate becomes exposed to the maternal microbiota, environmental bacteria and microbial constituents such as lipopolysaccharide (LPS). This first exposure occurs prior to ingestion of breast milk, and thus encounters the naïve fetal intestinal mucosa. Intestinal epithelial cells have been shown to express innate immune receptors, such as TLRs throughout fetal, neonatal, and adult life. Both TLR2 and TLR4 expression were found in human fetal tissue from 18 weeks of gestation [77]. Also, in mice, TLR4 and the accessory protein MD2 are expressed in fetal IECs [78, 79] that are able to respond to LPS [49, 79].
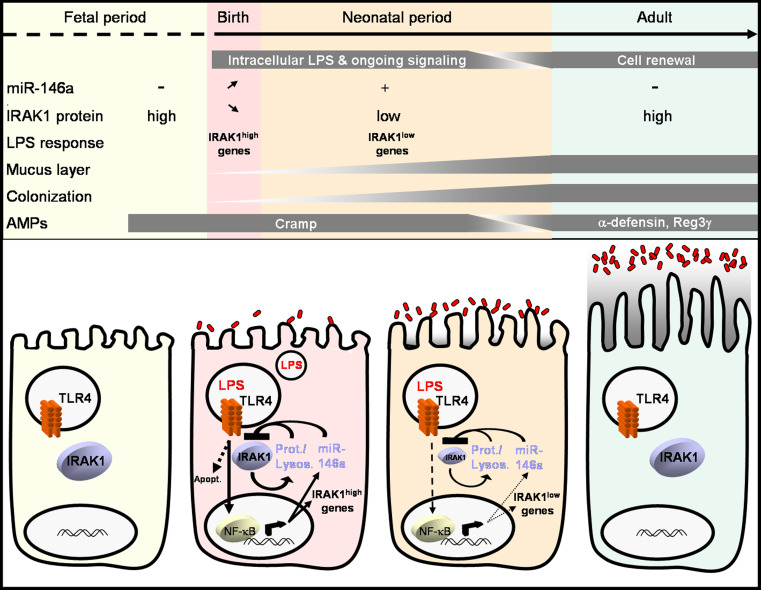
Interestingly, we observed a transient transcriptional postnatal activation of epithelial cells, with a peak of Cxcl2 chemokine expression between 2 and 4 h after birth followed by rapid normalization [79]. This transient transcriptional epithelial activation was induced by orally ingested LPS since it was absent in vaginally delivered TLR4-deficient mice or mice born by caesarian section and thus without exposure to the maternal mucosal secretions during birth. In accordance, low but detectable amounts of LPS were measured in the neonatal intestinal tissue shortly after birth and oral administration of LPS to cesarean section-born mice readily induced epithelial activation [49, 79]. Immunofluorescence studies confirmed epithelial stimulation demonstrating p65 nuclear translocation and IκB-α phosphorylation in small intestinal epithelial cells after vaginal delivery. These analyses also demonstrated epithelial internalization of orally administered LPS in accordance with the previous finding that TLR4 is localized intracellularly in intestinal epithelial cells and requires ligand internalization [80]. Surprisingly, intracellular epithelial LPS could be detected during the complete postnatal period. Since epithelial activation after vaginal delivery was transient and not accompanied by the recruitment of professional immune cells, the induction of negative regulators of the TLR4 signaling were subsequently studied. However, no increase in the expression of well-established regulator molecules such as Sigirr, ST-2, the spliced form of Myd88, or Tollip was detected in isolated IECs after birth. Yet, an almost complete disappearance of the essential TLR signalling molecule interleukin 1 receptor-associated kinase (IRAK) 1 in epithelial cells isolated from mice shortly after birth was noted. Epithelial IRAK1 down-regulation was observed in vaginally delivered mice but neither in caesarian section-delivered animals nor in TLR4-deficient mice, suggesting that it might be a direct consequence of the described postnatal epithelial activation [49, 79]. Also, IRAK1 downregulation might cause epithelial TLR hypo-responsiveness and thus contribute to the observed epithelial innate immune tolerance during the neonatal period. Indeed, significant apoptosis was observed in IRAK1 expressing intestinal epithelial cells from cesarean section-born neonates but not epithelial cells from vaginall delivered mice with reduced IRAK1 expression after oral administration of bacteria.
A similar effect of post-stimulatory IRAK1 downregulation associated with an impaired immune responsiveness had previously been demonstrated in macrophages and suggested to contribute to the well-known refractory state to secondary TLR4 stimulation named endotoxin tolerance [81]. Subsequent in vitro studies using a well-established murine intestinal epithelial cell line [82] confirmed the downregulation of IRAK1 protein expression following TLR4 activation associated with a lack of responsiveness upon secondary stimulation. In contrast to the situation in macrophages, both proteasomal and lysosomal degradative mechanisms were shown to contribute to IRAK1 downregulation in epithelial cells [49], and the functional relevance was also confirmed in vivo. Administration of proteasome and lysosome inhibitors to vaginally delivered newborns prevented the downregulation of epithelial IRAK1, and also caused epithelial apoptosis following oral administration of bacteria after vaginal delivery. In addition to the proteasomal/lysosomal degradation of IRAK1, translational repression of Irak1 mRNA by strongly enhanced miR-146a expression was identified in epithelial cells [49, 83, 84]. Epithelial miR-146a expression was induced by the initially observed postnatal epithelial activation and absent in caesarian section-delivered or TLR4-deficient mice. Although initial studies had described miR-146a-mediated Irak1 mRNA degradation, our results both in vitro and in vivo indicated solely transcriptional repression by miR-146a without any alteration in the level of Irak1 mRNA [49, 85, 86]. In accordance with a critical importance of enhanced postnatal epithelial miR-146a expression, administration of anti-miR-146a to vaginally delivered newborns restored epithelial IRAK1 expression and epithelial apoptosis upon oral bacterial challenge. Conversely, administration of a miR-146a homologue to caesarian section-delivered mice was sufficient to cause IRAK1 downregulation and protect the epithelium from bacteria-induced damage.
