Abstract
Alternative splicing generates multiple mRNAs from a single transcript and is a major contributor to proteomic diversity and to the control of gene expression in complex organisms. Not surprisingly, this post-transcriptional event is tightly regulated in different tissues and developmental stages. An increasing body of evidences supports a causative role of aberrant alternative splicing in cancer. However, very little is known about its impact on cellular processes crucially involved in tumor progression. The aim of this review is to discuss the link between alternative splicing and the epithelial-to-mesenchymal transition (EMT), one of the major routes by which cancer cells acquire invasive capabilities and become metastatic. We begin with a brief overview of alternative splicing. Next, we discuss alternative splicing factors that regulate EMT. Finally, we provide examples of target genes presenting alternative splicing changes that contribute to the morphological conversions in the EMT process.
Keywords: Alternative splicing, EMT, Splicing factors, Metastasis, RNA-binding proteins, Tumor progression
Introduction
More than 90% of all malignant tumors are of epithelial origin (carcinomas), indicating the key role of epithelial cells in human cancers [1]. The early steps in carcinoma metastases resemble a highly conserved developmental program called epithelial-to-mesenchymal transition (EMT), which is fundamental to organ morphogenesis and tissue remodeling during embryogenesis and is characterized by the loss of epithelial morphology and the acquisition of mesenchymal characteristics [2]. Terminally differentiated epithelial cells are polarized and display cohesive cell–cell junctions inhibiting the movement of individual cells and facilitating the formation of continuous cell layers [3]. As a result of EMT, epithelial cells undergo multiple changes in morphology, cellular architecture, adhesion, and motility [3]. These include: acquisition of elongated fibroblast-like shape with irregular structures that inhibit a rigid specialization, reduction of the intercellular junctions, enhanced migration, and acquisition of invasive properties [3]. In addition, phenotypic markers of EMT include an elevated resistance to anoikis/apoptosis and increased production of extracellular matrix (ECM) components [3].
In tumors, EMT occurs only in a subset of cells at the invasive front of the metastasizing primary carcinomas and is crucial for the ability to form metastases. According to the commonly accepted model, EMT dedifferentiated cells escape the primary tumor and, guided by signals in the tumor microenvironment, invade the surrounding tissues, enter into blood or lymphatic vessels, colonize distant organs and, finally, form metastases [2, 4]. Crucial to the metastatic process is the reversibility of EMT, namely the mesenchymal-to-epithelial transition (MET), that occurs at the final metastatic sites and involves redifferentiation programs and conversion of mesenchymal cells to an epithelial state [2].
EMT can be induced by distinct alternative signaling pathways, such as those involving the collaboration of TGF-β1 signaling with oncogenic Ras or receptor tyrosine kinases (RTKs) [5], Wnt [6], Notch [7] and the cascade controlled by Hedgehog [8]. These pathways activate a network of transcription factors, including Snail, Slug, Twist, Goosecoid, EF1/ZEB1, and SIP1/ZEB2 [2, 4, 9]. In turn, EMT-associated transcription factors promote the expression of mesenchymal markers such N-cadherin and vimentin, while inhibit E-cadherin production, a key component of adherens junctions but also a tumor suppressor frequently repressed, mutated or degraded during tumor transformation [2, 4].
Based on the above, it was initially proposed that EMT is regulated largely at the transcriptional level [2, 4]. However, we now know that many molecular mechanisms cooperate in altering tumor cell behavior. In particular, a role for post-transcriptional regulation of EMT is increasingly evident [10–13]. Post-transcriptional regulation of gene expression at the level of alternative splicing is important for normal physiology and in disease [14]. Recent studies have demonstrated an important contribution, often underestimated, of alternative splicing in the cascade of events that characterize tumor progression. Hence, the elucidation of the mechanisms that regulate alternative splicing decisions during EMT provides a useful new perspective for understanding tumorigenesis. Finally, evaluation of EMT-associated splicing signatures in diverse sets of carcinomas can be of medical relevance and opens the possibility of developing novel diagnostic/prognostic approaches and identifying possible therapeutic targets.
After a brief overview of the molecular mechanisms and the relevance of alternative splicing, in this review we will discuss specific examples of (a) the mechanisms governing alternative splicing decisions during EMT, (b) the differential functions of epithelial- and mesenchymal-specific splice variants, and (c) the biological consequences and the possible contribution to tumor metastasis associated with the aberrant splicing in some relevant EMT-regulated genes.
Alternative splicing
Protein coding genes are transcribed into precursor pre-mRNA molecules consisting of an ordered succession of exonic and intronic sequences.
Pre-mRNA splicing is an obligatory and highly regulated co-transcriptional process by which introns are removed and exons are joined together to reconstitute the mature mRNAs that are exported to the cytoplasm for translation. To meet the physiological requirements of cells and tissues, the splicing reaction must be rapid and precise at the single-nucleotide level so to avoid catastrophic errors of reading frame. This accuracy is ensured by the splicing apparatus (the spliceosome) that efficiently recognizes short and poorly conserved cis-acting sequences at the intron–exon boundaries (the 5′ and 3′ splice sites also known as “donor” and “acceptor” sites) (Fig. 1a) [15]. Essential components of the spliceosome are five small nuclear ribonucleoparticles (snRNPs U1, U2, U4, U5, and U6) and 100–200 non-snRNPs splicing factors such as RNA-binding proteins (U2AF, SF1, and SR proteins) and enzymes (helicases/RNPases, kinases, and phosphatases). The spliceosome undergoes a stepwise assembly and several compositional and structural changes aimed at ensuring: (a) the selection of proper splice sites among a multitude of similar sequences so to avoid inclusion of pseudo-exons, (b) the correct folding of intronic sequence with respect to the spliceosome catalytic activity that carries out the splicing reaction, and (c) the substrate release. The molecular details of the splicing reaction are discussed in several excellent reviews among which are Wahl et al. (2009) [16].
Fig. 1.
