Abstract
γ-Glutamyltranspeptidases (γ-GTs) are ubiquitous enzymes that catalyze the hydrolysis of γ-glutamyl bonds in glutathione and glutamine and the transfer of the released γ-glutamyl group to amino acids or short peptides. These enzymes are involved in glutathione metabolism and play critical roles in antioxidant defense, detoxification, and inflammation processes. Moreover, γ-GTs have been recently found to be involved in many physiological disorders, such as Parkinson’s disease and diabetes. In this review, the main biochemical and structural properties of γ-GTs isolated from different sources, as well as their conformational stability and mechanism of catalysis, are described and examined with the aim of contributing to the discussion on their structure–function relationships. Possible applications of γ-glutamyltranspeptidases in different fields of biotechnology and medicine are also discussed.
Keywords: Gamma-glutamyltranspeptidase, Glutathione, Autoproteolytic activation, Gamma-glutamylhydrolase, Biotechnological applications, Gamma-glutamyltransferase
Introduction
γ-Glutamyltranspeptidases: key enzymes involved in glutathione metabolism
Glutathione (GSH) is the most abundant anti-oxidant molecule in cells. This tri-peptide is involved in a series of important cellular functions, such as in the storage and transport of nitric oxide, control of sulfur assimilation, protection of cells against oxidative stress, and redox regulation of gene expression [1]. In general, the mechanisms of acquired resistance of tumors to many forms of treatment involve GSH [2]. Elevated GSH levels in tumors have been associated with resistance to chemotherapy and radiotherapy and prevent the initiation of the apoptotic cascade [3–7].
GSH contains an unusual peptide linkage between the amino group of cysteine and the carboxyl group of the glutamate side chain, so general peptidases are not able to hydrolyze it. In mammalian, the GSH metabolism is mediated by the so-called γ-glutamyl cycle [8] that includes two ATP-dependent GSH synthesis steps, catalyzed by γ-glutamyl Cys synthetase and GSH synthetase, and a specific GSH degradation pathway. In particular, GSH is synthesized in the cytosol and is then translocated out of cells, where it becomes a substrate for γ-glutamyltranspeptidase (γ-GT, EC 2.3.2.2), the first enzyme of the GSH breakdown pathway.
γ-GT, an evolutionary conserved enzyme, specifically catalyzes the cleavage of the γ-glutamyl bond of GSH and the transfer of the γ-glutamyl group to water or to some amino acids and peptides [9, 10].
Mammalian γ-GT is a glycoprotein integrated in the plasma membrane with its active site on the outside, where γ-glutamyl moieties of GSH are supposed to be hydrolyzed and transferred to other amino acids, leading to formation of γ-glutamyl amino acids, which are then transported into the cell. Therefore, the cleavage of the γ-glutamyl bond of extracellular GSH enables the cell to use this antioxidant compound as a source of cysteine for increased synthesis of intracellular GSH. Moreover, the recovery of cysteine, mediated by GSH metabolism becomes critical for protein synthesis especially in rapidly dividing neoplastic cells [11]. Human γ-GT is highly represented in kidney, liver, and brain and its expression is significantly increased in several tumors and is related to their enhanced resistance to cytotoxic drugs [12]. An increased level of γ-GT in the serum has also been associated with pancreatitis, type II diabetes, cardiovascular disease, and stroke, whereas γ-GT deficiency has been linked to disruptive glutathione homeostasis, DNA damage, reproductive defects, and cataract [13]. As γ-GT in serum is mainly derived from liver, the enzyme is used in blood tests as a marker of hepatic or biliary tract-associated diseases [14, 15]. An increase in serum levels of γ-GT has also been suggested to be a promising biohumoral predictor of atherosclerosis [16]. γ-GT is also implicated in many physiological disorders, such as neurodegenerative diseases [17, 18]. Finally, γ-GT is clinically significant because evaluation of its activity in obesity is associated with insulin resistance and the metabolic syndrome [19].
γ-GT has also been reported as a virulence factor associated with the colonization of the gastric mucosa by Helicobacter pylori, the pathogen responsible for gastritis, ulcer, and gastric cancer [20]. The reader is referred to other reviews that address these topics [12, 21–27].
Sequences and structures
Since its discovery in the sheep kidney [28], γ-GT has been isolated from various sources, ranging from bacteria to mammals. Table 1 is a representative list of organisms reported to produce γ-GTs. In general, these enzymes share >25 % sequence identity, suggesting a strong conservation of structure and function. Mammalian γ-GTs are heterologously glycosylated and embedded in the plasma membrane by a N-terminal trans-membrane peptide, whereas bacterial homologous are generally soluble and localized in the periplasmic space by an N-terminal signal peptide or secreted in the extracellular environment.
Table 1.
List of organisms known to produce γ-GTs
| Organism | References | Organism | References |
|---|---|---|---|
| Allium cepa | [33] | Homo sapiens | [53] |
| Aphidius ervis | [76] | Hordeum vulgare | [77] |
| Arabidopsis | [78] | Lycopersicon esculentum | [32] |
| Arabidopsis thaliana | [79] | Marthasterias glacialis | [80] |
| Ascaris suum | [81] | Morchella esculenta | [82] |
| Bacillus licheniformis | [47] | Mus musculus | [83] |
| Bacillus pumilus | [84] | Neisseria gonorroheae | [85] |
| Bacillus pumilus KS 12 | [36] | Oryctolagus cuniculus | [86] |
| Bacillus sp. KUN-17 | [87] | Ovis aries | [88] |
| Bacillus subtilis (natto) | [31] | Phaseolus vulgaris | [89] |
| Bacillus subtilis 168 | [90] | Proteus mirabilis | [29] |
| Bacillus subtilis NX-2 | [91] | Pseudomonas nitroreducens | [56] |
| Bacillus subtilis SK11.004 | [57] | Raphanus sativus | [92] |
| Bacillus subtilis TAM-4 | [58] | Rattus norvegicus | [93] |
| Bos Taurus | [53] | Saccharomyces cerevisiae | [94] |
| Campylobacter jejuni | [95] | Schizoaccharomyces pombe | [96] |
| Deinococcus radiodurans | [55] | Setaria cervi | [97] |
| Equus caballus | [88] | Solanum lycopersicum | [98] |
| Escherichia coli K-12 | [30] | Sus scrofa | [99] |
| Geobacillus thermodenitrificans | [49] | Treponema denticola | [100] |
| Helicobacter pylori | [20] | Thermus thermophilus | [55] |
All γ-GTs are encoded by a single gene and are translated as a unique polypeptide, which then undergoes an auto-proteolytic cleavage into a large and a small subunit (see below). The molecular weights of the two chains are generally found to be within 38–72 kDa for the large subunit and from 20 to 66 kDa for the small subunit [20, 29–31], respectively. Such a high variation in the molecular masses can be explained by the high glycosylation of the animal and plant enzymes [32, 33].
Analysis of the primary structure of γ-GTs based on multiple alignments revealed that the sequence of the small subunit, which contains most of the residues forming the catalytic site, is slightly more conserved than that of the large subunit [34]. Sequence alignments of the small subunits emphasize the presence of highly conserved residues, such as the catalytic threonine (Thr391 in Escherichia coli γ-GT, EcGT), the nucleophile responsible for both the autoprocessing and the enzymatic activity, and the two Gly residues (Gly483–Gly484 in EcGT) proposed to have a role in the stabilization of the tetrahedral transition state of the enzyme (Fig. 1) [34, 35].
Fig. 1.
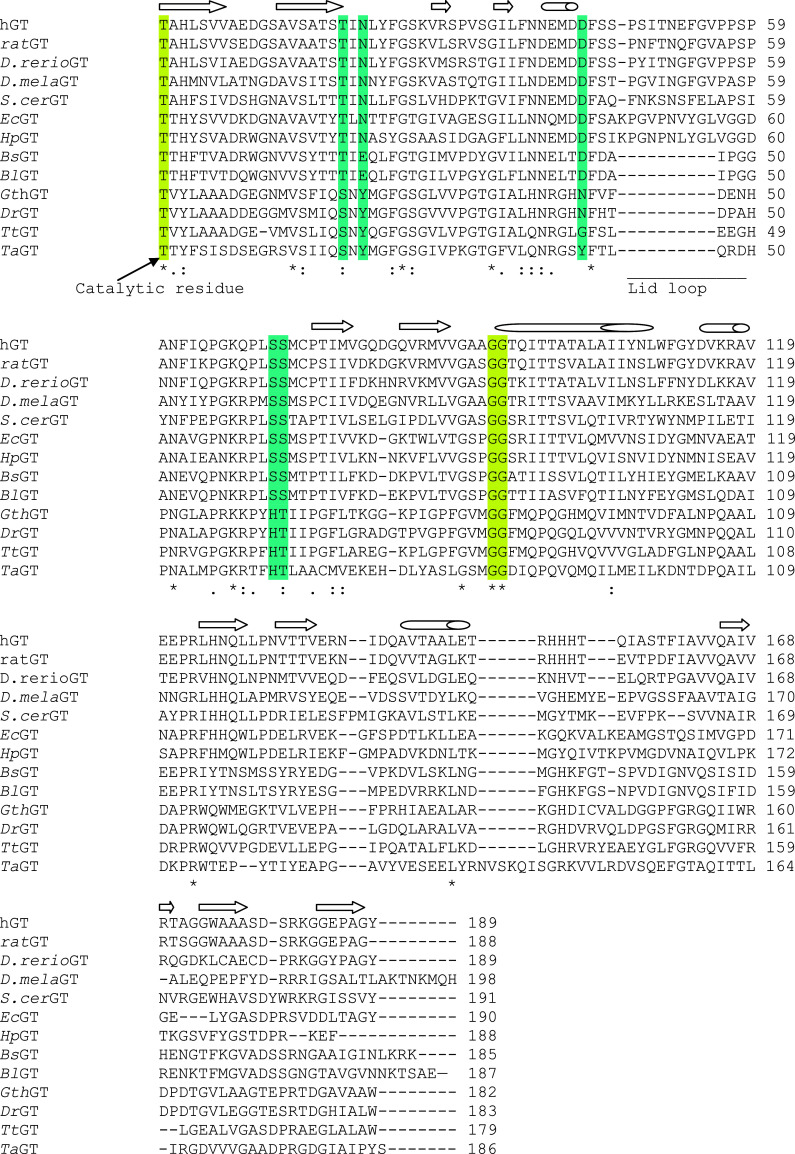
Multiple alignment of amino acid sequences of the small subunits of some γ-GTs. The amino acid sequences of γ-GT from H. sapiens (Swiss-Prot P19440), R. norvegicus (Swiss-Prot P07314), D. rerio (Swiss-Prot Q7T2A1), D. melanogaster (Swiss-Prot Q9VWT3), S. cerevisiae (Swiss-Prot Q05902) E. coli (Swiss-Prot P18956), H. pylori (Swiss-Prot O25743), B. subtilis (Swiss-Prot P54422), B. licheniformis (Swiss-Prot Q62WE3), G. thermodenitrificans (YP001127364.1), D. radiodurans (PIR: D75385), T. thermophylum (NCBI Reference Sequence: YP_144021.1) and T. acidophilum (NCBI Reference Sequence: NP_394454.1) are included. The alignment was performed by using the ClustalW method (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The conserved threonine residues responsible for autoprocessing of the enzyme and the two strictly conserved glycine residues involved in binding of the γ-glutamyl moiety (T391, G483, and G484 in EcGT) are highlighted in yellow. The other residues responsible for substrate binding and catalytic activity of γ-GTs are highlighted in cyan (T409, N411, D433, S462, and S463 in EcGT). The lid loop extending towards the active site (spanning from P438 to G449 of EcGT) and absent in some γ-GTs is underlined. The secondary structure elements are shown above the alignment. The numbering scheme reported in the figure refers to the small subunit
The N-terminal region of the protein is highly variable and its truncation does not hamper the auto-processing [36]; the C-terminus, which in Helicobacter pylori (HpGT) and in Bacillus licheniformis (BlGT) has been demonstrated to contribute to optimal enzymatic efficiency and autoprocessing [37, 38], shows a low sequence conservation.
