Abstract
Bacteriocin AS-48 is an intriguing molecule because of its unique structural characteristics, genetic regulation, broad activity spectrum, and potential biotechnological applications. It was the first reported circular bacteriocin and has been undoubtedly the best characterized for the last 25 years. Thus, AS-48 is the prototype of circular bacteriocins (class IV), for which the structure and genetic regulation have been elucidated. This review discusses the state-of-the-art in genetic engineering with regard to this circular protein, with the use of site-directed mutagenesis and circular permutation. Mutagenesis studies have been used to unravel the role of (a) different residues in the biological activity, underlining the relevance of several residues involved in membrane interaction and the low correlation between stability and activity and (b) three amino acids involved in maturation, providing information on the specificity of the leader peptidase and the circularization process itself. To investigate the role of circularity in the stability and biological properties of the enterocin AS-48, two different ways of linearization have been attempted: in vitro by limited proteolysis experiments and in vivo by circular permutation in the structural gene as-48A. The results summarized here show the significance of circularization on the secondary structure, potency and, especially, the stability of AS-48 and point as well to a putative role of the leader peptide as a protecting moiety in the pre-proprotein. Taken all together, the data available on circular bacteriocins support the idea that AS-48 has been engineered by nature to make a remarkably active and stable protein with a broad spectrum of activity.
Keywords: Protein engineering, Site-directed mutagenesis, Circular permutation, Limited proteolysis, Circular dichroism, Biological activity, Antimicrobial proteins, Enterocin, Circular protein
Introduction
Most proteins are synthesized as linear polymers of amino acids, in which the α-amino group of one residue is linked to the α-carboxyl group of the next by a peptide bond, with a definite three-dimensional structure under physiological conditions. However, the free ends of these proteins are routinely targeted by exopeptidases, which weaken the stability of the molecule. Also, the ends of linear proteins are often flexible or poorly defined, in contrast to their highly structured interior. The post-translational linkage of the N- and C-termini via a peptide bond creates a continuous peptide backbone that provides these proteins with extraordinary structural and proteolytic stability, compared to their conventional linear counterparts [1, 2]. Currently, numerous groups are working on diverse families of natural, ribosomally synthesized circular proteins, found in bacteria, plants, fungi, and animals. Eukaryotic circular proteins with antimicrobial activities include those produced by mammals, the theta-defensins, expressed as part of the innate immune system for combating infection, and the retrocyclins, which inhibit the entry of viruses into cells by binding to glycoproteins at the early stage of the viral infection. In plants, two types of circular proteins have also been described, the sunflower trypsin inhibitor (SFTI-1) and the cyclotides from Violaceae, Rubiaceae, and Cucurbitaceae families. The cyclotides constitute the largest known family of circular peptides (around 50,000 members, most of which are registered in the database CyBase [3], http://www.cybase.org.au/). Cyclotides are small ultra-stable plant peptides, comprising 28–37 amino acids, which have a circular peptide backbone cross-linked by a cystine knot formed by six conserved Cys residues [4]. They have a wide range of biological activities (uterotonic, anti-HIV, hemolytic, neurotensin antagonism, antimicrobial, antifouling, cytotoxic, and trypsin inhibition). Nevertheless, their natural function appears to be related to plant defense against insect predation [4–6]. More recently, two groups of small ribosomally synthesized cyclic peptides have been described in fungi, the amatoxins (octapeptides that inhibit the RNA polymerase II) and phallocidins (heptapeptides that stabilize the F-actin) [5]. In addition to their head-to-tail cyclic backbone, they have an unusual cross-link formed by the condensation of cysteine and tryptophan residues. The prokaryotic circular proteins include some bacteriocins and pilins. The latter correspond to the largest circular proteins known to date, involved in the transfer of genetic material between bacteria [4, 5]. Bacteriocins constitute a family of ribosomally synthesized antimicrobial peptides and proteins, showing a variable spectrum, mode of action, molecular weight, genetic origin, and biochemical properties. These inhibitory molecules are secreted by all major lineages of the Bacteria domain [7] and in all cases the producer strain shows a specific immunity mechanism. These peptides are nearly all cationic and very often amphiphilic, as reflected in the fact that many of them kill their target cells by accumulation or insertion in the membrane, thereby causing increased permeability and loss of barrier functions. Although there is not a definitive classification for bacteriocins from Gram-positive bacteria, it is generally accepted the division in Class I, composed by post-translationally modified peptides containing lanthionine or methyl-lanthionine (reviewed by [8]); Class II, which groups small thermostable, non-modified proteins (with the exception of disulfide bridges linkage), with or without leader peptide; and Class III, which includes secreted heat-labile, cell-wall-degrading enzymes [9]. Over the past decade, different research groups have described an intriguing family of circular, post-translationally modified bacteriocins recently grouped as a new class of bacteriocins (reviewed by [10]). They were initially considered as a subclass in Class II bacteriocins, but their predominantly helical structure in solution and the modifications they undergo clearly make a difference with members of the other class. Thus, Class IV encompasses globular, thermostable, helical, and post-translationally modified proteins, ranging between 35 and 70 amino acids, with the N- and C-termini linked by a peptide bond [10]. It has been suggested that the circular bacteriocins should be divided into two subclasses according to their sequence [11]: the group of disparate bacteriocins carnocyclin A [12], lactocyclicin Q [13], circularin A [14], AS-48 [15], uberolysin [16], and the recently described garvicin ML [17], and a second group that comprises the chemically identical gassericin A/reutericin 6 [18], which differ from acidocin B only by one residue (M24V) [19] and butyrivibriocin AR10 [20]. In addition, subtilosin A, from Bacillus subtilis, is another circular bacteriocin not included in these subclasses because of its atypical characteristics: it is anionic and contains thioether bridges linking cysteine sulfurs to the α-carbon of other residues. Thus, subtilosin A may represent a unique class of bacteriocins [21].
Despite the unquestionable interest of these molecules, only the three-dimensional structure of AS-48 [15], carnocyclin A [12], and subtilosin A [22] are available. The comparison of AS-48 and carnocyclin A shows that even though both bacteriocins have low sequence identity, they have a well-defined three-dimensional structure that resembles the saposin fold. Additional secondary structure prediction and homology modeling analysis of the rest of the circular bacteriocins indicate that all of them contain helical secondary structural elements (four or five helices) enclosing a compact hydrophobic core [11, 22]. Subtilosin A structure differs, because it contains additional thiol linkages and just one helical fragment [21, 22].
One of the advantages of the circular form over conventional linear proteins is that the joining of the ends removes the major degradation pathway by exopeptidase enzymes, therefore increasing notably their stability. What is particularly impressive about circular proteins is their highly resistant nature to a wide range of pH and temperatures. Moreover, enhanced stability and many of the biological activities of circular proteins are dependent on the circular peptide backbone [4]. One of the main objectives of protein engineering is to generate molecules with new and/or improved features with practical chemical, pharmaceutical, or agricultural applications. Circular proteins, and circularization of proteins itself, show interesting biotechnological properties and applications. This design can take different approaches: (a) de novo protein synthesis by chemical methods, (b) the search for clones with desired properties based on directed evolution through genetic libraries, and (c) rational modification with predictable structural and/or mechanistic consequences. This latter option requires a comprehensive understanding of the structure–function relationship in proteins but is one of the most promising, since it benefits from the features of proteins for which stability, functionality, and application have been selected during evolution. From a biological standpoint, modifying a protein requires the availability of genetic-engineering techniques that can change the DNA in which the information is accurate, giving rise to the synthesis of the modified protein. Notable among the many methods developed for gene manipulation are in vitro site-directed mutagenesis and circular permutation. Diverse applications and consequences of the mutagenesis make it possible to establish the role of a residue in the biological activity and/or in the folding of a protein, and to design an amino acid sequence for any desired 3D structure.