Strikingly, enhanced miR-146a, IRAK1 downregulation and lack of LPS-induced chemokine expression persisted in epithelial cells throughout the postnatal period until weaning, associated with the above-mentioned persistence of intraepithelial LPS. Further analyses revealed that continuous TLR4 stimulation and signal transduction possibly provided by the intraepithelial LPS maintained elevated miR-146a levels and ongoing IRAK1 degradation. In addition, this constant signaling under conditions of high miR-146a and low IRAK1 protein (IRAK1low) induced a discrete program of gene transcription, different from the gene pattern induced in naïve, high IRAK1 protein-expressing cells. This gene expression included genes associated with epithelial cell survival, proliferation, cell differentiation, and metabolism [49, 83, 84]. Of note, a similar change in the gene expression pattern after acute (M1 state) versus chronic stimulation (M2) has also been observed in macrophages [87]. In the intestine, the described adaptive process might thus simultaneously protect from inappropriate pro-inflammatory innate immune activation during bacterial colonization of the naive fetal mucosa and drive maturation of the epithelium to establish host–microbial homeostasis.
During the third week after birth, profound changes occur with enhanced stem cell proliferation, crypt formation and the start of the continuous crypt–villus migration and constant renewal of the epithelium. The loss of intraepithelial LPS coincided with reduced miR-146a expression, reappearance of high epithelial IRAK1 levels and inducible chemokine expression, thus providing a fully competent epithelial innate immune system to protect from enteropathogens that might encounter the adult host upon uptake of solid food.
Antimicrobial peptides as a host defense mechanism of the intestinal epithelium
Antimicrobial peptides are ancient gene-encoded natural peptide antibiotics. In mammals, two dominant antimicrobial peptide families are found: defensins and cathelicidins. Defensins are characterized by the presence of three intramolecular disulfide bonds and can be subcategorized into α- and β-defensins based on the interlinkage of the cysteine bonds. Mature defensins consist of approximately 30 amino acids and form a triple-stranded β-sheet structure. In the gastrointestinal tract, expression of α-defensins is confined to Paneth cells which are located at the base of the crypts of Lieberkühn in the small intestine and display a highly secretory phenotype filled with granules [88]. Whereas only two α-defensins, human α-defensins 5 (HD5) and HD6, are expressed in the human small intestinal tissue, more than 20 α-defensins (also named cryptdins) have been sequenced from murine small intestinal tissue. In addition, murine Paneth cells express a related large family of covalently linked homo- or hetero-dimeric antimicrobial peptides, the cryptdin-related sequence (CRS) peptides [89]. The distribution of β-defensins includes the stomach and colon. Although β-defensin mRNA has been detected in small intestinal tissue, its expression on the protein level has not been confirmed. β-defensins are regulated on the transcriptional level and their expression occurs either constitutively or after stimulation by endogenous proinflammatory stimuli or innate immune activation. In contrast, α-defensins are constitutively produced by Paneth cells and posttranscriptionally regulated by proteolytic processing. Proteolytic cleavage in mice is performed by the matrix metalloproteinase 7 (MMP7, also named matrilysin) prior to secretion, whereas human α-defensins are cleaved by the endoprotease trypsin only after secretion within the intestinal lumen. Paneth cells express a selection of PRRs and α-defensin secretion is induced by endogenous or microbial stimuli. In addition to α-defensins, which account for around 70% of the secreted bactericidal activity [90], Paneth cells also secrete the antimicrobial proteins lysozyme P, secretory phospholipase A2, and the recently discovered C-type lectins, Reg3γ and Reg3β [88, 91].
Defensins display broad spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria with some additional activity against fungi, viruses and protozoa. They are highly cationic and are believed to disrupt the membrane integrity of their bacterial targets by interaction with negatively charged phospholipid groups on the outer membrane. Displacement of lipids by integration between phospholipid groups alters the membrane stability and finally leads to membrane disintegration and potentially pore formation [92]. The biological importance of defensins has been demonstrated by the generation of MMP7-deficient mice, which are more susceptible against oral infection with Salmonella enterica ssp. enterica sv. Typhimurium (S. Typhimurium) [93] and display significant alterations of the enteric microbiota composition [17]. Conversely, transgenic mice expressing the human α-defensin 5 (HD5) display enhanced resistance against S. Typhimurium infection [94].
Cathelicidins also represent cationic amphipathic peptides but in contrast to α-defensins form α-helical or β-hairpin structures. They are produced by proteolysis of the C terminus of cathelin-domain-containing protein precursors. In humans and mice, only one cathelicidin precursor, hCAP18 and CRAMP, respectively, is produced, whereas in cattle and pigs this peptide family comprises a large number of members and is highly diverse. Cathelicidins are expressed in the skin, lung and intestinal tract by a variety of cell types including neutrophils, mast cells and epithelial cells.
