Abstract
The neuronal Cdk5 activator p35 is involved in a multitude of neuronal activities, including cytoskeletal organization. We show here that p35 directly interacts with filamentous actin (F-actin) but not with monomeric actin (G-actin). Through binding, p35 induces the formation of actin bundles and stabilizes F-actin against dilution-induced depolymerization. p35 forms intermolecular self-associations, suggesting that p35 cross-links actin filaments into bundles via its intermolecular self-association. p35 dimerization and association with F-actin occur at the N-terminal region that is absent in the calpain-cleaved product p25, indicating that such p35 properties are lost by its truncation induced under neurotoxic conditions. Using p35 phosphorylated by Cdk5 and a mutational approach, we demonstrate that the phosphorylation of p35 promotes its homodimerization and p35-induced formation of F-actin bundles. In addition, the phosphorylation regulates p35 distribution to microtubule and actin cytoskeletons. Together, these observations define a novel function for p35 in cytoskeletal regulation.
Keywords: Cdk5, Cdk5 activator, Actin, Actin-binding proteins, Protein phosphorylation
Introduction
Cdk5 and its neuron-specific activator, p35, play indispensable roles in developing the central nervous system. Mice deficient in either gene display disorganized cortical structures [1, 2]. In cultured neurons, depleting p35 or blocking Cdk5 kinase activity inhibits neurite outgrowth [3]. Therefore, both Cdk5 and p35 are required for neuronal migration and morphogenesis. A growing body of evidence has revealed their involvement in a wide variety of cellular functions, including the organization of actin cytoskeleton [4, 5]. In addition, deregulating Cdk5 kinase activity has been implicated in neuronal degeneration and cell death. Neurotoxins induce the truncation of p35 to p25, which loses N-terminal 98 amino acids, namely, p10, because of calpain-catalyzed cleavage [6, 7]. The p25 and p35 proteins show a number of distinct properties, including subcellular localization, even though p25 retains the Cdk5-activating domain and can activate Cdk5 kinase activity [8]. Moreover, p25 has been suggested to deregulate Cdk5 and cause cytotoxicity [8].
Both Cdk5 and p35 localize on actin filaments in the growth cones of extending neurites and are extracted from the filamentous actin fraction of rat brains [3, 9]. In addition, p39, a homolog of p35 in neurons, has been shown to interact with actin [10]. Therefore, Cdk5 and its neuronal activators, p35 and p39, associate with actin cytoskeleton. Among the growing list of Cdk5 substrates are several proteins acting as regulators of actin organization. Such Cdk5 substrates include PAK1 (a Rac1 effector), WAVE1 (an activator of the actin-nucleating Arp2/3 complex), ephexin1 (a guanine nucleotide exchanging factor of RhoA), and p27kip1 [11–14]. These phosphorylation events exert various effects on actin remodeling. For example, Cdk5-p35 phosphorylates PAK1 to suppress its activity, and its phosphorylation of p27kip1 suppresses the phosphorylation of the filamentous actin-severing protein cofilin [12, 14].
In eukaryotic cells, actin exists in a dynamic equilibrium between monomeric (G-actin) and filamentous polymeric (F-actin) forms. The dynamic organization of actin cytoskeleton is regulated by actin-binding proteins, which bind to G- or F-actin and show diverse functions, such as polymerization, depolymerization, severing, capping, and bundling of actin filaments [15, 16]. In various cellular processes, F-actin is assembled into a higher order of structures such as bundles. A number of proteins have been identified to promote F-actin bundling, exerting effects on cellular activities such as cell movement and morphogenesis [15–17]. For example, forming and maintaining dendritic spine morphology require the actin-bundling function of several proteins, including inositol trisphosphate 3-kinase A, espin, and Ca2+-calmodulin-dependent protein kinase II [18–20].
In the present study, we set out to investigate the association of p35 and Cdk5 with actin cytoskeleton and find that the former binds directly to filamentous actin. Moreover, p35 induces F-actin bundling and stabilization via its intermolecular self-association. The F-actin-bundling activity is enhanced by p35 phosphorylation. This work reveals a phosphorylation-regulated and direct effect of p35 on actin cytoskeletal organization.
Materials and methods
Plasmids and antibodies
p35 and Cdk5 constructs used in this work have been described previously [21]. p35 was expressed with GFP, SBP-CBP, V5, or His6 at the C-terminus or with FLAG in fusion to the N-terminus. Cdk5N144 was tagged with FLAG at the N-terminus. The anti-p35 (C-19) and anti-Cdk5 (DC17) antibodies were purchased from Santa Cruz Biotechnology. The following antibodies were from Sigma: anti-α-actin, anti-β-actin, anti-GST, anti-V5, and anti-FLAG (M2).
Protein preparation
Recombinant proteins containing a GST or His6 tag were prepared using E. coli BL21 (DE3) and isolated following the previously published protocols using GSH-Sepharose (GE Healthcare) or Ni2+-nitrilotriacetic acid beads (Qiagen), respectively [21]. Rabbit skeletal muscle actin was purified from acetone powder and chromatographed over a Sephacryl S-200 column [22, 23]. Prior to use in actin assays, proteins were dialyzed against G-buffer (2 mM Tris-HCl, pH 8.0, 0.2 mM ATP, 0.1 mM CaCl2, 0.5 mM DTT, and 0.5 mM NaN3) and centrifuged at 150,000g (1 h; 4°C) to remove aggregates.
Cell culture, transfection, and immunocytochemistry
COS-7 cells, maintained in DMEM supplemented with 10% fetal bovine serum, were transfected with plasmids using the Lipofectamine and Plus reagents (Invitrogen). Fifteen hours post-transfection, cells were fixed with 4% paraformaldehyde/phosphate-buffered saline at room temperature for 20 min and were then subjected to immunostaining. To disrupt actin fibers, cells were treated with 5 μM cytochalasin B (Sigma) for 10 min at 37°C before fixation. Images were captured with an inverted microscope (Nikon Eclipse TE2000). Fluorescence intensities were measured using MetaMorph software (MDS Analytical Technologies). After background subtraction, the fluorescence signals of rhodamine-phalloidin were plotted versus those of p35.
