Abstract
It has become apparent that ubiquitination plays a critical role in cell survival and cell death. In addition, deubiquitinating enzymes (DUBs) have been determined to be highly important regulators of these processes. Cells can be subjected to various stresses and respond in a variety of different ways ranging from activation of survival pathways to the promotion of cell death, which eventually eliminates damaged cells. The regulatory mechanisms of apoptosis depend on the balanced action between ubiquitination and deubiquitination systems. There is a growing recognition that DUBs play essential roles in regulating several binding partners to modulate the process of apoptosis. Thus, the interplay between the timing of DUB activity and the specificity of ubiquitin attachment and removal from its substrates during apoptosis is important to ensure cellular homeostasis. This review discusses the role of a few ubiquitin-specific DUBs that are involved in either promoting or suppressing the process of apoptosis.
Keywords: Apoptotic signal, Cell death, Cell proliferation, Cell survival, Deubiquitinating enzyme
Ubiquitin proteasome pathway
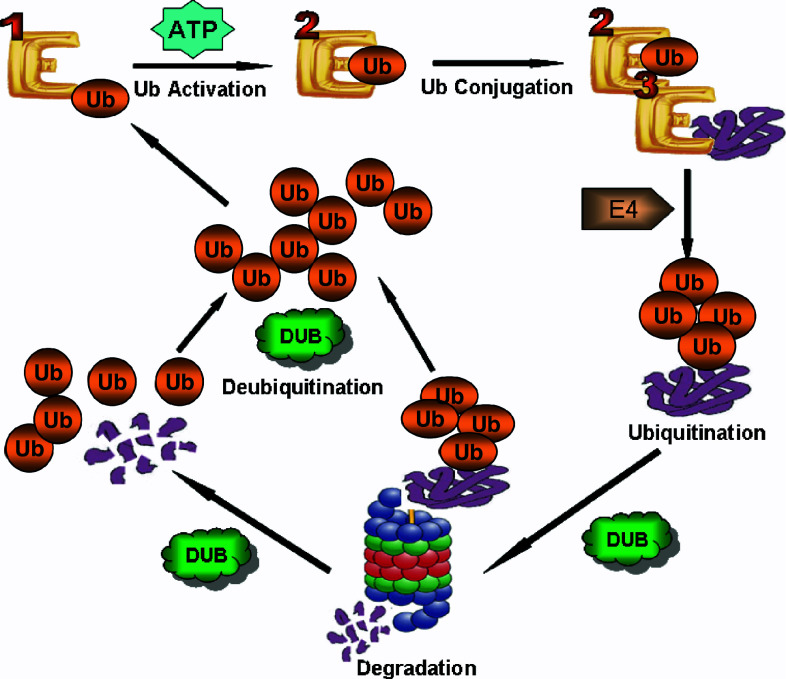
Ubiquitination is a posttranslational modification that involves the covalent attachment of ubiquitin molecules to targeted proteins that are directed towards the ubiquitin proteasome pathway (UPP). UPP is the major system responsible for elimination of intracellular proteins especially misfolded cellular proteins in eukaryotes [1, 2]. This process regulates a number of cellular processes such as stability, function and localization of the targeted proteins and is catalyzed by the sequential action of three enzymes, a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3) [2]. However, some ubiquitin elongation factors, referred to as E4 enzymes, are involved in the formation of multiubiquitin chains on targeted proteins [3]. E1 activates ubiquitin through the formation of ATP-dependent thiol ester bond between the C terminus of ubiquitin and the active cysteine site of the E1, which is then transferred to active cysteine site of E2. Finally, E3 catalyzes the transfer of ubiquitin to a lysine residue on the targeted protein. The conjugation of polyubiquitin chains on targeted protein is directed towards ATP-dependent hydrolysis by the 26S proteasome (Fig. 1).
Fig. 1.
The ubiquitin proteolytic pathway (UPP). The process of ubiquitination is regulated by organized milieu of E1, E2 and E3 enzymes, which mediate the ligation of ubiquitin to the lysine residues in proteins targeted to the 26S proteasome for degradation. Ubiquitins are recycled by the action of DUB enzymes
These ubiquitin chains can assemble in several different ways depending on the lysine site used to form the polyubiquitin chains including lysine (K)6, K11, K29, K48 and K63 [4]. It has been reported that ubiquitin chains can be formed from all these lysine residues on the substrate with various lengths and shapes in vitro and in vivo [5, 6]. Among them, polyubiquitin chains formed through K48 and K63 appear to be more frequent and have been extensively studied [5, 7]. K48-branched polyubiquitination is known to regulate protein stability and signals for proteasomal degradation of the substrate [2, 5]. Recently, K29 and K33-branched mixed chains have been implicated in the regulation of AMP-activated protein kinase-related kinases [8]. Additionally, K29-branched ubiquitin chains promote the proteasomal and lysosomal degradation of proteins [9–11], whereas K63-branched polyubiquitination plays a key role in the regulation of endocytosis, DNA repair, protein kinase activation [12, 13], signal transduction [14], intracellular trafficking of membrane proteins [14], and stress responses [15].
Monoubiquitination and polyubiquitination have also been implicated in non-proteasomal regulatory functions like targeting proteins to the nucleus, cytoskeleton and endocytic machinery, or modulating enzymatic activity and protein–protein interactions [14, 16]. Recent reports have indicated that the substrate modules consist of ubiquitin-binding domains (UBDs) with diverse structural folds, by which specific ubiquitin modification can be recognized [14]. The nonproteasomal functions of ubiquitination was first recognized in the process of endocytosis in yeast [17], where monoubiquitination is responsible for an endocytic internalization signal [18] and K63-branched ubiquitin promotes endocytosis [14, 19]. The K63-branched chains have a linear topology and increase the avidity of binding to UBDs [7, 14] which results in productive internalization of K63-conjugated proteins. In yeast, several plasma membrane proteins depend mainly on their ubiquitination for internalization [20]. Ubiquitination is also known to function as an endosomal sorting signal to target its substrate protein to the interior of the multivesicular body (MVB), which is eventually transported to lysosomes [14, 21]. For example, this ubiquitination targets the MHC I molecules for degradation through the MVB pathway [22]. Taken together, UPP plays a major role in balancing the levels of critical proteins involved in major cellular processes such as cell cycle progression, DNA replication and repair, transcription, immune responses, and apoptosis [23–25]. In addition, disruption of the UPP system has been associated with a number of disorders and tumors [26].
Deubiquitination
Deubiquitination is a process where ubiquitinated proteins can be reversed to counterbalance the ubiquitination process by cleaving ubiquitin from ubiquitin-conjugated protein substrates with the help of DUBs [27–29]. DUBs are involved in four major events: First, they activate and process ubiquitin proproteins into mature ubiquitin monomers [30]. Second, they are involved in recycling the utilized ubiquitin molecules during the ubiquitination process [31]. Third, they act as an antagonist for ubiquitinated proteins by reversing the process of ubiquitination or ubiquitin like modification of proteins [24, 29] and regenerating monoubiquitin molecules from the polyubiquitin chains that have been synthesized or released during the ubiquitination process [32]. Lastly, DUBs plays a major role in removing ubiquitin molecules that are not associated with proteasomal degradation during the ubiquitination process [24]. To date, nearly 100 DUBs have been identified from the human genome and have been divided into at least five major families: UBP or USP (ubiquitin-specific processing proteases), UCH (ubiquitin carboxy terminal hydrolases), JAMM (Jad1/Pad/MPN-domain-containing metallo enzymes), OTU (Otu-domain ubiquitin–aldehyde-binding proteins) and Ataxin-3/Josephin [24, 29].
