Abstract
Increasing evidence demonstrates that Na+, K+-ATPase plays an important role in pulmonary inflammation, but the mechanism remains largely unknown. In this study, we used cardiotonic steroids as Na+, K+-ATPase inhibitors to explore the possible involvement of Na+, K+-ATPase in pulmonary epithelial inflammation. The results demonstrated that mice after ouabain inhalation developed cyclooxygenase-2-dependent acute lung inflammation. The in vitro experiments further confirmed that Na+, K+-ATPase inhibitors significantly stimulated cyclooxygenase-2 expression in lung epithelial cells of human or murine origin, the process of which was participated by multiple cis-elements and trans-acting factors. Most importantly, we first described here that Na+, K+-ATPase inhibitors could evoke a significant Hu antigen R nuclear export in lung epithelial cells, which stabilized cyclooxygenase-2 mRNA by binding with a proximal AU-rich element within its 3′-untranslated region. In conclusion, HuR-mediated mRNA stabilization opens new avenues in understanding the importance of Na+, K+-ATPase, as well as its inhibitors in inflammation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0444-1) contains supplementary material, which is available to authorized users.
Keywords: Na+, K+-ATPase; Cardiotonic steroids; Acute lung inflammation; Cyclooxygenase-2; Hu antigen R
Introduction
Alveolar epithelial Na+, K+-ATPase plays an important role in the pathogenesis of acute lung injury (ALI), and its most severe manifestation acute respiratory distress syndrome (ARDS) [1]. The impairment of this pump normally leads to decreased active Na+ transport across the alveolar epithelium, and inefficient lung edema clearance [2]. Besides, disruption of membrane tight junction integrity by Na+, K+-ATPase impairment results in altered lung permeability, accumulation of pulmonary edema, and hindered gas exchange. Many pathophysiologic perturbations such as hypoxia, mechanic ventilation can lead to the decreased Na+, K+-ATPase function in ALI [1, 2].
Overexpression of Na+, K+-ATPase subunits in lung epithelial cells has been proven to be an effective approach for improving lung fluid clearance, at least in animal tests [3, 4]. However, a recent study demonstrated that electroporation delivery of Na+, K+-ATPase α1 and β1 subunits into rat’s lung not only prevented lipopolysaccharide (LPS)-induced pulmonary edema but also reduced lung inflammation [5]. This result suggests the possible involvement of Na+, K+-ATPase in lung inflammation. Cytokine-mediated inflammatory reaction in lungs is the major characteristic of ALI and ARDS. Injured lung epithelial, endothelial cells, or immune cells are all responsible for the abnormal cytokine production [6]. Whether Na+, K+-ATPase engages in the pulmonary inflammation through modulating cytokine production from these cells constitutes intriguing questions.
To address this concern, suppression of Na+, K+-ATPase or deletion of Na+, K+-ATPase gene in lung cells is necessary. However, limited by the ability to develop Na+, K+-ATPase knockout models, the use of chemical inhibitors has become an alternative tactic in many studies. So far, many cardiotonic steroids (CTS) are proven to be Na+, K+-ATPase-specific inhibitors that can bind with the extracellular portion of Na+, K+-ATPase α subunits, and compete for the K+ binding site. The pharmacological outcomes of CTS include intracellular [Ca2+] increase, reactive oxygen species (ROS) generation, and Src activation [7]. Until now, digitoxin, one of the CTS isolated from plants, is still frequently used in the clinical treatment of congestive heart failure [8]. In addition to these exogenously produced cardiotonic substances, endogenous CTS were also successfully isolated and identified from human plasma [9, 10], which spurs the enthusiasm in exploring their new biological roles. Notably, it is increasingly appreciated that these materials belong to a novel class of mammalian endocrine hormones [7].
Ouabain is a well-studied CTS with a high binding specificity for Na+, K+-ATPase. Amazingly, ouabain was recently identified to be identical with one of the mammalian inhibitors of Na+, K+-ATPase, called endogenous ouabain (EO) [10]. Therefore, determination of the biological activity of ouabain will have far-reaching effects on understanding the pathophysiologic significance of Na+, K+-ATPase. In fact, many investigations consider that besides being a cardiotonic substance, ouabain can also act as a potential immunomodulator [11]. For example, ouabain was shown to affect various immunological processes, such as lymphocyte proliferation, apoptosis, and monocyte function [11]. However, the most prominent role of ouabain involved in the immune system is its ability to modulate cytokine production. Ouabain was found to increase IL-1, IL-6, and tumor necrosis factor α (TNF-α) production in mononuclear cells [12–14], or stimulate nitric oxide (NO) production in rat peritoneal macrophages [15]. Besides these in vitro results, ouabain was also shown to modulate cytokine production in vivo [13, 16].
Surprisingly, although the function of ouabain in regulating cytokine production has been long appreciated, the underlying mechanism remains poorly understood. In this study, we observed that ouabain, as well as other Na+, K+-ATPase inhibitors, could induce acute lung inflammation by increasing cyclooxygenase-2 (COX-2) expression in lung epithelial cells, both in vitro and in vivo. Furthermore, Hu antigen R (HuR) translocation is an important regulatory mechanism for COX-2 production elicited by the Na+, K+-ATPase inhibitor.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and lipofectamine were from Invitrogen (California, USA). Ouabain (purity > 99%), foliandrin (purity > 99%), actinomycin D (Act D) and LPS were from Sigma (St. Louis, USA). Oleandrin (purity > 99.5%) was provided by Ronghe Chemicals (Kunming, China). SP600125, SB203580, and rofecoxib were from Calbiochem (San Diego, California, USA). U0126 was from Cell Signaling Technology (Beverly, Massachusetts, USA). [32P] ATP was from Radioactive Materials Center of China (Beijing, China). Nitrocellulose membranes were from Schleicher & Schuell (Keene, New Hampshire, USA).
Cell culture
Human type II lung adenocarcinoma epithelial cells (A549), transformed human embryonic kidney cells (HEK 293), and mouse lung epithelial cells (MLE12) were purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA), and cultured in DMEM supplemented with 10% FBS and 50 U/ml penicillin/streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2, trypsinized until 70% confluence and plated for experimental use.