Cis-acting sequences controlling splicing reaction and five different types of alternative splicing. a Within the intron, many cis-acting sequences are required for pre-mRNA splicing: the 5′ splice site (donor), the 3′ splice site (acceptor), the polypyrimidine tract and the branchpoint sequence, which includes an adenine nucleotide representing the nucleophile for the first step of splicing. b During transcription, through pre-mRNA splicing, introns are precisely removed and exons are joined together to reconstitute the reading frame and to generate translatable mRNAs. c–g Alternatively spliced mRNAs can result through the usage of various combinations of donor and acceptor sites from different exons. The five types of alternative splicing are therefore called: exon skipping (or cassette exon), intron retention, mutually exclusive exons, alternative 5′ splice sites and alternative 3′ splice sites. At the protein level, alternative splicing drastically affects the amino acid sequence by deletion or insertion of domains, frame-shifts, or stop codons. A single pre-mRNA can often exhibit multiple regions that undergo alternative splicing, giving rise to complex splicing patterns and, as a consequence, to many different final mRNAs
While the majority of the exons are constitutively spliced and their sequences are always present in the mature mRNA molecule (Fig. 1b), splicing of other exons is modulated through a process called alternative splicing to generate multiple, distinct mature mRNAs all encoded by the same gene [15, 17]. Frequently, alternative splicing leads to the expression of protein isoforms with distinct structural and functional properties (Fig. 1c–g). In some cases, splicing isoforms may have very different or even opposite functions. Thus, for instance, some apoptotic genes through alternative splicing may encode either pro- or anti-apoptotic factors [18]. Together with other regulatory mechanisms, such as the alternative usage of promoters and polyadenylation sites, RNA editing, and post-translational modifications, alternative splicing contributes to the complexity of the proteome. Recent data support a direct correlation between organism complexity and the proportion of genes that are alternatively spliced. In humans, more than 90% of pre-mRNAs undergo alternative splicing and the human genome can give rise to an estimated number of at least 100,000 different protein products [19, 20]. On the contrary, alternative splicing is far less frequent in lower eukaryotes [21, 22].
Appropriate spatio-temporal generation of splicing variants is important for tuning the cellular response to physiological stimuli, during development and cellular differentiation, or for maintaining the cellular homeostasis in different tissues and organs [15]. There are five distinct major alternative splicing patterns (Fig. 1c–g). About 80% of alternative splicing events occur within the open reading frame, increasing the functional diversity of the human proteome [23]. The remaining 20% affects untranslated regions, frequently influencing regulatory elements that control the mRNA stability, the efficiency of the translation process, or the cellular localization of the mRNA. In addition, alternative splicing may affect the stability of transcripts also by introducing premature stop codons and directing the mRNA to degradation via the nonsense-mediated mRNA decay (NMD) pathway, an mRNA quality-control mechanism that eliminates transcripts containing premature translation termination codons (PTCs) [24–26]. This process is called alternative splicing-activated NMD [AS-NMD] or regulated unproductive splicing and translation [11, 27].
Alternatively spliced exons are often flanked by short and degenerate 5′ and 3′ splice sites with a low affinity for the spliceosome. Recognition of these splicing sites is modulated by different classes of auxiliary cis-acting elements (non-splice site RNA elements), referred to as enhancers and silencers of splicing that, respectively, promote and inhibit splice sites recognition by several mechanisms (Fig. 2) [28]. Also secondary and tertiary pre-mRNA structures may contribute to exon recognition and constitute additional elements of the “splicing code” [29, 30].
Fig. 2.
Cis- and trans-acting elements controlling alternative splicing decisions. Alternatively spliced exons are usually characterized by weak splice sites (short and degenerate). Recognition of these sites is influenced by the presence of four types of regulatory RNA sequences: exonic splicing enhancers (ESE) and silencers (ESS) and intronic splicing enhancers (ISE) and silencers (ISS). Splicing enhancer elements are most commonly bound by splicing factors of the SR family, whereas among the proteins interacting with splicing silencers there are the heterogeneous nuclear ribonucleoproteins (hnRNPs). These ubiquitous splicing factors act by a enhancing or b preventing the binding of specific spliceosomal components to the pre-mRNA, such as snRNP Ul to the 5′ splice site, snRNP U2 to the branchpoint and the U2AF heterodimer that recognizes the polypyrimidine tract and the conserved dinucleotide AG at the 3′ splice site. c Specific splicing patterns result from cooperative as well as antagonistic effects (“combinatorial control”) of multiple RNA-binding proteins. Thus, exon inclusion or skipping is determined by balance of these competing activities, which in turn reflect the relative abundance and/or cellular localization of the cognate RNA-binding activator and repressor proteins
Splicing enhancers and silencers are short regulatory elements (~10 nucleotides) either dispersed or clustered on the pre-mRNA; they can be found both in introns (ISE, intronic splicing enhancer and ISS, intronic splicing silencer) and exons (ESE, exonic splicing enhancer and ESS, exonic splicing silencer) [31]. Splicing enhancers are the binding sites for splicing activators such as the members of the SR family of splicing regulators, which comprises a dozen of highly conserved RNA-binding proteins. SR factors share a modular structure in which one or two N-terminal RNA-recognition motifs (RRMs) are followed by a C-terminal domain rich in serine–arginine dipeptides (the RS domain) and involved in specific interactions with proteins of the spliceosome [32]. Splicing silencers correspond to the binding sites for splicing repressors. The majority of splicing repressors belongs to the group of heterogeneous nuclear ribonucleoproteins (hnRNPs) that, similarly to SR factors, have a modular structure in which one or more RNA-binding domains are associated with different “auxiliary” domains with different functions [33].
Alternative splicing patterns are generated through a combinatorial mechanism in which several splicing activators and repressors are recruited to regulatory RNA sequences on the pre-mRNA [28, 34]. Most models predict that SR factors and hnRNP proteins act by enhancing or preventing the binding of specific spliceosomal components to the pre-mRNA (Fig. 2a, b). Importantly, the ability of these trans-acting factors to bind pre-mRNA elements and regulate alternative splicing decisions depends on their abundance, activity, and nuclear localization, which can also be modulated through post-translational modifications [14]. Thus, the balance of positive and negative regulatory splicing factors determines the final outcome of the splicing reaction (Fig. 2c).