Although, the mechanism of deubiquitination is less well understood, the removal of ubiquitin appears to be a highly regulated process that has been implicated in numerous cellular functions. DUBs are involved in the regulation of cell cycle progression and chromosome segregation [33], preventing proteins from degradation [24, 28], gene expression [24], proteasome or lysosome-dependent protein degradation [34], DNA repair [35, 36], kinase activation [34], apoptosis [37, 38], localization and degradation of signaling intermediates, and microbial pathogenesis [39]. Furthermore, disruptions in deubiquitination events have been shown to lead to tumorigenesis and several disorders [40]. Earlier reviews have mainly discussed DUBs from pathogens [39], specificity for ubiquitin-like proteins [27], diverse cellular roles [24], and its association with diseases [40]. This review concentrates on those DUBs involved in either promoting or inhibiting apoptosis due to their response to several exogenous factors.
Cellular stress responses
Cells react in various ways to cellular stress either by activating several pathways that accelerate cell proliferation and promote survival or by eliciting programmed cell death that removes damaged cells. Most of the time, cells tend to defend against the stressful stimulus and recover from the insult. There are many different types of responses that cells undergo to cope with stress such as DNA damage response, cell death response, heat shock response, unfolded protein response and oxidative stress response. However, the cellular response depends on the type and the level of stress factors, if cells fail to defend against noxious stress stimulus, and then the cells may activate death signaling pathways to eliminate the damaged cells from the organism. UPP plays a key role in coping with stress and regulating the process of cell death [41]. The Bcl-2 superfamily members play a critical role in regulating the balance of cell fate, which are degraded by the UPP system. The phosphorylation of Bcl-2 on Ser70 and Ser87 is required for ubiquitination and deubiquitination [42]. Bax, Bid and Bim protein levels are also regulated by the UPP system [43]. Thus, specific regulation of individual Bcl-2 family members by ubiquitination can lead to promoting or preventing cell death. Ubiquitination of receptor interacting protein 1 (RIP1), which is a death receptor-interacting protein, prevents TNF-induced cell death, whereas monoubiquitinated RIP1 serves as a pro-apoptotic-signaling molecule by engaging caspase 8 [44]. Therefore, the function of RIP1 is regulated by the UPP system. In addition, ubiquitination can regulate the function of caspase-3 and -7 through direct interaction with inhibitor of apoptosis proteins (IAP) E3 ligases [45]. The proteasome inhibitors can decrease the protein expression of the Fas-like inhibitor protein (FLIP) in tumors, resulting in increased apoptosis signaling due to increased caspase-8 activation [46]. p53, which is a tumor suppressor, plays a critical role in the induction of a pleiotropic apoptotic program in response to various cellular stresses via transcription-dependent and -independent mechanisms. Regulation of p53 protein levels and its susceptibility to apoptosis can be deregulated by the human homolog Hdm2 (Mdm2) E3 ligase [47]. Activation of p53 increases the expression of cell death genes such as Bax, Noxa and Puma, which may lead to apoptosis. However, Mdm2-mediated monoubiquitination of p53 promotes its mitochondrial translocation and thus it directs mitochondrial apoptosis [48]. Therefore, activation of cell death signaling cascades can be regulated at multiple sites following the degradation of key regulatory proteins by the UPP system. Although significant attention has been given to the UPP system during cell death signaling, we must also consider the role of DUBs in regulating these processes.
DUBs in regulating apoptosis
There is a growing recognition that DUBs are involved in the process of switching from prosurvival signaling to cell death signaling. These DUBs either up- or down-regulate their protein level or actively regulate their substrates when a cell encounters stressors such as DNA damage, unfolded protein, and oxidative stress signaling eventually resulting in cell death. The process of cell death is associated with a series of biochemical and morphological changes that are relevant to development, degenerative disease and cancer [49, 50]. Thus, the study of factors regulating programmed cell death has made a strong impact in various fields of biology and medicine [51].
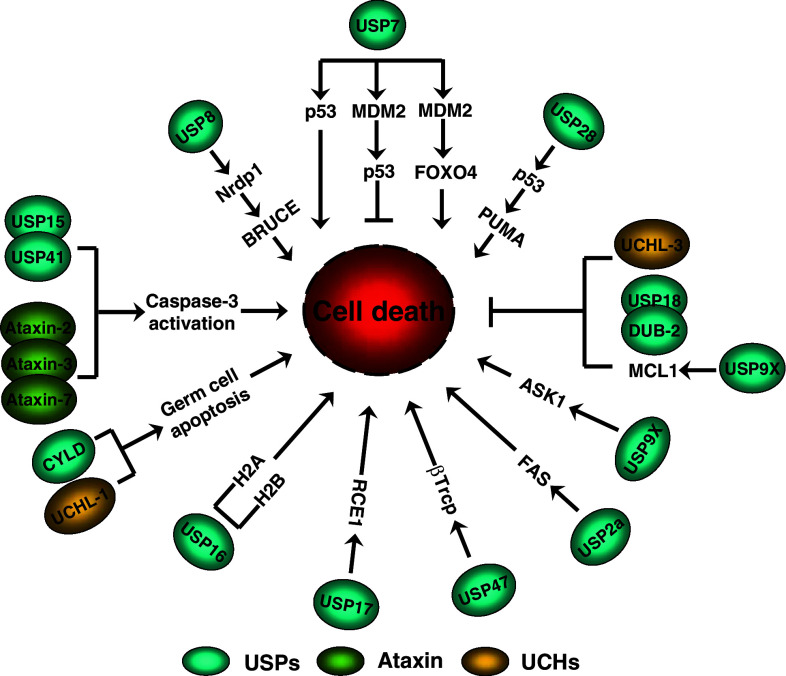
Apoptosis is a physiological process for killing cells and is critical for the normal development and function of multicellular organisms. Currently, apoptosis and delineation of the underlying biochemical pathway like post-translational modifications have dominated cell death research. Several studies have proposed that the proteasome complex itself plays a major role in the regulation of the apoptotic cascade [52]. Recent studies on the role of DUBs have proven productive in revealing its role in the process of apoptosis (Fig. 2). A few illustrative examples are discussed below.
Fig. 2.
Schematic overview of DUBs involved in the process of cell death by regulating its substrates. Activities involved in the inhibition of apoptosis are represented with lines ending in a ‘T’ symbol. Activities involved in inducing apoptosis are represented with arrowheads. USP family proteins are represented in blue, UCH family proteins are represented in yellow, and ataxin family proteins are represented in green. A detailed explanation of the figure is given in the text
DUB-1, 2 and 3
The specificity of DUB in regulating cell proliferation and apoptosis has been well studied in cytokine-inducible DUBs of murine lymphocytes [53–55]. The DUB family of USP was initially identified in mice as hematopoietic-specific DUBs, which are rapidly induced in response to a range of cytokines. DUB-1 is induced by IL-3, IL-5 and GM-CSF and ubiquitously expressed in a number of hematopoietic cell types [55], while DUB-2 is an immediate early gene that is induced by IL-2 in CTLL-2 and its expression is confined to T cells [54]. DUB-2A is also a member of the DUB family and is expressed primarily in hematopoietic cells [53]. Human DUB-3, a member of the DUB protein family induced by IL-4 and IL-6, is expressed in a number of hematopoietic tumors [37]. These genes are believed to form a part of the head-to-tail repeat of DUB genes on mouse chromosome 7, which resulted from a tandem duplication event [54]. Several lines of evidence also suggest that this family regulates cell growth and survival. Constitutive expression of DUB-1 from a steroid inducible promoter induces IL-3-specific signal transduction leading to growth arrest in the G1 phase of the cell cycle. Growth suppression by DUB-1 was specific to hematopoietic cells, indicating a hematopoietic-specific substrate may be involved in promoting this inhibitory effect [55]. DUB-2 expression can inhibit apoptosis following cytokine withdrawal [54]. DUB-2 is expressed in human T cell lymphotropic virus-1 (HTLV-1) transformed T cells with constitutive activation of the IL-2 signaling pathway [56]. When DUB-2 is expressed in Ba/F3 cells, it significantly inhibits apoptosis induced by the withdrawal of cytokines [56], as well as prolonging STAT5 phosphorylation [57]. Thus, DUB-2 may have an influence on cell survival by modulating STAT5 activation, which is essential for the action of several oncogenes [57]. mRNAs for both DUB-1 and DUB-2 are rapidly degraded as the cytokine response is down-regulated [54, 55, 58]. Cytokine withdrawal most likely initiates polyubiquitination and directs DUBs to proteasomes [59, 60]. DUB-3, which is also known as USP17, is responsible for the regulation of cell growth and survival, and the constitutive expression of DUB-3 can block cell proliferation and initiate apoptosis [37]. Several studies have examined the mechanism by which DUB-3/USP17 induces apoptosis. First, USP17 was reported to have two hyaluronan binding motifs at its C terminus region that are responsible for blocking cell proliferation and inducing apoptosis through caspase-3 activation [38]. Several lines of evidence show that the presence of a few hyaluronan binding motifs in the protein sequence can trigger apoptotic signaling cascade [61]. Recent studies have demonstrated that USP17 regulates cell survival by modulating Ras processing and Ras/MEK/ERK signaling, by regulating the ubiquitination status of the ‘CAAX’ box protease Ras-converting enzyme 1 (RCE1). Furthermore, USP17 is responsible for a RCE1-dependent inhibitory effect on cell proliferation [62]. Recently, DUB-3 was reported to interact with Cdc25A and regulate its protein stability to promote oncogenic transformation in cancerous cells [63]. However, the effect of DUB-3 expression level on cell fate and growth properties might depend on the cell type and the substrate to which DUB-3 interacts.
