Abstract
Pattern recognition receptors are somatically encoded and participate in the innate immune responses of a host to microbes. It is increasingly acknowledged that these receptors play a central role both in beneficial and pathogenic interactions with microbes. In particular, these receptors participate actively in shaping the gut environment to establish a fruitful life-long relationship between a host and its microbiota. Commensal bacteria engage Toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs) to induce specific responses by intestinal epithelial cells such as production of antimicrobial products or of a functional mucus layer. Furthermore, a complex crosstalk between intestinal epithelial cells and the immune system is initiated leading to a mature gut-associated lymphoid tissue to secrete IgA. Impairment in NLR and TLR functionality in epithelial cells is strongly associated with chronic inflammatory diseases such as Crohn’s disease, cancer, and with control of the commensal microbiota creating a more favorable environment for the emergence of new infections.
Keywords: Commensal bacteria, Pattern recognition receptors, Innate immune response, Toll-like receptors, Nucleotide oligomerization domain-like receptors, Epithelial cells
Introduction
Different types and functions of intestinal epithelial cells (IECs)
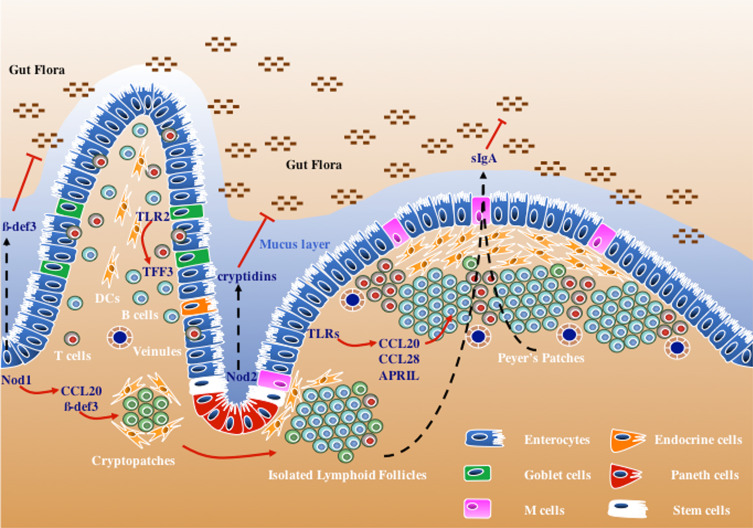
The intestinal epithelium is largely composed of a polarized monolayer of epithelial cells named enterocytes, which provide the gut with the necessary functionality to absorb nutrients. This is accomplished by the presence on the apical side of the polarized cell of microvilli rich in digestive enzymes, such as hydrolases, and multiple transporters. These cells form the biggest surface in the body in contact with the environment and therefore constitute the first layer of protection against foreign bodies. This is accomplished physically through very tight junctions separating the body from the luminal content. Besides the digestive function and the physical barrier, enterocytes also provide defensive mechanisms against microbes by producing antimicrobial peptides such as β-defensins and cathelicidins. Some antimicrobial mechanisms are constitutive, while others are inducible [1] (Fig. 1).
Fig. 1.
Role of different pattern recognition receptors (PRRs) in intestinal epithelial cell (IEC) signaling. Microbial recognition by IECs leads to the production of different effector molecules that are either secreted into the lumen, such as antimicrobial peptides (cryptidins and β-defensin 3), or at the basolateral side to establish a direct communication with stroma cells and cells of the immune system. These signaling cascades involve NOD1 in enterocytes to produce CCL20 and β-defensin 3 to induce cryptopatch maturation assisted by TLRs and NOD2, and TLR-mediated production of APRIL, CCL20, and CCL28, and recruitment of B cells to ILFs and Peyer’s patches. Goblet cells respond to TLR2 stimulation to produce TFF3, which supports the epithelial layer integrity both during homeostasis and tissue injury
The second most abundant epithelial cell is a specialized cell, the goblet cell, which has as its main function to secrete mucus into the lumen of the gut. The mucus is composed of a matrix of glycoproteins, the mucins, and glycolipids strongly associated with the epithelial cell layer that provide the gut with a protective slime. Furthermore, the mucus layer has an essential digestive function of providing a smooth gliding surface favoring the proper flow of nutrients along the digestive tract by peristalsis. Finally, the rare enteroendocrine cells constitute less than 1% of the epithelial cells and produce hormones such as serotonin, the P substance, and secretin [2]. There are up to 15 different types of enteroendocrine cells that can be characterized based on several criteria such as hormone expression, morphology of secretory granules, and spatial localization along the crypt-villus axis and from the duodenum to the distal colon. These three types of epithelial cells—enterocytes, goblet cells, and enteroendocrine cells—are renewed by a mechanism of differentiation and migration from the crypt to the extruding villus tip. The process takes 2–5 days from the precursor stem cells located in the crypts.
These same stem cells can migrate down to the villus to differentiate into Paneth cells [3], which are localized exclusively at the base of crypts. Paneth cells are involved in gut homeostasis by regulating the composition of the intestinal flora and defending against pathogens. These effects are mediated by the production of effector molecules such as secreted cryptidins also know as α-defensins, including DEFA-1 and DEFA-4, and other antimicrobial peptides (e.g., RegIIIγ) in response to bacteria. Of note, murine Paneth cells secrete more than 20 α-defensins, while only 2 α-defensins are secreted by human Paneth cells [4].
Furthermore, several effector enzymes such as phospholipase A2 (PLA2) and lysozyme are produced by Paneth cells. Finally, Paneth cells have a central role in controlling the homeostasis of the crypt-villus axis by providing a supporting role to the crypt epithelial stem cells. Most of these responses, such as the expression of antimicrobials [5], are induced by the presence of a microbiota, and deficiencies in these responses have been implicated in inflammatory bowel diseases such as Crohn’s [3].
In addition to the described epithelial cells that constitute the first cell monolayer, M cells are specialized epithelial cells of the gut that perform essential functions in translocating and presenting microbiota-derived antigens to antigen-presenting cells such as dendritic cells and macrophages. These cells localize above Peyer’s patches or above isolated lymphoid follicles (ILFs). These are organized lymphoid tissues known as gut-associated lymphoid tissues (GALT) composed essentially of B cell follicles surrounded by dendritic cells. Although these structures are not part of the epithelial cell layer, their maturation into functional IgA-producing follicles is dependent on signaling events occurring in epithelial cells [6, 7].