More recently, the C-type lectin Reg3γ was described as an antimicrobial protein expressed in the intestinal tract both by absorptive enterocytes and Paneth cells. Reg3γ is particularly active against Gram-positive bacteria [95]. Expression of this protein is induced by the presence of the microbiota [95], depends on the IL-1R and TLR adaptor molecule MyD88 and is at least in part mediated by an intrinsic regulatory loop mediated by IL-22 [96]. Activity of Reg3γ is further modulated by proteolystic cleavage of a negatively charged N-terminal inhibitory prosegment from the positively charged core protein by trypsin [97]. Although the processing of Reg3γ resembles the processing of α-defensins, the mechanism of action against bacteria is different. While mature cationic α-defensins bind to negatively charged bacterial phospholipids, Reg3γ specifically interacts with native peptidoglycan at the bacterial surfaces [97, 98]. Reg3γ expression was shown to also contribute to the antimicrobial host defence using the model of oral infection with Listeria monocytogenes [96]. In addition to their direct antimicrobial function, antimicrobial peptides have been shown to exert immunomodulatory functions. They bind to LPS and neutralize its proinflammatory activity, exhibit chemoattractive activity, promote wound healing and modulate dendritic cell responses [99].
A number of studies have shown that expression of antimicrobial peptides is also under developmental control. As outlined before, morphogenesis of the mouse small intestine is not completed until the the third week after birth. Crypts form from epithelial cells at the intervillus region after the first week and undergo proliferation by crypt fission between the second and third weeks [100]. The emergence of Paneth cells coincides with crypt morphogenesis and a dramatic increase in epithelial stem cell proliferation and is independent of the presence of the microbiota. The differentiation of Paneth cells includes the sequential expression of α-defensins, phospholipases and lysozyme [101]. The regulation α-defensin expression is generally considered not to occur on the transcriptional level, and total α-defensin mRNA levels were found to be similar in the presence or absence of the intestinal microbiota [102, 103]. Yet, some α-defensins, especially α-defensin 6, might be expressed by epithelial precursor cells before the emergence of Paneth cells [104], although at a much lower level [105]. Also, a significantly higher gene expression of a small group of α-defensins was reported in conventional as compared to germ-free mice [106]. In particular, α-defensin 4 and 5 were found to be significantly reduced in germ-free mice. Differences were also noted in the course of mRNA expression during postnatal development between individual α-defensin isoforms. One group, including α-defensin 1, 3 and 6, show a more gradual increase during the postnatal period whereas another group, including α-defensin 2 and 5, exhibit a rapid increase in gene expression accompanying the onset of Paneth cells. Differences in the experimental approaches (e.g., m-RNA vs. protein level) and the use of oligonucleotide probes that detect several members of this highly conserved large group of peptides simultaneously might account for some observed discrepancies [102, 106, 107]. Although generally coregulated, individual α-defensins might be influenced by endogenous or exogenous factors and play a distinct role in intestinal homeostasis and antimicrobial host defense [106].
In contrast to the delayed appearance of α-defensin expression in Paneth cells of the murine small intestine, high expression of the cathelicidin CRAMP by the intestinal epithelium is found at birth. Strikingly, CRAMP expression is restricted to the first postnatal 2 weeks and gradually disappeares with the appearance of crypts, Paneth cells, and α-defensins [105]. Again, the downregulation of CRAMP during postnatal development is independent of the enteric microflora. CRAMP-deficient neonates are more susceptible to oral infection with the Gram-positive enteric pathogen L. monocytogenes. Whether CRAMP also contributes to the postnatal establishment of the enteric microflora still needs further investigation.
Postnatal development of the intestinal microbiota
The fetus develops in a sterile and environmentally protected environment within the amniotic membranes in the uterus. Microbial exposure and bacterial colonization of mucosal surfaces, however, start immediately at birth. Already with passage through the birth canal and during the immediate postnatal period, maternal and environmental bacteria are transferred to the neonate’s body surfaces. The intestinal tract provides a favorable environment for commensal bacteria providing essential nutriments for their metabolism [14, 108]. A number of studies have investigated the postnatal development of the intestinal flora in mice [109–112]. Although neonatal rodents are exposed to greater numbers of environmental microbes than human neonates and differences have been observed on the species level, the principal scenario of the succession of intestinal microbial colonization in rats and mice resembles that in human neonates. Facultative anaerobic or microaerophilic bacteria like Lactobacilli and Streptococci dominate during the first week after birth, followed a few days later by Enterococci and members of the Enterobacteriaceae. These bacteria reduce the local oxygen concentration by their metabolic activity and thereby establish the milieu for the subsequent colonization by strictly anaerobic bacteria like Bifidobacteria, Bacteroides spp. and Clostridium spp. [109–113].
Colonization of the newborn intestinal mucosa is influenced by a variety of factors including the mode of delivery, gestational age, environmental factors such as hygiene and lifestyle and diet (i.e. formula vs. breast milk) [30, 31, 33, 114, 115]. Caesarean section-born infants, for example, undergo delayed colonization with an altered flora compared to vaginally delivered infants [31]. Significant differences in the enteric microbial colonization have also been found between breast-fed infants in which Bifidobacteria represent the dominant group whereas formula-fed infants harbor high numbers of Bacteroides spp., enterobacteria, Clostridium spp. and Lactobacilli [30]. The diversity of the infant’s intestinal microbiota increases gradually over time with major shifts at weaning or with changes in their diet [33, 116]. In addition to alterations in the environmental exposure, the increased diversification might also be influenced by the decline in maternal SIgA [117]. Most of the intestinal bacteria of adult mice establish within 3–5 weeks after birth [112], and obligate anaerobes of the phylum Bacteroides and Clostridiales represent the most abundant species after weaning.