Protein binding assays
The actin binding assays were performed either in G-buffer or under an actin polymerization condition by adding 10× KMEI (500 mM NaCl, 10 mM MgCl2, 10 mM EGTA, and 100 mM imidazole pH 7.0) to the concentration of 1×. In both assay conditions, 0.02% Triton X-100 and 0.5 mg/ml bovine serum albumin were added. GST or GST-p35 (3 μg) was incubated with G-actin (10 μg) in the assay buffers (250 μl) for 1 h at room temperature. After the absorption of GST proteins to GSH-Sepharose, the beads were washed and then analyzed by immunoblotting. To test the binding of p35 fragments to p35, GST-tagged p35 fragments (5 μg) were mixed with p35-His6 (1 μg) in a binding buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.2% Triton X-100, 1 mM DTT, and the Roche protease inhibitor cocktail) and were incubated for 2 h at 4°C. After retrieving the GST proteins, the coprecipitated proteins were analyzed.
Immunoprecipitation
p35 was ectopically expressed with a FLAG tag (FLAG-p35) or with a consecutive streptavidin-binding peptide and calmodulin-binding peptide (p35-SBP-CBP). HEK293T double-transfected with the FLAG and the SBP-CBP constructs was treated with 200 μg/ml of the protein cross-linker dithiobis (succinimidyl propionate) in phosphate-buffered saline at room temperature for 20 min and then extracted in a lysis buffer (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.2% Triton X-100, 1 mM DTT, and the protease inhibitor cocktail). After centrifugation (20,000g for 15 min; 4°C) to remove cell debris, the extracts were used for anti-FLAG immunoprecipitation. The immunoprecipitates were analyzed on anti-p35 immunoblots.
High- and low-speed pelleting assays
Actin filaments were formed by assembling 10 μM of G-actin for 2 h at room temperature in a Na-polymerization buffer (G buffer plus 50 mM NaCl, 1 mM MgCl2, 1 mM EGTA, and 10 mM imidazole pH 7.0 from a 10× stock) and stabilized with 1 μM of phalloidin (Invitrogen). To prepare p35 in phosphorylated and nonphosphorylated forms, p35-His6 was incubated with equal molar GST-Cdk5 or GST-Cdk5N144 in the kinase buffer (20 mM MOPS, pH 7.4, 5 mM MgCl2, and 1 mM ATP) at 30°C for 30 min. In the pelleting assays, p35 was incubated with 2 μM of polymerized actin in the polymerization buffer supplemented with 100 mM NaCl for 1 h at room temperature. Following the incubation, the samples were subjected to high- or low-speed centrifugation to test F-actin binding or bundling activities, respectively [24]. In the high-speed pelleting assay, the samples were spun at 150,000g (20 min; 4°C). After centrifugation, both supernatants and pellets were analyzed on immunoblots. Quantifications were acquired on the ChemiDoc XRS system (Bio-Rad) using the Quantity One software (Bio-Rad). To determine dissociation constants (K d), the ratios of F-actin-bound to unbound p35 were plotted and curves were fitted to the experimental data with GraphPad prism 5.0 software (GraphPad Software). Student’s t test was used to evaluate the differences between binding parameters. In the low-speed pelleting assay, the samples were spun at 10,000g (20 min; 4°C) through a cushion of 10% glycerol in the assay buffer.
Electron microscopy
To prepare for electron microscopy analysis, actin filaments were adsorbed onto freshly glow-discharged, carbon-coated copper grids and were then negatively stained with 2% uranyl acetate. The grids were examined on a Philips CM100 transmission electron microscope operated at 80 kV.
F-actin depolymerization
A mixture of pyrene-labeled (25%; Cytoskeleton) and unlabeled G-actin was dialyzed overnight against G-buffer and was precleared by centrifugation at 150,000g (1 h; 4°C). After clarification, actin (2 μM) was polymerized for ≥2 h at room temperature in G-buffer supplemented with 1× KMEI from the 10× stock. Depolymerization started when the filaments were diluted to 0.2 μM in the polymerization buffer. Pyrene fluorescence was recorded in a fluorescence spectrometer (excitation at 365 nm and emission at 405 nm; PerkinElmer).
Subcellular fractionation
COS-7 cells were differentially extracted for fractions of free tubulin and actin, microtubules, or F-actin by a protocol modified from published methods [25, 26]. Briefly, cells were first extracted for 2 min with a microtubule-preserving buffer (80 mM PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA, 0.2% Triton X-100, 10% glycerol, and protease inhibitors) prewarmed to 37°C. The extract was collected as the free tubulin and actin fraction (Fr. 1). The cell remnants were briefly rinsed before the extraction on ice with a microtubule-disrupting buffer (20 mM Tris-HCl, pH 7.4, 100 mM KCl, 1 mM CaCl2, and protease inhibitors) for 10 min. This extract was collected as the microtubule fraction (Fr. 2). Subsequently, the remnants were extracted for 1 h with an F-actin-depolymerizing buffer (10 mM Tris-HCl, pH 7.4, 2% Triton X-100, 0.6 M KCl, 2 mM EDTA, 250 mM sucrose, 0.2 mM ATP, 1 μM DNase I, and protease inhibitors) to obtain F-actin-associated proteins (Fr. 3). All extracts were clarified by centrifugation.