Plasmids
The COX-2 5′-untranslated region (UTR) full-length promoter construct (−942/+71) [17] was a generous gift of Dr. Michael James (Royal Adelaide Hospital, Australia). Primers used to amplify COX-2 promoter variants (−528/+71, −391/+71, −262/+71, −210/+71, −121/+71, −51/+71), or to amplify COX-2 full-length 3’-UTR and its variants are listed in Table E1 (supplementary data). The expression vector encoding inactive p38α MAPK (p38α AGF) [18] was generously gifted by Prof. Roger Davis (University of Massachusetts, USA). The MAPK-activated protein kinase 2 (MK2) constitutively active expression plasmid (pMK2 AspX3), and two MK2 dominant negative (DN) vectors (pMK2 Ala and pMK2 kin-) [19] were kindly gifted by Prof. Chris J. Marshall (Royal Cancer Hospital, London, UK). The sequence of N-terminal truncated form of human IκBα (IκBα DN, 37–317 amino acid) was amplified by RT-PCR using the primers as follows, forward: 5′-cccggatccatgaaagacgaggagtacg-3′; reverse: 5′-cgcgaagctttcataacgtcagacg ctggcctcc-3′. After digestion, the PCR product was subcloned into hemagglutinin (HA)-tagged pRK5 mammalian expression vectors. By Western-blot analysis, this IκBα DN plasmid was confirmed to have a high expression level in A549 cells, further, TNF-α-induced NF-κB activation in A549 cells could be completely suppressed under the overexpression of IκBα DN.
Transient transfection and luciferase assays
Transient transfection of cells with the human COX-2 promoter, COX-2 3′-UTR or their variants cloned into pGL3-Basic luciferase reporter plasmid were conducted in 24-well plates as described previously [20]. The luciferase activity in samples was measured following the manufacture’s instructions (Promega, USA).
Murine model of ouabain-induced acute lung injury [21]
All animal procedures were approved in advance by the Animal Care Committee of Jiangsu Province of China. Mice (ICR, 18.2 ± 1.2 g) were randomly assigned to experimental groups. In the control group, animals inhaled sterile 0.9% NaCl. In the LPS group, animals inhaled aerosolized LPS to induce ALI. In the ouabain group, aerosolized ouabain was administered, whereas in the ouabain/rofecoxib group, animals were intraperitoneally (i.p.) injected with rofecoxib (10 mg/kg) at 60 min before aerosolized ouabain inhalation. Animals inhaled 0.9% NaCl, ouabain or LPS for 30 min at 2-h intervals over a 4-h period (Fig. 1a-1). For each drug inhalation, a solution of 0.9% NaCl containing 1 mg/ml ouabain, or 500 μg/ml LPS (pH 7.2, 295 mosmol/kg) was aerosolized in room air by an ultrasonic nebulizer (Optineb, Nebu-Tec, Elsenfeld, Germany).
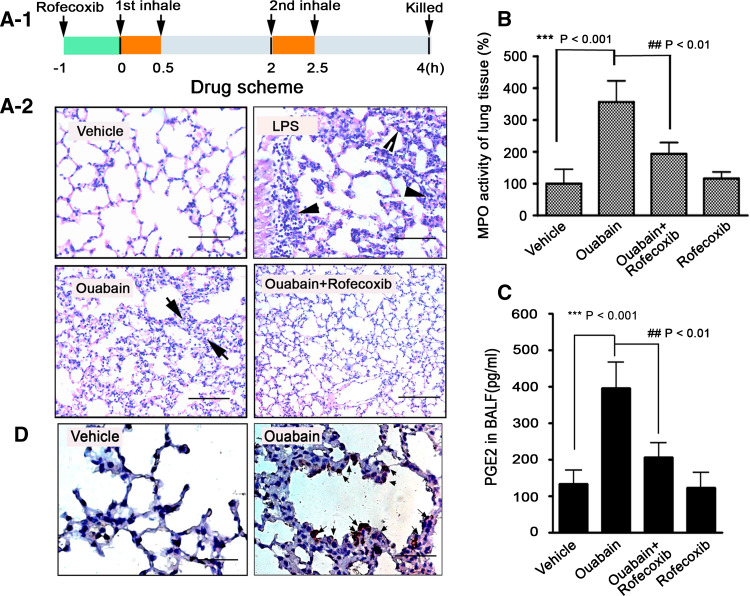
Fig. 1.
Ouabain induces acute lung inflammation in mice. a-1 Schematic of drug administration. a-2 Inhaled ouabain developed COX-2-dependent acute lung inflammation. Mice challenged with inhaled LPS, or 0.9% NaCl alone served as controls. Lung sections were subjected to H&E staining. Representative photomicrographs from six lung sections of mice having similar results are shown. Scale bars: 25 μm. The black arrows indicate the thickness of lung interstitial space, and neutrophils infiltration as well. b Inhaled ouabain increased the MPO activity in the lung tissue of mice. MPO activity of lung tissue from control animals was arbitrarily set at 100%. ***p < 0.001, vehicle versus ouabain, ## p < 0.01, ouabain versus ouabain + rofecoxib. c Inhaled ouabain stimulated the PGE2 release in BALF of mice. PGE2 content in BALF was measured by ELISA method, and expressed as pg/ml. ***p < 0.001, vehicle versus ouabain, ## p < 0.01, ouabain versus ouabain + rofecoxib. d Inhaled ouabain stimulated COX-2 expression in mice lung epithelial cells, as assessed by histological analysis. Whole lungs were fixed, sectioned, and stained with anti-mouse COX-2 (n = 4–6 mice/group). Representative photomicrographs having similar results are shown. Scale bars: 20 μm
Analysis of myeloperoxidase activity
Myeloperoxidase (MPO) activity in lung homogenates was determined by a 3,3′–5,5′-tetramethylbenzidine-based photometric assay [22].
RNA interference
A549 cells were transfected with the small interference RNA (siRNA) for human HuR as previously described [23]. The siRNAs targeting the human or murine Na+, K+-ATPase α1 subunit, or murine HuR, were purchased from Santa Cruz Biotechnology (USA), and cell transfection was performed based on the manufacturer’s instructions.
Determination of prostaglandin E2 level in culture or bronchoalveolar lavage fluid
5 × 104 cells/well were plated in six-well dishes and grown to 60% confluence in the growth medium. After treatment, the medium was collected, centrifuged, and stored at −80°C until assay. To measure prostaglandin E2 (PGE2) release in bronchoalveolar lavage fluid (BALF), 1 ml BALF was collected, centrifuged, and assayed. The ELISA method was used to measure the level of PGE2 in the cell culture, or in BALF based on the manufacturer’s instructions (Cayman, Ann Arbor, Michigan, USA).
Quantitative RT-PCR and conventional RT-PCR
Total RNA was isolated using TRIZOL agent and chloroform extraction according to the seller’s RNA extraction protocol. The PCR primers used to amplify COX-2, 28S, GAPDH are listed in Table E1 (supplementary data). Quantitative RT-PCR (qRT-PCR) was performed as previously described [24]. The oligonucleotides used for COX-2 and GAPDH amplification by qRT-PCR are listed in Table E1 (supplementary data). The COX-2 and GAPDH mRNA levels were calculated based on the standard curve, and COX-2 mRNA level was normalized to GAPDH mRNA level in the same sample.