The mature microbiota of an adult individual consists of 1014 bacteria representing approximately 500 species. Its composition stays relatively stable throughout the whole life. It fulfils a variety of important biological functions. Microbial enzymes help to process ingested nutrients and thereby influence the metabolism and digestive efficiency and regulate host fat storage. The dense population of commensal bacteria at the mucosal surface prevents adhesion and subsequent colonization by pathogenic species, a mechanism termed colonization resistance. Finally, the presence of the microbiota stimulates mucosal angiogenesis and significantly contributes to the maturation of the gut innate and adaptive immune system particularly during the postnatal period [118–121].
Susceptibility of neonates to inflammatory and infectious diseases
The most common inflammatory diseases of the gastrointestinal tract of preterm infants is NEC. Several contributing factors have been identified including breaches in the intestinal mucosal barrier leading to bacterial translocation, transient mucosal ischemia, cytokine induction and enteral feeding. The precise mechanism underlying the pathogenesis, however, is still unclear [122]. Increased adhesion of disease-promoting bacterial species to the immature mucosal surface was identified as possible risk factor for NEC [122]. Also, increased epithelial expression of the lipopolysaccharide (LPS) receptor TLR4 and enhanced TLR4-mediated signaling in response to hypoxia have been associated with NEC in humans and mice [123, 124]. The critical role of TLR4 has been illustrated by the finding that gene-deficient mice are protected against disease in a murine NEC model [124]. More recently, reciprocal expression patterns of TLR4 and TLR9, the receptor for bacterial CpG DNA, have been observed in the developing mouse intestine. TLR9 signalling inhibits TLR4-mediated cell activation in an IRAK-M-dependent manner. In accordance, activation of TLR9 in a murine NEC model ameliorated the tissue damage whereas TLR9-deficient mice exhibited enhanced disease severity [125]. Furthermore, induction of proinflammatory cytokines and reactive oxygen species (ROS), generated as a result of ischemia/reperfusion injury in the gut, have been linked to the development of NEC in premature infants. An important role may be played by the proinflammatory cytokine TNF-α. Whereas TNF-α via the TNF receptor (TNFR)2 induces Muc2 and Muc3 expression by goblet cells in the mature intestinal mucosa, it causes loss of Muc2-containing goblet cells in a TNFR1-dependent manner in immature pre-weaning mice. Of note, reduced goblet cell numbers were also found in the intestinal mucosa of human infants with NEC [126]. Additionally, TNF-α stimulation induces an increase of intestinal permeability by the degradation of occludin, a component of the tight junction [127].
In both mouse and human neonates, the immune response towards microbial infection is generally reduced as compared to mature adult individuals illustrated by an enhanced susceptible to gastrointestinal infection. A number of enteropathogenic microorganisms including rotavirus, Shigella, Listeria monocytogenes and Salmonella enterica affect neonates and infants more severely.
Rotavirus infection represents one of the leading causes of dehydrating diarrhea among children worldwide with, according to the World Health Organization, approximately half a million deaths per year particularly in areas with insufficient access to medical care [128]. It mainly affects children under the age of 6 years. Similarly, the susceptibility to rotavirus is highest between days 3 and 11 after birth in mice and decreases abruptly at weaning. The age-dependent susceptibility to infection has been associated with postnatal maturation of the intestinal mucosa and can be modulated by administration of glucocorticoids, which induce premature intestinal maturation [129]. The antiviral innate immune response of the neonate intestinal mucosa largely relies on the production of type III interferon and the inhibition of viral spread at the intestinal epithelium [130]. An adaptive T cell-mediated antiviral host response, however, is required to terminate viral replication and eliminate the virus.
The ubiquitous bacterium Listeria monocytogenes represents an opportunistic human pathogen which predominantely infects immunocompromised patients, pregnant women, elderly people and neonates [131]. In a murine infection model; increased levels of systemic IL-10 were detected in neonate animals compared to adult mice. Anti-IL-10 treatment decreased the bacterial burden in neonates at early and late time points after infection whereas this treatment was only effective at early stages of infection in adult mice [132]. More recently, lack of cytotoxic T cell activation and poor IFN-γ secretion was shown in neonatal mice after intraperitoneal infection with L. monocytogenes. Reduced immune activation was correlated with low expression of the mannose-bind lectin (MBL) and PRRs such as TLRs required to mount an efficient TH1 response [133]. Due to an amino acid exchange in the mouse gene encoding epithelial E-cadherin, an important epithelial host receptor for enteric L. monoctogenes invasion, oral infection of mice requires high doses of infection [134]. Therefore, tudies comparing neonate and adult animals in humanized transgenic mice expressing human E-cadherin still need to be performed [135].
Adult mice are largely resistant to infection with Shigella flexneri, the causative agent of human bacillary dysentery characterized by an acute colonic inflammation. In contrast, newborn mice are highly susceptible and develop acute, lethal enteritis [136]. The enhanced susceptibility of neonatal mice during the first week after birth was explained by the lack of intestinal Paneth cells during early postnatal development and thus reduced production of antimicrobial peptides. Accordingly, depletion of Paneth cells in adult animals rendered adult animals susceptible to Shigella infection [137]. Also, MMP7-deficient mice unable to proteolytically process mature enteric α-defensins displayed a higher bacterial load and increased inflammation than wild-type animals after oral Shigella challenge [138].
