Abstract
Antimicrobial agents are toxic to bacteria by a variety of mechanisms. One mechanism that is very dependent on the lipid composition of the bacterial membrane is the clustering of anionic lipid by cationic antimicrobial agents. Certain species of oligo-acyl-lysine (OAK) antimicrobial agents are particularly effective in clustering anionic lipids in mixtures mimicking the composition of bacterial membranes. The clustering of anionic lipids by certain cationic antimicrobial agents contributes to the anti-bacterial action of these agents. Bacterial membrane lipids are a determining factor, resulting in some species of bacteria being more susceptible than others. In addition, lipids can be used to increase the effectiveness of antimicrobial agents when administered in vivo. Therefore, we review some of the structures in which lipid mixtures can assemble, to more effectively be utilized as antimicrobial delivery systems. We describe in more detail the complexes formed between mixtures of lipids mimicking bacterial membranes and an OAK and their usefulness in synergizing with antibiotics to overcome bacterial multidrug resistance.
Keywords: Antimicrobial peptides, Drug delivery, Synergistic action, Cochleates, Multidrug resistance
Introduction
There is considerable interest in developing new strategies to combat bacterial infections because of the increasing occurrence of clinical cases caused by drug-resistant bacteria for which traditional antibiotic therapy becomes ineffective [1]. An important aspect of the effort to develop novel antimicrobial strategies is the involvement of lipids in several different contexts. It has long been recognized that lipids play a role in the selectivity of cationic antimicrobial agents for bacteria over eukaryotic cells as the result of the attraction to the exposed anionic lipids present on the surface of bacteria, but not in eukaryotic membranes. In addition, the lipid composition of bacterial membranes varies widely, allowing for the development of agents with selectivity against certain species of bacteria and not others [2, 3]. Many of the mechanisms of action of antimicrobial agents occur at the membrane and involve membrane lipids. Membranes are also important for agents that act intracellularly since they have to first enter the cell across the membrane. Finally, various forms of complexes of antimicrobial agents with lipids or with these drugs entrapped in lipid structures may be used for drug delivery. The present review will consider the role of microbial membrane lipids in the action of antimicrobial agents as well as discuss the potential of using systems composed of antimicrobial agents with lipid mixtures for drug delivery. We will place particular emphasis on the newly discovered role of cochleates in causing a synergistic action between a class of cationic antimicrobial agents, the OAKs (see Fig. 1 for OAK structures), and certain antibiotics in overcoming bacterial multidrug resistance.
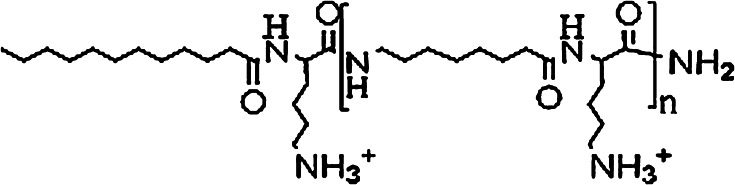
Fig. 1.
General structure of oligo-acyl-lysines (OAK). The value of n varies. Most of the studies discussed here were done with OAKs having n = 4–12
Antimicrobial agents
There is considerable interest in developing therapeutic agents that can be used clinically against bacterial infections, particularly in cases in which traditional antibiotics have lost their efficacy. There have been many approaches to this problem but one of the most actively studied is the use of antimicrobial peptides [1, 4]. Peptides used for this purpose range from host defense peptides that are found in most organisms, to designed peptides with various degrees of variation from known structures. Peptides have certain advantages for use as therapeutic agents. They offer a large range of structures, generally have a low toxicity, and are relatively inexpensive to produce. Of course, part of the reason for the low toxicity is that they tend to be rapidly degraded by proteolysis after administration, which also results in lowering their efficacy and increasing the difficulty of successfully regulating the oral/systemic delivery of peptides.
It is not surprising that such a large and diverse group of peptides also has several mechanisms by which they exert their antimicrobial action. With few exceptions, these peptides are not as potent as classical antibiotics and exhibit their antimicrobial effects at micromolar concentrations. In addition, the action of these agents is not lost by small changes in their structure. Thus, antimicrobial peptides generally have a relatively low potency and specificity and likely function by more than one mechanism. Despite this lack of specific targeting, antimicrobial peptides generally have in common that they interact with the membranes of bacteria.
There are two different ways in which the membranes of bacteria are involved in the action of antimicrobial agents. The one mechanism that has received the most attention is one where the antimicrobial agent breaks the permeability barrier provided by the membrane and allows leakage of polar materials across the membrane. Several molecular mechanisms have been proposed for this process that has been extensively discussed. These mechanisms include the formation of pores [5] lined by both lipids and peptides [6] or by a more general "carpet mechanism" [7], but other specific mechanisms have also been suggested [8–12]. This breaching of the membrane barrier may involve small perturbations that simply result in the loss of the large electrochemical gradient that exists across the bacterial membrane or it may result in the uncontrolled movement of larger molecules with more extensive membrane damage.
Antimicrobial agents can also pass through the bacterial membrane and interact with an intracellular target. Several antimicrobial peptides are known to interact with DNA, leading to a cytotoxic action [13–15]. This is not surprising since these peptides carry multiple positive charges and the nucleic acid is polyanionic.
Interaction of antimicrobial agents with lipids
Importance of charge
It has long been recognized that most, although not all, antimicrobial peptides are cationic. This property has been suggested to contribute to the specificity of these agents for targeting to bacteria since their membranes are enriched in exposed negatively charged lipids. In comparison, most of the anionic lipids in the plasma membranes of eukaryotic cells are sequestered to the cytoplasmic surface. This would provide cationic antimicrobial peptides with a degree of therapeutic efficacy because when administered to humans they would be selectively toxic to bacteria. It has been recently pointed out that this apparent selectivity for bacteria is also a consequence of the larger amount of mammalian membranes used for the assays, resulting in a greater dilution of the antimicrobial agent in the mammalian compared with the bacterial membrane [16].
Anionic lipid clustering: relationship to cochleate formation
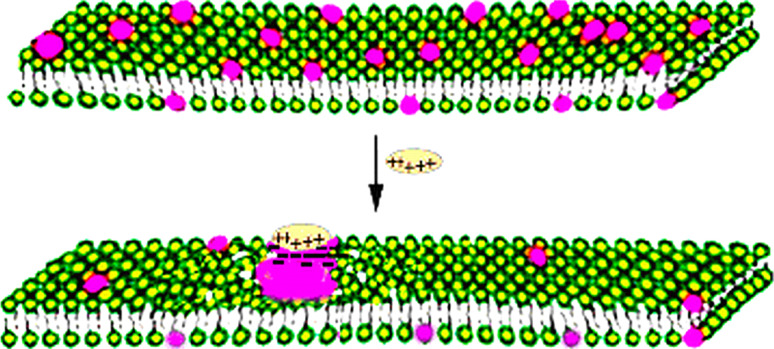
The preferential interaction of cationic antimicrobial peptides with anionic lipids can result in the segregation of anionic lipids into a membrane domain from bilayers containing both anionic and zwitterionic lipid (Fig. 2). This phenomenon can be clearly shown in membranes mimicking the lipid composition of typical Gram-negative bacteria, as has been demonstrated with homologous antimicrobial cationic arginine-rich nonapeptides [17], with some antimicrobial amphipathic cationic peptides [18], as well as with oligo-acyl-lysines [19], and has been recently reviewed [2, 3]. Although there is evidence that cationic antimicrobial peptides can segregate two different anionic lipids, when this clustering occurs in bacterial membranes that contain both anionic and zwitterionic lipid, the clustering of anionic lipids appears to be an important factor in determining the toxicity of selective antimicrobial agents to certain species of bacteria. In addition, in an attempt to visualize the reordering of the membrane by freeze-fracture electron microscopy, it was observed that some antimicrobial agents with a strong capacity to cluster anionic lipids in mixtures with zwitterionic lipids, can also form cochleate structures (see below), which can be exploited for drug delivery. However, not all antimicrobial peptides that cluster anionic lipids can form cochleates, as many factors enter into the requirement for cochleate formation [20]. The relationship between anionic lipid clustering by one specific OAK, the C12K-7α8, in lipid mixtures mimicking the composition of bacterial membranes, and the formation of cochleates that have in vivo biological activity, has been recently studied (Sarig et al., manuscript in preparation).