This review focuses on the current knowledge concerning the engineered AS-48 molecule, which is the most representative circular bacteriocin and a model in order to explore the relevant features of this striking group. Residues particularly important for the inhibitory action and for interaction with membranes have thereby been identified, and the effects on bacteriocin activity caused by altering those residues by site-directed mutagenesis have been determined. In addition, we compare wild-type AS-48 with fragments and linear counterparts, obtained in vivo by circular permutation and in vitro by limited proteolysis, thus providing valuable information on the role of circularization and the biological significance of AS-48 composition.
An overview of AS-48, prototype of circular bacteriocins
AS-48 is a 70-residue, gene-encoded, α-helical circular cationic bacteriocin produced by different Enterococcus species. Its antimicrobial activity against food-borne Gram-positive and Gram-negative pathogenic (E. coli, Salmonella spp., Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus) and food-spoilage bacteria (Bacillus spp., Paenibacillus spp.) has been extensively documented [15, 23, 24]. These characteristics, together with its stability and solubility over wide pH and temperature ranges, confer a clear potential to be used as food biopreservative. In fact, this bacteriocin has been successfully employed against different pathogens in a broad array of products, including salads, desserts, milk, cheeses, fruit juices, vegetables sauces, acid-fermented sausages, rice-based foods, soybean sprouts, canned food, and coconut milk (reviewed by [23] and [24]). Besides this, AS-48 could also have a veterinary application, since liposome-encapsulated AS-48 inhibits the growth of a S. aureus strain isolated from mastitis in dairy cows [25]. New clinical applications are currently under investigation, underscoring its potential as an antimicrobial agent in some disease treatments.
There is extensive and detailed information on the genetic determinants and physicochemical characteristics of AS-48 (reviewed by [10], [15]). The as-48 biosynthetic gene cluster was found in the conjugative pMB2 plasmid from E. faecalis S-48 strain, raising questions about the importance of horizontal gene transfer. The full expression of AS-48 trait depends upon the co-ordinated expression of ten genes organized in two operons, as-48ABC and as-48C 1 DD 1 EFGH, where genes encoding processing, secretion, and immunity functions are adjacent to the structural as-48A gene. Unmodified AS-48 pre-proprotein consists of an N-terminal leader peptide (35 residues), which appears to be related to the protection of unmodified AS-48 against proteases, followed by a proprotein moiety that undergoes post-translational modifications consisting of the leader peptide-cleavage reaction between His-1 and Met1 and the peptide bond formation between the N- and C-ends (i.e., Met1 and Trp70) to yield the mature circular AS-48 protein (Fig. 1). Expression of as-48ABC operon is controlled at the post-transcriptional level and is uncoupled from the as-48BC translation [26]. This mechanism provides the enterococcal cells with maximized production of functional AS-48 without deleterious effects, before the entire immunity machinery—the as-48D 1 immunity gene and the as-48EFGH transporter—begins to work. Besides this, the as-48 cluster contains another ABC transport system, As-48C1D, most probably involved in secretion of newly synthesized enterocin and providing low levels of immunity [27].
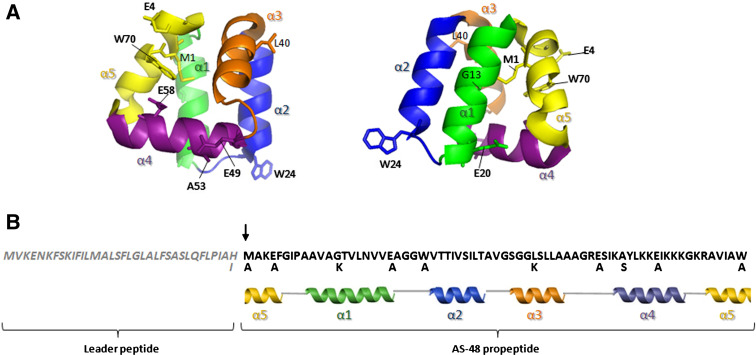
Fig. 1.
AS-48 derivatives obtained by site-directed mutagenesis. a Two different sights of the three-dimensional structure of AS-48 derived from the NMR structure of the protein (file 1E68) showing the position of the mutated residues. The five major helical regions of the protein are indicated with different colors. b Amino acid sequence of pre-proprotein AS-48 and the secondary structure. The leader peptide sequence is shown in italics and the arrow indicates the processing site. Mutations in single residues are indicated
The most distinctive structural feature of enterocin AS-48 is, unquestionably, its circular structure, which contributes to the stability of the native form, because of the reduction in conformational entropy. AS-48 is a strongly basic molecule (pI ~ 10.5) that contains a high proportion (49%) of hydrophobic amino acids and uncharged hydrophilic residues. Both the NMR and X-ray structures of AS-48 have been resolved [15]. The molecule consists of a fairly compact globular arrangement of five α-helices with a highly asymmetrical distribution of the positive charges, where all basic residues are clustered at the level of helices α4 and α5, whereas a hydrophobic surface is located in helices α1-3. In other circular molecules (carnocyclin A, uberolysin, circularin A, lactocyclicin Q, and garvicin ML) basic residues appear clustered as well [17]. All these effects may be complemented by an efficient interdigitation of the hydrophobic side chains at the core of the globular structure, as manifested by the large number of contacts among them.
The NMR structure at pH 3.0 suggests that this molecule forms complexes in the membrane by electrostatic interaction between its large positively charged patch and anionic membrane phospholipids, thus destabilizing the membrane potential and leading to the formation of non-specific pores and cell leakage in a process known as molecular electroporation. However, the high-resolution crystal structure showed that the protein molecules are arranged in dimers due to either hydrophobic (dimer DF-I), or hydrophilic (dimer DF-II) interactions (reviewed by [15]). The crystal structure of AS-48 at higher pH values shows that AS-48 protomers have a remarkable amphipathic surface due to a hypothetical plane containing the Cα atoms of Glu4, Glu20, Glu49, and Glu58 that segregates a patch of positively charged residues from the rest of the hydrophobic or uncharged surface residues. On the basis of these data, it has been proposed that DF-I mimics the solution form of the protein, whereas DF-II mimics the membrane-bound form. It is notable that the backbone circularization of pro-AS-48 occurs in the middle of α5-helix, and this is believed to have a marked effect on the extreme stability of the three-dimensional structure, as has been described in previous differential scanning calorimetric works [15]. However, this is not exclusive of AS-48, because in carnocyclin A the covalent linkage between Leu1 and Leu60 termini also occurs in the last helix [12].
The mode of action of enterocin AS-48 has been elucidated. This bacteriocin makes approximately 0.7-nm pores in the bacterial cytoplasmic membrane, thereby disrupting the proton motive force and causing cell death. Based on its crystal structure, the proposed mechanism of insertion into bacterial membranes suggests that the two different stages of molecular association, DF-I and DF-II, are involved in changing from the water-soluble DF-I to the membrane-bound DF-II stage at the membrane surface. This transition implies a 90° rotation of each protomer within DF-I, in such a way that the partially hidden hydrophobic helices α1 and α2 become solvent accessible [15].
Protein engineering on AS-48
Generation of single AS-48 derivatives by site-directed mutagenesis
Several studies have recently been performed to manipulate the structure and biological activity of AS-48 by site-directed mutagenesis within the structural as-48A gene. In these works, mutational analyses with three kinds of single AS-48-derivatives were obtained (Figs. 1, 2):
-
i)
Replacement of all negatively charged Glu residues individually, with a hydrophobic small residue (E4A, E20A, E49A, and E58A) to investigate their roles in the biological activity and stability of the AS-48 molecule [28].
-
ii)
Four mutants in the solvent-exposed residues (W24A, G13K, L40K, and A53S) to study their influence upon target-cell specificity [29].
-
iii)
Three substitutions (H-1I, M1A, and W70A) in the residues that constitute the recognition site for the cleavage enzyme (His-1, Met1) and in those involved in circularization (Met1, Trp70) to determine the critical positions involved in these maturation reactions [30].
Fig. 2.
