Abstract
Chemokines are a vertebrate-specific group of small molecules that regulate cell migration and behaviour in diverse contexts. So far, around 50 chemokines have been identified in humans, which bind to 18 different chemokine receptors. These are members of the seven-transmembrane receptor family. Initially, chemokines were identified as modulators of the immune response. Subsequently, they were also shown to regulate cell migration during embryonic development. Here, we discuss the influence of chemokines and their receptors on angiogenesis, or the formation of new blood vessels. We highlight recent advances in our understanding of how chemokine signalling might directly influence endothelial cell migration. We furthermore examine the contributions of chemokine signalling in immune cells during this process. Finally, we explore possible implications for disease settings, such as chronic inflammation and tumour progression.
Keywords: Chemokines, Angiogenesis, Seven-transmembrane receptors, Embryonic development, Immune response, Tumour, Cancer
Introduction
Angiogenesis is commonly defined as the sprouting of new blood vessels from pre-existing ones [1]. It is extensive in the developing embryo, in which the newly forming organs need to be vascularized. Work over the last decade has identified several key players important for proper angiogenesis to occur. Members of the vascular endothelial growth factor (VEGF) family have been shown to control many aspects of endothelial biology, including proliferation, differentiation, migration and survival [2]. Loss of VEGF function in the mouse [3, 4] and in the zebrafish [5] leads to severe vascular defects and reduced angiogenic sprouting. Mutants in components of the Notch signalling pathway also show severe vascular defects [6]. However, these are associated with an increase in angiogenic sprouting. In addition to these genetic pathways, changes in haemodynamics [7] and tissue hypoxia [8] are also important regulators of angiogenesis. In contrast to the embryo, angiogenesis in the adult is limited and is often associated with pathological situations. Accordingly, many disease settings, such as cancer, stroke and diabetes have a central vascular component [9]. Because of this, targeting newly forming blood vessels pharmacologically in order to slow disease progression has received increasing attention and several antiangiogenic strategies have been approved for human treatment. For instance, anti-VEGF therapy has proven beneficial in treating metastatic colorectal cancer [10] and age-related macular degeneration [11]. While many parallels exist between the mechanisms and molecules regulating angiogenesis in the early embryo and the adult, several differences have been elucidated recently. During early stages of blood vessel development, endothelial cells respond to proangiogenic factors present in the surrounding tissue. Later, mobile cells of the immune system appear to play an important role during blood vessel formation. They regulate angiogenesis in diverse settings, such as the embryonic brain, where they mediate fusion events between endothelial tip cells [12]. In pathological situations, such as wound healing [13] and tumour progression [14], immune cells secrete proangiogenic factors that act on the surrounding endothelium. They furthermore control the separation between blood endothelial cells and the forming lymphatic vasculature [15, 16]. Therefore, understanding the signals and molecules that mediate the interaction between endothelial and immune cells would be of great importance for our further understanding of the mechanisms controlling angiogenesis.
One class of molecules that has received increasing attention due to their action on both endothelial cells and cells of the immune system are chemokines (chemotactic cytokines). They were originally identified due to their role in directing cell migration and proliferation during the immune response. So far, 47 members have been described in humans, which can be categorized into four subgroups depending on the presence and position of conserved cysteine residues [17]. Typically, chemokines possess four cysteine residues that mediate the formation of intramolecular disulphide bonds. Members of the CXC group are characterized by the presence of a single amino acid between the first two N-terminal cysteines, while members of the CC class lack this amino acid. CXC chemokines can be further subdivided into ELR− and ELR+ classes, based on the presence of a glu-leu-arg (ELR) amino acid sequence at their N-terminus. The two other subfamilies consist of only one (CX3C) or two (XC) members each. In CX3C chemokines, three amino acids are present between the first two cysteines. The fourth class, XC chemokines, lack cysteines one and three of the typical chemokine structure [17].
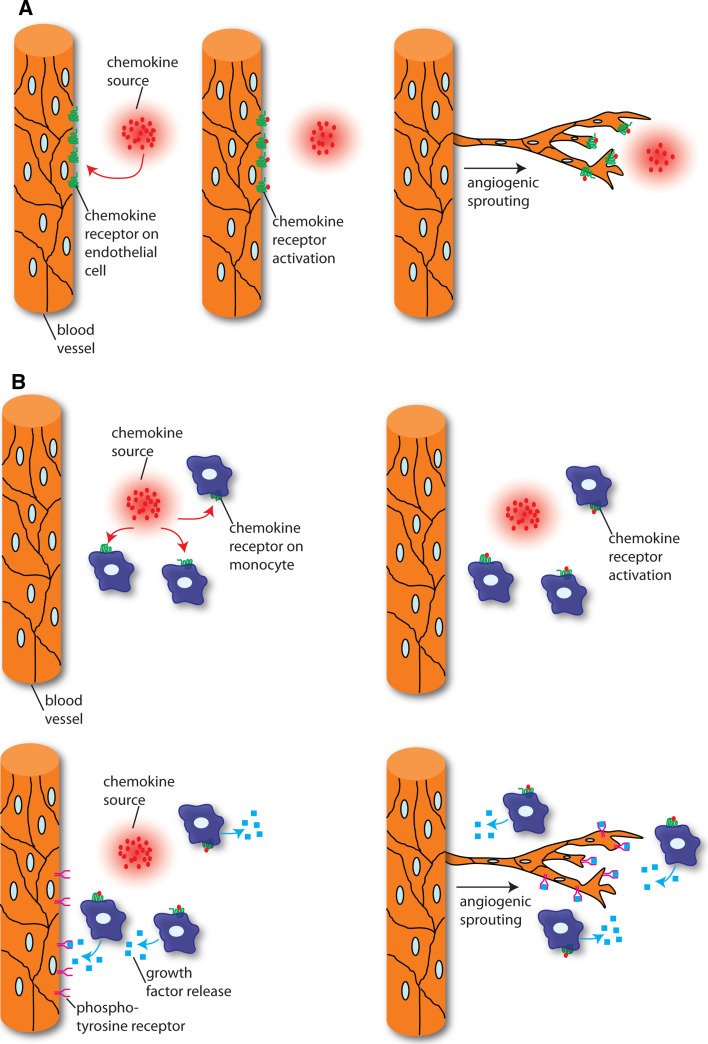
Chemokines bind to chemokine receptors, which belong to the G-protein coupled receptor family. So far, ten CCR family receptors and seven CXCR family receptors in addition to CX3CR1 and XCR1 have been identified. In addition, several decoy receptors exist that bind chemokine ligands without eliciting signal transduction. These include D6, DARC and CCX-CKR [18]. While there is a high degree of promiscuity between ligands and receptors, the biological relevance of this is poorly understood. Differential ligand/receptor interactions might regulate receptor trafficking and thereby signalling outcomes (see below). Upon ligand binding, receptors signal via heterotrimeric G-proteins, leading to the activation of several downstream signalling pathways, such as the MAP (mitogen-activated protein) kinase, RAS and Rho GTPases and phosphoinositide 3-kinase (PI 3-kinase) signalling cascades, which directly influence cell migration and proliferation [19]. In this review, we discuss the different classes of chemokines and illustrate how they influence angiogenesis in several settings, both in the embryo and the adult. We furthermore examine the autonomous effects of chemokines on endothelial cells and compare them with non-autonomous effects elicited via the recruitment of immune cells (Fig. 1). We conclude by exploring the implications of these findings for disease treatment.
Fig. 1.
Endothelial autonomous and non-autonomous roles of chemokines during angiogenesis. a Chemokines act directly on endothelial cells expressing chemokine receptors and thereby influence endothelial migration and angiogenesis. b Chemokines indirectly influence endothelial cell behaviours by attracting chemokine receptor-expressing leucocytes. These subsequently secrete proangiogenic factors, such as VEGF, that act on endothelial cells and initiate angiogenesis
Angiogenic versus angiostatic chemokines
The aforementioned distinction between the CXC chemokines ELR+ and ELR− chemokines is also a functional one (Table 1). All identified ELR+ chemokines mediate angiogenesis [20]. CXCR2 is the main ELR+ chemokine receptor expressed on endothelial cells and shows promiscuity in its ligand binding [21]. In humans, CXCR1 has been identified as a second CXC receptor expressed on endothelial cells and mediating angiogenic responses. It selectively binds to CXCL8/IL-8 and CXCL6/GCP-2 [22]. Blocking either CXCR1 or CXCR2 function in human microvascular dermal endothelial cells strongly affects chemotaxis [23]. This effect is further enhanced by simultaneous application of antibodies blocking both receptors. Further studies on the function of each receptor suggest that CXCR1 affects early steps during chemotaxis, such as the appearance of stress fibres, while signalling through CXCR2 is involved in the later occurring accumulation of cortical actin and cell retraction [24]. Interestingly, human intestinal microvascular endothelial cells exclusively express CXCR2 [25]. Here, CXCR2-neutralizing antibodies similarly affect stress fibre assembly and chemotaxis. These findings suggest tissue specificity in the expression of CXCR1 in different endothelial cell populations. They also argue that CXCR2 is the major ELR+ chemokine receptor mediating angiogenic responses.
Table 1.