Fig. 2.
Clustering of anionic lipid in a bilayer composed of anionic (red headgroups) and zwitterionic lipids (green headgroups) in the presence of a cationic agent, causes defects in the surrounding area of the bilayer, increasing permeability
Relationship to bacterial species specificity of toxicity
The clustering of anionic lipids in the membranes of bacteria that contain both zwitterionic as well as anionic lipids will be more sensitive to agents that act by this clustering mechanism than for bacterial membranes that are comprised predominantly of anionic lipids. For many bacteria, this difference in lipid composition also correlates with the bacteria being either Gram-positive or Gram-negative. Many Gram-positive bacteria contain mostly anionic lipids and are more resistant to the action of agents that can cluster anionic lipids away from zwitterionic ones. In contrast, almost all Gram-negative bacteria have a high content of the zwitterionic lipid, phosphatidylethanolamine (PE). There are, however, exceptions to this generalization. Gram-positive bacteria from the genus Bacillus and from some members of the genus Clostridium have a higher content of zwitterionic PE, while the Gram-negative bacterial species Caulobacter crescentus has little if any PE. PE is not the only bacterial lipid present that is not anionic. Several bacterial species have a significant content of other zwitterionic or uncharged lipids. Gram-positive bacteria have a thick outer peptidoglycans layer with lipoteichoic acid as the main constituent. The membrane anchor of the lipoteichoic acid is glucosyl diacylglycerol but by being incorporated into the lipoteichoic acid results in glucosyl diacylglycerol not behaving as an uncharged lipid component of the bacterial membrane. However, the species S. pyogenes has a high content in its membrane of glucosyl diacylglycerols, a fraction of which is not incorporated into lipoteichoic acid and acts as an uncharged lipid, making this species susceptible to agents that cluster anionic lipids in the presence of zwitterionic or uncharged lipids [17].
In addition to lipids common to most bacterial species, some bacteria have other lipids including cationic lysyl-phosphatidylglycerol, zwitterionic aminoacyl-phosphatidylglycerol, as well as zwitterionic lysyl-cardiolipin. The most common of these lipid species is lysyl-phosphatidylglycerol, which is a cationic lipid. At higher concentrations, such cationic lipids will prevent the binding of cationic antimicrobial agents to the membrane because of electrostatic repulsion and hence this will make the bacteria more resistant. This is a known mechanism of acquired resistance of S. aureus [21, 22]. However, in non-resistant strains of S. aureus, most of the lysyl-phosphatidylglycerol is found on the cytoplasmic surface of the bacterial membrane. Resistance to antimicrobial agents occurs only in those strains of S. aureus in which a protein facilitates the translocation of this lipid to the cell exterior [23, 24]. Although the membrane sidedness of lysyl-phosphatidylglycerol in S. aureus has been determined, in general, there is little information about the sidedness of membrane lipids in bacteria.
In the case of Gram-negative bacteria, antimicrobial agents have to pass through the outer membrane in order to access the cytoplasmic bacterial membrane. Outer membrane permeability can be directly tested using E. coli ML-35p, a strain that was engineered specifically to simultaneously probe penetration across the inner and outer membranes of Gram-negative bacteria [25]. Some antimicrobial peptides that can cluster anionic lipids are less toxic than expected against certain strains of Gram-negative bacteria [17] even though these peptides are capable of clustering anionic lipids. One reason for this appears to be the inability of less hydrophobic agents to penetrate the outer membrane of Gram-negative bacteria.
Anionic lipid clustering is a feature of some cationic antimicrobial agents [2, 3]. Among Gram-positive bacteria, such agents will be most toxic to species that contain significant quantities of neutral or zwitterionic lipids in the membrane. Generally, these agents are expected to be more toxic to Gram-negative than Gram-positive bacteria, since most species of Gram-negative bacteria have high concentrations of the zwitterionic lipid PE, provided that these agents are capable of penetrating the outer membrane. Since infections with Gram-negative bacteria are becoming an increasingly important clinical problem [26, 27], focus on lipid clustering can specifically contribute to developing new strategies to counter this serious clinical problem.
Use in drug delivery
Several systems have been explored for the purpose of entrapping drugs in nanoparticles to delay their degradation after administration as well as to increase their delivery to target cells or organs [28]. One of the most active efforts is in the use of lipids in forming drug-containing nanoparticles for drug delivery. There are several forms that these particles can take.
Liposomes
The most extensively studied lipid nanoparticle for drug delivery is the liposome. Liposomes have the advantage that they entrap drugs using phospholipids that have a low toxicity and are eventually degraded in the body. For drug delivery, liposomes are made as unilamellar vesicles, spherical particles composed of a shell of phospholipid and an aqueous interior. The drug is generally entrapped in the aqueous compartment inside the liposomes and is therefore protected from external degradation mechanisms. There are also methods to concentrate the drug within liposomes, for example with the use of pH gradients [29]. However, some more hydrophobic drugs likely also partition into the surrounding phospholipid membrane. This membrane can also contain a targeting ligand such as growth factors, hormones, toxins, antibodies, or cytokines. In addition, polyethyleneglycol linked to PE (PEG-PE) can be incorporated into the bilayer. PEG-PE is a lipid anchored hydrophilic flexible polymer that has been shown to reduce the clearance of liposomes by the reticuloendothelial system. Liposomes with PEG-PE have been called "stealth liposomes" because of their ability to avoid clearance from circulation [30, 31]. They have recently been discussed as potential vehicles for delivering anti-cancer drugs [32]. However, the effective increase in circulation time of these stealth liposomes does not always result in more drug delivery to target tumors [33]. Liposome drug delivery is also being investigated as a mechanism to pass the blood–brain barrier [34].
Cochleates
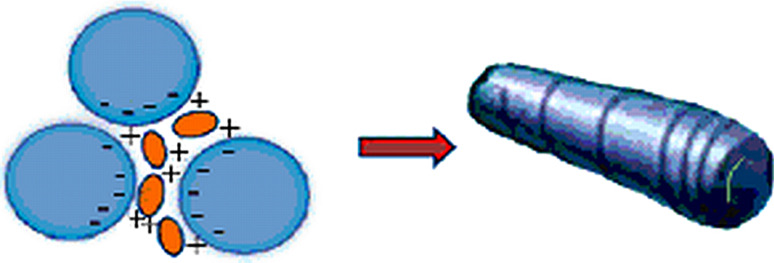
Cochleates are cylinders made of continuous solid bilayer sheets rolled up in multilayered spiral structures, consisting of anionic lipid molecules bridged by divalent or polyvalent cations (Fig. 3). Most of the cochleates studied up to the present time were structures formed mainly from phosphatidylserine (PS) and Ca2+. These Ca–PS cochleates revert back to liposomes upon addition of EDTA.
Fig. 3.
