Abstract
Endocytosis is increasingly understood to play crucial roles in most signaling pathways, from determining which signaling components are activated, to how the signal is subsequently transduced and/or terminated. Whether a receptor-ligand complex is internalized via a clathrin-dependent or clathrin-independent endocytic route, and the complexes’ subsequent trafficking through specific endocytic compartments, to then be recycled or degraded, has profound effects on signaling output. This review discusses the roles of endocytosis in three markedly different signaling pathways: the Wnt, Notch, and Eph/Ephrin pathways. These offer fundamentally different signaling systems: (1) diffusible ligands inducing signaling in one cell, (2) membrane-tethered ligands inducing signaling in a contacting receptor cell, and (3) bi-directional receptor-ligand signaling in two contacting cells. In each of these systems, endocytosis controls signaling in fascinating ways, and comparison of their similarities and dissimilarities will help to expand our understanding of endocytic control of signal transduction across multiple signaling pathways.
Keywords: Endocytosis, Clathrin, Dynamin, Caveolin, Primary cilium, Signaling, Wnt, Notch, Eph, Ephrin, EGF
Introduction
In order for multi-cellular organisms to thrive, communication between cells is necessary to co-ordinate such disparate processes as proliferation, patterning, migration, cell-cycle exit, and differentiation. As such, communication between cells is precisely regulated by a number of different signaling mechanisms. Endocytosis, a process by which eukaryotic cells internalize plasma membrane (PM), along with cell-surface receptors and diverse soluble molecules, is used by the cell for a number of different purposes. Within the realm of cellular signaling, it plays critical roles in initiating and spreading signals, determining which specific sub-pathway to activate, and in terminating signaling. In some systems, the very outcome of cell signaling depends on which endocytic routes are used, and how the various components of the signaling machinery are trafficked.
The cell has a multitude of mechanisms it can employ to endocytose signaling components, including clathrin-dependent and clathrin-independent endocytic mechanisms such as caveolin-mediated endocytosis, arf6-dependent endocytosis, the clathrin-independent carrier/glycosylphosphatidylinositol (GPI)-anchored protein-enriched early endosomal compartment (CLIC-GEEC) endocytic pathway, and flotillin-dependent endocytosis (for review, see [1]). Clathrin-independent endocytic pathways used for internalization of large-sized particles, such as phagocytosis and macropinocytosis, are outside the scope of this review and have been reviewed elsewhere [1].
Originally, endocytosis was thought to merely down-regulate signaling. Some of the earliest indications that endocytosis plays a role outside of signal down-regulation came from studies on epidermal growth factor (EGF) signaling, in which dynamin-mediated endocytosis was shown to be necessary for the EGF-induced phosphorylation of the EGF receptor (EGFR) [2]. Later studies also revealed that while lower doses of EGF are internalized through clathrin-mediated endocytosis, higher doses of EGF are associated with ubiquitination of EGFR, endocytosis by lipid-raft- and, possibly, caveolin-mediated endocytosis and degradation [3]. Therefore, endocytosis appears to be crucial in the regulation of EGF signaling. However, caveolin knock-out mice are viable and fertile [4–8], and caveolin-deficient brown adipocytes do not show perturbations in EGF-induced ERK/MAPK signaling at high or low doses of EGF [9], so the requirement for caveolin in EGF signaling is not perfectly clear. Similar to EGFR, the transforming growth factor beta (TGF-β) receptor is endocytosed both by clathrin-mediated endocytosis into EEA1-positive early endosomes, and by clathrin-independent endocytosis into caveosomes, endosomes enriched in caveolin [10]. Signaling induced by TGF-β also depends on the endocytic route utilized, wherein uptake by clathrin-mediated endocytosis promotes TGF-β signaling, but incorporation into caveosomes leads to receptor turnover and signal downregulation [10]. These examples demonstrate that ligands can induce different endocytic pathways, depending for example on concentration, and thus activate different signaling pathways within the cell.
It is becoming increasingly evident that specific endocytic pathways are essential for the appropriate cellular responses to different signaling cues. This review aims to examine the diverse ways in which the cell utilizes endocytosis to achieve signaling. Specifically, the role of endocytosis in assembling “signalosomes” in a single “signal-receiving” cell (Wnt signaling), the role of endocytosis in cell pairs to activate signaling in one signal-receiving cell (Notch signaling), and the role of endocytosis in cell pairs during bi-directional signaling (Eph/ephrin signaling) will be addressed.
Types of endocytic pathways
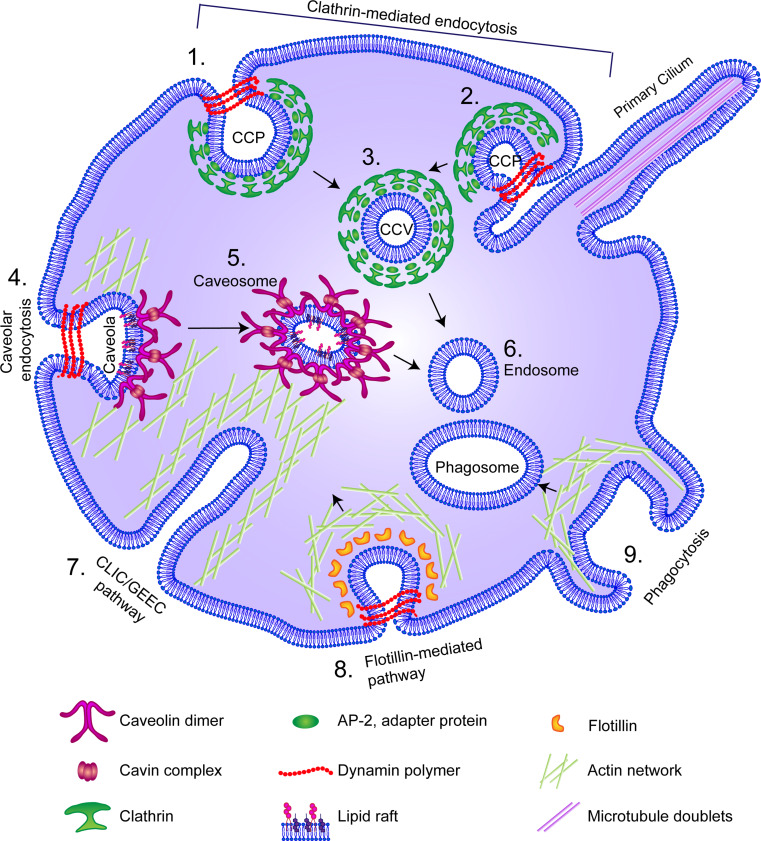
There is a growing roster of recognized endocytic pathways (Fig. 1). Moreover, there are a number of ways in which to classify these endocytic pathways, including distinctions based on the proteins required for a certain pathway (for example dynamin, clathrin, caveolin, or actin), the type of cargo being internalized (large versus small, specific receptors or extracellular fluid sampling), the morphological appearance of the endocytic process (vesicular or tubular appearance, or a ruffled appearance as seen in circular dorsal ruffles), or the sub-cellular compartment/organelle it is associated with (such as the primary cilium). As such, there is a degree of overlap between different definitions and it is important to accurately define each endocytic mechanism as completely as possible. Therefore, this review begins with a description of some of the different endocytic pathways available to the cell, with a focus on the two most thoroughly characterized pathways, the clathrin- and caveolin-mediated endocytic pathways.
Fig. 1.
Endocytic mechanisms employed by the cell. Mammalian cells can internalize plasma membrane and receptors through a number of different routes, which nevertheless may utilize some of the same cellular machinery. Clathrin-mediated (1, 2) and caveolin-mediated (4) routes both require dynamin. Clathrin-mediated endocytosis begins with the coating of clathrin-coated pits (CCP) from the cell membrane (1) or from clathrin-coated pits at the base of the primary cilium (2), which are pinched off by dynamin to yield clathrin-coated vesicles (CCVs) (3). Caveolae (4) are flask-shaped invaginations in the plasma membrane formed by caveolins and cavins, which are enriched in lipid rafts, and which require actin. Endosomes enriched in caveolin are termed caveosomes or cavicles (5). In some cases, caveosomes and CCVs can converge (6). The clathrin-independent carrier (CLIC)/GPI-anchored protein-enriched early endosomal compartment (GEEK) pathway is dependent on actin, but is clathrin and caveolin-independent (7). The flotillin-dependant pathway (8) has been suggested as an alternative route to caveolin-mediated pathways, since cells devoid of caveolin still manifest flask-shaped invaginations, in which flotillins can be found. Phagocytosis (9), which can endocytose large cargo such as apoptotic cells, also relies on actin and forms large endosomes termed phagosomes, but is primarily used by specialized cells such as neutrophils and macrophages (for further details, please see the text)
Clathrin-mediated endocytosis
Clathrin-mediated endocytosis (CME) is considered the best characterized endocytic pathway. Clathrin, which was first described in 1976 [11], is recruited from the cytoplasm by adapter proteins to form a polygonal lattice coating the plasma membrane (PM) intracellularly, resulting in the formation of clathrin-coated pits (CCPs) [12]. These subsequently pinch off in a dynamin-dependent fashion and form clathrin-coated vesicles (CCVs) of 100–200 nm in diameter. CCVs are uncoated after endocytosis and can then fuse with early endosomes for further sorting. Clathrin is used for endocytosis from several sub-cellular compartments, including the PM and the ciliary pocket. It is important to note that dynamin is used by several endocytic pathways in addition to CME, including caveolin-mediated endocytosis, the flotillin pathway, and circular dorsal ruffles [1].