Angiogenic and angiostatic chemokines and their receptors
| Systematic nomenclature | Old nomenclature | ELR motif | Receptor | Reference |
|---|---|---|---|---|
| Angiogenic | ||||
| CXCL1 | Gro-α | + | CXCR2 | [206] |
| CXCL2 | Gro-β | + | CXCR2 | [207] |
| CXCL3 | Gro-γ | + | CXCR2 | [208] |
| CXCL5 | ENA-78 | + | CXCR2 | [209] |
| CXCL6 | GCP-2 | + | CXCR1, 2 | [210] |
| CXCL7 | NAP-2 | + | CXCR2 | [211] |
| CXCL8 | Il-8 | + | CXCR1, 2 | [212] |
| CXCL12 | SDF-1 | – | CXCR4, 7 | [80] |
| CCL2 | MCP-1 | N/A | CCR2, 4 | [170] |
| CCL11 | Eotaxin | N/A | CCR3 | [213] |
| CCL16 | HCC-4/LEC | N/A | CCR1 | [166] |
| Angiostatic | ||||
| CXCL4, CXCL4L1 | PF-4, PF-4var | – | CXCR3B | [37, 41] |
| CXCL9 | Mig | – | CXCR3B | [52] |
| CXCL10 | IP-10 | – | CXCR3B | [214] |
| CXCL11 | I-TAC | – | CXCR3B, 7 | [52] |
| CXCL14 | BRAK | – | Unknown | [215] |
N/A non applicable
This is further supported by studies analysing CXCR2 function in mice. Angiogenesis in settings such as the cornea micropocket assay [21] and during wound repair [26] is compromised in CXCR2 knockout mice. When Addison et al. applied Hydron pellets containing the CXCR2 ligand CXCL8/IL-8 in the cornea micropocket assay, they observed a robust angiogenic response [21]. This response was strongly reduced in mice either treated with CXCR2 blocking antibodies or in CXCR2 homozygous mutant mice. The same was true for pellets containing CXCL2/MIP-2. In contrast to this, bFGF-driven angiogenesis was unaffected by loss of CXCR2 function. Thus, CXCL8/IL-8 and CXCL2/MIP-2 stimulate angiogenesis in a CXCR2-dependent manner. During wound healing, CXCR2 is necessary for epithelialization and neovascularization [26]. Loss of CXCR2 affects the recruitment of neutrophils and macrophages. Fewer neutrophils are recruited to the wound, while the number of macrophages is increased. Consistently, the expression profiles for several cytokines, such as interleukin-1beta (IL-1β) are changed. In addition, in vitro experiments using cultured keratinocytes have shown a decreased extent of wound closure after inducing a round wound in confluent cultures lacking CXCR2. These results suggest that CXCR2 function is necessary in different cell populations during wound closure.
It would be of interest to determine if the effect on wound neovascularization is due to an autonomous role of CXCR2 in endothelial cells or due to impaired neutrophil and macrophage recruitment (see Fig. 1). Analysis of endothelial specific CXCR2 knockout mice will help answer this question. Interestingly, CXCR2 knockout mice are outwardly healthy and viable, suggesting no major influence of this chemokine receptor during developmental angiogenesis [27]. This raises the interesting possibility that the mechanisms regulating angiogenesis in the embryo and during pathological conditions in the adult might differ in the set of chemokine receptor/ligands used. However, a detailed examination of embryonic angiogenesis in CXCR2 knockout mice has not been performed so far.
An exception to the rule is the ELR− chemokine CXCL12/SDF-1, which has been shown to induce angiogenesis via binding to its receptors CXCR4 [28] and CXCR7 [29]. It plays important roles during embryonic vascular development and during tumour growth and metastasis (see below). Stimulation of endothelial cells with VEGF leads to the induction of CXCR4 expression, while stimulating human umbilical vein endothelial cells (HUVECs) with TNF-α or IL-1β induces CXCR7 expression [29], suggesting that activation of endothelial cells is necessary for the expression of both CXCL12 receptors. These findings are in line with a recent study showing that in the adult tumour endothelial cells upregulate CXCR7 expression, while normal endothelial cells do not express CXCR7 [30]. Interestingly, while CXCR7 mRNA can be found in cells of many transformed and non-transformed tissues, CXCR7 protein is mainly detected in transformed tissues [29]. This suggests that CXCR7 expression is post-transcriptionally regulated.
The mode of action of both CXCL12 receptors is not clear to date. CXCR4 acts as a canonical chemokine receptor, since CXCL12 binding to CXCR4 induces calcium release and chemotaxis in various cell types, such as neurons [31] and lymphatic cells [29]. CXCL12 binding to CXCR7 does not induce these cellular responses, but leads to an increase in cell survival and cell adhesion [29]. These distinct cellular responses might result from the differential activation of downstream signalling events. Rajagopal et al. recently showed that CXCR7 activation preferentially results in signalling via β-arrestin, and not via canonical G-protein signalling [32]. In addition to the activation of different signalling pathways via CXCR4 and CXCR7, previous studies have suggested that CXCR7 might modulate CXCR4 function by forming heterodimers [33, 34]. Levoye et al. have proposed that in this setting CXCR7 modulates the activation of CXCR4 downstream signalling components, such as different G-proteins. Work in zebrafish has furthermore shown that somatic cells expressing CXCR7 can act as a sink for CXCL12 and thereby shape the CXCL12 gradient [35, 36]. Thus, in addition to directly acting via β-arrestins, CXCR7 can influence CXCL12-CXCR4 signalling by influencing ligand distribution and receptor downstream signalling.
ELR− chemokines are angiostatic and include CXCL4/PF-4, CXCL4L1/PF-4var, CXCL9/Mig, CXCL10/IP-10, CXCL11/I-TAC and CXCL14/BRAK. The first angiostatic chemokine described was CXCL4/PF-4 [37, 38]. CXCL4/PF-4 is, besides β thrombomodulin (CXCL7/β-TG), the second major platelet chemokine [39]. It is stored along with other secretable platelet proteins and notably the proangiogenic chemokines CXCL7/NAP-2 and CXCL12/SDF-1 in α-granules (see [40] for a recent review). CXCL4/PF-4 exists as two non-allelic variants, CXCL4/PF-4 and CXCL4L1/PF-4var, which differ from each other in three amino acids. Studies addressing human microvascular endothelial cell chemotaxis showed that CXCL4L1/PF-4var inhibits both bFGF and CXCL8/IL-8 driven angiogenesis more efficiently than CXCL4/PF-4 [41]. The same is true for bFGF-induced angiogenesis in the rat cornea micropocket assay. The mode of action of CXCL4/PF-4 or CXCL4L1/PF-4var during angiogenesis is not well understood. In addition to influencing chemotaxis, CXCL4/PF-4 has also been shown to inhibit cell cycle progression in endothelial cells [42]. Both effects are mediated in part by the ability of CXCL4/PF-4 to inhibit binding of the two proangiogenic factors VEGF and bFGF to their respective receptors [43–45]. For instance, CXCL4/PF-4 can heterodimerize with bFGF, preventing bFGF homodimerization necessary for receptor binding [45, 46]. Similarly, CXCL4/PF-4 inhibits binding of VEGF165 to VEGF receptors on endothelial cells and to soluble VEGF receptors [44]. In contrast to this, receptor binding of the VEGF121 isoform is not inhibited by CXCL4/PF-4. The VEGF165 and VEGF121 isoforms differ in their ability to bind surface heparan sulphates. While VEGF161 efficiently associates with heparan sulphates and therefore remains bound to the extracellular matrix, VEGF121 does not [47]. This might indicate that CXCL4/PF-4 specifically inhibits VEGF165 receptor binding by interfering with heparan sulphates. However, CXCL4/PF-4 mutants lacking heparin-binding capacity still inhibit angiogenesis [44]. In addition, even though CXCL4/PF-4 does not bind VEGF121, it still inhibits VEGF121-induced endothelial proliferation. These findings argue that CXCL4/PF-4 can inhibit VEGF signalling via a mechanism that does not involve direct binding to either VEGF ligands or receptors.
The main receptor for angiostatic CXC ELR− chemokines is CXCR3. It can bind to CXCL4/PF-4, CXCL9/Mig, CXCL10/IP-10 and CXCL11/I-TAC [48]. It exists in three different splice isoforms, CXCR3A, CXCR3B and CXCR3-alt. While CXCR3A is primarily responsible for the recruitment of lymphocytes [49, 50], CXCR3B is expressed on endothelial cells [23, 51, 52]. CXCR3B mediates the angiostatic effect of CXCL10/IP-10 in human melanoma progression [53]. Of interest, a recent study has provided evidence that CXCR3B does not exist in mice and that CXCL10/IP-10 can inhibit endothelial proliferation independently of CXCR3 function [54]. Together, these studies illustrate that the mode of action of the antiangiogenic chemokine CXCL10/IP-10 is context- and organism-dependent.
Chemokine receptor signalling
The pro- and antiangiogenic functions of the different chemokine classes are probably due to differential downstream signalling events elicited after receptor activation. Chemokine receptors are G-protein-coupled seven-transmembrane-spanning cell surface receptors (GPCRs) that belong to the family of class A rhodopsin-like GPCRs. Reminiscent of the mode of activation reported for receptor tyrosine kinases, GPCRs have been demonstrated to undergo homo- or heterodimerization following ligand engagement (reviewed in [55]). More recently, receptor trafficking has taken centre stage in the analysis of GPCR signal regulation and initiation. Endocytosis via a classic clathrin- and dynamin-dependent pathway (clathrin-coated pits) has been described for a number of chemokine receptors, including CXCR1, CXCR2, CXCR4 and CCR7. Other receptors, including DARC and CCX-CKR, are internalized via a caveolin-dependent pathway and a third group that includes CCR2, 4 and 5 appears to use both routes (reviewed in [56]). Interestingly, different chemokines can cause differential internalization of identical receptors. For instance, the receptor CCR4 is efficiently internalized after CCL22/MDC binding, but only poorly after CCL17/TARC binding [57]. Similarly, interaction of CCL3/MIP-1α and CCL5/RANTES with CCR5 results in rapid internalization, while binding of CCL4/MIP-1β to CCR5 does not [58]. Such differential effects can go along with and perhaps even be caused by differential activation of intracellular effectors, such as G-protein-receptor-coupled kinases (GRKs). This has been demonstrated for the interaction of CCR7 and CCL19/ELC, which in contrast to the interaction of CCR7 and CCL21/SLC, induces strong activation of GRK3 and GRK6 as well as β-arrestin recruitment [59]. For the vast majority of receptors, however, the molecular mechanisms underlying differential ligand-induced effects remain unknown.
As are immunoreceptors, for example, GPCRs are not only internalized following receptor engagement in an agonist-dependent fashion, but are also subject to constitutive uptake and recycling. Different ligands not only have divergent effects on receptor uptake, but they can also regulate the extent to which a receptor is internalized and degraded versus how much receptor is being recycled to the surface. For example, CCL5/RANTES induces efficient internalization of the three receptors CCR1, CCR3 and CCR5. However, while CCR5 is completely recycled to the surface, CCR3 is partly degraded and CCR1 is entirely degraded [60–62]. Thereby, different chemokines fine-tune the amount of receptor present on the surface of a target cell. In addition, ligand-induced GPCR internalization is not only a means to negatively regulate GPCR signalling by removal of active receptor from the surface. Signalling may also occur from endosomes, as internalized receptors continue to transmit signals initiated at the plasma membrane. Or receptors may even generate novel or stronger signals as they assemble “en route” newly formed complexes intracellularly (reviewed in [63, 64]). Taken together, these mechanisms, in addition to the large number of chemokines and receptors and the receptor promiscuity, add a further level of complexity to the regulation of cellular behaviour by chemokines.
