Abstract
Embryonic stem cells (ESCs) are derived from blastocysts and are capable of differentiating into whole tissues and organs. Transplantation of ESCs into recipient blastocysts leads to the generation of germline-competent chimeras in mice. Transgenic, knockin, and knockout gene manipulations are available in mouse ESCs, enabling the production of genetically modified animals. Rats have important advantages over mice as an experimental system for physiological and pharmacological investigations. However, in contrast to mouse ESCs, rat ESCs were not established until 2008 because of the difficulty of maintaining pluripotency. Although the use of signaling inhibitors has allowed the generation of rat ESCs, the production of genetically modified rats has been difficult due to problems in rat ESCs after gene introduction. In this review, we will focus on some well-documented examples of gene manipulation in rat ESCs.
Keywords: ES cell, Rat, Transgenic, Pluripotency, Chimera
Introduction
Embryonic stem cells (ESCs) established from the inner cell mass (ICM) of preimplantation blastocysts [1] have been routinely derived from mice since 1981 [2, 3]. These cells have a stable developmental potential to form derivatives of all three embryonic germ layers, the endoderm, mesoderm, and ectoderm, even after prolonged culture [4] and have been used to study the mechanism of cell differentiation. Moreover, they are capable of generating germline chimeras following injection into the blastocyst [5]. Gene manipulation is available, and germline transmission of transgenic ESCs was achieved in 1986 [6]. Soon after this achievement was reported, gene-targeting mice were generated via homologous recombination in ESCs [7]. So far, a huge number of genetically modified mice have been produced via the manipulation of ESCs and used in a range of biomedical researches. However, this technique is unavailable in species other than mice because of a lack of stable ESCs.
The laboratory rat, the first mammalian species domesticated for scientific research, has been used as an animal model for research in physiology, toxicology, nutrition, behavior, immunology, and neoplasia for over 150 years [8–12]. Despite the utility to use rats in experiments, rat ESCs were not established until 2008. The reasons for the failure to develop ESCs in rats are related to the difficulty in maintaining pluripotency in culture despite trials using numerous strategies [13–17]. Our group generated rat ESCs harboring a potential to contribute to chimera but not to develop germ cells [18]. On the other hand, despite the lack of authentic ESCs, several technologies have been developed to alter rats genetically [19–26].
Rat transgenesis from ESCs with 2i+LIF medium
In 2008, germline-competent rat ESCs were first established from blastocysts by using a 2i+LIF medium composed of two signaling inhibitors (MEK inhibitor PD0325901; GSK3 inhibitor CHIR99021), a leukemia inhibitory factor (LIF), and a defined basal culture medium containing no fetal bovine serum (FBS) (Fig. 1) [27, 28]. The results of the two studies showed that FBS was a key factor in the induction of differentiation in rat ESCs [29]. This culture medium was also used for the generation of mouse ESCs [30]. Generally, mouse ESCs are cultured on feeder layers of mouse embryonic fibroblasts (MEFs). Further, it was found that the use of DIA-M cells [27] or a mixture of MEFs and L-cells as feeder layers [28] was optimal for isolating rat ESCs. In these conditions, although ESCs maintained pluripotency and contributed to chimeras, only two of nine cell lines achieved germline transmission. Rat-induced pluripotent stem cells (iPSCs) with the potential to contribute to chimeras were also generated by the addition of A-83-01 (Type 1 Tgf β receptor inhibitor) to 2i+LIF in a mouse ESC basal culture medium containing 20% knockout serum replacement (KSR). However, these iPSCs did not achieve germline transmission [31].
Fig. 1.