Although CME is an umbrella-term for all endocytic pathways utilizing clathrin, there are a remarkable number of different adapter proteins coupling clathrin to the cargo being endocytosed [13], which help to provide signaling specificity. Endocytic adapters comprise two groups based on their multimerization capacity: multimeric adapter proteins such as adaptor protein 2 (AP-2) [14], and monomeric or dimeric adapter proteins, such as the clathrin-associated sorting proteins (CLASPs), which include proteins such as epsin [15–17], β-arrestin [18], and Numb [19, 20]. AP2 binds to lipids, cargo, accessory proteins and clathrin, while CLASPs may bind to all or only some of the above. For example, internalization can be elicited by ubiquitination of transmembrane receptors, which is recognized by epsin, a protein that contains an ubiquitin-interacting motif, a phospholipid-binding motif, a clathrin-binding motif, an accessory-protein-binding motif, and an AP-2 binding motif [15, 16]. Thanks to this vast array of clathrin-binding adapters, CME is a very adaptable endocytic pathway, which can target endosomes to different compartments in order to achieve specific signaling aims.
Clathrin-mediated endocytosis, the primary cilium
The primary (non-motile) cilium was first identified in 1898, but its signaling role in signaling has not been appreciated until more recently. This structure consists of a cellular protrusion containing a cytoskeleton of nine microtubule doublets, and is found on most differentiated cells in vertebrates (for review, see [21]). At its base is a ciliary pocket, an invagination in which the cilium is deeply rooted, which is rich in clathrin-coated pits [22]. As such, the ciliary pocket has been proposed to be a specialized endocytic membrane domain. Interestingly, in Trypanosomatids, the flagellar pocket (which shares many characteristics with the vertebrate ciliary pocket) is the obligate site of endocytosis and exocytosis [23]. While the same is not true for vertebrate cells, which can endocytose from the rest of the plasma membrane as well, it is interesting to note that ciliary proteins undergo very slow exchange with the rest of the cell due to the presence of a diffusion barrier [24], which may complicate analysis of endocytosis and trafficking in this region. The importance of primary cilia to signaling is well demonstrated by its requirement for activation of Sonic hedgehog (Shh) signaling [25]. However, while the Shh receptor patched (Ptc) can endocytose Shh, in a dynamin-dependant manner [25], it is not yet clear whether endocytosis from the primary cilium/ciliary pocket itself is required for Shh signaling. Thus, it is important to note that some clathrin-dependent signaling pathways may be specifically initiated at or near the primary cilium.
Raft-dependent endocytosis/caveolin-mediated endocytosis
One of the most widely studied endocytic pathways requiring dynamin, but not clathrin, is the caveolin-mediated endocytic pathway. This pathway is named for the appearance of flask-shaped PM invaginations termed caveolae, from the Latin for “little caves”. Found in most cells, they are especially abundant in adipocytes and endothelial cells [1]. Caveolae are enriched in lipid rafts, membrane microdomains containing cholesterol, glycosphingolipids, sphingomyelin, phospholipids, and glycosylphosphatidylinositol (GPI)-linked proteins. The formation of caveolae requires caveolin (Cav), of which there are three in mammals (Cav1-3). Cav1 appears to be required for caveolae formation in most cells, as Cav2 is not required, and Cav3 is found predominantly in muscle cells [26]. Cav1 binds to cholesterol and fatty acids and forms oligomers to induce the caveosome structure. In addition to caveolin, a second family of proteins, named cavins, (four subtypes in mammals) is required for caveolae formation [27, 28]. Cavin1 (or PTRF, polymerase I and transcript release factor), cavin2 (or SDPR, serum deprivation protein response) [29], cavin3 (or SRBC, sdr-related gene product that binds to-c-kinase) [30] and cavin4 (or MURC, muscle restricted coiled–coiled protein) are found in caveolae and contain putative leucine zipper-like domains and PEST (proline, glutamic acid, serine, and threonine-rich) domains, which target proteins towards proteolytic degradation. Intriguingly, cavin1 contains nuclear localization signals (NLSs) while cavin2 and cavin3 have been associated with protein kinase C (PKC) α and δ. Cavins may thus contribute to directing signaling in a specific direction from caveolae depending on cavin composition.
Caveolae themselves are highly reliant on the presence of cholesterol-rich lipid rafts and can be disrupted by depleting cholesterol from the cell membrane. While caveolin is required for the formation of caveolae, it is less clear what its role is in endocytosis per se. It was previously thought that caveolin was required for endocytosis of caveosomes, but this view is more recently being challenged. Budding off of caveolin positive vesicles, termed caveosomes or cavicles, requires dynamin [31], but the rate of caveolar endocytosis is negatively regulated by cav1 [32, 33] and positively regulated by increased raft-lipid levels. Similarly, increasing the levels of cav1 increases the number of caveolae but not endocytosis of these. Therefore, it has been suggested that caveolins stabilize caveolae at the plasma membrane but inhibit their endocytosis. Also, some caveosomal structures, which appear to be distant from the plasma membrane may, in fact, still be connected to the plasma membrane [34]. In addition, non-clathrin/cavaeolar and clathrin-derived endosomes can converge [35, 36], adding yet another layer of complexity to deciphering endocytic routes.
It is important to note that much of the information we have regarding trafficking and signaling by different pathways is derived from studies utilizing over-expression in mammalian cell culture or from studies performed in Drosophila. Unfortunately, many of the central players in clathrin-mediated endocytosis are so crucial to life itself that murine knockouts fail to thrive, and die during embryonic development, as in the case of AP1 [37] and AP2 [38], making analyses of these mammalian counterparts more difficult. In contrast to clathrin-related proteins, cav1, cav2, and cav3 appear to be dispensable for embryonic development [6–8], arguing that caveolin cannot be required for mediating signaling pathways during development.
Other endocytic pathways, circular dorsal ruffles, flotillin-mediated endocytosis, the CLIC-GEEC pathway
Besides clathrin and caveolin-mediated endocytosis, additional internalization pathways are being unveiled, which display quite different characteristics, from different morphologies to different requirements for dynamin or small GTPases (for review see [1]). While these are, as yet, less well characterized, it is important to bear these alternative routes in mind when considering the different endocytic conduits employed by signaling pathways.
Circular dorsal ruffles
Circular dorsal ruffles (CDRs), or waves, are transient structures that form and propagate across the dorsal PM of cells grown in vitro in response to growth factors such as EGF [39], hepatocyte growth factor (HGF) [40], and platelet-derived growth factor (PDGF) [41, 42], which causes integrins to re-localize to CDRs to be endocytosed by micropinocytosis [43]. CDR-associated endocytosis is independent of clathrin and caveolin [44] but requires dynamin and cortactin, an actin-binding protein that is important for actin remodeling [45]. CDRs are thought to be regulated by phosphoinositide metabolism [46] and activation of actin polymerization and curved membrane-bound proteins [43]. PDGF-induced CDRs require N-WASP and Arp2/3 [47], as well as small GTPases, as Vav2 and Rac drive CDR formation in response to PDGF [48].
Flotillin-mediated endocytosis
Another endocytic pathway independent of clathrin and caveolin is instead dependent on flotillin (also known as reggie in zebrafish), a protein that shares homology with caveolin [49] and that requires actin for its raft/membrane association and lateral mobility [50, 51]. Despite this homology, over-expression of flotillin-1 (Flot1) and -2 (Flot2) cannot rescue caveolae production in cav-null cells [52]. These results may be due to the fact that reggies appear to hetero-oligomerize, and the stoichiometry of this hetero-oligomerization regulates endocytosis [53]. In any case, flotillin/reggie appears to share some functions with caveolin, as it is responsible for some of the internalization of cholera-toxin B [49, 54], which was previously described to be endocytosed through caveolin-dependent mechanisms [55, 56]. In addition, flotillins are required for a number of different cellular processes, including PKC-mediated endocytosis of the dopamine transporter (DAT) and excitatory amino acid transporter 2 (EAAT2) [57], axon regeneration in zebrafish (reviewed in [58]), and matrix degradation by macrophage podosomes [59]. Interestingly, it has been shown that flotillin requires cholesterol to cluster amyloid precursor protein (APP) at the cell membrane, which is required for its subsequent endocytosis through clathrin-mediated routes [60]. EGF, which has been shown to traffic through caveolin and clathrin-dependent routes, as mentioned above, also lead to the phosphorylation of reggie and endocytosis of reggie-positive endosomes [61]. Finally, it is important to note that reggie has also been implicated in exocytosis, as it is required for secretion and spreading of Wnt and hedgehog in Drosophila [62].