In the recent years, the relevance of alternative splicing for tumor progression has become evident. Indeed, all major aspects of cell biology that are deregulated in cancer (including cell cycle control, differentiation, signal transduction pathways, cell death, angiogenesis, motility, and invasion) involve changes in the alternative splicing profile of specific genes [14, 35]. Modifications in the expression and/or activity of splicing regulators, such as SR and hnRNP factors, provide the primary source of alteration of splicing programs observed in cancer cells. Notably, the identification of 15,000 cancer-specific alternative variants that most likely contribute to the phenotype of the cancer cells was recently made [36]. An appealing possibility is that these could be used as new diagnostic and prognostic markers for human cancers.
EMT plays a critical role during malignant transformation and much is known about transcriptional regulation of this process [37]. Interestingly, in accord with the fact that alternative splicing and transcription predominantly regulate independent sets of genes to define tissue-specific characteristics [38, 39], an alternative splicing signature of EMT has recently been discovered [10–13]. This alternative splicing program affects key regulators of cell phenotype, including proteins that control cell adhesion and cytoskeletal dynamics. Thus, an increasing body of evidence indicates that splicing regulation alone can drive critical aspects of EMT-associated phenotypic changes. In the following section, we will discuss the mechanisms governing alternative splicing decisions during EMT.
SRSF1 affects the EMT program by modulating alternative splicing of the Ron proto-oncogene transcripts
The first example of a role of alternative splicing in EMT programs was provided by the analysis of the Ron proto-oncogene (also known as MST1R), which encodes the human tyrosine kinase receptor for the macrophage-stimulating protein (MSP) [10]. It is plausible that the analysis of other members of the receptor tyrosine kinase (RTK) superfamily could provide further examples of this link. Ron belongs to the scatter factor receptor family, which includes Met, the hepatocyte growth factor (HGF) receptor [40]. Like Met, Ron mediates multiple signaling cascades that, in addition to promoting cell growth, proliferation, and protection from apoptosis, are involved in the control of cell dissociation, motility, and invasion of extracellular matrices, a process known as “epithelial cell scattering” that, at least in defined cellular models, is equivalent to EMT [40]. Ron is synthesized as a single chain precursor protein and then cleaved to produce an extracellular 40-kDa α subunit and 145-kDa transmembrane β subunit, which are disulfide linked to form the mature heterodimer (p185-Ron) [41]. The Ron ligand MSP is a modulator of the growth of several cell types [42, 43]. MSP binding causes Ron autophosphorylation on internal tyrosine residues and the ensuing phosphorylation of its multisubstrate docking site. These phosphorylation events promote the interaction with several adapter proteins and lead to activation of downstream signaling pathways, often implicated in tumor progression and metastasis, including the Ras/mitogen-activated protein kinase (MAPK), phosphatidyl inositol 3 kinase (PI-3K)/Akt, focal adhesion kinase (FAK) and c-Src [44].
Ron transcripts undergo several alternative splicing events that affect the activity of the receptor [41]. The frequent alteration of the Ron splicing profile in epithelial cancers, such as colorectal and breast cancers [10, 45], raises the possibility that Ron splicing isoforms could contribute to metastatic process. Four splicing isoforms have been described so far [41]: ΔRon (skipping of exon 11), RonΔ160 (skipping of exons 5 and 6), RonΔ155 (skipping of exons 5, 6 and 11) and RonΔ170 (skipping of exon 19). With the exception of RonΔ170, over-expression of any of these isoforms increases the motile and invasive phenotypes of tumor cells, while only RonΔ160 and RonΔ155 have the ability to transform rodent fibroblasts in vitro and cause tumor growth in athymic nude mice [45]. Interestingly, RonΔ170 acts as dominant negative and has a great therapeutic potential being capable of inhibiting tumorigenesis of epithelial cancers with altered Ron expression [46].
Our group has characterized the molecular mechanisms underlying the production of the ΔRon isoform during EMT [10, 11]. This isoform lacks of 49 amino acids (aa) in the extracellular β-chain and originates from skipping of exon 11 [47]. The 49-aa deletion has several consequences on the protein structure and function: (a) it prevents the proteolytic processing of the pro-ΔRon precursor; (b) ΔRon accumulates in the cytoplasm and does not reach the plasma membrane; (c) ΔRon is constitutively phosphorylated and active even in the absence of the ligand MSP, and finally (d) ΔRon over-expression is sufficient to confer invasive properties to recipient cells [47]. Intriguingly, a basal level of ΔRon transcripts is visible in normal tissues but significantly increases in colon and breast cancers, indicating that the balance between splicing isoforms is relevant for the phenotype of cancer cells [10, 45]. We showed that the choice between inclusion and skipping of exon 11 is controlled by two adjacent regulatory elements, a silencer and an enhancer of splicing, both located in the constitutive downstream exon 12 [10]. The activity of the enhancer is governed by the splicing factor and proto-oncogene SRSF1 (previously known as SF2/ASF). SRSF1 affects the competition between acceptor sites in introns 10 and 11 and modulates the Ron/ΔRon ratio. This was the first example in which binding of SRSF1 to an exonic sequence leads to skipping of the upstream exon. By increasing the level of ΔRon, over-expression of SRSF1 in epithelial cells promotes morphological and molecular hallmarks of an activated EMT program [10]. This effect can be reverted by selective knock-down of ΔRon mRNA, implicating this isoform in EMT [10]. It is remarkable, therefore, that the SRSF1 gene is frequently amplified and SRSF1 protein up-regulated in various human tumors [48]. Even more interesting is the fact that SRSF1 may control the activity of important players in the signal transduction cascade such as mTOR [49].
Recently, Cartegni and colleagues showed that hnRNP H, also frequently upregulated in gliomas, is involved in controlling splicing of Ron transcripts through an exonic splicing silencer (ESS) located at the 5′ end of exon 11 [50]. Finally, the expression of hnRNP A2/B1, an important regulator of EMT that is upregulated in various types of human cancer [51], also affects splicing of Ron exon 11 [52].