Some cationic antimicrobial agents can bridge anionic lipids, thereby functioning effectively to promote the formation of rolled-up lipidic sheets or cochleates, as divalent cations do. Cationic OAKs can bridge multiple liposomes, in this case MLVs, leading to the formation of cochleates, much like when Ca2+ is added to PS
The cylindrical spiral lipid structures of cochleates were observed by freeze-etch electron microscopy by Verkleij et al. in 1974 [35] using dilauryl phosphatidylglycerol, when Ca2+ was added. They were also observed in the presence of Mg2+ [36, 37]. Fusion of unilamellar PS vesicles with Ca2+ was observed in 1974 by negative staining electron microscopy [38] and in 1975, the fusion of sonicated PS vesicles with Ca2+ to form rolled-up cylindrical structures was observed by freeze-fracture electron microscopy [39] and were called cochleate cylinders, from the Greek meaning "snail with spiral shell". Subsequently, lipid suspensions of PS or other anionic lipids and divalent cations were studied by several techniques in numerous laboratories. The ability of these structures to entrap solutes represented a new platform for studies regarding their capacity to deliver therapeutic agents.
Vesosomes
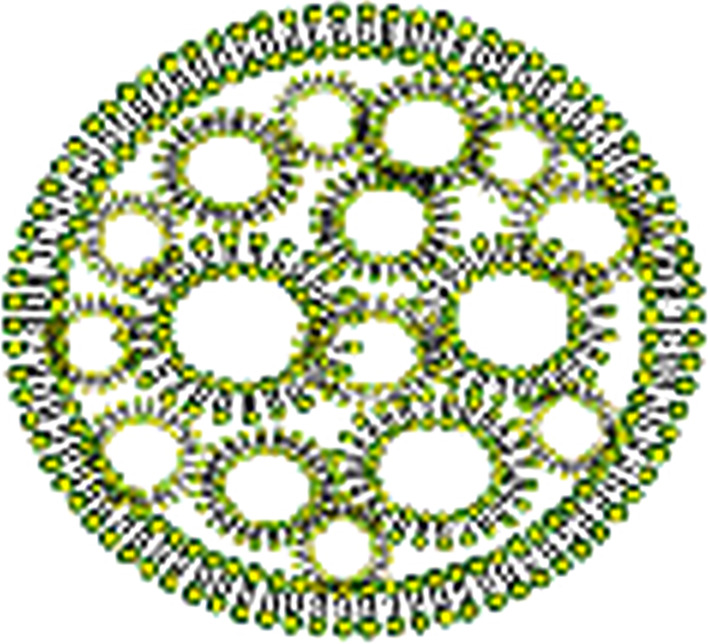
Vesosomes are unique structures comprised of unilamellar liposomes entrapped within an outer membrane, much like a eukaryotic cell containing multiple organelles [40, 41]. This morphology results in two membrane bilayers separating any drug entrapped within a liposome and the outside solution. As a consequence of this double membrane barrier, the interior membranes are protected from degradation by lipases and drug release is reduced by two orders of magnitude, resulting in increased drug retention from minutes to hours [42] (Fig. 4). Vesosomes have been produced by forming tightly packed cochleates upon addition of Ca2+ to a high concentration of unilamellar liposomes of dioleoyl phosphatidylserine (DOPS), causing a number of liposomes, often aggregated, to coexist with the newly formed cochleates; addition of excess EDTA causes cochleates to unravel, and unfavorable excess edge energy is eliminated by closure of the sheets back into spheres trapping some of the unilamellar liposomes inside [40, 41]. They have also been produced by use of the ethanol-induced interdigitation of saturated phospahtidylcholine (PC) bilayers below the main phase transition, i.e., membranes in the Lβ′ phase, which increases membrane rigidity, with the formation of bilayer sheets; by raising the temperature above the phase transition the acyl chains melt and the membrane reverts to a disordered bilayer phase (Lα), with reduced bending rigidity, allowing the bilayers to close, trapping small vesicles and/or nanoparticles [42]. Vesosomes themselves have not been extensively studied, but they may have a role as an intermediate in cochleate drug delivery, since these structures can arise spontaneously during the breakdown of cochleates or the fusion of liposomes with cochleates.
Fig. 4.
Structure of a vesosome. One of the ways in which vesosomes can form is when cochleates bind to liposomal aggregates, resulting in the opening up of the rolled-up sheets and the formation of an outer membrane, which entraps the aggregated liposomes
Cochleate formation
Methods of cochleate preparation, particularly those formed with PS and Ca2+, have already been discussed in published reviews [43, 44]. Up to the present time, focus has been on the preparation of small or nanocochleates for drug delivery by the trapping method, the solvent-drip method, the dialysis method, or the hydrogel-isolation method, containing PS and Ca2+. Recently, rapid formation of cochleates from multilamellar vesicles (MLVs) composed of zwitterionic and anionic lipids mixed with antimicrobial OAKs by simply adding a solution of peptide to a suspension of lipid mixtures in the form of MLVs, has been reported [45]. The biophysical characteristics of these systems were subsequently studied in detail [20].
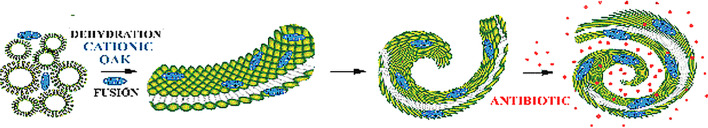
Charge neutralization of anionic lipids by divalent or multivalent cationic hydrophobic compounds to form multilayered systems, with dehydration at the membrane surface to allow close approach of bilayers, followed by membrane fusion leading to formation of rolled-up sheets, are the dominant processes that have been identified in cochleate assembly. Cochleates made with PS and Ca2+ are examples of tightly rolled-up structures with little or no aqueous interior space. Other cations, like Mg2+ for example, or cationic antimicrobial OAKs, cause water entrapment into the structure leading to the formation of larger-size cochleates in some systems [20, 45, 46]. Cochleate structures made with PS and Ca2+ revert back to liposomes upon addition of EDTA. Addition of high concentrations of EDTA produces an osmotic effect, disrupting cochleate structures. Cations can also be replaced by multicationic substances as bridging agents. The combined ability to revert back to liposomes when the bridging agent is removed that can entrap aqueous solutes and their capacity to form highly curved membrane fusion intermediates leading to rolled-up cylindrical structures, are the foundations for their ability to function as drug delivery agents (Fig. 5).
Fig. 5.
Cationic antimicrobial s02t1ructures like OAKs bind to the anionic lipids in MLVs. Binding to negatively charged lipids leads to the bridging of bilayers, eliminating electrostatic repulsion. Fusion of the bridged layers leads to formation of progressively bent bilayer sheets as fusion intermediates, finally achieving a rolled-up cylindrical state. OAK cochleates are not as tightly rolled up as Ca2+ bridged cochleates of PS and they allow for efficient entrapment of molecules like the antibiotic erythromycin, which can be effectively delivered in systemic therapy in vivo
Methods to identify and characterize cochleates
Freeze-fracture electron microscopy remains the method of choice for visualizing cochleates, as well as identifying the presence of other structures in the sample, particularly non-bilayer structures [39, 47, 48]. Cryo-EM is also a powerful technique for visualizing these assemblies, but although it is of higher resolution than freeze-fracture-EM and allows for the monitoring of the inner volume, it is restricted to smaller structures. In addition, the required concentration of dispersions and sample deterioration from the electron beam are intrinsic complications of the method [49]. Other techniques have also been employed successfully in the identification and characterization of cochleates. Dynamic light scattering was used [50], mostly to determine cochleate particle size distribution [51]. Scanning electron microscopy has also been employed to visualize these structures [51]. Confocal fluorescence microscopy has been used to visualize binding and transfer of fluorescent lipids from cochleates to membranes and fusion with cell membranes [43, 51]. Light microscopy shows different patterns depending on the molecule bridging liposomes [20]. FT-IR spectroscopy was used to identify an absorption band characteristic for cochleate phases made with PS and Ca2+, which undergoes an 8 cm−1 increase upon cochleate formation, from 1,386 cm−1 to 1,344 cm−1 [46]. Laurdan fluorescence has also been developed to successfully characterize cochleates [45, 52].
