Abstract
Rare human primary immunodeficiency disorders with extreme susceptibility to infections in infancy have provided important insights into immune function. Increasingly, however, primary immunodeficiencies are also recognized as a cause of other more common, often discrete, infectious susceptibilities. In a wider context, loss-of-function mutations in immune genes may also cause disorders of immune regulation and predispose to cancer. Here, we review the associations between human diseases and mutations in genetic elements affecting natural killer (NK) cell development and function. Although many such genetic aberrations significantly reduce NK cell numbers or severely impair NK cell responses, inferences regarding the role of NK cells in disease are confounded by the fact that most mutations also affect the development or function of other cell types. Still, data suggest an important role for NK cells in diseases ranging from classical immunodeficiency syndromes with susceptibility to viruses and other intracellular pathogens to cancer, autoimmunity, and hypersensitivity reactions.
Keywords: Natural killer cells, Primary immunodeficiency, Cytotoxic lymphocytes
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that also interact with, and influence, adaptive immunity [1]. NK cells and cytotoxic T lymphocytes (CTL) share a common mechanism for the killing of infected or neoplastic cells, utilizing polarized exocytosis of secretory lysosomes [2, 3]. NK cells also contribute to immunity through the secretion of cytokines and chemokines [4]. Additionally, NK cells may kill autologous immune cells including activated T cells and dendritic cells (DCs), thus affecting the balance between tolerance and adaptive immune responses [5].
The role of NK cells in human diseases has been enigmatic. In a seminal paper, Biron and colleagues [6] described an adolescent with severe herpesvirus infections linked to a total absence of peripheral blood NK cells. The role of NK cells in the defense against herpes viruses is also underscored by the evolutionary interplay between NK cell receptors and viral immune evasion strategies [7]. Furthermore, aberrations in NK cell function may manifest as an increased susceptibility to multiple intracellular pathogens, cancer, autoimmunity, and hypersensitivity reactions [8–11]. Here, we first provide a framework for the understanding of human NK cell biology and, subsequently, review the associations between genes affecting NK cell development, activation, and effector function, and specific diseases linked to these. The disease associations observed in the wide spectrum of patients described provide important clues to the role of NK cells in human health.
NK cell development and differentiation
The development of human NK cells from hematopoietic stem cells occurs in the bone marrow and involves cytokines as well as stromal cell interactions [12]. In in vitro cultures, interleukin (IL)-2 or IL-15 can differentiate CD34+ stem cells into bona fide NK cells, commonly defined as CD3−CD56+ cells. The expression of CD117 (c-kit) marks NK cell progenitors, and the addition of c-kit ligand to cultures augments NK cell expansion [12]. The final stages of human NK cell differentiation are marked by the expression of CD56. Secondary lymphoid organs mainly harbor CD56bright NK cells that all express the inhibitory NKG2A (CD159a) receptor [13]. In contrast, CD56bright NK cells constitute on average only 10% of NK cells in peripheral blood, where CD56dim NK cells are the major NK cell population. The latter display variegated expression of inhibitory NKG2A and polymorphic killer cell immunoglobulin-like receptors (KIR; CD158). IL-2, IL-15, or IL-12 are sufficient to induce proliferation of CD56bright NK cells and expression of KIR receptors [14]. Based on these and other experiments, CD56bright NK cells are considered to be precursors of CD56dim NK cells. Functionally, CD56bright NK cells are highly responsive to exogenous cytokines such as IL-2, IL-15, IL-12, and IL-18, whereas CD56dim NK cells are more cytotoxic and the major producers of cytokines in response to target cell recognition [15]. Further stages of CD56dim NK cell differentiation have recently been defined based on a number of phenotypical markers including CD117, CD94, CD62L, CD57, and KIR [16–19]. Cells in early stages express CD117, CD62L, and CD94 and retain responsiveness to exogenous cytokines, whereas cells in later stages that express CD57 are poorly responsive to exogenous cytokines, have limited proliferative capacity, but express high levels of cytotoxic proteins. The expression of inhibitory NKG2A or KIR receptors for self-MHC class I confers stronger NK cell responses upon target cell recognition, a process often referred to as “licensing” or education [20]. Finally, underlining NK cell phenotypical and functional plasticity, the properties of uterine NK cells differ in several ways from those of peripheral blood NK cells [21].
NK cell activation
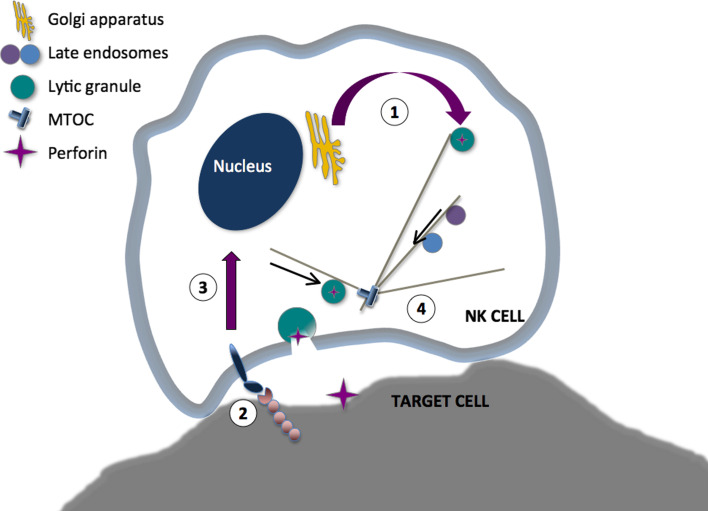
Unlike T or B cells that undergo gene rearrangement to generate a vast repertoire of receptors with unique antigen specificity, NK cell activation relies on germline-encoded receptors [22, 23]. Upon recognition of target cells, NK cells may release perforin and granzyme-containing secretory lysosomes, which are specialized lysosomal organelles that support conventional lysosomal functions in addition to mediating target cell killing. The process of target cell recognition and elimination includes cell–cell contact, adhesion, formation of an immunological synapse, granule polarization, granule exocytosis, and finally target cell detachment [24, 25]. Contact and formation of an immune synapse (IS) by human peripheral blood NK cells can be instigated by a number of activating receptors that induce inside-out signals for activation of the β2-integrin LFA-1 (CD11a/CD18) [26], promoting adhesion. LFA-1 signaling also induces convergence of granules to the microtubule organizing center (MTOC) and actin and microtubule-dependent reorientation of the MTOC to the IS [27, 28]. Engagement of inhibitory receptors for MHC class I molecules blocks inside-out signals for LFA-1-mediated adhesion and disrupts formation of an IS [26, 29]. Engagement of a few NK cell receptors such as the low affinity Fc receptor CD16 can suffice for triggering of granule exocytosis, while other receptors co-activate NK cell degranulation through synergistic interactions that overcome thresholds for activation [30, 31]. Notably, neither adhesion nor granule polarization is required for release of lytic granules by NK cells, but engagement of LFA-1 significantly enhances both CD16-mediated antibody-dependent cellular cytotoxicity (ADCC) and natural cytotoxicity [26, 28]. Remarkably, live-cell imaging of NK cell cytotoxic synapses has revealed dynamic spatio-temporal patterning of immune receptors at the synapse and, controlled by LFA-1 engagement, convergence of lytic granule exocytosis to a single site [32]. Endocytosis occurs immediately adjacent to the site of exocytosis. Thus, a versatile molecular machinery with bidirectional vesicular trafficking underlies NK cell-elimination of target cells. Perforin facilitates granzyme entry into target cells, where they induce apoptosis [33]. Apoptosis-inducing ligands such as FasL (CD178) and TRAIL (CD253) may also localize to secretory lysosomes and be expressed on the NK cell surface upon exocytosis [28, 34, 35]. These molecules may induce apoptosis by engagement of death receptors on the target cell. For effective immunity, cell contacts must be terminated in a timely fashion, yet much remains to be understood in regards to factors that control the duration of cellular contacts and the detachment process [36].
Concomitant with degranulation, engagement of activating receptors can also induce secretion of chemokines such as macrophage inflammatory protein (MIP)-1α, MIP-1β and RANTES, and, after several hours, release of cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ, both of which modulate the immune response [15]. In addition to target cell recognition, NK cells respond to cues from sentinel immune cells, including dendritic cells (DC), macrophages, and pathogen-infected tissue cells [37, 38]. These cues are communicated by the release of cytokines, including, IL-1, IL-10, IL-12, IL-15, and IL-18. In addition to the canonical cytokines induced by NK cell recognition of target cells, NK cells have been reported to secrete a number of other factors, including immunoregulatory cytokines such as IL-5, IL-10, IL-13, and the growth factor GM-CSF [39].