Intracellularly, signalling through GPCRs, including chemokine receptors, is tightly coupled to the activation of an associated heterotrimeric G-protein. The heterotrimer of Gα, Gβ and Gγ, in its resting state, binds the guanine nucleotide GDP. Upon receptor occupancy, GDP is released from the Gα subunit, which now binds GTP. The resulting allosteric change causes dissociation of the complex into a βγ dimer and the GTP-bound α monomer. Rapid hydrolysis of GTP is followed by re-association of the heterotrimer and the receptor, functionally causing cessation of receptor signalling. Chemokine receptors preferentially associate with Gαi subunits, which inhibit adenylate cyclase and render chemokine receptors susceptible to pertussis toxin. Recent data, however, suggest that at least signalling through the CXCR4 receptor may also utilize other Gα proteins including Gαq, Gα0 and Gαs [65].
The preeminent function of chemokines is the induction of directed cell migration along a concentration gradient towards the source of the chemokine. Cell migration relies on prior cell polarization, leading to functionally distinct processes happening at the front (leading edge) and the rear (uropod) of the cell. Several classes of molecules are essentially involved in this process, including PI 3-kinases, Rho-family members, integrins, cytoskeletal elements and vesicular transporters. In particular, PI 3-kinases, that are divided into class I and class II enzymes, regulate various aspects of the migratory machinery, including actin reorganization and gradient sensing [66]. Class I PI 3-kinases have a broad substrate specificity and their 3′ phosphorylated products (phosphatidylinositol PtdIns(3)P, PtdIns(3,4)P2 and PtdIns(3,4,5)P2) provide docking sites for lipid binding effector proteins containing pleckstrin homology and phox homology domains (reviewed in [67]). In leucocytes, the p110γ PI 3-kinase isoform is essential for myeloid chemotaxis [68], while it is dispensable for T-cell chemotaxis [69, 70], indicating a high degree of cell-type specificity. Class I PI 3-kinase activity is counteracted by the lipid phosphatases PTEN and SHIP. In neutrophils, the PI 3-kinase product PtdIns(3,4,5)P3 localizes to the leading edge, because in response to a chemokine gradient, PI 3-kinases concentrate at the leading edge, while PTEN is localized to the side and rear of the cell [71]. Class II PI 3-kinases are associated with membranes and nuclei. Chemokines efficiently stimulate class II PI 3-kinases, however, their activation mechanism and biological functions are less well investigated.
An important outcome of PI 3-kinase activation is the activation of protein kinase B (PKB/AKT), which shows antiapoptotic activity and fosters survival in many cell types, including cancer cells [72]. In addition, chemokines can cause the upregulation of genes associated with cell survival [73]. This is of particular relevance for protumorigenic chemokines.
Chemokines in embryonic angiogenesis
The earliest chemokine/receptor pair expressed during embryogenesis is CXCL12/SDF-1 and CXCR4, which starts to be expressed at around E7.5 in the mouse. Expression domains include embryonic and extraembryonic mesodermal and endodermal tissues [74]. Later, between E10.5 and E12.5, neural as well as cardiac and endothelial cells express CXCR4. Other chemokine receptors start to be expressed at around E12.5, at the onset of definitive haematopoiesis. In the zebrafish, two CXCR4 homologues are present, both of which are maternally provided [75]. Of these, only CXCR4A has been shown to be expressed in endothelial cells [75, 76]. These expression data from the mouse and the zebrafish suggest that CXCR4 is the only chemokine receptor acting autonomously in endothelial cells during early embryonic development. In line with this, mice deficient in either CXCL12/SDF-1 or CXCR4 show defects during embryonic blood vessel development [77]. In particular, vascularization of the gastrointestinal tract is affected, suggesting that CXCR4 plays a role during organ-specific blood vessel formation. An identical vascular phenotype has been observed in an endothelial specific knockout of CXCR4 [78]. The observed defects in the gastrointestinal tract vasculature were shown to be due to the absence of small interconnecting blood vessels between the superior mesenteric artery and the venous network. Recently, Takabatake et al. also found defects in the renal vasculature in mice mutant for CXCR4 [79]. Here, glomeruli in CXCR4 knockout mice exhibited aneurismal dilation of the capillaries, leading to a loss of the complex capillary tuft patterning. Work in the zebrafish has further revealed a function of CXCR4A during the formation of the first major artery in the body, the lateral dorsal aorta [76]. In this setting, only the anterior, bilateral aortae are affected in mutant embryos, while development of the single aorta in the tail proceeds normally. Using time-lapse microscopy, the authors also showed that endothelial migration per se is not affected in mutant zebrafish, but that mutant endothelial cells now migrate into ectopic locations within the embryo. Therefore, CXCR4 signalling appears to be crucial for the proper pathfinding of endothelial cells and not for their motility per se. Of interest, despite also being expressed in the forming intersomitic blood vessels in zebrafish [76] and mice [74], these vessels form normally in mutant embryos [76], suggesting the presence of compensatory mechanisms in these vascular beds. Together, these findings underscore the organ- and region-specific function of CXCR4 in the endothelium.
In addition to the specific defects in organ vascularization, CXCR4 and CXCL12 mutants also show defects in heart development, including ventricular septal defects [77, 80]. Similar defects in heart development have also been reported for mutants in CXCR7 [34, 81]. Of interest, CXCR7 is strongly expressed in cardiac microvessels. An endothelial specific knockout reproduced the observed cardiac defects [34], suggesting that CXCR7 function is specifically needed in endothelial cells during heart development. In contrast to these similarities between the knockout phenotypes for CXCR4, CXCL12 and CXCR7, both studies on CXCR7 failed to detect defects in organ-specific vascularization. Therefore, CXCR7 function in the endothelium might be more crucial for heart development than it is for other organs. Further research is needed to clarify the precise cardiac vascular phenotype of CXCR7 mutants and the contribution of the vasculature to the observed heart defects. Knockdown of CXCR7 in zebrafish embryos by injection of morpholino (MO) antisense oligonucleotides also causes heart defects [30]. The authors also observed defects in the sprouting of intersegmental blood vessels in the trunk of MO injected embryos, suggesting that in the zebrafish CXCR7 might play a role during angiogenesis. However, the authors monitored intersegmental blood vessel formation by performing angiography with fluorescent dextrans. Since CXCR7 MO-injected embryos display a strong cardiac defect, distribution of the fluorescent dye might be compromised and not fill intersegmental vessels properly. As discussed previously, CXCR4 mutant embryos form intersegmental blood vessels normally [76]. If CXCR7 and CXCR4 play independent roles in intersegmental blood vessel formation remains an interesting question for future research.
About half of the mouse mutants for CXCR4 or CXCL12/SDF1 die at around E18.5 and neonates die within 1 h [77, 80]. In zebrafish, about half of the CXCR4A mutants die during embryogenesis, while the rest of the homozygous mutants survive to adulthood and are fertile [76]. This is in contrast to mutants in other signalling pathways implicated in vascular development, such as VEGF or Notch, which die at earlier developmental stages. Mice mutant for VEGF-A show vascular abnormalities starting at E8.5 and die at E10.5 [3]. Mutants in Notch pathway components die at comparable stages [82]. This indicates that mutants in CXCR4 and CXCL12/SDF1 might either affect later aspects of endothelial development or coordinate the fine-tuning of the developing vasculature. In addition, compensatory mechanisms might be in place to balance out the defects in endothelial cell migration observed in CXCR4 and CXCL12/SDF1 mutants. So far, early embryonic vascular defects in chemokine mutant mice or zebrafish have only been described for CXCL12/SDF1. In addition, defects have only been reported in embryonic blood vessels expressing the CXCL12/SDF1 receptor CXCR4, arguing for an autonomous role of chemokine signalling during early embryonic blood vessel formation.
A recent report has highlighted the potential role of macrophages during the anastomosis of angiogenic tip cells in mouse and zebrafish embryos [12]. Mice lacking tissue macrophages display a reduction in the number of fusion events between neighbouring tip cells, leading to a reduction of intersections between brain blood vessels. These findings highlight the potential role of myeloid cells in regulating embryonic angiogenesis. Although a role for chemokine signalling has not been reported, it is tempting to speculate that macrophages might be recruited to sites of anastomosis via chemokine signalling. Of interest, Strasser et al. reported CXCR4 expression in the tips of sprouting retinal blood vessels, while CXCL12/SDF1 expression was restricted to endothelial cells of the larger vessels and to the underlying astrocyte layer [83]. When the authors blocked CXCR4 signalling in the retina, they observed an absence of interconnections between endothelial cell sprouts, similar to what was observed in macrophage-deficient retinae [84]. Thus, CXCL12/SDF1 could serve a double role by guiding the migration of endothelial tip cells and via recruitment of accessory cells to facilitate anastomosis. Further experiments are required to address this relationship.
Regulation of CXCR4 and CXCL12/SDF1 expression
Several signalling pathways which are important for regulating angiogenesis have been shown to influence CXCR4 expression. The Notch signalling pathway is necessary to control endothelial responses to the proangiogenic factor VEGF. In this setting, Notch acts to downregulate the expression of VEGF receptors and thereby attenuates angiogenic sprouting [85]. In sprouting blood vessels, tip cells are characterized by low Notch signalling pathway activation corresponding with increased angiogenic behaviour, while stalk cells with activated Notch signalling show decreased angiogenic responses [86]. Williams et al. found that activating the Notch signalling pathway by expressing the Notch ligand dll4 negatively influences CXCR4A expression and thereby inhibits migration of endothelial cells towards CXCL12/SDF1 [87]. These observations are in agreement with the reported expression of CXCR4 in endothelial tip cells in the mouse retina [83]. Therefore, Notch signalling might negatively influence angiogenic sprouting by simultaneously downregulating receptor expression of two promigratory pathways. Curiously, in addition to tip cells, expression of CXCR4 is highest in arterial endothelial cells [76, 78, 83]. This is hard to reconcile with the reported high activation of Notch signalling in arterial endothelial cells and the requirement of Notch signalling to induce arterial gene expression [82]. Therefore, additional mechanisms must exist in arteries that allow the expression of CXCR4 in the presence of high Notch signalling.