USP2
USP2 has two isoforms, USP2a and USP2b, which were initially detected in rat testis [64]. The functional role of USP2a is to stabilize and extend the half-life of fatty acid synthase (FAS) [65]. FAS is an androgen-regulated gene that has been recognized as an emerging oncology target [66]. Inhibition of FAS results in a reduction in cell proliferation and induction of apoptosis in several tumor cell lines [67]. RNA interference of USP2a increases polyubiquitinated FAS levels and induces apoptosis [65]. USP2a regulates the p53 pathway by targeting Mdm2, and the suppression of USP2a increases the mRNA levels of p53-responsive genes involved in both cell cycle arrest and apoptosis including Mdm2 itself [68]. Recently, USP2a was reported to be a deubiquitinating enzyme for MdmX and was shown to be involved in stabilizing the MdmX protein level [69]. Thus, USP2a influences cell survival through the regulation of the p53 pathway by stabilizing the activity of Mdm2 and MdmX.
USP7 and USP10
USP7, also known as Herpes Associated Ubiquitin Specific Protease (HAUSP), was originally identified as an ubiquitin-specific protease that binds to a viral-encoded protein called Vmw110, which is required for the lytic cycle of the herpes simplex virus [70]. USP7 plays a key role in stabilizing the half-life of p53 by regulating both p53 and its ubiquitin E3 ligase Mdm2 [71, 72]. In contrast, knockdown of USP7 increased p53 stability because USP7 was reported to be a deubiquitinating enzyme for Mdm2. Thus, a lower expression of USP7 results in the accumulation of non-ubiquitinated p53 [72, 73]. Different types of ubiquitination of p53 by various E3 ligases have been linked to its differential effects on p53-mediated stress responses, and ubiquitinated p53 may actively regulate cell proliferation or death [74]. The deubiquitinating action of USP7 was inhibited by the Epstein–Barr nuclear antigen 1 (EBNA1) protein of Epstein–Barr virus (EBV). Consequently, cells were protected from apoptotic challenges by lowering p53 levels [75]. HAUSP is highly expressed in organs that rely on apoptosis for development and is specifically processed upon dexamethasone and gamma-irradiation-induced cell death [76]. The overexpression of mHAUSP induces apoptosis in cervical adenocarcinoma cells [77]. Recently, inhibitors that were developed for USP7 induced p53-dependent apoptosis in cancer cell lines [78]. The Forkhead box O (FOXO) class of transcription factors are involved in the several cellular responses including cell cycle progression and apoptosis [79]. Overexpression of FOXO4 induces growth suppression in cell lines, including a Ras-transformed cell line, through the transcriptional activation of cyclin-dependent kinase inhibitor p27 [80]. HAUSP deubiquitinates FOXO4 in a p53-independent manner and inactivates FOXO4 [81]. Recently, Mdm2 was identified as an ubiquitin E3 ligase for FOXO, which regulates its transcription activity [82]. Thus, USP7 as a DUB enzyme for Mdm2 and FOXO plays a major role in regulating cellular stress responses. Recently, a new DUB enzyme called USP10 was identified to be a key regulator of the stability of the p53 tumor suppressor protein. In unstressed cells, USP10 remains in the cytoplasm and directly deubiquitinates p53 and mediates re-entry of p53 into the nucleus. Upon cellular stress, USP10 gets phosphorylated and is transported to the nucleus along with USP7 to stabilize p53 via p53 deubiquitination. Indeed, depletion of USP10 results in increased p53 degradation [83]. These combined results demonstrate that a central component of the DNA damage response is the activation of p53, which regulates processes such as cell cycle arrest, apoptosis, or senescence. Thus, USP7 and USP10 play a major role in apoptosis by regulating the p53 responsive genes and p53 pathway.
USP8
USP8 (UBPY) was initially characterized as a growth-regulated UBP and appears to play a critical role in controlling mammalian cell proliferation. Inhibition of UBPY accumulation prevents fibroblasts from entering the S-phase in response to serum stimulation. Overexpression or suppression of UBPY was directly correlated with the regulation of cell proliferation [84]. Nrdp1 is an E3 ligase, which promotes ubiquitination and proteasomal degradation of BRUCE leading to the induction of apoptosis [85]. USP8 acts as a DUB enzyme for Nrdp1 and augments Nrdp1 activity by mediating its stability [86]. Thus, USP8 may be involved in the induction of apoptosis by regulating Nrdp1-mediated ubiquitination of BRUCE.
USP9
USP9 is one of the largest members of the USP family and was initially identified in Drosophila, where mutations caused characteristic eye defects called fat facets (FAF). In mammals, USP9 is known as FAM [87]. USP9X has recently been identified as a DUB enzyme for apoptosis signal-regulating kinase 1 (ASK1). ASK1 is a mitogen-activated protein kinase kinase kinase (MAP3K) family member that mediates oxidative stress-induced cell death and inflammation through activation of the JNK and p38 MAPK pathways [88, 89]. USP9X was found to interact and deubiquitinate ASK1, and as a functional consequence, it antagonizes oxidative stress-induced ubiquitination and stabilizes ASK1 by preventing it from undergoing proteasomal degradation. USP9-deficient cells exhibited resistance to oxidative stress-induced cell death and a reduced level of JNK pathway activation was observed in these USP9-deficient cells [90]. In contrast, USP9X interacts and stabilizes MCL1, and promotes tumor cell survival. MCL1 is required for the survival of stem and progenitor cells of multiple lineages, and belongs to pro-survival BCL2 family [91]. Overexpression of MCL1 is associated with various disorders such as multiple myeloma, breast cancer, relapsing acute myeloid leukemia and acute lymphocytic leukemia [92]. USP9X deubiquitinates and prevents myeloid cell leukemia-1 (MCL1) protein degradation by removing K48-linked polyubiquitin chains; thus, USP9X-mediated stabilization of MCL1 is expected to increase survival when used to treat degenerative conditions where excessive apoptosis occurs [93].
USP15
USP15 is a member of the USPs that are involved in processing inactive ubiquitin precursors and maintaining 26S proteasomes free of inhibitory ubiquitin chains [94, 95]. USP15 has been characterized as a paclitaxel sensitivity-related gene in HeLa cells, and is responsible for the activation of caspase-3 during paclitaxel-induced apoptosis. USP15 regulates the stability of procaspase-3 and binding between procaspase-3 and the SCF complex (Skp1-CUL1-F-box) in such a way that procaspase-3 is deubiquitinated, released from the SCF complex and cleaved to cause apoptotic cell death [96].