The CLIC/GEEC pathway
Glycosylphosphatidylinositol-anchored proteins (GPI-APs) are internalized via a route not requiring caveolin, clathrin or dynamin, but requiring cdc42 [63–65]. This route has been termed the CLIC/GEEC pathway since it is mediated by clathrin-independent carriers (CLIC), and the endocytosed GPI-APS can be found in GPI-AP enriched early endosomal compartments (GEECs). The CLIC/GEEC pathway depends on actin instead [63] to form long tubular structures that are budded off by myosin motors [66]. It is unclear at present how, or whether, the CLIC/GEEC pathway overlaps with the flotillin pathway, which is also responsible for uptake of GPI-anchored proteins.
Endocytosis in signaling
The functions of endocytosis in signaling are numerous. In its simplest form, endocytosis can act to down-regulate the levels of a receptor at the cell-surface to inhibit further signaling. However, it is becoming increasingly clear that endocytosis can modulate signaling in a number of other manners. Compartmentalization of the plasma membrane, for example in cholesterol-rich lipid microdomains, serves to cluster receptors and signaling components in specific constellations to accomplish particular signaling aims. Endocytosis can direct signaling-active endosomes to different intracellular compartments for degradation, modification of the signal, or recycling of the signaling components. It can act as a sheer mechanical force to move or “pull” on membrane-bound proteins. In this review, three examples of signaling pathways will be discussed in light of the specific properties of each signaling pathway and the role that endocytosis plays in mediating signaling. In the first example, the Wnt pathway, a diffusible ligand activates signaling by binding to a membrane-bound receptor and co-receptors. Endocytosis is required to activate signaling, even though (at first glance) this would appear to be an unnecessary constraint. In the second example, the Notch pathway, a membrane-bound ligand, binds to a membrane-bound receptor on an adjacent cell. Here, endocytosis is required in both the receptor- and the ligand-expressing cell to activate signaling in the receptor-expressing cell. In the last example, the Eph/ephrin pathway, a membrane-bound ligand binds a membrane-bound receptor. Signaling by Eph/ephrins requires endocytosis and can occur both in a forwards and in a reverse manner, into the receptor or the ligand expressing cell, or both. These three examples offer different perspectives of how endocytosis may be utilized during signaling to accomplish specific objectives.
Ligand-induced endocytosis in order to signal: Wnt signaling
Wnts comprise a family of 19 secreted ligands that can activate multiple intracellular signaling pathways through stabilization and activation of β-catenin (Wnt/β-catenin), activation of small GTPases (Wnt/planar cell polarity [PCP]), or calcium signaling, among others [67] and with a degree of cross-talk between different Wnt pathways [68]. In Wnt/β-catenin signaling, a Wnt ligand binds to a frizzled (Fzd) receptor and the low-density lipoprotein 5 or 6 (Lrp5/6) co-receptor to destabilize the β-catenin destruction complex, which is comprised of axin, adenomatous polyposis coli (APC), casein kinase 1 alpha (CK1α) and glycogen synthase 3 kinase beta (GSK3β) [67, 68]. β-catenin thus accumulates and translocates to the nucleus where it associates with the transcription factors T cell factor (Tcf) and lymphoid enhancer-binding factor 1 (Lef1) to induce transcription of Wnt target genes. In Wnt/β-catenin-independent pathways, Wnt ligands can also utilize frizzled receptors, but other co-receptors have been implicated, including atypical receptor-related tyrosine kinase (Ryk) [69, 70], receptor tyrosine kinase-like orphan receptor 2 (Ror 2) [71, 72], van gogh/vang gogh-like (vang in Drosophila/vangl in mammals) and starry night (Stan), previously known as flamingo (Fmi) in Drosophila (cadherin, EGF LAG seven-pass G-type receptor [Celsr] in mammals) [73]. This leads to the activation of small GTPases, which are required for the actin cytoskeleton rearrangements necessary for the organization of PCP or convergent extension (CE) movements. Wnt pathways act through the scaffolding protein disheveled (Dsh in Drosophila, Dvl in vertebrates), which also plays a role in receptor endocytosis [74].
Both clathrin and caveolin-mediated endocytosis have been implicated in Wnt/β-catenin signaling, although most studies point to a predominant role for clathrin. Early studies in Drosophila showed that clathrin was required to degrade wingless (Drosophila Wnt) in a gradient [75] over the ventral cuticle, which supported the classical view of endocytosis as contributing to down-regulating signaling. Later studies revealed the positive role of endocytosis in activation of signaling itself; dynamin and Rab5 are required for activation of a Tcf/Lef luciferase reporter (TopFlash) in Drosophila S2R + cells [76], and clathrin and dynamin were found to be required for Wnt/β-catenin signaling in mouse L cells (L cells stably harboring the TOPFLASH and LacZ constructs, “LSL cells”) [77]. One hint as to the function of clathrin-mediated endocytosis is offered by studies on β-arrestin, a CLASP that binds to clathrin, AP-2, and G-protein coupled receptors, in Wnt/β-catenin signaling. Co-expression of β-arrestin-1 and Dvl is sufficient to induce LEF-mediated transcription [78], and β-arrestin can bind both Dvl and axin [79], thus it is possible that β-arrestin couples frizzled receptors to phosphorylated Dvl, forming the complexes required for active signaling while disrupting the destruction complex. Importantly, β-arrestin is required for Wnt/β-catenin signaling since β-arrestin morpholinos reduce the activation of endogenous β-catenin and block the axis duplication otherwise induced by X-Wnt-8 [79].
With regards to caveolin-mediated endocytosis in Wnt/β-catenin signaling, both positive and negative regulation has been observed. Wnt3a treatment of HEK 293 or HeLa S3 cells induces trafficking of LRP6 from lipid rafts at the PM to caveosomes, and caveolin is required for this trafficking and the stabilization of β-catenin [80]. However, in the absence of co-expressed LRP6, Wnt3a-induced internalized FZD5 co-localizes with clathrin, but not caveolin, and is inhibited by clathrin siRNA, suggesting that multiple endocytic routes may be activated by Wnt3a, depending on which receptors are present. When both LRP6 and FZD5 are expressed, these co-localize with caveolin upon Wnt3a treatment. These results were extended by the finding that Wnt3a-stimulation of HeLa cells induces DVL oligomerization at the PM and the formation of LRP6 aggregates, which then induce CK1γ phosphorylation of LRP6 and the recruitment of AXIN to lipid rafts. These “signalosomes”, which include FZD, GSK3-β, DVL, and AXIN, co-localize with caveolin but not clathrin and are thought to promote the stabilization of β-catenin by AXIN sequestration and inhibition of GSK3 activity [81].
Pharmacological disruption of lipid rafts and caveolae by filipin has revealed a role for caveolin-mediated endocytosis in LRP6 turnover, while inhibition of clathrin using monodansylcadaverine had no effect [82]. These findings highlight the importance of studying not only how signal transduction is affected by different endocytic routes but also how steady-state levels of signaling components are affected by increased or decreased endocytic capacity, as these will affect signal transduction indirectly.
Endocytosis is also important for β-catenin-independent Wnt signaling, as seen during Xenopus laevis gastrulation. Both Wnt5a and Wnt11 are well characterized as non-canonical Wnt ligands that act through β-catenin-independent pathways. During gastrulation, Wnt11 signals through the receptor Ryk to induce β-arrestin2-mediated endocytosis of Fz7 and Dsh. Accordingly, depletion of Ryk and Wnt11 prevents Dsh endocytosis in dorsal marginal zone tissues [83] and the endocytic function of β-arrestin2 is required for convergent extension movements [84]. Fz4 internalization, which can be induced by Wnt5a, is regulated in a clathrin-dependent manner together with AP-2, β-arrestin-2 and Dsh during convergent extension in Xenopus [85, 86]. Wnt5a can also activate the small GTPase Rac1 in several cell types [87, 88], and this too, is endocytosis-dependent, acting via clathrin-dependent mechanisms through the receptors Fzd2 and Ror1/2 [88].
In sum, endocytosis is required for Wnts to signal through both β-catenin-dependent and -independent pathways. Further studies would be of great interest to elucidate the degree to which endocytosis contributes to Wnt pathway specificity; for example whether all Fzd receptors are targeted through the same endocytic pathways, regardless of the Wnt activating them, or whether some of the pathway specificity arises from specific endocytic adapters and endocytic targeting. It would be interesting to determine whether specific Wnts are preferentially endocytosed through various endocytic routes. Unfortunately, the current paucity of commercially available Wnts, and the difficulty associated with purifying Wnts, makes such analyses difficult. An additional consideration is that Wnts, for example Wnt3a and Wnt5a, appear to have relatively different activation concentrations (from 10 ng to 300 ng/ml) and thus it is difficult to assess whether the pathway activated and the endocytic route employed is a high- or low-dose pathway, making comparisons between Wnts difficult. Nevertheless, further investigation into this and other questions surrounding Wnt endocytosis and pathway activation will certainly help to improve our understanding of pathway specificity.