Immunoprecipitation of mRNP complex and RT-PCR
To explore the interaction between COX-2 mRNA and HuR protein, a combination of immunoprecipitation and RT-PCR was used as previously described [23, 24]. Briefly, A549 cells treated with ouabain were lysed, and immunoprecipitated with 3 μg of either a monoclonal HuR Ab, or the same amount of control IgG overnight at 4°C. To normalize equal input of RNA before subsequent immunoprecipitation step, the same amount of extract was subjected to total RNA isolation by TRIZOL agent. Subsequently, protein G-Sepharose CL-4B beads were added and incubated for another 3 h. After centrifugation, the beads were successively washed with low- and high-salt buffer before total RNA extraction, the following RT-PCR was performed as described in the “conventional RT-PCR”.
Indirect immunofluorescence microscopy
Immunofluorescence was performed based on previously described protocol with minor changes [25]. Briefly, cells were fixed using 4% formaldehyde, and incubated overnight at 4°C with a monoclonal anti-human, or anti-mouse HuR Ab (Santa Cruz Biotechnology, USA) diluted at 1:200 in blocking buffer. The secondary Ab conjugated with FITC (Molecular Probes, Eugene, OR) was added for 2 h at room temperature. After rinsing, the slides were incubated with DAPI (Molecular Probes, USA) for 5 min to stain nuclei, and viewed through a Carl Zeiss fluorescent microscope. Images were processed using Photoshop software (Adobe, San Jose, CA).
Immunohistochemical analysis
The isolated mice lung tissue samples were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-mm-thick sections. The sections were stained with COX-2 using a 1:200 dilution of purified rabbit anti-mouse COX-2 Ab (Santa Cruz Biotechnology, USA). Signal was developed using liquid diaminobenzidine tetrahydrochloride and counterstained with hematoxylin and eosin stain (H&E).
Western-blot analysis
The immunoblot analysis was performed as previously described [26]. Briefly, A549 cells after treatments were harvested and lysed, and the cleared lysate was separated by SDS-PAGE. After electrophoresis, proteins were transferred to PVDF membranes. The membranes were first hybridized with primary antibodies, and then with a horseradish peroxidase (HRP)-conjugated anti-mouse, or anti-rabbit IgG secondary Ab (Sigma, St. Louis, MO). Human COX-2 monoclonal Ab was from BD Pharmingen (San Diego, California, USA), HuR (3A2), FLAG, GAPDH, and tubulin Abs were from Santa Cruz Biotechnology (Santa Cruz, California, USA). Abs to extracellular regulated protein kinases 1/2 (ERK1/2), phosphor-ERK1/2, p38 MAPK, phosphor-p38 MAPK and phosphor-MK2 were from Cell Signaling Technology (Beverly, Massachusetts, USA). The immune blots were developed using enhanced chemiluminescence system (Amersham Pharmacia Biotech, Amersham, UK).
Statistical analysis
The results were expressed as mean ± SEM. The statistical analysis involving two groups was performed by means of Student’s t test. Analysis of variance followed by Dunnett’s multiple comparison test was used to compare more than two groups. All data were processed with SPSS 10.0 software.
Results
Ouabain inhalation induces acute lung inflammation in mice
Experimental inhalation is a commonly used approach to investigate the pathological mechanism of acute lung epithelial inflammation in mice [21]. In this study, with the purpose of examining the direct pathological effect of Na+, K+-ATPase inhibitor on mice lung, animals were challenged with aerosolized ouabain via inhalation (Fig. 1a-1). As a result, acute inflammatory response occurred in mice lung after exposure to aerosolized ouabain, the severity of which was evidenced by massive neutrophil infiltration into the pulmonary interstitium and airspace (see black arrows, Fig. 1a-2). Interestingly, these pathological abnormalities were largely attenuated when mice were preinjected (i.p.) with rofecoxib, a selective COX-2 inhibitor, since neutrophil recruitment was greatly reduced in lung tissue from animals treated with ouabain plus rofecoxib. As a positive control, inhaled LPS induced a stronger inflammation than that of ouabain.
The improvement of ouabain-induced lung inflammation by rofecoxib was confirmed by MPO activity of lung tissue (Fig. 1b, ## p < 0.01). PGE2 is an active metabolite of COX-2, we noted a significant increase of PGE2 production in BALF isolated from mice lung in the ouabain-treated group (Fig. 1c, ***p < 0.001), which was largely attenuated in the presence of rofecoxib (## p < 0.01). To characterize the lung cells responsible for COX-2 overexpression, immunohistochemistry experiment by using COX-2 antiserum was performed. The result showed the upregulation of COX-2 expression by ouabain mostly occurred in lung epithelial cells, especially in the type II cells surrounding the alveolar space (see black arrows, Fig. 1d). Notably, inhaled ouabain used in this study did not cause lung epithelial cell death, as examined by TUNEL staining (data not shown).
CTS stimulate Na+, K+-ATPase-dependent COX-2 expression in lung epithelial cells
To support in vivo results, we treated A549 cells with multiple CTS. A549 cells are a well-established alveolar epithelial cell line, and are often used as a laboratory model for characterizing the relationship between Na+, K+-ATPase and ALI [27–31]. In addition, this cell line is widely used to explore the molecular mechanisms underlying pulmonary epithelial inflammation induced by various stimuli [32–35]. CTS used in this study include ouabain, foliandrin, and oleandrin. The results, as shown in Fig. 2a, displayed that ouabain, foliandrin, and oleandrin time-dependently stimulated COX-2 mRNAs expression in A549 cells. Among them, ouabain also induced COX-2 mRNA expression dose-dependently, with the minimal concentration needed was at 10 nM (Fig.2b-1, b-2). The elevation of COX-2 mRNA levels by CTS led to strengthened COX-2 protein expressions (Fig. 2c). Of note, we performed a time-course experiment and found that COX-2 protein expression reached a plateau after ouabain treatment for 12 h (Fig. 2d). Consistent with in vivo experiments (Fig. 1c), CTS also led to significant induction of PGE2 in A549 cells (Fig. E1, supplementary data). Some reports suggest the biological activity of CTS, such as ouabain, is sometimes Na+, K+-ATPase independent [36]. In this study, we transfected A549 cells with Na+, K+-ATPase α1 subunit siRNA to knockdown α1 subunit expression. As expected, the siRNA transfection led to a significant decrease in Na+, K+-ATPase α1 subunit expression (Fig. 2e). Under this circumstance, ouabain-enhanced COX-2 protein expression, as well as PGE2 production (Fig. 2f) was significantly suppressed. Of note, in agreement with other investigations [37], most A549 cells (95% or so) remained healthy under the lessened expression of Na+, K+-ATPase α1 subunit (data not shown). Ouabain also increased COX-2 mRNA production in MLE cells, as that in A549 cells (Fig. E2, supplementary data). Similarly, ouabain-induced PGE2 production in MLE cells was relieved under the lessened Na+, K+-ATPase α1 subunit expression by siRNA transfection (Fig. E3, supplementary data).
Fig. 2.