Schematic depicture of mutants activity with letter height showing the relative activity of each mutant with respect to wild type AS-48. In solid grey letters amino acids of wild-type AS-48 are shown. Met1 and Trp70 are underlined. The inner circle shows substituted amino acids
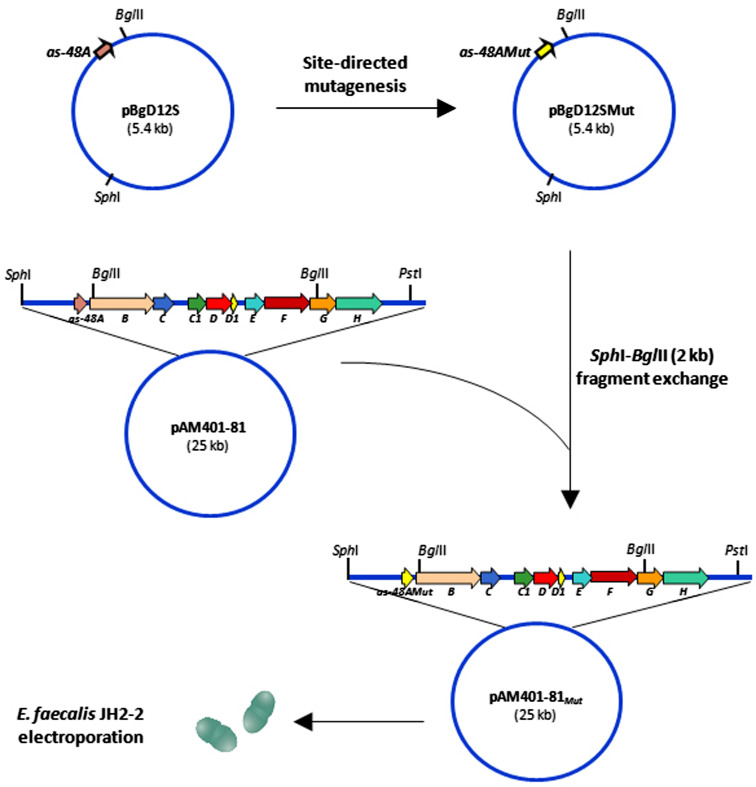
Each substitution was separately inserted into the as-48A structural gene by site-directed mutagenesis [28], thus leading to different pAM401-81Mut plasmids that were separately expressed in Enterococcus faecalis JH2-2 strain to investigate the significance of the substitutions introduced (Fig. 3). This cloning strategy altered the secondary structure of mRNA in the intergenic region between as-48A and as-48BC modifying the post-transcriptional processing and reducing the production of the first two groups of mutants described above [26]. Despite the low production levels, their biosynthesis was not completely inhibited and the AS-48 maturation was not affected.
Fig. 3.
Construction of pAM401-81Mut plasmids by site-directed mutagenesis and gene replacement strategy
A different approach was then taken for mutation of three residues involved in maturation [30]. A somewhat surprising result was that substitution H-1I hindered any circular, linear, or immature AS-48 production, while the yield of the M1A and W70A derivatives was diminished to values of 2 and 12%, respectively, in relation to the wild-type AS-48. Therefore, purification of similar quantities of each mutated AS-48 protein was mandatory to compare their antibacterial activity against different susceptible bacteria and to characterize their stability and secondary structure. Similar results have been described in other genetically modified antibiotic peptides [31, 32] where the introduced changes adversely affected the activity of enzymes involved in the post-translational modifications of these lantibiotics. In addition, it has long been acknowledged that some specificity of the cleavage reaction resides in the last residue of the leader sequence [33, 34] and the importance of leader peptide for bacteriocins production has been discussed [30]. In summary, a total of ten different derivatives were produced, purified, and analyzed by mass spectrometry. Notably, none of the replacements altered the immunity of the mutant producer strains, a complex mechanism in which, as it has been already commented, several determinants from the as-48 gene cluster are involved.
Antibacterial spectra of AS-48 derivatives
The minimal inhibitory concentrations (MICs) for highly purified E4A, E20A, E49A, and E58A, W24A, G13 K, L40K, and A53S derivatives at different pH values, were determined using different indicator strains, and the wild-type AS-48 as control. The results demonstrated that some of the introduced changes drastically altered the antimicrobial activity, depending on their nature, the pH, and their situation in the 3D structure of the molecule. This occurred with the glutamic acid derivatives, where the E20A mutant remained almost as active as AS-48 against the majority of the Gram-positive bacteria tested, while the loss of a negatively charged Glu residue, particularly at positions 4, 49, and 58, had a somewhat more negative effect (MIC values two- to ten-fold higher than that of wild-type AS-48), except for the most susceptible bacteria (B. megaterium and Listeria species) [28]. This is in accordance with the putative interactions of the changed residues in the DF-I and II forms that AS-48 adopts in solution, even after addition of an extra positive charge at residues 13 and 40, due to be located in a domain opposite to the active site of the molecule [29]. This behavior agrees with that found with AS48RJ, a natural variant of AS-48 with an E20V substitution produced by the E. faecium RJ16 strain [35]. These data rule out the loss of a negative charge in the molecule as the only cause of the activity reduction and reinforce the idea of the importance of intra- and inter-molecular interactions. Interestingly, the surface exposed A53S, G13K, and L40K mutants exhibited similar inhibitory activity against the majority of the Gram-positive bacteria tested. Substitution W24A was, however, the most deleterious, since significantly higher MICs (14- to 84-fold higher than that of wild-type AS-48) were needed for inhibition according to the Gram-positive bacteria tested. It has been proposed that Trp plays a role in the interaction and orientation of the bacteriocin in the membrane, although the effect depends on the situation of such residues in the molecule [36]. Thus, the MIC of the circular W70A mutant, which is directly involved in the formation of the head–tail peptide bond during the circularization of the molecule, was very similar to that of the wild-type AS-48 [30]. This behavior agrees with that found with peptides that are active at the membrane level, where Trp residues are considered essential, especially those that, by their location in the molecule, have a strong preference for a well-defined position near the lipid carbonyls, while those located in amphiphilic helices seem rather to be involved in interactions over wider interfacial regions [37, 38]. Finally, MIC determination of the M1A against different strains revealed that the effect of replacing Met by Ala rendered MICs much higher than in the W70A derivative [30]. Since the mutation is the same (Ala in both cases) and at nearby positions, the effect observed must be due to structural questions (see below).
Effect of single mutations in residues involved in AS-48 maturation
The general trend in the biosynthesis of circular bacteriocins involves the synthesis of an N-terminal-extended pre-propeptide that undergoes post-translational circularization after the proteolytic cleavage of the leader sequence. Transport through the membrane is carried on by a dedicated ABC-transporter [10, 11, 15, 17] although there is no clear evidence of the processing of the leader peptide, if it happens concomitantly with the transport or in a different step. Moreover, different mechanisms as in the case of type I and II lantibiotics [8] cannot be ruled out. Notably, among the unanswered questions regarding the machinery involved in processing and maturation of the circular bacteriocins stands out the absence of conserved motifs among them [12], precluding any consensus for cleavage/circularization sites. In addition, their size is highly variable, ranging in length between the longest ones of AS-48 (35 residues) and acidocin B/gassericin A/reutericin 6 (33 residues) to the shortest ones of lactocyclicin Q (ME), circularin A (MFL), garvicin ML (MFD), carnocyclin A (MLYE), and subtilosin A (MKKAVIVE). Moreover, the amino acids involved in the removal of the leader peptide and/or maturation also differ in most cases. Thus, the couple His-1Met1 found in AS-48 is unique, as only the lasso-peptide capistruin has a His at the carboxyl terminus of the leader peptide, although its replacement does not affect its maturation [39]. Conversely, in the 50% of circular bacteriocins, an aromatic residue (Trp or Tyr) is found in the carboxyl terminal region of the linear propeptide, either as the last amino acid (AS-48, lactocyclicin Q, uberolysin, and circularin A) or in subterminal position (subtilosin A). This suggests that this type of residue may play a role in the secretion/transport or acting as a recognition site for the enzyme involved in circularization. By contrast, AS-48 is the only circular bacteriocin that has a Met in position +1 directly involved in cleavage and propeptide circularization. With this in mind, it would be of interest to characterize the three new single derivatives in residues directly involved in the leader peptide cleavage (H-1I, M1A) and circularization (M1A, W70A).