Results
p35 is an F-actin-binding protein
A body of evidence from the literature has suggested that Cdk5 and p35 are involved in reorganizing the actin cytoskeleton [4, 5]. To investigate further the p35 function, we transfected p35-GFP into COS-7 cells and examined the F-actin patterns. In a significant population of transfected cells, the ectopic expression induced the formation of clusters of bundled actin filaments in various amounts, and the p35 protein was enriched on the actin bundles (Fig. 1a). Treating the cells with cytochalasin B completely disrupted the actin filaments (Fig. 1a), verifying the formation of actin clusters. In a control experiment, the expression of GFP in fusion with an irrelevant protein (i.e., GST) did not induce such actin structures (Fig. 1a). Therefore, p35, but not the tag moiety, promotes the formation of actin bundles in transfected cells.
Fig. 1a, b.
Transfection of p35 induces the formation of actin bundle clusters. a COS-7 cells were transiently transfected with p35-GFP or GFP-GST. The cells were treated with or without 5 μM cytochalasin B and then stained for F-actin. Solvent refers to the solvent of cytochalasin B. Higher magnification of the boxed areas is shown on the far right. b COS-7 was double transfected with p35-GFP and Cdk5N144 or was single transfected with p35(D288A/L289A)-GFP (abbreviated as D288A/L289A ). Scale bar 10 μm
To address whether the kinase activity of Cdk5 is required for the p35-induced formation of F-actin bundles, the kinase-dead mutant Cdk5N144 [3] was co-expressed with p35. The expression of Cdk5N144 did not block the formation of F-actin bundles (Fig. 1b). Moreover, Cdk5N144 co-localized with p35 on the actin bundles (Fig. 1b). We also tested a Cdk5-binding-deficient mutant, p35(D288A/L289A) [21]. When expressed, p35(D288A/L289A) induced the formation of such actin clusters (Fig. 1b). Therefore, p35 can induce the formation of F-actin bundles in the absence of Cdk5 activity. However, we found that the phosphorylation of p35 promotes such an effect of p35 (see below).
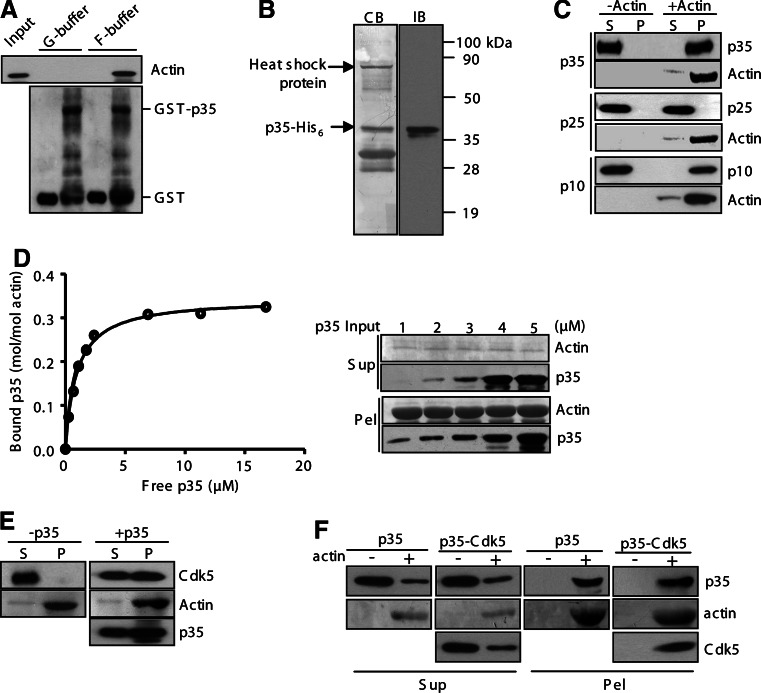
We then investigated whether p35 binds to actin. The potential binding was first tested in a pull-down assay conducted either in G-buffer to prevent actin polymerization or under an actin-polymerizing condition. The recombinant protein of p35 associated with actin under the actin-polymerizing condition but not in G-buffer (Fig. 2a). Under both conditions, the GST of the fusion protein did not bind to actin (Fig. 2a). These experiments revealed that p35 interacts with F-actin but not with G-actin. We then probed the F-actin-binding activity in a high-speed sedimentation assay performed with or without prepolymerized actin. The recombinant protein p35-His6 was expressed in bacteria and purified. The preparation contained heat shock proteins and p35 fragments, in addition to the full-length p35 protein (~15% of the preparation, Fig. 2b). Two p35 fragments, the truncated p25 form and the N-terminal p10 region, were also prepared and tested. Neither p35 nor any of its derived proteins were sedimented in the absence of F-actin (Fig. 2c). Both p35 and p10 showed strong cosedimentation with F-actin, whereas p25 failed cosedimentation (Fig. 2c), indicating that p35 associates with F-actin via the N-terminal region. The F-actin-binding affinity of p35 was determined in the sedimentation assay performed with various amounts of p35. The fitting curve revealed that p35 bound to F-actin in a saturable manner with the maximum binding ratio of approximately 0.35 mol of p35 bound per mole of actin (B max = 0.35 ± 0.01) and with the K d value of 0.95 ± 0.06 μM (Fig. 2d). These results indicate a high binding affinity between p35 and F-actin.