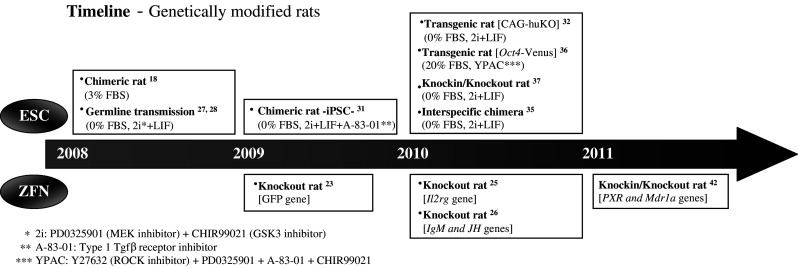
Timeline of rat transgenesis using ESC or ZFN technology since 2008. The parentheses indicate the culture conditions. The brackets indicate transgenes or targeted genes
Gene introduction was available in the rat ESCs cultured in 2i+LIF medium. However, they were sensitive to electro-physical stimulation induced by the conventional electroporation method, which led to cell death. A nucleofection method was found to be more efficient and convenient for gene introduction in rat ESCs [28]. Furthermore, FBS was temporally added into an electroporation medium as well as a 2i+LIF cell-culture medium to aid viability [27]. Each group obtained stable transfectant clones in which the CAG-eGFP-IRES-pac plasmid was randomly integrated in their genome after selection with puromycin [27, 28]. Although five overt coat color chimeras were born after injection of the clone, they either died perinatally or were euthanized due to jaw abnormalities. The reasons for their abnormalities might have been chromosomal instability in the transfectant ESC line [27]. On the other hand, Hirabayashi et al. [32] succeeded in the germline transmission of a transfectant rat ESC line harboring a humanized Kusabira-Orange (huKO) gene using the 2i+LIF culture medium. A CAG/huKO-neo plasmid was introduced into ESCs by electroporation, and then stable clones were obtained by neomycin selection. In the 2i+LIF medium, 1,000 U/ml of rat LIF [33] was substituted for the human LIF used in the previous works (100 U/ml [27]; 10 U/ml [28]). It is possible that the rat LIF is better for the maintenance of rat ESCs [34]. Kobayashi et al. [35] overcame the difficulty to generate interspecific chimeras between rats and mice using rat ES or iPS cells cultured in a 2i+rat LIF medium. Thus, using rat LIF might be an option to keep rat ESCs stable.
Rat transgenesis from ESCs with YPAC medium
Our group developed a new culture medium (YPAC medium) including the additional signaling inhibitors of Rho-associated kinase (Y-27632) and A-83-01 to the 2i [36]. The four inhibitors, Y-27632, PD0325901, A-83-01, and CHIR99021, are collectively referred to as YPAC. A mouse ESC basal culture medium containing FBS (20% vol/vol) and MEFs was used, but LIF was not necessary in our study (Fig. 1). In the culture condition, the majority of cell lines (six out of six) demonstrated chimerism and germline transmission and could be stably transfected with a reporter transgene to produce genetically modified rats. These three cell lines were derived from each of the following strains: Wistar, LEA (Long Evans Agouti), and hybrid Wistar/LEA [36].
Since the medium contained 20% serum, the ESCs were tolerant to the damage induced by electric stimuli during gene introduction. In our procedure, a transgene in which the Venus gene was transcribed by the Oct4-promoter (Oct4-Venus) was introduced in the ESCs by the nucleofection method. When the manipulated cells were plated, the use of matrigel (2% at final concentration) was effective for selecting stable clones because they are retained to adhere on MEFs [36]. It is generally known that rat ESC colonies tend to detach from MEFs [27, 28, 36]. This phenomenon enhances the ESCs’ attachment to each other, leading to clone contamination. As the transgene did not include a selection cassette, Venus-positive clones were picked and expanded without drugs. In this cloning process, we found an advantage of using ESCs for the generation of transgenic rats because we were able to choose high-quality clones mimicking an endogenous Oct4 expression pattern. While the majority of the clones exhibited a heterogeneous expression pattern in undifferentiated cells, only a few clones maintained homogenous expression after long-term culture (Fig. 2a). This homogeneity corresponds to the expression pattern of endogenous OCT4 protein. Oct4-Venus transgenic rats were generated through germline transmission of the selected clones without any adverse effects of gene introduction on chimera contribution (Fig. 2b). The Venus fluorescence was also detected in germ cells of the transgenic fetal gonads (Fig. 2c). Moreover, we could trace the fluorescence only in undifferentiated ESCs from their blastocysts during the establishment process and long-term culture (Fig. 2d) [36].
Fig. 2.