Endocytosis in two cells to elicit signaling in one signal-receiving cell: Notch signaling
The Notch pathway represents an especially interesting example of endocytic regulation of signaling in that both the receptor and the ligand are membrane-bound on contacting cells and both require endocytosis for functional signaling to occur. Notch signaling is initiated when a Notch receptor on one cell is bound by a Notch ligand on a contacting cell. The Notch receptor is then sequentially cleaved to generate the Notch extracellular truncation (NEXT) and then release the Notch intracellular domain (NICD), which translocates to the nucleus to initiate transcription of Notch target genes together with the transcription factor CSL (CBF1/suppressor of hairless/LAG-1). Vertebrates have four Notch receptors (Notch 1–4) and five canonical Notch ligands (jagged 1,2 [Jag1, Jag2], and delta-like-1,3,4 [Dll1, Dll3, Dll4]), while Drosophila has one Notch receptor and two ligands, delta and serrate (for review, see [89]).
The first indication that endocytosis was required for functional Notch signaling came from the identification of a temperature-sensitive Drosophila dynamin mutant (shibire, shi ts), which displayed a Notch loss-of-function phenotype [90] with an excess of neuroblasts and neurons [91, 92]. Later studies revealed that dynamin-mediated endocytosis was required in both the ligand-expressing, “signal-sending”, and the receptor-expressing, “signal-receiving”, cells [93].
On the signal-sending side, mutational analyses of delta [94] and serrate [95] revealed the requirement of intracellular lysine residues in these ligands for proper Notch signaling. Lysine residues on transmembrane proteins are targets for monoubiquitination and thus serve as a signal for endocytosis [96]. Neuralized (Neur) [97–100] and mind bomb (Mib) [101, 102] have been identified as two E3 ligases required for ubiquitination of Notch ligands and thus activation of Notch signaling. Epsin, a CLASP mentioned previously, which is also known as liquid facets (Lqf) in Drosophila, recognizes the ligand monoubiquitination and is additionally required for ligand endocytosis and initiation of Notch signaling [17, 103–105], and loss of epsin results in Notch loss of function phenotypes in mouse [106], Drosophila [17], and C. elegans [104]. Epsin was previously regarded as an adapter protein mainly involved in clathrin-mediated endocytosis, and thus it is tempting to assume that Notch ligands are internalized via clathrin-dependent routes. However, more recent studies have shown that, at least in Drosophila oogenesis, ligand endocytosis does not require clathrin, but does require dynamin [107].
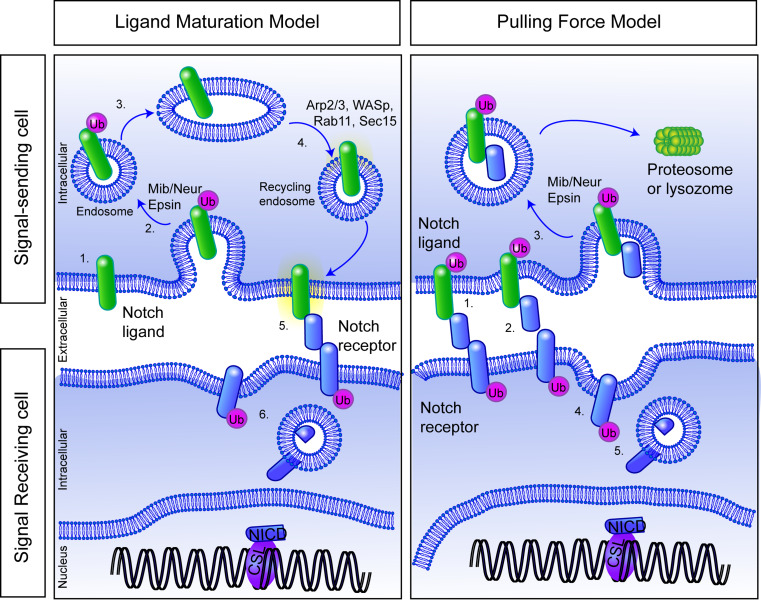
While it is clear that endocytosis is required in the signal-sending cell, its actual function in this cell is still debated. Two main models have been proposed to explain the role of endocytosis in signal-sending cells (Fig. 2): the ligand-maturation or recycling model, and the pulling-force model [108]. In the ligand-maturation model, endocytosis and recycling of the ligand is required for its final maturation into a Notch-activating ligand, either through modification(s) of the ligand itself, or through appropriate sub-cellular re-distribution, thus being presented either at the cell surface in specific microdomains, or packaged into exosomes, which are capable of signaling [109]. In the pulling-force model, the binding of Notch receptor by the ligand, and subsequent pulling away of the extracellular domain through endocytic force, is required for the successive cleavage events of the Notch receptor. There is some evidence to support each of these models, which are, in fact, not mutually exclusive.
Fig. 2.
Alternative roles for endocytosis in the ligand-expressing cell during Notch signaling. In the ligand maturation model, a Notch ligand (1), undergoes ubiquitination, for example by mind bomb or neuralized (2), is endocytosed and is sent to an as-yet undefined compartment (3) to be modified to be activation-competent. The ligand is then recycled back (4) to the membrane where it can now activate Notch on an adjacent cell (6). b In the pulling-force model, the Notch ligand binds to the Notch receptor (1) and when both are subject to endocytic forces, the ligand “pulls” on the receptor (2), revealing cleavage sites for ADAM secretase. The ligand is endocytosed into the ligand-expressing cell, carrying the Notch extracellular domain with it (3), while the Notch receptor is now further cleaved and activated, either at the plasma membrane (4) or in endosomes (5) (for further information, please see the text)
In the case of ligand maturation, there is some evidence to support this model, but also some evidence that speaks against it. For example, Dll4 has been found in exosomes from endothelial cells and Dll4-overexpressing tumors, but these exosomes do not activate Notch signaling. Instead, they are incorporated into the Notch-expressing cells and cis-inhibit Notch signaling there [110]. Epsin, as mentioned previously, is necessary for ligand endocytosis and activation of Notch signaling, but not for bulk endocytosis of ligand [105], and replacing the intracellular domain of delta with an LDL internalization signal bypasses the requirement for epsin. This led to the suggestion that monoubiquitination of the ligand is required for its endocytosis and activation of Notch signaling. Furthermore, delta is proteolytically cleaved in wild-type cells, which contain a full-length (~105 kDa) and a cleaved fragment (~50 kDa), while lqf- cells only contain the full-length version [105]. However, studies of ligand cleavage are complicated by the fact that different groups report different sized fragments, and a more recent investigation of the role of the actin-related protein 3 (Arp3) and Wiskott–Aldrich syndrome protein (WASp) found protein bands for delta of multiple sizes, including a doublet at 98 kDa, one band at 68 kDa, and one at ~55 kDa. There were no dramatic differences in the levels of each of these in wild-type or arp3- cells, although this is not overly surprising considering Arp3 was proposed to regulate a step in the post-endocytic sorting and recycling of delta, and thus delta may have been cleaved prior to its interaction with the Arp3+ compartment [111]. Therefore, Arp3 is required for the appropriate sub-cellular distribution of delta [111], as are the recycling proteins Rab11 [112] and Sec15 [113]. Sucrose-gradient fractionation has shown that wild-type Dll1 is enriched in detergent-insoluble lipid raft microdomains, rich in caveolin, while mutated versions of Dll1, which are not found in lipid raft fractions, are incapable of eliciting Notch signaling [114]. Jagged1, however, is not enriched in lipid rafts [115]. In sum, Notch ligands can be recycled from the plasma membrane and can be found in specific sub-cellular domains, which includes apical versus. basal redistribution and membrane microdomain targeting, which may be especially important in polarized cells.
In support of the pulling-force model, Notch ligands are ubiquitinated in response to interaction with the receptor [116], a signal which induces endocytosis [96], and the Notch extracellular domain is trans-endocytosed into the ligand-expressing cell [117, 118], dissociating the Notch receptor and unfolding the negative regulator region (NRR) of Notch to reveal cleavage sites for a disintegrin and metallo-protease (ADAM) secretases [119, 120]. Thus, both models are supported by a number of observations and it is possible that both mechanisms are used in different contexts.
On the Notch receptor-expressing, signal-receiving side, endocytosis via dynamin, epsin and clathrin is required for functional Notch signaling. In response to ligand interaction, the Notch receptor can be monoubiquitinated at lysine 1749 [121], and then de-ubiquitinated by elF3f (a subunit of the elongation factor 3 [Elf3]) which is required for Notch to be processed by γ-secretase [122]. In fact, there are a growing number of E3 ligases associated with Notch ubiquitination and signaling. For example, Numb, a CLASP also mentioned previously, acts as a suppressor of Notch signaling (for review, see [123, 124]) by recruiting the E3 ubiquitin ligase itchy (Itch), the mammalian homolog of Drosophila suppressor of deltex (Su(Dx)), to regulate post-endocytic sorting events for Notch [125], and degradation of the Notch receptor [126]. There is also the putative E3 ubiquitin ligase deltex, which regulates Notch internalization and processing [127–131], and acts as a bridging protein between elF3f and Notch in early endosomes [122]. Deltex has been ascribed both positive [129, 130, 132, 133] and negative [134, 135] effects on Notch signaling, and perhaps some of the differences can be explained by the recent finding that canonical Notch signaling, activated by interaction with ligand, and noncanonical, deltex-activated, Notch signaling may be separate events activated in different endocytic compartments [131]. In sum, there appear to be a number of different E3 ligases that may regulate Notch receptor ubiquitination and thus its endocytosis, targeting, and activity.