The sequence of human γ-GT contains seven N-glycosylation sites [39]. In particular, the large subunit contains six of these sites (Asn95, 120, 230, 266, 297, and 344), whereas the small subunit contains a single site (Asn511). Four of the N-glycosylation sites (Asn95, 120, 344, and 511) are highly conserved among eukaryotes and two additional sites (Asn230 and 297) are conserved among mammalian γ-GTs. Furthermore, four cysteines (Cys50 and 74, and Cys192 and 196) are likely involved in the formation of disulphide bonds. Cys192–196 pair is only found in a subset of mammalian γ-GTs (see for example [40]), whereas the Cys50–Cys74 pair is highly conserved in non-bacterial homologues. Since these residues do not appear to be involved in the catalytic mechanism, the Cys50–Cys74 pair has been supposed to have a structural role and/or a regulatory function [35].
So far, no structure of mammalian γ-GTs has been determined, probably because of difficulty in crystallization of the heavily and heterogeneously glycosylated mammalian proteins, but their biochemical properties can be partially explained from sequence alignment, by referring to the few three-dimensional structures of bacterial γ-GTs reported so far. Crystal structures have been solved for EcGT (PDB code 2DBU) [34], HpGT (PDB code 2NQO) [35], and Bacillus subtilis GT (BsGT, PDB code 2V36). The structures of the putative γ-GTs from Bacillus halodurans (BhGT, PDB code 2NLZ, annotated as cephalosporin acylase) and from the thermoacidophilic archaeon Thermoplasma acidophilum (TaGT, PDB code 2I3O) have been reported in the Protein Databank, but have not been described in literature. All these γ-GTs exhibit a similar molecular architecture that consists of a four-layer αββα-structure, with two antiparallel β-sheets between α-helical layers. In the central β-sheet sandwich, strands are arranged in an antiparallel fashion, and both large and small subunits contribute strands to the nearly flat β-sheets (Fig. 2). These features demonstrate that γ-GTs belong to the structural superfamily of the N-terminal nucleophilic (Ntn) hydrolases [41, 42].
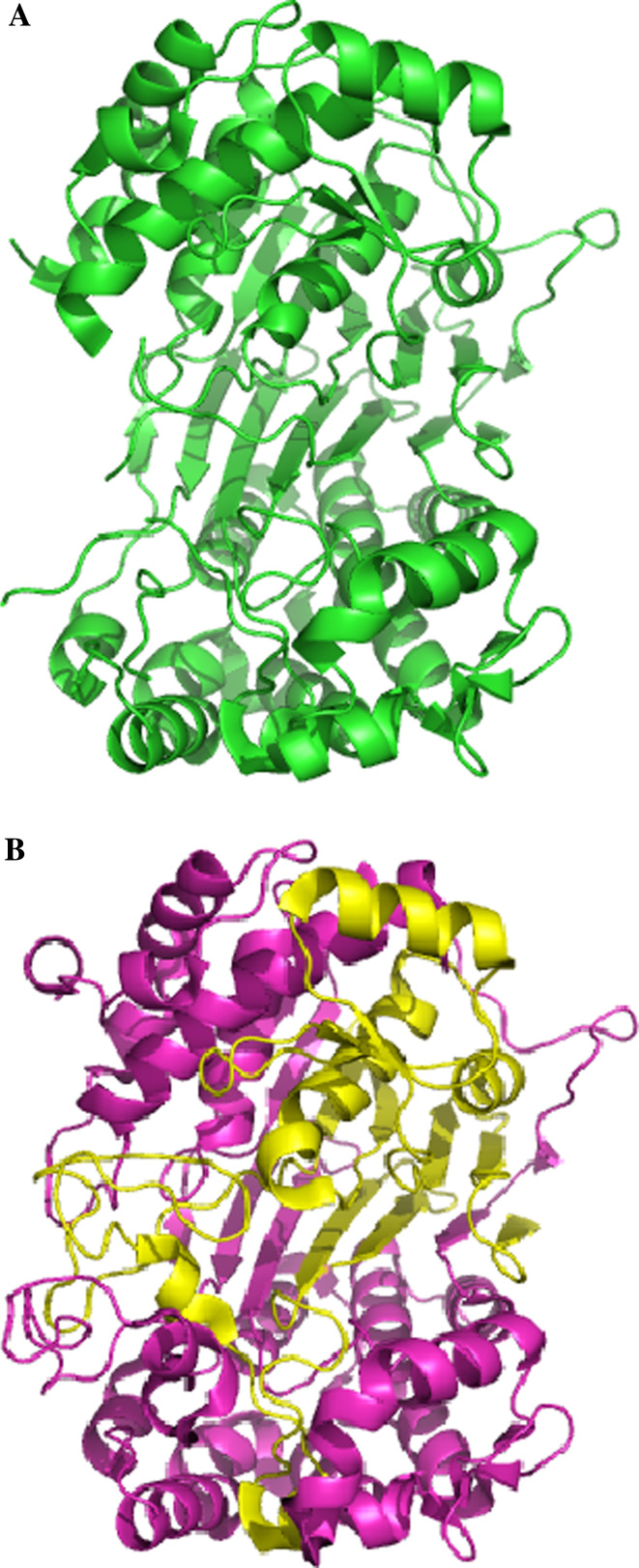
Fig. 2.
Diagram showing a schematic representation of the precursor (a) and mature (b) forms of EcGT. In panel b, the large subunit is shown in violet and the small subunit in yellow
In the studied γ-GTs, the small and the large subunits are highly intertwined throughout the structure with a 9,000–10,000 Å2 surface area buried between them.
The crystal structure of EcGT has been solved both for the unprocessed T391A mutant (PDB code 2E0W) [43] and for the mature enzyme (PDB code 2DBU; [34]). A detailed comparison of these structures is reported below. Finally, the complexes of EcGT and BsGT with glutamate (PDB codes 2DBX and 3A75, [34, 44]) and those of HpGT with the inhibitor acivicin (PBD code 3FNM; [37]) and of E. coli GT with azaserine (PDB code 2Z8IA) and acivicin (PDB code 2Z8K) have also been solved [45].
A complete list of X-ray structures of γ-GTs deposited in the Protein Data Bank is reported in Table 2.
Table 2.
