Abstract
Cytochrome P450 enzymes (P450s) are important targets in cancer, due to their role in xenobiotic metabolism. Since P450s are the “bridges” between the environment and our body, their function can be linked in many ways to carcinogenesis: they activate dietary and environmental components to ultimate carcinogens (i), the cancer tissue maintains its drug resistance with altered expression of P450s (ii), P450s metabolize (sometimes activate) drugs used for cancer treatment (iii) and they are potential targets for anticancer therapy (iiii). These highly polymorphic enzymes are regulated at multiple molecular levels. Regulation is as important as genetic difference in the existing individual variability in P450 activity. In this review, examples of the transcriptional (DNA methylation, histone modification, modulation by xenosensors) and post-transcriptional (miRNA) regulation will be presented and thereby introduce potential molecular targets at which the metabolism of anticancer drugs, the elimination of cancerogenes or the progress of carcinogenesis could be affected.
Keywords: Drug metabolism, P450, AHR, CAR, PXR, MiRNA
Introduction
The human body is exposed to a wide range of external chemicals; both purposely (e.g., medicines) and accidentally (e.g., environmental contaminants) and these foreign molecules (xenobiotics) are metabolized by enzymes called cytochrome P450s (P450). P450s are heme-thiolate enzymes that participate in the biotransformation of xenobiotics and production of many important endogenous compounds including steroid hormones, prostaglandins, and leukotrienes [1, 2]. Their expression is believed to be highly regulated; some P450s are expressed only in specific tissues and at times in specific cells within the tissue. Similarly, the expression pattern of a number of P450s is different at each developmental stage and different in females and males. Paradoxically, these enzymes catalyze the formation of reactive intermediates of thousands of chemicals that can damage DNA, as well as lipids and proteins. P450 expression can also affect the production of proliferation-promoting molecules derived from arachidonic acid, and alters various downstream signal-transduction pathways. Reactive intermediates, activated carcinogens, and arachidonic acid derivatives can be precursors to malignancy and cause chemical cancer [3]. In a developed cancer, these enzymes can determine the outcome of anticancer therapy and altered expression of P450s can support cancer progression and cause drug resistance [4, 5].
Since P450s are the “bridges” between the environment and our body, their function can be linked in many ways to carcinogenesis: they activate xenobiotics to ultimate carcinogens but at the same time they metabolize (and in some cases activate) drugs used for cancer treatment (Table 1). In some tumors, the cancer tissue maintains its drug resistance with altered expression of P450s and this explains why they are potential targets for anticancer therapy. Mostly, dose P450s could be linked to chemical cancer, which are involved in xenobiotic metabolism (CYP1-4 family), and their regulation is discussed in the following sections.
Table 1.
Role of xenobiotic metabolism in cancer
| Result of xenobiotic metabolism by P450s | Changes in P450 activity | |
|---|---|---|
| Promotes carcinogenesis | Promotes anticancer effect | |
| Carcinogen deactivation | No | Yes |
| Precarcinogen activation | Yes | No |
| Anticancer drug deactivation | Yes | No |
| Anticancer prodrug activation | No | Yes |
Until the last decade, it was thought that genetic polymorphism of cancer-related genes is responsible for tumor development. New findings of epigenetics and signal transduction suggest that during carcinogenesis, these factors (e.g., chromatin conformation, regulatory mechanisms) are as important as the genetic background.
There are many published reviews discussing the epigenetics of P450s and their role in cancer [3, 6, 7] and there are many introducing the function of nuclear receptors in this disease [8–10].
In the present manuscript, both regulation types—transcriptional and miRNA regulation mechanisms—were described from an aspect of xenobiotic metabolizing P450s and their role in cancer. In the past it was thought that mainly genetic polymorphisms were due to the variation of P450 activities and some genotypes increase the risk of cancer. With this review we would like to highlight the importance of regulatory mechanisms in cancer, which harmonize the genetically determined P450 activity with the environmental effects and could be beneficially altered by anticancer drugs in the future.
Molecular aspects of p450 regulation
Transcriptional regulation
Cancer is a complex disease where genetics, lifestyle, and environment play important roles in dictating susceptibility to the disease. Environmental factors are transmitted to the cell by different regulatory mechanisms. Although the major regulation of P450s through xenosensors, nuclear receptors have been well studied. We provide an overview of new results in the field related to regulation by DNA methylation and histone modulation, since they are topics of considerable current interest, which may describe the large variation in expression seen for several important P450s in cancer.
DNA methylation
In mammals, the majority of CpG pairs are chemically modified by the covalent attachment of a methyl group to the C5 position of the cytosine ring. DNA methylation occurs predominantly at CpG sites in the mammalian genome [11] by the DNA methyltransferase (DNMT) enzymes [12]. Methylation of DNA is regarded as a means of regulating gene expression through two general mechanisms. First, DNA methylation of gene promoters may prevent the physical binding of some transcription factors to their DNA-binding sites [13]. Second, the transcriptional silencing capability of DNA methylation may occur via indirect mechanisms involving changes in chromatin conformation. There is extensive evidence to support a functional role for promoter-CGI methylation in transcriptional repression [14–16]. DNA methylation of CpG-rich promoters of some genes correlates with tissue-specific gene silencing [17, 18]. Tumors are often characterized by an imbalance in cytosine methylation having both hypomethylation and hypermethylation of various regions of the genome. To date, several studies show altered DNA methylation of P450s in cancer, mostly of those P450s, which are involved in the metabolism of endogenous compounds [6]. Examples of such regulation are shown in the following section.
CYP1A1
Both hypermethylation (less active CYP1A1, altered metabolism of carcinogens) and hypomethylation (more active enzyme, higher activation of precarcinogens) of CYP1A1 promotes carcinogenesis.
In prostate cells, CpG islands in CYP1A1 show segmented/selective methylation patterns; CpG sites from 1 to 36 are not methylated; this DNA region contains the CYP1A1 promoter and is responsible for correct initiation of gene transcription, CpG sites 37 to 90, which corresponds to the CYP1A1 enhancer region that mediates TCDD inducibility, exhibits cancer cell-dependent hypermethylation, and CpG sites 91 to 125 are commonly methylated, but no known regulatory function has been associated with this DNA region, possibly due to its positive methylation status [19]. In noncancerous prostate tissue, CYP1A1 enhancer methylation could not be detected, but in prostate tumors, 36% (11 of 30) of the DNA samples was methylated (Table 2).
Table 2.
CpG islands found in various P450 enzymes and their role of cancer
| CPG islands | |||||
|---|---|---|---|---|---|
| P450 | Region | Methylation status | Consequences in activity | Tissue/cell line | References |
| CYP1A1 | Enhancer/promoter | Hypermethylated | ↓ | Prostate cancer | [19] |
| Enhancer/promoter | Hypomethylated | ↑ | Lung of smokers | [20] | |
| CYP1B1 | Enhancer/promoter | Hypomethylated | ↑ | Prostate cancer | [25] |
| Enhancer/promoter | Hypermethylated | ↓ | Colorectal cancer | [26] | |
| CYP2E1 | Region not described | Hypomethylated | ↓ | Lung tumor | [31] |
| CYP2W1 | I. exon/I. intron | Hypermethylated | ↓ | Colon cancer | [35–37] |
| CYP3A4 | Enhancer/promoter | Hypomethylated | ↑ | HepG2 | [38] |
Environmental factors such as tobacco smoke have been shown to influence the DNA methylation of CYP1A1. Smokers' DNA was hypomethylated compared to non-smokers on the upstream regions, containing functional XREs. In addition, there was an inverse correlation between methylation and the number of cigarettes smoked daily. Cessation of smoking results in the methylation of CYP1A1 promoter being increased at 1–7 days after the last cigarette [20]. Although there was no correlation between EROD activity and the percentage of methylated DNA in a sample in either smokers or nonsmokers, a decrease in methylation caused significant higher CYP1A1 activity. A high inducibility of CYP1A1 has been connected with increased susceptibility to smoking-associated lung cancer [21, 22].
CYP1B1
CYP1B1 is overexpressed in a variety of human tumor cells such as the lung, breast, liver, gastrointestinal tract, and ovarian cancer [23, 24]. CYP2B1 may be an important tumor marker because it hydroxylates estrogens and activates many procarcinogens. CYP1B1 enzyme could be methylated both on the promoter and on the enhancer of the gene. The promoter region contains the CpG sites of the core promoter region including SP1-binding sites and the enhancer region including AHR/ARNT-binding sites DRE2 and DRE3. Aberrant methylation in the CYP1B1 gene affects binding of transcription factors and enhancer molecules. In cancer, the methylation of this region is differently altered, depending on the type and original tissue of the tumor. Tokizane et al. [25] showed that increased CYP1B1 expression in clinical prostate cancer tissues is caused by hypomethylation both of the promoter and enhancer regions of the CYP1B1 gene.
The same methylation pattern as in prostate cancer was observed in other cancers, for example, colorectal cancer. Here, inhibited activity of CYP1B1 was observed due to complete hypermethylation of the promoter region [26]. Because expression of CYP1B1 is regulated by the methylation of its promoter/enhancer, this region may be a useful target for anticancer drugs and in preventive medicine (Table 2).
CYP2E1
Methylation of the CYP2E1 gene inhibits the expression of this enzyme in prenatal period [27]. In adult tissues, the methylation pattern of the CYP2E1 gene differs among various tissue types such as lung, kidney, placenta, liver, and skin, indicating that DNA methylation results in tissue-specific regulation [28, 29]. CYP2E1 metabolizes ethanol to its carcinogenic metabolite acetaldehyde and it is involved in the bioactivation of other small-molecule precarcinogens [30]. In lung tumors, lower methylation was observed and hypomethylation has been associated with underexpression of the CYP2E1 gene [31].