We will focus on the role of innate immune receptors in the epithelial cell response and their functional importance both in homeostasis as well as during disease.
Innate immune receptors
TLRs, NLRs, and RLRs
Innate immune receptors are best known as pattern recognition receptors (PRRs) involved in the recognition of microbial-associated molecular patterns (MAMPs) as well as danger-associated molecular patterns (DAMPs). PRRs are composed of two major families: those that are mainly located at the cell membrane and those localized in the cytosol. The cell membrane PRRs include only the Toll-like receptor (TLR) family. These can be on the cell surface, such as TLR4, TLR2, or TLR5, or in endosomes, such as TLR3, TLR7, and TLR9. Most of the cell surface TLRs recognize bacterial-surface-exposed structures such as lipopolysaccharide (LPS), lipoproteins, or flagellin, while the TLRs present in cellular compartments recognize nucleic acids (dsRNA, ssRNA, and dsDNA).
The cytosolic receptors can be classified into two major families: the nucleotide oligomerization domain (NOD)-like receptors (NLRs) and the retinoic acid inducible gene I (RIG-1)-like receptors (RLRs). Although there are more than 20 members of the NLR family, in the intestine only the NOD1 and NOD2 functions have been well characterized. NOD1 and NOD2 have been shown to recognize fragments of peptidoglycan (PGN). PGN is a unique and major component of the bacterial cell wall. It is a heteropolymer composed of glycan chains of repeating disaccharide units of N-acetylglucosaminyl-β1,4-N-acetyl muramic acid, linked by short peptides. While NOD1 senses meso-diaminopimelic-type of PGN [8, 9], most frequently found in Gram-negative bacteria and some species of the Bacillales order such as Bacillus and Listeria sp., NOD2 has a broader spectrum of sensing as it recognizes the smallest fragment common to both Gram-negative and Gram-positive bacteria, the muramyldipeptide N-acetylmuramoyl-l-alanyl-d-glutamate [9, 10]. Other members of the NLR family such as NLRP1 and NLRP3 have been studied particularly in the context of the inflammasome required for IL-1β secretion. However, their role in intestinal epithelial function remains poorly studied [11], although NLRP1 can be found expressed in glandular epithelial structures in the gut [12]. NLRP1 has been shown to detect toxins, while NLRP3 senses danger signals such as potassium imbalance or ATP release [13]. Finally, the RLRs are a family of RNA helicases that recognize viral RNAs and induce innate antiviral responses, including activation of pro-inflammatory cytokines and type-I IFN [14].
Expression and localization
The gut is exposed continuously to a huge commensal flora. Although the thick mucus layer physically separates the commensal flora from the epithelial cells, bacteria constantly shed surface components such LPS, PGN fragments, or flagellin as part of their growth cycle. Thus, the most intriguing aspect of PRR function has been to decipher the mechanisms by which cells discriminate friend from foe. Part of the answer lies in the expression and localization of these PRRs, in particular of TLRs, which can be surface exposed and directly in contact with bacteria or bacterial components. Although the information about TLR expression and compartmentalization is incomplete [15], some of these appear to be expressed and localized exclusively in the crypt epithelial cells both in the stomach and the intestine [14–17] far from the commensal bacteria. Furthermore, in the case of TLR4, the essential co-receptor MD2 is poorly expressed [16]. Finally, TLR4 seems to be sequestered in the Golgi [18] and requires prior internalization of LPS to induce an immune response [19]. In contrast, TLR9, the receptor of CpG DNA, is found on the surface of intestinal epithelial cells but displays a differential response depending on its localization on the apical or the basolateral side. While TLR9 on the apical side activates a tolerogenic response, TLR9 on the basolateral side triggers the translocation of NF-κB into the nucleus [20] suggesting that the TLR pathways on polarized cells are regulated differently with respect to their location (apical vs. basolateral membrane). Alternatively, some TLRs might be localized exclusively to the basolateral side where invading microbes can be detected triggering an appropriate response.
Thus, at the beginning of this century, the discovery of the NLRs [21, 22] led to the hypothesis that surface-exposed TLR signaling was dampened in the intestinal track to avoid overstimulation by the commensal flora and that the NLRs would be the cytosolic sentinels of the innate immune system to detect the intrusion of foreign organisms such as intracellular pathogens. Thus, tolerance to the gut flora would rely on compartmentalization as most PRRs would be either downregulated or localized in cellular compartments out of reach of the normal flora. However, this view has been challenged as both NOD1 and NOD2 appear to have central roles in beneficial interactions with the commensal flora [7].
Expression of NLRs has mainly been studied for NOD1 and NOD2. NOD1 is constitutively expressed in a broad range of tissue types, including intestinal cells such as caco-2 cell line, primary mouse intestinal epithelial cells, freshly isolated human colon epithelial cells, human embryonic kidney cells (HEK293T), and HeLa cells [8, 21, 23, 24]. In stomach and intestinal epithelial cells, it appears indeed that NOD1 is the first line of defense against pathogens [23, 24]. In contrast, NOD2 expression is absent in epithelial cell lines although it can be upregulated after NF-κB activation [25, 26]. NOD2 seems to be confined in the gut to Paneth cells in the terminal ileum as well as in metaplastic Paneth cells in the colon [27, 28].
Signaling
Activation of the PRRs leads to a signaling cascade that triggers a transcriptional program. The master transcription factor involved in immune signaling is NF-κB, which is sequestered in the cytosol. The activation of the NF-κB cascade releases NF-κB from its inhibitors leading to nuclear translocation and gene transcription. TLR signaling also leads to MAPK activation acting synergistically with NF-κB to express antimicrobial effectors, chemokines, and cytokines. TLRs, except TLR3, transmit the signal information through the recruitment of an adaptor molecule, MyD88. TLR4 and TLR3 can also engage another adaptor molecule, TRIF, which instead activates the IFN response factor 3 and type-I interferon production. Interestingly, while TLR4 signaling through MyD88 does not necessarily require trafficking from the plasma membrane to endocytic vesicles, it appears that TRIF-mediated signaling requires internalization of TLR4 [29].