Na+, K+-ATPase inhibitors increase COX-2 expressions in human and murine lung alveolar epithelial cells. a RT-PCR analysis of COX-2 mRNA levels in A549 cells after treatment with Na+, K+-ATPase inhibitors, ouabain, oleandrin, and foliandrin. Cells were challenged with these inhibitors at a concentration of 100 nM for 0, 1, 2, 3, 4, and 6 h, respectively. 28S was included as a control. b-1 RT-PCR analysis of COX-2 mRNA level in A549 cells after treatment with ouabain at varied concentrations. The quantification result is shown (b-2). Cells were challenged with ouabain for 6 h at 0, 10, 25, 50, and 100 nM, respectively. **p < 0.01, ***p < 0.001, as compared to the vehicle group. The mRNA expression level in the vehicle group was arbitrarily set at 100%. c Western-blot analysis of COX-2 protein levels in A549 cells after treatment with ouabain, oleandrin, and foliandrin. Cells were treated with these chemicals at 100 nM for 12 h. d Ouabain dose-dependently induced COX-2 protein expression in A549 cells. Cells were treated with ouabain at 100 nM for 0, 4, 8, 12, and 16 h, respectively. e, f Ouabain-induced COX-2 protein expression and PGE2 production were suppressed in A549 cells after knockdown of Na+, K+-ATPase α1 subunit expression. Cells were left untransfected, or transfected with the scramble or Na+, K+-ATPase α1 siRNA for 48 h, then treated with ouabain at 100 nM for an additional 12 h. Western-blot analysis was performed to measure COX-2, Na+, K+-ATPase α1 subunit and GAPDH protein levels. PGE2 content in the culture medium was measured by ELISA method, and expressed as pg/ml. ### p < 0.001 as compared with Na+, K+-ATPase α1 siRNA group. All experiments were performed at least in triplicate
Identification of cis-elements within the COX-2 promoter responsible for ouabain-induced COX-2 gene transcription
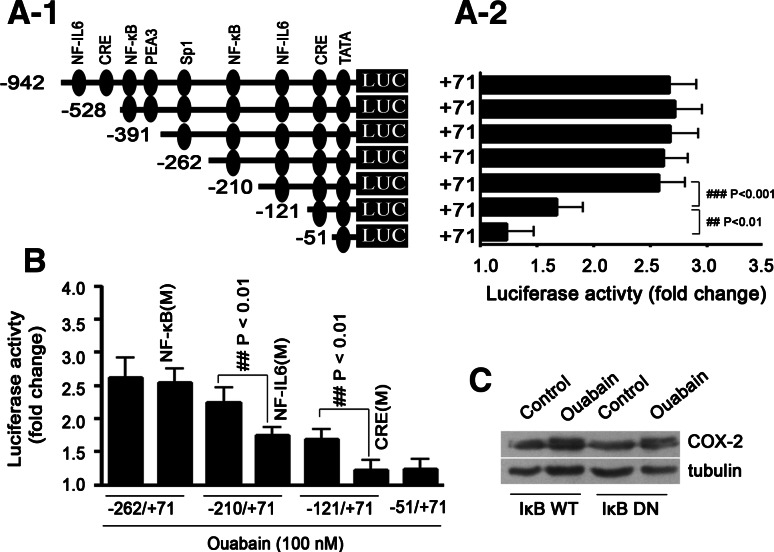
Preliminary experiments demonstrated that ouabain could stimulate COX-2 gene transcription in A549 cells (Fig. E4, supplementary data). In an attempt to identify the cis-elements within a COX-2 promoter that are important for mediating the inductive effect of ouabain, transient transfections were performed with a series of human COX-2 5′-promoter deletion constructs (Fig. 3a-1, a-2). As a result, we found the magnitude of induction by ouabain remained essentially constant with all promoter deletion constructs, except the −121/+71 and −51/+71 constructs. Based on computational analysis, an NF-IL6/C/EBP binding site is present within nucleotides between −210 and −121, and a CRE binding site is present within nucleotides between −121 and −51. Transient transfections were thus performed using the COX-2 promoter including the mutagenized NF-IL6/C/EBP or CRE binding site. As shown in Fig. 3b, stimulating COX-2 promoter by ouabain was attenuated under the condition of NF-IL6/C/EBP or CRE binding site mutation. Unexpectedly, mutagenizing of the NF-κB site has no significant effect, and accordingly, overexpression of IκBα DN in A549 cells failed to suppress ouabain-induced COX-2 upregulation in protein level (Fig. 3c). DNA-EMSA experiments further confirmed the importance of NF-IL6/C/EBP or CRE binding site in COX-2 gene transcription by ouabain (Fig. E5, supplementary data).
Fig. 3.
NF-IL6/C/EBP and CRE-binding sites are cis-elements critically involved in ouabain-induced COX-2 gene transcription. a-1 Identification of ouabain-responsive elements within the COX-2 promoter by sequential deletion analysis. Cells were transfected with a series of COX-2 promoter variants, further treated with ouabain for 12 h. The fold increase of luciferase activity (firefly/renilla) of each transfection group after ouabain treatment is indicated (a-2). ### p < 0.001, −210 to +71 vs. −121 to +71; ## p < 0.01, −121 to +71 vs. −51 to +71. b Ouabain-induced COX-2 promoter activation was suppressed when NF-IL6/C/EBP or CRE-binding site, but not NF-κB-binding site was mutated. Cells were transiently transfected with the intact, or mutated human COX-2 promoter variants. M: mutation, the mutagenesis of NF-κB, NF-IL6, or CRE-binding sites within the COX-2 promoter were performed by PCR, and the primers are listed in Table E1 (supplementary data). The fold increase of luciferase activity in the cell transfection group after ouabain treatment is indicated. c Overexpression of IκBα DN failed to suppress ouabain-induced COX-2 protein expression in A549 cells. Cells were transiently transfected with 2 μg wild-type (WT), or IκBα DN overexpressing plasmid, further treated with ouabain at 100 nM for 12 h. All experiments were performed at least in triplicate