List of γ-GT structures deposited in the Protein Databank
| Enzyme | Structure description | PDB code | References |
|---|---|---|---|
| EcGT | Ligand-free form | 2DBU | [34] |
| EcGT | Complex with hydrolyzed glutathione | 2DG5 | [34] |
| EcGT | Complex with acivicin | 2Z8K | [45] |
| EcGT | Complex with L-glutamate | 2DBX | [34] |
| EcGT | Complex with azaserine | 2Z8I | [45] |
| EcGT | Acyl-enzyme intermediate | 2DBW | [34] |
| EcGT | Monoclinic form | 2E0X | [43] |
| EcGT | Samarium derivative | 2E0Y | [43] |
| EcGT | Complex with azaserine prepared in the dark | 2Z8J | [45] |
| EcGT | T391A mutant | 2E0W | [43] |
| HpGT | Ligand-free form | 2NQO | [35] |
| HpGT | Complex with glutamate | 2QM6 | [101] |
| HpGT | Complex with acivicin | 3FNM | [37] |
| HpGT | Mature T380A mutant in complex with S(nitrobenzyl)glutathione | 2QMC | [101] |
| BsGT | Ligand-free form | 2V36 | Sharath et al., to be published |
| BsGT | Complex with glutamate | 3A75 | [44] |
| TaGT | Ligand-free form | 2I3O | Rao et al., to be published |
| BhGT | Ligand-free form | 2NLZ | Patskovsky et al., to be published |
Autoproteolytic activation
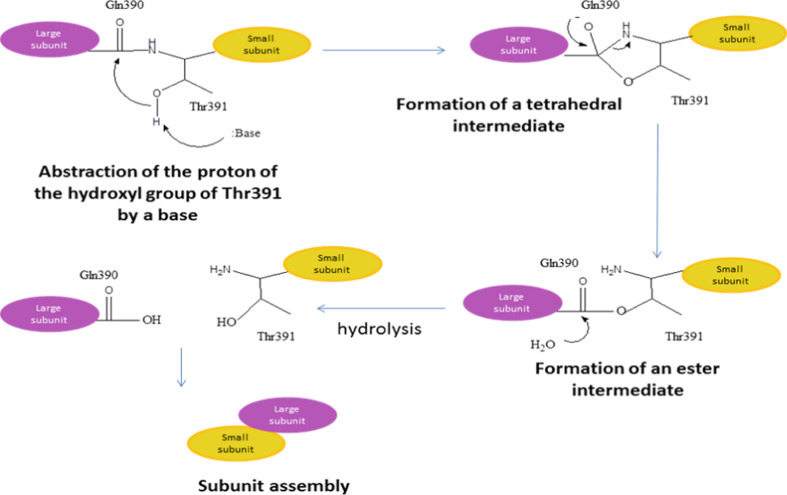
A common feature of the members of the Ntn hydrolase superfamily is the autoproteolytic activation of inactive precursors to release a catalytic serine, threonine, or cysteine at the N-terminal position [41, 42]. The proposed mechanism for autoprocessing of γ-GT is shown in Fig. 3 [46]. In detail, the hydroxyl group of the side chain of a strictly conserved threonine (Thr391 in EcGT) of the precursor protein acts as the nucleophile for the cleavage, attacking the carbonyl group of the preceding glutamine residue (Gln390 in EcGT) and forming a transitional tetrahedral intermediate. The cleavage of the C–N bond through protonation of the amino group of the Thr yields an ester intermediate (N–O acyl shift), then hydrolyzed by a water molecule to form a large subunit and a small subunit with the Thr as the new N-terminal residue.
Fig. 3.
Proposed mechanism for autocatalytic processing of γ-GTs
Recently, a water molecule has been suggested to play a critical role in the autocatalytic activation by enhancing the nucleophilicity of the hydroxyl group of the Thr [38, 43, 47]. Finally, in human γ-GT, glycosylation is required to produce the mature enzyme [39, 48].
The proenzyme and the activated γ-GT have distinct features in different organisms. In mammalians, as well as in many bacteria like E. coli, the proenzyme is a dimer of two identical monomers (αβ)2, whereas the activated γ-GT is a heterodimer of the small and large subunits (α–β) [34]. However, it has been recently found that γ-GT from Geobacillus thermodenitrificans (GthGT) assumes a homotetrameric structure as precursor (αβ)4 and a heterotetrameric structure formed by two small and two large subunits (α2–β2) as mature enzyme [49]. Interestingly, evidences for the existence of heterotetrameric mature γ-GTs also come from gel filtration and dynamic light scattering data on HpGT [50] and from the X-ray structures of both EcGT and HpGT that revealed the presence of two heterodimeric molecules in the asymmetric unit with identical relative arrangement of heterodimers [34, 35]. A detailed analysis of the structures shows that, if the heterotetramer were the biologically relevant form of these enzymes, this arrangement would be scarcely stable since the two heterodimers bury a surface area of only about 1,600 Å2.
The substitution of the conserved Thr results in a protein unable to undergo the autocatalytic maturation: the T → A substitution leads to the formation of an inactive and homodimeric precursor in HpGT, EcGT and BlGT [35, 43, 47], whereas the same replacement leads to a homotetrameric pro-enzyme, which keeps a reduced but significant hydrolase activity, in GthGT [49]. In this case, the small observed catalytic activity might be due to a solvent molecule that mimics the hydroxyl group of the catalytic threonine side chain [49].
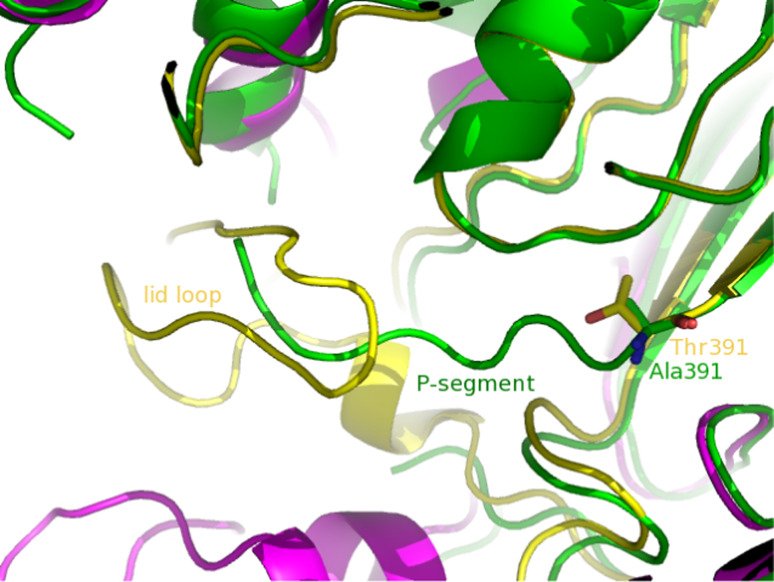
Crystal structure of mature γ-GTs from E. coli and H. pylori showed that the C-terminal regions of the large subunits are far from the N-terminal region of the small subunits (>35Å) [34, 35], thus suggesting that large conformational changes are necessary upon processing. Structural comparison of T391A unprocessed enzyme and mature EcGT demonstrated that the structures of the core regions in the two proteins are unchanged, but marked differences are found near the active site [43]. In particular, in the precursor analog, the segment corresponding to the C-terminal region of the large subunit occupies the site where a loop (residues 438–449 in EcGT, hereafter denoted as lid loop) forms the lid of the γ-glutamyl group-binding pocket in the mature γ-GT (Fig. 4). This feature demonstrates that, upon cleavage of the N-terminal peptide bond of Thr391, the newly produced C-terminus (residues 375–390 in EcGT), hereafter denoted as P-segment, flips out, allowing the formation of the γ-glutamyl group-binding pocket. The mobile P-segment has been shown to be positioned by several electrostatic interactions. Although this latter region shows low conservation among γ-GT members, mutational studies have confirmed that it can play an important role for autoprocessing in HpGT [37] and BlGT [38].
Fig. 4.
Superimposed structure of precursor (green) and mature forms of EcGT. In the mature enzyme, the large subunit is shown in violet and the small subunit in yellow
The above described conformational changes associated with autoprocessing could be not a general property of γ-GTs, since the determination of the structure of the enzyme from B. subtilis has recently revealed that the C-terminal segment of the large subunit, which in this enzyme has extra residues when compared to EcGT, appears to be changed little after autocatalytic processing, being located close to the N-terminal region of the small subunit [44].
The reaction mechanism
The highly conserved Thr is not only responsible for autoproteolytic processing into the small and large subunits, but also for the catalysis. This residue is essential for enzymatic activity, because most of the unprocessed proteins, in which this residue was mutated, were found to become completely or partially inactive [43, 50].
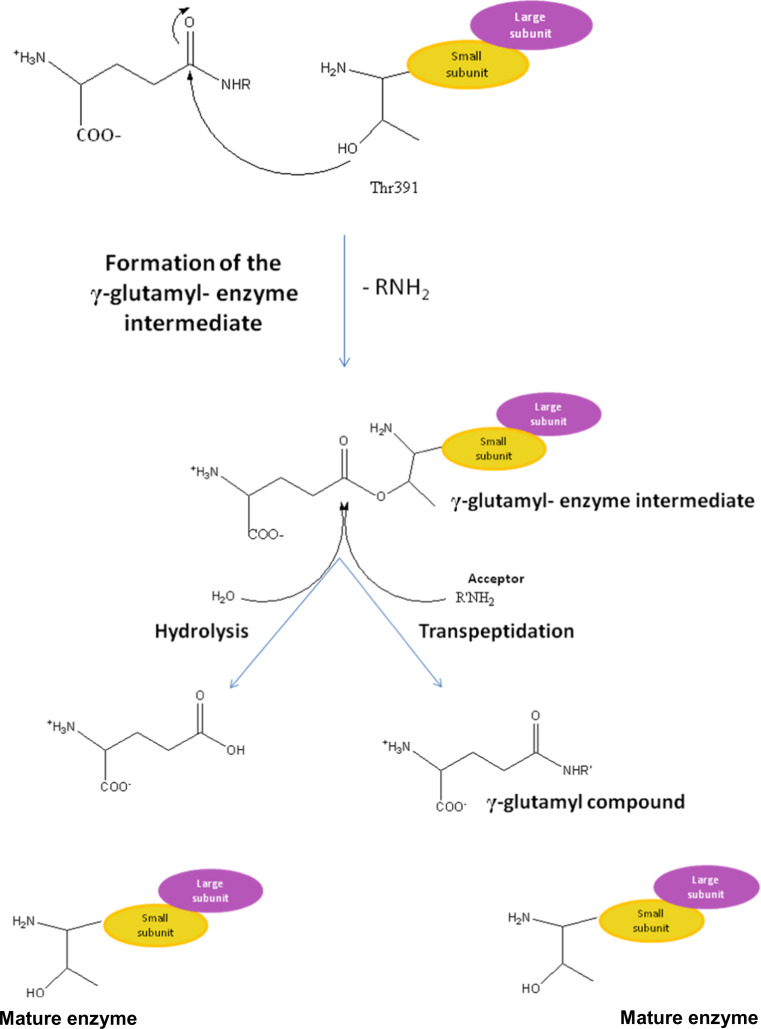
The enzymatic reactions catalyzed by γ-GTs are reported in Fig. 5. These enzymes catalyze the cleavage of the γ-glutamyl linkage of γ-glutamyl compounds, such as GSH, and the transfer of the γ-glutamyl moiety to other amino acids or short peptides. In vivo, the primary reaction catalyzed by human γ-GT is the hydrolysis, in which water, rather than amino acids, acts as the acceptor molecule during the cleavage of the γ-glutamyl bond [39].