Genetic defects impairing NK cell development
A number of genes have been associated with impairments in NK cell development (Table 1). Mutations in genes associated with severe combined immunodeficiencies (SCID) generally affect lymphocyte development [40]. SCID patients present with recurrent infections, typically in infancy. Several genes associated with SCID selectively affect T cell and B cell development by interfering with antigen receptor gene rearrangement [41]. Other SCIDs are caused by mutations in genes required for early lymphocyte development. In addition to abrogating T cell development and impairing B cell function, X-linked mutations in IL2RG, encoding the common cytokine receptor γ-chain (CD132), constituent of the IL-2 and IL-15 receptor complex, also markedly decrease the number of NK cells in peripheral blood [42]. Moreover, a single patient with defective IL2RB transcription and expression of the β-chain (CD122) has been described [43]. This patient presented with recurrent respiratory syncytial virus bronchiolitis and Candida enteritis and demonstrated diminished numbers of T cells and an almost complete absence of NK cells, but normal B cell numbers. Notably, in mice, a link between the thioredoxin interacting protein (TXNIP), CD122 expression, and NK cell development has been described. Txnip-deficient mice display a selective defect in NK and NKT cell development, caused by attenuated CD122 expression [44]. The mice also exhibit lymphoid hyperplasia in the intestine. Downstream of the IL-2 and IL-5 receptor complex, autosomal recessive mutations in JAK3, encoding Janus kinase 3 (JAK3), are associated with SCID in infancy and also impair T cell and NK cell development [45, 46]. Upon IL-2 receptor occupancy, JAK3 phosphorylates STAT1, STAT3, and STAT5, whereas occupancy of the IL-15 receptor selectively leads to STAT5 phosphorylation [47]. Autosomal recessive mutations in STAT5B yield susceptibility to severe herpes virus infections, pruritic skin infections, and recurrent pulmonary infections in childhood [48–50]. Moreover, STAT5b-deficiency results in impaired development and function of NK cells, γδ T cells, and regulatory T cells. STAT5b-deficient patients also display growth-retardation due to impaired growth hormone signaling. STAT1 is not required for NK cell development, but autosomal recessive mutations are associated with immunodeficiency due to defects in IFN-γ production that cause susceptibility to mycobacterial infections. Genetic deletion of Stat3 in mice results in embryonic lethality.
Table 1.
Genetic defects affecting human NK cell development
| Disease | Gene | Protein | Locus | Protein function | Cells affected |
|---|---|---|---|---|---|
| SCID | IL2RG | IL-2 receptor γ-chain | Xq13.1 | Cytokine receptor | NK, T |
| SCID | JAK3 | JAK3 | 19p13.1 | Tyrosine kinase | NK, T |
| SCID | STAT5B | STAT5B | 17q11.2 | Transcription factor | NK, T |
| SCID | ADA | ADA | 20q13.11 | Catalyses purine salvage, methylation | NK, T, B |
In addition to mutations in the IL-2 and IL-15 signaling pathway, autosomal recessive mutations in ADA, encoding the enzyme adenosine deaminase (ADA), result in SCID in infancy [51]. ADA-deficient patients cannot metabolize purine and display severely reduced numbers of T cells, B cells, and NK cells in addition to costochondral abnormalities, skeletal dysplasias, neurologic deficits, and hepatic dysfunction. Studies of ADA-deficient mice have indicated that their defect in lymphocyte development and function is due to high systemic levels of adenosine, which attenuates lymphocyte development and function [52].
The above-listed immunodeficiencies all affect multiple cell lineages. Thus, disease cannot be attributed to absence of NK cells alone. Other patients with selective NK cell deficiencies and susceptibility to severe cytomegalovirus (CMV), Epstein–Barr virus (EBV), herpes simplex virus (HSV), or varicella zoster virus (VZV) infections, all members of the herpes virus family, have been reported [6, 53–55], but the genetic causes are not known. Mice with targeted deletion of Il15 or Il15ra are deficient in NK cells, NKT cells, CD8+ T cells, and TCRγδ intraepithelial lymphocytes [56]. In mouse models, the transcription factors Id2 and E4bp4 are required for NK cell development [57, 58], whereas T-bet is required for proper development and maturation of NK cells [59]. However, humans with mutations in the genes encoding these transcription factors have not been reported and it is not clear how these and other transcription factors collectively control NK cell development.
Together, these immunodeficiencies highlight a central role for γ-chain-dependent cytokine signaling in NK cell development. Future studies might uncover patients with mutations in IL15, IL15RA, and transcription factors required for NK cell differentiation, hopefully providing further insights into the transcriptional regulation of human NK cell development and maturation.
Genetic defects and variations affecting NK cell receptors for target cell recognition
NK cells express a multitude of germline-encoded receptors implicated in regulation of NK cell effector functions. Mutations in some such receptors have been associated with primary immunodeficiencies (Table 2; as summarized in Fig. 1), whereas polymorphisms in other receptors have been linked to other more complex human diseases.
Table 2.
Genetic defects affecting human NK cell receptors
| Disease | Gene | Protein | Locus | Protein function | Cells affected |
|---|---|---|---|---|---|
| LAD |
ITGB2 FCGR3A |
CD18 CD16/FcγRIIIA |
21q22.3 1q23 |
Adhesion, polarization Fc receptor |
Leukocytes NK, myeloid |
| BLS | TAP1 | TAP1 | 6p21.3 | Peptide transporter, MHC class I expression | NK, T |
| BLS | TAP2 | TAP2 | 6p21.3 | Peptide transporter, MHC class I expression | NK, T |
Fig. 1.
Four stages in NK cell cytotoxicity affected by disease-causing mutations. (1) Granule biogenesis, which can be affected by LYST- or AP3BP1-deficiency, or to absence of cathepsin C or perforin. Granules are synthesized prior to activation. (2) NK cells recognize expression of a range of ligands for activating and inhibitory receptors on target cells. Mutations in the genes encoding the b2-integrin subunit CD18, the Fc receptor CD16, or in TAP that is required for peptide-loading and target cell expression of MHC class I molecules. (3) NK cell activation can be affected by mutations in the genes encoding CD3ζ, WASP, WASP, caspase 8, IKKγ, SAP or XIAP. Signals induce polarization of lytic granules to the MTOC, repolarization of the MTOC and granules to the immune synapse, fusion of lytic granules with other endosomal compartments, and transcriptional activation. (4) Lytic granule exocytosis is affected by mutations in the genes encoding Rab27a, Munc13-4, syntaxin-11, or Munc18-2
Leukocyte adhesion deficiency (LAD) describes a group of autosomal recessive immunodeficiencies caused by mutations in ITGB2, SLC35C1, or FERMT3 [60]. These genes encode the β2-integrin subunit (CD18), the Golgi-resident enzyme that fucosylates selectins, and the cytoplasmic protein KINDLIN3 that together with talin is crucial for integrin activation, respectively. LAD is associated with delayed separation of the umbilical cord at birth, persistent leukocytosis, and recurring bacterial and fungal infections in infancy involving the skin and mucosa. LAD affects extravasation of all immune cells in addition to lymphocyte synapse formation. To date, the function of NK cells has been studied only in LAD patients lacking CD18. NK cells from these patients display reduced ADCC and natural cytotoxicity [61, 62], but are phenotypically normal and can kill target cells upon activation with IL-2 [63].
Loss-of-function mutations and single nucleotide polymorphisms in FCGR3A, encoding the low-affinity Fc receptor CD16, have been associated with immunodeficiency or autoimmunity, in addition to diminished clinical responses to antibody-mediated therapy [8, 64, 65]. A patient with single nucleotide deletion in FCRG3A, leading to a complete loss of CD16 expression on NK cells, has been reported [66]. This patient presented with polyneuropathy. Although patients presenting with severe recurrent herpes virus- and respiratory infections and carrying the homozygous missense mutations L48H or L48R in CD16 have been previously reported [8], the consequence of these mutations for ADCC and relevance to disease is not clear [67].