Ouabain stabilizes COX-2 mRNA
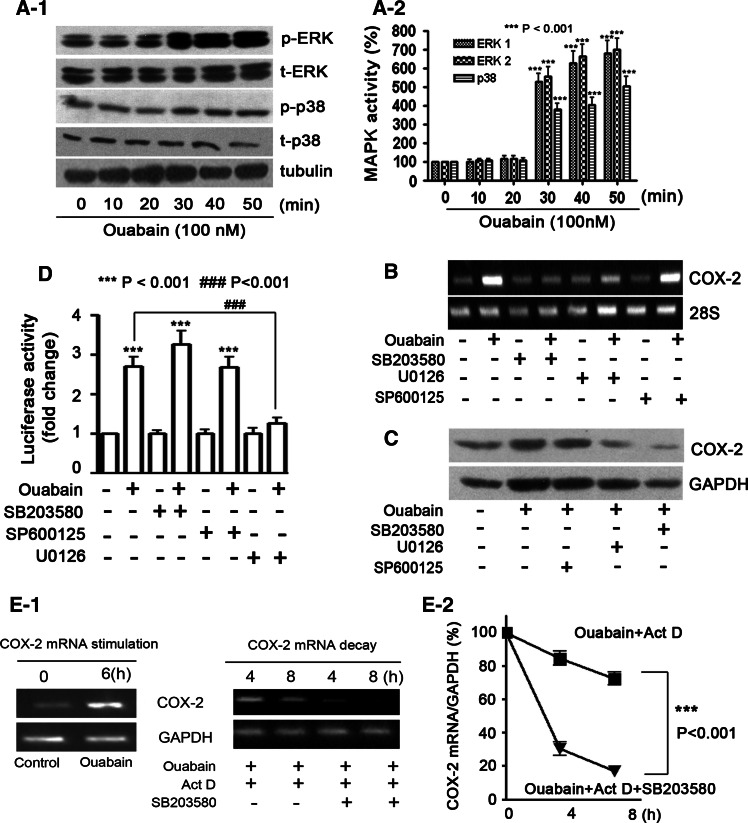
From the above results, we noticed that ouabain-induced COX-2 promoter activation is indeed not fully compatible with COX-2 mRNA production. This conflict invites us to speculate that the transcriptional upregulation is likely not the sole mechanism underlying COX-2 mRNA production by ouabain. As COX-2 is a short-lived protein and its mRNA is unstable, we thus sought to examine whether ouabain could influence the mRNA stability of COX-2. Cells were treated with ouabain (100 nM) for 6 h to induce COX-2 transcription, and then transcription was stopped with the addition of Act D at 5 μg/ml (Fig. 4a-1). The total cellular RNA was isolated at 4 and 8 h after treatment with Act D, and subjected to real-time qRT-PCR analysis (Fig. 4a-2). The results demonstrated that ouabain treatment caused a significant increase in the half-life of COX-2 mRNA (***p < 0.001).
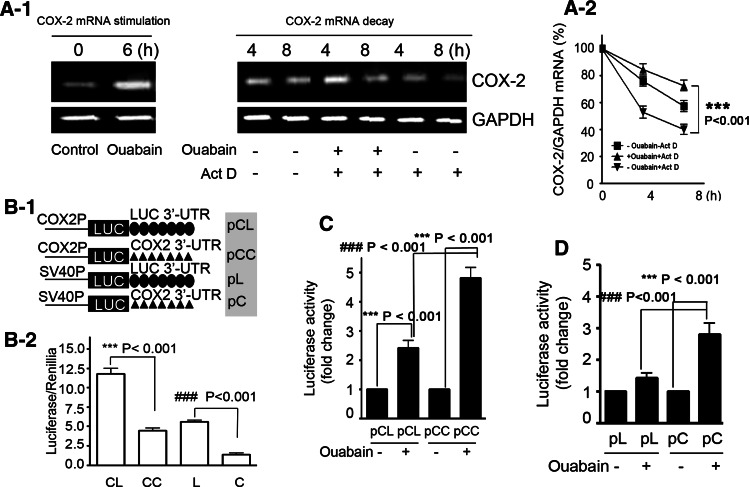
Fig. 4.
Ouabain prevents COX-2 mRNA decay by stabilization of COX-2 3′-UTR. a-1 COX-2 mRNA decay was prevented by ouabain treatment. A549 cells were stimulated with ouabain (100 nM) for 6 h, and washed twice before 5 μg/ml Act D was added. After a short preincubation with Act D for 30 min, cells were additionally treated for the indicated times (4 and 8 h) with vehicle or with 100 nM ouabain before being harvested and extracted for total cellular RNA. At the indicated time, COX-2 mRNA levels were quantified by qRT-PCR by using GAPDH as normalization control (a-2). ***p < 0.001, ouabain + Act D versus-ouabain + Act D. b-1 COX-2 3′-UTR decreased the basal level of luciferase activity without ouabain treatment. Construction of firefly luciferase reporter plasmid harboring COX-2 or luciferase 3′-UTR, and the abbreviations for these constructs are indicated. COX2P: COX-2 promoter; SV40P, SV40 promoter. A549 cells were transiently transfected with 1 μg firefly luciferase plasmids (pCL, pCC, pL, and pC) and 50 ng pRL-TK. The luciferase activity (firefly/renilla) of each transfection group is indicated (b-2). ***p < 0.001, pCL versus pCC, ### p < 0.001, pL versus pC. c, d Ouabain treatment led to enhanced luciferase activity in A549 cells after transfection with reporter plasmids containing COX-2 3′-UTR. The plasmids used in C are driven by a COX-2 promoter, used in D are driven by an SV40 promoter. After transfection, A549 cells were treated with ouabain (100 nM) for an additional 12 h. The luciferase activity of A549 cells without ouabain treatment was arbitrarily set at 1.0. All experiments were performed at least in triplicate
COX-2 3′-UTR plays an important role in regulating mRNA stability [20], transient transfections were thus carried out in A549 cells with COX-2 3′-UTR luciferase reporter construct, or with luciferase 3′-UTR construct as a control. To simplify the interpretation, the four luciferase reporter plasmids used in the present study are pictured in Fig. 4b-1, and abbreviated as pCL, pCC, pL, and pC, respectively. Without ouabain stimulation, we found COX-2 3′-UTR resulted in a significant decrease of the basal luciferase activity (Fig. 4b-2, ***p < 0.001, pCL vs. pCC; ### p < 0.01, pL vs. pC), suggesting the existence of special cis-elements within COX-2 3′-UTR that renders the luciferase mRNA transcripts easily degradable.
In contrast, when ouabain was present, a one-fold increase of luciferase activity was observed in pCC transfectant as compared to that in pCL transfectant (### p < 0.001, pCC vs. pCL, Fig. 4c). Please note the only difference between pCL and pCC is the 3′-UTR cloned (Fig. 4b-1). Therefore, besides gene transcriptional activation, stabilization of COX-2 3′-UTR could be another important mechanism for ouabain to increase COX-2 mRNA steady level. Similar results were also obtained in cells transfected with pL or pC plasmid harboring an SV40 promoter (Fig. 4d, ### p < 0.001, pL vs. pC). In this experiment, the stimulatory effect of ouabain on luciferase activity of pL-transfected cells was negligible, probably because SV40 promoter is insensitive to ouabain stimulation.