Fig. 5.
Proposed reaction mechanism of γ-GTs
In the first step of a ping-pong mechanism, the oxygen atom of the N-terminal Thr residue attacks the carbonyl of the γ-glutamyl-compound to form a γ-glutamyl-enzyme intermediate. This intermediate can then react with water to release glutamate in a hydrolysis reaction or with an amino acid or di-peptide to give a transpeptidation reaction forming new γ-glutamyl compounds (Fig. 5).
Barycki and coworkers [35] have proposed a mechanism for hydrolysis of GSH by γ-GT, in which the residues adjacent to the catalytic Thr affect the enzymatic activity. This hypothesis is supported by kinetic and mutagenesis studies on HpGT and on BlGT [51], which reveal that a Thr–Thr dyad is critical for efficient cleavage of the γ-glutamyl peptide bond of GSH. In particular, the hydroxyl group of a Thr (the second one in the sequence of the small subunit) forms two hydrogen bonds with the catalytic Thr, by increasing the reactivity of its hydroxyl group. Then, this hydroxyl group attacks the γ-glutamyl peptide bond of GSH and leads to the formation of a tetrahedral transition state that is stabilized through interactions with two conserved glycines. The two threonines are rather well conserved in γ-GT family (see Fig. 1). Interestingly, in BlGT, the replacement of the second Thr of the catalytic dyad with lysine, also impairs the autoprocessing of the enzyme [51].
Since the mechanism of transpeptidation has not been clarified yet, further studies are needed to verify the speculative aspects of this model on the basis of new structural insights. Moreover, it remains to discover why the absence of any single glycosylation in mammalian enzymes affects both the transpeptidation and hydrolysis reactions [39].
The γ-glutamyl-enzyme intermediate structure
As described above, the reaction catalyzed by γ-GTs proceeds via the formation of a γ-glutamyl-enzyme intermediate followed by nucleophilic substitution by water, amino acids or short peptides.
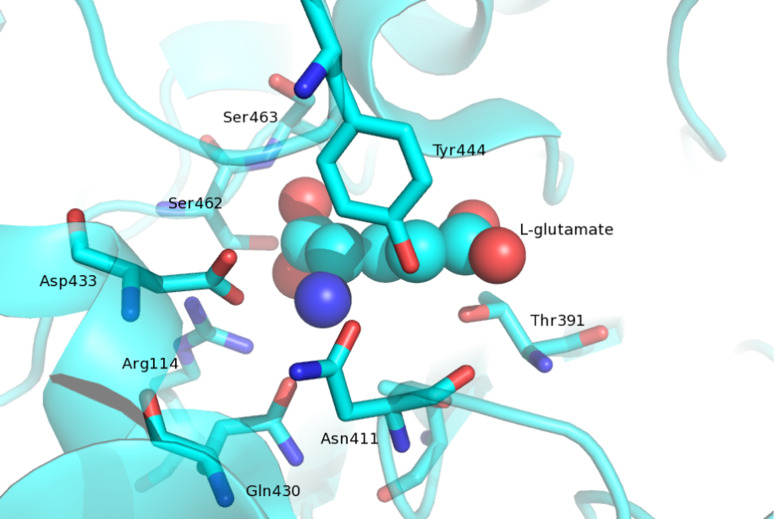
The structures of EcGT crystals soaked in the solutions containing GSH (γ-glutamyl-enzyme intermediate, PDB code 2D5G) and l-glutamate (PDB code 2DBX) have provided a clear illustration of the γ-glutamyl-enzyme intermediate (Fig. 6). The substrate-binding pocket is located at the bottom of a deep groove, where the catalytic Thr resides (Fig. 6). When bound within the enzymatic pocket, the carbonyl group of the γ-glutamyl moiety is covalently linked to the Oγ atom of the catalytic Thr. The γ-glutamyl moiety is held in position by many hydrogen bonds and salt bridges (Fig. 7). In particular, in the complex of EcGT with l-glutamate, the carboxyl group of the ligand is bonded with Arg114, Ser462 and Ser463, whereas the amino group interacts with Asn411, Gln430 and Asp433. The γ-glutamyl carbonyl oxygen is hydrogen bonded with the main-chain atoms of Gly483 and Gly484. Except for Arg114, all residues involved in γ-glutamyl binding belong to the small subunit.
Fig. 6.
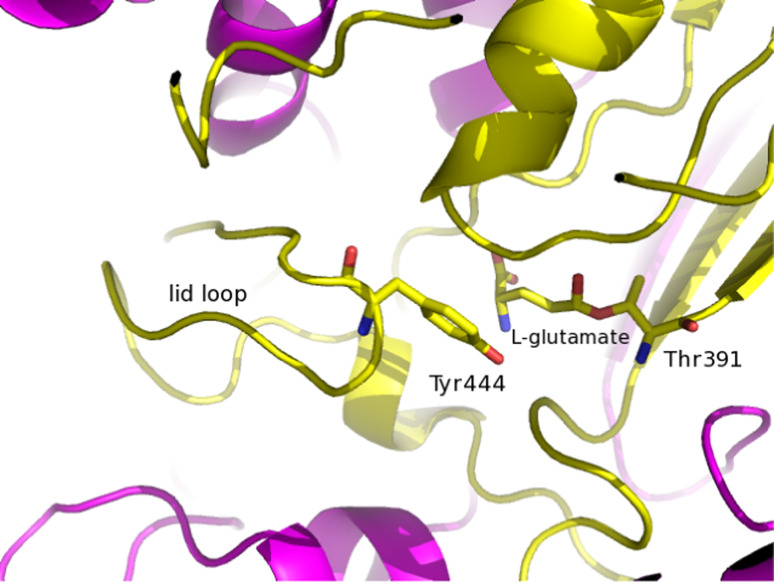
Glutamate binding in the active site of EcGT. The stick model of l-glutamate, nucleophile Thr391 and of Tyr444 are shown. The large subunit is shown in violet and the small subunit in yellow
Fig. 7.
Binding mode of l-glutamate in the catalytic pocket of EcGT. Carbon, nitrogen and oxygen atoms are colored cyan, blue and red. The stick models of residues involved in the ligand binding and enzyme reaction are shown
Role of the lid loop
The lid loop, conserved in all eukaryotic γ-GTs and only in some bacterial counterparts, has been proposed to play a role in regulating the access of the substrate to the active site or the binding of the substrate to the active site cleft [44, 49, 52]. Upon cleavage, the C-terminal segment of the large subunit seems to move away from the N-terminal threonine of the small subunit, thus forming the γ-glutamyl binding pocket. In the meanwhile the lid loop could form a lid upon the pocket. When the pocket is occupied by a substrate or inhibitor, the lid loop shields the catalytic pocket from the solvent, otherwise, when the pocket is empty the lid loop is flexible. In EcGT, Tyr444, located at the middle of this loop, is hydrogen-bonded with Asn411, thus forming the wall of the substrate binding pocket.
In BsGT [44] and in several extremophilic γ-GTs, such as GthGT, BhGT, TaGT and those from Deinococcus radiodurans (DrGT) and Thermus thermophilus (TtGT) the lid loop is absent [49, 52].
Recently, the structure of the l-glutamate-bound BsGT revealed that, in this protein, neither the lid loop nor alternative ordered segments cover that active site. Therefore, in this case, the binding pocket remains exposed to solvent [44] and could accept other potential substrates. This result prompts questions about the role and significance of the lid loop in γ-GT catalysis.
Biochemical properties of γ-glutamyltranspeptidases
A direct comparison of the biochemical features of the enzymatic activity of different γ-GTs is difficult, because of the different experimental conditions and substrate molecules used to study each enzyme.
The enzymatic activity determination has been generally performed using the substrate analogous l-glutamic acid p-(4-nitroanilide) [53, 54]. The release of 4-nitroaniline was monitored by spectrophotometer at 412 nm. When the activity of the recombinant proteins was too low to allow continuous monitoring, the release of 4-nitroaniline was assessed by end point assay after 10 min of incubation with the substrate [49, 52]. Transpeptidase activity is generally defined as the ratio between the activity of the enzyme in the presence of an acceptor substrate and the activity of the enzyme in the absence of this acceptor (hydrolase activity).
Despite the considerable sequence identity, significant catalytic differences exist between γ-GTs. In particular, plant γ-GTs are similar to the mammalian enzymes in their biochemical characteristics [55], whereas bacterial γ-GTs have been shown to react poorly with amino acid acceptor substrates compared to plant and mammalian enzymes. For example, HpGT is 100-fold less and EcGT is several hundred-fold less effective at catalyzing transpeptidation with respect to human γ-GT [50]. Large differences in catalytic activities also exist when bacterial homologues are compared. For example, EcGT is 33-fold less active than BsGT [51]; furthermore, extremophilic bacterial γ-GTs display reduced hydrolase activities, when compared to the other bacterial and eukaryal counterparts, and do not display any ability to transfer the γ-glutamyl group to several acceptors [49, 52]. Under this aspect, transpeptidase activity could be a feature appeared later during the enzyme evolution [52].
The optimal pH of the reactions catalyzed by γ-GTs is generally between 8 and 9, with the exceptions of the enzymes from Bacillus subtilis SK 11.004 and Pseudomonas nitroreducens which have an optimum pH of the transfer reaction of 10 and 10.5, respectively [56, 57]. It is interesting to note that, in many cases, the pH optima of the hydrolysis and transfer reactions are different. Therefore, by adjusting the pH of the reaction mixture, it is possible to make the enzyme able to catalyze one of the two reactions selectively.