NOD1 and NOD2 are cytosolic PRRs that also lead to MAPK and NF-κB activation. However, activation requires recruitment of both molecules to the plasma membrane [30–32]. NF-κB activation requires the adaptor molecule RIP2 [33], while the MAPK pathway is mediated by CARD9 [34]. In parallel with the transcriptional response to NOD1 and NOD2 stimulation, it has been shown recently that control of bacterial infection by NOD1 and NOD2 can also induce autophagy as a response to pure MAMPs as well as to bacterial infections with Listeria and Shigella [35]. Interestingly, this response is RIP2-independent and requires the recruitment of ATG16L1 [35]. This is in sharp contrast to TLRs, which are also able to induce autophagy but use the same adaptor molecules MyD88 and TRIF [36]. Autophagy targets bacteria to the lysosomal compartment for degradation, enhancing bacterial clearance.
The TLR and NLR pathways use distinct adaptor molecules but lead to similar downstream signaling events with activation of NF-κB, MAPK, the inflammasome, and autophagy. Hence, cross-regulation of TLRs and NLRs is likely to exist. In fact, there is a wealth of information on the crosstalk between TLRs and NLRs that can be synergistic as well as antagonistic. However, most of the available data concerns cells of the immune system [37], and few data exist on IECs [26]. Recently, NOD2 activation in IECs was shown to be crucial to dampen TLR2 and TLR4 signaling in the gut and prevent enhanced inflammation [38]. As most of the PRRs (except NOD1) can be upregulated by NF-κB activation, it is crucial that their function in epithelial cells must be controlled to avoid pathological responses.
Regulation of the TLR and NLR response in intestinal epithelial cells appears to be governed by a variety of inhibitors such as IRAK-M, Tollip, SIGIRR, and A20 [39]. These inhibitors have been mostly studied in the TLR context and dampen their responses, regulating the potential of chronic inflammation in the gut. Most of these molecules are active in cells of the immune system while expression in IECs has not been assessed. SIGIRR, on the contrary, seems to be specifically active in IECs and not in immune cells [39]. Some of these inhibitors such as A20 [40] have been shown to also regulate NLRs although it has not been demonstrated yet to occur in IECs. Beyond direct inhibitors, regulation by miRNAs has also been emerging as a central regulatory mechanism in inducing tolerance in the gut to MAMPs. The miR-146a was shown to mediate protection by inducing IRAK1 degradation to bacterial-induced damage in early life during the transition from the sterile environment of the uterus to bacterial exposure after birth [41]. Among other miRNAs, miR-155 has a central role in modulating the immune response [42], although little is currently known about its role in intestinal epithelial cells. It has been suggested to play an important role in dampening Helicobacter pylori–induced inflammation in gastric epithelial cells [43].
Function of innate immune receptors for gut mucosal homeostasis
Triggering of innate immune receptors in IECs leads to the expression of pro-inflammatory mediators and antimicrobial peptides, as well as the direct induction of IgA class switching by B cells. Some of these downstream effects require the sensing by IECs of microbes to establish a crosstalk between the epithelium layer and the adjacent cells. IECs can interact with intact microbes or with molecules released by them such as components of the bacteria cell wall or metabolites. This interaction occurs via PRRs (despite the mechanisms described above to modulate their activation) that trigger the expression of a number of immune modulators including defensins, cytokines, and chemokines. These mediators will have an impact on the regulation of the immune function of other cells in the mucosal site and are necessary to maintain intestinal homeostasis. Deregulation of this crosstalk results in abnormal mucosal response and concomitant pathology.
Regulating homeostasis
A number of mouse models deficient in different PRRs show a rupture in this equilibrium. However in most of these mouse models, the deficiency is not epithelial cell specific. To discriminate the role of hematopoietic versus non-hematopoietic cells (which include, but are not restricted to epithelial cells) it is common to conduct reciprocal bone marrow transplants. Mice in which MyD88 is expressed exclusively on Paneth cells show that the production of several antimicrobial factors, such as RegIIIγ, RegIIIβ, CRP-ductin, and RELMβ, is MyD88-dependent and is activated by the microbiota in a reversible fashion. Importantly, Paneth cell–specific MyD88 activation is sufficient to limit microbial penetration to mesenteric lymph nodes [44]. Similarly, NOD2 deficiency leads to reduced expression of cryptidins in Paneth cells [45]. Additionally, NOD2 regulates transcellular permeability and bacterial translocation into Peyer’s patches [38]. In addition to Paneth cells, goblet cells have an important role generating a functional mucus layer that separates the epithelial layer from the commensal flora. Mice devoid of Muc2, the major mucin in the intestinal track, spontaneously develop inflammation and cancer [46]. TLR2 seems to perform an important role in goblet cells by inducing trefoild factor 3 (TFF3) required for mucosal repair [47]. However, most mice deficient in different PRRs do not develop spontaneous inflammatory diseases unless induced by certain enteropathogens or chemical damage (see below), which is in sharp contrast with mice selectively deficient in the NF-κB pathway.
Signaling through PRRs initiates signaling cascades that lead to the activation of pro-inflammatory pathways including NF-κB. Although several mouse models of colitis show excessive NF-κB activation, a beneficial role of NF-κB has been shown in other models. [48]. Mice in which NEMO—a molecule required for activation of canonical NF-κB signaling—was specifically deleted in IECs developed spontaneous severe chronic colitis characterized by epithelial ulceration, impaired expression of antimicrobial peptides, elevated expression of proinflammatory mediators, infiltration of immune cells, and translocation of bacteria into the mucosa, demonstrating that activation of the canonical NF-κB pathway in IECs is essential for colonic homeostasis. The NF-κB pathway is common to both TLR and NLR signaling. Importantly, MyD88 deficiency prevented intestinal inflammation indicating that TLR signaling in this model is essential for disease pathogenesis. Moreover, TNF receptor-1 signaling was shown to be crucial for disease induction [49]. Furthermore mice lacking TAK1, a molecule that acts upstream of the IKK complex, specifically in IECs, also developed spontaneous intestinal inflammation (more extensive and more severe than the NEMO) supporting the role of NF-κB activation as essential for the maintenance of intestinal mucosal homeostasis [48]. Interestingly, humans carrying hypomorphic NEMO mutations present a variety of immunological disorders including inflammatory diseases in 23% of the patients [50]. The L153R mutation was frequently associated with excessive inflammation despite an impaired TLR and TNF-α mediated activation of NF-κB. The clinical association of this mutation with inflammatory diseases seemed to be related to the increased TNF-α-induced programmed cell death due to a downregulation of A20 [50], again highlighting the importance of maintaining the check-and-balance mechanisms in IECs to establish homeostasis.