Importantly, CXCR4 has also been shown to be responsive to changes in blood flow dynamics. Packham et al. showed by gene expression profiling that CXCR4A mRNA becomes downregulated after the onset of blood flow, suggesting that blood flow negatively regulates CXCR4A expression [88]. Accordingly, blocking blood flow in zebrafish embryos leads to an upregulation of CXCR4A expression in the vasculature. The same is true for embryos mutant for gridlock (hey-2), in which the distal artery is occluded. Importantly, this upregulation of CXCR4A expression is only observed in arterial endothelial cells. Similar results have been obtained in HUVECs. Exposing HUVECs to high laminar shear stress leads to a significant downregulation of CXCR4A expression [89]. Whether shear stress and Notch independently regulate CXCR4A expression remains an important unanswered question.
Chemokines during lymphatic development
In addition to their effects on blood endothelial cells, chemokines also influence the formation of the lymphatic system. Lymph endothelial cells differentiate in the mouse (at embryonic day 9.5) and human fetuses (around 6–7 weeks) from venous endothelium after the primitive cardiovascular system is formed and circulation has started [90]. The first lymphatic progenitors are detected in the anterior cardinal vein and are characterized by expression of the homeobox transcription factor PROX1 [91]. Soon after their appearance, PROX1-positive cells bud from the vein and migrate away in a polarized fashion to form the primary lymph sacs. This migration is largely directed by a gradient of VEGF-C [92]. However, additional locally provided spatial cues that govern the positioning of the primary vascular plexus in mammals have yet to be defined. In the zebrafish, arteries have recently been reported to provide indispensable guidance functions for lymphatic endothelial cells [93].
In the mouse, platelets are involved in the process of initial separation of the blood and lymphatic circulation [94–98]. While it is clear that recognition of the endothelial surface glycoprotein podoplanin (PDPN) by the platelet c-type lectin receptor 2 (CLEC-2) is essential for this process, the exact action of platelets remains to be elucidated. Genetic mouse mutants in PDPN, CLEC-2 or its downstream signalling components Syk, SLP-76 and PLC-γ suffer from defects in separation of the blood and lymphatic circulation [94, 96, 99]. In addition, Syk-deficient mice have been demonstrated to develop shunts between the otherwise strictly separated peripheral blood and lymphatic vessels [15]. Such illegitimate connections are the consequence of a strong lymphatic hyperplasia that is caused by a CCL2/MCP-1-driven accumulation of highly prolymphangiogenic macrophages in the skin of Syk-deficient mice. At sites of intimate contact between blood and lymphatic vessels, peripheral shunt formation between previously separated vessels is frequently observed [15]. The inappropriate expression of the inflammatory chemokine CCL2/MCP-1 in Syk-deficient monocytes may be a consequence of a lack of inhibitory environmental signals that are mediated by receptors, which signal via immune receptor tyrosine-based activation motifs [100].
Once the lymphatic sacs are established in the fetus, a primary lymphatic plexus that is subject to intense remodelling forms by sprouting lymphangiogenesis [101]. Cells expressing CD45, CD4, IL-7 receptor α (IL-7Rα), but not CD3, which have not undergone T-cell receptor (TCR) rearrangement and hence do not express a TCR, appear at the site of future lymph node formation. These lymphoid tissue inducer cells (LTIs) are probably of haematopoietic origin and share the expression of many surface proteins found on natural killer cells [102]. They differentiate from CD45pos, CD4pos and CD3neg precursor cells in response to tumour necrosis factor-related activation-induced cytokine (TRANCE) in a process that is absolutely dependent on the retinoid-related orphan receptor (ROR) γt [103, 104]. Mice deficient for both CXCL13/BCA-1 and the IL-7Rα lack the development of all lymph nodes. CXCL13/BCA-1 has been demonstrated to be induced by retinoic acid and neuronal stimulation and to be essential for lymph node initiation [105]. Furthermore, CCL21/SLC and CCL19/ELC have been shown to function in the development of facial, cervical, brachial, and axillary lymph nodes [106].
Therefore, CXCL13/BCA-1 and CCR7 ligands, in cooperation with IL-7Rα, likely promote the recruitment and accumulation of LTIs to the sites of emerging lymph nodes. This recruitment is mostly driven via the chemokine receptor CXCR5, which is present on LTIs. In the absence of CXCR5, LTI cells in mesenteric lymph nodes lack activation of β1 integrins, suggesting that CXCL13/BCA-1 signalling is crucial for the initiation of tight interactions with stromal cells [107]. Further lymph node development is coordinated by a set of complex interactions between LTIs and local stromal cells also referred to as organizer cells [108]. Signals through the IL-7R and TRANCE receptor on LTIs cause high level expression of the surface lymphotoxin (LT) α1β2, which engages the LTβ receptor (LTβR) on adjacent stromal cells [109]. Stromal cell activation through LTβR ligation causes a profound upregulation of the adhesion molecules ICAM-1 and VCAM-1 [110]. ICAM-1 and VCAM-1 on stromal cells interact tightly with the integrin α4β1 expressed on LTIs (reviewed in [111]). In addition, activation of stromal cells through the LTβR results in subsequent expression of the lymphoid chemokines CXCL13/BCA-1, CCL21/SLC and CCL19/ELC [112, 113]. This supports the recruitment of further LTIs to the site of the emerging secondary lymphatic organ and initiates the segregation of T and B cell areas [114]. Absence of CCL21/SLC and CCL19/ELC in mice deficient for CXCL13/BCA-1, results in failure to form any peripheral lymph nodes, while mesenteric lymph nodes can still form [106]. Related defects were detected in the absence of CXCR5 and CCR7, suggesting a highly cooperative function of the chemokines CXCL13/BCA-1, CCL19/ELC and CCL21/SLC during lymph node formation [115]. Similar mechanisms involving CXCL13/BCA-1 and its receptor CXCR5 are active during the formation of tertiary lymphoid organs at sites of chronic inflammation [116].
Chemokines in tumour angiogenesis and metastatic spread
The capacity to usurp and abuse normal cellular control mechanisms is a hallmark of malignantly transformed cells. Given the central role of chemokines in the regulation of tissue homeostasis and cell migration, it does not come as a big surprise that chemokines play essential roles during tumour progression and metastasis. Important steps in tumour development include the so-called “angiogenic switch” that describes the transition from a dormant hyperplasia to a vascularized, growing tumour [117] and the metastatic spread to distant sites. Several modes of action of chemokine subversion leading to tumour progression have been defined. Here we discuss four aspects: (1) tumours may express chemokines that attract leucocytes which are converted by the tumour environment into proangiogenic mediators. This is probably the most relevant action of chemokines in tumour angiogenesis; (2) tumour cells may produce chemokines that act directly on endothelial cells to stimulate angiogenesis; (3) tumour cells may produce chemokines that act in a paracrine fashion and thereby foster tumour growth and progression ultimately leading to an angiogenic outcome; and (4) tumour cells may express receptors for homeostatic chemokines that enable them to spread via access to the tumour vasculature.
Tumours may express chemokines that attract leucocytes
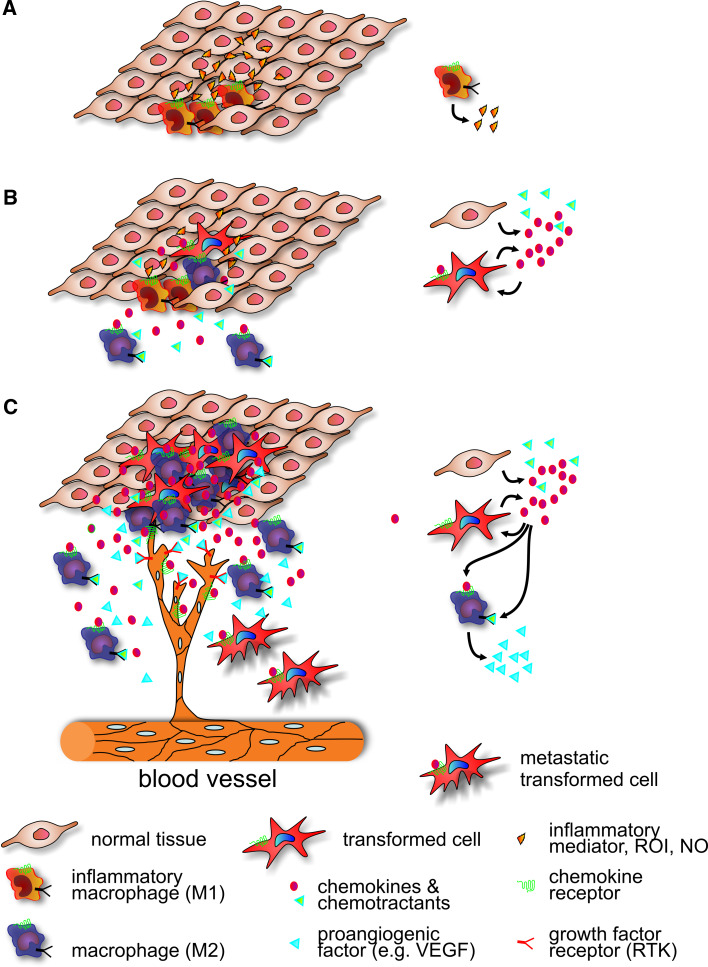
Tumours can utilize inflammatory chemokines to attract leucocytes and control the extent and nature of the infiltrate. It has long been appreciated that chronic inflammation is a predisposing risk factor to cancer [118]. Microbial infections, such as Helicobacter pylori infection, are associated with gastric cancer and mucosal lymphoma, while autoimmune disease of the digestive tract can cause colitis-associated cancer. Indeed, the presence of smouldering inflammation in the tumour environment has been considered the seventh hallmark of cancer [119] and tumour-associated macrophages (TAMs) constitute a major fraction of the leucocyte infiltrate (Fig. 2a). Production of CCL5/RANTES by mammary carcinoma cells correlates with macrophage infiltration and lymph node metastasis [120]. Similarly CCL2/MCP-1 expression in oesophageal carcinoma fosters macrophage infiltration, and tumour angiogenesis and spread [121]. Blockade of CCL2/MCP-1, on the other hand, reduces chronic colitis-associated carcinogenesis in mice [122]. Interestingly, the finding that the D6 decoy and scavenger receptor for inflammatory cytokines exerts a protective role against colon and skin cancer in mice provides direct genetic evidence for a protumorigenic action of inflammatory leucocytes [123–125]. Besides chemokines, other factors including colony-stimulating factor-1 (reviewed in [126]) and VEGF-A (reviewed in [127]) contribute to the recruitment of TAMs into tumours (Fig. 2).