Once the NECD has been trans-endocytosed into the ligand-expressing cell, the remaining membrane-tethered portion of Notch, the Notch extracellular truncation (NEXT), becomes a substrate for subsequent site 2 (S2) cleavage by ADAM protease and site 3 (S3) cleavage by a multi-subunit protease complex known as the γ-secretase complex [136].
Different groups have observed different localizations for Notch cleavage; either occurring predominantly at the cell surface [137 – 139] or mostly after internalization of the receptor by endocytosis [140]. In fact, S3 cleavage itself may be regulated by endocytosis: Notch ICD fragments generated from S3 cleavage will have an amino-terminal valine (Val) if cleaved at the plasma membrane, but have an amino-terminal serine/leucine (Ser/Leu) if cleaved in endosomes [141]. Ser/Leu-NICD fragments have a shorter half-life than Val-NICD fragments, which should therefore affect the duration of Notch signaling.
While endocytosis of NEXT requires clathrin and dynamin, its cleavage by γ-secretase does not, and in fact inhibition of endocytosis enhances S3 cleavage and Notch target gene transcription [138], which could be because of increased plasma-membrane cleavage rather than endosomal cleavage of NEXT. Meanwhile, loss of caveolin-1 expression results in an increase in γ-secretase-mediated cleavage of Notch, and over-expression of caveolin-1 attenuates γ-secretase-mediated proteolysis of Notch [142]. It is worth noting that γ-secretase is most active in lipid raft-like membrane domains [143].
All in all, clathrin-mediated endocytosis appears to be the predominant endocytic route required for Notch signaling, a signaling pathway that requires active endocytosis by both the receptor- and the ligand-expressing cell. Notch endocytosis and signaling is fine-tuned by the action of different CLASPs, such as epsin and Numb, and signaling longevity may be determined by whether the Notch receptor is cleaved at the PM or in endosomes. It is therefore clear that endocytosis plays several important roles in Notch signaling, and it would seem that endocytic regulation of Notch signal initiation, and transduction, may be one way in which complex signaling is achieved from an otherwise relatively straightforward signaling pathway [89].
An additional point of interest is a recent finding describing a role for primary cilia in Notch signaling. Knockdown of intra-flagellar transport proteins during embryonic development resulted in the loss of cilia and causes differentiation defects that are Notch-dependent but Shh-independent [144], adding yet another dimension to endocytosis in Notch signaling.
Trans-endocytosis and bi-directional signaling: Eph receptors and ephrin ligands
Trans-endocytosis is the process by which one cell endocytoses material from another cell. In some cases, cells can trans-endocytose entire membrane-bound molecules from one cell to the other, sometimes bringing over portions of the plasma membrane and other associated proteins. This is not to be confused with the process of trogocytosis, implemented by immune cells, in which lymphocytes extract cell surface molecules from antigen-presenting cells to present them on their own cell surface (reviewed in [145]). Examples of membrane-bound receptor/ligand pairs that display trans-endocytosis include Eph receptors/ephrin ligands [146], the sevenless (sev) receptor/bride of sevenless (boss) ligand [147], and CD47/Src-homology-2-domain-containing protein tyrosine phosphatase substrate 1 (Shps-1) [148]. Of these, the most thoroughly studied group is the Eph/ephrin family.
Eph receptors are a family of receptor tyrosine kinases, activated by interaction with ephrin ligands, which are especially important in adhesion and repulsion during axon guidance (reviewed in [149]). Eph-ephrin interactions can result in forward-signaling in the Eph receptor cell, and reverse-signaling in the ephrin ligand cell, which is known as bi-directional signaling. Both forward and reverse signaling are of importance in vivo, for example EphB1/EphB2 forward-signaling and ephrin-B2 reverse signaling guide retinal ganglion cells axons to their proper termination zone in the superior colliculus [150]. Similarly to Notch signaling [151], co-expression of receptor and ligand in the same cell (in cis), inhibits trans-activation of the receptor by ligand on an adjacent cell [152, 153].
With regards to which endocytic pathway Eph/ephrins utilize, there appears to be some controversy as to whether clathrin- or caveolin-mediated endocytosis plays a more important role. Ephrin-B1 endocytosis of EphB1-Fc is dynamin-dependent, indicating that either caveolin or clathrin could be involved [154]. Supporting a role for caveolin-mediated endocytosis, ephrin-B1 can be found in lipid raft microdomains [155] and colocalizes with caveolin [154]. Also, EphB1 co-fractionates, co-immunoprecipitates and co-localizes with Cav1 upon ephrin-B2-Fc stimulation, and EphA2 similarly binds Cav1 in response to ephrin-A1-fc [156]. In support of a role for clathrin-mediated endocytosis, EphA8-mediated endocytosis of ephrin-A5-Fc co-localizes with transferrin, clathrin and EEA1 [157], and EphB2 co-localizes with transferrin, but not EEA1, upon ephrin-B2 treatment [158]. In vivo, EphA4 can be found in clathrin-coated vesicles [159], and functionally, ephrin-B1 endocytosis of EphB1-Fc is disrupted by potassium depletion, which inhibits clathrin-mediated endocytosis [154]. However, potassium depletion affects several other endocytic pathways [160, 161], and it will be important to further investigate the functional roles of caveolin-mediated, clathrin-mediated, and other endocytic pathways in Eph/ephrin internalization.
While the relative roles of caveolin- and clathrin-mediated endocytosis have yet to be fully elucidated, a large number of small GTPases have been linked to trans-endocytosis of Eph/ephrin complexes. The Rho family of small GTPases, which includes the Ras, Ran, Rad, Rab, Arf, and Rho families, are mainly associated with regulation of the cytoskeleton, but also play important roles in endocytosis and trafficking [162]. EphB4+ fibroblasts trans-endocytose full-length ephrin-B2 in a Rac, Arp2/3 and dynamin-dependent manner [163], which is correlated with phosphorylation of EphB4 and activation of signaling in the receptor cell (forwards signaling). Similarly, AphA8+ 293 cells internalize ephrin-A5 into early endosomal antigen (EEA1)+, clathrin+ endosomes, in a process requiring Tiam1, a Rac-specific guanine nucleotide exchange factor [157]. Vav2, another Rho family GEF, physically interacts with both EphA and EphB receptors and is required for endocytosis of ephrin-A1-Fc by retinal ganglion cells [164], and EphA2-mediated activation of Rac1 during angiogenesis [165]. Rin1, a Rab5 GEF that targets phosphorylated receptors to Rab5 compartments during endocytosis, is required for EphA4 endocytosis upon ephrin-B3 stimulation [166]. EphA4 interacts directly with the Rho family guanosine exchange factor (GEF) ephexin upon ephrin-A1 stimulation, which upregulates RhoA activation [167] while EphA2 interacts with ephexin4, in a ligand-independent manner, to activate RhoG, but not Rac1 or RhoA [168]. Interestingly, ephexin1 constitutively activates RhoA, Rac1, and cdc42 in the absence of ephrin-A, but when ephrin-A is present, tyrosine phosphorylation of ephexin1 by EphA receptors enhances ephexin1’s exchange activity specifically toward RhoA [169].
It is of note that early studies found that Rac1 and RhoA both inhibit uptake of transferrin, implying that they inhibit clathrin-mediated endocytosis [170]. However, local rearrangements of the actin cytoskeleton, possibly mediated by RhoA or Rac, are required for clathrin-mediated endocytosis [171]. This reinforces the idea that further studies of Eph/ephrin endocytosis must aim to determine (1) which specific GTPases are involved in clathrin or caveolin-mediated endocytosis in forward and reverse-signaling, (2) whether they act on endocytosis and activation of signaling and/or (3) whether they act on the actin cytoskeleton to mediate, for example, axon guidance.
The directionality of trans-endocytosis is regulated by cytoplasmic determinants. An ephrin-B1 mutant lacking its cytoplasmic domain is preferentially trans-endocytosed into the EphB2 cell, while an EphB2 mutant lacking its cytoplasmic tail is preferentially trans-endocytosed into the ephrin-B1 cell [172]. Importantly, a kinase-dead version of EphB2 displays similar trafficking as the cytoplasmic truncation, demonstrating that phosphorylation is important for its endocytosis into the EphB2 cell. In this study, when both ligand and receptor are full-length, the majority of ephrin-B1 is internalized into the ephrin-B1 cell [172].
Many of these studies have been performed using Fc-fusion constructs, and as such, they frequently lack transmembrane or cytoplasmic domains and may not reflect true signaling states observable in vivo. Like the Notch receptor, Eph receptors and ligands can be cleaved by ADAM secretases and γ-secretase to release signaling fragments [173 –178]. Therefore, it will be of paramount importance to confirm the trans-endocytosis studies using full-length proteins and antibodies directed towards N- and C-termini of both Eph receptors and ephrin ligands to accurately map cleavage and/or trans-endocytosis of receptor and ligand. This would enable accurate assessment of the role of endocytosis and ligand/receptor cleavage in mediating such disparate events as cellular adhesion and repulsion.