Most of the antimicrobial responses that keep the commensal flora in check occur locally in IECs, particularly in Paneth cells. A second major mechanism contributing to homeostasis involves IgA class switching, which requires instead a complex dialog between IECs and the immune system. Plasma cells in the intestine secrete IgA, which is transported into the intestinal lumen by IECs (at a rate of 0.8 g/m of intestine per day). The signals required for class switch recombination (CSR) from IgM to IgA positive B cells are typically induced in the germinal centers of Peyer’s patches (PP), and IgA plasma B cells recirculate through the thoracic duct lymph to enter the bloodstream and return back to the intestinal lamina propria, where they secrete IgA. Secretory IgA (sIgA) is then transported to the intestinal lumen via the polymeric immunoglobulin receptor (pIgR) expressed on IECs [51]. However, IEC secretion of cytokines upon TLR activation can trigger IgA class switching locally in the lamina propria through a T-cell independent process. Indeed, TLR signaling in IECs leads to the secretion of APRIL and BAFF by these cells. APRIL directly induces IgA CSR, whereas BAFF enhances B cell survival and proliferation. Furthermore, IECs can also induce CSR indirectly through secretion of TSLP, which will stimulate lamina propria DC to secrete APRIL [52]. Furthermore, transgenic mice expressing a constitutively active form of TLR4 in IECs showed a marked increase in intestinal B cells and IgA production, which resulted from the epithelial production of the chemokines CCL20 and CCL28, which promote B cell recruitment and APRIL-mediated IgA class switch [53] in intestinal lymphoid tissues, which include PP and ILFs.
Interestingly, the organogenesis of intestinal lymphoid tissues such as PP and lymph nodes (LNs) is programmed to develop during embryonic stages. However, ILFs are induced after birth by the commensal flora and require the nuclear hormone receptor RORγt [7]. IEC triggering of NOD1 was recently shown to be necessary and sufficient for the maturation of cryptopatches into ILFs [6]. Cryptopatches are small clusters of lymphoid tissue inducer cells located between the crypts of the intestinal lamina propria. The intestine contains several hundred of these structures. Upon bacterial colonization, CPs recruit B cells and develop into ILFs. Bone marrow transplants revealed that expression of NOD1 in nonhematopoietc cells was responsible for the induction of ILFs [6]. Triggering of NOD1 leads to the release of CCR6 ligands CCL20 and β-defensin 3 by the IECs. CCR6 is expressed by B cells that are recruited to ILFs [6]. However, full maturation of ILFs into sIgA-producing germinal centers requires additional signals involving TLRs and NOD2 [6, 53].
Inflammatory bowel diseases
Genetic associations of TLRs
Inflammatory bowel diseases (IBD) include Crohn’s disease (CD) and ulcerative colitis. Both are characterized by chronic inflammatory response to commensal bacteria. IBD patients have been shown to have increased expression of multiple TLRs although a decrease in TLR3 mucosal expression has also been shown in a subset of patients with Crohn’s disease [54]. Furthermore, several TLR polymorphisms have been associated with IDB, such as the TLR4 polymorphism D299G, the TLR9 polymorphism-1237C, or the TLR2 polymorphism R753Q. The strongest association with Crohn’s disease is with the TLR4 polymorphism D299G, which is located within the extracellular domain of TLR4 suggesting a decrease in recognition of ligands [55].
TLR expression is not restricted to epithelial cells but extends to myeloid and lymphoid cells. Human association studies do not define which cell types are involved in susceptibility to disease. In this respect, mouse studies can shed some light on cell-specific contributions to disease.
Mouse models have clearly shown that intact TLR signaling is necessary for homeostasis [56, 57]. One of the mechanisms by which TLR polymorphisms contribute to susceptibility to IBD is by causing impairment in epithelial repair. For example, TLR2 has been shown to have an important role in inducing gap junctional intercellular communication via Cx43. This controls IEC barrier function and restitution during acute and chronic inflammatory damage [58]. However, whereas some studies have shown a protective role of TLRs and MyD88 in intestinal inflammation by promoting epithelial repair [57, 59], others suggest that MyD88 signaling can promote intestinal inflammation. Maloy and colleagues have looked at the contribution of MyD88 signaling in distinct cellular compartments in the Helicobacter hepaticus model of intestinal immune pathology. In the absence of adaptive immunity, the study revealed that MyD88 signaling from hematopoietic cells and not epithelial cells was required for inflammation. Epithelial MyD88 signaling however was important for host survival by driving antimicrobial peptide expression, demonstrating that MyD88 signaling has distinct roles in immune and epithelial cells [60]. Thus, it is likely that TLR and MyD88 signaling will have a differential impact on the control of the host response depending on the model of infection used and whether the epithelial or immune compartment has a dominant role in the pathology. Moreover, establishing the contribution of MyD88 in PRR versus IL-1R/IL18R signaling pathway is also important.
This balance between the epithelial and immune compartment can also be assessed by the contribution of PRRs in chemically induced models of IDB. In the chemically induced model of intestinal inflammation by administration of DSS, MyD88-dependent signaling pathway was shown to be protective against colonic injury. This protection was given by hematopoietic cells [61]. Using the same protocol, i.e., 2% (wt/vol) DSS in drinking water for 7 days, Brandl et al. [62] found divergent results. The authors found that protection was given by nonhematopoietic cells. Although in this study MyD88/TRIF-deficient mice were used, the differences could not be accounted for by this since TRIF alone had no role in DSS colitis. The authors further show that protection is given by the epidermal growth factor (EGF) receptor ligands amphiregulin (AREG) and epiregulin (EREG) [62].