Fig. 2a–f.
p35 associates directly with F-actin. a A pull-down assay was performed with GST-p35 and G-actin in G-buffer or the actin polymerization buffer (F-buffer). The pull-downs of the GST proteins were analyzed on anti-α-actin and anti-GST immunoblots. b The purified protein of p35-His6 was stained by Coomassie Blue (CB) on an SDS-PAGE gel and immunoblotted with p35 antibody (IB). c A high-speed pelleting assay was performed with p35 and its fragments. After centrifugation, supernatants (S) and pellets (P) were immunoblotted for actin and other proteins as indicated. d In a high-speed pelleting assay, p35 at various amounts was incubated with F-actin (10 μM). After sedimentation, supernatants (Sup) and pellets (Pel) were collected for immunoblotting. The binding cure was obtained with the quantification data of p35 and actin. e Cdk5 was tested in the high-speed pelleting assay with or without p35. f p35 and the p35-Cdk5 complex were tested in the high-speed pelleting assay
The potential interaction of Cdk5 with F-actin was also investigated using the sedimentation assay. Cdk5 alone did not have any detectable binding activity toward F-actin (Fig. 2e, left panels). However, the co-sedimentation of Cdk5 with F-actin was readily detected in the presence of p35 (Fig. 2e, right panels). We conclude that Cdk5 associates with F-actin indirectly through bound p35. In addition, p35 in the absence of Cdk5 and p35 in the complex with Cdk5 displayed similar F-actin-cosedimenting activities (Fig. 2f), indicating that the F-actin-binding activity is unaffected upon its association with Cdk5.
p35 bundles and stabilizes F-actin
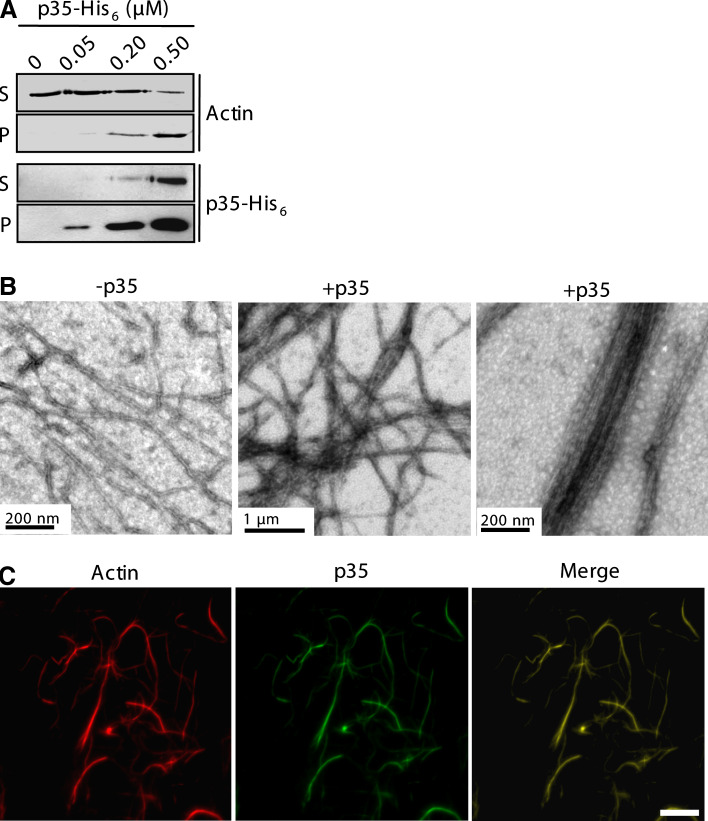
F-actin bundling was examined by the low-speed sedimentation assay, in which F-actin bundles and networks, but no single filaments, were pelleted by a low-speed centrifugation [27]. The incubation of preassembled F-actin and the p35 protein resulted in their cosedimentation in a p35-dependent manner (Fig. 3a). In the absence of p35, actin appeared exclusively in the supernatant of the centrifugation (Fig. 3a). We then examined actin filaments by electron microscopy. Most actin filaments of the p35-containing samples existed in closely packed bundles, which were not observed in the samples of F-actin alone (Fig. 3b). We also performed immunostaining of the p35-containing samples and found that p35 was stained to the entirety of the actin filaments (Fig. 3c). These results show that p35 binds along the entire length of actin filaments and organizes them into bundles.
Fig. 3a–c.
p35 bundles F-actin in vitro. a The low-speed pelleting assay was performed with phalloidin-stabilized actin filaments and p35-His6. Sedimented proteins (P) as well as the supernatants (S) were analyzed by anti-α-actin and anti-His6 immunoblotting. b F-actin preassembled from 4 μM G-actin in 1× KMEI buffer was incubated with or without 1 μM p35-His6. Shown are electron micrographs of actin filaments. c Aliquots of the samples from b containing p35-His6 were applied onto poly-l-lysine-coated cover glasses and fixed in 1% glutaraldehyde. The samples were then stained with rhodamine-labeled phalloidin and an anti-p35 antibody for fluorescence microscopy. Scale bar 10 μm
Given the F-actin binding and bundling activities, we examined whether p35 affects the kinetics of actin polymerization and depolymerization using the pyrene-actin assays. Actin polymerization was initiated by adding a polymerization buffer into G-actin, while depolymerization of preassembled F-actin was induced by tenfold dilution of F-actin samples. Fluorescence changes of pyrene-labeled actin were monitored during polymerization or depolymerization. In the polymerization assays, adding p35 did not change the polymerization kinetics (data not shown). However, the incubation of p35 with pre-assembled actin filaments largely inhibited dilution-induced depolymerization, which is similar to the effect of phalloidin (Fig. 4). Therefore, p35 stabilizes F-actin against dilution-induced depolymerization. As a substrate of Cdk5, p35 is mainly phosphorylated at Ser8 and Thr138 [28]. We also tested the phosphomimetic and nonphosphorylatable mutants p35(S8E/T138E) and p35(S8A/T138A) in the assays. Both mutants showed similar effects as the wild-type protein in protecting F-actin against dilution-induced depolymerization (Fig. 4).
Fig. 4.