CYP2W1
This enzyme has been shown to metabolize arachidonic acid and benzphetamine, as well as being able to metabolically activate several procarcinogens, including polycyclic aromatic hydrocarbon dihydrodiols, aflatoxin B1, or sterigmatocystin. CYP2W1 is expressed at relatively low levels (mRNA) in the human adult non-transformed tissues, whereas the expression in colorectal cancer tissues was significantly higher (both at mRNA and protein levels) [32–34]. This suggests that the extent of CYP2W1 expression in colorectal cancers might be a prognostic marker for malignancy and survival (Edler et al. 2009). CYP2W1 gene expression appears to be governed by gene methylation. The CYP2W1 gene was shown to contain one functional CpG island in the exon 1-intron 1 region, which was methylated in cell lines lacking CYP2W1 expression, but unmethylated in cells expressing CYP2W1 [35–37].
CYP3A
A different DNA methylation pattern was found between primary hepatocytes and hepatocyte cell lines. HepG2 cells exhibit many cellular features of normal human hepatocytes, but also display characteristics resembling those of a cancerous or fetal hepatocyte. CYP3A expression in untreated HepG2 cells is fairly low, suggesting that their expression is reduced in these partially dedifferentiated cells. Dannenberg and coworkers were interested in determining whether CYP3A genes are regulated by DNA methylation in HepG2 cells. Their microarray experiments showed that after 5-aza-dC treatment (5-aza-2′-deoxycytidine, methylation inhibitor), expression of CYP3A4, CYP3A5, and CYP3A7 was two- to fourfold higher, suggesting a regulatory role of methylation with these P450s. The mentioned increase in expression was confirmed by real-time RT–PCR only for CYP3A7 [38]. Since the CYP3A enzyme catalyzes the transformation of many drugs, understanding its regulation would open new possibilities in cancer therapy.
Histone modification
Post-translational modifications such as phosphorylation, acetylation, methylation, and ubiquitination on the N-termini of histones have been shown to play critical roles in gene regulation [39]. It is believed that the combination of modifications of the chromatin-associated histone and non-histone proteins and the interplay between these modifications create a marking system (“histone code”), which is part of the epigenetic mechanism for gene regulation [40]. The modified histones and methylated DNA sequences may interact in a synergistic manner, including methyl-CpG binding protein, nuclear receptor corepressor (NCoR), associated histone deacetylases, and histone methyl transferases, to regulate gene expression [41]. There are already several indicators of an association between histone-modifying enzymes and cancer [42]. Histone modifications are involved in the regulation of the two cancer-related P450s: CYP1A and CYP3A4.
Chromatin structure has been suggested to play an essential role in CYP1A1 transcription. In the basal state, HDAC1 is bound to the CYP1A1 promoter and is released in concert with the recruitment of p300 upon B[a]P ligand activation of the receptor. HDAC1 removal allows for several histone-modification steps associated with the AHR-mediated induction of CYP1A1 expression. Removal of HDAC1 is necessary, but it is not sufficient to activate CYP1A1 expression [43]. CYP3A4 transcription is also regulated by a histone methyltransferase enzyme called protein arginine methyltransferase 1 (PRMT1). PRMT1 is required for the transcriptional activity of PXR. It is recruited to the 5′-region of the CYP3A4 gene to methylate histone H4 as a response to the PXR agonist rifampicin [44]. CYP3A4 is also regulated with PXR and is regulated by CAR albeit it at a lower rate of expression. Assenat and coworkers reported that the synthetic glucocorticoid, dexamethasone, induces histone H4 acetylation at the proximal CAR promoter region, and indirectly affects CYP3A4 induction by regulating CAR expression [45]. Interestingly, experiments for H4 acetylation on PXR promoter were not performed.
Regulation with xenosensors
A specific group of transcription factors and receptors that are specialized to regulate genomic processes to protect the body against the innumerable chemicals found in the environment are called xenobiotic-activated receptors (XARs) or xenosensors. XARs regulate xenobiotic metabolism and disposition by controlling the expression and induction of drug-metabolizing enzymes and transporters. As the primary means of xenobiotic sensing and defense, XARs are intimately involved in drug disposition, polymorphic drug clearance, drug–drug interaction, and pathogenesis of some chemically induced diseases such as cancer. Furthermore, XARs integrate a broad range of protective mechanisms (such as antioxidative response and immune/inflammatory functions) to antagonize foreign chemicals [46]. Regulation of P450 expression has a high impact on both carcinogenesis (activation of precarcinogens, slow metabolism of toxic compounds) and on cancer therapy by anticancer drugs (narrows therapeutic index, a steep dose-toxicity curve).
The expression of P450s is primary regulated by two nuclear hormone receptor type xenosensors [8, 47–49], the constitutive androstane receptor (CAR), the pregnane X receptor (PXR), and two other type of xenosensors, namely the aryl hydrocarbon receptor (AHR) and nuclear factor (erythroid-derived 2)-like 2 (NRF2).
The prototype transcriptional circuit of an XARs for P450 induction is as follows: XARs are inactivated with associated proteins in the cytoplasm; binding with an agonist activates XARs; activated XAR translocates into the nucleus and heterodimerizes with a partner protein; and the XAR dimer binds to specific DNA sequences located in the enhancers of a battery of foreign-compound metabolizing genes. These receptors can alter the action of other nuclear receptors or transcription factors, thereby controlling the signaling pathways that regulate the homeostasis of bile acids, lipids, glucose, inflammation, vitamins, hormones, and others (Table 3).
Table 3.
Regulation of human P450s by XAR crosstalks
| P450 | Crosstalk | Consequences | References | Mechanisms |
|---|---|---|---|---|
| CYP1A | AHR and GR | ↓ | [113, 114] | Direct interaction of AHR and activated GR |
| AHR and ER | ↑ | [118–120, 122] | ER coactivates AHR, ER interacts with AHR/ARNT complex | |
| AHR and HIF1 | ↓ | [123, 124] | Sharing ARNT partner | |
| AHR and NRF2 | ↑ | [138, 139] | Transactivating the same responsive element | |
| CYP2B6 | CAR and GR | ↑ | [101] | Direct interaction of CAR and GR, glucocorticoid responsive element (GRE) in CAR distal region |
| CAR and FOXO | ↑ | [98, 99] | FOXO binds directly with CAR | |
| CAR and PXR | ↑ | [65–68, 70] | Transactivating the same responsive element | |
| PXR and VDR | ↑ | [72–74] | Transactivating the same responsive element | |
| CYP2C9 | CAR and HNF-4α | ↑ | [79] | Synergizing the effect of CAR |
| PXR and HNF-4α | ↑ | [79] | Synergizing the effect of PXR | |
| PXR and VDR | ↑ | [72–74] | Transactivating the same responsive element | |
| CYP2J2 | NRF2 and c-Jun | ↑ | [144] | NRF2/c-Jun binds to the same responsive element as c-Jun/c-Jun |
| CYP2S1 | AHR and HIF1 | ↑ | [127] | AHR and HIF1 binds to the same responsive element |
| CYP3A4 | PXR and CAR | ↑ | [65–68, 70] | Binding to the same responsive element |
| PXR and VDR | ↑ | [72–74] | Transactivating the same responsive element | |
| PXR and GR | ↑ | [79] | Cofactor, facilitate the binding of PXR | |
| PXR and HNF4 α | ↓ | [79, 80] | Binding the same coactivators |
Xenosensors are also able to crosstalk with other signalling pathways. The consequences of crosstalks are that the expression of a given battery of target genes that is expected to be regulated by a given signalling pathway in fact are dependent on the function of other signalling pathways as well. Given the multitude of biological partners with which xenosensors might interact, xenobiotic metabolism and its influence on carcinogenesis appear to be dependent on a very complex network of regulatory pathways. Some of the cancer-related crosstalks are listed in the following section with each xenosensor.
PXR
Pregnane X receptor is one of the most frequent target receptors for xenobiotics and therefore its role in the regulation and control of drug metabolism is important to consider in relationship to drug-drug interactions. PXRs have been shown to be activated by various xenobiotics (e.g., rifampicin, clotrimazole, hyperforin), natural and synthetic steroids (e.g., 5ß-pregnane-3,20-dione, pregnenolone 16α-carbonitrile, dexamethasone, dehydroepiandrosterone), and bile acids (e.g., lithocholic acid and 6-keto lithocholic acid) [50]. After binding with the xenobiotic compound, PXR translocates to the nucleus and heterodimerizes with retinoid X receptor (RXR). The heterodimeric complex can bind to several responsive element types, such as direct repeats (DR-3, DR-4, and DR-5) and everted repeats (ER-6, ER-8) [51–53]. The modulation of basal activity and induction of transcriptional events are mediated by recruitment of coregulators: activators [SRC-1, SRC-2, SRC-3 (steroid receptor coactivator-1, 2 or 3), TUF2 (transcriptional intermediary factor 2), GRIP1 (glucocorticoid receptor interacting protein 1), CREBBP (CREB binding protein), AIB1 (amplified in breast cancer 1], or corepressors [NCoR (nuclear receptor corepressor), SMRT (silencing mediator for retinoid and thyroid receptor)] [54–58]. The signaling pathway is even more complex if the crosstalk mechanisms with other receptors are taken into consideration. Major interactions are summarized in Table 3 and described briefly below.
It has been observed that the expression of PXR in prostate, ovarian, and colon cancer cells can modulate tumor cell metabolism and sensitivity to several cytotoxins. For instance, PXR ligands such as rifampicin and hyperforin (the active constituent of the herbal antidepressant St. John’s Wort) significantly enhance both the hepatic elimination of irinotecan in patients, and the intratumoral glucuronosylation and inactivation of the drug. Cyclophosphamide is widely used to treat a variety of cancers and it is activated mostly by CYP2B6 and CYP3A4 [59]. Treatment of hepatocytes with mentioned PXR inducers markedly increased hydroxylation of cyclophosphamide and its isomer ifosfamide [60]. Tamoxifen is mainly metabolized by CYP3A4, and at the same time, induces this enzyme [61], and with this, accelerates its own clearance as a result of autoinduction [62]. PXR and CYP3A4 is also activated by the microtubule stabilizing agent paclitaxel, but not with its structurally related molecule docetaxel. The mechanism behind the two structurally related drugs is as follows: paclitaxel stimulates the displacement of SMRT cofactor on the PXR/RXR complex while docetaxel does not [63]. In contrast to paclitaxel, cyclophosphamide, or tamoxifen, ecteinascidin (ET)-743 anticancer has been shown to inhibit the transcriptional activation of CYP3A4 by direct antagonizing PXR [64].