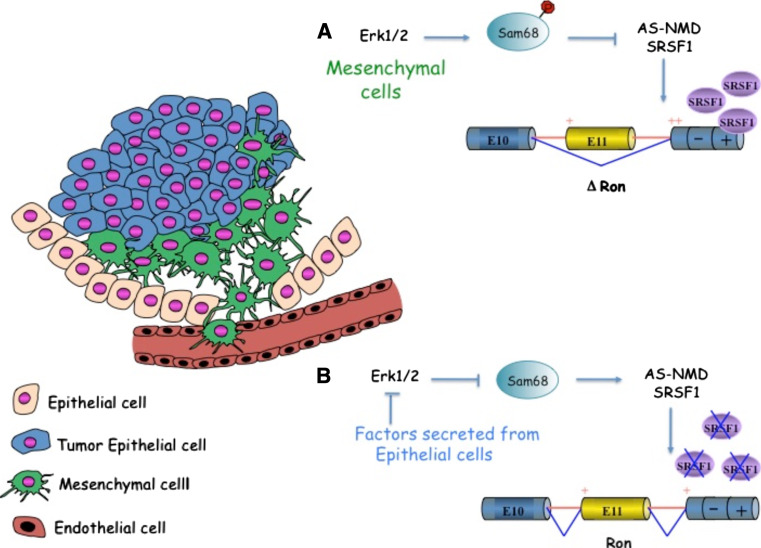
To understand how alternative splicing events and the activity/expression level of splicing factors are coordinated with other mechanisms of gene expression regulation, particularly transcription and signal transduction, remains a major challenge in this area of research. We recently exploited an in vitro model of EMT based on the density of mesenchymal SW480 cells that acquire epithelial features when grown at over-confluence [11]. In this experimental system, the levels of SRSF1 transcripts and proteins are regulated during EMT through the alternative splicing of the 3′UTR-intron associated with the nonsense-mediated mRNA decay pathway (AS-NMD). Interestingly, splicing of the 3′UTR-intron is frequently inhibited in colon cancer [11]. AS-NMD of SRSF1 transcripts is controlled by soluble factors expressed by epithelial cells that act through the extracellular signal-regulated kinase 1/2 (ERK1/2) to modulate the phosphorylation status of another splicing regulator, Sam68 (Src associated in mitosis, 68 kDa). In turn, phosphorylated Sam68, which occurs only in mesenchymal cells, represses splicing of the 3′UTR-intron. Inhibition of ERK activity either with small molecules or with pre-conditioned medium from epithelial cells blocks Sam68 phosphorylation, decreases SRSF1 mRNA and protein levels, promotes inclusion of Ron exon 11, and induces the mesenchymal-to-epithelial cell transition [11] (Fig. 3).
Fig. 3.
Alternative splicing of Ron proto-oncogene during epithelial-to-mesenchymal transitions (EMT). Skipping of Ron exon 11 results in the production of ∆Ron and is controlled by a splicing silencer (–) and an enhancer (+) located in the constitutive exon 12 [10]. The splicing factor SRSF1, by promoting ∆Ron splicing, activates EMT, leading to acquisition of an invasive phenotype. Cells that undergo EMT (green) during tumor progression are characterized by the loss of cell–cell adhesion, cytoskeleton rearrangements, and increased cell motility. Interestingly, morphological and molecular hallmarks of EMT are transient and are restricted to the invasive front of metastasizing carcinomas. SRSF1 levels are regulated during EMT through alternative splicing associated with nonsense-mediated RNA decay (AS-NMD) by another splicing factor (Sam68) that binds within the 3′ UTR-intron of the SRSF1 transcript [11]. a In mesenchymal cells, Sam68 phosphorylation by the extracellular signal regulated kinase 1/2 (ERK1/2) is sufficient to increase the full-length transcript of SRSF1 thus increasing SRSF1 protein levels that in turn drive a ∆Ron-mediated EMT. b Diffusible factors specifically secreted by epithelial cells (blu) repress ERK 1/2 activity by this means inhibiting Sam68 phosphorylation, which reduces SRSF1 protein levels through increased NMD of its transcript
Cancer-specific splicing variants involved in EMT are potential targets for the development of new anti-metastatic therapeutic strategies. In the case of Ron, we explored the possibility to inhibit skipping of exon 11 and the production of ΔRon either with bifunctional oligonucleotides––TOES (Targeted Oligonucleotide Enhancers of Splicing)––or with small-molecule inhibitors of SRSF1, namely indole-derived compounds (IDCs), a new class of splicing inhibitors that selectively block the ESE-dependent splicing activity of individual SR proteins [53]. Both treatments efficiently correct ΔRon splicing and increase exon 11 inclusion. In addition, inhibitors of SRSF1 activity also affect the invasive phenotype of the mesenchymal cells [53]. These constitute important proof-of-concept of strategies toward the development of effective anti-cancer therapeutic approaches. However, considering the multiple roles of the Ron-activated pathway, it will be interesting to investigate in the future whether alteration in Ron splicing levels not only affects invasion but also influences other processes such proliferation and protection from apoptosis.
Tumor microenvironment, EMT, and alternative splicing of the Rac1 gene
Rac1 is a small (~21 kDa) GTPase of the mammalian Rho (Ras-homologous) family [54]. It uses the energy from GTP hydrolysis to modulate and control numerous cellular processes primarily associated with actin dynamics such as cell adhesion and production of polarized protrusions of lamellipodia leading to directional cell migration [54]. Other Rac1 functions include control of the cell cycle, cell transformation, DNA synthesis, and ROS (reactive oxygen species)-mediated genomic instability [54]. The activated GTP-bound Rac1 associates with effector molecules, including PAK kinases, IRSp53/WAVE, MLK2/3, and p67phox, which stimulate several signaling cascades [54].
As with Ron, alternative splicing plays an important role in controlling Rac1 activity. The splice variant Rac1b is generated by inclusion of a highly conserved 57-nucleotide cassette exon (exon 3b) resulting in an in-frame insertion of 19 new amino acids near a protein domain important for the interaction with regulators and effectors [55]. Because of an enhanced intrinsic GDP/GTP exchange activity and an impaired GTPase activity, Rac1b isoform is constitutively active and able to transform NIH 3T3 cells [56]. Furthermore, Rac1b exhibits distinctive functional properties. For example, it does not stimulate several classical Rac1 signaling pathways such as PAK and JNK kinases, while it is able to stimulate the NF-κB pathway [57]. Interestingly, Rac1b is upregulated in breast cancers [58] and in colorectal tumors at various stages of tumor progression, compared to adjacent normal tissues [55].