HuR is necessary for COX-2 mRNA stabilization induced by CTS in A549 cells
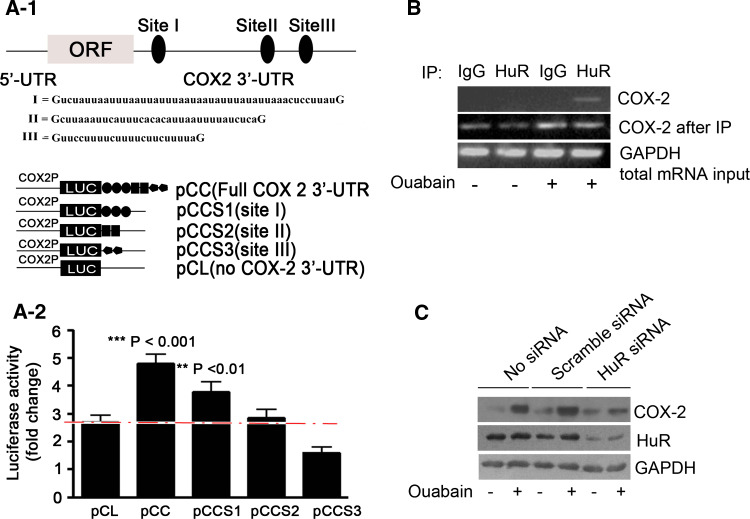
Recently, three major AU-rich elements (AREs) have been identified in the proximal end of the 3′-UTR of COX-2 mRNA, which are important for modulating COX-2 mRNA stability [38] (Fig. 5a-1). Experiments were thus performed to determine whether they also mediate the mRNA-stabilizing effect of ouabain. To simplify the interpretation, three luciferase constructs, each containing a COX-2 promoter and one of the three AREs of COX-2 3′-UTR, are pictured (Fig. 5a-1). Luciferase constructs with full-length of COX-2 3′-UTR, or with luciferase 3′-UTR served as controls. The abbreviations for these constructs are pCC, pCCS1, pCCS2, pCCS3, and pCL, respectively. The result demonstrated that among the COX-2 3′-UTR variants used, only the luciferase activity increase in pCCS1 was comparable to that of full-length 3′-UTR (Fig. 5a-2). Therefore, ARE site I is mainly responsible for COX-2 3′-UTR stabilization by ouabain. Interestingly, ouabain even suppressed the luciferase activity in pCCS3 when compared to control (pCL), suggesting that AREs within COX-2 3′-UTR may have different responsiveness to ouabain. As such, the protective effect of ouabain on COX-2 mRNA is probably a combined result of 3′-UTR stabilization and destabilization.
Fig. 5.
HuR silencing prevents ouabain-induced COX-2 upregulation. a-1 ARE site I within COX-2 3′-UTR was mainly responsible for ouabain-induced stabilization of COX-2 mRNA. Three major AREs within human COX-2 3′-UTR are indicated. Construction of luciferase reporter plasmids harboring a COX-2 promoter and ARE site of COX-2 3′-UTR, and the abbreviations for these constructs are shown in the lower panel. A549 cells were transiently transfected with 1 μg of the indicated firefly luciferase reporter plasmid and 50 ng pRL-TK, further treated with ouabain at 100 nM for 12 h. The fold increase of luciferase activity in cells after ouabain treatment is shown (a-2). **p < 0.01, ***p < 0.001, as compared to pCL transfectant. b Ouabain increased the intracellular binding of HuR to COX-2 mRNA. The immunoprecipitation of mRNP complex was performed as described in the “Materials and methods” section. Representative RT-PCR results of three independent experiments showing similar results are shown. GAPDH was used to normalize the total RNA input. c HuR silencing suppressed Na+, K+-ATPase inhibitors-induced COX-2 protein expression in A549 cells. Cells were left untransfected, or transfected with HuR siRNA or scramble siRNA (100 pmol) for 36 h, treated with ouabain (c) at 100 nM for an additional 12 h. GAPDH was included as a control. All experiments were performed at least in triplicate
Various trans-acting factors binding on the AREs facilitate the post-transcriptional regulation mediated by AREs [39]. Among these factors, HuR was a key RNA-binding protein responsible for increased COX-2 mRNA stability by CTS. The reasons are as follows, when in vivo RNA pull down experiment was performed, HuR co-immunoprecipitated with COX-2 mRNA in A549 cells after challenge of ouabain (Fig. 5b). In contrast, normal IgG did not pull down COX-2 mRNA. Further, transfection with HuR siRNA in A549 cells resulted in a lessened HuR, and consequently suppressed the ouabain-induced COX-2 protein upregulation (Fig. 5c). Comparable results were also obtained in A549 cells after foliandrin or oleandrin treatment (Fig. E6, supplementary data).
CTS regulate the nucleo-cytoplasmic shuttling of HuR
HuR is a nuclear-to-cytoplasmic shuttling protein [39, 40]. In this study, HuR remained exclusively nuclear in control cells, as examined by indirect immunofluorescence analysis (Fig. 6a). However, after ouabain treatment, HuR altered its cellular distribution, and was predominantly present in the cytoplasm. Ouabain-induced HuR translocation was specific, because HnRNP A1 or AUF1, two other nuclear RNA-binding proteins, failed to alter their subcellular distribution after ouabain treatment (Fig. E7, supplementary data). CTS also time-dependently triggered the HuR translocation (Fig. 6b-1). To biochemically confirm the stimulated HuR redistribution, cytoplasmic fractions were carefully separated, and subjected to a Western-blot analysis. Ouabain time-dependently increased the HuR content in cytoplasmic extracts, with the minimal induction time was 2 h (Fig. 6b-2).
Fig. 6.