The optimum temperatures for γ-GT activities range from 37 to 60 °C. Some enzymes are highly stable around 50 °C [57], whereas others are highly sensitive to thermal inactivation [31, 58]. For example, the enzyme from Bacillus pumilus KS is highly thermostable retaining 50 % of the original activity at 70 °C [36], whereas BlGT shows ~40 % of the original catalytic activity at 45 °C [47]. GthGT retains 83 % of activity even after 24 h at 45 °C [49].
Altogether these features could reflect different mechanisms of adaptation of the enzyme to colonize different niches. Otherwise, they could remark different evolutionary relationships in γ-GT family, with extremophilic γ-GTs as the ancient progenitors [52].
Since salt-tolerant γ-GTs may play a potential role in industrial processes that require high-salt conditions, such as in the manufacture of fermented foods, the activity of some γ-GTs has been studied in presence of different salt concentrations. It has been found that very few γ-GTs are halotolerant: examples are limited to BsGT, BlGT and a monomeric 30 kDa γ-GT purified from B. licheniformis, probably generated by proteolytic digestion of the mature BlGT by subtilisin [59–62]. A hypothesis on the structural basis for the salt tolerance of BsGT has also been proposed: the protein possesses strong acid patches on the surface that may allow it to remain in the hydrated state and avoid self-aggregation even under high-salt conditions [44]. This finding is in line with the results of several independent studies which suggest that halophilic enzymes present higher proportion of acid residues on the surface than their non-halophilic homologues [63, 64].
The catalytic assays of many γ-GTs have also been performed in the presence of metal ions. EcGT was reported to be activated by several cations, for example Li+, Rb+, K+, Na+, Cs+, Mg2+, Ca2+, Co2+and Mn2+ [65]. On the contrary, the enzymatic activity of BsGT enhances in the presence of Al3+, Mg2+, K+, Na+, whereas it is inhibited by Cu2+, Fe2+ and Zn2+ [57]. Addition of Co2+ has no effects on the enzymatic activity of BlGT, while Hg2+, Zn2+, Pb2+ and Ni2+ have a inhibitory effect and Mg2+, K+ and Na+ enhance its original activity [47].
Conformational stability of γ-glutamyltranspeptidases
Few reports deal with biophysical characterization of precursors and mature forms of γ-GTs. This is in part due to the fact that it is difficult to purify γ-GTs at homogeneity, since a mixture of the unprocessed and mature protein is often obtained [36, 49, 52]. Unfolding analyses using circular dichroism and tryptophan emission fluorescence have revealed that members of the γ-GT family display different sensitivity towards temperature and guanidinium hydrochloride induced-denaturation [62, 66, 67]. Thermal denaturation of EcGT and BlGT follows a simple irreversible two-state mechanism [62], whereas that of GthGT was described using a three-state model involving the formation of a stable intermediate [67]. T m values of both precursor analogues and mature forms of the studied γ-GTs and of some mutants are reported in Table 3, and the values of denaturant concentration at half-completion of the transition characterizing the chemical-induced denaturation of the same proteins are summarized in Table 4. Among the characterized enzymes, GthGT is the most thermostable, in line with its thermophilic origin, whereas BlGT is the most resistant to the chemical denaturation with guanidinium hydrochloride.
Table 3.
Values of T m1 and T m2 characterizing the thermal-induced denaturation of γ-GTs from different sources
| Mature protein | T m1 (°C) | T m2 (°C) | References |
|---|---|---|---|
| EcGT |
50.1 52.5 |
[62, 66] | |
| S463T-EcGT | 48.9 | [62] | |
| BlGT |
61.4 64.8 |
[38, 62] | |
| Δ(581-585)BlGT | 64.7 | [38] | |
| Δ(577-585)BlGT | 51.0 | [38] | |
| GthGT | 63.2 | 95.4 | [67] |
| Precursor | |||
| S463D-EcGT | 36.1 | [66] | |
| S463 K-EcGT | 38.2 | [66] | |
| T399A-BlGT | 68.1 | [38] | |
| Δ(576-585)BlGT | 45.1 | [38] | |
| Δ(566-585)BlGT | 44.9 | [38] | |
| Δ(558-585)BlGT | 43.9 | [38] | |
| Δ(523-585)BlGT | 32.6 | [38] | |
| Δ(479-585)BlGT | 31.7 | [38] | |
| T353A-GthGT | 66.5 | 88 | [67] |
Table 4.
Values of denaturant concentration at half-completion of the transition characterizing the chemical-induced denaturation of γ-GTs from different sources
| Mature protein | GdnHCl, pH 8.0 C1/2 (M) |
References |
|---|---|---|
| EcGT | 0.8 | [62] |
| BlGT | 2.7–2.8 | [38] |
| Δ(581–585)BlGT | 2.9 | [38] |
| Δ(577–585)BlGT | 1.4 | [38] |
| GthGT | 1.4 | [67] |
| Precursor | ||
| T399A-BlGT | 3.7–3.9 | [62] |
| Δ(576–585)BlGT | 0.4 | [38] |
| Δ(566–585)BlGT | 0.6 | [38] |
| Δ(558–585)BlGT | 0.6 | [38] |
| Δ(523–585)BlGT | 10−6 | [38] |
| Δ(479–585)BlGT | 10−9 | [38] |
| T353A-GthGT | 1.7 | [67] |
In recent years, demands for thermostable enzymes have increased in industrial fields. Thermostability of biocatalyst allows a higher operation temperature, which is clearly advantageous because of a higher reactivity and process yield (increased solubility of substrates and products and favorable equilibrium displacement in endothermic reactions), lower viscosity, and fewer contamination problems. Thus, it is important to purify and characterize thermostable γ-GTs.
Pharmaceutical and biotechnological applications
The biochemical properties of γ-GTs to cleave the unusual peptide bond of GSH and to transfer the γ-glutamyl moiety to some acceptors for producing γ-glutamyl compounds can be exploited in different ways for pharmaceutical and biotechnological interests.
First of all, the increasing synthesis of γ-GT in many human tumors has been correlated with their enhanced resistance to chemotherapy [68]. Therefore, it has been suggested that inhibiting γ-GT prior to chemotherapy or radiation would sensitize γ-GT-positive tumors to treatment [6]. In other words, inhibitors of γ-GT activity could be used prior to the administration of chemotherapy to limit the supply of cysteine to the tumor, thereby blocking the tumor’s ability to maintain high levels of intracellular GSH. Inhibitors of γ-GTs include the glutamine analogues acivicin and azaserine, that compete with the substrate for the γ-glutamyl binding site. However, the glutamine analogues evaluated in clinical trials are too toxic: acivicin, the most potent inhibitor of γ-GT is a neurotoxin [69]. Recently, rational design of γ-GT inhibitors based on studies of the active site has led to the identification of other γ-glutamyl analogues: sulfur derivatives of l-glutamic acid, γ-(monophenyl)phosphono glutamate analogues and a novel class of uncompetitive inhibitors of γ-GT [70], less toxic than the glutamine analogues.
Recently, the exploitation of γ-GT has also been proposed to improve the therapeutic efficacy of selected drugs. As γ-GTs are differently expressed in several tumors, it is possible to speculate on the possibility to design pro-drugs that can be activated by γ-GT expressed over the surface of the γ-GT-positive tumors. Since the γ-glutamyl linkage cannot be cleaved off by normal peptidase in serum, the half lives of such compounds become much longer with γ-glutamylation. Indeed, γ-glutamyl compounds are more soluble and stable in blood respect to the non modified precursors [65]. The goal is to add a γ-glutamyl group at a site making the drug inactive until the γ-GT cleaves it off.
Bacterial γ-GTs display a lot of advantageous industrial properties; they are soluble (periplasmic or extracellular) and non-glycosylated proteins and have a broad substrate specificity for γ-glutamyl acceptors, with respect to the mammalian counterparts. Thus, employing various acceptors, one can synthesize various γ-glutamyl compounds using bacterial γ-GTs. These γ-GTs have been used as catalysts for the synthesis of pharmaceutically important peptides through the transpeptidation reaction [65, 71]. For example, γ-Glu-Trp (SCV-07) and γ-glutamyltaurine were synthesized with EcGT using inexpensive l-glutamine as a γ-glutamyl donor [65, 71]. These γ-glutamyl compounds have been reported to have physiological effects on mammals: for example, SCV-07 and γ-glutamyltaurine have a broad spectrum of immunostimulatory activities against murine tuberculosis [72] and an antagonistic effect against excitatory amino acids [73], respectively. Another example is the production of γ-l-glutamyl-dihydroxyphenylalanine (L-DOPA), a molecule able to increase the concentration of dopamine in the brain, and for this reason a promising pro-drug for Parkinson’s disease [74].
Finally, bacterial γ-GTs can also be employed as glutaminase in food industry [75]. In fact, γ-GT catalyzes the hydrolysis of glutamine to glutamic acid, an important flavor component during wheat fermentation in the manufacture of bread and soy sauce. In particular, BsGT was found resistant to high salt concentrations, used during these processes, and a mutant specialized in hydrolase activity was produced thus avoiding the production of undesired by-products [59]. Recently, γ-GTs isolated from extremophilic sources, such as GthGT, DrGT and TtGT, have been found to naturally display the advantage of lacking transpeptidase activity [49, 52].
Conclusions and perspectives
In the last few years, several γ-GTs from different organisms have been studied. Consequently, our knowledge of γ-GTs at enzymatic and structural level, has increased.
γ-GTs are synthesized as a single polypeptide that undergoes autocatalytic cleavage, which results in the formation of the large and small subunits that comprise the mature enzyme. The two subunits remain associated with each other after the cleavage. The structural features of the precursor and of the catalytically competent protein, which can adopt different oligomeric states, have been elucidated. However, γ-GTs have received scarce attention from investigators when compared to other potentially important industrial enzymes and a number of questions on these proteins remain unanswered.
First of all, the interplay between the small and the large subunits needs to be addressed and further studies on the role of the unprocessed precursor are required to obtain a deeper insight into the properties and functions of these enzymes, which could be also involved in different metabolic pathways.