Age-dependent differences in susceptibility to infection have also been reported for oral challenge of mice with S. Typhimurium [50, 139]. Additionally, mutant bacteria that exhibit an attenuated phenotype in adult mice are still able to infect neonatal and suckling mice systemically [139]. Neonatal mice show an attenuated inflammatory response and a higher systemic bacterial burden. IFN-γ in adult mice is required for an efficient host defence against Salmonella and the increased resistance of adult mice was correlated with an age-dependent increase of IFN-γ- and IFN-γ-regulated genes. The source of developmentally regulated IFN-γ most likely is not of epithelial nature. This cytokine, however, targets a number of cell types including epithelial cells and induces a variety of epithelial defence mechanisms against intracellular pathogens [50].
Strikingly, neonatal mice were shown to be more resistant than adult mice to an oral infection with Yersinia enterocolitica, an enteric pathogen causing gastroenteritis in humans [140]. These results differ from the situation in humans, in which two-thirds of Y. enterocolitica infections occur among infants. After oral infection, Y. enterocolitica disseminated to spleen and liver in adult mice whereas the spread to these organs was restricted in neonates. The lower bacterial load in spleen and liver of neonate mice correlated with an enhanced survival. The enhanced resistance of neonates was only observed after oral infection. Since bacterial spread to spleen and liver was largely controlled by neutrophils and the percentage of neutrophils and macrophages was increased in neonatal mesenteric lymph nodes compared to adult tissue. the authors of this study speculated that neonates maybe more resistant due their ability to rapidly mobilize innate phagocytes to the site of infection [140]. Additionally, the strong innate immune response in neonates orally infected with Y. enterocolitica promotes a robust protective CD4+ T cell-dependent immune responses [141]. It is unclear, however, whether this rapid mobilization of neutrophils is restricted to Y. enterocolitica infection and why this mechanism is not protective in other infection models.
Conclusions
Many aspects of the intestinal innate and adaptive immune system as well as the intestinal epithelial barrier undergo significant changes during the postnatal period. This includes the rate of epithelial cell proliferation, cell differentiation and gene expression, the spectrum of synthesized antimicrobial peptides, and maturation of the mucosal immune system, and also environmental factors such as bacterial colonization, nutrient composition, and exposure to immunomodulatory factors in breast milk. Whereas many adaptive changes are induced by exogenous stimuli such as the microbial colonization, developmental regulatory circuits are also involved. Together, the changes characterize a unique adaptive process that governs the transition from a sterile, environmentally protected site in utero to the situation of the adult intestine, densely populated by a highly diverse microbiota and exposed to a large variety of nutritional and environmental substrates (Fig. 1). Further characterization of the mechanisms involved will illustrate the enormous challenge of the mucosal surface to establish the delicate host–microbial interaction and unravel new factors critical to establish, but also to maintain and restore, intestinal mucosal homeostasis. Thus, the analysis of the processes that occur at the intestinal mucosa during the postnatal period might ultimately also lead to a better understanding of inflammatory diseases in the adult host and help to develop strategies to restore a beneficial homeostatic mucosal host–microbial interaction.
Fig. 1.
Summary of changes taking place in the intestine from the fetal period to adulthood
Acknowledgments
S.S. was recipient of an APART fellowship at the Institute for Medical Microbiology at Hanover Medical School. S.S. present address is: Institute of Animal Breeding and Genetics, University of Veterinary Medicine, Vienna, Austria. C.C. was supported by a post-doctoral fellowship from the Alexander Von Humboldt foundation. M.W.H. was supported by the German Research Foundation (Ho2236/5-3), the Federal Ministry of Education and Research (DLR 01GU0825) as well as the Collaborative Research Center SFB 621 and SFB900.