Ouabain induces HuR nuclear export specifically involved in COX-2 upregulation. a Immunofluorescence analysis of the cellular distribution of HuR in A549 cells before or after ouabain treatment. Cells were challenged with either vehicle or ouabain (100 nM) for 12 h. Representative fluorescent images of six independent experiments showing similar results are shown. Scale bars: 20 μm. b-1 Na+, K+-ATPase inhibitors time-dependently induced HuR nuclear export in A549 cells. Cells were treated with 100 nM ouabain, oleandrin, and foliandrin for 0, 4, 8, and 12 h, respectively. Representative fluorescent images of three independent experiments showing similar results are shown. Scale bars: 20 μm. b-2 Western-blot analysis of cytosolic HuR in A549 cells after exposure to ouabain at 100 nM for 1, 2, 3, 4, and 6 h, respectively, tubulin and lamin B were used to distinguish cytoplasmic and nuclear protein. c-1 Ouabain induced HuR nuclear export in A549, but not in HEK 293 cells. Scale bars: 20 μm. c-2 RT-PCR analysis of COX-2 mRNA level in HEK293 cells after exposure to ouabain at 100 nM for the indicated time. Representative semi-quantitative RT-PCR results of three independent experiments with similar results are shown. d Ouabain-induced PGE2 release in MLE 12 cells was prevented by HuR silencing. Cells were left untransfected, or transfected with HuR siRNA for 48 h, treated with ouabain at 50 nM for an additional 12 h. PGE2 content in the culture medium was measured by the ELISA method, and expressed as pg/ml. **p < 0.01, as compared with the control group. All experiments were performed at least in triplicate
Given the above results, whether the translocated cytoplasmic HuR by ouabain is functional to bind with ARE site I of COX-2 3′-UTR remains unsolved. We therefore incubated cytoplasmic lysates with biotin-labeled RNA containing the COX-2 ARE site I. The results demonstrated that CTS increased the binding of cytoplasmic proteins to COX-2 ARE site I, the intensity of which well correlated with cytoplasmic HuR (Fig. E8, supplementary data). Supershift analysis revealed that HuR was present in the binding complex (data not shown). The increased binding of cytoplasmic extracts to COX-2 ARE site I was competed by an excess of cold ARE site I (1,000 fold), which confirmed the specificity of the protein binding. Of note, the stabilizing effect of ouabain on COX-2 mRNA by HuR was cell-type-specific, because ouabain failed to trigger nuclear export of HuR in HEK293 cells, an epithelial cells line originally derived from human embryonic kidney (Fig. 6c-1). As a result, ouabain time-dependently decreased, but did not increase the steady level of COX-2 mRNA (Fig. 6c-2). This result is similar to previous findings that COX-2 3′-UTR alone was found to destabilize luciferase mRNA without ouabain (Fig. 4b-2). Taken together, HuR is both sufficient and necessary for COX-2 mRNA stabilization by ouabain. Ouabain also significantly induced the HuR nuclear export in MLE cells (Fig. E9, supplementary data), meanwhile, ouabain-induced PGE2 production in MLE cells was abrogated under the lessened HuR expression by siRNA (**p < 0.01, Fig. 6d).
Differential effects of ERK and p38 MAPKs on CTS-induced COX-2 upregulation
To sketch the signaling mechanisms, we found that ouabain time-dependently stimulated the phosphorylation of ERK1/2 and p38 MAPKs in A549 cells (Fig. 7a-1, a-2). In an attempt to determine whether the increased MAPKs activity is associated with COX-2 upregulation in ouabain-treated cells, U0126, SP600125, and SB203580 were used to suppress ERK1/2, JNK, and p38 MAPK activation, respectively. As a result, ouabain-mediated induction of COX-2 mRNA (Fig. 7b), or protein level (Fig. 7c) was suppressed by U0126 or SB203580, but not SP600125. Unexpectedly, U0126 was effective in suppressing ouabain-mediated activation of COX-2 promoter (### p < 0.001, Fig. 7d), but not COX-2 3’-UTR stabilization (Fig. E10, supplementary data). By contrast, SB203580 abrogated the protective effect of ouabain on COX-2 mRNA stability (***p < 0.001, Fig. 7e-1, e-2), while having a weak effect on the activation of the COX-2 promoter (Fig. 7d). Notably, neither COX-2 transcription nor 3′-UTR stabilization was influenced by JNK inhibitor.
Fig. 7.
Differential effects of ERK1/2 and p38 MAPKs on ouabain-induced COX-2 gene transcription and mRNA stabilization. a-1 Ouabain time-dependently increased ERK1/2 and p38 MAPK phosphorylation in A549 cells. p-ERK: phosphorylated ERK; t-ERK: total ERK; p-p38: phosphorylated p38; t-p38: total p38. The quantitative analysis of ERK1/2 and p38 MAPK phosphorylation is provided (a-2). ***p < 0.001, as compared to the control group. Ouabain-induced COX-2 mRNA (b), and protein (c) expressions were attenuated in the presence of ERK1/2 and p38 MAPK inhibitors, but not JNK inhibitor. d Differential effects of ERK1/2 and p38 MAPK inhibitors on ouabain-induced COX-2 promoter activity. The luciferase activity of A549 cells without ouabain treatment was arbitrarily set at 1.0. ***p < 0.001, as compared to control cells; ### p < 0.001, ouabain versus ouabain + U0126. Cells were transfected with 1 μg full-length COX-2 promoter and 50 ng pRL-TK. Cells were preincubated with 20 μM SP600125, U0126, and SB203580 for 1 h (b, c, and d), further treated with ouabain at 100 nM for 6 h (b), or 12 h (c, d). e-1 Ouabain-induced COX-2 mRNA stabilization was abrogated in the presence of p38 MAPK inhibitor, SB203580. The experimental procedure was the same as that described in Fig. 4a-1. The quantitative analysis of mRNA content by qRT-PCR is shown (e-2). ***p < 0.001, ouabain + Act D versus ouabain + ActD + SB203580. All experiments were performed at least in triplicate
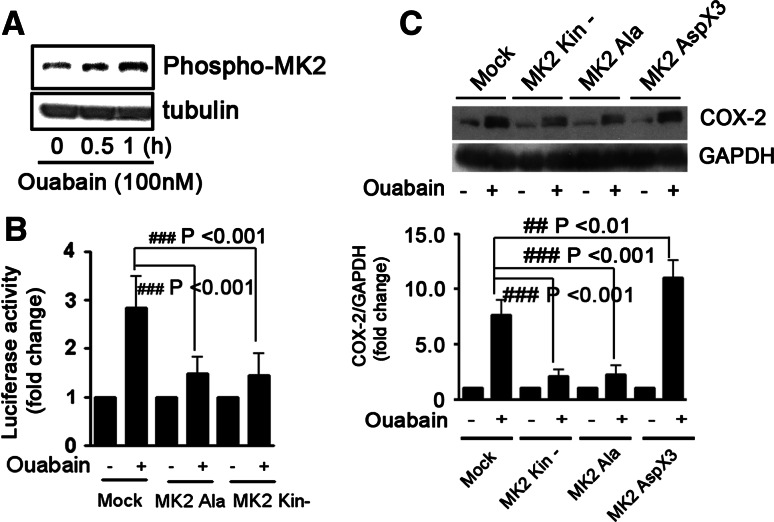
Lines of evidence suggest that MK2 is a substrate for p38 MAPK [41]. In this study, we found that ouabain treatment led to MK2 phosphorylation (Fig. 8a). MK2 was also critically involved in COX-2 mRNA stabilization and protein expression by ouabain, because overexpression of two MK2 DN plasmids (MK2 kin- and MK2 Ala) suppressed ouabain-induced stabilization of COX-2 3′-UTR (Fig. 8b), and protein upregulation (Fig. 8c). MK2AspX3 is the permanent active form of MK2 [19]. In contrast to MK2 DNs, ouabain-induced COX-2 protein expression was strengthened under the forced expression of MK2 AspX3 (## p < 0.01, Fig. 8c), suggesting a combined effect between ouabain and MK2 AspX3 on COX-2 protein upregulation.
Fig. 8.