The molecular mechanisms and signaling pathways that modulate Rac1 splicing have recently been clarified by Jordan and colleagues [59]. Splicing of Rac1 transcripts is controlled by SRSF1 and SRSF3 (already known as SRp20) in an antagonistic manner: SRSF1 increases inclusion, while SRSF3 promotes skipping of exon 3b. As in the case of Ron, splicing decisions are governed by upstream signaling pathways. Thus, the Wnt signaling, activated in many colorectal tumors [60], increases SRSF3 and decreases Rac1b levels while PI3K/AKT kinase (but not of ERK, JNK, or p38 MAP pathways) decreases SRSF1 protein levels and, as a consequence, the Rac1b isoform [59].
Alternative splicing of Rac1 is involved in the tumor promotion activity of matrix metalloproteinases (MMPs), important regulators of tumor microenvironment that modulate proliferation, cell–cell adhesion, angiogenesis, invasion, and the formation of metastases [61]. MMPs expression increases in nearly every type of human cancer and is often linked with poor survival [61]. In particular, MMP-3 (also known as Stromelysin-1) enhances the levels of the transcription factor Snail1 and of vimentin thus promoting the EMT process [62]. Interestingly, exposure of mouse mammary epithelial cells to MMP-3 results in DNA damage and genomic instability that can drive tumorigenesis in transgenic mice [62]. All these processes require the expression of Rac1b, which causes an increase in cellular reactive oxygen species (ROS) by stimulating the release of mitochondrial superoxide into the cytoplasm [62].
Differential expression of CD44 splicing isoforms controls the execution of EMT
An interesting example of how the regulation of multiple alternative splicing events at different levels may impact EMT is provided by CD44.
CD44 is a cell surface glycoprotein with an N-terminal extracellular region, a short hydrophobic transmembrane region, and a cytoplasmic tail. It acts as the receptor for several components of the extracellular matrix (ECM), including hyaluronan, collagen, laminin, and fibronectin. CD44 is primarily involved in cell–cell and cell–matrix interactions [63]. In addition, similarly to other cell-adhesion molecules, it has other important functions in cell signaling cascades activated by alterations in the ECM that influence cell growth, survival, and differentiation [64].
CD44 is expressed from a wide group of cell types, including hematopoietic, epithelial, and mesenchymal cells such as fibroblasts, skeletal muscle, and glial cells. Hematopoietic cells, fibroblasts, and glial cells mainly express a low-molecular-weight CD44 form (90-kDa standard form also known as CD44s), whereas higher-molecular-weight forms (140–230 kDa variants) are expressed only from epithelial tissues [64].
The considerable heterogeneity of CD44 isoforms is predominantly due to alternative splicing events that affect the extracellular membrane-proximal domain. The CD44 gene consists of 21 constitutive exons and ten consecutive variant exons (called v1–v10) located between the constitutive exons 5 and 6. Variant exons are subjected to extensive alternative splicing leading to the production of several isoforms [63, 64], whose physiological functions are still poorly understood. Notably, CD44 splicing variants that contain exon v5 and/or v6 are mainly found in proliferating cells and tumors and their expression often correlates with enhanced malignancy and invasiveness [65, 66]. In particular, isoforms including exon v6 (CD44v6) are bad prognostic markers of gastric cancer and are frequently up-regulated in squamous cell carcinomas and in a proportion of adenocarcinomas of different origin [67, 68]. This association between CD44v6 and cancer paved the way to the development of new therapeutic tools and radiolabeled anti-v6 antibodies were used in clinical trials for the treatment of head and neck cancers [67].
Alternative splicing of CD44 is stimulated by mitogenic signals through the Ras–MEK–ERK pathway and is regulated by several trans-acting splicing factors, including Sam68 and SRm160 [69, 70]. Upon phosphorylation by ERK, Sam68 promotes inclusion of exon v5 probably through protein–protein contacts with factors implicated in splice-site recognition and/or in spliceosome assembly. The link with signaling pathways is suggested also by the ability of CD44v6 to induce Ras activation through formation of a complex with the hepatocyte growth factor HGF and its tyrosine kinase receptor Met [71]. Thus, signaling pathways and CD44 variant expression might be coordinately regulated through a positive feedback loop [72].
Surprisingly, a switch from CD44v to CD44 standard (CD44s) isoforms has been recently reported to be required for the execution of EMT. The switch from CD44v to CD44s was prevented by the splicing factor ESRP1 (Epithelial Splicing Regulatory Protein 1, described in more detail in the following paragraphs) that also negatively regulates EMT [13]. CD44s was shown to drive the formation of breast tumors in mice and be upregulated in high-grade human breast tumors [13]. Finally, the same authors found that CD44s, but not CD44v, activates the PI3K/Akt pathway in TGF-β-induced EMT resulting in impaired expression of E-cadherin and thus accelerating the EMT process. Thus, CD44v and CD44s isoforms appear to have different relevance for invasiveness in different tumor types. Moreover, different CD44 isoforms may interact with different RTKs on the cell surface, thus affecting distinct downstream signaling cascades. These observations highlight the complex role of CD44 and its isoforms in tumor progression. Hence, isoform specificity must be considered when dissecting the role of CD44 in tumorigenesis.
Recent findings indicate that changes in the epigenome enhance the probability of the transformed cell to metastasize [73, 74]. While a genetic mutation initiates the cancer process, the epigenetic change would be necessary to promote cancer progression [74]. As discussed in this review, the same argument can be raised for alternative splicing. It is not surprising, therefore, that chromatin structure and histone modifications can modulate specific alternative splicing decisions [75, 76]. This has been shown for the CD44 gene where the information stored in the histones bound to variant exons through HP1γ can influence splicing decision modulating the elongation rate of RNA polymerase II [77]. Interestingly, several studies have identified within tumors a small population of neoplastic cells defined operationally by their ability to seed new tumors, called cancer stem cells (CSCs) [78]. Notably, an important association between EMT and the formation of stem cells has recently been provided [79]. Since, a high expression level of CD44 is used as a functional CSC marker, one could speculate that alternative splicing of this cell surface protein could have also been involved in the acquisition of epithelial stem cell properties.