Fig. 2.
The role of chemokine signalling during tumour formation. a Immune evasion. Chronic local inflammation caused by irritants or pathogens is accompanied by persistent leucocyte infiltration. Activated inflammatory macrophages secrete abundant growth factors and produce reactive oxygen and nitrogen species, which generate a mutagenic environment that ultimately initiates transforming events. b Angiogenesis. The forming hyperplastic tissue is a source of chemoattractants, including CCL2/MCP-1 and CSF-1, which recruit circulating monocytes. As these monocytes colonize the tumour, they are instructed by the tumour environment to differentiate into M2 type TAMs. Tumour cells may also express chemokine receptors and respond to autocrine and paracrine stimulation. c Tissue destruction/remodelling. Tumour growth is associated with accumulation of M2-type TAMs, which not only produce additional chemoattractants, but also actively suppress inflammatory and cytotoxic responses to the tumour. TAMs secrete proangiogenic factors that in addition to proangiogenic chemokines support tumour vascularization. TAMs also provide proteolytic activities that help the tumour to overcome constraining tissue borders and enhance metastasis. Throughout the entire development, new macrophages are recruited and subverted to serve the major objectives of the developing tumour. The major chemoattractant/growth factor producing cell types are highlighted on the right
The overwhelming majority of clinical studies have shown protumorigenic effects associated with increased macrophage tumour infiltration, but there is also evidence for tumoricidal activity of TAMs [128]. The disparate activities of TAMs raises the necessity to characterize functionally different subsets of this heterogeneous population. Macrophages are a highly diverse class of mononuclear phagocytic cell and a successful classification refers to their mode of activation and immunological response capacity (reviewed in [127, 129]). Classically activated macrophages respond to type 1 helper cell (Th1) stimulation, show strong proinflammatory activity, cause profound tissue destruction and are characterized by expression of the proinflammatory marker myeloperoxidase, high IL-1, IL-12 and IL-23 levels and the generation of toxic intermediates (NO, reactive oxygen species). This M1 phenotype is also characterized by profound NFκB-driven responses and the expression of opsonic surface receptors and inflammatory mediators such as TNFα and the chemokine CCL2/MCP-1. At the opposite side of the spectrum, there are alternatively activated or M2 macrophages that differentiate in response to IL-4 and IL-13 during Th2-driven responses and are low in IL-12 production, but high in IL-10. Alternatively activated macrophages are rich in angiogenic mediators such as VEGF and the cyclooxigenase-2-derived prostaglandin E2. The non-inflammatory M2 phenotype is characterized by the presence of non-opsonic surface receptors and high metalloprotease expression. Biologically, M2 macrophages are believed to induce tissue morphogenesis and wound healing, show proangiogenic activity and support high cell turnover. M2 macrophages are probably similar to tissue-resident macrophages found in the absence of inflammation or the trophic macrophages involved in developmental processes.
From their gene expression profile and biological activity, M2 macrophages would be well equipped to support tumour growth, progression and expansion into the surrounding tissue, and indeed TAMs associated with actively growing tumours nearly exclusively appear to be polarized towards the M2 phenotype [130]. Tumour cell products such as CSF-1, IL-10 and extracellular matrix compounds not only activate macrophages, but also induce differentiation towards the M2 phenotype [131]. In human lung cancer, cancer-derived versican, by macrophage activation through TLR2 and its co-receptors TLR6 and CD14 and induction of TNFα secretion, strongly enhances metastatic growth [132]. But also the chemokines CCL17/TARC and CCL22/MDC that bind the receptor CCR4 have been shown to promote M2 polarization [127]. The inflammatory chemokine CCL2/MCP-1 not only attracts tumour-promoting macrophages to carcinomas, but also promotes survival and surprisingly M2 polarization of this cell type [133]. M2 macrophages express not only low levels of opsonic surface receptors, but also major histocompatibility class II proteins, resulting in reduced antimicrobial and tumoricidal activity [134]. At least a fraction of TAMs express the receptor tyrosine kinase Tie2 [135]. These Tie2-expressing, tumour-infiltrating monocytes (TEMs), which appear particularly closely related to Tie2-expressing embryonic/fetal macrophages and tissue resident monocytes, share many properties with M2-polarized macrophages and may actually represent largely overlapping populations. While the exact relationship between the two populations remains to be determined, TEMs are also excellently equipped to execute physiological, proangiogenic and tissue-remodelling programs and their profound capacity to change tissue architecture has apparently been co-opted by tumours. Therefore, in the tumour microenvironment, chemokines influence angiogenesis by acting on non-endothelial cell populations, mainly TEMs.
Although the M1/M2 paradigm has been very successfully applied to TAMs, the M1 and M2 phenotypes represent extreme polarizations of macrophage identity [127]. TAMs are a highly heterogeneous cell population that is likely to contain cells of various stages between the M1/M2 extremes. Macrophages are extremely plastic and are able to adapt their responses to a multitude of microenvironmental conditions [136]. Consequently, their role in cancer development is complex and can even be ambivalent. During tumour initiation, the proinflammatory properties of M1 macrophages support a mutagenic and tumour growth-promoting environment (Fig. 2a). Subsequent polarization towards the M2 phenotype during tumour progression results in synergistic interactions between tumour cells and TAMs (Fig. 2b, c). While most TAM populations apparently have been subverted to support tumour growth [137], from regressing tumours, TAMs can be isolated that display M1 polarization, possess the hallmarks of inflammatory macrophages and are probably tumoricidal.
Similar populations probably exist in human tumours, in which expression of CCL2/MCP-1 by pancreatic carcinomas positively correlates with macrophage infiltration. Patients with high circulating levels of CCL2 have a significantly higher survival rate than those with low CCL2 levels and interestingly a direct effect of CCL2 on tumour proliferation and apoptosis has been excluded [138]. Certainly, M2-polarized TAMs, which display defective NFκB activation in response to proinflammatory signals [139], are an attractive target for tumour therapy. It has been speculated that dynamic changes of the tumour microenvironment drive a switch of associated TAMs from an initial M1 state to the M2 state, which is reflected by the inhibition of NFκB responses in infiltrating myeloid and lymphoid cells during advanced tumour stages [140]. M2-polarized TAMs show a massive nuclear accumulation of homodimers of the inhibitory NFκB family member p50, which appears to be responsible for the maintenance of the unresponsive phenotype [141, 142]. This raises the question as to whether it is possible to convert TAMs during tumour development from a pro- to an antitumorigenic action. The first results obtained by blockade of the NFκB pathway in a mouse model of ovarian cancer are promising [143]. In this model, either genetic deletion or inhibition of the NFκB-activating kinase IKKβ in TAMs results in the loss of their IL-10high/IL-12low status, which is a sign of the loss of M2 polarization. Increased expression of IL-12 and NOS-2 indicates a repolarization towards the M1 phenotype that goes along with a significantly enhanced tumoricidal activity. While reprogramming of M2-polarized TAMs and reactivation of associated innate immune functions might be the ultimate therapeutic goal, the intrinsic protumorigenic function associated with TAMs in many human cancers has led to approaches that rather aim at blocking their recruitment altogether. Strategies that have shown promising results include the targeting of chemokines and CSF-1 or the direct inhibition of TAMs [144, 145].
While the majority of studies on myeloid cells in tumour development have focused on TAMs, in patients with melanoma or colon carcinoma, CD15+ IL4 receptor-expressing polymorphonuclear neutrophils also functionally suppress the antitumour immune response [146]. In patients with advanced renal cell cancer or pancreatic cancer, granulocytes appear to dominate the tumour-infiltrating myeloid population [147]. Following the M1/M2 paradigm, the existence of N1 and N2 polarized tumour-associated neutrophils (TANs) has been proposed [148]. Depletion of N2 TANs from untreated tumours increases CD8+ T cell activity, suggesting that the tumour environment converts neutrophils into immunosuppressive cells. After pretreatment of tumours by TGF-β blockade, neutrophil depletion rather results in a decreased activation status of intratumoral CD8+ T cells, suggesting that the removed N1 TANs are predominantly immunostimulatory. Proinflammatory N1 TANs promote CD8+ T-cell recruitment by producing CCL3/MIP-1α, CXCL9/MIG and CXCL10/IP10 [149], while N2 TANs lack high levels of proinflammatory agents, but produce large amounts of arginase 1 that probably serves to inactivate T-cell effector functions [150].
Under certain conditions inflammation is protective against tumour development and it certainly contributes to the efficacy of chemotherapy [151]. Antigens from dying tumour cells are probably presented by inflammatory macrophages and dendritic cells (DCs) resulting in inflammasome activation, IL-1β production and the generation of protective immunity [152]. In breast carcinoma, mature DCs are restricted to peritumoral areas, while immature DCs are preferentially found in the tumour bed, where expression of CCL20/MIP-3α is high. Maturation and activation of these DC populations might be a route to positively influence the clinical outcome.