A number of other receptors that bind stress-induced ligands or regulate interaction with other immune cells are implicated in triggering of natural cytotoxicity. Recently, genome-wide associations studies have linked a single nucleotide polymorphism (SNP) in CD226, conferring an amino acid substitution in the DNAM-1 receptor, with development of multiple sclerosis, rheumatoid arthritis (RA), type 1 diabetes (T1D), and autoimmune thyroid disease [68]. In addition, two intronic SNPs in CD244 that likely increase 2B4 (CD244) receptor surface expression have been associated with development of RA in a Japanese cohort [69]. Together, these data implicate activating NK cell receptors in autoimmune disease susceptibility. Moreover, several lines of evidence point to an important role of NKG2D (CD314) and NKG2D ligands in mediating self-tolerance and protection from malignancy. SNPs in KLRK1, encoding NKG2D, are associated with development of cholangiocarcinoma in patients first diagnosed with primary sclerosing cholangitis [70]. Moreover, SNPs in MICA and MICB have been associated with a variety of autoimmune disorders [71], and the locus encoding the NKG2D ligands ULBP have recently been associated with alopecia areata [72]. Despite the often-used term “NK cell receptors”, it should be noted that these receptors are also expressed on other lymphocyte populations and, therefore, the extent to which genetic associations with disease specifically reflect defects in NK cell function is not entirely clear.
A number of the polymorphisms in the rapidly evolving KIR receptors have been associated resistance to infection but may also predispose to autoimmune diseases [73]. As KIR expression is generally restricted to NK cells and a small number of effector T cells, associations between these receptors and disease provide an interesting perspective on the role of NK cells in human health. For instance, a number of associations predisposing to autoimmune diseases have been identified where activating KIR or KIR/HLA genotypes are accompanied by lack of inhibition. Scleroderma and psoriatic arthritis have been linked to the presence of activating KIR in the absence of counteracting KIR inhibitory interaction [74, 75]. Similarly, an increased risk of RA and T1D is associated with the presence of the activating KIR2DS2 gene [76, 77]. Other KIR/HLA genotypes are associated with control of hepatitis C virus (HCV), human immunodeficiency virus (HIV), and human papilloma virus (HPV) [73]. Thus, the interplay between human NK cell inhibitory and activating receptor-MHC class I ligand pairs is another independent line of research that has provided significant insights with respect to the role of NK cells in human disease.
In addition to diseases directly affecting NK cell receptors, mutations in the ligands of NK cell receptors can affect NK cell maturation and differentiation. Autosomal recessive mutations in TAP1 and TAP2, which encode constituents of the transporter-associated antigen-processing (TAP) complex, are associated with bare lymphocyte syndrome [78, 79]. TAP-deficient patients typically present with sinusitis and recurrent pulmonary infections in childhood or adolescence. Strikingly, although patients express greatly diminished surface levels of MHC class I due to a defect in antigen loading of MHC class I molecules, asymptomatic carriers have been reported [80]. CTL numbers are slightly decreased in TAP-deficient individuals, whereas NK cell numbers are normal. Interestingly, NK cells from TAP-deficient patients display reduced natural cytotoxicity. Possibly, this impaired cytotoxic capacity is due to defective inhibitory receptor-mediated “licensing” of the NK cells [20].
In conclusion, only a few receptors that are broadly expressed on NK cells and other immune cells have been associated with primary immunodeficiencies. However, a susceptibility to a number of human diseases has been associated with polymorphisms in receptors that are more or less NK cell-specific.
Genetic defects affecting proximal signals for NK cell activation
Mutations affecting genes that encode membrane proximal signaling proteins may leave early stages of NK cell development unaffected but interfere with activation. Only a few such deficiencies have so far been described (Table 3; as summarized in Fig. 1).
Table 3.
Genetic defects affecting proximal signals for NK cell activation
| Disease | Gene | Protein | Locus | Protein function | Cells affected |
|---|---|---|---|---|---|
| WAS | WASP | WASP | Xp11.23-p22 | Actin polymerization | NK, T |
| N/A | CASP8 | Caspase 8 | 2q33-q34 | Mediates NF-κB signaling | NK, myeloid |
| HED-ID | IKBKG | IKKγ/NEMO | Xq28 | Mediates NF-κB signaling | NK, myeloid |
| XLP1 | SH2D1A | SAP | Xq25 | Signal transduction | NK, T, NKT |
| XLP2 | XIAP | XIAP | Xq25 | Signal transduction | NK, T, NKT |
| N/A | ITK | ITK | 5q32 | Tyrosine kinase | T, NK? |
| CID | STIM1 | STIM1 | 11p15.5 | Calcium channel act. | NK, T, B |
| CID | ORAI1 | ORAI1 | 12q24.31 | Calcium channel | NK, T, B |
To date, two unrelated patients with autosomal recessive mutations in CD3Z, encoding the CD3ζ-chain, which is part of the T cell receptor complex and mediates signaling through CD16 and receptors for natural cytotoxicity, have been reported [81, 82]. Both patients presented in infancy with recurrent severe infections, including CMV and HSV. CD3ζ deficiency impaired T cell numbers, whereas NK cell numbers were not affected. One report noted unusual CD16+CD56− NK cells [82]. Of note, autosomal recessive mutations in TYROBP, encoding the DAP12 adaptor chain of the activating NKG2C and KIR NK cell receptors, are associated with the autosomal recessive Nasu-Hakola syndrome. DAP12-deficient patients develop presenile dementia and bone cysts due to defects in TREM2-mediated nervous tissue immune homeostasis, but so far no increased susceptibility to infections has been reported [83].
X-linked mutations in WASP, encoding the Wiskott–Aldrich syndrome protein (WASP), are associated with Wiskott–Aldrich syndrome. WASP-deficient patients are characterized by eczema, thrombocytopenia, and susceptibility to severe viral, including herpesviruses, and bacterial infections in infancy and childhood, and a propensity for autoimmune disorders and hematopoietic malignancies [84]. WASP is widely expressed in hematopoietic cells and involved in actin polymerization through binding to monomeric actin and activation of the Arp2/3 complex that catalyzes actin branching. WASP-deficient NK cells display defective NK cell cytotoxicity and degranulation that can be partly restored by IL-2 [85, 86]. WASP is also required for chemokine-induced inside-out signals for LFA-1 and chemotaxis [87].
Autosomal recessive mutations in CASP8, encoding caspase-8, are associated with a rare syndrome characterized by lymphadenopathy and splenomegaly in childhood reminiscent of autoimmune lymphoproliferative syndrome (caused by defects in Fas-mediated apoptosis), in addition to recurrent sinopulmonary and HSV infections and poor responses to immunization [88]. In addition to defective Fas-mediated apoptosis, studies of caspase 8-deficient patients have revealed a role for caspase 8 in CD16 or 2B4-induced signals for NF-κB activation in NK cells, as well as antigen receptor-induced signals for NF-κB activation in T cells and B cells [89]. In T cells, caspase 8 has been shown to link the Bcl10-MALT1 complex to activation of IKKα and IKKβ, which is required for nuclear translocation of NKκB [89].
X-linked mutations in IKBKG, encoding the inhibitor of NF-κB kinase subunit γ (IKK-γ) and also known as NF-κB essential modulator (NEMO), are associated with a syndrome termed hypohidrotic ectodermal dysplasia with immunodeficiency (HED-ID) [90, 91]. A spectrum of missense mutations are found in affected males, while complete loss-of-function mutations affect females, whose male offspring die in utero. HED-ID affects hair, tooth, and sweat gland development and is associated with susceptibility to bacterial and viral infections, and dysgammaglobulinemia. NEMO deficiency results in reduced natural cytotoxicity [92]. Interestingly, IL-2 stimulation of NEMO-deficient NK cells can rescue NF-κB activation and partially restore NK cell function [92]. Moreover, NEMO deficiency results in defects in cytokine production by APCs and immunoglobulin class switching by B cells [91, 93].