Effect of p35 on F-actin depolymerization. Preassembled F-actin was diluted in KMEI alone or with phalloidin (1 μM) or p35-His6 (1 μM). WT p35 wild-type, 2E p35(S8E/T138E), 2A p35(S8A/T138A)
p35 forms a homodimer or oligomers
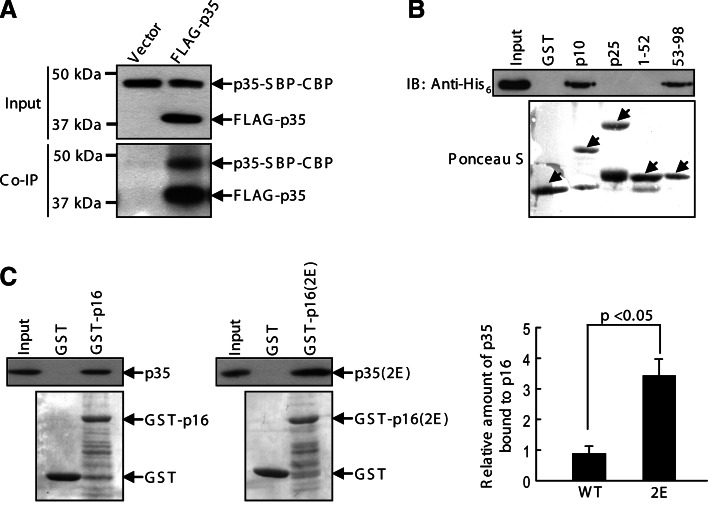
The observation of actin bundling prompted us to investigate if p35 forms a homodimer or oligomers, as several actin-bundling proteins cross-link actin filaments via their dimerization or oligomerization [29, 30]. We double-transfected p35 into HEK293T with different ectopic tags, namely: FLAG-p35 and p35-SBP-CBP. Anti-FLAG immunoprecipitation readily co-precipitated p35-SBP-CBP (Fig. 5a). In a control experiment, p35-SBP-CBP was not precipitated by anti-FLAG immunoprecipitation from the lysates in which the FLAG vector was transfected instead of FLAG-p35 (Fig. 5a). To examine whether the interaction between p35 molecules is direct or indirect and to gain information on the binding region, we performed an in vitro binding assay of p35-His6 with the GST fusion proteins of p35 fragments. After pulling down GST proteins, the p35-His6 protein was detected to bind to p10 and 53–88 but not to p25 and 1–52 (Fig. 5b). Collectively, p35 forms intermolecular self-interaction through a p10 region, which is absent in p25.
Fig. 5a–c.
Intermolecular self-association of p35. a HEK293T was double transfected with p35-SBP-CBP and FLAG-p35 or the FLAG vector. Anti-FLAG immunoprecipitates as well as the lysate inputs (10%) were analyzed on anti-p35 immunoblots. b p35 fragments tagged with GST were subjected to a GST pull-down assay with p35-His6. The pull-downs and the p35 input were detected by anti-His6 immunoblotting and Ponceau S staining of the membranes. Arrows point to the GST proteins. c HEK293T extracts transiently expressing p35 and p35(S8E/T138E) were subjected to GST pull-down using the wild-type and the phosphomimetic mutant of GST-p16 (5 μg), respectively. 2E S8E/T138E. p35 proteins coprecipitated with the GST proteins as well as the inputs (5%) were analyzed on anti-p35 immunoblots and quantified. The GST proteins were visualized by staining the membranes with Ponceau S. A representative of three experiments is shown. In the histogram, p35 coprecipitated with GST-p16 is presented as amounts relative to those of the respective inputs
To investigate whether p35 phosphorylation affects its self-association, we generated a recombinant protein of p35(1–149) (hereinafter referred to as p16), which contains the self-association domain and the phosphorylation sites Ser8 and Thr138. In addition, the phosphomimetic mutations S8E and T138E were engineered into p35 and p16. In an assay, p35 wild-type and mutant expressed in HEK293T were subjected to binding with p16 wild-type and mutant, respectively. The binding activities were compared between the wild-type proteins and the phosphomimetic mutants. With similar inputs of the wild-type and mutant proteins, the phosphomimetic mutants displayed a ~twofold higher binding activity than the wild-type ones (Fig. 5c). These results suggest that p35 phosphorylation promotes the formation of a homodimer or oligomers.
Phosphorylation of p35 regulates its actin-bundling activity
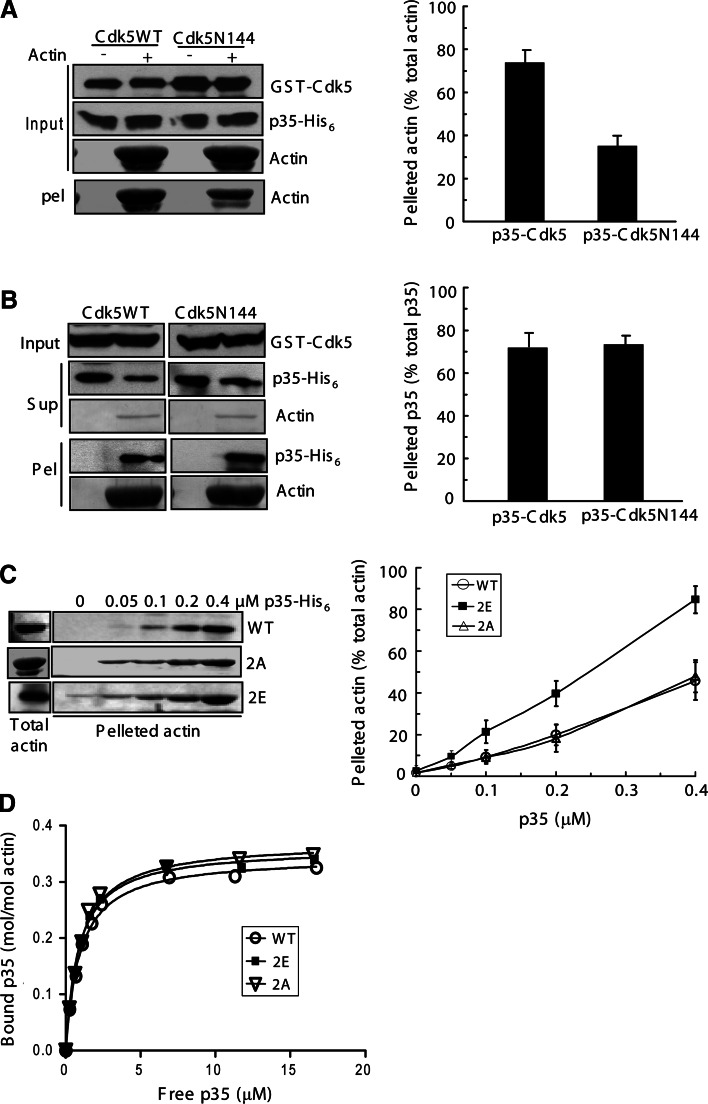
We assessed the effects of p35 phosphorylation on its F-actin-bundling and binding activities in the low-speed and high-speed pelleting assays, respectively. In the assays, p35 proteins were incubated with preassembled and phalloidin-stabilized F-actin. The samples were then centrifuged either at a low speed to sediment F-actin bundles and networks or at a high speed to pellet all F-actin filaments. Before assays, p35 was phosphorylated by Cdk5 in a phosphorylation reaction. For comparison, nonphosphorylated p35 was generated from the reaction using Cdk5N144 instead of the wild-type protein. In the low-speed pelleting assay, phosphorylated p35 displayed a significantly stronger activity of F-actin bundling than the nonphosphorylated protein (Fig. 6a). However, both forms of p35 showed similar F-actin-binding activities in the high-speed pelleting assay (Fig. 6b).