Tumour cells may produce chemokines that act directly on endothelial cells
A more direct mechanism by which tumour cells foster angiogenesis and thereby support their own growth and survival is the production of proangiogenic chemokines by the tumour that can act on endothelial cells. Candidate receptors on endothelial cells are the CXCL8/IL-8 receptors CXCR1 and CXCR2. These appear to be differentially regulated in different endothelial cell types. While HUVECs apparently do not express CXCR1, 2 and 3, human microvascular dermal endothelial cells (HMECs) consistently express high levels of CXCR1 and CXCR4 and lower levels of CXCR2 and CXCR3 [23]. Increased expression of the ELR+ CXC chemokine CXCL8/IL-8 has been reported in various tumour settings including aggressive ovarian cancer [153]. Upon intraperitoneal transplantation of human ovarian carcinoma cells into nude mice, CXCL8 levels produced by the tumour directly correlate with the extent of neoangiogenesis and inversely correlate with survival [154]. Non-small-cell lung cancer cell (NSCLC) lines that express elevated levels of CXCL8/IL-8 display increased angiogenic activity in mice [155] while inhibition of CXCL8/IL-8 attenuated NSCLC development [156, 157]. Besides CXCL8/IL-8, CXCL5/ENA-78 which also engages the CXCR2 receptor is upregulated in NSCLC, and tissue levels directly correlate with the extent of tumour angiogenesis and even clinical outcome [158]. It should be pointed out, that in patients with NSCLC high CXCL8/IL-8 levels are also associated with extensive TAM infiltration. Also in prostate cancer, upregulation of ELR+ CXC chemokines results in angiogenesis-mediated tumour progression [159]. Here suppression of ELR+ CXC chemokine production by the tumour inhibited prostate cancer growth in SCID mice, which was largely attributed to inhibition of tumour angiogenesis [160]. Similar to the D6 decoy receptor, absence of the Duffy receptor for chemokines (DARC) in a mouse model for prostate cancer results in larger more aggressive tumours with higher vascularization [161]. Finally, elevated levels of CXCR2 ligands have been found in renal cell carcinoma biopsies, whereas CXCR2 has been found to be expressed on endothelial cells within the tumours. Again, elevated plasma levels of the CXCR2 ligands CXCL1/Groα, CXCL3/Groγ, CXCL5/ENA-78, and CXCL8/IL-8 correlate with a high metastatic spread in patients [162].
In addition to CXC chemokines, the CC chemokines CCL2/MCP-1, CCL11/eotaxin and CCL16/LEC have also been implicated in direct stimulation of angiogenesis (reviewed in [163]) based on the expression of CCR1 and CCR2 in endothelial cells [164–166]. CCL-2/MCP-1 has also been found to be upregulated in human breast cancer, where it is associated with neovascularization and survival [167, 168]. CCL2/MCP-1 is highly efficient in provoking neovascularization [169–171]. A recent study of the cytokine expression profile of breast cancer samples found an inverse correlation between oestrogen receptor status and the expression of a large number of cytokines [172]. In particular, CCL2/MCP-1, CCL4/MIP-1ß and CCL8/IL-8 were found to be elevated in high-grade tumours and to be associated with massive infiltration of proangiogenic macrophages.
Hence, due to the potent activity of chemokines on leucocytes and the simultaneous upregulation of multiple factors during tumour progression, it is impossible to distinguish in vivo between the direct effects of chemokines on endothelial cells and the proangiogenic activity of tumour-associated leucocytes.
Tumour cells may produce chemokines that act in a paracrine fashion
Production of chemokine receptors by tumour cells allows chemokine-driven support of tumour growth in an either autocrine or paracrine fashion. A clear pathophysiological role for CXCL8/IL-8 has been proposed for human melanoma [173]. However, in these studies, tumour cells in addition to the chemokine also expressed the corresponding receptors CXCR1 and CXCR2 [174–176]. Autocrine signalling via CXCR2 is suspected to induce cell growth in a number of tumour types (reviewed in [177]). Interestingly, tumorigenic viruses such as Kaposi’s sarcoma-associated herpes virus 8 (HHV-8) also encode G-protein-coupled receptors that bind CXCR2 ligands. Transgenic mice that express the HHV-8-encoded receptor in haematopoietic cells develop lesions that resemble Kaposi’s sarcoma [178]. The development of angioproliferative defects in this transgenic model suggests that the CXCR2-like viral receptor is involved in oncogenic cellular transformation. Furthermore, in mouse melanoma cells that constitutively express the chemokine receptor CXCR3, and its ligands CXCL9/Mig, CXCL10/IP-10 and CXCL11/I-TAC, induce cell survival, actin polymerization, migration and invasion [179].
Another chemokine receptor, CXCR4, is particularly well studied due to its essential role as a coreceptor for HIV entry [180]. The CXCR4 C-terminal domain plays a major role in receptor regulation, which appears to augment the process of epithelial-to-mesenchymal transition during breast cancer formation [181]. CXCR4 expression has also been detected on human glioma cell lines, where the corresponding ligand CXCL12/SDF-1α not only mediates AKT phosphorylation and resistance against serum withdrawal, but also chemotaxis [182]. CXCR4 is a prognostic marker in a number of cancer types including leukaemia, breast cancer and prostate cancer. Furthermore the SDF1/CXCL12–CXCR4 axis contributes to tumour progression (reviewed in [183]). Enhanced VEGF-A and NF-κB signalling increases CXCR4 expression during breast cancer progression and thereby promotes invasion and metastasis [184, 185]. It is interesting in this context that CXCR4 expression is increased in ductal carcinoma in situ (DCIS) lesions as compared to normal breast tissue and is further increased in invasive carcinoma [186].
Tumour cells may express receptors for homeostatic chemokines
The potent chemoattractant activity of chemokines and the capacity of chemokine receptors to mediate leucocyte migration during homeostasis and inflammation enables chemokine receptor-expressing tumour cells to respond to chemokine gradients and the use certain chemokines as tissue-specific attractants. Conceptually, it is tempting to postulate that tumours misuse stem cell and leucocyte homeostatic chemokines to metastasize to lymph nodes and the bone marrow. Indeed, CXCR4 is found more highly expressed in mammary carcinoma tissue than in normal mammary tissue [187]. The corresponding ligand CXCL12/SDF-1α is expressed in lymph nodes, bone marrow, liver and lungs, where it induces homeostatic homing of haematopoietic cells [188, 189]. These are exactly the tissues to which mammary carcinoma cells preferentially metastasize and indeed a functional axis between CXCL12/SDF-1 and its receptor CXCR4 has been demonstrated for breast cancer metastasis [187]. CXCR4 is expressed in a large variety of human tumours including prostate cancer, leukaemia, brain tumours, breast cancer, Wilms’ tumour, retinoblastoma, hepatoblastomas, ovarian cancers and cervical cancers [190]. The fact that CXCR4 is the chemokine receptor most commonly found in human tumours may provide an explanation for the frequent metastasis formation to CXCR4 ligand-expressing tissues [191]. Due to their diverse biological activity in many aspects of tumour formation, chemokines are attractive targets for the inhibition of tumour growth and metastasis formation.
The manifold actions of chemokines are also reflected in the higher efficacy of receptor neutralization in comparison to ligand neutralization. Monoclonal antibodies against CXCR4 inhibit growth and metastasis formation of human mammary carcinoma cells to the lymph nodes in SCID mice [187]. The growth of non-Hodgkin’s lymphomas in immunodeficient mice is severely impaired by anti CXCR4 antibody treatment [192]. The CXCR4 small molecule inhibitor AMD3100, which was originally designed to block entry of human immunodeficiency virus, shows good efficacy in inhibiting brain tumours [193]. Besides CXCR4, CCR7, the receptor for the major T zone chemokines CCL21/SLC and CCL19/ELC, has also been implicated in tumour metastasis to the lymph nodes [194]. Also CCR7 has been shown to be highly expressed by human breast cancer cells and induces actin polymerization, chemotaxis and invasion [187]. In line with these findings, CCR7 is a biomarker predicting axillary lymph node metastasis in T1 breast cancer [195] and the CCR7 ligands CCL19 and CCL21 are significantly higher in lymph node-positive than lymph node-negative patients [196]. The CCR7-dependent lymphatic migration of tumour cells resembles the physiological migration of DCs into the draining lymph nodes [197]. CCR7-expressing gastric carcinoma cells metastasize with a high frequency to the lymph nodes and patients with CCR7-expressing tumours have a significantly poorer prognosis [198]. Apparently, this also holds true for oesophageal squamous cells carcinomas, where CCR7 expression directs oesophageal lymphatic permeation, lymph node metastasis, tumour depth and tumour-node-metastasis stage and is associated with a poor survival [199].
The chemokine receptors CCR10 and CCR4 have been implicated in metastasis formation of melanoma cells and localization of T cell leukaemia to the skin [200]. The CCR10 ligand CCL27/ESkine is constitutively expressed in the keratinocytes of normal skin [201]. In melanoma, CCR10 expression appears to have a dual role in conferring immune resistance and being associated with lymph node metastasis [202, 203]. CCR4, the second skin-homing chemokine receptor is frequently coexpressed with CCR10 in T-cell leukaemia/lymphoma and both receptors together are probably responsible for the invasion of acute T-cell leukaemia/lymphoma into the skin [204]. Finally, CCR3 has been found to be expressed in tumour samples and tumour cell suspensions from patients with CD30+ T-cell lymphoma, where together with its ligand CCL11/eotaxin CCR3 appears to play a role in the recruitment and retention of malignant T cells in the skin [205].
At first glance one might be led to believe that chemotaxis is the dominant mechanism for organ-specific metastasis. However, metastasis involves many different aspects and types of molecules. It is therefore most likely that the attractive forces exerted by chemokines need the cooperation of many additional players during tumour dissemination. Still, the chemokine/chemokine receptor axis will remain an important target for antitumour therapy. This will hold true irrespective of the mechanism of anti-chemokine-receptor efficacy, to which true receptor inhibition could contribute as much as Fc-receptor-mediated killing and presentation of opsonized travelling tumour cells by the innate immune system.
Concluding remarks
The formation of a functional vasculature via angiogenesis plays an important part in embryonic development and during disease progression. Work over the last several years has unravelled a complex interplay between endothelial cells and accessory cells of the immune system, such as macrophages, in controlling angiogenic processes. Chemokines play an important role in coordinating this interplay via direct mechanisms, acting on endothelial cells, and by influencing immune cells to secrete proangiogenic factors. Deeper insights into these interactions will help us in understanding the mechanisms at play during situations where an immune response needs to be coordinated with the formation of new blood vessels, for instance during wound healing or tumour formation. Applying these insights in the clinic will also provide new avenues for disease treatments.