The X-linked lymphoproliferative syndrome (XLP1) and XLP2 are caused by mutations in SH2D1A and XIAP, encoding SLAM-associated protein (SAP) and X-linked inhibitor of apoptosis (XIAP), respectively [94, 95]. XLP1 patients typically present in adolescence with life-threatening EBV infections. One-third of XLP1 patients develop the hyperinflammatory syndrome hemophagocytic lymphohistiocytosis (HLH), whereas other patients may present with lymphoma or hypogammaglobulinemia [94, 96]. XLP2 patients generally present with HLH [95, 97]. SAP is expressed is expressed in lymphocytes, including NK cells, and signals for activation via SLAM receptors by recruitment of the Fyn kinase, which in turn recruits and phosphorylates downstream signaling proteins such as Vav1. SAP-deficient NK cells display defective activation induced by SLAM family receptors such as 2B4, NTB-A, and CRACC [98]. Cytotoxicity is reduced to, on average, half normal levels in NK cells from these patients, with mild improvement upon interferon stimulation in vitro [99, 100]. In the case of SAP-deficient XLP1 patients, accumulating evidence suggests not only a defect of cytotoxic lymphocyte effector function but also target cell intrinsic defects in signaling for apoptosis in the absence of SAP [101]. XIAP binds to and inhibits caspases, thereby inhibiting cellular apoptosis, but the mechanism whereby XIAP deficiency causes HLH is not clear.
Of note, autosomal recessive mutations in ITK, encoding the IL-2 inducible T-cell kinase (ITK), were recently identified along with fatal EBV-induced lymphoproliferation in a single family with two affected siblings [102]. Immunological evaluation of the two ITK-deficient patients revealed slightly reduced numbers of T cells with an increased memory phenotype but proliferation in response to mitogens, and similarly reduced numbers of NK cells, which were able to kill K562 target cells. Experiments with overexpression or knock-down of ITK in NK cells suggested that ITK is involved in positive regulation of CD16 signaling, but might negatively regulate receptors for natural cytotoxicity [103].
Finally, autosomal recessive mutations in ORAI1 and STIM1, encoding ORAI1 and the stromal interaction molecule-1 (STIM1), result in a combined immunodeficiency syndrome [104]. STIM1 transactivates the Ca2+-release activated Ca2+-channel ORAI1 upon depletion of the endoplasmic reticulum Ca2+ stores and both proteins are required for store-operated Ca2+ entry. In ORAI1- or STIM1-deficient patients, lymphocytes develop but activation by antigen receptors does not induce cytokine secretion [104]. Recently, a requirement for store-operated Ca2+-entry by ORAI1 and STIM1 in NK cell lytic granule exocytosis as well as cytokine and chemokine production has been established [105].
Clearly, there is a paucity of data associating defects in lymphocyte signaling to disease. This fact may be the result of challenges in studying immune cell signaling in human lymphocytes. Lymphocyte signaling is complex, but new techniques of genome analysis and biochemical characterization of signaling in lymphocyte subsets promises to provide further insights into the link between defects in immune cell signaling and disease.
Genetic defects specifically impairing NK cell cytotoxicity
Understanding of the molecular mechanisms of lymphocyte cytotoxicity has been provided by studies of patients with lymphoproliferative disorders that often are triggered by viral infections [106]. Autosomal recessive nonsense mutations in PRF1, encoding perforin, are associated with an early onset, often fatal familial HLH type 2 (FHL2), whereas other nonsense mutations in PRF1 may predispose to hematological malignancies [107, 108]. Furthermore, data from other patients with similar clinical presentations caused by mutations in a number of genes encoding intracellular proteins have elucidated the molecular mechanisms controlling biogenesis, maturation, trafficking, and the release of secretory lysosomes for target cell killing by NK cells and cytotoxic T cells (Table 4; as summarized in Fig. 1, detailed in Fig. 2). In the following sections, diseases associated with loss of lytic granule biogenesis and exocytosis are discussed.
Table 4.
Genetic defects affecting NK cell cytotoxicity
| Disease | Gene | Protein | Locus | Protein function | Cells affected |
|---|---|---|---|---|---|
| FHL2 | PRF1 | Perforin | 10q21-22 | Target cell lysis | NK, CTL |
| CHS | LYST | LYST | 1q42.1-2 | Granule biogenesis | NK, CTL, melanosomes |
| HPS2 | AP3B1 | AP3 | 5q14.1 | Granule trafficking | NK, CTL, melanosomes |
| PLS | CTSC | Cathepsin C | 11q14.2 | Granzyme activation | Leukocytes |
| FHL3 | UNC13D | Munc13-4 | 17q25 | Granule exocytosis | NK, CTL, platelets? |
| FHL4 | STX11 | Syntaxin 11 | 6q24 | Granule exocytosis | NK, CTL |
| FHL5 | STXBP2 | Munc18-2 | 19p13.3-2 | Granule exocytosis | NK, CTL, platelets? |
| GS2 | RAB27A | Rab27a | 15q15-21 | Granule exocytosis and motility | NK, CTL, platelets, melanosomes |
| MYH9 | Myosin IIA | 22q13.1 | Granule exocytosis | Leukocytes |
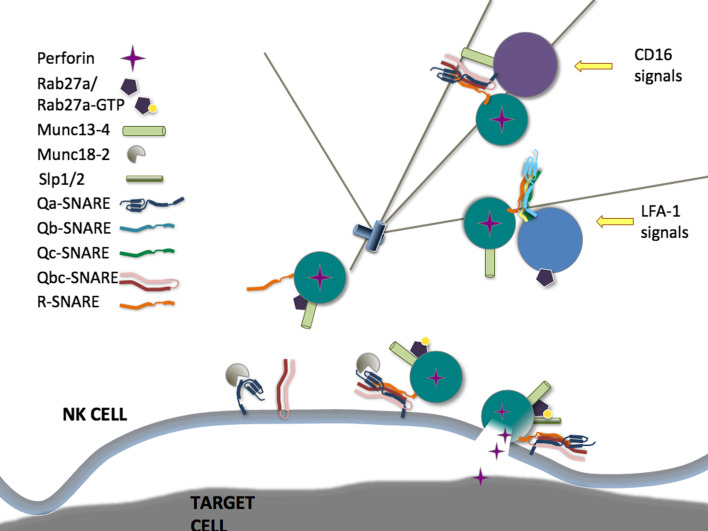
Fig. 2.
Regulation of lytic granule exocytosis. CD16 signals for co-localization of Munc13-4 to perforin granules, while LFA-1 signals for co-localization of Rab27a to perforin granules. Mature granules at the immune synapse are positive for Munc13-4 and Rab27a. Rab27a has multiple effectors during exocytosis, including Munc13-4 and Slp1 and/or Slp2. Unknown SNARE complexes mediate these fusion events, and Stx11 and Munc18-2, which interact directly, are required for exocytosis at an unknown stage
Genetic defects in granule biogenesis and cytolytic mediators
Two autosomal recessive syndromes, Chediak–Higashi syndrome (CHS) and Hermansky–Pudlak syndrome type 2 (HPS2), are caused by mutations in LYST and AP3BP1, respectively [109, 110]. These genes encode the lysosomal trafficking regulator protein LYST and the adaptor protein-3 (AP-3) binding protein β-subunit. CHS patients often present with HLH, but also display hypopigmentation, a bleeding disorder, and neuropathy, as lysosomes in all cell types are affected. Similarly, HPS2 patients display hypopigmentation and a bleeding disorder, but rarely develop HLH [111]. Thirty years ago, seminal work by Fauci and colleagues [112] demonstrated that LYST-deficient patients are defective in NK cell cytotoxicity. Lymphocytes from CHS patients contain abnormal enlarged lytic granules that are reduced in number, do not polarize to the MTOC, and fail to undergo exocytosis [113]. LYST is a member of a BEACH domain-containing protein family which may regulate a number of aspects of protein sorting and trafficking in the endosomal system [114]. The Ca2+-dependent proteolysis of activated PKC by calpain is increased in LYST-deficient cells, as is the activity of sphingomyelinase, suggesting that these enzymes may mediate LYST regulation of trafficking and lysosomal permeability [115]. HPS2 patients lack function of the ubiquitous AP3 complex, which regulates sorting at the trans-Golgi network of cargo destined for endosomal and lysosomal organelles. AP3-deficiency results in mis-sorting to the plasma membrane of proteins including CD63 and LAMPs, and slightly enlarged granules [111]. While early reports did not demonstrate reduced NK cell activity or counts, more recent studies have reported reduced NK and NKT cell counts, reduced perforin expression, and lymphocyte cytotoxicity in freshly isolated patient NK cells, with some recovery of cytotoxicity upon IL-2 treatment [116–118].