Future experiments must be performed to elucidate the thermodynamics of folding/unfolding processes and subsequently evaluate the contributions of the small and large subunits and of specific amino acid residues on them. Further thermodynamic data on the separated subunits could also provide useful information on the energetic forces that drive the assembly of the subunits upon the autoprocessing.
Moreover, the catalytic differences in γ-GT activities between bacterial and eukaryal counterparts emphasize the importance of further kinetic and structural studies on these enzymes to determine the mechanisms and the relative importance of the transpeptidation and hydrolysis reactions. In this respect, it is important to remark that the mechanism of transpeptidation still remains unclear. The structural comparison between bacterial mesophilic and extremophilic γ-GTs, the first displaying and the latter lacking transpeptidase activity, could be fundamental to unravel the structural mechanism of such a reaction and the molecular bases of their different behavior.
Finally, while the physiological role of the hydrolysis reaction seems to be universally accepted, the physiological role of transpeptidation remains controversial. In mammals, the transfer reaction catalyzed by γ-GTs out of the cell, that is γ-glutamylation of the amino acids, produced upon the hydrolysis of GSH, seems to be a strategy adopted to favor their re-uptake in the cell. In unicellular organisms, transpeptidase reaction is poor or absent and could play a marginal role. These speculations need further studies to be confirmed.
Contributor Information
Immacolata Castellano, Phone: +39-81-6132293, Phone: +39-81-5833276, FAX: +39-81-6132277, Email: i.castellano@ibp.cnr.it, Email: immacolata.castellano@szn.it.
Antonello Merlino, Phone: +39-81-674276, FAX: +39-81-674090, Email: antonello.merlino@unina.it.
References
- 1.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17(7):1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King JB, West MB, Cook PF, Hanigan MH. A novel, species-specific class of uncompetitive inhibitors of gamma-glutamyl transpeptidase. J Biol Chem. 2009;284(14):9059–9065. doi: 10.1074/jbc.M809608200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad S, Okine L, Wood R, Aljian J, Vistica DT. gamma-Glutamyl transpeptidase (gamma-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol. 1987;131(2):240–246. doi: 10.1002/jcp.1041310214. [DOI] [PubMed] [Google Scholar]
- 4.Ruoso P, Hedley DW. Inhibition of gamma-glutamyl transpeptidase activity decreases intracellular cysteine levels in cervical carcinoma. Cancer Chemother Pharmacol. 2004;54(1):49–56. doi: 10.1007/s00280-004-0776-3. [DOI] [PubMed] [Google Scholar]
- 5.Benlloch M, Ortega A, Ferrer P, Segarra R, Obrador E, Asensi M, Carretero J, Estrela JM. Acceleration of glutathione efflux and inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells to endothelium-induced cytotoxicity. J Biol Chem. 2005;280(8):6950–6959. doi: 10.1074/jbc.M408531200. [DOI] [PubMed] [Google Scholar]
- 6.Mena S, Benlloch M, Ortega A, Carretero J, Obrador E, Asensi M, Petschen I, Brown BD, Estrela JM. Bcl-2 and glutathione depletion sensitizes B16 melanoma to combination therapy and eliminates metastatic disease. Clin Cancer Res. 2007;13(9):2658–2666. doi: 10.1158/1078-0432.CCR-06-2642. [DOI] [PubMed] [Google Scholar]
- 7.Lee CY, Wey SP, Liao MH, Hsu WL, Wu HY, Jan TR. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int Immunopharmacol. 2008;8(5):732–740. doi: 10.1016/j.intimp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 9.Folk JE. Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem. 1969;244(13):3707–3713. [PubMed] [Google Scholar]
- 10.Meister A. On the enzymology of amino acid transport. Science. 1973;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- 11.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71(3):231–238. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30(4):1169–1181. [PubMed] [Google Scholar]
- 13.Zhang H, Forman HJ. Redox regulation of gamma-glutamyl transpeptidase. Am J Respir Cell Mol Biol. 2009;41(5):509–515. doi: 10.1165/rcmb.2009-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betro MG, Oon RC, Edwards JB. Gamma-glutamyl transpeptidase in diseases of the liver and bone. Am J Clin Pathol. 1973;60(5):672–678. doi: 10.1093/ajcp/60.5.672. [DOI] [PubMed] [Google Scholar]
- 15.Betro MG, Oon RC, Edwards JB. Gamma-glutamyl transpeptidase and other liver function tests in myocardial infarction and heart failure. Am J Clin Pathol. 1973;60(5):679–683. doi: 10.1093/ajcp/60.5.679. [DOI] [PubMed] [Google Scholar]
- 16.Turgut O, Yilmaz MB, Yalta K, Tandogan I. Gamma-glutamyltransferase as a useful predictor for cardiovascular risk: clinical and epidemiological perspectives. Atherosclerosis. 2009;202(2):348–349. doi: 10.1016/j.atherosclerosis.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Owen AD, Schapira AH, Jenner P, Marsden CD. Oxidative stress and Parkinson’s disease. Ann N Y Acad Sci. 1996;786:217–223. doi: 10.1111/j.1749-6632.1996.tb39064.x. [DOI] [PubMed] [Google Scholar]
- 18.Paolicchi A, Minotti G, Tonarelli P, Tongiani R, De Cesare D, Mezzetti A, Dominici S, Comporti M, Pompella A. Gamma-glutamyl transpeptidase-dependent iron reduction and LDL oxidation—a potential mechanism in atherosclerosis. J Investig Med. 1999;47(3):151–160. [PubMed] [Google Scholar]
- 19.Marchesini G, Avagnina S, Barantani EG, Ciccarone AM, Corica F, Dall’Aglio E, Dalle Grave R, Morpurgo PS, Tomasi F, Vitacolonna E. Aminotransferase and gamma-glutamyltranspeptidase levels in obesity are associated with insulin resistance and the metabolic syndrome. J Endocrinol Invest. 2005;28(4):333–339. doi: 10.1007/BF03347199. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31(5):1359–1372. doi: 10.1046/j.1365-2958.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 21.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112(14):2078–2080. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- 22.Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7(4):360–366. doi: 10.1016/j.coph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Emdin M, Passino C, Franzini M, Paolicchi A, Pompella A. gamma-glutamyltransferase and pathogenesis of cardiovascular diseases. Future Cardiol. 2007;3(3):263–270. doi: 10.2217/14796678.3.3.263. [DOI] [PubMed] [Google Scholar]
- 24.Mason JE, Starke RD, Van Kirk JE. Gamma-glutamyl transferase: a novel cardiovascular risk biomarker. Prev Cardiol. 2010;13(1):36–41. doi: 10.1111/j.1751-7141.2009.00054.x. [DOI] [PubMed] [Google Scholar]
- 25.Mistry D, Stockley RA. Gamma-glutamyl transferase: the silent partner? Copd. 2010;7(4):285–290. doi: 10.3109/15412555.2010.496819. [DOI] [PubMed] [Google Scholar]
- 26.Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer—a narrative review. Clin Chem Lab Med. 2010;48(2):147–157. doi: 10.1515/cclm.2010.031. [DOI] [PubMed] [Google Scholar]
- 27.Turgut O, Tandogan I. Gamma-glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward. J Atheroscler Thromb. 2011;18(3):177–181. doi: 10.5551/jat.6189. [DOI] [PubMed] [Google Scholar]
- 28.Hanes CS, Hird FJ. Synthesis of peptides in enzymic reactions involving glutathione. Nature. 1950;166(4216):288–292. doi: 10.1038/166288a0. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama R, Kumagai H, Tochikura T. Purification and properties of gamma-glutamyltranspeptidase from Proteus mirabilis . J Bacteriol. 1984;160(1):341–346. doi: 10.1128/jb.160.1.341-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki H, Kumagai H, Tochikura T. gamma-Glutamyltranspeptidase from Escherichia coli K-12: purification and properties. J Bacteriol. 1986;168(3):1325–1331. doi: 10.1128/jb.168.3.1325-1331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa Y, Hosoyama H, Hamano M, Motai H. Purification and properties of gamma-glutamyltranspeptidase from Bacillus subtilis (natto) Agric Biol Chem. 1991;55(12):2971–2977. doi: 10.1271/bbb1961.55.2971. [DOI] [PubMed] [Google Scholar]
- 32.Martin MN, Slovin JP. Purified gamma-glutamyl transpeptidases from tomato exhibit high affinity for glutathione and glutathione S-conjugates. Plant Physiol. 2000;122(4):1417–1426. doi: 10.1104/pp.122.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancaster JE, Shaw ML. Characterization of purified gamma-glutamyl transpeptidase in onions: evidence for in vivo role as peptidase. Phytochemistry. 1994;36:1351–1358. doi: 10.1016/S0031-9422(00)89723-X. [DOI] [Google Scholar]