PXR and CAR
Because PXR-responsive elements are recognized and transactivated by CAR and vice versa, PXR and CAR can activate the overlapping series of genes. In addition, these receptors compete for a common partner, RXRalpha; for common coactivators, such as SRC-1 and PGC-1alpha and for the same ligands [65–68], but there are differences in the degree to which specific genes are activated by either CAR or PXR agonists, which undoubtedly contribute to the distinct pharmacologies of xenobiotics. Retinoids that are used clinically to treat acute promyelocytic leukemia, skin cancer, Kaposi’s sarcoma, and cutaneous T-cell lymphoma were shown to activate CYP3A4 mainly via CAR and not PXR. These findings suggest that retinoids may have complex and variable effects on xenobiotic responses in these patients [69].
PXR and VDR
VDR mediates the effect of 1α, 25-dihydroxyvitamin D3, retinoids and carotenoids to genes that are involved in mineralization, epidermal development, etc. After binding the ligand, VDR/RXR form a heterodimer and then bind to and transactivate the vitamin D responsive elements (VDREs) that are present in the regulatory region of target genes [70]. Wang’s experiments prove that certain retinoids and carotenoids transactivate the PXR/RXR-mediated pathway and upregulate CYP3A4 gene expression in a human hepatoma cell line [71]. VDR binds and activates other PXR/CAR-target genes such as CYP3A4, CYP2C9, and CYP2B6 [72–74]. Because vitamin D is a common component of food products and it is also produced in the skin, it is possible that its activating influence on P450s may contribute to some of the interindividual variations of these enzymes. Induction of CYP3A4 by VDR ligands could have importance in cancer prevention, since a higher level of CYP3A4 gives better protection in carcinogen deactivation or in cancer therapy, and faster activation of anticancer prodrugs [75].
PXR and GR
Dexamethasone was shown to induce CYP3A4 transcription in primary human hepatocytes [76], suggesting a functional cross talk between PXR and GR (glucocorticoid receptor). The cross talk potentially regulates metabolism of steroid hormone and sterol homeostasis. Induction of CYP2B6 by PXR is also augmented by dexamethasone, suggesting that GR regulates PXR expression. Besides modulating PXR expression, activated GR (but not the receptor alone) acts as a cofactor to facilitate the binding of PXR to CYP2B6 [77]. Based on these findings, active GR can serve as an enhancer in the induction of the CYP2B6 gene and this could be useful in cancer therapy. Ketoconazole and miconazole, which were identified as GR antagonists [78], can block the effect of GR, and treatment with these imidazole derivatives might improve the pharmacokinetic properties of some CYP2B6 dependent, anticancer drugs (for example paclitaxel and tamoxifen).
PXR and HNF-4α
PXR/HNF-4α crosstalk is characterized as an enhancer as well as regulator of constitutive expression of P450s. CYP2C9 has many anticancer substrates (e.g., cyclophosphamide, ifosfamide, or tamoxifen) and it is regulated by both CAR and PXR. Additionally, two proximal HNF-4α-binding sites functionally control the transcriptional activation of CYP2C9. HNF-4α synergizes with PXR or CAR to enhance promoter activity of CYP2C9 and HNF-4α sites are essential for maximal induction of CYP2C9 promoter [79]. Recent studies for CYP3A4 indicate that a distant enhancer module containing a PXR response element (F-ER6) is a recognized binding site for HNF-4α [80]. This element supports constitutive expression of CYP3A4. The applications of drugs that target these interactions with PXR will continue to be a focus of research in cancer prevention in the future.
CAR
In addition to the pregnane X receptor (PXR, NR1I2), the constitutive androstane receptor (CAR, NR1I3) is the other nuclear receptor that plays a role in the transcriptional activation of P450 genes after exposure to anticancer drugs (e.g., tamoxifen, cyclophosphamide, ifosfamide). CAR is constitutively active without ligand, and many xenobiotics (phenobarbital and phenytoin) act primarily by causing its nuclear translocation rather than serving as ligands [81, 82].
Induction of many P450s, like the CYP2B6, CYP2C9, CYP2C8, and CYP2C19 is mediated with the nuclear receptor CAR. Phenobarbital-type CYP2B6 inducers activate LKB1 (serine/threonine kinase 11), which can phosphorylate AMPK in human hepatocytes and LMH cells [83, 84]. Activation of cytosolic CAR by AMPK results in its dissociation from chaperone partners, such as cytoplasmic CAR retention protein (CCRP) and HSP90. Translocation of CAR to the nucleus, presumably dependent on the activity of the protein phosphatase PP2A, is followed by association with the retinoid X receptor (RXR) and binding to the PBREM (phenobarbital responsive enhancer module). Transcriptional activation occurs upon CAR binding to the PBREM, which contains two nuclear receptor DR4 sites (NR1 and NR2). CAR coactivators identified to date that facilitate transactivation include GRIP1/TIF2, peroxisome proliferator-activated receptor-γ coactivator (PGC-1γ), SRC-1, Sp1, activating signal cointegrator 2 (ASC-2) and more recently, structural maintenance of chromosomes 1-like 1 (SMC-1) [85–90]. Modulating CAR activity could be useful to adjust metabolism of some cancer-related drugs.
It was reported that CYP bioactivates anti-cancer prodrug ifosfamide [9] in breast cancers and that the expression of CYPs including CYP2B6 was lower in the tumor tissue than in the adjacent normal tissue [91, 92]. Coadministration of phenytoin (CAR activator) in an anticancer therapy has been reported to decrease the level of the prodrug cyclophosphamide and increase the plasma levels of its cytotoxic metabolite 4-hydroxycyclophosphamide through the hepatic induction of CYP2B6 and CYP3A4. The problem is, that at the same time, induced CAR increases cancer cell resistance to 4-hydroxycyclophosphamide by increasing the detoxification rate of this compound.
CAR and PXR could serve as carcinogenesis promoters; it has been proposed that they directly affect tumor growth by controlling the expression of genes involved in motility, apoptosis, and cell–cycle progression in cancer cells [93–95]. CYP2B6 mRNA was also diminished in hepatocellular carcinoma with venous invasion but not in hepatocellular carcinoma without venous invasion [96]. Also, testosterone metabolizer CYP2B6 was found to decrease in prostate cancer. Decreased expression of CYP2B6 significantly correlated with faster cancer progression and poor prognosis in human prostate cancer. These findings suggest that CYP2B6 expression may be decreased in hormonal cancers, where the hormone is closely related to substrates of CYP2B6.
CAR and FOXO1
Forkhead box O (FOXO) transcription factors play an important role in modulating metabolic functions. When nutrient and insulin levels are low, FOXO1 promotes expression of gluconeogenic enzymes and its level is also higher in diabetic patients [97]. It is known that diabetes elevates the activity of hepatic drug metabolism in humans [98]. During the last decade, many scientific groups wanted to resolve the molecular connection between insulin regulation and drug metabolism. As a result, information on potential crosstalk between FOXO1 and xenobiotic metabolism was provided by Kodama and coworkers, who showed that FOXO1 binds to CAR and activates its transcriptional activity [99].
Increasing the level of P450s such as CYP2B6 or CYP3A4 in diabetes, increases production of sex hormones, leading to high plasma-free estrogen concentrations, which in turn activate the estrogen receptor (ER). Activation of these pathways can lead to proliferation, invasiveness, angiogenesis, and decreased apoptosis and will increase the chances of breast cancer in diabetic patients [100].
CAR and GR
Although submicromolar concentrations of dexamethasone (DEX) are known to activate GR, it does not appear to induce CYP2B6 expression at the mRNA level [101] in primary hepatocytes. GR plays an indirect role in CYP2B6 regulation by inducing the expression of CAR itself. In huh7 cell transfection experiments, activated GR increased the constitutive activation of a CYP2B6-PBREM reporter construct by CAR alone, in the absence of inducers. In the presence of ligand activated GR, only PB treatment caused a further twofold CAR-mediated CYP2B6-PBREM activation, compared to control cells. These studies show that GR is involved synergistically in the xenobiotic-responsive regulation of human CYP2B6 by CAR [102]. Increasing CAR levels with GR agonists, such as imidazole derivatives would increase the level of CAR-dependent drug metabolism, which could be beneficial in certain cancer diseases.
CAR and HNF-4α. See PXR and HNF-4
CAR and VDR
1α,25-(OH)2D3 induces the expression of the CYP2B6, CYP2C9, and CYP3A4 in primary human hepatocytes. The induction is mediated by VDR receptor, which binds to the same transactivating responsive elements (ER6, DR3, and DR4) shown to be targeted by PXR (see PXR/VDR cross talk) or CAR [103]. Since VDR activation increases CAR target P450s and with this its metabolic capacity, it could be established that VDR activators can serve as protectors from toxic compounds, which are inactivated by these enzymes, and on the other hand, vitamin D coadministration can increase the level of enzymes involved in anticancer drug activation.
CAR and PXR (see chapter PXR)
AHR
The induction of CYP1 family is mediated mainly through a specific cytosolic receptor, the aryl hydrocarbon receptor (AHR) [104]. Although AHR has long been recognized as a ligand-induced transcription factor, which is responsible for the xenobiotic activating pathway of several phase I and phase II metabolizing enzymes, recent evidence suggests that the AHR is involved in various cell signaling pathways critical to cell cycle regulation and normal homeostasis. Dysregulation of these pathways is implicated in tumor progression [105]. AHR not only mediates tumor promotion but also plays a role in tumor progression. It is also known that activation of the AHR may lead to deregulation of cell–cell contacts, thereby inducing unbalanced proliferation, dedifferentiation, and enhanced motility [106].