Fig. 6a–d.
Effect of p35 phosphorylation on its F-actin-bundling and binding activities. a–b p35 was subjected to a phosphorylation reaction with Cdk5 or Cdk5N144. After reaction, the samples were tested in the low-speed (a) and high-speed (b) pelleting assays. c F-actin, prepolymerized with phalloidin, was incubated with wild-type (WT) p35 or its mutants in various amounts. 2E p35(S8E/T138E), 2A p35(S8A/T138A). After low-speed centrifugation, sedimented actin was analyzed by immunoblotting (anti-α-actin) and quantified. d High-speed pelleting assays were performed with p35 and its mutants to plot binding curves. p35 WT: K d = 0.95 ± 0.06 μM and B max = 0.35 ± 0.01 mol/mol (n = 9); p35(S8E/T138E): K d = 0.87 ± 0.06 μM and B max = 0.36 ± 0.01 mol/mol (n = 9); p35(S8A/138A): K d = 0.93 ± 0.08 μM and B max = 0.37 ± 0.01 mol/mol (n = 9)
To further investigate the phosphorylation effect, the phosphomimetic mutant p35(S8E/T138E) and the nonphosphorylatable mutant p35(S8A/T138A) were tested in F-actin bundling and binding assays for comparison with wild-type p35. In the low-speed pelleting assay, actin was sedimented in a manner dependent on the p35 proteins (Fig. 6c). Moreover, the phosphomimetic mutant exhibited a significantly stronger activity than the wild-type and the nonphosphorylatable mutant in inducing actin sedimentation by centrifugation (Fig. 6c). When 0.4 μM of the p35 proteins was applied, p35(S8E/T138E) and wild-type p35 caused the sedimentation of 85 and 46% actin, respectively (Fig. 6c). The nonphosphorylatable mutant exhibited an almost identical activity as the wild-type to induce F-actin bundling (Fig. 6c). In the high-speed pelleting assay, the wild-type and mutant proteins were detected to have similar K d and B max values (Fig. 6d), revealing similar F-actin-binding activities of the p35 proteins. Taken together, the phosphomimetic mutation of p35 augments its F-actin-bundling activity but does not affect its F-actin-binding activity.
Given the observation that p35 induces the formation of F-actin bundles in transfected cells (Fig. 1), we transfected its phosphomimetic and nonphosphorylatable mutants to evaluate their effects. After staining of p35 and F-actin, the fluorescence intensities were quantified to plot F-actin fluorescence versus that of p35. The amount of F-actin correlated to that of the expressed p35 proteins (Fig. 7a). The wild-type p35 and the nonphosphorylatable mutant p35(S8A/T138A) showed similar activities of inducing F-actin clusters (Fig. 7a). This was plausibly due to a low phosphorylation stoichiometry of overexpressed p35. The phosphomimetic mutant p35(S8E/T138E) displayed a significantly stronger activity than the wild-type and the nonphosphorylatable mutant (Fig. 7a). These results are in agreement with those of the low-speed sedimentation assay.
Fig. 7a, b.
The phosphomimetic mutation of p35 promotes its effect on actin cytoskeleton. a COS-7 cells expressing V5-tagged p35 or its mutants were stained for p35 (anti-V5) and F-actin (phalloidin). Shown are representative fluorescence micrographs (left) and a plot of F-actin fluorescence versus p35 fluorescence (right) from 50 cells analyzed for each sample. p35(2A) p35(S8A/T138A), p35(2E) p35(S8E/T138E). Scale bar 10 μm. b Transfected cells were subjected to fractionation to extract the fractions of free tublin and actin (Fr.1), microtubules (Fr. 2), and F-actin (Fr. 3). Each fraction and the whole cell extracts (WCEs) were examined on immunoblots
p35 is a microtubule-associated protein and its microtubule association is regulated by phosphorylation [31, 32]. To examine whether p35 phosphorylation alters its distribution between microtubule and actin cytoskeletons, we performed subcellular fractionation and immunoblotting on cells expressing the phosphomimetic and nonphosphorylatable mutants of p35. A significant amount of p35(S8A/T138A) was detected in the microtubule fraction (9.67 ± 2.49% of the protein), while the amount was reduced to 1.53 ± 0.41% for p35(S8E/T138E) (Fig. 7b). These results further support the notion that the phosphorylation of p35 blocks its binding to microtubules [31]. Both mutants associated with the F-actin fraction at high levels (41.67 ± 7.04% of the S8A/T138A mutant and 46.33 ± 5.79% of the S8E/T138E mutant) and the phosphomimetic mutation slightly increased the distribution of p35 to the F-actin fraction (Fig. 7b). Together, these results suggest that p35 phosphorylation facilitates its localization to F-actin and enhances its activity of inducing F-actin bundling.