USP16
USP16, also known as ubiquitin-processing protease Ubp-M, is phosphorylated at the onset of mitosis and is dephosphorylated during the metaphase/anaphase transition. Ubp-M-transfected cells eventually stop dividing and trigger apoptosis signaling. Ubp-M may deubiquitinate several critical proteins that are involved in the condensation of mitotic chromosomes, mainly on ubiquitinated proteins of the chromatin such as histones H2A and H2B [97]. Further studies revealed that the deubiquitination action of Ubp-M on histone H2A regulates Hox gene expression during cell cycle progression [98]. Deubiquitination of monoubiquitinated nucleosomal H2A and H2B is closely associated with mitotic chromatin condensation, appearance of plasma blebbing, and nuclear pyknosis in cells, which lead to apoptosis. The apoptotic stimulus by deubiquitination of H2A is prevented by the pan-caspase inhibitor or by Bcl-xL overexpression. Thus, H2A deubiquitination is a downstream consequence of procaspase activation and may function as a cellular sensor to undergo apoptosis in response to various stresses [99].
USP18
USP18 acts as an antiapoptotic protein in cells treated with drugs such as bortezomib and IFN-α. The ectopic expression of USP18 results in repressed apoptotic signaling caused by IFN-α, TRAIL, or bortezomib. Ablation of USP18 increases tumor necrosis factor-related apoptosis-inducing ligand production along with an elevation of certain transcription factors like IFN-regulatory factor (IRF)-1, IRF-7, and IR-9, which aid in triggering extrinsic pathways of apoptosis [100].
USP28
USP28 has been identified as a DUB enzyme that regulates the Chk2-p53-PUMA pathway, which is a major in vivo regulator of DNA-damage-induced apoptosis in response to double-strand breaks. USP28 plays a critical role in stabilizing Chk2 and 53BP1 in response to DNA-damage-induced apoptosis by regulating p53 induction of proapoptotic genes like PUMA. Knockdown of USP28 renders cells resistant to both IR-induced killing and IR-induced apoptosis [101].
USP41
USP41 was pegged as a proapoptotic ubiquitin-specific protease due to its ability to induce apoptosis in transfected mammalian cells. Overexpression of USP41 results in activation of caspase-3, which was revealed by the observation that apoptosis was inhibited when cells were treated with the caspase inhibitor zVAD-fmk. USP21 and USP18, the two homologous genes of USP41, did not show any signs of apoptosis when stably transfected in 293T cells, indicating that they are highly specific towards the substrates responsible for apoptotic induction. Furthermore, overexpression of USP41 has been shown to down-regulate cell cycle proteins such as p21, p27 and cyclin B resulting in the induction of apoptosis [102].
USP47
USP47 interacts with β-Trcp to regulate cell survival [103]. β-Trcp plays a critical role in controlling cell survival in addition to various other biological functions [104]. β-Trcp produces a pro-apoptotic effect by accumulating several substrates that negatively regulate cell survival [104]. In contrast, modulation or knockdown of β-Trcp did not affect expression of USP47 under various cellular stress conditions. However, depletion of USP47 results in suppression of cell survival and elevates the anti-proliferative effect of anticancer drugs in several tumor cell lines. Thus, USP47 may be involved in sensitizing tumor cells to undergo apoptosis when they are treated with chemotherapic agents [103].
CYLD
The cylindromatosis gene (CYLD) was first identified in humans affected with familial cylindromatosis (FC), a genetic condition that predisposes patients to the development of tumors on skin appendages, termed cylindroma [105]. The human CYLD gene is located on chromosome 16q12.1 and encodes a protein that is 956 amino acids in length. The C-terminal region of CYLD possesses a catalytic domain that has sequence homology to USP family members [105]. The tumor-suppressing function of this protein has been extensively studied using animal models. Loss of CYLD in mice makes them more prone to chemical tumorigenesis [106], and also CYLD levels are down-regulated in many tumor types [107]. CYLD plays a critical role in germ cell apoptosis and spermatogenesis [108]. The early wave of germ cell apoptosis is a controlled biological process that occurs during spermatogenesis, which is important for removing excess germ cells to maintain the proper balance between the germ cells and the supporting Sertoli cells [109]. CYLD knockout male mice showed attenuation in the early wave of germ cell apoptosis, which resulted in blocking spermatogenesis [108]. The loss of CYLD in testicular cells results in the activation of the transcription factor NF-κB in germ cells, which leads to aberrant expression of antiapoptotic genes such as Bcl-2. In addition, CYLD is involved in negative regulation of RIP1, which is an ubiquitin-dependent activator of NF-κB. CYLD binds to RIP1 and inhibits its ubiquitination, which is required for not only NF-κB activation but also to prevent RIP1 from accessing caspase-8 and activating apoptosis [110]. Thus, CYLD regulates germ cell apoptosis by regulating RIP1 ubiquitination to control the RIP1/NF-κB signaling axis in the testis.
USP19, USP21, and USP36
There are several USPs that modulate cell proliferation but are not directly involved in the induction of apoptosis. USP19 promotes cell proliferation by stabilizing the Kip1-promoting complex (KPC). KPC is an ubiquitin ligase for p27Kip1, and it regulates p27Kip1 ubiquitination during normal cellular processes [111]. Knockdown of USP19 results in the accumulation of p27Kip1 and thus inhibits cell proliferation by slowing the G0/G1 to S-phase transition during the cell cycle [112]. USP21 has a dual specificity for ubiquitin and NEDD8-conjugated proteins inhibit U2OS cell growth [113]. Knockdown of USP36 results in reduced cell proliferation in HeLa cells, which did not involve induction of apoptosis [114].
UCH
Ubiquitin C-terminal hydrolases (UCHs) is a member of the DUB family. Among mammalian UCHs, UCHL-1 and UCHL-3 have been extensively studied and share >40% amino acid sequence identity [115]. UCHL-3 is ubiquitously expressed, whereas UCHL-1 is selectively expressed in neuronal cells and in the testis/ovary [115]. UCHL-1 and UCHL-3 play distinct roles in spermatogenesis and regulate germ cell apoptosis to maintain testicular homeostasis [109, 116]. The pathophysiological roles of UCHL-1 and UCHL-3 were extensively studied in gad and Uchl3 knockout mice during cryptorchid injury. Upon cryptorchid injury, a balance between the expression of apoptosis-inducing and preventing proteins is regulated by UCHs. In gad mice, cryptorchid injury promotes antiapoptotic proteins Bcl-2, Bcl-xL and XIAP along with expression of prosurvival proteins like pCREB and BDNF [117]. In contrast, Uchl3 knockout mice showed a slight increase in the expression of apoptotic proteins p53, Bax and caspase-3 [117]. Thus, during cryptorchid injury, UCHL-1 maintains the balance between the expression of apoptotic and antiapoptotic proteins, whereas UCHL-3 appears to prevent germ cell apoptosis. In addition, high levels of UCHL-1 expression in transgenic mice promote the arrest of spermatogenesis through massive germ cell death, leading to male sterility [117]. UCHL-1 is essential for the early apoptotic wave and for normal sperm production, motility and morphology [117].
Ataxin-3/Josephin
Ataxin-3 (ATX3) is one of the best characterized members among the subclasses of DUBs [29]. The deubiquitinating activity of ATX3 is due to the cysteine residue at position 14 in its N-terminal josephin domain, which is responsible for protease activity [118]. Several lines of evidence suggest that ATX3 plays a major role in the ubiquitin proteasomal system, by interacting with ubiquitin and an ubiquitin-like protein called NEDD8 [119]. ATX3 was reported to bind and hydrolyze polyubiquitin chains in vitro [120] and has been shown to be involved in the regulation of proteasome by interacting with various substrates such as p45, VCP/p97, and Ubxn-5 [121–123]. Spinocerebellar ataxia type 3 (SCA3) is an autosomal-dominant neurodegenerative disease caused by polyglutamine-expanded ataxin-3. Normal ataxin-3 contains 12–44 glutamines, but it is expanded to ~60–87 glutamines in Machado–Joseph disease (MJD) [124].