In sum, Eph receptors and ephrin ligands appear to traffic through both clathrin- and caveolin-mediated endocytic routes, activating small GTPases, which are required for these endocytic events but also for actin remodeling. Determining which components control whether endocytosis occurs in a forwards or a reverse direction will doubtless increase our understanding of the fine-tuning of Eph/ephrin signaling, as well as other signaling pathways.
Concluding remarks
Although the three signaling pathways described here are very different mechanistically, they all require endocytosis for signaling to proceed in a correct fashion. In some cases, endocytosis may be required to assemble the correct signaling components into signalosomes, as described for Wnt signaling. In other cases, endocytosis may be required to exert a pulling force of the ligand on the receptor to reveal crucial cleavage sites required for activation, as suggested for Notch signaling. Additionally, correct targeting of the receptor, as in the case of Notch, may dictate at which amino acid and at what rate it is further processed/cleaved. In the case of Eph/ephrins, bi-directional signaling is finely regulated by endocytosis in each cell. Although these examples are quite dissimilar, certain questions and considerations may be applicable across several signaling pathways.
Eph/ephrins rely heavily on small GTPases for their endocytosis and for further signaling. Wnts can activate small GTPases, but the extent to which this is linked to endocytosis remains to be further explored. This is of particular interest since small GTPases link endocytosis with cytoskeletal rearrangements; in the case of Eph/ephrins to regulate axon guidance, and in the case of Wnts, to regulate convergent extension movements. Even Notch has been described to regulate axon guidance, in a non-canonical manner, through the activation of the small GTPase Rac1 [179]. Wnts activate a multitude of small GTPases [180], and therefore it would be of tremendous interest to determine which are required for endocytosis, which are required for transmitting the signal, and which are required for actin remodeling. Wnts also signal to the nucleus via small GTPases, which begs the question of whether Eph/ephrins or Notch can also signal to the nucleus via small GTPases, although it is thought that the main target of Eph/ephrin signaling is actin rearrangements, not transcriptional regulation.
Notch signaling shares several interesting features with Eph/ephrin signaling. Both of these pathways utilize membrane-bound receptors and ligands, and both pathways utilize ADAM and γ-secretase to cleave the receptors and ligands [175, 177, 181, 182]. It would be beneficial to determine whether the ligands and receptors are similarly targeted for their respective cleavage events. Additionally, it is still debated whether backwards-signaling occurs in the Notch ligand-expressing cell, similar to the ephrin-expressing cell. In support of bi-directional signaling in the Notch pathway, Notch ligands are cleaved and release signaling fragments, which have been described to activate an Ap-1 reporter [183], although this remains to be further investigated.
In sum, our knowledge of the vast array of possible endocytic routes is expanding rapidly, as is our knowledge of the complexity of the signaling networks activated by separate signaling pathways. The more we discover about each route and pathway, the more apparent it is that the cell reuses the same components to achieve slightly different goals in different contexts. It is therefore fruitful to compare signaling pathways with respect to, not only which components are activated but also which endocytic pathways are utilized and how the endocytosed components are subsequently routed. Doing so will hopefully reveal cellular machineries linking initiation of signaling with signaling outputs, as well as divulge more universal paradigms applicable to signal specificity.
Acknowledgments
E.R.A. thanks Professor Urban Lendahl for scientific discussions and support, and Dr. Nicolas Fritz for fruitful discussions and constructive criticism of the manuscript.
Abbreviations
- ADAM
A disintegrin and metallo-protease
- AP1/2
Adaptor protein one or two
- Arf
ADP-ribosylation factor
- Arp2/3
Actin-related protein 2/3
- Cav1/2
Caveolin one or two
- CCP
Clathrin-coated pit
- CCV
Clathrin-coated vesicle
- CDR
Circular dorsal ruffles (also known as waves)
- CE
Convergent extension
- CLASP
Clathrin-associated sorting proteins
- CLIC-GEEC
Clathrin-independent carrier/GPI-anchored protein-enriched early endosomal compartment
- CME
Clathrin-mediated endocytosis
- CSL
CBF1/Suppressor of Hairless/LAG-1
- Dll1/3/4
Delta-like one, three or four (Notch ligands)
- Dsh/Dvl
Disheveled
- EEA1
Early endosomal antigen one
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- Fc
Fragment crystallizable region (tail region of antibody)
- Flot1/2
Flotillin one or two
- GPI
Glycosylphosphatidylinositol
- GTPase
Guanosine triphosphate hydrolase enzyme
- Hek cells
Human embryonic kidney cells
- HeLa cells
Cervical cancer cells from Henrietta Lacks
- HGF
Hepatocyte growth factor
- Jag1/2
Jagged one or two (Notch ligands)
- LacZ
Beta-d-galactosidase
- LDL
Low-denstity lipoprotein
- Lef1
Lymphoid enhancer-binding factor 1
- Lqf
Liquid facets (Drosophila epsin homolog)
- NECD
Notch extracellular domain
- NEXT
Notch extracellular truncation
- NICD
Notch intracellular domain
- N-WASP
Neural Wiskott-Aldrich syndrome protein (aka WASL, Wiskott-Aldrich syndrome-like)
- PCP
Planar cell polarity
- PDGF
Platelet-derived growth factor
- PKC
Protein kinase C
- PM
Plasma membrane
- Ptc
Patched (Shh receptor)
- Rab11
Rab-protein 11
- Rac1
RAS-related C3 botulinum substrate 1
- Rin1
Ras and Rab interactor one
- Ror1/2
Receptor tyrosine kinase-like orphan receptor one or two
- Ryk
Receptor-like tyrosine kinase
- Shh
Sonic hedgehog
- TCF
T-cell factor
- TGF-β
Transforming growth factor beta
- Vav2
Vav 2 guanine nucleotide exchange factor
References
- 1.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 2.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 3.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 5.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–3054. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- 7.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 8.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattsson CL, Andersson ER, Nedergaard J. Differential involvement of caveolin-1 in brown adipocyte signaling: impaired beta3-adrenergic, but unaffected LPA, PDGF and EGF receptor signaling. Biochim Biophys Acta. 2010;1803:983–989. doi: 10.1016/j.bbamcr.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 11.Pearse BM. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci USA. 1976;73:1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 13.Reider A, Wendland B. Endocytic adaptors-social networking at the plasma membrane. J Cell Sci. 2011;124:1613–1622. doi: 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearse BM, Robinson MS. Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 1984;3:1951–1957. doi: 10.1002/j.1460-2075.1984.tb02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 16.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4, 5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 17.Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote delta endocytosis and delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 18.Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dho SE, French MB, Woods SA, McGlade CJ. Characterization of four mammalian Numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J Biol Chem. 1999;274:33097–33104. doi: 10.1074/jbc.274.46.33097. [DOI] [PubMed] [Google Scholar]
- 20.Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP. Numb is an endocytic protein. J Cell Biol. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghossoub R, Molla-Herman A, Bastin P, Benmerah A. The ciliary pocket: a once-forgotten membrane domain at the base of cilia. Biol Cell. 2011;103:131–144. doi: 10.1042/BC20100128. [DOI] [PubMed] [Google Scholar]
- 22.Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 23.Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- 24.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Res. 2006;66:6463–6467. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- 26.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 27.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314–4322. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 29.Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11:807–814. doi: 10.1038/ncb1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, Luby-Phelps K, Anderson RG. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28:1001–1015. doi: 10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059–1071. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- 33.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parton RG, Molero JC, Floetenmeyer M, Green KM, James DE. Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J Biol Chem. 2002;277:46769–46778. doi: 10.1074/jbc.M205683200. [DOI] [PubMed] [Google Scholar]
- 35.Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14:417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsunari T, Nakatsu F, Shioda N, Love PE, Grinberg A, Bonifacino JS, Ohno H. Clathrin adaptor AP-2 is essential for early embryonal development. Mol Cell Biol. 2005;25:9318–9323. doi: 10.1128/MCB.25.21.9318-9323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinkers M, McKanna JA, Cohen S. Rapid induction of morphological changes in human carcinoma cells A-431 by epidermal growth factors. J Cell Biol. 1979;83:260–265. doi: 10.1083/jcb.83.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- 41.Mellstrom K, Heldin CH, Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988;177:347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- 42.Mellstroom K, Hoglund AS, Nister M, Heldin CH, Westermark B, Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J Muscle Res Cell Motil. 1983;4:589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- 43.Peleg B, Disanza A, Scita G, Gov N. Propagating cell-membrane waves driven by curved activators of actin polymerization. PLoS One. 2011;6:e18635. doi: 10.1371/journal.pone.0018635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orth JD, McNiven MA. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 2006;66:11094–11096. doi: 10.1158/0008-5472.CAN-06-3397. [DOI] [PubMed] [Google Scholar]