Pharmacological applications of cochleates
One of the main applications of cochleate formation has been their use as adjuvants to enhance the immune response. Cochleate-based vaccine research involved studying circulating antibody responses to PS–Ca2+ cochleates as well as their effectiveness in mucosal and systemic immunization [53]. PS–Ca2+ cochleates loaded with amphotericin B were successfully tested against Leishmania [41]; they also showed antifungal activity against Candida albicans in a mouse model [54, 55]. Development of vaccine adjuvants using cochleates was carried out with proteoliposomes obtained from N. meningitis B by extraction with Na deoxycholate [56, 57]. The proteoliposomes contained lipopolysaccharide (LPS) and all the bacterial outer membrane proteins inserted into the phospholipid structure. They were then transformed into cochleates by detergent elimination in the presence of Ca2+. These cochleates were potent adjuvants for parenteral and mucosal administration of vaccines, particularly nasal [58, 59]. The same system was also used to encapsulate recombinant glycoprotein D from herpes simplex virus type 2, for induction of protective immunity against genital herpes simplex infections in mice [60]. Proteoliposomes from Vibrio colerae O1 were also prepared with the same procedure to produce cochleates for oral administration against cholera, resulting in mucosal and systemic immune responses in mice [61].
Other applications were in the area of drug delivery. Amphotericin B is an amphipathic molecule insoluble in water, but mixes well with lipids. Fungizone, a commercial micellar antifungal (amphotericin B in deoxycholate), is very toxic and highly hemolytic. Liposome-based formulations (like Ambisone) were developed to reduce toxicity, but required much higher doses than micellar ones, and were unstable and expensive. PS–Ca2+ cochleates on the other hand were found to be nonhemolytic, stable, and were almost equally effective in inhibiting the growth of C. albicans cells in vivo as liposomally entrapped amphotericin B [55]. Delivery of genes to mammalian cells using cochleates [62] was attempted in 2004 by encapsulating GFP expression plasmids in rhodamine labeled PS–Ca2+ cochleates and visualizing cellular uptake in 4T1 adenocarcinoma cells and H36.12 macrophage hybridoma; cochleates were found to be an effective, non-toxic and non-immunogenic method to introduce transgenes in vivo and in vitro. Cochleates made of brain PS and Ca2+ encapsulating recombinant Factor VIII (deficiency in Factor VIII causes hemophilia) were effective in protecting mice from bleeding upon systemic exposure [63]. The antimicrobial activity of DOPS nanocochleates prepared by the hydrogel-isolation method (HIC) from dextran/PEG emulsions, in the presence of tobramycin, a pentacationic antibiotic which targets ribosomes, was tested in E. coli to assess their antimicrobial effectiveness compared to the antibiotic in solution and found this to be concentration dependent, with nanocochleates requiring higher concentrations [51].
Recently, we showed that antimicrobial polycationic OAKs in multilamellar mixtures of zwitterionic and anionic lipids formed cochleates. These could overcome drug resistance in bacteria when OAKs were coencapsulated with erythromycin and administered to neutropenic mice infected with a Multidrug Resistant (MDR) strain of E. coli [45]. This will be discussed below.
In 1997 the binding and transfer of fluorescent lipids from PS–Ca2+ cochleates to plasma and intracellular membranes of white blood cells was demonstrated [59]. Uptake of cochleates by macrophages was demonstrated with empty rhodamine labeled PS–Ca2+ cochleates and rhodamine labeled amphotericin B loaded cochleates, incubated with J774 macrophages by fluorescence microscopy and confocal laser scanning microscopy [43]. More recently, fusion with human fibroblast cells was demonstrated by incubation with nanocochleates based on the fluorescent lipid oleoyl(NBD)-hexanoyl PC added in small amounts to DOPS [51]. Cells treated with these nanocochleates exhibited a brighter contour under the fluorescent microscope than similarly treated liposomes. The line tension at the bilayer edges of the rolled-up sheets in cochleates, as opposed to the edge free spherical liposomes, was suggested to be the driving force for cochleate-membrane fusion.
Although the mechanism of drug release in cells by cochleates is not exactly known, it has been hypothesized that either entrapped molecules are released more slowly in oral delivery or, alternatively, that fusion with the membrane of cells delivers entrapped material to the cytoplasm [44]. On the other hand, liposomes, with aqueous space inside, showed poor drug encapsulating capability, poor drug retention, low stability, lack of cross-membrane diffusion in the gut. To improve stability and retention of entrapped material during circulation, derivatized liposomes were developed in recent years, which show greater promise in drug delivery and have superseded the use of cochleation.
In the example given above, with amphotericin B drug delivery as liposomes or cochleates in the mouse model of systemic candidiasis, entrapped cochleates required a slightly lower dose than entrapped liposomes, and both were more efficacious than micellar entrapment in producing a systemic effect [55].
OAK-lipid based cochleates with lipid mixtures: new strategies for drug delivery
A member of the family of oligo-acyl-lysyl (OAK), C12K-7α8, is particularly potent in clustering anionic lipids in mixtures of anionic-zwitterionic lipids and their toxicity against different species of bacteria correlates well with the lipid composition of these bacteria, independent of the presence of an outer membrane in Gram-negative bacteria [19]. This is likely to be the consequence of a combined high density of cationic charges together with a high degree of hydrophobicity. This same OAK, C12K-7α8, is also effective in forming cochleates [20, 45] and therefore it is pertinent to discuss here cochleate formation by OAKS and the relative importance of those factors which lead to cochleate formation.
Unique properties of cochleates formed with OAKs
There is particular interest in the study of cochleates made from OAKs and phospholipid mixtures since these complexes can be assembled rapidly and in the absence of divalent cations, thus also allowing replacement of PS by other lipids including mixtures containing zwitterionic–anionic or anionic–anionic lipids, by simply mixing multilamellar liposomes with OAKs at physiological pH and temperatures. In the type of cochleate we describe, addition of polyethylene glycol, dextran, or polymers to form hydrogels or emulsions, as well as reconstitution from detergents, are not required for their assembly.
Cochleates formed from C12K-7α8 and the lipid mixture 1-palmitoyl-2-oleoyl phosphatidylethanolamine (POPE): tetraoleoyl cardiolipin (TOCL) 75:25, which mimics the composition of Gram-negative bacteria, showed an increase in therapeutic efficacy when coadministered as cochleates together with erythromycin [45]. Systemic treatment of neutropenic mice infected with lethal inoculums of multidrug resistant E. coli and treated by a single intravenous dose of the OAK-phospholipid cochleate with entrapped erythromycin increased mice survival in a dose-dependent manner [45]. Unlike individual treatments with free erythromycin or cochleated OAK, the co-encapsulation of erythromycin in OAK-based cochleates can decrease drug toxicity and increase systemic therapeutic efficacy by orders of magnitude. We have followed up this initial observation with further studies comparing the protective effect of a combination of erythromycin and the OAK, C12K-7α8 in the presence and absence of various lipids using the model of neutropenic mice infected with lethal inoculums of multidrug resistant E. coli (Sarig, Ohana, Epand, Mor and Epand, manuscript in preparation). We demonstrated that several cochleate preparations that were shown to entrap erythromycin in the presence of this OAK, allowed for a much greater survival rate compared with erythromycin and the OAK free in solution (see Sec. “Lipid requirements for drug delivery”).
Dependence of efficiency of formation on OAK structure
The structure of the OAK is important for the formation of cochleates since it is necessary to achieve a balance between charge and hydrophobicity. Lengthening the chain from C12K-7α4 to C12K-7α8 improved cochleate formation, but further lengthening to C12K-7α12 results in the opposite effect (Table 1). This biphasic behavior is a consequence of increased hydrophobicity favoring cochleate assembly, but extending the distance between cationic groups disfavors it. A chain length of 8–9 carbon atoms is most favorable to increase the probability of assembly into rolled cylindrical structures.