Despite the fact that RT-PCR assays confirmed the presence of specific mRNA transcript, the absence of AS-48 in the JH2-2(pAM401-81H-1I) mutant suggests that His-1 is critically involved in the cleavage-site recognition. In fact, the H-1I substitution causes a processing blockage, thereby hampering a functional production of the AS-48 enterocin protein, as well as of pre-proprotein truncated product or lineal proprotein in the supernatants [30]. Furthermore, the striking low yield of the circular M1A mutant (2%) is interpreted as being due to an inefficient processing of the pre-proprotein, induced by the absence of this exclusive residue, in such a critical position [30]. Thus, we conclude that the N-terminal amino acid of the AS-48 proprotein, the Met1 residue, is directly involved in the processing of the pre-proprotein, most likely located at the cytoplasmic membrane. The results also confirmed the absence of alternatives leader protease cleavage sites in the AS-48 pre-proprotein.
Nevertheless, the most significant result has been the characterization of three active forms in the W70A supernatants, with theoretical masses corresponding to the circular (7,035.42 kDa), oxidized (7,050.93 kDa), and linear forms (7,053.35 kDa) [30]. The unambiguous identification of linear W70A forms indicates the requirement of a second biosynthetic enzyme/domain, responsible for the circularization reaction, the partial specificity of which resides in the last Trp70, since linear forms could be only detected from JH2-2(pAM401-81W70A) transformants. Therefore, the enzyme involved in this step could require the proximity of an -NH2 group at the N-terminal end of the proprotein, acting as a nucleophile in the circularization reaction with the specific, although not essential, C-terminal Trp70 residue, since circular forms are also produced [30].
Analysis of point mutations effects on AS-48 conformational stability and activity
The effect of single substitutions on the secondary structure of AS-48 has been evaluated by circular dichroism (CD) spectroscopy [29, 30]. Far-UV CD spectra showed very similar, high-molar ellipticity values, typical of extremely helical molecules, and confirmed that all of them were well structured in aqueous solution and that no significant structural rearrangements took place upon mutation (Fig. 4a). This behavior is quite different from that of the majority of α-helical linear peptides, which appear as disordered structures in aqueous media and become helical only upon interaction with hydrophobic solvents or phospholipid vesicles [40]. The CD studies conducted in lantibiotics indicate no relationship between the loss of activity and the secondary structure. For example, nisin Z mutants differ in their biological activity, stability, and action spectrum but changes in their secondary structure deduced from their CD spectra, are not significant [41]. In the case of nisin, structural changes are not so easily achieved due to the constraints caused by lanthionine rings, but the effect in the activity can easily be ascribed to the specific role of each residue in lipid II interaction and the conformational change afterwards [42]. However, mutants of mesentericin A and 105 are less active and also have a typical helical spectrum, albeit with less α-helix content (9–15%) than the wild-type molecule (24%) [40].
Fig. 4.
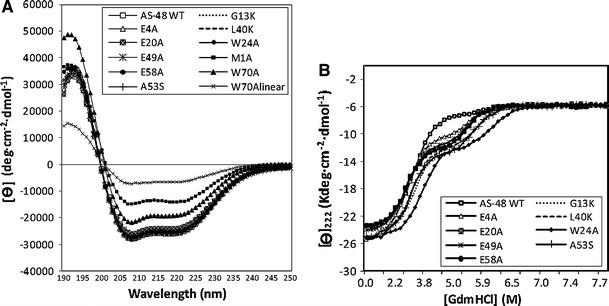
a Far-UV CD spectra of AS-48 and derivatives in 10 mM phosphate buffer at pH 7.0 and 25°C. b CD titration curves of AS-48 WT and eight derivatives at pH 3.0 in the presence of increasing GdmHCl concentrations. Best fittings to the three-state model are shown by solid lines
Notably, M1A derivative has a lower percentage of helical structure than does the wild-type AS-48 molecule. Thus, it appears that, upon mutation, there is a marked helical fraying towards a less-rigid turn-like structure. This agrees with the fact that M1A may have weaker membrane–protein interactions, as suggested by the higher MICs mentioned above [30]. As expected, the highest molar ellipticity value, as well as the lowest helical percentage, corresponds to the linear W70A form. In fact, the helical percentage of linear W70A was similar to those of the linear derivatives obtained in vitro [43] (see below).
Unfolding CD curves of solvent-exposed residues and glutamic mutants in the presence of increasing concentrations of guanidinium hydrochloride (GdmHCl) have illustrated a coincident unfolded state around 7M GdmHCl, and the refolding curves showed a reversible transition (Fig. 4b) [29]. The curves fit a three-state model, in which the intermediate state may correspond to a molten-globule related to an incomplete unfolded protein, partially retaining the secondary structure, which at acidic pH values could be stabilized by specific interaction with GdmHCl. In the circularization-related mutants (M1A and W70A), thermal denaturizing experiments showed that M1A species had no sigmoidal transition between 25 and 95°C, as in the case of AS-48 WT [30]. However, the W70A mutation affected the stability of the circular and linear species. This behavior differs from that of the mutation W24A, which clearly favored stability, although it was clearly unfavorable to the activity of the mutant [28].
The AS-48 dimer DF-II, which is assumed to be involved in membrane insertion (reviewed by [15]), was also structurally analyzed with Fold X [44] to explain the effect of mutations on the activity and stability. The effect of Trp replacements strongly depends on the position of the mutated residue rather than on the disruption of the helical structure caused by the mutation, indicating that these residues can interact with different environments in the target cell membrane. Indeed, Trp24 placed in a region that interacts with the hydrophobic part of the membrane, (α1-α2 helices), has access to the membrane interface/water in binding to host cells, influencing the precise interfacial position of the AS-48 in the membrane, whereas Trp70, located in the most-amphiphilic region (α4-α5 helices), is involved in the access to the membrane–water interface for the union to the host cells. Notably, the addition of an extra positive charge in mutants G13K and L40K did not improve the activity and/or stability of these mutants. In fact, these residues are located opposite the active site of the molecule (the charged exposed AS-48 surface α4-α5 helices), of which the maximum positive electrostatic potential will guide the initial approach to the negatively charged membranes and thus determines target-cell specificity [15]. In addition, residue Leu40 is an exposed solvent and does not cover a large hydrophobic area, while it has similar helical propensity to Lys. Mutation G13K stabilizes the protein, since Lys displays a stronger tendency to form helices. However, the replacement of a non-polar residue such as Ala53 (located at the center of helix α4 in a solvent-exposed orientation) by a non-charged polar residue (Ser) had no effect on the interaction with membranes. It is known that Ser has a weaker tendency to form α-helices and reduces stability but does not affect activity.
The structural analysis of DF-II revealed a significant stabilization effect after E4A, E58A, and E49A mutations. Residues Glu4 and Glu49 face each other, and the substitutions introduced may reduce the strong repulsive forces between them [28]. This explains the decreased antibacterial activity observed for these mutants. Otherwise, residue Glu58 is involved in ionic interactions with Tyr54 and Lys62 from different protomers in DF-II. By contrast, E20A substitution caused a destabilization that may well be due to its external position on the DF-II surface. Although the interaction of AS-48 with the membrane might appear to be mainly electrostatic, our results suggest that the DF-II interaction may facilitate penetration of most of the domain into the membrane interface. Thus, the behavior of the AS-48-mutated proteins could be explained in the light of the typical distribution of amino acid residues from membrane-inserted proteins at the membrane–water interface, and the more complex chemical nature of this area, which includes charged phosphate heads and ester bonds of phospholipids. All this is in accordance with the results published by Shental-Bechor et al. [45], which confirm that amphipathic and cationic peptides adopt two main membrane-associated states. In the first, the peptide resides mostly outside the polar headgroup region. In the second, energetically more favorable, the peptide assumes an amphipathic-helix conformation, with its hydrophobic face immersed in the hydrocarbon region of the membrane and the charged residues in contact with the surface of smeared charges.