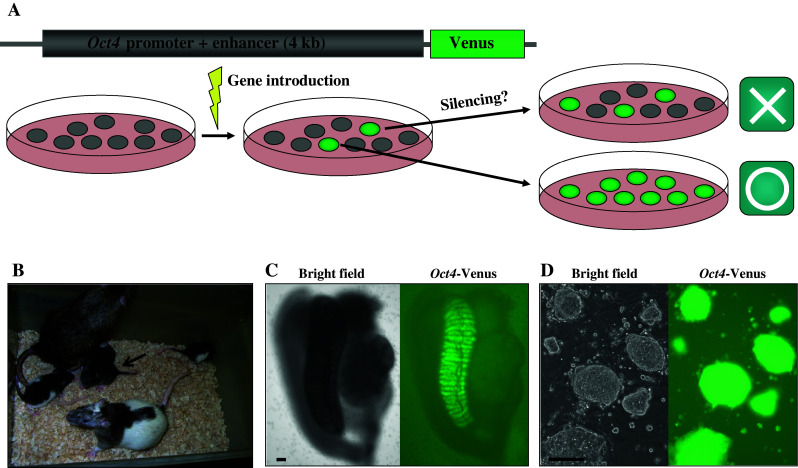
Transgenesis in rat ESCs. a Oct4-Venus transgene is introduced in rat ESCs by a nucleofection method. Some clones receive random integration of the transgene, with subsequent green fluorescence. After subcloning and passaging, Venus fluorescence was decreased in a majority of the clones (upper), while a minority of the clones expressed the fluorescence homogeneously. b The Oct4-Venus transgenic rat (arrow) was produced through germline transmission of the recombinant ESCs from a chimeric rat. c Oct4-Venus positive-germ cells in E16.0 gonad of the transgenic rats. d An ESC line derived from the Oct4-Venus transgenic rat. Venus fluorescence was kept in undifferentiated cells after 18 passages. All scale bars, 100 μm
Gene targeting rats from ESCs
Tong et al. [37] achieved for the first time the production of knockout rats via homologous recombination in rat ESCs. A targeting vector was constructed to disrupt the tumor suppressor gene p53 (also known as Tp53). Targeting efficiencies in two ESC lines derived from the DA (Dark Agouti) strain were 1.12–3.70%. Many properly targeted cell lines cultured in the 2i+LIF medium developed chromosome abnormalities. Over 65% of the cells were polyploid. This phenomenon is similar to that reported in previous works [27, 28]. However, after subcloning round and compact colonies, two out of 20 clones had euploid chromosome numbers leading to the production of a viable knockout [34]. This achievement is historic because the p53 knockout rat validates the culminated effort of many to enable targeted genetic engineering in rat ESCs. Another group also succeeded in gene targeting in a hypoxanthine phosphoribosyltransferase (hprt) locus by homologous recombination in rat ESCs [38]. Although these hprt heterozygous clones cultured in the 2i+LIF medium maintained pluripotency, aneuploid cells did emerge in the cultures. However, approximately 2% of geneticine-resistant colonies achieved recombination correctly. The efficiency was similar to that originally reported for mouse and human ESCs [39–41]. Thus, these reports suggest that rat ESCs are readily amenable to gene targeting by homologous recombination using the basic methodology that has proved so effective in mouse ESCs.
Discussion
Rat transgenesis via gene manipulation in ESCs was demonstrated in 2010, marking the beginning of a new era in rat genetics. Although some problems remain in the rat ESC handling, a combination of the methods described in this manuscript as well as newly devised techniques will lead to the discovery of a gold standard method to routinely generate genetically modified rats from ESCs. Recently, not only knockout but also knockin rats have been generated using ZFN-mediated homologous recombination [42]. This knockin strategy will make it possible to introduce temporal control and tissue-specific changes in genes in rat models by combining Cre/loxP and an inducible gene expression system. ZFN technology also possesses several advantages, such that the time frame to obtain mutant animals is short, ZFN-mediated homologous recombination in embryos does not require a selection marker, and time-consuming backcrossing is avoided [42]. However, this technology remains expensive to purchase, which is an obstacle for most researchers. In contrast, researchers can apply gene targeting by using ESCs, as is routinely done in mouse research. Therefore, ESC is also required to expand knockout rat lines. There is another advantage of using ESCs when generating transgenic rats. Useless transgenic animals are frequently generated with the conventional method. However, as described in this manuscript, we can choose ESC clones in which a transgene is correctly expressed, leading to the generation of high-quality transgenic rats [36]. Moreover, we can analyze gene function in chimeric animals by using ESCs. Recent reports have shown that this chimeric strategy is effective in identifying gene functions in vivo in terms of developing a more clinically relevant stochastic model [43]. Thus, we speculate that using both the ESC and ZFN strategies will be necessary for routine rat transgenesis.