Epithelial cell-specific splicing factors for a post-transcriptional regulated EMT program
A strong link between EMT and alternative splicing has been demonstrated in the case of the fibroblast growth factor receptors (FGFRs), a family of four highly conserved tyrosine kinase receptors that bind to members of the largest family of growth factors, namely fibroblast growth factors (FGFs) [80]. Binding of FGFs to their receptors activates a signaling cascade that controls an ample spectrum of biological functions, such as proliferation, cell survival, angiogenesis, migration, and differentiation [80]. The specificity of the FGF–FGFR interaction is in part determined by the tissue-specific expression of particular ligands and receptors, by the different affinity of the ligands for the receptor paralogues and, above all, by alternative splicing events in the third extracellular Ig domain (IgIII) of FGFRs. The IgIII domain is encoded by an invariant exon (IIIa) that is spliced to either exon IIIb or IIIc, two mutually exclusive exons that determine the ligand binding specificity, and are spliced to the exon that encodes the transmembrane region. Alternative splicing of IIIb/IIIc exons has been characterized in detail for the FGFR2 gene. This splicing event is regulated in a cell type-specific manner, with the FGFR2-IIIb and FGFR2-IIIc isoforms predominantly expressed in epithelial and mesenchymal tissues, respectively [81, 82]. By applying a minigene assay, the use FGFR2 exon IIIc was monitored in prostate tumors in rats. Skipping of exon IIIc takes place frequently within the primary tumor and metastasis revealing the occurrence of mesenchymal-to-epithelial transition (MET) and the plasticity of this aggressive tumor [83].
The choice between exons IIIb and IIIc is controlled by several splicing factors. In particular, hnRNP A1 and the polypyrimidine tract-binding protein (PTB) reduce the usage of the epithelial-specific IIIb exon [84, 85], whereas hnRNP H/F cause skipping of exon IIIc [86]. Interestingly, although expressed in both epithelial and mesenchymal cells, PTB is able to inhibit exon IIIb inclusion exclusively in mesenchymal cells [82]. This selectivity is linked to distinctive histone modification signatures, such as histone H3 trimethylation at lysine 36 (H3-K36me3), on chromatin of mesenchymal cells near exon IIIb. Binding of the chromatin-binding protein MRG15 to the H3K36me3 histone recruits PTB in close proximity to exon IIIb leading to exon skipping [76].
Alternative splicing of FGFR2 has been recently investigated by the group of Carstens who reported the isolation of two paralogous and phylogenetically conserved proteins, ESRP1 and ESRP2 (Epithelial Splicing Regulatory Proteins 1 and 2), as the primary factors for controlling the FGFR2-IIIb to FGFR2-IIIc switching during EMT. Similarly to their orthologs in chicken (G. gallus), D. melanogaster and worm (C. elegans), they contain three RRMs (RNA-recognition motifs) with a high identity percentage [87]. Furthermore, ESRPs exhibit strong homology to splicing regulators hnRNP F/H [87]. These RNA-binding proteins, which are expressed exclusively in epithelial cells, contemporary repress inclusion of exon IIIc and increase inclusion of exon IIIb [87]. Interestingly, downregulation of the ESRPs factors is accompanied by with loss of epithelial splicing of CD44 (exons V8–V10), p120-catenin (exons 2 and 3) and ENAH (exon 11a) genes, whereas ectopic expression of ESRP1 in mesenchymal cells restores the epithelial splicing programs.
More recently, by using a genome-wide based approach, the same group has deeply characterized the global splicing regulatory network for ESRPs [12, 87]. This includes several gene transcripts encoding for proteins with well-known roles in EMT such as organization of actin cytoskeleton, cell–cell adhesion, cell polarity, and migration [12, 87]. Interestingly, in some cases, the epithelial and mesenchymal isoforms of ESRPs-regulated transcripts exhibit distinct and even opposing functions in the balance of cell adhesion versus cell migration. That is the case of p120-catenin epithelial and mesenchymal splice variants, which promote respectively cell–cell adhesion and cell migration [88]. Another example is TCF7L2 that acts in association with nuclear β-catenin in EMT transcriptional regulation. The TCF7L2 isoform, which results from inclusion of exon 4, is able to reduce the transcriptional activation of β-catenin-target genes and its expression in epithelial cells is promoted by ESRPs [12, 89]. The analysis also showed that many of the ESRP targets encode for proteins participating in the same biological pathways and/or interacting with one another. For instance, splicing of both p120-catenin and EPB41L5 are regulated by ESRPs and EPB41L5 promotes EMT by inhibiting the binding of p120-catenin with E-cadherin [90]. In addition, ESRPs affect splicing of components and regulators of the vesicle-mediated transport system, involved in maintaining cell polarity and adhesion [12, 91].
Concluding remarks
Cancer is an acquired genetic disease due to mutations in genes that control the cellular homeostasis. Several cancer-associated mutations activate signal transduction pathways leading to alterations in cell proliferation and survival, DNA repair, motility, invasion, and angiogenesis.
Recently, alternative splicing has emerged as an important player in the development and progression of human cancers. Because of its ability to tune gene expression programs to growth conditions and environmental stimuli, deregulation of alternative splicing programs, as for instance caused by the imbalanced expression of trans-acting factors, deeply affects the biology of cancer cells and is causatively associated with multiple aspects of tumor progression, including resistance to treatments. A great deal of data, in addition to those reviewed here, indicates that the unscheduled expression of alternative splicing variants may be crucial for some features of cancers cells and for the metastatic process. This is the case of splicing isoforms promoting the epithelial-to-mesenchymal transition (EMT) or its reversal MET. Thus, deciphering alternative splicing programs and the control mechanisms that link them with signal transduction pathways may contribute to a better comprehension of the cancerous phenotype.