- 34.Okada T, Suzuki H, Wada K, Kumagai H, Fukuyama K. Crystal structures of gamma-glutamyltranspeptidase from Escherichia coli, a key enzyme in glutathione metabolism, and its reaction intermediate. Proc Natl Acad Sci USA. 2006;103(17):6471–6476. doi: 10.1073/pnas.0511020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boanca G, Sand A, Okada T, Suzuki H, Kumagai H, Fukuyama K, Barycki JJ. Autoprocessing of Helicobacter pylori gamma-glutamyltranspeptidase leads to the formation of a threonine–threonine catalytic dyad. J Biol Chem. 2007;282(1):534–541. doi: 10.1074/jbc.M607694200. [DOI] [PubMed] [Google Scholar]
- 36.Murty NA, Tiwary E, Sharma R, Nair N, Gupta R. gamma-Glutamyl transpeptidase from Bacillus pumilus KS 12: Decoupling autoprocessing from catalysis and molecular characterization of N-terminal region. Enzyme Microb Technol. 2011;50(3):159–164. doi: 10.1016/j.enzmictec.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Williams K, Cullati S, Sand A, Biterova EI, Barycki JJ. Crystal structure of acivicin-inhibited gamma-glutamyltranspeptidase reveals critical roles for its C-terminus in autoprocessing and catalysis. Biochemistry. 2009;48(11):2459–2467. doi: 10.1021/bi8014955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang HP, Liang WC, Lyu RC, Chi MC, Wang TF, Su KL, Hung HC, Lin LL. Effects of C-terminal truncation on autocatalytic processing of Bacillus licheniformis gamma-glutamyl transpeptidase. Biochemistry (Mosc) 2010;75(7):919–929. doi: 10.1134/S0006297910070151. [DOI] [PubMed] [Google Scholar]
- 39.West MB, Wickham S, Quinalty LM, Pavlovicz RE, Li C, Hanigan MH. Autocatalytic cleavage of human gamma-glutamyl transpeptidase is highly dependent on N-glycosylation at asparagine 95. J Biol Chem. 2011;286(33):28876–28888. doi: 10.1074/jbc.M111.248823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinlough CL, Poland PA, Bruns JB, Hughey RP. Gamma-glutamyltranspeptidase: disulfide bridges, propeptide cleavage, and activation in the endoplasmic reticulum. Methods Enzymol. 2005;401:426–449. doi: 10.1016/S0076-6879(05)01026-8. [DOI] [PubMed] [Google Scholar]
- 41.Brannigan JA, Dodson G, Duggleby HJ, Moody PC, Smith JL, Tomchick DR, Murzin AG. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature. 1995;378(6555):416–419. doi: 10.1038/378416a0. [DOI] [PubMed] [Google Scholar]
- 42.Oinonen C, Rouvinen J. Structural comparison of Ntn-hydrolases. Protein Sci. 2000;9(12):2329–2337. doi: 10.1110/ps.9.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada T, Suzuki H, Wada K, Kumagai H, Fukuyama K. Crystal structure of the gamma-glutamyltranspeptidase precursor protein from Escherichia coli. Structural changes upon autocatalytic processing and implications for the maturation mechanism. J Biol Chem. 2007;282(4):2433–2439. doi: 10.1074/jbc.M607490200. [DOI] [PubMed] [Google Scholar]
- 44.Wada K, Irie M, Suzuki H, Fukuyama K. Crystal structure of the halotolerant gamma-glutamyltranspeptidase from Bacillus subtilis in complex with glutamate reveals a unique architecture of the solvent-exposed catalytic pocket. FEBS J. 2010;277(4):1000–1009. doi: 10.1111/j.1742-4658.2009.07543.x. [DOI] [PubMed] [Google Scholar]
- 45.Wada K, Hiratake J, Irie M, Okada T, Yamada C, Kumagai H, Suzuki H, Fukuyama K. Crystal structures of Escherichia coli gamma-glutamyltranspeptidase in complex with azaserine and acivicin: novel mechanistic implication for inhibition by glutamine antagonists. J Mol Biol. 2008;380(2):361–372. doi: 10.1016/j.jmb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Kajimoto Y, Kumagai H. Improvement of the bitter taste of amino acids through the transpeptidation reaction of bacterial gamma-glutamyltranspeptidase. J Agric Food Chem. 2002;50(2):313–318. doi: 10.1021/jf010726u. [DOI] [PubMed] [Google Scholar]
- 47.Lin LL, Chou PR, Hua YW, Hsu WH. Overexpression, one-step purification, and biochemical characterization of a recombinant gamma-glutamyltranspeptidase from Bacillus licheniformis . Appl Microbiol Biotechnol. 2006;73(1):103–112. doi: 10.1007/s00253-006-0440-4. [DOI] [PubMed] [Google Scholar]
- 48.Angele C, Oster T, Visvikis A, Michels JM, Wellman M, Siest G. Different constructs for the expression of mammalian gamma-glutamyltransferase cDNAs in Escherichia coli and in Saccharomyces cerevisiae . Clin Chem. 1991;37(5):662–666. [PubMed] [Google Scholar]
- 49.Castellano I, Merlino A, Rossi M, La Cara F. Biochemical and structural properties of gamma-glutamyl transpeptidase from Geobacillus thermodenitrificans: an enzyme specialized in hydrolase activity. Biochimie. 2010;92(5):464–474. doi: 10.1016/j.biochi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Boanca G, Sand A, Barycki JJ. Uncoupling the enzymatic and autoprocessing activities of Helicobacter pylori gamma-glutamyltranspeptidase. J Biol Chem. 2006;281(28):19029–19037. doi: 10.1074/jbc.M603381200. [DOI] [PubMed] [Google Scholar]
- 51.Lyu RC, Hu HY, Kuo LY, Lo HF, Ong PL, Chang HP, Lin LL. Role of the conserved Thr399 and Thr417 residues of Bacillus licheniformis gamma-Glutamyltranspeptidase as evaluated by mutational analysis. Curr Microbiol. 2009;59(2):101–106. doi: 10.1007/s00284-009-9403-1. [DOI] [PubMed] [Google Scholar]
- 52.Castellano I, Di Salle A, Merlino A, Rossi M, La Cara F. Gene cloning and protein expression of gamma-glutamyltranspeptidases from Thermus thermophilus and Deinococcus radiodurans: comparison of molecular and structural properties with mesophilic counterparts. Extremophiles. 2011;15(2):259–270. doi: 10.1007/s00792-011-0355-6. [DOI] [PubMed] [Google Scholar]
- 53.Orlowski M, Meister A. Gamma-Glutamyl-P-Nitroanilide: a new convenient substrate for determination and study of L- and l-Gamma-glutamyltranspeptidase activities. Biochim Biophys Acta. 1963;73:679–681. doi: 10.1016/0926-6569(63)90197-4. [DOI] [PubMed] [Google Scholar]
- 54.Tate SS, Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/S0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- 55.Storozhenko S, Belles-Boix E, Babiychuk E, Herouart D, Davey MW, Slooten L, Van Montagu M, Inze D, Kushnir S. Gamma-glutamyl transpeptidase in transgenic tobacco plants. Cellular localization, processing, and biochemical properties. Plant Physiol. 2002;128(3):1109–1119. doi: 10.1104/pp.010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imaoka M, Yano S, Okumura M, Hibi T, Wakayama M. Molecular cloning and characterization of gamma-glutamyltranspeptidase from Pseudomonas nitroreducens IFO12694. Biosci Biotechnol Biochem. 2010;74(9):1936–1939. doi: 10.1271/bbb.100199. [DOI] [PubMed] [Google Scholar]
- 57.Shuai Y, Zhang T, Mu W, Jiang B. Purification and characterization of gamma-glutamyltranspeptidase from Bacillus subtilis SK11.004. J Agric Food Chem. 2011;59:6233–6238. doi: 10.1021/jf2003249. [DOI] [PubMed] [Google Scholar]
- 58.Abe K, Ito Y, Ohmachi T, Asada Y. Purification and properties of two isozymes of gamma-glutamyltranspeptidase from Bacillus subtilis TAM-4. Biosci Biotechnol Biochem. 1997;61(10):1621–1625. doi: 10.1271/bbb.61.1621. [DOI] [PubMed] [Google Scholar]
- 59.Minami H, Suzuki H, Kumagai H. A mutant Bacillus subtilis gamma-glutamyltranspeptidase specialized in hydrolysis activity. FEMS Microbiol Lett. 2003;224(2):169–173. doi: 10.1016/S0378-1097(03)00456-7. [DOI] [PubMed] [Google Scholar]
- 60.Tiwary E, Gupta R. Improved catalytic efficiency of a monomeric gamma-glutamyl transpeptidase from Bacillus licheniformis in presence of subtilisin. Biotechnol Lett. 2010;32(8):1137–1141. doi: 10.1007/s10529-010-0271-3. [DOI] [PubMed] [Google Scholar]
- 61.Tiwary E, Gupta R. Subtilisin-gamma-glutamyl transpeptidase: a novel combination as ungual enhancer for prospective topical application. J Pharm Sci. 2010;99(12):4866–4873. doi: 10.1002/jps.22199. [DOI] [PubMed] [Google Scholar]
- 62.Yang JC, Liang WC, Chen YY, Chi MC, Lo HF, Chen HL, Lin LL. Biophysical characterization of Bacillus licheniformis and Escherichia coli gamma-glutamyltranspeptidases: A comparative analysis. Int J Biol Macromol. 2011;48(3):414–422. doi: 10.1016/j.ijbiomac.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Madern D, Ebel C, Zaccai G. Halophilic adaptation of enzymes. Extremophiles. 2000;4(2):91–98. doi: 10.1007/s007920050142. [DOI] [PubMed] [Google Scholar]
- 64.Madern D, Ebel C, Mevarech M, Richard SB, Pfister C, Zaccai G. Insights into the molecular relationships between malate and lactate dehydrogenases: structural and biochemical properties of monomeric and dimeric intermediates of a mutant of tetrameric l-[LDH-like] malate dehydrogenase from the halophilic archaeon Haloarcula marismortui . Biochemistry. 2000;39(5):1001–1010. doi: 10.1021/bi9910023. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki H, Yamada C, Kato K. Gamma-glutamyl compounds and their enzymatic production using bacterial gamma-glutamyltranspeptidase. Amino Acids. 2007;32(3):333–340. doi: 10.1007/s00726-006-0416-9. [DOI] [PubMed] [Google Scholar]