Genetic associations of NLRs
Although TLR mutations have been implicated in IBD, the strongest associations are with mutations in NLRs. In particular, homozygous mutations in NOD2 are highly correlated with the incidence of Crohn’s disease (CD) [63, 64]. Although CD is characterized by a strong Th1-mediated inflammation, these NOD2 mutations associated with CD are clustered in the leucine-rich repeat, ligand-sensing domain, and are found to involve loss of function [10, 63, 64]. Thus, the physiological roles of NOD2 in intestinal immunity and CD remain unclear. Possible mechanisms involving IECs specifically are the regulation of antimicrobial peptides, epithelial permeability, and the regulation of commensal flora.
Cryptidins are a group of antimicrobial peptides preferentially produced by Paneth cells. NOD2-deficient mice have significantly lower expression of defensin-related cryptdin 4 (Defcr4) and Defcr-related sequence 10 (Defcr-rs10) when compared to wild-type [45]. Interestingly, ileal CD is characterized by a decrease in antimicrobial α-defensins produced by Paneth cells. In patients with an NOD2 mutation, a defective production of α-defensins DEFA5 and DEFA6 has been observed [65, 66]. Accordingly, monolayers of NOD2-deficient epithelial cells are more sensitive to Salmonella infection [67]. NOD2 also appears to regulate epithelial transcellular permeability indirectly through a crosstalk between T cells and epithelial cells leading to increased bacterial translocation. In NOD2-deficient mice, an increase in the permeability appears to be mediated by an increase in the long isoform of myosin light chain kinase (MLCK) that can be restored by injection of ML-7, a MLCK inhibitor [38].
Chemical models of IBD have shown that NOD2 is protective, in particular, in the TNBS model that induces an acute Th1-dependent inflammation similar to CD. NOD2 ligands can induce an NOD2-dependent protection [68, 69]. However, in these experimental models, protection appears to be conferred by hematopoietic cells, particularly dendritic cells, through a downregulation by NOD2 of the TLR pathways [68, 69]. These experimental models instead support a central role in the crosstalk mechanisms between NOD2 and TLRs in immune cells, in particular, TLR2 leading to a Th1 prone response [70].
As NOD2 has a direct impact on IEC function, in particular Paneth cell production of antimicrobial products, it is likely that these alterations have an impact on the existing flora. Indeed, NOD2- (and RIP2-) deficient mice have an increased load of certain commensal bacteria strains in the terminal ileum [71, 72]. These results are correlated with human studies [72]. The microbiota itself can regulate the expression of NOD2 as shown by the fact that germ-free mice have reduced expression of NOD2 (and RIP2) in the terminal ileum, compared with SPF mice. Furthermore, NOD2 expression could be restored by complementation of GF mice with a conventional commensal flora [71].
Besides NOD2, polymorphisms in the NOD1 gene have been associated with the age of IBD onset [73]. However, the precise mechanism governing this NOD1 association remains uncharacterized. As NOD1 is important for ILF induction in the ileum, the major provider of sIgA, and has a major impact on the gut flora composition, again the data point to an imbalance between the immune system and its gut flora. Similarly, NLRP3 single nucleotide polymorphisms (SNPs) have also been shown to contribute to CD susceptibility [74], but the precise role of the NLRP3 inflammasome in IBD is still a matter of debate [75]. Not surprisingly, NLRP3-deficient mice have altered microbiota composition [76].
Because NLRP3 is expressed in both immune and epithelial cells [12], bone marrow chimeric mice were used to determine that NLRP3 signaling in the nonhematopoietic compartment is crucial for NLRP3 inflammasome-mediated protection against colitis. Indeed, expression of NLRP3 [77] and caspase-1 [78] in this compartment prevented the disease symptoms seen in DSS-administered nlrp3 −/− and casp1 −/− mice. However, in the chronic azoxymethane (AOM)/DSS model of colitis, reconstitution of NLRP3-deficient mice with wild type bone marrow was shown to confer resistance against colon tumorigenesis [79] demonstrating a protective role of NLRP3 inflammasome signaling in myeloid cells. Taken together these results suggest that inflammasome-mediated protection against colitis relies on both hematopoietic and nonhematopoietic cells. Indeed, colitis-associated tumorigenesis was recently demonstrated to be more severe in casp1 −/− mice when compared to mice specifically deficient for caspase-1 in either the epithelial or myeloid compartments [80].
Other susceptibility genes for CD include ATG16L1, IL23R, and IRGM along with many new loci [81–84]. Interestingly, ATG16L1 and IRGM establish a direct link between CD and autophagy.
Autophagy and PRRs
Autophagy defines a general mechanism to target large particles in the cytosol to a lysosomal degradation pathway. It was initially described as an energy-saving mechanism during nutrient restriction of eukaryotic cells to target misfolded protein aggregates or dysfunctional mitochondria for recycling. However, in recent years, autophagy has also been implicated in innate immune regulation, antigen presentation, and microbial killing and clearing [85]. Targeting of microbes to the autophagic pathway can occur both for microbes that escape into the cytosol and for those that remain in a vacuole. The mechanisms that target the autophagic machinery to intact vacuoles remain poorly characterized. Part of the explanation resides in the ability of MAMPs to induce autophagy through their cognate PRRs. TLR stimulation has been shown to induce the autophagic pathway [86]. However, the role of TLRs in targeting particles (live or dead) to the autophagosome has been studied in the context of macrophages and dendritic cells, and their role in IECs has remained uncharacterized. In contrast, the NLRs, in particular NOD1 and NOD2, have been shown to mediate the induction of autophagy both in immune cells and epithelial cells [35, 87, 88]. Upon NOD1 and NOD2 stimulation, these PRRs recruit ATG16L1 to the membrane by a direct interaction initiating the autophagic pathway [35]. Interestingly, this pathway is independent of the adaptor RIP2 and NF-κB activation. Travassos and colleagues have shown that the NOD2 frame shift mutation associated with CD fails to recruit ATG16L1 [35]. In contrast, the ATG16L1 T300A mutation also associated with CD still interacted with NOD2 [35] despite an impaired autophagy and bacterial clearing in epithelial cells [88]. In fact, this impaired bacterial killing was restricted to colonic epithelial cells [88]. Consistent with an important role of the autophagic machinery in epithelial cells, ATG16L1 and ATG5, another component of the autophagic machinery, have a key role in antimicrobial degranulation by Paneth cells in both mouse and human [89].