More specifically affecting cytotoxicity by NK cells and T cells, perforin deficiency leads to an inability to lyse target cells without affecting granule exocytosis per se [107]. Upon exocytosis, perforin binds to calcium on the target cell membrane, oligomerizes, and enables the entry of apoptosis-inducing granzymes into the cytosol of the target through formation of pores [119]. The structure of perforin has recently been solved, revealing a thin ‘key-shaped’ molecule, comprising an N-terminal membrane attack complex followed by an epidermal growth factor (EGF) domain that, together with the extreme carboxy-terminal sequence, forms a central shelf-like structure for breaching of the target cell membrane and a C-terminal Ca2+-binding C2 domain [120]. Perforin pores consist of 20 monomers on average. Prior to release, perforin and granzymes are stored with other granule contents such as Fas ligand and cytokines in specialized lysosomal organelles [33]. Thus far, no patients have been identified with mutations in genes encoding for granzymes. Studies of knock-out mice indicate that single knockouts for granzyme A or B do not have increased disease susceptibility, whereas double knock-out of granzyme A and granzyme B are more susceptible to viral infections [121]. Interestingly, autosomal recessive mutations in CTSC, encoding the oligomeric lysosomal protease cathepsin C, cause Papillon–Lefevre syndrome, which is associated with palmoplantar keratoderma and periodontitis, in addition to susceptibility to viral infections [122]. Cathepsin C is required for processing of granzyme A and B [123, 124]. Consequently, NK cells from cathepsin C-deficient patients display defective NK cell cytotoxicity due to defective processing of granzymes [125]. In summary, while perforin deficiency is the most severe and penetrant disorder of lytic granule content expression or granule biogenesis, other defects may affect lymphocyte cytotoxicity and function of other immune cells, increasing susceptibility to infection.
Genetic defects in exocytosis of lytic granules
Other immunodeficiency syndromes caused by autosomal recessive mutations also manifest with HLH. These genes include RAB27A, UNC13D, STX11, and STXBP2, encoding Rab27a, Munc13-4, syntaxin 11 (Stx11), and Munc18-2, and associated with Griscelli syndrome type 2 (GS2), FHL3, FHL4, and FHL5, respectively [126–130]. Aside from typically presenting with HLH in infancy with a mean age at onset similar to that of Munc13-4-deficient patients, Rab27a-deficient patients also display hypopigmentation due to defects in melanosomes [131]. Stx11 and Munc18-2-deficient patients also generally present with HLH in infancy or childhood, with mean onset somewhat later than that of Rab27a- and Munc13-4-deficient patients. In addition to HLH, Munc18-2- and Munc13-4-deficient patients may manifest with hypogammaglobulinemia, whereas episodes of gastroenteritis are specific to Munc18-2-deficient patients [131, 132]. A common denominator of mutations in these different genes is impaired secretory lysosome exocytosis, although the granules form correctly [127, 129, 130, 133]. Interestingly, cytokine stimulation of NK cells in vitro can partially restore degranulation by Stx11- and Munc18-2-deficient NK cells [129, 130, 133], correlating with disease severity among the FHL subtypes. These proteins belong to families that regulate vesicle trafficking and membrane fusion and their roles in secretory lysosome exocytosis have been elucidated to varying degrees. Below, we briefly discuss how these proteins regulate NK cell cytotoxicity.
Ligands for LFA-1 are abundant during infection, and thus LFA-1-mediated signals likely represent an early signal in NK cell recognition [28]. In resting cells, Rab27a and Munc13-4 do not co-localize with perforin, but are recruited to secretory lysosomes following activation by target cells [134, 135]. Interestingly, engagement of LFA-1 on NK cells preferentially induces co-localization of Rab27a to secretory lysosomes [135]. Conversely, Munc13-4 is recruited to secretory lysosomes following engagement of CD16 or other receptor combinations that induce intracellular Ca2+-mobilization, and this recruitment is dependent on Rab27a. Recent data suggest that the protein MADD might be a guanine exchange factor responsible for activation of Rab27a [136], whereas Slp1, Slp2a, and Munc13-4 can bind to active, GTP-bound Rab27a and constitute effector proteins in immune cells [137–139]. Although overexpression of dominant negative forms of Slp2a impairs CTL degranulation, mutations in SYTL1 and SYTL2 have not been reported in HLH patients [138, 139]. Furthermore, CTL from Slp1 or Slp2a-deficient mice have no defect in degranulation, suggesting functional compensation by the two isoforms [138]. By contrast, Munc13-4 is essential for exocytosis of secretory lysosomes by NK cells and CTL [127, 133, 140].
In contrast to melanocytes, where melanophilin promotes Rab27a binding to myosin Va, facilitating microtuble plus-end directed transport of melanosomes, secretory lysosomes in cytotoxic lymphocytes undergo dynein-dependent, minus-end directed movement towards the MTOC, which then polarizes to the IS [27, 141]. In contrast, live cell imaging has indicated that Rab27a restricts secretory lysosome movement in the cytosol of mouse NK cells and human NK cell lines [142]. In human and mouse CTLs lacking functional Munc13-4 or Rab27a, perforin granules polarize normally to the IS, indicating a defect in the final stage of exocytosis [127, 143]. There is some observational evidence that granules in Rab27a-deficient mouse and human CTLs do not dock closely at the plasma membrane [143], however, quantitative measurements have not been published. Myosin IIA, associated with lytic granules, facilitates final transit through the actin-rich IS to the plasma membrane [144]. In NK cells from patients with a heterozygous truncation mutation in MYH9 or in NK cells where myosin IIA expression was knocked down, lytic granule penetration into F-actin at the IS, interaction of isolated granules with F-actin, lytic granule exocytosis, and target cell lysis are all decreased [144, 145]. The precise molecular function of Munc13-4 in lytic granule exocytosis is unclear. Munc13 proteins contain one C1 and two C2 domains, along with two Munc-homology domains. The neuronal homolog Munc13-1 binds Ca2+/calmodulin and diacylglycerol to regulate exocytosis, and interacts directly with Stx1 to prime vesicles by modulating SNARE conformation [146, 147]. No interaction has been demonstrated between Munc13-4 and Stx11 or other SNARE proteins expressed in lymphocytes.
Stx11 is 1 of 36 mammalian SNARE proteins, which act in complexes of 3–4 proteins to allow fusion between two membranes by overcoming the repulsion between two lipid bilayers [148]. Stx11 is an atypical SNARE in that it contains a Qa-SNARE motif but no transmembrane domain, though it still associates strongly with membranes through a cysteine-rich C-terminal domain. SNARE complex partners or a subcellular localization have not been identified for Stx11 in cytotoxic lymphocytes, though in B cells Stx11 binds to SNAP-23, and in HeLa cells overexpressed Stx11 localized to the same perinuclear membranes as the transferrin receptor and mannose-6-phosphate receptor but not LAMP1 or a Golgi-resident protein, suggesting a possible trans-Golgi network or recycling endosome localization [149–151]. Thus, while it can reasonably be hypothesized that Stx11 regulates a membrane fusion event in the exocytic pathway of perforin granules, this has not been demonstrated to date. In addition to Stx11, Munc18-2 can bind and stabilize other Qa-SNARE syntaxins, and regulate the binding of their cognate SNARE complex partners and effectors [152, 153]. As Munc18-2 is widely expressed, this may explain why Munc18-2-deficient patients suffer from gastrointestinal symptoms [131].
Through the study of genetic immunodeficiencies affecting lymphocyte degranulation, four critical mediators of exocytosis have been identified. Further studies are required to elucidate their precise molecular roles and interaction partners, in order to build a complete picture of lytic granule release.
Conclusions
As the expression of particular genes seldom is restricted to any specific cell type, caution must be exerted when making inferences from associations between genetic aberrations and human disease. Still, a number of defined primary immunodeficiency syndromes affecting several cellular lineages in addition to several more NK cell-specific, yet poorly defined, deficiencies have provided compelling evidence for a role of NK cells in protection from viral infections, particularly of the herpes virus family. Moreover, primary defects in cytotoxic function are associated with hyperproliferative disorders, suggesting that NK cells might exert an important role in mediating immune homeostasis. Furthermore, increasing evidence from human genetics as well as animal models suggests a role for NK cells in tumor immunosurveillance and in self-tolerance. Certain new associations are not fully understood. For example, mutations in CLEC16A, encoding a C-type lectin-like protein, have been associated with T1D and multiple sclerosis [154, 155]. CLEC16A is highly expressed in NK cells, but its function is as yet unknown. Not only does the study of these genetic associations with disease provide important insights into the role of NK cells in immune defense, they also provide crucial mechanistic insight into the mechanisms of human NK cell development and effector function.