References
- 1.Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100(18):10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 4.Sansonetti PJ. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol. 2011;4(1):8–14. doi: 10.1038/mi.2010.77. [DOI] [PubMed] [Google Scholar]
- 5.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 6.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 7.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 8.Eberl G, Boneca IG. Bacteria and MAMP-induced morphogenesis of the immune system. Curr Opin Immunol. 2010;22(4):448–454. doi: 10.1016/j.coi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446(7135):557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 10.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446(7135):552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 11.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K, Jobin C, Ninomiya-Tsuji J. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181(2):1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson GC, Johansson ME. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1(1):51–54. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Jr, Rothenberg ME, Finkelman FD. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182(5):3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 20.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203(13):2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8(6):435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1 + dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184(4):2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 23.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORgammat + innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12(4):320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu YX, Tumanov AV. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 2010;32(3):403–413. doi: 10.1016/j.immuni.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6(5):507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 26.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117(12):3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tapiainen T, Ylitalo S, Eerola E, Uhari M. Dynamics of gut colonization and source of intestinal flora in healthy newborn infants. Apmis. 2006;114(11):812–817. doi: 10.1111/j.1600-0463.2006.apm_488.x. [DOI] [PubMed] [Google Scholar]
- 30.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91(441):48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 33.Decker E, Hornef M, Stockinger S. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Gut Microbes. 2011;2(2):91–98. doi: 10.4161/gmic.2.2.15414. [DOI] [PubMed] [Google Scholar]
- 34.Lecuit M, Abachin E, Martin A, Poyart C, Pochart P, Suarez F, Bengoufa D, Feuillard J, Lavergne A, Gordon JI, Berche P, Guillevin L, Lortholary O. Immunoproliferative small intestinal disease associated with Campylobacter jejuni . N Engl J Med. 2004;350(3):239–248. doi: 10.1056/NEJMoa031887. [DOI] [PubMed] [Google Scholar]
- 35.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(5):685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fell JM. Neonatal inflammatory intestinal diseases: necrotising enterocolitis and allergic colitis. Early Hum Dev. 2005;81(1):117–122. doi: 10.1016/j.earlhumdev.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Frank DN, Zhu W, Sartor RB, Li E (2011) Investigating the biological and clinical significance of human dysbioses. Trends Microbiol (in press) [DOI] [PMC free article] [PubMed]
- 38.Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16(3):124–130. doi: 10.1016/S0168-9525(99)01957-5. [DOI] [PubMed] [Google Scholar]
- 39.Beaulieu JF, Menard D, Calvert R. Influence of epidermal growth factor on the maturation of the fetal mouse duodenum in organ culture. J Pediatr Gastroenterol Nutr. 1985;4(3):476–481. doi: 10.1097/00005176-198506000-00026. [DOI] [PubMed] [Google Scholar]
- 40.Hirano S, Kataoka K. Histogenesis of the mouse jejunal mucosa, with special reference to proliferative cells and absorptive cells. Arch Histol Jpn. 1986;49(3):333–348. doi: 10.1679/aohc.49.333. [DOI] [PubMed] [Google Scholar]
- 41.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7(5):349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 42.Dolowschiak T, Chassin C, Ben Mkaddem S, Fuchs TM, Weiss S, Vandewalle A, Hornef MW. Potentiation of epithelial innate host responses by intercellular communication. PLoS Pathog. 2010;6(11):e1001194. doi: 10.1371/journal.ppat.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amerongen HM, Mack JA, Wilson JM, Neutra MR. Membrane domains of intestinal epithelial cells: distribution of Na+ , K+ -ATPase and the membrane skeleton in adult rat intestine during fetal development and after epithelial isolation. J Cell Biol. 1989;109(5):2129–2138. doi: 10.1083/jcb.109.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80(4):1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- 45.Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci. 2010;96:207–229. doi: 10.1016/B978-0-12-381280-3.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175(3):505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermiston ML, Green RP, Gordon JI. Chimeric-transgenic mice represent a powerful tool for studying how the proliferation and differentiation programs of intestinal epithelial cell lineages are regulated. Proc Natl Acad Sci USA. 1993;90(19):8866–8870. doi: 10.1073/pnas.90.19.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustata RC, Van Loy T, Lefort A, Libert F, Strollo S, Vassart G, Garcia MI. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 2011;12(6):558–564. doi: 10.1038/embor.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chassin C, Kocur M, Pott J, Duerr CU, Gutle D, Lotz M, Hornef MW. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8(4):358–368. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Rhee SJ, Walker WA, Cherayil BJ. Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J Immunol. 2005;175(2):1127–1136. doi: 10.4049/jimmunol.175.2.1127. [DOI] [PubMed] [Google Scholar]
- 51.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 52.Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci USA. 2011;108(26):10585–10590. doi: 10.1073/pnas.1105852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139(5):1654–1664. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H, Kredo-Russo S, Avnit-Sagi T, Cojocaru G, Zreik F, Bentwich Z, Poy MN, Artis D, Walker MD, Hornstein E, Pikarsky E, Ben-Neriah Y. Epithelial microRNAs regulate gut mucosal immunity via epithelium—T cell crosstalk. Nat Immunol. 2011;12(3):239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 55.Zeng L, Carter AD, Childs SJ. miR-145 directs intestinal maturation in zebrafish. Proc Natl Acad Sci USA. 2009;106(42):17793–17798. doi: 10.1073/pnas.0903693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hino K, Fukao T, Watanabe M (2007) Regulatory interaction of HNF1-alpha to microRNA-194 gene during intestinal epithelial cell differentiation. Nucleic Acids Symp Ser (Oxf) (51):415–416 [DOI] [PubMed]