PRRs and intestinal cancer
The discovery in 1982 of Helicobacter pylori by Barry Marshall and Robin Warren as the etiological agent of duodenal and gastric ulcers as well as gastric adenoma and mucosal associated lymphoid tissue (MALT) lymphoma has modified our vision of the role that bacteria might play in the occurrence of certain cancers, particularly of the gastrointestinal tract. However, while the case of H. pylori and gastric cancer has been clearly established by epidemiological studies [90], the relationship between the intestinal flora (or certain of its members) and other cancers has remained uncertain. For example, intestinal MALT lymphomas have been associated with the presence of Campylobacter jejuni [91], and colorectal cancer with the presence of Streptococcus bovis/gallolyticcus (reviewed in [92]).
The role of the gut flora (and potentially of certain members in particular) has been addressed using animal models of intestinal cancers. Germline mutations on the adenomatous polyposis coli (APC) gene in humans are the cause of familial adenomatous polyposis (FAP), a dominant inherited colorectal cancer syndrome. APCmin/+ mice, which harbor a mutation in this gene, develop spontaneous intestinal tumors and are considered a model for human FAP. APCmin/+ mice deficient in MyD88 have dramatically reduced tumor-induced mortality compared to MyD88-sufficient littermate controls, indicating that MyD88 promotes tumorigenesis in this model [93]. Accordingly, in a different model of colon cancer by administration of azoxymethane, MyD88-deficient mice showed decreased incidence of tumor formation compared to normal mice [94]. Although the role of MyD88 expression specifically in epithelial cells was not assessed, one of the molecules that was differentially expressed in an MyD88-dependent manner was MMP7, which is expressed exclusively by epithelial cells [93]. Deficiency of MMP7 in this tumor system has been shown to reduce the incidence of tumors by approximately 60% [95]. Taken together, these results suggest a role for epithelial MyD88-mediated tumor progression. More recently, Lee et al. showed in the same APCmin/+ mouse model that MyD88-mediated tumorigenesis involved the activation of ERK, which resulted in post-transcriptional stabilization of the c-myc protein, a product of the oncogene Myc. C-myc then can activate genes involved in upregulation of proliferation, anti-apoptosis, and angiogenesis, enhancing IEC proliferation and tumor growth. Importantly, the authors show that tumor growth in the same model was dependent on MyD88 signaling in IECs [96]. The role of epithelial versus myeloid cells in colitis-associated tumor was assessed in another study where the authors compared IKKβ deletion in IEC and in myeloid cells in the azoxymethane/DSS model of intestinal cancer. In IEC, IKKβ deletion results in a decrease in tumor incidence without impacting inflammation [97].
In sharp contrast to the role of MyD88 (and presumably some of the TLRs) in promoting intestinal carcinogenesis, NOD1 protects against colonic tumor development in both azoxymethane/DSS and APCmin/+ models [98]. Tumorigenesis in the colon (but not in the anus) was dependent on the gut flora as antibiotic treatment greatly reduced the tumor burden. Protection was shown to be due to the role of NOD1 in maintaining an intestinal epithelial barrier against injury in the context of chemically induced chronic injury [98]. Although a role of NOD1 in nonepithelial cells cannot be excluded, this study supports a role of NOD1 in promoting survival of IEC in chemically induced injury. Similarly, certain NOD2 mutations significantly increase the risk of colorectal cancer in humans [99, 100], although none in NOD1 have been associated with cancer [100].
Angiogenesis and PRRs
Angiogenesis is part of the natural tissue repair mechanism after injury and inflammation, and yet it can be also deleterious in the cancer context by promoting tumor survival. Although data on the involvement of PRRs in angiogenesis are still limited, there is some evidence that TLR activation promotes angiogenesis [101]. The few data available mainly involve studies on macrophages or endothelial cells. Interestingly, germ-free (GF) mice have a defective vasculature, and this defect can be corrected by conventionalization or by colonization by Bacteroides thetaiotaomicron, suggesting a role for PRRs. This process occurs through an unknown mechanism involving Paneth cells. However it must be noted that GF mice deficient in Paneth cells have more microvasculature defects than GF with Paneth cells [102].
Impaired host resistance towards infection
The discovery of TLRs in mammals followed closely upon the characterization of the role of TOLL in drosophila immunity against fungi and Gram-positive bacteria [103]. Subsequently, TLR4 was the first mammalian TLR identified for its role in endotoxic shock [104–106]. Interestingly, as most TLR responses are dampened in the gut and TLR4 has a dominant role in septic shock, TLRs have historically been studied in immune cells and in models of systemic infection, TLR5 being one of the exceptions. In contrast, NLRs have been associated since their discovery with enteropathogens such as Shigella flexneri [107, 108], although their role has been often studied in vitro or ex vivo rather than in vivo. The central role of NOD1 in IECs was demonstrated in primary intestinal epithelial cells [8]. In this section, we will briefly discuss the role of PRRs in impaired resistance to intestinal infections as another review by Beth A. McCormick addresses in detail bacterial-epithelial interaction during microbial pathogenesis. These can be genetically determined deficiencies as well as impairments due to indirect effects from changing gut flora, such as may be induced by antibiotics, and thus relate strongly to inflammatory bowel diseases such as CD or opportunistic nosocomial infections such as vancomycin-resistant enterococci (VRE) or Clostridium difficile. In contrast, so far, no role has been demonstrated for IECs and their PRRs in antiparasitic and antiviral responses.