An increasing number of patients with loss-of-function mutations in genes required for immunity have been identified [156]. Remarkably, despite defects in crucial immune elements, these individuals may remain healthy for many years and control multiple infections. Thus, studies of immunodeficient patients also highlight resilience and redundancy in the immune system. Meticulous population-based studies of immune function in the context of environmental factors and genetic variation can provide more insights into the fascinating interplay of human genetic variation, disease, and parameters of NK cell function.
In a clinical setting, evaluation of NK cell function is useful for providing rapid diagnosis and facilitating prompt treatment in settings of life-threatening primary immunodeficiencies affecting lymphocyte cytotoxic function, such as FHL. Interestingly, the degree of defects in NK cell cytotoxicity and degranulation appear to correlate with disease severity [132, 133, 157]. Thus, further understanding of how NK cell development and function relates to human health can aid clinical decisions.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 3.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 10.Flodstrom-Tullberg M, Bryceson YT, Shi FD, Hoglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr Opin Immunol. 2009;21:634–640. doi: 10.1016/j.coi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 11.von Bubnoff D, Andres E, Hentges F, Bieber T, Michel T, Zimmer J. Natural killer cells in atopic and autoimmune diseases of the skin. J Allergy Clin Immunol. 2010;125:60–68. doi: 10.1016/j.jaci.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 14.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Munz C, Thiel A, Moretta L, Ferlazzo G. CD56brightCD16—killer Ig-like receptor—NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 15.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, Liu S, McClory S, Marcucci G, Trotta R, Caligiuri MA. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116:1299–1307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- 18.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK cell differentiation uncoupled from NK cell education. Blood. 2010;116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 22.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 23.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21:2241–2256. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culley FJ, Johnson M, Evans JH, Kumar S, Crilly R, Casasbuenas J, Schnyder T, Mehrabi M, Deonarain MP, Ushakov DS, Braud V, Roth G, Brock R, Kohler K, Davis DM. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7:e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Das A, Gross CC, Bryceson YT, Long EO. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity. 2010;32:175–186. doi: 10.1016/j.immuni.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 34.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 35.Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Naval J, Anel A. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- 36.Davis DM. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9:543–555. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- 37.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 38.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munz C. Non-cytotoxic protection by human NK cells in mucosal secondary lymphoid tissues. Eur J Immunol. 2008;38:2946–2948. doi: 10.1002/eji.200838849. [DOI] [PubMed] [Google Scholar]
- 40.Fischer A, Latour S, de Saint Basile G. Genetic defects affecting lymphocyte cytotoxicity. Curr Opin Immunol. 2007;19:348–353. doi: 10.1016/j.coi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Hammartrom L, Nonoyama S, Ochs HD, Puck J, Roifman C, Seger R, Wedgwood J. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 43.Gilmour KC, Fujii H, Cranston T, Davies EG, Kinnon C, Gaspar HB. Defective expression of the interleukin-2/interleukin-15 receptor beta subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood. 2001;98:877–879. doi: 10.1182/blood.v98.3.877. [DOI] [PubMed] [Google Scholar]
- 44.Lee KN, Kang HS, Jeon JH, Kim EM, Yoon SR, Song H, Lyu CY, Piao ZH, Kim SU, Han YH, Song SS, Lee YH, Song KS, Kim YM, Yu DY, Choi I. VDUP1 is required for the development of natural killer cells. Immunity. 2005;22:195–208. doi: 10.1016/j.immuni.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O’Shea JJ, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 46.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, Migone TS, Noguchi M, Markert ML, Buckley RH, O’Shea JJ, Leonard WJ. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 47.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349:1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 49.Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, Rosenfeld RG. Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b. J Clin Endocrinol Metab. 2005;90:4260–4266. doi: 10.1210/jc.2005-0515. [DOI] [PubMed] [Google Scholar]
- 50.Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, Ornani A, Paz R, Rivarola MA, Zelazko M, Belgorosky A. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118:e1584–e1592. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- 51.Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- 52.Apasov SG, Blackburn MR, Kellems RE, Smith PT, Sitkovsky MV. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling. J Clin Invest. 2001;108:131–141. doi: 10.1172/JCI10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146:423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, McMahon C, Smith O, Casanova JL, Abel L, Feighery C. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78:721–727. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eidenschenk C, Jouanguy E, Alcais A, Mention JJ, Pasquier B, Fleckenstein IM, Puel A, Gineau L, Carel JC, Vivier E, Le Deist F, Casanova JL. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177:8835–8843. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 56.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 57.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 58.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 59.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 60.Etzioni A. Genetic etiologies of leukocyte adhesion defects. Curr Opin Immunol. 2009;21:481–486. doi: 10.1016/j.coi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Kohl S, Loo LS, Schmalstieg FS, Anderson DC. The genetic deficiency of leukocyte surface glycoprotein Mac-1, LFA-1, p150,95 in humans is associated with defective antibody-dependent cellular cytotoxicity in vitro and defective protection against herpes simplex virus infection in vivo. J Immunol. 1986;137:1688–1694. [PubMed] [Google Scholar]
- 62.Lau YL, Low LC, Jones BM, Lawton JW. Defective neutrophil and lymphocyte function in leucocyte adhesion deficiency. Clin Exp Immunol. 1991;85:202–208. doi: 10.1111/j.1365-2249.1991.tb05705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castriconi R, Dondero A, Cantoni C, Della Chiesa M, Prato C, Nanni M, Fiorini M, Notarangelo L, Parolini S, Moretta L, Moretta A, Bottino C. Functional characterization of natural killer cells in type I leukocyte adhesion deficiency. Blood. 2007;109:4873–4881. doi: 10.1182/blood-2006-08-038760. [DOI] [PubMed] [Google Scholar]
- 64.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 65.Kastbom A, Ahmadi A, Soderkvist P, Skogh T. The 158V polymorphism of Fc gamma receptor type IIIA in early rheumatoid arthritis: increased susceptibility and severity in male patients (the Swedish TIRA project) Rheumatology (Oxford) 2005;44:1294–1298. doi: 10.1093/rheumatology/kei010. [DOI] [PubMed] [Google Scholar]
- 66.Takeuchi T, Nakagawa T, Ikemoto T, Sasaki M, Makino S, Shimizu A, Ohsawa N. A novel mutation in the FcgammaRIIIA gene (CD16) results in active natural killer cells lacking CD16. Autoimmunity. 1999;31:265–271. doi: 10.3109/08916939908994072. [DOI] [PubMed] [Google Scholar]
- 67.Lenart M, Trzyna E, Rutkowska M, Bukowska-Strakova K, Szaflarska A, Pituch-Noworolska A, Szczepanik A, Zembala M, Siedlar M. The loss of the CD16 B73.1/Leu11c epitope occurring in some primary immunodeficiency diseases is not associated with the FcgammaRIIIa-48L/R/H polymorphism. Int J Mol Med. 2010;26:435–442. [PubMed] [Google Scholar]
- 68.Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM, Maisuria M, Duley S, Coleman G, Gough SC, Worthington J, Kuchroo VK, Wicker LS, Todd JA. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10:5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki A, Yamada R, Kochi Y, Sawada T, Okada Y, Matsuda K, Kamatani Y, Mori M, Shimane K, Hirabayashi Y, Takahashi A, Tsunoda T, Miyatake A, Kubo M, Kamatani N, Nakamura Y, Yamamoto K. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat Genet. 2008;40:1224–1229. doi: 10.1038/ng.205. [DOI] [PubMed] [Google Scholar]