- 66.Hsu WH, Ong PL, Chen SC, Lin LL. Contribution of Ser463 residue to the enzymatic and autoprocessing activities of Escherichia coli gamma-glutamyltranspeptidase. Indian J Biochem Biophys. 2009;46(4):281–288. [PubMed] [Google Scholar]
- 67.Pica A, Russo Krauss I, Castellano I, Rossi M, La Cara F, Graziano G, Sica F, Merlino A. Exploring the unfolding mechanism of γ-glutamyltranspeptidases: the case of the thermophilic enzyme from Geobacillus thermodenitrificans. Biochimica et Biophysica Acta (BBA)-Proteins & Proteomics. 2012;1824(4):571–7. doi: 10.1016/j.bbapap.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 69.Antczak C, Karp DR, London RE, Bauvois B. Reanalysis of the involvement of gamma-glutamyl transpeptidase in the cell activation process. FEBS Lett. 2001;508(2):226–230. doi: 10.1016/S0014-5793(01)03057-5. [DOI] [PubMed] [Google Scholar]
- 70.King JB, West MB, Cook PF, Hanigan MH. A novel, species-specific class of uncompetitive inhibitors of gamma-glutamyl transpeptidase. J Biol Chem. 2009;284:9059–9065. doi: 10.1074/jbc.M809608200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki H, Izuka S, Minami H, Miyakawa N, Ishihara S, Kumagai H. Use of bacterial gamma-glutamyltranspeptidase for enzymatic synthesis of gamma-d-glutamyl compounds. Appl Environ Microbiol. 2003;69(11):6399–6404. doi: 10.1128/AEM.69.11.6399-6404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simbirtsey A, Kolobov A, Zabolotnych N, Pigareva N, Konusova V, Kotov A, Variouchina E, Bokovanov V, Vinogradova T, Vasilieva S, Tuthill C. Biological activity of peptide SCV-07 against murine tuberculosis. Russ J Immunol. 2003;8:11–22. [PubMed] [Google Scholar]
- 73.Jones AW, Smith DA, Watkins JC. Structure-activity relations of dipeptide antagonists of excitatory amino acids. Neuroscience. 1984;13:573–581. doi: 10.1016/0306-4522(84)90250-1. [DOI] [PubMed] [Google Scholar]
- 74.Di Stefano A, Sozio P, Cerasa LS. Antiparkinson prodrugs. Molecules. 2008;13:46–68. doi: 10.3390/molecules13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermeulen N, Gänzle MG, Vogel RF. Glutamine deamidation by cereal-associated lactic acid bacteria. J Appl Microbiol. 2007;103:1197–1205. doi: 10.1111/j.1365-2672.2007.03333.x. [DOI] [PubMed] [Google Scholar]
- 76.Falabella P, Riviello L, Caccialupi P, Rossodivita T, Teresa Valente M, Luisa De Stradis M, Tranfaglia A, Varricchio P, Gigliotti S, Graziani F, Malva C, Pennacchio F. A gamma-glutamyl transpeptidase of Aphidius ervi venom induces apoptosis in the ovaries of host aphids. Insect Biochem Mol Biol. 2007;37(5):453–465. doi: 10.1016/j.ibmb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Ferretti M, Destro T, Tosatto SC, La Rocca N, Rascio N, Masi A. Gamma-glutamyl transferase in the cell wall participates in extracellular glutathione salvage from the root apoplast. New Phytol. 2009;181(1):115–126. doi: 10.1111/j.1469-8137.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- 78.Ohkama-Ohtsu N, Radwan S, Peterson A, Zhao P, Badr AF, Xiang C, Oliver DJ. Characterization of the extracellular gamma-glutamyl transpeptidases, GGT1 and GGT2 Arabidopsis . Plant J. 2007;49(5):865–877. doi: 10.1111/j.1365-313X.2006.03004.x. [DOI] [PubMed] [Google Scholar]
- 79.Destro T, Prasad D, Martignago D, Bernet IL, Trentin AR, Renu IK, Ferretti M, Masi A. Compensatory expression and substrate inducibility of gamma-glutamyl transferase GGT2 isoform in Arabidopsis thaliana . J Exp Bot. 2011;62(2):805–814. doi: 10.1093/jxb/erq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glynn BP, Johnson DB. gamma-Glutamyltransferase from Marthasterias glacialis: purification procedures and enzyme characterisation. Comp Biochem Physiol B. 1985;80(4):941–948. doi: 10.1016/0305-0491(85)90488-2. [DOI] [PubMed] [Google Scholar]
- 81.Hussein AS, Walter RD. Purification and characterization of gamma-glutamyl transpeptidase from Ascaris suum . Mol Biochem Parasitol. 1996;77(1):41–47. doi: 10.1016/0166-6851(96)02573-X. [DOI] [PubMed] [Google Scholar]
- 82.Moriguchi M, Yamada M, Suenaga S, Tanaka H, Wakasugi A, Hatanaka S. Partial purification and properties of gamma-glutamyltranspeptidase from mycelia of Morchella esculenta . Arch Microbiol. 1986;144(1):15–19. doi: 10.1007/BF00454949. [DOI] [PubMed] [Google Scholar]
- 83.Orlowski M, Wilk S. Metabolism of gamma-glutamyl amino acids and peptides in mouse liver and kidney in vivo. Eur J Biochem. 1976;71(2):549–555. doi: 10.1111/j.1432-1033.1976.tb11144.x. [DOI] [PubMed] [Google Scholar]
- 84.Moallic C, Dabonne S, Colas B, Sine JP. Identification and characterization of a gamma-glutamyl transpeptidase from a thermo-alcalophile strain of Bacillus pumilus . Protein J. 2006;25(6):391–397. doi: 10.1007/s10930-006-9025-4. [DOI] [PubMed] [Google Scholar]
- 85.Riou JY, Buissiere J, Richard C, Guibourdenche M. gamma-Glutamyl-transferase activity in the family “Neisseriaceae” (author’s transl) Ann Microbiol (Paris) 1982;133(3):387–392. [PubMed] [Google Scholar]
- 86.Young JD, Ellory JC, Wright PC. Evidence against the participation of the gamma-glutamyltransferase-gamma-glutamylcylclotransferase pathway in amino acid transport by rabbit erythrocytes. Biochem J. 1975;152(3):713–715. doi: 10.1042/bj1520713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang SY, Ryang JH, Lim WJ, Yoo ID, Oishi K. Purification and properties of gamma-glutamyl transpeptidase from Bacillus sp. KUN-17. J Microbiol Biotechnol. 1996;6(4):238–244. [Google Scholar]
- 88.Braun JP, Benard P, Burgat V, Rico AG. Gamma Glutamyl Transferase in domestic animals. Vet Res Commun. 1983;6(2):77–90. doi: 10.1007/BF02214900. [DOI] [PubMed] [Google Scholar]
- 89.Goore MY, Thompson JF. Gamma-glutamyl transpeptidase from kidney bean fruit. I. Purification and mechanism of action. Biochim Biophys Acta. 1967;132:15–26. doi: 10.1016/0005-2744(67)90187-8. [DOI] [PubMed] [Google Scholar]
- 90.Xu K, Strauch MA. Identification, sequence, and expression of the gene encoding gamma-glutamyltranspeptidase in Bacillus subtilis . J Bacteriol. 1996;178(14):4319–4322. doi: 10.1128/jb.178.14.4319-4322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Q, Xu H, Zhang L, Yao J, Ouyang P. Production, purification and properties of γ-glutamyltranspeptidase from a newly isolated. NX-2 Mol Catal B Enzym. 2006;43:113–117. [Google Scholar]
- 92.Nakano Y, Okawa S, Yamauchi T, Koizumi Y, Sekiya J. Purification and properties of soluble and bound gamma-glutamyltransferases from radish cotyledon. Biosci Biotechnol Biochem. 2006;70:369–376. doi: 10.1271/bbb.70.369. [DOI] [PubMed] [Google Scholar]
- 93.Curthoys NP, Hughey RP. Characterization and physiological function of rat renal gamma-glutamyltranspeptidase. Enzyme. 1979;24(6):383–403. doi: 10.1159/000458694. [DOI] [PubMed] [Google Scholar]
- 94.Penninckx MJ, Jaspers CJ. Characterization of gamma-glutamylamidase-glutaminase activity in Saccharomyces cerevisiae . Biochimie. 1985;67(9):999–1006. doi: 10.1016/S0300-9084(85)80294-7. [DOI] [PubMed] [Google Scholar]
- 95.Barnes IH, Bagnall MC, Browning DD, Thompson SA, Manning G, Newell DG. Gamma-glutamyl transpeptidase has a role in the persistent colonization of the avian gut by Campylobacter jejuni . Microb Pathog. 2007;43(5–6):198–207. doi: 10.1016/j.micpath.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HG, Park HJ, Kang HJ, Lim HW, Kim K, Park EH, Ahn K, Lim CJ. The Schizosaccharomyces pombe gene encoding gamma-glutamyl transpeptidase I is regulated by non-fermentable carbon sources and nitrogen starvation. J Microbiol. 2005;43(1):44–48. [PubMed] [Google Scholar]
- 97.Singh SN, Srivastava AK, Chatterjee RK. Gamma-glutamyl transpeptidase activity in adult Setaria cervi and Acanthocheilonema viteae and the effect of inhibitors. J Parasit Dis. 1996;20(2):163–166. [Google Scholar]
- 98.Martin MN, Slovin JP. Purified γ-glutamyl transpeptidases from tomato exhibit high affinity for glutathione and glutathione S-conjugates. Plant Physiol. 2000;122:1417–1426. doi: 10.1104/pp.122.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura Y, Kato H, Suzuki F, Nagata Y. Some properties of gamma-glutamyltransferase from hog small intestine. Biomed Res. 1981;2:509–516. [Google Scholar]
- 100.Chu L, Xu X, Dong Z, Cappelli D, Ebersole JL. Role for recombinant gamma-glutamyltransferase from Treponema denticola in glutathione metabolism. Infect Immun. 2003;71(1):335–342. doi: 10.1128/IAI.71.1.335-342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morrow AL, Williams K, Sand A, Boanca G, Barycki JJ. Characterization of Helicobacter pylori gamma-glutamyltranspeptidase reveals the molecular basis for substrate specificity and a critical role for the tyrosine 433-containing loop in catalysis. Biochemistry. 2007;46(46):13407–13414. doi: 10.1021/bi701599e. [DOI] [PubMed] [Google Scholar]