AHR exists as part of a cytosolic protein complex, which consists of two Hsp90 heat-shock proteins; a Hsp-90-interacting cochaperone p23 and an immunophillin-like protein (XAP2). In the presence of an exogenous ligand, like polycyclic aromatic hydrocarbons, anticancer drugs, e.g., docetaxel, erlotinib, tamoxifen, or dacarbazine), the receptor complex translocates to the nucleus, where it heterodimerizes with another protein, the aryl hydrocarbon nuclear translocator (ARNT). This heterodimer binds to consensus regulatory sequences termed AHREs (Aryl hydrocarbon responsive elements), XREs (Xenobiotic responsive elements) or DREs (Dioxin responsive elements), located in the promoter region of CYP1A1 and CYP1A2 and initiates their transcription [107]. The transcription of CYP1A1 is inhibited by the AHR-related factor aryl hydrocarbon receptor repressor (AHRR), which localizes in the nucleus in the form of a dimeric protein along with ARNT [108]. The AHRR/ARNT heterodimer acts as a repressor both by stopping transcription initiated at the XREs and by competing with AHR for heterodimer formation with ARNT. All AHR, ARNT, and AHRR proteins are members of the bHLH (basic helix-loop-helix) PAS (Per-ARNT-Sim) family of proteins. The bHLH motif is shared by other transcription factors such as MYC and MYOD and is the protein part essential for DNA binding of the AHR complex [109]. Heterodimerization of AHR/ARNT is facilitated by interactions between bHLP and PAS domains. Further interactions of the AHR/ARNT heterodimer with transcription factors such as Sp1 and NF-1 are essential to enhance the expression of the CYP1A1 gene. The half-life of AHR depends on the regulated proteosomal degradation of AHR; AHR degradation is mediated by ADPF (AHR degradation promoting factor), which has been proposed to serve as an E3 ubiquitin ligase [110].
AHR and GR
AHR has been shown to interact with the glucocorticoid receptor (GR) both in vitro and in vivo; GR decreases AHR-mediated expressions by interacting with XRE-binded AHR [111]. Decreased expression of P450s means prolonged half-life of some anticancer drugs or slower activation of procarcinogens. Recent evidence from studies in HepG2 cells and primary cultures of human hepatocytes has shown that dexamethasone reduces both basal and inducible CYP1A1 EROD activity. Furthermore, dexamethasone was shown to have direct effects on the modulation of TCDD-induced transcriptional activation as well as the proteosomal degradation of AHR in HepG2 cells. Induction of CYP1A1 protein by 3-methylcholantrene (3-MC) was also down-regulated by dexamethasone although it was not observed at the mRNA level [112]. These findings show that dexamethasone controls CYP1A1 expression in human hepatocytes and HepG2 cells through interactive regulatory crosstalk between GR and AHR receptors. The cross-reaction occurs only when both the AHR and GR are activated [113, 114]. In extrahepatic tissues, gefitinib and erlotinib antineoplastic drugs are metabolized by CYP1A1 and CYP1A2. Coadministration of the mentioned anti-GR imidazole-derivatives would prolong the drug exposure in the tumor [115].
AHR and ER
Crosstalk of AHR and ARNT with the estrogen receptor α (ERα) has also been established in a number of different systems [116, 117] and could be important in estrogen-related cancers. TCDD does not bind to ERα, but it does inhibit ERα signaling. More importantly, ERα plays a significant role in modulating AHR activity; treatment of TCDD and E2 results in an increased induction of CYP1A1, compared to TCDD treatment alone. ERα has a direct interaction with the CYP1A1 promoter, suggesting that it acts as a coregulator of AHR-mediated transcriptional activation [118]. In human bronchial epithelial cells, ERα increased the basal mRNA levels of CYP1B1 and the inducible protein levels of CYP1A1, thus regulating the expression of these genes at both the transcriptional and translational level, respectively [119]. A long time increase in CYP1 activity will lead to elevated concentration of free estrogen in the plasma, which in turn activates the ERα. Constant activation of ERα will promote abnormal cell division and tumor growth. With this, AHR-ER crosstalk increases the chances for hormonal cancer. The interaction of ERβ with AHR and ARNT has also been demonstrated after 3-MC exposure [120].
ER- and AHR-signaling pathways have been demonstrated to share several coactivator proteins, such as SRC-1 and receptor-interacting protein 140 (RIP140) [121] and it is also known that AHR induces the proteasomal downregulation of ER, which means that there are other cross points where these two receptors can interact and with this regulate CYP1A1 activity [122].
AHR and HIF1α
Like AHR, HIF1α protein is a member of the PAS family and it also interacts with ARNT [123]. The fact that these proteins share the same dimerization partner suggests an interaction between these nuclear receptors.
During angiogenesis and tumor growth, HIF-1alpha dimerizes with ARNT, inducing expression of many genes, including vascular endothelial growth factor (VEGF).
In normal tissue, AHR sequesters ARNT, decreasing its interaction with HIF-1alpha and with that diminishing VEGF production. In many cancer tissues, increased VEGF is responsible for tumor growth and tumor vascularization. Stimulation of the AHR pathway with AHR inducers would enhance the sequestering effects of the AHR through interaction with ARNT and would inhibit carcinogenesis [124].
AHR/HIFα cross talk is important in the regulation of the human CYP2S1 enzyme. The function of CYP2S1 is still unknown; very few studies report that this enzyme can process naphthalene type xenobiotics [125, 126]. This enzyme is regulated primarily by AHR, but a crosstalk between AHR and HIF-1α was also discovered after dioxin and hypoxia exposure, respectively [127]. Selective expression of CYP2S1 in hypoxic tumors results from this crosstalk. The AHR/HIFα heterodimer can bind to the promoter containing three hypoxia response elements that respond to the hypoxia sensor hypoxia-inducible factor 1 [128]. Because the presence of CYP2S1 in tumors was associated with poor prognosis [129, 130] it could be used as a marker for hypoxic tumor diagnosis.
NRF2
NF-E2-related factor 2 (NRF2) is a member of the Cap‘n’Collar family of basic region-leucine zipper (bZIP) transcription factors and is related to the erythroid transcription factor NF-E2 [157]. Multiple chemical inducers have been shown to activate through this element, e.g., butylated hydroxytoluene (BHT), oltipraz, sulforaphane, isothiocyanates (abundant in cruciferous vegetables), metal salts, and Michael reaction acceptors [131, 132].
Beyond the classical response of catalyzing the detoxification of carcinogens and other xenobiotics through conjugation and trapping processes, the NRF2 protein is also involved in chemoresistance by increasing glutation levels in cancer cells. In particular, induction of NRF2 causes the overexpression of glutathione S-transferases (GSTs), the enzymes that catalyze the conjugation of reduced glutathione to electrophilic compounds [133], as well as efflux pumps may reduce the reactivity of various anticancer drugs [134].
NRF2 is localized in the cytosol and binds to protein KEAP-1 (Kelch-like ECH-associated protein 1). Exposure to a number of stressors and inducing agents leads to dissociation of one or both of the NRF2-interacting motifs from KEAP-1, thereby rescuing NRF2 from proteasomal degradation and allowing for import into the nucleus. Once inside the nucleus, NRF2 dimerizes with small MAF proteins leading to the transactivation of several hundred cytoprotective genes, each of which contains one or more antioxidant response elements (AREs) in their promoters. Interactions with additional proteins serve to either amplify or attenuate the transcriptional response [135, 136]. The NRF2/MAF complex regulates P450s such as CYP1A1 or CYP2A5 [137].
NRF2 and AHR
It is well recognized that both AHR and NRF2 signaling regulates the expression of genes affecting the metabolism of xenobiotics, and both of them are involved in cancer formation. Miao and coworkers demonstrated that NRF2 gene transcription is directly modulated by AHR activation, where the receptor directly binds to the NRF2 promoter. Moreover, silencing of AHR expression with siRNA obviated induction of NRF2 mRNA by TCDD [138]. In this way, a comprehensive set of responses to xenobiotic challenges by AHR and NRF2 can be mounted. A functional collaboration between the AHR and NRF2 pathways can also be important in cancer. Talalay and colleagues defined two types of activators of NRF2 signaling: monofunctional and bifunctional [139]. Monofunctional inducers only affect NRF2 signaling. Bifunctional inducers such as ßnaphthoflavone may interact with the AHR directly to activate AHR-regulated genes, such as CYP1A1, CYP1A2, and CYP1B1, and then undergo transformation by these enzymes to reactive intermediates that then trigger NRF2 signaling [140, 141]. The discovery of functional cooperation between AHR and NRF2 has significant biological implications. Historically, the AHR has been associated with carcinogenesis, whereas NRF2 is associated with cytoprotection against degenerative diseases. The future challenge is to determine whether crosstalk between these two transcription factors can be exploited to therapeutic advantage or to improve chemopreventive strategies [142].
NRF2 and c-jun
The interplay between members of the activator protein-1 (AP-1) complex of leucine zipper (bZIP) transcription factors, such as c-jun/c-jun, and c-fos/c-jun, has been found to regulate basal CYP2J2 expression in human liver-derived cells [143, 144]. Recent findings of Lee and coworkers now establish that heterodimers formed between NRF2 and c-Jun upregulate P450s, such as CYP2J2. In their experiments, −105/−88-base pair region of the CYP2J2 gene 5′-flank as an atypical bZIP binding sequence was responsive to c-Jun/NRF2 dimers. Since CYP2J2 is the only activator of the non-sedating H1 antihistamine, ebastine, and it is also involved in the oxidation of epoxyeicosatrienoic acid (EET), discovering CYP2J2 regulation will enable the therapeutic exploitation of some of the beneficial actions of EET isomers in human tissues. EETs regulate numerous homeostatic processes, including cytoprotective and proliferative responses against injurious stresses [145] and it is also known that CYP epoxygenase 2J2 plays a role in promotion of the neoplastic cellular phenotype and in the pathogenesis of a variety of human cancers. Recognition of regulatory effect of NRF2 and c-jun on CYP2J2 in carcinoma cells could be important in potential antitumor therapy [146].