- 70.Melum E, Karlsen TH, Schrumpf E, Bergquist A, Thorsby E, Boberg KM, Lie BA. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology. 2008;47:90–96. doi: 10.1002/hep.21964. [DOI] [PubMed] [Google Scholar]
- 71.Van Belle TL, von Herrath MG. The role of the activating receptor NKG2D in autoimmunity. Mol Immunol. 2009;47:8–11. doi: 10.1016/j.molimm.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, Kim H, Singh P, Lee A, Chen WV, Meyer KC, Paus R, Jahoda CA, Amos CI, Gregersen PK, Christiano AM. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008;20:343–352. doi: 10.1016/j.smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Momot T, Koch S, Hunzelmann N, Krieg T, Ulbricht K, Schmidt RE, Witte T. Association of killer cell immunoglobulin-like receptors with scleroderma. Arthritis Rheum. 2004;50:1561–1565. doi: 10.1002/art.20216. [DOI] [PubMed] [Google Scholar]
- 75.Nelson GW, Martin MP, Gladman D, Wade J, Trowsdale J, Carrington M. Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. J Immunol. 2004;173:4273–4276. doi: 10.4049/jimmunol.173.7.4273. [DOI] [PubMed] [Google Scholar]
- 76.Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM, Goronzy JJ. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001;193:1159–1167. doi: 10.1084/jem.193.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Slik AR, Alizadeh BZ, Koeleman BP, Roep BO, Giphart MJ. Modelling KIR-HLA genotype disparities in type 1 diabetes. Tissue Antigens. 2007;69(Suppl 1):101–105. doi: 10.1111/j.1399-0039.2006.762_5.x. [DOI] [PubMed] [Google Scholar]
- 78.de la Salle H, Hanau D, Fricker D, Urlacher A, Kelly A, Salamero J, Powis SH, Donato L, Bausinger H, Laforet M, et al. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science. 1994;265:237–241. doi: 10.1126/science.7517574. [DOI] [PubMed] [Google Scholar]
- 79.Furukawa H, Murata S, Yabe T, Shimbara N, Keicho N, Kashiwase K, Watanabe K, Ishikawa Y, Akaza T, Tadokoro K, Tohma S, Inoue T, Tokunaga K, Yamamoto K, Tanaka K, Juji T. Splice acceptor site mutation of the transporter associated with antigen processing-1 gene in human bare lymphocyte syndrome. J Clin Invest. 1999;103:755–758. doi: 10.1172/JCI5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerundolo V, de la Salle H. Description of HLA class I- and CD8-deficient patients: insights into the function of cytotoxic T lymphocytes and NK cells in host defense. Semin Immunol. 2006;18:330–336. doi: 10.1016/j.smim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Rieux-Laucat F, Hivroz C, Lim A, Mateo V, Pellier I, Selz F, Fischer A, Le Deist F. Inherited and somatic CD3zeta mutations in a patient with T-cell deficiency. N Engl J Med. 2006;354:1913–1921. doi: 10.1056/NEJMoa053750. [DOI] [PubMed] [Google Scholar]
- 82.Roberts JL, Lauritsen JP, Cooney M, Parrott RE, Sajaroff EO, Win CM, Keller MD, Carpenter JH, Carabana J, Krangel MS, Sarzotti M, Zhong XP, Wiest DL, Buckley RH. T-B+NK+ severe combined immunodeficiency caused by complete deficiency of the CD3zeta subunit of the T-cell antigen receptor complex. Blood. 2007;109:3198–3206. doi: 10.1182/blood-2006-08-043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 84.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 85.Orange JS, Ramesh N, Remold-O’Donnell E, Sasahara Y, Koopman L, Byrne M, Bonilla FA, Rosen FS, Geha RS, Strominger JL. Wiskott–Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gismondi A, Cifaldi L, Mazza C, Giliani S, Parolini S, Morrone S, Jacobelli J, Bandiera E, Notarangelo L, Santoni A. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104:436–443. doi: 10.1182/blood-2003-07-2621. [DOI] [PubMed] [Google Scholar]
- 87.Stabile H, Carlino C, Mazza C, Giliani S, Morrone S, Notarangelo LD, Notarangelo LD, Santoni A, Gismondi A. Impaired NK-cell migration in WAS/XLT patients: role of Cdc42/WASp pathway in the control of chemokine-induced beta2 integrin high-affinity state. Blood. 2010;115:2818–2826. doi: 10.1182/blood-2009-07-235804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 89.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 90.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, Shapira SK, Farndon PA, Wara DW, Emmal SA, Ferguson BM. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israel A, Courtois G, Casanova JL. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 92.Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, Nurko S, Rasmussen WL, Kohler JR, Gellis SE, Ferguson BM, Strominger JL, Zonana J, Ramesh N, Ballas ZK, Geha RS. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109:1501–1509. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 94.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 95.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 96.Arico M, Imashuku S, Clementi R, Hibi S, Teramura T, Danesino C, Haber DA, Nichols KE. Hemophagocytic lymphohistiocytosis due to germline mutations in SH2D1A, the X-linked lymphoproliferative disease gene. Blood. 2001;97:1131–1133. doi: 10.1182/blood.v97.4.1131. [DOI] [PubMed] [Google Scholar]
- 97.Marsh RA, Madden L, Kitchen BJ, Mody R, McClimon B, Jordan MB, Bleesing JJ, Zhang K, Filipovich AH. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan JL, Byron KS, Brewster FE, Purtilo DT. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 1980;210:543–545. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- 100.Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, van Hoff J, Dhar D, Nichols KE, Filipovich AH, Su HC, Bleesing JJ, Lenardo MJ. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagy N, Matskova L, Kis LL, Hellman U, Klein G, Klein E. The proapoptotic function of SAP provides a clue to the clinical picture of X-linked lymphoproliferative disease. Proc Natl Acad Sci USA. 2009;106:11966–11971. doi: 10.1073/pnas.0905691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, Telieps T, Knapp S, Wacker HH, Meindl A, Jumaa H, Borkhardt A. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119:1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khurana D, Arneson LN, Schoon RA, Dick CJ, Leibson PJ. Differential regulation of human NK cell-mediated cytotoxicity by the tyrosine kinase Itk. J Immunol. 2007;178:3575–3582. doi: 10.4049/jimmunol.178.6.3575. [DOI] [PubMed] [Google Scholar]
- 104.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maul-Pavicic A, Chiang SCC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM, Sjöqvist S, Hufnagel M, Schulze I, Bass T, Schamel WWA, Fuchs S, Pircher H, McCarl C-A, Mikoshiba K, Schwarz K, Feske S, Bryceson YT, Ehl S (2011) ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci USA (in press) [DOI] [PMC free article] [PubMed]
- 106.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–253. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 107.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 108.Chia J, Yeo KP, Whisstock JC, Dunstone MA, Trapani JA, Voskoboinik I. Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer. Proc Natl Acad Sci USA. 2009;106:9809–9814. doi: 10.1073/pnas.0903815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barbosa MD, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RC, Lovett M, Kingsmore SF. Identification of the homologous beige and Chediak–Higashi syndrome genes. Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky–Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 111.Badolato R, Parolini S. Novel insights from adaptor protein 3 complex deficiency. J Allergy Clin Immunol. 2007;120:735–741. doi: 10.1016/j.jaci.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 112.Roder JC, Haliotis T, Klein M, Korec S, Jett JR, Ortaldo J, Heberman RB, Katz P, Fauci AS. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980;284:553–555. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- 113.Baetz K, Isaaz S, Griffiths GM. Loss of cytotoxic T lymphocyte function in Chediak–Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J Immunol. 1995;154:6122–6131. [PubMed] [Google Scholar]