Table 1.
Comparison of cochleate assembly ability for OAKs with the same number of acyl-lysyl residues separated by different chain lengths
| Lipid mixtures (L/P = 10) | C12K-7α4 | C12K-7α8 | C12K-7α12 |
|---|---|---|---|
| POPE:TOCL 75:25 | G | G | N |
| DMPE:TOCL 75:25 | G | E | P |
Letters indicate the relative number of cochleates observed by microscopy
N none, P poor, G good, E excellent
Lipid requirements for the formation of OAK cochleates
Cochleates are not formed by all lipid mixtures and OAKs. However, they do form with many different lipid mixtures, which should allow optimization of their biological and pharmacological characteristics. One feature to consider is intrinsic monolayer curvature, since the morphology of the lipids changes from a flat structure to a curved one in the process of cochleate formation. We have observed [20] that the dimyristoyl phosphatidylcholine (DMPC):TOCL mixture, that has less negative curvature tendency than dimyristoyl phosphatidylethanolamine (DMPE):TOCL (PC headgroup larger than PE), is a poorer cochleate former. However, dioleoyl phosphatidylethanolamine (DOPE):TOCL has more negative curvature tendency (higher acyl chain unsaturation) than DMPE:TOCL but is a poorer cochleate former. There are also other such comparisons that can be made to indicate that curvature tendency is not the prime driving force in cochleate formation.
Another feature to consider is charge on the lipid. Increased charge would inhibit cochleate formation because of electrostatic repulsion, however, increased negative charge on the lipid would facilitate the extent and strength of binding of the OAKs. In several cases increased negative charge on the lipid leads to diminished cochleate formation, as for example when the mole percent of TOCL is increased in the bilayer. Although, C12K-5α8 does assemble into cochleates with the negatively charged lipid mixture, dimyristoyl phosphatidylglycerol (DMPG):TOCL 75:25, even though the OAK itself is less positively charged compared with C12K-7α8 or C12K-9α8, that do not form cochleates with these lipids. Although charge undoubtedly plays an important role in the formation of cochleates, there is no simple relationship to the overall charge on either the OAK or the lipid mixture. Clustering of anionic lipids in the presence of zwitterionic ones, as has been found for several antimicrobial agents [2], may play a role. As far as anionic–anionic mixtures forming cochleates with certain OAKs, it should be noted that this does not negate the possibility that there is segregation of the two anionic lipids that could facilitate cochleate formation, as this phenomenon has been observed with certain antimicrobial peptides [18].
Dehydration of the membrane interface appears to play a role in facilitating the coiling up of the bilayer when bridged by cationic molecules. Thus, PC is more hydrated than PE and has fewer tendencies to form cochleates in these lipid mixtures. Similarly, CL is internally hydrogen bonded [64] with an H-bonded ring structure formed between the two phosphates and the central OH group of CL making for a tighter packing of the four hydrophobic acyl chains on each molecule. Thus, CL as well as PE and PG have hydrogen bonding among the lipid headgroups but not to water. PE and CL have particularly small headgroups relative to their acyl chains and are poorly hydrated. We therefore suggest that a factor contributing to the ability to form cochleates with OAK-lipid mixtures is a consequence of a less hydrated membrane interface resulting from a stable hydrogen bonded network among the headgroups of these lipids. This feature also explains the very slow formation of cochleates upon incubation of DMPG at low temperature [65], since low temperature storage is known to slowly cause dehydration of this lipid as a consequence of forming more stable inter-lipid bonding.
Inter-related with dehydration is the melting temperature. Lipids with a more dehydrated interface tend to have a higher melting temperature. The PE headgroup also forms a compact rigid network of hydrogen bonds at the bilayer surface, giving it higher T m values than PC [66–70]. In the case of cochleates previously found to form with PS and Ca2+, the divalent cation also markedly raises the melting temperature of the lipid [71]. With regard to the lipid mixtures we have studied, there is a correlation between melting temperature and the tendency to form cochleates. Thus, the presence of DPPE or DMPE with an anionic lipid results in more cochleate formation than does POPE, which is better than DOPE, the lowest melting of the PE lipids used. It appears that the presence of a higher melting lipid contributes to a tendency to form these assemblies. With tetramyristoyl cardiolipin (TMCL), lipid mixtures also melt at higher T m; but the required exposure of the samples during preparation to higher temperatures has a somewhat disrupting effect in cochleate formation.
In summary, we suggest that the formation of OAK-based cochleates requires the use of an OAK with an appropriate number and spacing of positively charged amino groups and is also favoured by the use of a lipid mixture of both anionic and zwitterionic lipids that have a hydrogen-bonded network at the membrane surface. These lipid mixtures will also have a high melting temperature, which will also support cochleate formation as long as the temperature is not too high to disrupt the cochleates.
Finally, the role of anionic lipid clustering in cochleate assembly with an OAK has been recently studied and is being reported (Sarig, et al., manuscript in preparation).
Lipid requirements for drug delivery
We have shown that drug encapsulation with the OAK, C12K-7α8, is several fold more efficient when the mixture forms cochleates compared with entrapment in liposomes [45]. The in vivo activity against an MDR strain of E. coli can also be enhanced when the drugs are entrapped in cochleates compared with the drugs in free solution (Sarig et al., manuscript in preparation). Cochleates formed with other lipid mixtures are even more effective than POPE/TOCL used in the first demonstration of cochleate efficacy [45]. Interestingly, drugs entrapped in the lipid mixture DMPE/DOPG are no more effective than free drugs in the absence of lipids, even though the lipid mixture DMPE/DOPG forms large cochleates [20]. The difference in properties between cochleates that are effective delivery vehicles while other cochleates are not, is discussed in a manuscript in preparation.
Efflux pumps and bacterial multidrug resistance
Bacteria have several mechanisms to develop resistance to bactericidal drugs. These mechanisms include both acquired resistance to individual drugs as well as multidrug resistance. A principle mechanism of multidrug resistance is the expression of energy-dependent drug efflux transporters [72, 73]. Toxic compounds in the environment, such as bile salts in the intestine of hosts as well as toxins released by other bacteria are thought to be the original substrates for these pumps, prior to the use of antibiotics. However, these transport systems are now an important factor in microbial drug resistance [74]. There have been significant advances in our understanding of the structures and mechanisms of the proteins involved in this resistance mechanism [75]. The largest group of multidrug efflux pumps is the Major Facilitator Superfamily (MFS). These are proton symporters or antiporters. The proteins QacA and QacB were the first examples of this class. These proteins have 14 transmembrane segments and actively extrude cationic, hydrophobic compounds [76, 77]. There are also some MFS pumps with 12 transmembrane segments, such as the NorA transporter that is found in about half of the S. aureus present in the blood stream of patients [78]. In addition, there is a family of small multidrug resistance pumps [79]. A member of this family, the EmrE of E. coli, has only four transmembrane helices [80].
Drug efflux pumps have a particular requirement in Gram-negative bacteria since these bacteria have two membranes through which the drug must efflux. This is accomplished by a family of proteins, the Resistance-Nodulation-Division (RND) drug efflux pumps that have a predominant role in the multidrug resistance of Gram-negative bacteria. Many of these systems are proton antiporters with broad substrate specificity. These pumps become associated with two other proteins, one being an outer membrane channel such as the TolC protein of E. coli, and the other being a periplasmic adapter protein that can link the RND efflux pump with the outer membrane channel. This protein complex thus allows export of drug directly into the external media, rather than the periplasmic space. Some RND proteins are also capable of transporting drugs from the periplasmic space to the cell exterior. They can thus be effective in protecting resistant bacteria against the action of β-lactams that have targets in the periplasm [81] and there are also RND transport systems that remove toxic heavy metal ions from both the cytoplasm and periplasmic space [82].