Post-transcriptional regulation
Although there are other post-transcriptional mechanisms than regulation by miRNA (alternative splicing, nuclear degradation, processing, nuclear export), not much is known about their role in carcinogenesis and we will not discuss them in this review.
miRNA regulation
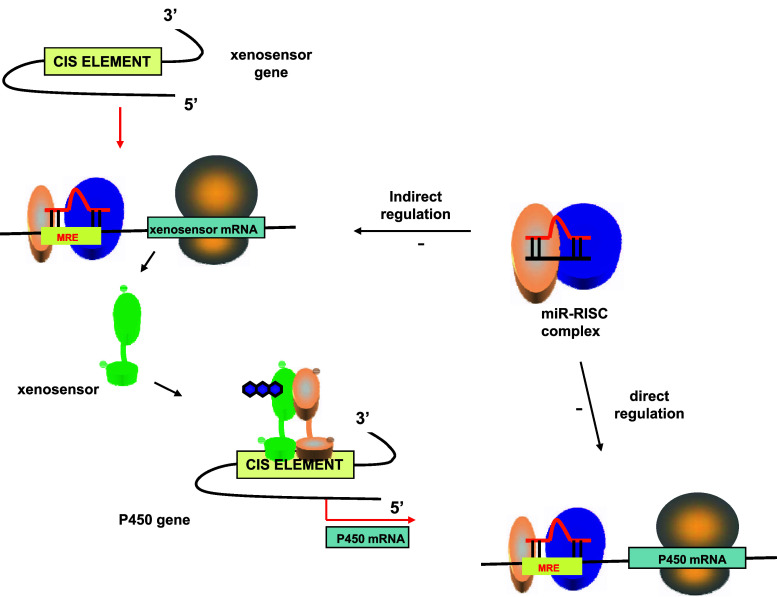
miRNAs are a large family of endogenous, small regulatory RNAs that are generated by a two-step process from long primary miRNAs (pri-miRNAs) [147]. They are processed by a complex comprised of the RNase III enzyme DROSHA and an RNA-binding domain possessing protein DGCR8 (DiGeorge syndrome critical region gene 8) to 60–70 nt precursor miRNA (pre-miRNA) intermediates. Subsequently, these hairpin-shaped pre-miRNAs are transported to the cytoplasm, where they are cleaved by DICER to generate 20- to 22-nucleotide duplexes bearing two nucleotide single-stranded 3′ extensions [148]. miRNAs control the expression of a gene by targeting the 3 ‘UTR of mRNAs as a miRNA/RISC complex (RISC-RNA Induced Silencing Complex), which result ultimately in the degradation of the mRNA [149]. Human cancers commonly exhibit an altered expression profile of miRNAs with oncogenic (miR-21, miR-106a, and miR-155) or tumor-suppressive (let-7, miR-15a/16, miR-34a, and miR-143/145) activity. P450s are also regulated by miRNAs; the miRNA/RISC complex directly represses P450 protein translation, by binding to sequences in the 3′ untranslated region of P450 mRNAs or indirectly, by binding of the MRE sequences in xenosensor mRNA (Fig. 1).
Fig. 1.
Direct and indirect regulation of P450 expression by miRNA. In the cytoplasm, mature miRNA associates with a protein complex called RNA Induced Silencing Complex (RISC). The miRNA/RISC complex represses P450 protein translation directly by binding to sequences in the 3′ untranslated region of P450 mRNAs or indirectly by binding of the MRE sequences in xenosensor mRNA
Post-transcriptional regulation by miRNA could be responsible for a portion of the significant amount of unexplained interindividual variability in P450 enzyme expression and activity. Since xenobiotic-converting P450s play a role in carcinogenesis, P450-related cancer phenotypes can be modified by targeting expression of miRNAs.
Here we summarize the recent knowledge of those miRNAs that could have importance in P450s regulation during carcinogenesis.
CYP1 family
Human CYP1B1, which is highly expressed in estrogen target tissues, catalyzes the metabolic activation of various procarcinogens and the 4-hydroxylation of 17-beta-estradiol. Tsuchiya and coworkers found an abundant amount of CYP1B1 protein in cancerous tissue and they identified a near-perfect matching sequence with miR-27b in the 3′-untranslated region of human CYP1B1 (Table 4). Human CYP1B1 is post-transcriptionally regulated by miR-27b [150]. Another brain-specific miRNA, miR-124, also downregulates CYP1B1 directly and modulate all AHR target genes by binding to both AHR and AHRR, but its cancer relations have not yet been investigated [151].
Table 4.
Regulation of P450s and XARs by validated miRNA molecules
| miRNA | P450 | miRNA response element (5′-MRE-3′) | Tissue/cell line | References |
|---|---|---|---|---|
| hsa-miR-27b | CYP1B1 | 3′UTR (+4358 to +4381) CAGAACUUAGCCUUUACCUGUGAA | Breast cancer | [150] |
| CYP3A4 | 3′UTR (+612 to +589) GGUGAAAGUUAAUCCACUGUGAC | HEK 293 | [155] | |
| VDR | 3′UTR (+1754 to +1782) UUUAUGGGGGGAGAACUUACAUUGUGAA | HEK 293 | [155] | |
| hsa-miR-148a | PXR | 3′UTR (+3359 to +3386) GCCACAGACUCUUACGUGGAGAGUGCACUGACCU | HepG2, Caco-2d LS180 | [156] |
| hsa-miR-378 | CYP2E1 | 3′UTR (+1559 to +1580) UCAAAUUGUUUGAGGUCAGGAU | HEK293 | [153] |
CYP2A
NNK (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone), another potent precarcinogen that can be found in tobacco products and smoke, is metabolized by CYP1A2, CYP2A6, and CYP3A4 in the liver and with CYP2A13 in the lung. NNK metabolites then form alkylating adducts with DNA, leading to genetic mutations. NNK induces tumors in multiple organs. The analogue rat enzyme for CYP2A13 is CYP2A3. Treating rats chronically with NNK, miR-34b, miR-101, miR-126, and miR-199 had lowered expression levels very early in the lungs of male F344 rats. Lowered miR-126 contributes to the increased production of CYP2A3, further activating NNK in a positive feedback loop and increasing the growth of the tumor [152].
CYP2E1
Mohri and coworkers recently found that miR-378 is involved in the post-transcriptional regulation of CYP2E1. The overexpression of miR-378 significantly decreased CYP2E1 protein levels and enzyme activity in the cells expressing CYP2E1, including 3′-UTR, but not in the cells expressing CYP2E1 without 3′-UTR, indicating that the 3′-UTR plays a role in the miR-378-dependent repression [153]. Chronically induced CYP2E1 with ethanol or other CYP2E1 inducers is a high-risk factor for esophageal and gastrointestinal cancers, which gives importance to investigate transcriptional and post-transcriptional CYP2E1 regulatory mechanisms, as basic targets in anticancer therapy.
CYP3A4
Epigenetic mechanisms provide new insight into the unsolved mechanism of the large interindividual variability of CYP3A4 expressions. Since CYP3A4 is involved in the metabolism of many anticancer drugs, from which many have a low therapeutic window (e.g., doxorubicine, ifosfamide, erlotinib), determining its metabolic capacity is very important. Lamba et al. reported that variability in hepatic CYP3A4 cannot be explained just by common CYP3A4 coding variants [154], suggesting the role of other mechanisms, such as regulation with miRNAs or inherited modifications in the miRNA gene itself. Until now, one miRNA, miR-27b, has been described to regulate CYP3A4 expression directly binding to the miRNA response element (MRE) within the 3’UTR region of CYP3A4 mRNA [155].
Some miRNAs, such as miR-148a, which is selectively and abundantly expressed in the liver, regulates other liver specific genes, for e.g., the human PXR receptor. miR-148a binds to the 3′-UTR region of PXR mRNA, thereby decreasing synthesis of PXR protein. Since CYP3A4 is a target for PXR, miR-148a indirectly modulates the inducible and/or constitutive levels of CYP3A4 expression [156]. Another example of indirect modulation would be the VDR receptor. VDR also regulates CYP3A4 (see cross talk VDR/PXR or VDR/CAR) and VDR could be down-regulated with miR-27b [153].
Conclusions
In this review, various examples of P450 regulation mechanisms were presented, which all contribute to the amount of finally translated P450 mRNA.
The P450s responsible for the activation or inactivation of precarcinogens and anticancer drugs are polymorphic. Considerable research has been directed to elucidate to what extent genetic polymorphism in the corresponding genes could affect susceptibility for environmentally induced cancer and for cancer therapy (for e.g., in a recent study, sex and polymorphisms in FoxA2, HNF4α, FoxA3, PXR, ABCB1, and the CYP3A4 promoter together explained 24.6% of the variation in hepatic CYP3A4 expression). The remaining 75.4% of variations depends on individual regulatory patterns (transcriptional, post-transcriptional or post-translational regulation) of CYP3A4 expression.
Understanding the molecular regulation of P450s with these receptors and enzymes, deducing new endogenous ligands for the nuclear receptors, and identification of novel miRNAs will be the next challenges to establish new markers of carcinogenesis and new therapeutical, cancer-related concepts.