Acknowledgments
The authors would like to thank their colleagues for stimulating discussions. This work was funded by the Max Planck Society, a DFG grant (SI-1374/3-1; A.F.S.), an ERC starting grant (260794-ZebrafishAngio; A.F.S.) and the DFG special priority program 629 (F.K.).
Contributor Information
Friedemann Kiefer, Phone: +49-251-70365230, FAX: +49-251-70365299, Email: friedemann.kiefer@gwdg.de.
Arndt F. Siekmann, Phone: +49-251-70365459, FAX: +49-251-70365499, Email: arndt.siekmann@mpi-muenster.mpg.de
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 5.Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17(4):294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.le Noble F, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS. Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc Res. 2005;65(3):619–628. doi: 10.1016/j.cardiores.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Germain S, Monnot C, Muller L, Eichmann A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol. 2010;17(3):245–251. doi: 10.1097/MOH.0b013e32833865b9. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 10.Jenab-Wolcott J, Giantonio BJ. Bevacizumab: current indications and future development for management of solid tumors. Expert Opin Biol Ther. 2009;9(4):507–517. doi: 10.1517/14712590902817817. [DOI] [PubMed] [Google Scholar]
- 11.Ozkiris A. Anti-VEGF agents for age-related macular degeneration. Expert Opin Ther Pat. 2010;20(1):103–118. doi: 10.1517/13543770902762885. [DOI] [PubMed] [Google Scholar]
- 12.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18(8):349–351. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 14.Hall K, Ran S. Regulation of tumor angiogenesis by the local environment. Front Biosci. 2010;15:195–212. doi: 10.2741/3615. [DOI] [PubMed] [Google Scholar]
- 15.Bohmer R, Neuhaus B, Buhren S, Zhang D, Stehling M, Bock B, Kiefer F. Regulation of developmental lymphangiogenesis by Syk(+) leukocytes. Dev Cell. 2010;18(3):437–449. doi: 10.1016/j.devcel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137(22):3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 18.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9(9):953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 20.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16(6):593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165(9):5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 22.Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol. 1998;28(1):164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, Wasserman K, Oppenheim JJ. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14(13):2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 24.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- 25.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 26.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115(2):234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265(5172):682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 28.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154(4):1125–1135. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104(40):15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 2007;38(3):365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107(2):628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 34.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez AC, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104(37):14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132(3):463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 38.Taylor S, Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982;297(5864):307–312. doi: 10.1038/297307a0. [DOI] [PubMed] [Google Scholar]
- 39.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96(6):612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 40.Flad HD, Brandt E. Platelet-derived chemokines: pathophysiology and therapeutic aspects. Cell Mol Life Sci. 2010;67(14):2363–2386. doi: 10.1007/s00018-010-0306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ Res. 2004;95(9):855–857. doi: 10.1161/01.RES.0000146674.38319.07. [DOI] [PubMed] [Google Scholar]
- 42.Gupta SK, Singh JP. Inhibition of endothelial cell proliferation by platelet factor-4 involves a unique action on S phase progression. J Cell Biol. 1994;127(4):1121–1127. doi: 10.1083/jcb.127.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato Y, Abe M, Takaki R. Platelet factor 4 blocks the binding of basic fibroblast growth factor to the receptor and inhibits the spontaneous migration of vascular endothelial cells. Biochem Biophys Res Commun. 1990;172(2):595–600. doi: 10.1016/0006-291x(90)90715-y. [DOI] [PubMed] [Google Scholar]
- 44.Gengrinovitch S, Greenberg SM, Cohen T, Gitay-Goren H, Rockwell P, Maione TE, Levi BZ, Neufeld G. Platelet factor-4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J Biol Chem. 1995;270(25):15059–15065. doi: 10.1074/jbc.270.25.15059. [DOI] [PubMed] [Google Scholar]
- 45.Perollet C, Han ZC, Savona C, Caen JP, Bikfalvi A. Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood. 1998;91(9):3289–3299. [PubMed] [Google Scholar]
- 46.Jouan V, Canron X, Alemany M, Caen JP, Quentin G, Plouet J, Bikfalvi A. Inhibition of in vitro angiogenesis by platelet factor-4-derived peptides and mechanism of action. Blood. 1999;94(3):984–993. [PubMed] [Google Scholar]
- 47.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21(5):687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28(11):1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2(2):123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 51.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197(11):1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107(1):53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J, Richmond A. The angiostatic activity of interferon-inducible protein-10/CXCL10 in human melanoma depends on binding to CXCR3 but not to glycosaminoglycan. Mol Ther. 2004;9(6):846–855. doi: 10.1016/j.ymthe.2004.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campanella GS, Colvin RA, Luster AD. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS One. 2010;5(9):e12700. doi: 10.1371/journal.pone.0012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mellado M, Rodriguez-Frade JM, Manes S, Martinez A. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 56.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 57.Mariani M, Lang R, Binda E, Panina-Bordignon P, D’Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur J Immunol. 2004;34(1):231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- 58.Mueller A, Strange PG. Mechanisms of internalization and recycling of the chemokine receptor, CCR5. Eur J Biochem. 2004;271(2):243–252. doi: 10.1046/j.1432-1033.2003.03918.x. [DOI] [PubMed] [Google Scholar]
- 59.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A. 2009;106(24):9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151(6):1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmermann N, Conkright JJ, Rothenberg ME. CC chemokine receptor-3 undergoes prolonged ligand-induced internalization. J Biol Chem. 1999;274(18):12611–12618. doi: 10.1074/jbc.274.18.12611. [DOI] [PubMed] [Google Scholar]
- 62.Elsner J, Mack M, Bruhl H, Dulkys Y, Kimmig D, Simmons G, Clapham PR, Schlondorff D, Kapp A, Wells TN, Proudfoot AE. Differential activation of CC chemokine receptors by AOP-RANTES. J Biol Chem. 2000;275(11):7787–7794. doi: 10.1074/jbc.275.11.7787. [DOI] [PubMed] [Google Scholar]
- 63.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borroni EM, Mantovani A, Locati M, Bonecchi R. Chemokine receptors intracellular trafficking. Pharmacol Ther. 2010;127(1):1–8. doi: 10.1016/j.pharmthera.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Rubin JB. Chemokine signaling in cancer: one hump or two? Semin Cancer Biol. 2009;19(2):116–122. doi: 10.1016/j.semcancer.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward SG. T lymphocytes on the move: chemokines, PI 3-kinase and beyond. Trends Immunol. 2006;27(2):80–87. doi: 10.1016/j.it.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3 K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 69.Cronshaw DG, Owen C, Brown Z, Ward SG. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J Immunol. 2004;172(12):7761–7770. doi: 10.4049/jimmunol.172.12.7761. [DOI] [PubMed] [Google Scholar]
- 70.Curnock AP, Sotsios Y, Wright KL, Ward SG. Optimal chemotactic responses of leukemic T cells to stromal cell-derived factor-1 requires the activation of both class IA and IB phosphoinositide 3-kinases. J Immunol. 2003;170(8):4021–4030. doi: 10.4049/jimmunol.170.8.4021. [DOI] [PubMed] [Google Scholar]
- 71.Merlot S, Firtel RA. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J Cell Sci. 2003;116(Pt 17):3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- 72.Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63(8):1969–1974. [PubMed] [Google Scholar]
- 73.Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J Immunol. 2001;167(6):3064–3073. doi: 10.4049/jimmunol.167.6.3064. [DOI] [PubMed] [Google Scholar]
- 74.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213(2):442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 75.Chong SW, Emelyanov A, Gong Z, Korzh V. Expression pattern of two zebrafish genes, cxcr4a and cxcr4b. Mech Dev. 2001;109(2):347–354. doi: 10.1016/s0925-4773(01)00520-2. [DOI] [PubMed] [Google Scholar]
- 76.Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23(19):2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 78.Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105(8):3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- 79.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, Imai E, Nagasawa T, Rakugi H, Isaka Y. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20(8):1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 81.Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, Koenen TB, Krajnc-Franken MA, Gossen JA. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46(5):235–245. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 82.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134(15):2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 83.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115(24):5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 84.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206(5):1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays. 2008;30(4):303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 86.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16(2):196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 87.Williams CK, Segarra M, Sierra Mde L, Sainson RC, Tosato G, Harris AL. Regulation of CXCR4 by the Notch ligand delta-like 4 in endothelial cells. Cancer Res. 2008;68(6):1889–1895. doi: 10.1158/0008-5472.CAN-07-2181. [DOI] [PubMed] [Google Scholar]
- 88.Packham IM, Gray C, Heath PR, Hellewell PG, Ingham PW, Crossman DC, Milo M, Chico TJ. Microarray profiling reveals CXCR4a is downregulated by blood flow in vivo and mediates collateral formation in zebrafish embryos. Physiol Genomics. 2009;38(3):319–327. doi: 10.1152/physiolgenomics.00049.2009. [DOI] [PubMed] [Google Scholar]
- 89.Melchionna R, Porcelli D, Mangoni A, Carlini D, Liuzzo G, Spinetti G, Antonini A, Capogrossi MC, Napolitano M. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J. 2005;19(6):629–631. doi: 10.1096/fj.04-2219fje. [DOI] [PubMed] [Google Scholar]
- 90.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 91.Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4(1):35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 92.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 93.Bussmann J, Bos FL, Urasaki A, Kawakami K, Duckers HJ, Schulte-Merker S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development. 2010;137(16):2653–2657. doi: 10.1242/dev.048207. [DOI] [PubMed] [Google Scholar]
- 94.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116(4):661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carramolino L, Fuentes J, Garcia-Andres C, Azcoitia V, Riethmacher D, Torres M. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res. 2010;106(7):1197–1201. doi: 10.1161/CIRCRESAHA.110.218073. [DOI] [PubMed] [Google Scholar]
- 96.Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, Nye E, Ju T, Ramirez MI, Carmeliet P, Cummings RD, Lupu F, Xia L. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118(11):3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, Hirashima M, Ozaki Y. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285(32):24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115(19):3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 99.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299(5604):247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7(2):155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 101.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 102.Mebius RE, Miyamoto T, Christensen J, Domen J, Cupedo T, Weissman IL, Akashi K. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+ CD4+ CD3− cells, as well as macrophages. J Immunol. 2001;166(11):6593–6601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- 103.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, Rennert PD, Choi Y. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192(10):1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 105.van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Hopken UE, Lipp M, Niederreither K, Blomhoff R, Sitnik K, Agace WW, Randall TD, de Jonge WJ, Mebius RE. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10(11):1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197(9):1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+ CD3− cells induce Peyer’s patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 2002;17(3):363–373. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 108.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 109.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9(1):71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 110.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, Nishikawa SI. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193(5):621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174(1):21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 112.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17(4):525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 113.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189(2):403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim MY, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V, Walker LS, Caamano J, Jenkinson E, Anderson G, Lane PJ. Function of CD4+ CD3− cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109(4):1602–1610. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 115.Ohl L, Henning G, Krautwald S, Lipp M, Hardtke S, Bernhardt G, Pabst O, Forster R. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197(9):1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winter S, Loddenkemper C, Aebischer A, Rabel K, Hoffmann K, Meyer TF, Lipp M, Hopken UE. The chemokine receptor CXCR5 is pivotal for ectopic mucosa-associated lymphoid tissue neogenesis in chronic Helicobacter pylori-induced inflammation. J Mol Med. 2010;88(11):1169–1180. doi: 10.1007/s00109-010-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21(1):27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 119.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 120.Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59(18):4681–4687. [PubMed] [Google Scholar]