One of the most puzzling aspects of resistance to infections is the selective pressure that might have led to the emergence of mutations in certain PRRs during human evolution. For example, around 20% of humans carry a premature stop codon in TLR5 leading to loss of function [109]. Loss of function of TLR5 might be deleterious in certain circumstances. For example, antibiotic treatment of patients in the hospital setting can have a major collateral impact by modifying the gut flora. Modifications of the normal resident flora can modify the normal gut response. It has been shown that antibiotic treatment can modulate the expression of antimicrobial responses [110], and these require stimulation by TLRs [44]. These modifications lead consequently to an impaired resistance to different bacterial infections [111, 112] if a normal gut flora is not restored. Interestingly, protection against VRE or C. difficile infection can be restored by TLR5-dependent stimulation by purified flagellin [113, 114]. Nevertheless, loss of function mutations are frequently found in humans. In fact, while TLR5-dependent stimulation might be essential under certain circumstances by enhancing RegIIIγ secretion and anti-apoptotic processes in IECs conferring resistance to enteric infections [113, 114], TLR5 appears deleterious for chronic Salmonella-induced typhoid-like disease [115], generating a potential positive selection for TLR5 null mutations in humans. Similar antagonistic effects can be observed in other experimental contexts. In a model of murine experimental ileitis induced by Toxoplasma gondii, sensing of LPS by TLR4 exacerbates the disease [116]. Thus, the same LPS signal that protects the gut against certain enteric infections [112] can become harmful in a different context, highlighting the double-edged sword “personality” of the PRRs and their complex role in host-microbe responses.
In the case of NLRs, the most frequently found mutations are those associated with NOD2 and CD. However, CD has emerged as a disease of developed countries during the 20th century and has been associated with the hygiene hypothesis [117]. But then which are the environmental pressures selecting for loss of function mutations in NLRs and in particular in NOD2? For example, mutations in NOD2 associated with CD were suggested to be selected by conferring an advantage against certain enteric infections [118]. But in general, NOD2 mutations have been associated with impaired response to infections. NOD2 function is important for resistance against enteric infections by Listeria monocytogenes [45] or Helicobacter hepaticus [119]. In both examples, some of the defects appear related to Paneth cell function. Accordingly, H. hepaticus was found in the crypts of the ileum, which are in general free of bacteria [119]. H. hepaticus induced a Th1-dependent granulamatous intestinal disease that could not be rescued by adoptive transfer of normal hematopoeitic cells, suggesting a central role for the IECs and/or stroma cells in the infection outcome. Accordingly, the transgenic expression of HD5 in NOD2-deficient mice by Paneth cells was sufficient to restore their bactericidal capabilities and control the infection [119]. Interestingly, NOD2 mutations in humans have been associated with an increased risk of developing H. pylori-induced gastric MALT lymphoma [120]. However, the cellular compartment involved in the increased risk has not been studied. H. pylori seems to rely primarily on NOD1 in epithelial cells to induce a potentially deleterious chronic inflammation [24, 121], although NOD1 deficiency leads to increased bacterial burden. Indeed, although NOD1 drives a strong cytokine and type-I interferon response [24, 121], it is central for production of the antimicrobial peptides and IP10 that control the bacteria load [122, 123]. Most importantly, the type-I interferon response seems to be epithelial cell–specific [121]. Similarly, NOD1 is an essential signal transducer in IECs infected with bacteria that avoid recognition by TLRs [23, 124]. The fact that NOD2 is permissive to mutations more frequently than NOD1 indicates that these two NLRs have distinct roles in gut homeostasis and resistance to infection. Accordingly, NOD1 seems to have a central role in the recruitment of neutrophils to the site of infection [24, 124, 125], which is not compensated for by NOD2 [124]. Indeed, NOD1 priming by the commensal flora was shown to be central for neutrophil activation and resistance even to systemic infections [126].
Conclusion
The 15 years since the discovery of Toll-like receptors, soon followed by the NOD-like receptors, have led to an explosion of new data on the role of innate immunity in all aspects of a host response to microbes. Initially, PRRs were seen as the first sentinels in the fight against infectious diseases, but soon it became evident that their functions had to be fine-tuned spatially and temporally to establish a tolerogenic response. In exchange, this large community of commensal bacteria performs essential functions for the host and primes the host at the same time to establish a vigorous response against pathogens. However, challenges in the field still lay ahead to fully understand the double-edged sword response of the immune system. It is becoming apparent that the same PRRs can have both beneficial and deleterious effects for the host depending on the time and location of their effects. These aspects are just being addressed as discussed in this review and will require sustained efforts to fully grasp the interacting balances between microbes and their hosts. Some of the future advances will probably come from the full use of genetic manipulations of the host (transgenic mice, fate mapping, selective ablation of genes in different cell types, etc.) to study in detail the contribution of different cell types both of the epithelial layer, of the immune system, and in particular of the stroma, which has been the forgotten child of the field despite a highly probable central role in orchestrating the entire gut environment. On the bacterial communities side, important advances are being made with the use of germ-free mice and monocolonization studies as well as metagenomic studies both in humans and in mice. The coming years will be the most exciting times to study and fully grasp the emerging concept of the superorganism: that the collective force of the host and its commensal flora in the gut, as well as in other environmentally exposed surfaces, is more than just the sum of the individual players.
Acknowledgments
Despite our attempt to be as exhaustive as possible, the vast amount of literature produced in the last 15 years and the limited space required us to make choices, and we apologize to all of those whose work was not cited in this review. Work in the lab of I.G.B. is supported by a European Research Council starting grant (PGNfromSHAPEtoVIR n° 202283).