Association of ATX3 with apoptosis was first observed when the C-terminal fragment of ATX3 containing an expanded polyglutamine tract was found to induce apoptotic signals in cultured cells [125]. It has been reported that full length expanded ataxin-3-containing cells were also prone to the staurosporine-induced apoptotic insult due to a significant decrease in Bcl-2 protein expression, leading to an increase in cytochrome c release from the mitochondria and subsequent activation of caspase-3 [126, 127]. However, the molecular mechanism behind neuronal cell death in the brain was revealed when polyglutamine-expanded ataxin-3 was shown to be responsible for the activation of the mitochondrial apoptotic pathway by up-regulating Bax and down-regulating Bcl-xL expression in cultured cerebellar, striatal and substantia nigra neurons [128]. Similarly, ataxin-7 or polyglutamine-expanded huntingtin were responsible for caspase-3 activation and apoptotic neuronal death by releasing cytochrome c and Smac from the mitochondria [129, 130]. Thus, mutant polyglutamine-expanded proteins may induce apoptotic cell death by promoting mitochondrial release of apoptogenic proteins in polyglutamine diseases. A cellular model for SCA3 using the rat mesencephalic dopaminergic cell line CSM14.1, which expresses a high level expanded full-length ataxin-3 protein, was shown to have a higher formation of nuclear inclusion bodies, which caused non-apoptotic cell death [131]. Ataxin-2, the product of the spinocerebellar ataxia type 2 (SCA2) gene, is a member of the protein family that causes human neurodegenerative diseases. Expression of full-length ataxin-2 with an expanded repeat disrupts normal morphology and localization of the Golgi complex and leads to cell death by promoting the expression of caspase-3 [132]. Ectopic expression of ataxin-2 was reported to be an important regulator in sensitizing neuroblastoma cells to apoptotic stimuli [133].
A20
A20 was first recognized as a cytokine-induced gene in human umbilical vein endothelial cells [134]. Initially, A20 was characterized as a primary response gene of the pro-inflammatory cytokines TNFα, IL-1β, and LPS. These cytokines significantly increased the A20 mRNA level but not protein synthesis [134]. Thus, A20 is also known as tumor necrosis factor-α-induced protein 3 (TNFAIP3). Analysis of the A20 domain revealed seven repeats of an A20-type zinc finger (ZnF-A20) in one single polypeptide chain, which exhibits E3 ligase activity, modulating the ubiquitination status of key adaptors in the NF-κB signaling cascade [135]. Additionally, an OTU domain that contained DUB protease activity was found at the N terminus of A20 [136]. It has been reported that overexpression of A20 inhibits TNF-mediated cell death and also down-regulates NF-κB signaling [135, 137]. NF-κB signaling is known to play a key role in host defense, immune response, cell survival, and vascular inflammation and injury [138]. Upon treatment with TNF, A20 expression was found to increase in variety of cells [139]. In addition, an increase in the transcription and protein synthesis of A20 was observed in TNF resistance MCF7 cell lines [139]. Mice deficient in A20 were prone to inflammation, and persistent activation of NF-κB by Toll-like and TNF receptors was observed in these mice [140]. Thus, A20 is critical for limiting inflammation by terminating TNF-induced NF-κB responses in vivo. The constitutive expression of A20 in different cell types prevents TNF-α-induced cell apoptosis [139, 141, 142]. Furthermore, it has been reported that a loss of A20 expression increased the lethality of TNF-α and lipopolysaccharides due to activation of NF-κB [143, 144]. Taken together, these results indicate that A20 plays a major role in the signaling mechanisms of cell survival.
Concluding remarks
Cellular stress response is an integral part of normal physiology to either activate the pathways that promote cell survival or alternatively to eliminate damaged or unwanted cells. UPP is involved in regulating cellular stress responses. While much attention has been given to the role of the ubiquitination system in regulating apoptosis, reversal of ubiquitination by DUBs plays an equally important role. DUBs are often found as a part of large multi-protein complexes that function directly in regulating the activation or suppression of apoptotic signaling cascades. In this review, we described the existence of a number of DUBs that actively regulate or suppress the process of apoptosis and which are illustrated in Table 1.
Table 1.
List of DUBs discussed in this review and their regulatory role in apoptosis
| DUBs | Regulatory action | References |
|---|---|---|
| DUB-2 | Prevent apoptosis | [54] |
| USP2 | Promote apoptosis | [65, 68] |
| USP7 | Promote apoptosis | [76–78, 81] |
| USP8 | Promote apoptosis | [84, 86] |
| USP9X | Promote apoptosis | [88–91] |
| USP15 | Promote apoptosis | [96] |
| USP16 | Promote apoptosis | [98, 99] |
| USP17 | Promote apoptosis | [38, 62] |
| USP18 | Prevent apoptosis | [100] |
| USP28 | Promote apoptosis | [101] |
| USP41 | Promote apoptosis | [102] |
| CYLD | Promote apoptosis | [108–110] |
| UCHL-1 | Promote apoptosis | [117] |
| UCHL-3 | Prevent apoptosis | [117] |
| Ataxin-2 | Promote apoptosis | [132, 133] |
| Ataxin-3 | Promote apoptosis | [126–128] |
| Ataxin-7 | Promote apoptosis | [129, 130] |
| A20 | Prevent apoptosis | [137, 141, 142] |
It is clear that the DUBs play a critical role in the regulation of apoptosis. Several important cellular proteins that are involved in apoptosis are degraded by the UPP. Aberrant cellular stress responses are directly linked to many human disorders. A better understanding of the molecular mechanism by which DUBs either promote or suppress apoptosis is expected to help in the development of more specific drugs that target the UPP. Additionally, new insights into the mechanistic basis of apoptosis will provide new insights for the development of DUB-targeted treatment approaches for various human disorders.
Acknowledgments
The authors thank members of Baek laboratory at CHA University and CHA General Hospital for their critical comments on the manuscript. This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (00001602).