Because of the digital nature of the genetic information, alternative splicing is an ideal target for innovative therapeutic approaches based on antisense oligonucleotides that specifically affect a single splicing isoform. Promising examples are coming from new strategies to correct genetic disorders due to defects in splicing decisions. Since cancer cells frequently show a global alteration of splicing programs, small molecules may be another interesting strategy to modulate the activity of splicing factors and correct the oncogenic phenotype. Hopefully, in the next few years, a better understanding of the contribution of alternative splicing to carcinogenesis, in particular in different sets of tumors, will be reached and novel methods will help identifying new targets for innovative anticancer therapeutic approaches. This will be obtained through detailed analysis of the alternative splicing program activated in different growth conditions and during development.
Acknowledgments
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC project number: 11913), the Association for International Cancer Research (AICR) to C.G. and grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Fondazione Cariplo and the European Union Network of Excellence on Alternative Splicing (EURASNET) to G.B.
Abbreviations
- AS
Alternative splicing
- AS-NMD
Alternative splicing-activated NMD
- ECM
Extracellular matrix
- IDC
Indole-derived compound
- MMP
Metalloproteinase
- PTC
Premature translation termination codon
- NMD
Nonsense-mediated RNA decay
- ROS
Reactive oxygen species
- RTK
Receptor tyrosine kinase
- UTR
Untranslated region
- TOES
Targeted oligonucleotide enhancers of splicing
Contributor Information
Giuseppe Biamonti, Phone: +39-0382-546322, FAX: +39-0382-422286, Email: biamonti@igm.cnr.it.
Claudia Ghigna, Phone: +39-0382-546375, FAX: +39-0382-422286, Email: arneri@igm.cnr.it.
References
- 1.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 5.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Natl Rev Mol Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisuaa-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 9.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, Green MR, Riva S, Biamonti G. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol Cell. 2005;20:881–890. doi: 10.1016/j.molcel.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Valacca C, Bonomi S, Buratti E, Pedrotti S, Baralle FE, Sette C, Ghigna C, Biamonti G. Sam68 regulates EMT through alternative splicing-activated nonsense-mediated mRNA decay of the SF2/ASF proto-oncogene. J Cell Biol. 2010;191:87–99. doi: 10.1083/jcb.201001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y, Carstens RP. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J. 2010;29:3286–3300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghigna C, Valacca C, Biamonti G. Alternative splicing and tumor progression. Curr Genomics. 2008;9:556–570. doi: 10.2174/138920208786847971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 16.Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Blencowe BJ. Alternative splicing: new insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Schwerk C, Schulze-Osthoff K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell. 2005;19:1–13. doi: 10.1016/j.molcel.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 20.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillier LW, Reinke V, Green P, Hirst M, Marra MA, Waterston RH. Massively parallel sequencing of the polyadenylated transcriptome of C. elegans . Genome Res. 2009;19:657–666. doi: 10.1101/gr.088112.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolc V, Gauhar Z, Mason C, Halasz G, van Batenburg MF, Rifkin SA, Hua S, Herreman T, Tongprasit W, Barbano PE, Bussemaker HJ, White KP. A gene expression map for the euchromatic genome of Drosophila melanogaster . Science. 2004;306:655–660. doi: 10.1126/science.1101312. [DOI] [PubMed] [Google Scholar]
- 23.Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30:13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- 24.Isken O, Maquat LE. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat Rev Genet. 2008;9:699–712. doi: 10.1038/nrg2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lareau LF, Inada M, Green RE, Wengrod JC, Brenner SE. Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature. 2007;446:926–929. doi: 10.1038/nature05676. [DOI] [PubMed] [Google Scholar]
- 26.Ni JZ, Grate L, Donohue JP, Preston C, Nobida N, O’Brien G, Shiue L, Clark TA, Blume JE, Ares M., Jr Ultraconserved elements are associated with homeostatic control of splicing regulators by alternative splicing and nonsense-mediated decay. Genes Dev. 2007;21:708–718. doi: 10.1101/gad.1525507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillman RT, Green RE, Brenner SE. An unappreciated role for RNA surveillance. Genome. 2004;5:R8. doi: 10.1186/gb-2004-5-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ. Deciphering the splicing code. Nature. 2010;465:53–59. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 29.Buratti E, Baralle FE. Influence of RNA secondary structure on the pre-mRNA splicing process. Mol Cell Biol. 2004;24:10505–10514. doi: 10.1128/MCB.24.24.10505-10514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McManus CJ, Graveley BR. RNA structure and the mechanisms of alternative splicing. Curr Opin Genet Dev. 2011;21:373–379. doi: 10.1016/j.gde.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Natl Rev Mol Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Burge CB. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA. 2008;14:802–813. doi: 10.1261/rna.876308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biamonti G, Riva S. New insights into the auxiliary domains of eukaryotic RNA-binding proteins. FEBS Lett. 1994;340:1–8. doi: 10.1016/0014-5793(94)80162-2. [DOI] [PubMed] [Google Scholar]
- 34.Smith CW, Valcarcel J. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem Sci. 2000;25:381–388. doi: 10.1016/S0968-0004(00)01604-2. [DOI] [PubMed] [Google Scholar]
- 35.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He C, Zhou F, Zuo Z, Cheng H, Zhou R. A global view of cancer-specific transcript variants by subtractive transcriptome-wide analysis. PLoS One. 2009;4:e4732. doi: 10.1371/journal.pone.0004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le K, Mitsouras K, Roy M, Wang Q, Xu Q, Nelson SF, Lee C. Detecting tissue-specific regulation of alternative splicing as a qualitative change in microarray data. Nucleic Acids Res. 2004;32:e180. doi: 10.1093/nar/gnh173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Q, Saltzman AL, Kim YK, Misquitta C, Shai O, Maquat LE, Frey BJ, Blencowe BJ. Quantitative microarray profiling provides evidence against widespread coupling of alternative splicing with nonsense-mediated mRNA decay to control gene expression. Genes Dev. 2006;20:153–158. doi: 10.1101/gad.1382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 2007;257:157–164. doi: 10.1016/j.canlet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Funakoshi H, Nakamura T. Identification of HGF-like protein as a novel neurotrophic factor for avian dorsal root ganglion sensory neurons. Biochem Biophys Res Commun. 2001;283:606–612. doi: 10.1006/bbrc.2001.4819. [DOI] [PubMed] [Google Scholar]