Design of linear AS-48 derivatives: how important is the circular backbone?
Proteolysis experiments on AS-48: nicked AS-48-forms
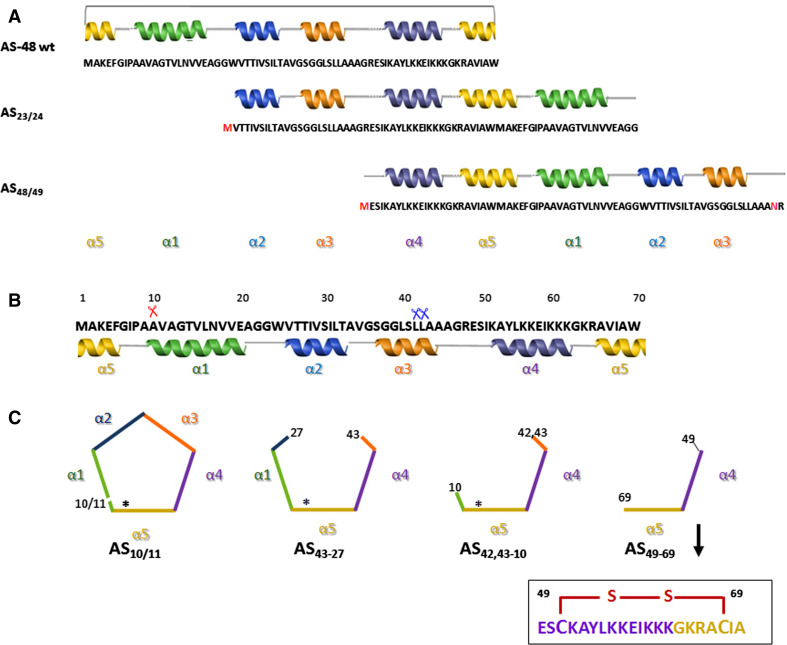
Proteolysis experiments on AS-48 were conducted using several proteases under different reaction conditions. Undoubtedly, the best results were found with thermolysin at a neutral pH, in the presence of TFE or SDS. Under such conditions, a protein species carrying a single nicking, AS10/11, and different fragments characterized by the stepwise deletion of helices α1–α3 (see below) were obtained and they were structurally as well as biologically analyzed (Fig. 5b) [43].
Fig. 5.
Schematic outline of the primary and secondary structures of wild-type AS-48, linear derivatives, and simplified fragments. a Linear derivatives obtained by circular permutation in the structural as-48A gene (AS23/24 and AS48/49). Changed or introduced residues in the permutated constructions are shown in red. b Amino acid sequence and helical regions of WT-AS-48. Scissors indicate the sites of initial proteolytic cleavage by thermolysin (Th) in 0.1% SDS (red) and 15% TFE (blue). c Schematic representation of the nicked AS10/11 and the AS43-27 and AS42,43-10 fragments produced by limited proteolysis on AS-48 or by chemical synthesis (AS49-69 fragment). Asterisks show the head-to-tail peptide bond formation
Nicking of the peptide bond between Ala10 and Val11 at the N-terminus of helix α1, leads to a consistent destabilization of the protein. Indeed, CD analysis shows a reduction in ellipticity in relation to the native protein, being less compact and rigid than the intact protein, because structural fluctuations and reductions in helix length can lower the measured helicity. Nevertheless, upon addition of SDS (0.1%) the helical content of the native and the nicked AS-48 became almost identical, demonstrating that AS10/11 can acquire a conformation similar to that of intact AS-48 in the presence of a membrane-like environment. This suggests that nicking is compatible with the insertion into the lipid bilayer required for the activity. The secondary structure of AS10/11 was also less stable to thermal denaturation for the favorable entropic contribution introduced by circularization. Despite the differences in helical content and stability, biological assays confirmed that the nicked form partly retains its functional properties even if it shows a inhibitory activity that is 300 times lower. Similar results have been found with synthetic non-cyclic homologues of cyclotides that rendered mostly inactive molecules and some proteins with a reduced activity compared with their cyclic counterparts [46], even though in this family of circular proteins, the importance of the head-to-tail peptide bond is reduced due to the mobility constraints introduced by the cyclic cystine knot [47]. Kawai et al. [48] were also able to generate a linear variant of gassericin A by limited proteolysis retaining activity although no structural or quantitative data of potency are provided.
Screening for permuted linear AS-48 derivatives produced in vivo as single and as fusion partners
A very important branch of protein engineering involves changing the backbone architecture of a polypeptide chain into an entirely different topology (e.g., circular permutants and/or acyclic structures). However, the consequence of linearization in the stability and ability to fold is still not clear and could be imperative for the rational development of engineering strategies on circular proteins. In general terms, closing the loops covalently should produce some entropic advantage during folding. In fact, it has been demonstrated that circularization introduces a favorable entropic contribution to the AS-48 structure, which compensates the unfavorable enthalpic one and increases the Gibbs energy to the abnormally high value found [49]. This stabilization has been put down to a reduction in entropy and the resulting destabilization of the unfolded state as a consequence of a loss of freedom in the movement of the polypeptide chain upon circularization. Therefore circularization does not always increase the stability, because an increase in the energy of the unfolded state could be compensated by another increase in the folded-state energy due to sterical tensions introduced by cross-linking. A way of circumventing this question is the comparison of naturally circularized proteins with its linear counterparts and AS-48 is a suitable model for these studies.
Production of a linear active form by limited proteolysis on AS-48 prompted the generation in vivo of two linear, permuted variants without leader peptide, using the E. coli genetic background. Consequently, the linear pro-protein encoded by as-48A gene was circularly permuted to produce the AS23/24 and AS48/49 linear variants. The choice of these residues was based on their location in regions connecting helical segments of the protein and therefore trying to preserve the overall structure and to favor a more stable thermodynamical state [50] (Fig. 5a). Unfortunately, none of the linear permuted proteins were detected in the E. coli cell extracts, whether as soluble proteins or inclusion bodies, in spite of the confirmed transcription of the two recombinant genes, thus demonstrating that there was no transcriptional blockage. A plausible justification for this failure in the expression is the degradation of the newly synthesized leaderless misfolded proteins by the host proteases. Leader peptide might function as an intramolecular chaperone avoiding the degradation of the unfolded proteins by selection of the best conformation among all competing local conformations [51, 52]. However, the inadequacy of the opening sites selected could be another critical question, because new sequences that resemble the original proteins in composition, but not in chain connectivity, could have problems to fold into the same native state as the original sequence [53]. Overall, the negative results show that we are facing a situation in which the leaderless linear polypeptide chains are unable to fold into the native conformation or at least into stable structures, perhaps because their fold could be under kinetic control, or their nuclei have shifted greatly from the wild-type position and the polypeptide chains undergoes proteolysis after their expression.