Abbreviations
- DUB
Deubiquitinating enzyme
- UPP
Ubiquitin proteasomal pathway
- USP
Ubiquitin specific protease
- UCH
Ubiquitin carboxy terminal hydrolases
- OTU
Ovarian tumor domain
- MJD
Machado–Joseph disease
- JAMM
Jab1/MPN domain-associated metalloisopeptidase
- UBDs
Ubiquitin-binding domains
- MVB
Multivesicular body
- IL
Interleukin
- IFN
Interferon
References
- 1.Glickman MH, Ciechanover A. The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 3.Hoppe T. Multiubiquitylation by E4 enzymes: ‘one size’ doesn’t fit all. Trends Biochem Sci. 2005;30:183–187. doi: 10.1016/j.tibs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Pickart C. Ubiquitin in chains. Trends Biochem Sci. 2000;25:544–548. doi: 10.1016/S0968-0004(00)01681-9. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem. 2007;282:17375–17386. doi: 10.1074/jbc.M609659200. [DOI] [PubMed] [Google Scholar]
- 7.Varadan R, Assfalg M, Haririnia A, Raasi S, Pickart C, Fushman D. Solution conformation of Lys63-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J Biol Chem. 2004;279:7055–7063. doi: 10.1074/jbc.M309184200. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hakim AK, Zagorska A, Chapman L, Deak M, Peggie M, Alessi DR. Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem J. 2008;411:249–260. doi: 10.1042/BJ20080067. [DOI] [PubMed] [Google Scholar]
- 9.Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell. 2008;14:10–21. doi: 10.1016/j.ccr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Chastagner P, Israel A, Brou C. Itch/AIP4 mediates Deltex degradation through the formation of K29-linked polyubiquitin chains. EMBO Rep. 2006;7(11):1147–1153. doi: 10.1038/sj.embor.7400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Cheng D, Peng J, Pickart CM. Molecular determinants of polyubiquitin linkage selection by an HECT ubiquitin ligase. EMBO J. 2006;25:1710–1719. doi: 10.1038/sj.emboj.7601061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spence J, Sadis S, Haas AL, Finley D. A ubiquitin mutant with specific defects in DNA repair and multiubiquitination. Mol Cell Biol. 1995;15:1265–1273. doi: 10.1128/mcb.15.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation. Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 15.Arnason T, Ellison MJ. Stress resistance in Saccharomyces cerevisiae is strongly correlated with assembly of a novel type of multiubiquitin chain. Mol Cell Biol. 1994;14:7876–7883. doi: 10.1128/mcb.14.12.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 17.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/S0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 18.Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/S1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 19.Galan JM, Haguenauer-Tsapis R. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 1997;16:5847–5854. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 21.Komada M, Kitamura N. The Hrs/STAM complex in the downregulation of receptor tyrosine kinases. J Biochem. 2005;137:1–8. doi: 10.1093/jb/mvi001. [DOI] [PubMed] [Google Scholar]
- 22.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstantinova IM, Tsimokha AS, Mittenberg AG. Role of proteasomes in cellular regulation. Int Rev Cell Mol Biol. 2008;267:59–124. doi: 10.1016/S1937-6448(08)00602-3. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorokin AV, Kim ER, Ovchinnikov LP. Proteasome system of protein degradation and processing. Biochemistry (Mosc) 2009;74:1411–1442. doi: 10.1134/S000629790913001X. [DOI] [PubMed] [Google Scholar]
- 26.Petroski MD. The ubiquitin system, disease, and drug discovery. BMC Biochem. 2008;9:S7. doi: 10.1186/1471-2091-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Bio Phys Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Baek KH. Cytokine-regulated protein degradation by the ubiquitination system. Curr Protein Pept Sci. 2006;7:171–177. doi: 10.2174/138920306776359740. [DOI] [PubMed] [Google Scholar]
- 29.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Baker RT, Board PG. The human ubiquitin gene family: structure of a gene and pseudogenes from the Ub B subfamily. Nucleic Acids Res. 1987;15:443–463. doi: 10.1093/nar/15.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickart CM, Rose IA. Ubiquitin carboxyl-terminal hydrolase acts on ubiquitin carboxyl-terminal amides. J Biol Chem. 1985;260:7903–7910. [PubMed] [Google Scholar]
- 32.Wilkinson KD, Tashayev VL, O’Connor LB, Larsen CN, Kasperek E, Pickart CM. Metabolism of the polyubiquitin degradation signal: structure, mechanism, and role of isopeptidase T. Biochemistry. 1995;34:14535–14546. doi: 10.1021/bi00044a032. [DOI] [PubMed] [Google Scholar]
- 33.Song L, Rape M. Reverse the curse––the role of deubiquitination in cell cycle control. Curr Opin Cell Biol. 2008;20:156–163. doi: 10.1016/j.ceb.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komada M. Controlling receptor downregulation by ubiquitination and deubiquitination. Curr Drug Discov Technol. 2008;5:78–84. doi: 10.2174/157016308783769469. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy RD, D’Andrea AD. The Fanconi anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 36.Rathaus M, Lerrer B, Cohen HY. DeubiKuitylation: a novel DUB enzymatic activity for the DNA repair protein, Ku70. Cell Cycle. 2009;8:1843–1852. doi: 10.4161/cc.8.12.8864. [DOI] [PubMed] [Google Scholar]
- 37.Burrows JF, McGrattan MJ, Rascle A, Humbert M, Baek KH, Johnston JA. DUB-3, a cytokine-inducible deubiquitinating enzyme that blocks proliferation. J Biol Chem. 2004;279:13993–14000. doi: 10.1074/jbc.M311291200. [DOI] [PubMed] [Google Scholar]
- 38.Shin JM, Yoo KJ, Kim MS, Kim D, Baek KH. Hyaluronan- and RNA-binding deubiquitinating enzymes of USP17 family members associated with cell viability. BMC Genom. 2006;7:292. doi: 10.1186/1471-2164-7-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rytkönen A, Holden DW. Bacterial interference of ubiquitination and deubiquitination. Cell Host Microbe. 2007;1:13–22. doi: 10.1016/j.chom.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain S, Zhang Y, Galardy PJ. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8:1688–1697. doi: 10.4161/cc.8.11.8739. [DOI] [PubMed] [Google Scholar]
- 41.Liu CH, Goldberg AL, Qiu XB. New insights into the role of the ubiquitin–proteasome pathway in the regulation of apoptosis. Chang Gung Med J. 2007;30:469–479. [PubMed] [Google Scholar]
- 42.Basu A, Haldar S. Signal-induced site specific phosphorylation targets Bcl2 to the proteasome pathway. Int J Oncol. 2002;21:597–601. [PubMed] [Google Scholar]
- 43.Thompson SJ, Loftus LT, Ashley MD, Meller R. Ubiquitin–proteasome system as a modulator of cell fate. Curr Opin Pharmacol. 2008;8:90–95. doi: 10.1016/j.coph.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang HG, Wang J, Yang X, Hsu HC, Mountz JD. Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene. 2004;23:2009–2015. doi: 10.1038/sj.onc.1207373. [DOI] [PubMed] [Google Scholar]
- 46.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 2007;26:923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Dou QP. Targeting apoptosis pathway with natural terpenoids: implications for treatment of breast and prostate cancer. Curr Drug Targets. 2010;11:733–744. doi: 10.2174/138945010791170842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 51.Reeve JL, Duffy AM, O’Brien T, Samali A. Don’t lose heart––therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med. 2005;9:609–622. doi: 10.1111/j.1582-4934.2005.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grimm LM, Osborne BA. Apoptosis and the proteasome. Results Probl Cell Differ. 1999;23:209–228. doi: 10.1007/978-3-540-69184-6_10. [DOI] [PubMed] [Google Scholar]
- 53.Baek KH, Mondoux MA, Jaster R, Fire-Levin E, D’Andrea AD. DUB-2A, a new member of the DUB subfamily of hematopoietic deubiquitinating enzymes. Blood. 2001;98:636–642. doi: 10.1182/blood.V98.3.636. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Lambert K, Corless C, Copeland NG, Gilbert DJ, Jenkins NA, D’Andrea AD. DUB-2 is a member of a novel family of cytokine-inducible deubiquitinating enzymes. J Biol Chem. 1997;272:51–57. doi: 10.1074/jbc.272.1.51. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Y, Pless M, Inhorn R, Mathey-Prevot B, D’Andrea AD. The murine DUB-1 gene is specifically induced by the betac subunit of interleukin-3 receptor. Mol Cell Biol. 1996;16:4808–4817. doi: 10.1128/mcb.16.9.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migone TS, Humbert M, Rascle A, Sanden D, D’Andrea A, Johnston JA. The deubiquitinating enzyme DUB-2 prolongs cytokine-induced signal transducers and activators of transcription activation and suppresses apoptosis following cytokine withdrawal. Blood. 2001;98:1935–1941. doi: 10.1182/blood.V98.6.1935. [DOI] [PubMed] [Google Scholar]