- 57.Liao Y, Lonnerdal B. Global microRNA characterization reveals that miR-103 is involved in IGF-1 stimulated mouse intestinal cell proliferation. PLoS One. 2010;5(9):e12976. doi: 10.1371/journal.pone.0012976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Obertone TS, Sitaraman SV, Merlin D. MicroRNA-92b regulates expression of the oligopeptide transporter PepT1 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;300(1):G52–G59. doi: 10.1152/ajpgi.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Husband AJ, Gleeson M. Ontogeny of mucosal immunity—environmental and behavioural influences. Brain Behav Immun. 1996;10(3):188–204. doi: 10.1006/brbi.1996.0018. [DOI] [PubMed] [Google Scholar]
- 60.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92(2):377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor alpha + CD3(−) cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11(5):643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 62.Friedberg SH, Weissman IL. Lymphoid tissue architecture. II. Ontogeny of peripheral T and B cells in mice: evidence against Peyer’s patches as the site of generation of B cells. J Immunol. 1974;113(5):1477–1492. [PubMed] [Google Scholar]
- 63.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Invest. 2010;39(4–5):303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 64.Cummins AG, Thompson FM. Postnatal changes in mucosal immune response: a physiological perspective of breast feeding and weaning. Immunol Cell Biol. 1997;75(5):419–429. doi: 10.1038/icb.1997.67. [DOI] [PubMed] [Google Scholar]
- 65.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. “Default” generation of neonatal regulatory T cells. J Immunol. 2010;185(1):71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 67.Pabst R, Russell MW, Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 2008;29(5):206–208. doi: 10.1016/j.it.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin Rev Allergy Immunol. 2008;34(2):191–204. doi: 10.1007/s12016-007-8032-3. [DOI] [PubMed] [Google Scholar]
- 69.Minekawa R, Takeda T, Sakata M, Hayashi M, Isobe A, Yamamoto T, Tasaka K, Murata Y. Human breast milk suppresses the transcriptional regulation of IL-1beta-induced NF-kappaB signaling in human intestinal cells. Am J Physiol Cell Physiol. 2004;287(5):C1404–C1411. doi: 10.1152/ajpcell.00471.2003. [DOI] [PubMed] [Google Scholar]
- 70.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1(1):7. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, Labeta MO. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J Immunol. 2006;176(6):3742–3752. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- 72.Robinson G, Volovitz B, Passwell JH. Identification of a secretory IgA receptor on breast-milk macrophages: evidence for specific activation via these receptors. Pediatr Res. 1991;29(5):429–434. doi: 10.1203/00006450-199105010-00004. [DOI] [PubMed] [Google Scholar]
- 73.Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62(12):1297–1307. doi: 10.1007/s00018-005-5034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137(3 Suppl 2):847S–849S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- 75.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36(1):126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 76.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 77.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49(4):589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 78.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101(12):4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203(4):973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195(5):559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L, Cousart S, Hu J, McCall CE. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J Biol Chem. 2000;275(30):23340–23345. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- 82.Bens M, Bogdanova A, Cluzeaud F, Miquerol L, Kerneis S, Kraehenbuhl JP, Kahn A, Pringault E, Vandewalle A. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am J Physiol. 1996;270(6 Pt 1):C1666–C1674. doi: 10.1152/ajpcell.1996.270.6.C1666. [DOI] [PubMed] [Google Scholar]
- 83.Barbalat R, Barton GM. MicroRNAs and LPS: developing a relationship in the neonatal gut. Cell Host Microbe. 2010;8(4):303–304. doi: 10.1016/j.chom.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Leavy O. (micro)Tolerance in the gut. Nat Rev Immunol. 2010;10(12):810. doi: 10.1038/nri2894. [DOI] [PubMed] [Google Scholar]
- 85.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208(6):1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mege JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis. 2011;24(3):230–234. doi: 10.1097/QCO.0b013e328344b73e. [DOI] [PubMed] [Google Scholar]
- 88.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 89.Hornef MW, Putsep K, Karlsson J, Refai E, Andersson M. Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nat Immunol. 2004;5(8):836–843. doi: 10.1038/ni1094. [DOI] [PubMed] [Google Scholar]
- 90.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 91.Narushima Y, Unno M, Nakagawara K, Mori M, Miyashita H, Suzuki Y, Noguchi N, Takasawa S, Kumagai T, Yonekura H, Okamoto H. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII alpha, RegIII beta, RegIII gamma. Gene. 1997;185(2):159–168. doi: 10.1016/S0378-1119(96)00589-6. [DOI] [PubMed] [Google Scholar]
- 92.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19(2):70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286(5437):113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 94.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422(6931):522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 95.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204(8):1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukherjee S, Partch CL, Lehotzky RE, Whitham CV, Chu H, Bevins CL, Gardner KH, Hooper LV. Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem. 2009;284(8):4881–4888. doi: 10.1074/jbc.M808077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehotzky RE, Partch CL, Mukherjee S, Cash HL, Goldman WE, Gardner KH, Hooper LV. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc Natl Acad Sci USA. 2010;107(17):7722–7727. doi: 10.1073/pnas.0909449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30(3):131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calvert R, Pothier P. Migration of fetal intestinal intervillous cells in neonatal mice. Anat Rec. 1990;227(2):199–206. doi: 10.1002/ar.1092270208. [DOI] [PubMed] [Google Scholar]