Discussion
Actin cytoskeleton plays an essential role in various neuronal activities, including cell migration and morphogenesis. In this study, we describe the p35 activator of Cdk5 as a structural protein that binds selectively to and bundles F-actin. This is in line with previous reports suggesting that p35 and Cdk5 localize in the actin cytoskeleton and regulate actin reorganization [3, 9–14]. p35 contains an F-actin-binding domain and a dimerization domain within the N-terminal 98 amino acids (i.e., p10), which are absent in p25. p25 displays a number of properties distinct from those of p35, including subcellular localization [8, 33]. The formation of actin bundles depends on proteins that bind to and cross-link adjacent actin filaments. We have found that p35 forms a homodimer or oligomers both in vitro and in transfected cells. Therefore, we propose that the intermolecular self-association of p35 cross-links its bound actin filaments to form actin cables. Consistent with this idea, p35 was stained along the sides of bundled actin filaments in the actin bundling assays and in the transfected cells (Figs. 1, 3c).
In a previous study, we showed that p35 is a microtubule-associated protein (MAP) [32]. Several MAPs are known to associate with actin filaments; among them, MAP2 and tau act as cross-linking proteins to bundle F-actin, which implies a coordinated regulation of microtubule and actin cytoskeletons by MAPs, such as MAP2 and tau [34–37]. The p10 region located at the N-terminus of p35 is responsible for both microtubule and F-actin associations. In addition, this region binds to several other proteins [21, 38, 39]. Given the wide differences in the geometry of F-actin, microtubules, and the other binding proteins, p10 may adopt different conformations after binding to partners. Presumably, the structural flexibility of p10 permits its adoption of different conformations to form stable complexes with its binding partners.
In neurons, p35 is mainly phosphorylated by Cdk5 at Ser-8 and Thr-138 [28]. Such phosphorylation suppresses calpain-catalyzed truncation of p35 but increases its susceptibility to proteasome-mediated degradation [28, 40, 41]. We have found that the phosphomimetic mutation of Ser-8 and Thr-138 potentiates the self-association and F-actin-bundling activities of p35. In addition, we ruled out the effect of phosphorylation on p35 binding with F-actin. Currently, the mechanism by which p35 phosphorylation modulates the affinity of its self-association remains unclear. Such an effect on p35 self-association may increase its cross-linking activity of F-actin. Therefore, a plausible explanation for the alteration of the F-actin-bundling activity by p35 phosphorylation is the enhancement of its dimerization or oligomerization. We have previously reported that p35 phosphorylation inhibits its association with microtubules [31]. Therefore, Cdk5-mediated phosphorylation regulates p35 actions on cytoskeletons by suppressing the effect of p35 on microtubules and promoting its effect on actin. It would be interesting to determine the consequence of such effects, as the cross-talk between and the coordinated regulation of actin and microtubule networks underlie various cellular activities.
A body of evidence suggests that Cdk5 and p35 participate in actin-based neuronal activities such as morphogenesis and plasticity of dendritic spines, structures rich in actin cables and networks [4, 42]. Active Cdk5-p35 kinase phosphorylates several substrates to regulate actin remodeling and thus dendritic morphology. The phosphorylation of WAVE1 and ephexin1 regulates their activating activity towards the Arp2/3 complex and guanine-nucleotide exchanging activity towards RhoA, respectively [11, 13]. Our present study reveals a novel function of p35 in addition to Cdk5 activation in regulating actin reorganization. We suggest that the dimerization or oligomerization of p35 allows for the formation of F-actin bundles, providing the mechanical strength required to generate and support membrane protrusions during neuronal morphogenesis and migration. In addition, Cdk5 acts through p35 phosphorylation to potentiate the p35 activity of F-actin bundling and thus stabilization. These results, together with other studies [31, 32, 43], suggest that p35 is a cytoskeletal protein whose functions are regulated by Cdk5 phosphorylation.
Acknowledgments
We thank Dr. Henry N. Higgs (Dartmouth Medical School) for advice on actin polymerization assays. This work was supported by the Research Grants Council (General Research Fund and Collaborative Research Fund) and the University Grants Committee (Area of Excellence Scheme and Special Equipment Grant) of Hong Kong.