Abbreviations
- ADPF
AHR degradation promoting factor
- AHR
Aromatic hydrocarbon receptor
- AHRR
Aromatic hydrocarbon receptor repressor
- ARNT
Aromatic hydrocarbon receptor nuclear translocator
- ARE
Antioxidant response element
- ASC2
Activating signal cointegrator 2
- B[a]P
Benzo[a]pyrene
- CAR
Constitutive androstane receptor
- CREBBP
CREB-binding protein
- DNMT
DNA methyltransferase
- ER
Estrogen receptor
- FOXO
Forkhead box O
- GR
Glucocorticoid receptor
- GRIP1
Glucocorticoid receptor interacting protein 1
- HDAC1
Histone deacetylase-1
- HIF-1
Hypoxia induced factor
- HNF-4α
Hepatocyte nuclear factor
- KEAP-1
Kelch-like ECH-associated protein 1
- 3-MC
3-Methylcholantrene
- NcoR
Nuclear receptor corepressor
- NF-κb
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NRF2
NF-E2-related factor 2
- PRMT1
Arginine methyltransferase 1
- PXR
Pregnane X receptor
- RXR
Retinoid X receptor
- SMC-1
Structural maintenance of chromosomes 1-like 1
- SRC-1
Steroid receptor coactivator-1
- SMRT
Silencing mediator for retinoid and thyroid receptor
- TUF2
Transcriptional intermediary factor 2
- VDR
Vitamin D receptor
- XAP2
Immunophillin like X protein XAP2
- XAR
Xenobiotic activated receptors
References
- 1.Waxman DJ, Azaroff L. Phenobarbital induction of P-450 gene expression. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25(11):1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 4.McFadyen MC, Cruickshank ME, Miller ID, McLeod HL, Melvin WT, Haites NE, Parkin D, Murray GI. Cytochrome P450 CYP1B1 over-expression in primary and metastatic ovarian cancer. Br J. 2001;Cancer85(2):242–246. doi: 10.1054/bjoc.2001.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFadyen MC, McLeod HL, Jackson FC, Melvin WT, Doehmer J, Murray GI. Cytochrome P450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochem Pharmacol. 2001;62(2):207–212. doi: 10.1016/s0006-2952(01)00643-8. [DOI] [PubMed] [Google Scholar]
- 6.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116(3):496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Antona C, Gomez A, Karlgren M, Sim SC, Ingelman-Sundberg M. Molecular genetics and epigenetics of the cytochrome P450 gene family and its relevance for cancer risk and treatment. Hum Genet. 2010;127(1):1–17. doi: 10.1007/s00439-009-0748-0. [DOI] [PubMed] [Google Scholar]
- 8.Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev. 2003;55(4):649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- 9.Pascussi JM, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- 10.Lim YP, Huang JD. Interplay of pregnane X receptor with other nuclear receptors on gene regulation. Drug Metab Pharmacokinet. 2008;23(1):14–21. doi: 10.2133/dmpk.23.14. [DOI] [PubMed] [Google Scholar]
- 11.Takai D, Jones PA. The CpG island searcher: a new WWW resource. In Silico Biol. 2003;3(3):235–240. [PubMed] [Google Scholar]
- 12.Tate PH, Bird AP. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993;3:226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- 13.Rountree MR, Bachman KE, Herman JG, Baylin SB. DNA methylation, chromatin inheritance and cancer. Oncogene. 2001;20:3156–3165. doi: 10.1038/sj.onc.1204339. [DOI] [PubMed] [Google Scholar]
- 14.Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 15.Smet C, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19(11):7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein R, Razin A, Cedar H. In vitro methylation of the hamster adenine phosphoribosyltransferase gene inhibits its expression in mouse L cells. Proc Natl Acad Sci USA. 1982;79:3418–3422. doi: 10.1073/pnas.79.11.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futscher BW, Oshiro MM, Wozniak RJ, Holtan N, Hanigan CL, Duan H, Domann FE. Role for DNA methylation in the control of cell type specific maspin expression. Nat Genet. 2002;31:175–179. doi: 10.1038/ng886. [DOI] [PubMed] [Google Scholar]
- 18.Song F, Smith JF, Kimura MT, Morrow AD, Matsuyama T, Nagase H, Held WA. Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression. Proc Natl Acad Sci USA. 2005;102:3336–3341. doi: 10.1073/pnas.0408436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okino ST, Pookot D, Li LC, Zhao H, Urakami S, Shiina H, Dahiya R. Epigenetic inactivation of the dioxin-responsive cytochrome P4501A1 gene in human prostate cancer. Cancer Res. 2006;66:7420–7428. doi: 10.1158/0008-5472.CAN-06-0504. [DOI] [PubMed] [Google Scholar]
- 20.Anttila S, Hakkola J, Tuominen P, Elovaara E, Husgafvel-Pursiainen K, Karjalainen A, Hirvonen A, Nurminen T. Methylation of cytochrome P4501A1 promoter in the lung is associated with tobacco smoking. Cancer Res. 2003;63:8623–8628. [PubMed] [Google Scholar]
- 21.Kellermann G, Shaw CR, Luyten-Kellermann M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N Engl J Med. 1973;289:934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- 22.Stücker I, Jacquet M, de Waziers I, Cénée S, Beaune P, Kremers P, Hémon D. Relation between inducibility of CYP1A1, GSTM1 and lung cancer in a French population. Pharmacogenetics. 2000;10:617–627. doi: 10.1097/00008571-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Murray GI, Taylor MC, McFadyen MCMcKay JA, Greenlee WF, Burke MD, Melvin WT. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–3031. [PubMed] [Google Scholar]
- 24.McFadyen MC, Breeman S, Payne S, Stirk C, Miller ID, Melvin WT, Murray GI. Immunohistochemical localization of cytochrome P450 CYP1B1 in breast cancer with monoclonal antibodies specific for CYP1B1. J Histochem Cytochem. 1999;47:1457–1464. doi: 10.1177/002215549904701111. [DOI] [PubMed] [Google Scholar]
- 25.Tokizane T, Shiina H, Igawa M, Enokida H, Urakami S, Kawakami T, Ogishima T, Okino ST, Li LC, Tanaka Y, Nonomura N, Okuyama A, Dahiya R. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin Cancer Res. 2005;11:5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- 26.Habano W, Gamo T, Sugai T, Otsuka K, Wakabayashi G, Ozawa S. CYP1B1, but not CYP1A1, is downregulated by promoter methylation in colorectal cancers. Int J Oncol. 2009;34(4):1085–1091. doi: 10.3892/ijo_00000235. [DOI] [PubMed] [Google Scholar]
- 27.Vieira I, Pasanen M, Raunio H, Cresteil T. Expression of CYP2E1 in human lung and kidney during development and in full-term placenta: a differential methylation of the gene is involved in the regulation process. Pharmacol Toxicol. 1998;83(5):183–187. doi: 10.1111/j.1600-0773.1998.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 28.Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver. Hypermethylation control of gene expression during the neonatal period. Eur J Biochem. 1996;238(2):476–483. doi: 10.1111/j.1432-1033.1996.0476z.x. [DOI] [PubMed] [Google Scholar]
- 29.Botto F, Seree E, el Khyari S, de Sousa G, Massacrier A, Placidi M, Cau P, Pellet W, Rahmani R, Barra Y. Tissue-specific expression and methylation of the human CYP2E1 gene. Biochem Pharmacol. 1994;48(6):1095–1103. doi: 10.1016/0006-2952(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 30.Ghanayem BI, Hoffler U. Investigation of xenobiotics metabolism, genotoxicity and carcinogenicity using cyp2e1( −/−) mice. Curr Drug Metab. 2007;8:728–749. doi: 10.2174/138920007782109760. [DOI] [PubMed] [Google Scholar]
- 31.Botto F, Seree E, el Khyari S, Cau P, Henric A, De Meo M, Bergeron P, Barra Y. Hypomethylation and hypoexpression of human CYP2E1 gene in lung tumors. Biochem Biophys Res Commun. 1994;205(2):1086–1092. doi: 10.1006/bbrc.1994.2777. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Tang Y, Hoshino T, Neya S. Molecular modeling of human cytochrome P450 2W1 and its interactions with substrates. Mol Graph Model. 2009;28(2):170–176. doi: 10.1016/j.jmgm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Gomez A, Nekvindova J, Travica S, Lee MY, Johansson I, Edler D, Mkrtchian S, Ingelman-Sundberg M (2010) Colorectal cancer specific cytochrome P450 2W1 (CYP2W1): intracellular localization, glycosylation, and catalytic activity. Mol Pharmacol. doi: 10.1124/mol.110.067652 [DOI] [PubMed]
- 34.Edler D, Stenstedt K, Ohrling K, Hallstrom M, Karlgren M, Ingelman-Sundberg M, Ragnhammar P. The expression of the novel CYP2W1 enzyme is an independent prognostic factor in colorectal cancer—a pilot study. Eur J Cancer. 2009;45(4):705–712. doi: 10.1016/j.ejca.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Karlgren M, Gomez A, Stark K, Svärd J, Rodriguez-Antona C, Oliw E, Bernal ML, Ramón y Cajal S, Johansson I, Ingelman-Sundberg M. Tumor-specific expression of the novel cytochrome P450 enzyme, CYP2W1. Biochem Biophys Res Commun. 2006;341(2):451–458. doi: 10.1016/j.bbrc.2005.12.200. [DOI] [PubMed] [Google Scholar]
- 36.Karlgren M, Ingelman-Sundberg M. Tumour-specific expression of CYP2W1: its potential as a drug target in cancer therapy. Expert Opin Ther Targets. 2007;11(1):61–67. doi: 10.1517/14728222.11.1.61. [DOI] [PubMed] [Google Scholar]
- 37.Gomez A, Karlgren M, Edler D, Bernal ML, Mkrtchian S, Ingelman-Sundberg M. Expression of CYP2W1 in colon tumors: regulation by gene methylation. Pharmacogenomics. 2007;8(10):1315–1325. doi: 10.2217/14622416.8.10.1315. [DOI] [PubMed] [Google Scholar]
- 38.Dannenberg LO, Edenberg HJ. Epigenetics of gene expression in human hepatoma cells: expression profiling the response to inhibition of DNA methylation and histone deacetylation. BMC Genomics. 2006;7:181. doi: 10.1186/1471-2164-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 41.Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein. Kaiso Mol Cell. 2003;12:723–734. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Chi P, Allis CD, Wang GG. Covalent histone modifications–miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10(7):457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnekenburger M, Peng L, Puga A. HDAC1 bound to the Cyp1a1 promoter blocks histone acetylation associated with Ah receptor-mediated trans-activation. Biochim Biophys Acta. 2007;1769(9–10):569–578. doi: 10.1016/j.bbaexp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y, Ke S, Ouyang N, He J, Xie W, Bedford MT, Tian Y. Epigenetic regulation of transcriptional activity of pregnane X receptor by protein arginine methyltransferase 1. J Biol Chem. 2009;284(14):9199–9205. doi: 10.1074/jbc.M806193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assenat E, Gerbal-Chaloin S, Larrey D, Saric J, Fabre JM, Maurel P, Vilarem MJ, Pascussi JM. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40(4):951–960. doi: 10.1002/hep.20387. [DOI] [PubMed] [Google Scholar]