- 43.Stella MC, Vercelli A, Repici M, Follenzi A, Comoglio PM. Macrophage stimulating protein is a novel neurotrophic factor. Mol Biol Cell. 2001;12:1341–1352. doi: 10.1091/mbc.12.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann Surg Oncol. 2005;12:273–281. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 46.Wang MH, Lao WF, Wang D, Luo YL, Yao HP. Blocking tumorigenic activities of colorectal cancer cells by a splicing RON receptor variant defective in the tyrosine kinase domain. Cancer Biol Ther. 2007;6:1121–1129. doi: 10.4161/cbt.6.7.4337. [DOI] [PubMed] [Google Scholar]
- 47.Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518–5526. doi: 10.1128/mcb.16.10.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karni R, Hippo Y, Lowe SW, Krainer AR. The splicing-factor oncoprotein SF2/ASF activates mTORC1. Proc Natl Acad Sci USA. 2008;105:15323–15327. doi: 10.1073/pnas.0801376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefave CV, Squatrito M, Vorlova S, Rocco GL, Brennan CW, Holland EC, Pan YX, Cartegni L. Splicing factor hnRNPH drives an oncogenic splicing switch in gliomas. EMBO J. 2011;30:4084–4097. doi: 10.1038/emboj.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tauler J, Zudaire E, Liu H, Shih J, Mulshine JL. hnRNP A2/B1 modulates epithelial-mesenchymal transition in lung cancer cell lines. Cancer Res. 2010;70:7137–7147. doi: 10.1158/0008-5472.CAN-10-0860. [DOI] [PubMed] [Google Scholar]
- 52.Golan-Gerstl R, Cohen M, Shilo A, Suh SS, Bakacs A, Coppola L, Karni R. Splicing factor hnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 2011;71:4464–4472. doi: 10.1158/0008-5472.CAN-10-4410. [DOI] [PubMed] [Google Scholar]
- 53.Ghigna C, De Toledo M, Bonomi S, Valacca C, Gallo S, Apicella M, Eperon I, Tazi J, Biamonti G. Pro-metastatic splicing of Ron proto-oncogene mRNA can be reversed: therapeutic potential of bifunctional oligonucleotides and indole derivatives. RNA Biol. 2010;7:495–503. doi: 10.4161/rna.7.4.12744. [DOI] [PubMed] [Google Scholar]
- 54.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a “Rac” of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan P, Brazao R, Boavida MG, Gespach C, Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18:6835–6839. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 56.Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene. 2004;23:9369–9380. doi: 10.1038/sj.onc.1208182. [DOI] [PubMed] [Google Scholar]
- 57.Matos P, Jordan P. RAC1, but not RAC1B, stimulates RELB-mediated gene transcription in colorectal cancer cells. J Biol Chem. 2006;281:13724–13732. doi: 10.1074/jbc.M513243200. [DOI] [PubMed] [Google Scholar]
- 58.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 59.Goncalves V, Matos P, Jordan P. Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways. Hum Mol Genet. 2009;18:3696–3707. doi: 10.1093/hmg/ddp317. [DOI] [PubMed] [Google Scholar]
- 60.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 62.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 64.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Natl Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 65.Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003;46:165–186. doi: 10.1016/S1040-8428(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 66.Sonobe S, Miyamoto H, Nobukawa B, Izumi H, Futagawa T, Ishikawa N, Yamazaki A, Uekusa T, Abe H, Suda K. Prognostic value of CD44 isoform expression in thymic epithelial neoplasms. Cancer. 2005;103:2015–2022. doi: 10.1002/cncr.21046. [DOI] [PubMed] [Google Scholar]
- 67.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 68.Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004;53:567–579. doi: 10.1007/s00262-003-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 70.Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Mol Cell Biol. 2006;26:362–370. doi: 10.1128/MCB.26.1.362-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 74.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 75.Kornblihtt AR. Chromatin, transcript elongation and alternative splicing. Nat Struct Mol Biol. 2006;13:5–7. doi: 10.1038/nsmb0106-5. [DOI] [PubMed] [Google Scholar]
- 76.Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saint-André V, Batsché E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 78.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 79.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 81.Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993;13:4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner EJ, Garcia-Blanco MA. RNAi-mediated PTB depletion leads to enhanced exon definition. Mol Cell. 2002;10:943–994. doi: 10.1016/S1097-2765(02)00645-7. [DOI] [PubMed] [Google Scholar]
- 83.Oltean S, Sorg BS, Albrecht T, Bonano VI, Brazas RM, Dewhirst MW, Garcia-Blanco MA. Alternative inclusion of fibroblast growth factor receptor 2 exon IIIc in Dunning prostate tumors reveals unexpected epithelial mesenchymal plasticity. Proc Natl Acad Sci USA. 2006;103:14116–14121. doi: 10.1073/pnas.0603090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Del Gatto-Konczak F, Olive M, Gesnel M, Breathnach R. hnRNP A1 recruited to an exon in vivo can function as an exon splicing silencer. Mol Cell Biol. 1999;19:251–260. doi: 10.1128/mcb.19.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carstens RP, Wagner EJ, Garcia-Blanco MA. An intronic splicing silencer causes skipping of the IIIb exon of fibroblast growth factor receptor 2 through involvement of polypyrimidine tract binding protein. Mol Cell Biol. 2000;20:7388–7400. doi: 10.1128/MCB.20.19.7388-7400.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mauger DM, Lin C, Garcia-Blanco MA. hnRNP H and hnRNP F complex with Fox2 to silence fibroblast growth factor receptor 2 exon IIIc. Mol Cell Biol. 2008;28:5403–5419. doi: 10.1128/MCB.00739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, Copland JA, Anastasiadis PZ. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. J Biol Chem. 2008;283:18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weise A, Bruser K, Elfert S, Wallmen B, Wittel Y, Wöhrle S, Hecht A. Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/beta-catenin targets. Nucleic Acids Res. 2010;38:1964–1981. doi: 10.1093/nar/gkp1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirano M, Hashimoto S, Yonemura S, Sabe H, Aizawa S. EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. J Cell Biol. 2008;182:1217–1230. doi: 10.1083/jcb.200712086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Natl Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]