We now have an opportunity to find new gene functions that have been concealed or questioned in mutant mice. We have accumulated genetic information and a vast amount of research data on physiology and pharmacology in rats. Thus, a combination of these studies will lead to the discovery of new and profound mechanisms of human diseases and the manufacture of medicines to cure patients. Furthermore, rats with their larger sizes make it possible to extract sufficient quantities of samples, such as blood, without killing the animals and to perform difficult surgeries, such as those in brain tissue; all of this emphasizes the advantages of gene-modified rats. We hope that researchers will create many genetically modified rats and open up a powerful new platform for the study of human diseases.
Acknowledgments
This work is supported by a Grant-in-Aid from the Third-Term Comprehensive 10-Year Strategy for Cancer Control.
References
- 1.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson JA, Marshall VS. Primate embryonic stem cells. Curr Top Dev Biol. 1998;38:133–165. doi: 10.1016/S0070-2153(08)60246-X. [DOI] [PubMed] [Google Scholar]
- 5.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 6.Robertson E, Bradley A, Kuehn M, Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986;323:445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- 7.Koller BH, Hagemann LJ, Doetschman T, Hagaman JR, Huang S, Williams PJ, First NL, Maeda N, Smithies O. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci USA. 1989;86:8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill TJ, 3rd, Smith GJ, Wissler RW, Kunz HW. The rat as an experimental animal. Science. 1989;245:269–276. doi: 10.1126/science.2665079. [DOI] [PubMed] [Google Scholar]
- 9.Jacob HJ. Functional genomics and rat models. Genome Res. 1999;9:1013–1016. doi: 10.1101/gr.9.11.1013. [DOI] [PubMed] [Google Scholar]
- 10.Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet. 2002;3:33–42. doi: 10.1038/nrg702. [DOI] [PubMed] [Google Scholar]
- 11.Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, Gauguier D, Geurts AM, Gould M, Harris PC, Holmdahl R, Hubner N, Izsvák Z, Jacob HJ, Kuramoto T, Kwitek AE, Marrone A, Mashimo T, Moreno C, Mullins J, Mullins L, Olsson T, Pravenec M, Riley L, Saar K, Serikawa T, Shull JD, Szpirer C, Twigger SN, Voigt B, Worley K. Progress and prospects in rat genetics: a community view. Nat Genet. 2008;40:516–522. doi: 10.1038/ng.147. [DOI] [PubMed] [Google Scholar]
- 12.Jacob HJ, Lazar J, Dwinell MR, Moreno C, Geurts AM. Gene targeting in the rat: advances and opportunities. Trends Genet. 2010;26:510–518. doi: 10.1016/j.tig.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenin D, Look J, Bader M, Hübner N, Levan G, Iannaccone P. Rat embryonic stem cells: a progress report. Transplant Proc. 1997;29:1761–1765. doi: 10.1016/S0041-1345(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 14.Buehr M, Nichols J, Stenhouse F, Mountford P, Greenhalgh CJ, Kantachuvesiri S, Brooker G, Mullins J, Smith AG. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol Reprod. 2003;68:222–229. doi: 10.1095/biolreprod.102.006197. [DOI] [PubMed] [Google Scholar]
- 15.Demers SP, Yoo JG, Lian L, Therrien J, Smith LC. Rat embryonic stem-like (ES-like) cells can contribute to extraembryonic tissues in vivo. Cloning Stem Cells. 2007;9:512–522. doi: 10.1089/clo.2007.0029. [DOI] [PubMed] [Google Scholar]
- 16.Fändrich F, Dresske B, Bader M, Schulze M. Embryonic stem cells share immune-privileged features relevant for tolerance induction. J Mol Med. 2002;80:343–530. doi: 10.1007/s00109-002-0342-6. [DOI] [PubMed] [Google Scholar]
- 17.Vassilieva S, Guan K, Pich U, Wobus AM. Establishment of SSEA-1- and Oct-4-expressing rat embryonic stem-like cell lines and effects of cytokines of the IL-6 family on clonal growth. Exp Cell Res. 2000;258:361–373. doi: 10.1006/excr.2000.4940. [DOI] [PubMed] [Google Scholar]