- 46.Ma Quiang. Xenobiotic-activated receptors: from transcription to drug metabolism to disease. Chem Res Toxicol. 2008;21(9):1651–1671. doi: 10.1021/tx800156s. [DOI] [PubMed] [Google Scholar]
- 47.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamási V, Vereczkey L, Falus A, Monostory K. Some aspects of interindividual variations in the metabolism of xenobiotics. Inflamm Res. 2003;52(8):322–333. doi: 10.1007/s00011-003-1186-4. [DOI] [PubMed] [Google Scholar]
- 50.Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16(5):977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- 51.Goodwin B, Hodgson E, Liddle C. The orphan human Pregnane X Receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 52.Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- 53.Gerbal-Chaloin S, Pascussi JM, Pichard-Garcia L, Daujat M, Waechter F, Fabre JM, Carrere N, Maurel P. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos. 2001;29:242–251. [PubMed] [Google Scholar]
- 54.Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 55.Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 56.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 57.Ordentlich P, Downes M, Xie W, Genin A, Spinner NB, Evans RM. Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc Natl Acad Sci USA. 1999;96:2639–2644. doi: 10.1073/pnas.96.6.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park EJ, Schroen DJ, Yang M, Li H, Li L, Chen JD. SMRT, a silencing mediator for retinoid and thyroid hormone receptors-extended isoform that is more related to the nuclear receptor corepressor. Proc Natl Acad Sci USA. 1999;96:3519–3524. doi: 10.1073/pnas.96.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–5637. [PubMed] [Google Scholar]
- 60.Chang TK, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57:1946–1954. [PubMed] [Google Scholar]
- 61.Crewe HK, Ellis SW, Lennard MS, Tucker GT. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol. 1997;53:171–178. doi: 10.1016/s0006-2952(96)00650-8. [DOI] [PubMed] [Google Scholar]
- 62.Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR. Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos. 2002;30:608–612. doi: 10.1124/dmd.30.5.608. [DOI] [PubMed] [Google Scholar]
- 63.Mani S, Huang H, Sundarababu S, Liu W, Kalpana G, Smith AB, Horwitz SB. Activation of the steroid and xenobiotic receptor (human pregnane X receptor) by nontaxane microtubule stabilizing agents. Clin Cancer Res. 2005;11:6359–6369. doi: 10.1158/1078-0432.CCR-05-0252. [DOI] [PubMed] [Google Scholar]
- 64.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- 66.Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- 67.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 68.Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp. 2006;317(3):1200–1209. doi: 10.1124/jpet.105.098160. [DOI] [PubMed] [Google Scholar]
- 69.Njar VC, Gediya L, Purushottamachar P, Chopra P, Vasaitis TS, Khandelwal A, Mehta J, Huynh C, Belosay A, Patel J. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem. 2006;14(13):4323–4340. doi: 10.1016/j.bmc.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 70.Kato S. The function of vitamin D activation. J Biochem. 2000;127:717–722. doi: 10.1093/oxfordjournals.jbchem.a022662. [DOI] [PubMed] [Google Scholar]
- 71.Wang K, Mendy AJ, Dai G, Luo HR, He L, Wan YJ. Retinoids activate the RXR/SXR-mediated pathway and induce the endogenous CYP3A4 activity in Huh7 human hepatoma cells. Toxicol Sci. 2006;92:51–60. doi: 10.1093/toxsci/kfj207. [DOI] [PubMed] [Google Scholar]
- 72.Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, Ishizuka H, Kharasch E, Schuetz J, Schuetz E. Transcriptional control of intestinal cytochrome P-4503A by 1alpha, 25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- 73.Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277:25125–25132. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 74.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 75.Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annu Rev Pharmacol Toxicol. 2001;41:421–442. doi: 10.1146/annurev.pharmtox.41.1.421. [DOI] [PubMed] [Google Scholar]
- 76.Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol. 2000;58:361–372. doi: 10.1124/mol.58.2.361. [DOI] [PubMed] [Google Scholar]
- 77.Wang H, Faucette SR, Gilbert D, Jolley SL, Sueyoshi T, Negishi M, LeCluyse EL. Glucocorticoid receptor enhancement of pregnane X receptor-mediated CYP2B6 regulation in primary human hepatocytes. Drug Metab Dispos. 2003;31:620–630. doi: 10.1124/dmd.31.5.620. [DOI] [PubMed] [Google Scholar]
- 78.Loose DS, Stover EP, Feldman D. Ketoconazole binds to glucocorticoid receptors and exhibits glucocorticoid antagonist activity in cultured cells. J Clin Invest. 1983;72:404–408. doi: 10.1172/JCI110982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Kissling G, Negishi M, Goldstein JA. The nuclear receptors constitutive androstane receptor and pregnane X receptor cross talk with hepatic nuclear factor 4alpha to synergistically activate the human CYP2C9 promoter. J Pharmacol Exp Ther. 2005;314:1125–1133. doi: 10.1124/jpet.105.087072. [DOI] [PubMed] [Google Scholar]
- 80.Liu FJ, Song X, Yang D, Deng R, Yan B. The far and distal enhancers in the CYP3A4 gene coordinate the proximal promoter in responding similarly to the pregnane X receptor but differentially to hepatocyte nuclear factor-4alpha. Biochem J. 2008;409:243–250. doi: 10.1042/BJ20070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawana K, Ikuta T, Kobayashi Y, Gotoh O, Takeda K, Kawajiri K. Molecular mechanism of nuclear translocation of an orphan nuclear receptor, SXR. Mol Pharmacol. 2003;63(3):524–531. doi: 10.1124/mol.63.3.524. [DOI] [PubMed] [Google Scholar]
- 82.Zelko I, Sueyoshi T, Kawamoto T, Moore R, Negishi M. The peptide near the C terminus regulates receptor CAR nuclear translocation induced by xenochemicals in mouse liver. Mol Cell Biol. 2001;21(8):2838–2846. doi: 10.1128/MCB.21.8.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da Silva Xavier G, Rutter GA, Viollet B, Meyer UA. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70(6):1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- 84.Blättler SM, Rencurel F, Kaufmann MR, Meyer UA. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc Natl Acad Sci USA. 2007;104(3):1045–1050. doi: 10.1073/pnas.0610216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi E, Lee S, Yeom SY, Kim GH, Lee JW, Kim SW. Characterization of activating signal cointegrator-2 as a novel transcriptional coactivator of the xenobiotic nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1711–1719. doi: 10.1210/me.2005-0066. [DOI] [PubMed] [Google Scholar]
- 86.Kim HJ, Lee SK, Na SY, Choi HS, Lee JW. Molecular cloning of xSRC-3, a novel transcription coactivator from Xenopus, that is related to AIB1, p/CIP, and TIF2. Mol Endocrinol. 1998;12:1038–1047. doi: 10.1210/mend.12.7.0139. [DOI] [PubMed] [Google Scholar]
- 87.Min G, Kemper JK, Kemper B. Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J Biol Chem. 2002;277:26356–26363. doi: 10.1074/jbc.M200051200. [DOI] [PubMed] [Google Scholar]
- 88.Muangmoonchai R, Smirlis D, Wong SC, Edwards M, Phillips IR, Shephard EA. Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcription factor Sp1. Biochem J. 2001;355:71–78. doi: 10.1042/0264-6021:3550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shiraki T, Sakai N, Kanaya E, Jingami H. Activation of orphan nuclear constitutive androstane receptor requires subnuclear targeting by peroxisome proliferator-activated receptor γ coactivator-1α. A possible link between xenobiotic response and nutritional state. J Biol Chem. 2003;278:11344–11350. doi: 10.1074/jbc.M212859200. [DOI] [PubMed] [Google Scholar]
- 90.Inoue K, Borchers CH, Negishi M. Cohesin protein SMC1 represses the nuclear receptor CAR-mediated synergistic activation of a human P450 gene by xenobiotics. Biochem J. 2006;398:125–133. doi: 10.1042/BJ20060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt R, Baumann F, Knupfer H, Brauckhoff M, Horn LC, Schonfelder M, Kohler U, Preiss R. CYP3A4, CYP2C9 and CYP2B6 expression and ifosfamide turnover in breast cancer tissue microsomes. Br J Cancer. 2004;90(4):911–916. doi: 10.1038/sj.bjc.6601492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El-Rayes BF, Ali S, Heilbrun LK, Lababidi S, Bouwman D, Visscher D, Philip PA. Cytochrome p450 and glutathione transferase expression in human breast cancer. Clin Cancer Res. 2003;9(5):1705–1709. [PubMed] [Google Scholar]
- 93.Williams JA, Phillips DH. Mammary expression of xenobiotic metabolizing enzymes and their potential role in breast cancer. Cancer Res. 2000;60(17):4667–4677. [PubMed] [Google Scholar]
- 94.de Jonge ME, Huitema AD, van Dam SM, Beijnen JH, Rodenhuis S. Significant induction of cyclophosphamide and thiotepa metabolism by phenytoin. Cancer Chemother Pharmacol. 2005;55(5):507–510. doi: 10.1007/s00280-004-0922-y. [DOI] [PubMed] [Google Scholar]
- 95.Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev. 2007;33(4):369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Tsunedomi R, Iizuka N, Hamamoto Y, Uchimura S, Miyamoto T, Tamesa T, Okada T, Takemoto N, Takashima M, Sakamoto K, Hamada K, Yamada-Okabe H, Oka M. Patterns of expression of cytochrome P450 genes in progression of hepatitis C virus associated hepatocellular carcinoma. Int J Oncol. 2005;27(3):661–667. [PubMed] [Google Scholar]
- 97.Puigserver PJ, Rhee J, Donovan CJ, Walkey J, Cliff Yoon F, Oriente Y, Kitamura J, Altomonte H, Dong D, Accili B, Spiegelman M. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 98.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 99.Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;2(18):7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf I, Rubinek T. Diabetes mellitus and breast cancer. Front Diabetes. 2008;19:97–113. [Google Scholar]