The absence of protein expression in E. coli as “free” proteins prompted the production of these variants fused to a partner, which is so far the most successful approach overcoming the trouble of expressing cationic peptides [54]. This approach was sufficient to prevent cellular proteolysis of the misfolded proteins. In fact, gassericin A could also be expressed in E. coli as a linear molecule with the presence of an attached tag, although with reduced activity after proteolysis (173-fold less) [48]. Thus, the two permuted mature non-circular AS-48 variants were tagged to the choline-binding domain of l-acetylmuramoyl l-alanine amidase (C-LytA) of Streptococcus pneumoniae and expressed in E. coli. This system rendered such high expression levels that favored their intracellular accumulation as inclusion bodies. These bodies showed a propensity to aggregate after solubilization, and thus hindered the specific enterokinase cleavage. The influence of the amino acid sequence was also clearly observed. Thus, the opening of the AS-48 molecule between residues Gly23 and Trp24 (Fig. 5a) conferred an increased stability and suggested that the sequence and/or the terminal amino acid in the polypeptide chain are critical questions in the design of new variants. It bears noting that the fusion proteins were recognized by both anti-AS-48 and anti-tag antibodies, but were unable to inhibit the growth of L. monocytogenes CECT 4032 and E. faecalis S-47 due to the huge size and different polarity relative to the attached hydrophobic bacteriocins.
The marked tendency of these proteins to aggregate hindered the specific cleavage to release the tag moiety. Interestingly, the MALDI-TOF and fingerprinting analysis confirmed that the chimeric proteins obtained after non-specific cleavage with enterokinase contained a mixture of hybrid proteins having a part of the C-LytA moiety and another one of the AS-48 derivative [50]. The conserved distribution of the hydrophobic and hydrophilic surfaces is responsible for their antibacterial activity. Significantly, neither of the two derivatives lacked the positively charged region comprising helices α4 and α5 from the native AS-48 molecule.
Simplified AS-48-derivative peptides
Different peptides, AS43-27, the two AS42,43-10 fragments derived from proteolysis experiments on AS-48 with thermolysin [43], and a synthetic peptide comprising residues 49–69 (here named as AS49-69) [55] have also been analyzed. AS49-69 is a 21-residue peptide fragment derived from chemical synthesis, in which the N-terminal amino group was acetylated and the C-terminal carboxylate group was amidated. For the helix-turn-helix arrangement of the parent molecule to be maintained, residues Ile59 and Val67 were replaced with cysteinyl residues and linked by a disulfide bond (Fig. 5c). All these simplified peptides comprised the helix α4 and the N-terminal region of helix α5, where the large patch of positive charges, considered responsible for the membrane interaction, were present. Additionally, the AS43-27 and AS42,43-10 fragments also conserved the hydrophobic α1/α2 helices of the wild-type AS-48 molecule (Fig. 5c). It was confirmed by 1H and 13C NMR analysis that AS49-69 adopts the same secondary structure as the analogous region in the intact bacteriocin AS-48 [55]. The spectroscopic characterization by circular dichroism of AS43-27 and AS42,43-10 linear peptides were instead quite different. Hence, fragment AS43-27 showed a helicity of 71% (~9% less than native AS-48), displaying the features of α-helical polypeptides, whereas AS42,43-10 exhibited a far-UV CD spectrum characteristic of a more random conformation, showing a much lower helical content [43].
The biological activity of these AS-48-fragments was evaluated by inhibition of the most sensitive L. monocytogenes bacteria. It has been suggested that the positive charge domain of AS-48 was essential for the membrane insertion through a mechanism known as molecular electroporation [15]. According to this mechanism, when a peptide carrying sufficient charge density binds to the surface of a membrane, the local electrical field associated with the peptide might destabilize the membrane, allowing insertion of the peptide. However, the synthetic AS49-69 peptide does not conserve any of the inhibitory activity of the circular polypeptide, nor does it show the immunological properties of the native molecule, although it competed with AS-48 when added to exponential growth cultures prior to bacteriocin [55]. This behavior suggests that this region alone is not sufficient for antibacterial activity because this positively charged molecule could bind to the cell surface, but once there it remains inoperative, being unable to produce the expected membrane-permeabilization effect. Therefore, this charged portion of the peptide must act in concert with other portions of the bacteriocin to exert the killing effect on target bacteria. This might for instance be the case for AS43-27 and AS42,43-10 fragments, which retained certain anti-listerial activity, although the MIC required for a complete inhibition of the sensitive strain was 500 or 1,000 times higher, respectively, than that of AS-48 used as control [43]. Additionally, the higher activity of the AS43-27 fragment could be explained by the existence of the Trp24 unquestionably involved in the biological activity of AS-48 [29] and the presence of the additional hydrophobic α2 helix that confers a more structured conformation, maintaining the cluster of positive charges in a native-like arrangement. Indeed, the helical content has been demonstrated to be important for the activity of anti-bacterial peptides. It is also remarkable that in the AS43-27 peptide, the α3 helix is removed, as occurred in carnocyclin A [11].
Concluding remarks
Enterocin AS-48, an extremely stable molecule with high activity against Gram-positive and some Gram-negative bacteria, serves as a useful model to study the role of circularization in natural proteins. A considerable number of engineered bacteriocins from Gram-positive bacteria have been designed and characterized, but only a few examples belong to class IV of LAB bacteriocins. Nevertheless, the past years had seen great leaps in our understanding of AS-48 engineering possibilities. By producing single mutants in the AS-48 sequence through site-directed mutagenesis, we have detected neutral or negative effects in relation to the helical content, stability, and antibacterial activity of the majority of the single derivatives. In fact, these investigations highlight the tolerance of certain mutations for the potency, stability, and functionality in AS-48, being more restricted than those performed on residues involved in the maturation process of this enterocin. Thus, the analysis of eight single mutants (E4A, G13K, E20A, W24A, L40K, E49A, A53S, and E58A) reveals that overall, the majority of the replacements slightly reduced the biological activity of the derivative molecules, without correlation with the changes noted in their stability. Our results are consistent with the spatial location of the residues in the three-dimensional structure of AS-48. We conclude that the different replacements affect the interactions between the side chains of neighboring residues and with the solvent, disturbing the stability pattern of the AS-48-derivatives and causing slight variations in the activity levels against identical organisms. The most significant outcome has been the identification of Trp24, unquestionably involved in the biological activity, because of its location in a region that interacts with the hydrophobic part of the membrane.
In addition, the importance of the His-1, Met1, and Trp70 residues on AS-48 maturation (elimination of the leader peptide and circularization itself) must also be underlined. The results confirm that residue His-1 plays a critical role in the cleavage process as no AS-48 molecules could be purified from cultures of the H-1I mutant. More important is the identification of linear forms in the supernatants of the W70A mutants never before described in the wild-type strain. These data and the analysis of the other circular bacteriocins (lactocyclicin Q, uberolysin, and circularin A), in which an aromatic residue is present in the C-terminus pose the question of its importance to favor circularization, a fact requiring more extensive study.
Site-specific mutagenesis/modification, using either recombinant DNA technology or chemical synthesis, has been successfully applied to countless proteins and continues to bear an enormous impact on basic research. To investigate the role of circularity in the stability and biological properties of enterocin AS-48, we have focused on the linear AS-48-derivatives, obtained either in vitro by limited proteolysis experiments or in vivo by circular permutation in the structural as-48A gene. Consistent with earlier results, isolation of linear active proteins after controlled proteolytic cleavage represents a major step forward in the bioengineering of AS-48 and confirms that circularization may not be essential for activity, but stabilizes the three-dimensional structure of the protein, therefore increasing its effect. However, we conclude that a proper distribution of electrostatic and hydrophobic surfaces in the protein species is required for activity and for an efficient insertion into the sensitive membranes. The unsuccessful expression of the two permuted genes coding for linear molecules (AS23/24 and AS48/49), warns of the importance of AS-48 leader peptide to stabilize the structure and to prevent cellular proteolysis. Hence, the greater stability of the whole linear proteins by fusion to a tag, successfully prevents cellular proteolysis, providing useful clues on more complex proteins with new activities.
A possible structure–function relationship can be deduced from a detailed analysis of the primary structure of the simplified AS-48-derivatives. In fact, we noted that AS49-69 differed from AS42,43-10 in the absence of the last 11 residues 70–10 (WMAKEFGIPAA) from α5 helix, as well as in the replacements I59C and V67C introduced to maintain the relative orientation of the helices. This suggests that the presence of some residues, mainly the aromatic ones and Glu4, which replacement determines instability and lack of activity against the majority of the Gram-positive strains [28] could be essential for activity, and that the AS42,43-10 peptide could be considered as the minimal derivative-AS-48 fragment retaining activity.