- 57.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Y, Carroll M, Papa FR, Hochstrasser M, D’Andrea AD. DUB-1, a deubiquitinating enzyme with growth-suppressing activity. Proc Natl Acad Sci USA. 1996;93:3275–3279. doi: 10.1073/pnas.93.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baek KH, Kim MS, Kim YS, Shin JM, Choi KH. DUB-1A, a novel subfamily member of deubiquitinating enzyme, is polyubiquitinated and cytokine inducible in B-lymphocytes. J Biol Chem. 2004;279:2368–2376. doi: 10.1074/jbc.M304774200. [DOI] [PubMed] [Google Scholar]
- 60.Lee MY, Ajjappala BS, Kim MS, Oh YK, Baek KH. DUB-1, a fate determinant of dynein heavy chain in B-lymphocytes, is regulated by the ubiquitin–proteasome pathway. J Cell Biochem. 2008;105:1420–1429. doi: 10.1002/jcb.21961. [DOI] [PubMed] [Google Scholar]
- 61.Xu XM, Chen Y, Chen J, Yang S, Gao F, Underhill CB, Creswell K, Zhang L. A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res. 2003;63(18):5685–5690. [PubMed] [Google Scholar]
- 62.Burrows JF, Kelvin AA, McFarlane C, Burden RE, McGrattan MJ, De la Vega M, Govender U, Quinn DJ, Dib K, Gadina M, Scott CJ, Johnston JA. USP17 regulates Ras activation and cell proliferation by blocking RCE1 activity. J Biol Chem. 2009;284:9587–9595. doi: 10.1074/jbc.M807216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pereg Y, Liu BY, O’Rourke KM, Sagolla M, Dey A, Komuves L, French DM, Dixit VM. Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat Cell Biol. 2010;12:400–406. doi: 10.1038/ncb2041. [DOI] [PubMed] [Google Scholar]
- 64.Lin H, Keriel A, Morales CR, Bedard N, Zhao Q, Hingamp P, Lefrançois S, Combaret L, Wing SS. Divergent N-terminal sequences target an inducible testis deubiquitinating enzyme to distinct subcellular structures. Mol Cell Biol. 2000;20:6568–6578. doi: 10.1128/MCB.20.17.6568-6578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/S1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- 66.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 67.Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, Lupu R. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevenson LF, Sparks A, Allende-Vega N, Xirodimas DP, Lane DP, Saville MK. The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J. 2007;26:976–986. doi: 10.1038/sj.emboj.7601567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allende-Vega N, Sparks A, Lane DP, Saville MK. MdmX is a substrate for the deubiquitinating enzyme USP2a. Oncogene. 2010;29:432–441. doi: 10.1038/onc.2009.330. [DOI] [PubMed] [Google Scholar]
- 70.Meredith M, Orr A, Everett R. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology. 1994;200:457–469. doi: 10.1006/viro.1994.1209. [DOI] [PubMed] [Google Scholar]
- 71.Cheon KW, Baek KH. HAUSP as a therapeutic target for hematopoietic tumors (review) Int J Oncol. 2006;28:1209–1215. [PubMed] [Google Scholar]
- 72.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13:879–886. doi: 10.1016/S1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 73.Cummins JM, Vogelstein B. HAUSP is required for p53 destabilization. Cell Cycle. 2004;3:689–692. [PubMed] [Google Scholar]
- 74.Lee JT, Gu W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 2010;17:86–92. doi: 10.1038/cdd.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saridakis V, Sheng Y, Sarkari F, Holowaty MN, Shire K, Nguyen T, Zhang RG, Liao J, Lee W, Edwards AM, Arrowsmith CH, Frappier L. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein–Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol Cell. 2005;18:25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 76.Vugmeyster Y, Borodovsky A, Maurice MM, Maehr R, Furman MH, Ploegh HL. The ubiquitin–proteasome pathway in thymocyte apoptosis: caspase-dependent processing of the deubiquitinating enzyme USP7 (HAUSP) Mol Immunol. 2002;39:431–441. doi: 10.1016/S0161-5890(02)00123-2. [DOI] [PubMed] [Google Scholar]
- 77.Yoo KJ, Lee HJ, Lee H, Lee KY, Lee SH, Chung HM, Baek KH. Expression and functional analyses of mHAUSP regulating apoptosis of cervical adenocarcinoma cells. Int J Oncol. 2005;27:97–104. [PubMed] [Google Scholar]
- 78.Colland F, Formstecher E, Jacq X, Reverdy C, Planquette C, Conrath S, Trouplin V, Bianchi J, Aushev VN, Camonis J, Calabrese A, Borg-Capra C, Sippl W, Collura V, Boissy G, Rain JC, Guedat P, Delansorne R, Daviet L. Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol Cancer Ther. 2009;8:2286–2295. doi: 10.1158/1535-7163.MCT-09-0097. [DOI] [PubMed] [Google Scholar]
- 79.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/S0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 80.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 81.van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice M, Burgering BM. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–1073. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 82.Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3:e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–396. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naviglio S, Mattecucci C, Matoskova B, Nagase T, Nomura N, Di Fiore PP, Draetta GF. UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J. 1998;17:3241–3250. doi: 10.1093/emboj/17.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiu XB, Markant SL, Yuan J, Goldberg AL. Nrdp1-mediated degradation of the gigantic IAP, BRUCE, is a novel pathway for triggering apoptosis. EMBO J. 2004;23:800–810. doi: 10.1038/sj.emboj.7600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu X, Yen L, Irwin L, Sweeney C, Carraway KL. Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol Cell Biol. 2004;24:7748–7757. doi: 10.1128/MCB.24.17.7748-7757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y, Baker RT, Fischer-Vize JA. Control of cell fate by a deubiquitinating enzyme encoded by the fat facets gene. Science. 1995;270:1828–1831. doi: 10.1126/science.270.5243.1828. [DOI] [PubMed] [Google Scholar]
- 88.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 89.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1–p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 90.Nagai H, Noguchi T, Homma K, Katagiri K, Takeda K, Matsuzawa A, Ichijo H. Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death. Mol Cell. 2009;36:805–818. doi: 10.1016/j.molcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 91.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O’Rourke K, Bazan F, Eastham-Anderson J, Yue P, Dornan D, Huang DC, Dixit VM. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 94.Hetfeld BK, Helfrich A, Kapelari B, Scheel H, Hofmann K, Guterman A, Glickman M, Schade R, Kloetzel PM, Dubiel W. The zinc finger of the CSN-associated deubiquitinating enzyme USP15 is essential to rescue the E3 ligase Rbx1. Curr Biol. 2005;15:1217–1221. doi: 10.1016/j.cub.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 95.Huang X, Langelotz C, Hetfeld-Pechoc BK, Schwenk W, Dubiel W. The COP9 signalosome mediates beta-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J Mol Biol. 2009;391:691–702. doi: 10.1016/j.jmb.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 96.Xu M, Takanashi M, Oikawa K, Tanaka M, Nishi H, Isaka K, Kudo M, Kuroda M. USP15 plays an essential role for caspase-3 activation during paclitaxel-induced apoptosis. Biochem Biophys Res Commun. 2009;388:366–371. doi: 10.1016/j.bbrc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 97.Cai SY, Babbitt RW, Marchesi VT. A mutant deubiquitinating enzyme (Ubp-M) associates with mitotic chromosomes and blocks cell division. Proc Natl Acad Sci USA. 1999;96:2828–2833. doi: 10.1073/pnas.96.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P, Chang C, Wang H. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature. 2007;449:1068–1072. doi: 10.1038/nature06256. [DOI] [PubMed] [Google Scholar]
- 99.Mimnaugh EG, Kayastha G, McGovern NB, Hwang SG, Marcu MG, Trepel J, Cai SY, Marchesi VT, Neckers L. Caspase-dependent deubiquitination of monoubiquitinated nucleosomal histone H2A induced by diverse apoptogenic stimuli. Cell Death Differ. 2001;8:1182–1196. doi: 10.1038/sj.cdd.4400924. [DOI] [PubMed] [Google Scholar]