- 101.Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci USA. 1994;91(22):10335–10339. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Putsep K, Axelsson LG, Boman A, Midtvedt T, Normark S, Boman HG, Andersson M. Germ-free and colonized mice generate the same products from enteric prodefensins. J Biol Chem. 2000;275(51):40478–40482. doi: 10.1074/jbc.M007816200. [DOI] [PubMed] [Google Scholar]
- 103.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 104.Darmoul D, Brown D, Selsted ME, Ouellette AJ. Cryptdin gene expression in developing mouse small intestine. Am J Physiol. 1997;272(11):G197–G206. doi: 10.1152/ajpgi.1997.272.1.G197. [DOI] [PubMed] [Google Scholar]
- 105.Menard S, Forster V, Lotz M, Gutle D, Duerr CU, Gallo RL, Henriques-Normark B, Putsep K, Andersson M, Glocker EO, Hornef MW. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. 2008;205(1):183–193. doi: 10.1084/jem.20071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue R, Tsuruta T, Nojima I, Nakayama K, Tsukahara T, Yajima T. Postnatal changes in the expression of genes for cryptdins 1–6 and the role of luminal bacteria in cryptdin gene expression in mouse small intestine. FEMS Immunol Med Microbiol. 2008;52(3):407–416. doi: 10.1111/j.1574-695X.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- 107.Ouellette AJ, Greco RM, James M, Frederick D, Naftilan J, Fallon JT. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1(3):183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schaedler RW, Dubos R, Costello R. The development of the bacterial flora in the gastrointestinal tract of mice. J Exp Med. 1965;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savage DC, Dubos R, Schaedler RW. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis CP, McAllister JS, Savage DC. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973;7(4):666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirayama K, Miyaji K, Kawamura S, Itoh K, Takahashi E, Mitsuoka T. Development of intestinal flora of human-flora-associated (HFA) mice in the intestine of their offspring. Exp Anim. 1995;44(3):219–222. doi: 10.1538/expanim.44.219. [DOI] [PubMed] [Google Scholar]
- 113.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98(2):229–238. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 114.Rook GA. Hygiene and other early childhood influences on the subsequent function of the immune system. Dig Dis. 2011;29(2):144–153. doi: 10.1159/000323877. [DOI] [PubMed] [Google Scholar]
- 115.Hascoet JM, Hubert C, Rochat F, Legagneur H, Gaga S, Emady-Azar S, Steenhout PG. Effect of formula composition on the development of infant gut microbiota. J Pediatr Gastroenterol Nutr. 2011;52(6):756–762. doi: 10.1097/MPG.0b013e3182105850. [DOI] [PubMed] [Google Scholar]
- 116.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inoue R, Otsuka M, Ushida K. Development of intestinal microbiota in mice and its possible interaction with the evolution of luminal IgA in the intestine. Exp Anim. 2005;54(5):437–445. doi: 10.1538/expanim.54.437. [DOI] [PubMed] [Google Scholar]
- 118.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99(24):15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacDonald TT, Gordon JN. Bacterial regulation of intestinal immune responses. Gastroenterol Clin North Am. 2005;34(3):401–412. doi: 10.1016/j.gtc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 121.Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, Backhed F. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51(5):1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neu J, Chen M, Beierle E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14(3):137–144. doi: 10.1053/j.sempedsurg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 124.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177(5):3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182(1):636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McElroy SJ, Prince LS, Weitkamp JH, Reese J, Slaughter JC, Polk DB (2011) Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol (in press) [DOI] [PMC free article] [PubMed]
- 127.Ye D, Guo S, Al-Sadi R, Ma TY (2011) MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology (in press) [DOI] [PMC free article] [PubMed]
- 128.WHO . Global deaths under age five attributable to rotavirus infection. Geneva: World Health Organization; 2004. [Google Scholar]
- 129.Wolf JL, Cukor G, Blacklow NR, Dambrauskas R, Trier JS. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect Immun. 1981;33(2):565–574. doi: 10.1128/iai.33.2.565-574.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA. 2011;108(19):7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Genovese F, Mancuso G, Cuzzola M, Biondo C, Beninati C, Delfino D, Teti G. Role of IL-10 in a neonatal mouse listeriosis model. J Immunol. 1999;163(5):2777–2782. [PubMed] [Google Scholar]
- 133.Byun HJ, Jung WW, Lee JB, Chung HY, Sul D, Kim SJ, Park CG, Choi I, Hwang KW, Chun T. An evaluation of the neonatal immune system using a listeria infection model. Neonatology. 2007;92(2):83–90. doi: 10.1159/000100806. [DOI] [PubMed] [Google Scholar]
- 134.Bonazzi M, Lecuit M, Cossart P. Listeria monocytogenes internalin and E-cadherin: from structure to pathogenesis. Cell Microbiol. 2009;11(5):693–702. doi: 10.1111/j.1462-5822.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 135.Lecuit M, Vandormael-Pournin S, Lefort J, Huerre M, Gounon P, Dupuy C, Babinet C, Cossart P. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 2001;292(5522):1722–1725. doi: 10.1126/science.1059852. [DOI] [PubMed] [Google Scholar]
- 136.Fernandez MI, Thuizat A, Pedron T, Neutra M, Phalipon A, Sansonetti PJ. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 2003;5(7):481–491. doi: 10.1046/j.1462-5822.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 137.Fernandez MI, Regnault B, Mulet C, Tanguy M, Jay P, Sansonetti PJ, Pedron T. Maturation of paneth cells induces the refractory state of newborn mice to Shigella infection. J Immunol. 2008;180(7):4924–4930. doi: 10.4049/jimmunol.180.7.4924. [DOI] [PubMed] [Google Scholar]
- 138.Shim DH, Ryu S, Kweon MN. Defensins play a crucial role in protecting mice against oral Shigella flexneri infection. Biochem Biophys Res Commun. 2010;401(4):554–560. doi: 10.1016/j.bbrc.2010.09.100. [DOI] [PubMed] [Google Scholar]
- 139.Burns-Guydish SM, Olomu IN, Zhao H, Wong RJ, Stevenson DK, Contag CH. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr Res. 2005;58(1):153–158. doi: 10.1203/01.PDR.0000157725.44213.C4. [DOI] [PubMed] [Google Scholar]
- 140.Echeverry A, Schesser K, Adkins B. Murine neonates are highly resistant to Yersinia enterocolitica following orogastric exposure. Infect Immun. 2007;75(5):2234–2243. doi: 10.1128/IAI.01681-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Echeverry A, Saijo S, Schesser K, Adkins B. Yersinia enterocolitica promotes robust mucosal inflammatory T-cell immunity in murine neonates. Infect Immun. 2010;78(8):3595–3608. doi: 10.1128/IAI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]