References
- 1.Muller CA, et al. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62:1297–1307. doi: 10.1007/s00018-005-5034-2. [DOI] [PubMed] [Google Scholar]
- 2.Hocker M, Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci. 1998;859:160–174. doi: 10.1111/j.1749-6632.1998.tb11120.x. [DOI] [PubMed] [Google Scholar]
- 3.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 4.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 5.Salzman NH, et al. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Bouskra D, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 7.Eberl G, Boneca IG. Bacteria and MAMP-induced morphogenesis of the immune system. Curr Opin Immunol. 2010;22:448–454. doi: 10.1016/j.coi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Girardin SE, et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 9.Girardin SE, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 10.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 11.Cario E. Heads up! How the intestinal epithelium safeguards mucosal barrier immunity through the inflammasome and beyond. Curr Opin Gastroenterol. 2010;26:583–590. doi: 10.1097/MOG.0b013e32833d4b88. [DOI] [PubMed] [Google Scholar]
- 12.Kummer JA, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 13.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehwinkel J, Reis e Sousa C. RIGorous detection: exposing virus through RNA sensing. Science. 2010;327:284–286. doi: 10.1126/science.1185068. [DOI] [PubMed] [Google Scholar]
- 15.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 16.Abreu MT, et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 18.Hornef MW, et al. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornef MW, et al. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 21.Inohara N, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 23.Kim JG, et al. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by Toll-like receptors. Infect Immun. 2004;72:1487–1495. doi: 10.1128/IAI.72.3.1487-1495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viala J, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez O, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstiel P, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 27.Ogura Y, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lala S, et al. Crohn’s disease and the NOD2 gene: a role for Paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/S0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 29.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnich N, et al. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-{kappa}B activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–26. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kufer TA, et al. The pattern-recognition molecule Nod1 is localized at the plasma membrane at sites of bacterial interaction. Cell Microbiol. 2008;10:477–486. doi: 10.1111/j.1462-5822.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 32.Lecine P, et al. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282:15197–15207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 34.Hsu YM, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 35.Travassos LH, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 36.Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci. 2010;67:1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill LA. When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Barreau F, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut. 2010;59:207–217. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 39.Shibolet O, Podolsky DK. TLRs in the gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1469–G1473. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hitotsumatsu O, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chassin C, et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8:358–368. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Coll RC, O’Neill LA. New insights into the regulation of signalling by Toll-like receptors and nod-like receptors. J Innate Immun. 2010;2:406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- 43.Xiao B, et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200:916–925. doi: 10.1086/605443. [DOI] [PubMed] [Google Scholar]
- 44.Vaishnava S, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host–microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 46.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podolsky DK, et al. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209–220. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wullaert A, et al. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 50.Hanson EP, et al. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122:1169–1177. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A (2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Shang L, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukata M, et al. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Fukata M, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 57.Rakoff-Nahoum S, et al. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Ey B, et al. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284:22332–22343. doi: 10.1074/jbc.M901619200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibson DL, et al. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:618–631. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 60.Asquith MJ, et al. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rakoff-Nahoum S, et al. Role of Toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Brandl K, et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci USA. 2010;107:19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 64.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 65.Wehkamp J, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wehkamp J, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hisamatsu T, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez EM, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe T, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe T, et al. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type-1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 71.Petnicki-Ocwieja T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rehman A, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 73.McGovern DP, et al. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet. 2005;14:1245–1250. doi: 10.1093/hmg/ddi135. [DOI] [PubMed] [Google Scholar]
- 74.Villani AC, et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaki MH, et al. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirota SA, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson CA, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Momozawa Y, et al. Resequencing of positional candidates identifies low frequency IL23R coding variants protecting against inflammatory bowel disease. Nat Genet. 2011;43:43–47. doi: 10.1038/ng.733. [DOI] [PubMed] [Google Scholar]
- 83.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 84.Parkes M, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levine B, et al. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 87.Cooney R, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 88.Homer CR, et al. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology. 2010;139:1630–1641. doi: 10.1053/j.gastro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cadwell K, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parsonnet J, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 91.Lecuit M, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni . N Engl J Med. 2004;350:239–248. doi: 10.1056/NEJMoa031887. [DOI] [PubMed] [Google Scholar]
- 92.Abdulamir AS, et al. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res. 2011;30:11. doi: 10.1186/1756-9966-30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 94.Uronis JM, et al. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson CL, et al. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee SH, et al. ERK activation drives intestinal tumorigenesis in Apc (min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 98.Chen GY, et al. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian Y, et al. Differential effects of NOD2 polymorphisms on colorectal cancer risk: a meta-analysis. Int J Colorectal Dis. 2010;25:161–168. doi: 10.1007/s00384-009-0809-9. [DOI] [PubMed] [Google Scholar]
- 100.Mockelmann N, et al. Investigation of innate immunity genes CARD4, CARD8 and CARD15 as germline susceptibility factors for colorectal cancer. BMC Gastroenterol. 2009;9:79. doi: 10.1186/1471-230X-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grote K, et al. Toll-like receptors in angiogenesis. Sci World J. 2011;11:981–991. doi: 10.1100/tsw.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stappenbeck TS, et al. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lemaitre B, et al. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 104.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 105.Qureshi ST, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 107.Girardin SE, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri . EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Philpott DJ, et al. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr Opin Immunol. 2001;13:410–416. doi: 10.1016/S0952-7915(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 109.Wlasiuk G, et al. A history of recurrent positive selection at the toll-like receptor 5 in primates. Mol Biol Evol. 2009;26:937–949. doi: 10.1093/molbev/msp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cash HL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brandl K, et al. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brandl K, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kinnebrew MA, et al. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201:534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jarchum I, et al. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun. 2011;79:1498–1503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vijay-Kumar M, et al. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect Immun. 2008;76:1276–1281. doi: 10.1128/IAI.01491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heimesaat MM, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli . Gut. 2007;56:941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bufford JD, Gern JE. The hygiene hypothesis revisited. Immunol Allergy Clin North Am. 2005;25:247–262. doi: 10.1016/j.iac.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Meinzer U, et al. Nod2 mediates susceptibility to Yersinia pseudotuberculosis in mice. PLoS One. 2008;3:e2769. doi: 10.1371/journal.pone.0002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Biswas A, et al. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci USA. 2010;107:14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosenstiel P, et al. Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori infection. Cell Microbiol. 2006;8:1188–1198. doi: 10.1111/j.1462-5822.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe T, et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type-I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645–1662. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boughan PK, et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infection. J Biol Chem. 2006;281:11637–11648. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 123.Grubman A, et al. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol. 2010;12:626–639. doi: 10.1111/j.1462-5822.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 124.Hasegawa M, et al. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol. 2011;186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- 125.Masumoto J, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clarke TB, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]