- 18.Ueda S, Kawamata M, Teratani T, Shimizu T, Tamai Y, Ogawa H, Hayashi K, Tsuda H, Ochiya T. Establishment of rat embryonic stem cells and making of chimera rats. PLoS One. 2008;3:e2800. doi: 10.1371/journal.pone.0002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zan Y, Haag JD, Chen KS, Shepel LA, Wigington D, Wang YR, Hu R, Lopez-Guajardo CC, Brose HL, Porter KI, Leonard RA, Hitt AA, Schommer SL, Elegbede AF, Gould MN. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol. 2003;21:645–651. doi: 10.1038/nbt830. [DOI] [PubMed] [Google Scholar]
- 20.Kitada K, Ishishita S, Tosaka K, Takahashi R, Ueda M, Keng VW, Horie K, Takeda J. Transposon-tagged mutagenesis in the rat. Nat Methods. 2007;4:131–133. doi: 10.1038/nmeth1002. [DOI] [PubMed] [Google Scholar]
- 21.Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–346. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 22.Cozzi J, Anegon I, Braun V, Gross AC, Merrouche C, Cherifi Y. Pronuclear DNA injection for the production of transgenic rats. Methods Mol Biol. 2009;561:73–88. doi: 10.1007/978-1-60327-019-9_5. [DOI] [PubMed] [Google Scholar]
- 23.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izsvák Z, Fröhlich J, Grabundzija I, Shirley JR, Powell HM, Chapman KM, Ivics Z, Hamra FK. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat Methods. 2010;7:443–445. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménoret S, Iscache AL, Tesson L, Rémy S, Usal C, Osborn MJ, Cost GJ, Brüggemann M, Buelow R, Anegon I. Characterization of immunoglobulin heavy chain knockout rats. Eur J Immunol. 2010;40:2932–2941. doi: 10.1002/eji.201040939. [DOI] [PubMed] [Google Scholar]
- 27.Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, Pera MF, Ying QL. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamata M, Ochiya T. Establishment of embryonic stem cells from rat blastocysts. Methods Mol Biol. 2010;597:169–177. doi: 10.1007/978-1-60327-389-3_12. [DOI] [PubMed] [Google Scholar]
- 30.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi M, Kato M, Sanbo M, Kobayashi T, Hochi S, Nakauchi H. Rat transgenesis via embryonic stem cells electroporated with the Kusabira-orange gene. Mol Reprod Dev. 2010;77:474. doi: 10.1002/mrd.21181. [DOI] [PubMed] [Google Scholar]
- 33.Takahama Y, Ochiya T, Sasaki H, Baba-Toriyama H, Konishi H, Nakano H, Terada M. Molecular cloning and functional analysis of cDNA encoding a rat leukemia inhibitory factor: towards generation of pluripotent rat embryonic stem cells. Oncogene. 1998;16:3189–3196. doi: 10.1038/sj.onc.1201826. [DOI] [PubMed] [Google Scholar]
- 34.Hirabayashi M, Kato M, Kobayashi T, Sanbo M, Yagi T, Hochi S, Nakauchi H. Establishment of rat embryonic stem cell lines that can participate in germline chimerae at high efficiency. Mol Reprod Dev. 2010;77:94. doi: 10.1002/mrd.21181. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS, Hirabayashi M, Nakauchi H. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Kawamata M, Ochiya T. Generation of genetically modified rats from embryonic stem cells. Proc Natl Acad Sci USA. 2010;107:14223–14228. doi: 10.1073/pnas.1009582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meek S, Buehr M, Sutherland L, Thomson A, Mullins JJ, Smith AJ, Burdon T. Efficient gene targeting by homologous recombination in rat embryonic stem cells. PLoS One. 2010;5:e14225. doi: 10.1371/journal.pone.0014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 40.Doetschman T, Maeda N, Smithies O. Targeted mutation of the Hprt gene in mouse embryonic stem cells. Proc Natl Acad Sci USA. 1988;85:8583–8587. doi: 10.1073/pnas.85.22.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- 42.Cui X, Ji D, Fisher DA, Wu Y, Briner DM, Weinstein EJ. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotechnol. 2011;29:64–67. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Rideout WM, 3rd, Zi T, Bressel A, Reddypalli S, Rancourt R, Woo JK, Horner JW, Chin L, Chiu MI, Bosenberg M, Jacks T, Clark SC, Depinho RA, Robinson MO, Heyer J. Chimeric mouse tumor models reveal differences in pathway activation between ERBB family- and KRAS-dependent lung adenocarcinomas. Nat Biotechnol. 2010;28:71–78. doi: 10.1038/nbt.1595. [DOI] [PubMed] [Google Scholar]