- 114.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 115.Tanabe F, Kasai H, He L, Kin T, Fujikado T, Kumamoto T, Hara T, Iwata T, Ito M. Improvement of deficient natural killer activity and delayed bactericidal activity by a thiol proteinase inhibitor, E-64-d, in leukocytes from Chediak–Higashi syndrome patients in vitro. Int Immunopharmacol. 2009;9:366–370. doi: 10.1016/j.intimp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Jung J, Bohn G, Allroth A, Boztug K, Brandes G, Sandrock I, Schaffer AA, Rathinam C, Kollner I, Beger C, Schilke R, Welte K, Grimbacher B, Klein C. Identification of a homozygous deletion in the AP3B1 gene causing Hermansky–Pudlak syndrome, type 2. Blood. 2006;108:362–369. doi: 10.1182/blood-2005-11-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fontana S, Parolini S, Vermi W, Booth S, Gallo F, Donini M, Benassi M, Gentili F, Ferrari D, Notarangelo LD, Cavadini P, Marcenaro E, Dusi S, Cassatella M, Facchetti F, Griffiths GM, Moretta A, Notarangelo LD, Badolato R. Innate immunity defects in Hermansky–Pudlak type 2 syndrome. Blood. 2006;107:4857–4864. doi: 10.1182/blood-2005-11-4398. [DOI] [PubMed] [Google Scholar]
- 118.Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle EM, Kontny U, Muller C, Nurden A, Rohr J, Henschen M, Pannicke U, Niemeyer C, Nurden P, Ehl S. Lethal hemophagocytic lymphohistiocytosis in Hermansky–Pudlak syndrome type II. Blood. 2006;108:81–87. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- 119.Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: structure, function, and role in human immunopathology. Immunol Rev. 2010;235:35–54. doi: 10.1111/j.0105-2896.2010.00896.x. [DOI] [PubMed] [Google Scholar]
- 120.Law RH, Lukoyanova N, Voskoboinik I, Caradoc-Davies TT, Baran K, Dunstone MA, D’Angelo ME, Orlova EV, Coulibaly F, Verschoor S, Browne KA, Ciccone A, Kuiper MJ, Bird PI, Trapani JA, Saibil HR, Whisstock JC. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468:447–451. doi: 10.1038/nature09518. [DOI] [PubMed] [Google Scholar]
- 121.Simon MM, Hausmann M, Tran T, Ebnet K, Tschopp J, ThaHla R, Mullbacher A. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A x B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J Exp Med. 1997;186:1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toomes C, James J, Wood AJ, Wu CL, McCormick D, Lench N, Hewitt C, Moynihan L, Roberts E, Woods CG, Markham A, Wong M, Widmer R, Ghaffar KA, Pemberton M, Hussein IR, Temtamy SA, Davies R, Read AP, Sloan P, Dixon MJ, Thakker NS. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet. 1999;23:421–424. doi: 10.1038/70525. [DOI] [PubMed] [Google Scholar]
- 123.Smyth MJ, McGuire MJ, Thia KY. Expression of recombinant human granzyme B. A processing and activation role for dipeptidyl peptidase I. J Immunol. 1995;154:6299–6305. [PubMed] [Google Scholar]
- 124.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meade JL, de Wynter EA, Brett P, Sharif SM, Woods CG, Markham AF, Cook GP. A family with Papillon–Lefevre syndrome reveals a requirement for cathepsin C in granzyme B activation and NK cell cytolytic activity. Blood. 2006;107:3665–3668. doi: 10.1182/blood-2005-03-1140. [DOI] [PubMed] [Google Scholar]
- 126.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 127.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, de Saint Basile G. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 128.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, Schneppenheim R, Nurnberg P, Janka G, Hennies HC. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 129.Cote M, Menager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al-Manjomi F, Al-Harbi M, Alangari A, Le Deist F, Gennery AR, Prince N, Cariou A, Nitschke P, Blank U, El-Ghazali G, Menasche G, Latour S, Fischer A, de Saint Basile G. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nurnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S, Hennies HC. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meeths M, Entesarian M, Al-Herz W, Chiang SC, Wood SM, Al-Ateeqi W, Almazan F, Boelens JJ, Hasle H, Ifversen M, Lund B, van den Berg JM, Gustafsson B, Hjelmqvist H, Nordenskjold M, Bryceson YT, Henter JI. Spectrum of clinical presentations in familial hemophagocytic lymphohistiocytosis (FHL) type 5 patients with mutations in STXBP2. Blood. 2010;116:2635–2643. doi: 10.1182/blood-2010-05-282541. [DOI] [PubMed] [Google Scholar]
- 132.Rohr J, Beutel K, Maul-Pavicic A, Vraetz T, Thiel J, Warnatz K, Bondzio I, Gross-Wieltsch U, Schundeln M, Schutz B, Woessmann W, Groll AH, Strahm B, Pagel J, Speckmann C, Janka G, Griffiths G, Schwarz K, Zur Stadt U, Ehl S. Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases. Haematologica. 2010;95:2080–2087. doi: 10.3324/haematol.2010.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, Winiarski J, Roche PA, Nordenskjold M, Henter JI, Long EO, Ljunggren HG. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint Basile G. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 135.Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, Horiuchi H, Rosthoj S, Rutynowska O, Winiarski J, Stow JL, Nordenskjold M, Henter JI, Ljunggren HG, Bryceson YT. Different NK cell activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117–4127. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- 136.Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol. 2010;191:367–381. doi: 10.1083/jcb.201008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shirakawa R, Higashi T, Tabuchi A, Yoshioka A, Nishioka H, Fukuda M, Kita T, Horiuchi H. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J Biol Chem. 2004;279:10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- 138.Holt O, Kanno E, Bossi G, Booth S, Daniele T, Santoro A, Arico M, Saegusa C, Fukuda M, Griffiths GM. Slp1 and Slp2-a Localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic. 2008;9:446–457. doi: 10.1111/j.1600-0854.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Menasche G, Menager MM, Lefebvre JM, Deutsch E, Athman R, Lambert N, Mahlaoui N, Court M, Garin J, Fischer A, de Saint Basile G. A newly identified isoform of Slp2a associates with Rab27a in cytotoxic T cells and participates to cytotoxic granule secretion. Blood. 2008;112:5052–5062. doi: 10.1182/blood-2008-02-141069. [DOI] [PubMed] [Google Scholar]
- 140.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–2323. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 141.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 142.Liu D, Meckel T, Long EO. Distinct role of rab27a in granule movement at the plasma membrane and in the cytosol of NK cells. PLoS One. 2010;5:e12870. doi: 10.1371/journal.pone.0012870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanborn KB, Rak GD, Maru SY, Demers K, Difeo A, Martignetti JA, Betts MR, Favier R, Banerjee PP, Orange JS. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol. 2009;182:6969–6984. doi: 10.4049/jimmunol.0804337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–2291. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 147.Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 148.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 149.Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- 150.Valdez AC, Cabaniols JP, Brown MJ, Roche PA. Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network. J Cell Sci. 1999;112(Pt 6):845–854. doi: 10.1242/jcs.112.6.845. [DOI] [PubMed] [Google Scholar]
- 151.Prekeris R, Klumperman J, Scheller RH. Syntaxin 11 is an atypical SNARE abundant in the immune system. Eur J Cell Biol. 2000;79:771–780. doi: 10.1078/0171-9335-00109. [DOI] [PubMed] [Google Scholar]
- 152.Riento K, Kauppi M, Keranen S, Olkkonen VM. Munc18-2, a functional partner of syntaxin 3, controls apical membrane trafficking in epithelial cells. J Biol Chem. 2000;275:13476–13483. doi: 10.1074/jbc.275.18.13476. [DOI] [PubMed] [Google Scholar]
- 153.Fukuda M, Imai A, Nashida T, Shimomura H. Slp4-a/granuphilin-a interacts with syntaxin-2/3 in a Munc18-2-dependent manner. J Biol Chem. 2005;280:39175–39184. doi: 10.1074/jbc.M505759200. [DOI] [PubMed] [Google Scholar]
- 154.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Lawson ML, Robinson LJ, Skraban R, Lu Y, Chiavacci RM, Stanley CA, Kirsch SE, Rappaport EF, Orange JS, Monos DS, Devoto M, Qu HQ, Polychronakos C. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 155.Rubio JP, Stankovich J, Field J, Tubridy N, Marriott M, Chapman C, Bahlo M, Perera D, Johnson LJ, Tait BD, Varney MD, Speed TP, Taylor BV, Foote SJ, Butzkueven H, Kilpatrick TJ. Replication of KIAA0350, IL2RA, RPL5 and CD58 as multiple sclerosis susceptibility genes in Australians. Genes Immun. 2008;9:624–630. doi: 10.1038/gene.2008.59. [DOI] [PubMed] [Google Scholar]
- 156.Notarangelo LD, Casanova JL. Primary immunodeficiencies: increasing market share. Curr Opin Immunol. 2009;21:461–465. doi: 10.1016/j.coi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 157.Rudd E, Bryceson YT, Zheng C, Edner J, Wood SM, Ramme K, Gavhed S, Gurgey A, Hellebostad M, Bechensteen AG, Ljunggren HG, Fadeel B, Nordenskjold M, Henter JI. Spectrum, and clinical and functional implications of UNC13D mutations in familial haemophagocytic lymphohistiocytosis. J Med Genet. 2008;45:134–141. doi: 10.1136/jmg.2007.054288. [DOI] [PubMed] [Google Scholar]