- 45.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa J, Tokuda E, Tenno T, Tsujita K, Sawai H, Hiroaki H, Takenawa T, Itoh T. SH3YL1 regulates dorsal ruffle formation by a novel phosphoinositide-binding domain. J Cell Biol. 2011;193:901–916. doi: 10.1083/jcb.201012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legg JA, Bompard G, Dawson J, Morris HL, Andrew N, Cooper L, Johnston SA, Tramountanis G, Machesky LM. N-WASP involvement in dorsal ruffle formation in mouse embryonic fibroblasts. Mol Biol Cell. 2007;18:678–687. doi: 10.1091/mbc.E06-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang M, Satchell L, Duhadaway JB, Prendergast GC, Laury-Kleintop LD. RhoB links PDGF signaling to cell migration by coordinating activation and localization of Cdc42 and Rac. J Cell Biochem. 2011;112:1572–1584. doi: 10.1002/jcb.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 50.Affentranger S, Martinelli S, Hahn J, Rossy J, Niggli V. Dynamic reorganization of flotillins in chemokine-stimulated human T-lymphocytes. BMC Cell Biol. 2011;12:28. doi: 10.1186/1471-2121-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langhorst MF, Solis GP, Hannbeck S, Plattner H, Stuermer CA. Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett. 2007;581:4697–4703. doi: 10.1016/j.febslet.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 52.Kirkham M, Nixon SJ, Howes MT, Abi-Rached L, Wakeham DE, Hanzal-Bayer M, Ferguson C, Hill MM, Fernandez-Rojo M, Brown DA, et al. Evolutionary analysis and molecular dissection of caveola biogenesis. J Cell Sci. 2008;121:2075–2086. doi: 10.1242/jcs.024588. [DOI] [PubMed] [Google Scholar]
- 53.Babuke T, Ruonala M, Meister M, Amaddii M, Genzler C, Esposito A, Tikkanen R. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signal. 2009;21:1287–1297. doi: 10.1016/j.cellsig.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Saslowsky DE, Cho JA, Chinnapen H, Massol RH, Chinnapen DJ, Wagner JS, De Luca HE, Kam W, Paw BH, Lencer WI. Intoxication of zebrafish and mammalian cells by cholera toxin depends on the flotillin/reggie proteins but not derlin-1 or -2. J Clin Invest. 2010;120:4399–4409. doi: 10.1172/JCI42958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 57.Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuermer CA. Microdomain-forming proteins and the role of the reggies/flotillins during axon regeneration in zebrafish. Biochim Biophys Acta. 2011;1812:415–422. doi: 10.1016/j.bbadis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Cornfine S, Himmel M, Kopp P, El Azzouzi K, Wiesner C, Kruger M, Rudel T, Linder S. The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes. Mol Biol Cell. 2011;22:202–215. doi: 10.1091/mbc.E10-05-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, Hell SW, Simons M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28:2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neumann-Giesen C, Fernow I, Amaddii M, Tikkanen R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci. 2007;120:395–406. doi: 10.1242/jcs.03336. [DOI] [PubMed] [Google Scholar]
- 62.Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, Stuermer CA, Basler K. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of wingless and hedgehog in Drosophila . EMBO J. 2008;27:509–521. doi: 10.1038/sj.emboj.7601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chadda R, Howes MT, Plowman SJ, Hancock JF, Parton RG, Mayor S. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic. 2007;8:702–717. doi: 10.1111/j.1600-0854.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 66.Geli MI, Riezman H. Role of type I myosins in receptor-mediated endocytosis in yeast. Science. 1996;272:533–535. doi: 10.1126/science.272.5261.533. [DOI] [PubMed] [Google Scholar]
- 67.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 68.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 69.Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, Katz WS, Sternberg PW. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 72.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–997. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 75.Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 76.Seto ES, Bellen HJ. Internalization is required for proper wingless signaling in Drosophila melanogaster . J Cell Biol. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci USA. 2001;98:14889–14894. doi: 10.1073/pnas.211572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:6690–6695. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Lu W, King TD, Liu CC, Bijur GN, Bu G. Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One. 2010;5:e11014. doi: 10.1371/journal.pone.0011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim GH, Her JH, Han JK. Ryk cooperates with frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 86.Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T. Association of dishevelled with the clathrin AP-2 adaptor is required for frizzled endocytosis and planar cell polarity signaling. Dev Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andersson ER, Prakash N, Cajanek L, Minina E, Bryja V, Bryjova L, Yamaguchi TP, Hall AC, Wurst W, Arenas E. Wnt5a regulates ventral midbrain morphogenesis and the development of A9–A10 dopaminergic cells in vivo. PLoS One. 2008;3:e3517. doi: 10.1371/journal.pone.0003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 90.Poulson D. Chromosomal deficiencies and the embryonic development of Drosophila melanogaster . PNAS. 1937;23:133–137. doi: 10.1073/pnas.23.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 92.Poodry CA. Shibire, a neurogenic mutant of Drosophila . Dev Biol. 1990;138:464–472. doi: 10.1016/0012-1606(90)90212-2. [DOI] [PubMed] [Google Scholar]
- 93.Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- 94.Parks AL, Stout JR, Shepard SB, Klueg KM, Dos Santos AA, Parody TR, Vaskova M, Muskavitch MA. Structure-function analysis of delta trafficking, receptor binding and signaling in Drosophila . Genetics. 2006;174:1947–1961. doi: 10.1534/genetics.106.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glittenberg M, Pitsouli C, Garvey C, Delidakis C, Bray S. Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and endocytosis. EMBO J. 2006;25:4697–4706. doi: 10.1038/sj.emboj.7601337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Deblandre GA, Lai EC, Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates notch signaling. Dev Cell. 2001;1:795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- 98.Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001;1:783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- 99.Pavlopoulos E, Pitsouli C, Klueg KM, Muskavitch MA, Moschonas NK, Delidakis C. Neuralized encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev Cell. 2001;1:807–816. doi: 10.1016/s1534-5807(01)00093-4. [DOI] [PubMed] [Google Scholar]
- 100.Yeh E, Dermer M, Commisso C, Zhou L, McGlade CJ, Boulianne GL. Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr Biol. 2001;11:1675–1679. doi: 10.1016/s0960-9822(01)00527-9. [DOI] [PubMed] [Google Scholar]
- 101.Chen W, Casey Corliss D. Three modules of zebrafish mind bomb work cooperatively to promote delta ubiquitination and endocytosis. Dev Biol. 2004;267:361–373. doi: 10.1016/j.ydbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 103.Overstreet E, Chen X, Wendland B, Fischer JA. Either part of a Drosophila epsin protein, divided after the ENTH domain, functions in endocytosis of delta in the developing eye. Curr Biol. 2003;13:854–860. doi: 10.1016/s0960-9822(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 104.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans . Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 105.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 106.Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci USA. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Windler SL, Bilder D. Endocytic internalization routes required for delta/notch signaling. Curr Biol. 2010;20:538–543. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le Borgne R, Schweisguth F. Notch signaling: endocytosis makes delta signal better. Curr Biol. 2003;13:R273–R275. doi: 10.1016/s0960-9822(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 109.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 111.Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ. The Arp2/3 complex and WASp are required for apical trafficking of delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol. 2009;11:815–824. doi: 10.1038/ncb1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 113.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes Notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 114.Heuss SF, Ndiaye-Lobry D, Six EM, Israel A, Logeat F. The intracellular region of Notch ligands Dll1 and Dll3 regulates their trafficking and signaling activity. Proc Natl Acad Sci USA. 2008;105:11212–11217. doi: 10.1073/pnas.0800695105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parr-Sturgess CA, Rushton DJ, Parkin ET. Ectodomain shedding of the Notch ligand Jagged1 is mediated by ADAM17, but is not a lipid-raft-associated event. Biochem J. 2010;432:283–294. doi: 10.1042/BJ20100321. [DOI] [PubMed] [Google Scholar]
- 116.Hansson EM, Lanner F, Das D, Mutvei A, Marklund U, Ericson J, Farnebo F, Stumm G, Stenmark H, Andersson ER, et al. Control of Notch-ligand endocytosis by ligand-receptor interaction. J Cell Sci. 2010;123:2931–2942. doi: 10.1242/jcs.073239. [DOI] [PubMed] [Google Scholar]