- 121.Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102(3):220–224. doi: 10.1002/ijc.10705. [DOI] [PubMed] [Google Scholar]
- 122.Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69(19):7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 123.Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117(7):1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, Mantovani A, Locati M, Danese S. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut. 2010;59(2):197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 125.Collins CB, McNamee EN, Wermers JD, Lebsack MD, Rivera-Nieves J. Chemokine decoy receptor D6 in inflammatory bowel disease (IBD) and IBD-associated colon cancer. Gut. 2010;59(2):151–152. doi: 10.1136/gut.2009.192708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 128.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 129.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 130.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176(8):5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 132.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284(49):34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 135.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109(12):5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 136.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 137.Ruhrberg C, De Palma M. A double agent in cancer: deciphering macrophage roles in human tumors. Nat Med. 2010;16(8):861–862. doi: 10.1038/nm0810-861. [DOI] [PubMed] [Google Scholar]
- 138.Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, Di Carlo V, Allavena P, Piemonti L. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63(21):7451–7461. [PubMed] [Google Scholar]
- 139.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18(1):3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66(23):11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 142.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106(35):14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC, Metelitsa LS. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119(6):1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116(8):2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182(10):6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 147.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 150.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 153.Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol. 1997;28(3):321–331. doi: 10.1016/s0046-8177(97)90131-3. [DOI] [PubMed] [Google Scholar]
- 154.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90(6):447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 155.Yatsunami J, Tsuruta N, Ogata K, Wakamatsu K, Takayama K, Kawasaki M, Nakanishi Y, Hara N, Hayashi S. Interleukin-8 participates in angiogenesis in non-small cell, but not small cell carcinoma of the lung. Cancer Lett. 1997;120(1):101–108. doi: 10.1016/s0304-3835(97)00296-6. [DOI] [PubMed] [Google Scholar]
- 156.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97(12):2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179(5):1409–1415. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9(2):853–860. [PubMed] [Google Scholar]
- 159.Bostwick DG, Iczkowski KA. Microvessel density in prostate cancer: prognostic and therapeutic utility. Semin Urol Oncol. 1998;16(3):118–123. [PubMed] [Google Scholar]
- 160.Moore BB, Arenberg DA, Stoy K, Morgan T, Addison CL, Morris SB, Glass M, Wilke C, Xue YY, Sitterding S, Kunkel SL, Burdick MD, Strieter RM. Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol. 1999;154(5):1503–1512. doi: 10.1016/S0002-9440(10)65404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. FASEB J. 2006;20(1):59–64. doi: 10.1096/fj.05-4764com. [DOI] [PubMed] [Google Scholar]
- 162.Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175(8):5351–5357. doi: 10.4049/jimmunol.175.8.5351. [DOI] [PubMed] [Google Scholar]
- 163.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res. 2011;317(5):685–690. doi: 10.1016/j.yexcr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galvez BG, Genis L, Matias-Roman S, Oblander SA, Tryggvason K, Apte SS, Arroyo AG. Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. J Biol Chem. 2005;280(2):1292–1298. doi: 10.1074/jbc.M408673200. [DOI] [PubMed] [Google Scholar]
- 165.Stamatovic SM, Keep RF, Mostarica-Stojkovic M, Andjelkovic AV. CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. J Immunol. 2006;177(4):2651–2661. doi: 10.4049/jimmunol.177.4.2651. [DOI] [PubMed] [Google Scholar]
- 166.Strasly M, Doronzo G, Cappello P, Valdembri D, Arese M, Mitola S, Moore P, Alessandri G, Giovarelli M, Bussolino F. CCL16 activates an angiogenic program in vascular endothelial cells. Blood. 2004;103(1):40–49. doi: 10.1182/blood-2003-05-1387. [DOI] [PubMed] [Google Scholar]
- 167.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92(5):1085–1091. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 168.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–3289. [PubMed] [Google Scholar]
- 169.Barcelos LS, Talvani A, Teixeira AS, Cassali GD, Andrade SP, Teixeira MM. Production and in vivo effects of chemokines CXCL1–3/KC and CCL2/JE in a model of inflammatory angiogenesis in mice. Inflamm Res. 2004;53(10):576–584. doi: 10.1007/s00011-004-1299-4. [DOI] [PubMed] [Google Scholar]
- 170.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82(5):765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 171.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96(1):34–40. [PubMed] [Google Scholar]
- 172.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9(1):R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Singh S, Singh AP, Sharma B, Owen LB, Singh RK. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010;6(1):111–116. doi: 10.2217/fon.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. 1997;174(5):507–512. doi: 10.1016/s0002-9610(97)00165-7. [DOI] [PubMed] [Google Scholar]
- 175.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54(12):3242–3247. [PubMed] [Google Scholar]
- 176.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19(2):577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 177.Arya M, Patel HR, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin. 2003;19(6):557–564. doi: 10.1185/030079903125002216. [DOI] [PubMed] [Google Scholar]
- 178.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, Lira SA. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med. 2000;191(3):445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64(11):4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 180.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 181.Ueda Y, Neel NF, Schutyser E, Raman D, Richmond A. Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res. 2006;66(11):5665–5675. doi: 10.1158/0008-5472.CAN-05-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277(51):49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 183.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25(4):345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 184.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62(24):7203–7206. [PubMed] [Google Scholar]
- 185.Helbig G, Christopherson KW, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 186.Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, Bianchi R, Basik M. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97(3):275–283. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 187.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 188.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 189.Nagasawa T. A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol. 2000;72(4):408–411. [PubMed] [Google Scholar]
- 190.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60(7):497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 191.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14(3):171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 192.Bertolini F, Dell’Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, Gobbi A, Pruneri G, Martinelli G. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin’s lymphoma. Cancer Res. 2002;62(11):3106–3112. [PubMed] [Google Scholar]
- 193.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100(23):13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93(21):1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 195.Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11(16):5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 196.Wilson JL, Burchell J, Grimshaw MJ. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res. 2006;66(24):11802–11807. doi: 10.1158/0008-5472.CAN-06-1222. [DOI] [PubMed] [Google Scholar]
- 197.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80(5):448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 198.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62(10):2937–2941. [PubMed] [Google Scholar]
- 199.Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H, Imamura M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9(9):3406–3412. [PubMed] [Google Scholar]
- 200.Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, Takeuchi G, Shimizu S, Ito M, Komatsu H, Wakita A, Eimoto T, Matsushima K, Ueda R. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10 Pt 1):3625–3634. [PubMed] [Google Scholar]
- 201.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnik A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164(7):3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 202.Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, Biagini G, Offidani A. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer. 2006;42(8):1181–1187. doi: 10.1016/j.ejca.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 203.Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, Castillo SS, Dennis PA, Hwang ST. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med. 2003;198(9):1337–1347. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Harasawa H, Yamada Y, Hieshima K, Jin Z, Nakayama T, Yoshie O, Shimizu K, Hasegawa H, Hayashi T, Imaizumi Y, Ikeda S, Soda H, Atogami S, Takasaki Y, Tsukasaki K, Tomonaga M, Murata K, Sugahara K, Tsuruda K, Kamihira S. Survey of chemokine receptor expression reveals frequent co-expression of skin-homing CCR4 and CCR10 in adult T-cell leukemia/lymphoma. Leuk Lymphoma. 2006;47(10):2163–2173. doi: 10.1080/10428190600775599. [DOI] [PubMed] [Google Scholar]
- 205.Kleinhans M, Tun-Kyi A, Gilliet M, Kadin ME, Dummer R, Burg G, Nestle FO. Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T-cell lymphoma. Blood. 2003;101(4):1487–1493. doi: 10.1182/blood-2002-02-0475. [DOI] [PubMed] [Google Scholar]
- 206.Martins-Green M, Tilley C, Schwarz R, Hatier C, Bissell MJ. Wound-factor-induced and cell cycle phase-dependent expression of 9E3/CEF4, the avian gro gene. Cell Regul. 1991;2(9):739–752. doi: 10.1091/mbc.2.9.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cao Y, Chen C, Weatherbee JA, Tsang M, Folkman J. gro-beta, a -C-X-C- chemokine, is an angiogenesis inhibitor that suppresses the growth of Lewis lung carcinoma in mice. J Exp Med. 1995;182(6):2069–2077. doi: 10.1084/jem.182.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62(5):588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 209.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102(3):465–472. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Van Coillie E, Van Aelst I, Wuyts A, Vercauteren R, Devos R, De Wolf-Peeters C, Van Damme J, Opdenakker G. Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. Am J Pathol. 2001;159(4):1405–1414. doi: 10.1016/S0002-9440(10)62527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271(34):20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 212.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 213.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, Oppenheim JJ. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166(12):7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 214.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182(1):219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shellenberger TD, Wang M, Gujrati M, Jayakumar A, Strieter RM, Burdick MD, Ioannides CG, Efferson CL, El-Naggar AK, Roberts D, Clayman GL, Frederick MJ. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64(22):8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]