In addition to the transporters discussed above that obtain the energy for drug transport from the dissipation of a proton gradient, there are also bacterial multidrug efflux pumps that utilize the influx of Na+ [83, 84]. There are also ATP-Binding Cassette (ABC) transporters. ABC transporters play a major role in multidrug resistance in cancer cells in humans, but they have a more limited role in bacteria [85].
OAK cochleates to overcome bacterial efflux pumps
A major mechanism of drug resistance in bacteria is through the expression of multidrug efflux pumps. A direct approach to overcome the action of these efflux pumps is to develop specific inhibitors to their actions [86–88]. Alternatively, if the rate of drug influx could be accelerated, this would eventually overcome the capacity of the efflux pumps and thereby reverse multidrug resistance. We suggest that this mechanism can explain the synergistic action of OAK cochleates in combination with erythromycin in killing multidrug resistant strains of E. coli [45]. As indicated above, cochleates have been shown to have potential to be developed into agents for drug delivery. Traditional antibiotics have been shown in some cases to sensitize resistant bacteria [89] although, in most cases, the molecular basis for these phenomena has not been addressed experimentally. It has been demonstrated that there is a synergistic action as a cochleate, between the OAK, C12K-7α8, and several antibiotics, thus lowering their MICs by several orders of magnitude [45]. The results suggest that bacterial sensitization to antibiotics was derived mainly from the OAK’s capacity to overcome the efflux-enhanced resistance mechanism. The delivery of erythromycin entrapped in cochleates to overcome infection with lethal inoculums of MDR E. coli was successfully demonstrated in vivo using neutropenic mice [45].
Conclusions and future perspectives
Most antimicrobial agents interact with lipids. This interaction is an important aspect of their mechanism of action as antimicrobial agents. In addition, certain antimicrobial agents can cluster anionic lipids and this forms the basis for their species selectivity. Because this mechanism is more important in Gram-negative bacteria, it provides a platform for investigating the specific targeting of such a species of bacteria. Some antimicrobial agents that cluster anionic lipids can also form cochleate structures when complexed with lipid mixtures. Cochleates can both entrap other drugs and can facilitate drug delivery by protecting these drugs from enzymes in the serum and they can also effectively deliver drugs to tissues. The efficacy of OAK–erythromycin–lipid cochleates against multidrug resistant E. coli has been demonstrated [45]. These exciting findings provide stimulus for optimizing cochleate assemblies and further investigating the generality and efficacy of this phenomenon, as many other polycationic antimicrobial agents with a certain degree of hydrophobicity may share properties such as those which have recently been demonstrated for the antimicrobial OAKs.
Abbreviations
- ABC
ATP-binding cassette
- DMPC
Dimyristoyl phosphatidylcholine
- DMPG
Dimyristoyl phosphatidylglycerol
- DMPE
Dimyristoyl phosphatidylethanolamine
- DOPE
Dioleoyl phosphatidylethanolamine
- DOPS
Dioleoyl phosphatidylserine
- EM
Electron microscopy
- MDR
Multidrug resistant
- MIC
Minimum inhibitory concentration
- MLVs
Multilamellar vesicles
- OAK
Oligo-acyl-lysine
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PEG-PE
Polyethylene glycol linked covalently to the amino group of PE
- POPE
1-palmitoyl-2-oleoyl phosphatidylethanolamine
- PS
Phosphatidylserine
- RND
Resistance-nodulation-division
- TMCL
Tetramyristoyl cardiolipin
- TOCL
Tetraoleoyl cardiolipin
References
- 1.Wang G (2010) Antimicrobial peptides: discovery, design and novel therapeutic strategies. 1–230
- 2.Epand RM, Epand RF (2010) Biophysical analysis of membrane-targeting antimicrobial peptides: membrane properties and the design of peptides specifically targeting Gram-negative bacteria. In: Wang G (ed) Antimicrobial peptides: discovery, design and novel therapeutic strategies, pp 116–127
- 3.Epand RM, Epand RF. Bacterial membrane lipids in the action of antimicrobial agents. J Peptide Sci. 2010;17:298–305. doi: 10.1002/psc.1319. [DOI] [PubMed] [Google Scholar]
- 4.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/S0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen FY, Lee MT, Huang HW. Sigmoidal concentration dependence of antimicrobial peptide activities: a case study on alamethicin. Biophys J. 2002;82:908–914. doi: 10.1016/S0006-3495(02)75452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki K. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim Biophys Acta. 1998;1376:391–400. doi: 10.1016/s0304-4157(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 7.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Gordon VD, Mishra A, Som A, Purdy KR, Davis MA, Tew GN, Wong GCL. Synthetic antimicrobial oligomers induce a composition-dependent topological transition in membranes. J Am Chem Soc. 2007;129:12141–12147. doi: 10.1021/ja072310o. [DOI] [PubMed] [Google Scholar]
- 9.Epand RF, Sarig H, Mor A, Epand RM. Cell-wall interactions and the selective bacteriostatic activity of a miniature oligo-acyl-lysyl. Biophys J. 2009;97:2250–2257. doi: 10.1016/j.bpj.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchand C, Krajewski K, Lee HF, Antony S, Johnson AA, Amin R, Roller P, Kvaratskhelia M, Pommier Y. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006;34:5157–5165. doi: 10.1093/nar/gkl667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl HG, de Kruijff B. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science. 1999;286:2361–2364. doi: 10.1126/science.286.5448.2361. [DOI] [PubMed] [Google Scholar]
- 12.Almeida PF, Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S, Bisht GS, Rawat DS, Kumar A, Kumar R, Maiti S, Pasha S. Interaction studies of novel cell selective antimicrobial peptides with model membranes and E. coli ATCC 11775. Biochim Biophys Acta. 2010;1798:1864–1875. doi: 10.1016/j.bbamem.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Shin S, Kim JK, Lee JY, Jung KW, Hwang JS, Lee J, Lee DG, Kim I, Shin SY, Kim Y. Design of potent 9-mer antimicrobial peptide analogs of protaetiamycine and investigation of mechanism of antimicrobial action. J Pept Sci. 2009;15:559–568. doi: 10.1002/psc.1156. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Epand RM, Epand RF, Arnusch CJ, Papahadjopoulos-Sternberg B, Wang G, Shai Y. Lipid clustering by three homologous arginine-rich antimicrobial peptides is insensitive to amino acid arrangement and induced secondary structure. Biochim Biophys Acta-Biomembranes. 2010;1798:1272–1280. doi: 10.1016/j.bbamem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Epand RF, Maloy L, Ramamoorthy A, Epand RM. Amphipathic helical cationic antimicrobial peptides promote rapid formation of crystalline states in the presence of phosphatidylglycerol: lipid clustering in anionic membranes. Biophys J. 2010;98:2564–2573. doi: 10.1016/j.bpj.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epand RM, Rotem S, Mor A, Berno B, Epand RF. Bacterial membranes as predictors of antimicrobial potency. J Am Chem Soc. 2008;130:14346–14352. doi: 10.1021/ja8062327. [DOI] [PubMed] [Google Scholar]