In short, all the engineering studies performed with AS-48 have provided insights into the structural function of the circular wild-type backbone conformation. In addition, they afford experimental basis to affirm that the wild-type sequence is the most active and stable form among all the designed and natural derivatives studied to date. It is possible, however, to reduce in vitro the sequence to a minimal AS-48 domain, without total inactivation of this enterocin. Thus, we conclude that enterocin AS-48 has already been well optimized, so it could be improbable to develop a better molecule than the one so perfectly designed by nature.
Acknowledgments
Work in our laboratory was supported by grants BIO2005-01544 and BIO2008-01708 (Ministerio de Ciencia e Innovación, Spain) and PAI CVI 160 (Junta de Andalucía).
Footnotes
Marina Sánchez-Hidalgo and Manuel Montalbán-López contributed equally to this work.
References
- 1.Scott CP, Abel-Santos E, Wall M, Wahnon DC, Benkovic SJ. Production of cyclic peptides and proteins in vivo. Proc Natl Acad Sci USA. 1999;96:13638–13643. doi: 10.1073/pnas.96.24.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwai H, Lingel A, Pluckthun A. Cyclic green fluorescent protein produced in vivo using an artificially split PI-PfuI intein from Pyrococcus furiosus . J Biol Chem. 2001;276:16548–16554. doi: 10.1074/jbc.M011639200. [DOI] [PubMed] [Google Scholar]
- 3.Mulvenna JP, Wang C, Craik DJ. CyBase, a database of cyclic protein sequence structure. Nucleic Acid Res. 2006;34:D192–D194. doi: 10.1093/nar/gkj005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlan BF, Gillon AD, Craik DJ, Anderson MA. Circular proteins and mechanisms of cyclization. Biopolymers. 2010;94:573–583. doi: 10.1002/bip.21422. [DOI] [PubMed] [Google Scholar]
- 5.Cascales L, Craik DJ. Naturally occurring circular proteins: distribution, biosynthesis and evolution. Org Biomol Chem. 2010;8:5035–5047. doi: 10.1039/c0ob00139b. [DOI] [PubMed] [Google Scholar]
- 6.Garcia AE, Camarero JA. Biological activities of natural and engineered cyclotides, a novel molecular scaffold for peptide-based therapeutics. Curr Mol Pharmacol. 2010;3:153–163. doi: 10.2174/1874467211003030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley MA, Wertz JE. Bacteriocins: evolution, ecology, and application. Ann Rev Microbiol. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 8.Willey JM, van der Donk WA. Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol. 2007;61:477–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]
- 9.Heng NCK, Wescombe PA, Burton JP, Jack RW, Tagg JR. The diversity of bacteriocins produced by Gram-positive bacteria. In: Riley MA, Chavan MA, editors. Bacteriocins. Ecology and evolution. Berlin Heidelberg New York: Springer; 2007. pp. 45–92. [Google Scholar]
- 10.Maqueda M, Sánchez-Hidalgo M, Fernández M, Montalbán-López M, Valdivia E, Martínez-Bueno M. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:2–22. doi: 10.1111/j.1574-6976.2007.00087.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Visscher LA, Gong X, Duszyk M, Vederas JC. The three-dimensional structure of carnocyclin A reveals that many circular bacteriocins share a common structural motif. J Biol Chem. 2009;284:28674–28681. doi: 10.1074/jbc.M109.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Visscher LA, van Belkum MJ, Garneau-Tsodikova S, Whittal RM, Zheng J, McMullen LM, Vederas JC. Isolation and characterization of carnocyclin A, a novel circular bacteriocin, produced by Carnobacterium maltaromaticum UAL307. Appl Environ Microbiol. 2008;74:4756–4763. doi: 10.1128/AEM.00817-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawa N, Zendo T, Kiyofuji J, Fujita K, Himeno K, Nakayama J, Sonomoto K. Identification and characterization of lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp strain QU 12. Appl Environ Microbiol. 2009;75:1552–1558. doi: 10.1128/AEM.02299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemperman R, Jonker M, Nauta A, Kuipers OP, Kok J. Functional analysis of the gene cluster involved in production of the bacteriocin circularin A by Clostridium beijerinckii ATCC 25752. Appl Environ Microbiol. 2003;69:5839–5848. doi: 10.1128/AEM.69.10.5839-5848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maqueda M, Gálvez A, Martínez-Bueno M, Sánchez-Barrena MJ, González C, Albert A, Rico M, Valdivia E. Peptide AS-48, prototype of a new class of cyclic bacteriocins. Curr Prot Pept Sci. 2004;5:399–416. doi: 10.2174/1389203043379567. [DOI] [PubMed] [Google Scholar]
- 16.Wirawan RE, Swanson KM, Kleffmann T, Jack RW, Tagg JR. Uberolysin, a novel cyclic bacteriocin produced by Streptococcus uberis . Microbiology. 2007;153:1619–1630. doi: 10.1099/mic.0.2006/005967-0. [DOI] [PubMed] [Google Scholar]
- 17.Borrero J, Brede DA, Skaugen M, Diep DB, Herranz C, Nes IF, Cintas LM, Hernández P. Characterization of garvicin ML, a novel circular bacteriocin produced by Lactococcus garvieae DCC43, isolated from mallard ducks (Anas platyrhynchos) Appl Environ Microbiol. 2011;77:369–373. doi: 10.1128/AEM.01173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arakawa K, Kawai Y, Ito Y, Nakamura K, Chujo T, Nishimura J, Kitazawa H, Saito T. HPLC purification and re-evaluation of chemical identity of two circular bacteriocins, gassericin A and reutericin 6. Lett Appl Microbiol. 2010;50:406–411. doi: 10.1111/j.1472-765X.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawai Y, Kemperman R, Kok J, Saito T. The circular bacteriocins gassericin A and circularin A. Curr Prot Pept Sci. 2004;5:393–398. doi: 10.2174/1389203043379549. [DOI] [PubMed] [Google Scholar]
- 20.Kalmokoff ML, Teather RM. Isolation and characterization of a bacteriocin (butyrivibriocin AR10) from the ruminal anaerobe Butyrivibrio fibrisolvens AR10: evidence in support of the widespread occurrence of bacteriocin-like activity among ruminal isolates of B. fibrisolvens . Appl Environ Microbiol. 1997;63:394–402. doi: 10.1128/aem.63.2.394-402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawulka K, Sprules T, McKay RT, Mercier P, Diaper CM, Zuber P, Vederas JC. Structure of subtilosin A, an antimicrobial peptide from Bacillus subtilis with unusual post-translational modifications linking cysteine sulfurs to alpha-carbons of phenylalanine and threonine. J Am Chem Soc. 2003;125:4726–4727. doi: 10.1021/ja029654t. [DOI] [PubMed] [Google Scholar]
- 22.Kawulka KE, Sprules T, Diaper CM, Whittal RM, McKay RT, Mercier P, Zuber P, Vederas JC. Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to alpha-carbon cross-links: formation and reduction of alpha-thio-alpha-amino acid derivatives. Biochemistry. 2004;43:3385–3395. doi: 10.1021/bi0359527. [DOI] [PubMed] [Google Scholar]
- 23.Abriouel H, Lucas R, Ben Omar N, Valdivia E, Gálvez A. Potential applications of the cyclic peptide enterocin AS-48 in the preservation of vegetable foods beverages. Probiotics Antimicrob Prot. 2010;2:77–89. doi: 10.1007/s12602-009-9030-y. [DOI] [PubMed] [Google Scholar]
- 24.Khan H, Flint S, Pak-Lam Yu. Enterocins in food preservation. Intern J Food Microbiol. 2010;141:1–10. doi: 10.1016/j.ijfoodmicro.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Davidse EK, Balla E, Holzapfel WH, Muller CJC, Cloete SWP, Dicks LMT. Peptide AS-48 from Enterococcus faecalis for prevention and treatment of mastitis in dairy cows. Online J Veter Res. 2004;8:22–32. [Google Scholar]