- 117.Nichols JT, Miyamoto A, Olsen SL, D’Souza B, Yao C, Weinmaster G. DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol. 2007;176:445–458. doi: 10.1083/jcb.200609014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 119.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 120.Sanchez-Irizarry C, Carpenter AC, Weng AP, Pear WS, Aster JC, Blacklow SC. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol Cell Biol. 2004;24:9265–9273. doi: 10.1128/MCB.24.21.9265-9273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta-Rossi N, Six E, LeBail O, Logeat F, Chastagner P, Olry A, Israel A, Brou C. Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor. J Cell Biol. 2004;166:73–83. doi: 10.1083/jcb.200310098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moretti J, Chastagner P, Gastaldello S, Heuss SF, Dirac AM, Bernards R, Masucci MG, Israel A, Brou C. The translation initiation factor 3f (eIF3f) exhibits a deubiquitinase activity regulating Notch activation. PLoS Biol. 2010;8:e1000545. doi: 10.1371/journal.pbio.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cayouette M, Raff M. Asymmetric segregation of Numb: a mechanism for neural specification from Drosophila to mammals. Nat Neurosci. 2002;5:1265–1269. doi: 10.1038/nn1202-1265. [DOI] [PubMed] [Google Scholar]
- 124.Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- 125.McGill MA, Dho SE, Weinmaster G, McGlade CJ. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem. 2009;284:26427–26438. doi: 10.1074/jbc.M109.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beres BJ, George R, Lougher EJ, Barton M, Verrelli BC, McGlade CJ, Rawls JA, Wilson-Rawls J. Numb regulates Notch1, but not Notch3, during myogenesis. Mech Dev. 2011;128(5–6):247–257. doi: 10.1016/j.mod.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 127.Diederich RJ, Matsuno K, Hing H, Artavanis-Tsakonas S. Cytosolic interaction between deltex and Notch ankyrin repeats implicates deltex in the Notch signaling pathway. Development. 1994;120:473–481. doi: 10.1242/dev.120.3.473. [DOI] [PubMed] [Google Scholar]
- 128.Hori K, Fostier M, Ito M, Fuwa TJ, Go MJ, Okano H, Baron M, Matsuno K. Drosophila deltex mediates suppressor of hairless-independent and late-endosomal activation of Notch signaling. Development. 2004;131:5527–5537. doi: 10.1242/dev.01448. [DOI] [PubMed] [Google Scholar]
- 129.Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995;121:2633–2644. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- 130.Wilkin M, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, Hori K, Taniguchi-Kanai M, Franklin E, Matsuno K, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev Cell. 2008;15:762–772. doi: 10.1016/j.devcel.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 131.Yamada K, Fuwa TJ, Ayukawa T, Tanaka T, Nakamura A, Wilkin MB, Baron M, Matsuno K. Roles of Drosophila deltex in Notch receptor endocytic trafficking and activation. Genes Cells. 2011;16:261–272. doi: 10.1111/j.1365-2443.2011.01488.x. [DOI] [PubMed] [Google Scholar]
- 132.Fuwa TJ, Hori K, Sasamura T, Higgs J, Baron M, Matsuno K. The first deltex null mutant indicates tissue-specific deltex-dependent Notch signaling in Drosophila . Mol Genet Genomics. 2006;275:251–263. doi: 10.1007/s00438-005-0087-3. [DOI] [PubMed] [Google Scholar]
- 133.Matsuno K, Ito M, Hori K, Miyashita F, Suzuki S, Kishi N, Artavanis-Tsakonas S, Okano H. Involvement of a proline-rich motif and RING-H2 finger of deltex in the regulation of Notch signaling. Development. 2002;129:1049–1059. doi: 10.1242/dev.129.4.1049. [DOI] [PubMed] [Google Scholar]
- 134.Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol. 2005;7:1191–1201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- 135.Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by Notch signaling. Science. 1999;286:741–746. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 136.Jorissen E, De Strooper B. Gamma-secretase and the intramembrane proteolysis of Notch. Curr Top Dev Biol. 2010;92:201–230. doi: 10.1016/S0070-2153(10)92006-1. [DOI] [PubMed] [Google Scholar]
- 137.Kaether C, Schmitt S, Willem M, Haass C. Amyloid precursor protein and Notch intracellular domains are generated after transport of their precursors to the cell surface. Traffic. 2006;7:408–415. doi: 10.1111/j.1600-0854.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 138.Sorensen EB, Conner SD. Gamma-secretase-dependent cleavage initiates Notch signaling from the plasma membrane. Traffic. 2010;11:1234–1245. doi: 10.1111/j.1600-0854.2010.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tarassishin L, Yin YI, Bassit B, Li YM. Processing of Notch and amyloid precursor protein by gamma-secretase is spatially distinct. Proc Natl Acad Sci USA. 2004;101:17050–17055. doi: 10.1073/pnas.0408007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster . J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tagami S, Okochi M, Yanagida K, Ikuta A, Fukumori A, Matsumoto N, Ishizuka-Katsura Y, Nakayama T, Itoh N, Jiang J, et al. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol Cell Biol. 2008;28:165–176. doi: 10.1128/MCB.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kapoor A, Hsu WM, Wang BJ, Wu GH, Lin TY, Lee SJ, Yen CT, Liang SM, Liao YF. Caveolin-1 regulates gamma-secretase-mediated AbetaPP processing by modulating spatial distribution of gamma-secretase in membrane. J Alzheimers Dis. 2010;22:423–442. doi: 10.3233/JAD-2010-100531. [DOI] [PubMed] [Google Scholar]
- 143.Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med. 2011;15:1458–1473. doi: 10.1111/j.1582-4934.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pitulescu ME, Adams RH. Eph/ephrin molecules–a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cagan RL, Kramer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992;69:393–399. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- 148.Kusakari S, Ohnishi H, Jin FJ, Kaneko Y, Murata T, Murata Y, Okazawa H, Matozaki T. Trans-endocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci. 2008;121:1213–1223. doi: 10.1242/jcs.025015. [DOI] [PubMed] [Google Scholar]
- 149.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 150.Thakar S, Chenaux G, Henkemeyer M. Critical roles for EphB and ephrin-B bidirectional signalling in retinocollicular mapping. Nat Commun. 2011;2:431. doi: 10.1038/ncomms1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.del Alamo D, Rouault H, Schweisguth F. Mechanism and significance of cis-inhibition in Notch signalling. Curr Biol. 2011;21:R40–R47. doi: 10.1016/j.cub.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 152.Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- 153.Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48:285–296. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 154.Parker M, Roberts R, Enriquez M, Zhao X, Takahashi T, Pat Cerretti D, Daniel T, Chen J. Reverse endocytosis of transmembrane ephrin-B ligands via a clathrin-mediated pathway. Biochem Biophys Res Commun. 2004;323:17–23. doi: 10.1016/j.bbrc.2004.07.209. [DOI] [PubMed] [Google Scholar]
- 155.Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 156.Vihanto MM, Vindis C, Djonov V, Cerretti DP, Huynh-Do U. Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J Cell Sci. 2006;119:2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- 157.Yoo S, Shin J, Park S. EphA8-ephrinA5 signaling and clathrin-mediated endocytosis is regulated by Tiam-1, a Rac-specific guanine nucleotide exchange factor. Mol Cells. 2010;29:603–609. doi: 10.1007/s10059-010-0075-2. [DOI] [PubMed] [Google Scholar]
- 158.Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bouvier D, Tremblay ME, Riad M, Corera AT, Gingras D, Horn KE, Fotouhi M, Girard M, Murai KK, Kennedy TE, et al. EphA4 is localized in clathrin-coated and synaptic vesicles in adult mouse brain. J Neurochem. 2010;113:153–165. doi: 10.1111/j.1471-4159.2010.06582.x. [DOI] [PubMed] [Google Scholar]
- 160.Carpentier JL, Sawano F, Geiger D, Gorden P, Perrelet A, Orci L. Potassium depletion and hypertonic medium reduce “non-coated” and clathrin-coated pit formation, as well as endocytosis through these two gates. J Cell Physiol. 1989;138:519–526. doi: 10.1002/jcp.1041380311. [DOI] [PubMed] [Google Scholar]
- 161.Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC, Sanders NN, Braeckmans K. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther. 2010;18:561–569. doi: 10.1038/mt.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ellis S, Mellor H. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 2000;10:85–88. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- 163.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 164.Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 165.Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, Chen J. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol Cell Biol. 2006;26:4830–4842. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Deininger K, Eder M, Kramer ER, Zieglgansberger W, Dodt HU, Dornmair K, Colicelli J, Klein R. The Rab5 guanylate exchange factor Rin1 regulates endocytosis of the EphA4 receptor in mature excitatory neurons. Proc Natl Acad Sci USA. 2008;105:12539–12544. doi: 10.1073/pnas.0801174105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 168.Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, Katoh H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol. 2010;190:461–477. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 170.Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 171.Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 172.Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 173.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 176.Janes PW, Wimmer-Kleikamp SH, Frangakis AS, Treble K, Griesshaber B, Sabet O, Grabenbauer M, Ting AY, Saftig P, Bastiaens PI, et al. Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol. 2009;7:e1000215. doi: 10.1371/journal.pbio.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J Biol Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song JK, Giniger E. Noncanonical Notch function in motor axon guidance is mediated by Rac GTPase and the GEF1 domain of Trio. Dev Dyn. 2011;240:324–332. doi: 10.1002/dvdy.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 181.Steiner H, Fluhrer R, Haass C. Intramembrane proteolysis by gamma-secretase. J Biol Chem. 2008;283:29627–29631. doi: 10.1074/jbc.R800010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zolkiewska A. ADAM proteases: ligand processing and modulation of the Notch pathway. Cell Mol Life Sci. 2008;65:2056–2068. doi: 10.1007/s00018-008-7586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.LaVoie MJ, Selkoe DJ. The Notch ligands, jagged and delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments. J Biol Chem. 2003;278:34427–34437. doi: 10.1074/jbc.M302659200. [DOI] [PubMed] [Google Scholar]