Footnotes
L. He and Z. Zhang contributed equally to this work.
References
- 1.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/S0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 3.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 4.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 5.Lim AC, Qu D, Qi RZ. Protein–protein interactions in Cdk5 regulation and function. Neurosignals. 2003;12:230–238. doi: 10.1159/000074625. [DOI] [PubMed] [Google Scholar]
- 6.Kusakawa G, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga S. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- 7.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 8.Patrick GN, Zukerberg L, Nikolic M, de la MS, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 9.Paglini G, Peris L, ez-Guerra J, Quiroga S, Caceres A. The Cdk5–p35 kinase associates with the Golgi apparatus and regulates membrane traffic. EMBO Rep. 2001;2:1139–1144. doi: 10.1093/embo-reports/kve250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbert S, Dhavan R, Tsai L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- 11.Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, Bikoff JB, Lai KO, Yung WH, Fu AK, Greenberg ME, Ip NY. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- 12.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho RS, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 14.Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 15.Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 16.Pollard TD, Cooper JA. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- 17.Otto JJ. Actin-bundling proteins. Curr Opin Cell Biol. 1994;6:105–109. doi: 10.1016/0955-0674(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 18.Johnson HW, Schell MJ. Neuronal IP3 3-kinase is an F-actin bundling protein: role in dendritic targeting and regulation of spine morphology. Mol Biol Cell. 2009;20:5166–5180. doi: 10.1091/mbc.E09-01-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekerkova G, Loomis PA, Changyaleket B, Zheng L, Eytan R, Chen B, Mugnaini E, Bartles JR. Novel espin actin-bundling proteins are localized to Purkinje cell dendritic spines and bind the Src homology 3 adapter protein insulin receptor substrate p53. J Neurosci. 2003;23:1310–1319. doi: 10.1523/JNEUROSCI.23-04-01310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu X, Choi YK, Qu D, Yu Y, Cheung NS, Qi RZ. Identification of nuclear import mechanisms for the neuronal CDK5 activator. J Biol Chem. 2006;281:39014–39021. doi: 10.1074/jbc.M512663200. [DOI] [PubMed] [Google Scholar]
- 22.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin–troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 23.Lean-Fletcher S, Pollard TD. Mechanism of action of cytochalasin B on actin. Cell. 1980;20:329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- 24.Pollard TD, Cooper JA. Methods to characterize actin filament networks. Methods Enzymol. 1982;85:211–233. doi: 10.1016/0076-6879(82)85022-2. [DOI] [PubMed] [Google Scholar]
- 25.Lim AC, Tiu SY, Li Q, Qi RZ. Direct regulation of microtubule dynamics by protein kinase CK2. J Biol Chem. 2004;279:4433–4439. doi: 10.1074/jbc.M310563200. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JA, Pollard TD. Methods to measure actin polymerization. Methods Enzymol. 1982;85:182–210. doi: 10.1016/0076-6879(82)85021-0. [DOI] [PubMed] [Google Scholar]
- 27.Sandrock TM, Brower SM, Toenjes KA, Adams AE. Suppressor analysis of fimbrin (Sac6p) overexpression in yeast. Genetics. 1999;151:1287–1297. doi: 10.1093/genetics/151.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamei H, Saito T, Ozawa M, Fujita Y, Asada A, Bibb JA, Saido TC, Sorimachi H, Hisanaga S. Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J Biol Chem. 2007;282:1687–1694. doi: 10.1074/jbc.M610541200. [DOI] [PubMed] [Google Scholar]
- 29.George SP, Wang Y, Mathew S, Srinivasan K, Khurana S. Dimerization and actin-bundling properties of villin and its role in the assembly of epithelial cell brush borders. J Biol Chem. 2007;282:26528–26541. doi: 10.1074/jbc.M703617200. [DOI] [PubMed] [Google Scholar]
- 30.Bunai F, Ando K, Ueno H, Numata O. Tetrahymena eukaryotic translation elongation factor 1A (eEF1A) bundles filamentous actin through dimer formation. J Biochem. 2006;140:393–399. doi: 10.1093/jb/mvj169. [DOI] [PubMed] [Google Scholar]
- 31.He L, Hou Z, Qi RZ. Calmodulin binding and Cdk5 phosphorylation of p35 regulate its effect on microtubules. J Biol Chem. 2008;283:13252–13260. doi: 10.1074/jbc.M706937200. [DOI] [PubMed] [Google Scholar]
- 32.Hou Z, Li Q, He L, Lim HY, Fu X, Cheung NS, Qi DX, Qi RZ. Microtubule association of the neuronal p35 activator of Cdk5. J Biol Chem. 2007;282:18666–18670. doi: 10.1074/jbc.C700052200. [DOI] [PubMed] [Google Scholar]
- 33.O’Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selden SC, Pollard TD. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983;258:7064–7071. [PubMed] [Google Scholar]
- 35.Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol. 2004;14:363–371. doi: 10.1016/j.cub.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 37.Togel M, Wiche G, Propst F. Novel features of the light chain of microtubule-associated protein MAP1B: microtubule stabilization, self interaction, actin filament binding, and regulation by the heavy chain. J Cell Biol. 1998;143:695–707. doi: 10.1083/jcb.143.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou Z, He L, Qi RZ. Regulation of S6 kinase 1 activation by phosphorylation at ser-411. J Biol Chem. 2007;282:6922–6928. doi: 10.1074/jbc.M607836200. [DOI] [PubMed] [Google Scholar]
- 39.Qu D, Li Q, Lim HY, Cheung NS, Li R, Wang JH, Qi RZ. The protein SET binds the neuronal Cdk5 activator p35nck5a and modulates Cdk5/p35nck5a activity. J Biol Chem. 2002;277:7324–7332. doi: 10.1074/jbc.M107270200. [DOI] [PubMed] [Google Scholar]
- 40.Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin–proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- 41.Saito T, Onuki R, Fujita Y, Kusakawa G, Ishiguro K, Bibb JA, Kishimoto T, Hisanaga S. Developmental regulation of the proteolysis of the p35 cyclin-dependent kinase 5 activator by phosphorylation. J Neurosci. 2003;23:1189–1197. doi: 10.1523/JNEUROSCI.23-04-01189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung ZH, Ip NY. The roles of cyclin-dependent kinase 5 in dendrite and synapse development. Biotechnol J. 2007;2:949–957. doi: 10.1002/biot.200700056. [DOI] [PubMed] [Google Scholar]
- 43.Qi Z, Tang D, Zhu X, Fujita DJ, Wang JH. Association of neurofilament proteins with neuronal Cdk5 activator. J Biol Chem. 1998;273:2329–2335. doi: 10.1074/jbc.273.4.2329. [DOI] [PubMed] [Google Scholar]