- 20.Epand RF, Sarig H, Ohana D, Papahadjopoulos-Sternberg B, Mor A, Epand RM. Physical properties affecting cochleate formation and morphology using antimicrobial oligo-acyl-lysyl peptide mimetics and mixtures mimicking the composition of bacterial membranes in the absence of divalent cations. J Phys Chem B. 2011;115:2287–2293. doi: 10.1021/jp111242q. [DOI] [PubMed] [Google Scholar]
- 21.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KP, van Strijp JA. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo IL, Neoh HM, Cui L, Hiramatsu K. Serial daptomycin selection generates daptomycin-nonsusceptible Staphylococcus aureus strains with a heterogeneous vancomycin-intermediate phenotype. Antimicrob Agents Chemother. 2008;52:4289–4299. doi: 10.1128/AAC.00417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra NN, Yang SJ, Sawa A, Rubio A, Nast CC, Yeaman MR, Bayer AS. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2009;53:2312–2318. doi: 10.1128/AAC.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst CM, Staubitz P, Mishra NN, Yang SJ, Hornig G, Kalbacher H, Bayer AS, Kraus D, Peschel A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS.Pathog. 2009;5:e1000660. doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrer RI, Barton A, Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- 26.Gould D. Effective strategies for prevention and control of Gram-negative infections. Nurs Stand. 2009;23:42–46. doi: 10.7748/ns2009.08.23.48.42.c7204. [DOI] [PubMed] [Google Scholar]
- 27.Maragakis LL. Recognition and prevention of multidrug-resistant Gram-negative bacteria in the intensive care unit. Crit Care Med. 2010;38:S345–S351. doi: 10.1097/CCM.0b013e3181e6cbc5. [DOI] [PubMed] [Google Scholar]
- 28.Singh S. Nanomedicine-nanoscale drugs and delivery systems. J Nanosci Nanotechnol. 2010;10:7906–7918. doi: 10.1166/jnn.2010.3617. [DOI] [PubMed] [Google Scholar]
- 29.Waterhouse DN, Madden TD, Cullis PR, Bally MB, Mayer LD, Webb MS. Preparation, characterization, and biological analysis of liposomal formulations of vincristine. Methods Enzymol. 2005;391:40–57. doi: 10.1016/S0076-6879(05)91002-1. [DOI] [PubMed] [Google Scholar]
- 30.Parr MJ, Ansell SM, Choi LS, Cullis PR. Factors influencing the retention and chemical stability of poly(ethylene glycol)-lipid conjugates incorporated into large unilamellar vesicles. Biochim Biophys Acta. 1994;1195:21–30. doi: 10.1016/0005-2736(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 31.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 32.Paliwal SR, Paliwal R, Mishra N, Mehta A, Vyas SP. A novel cancer targeting approach based on estrone anchored stealth liposome for site-specific breast cancer therapy. Curr Cancer Drug Targets. 2010;10:343–353. doi: 10.2174/156800910791190210. [DOI] [PubMed] [Google Scholar]
- 33.Parr MJ, Masin D, Cullis PR, Bally MB. Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: the lack of beneficial effects by coating liposomes with poly(ethylene glycol) J Pharmacol Exp Ther. 1997;280:1319–1327. [PubMed] [Google Scholar]
- 34.Koziara JM, Lockman PR, Allen DD, Mumper RJ. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6:2712–2735. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- 35.Verkleij AJ, de Kruijff B, Ververgaert PH, Tocanne JF, van Deenen LL. The influence of pH, Ca2+ and protein on the thermotropic behaviour of the negatively charged phospholipid, phosphatidylglycerol. Biochim Biophys Acta. 1974;339:432–437. doi: 10.1016/0005-2736(74)90171-0. [DOI] [PubMed] [Google Scholar]
- 36.Tocanne JF, Ververgaert PH, Verkleij AJ, van Deenen LL. A monolayer and freeze-etching study of charged phospholipids. I. Effects of ions and pH on the ionic properties of phosphatidylglycerol and lysylphosphatidylglycerol. Chem Phys Lipids. 1974;12:201–219. doi: 10.1016/0009-3084(74)90075-9. [DOI] [PubMed] [Google Scholar]
- 37.Ververgaert PH, de KB, Verkleij AJ, Tocanne JF, Van Deened LL. Calorimetric and freeze-etch study of the influence of Mg2+ on the thermotropic behaviour of phosphatidylglycerol. Chem Phys Lipids. 1975;14:97–101. doi: 10.1016/0009-3084(75)90021-3. [DOI] [PubMed] [Google Scholar]
- 38.Papahadjopoulos D, Poste G, Schaeffer BE, Vail WJ. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim Biophys Acta. 1974;352:10–28. doi: 10.1016/0005-2736(74)90175-8. [DOI] [PubMed] [Google Scholar]
- 39.Papahadjopoulos D, Vail WJ, Jacobson K, Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975;394:483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- 40.Evans CC, Zasadzinski J. Encapsulating vesicles and colloids from cochleate cylinders. Langmuir. 2003;19:3109–3113. doi: 10.1021/la0265171. [DOI] [Google Scholar]
- 41.Walker SA, Kennedy MT, Zasadzinski JA. Encapsulation of bilayer vesicles by self-assembly. Nature. 1997;387:61–64. doi: 10.1038/387061a0. [DOI] [PubMed] [Google Scholar]
- 42.Boyer C, Zasadzinski JA. Multiple lipid compartments slow vesicle contents release in lipases and serum. ACS Nano. 2007;1:176–182. doi: 10.1021/nn7002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarif L. Elongated supramolecular assemblies in drug delivery. J Control Release. 2002;81:7–23. doi: 10.1016/S0168-3659(02)00010-X. [DOI] [PubMed] [Google Scholar]
- 44.Rao R, Squillante E, III, Kim KH. Lipid-based cochleates: a promising formulation platform for oral and parenteral delivery of therapeutic agents. Crit Rev Ther Drug Carrier Syst. 2007;24:41–61. doi: 10.1615/critrevtherdrugcarriersyst.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 45.Livne L, Epand RF, Papahadjopoulos-Sternberg B, Epand RM, Mor A. OAK-based cochleates as a novel approach to overcome multidrug resistance in bacteria. FASEB J. 2010;24:5092–5101. doi: 10.1096/fj.10-167809. [DOI] [PubMed] [Google Scholar]
- 46.Flach CR, Mendelsohn R. A new infrared spectroscopic marker for cochleate phases in phosphatidylserine-containing model membranes. Biophys J. 1993;64:1113–1121. doi: 10.1016/S0006-3495(93)81477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sternberg B (1996) Liposomes as a model for membrane structure and structural transformations: a liposome album. In: Lasic DD, Barenholz Y (eds) Handbook of nonmedical applications of liposomes, vol IV. From gene delivery and diagnosis to ecology. CRC Press, Boca Raton, pp .271–297
- 48.Sternberg B (1998) Ultrastructural morphology of cationic liposome-DNA complexes for gene therapy. 395–427
- 49.Papahadjopoulos-Sternberg B. Comparison of freeze-fracture- with cryo-electron microscopy on molecular assemblies suitable for drug and gene delivery. Microsc Microanal. 2005;11:1048–1049. doi: 10.1017/S1431927605509139. [DOI] [Google Scholar]
- 50.Day EP, Ho JT, Kunze RK, Jr, Sun ST. Dynamic light scattering study of calcium-induced fusion in phospholipid vesicles. Biochim Biophys Acta. 1977;470:503–508. doi: 10.1016/0005-2736(77)90142-0. [DOI] [PubMed] [Google Scholar]
- 51.Syed UM, Woo AF, Plakogiannis F, Jin T, Zhu H. Cochleates bridged by drug molecules. Int J Pharm. 2008;363:118–125. doi: 10.1016/j.ijpharm.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 52.Ramani K, Balasubramanian SV. Fluorescence properties of Laurdan in cochleate phases. Biochim Biophys Acta. 2003;1618:67–78. doi: 10.1016/j.bbamem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Mannino RJ, Canki M, Feketeova E, Scolpino AJ, Wang Z, Zhang F, Kheiri MT, Gould-Fogerite S. Targeting immune response induction with cochleate and liposome-based vaccines. Adv Drug Deliv Rev. 1998;32:273–287. doi: 10.1016/S0169-409X(98)00014-3. [DOI] [PubMed] [Google Scholar]