- 101.Pascussi J, Busson-Le Coniat M, Maurel P, Vilarem M. Transcriptional analysis of the orphan nuclear receptor constitutive androstane receptor (NR1I3) gene promoter: identification of a distal glucocorticoid responsive element. Mol Endocrinol. 2003;17:42–55. doi: 10.1210/me.2002-0244. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Faucette SR, Gilbert D, Jolley SL, Sueyoshi T, Negishi M, LeCluyse EL. Glucocorticoid receptor enhancement of pregnane X receptor-mediated CYP2B6 regulation in primary human hepatocytes. Drug Metab Dispos. 2003;31(5):620–630. doi: 10.1124/dmd.31.5.620. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Goldstein JA. The transcriptional regulation of the human CYP2C genes. Curr Drug Metab. 2009;10(6):567–578. doi: 10.2174/138920009789375397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hankinson O. The aryl hydrocarbon receptor complex. Ann Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 105.Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer. 2009;16(9):187. doi: 10.1186/1471-2407-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31(8):1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 110.Ma Q. Aryl hydrocarbon receptor degradation-promoting factor (ADPF) and the control of the xenobiotic response. Mol Interv. 2007;7(3):133–137. doi: 10.1124/mi.7.3.4. [DOI] [PubMed] [Google Scholar]
- 111.Wang SH, Liang CT, Liu YW, Huang MC, Huang SC, Hong WF, Su JG. Crosstalk between activated forms of the aryl hydrocarbon receptor and glucocorticoid receptor. Toxicology. 2009;262(2):87–97. doi: 10.1016/j.tox.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 112.Monostory K, Kohalmy K, Prough RA, Kóbori L, Vereczkey L. The effect of synthetic glucocorticoid, dexamethasone on CYP1A1 inducibility in adult rat and human hepatocytes. FEBS Lett. 2005;579(1):229–235. doi: 10.1016/j.febslet.2004.11.080. [DOI] [PubMed] [Google Scholar]
- 113.Dvoøak Z, Vrzal R, Pávek P, Ulrichová J. An evidence for regulatory cross-talk between aryl hydrocarbon receptor and glucocorticoid receptor in HepG2 cells. Physiol Res. 2008;57:427–435. doi: 10.33549/physiolres.931090. [DOI] [PubMed] [Google Scholar]
- 114.Vrzal R, Stejskalova L, Monostory K, Maurel P, Bachleda P, Pavek P, Dvorak Z. Dexamethasone controls aryl hydrocarbon receptor (AhR)-mediated CYP1A1 and CYP1A2 expression and activity in primary cultures of human hepatocytes. Chem Biol Interact. 2009;179:288–296. doi: 10.1016/j.cbi.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 115.van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35(8):692–706. doi: 10.1016/j.ctrv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 116.Pearce ST, Liu H, Radhakrishnan I, Abdelrahim M, Safe S, Jordan VC. Interaction of the aryl hydrocarbon receptor ligand 6-methyl-1, 3, 8-thrichlorodibenzofuran with oestrogen receptor alpha. Cancer Res. 2004;64:2889–2897. doi: 10.1158/0008-5472.can-03-1770. [DOI] [PubMed] [Google Scholar]
- 117.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 118.Matthews J, Wihlén B, Thomsen J, Gustafsson J. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor α to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Biol. 2005;25:5317–5328. doi: 10.1128/MCB.25.13.5317-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han W, Pentecost BT, Pietropaolo RL, Fasco MJ, Spivack SD. Estrogen receptor α increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1 in normal human bronchial epithelial cells. Mol Carcinogen. 2005;44:202–211. doi: 10.1002/mc.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ohtake F, Takeyama K, Matsumoto H, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signaling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 121.Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, O’Malley BW. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- 122.Monostory K, Pascussi JM, Kóbori L, Dvorak Z. Hormonal regulation of CYP1A expression. Drug Metab Rev. 2009;41(4):547–572. doi: 10.1080/03602530903112284. [DOI] [PubMed] [Google Scholar]
- 123.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fritz WA, Lin TM, Peterson RE. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis. 2008;29(5):1077–1082. doi: 10.1093/carcin/bgn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nishida CR, Lee M, de Montellano PR. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol Pharmacol. 2010;78(3):497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karlgren M, Miura S, Ingelman-Sundberg M. Novel extrahepatic cytochrome P450s. Toxicol Appl Pharmacol. 2005;207:57–61. doi: 10.1016/j.taap.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 127.Rylander T, Neve EP, Ingelman-Sundberg M, Oscarson M. Identification and tissue distribution of the novel human cytochrome P450 2S1 (CYP2S1) Biochem Biophys Res Commun. 2001;281:529–535. doi: 10.1006/bbrc.2001.4390. [DOI] [PubMed] [Google Scholar]
- 128.Rivera SP, Wang F, Saarikoski ST, Taylor RT, Chapman B, Zhang R, Hankinson O. A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of P4502S1 by both dioxin and hypoxia. J Biol Chem. 2007;282:10881–10893. doi: 10.1074/jbc.M609617200. [DOI] [PubMed] [Google Scholar]
- 129.Downie D, McFadyen MC, Rooney PH, Cruickshank ME, Parkin DE, Miller ID, Telfer C, Melvin WT, Murray GI. Profiling cytochrome P450 expression in ovarian cancer: identification of prognostic markers. Clin Cancer Res. 2005;11:7369–7375. doi: 10.1158/1078-0432.CCR-05-0466. [DOI] [PubMed] [Google Scholar]
- 130.Kumarakulasingham M, Rooney PH, Dundas SR, Telfer C, Melvin WT, Curran S, Murray GI. Cytochrome p450 profile of colorectal cancer: identification of markers of prognosis. Clin Cancer Res. 2005;11:3758–3765. doi: 10.1158/1078-0432.CCR-04-1848. [DOI] [PubMed] [Google Scholar]
- 131.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25(44):10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tamasi V, Jeffries JM, Arteel GE, Falkner KC. Ebselen augments its peroxidase activity by inducing nrf-2-dependent transcription. Arch Biochem Biophys. 2004;431(2):161–168. doi: 10.1016/j.abb.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 133.Douglas KT (1987) Mechanism of action of glutathione-dependent enzymes. Adv Enzymol Relat Areas Mol Biol 59103–59167 [DOI] [PubMed]
- 134.Sau F, Pellizzari Tregno F, Valentino F, Federici G, Caccuri AM (2010) Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys 500116–122 [DOI] [PubMed]
- 135.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 136.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 137.Lämsä V, Levonen AL, Leinonen H, Ylä-Herttuala S, Yamamoto M, Hakkola J. Cytochrome P450 2A5 constitutive expression and induction by heavy metals is dependent on redox-sensitive transcription factor Nrf2 in liver. Chem Res Toxicol. 2010;23(5):977–985. doi: 10.1021/tx100084c. [DOI] [PubMed] [Google Scholar]
- 138.Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 139.Prochaska HJ, Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988;48:4776–4782. [PubMed] [Google Scholar]
- 140.Kohle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 141.Kensler TW, Wakabayashi N, Slocum SL, Skoko JJ, Shin S. When Nrf2 talks, who’s listening? Antioxid Redox Signal. 2010;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayes JD, Dinkova-Kostova AT, McMahon M. Cross-talk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicol Sci. 2009;111(2):199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 143.Marden NY, Fiala-Beer E, Xiang SH, Murray M. Role of activator protein-1 in the down-regulation of the human CYP2J2 gene in hypoxia. Biochem J. 2003;373:669–680. doi: 10.1042/BJ20021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marden NY, Murray M. Characterization of a c-Jun-responsive module in the 5′flank of the human CYP2J2 gene that regulates transactivation. Biochem J. 2005;391:631–640. doi: 10.1042/BJ20050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee AC, Murray M. Up-regulation of human CYP2J2 in HepG2 cells by butylated hydroxyanisole is mediated by c-Jun and Nrf2. Mol Pharmacol. 2010;77(6):987–994. doi: 10.1124/mol.109.062729. [DOI] [PubMed] [Google Scholar]
- 146.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65(11):4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 147.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 148.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 149.Molnár V, Tamási V, Bakos B, Wiener Z, Falus A. Changes in miRNA expression in solid tumors: an miRNA profiling in melanomas. Semin Cancer Biol. 2008;18(2):111–122. doi: 10.1016/j.semcancer.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 150.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66(18):9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 151.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Title microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 152.Kalscheuer S, Zhang X, Zeng Y, Upadhyaya P. Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 2008;29(12):2394–2399. doi: 10.1093/carcin/bgn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mohri T, Nakajima M, Fukami T, Takamiya M, Aoki Y, Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol. 2010;79(7):1045–1052. doi: 10.1016/j.bcp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 154.Lamba V, Panetta JC, Strom S, Schuetz EG. Genetic predictors of interindividual variability in hepatic CYP3A4 expression. J Pharmacol Exp Ther. 2010;332:1088–1099. doi: 10.1124/jpet.109.160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pan YZ, Gao W, Yu AM. MicroRNAs regulate CYP3A4 expression via direct, indirect targeting. Drug Metab Dispos. 2009;37(10):2112–2117. doi: 10.1124/dmd.109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takagi S, Nakajima M, Mohri T, Yokoi T. Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem. 2008;283(15):9674–9680. doi: 10.1074/jbc.M709382200. [DOI] [PubMed] [Google Scholar]