- 26.Fernández M, Sánchez-Hidalgo M, García-Quintáns N, Martínez-Bueno M, Valdivia E, López P, Maqueda M. Processing of as-48ABC RNA in AS-48 enterocin production by Enterococcus faecalis . J Bacteriol. 2008;190:240–250. doi: 10.1128/JB.01528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Bueno M, Valdivia E, Gálvez A, Coyette J, Maqueda M. Analysis of the gene cluster involved in production and immunity of the peptide antibiotic AS-48 in Enterococcus faecalis . Mol Microbiol. 1998;27:347–358. doi: 10.1046/j.1365-2958.1998.00682.x. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Hidalgo M, Martínez-Bueno M, Fernández-Escamilla AM, Valdivia E, Serrano L, Maqueda M. Effect of replacing glutamic residues upon the biological activity and stability of the circular enterocin AS-48. J Antimicrob Chemother. 2008;61:1256–1265. doi: 10.1093/jac/dkn126. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Hidalgo M, Fernández-Escamilla AM, Martínez-Bueno M, Valdivia E, Serrano L, Maqueda M. Conformational stability and activity of circular Enterocin AS-48 derivatives. Prot Pept Let. 2010;17:708–714. doi: 10.2174/092986610791190390. [DOI] [PubMed] [Google Scholar]
- 30.Cebrián R, Maqueda M, Neyra JL, Valdivia E, Martinez-Bueno M, Montalbán-López M. Insights into the functionality of the putative residues involved in AS-48-maturation. Appl Environ Microbiol. 2010;76:7268–7276. doi: 10.1128/AEM.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plat A, Kluskens LD, Kuipers A, Rink R, Moll GN. Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage. Appl Environ Microbiol. 2011;77:604–611. doi: 10.1128/AEM.01503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szekat C, Jack RW, Skutlarek D, Farber H, Birbaum G. Construction of an expression system for site-directed mutagenesis of the lantibiotic mersacidin. Appl Environ Microbiol. 2003;69:378–3777. doi: 10.1128/AEM.69.7.3777-3783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyndall JDA, Nall T, Fairlie DP. Proteases universally recognize beta strands in their active sites. Chem Rev. 2005;105:973–999. doi: 10.1021/cr040669e. [DOI] [PubMed] [Google Scholar]
- 34.Phan UT, Lackman RL, Cresswell P. Role of the C-terminal propeptide in the activity and maturation of gamma -interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci USA. 2002;99:12298–12303. doi: 10.1073/pnas.182430499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abriouel H, Lucas R, Ben Omar N, Martínez-Bueno M, Valdivia E, Maqueda M, Martínez-Cañamero M, Gálvez A. Enterocin AS-48RJ, a variant of enterocin AS-48 chromosomally encoded by the food isolate Enterococcus faecium RJ16. Syst Appl Microbiol. 2005;28:383–397. doi: 10.1016/j.syapm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Fimland G, Eijsink VGH, Nissen-Meyer J. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry. 2002;41:9508–9515. doi: 10.1021/bi025856q. [DOI] [PubMed] [Google Scholar]
- 37.Killian JA, von Heijne G. How proteins adapt to a membrane-water interface. Trends Biochem Sci. 2000;25:429–434. doi: 10.1016/S0968-0004(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 38.Ridder ANJA, Morein S, Stam JG, Kuhn A, de Kruijff B, Killian JA. Analysis of the role of interfacial tryptophan residues in controlling the topology of membrane proteins. Biochemistry. 2000;39:6521–6528. doi: 10.1021/bi000073v. [DOI] [PubMed] [Google Scholar]
- 39.Knappe TA, Linne U, Robbel L, Marahiel MA. Insights into the biosynthesis and stability of the lasso peptide capistruin. Chem Biol. 2009;16:1290–1298. doi: 10.1016/j.chembiol.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Morisset D, Berjeaud JM, Marion D, Lacombe C, Frère J. Mutational analysis of mesentericin Y105, an anti-Listeria bacteriocin, for determination of impact on bactericidal activity, in vitro secondary structure, and membrane interaction. Appl Environ Microbiol. 2004;70:4672–4680. doi: 10.1128/AEM.70.8.4672-4680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, Zhang ZZ, Chen XZ, Yang W, Huan ZLD. Site-directed mutagenesis of the hinge region of nisin Z and properties of nisin Z mutants. Appl Microbiol Biotechnol. 2004;64:806–815. doi: 10.1007/s00253-004-1599-1. [DOI] [PubMed] [Google Scholar]
- 42.Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science. 2006;313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 43.Montalbán-López M, Spolaore B, Pinato O, Martínez-Bueno M, Valdivia E, Maqueda M, Fontana A. Characterization of linear forms of the circular enterocin AS-48 obtained by limited proteolysis. FEBS Lett. 2008;582:3237–3242. doi: 10.1016/j.febslet.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Schymkowitz J, Borg J, Stricher F, Nys R, Rousseau F, Serrano L. The FoldX web server, an online force field. Nucleic Acids Res. 2005;33:W382–W388. doi: 10.1093/nar/gki387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shental-Bechor D, Haliloglu T, Ben-Tal N. Interactions of cationic-hydrophobic peptides with lipid bilayers, a Monte Carlo simulation method. Biophys J. 2007;93:1858–1871. doi: 10.1529/biophysj.106.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonsen SM, Daly NL, Craik DJ. Capped acyclic permutants of the circular protein kalata B1. FEBS Lett. 2004;577:399–402. doi: 10.1016/j.febslet.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 47.Ireland DC, Colgrave ML, Nguyencong P, Daly NL, Craik DJ. Discovery and characterization of a linear cyclotide from Viola odorata, implications for the processing of circular proteins. J Mol Biol. 2006;357:1522–1535. doi: 10.1016/j.jmb.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 48.Hawai Y, Arakawa K, Itoh A, Saitoh B, Ishii Y, Nishimura J, Kitazawa H, Itoh T, Saito T. Heterologous expression of gassericin A, a bacteriocins produced by Lactobacillus gasseri LA39. Anim Sci J. 2003;74:45–51. doi: 10.1046/j.1344-3941.2003.00085.x. [DOI] [Google Scholar]
- 49.Cobos ES, Filimonov VV, Gálvez A, Valdivia E, Maqueda M, Martínez JC, Mateo PL. The denaturation of circular enterocin AS-48 by urea and guanidinium hydrochloride. Biochim Biophys Acta. 2002;1598:98–107. doi: 10.1016/s0167-4838(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 50.Montalbán-López M, Martínez-Bueno M, Valdivia E, Maqueda M. Expression of linear permutated variants from circular enterocin AS-48. Biochimie. 2011;93:549–555. doi: 10.1016/j.biochi.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Oman TJ, van der Donk WA. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat Chem Biol. 2010;6:9–18. doi: 10.1038/nchembio.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai CJ, Ma B, Nussinov R. Intra-molecular chaperone: the role of the N-terminal in conformational selection and kinetic control. Phys Biol. 2009;6:013001–013009. doi: 10.1088/1478-3975/6/1/013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Shakhnovich EI. Different circular permutations produced different folding nuclei in proteins: a computational study. J Mol Biol. 2001;306:121–132. doi: 10.1006/jmbi.2000.4375. [DOI] [PubMed] [Google Scholar]
- 54.Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2003;60:523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- 55.Jiménez MA, Barrachi-Saccilotto AC, Valdivia E, Maqueda M, Rico M. Design, NMR characterization and activity of a 21-residue peptide fragment of bacteriocin AS-48 containing its putative membrane interacting region. J Pept Sci. 2005;11:29–36. doi: 10.1002/psc.589. [DOI] [PubMed] [Google Scholar]