- 100.Potu H, Sgorbissa A, Brancolini C. Identification of USP18 as an important regulator of the susceptibility to IFN-alpha and drug-induced apoptosis. Cancer Res. 2010;70:655–665. doi: 10.1158/0008-5472.CAN-09-1942. [DOI] [PubMed] [Google Scholar]
- 101.Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell. 2006;126:529–542. doi: 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 102.Gewies A, Grimm S. UBP41 is a proapoptotic ubiquitin-specific protease. Cancer Res. 2003;63:682–688. [PubMed] [Google Scholar]
- 103.Peschiaroli A, Skaar JR, Pagano M, Melino G. The ubiquitin-specific protease USP47 is a novel beta-TRCP interactor regulating cell survival. Oncogene. 2010;29:1384–1393. doi: 10.1038/onc.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 105.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 106.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-κB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 107.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M, Sun SC. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13:705–716. doi: 10.1016/j.devcel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 110.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 111.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41–47. doi: 10.1016/S1044-579X(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 112.Lu Y, Adegoke OA, Nepveu A, Nakayama KI, Bedard N, Cheng D, Peng J, Wing SS. USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol Cell Biol. 2009;29:547–558. doi: 10.1128/MCB.00329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gong L, Kamitani T, Millas S, Yeh ET. Identification of a novel isopeptidase with dual specificity for ubiquitin- and NEDD8-conjugated proteins. J Biol Chem. 2000;275:14212–14216. doi: 10.1074/jbc.275.19.14212. [DOI] [PubMed] [Google Scholar]
- 114.Endo A, Matsumoto M, Inada T, Yamamoto A, Nakayama KI, Kitamura N, Komada M. Nucleolar structure and function are regulated by the deubiquitylating enzyme USP36. J Cell Sci. 2009;122:678–686. doi: 10.1242/jcs.044461. [DOI] [PubMed] [Google Scholar]
- 115.Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 116.Kwon J, Wang YL, Setsuie R, Sekiguchi S, Sakurai M, Sato Y, Lee WW, Ishii Y, Kyuwa S, Noda M, Wada K, Yoshikawa Y. Developmental regulation of ubiquitin C-terminal hydrolase isozyme expression during spermatogenesis in mice. Biol Reprod. 2004;71:515–521. doi: 10.1095/biolreprod.104.027565. [DOI] [PubMed] [Google Scholar]
- 117.Kwon J. The new function of two ubiquitin C-terminal hydrolase isozymes as reciprocal modulators of germ cell apoptosis. Exp Anim. 2007;56:71–77. doi: 10.1538/expanim.56.71. [DOI] [PubMed] [Google Scholar]
- 118.Chow MK, Mackay JP, Whisstock JC, Scanlon MJ, Bottomley SP. Structural and functional analysis of the Josephin domain of the polyglutamine protein ataxin-3. Biochem Biophys Res Commun. 2004;322:387–394. doi: 10.1016/j.bbrc.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 119.Ferro A, Carvalho AL, Teixeira-Castro A, Almeida C, Tomé RJ, Cortes L, Rodrigues AJ, Logarinho E, Sequeiros J, Macedo-Ribeiro S, Maciel P. NEDD8: a new ataxin-3 interactor. Biochim Biophys Acta. 2007;1773:1619–1627. doi: 10.1016/j.bbamcr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet. 2003;12:3195–3205. doi: 10.1093/hmg/ddg344. [DOI] [PubMed] [Google Scholar]
- 121.Rodrigues AJ, Neves-Carvalho A, Ferro A, Rokka A, Corthals G, Logarinho E, Maciel P. ATX-3, CDC-48 and UBXN-5: a new trimolecular complex in Caenorhabditis elegans . Biochem Biophys Res Commun. 2009;386:575–581. doi: 10.1016/j.bbrc.2009.06.092. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, Jia N, Fei E, Wang Z, Liu C, Zhang T, Fan J, Wu M, Chen L, Nukina N, Zhou J, Wang G. p45, an ATPase subunit of the 19S proteasome, targets the polyglutamine disease protein ataxin-3 to the proteasome. J Neurochem. 2007;101:1651–1661. doi: 10.1111/j.1471-4159.2007.04460.x. [DOI] [PubMed] [Google Scholar]
- 123.Wang Q, Li L, Ye Y. Regulation of retrotranslocation by p97-associated deubiquitinating enzyme ataxin-3. J Cell Biol. 2006;174:963–971. doi: 10.1083/jcb.200605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kobayashi T, Kakizuka A. Molecular analyses of Machado–Joseph disease. Cytogenet Genome Res. 2003;100:261–275. doi: 10.1159/000072862. [DOI] [PubMed] [Google Scholar]
- 125.Yoshizawa T, Yamagishi Y, Koseki N, Goto J, Yoshida H, Shibasaki F, Shoji S, Kanazawa I. Cell cycle arrest enhances the in vitro cellular toxicity of the truncated Machado–Joseph disease gene product with an expanded polyglutamine stretch. Hum Mol Genet. 2000;9:69–78. doi: 10.1093/hmg/9.1.69. [DOI] [PubMed] [Google Scholar]
- 126.Tsai HF, Tsai HJ, Hsieh M. Full-length expanded ataxin-3 enhances mitochondrial-mediated cell death and decreases Bcl-2 expression in human neuroblastoma cells. Biochem Biophys Res Commun. 2004;324:1274–1282. doi: 10.1016/j.bbrc.2004.09.192. [DOI] [PubMed] [Google Scholar]
- 127.Berke SJ, Schmied FA, Brunt ER, Ellerby LM, Paulson HL. Caspase-mediated proteolysis of the polyglutamine disease protein ataxin-3. J Neurochem. 2004;89:908–918. doi: 10.1111/j.1471-4159.2004.02369.x. [DOI] [PubMed] [Google Scholar]
- 128.Chou AH, Yeh TH, Kuo YL, Kao YC, Jou MJ, Hsu CY, Tsai SR, Kakizuka A, Wang HL. Polyglutamine-expanded ataxin-3 activates mitochondrial apoptotic pathway by upregulating Bax and downregulating Bcl-xL. Neurobiol Dis. 2006;21:333–345. doi: 10.1016/j.nbd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 129.Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 130.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Evert BO, Wüllner U, Schulz JB, Weller M, Groscurth P, Trottier Y, Brice A, Klockgether T. High level expression of expanded full-length ataxin-3 in vitro causes cell death and formation of intranuclear inclusions in neuronal cells. Hum Mol Genet. 1999;8:1169–1176. doi: 10.1093/hmg/8.7.1169. [DOI] [PubMed] [Google Scholar]
- 132.Huynh DP, Yang HT, Vakharia H, Nguyen D, Pulst SM. Expansion of the polyQ repeat in ataxin-2 alters its Golgi localization, disrupts the Golgi complex and causes cell death. Hum Mol Genet. 2003;12:1485–1496. doi: 10.1093/hmg/ddg175. [DOI] [PubMed] [Google Scholar]
- 133.Wiedemeyer R, Westermann F, Wittke I, Nowock J, Schwab M. Ataxin-2 promotes apoptosis of human neuroblastoma cells. Oncogene. 2003;22:401–411. doi: 10.1038/sj.onc.1206150. [DOI] [PubMed] [Google Scholar]
- 134.Dixit VM, Green S, Sarma V, Holzman LB, Wolf FW, O’Rourke K, Ward PA, Prochownik EV, Marks RM. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem. 1990;265:2973–2978. [PubMed] [Google Scholar]
- 135.Heyninck K, Beyaert R. A20 inhibits NF-kappaB activation by dual ubiquitin-editing functions. Trends Biochem Sci. 2005;30:1–4. doi: 10.1016/j.tibs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Makarova KS, Aravind L, Koonin EV. A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae . Trends Biochem Sci. 2000;25:50–52. doi: 10.1016/S0968-0004(99)01530-3. [DOI] [PubMed] [Google Scholar]
- 137.Cooper JT, Stroka DM, Brostjan C, Palmetshofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J Biol Chem. 1996;271:18068–18073. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 138.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 139.Opipari AW, Jr, Hu HM, Yabkowitz R, Dixit VM. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- 140.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daniel S, Arvelo MB, Patel VI, Longo CR, Shrikhande G, Shukri T, Mahiou J, Sun DW, Mottley C, Grey ST, Ferran C. A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation. Blood. 2004;104:2376–2384. doi: 10.1182/blood-2003-02-0635. [DOI] [PubMed] [Google Scholar]
- 142.Longo CR, Arvelo MB, Patel VI, Daniel S, Mahiou J, Grey ST, Ferran C. A20 protects from CD40-CD40 ligand-mediated endothelial cell activation and apoptosis. Circulation. 2003;108:1113–1118. doi: 10.1161/01.CIR.0000083718.76889.D0. [DOI] [PubMed] [Google Scholar]
- 143.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 144.Li HL, Zhuo ML, Wang D, Wang AB, Cai H, Sun LH, Yang Q, Huang Y, Wei YS, Liu PP, Liu DP, Liang CC. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115:1885–1894. doi: 10.1161/CIRCULATIONAHA.106.656835. [DOI] [PubMed] [Google Scholar]