- 54.Zarif L, Graybill JR, Perlin D, Najvar L, Bocanegra R, Mannino RJ. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob Agents Chemother. 2000;44:1463–1469. doi: 10.1128/AAC.44.6.1463-1469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santangelo R, Paderu P, Delmas G, Chen ZW, Mannino R, Zarif L, Perlin DS. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob Agents Chemother. 2000;44:2356–2360. doi: 10.1128/AAC.44.9.2356-2360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez O, Bracho G, Lastre M, Mora N, Del CJ, Gil D, Zayas C, Acevedo R, Gonzalez D, Lopez JA, Taboada C, Turtle C, Solis RL. Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen-associated molecular pattern. Immunol Cell Biol. 2004;82:603–610. doi: 10.1111/j.1440-1711.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- 57.Campo JD, Zayas C, Romeu B, Acevedo R, Gonzalez E, Bracho G, Cuello M, Cabrera O, Balboa J, Lastre M. Mucosal immunization using proteoliposome and cochleate structures from Neisseria meningitidis serogroup B induce mucosal and systemic responses. Methods. 2009;49:301–308. doi: 10.1016/j.ymeth.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Perez O, Bracho G, Lastre M, Zayas C, Gonzalez D, Gil D, del CJ, Acevedo R, Taboada C, Rodriguez T, Fajardo ME, Sierra G, Campa C, Mora N, Barbera R, Solis RL. Proteliposome-derived cochleate as an immunomodulator for nasal vaccine. Vaccine. 2006;24(Suppl 2):S2–S3. doi: 10.1016/j.vaccine.2005.01.127. [DOI] [PubMed] [Google Scholar]
- 59.Gil D, Bracho G, Zayas C, del Campo J, Acevedo R, Toledo A, Lastre M, Perez O. Strategy for determination of an efficient cochleate particle size. Vaccine. 2006;24:S92–S93. doi: 10.1016/j.vaccine.2005.01.138. [DOI] [PubMed] [Google Scholar]
- 60.del Campo J, Lindqvist M, Cuello M, Backstrom M, Cabrerra O, Persson J, Perez O, Harandi AM. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine. 2010;28:1193–1200. doi: 10.1016/j.vaccine.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 61.Acevedo R, Callico A, del CJ, Gonzalez E, Cedre B, Gonzalez L, Romeu B, Zayas C, Lastre M, Fernandez S, Oliva R, Garcia L, Perez JL, Perez O. Intranasal administration of proteoliposome-derived cochleates from Vibrio cholerae O1 induce mucosal and systemic immune responses in mice. Methods. 2009;49:309–315. doi: 10.1016/j.ymeth.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 62.Gibson B, Duffy AM, Gould FS, Krause-Elsmore S, Lu R, Shang G, Chen ZW, Mannino RJ, Bouchier-Hayes DJ, Harmey JH. A novel gene delivery system for mammalian cells. Anticancer Res. 2004;24:483–488. [PubMed] [Google Scholar]
- 63.Miclea RD, Varma PR, Peng A, Balu-Iyer SV. Development and characterization of lipidic cochleate containing recombinant factor VIII. Biochim Biophys Acta. 2007;1768:2890–2898. doi: 10.1016/j.bbamem.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kates M, Syz JY, Gosser D, Haines TH. pH-dissociation characteristics of cardiolipin and its 2-deoxy analogue. Lipids. 1993;28:877–882. doi: 10.1007/BF02537494. [DOI] [PubMed] [Google Scholar]
- 65.Garidel P, Richter W, Rapp G, Blume A. Structural and morphological investigations of the formation of quasi-crystalline phases of 1, 2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG) Phys Chem Chem Phys. 2001;3:1504–1513. doi: 10.1039/b009881g. [DOI] [Google Scholar]
- 66.Matsumoto K, Kusaka J, Nishibori A, Hara H. Lipid domains in bacterial membranes. Mol Microbiol. 2006;61:1110–1117. doi: 10.1111/j.1365-2958.2006.05317.x. [DOI] [PubMed] [Google Scholar]
- 67.Elder M, Hitchcock P, Mason FRS, Shipley GG. A refinement analysis of the crystallography of the phospholipid, 1, 2-Dilauroyl-DL-phosphatidylethanolamine, and some remarks on lipid–lipid and lipid-protein interactions. Proc Royal Soc London A. 1977;354:157–170. doi: 10.1098/rspa.1977.0062. [DOI] [Google Scholar]
- 68.Hauser H, Pascher I, Pearson RH, Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981;650:21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 69.Boggs JM. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim Biophys Acta. 1987;906:353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- 70.Pascher I, Sundell S, Harlos K, Eibl H. Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim Biophys Acta. 1987;896:77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- 71.Jacobson K, Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975;14:152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- 72.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009;69:1555–1623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 75.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 76.Paulsen IT, Brown MH, Skurray RA. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paulsen IT, Brown MH, Littlejohn TG, Mitchell BA, Skurray RA. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci USA. 1996;93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeMarco CE, Cushing LA, Frempong-Manso E, Seo SM, Jaravaza TA, Kaatz GW. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus . Antimicrob Agents Chemother. 2007;51:3235–3239. doi: 10.1128/AAC.00430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paulsen IT, Skurray RA, Tam R, Saier MH, Jr, Turner RJ, Weiner JH, Goldberg EB, Grinius LL. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 80.Schuldiner S, Granot D, Mordoch SS, Ninio S, Rotem D, Soskin M, Tate CG, Yerushalmi H. Small is mighty: EmrE, a multidrug transporter as an experimental paradigm. News Physiol Sci. 2001;16:130–134. doi: 10.1152/physiologyonline.2001.16.3.130. [DOI] [PubMed] [Google Scholar]
- 81.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su CC, Long F, Yu EW. The Cus efflux system removes toxic ions via a methionine shuttle. Protein Sci. 2011;20:6–18. doi: 10.1002/pro.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyamae S, Ueda O, Yoshimura F, Hwang J, Tanaka Y, Nikaido H. A MATE family multidrug efflux transporter pumps out fluoroquinolones in Bacteroides thetaiotaomicron . Antimicrob Agents Chemother. 2001;45:3341–3346. doi: 10.1128/AAC.45.12.3341-3346.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morita Y, Kataoka A, Shiota S, Mizushima T, Tsuchiya T. NorM of vibrio parahaemolyticus is an Na(+)-driven multidrug efflux pump. J Bacteriol. 2000;182:6694–6697. doi: 10.1128/JB.182.23.6694-6697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kristiansen JE, Thomsen VF, Martins A, Viveiros M, Amaral L. Non-antibiotics reverse resistance of bacteria to antibiotics. In Vivo. 2010;24:751–754. [PubMed] [Google Scholar]
- 87.Pages JM, ibert-Franco S, Mahamoud A, Bolla JM, vin-Regli A, Chevalier J, Garnotel E. Efflux pumps of Gram-negative bacteria, a new target for new molecules. Curr Top Med Chem. 2010;10(18):1848–1857. doi: 10.2174/156802610793176620. [DOI] [PubMed] [Google Scholar]
- 88.Zechini B, Versace I. Inhibitors of multidrug resistant efflux systems in bacteria. Recent Pat Antiinfect Drug Discov. 2009;4:37–50. doi: 10.2174/157489109787236256. [DOI] [PubMed] [Google Scholar]
- 89.Anantharaman A, Rizvi MS, Sahal D. Synergy with rifampin and kanamycin enhances potency, kill kinetics, and selectivity of de novo-designed antimicrobial peptides. Antimicrob Agents Chemother. 2010;54:1693–1699. doi: 10.1128/AAC.01231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]