MK2 is involved in ouabain-induced COX-2 expression in A549 cells. a Ouabain-induced phosphorylation of MK2 in A549 cells. Cells were treated with ouabain at 100 nM for 0, 0.5, and 1 h, respectively. b Overexpression of MK2 DNs suppressed ouabain-induced stabilization of COX-2 3′-UTR. A549 cells were transfected with 2 μg mock, MK2 Ala, or MK2 Kin- plasmid for 12 h, then co-transfected with 1 μg COX-2 3′-UTR firefly luciferase reporter plasmid and 0.5 μg pRL-TK. After transfection, cells were treated with ouabain at 100 nM for an additional 12 h. The luciferase activity of A549 cells without ouabain treatment was arbitrarily set at 1.0. ### p < 0.001, as compared to cells transfected with mock plasmid. c Effect of ouabain on COX-2 protein expression in A549 cells overexpressing MK2 DNs or MK2AspX3. MK2AspX3 is the permanent active form of MK2. Western-blot analysis was performed to determine COX-2 protein expression. GAPDH was included as a control. The quantitative analysis of COX-2 protein expression is shown in the bottom panel. The COX-2 expression in control cells was arbitrarily set at 1.0. ## p < 0.01, ### p < 0.001, as compared to cells transfected with mock plasmid. All experiments were performed at least in triplicate
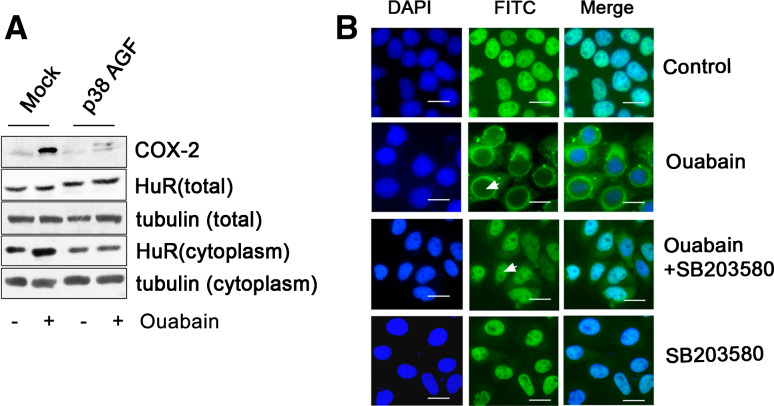
To further understand the mechanism by which ouabain stabilized COX-2 mRNA via p38 MAPK signaling, we transfected A549 cells with p38α MAPK DN plasmid (p38α MAPK AGF, Fig. 9a). Consistently, ouabain-induced COX-2 protein expression was largely suppressed by p38 MAPK DN transfection (Fig. 9a). Notably, p38 MAPK DN overexpression failed to influence the total HuR content, but led to a significant decrease of cytosolic HuR in both the control and ouabain-treated cells. These results suggest that HuR translocation in A549 cells is regulated by p38 MAPK. In support of this notion, ouabain-induced HuR translocation was partially suppressed in the presence of p38 MAPK inhibitor, SB203580, as viewed by immunofluorescence analysis (Fig. 9b). Similar results were also obtained in A549 cells under the treatment with foliandrin (Fig. E11, supplementary data).
Fig. 9.
p38 MAPK regulates the HuR translocation in A549 cells challenged with Na+, K+-ATPase inhibitors. a Ouabain-induced HuR nuclear export in A549 cells was attenuated under the forced expression of p38α MAPK DN. Cells were transiently transfected with 2 μg mock plasmid or p38α MAPK AGF (DN) plasmid. After transfection and subsequent treatment with ouabain at 100 nM for 12 h, whole cells protein extracts or cytoplasmic extracts were subjected to the Western-blot analysis for measurement of COX-2 and HuR protein expressions. Total tubulin and cytosolic tubulin were used to normalize protein loading of whole cell extracts and cytoplasmic extracts, respectively. Representative Western-blot images of three independent experiments with similar results are shown. b Ouabain-induced HuR nuclear export was attenuated in the presence of SB203580. A549 cells were preincubated with SB203580 at 20 μM for 1 h, treated with ouabain (100 nM) for an additional 12 h. HuR cellular distribution was examined by indirect immunofluorescence analysis, as described in the “Materials and methods”. The white arrows indicate the absence of HuR protein in the nucleus of A549 cells after ouabain treatment, and HuR build-up in the nucleus of A549 cells after challenge of ouabain and SB203580. Cells treated with SB203580 alone served as a control. Representative fluorescent images of three independent experiments with similar results are shown. Scale bars: 20 μm
Ca2+ signaling is involved in ouabain-induced COX-2 gene transcription, but not in mRNA stabilization
An important feature of CTS-mediated signaling is Ca2+ increase [37]. To examine whether Ca2+ signaling is involved in CTS-induced COX-2 upregulation, A549 cells were preincubated with BAPTA/AM to chelate intracellular Ca2+. The results displayed that ouabain-induced COX-2 mRNA upregulation was significantly, but not completely suppressed in the presence of BAPTA/AM (Fig. 10a). Accordingly, ouabain-induced PGE2 increase in either A549 cells (### p < 0.001, Fig. 10b-1), or MLE cells (### p < 0.001, Fig. 10b-2) was also partially abrogated by BAPTA/AM. Notably, the stimulatory effect of ouabain on COX-2 mRNA was mimicked by the sodium ionophore monensin, or calcium ionophore A23187 [14] (data not shown). To examine the concrete role of Ca2+ in COX-2 upregulation by ouabain, cells transected with luciferase construct harboring full-length COX-2 promoter, or COX-2 3′-UTR were used. The results demonstrated that ouabain-induced COX-2 transcriptional activation (Fig. 10c), but not COX-2 mRNA stabilization (Fig. 10d) was blocked in the presence of BAPTA/AM, suggesting that Ca2+ is specifically involved in COX-2 gene transcription. A previous study demonstrated that ERK1/2 and p38 MAPKs were differentially involved in COX-2 gene transcription and mRNA stabilization (Fig. 7d, e-1, e-2). Consistently, BAPTA/AM inhibited ouabain-induced ERK1/2, but not p38 MAPK phosphorylation (Fig. E12 supplementary data). Finally, ouabain-induced HuR nuclear export in A549 cells remained unaffected in the presence of BAPTA/AM (Fig. 10e).
Fig. 10.
Ca2+ is involved in ouabain-induced COX-2 upregulation in lung epithelial cells. a Ouabain-induced COX-2 mRNA upregulation was suppressed in the presence of BAPTA/AM. RT-PCR analysis was applied to measure the COX-2 mRNA level in A549 cells after treatment of ouabain at 100 nM for 6 h, 28S was used as a control. Ouabain-induced PGE2 increase in A549 cells (b-1), or in MLE 12 cells (b-2) was partially suppressed by BAPTA/AM. The PGE2 content in the culture medium was measured by the ELISA method, and expressed as pg/ml. ## p < 0.01, ### p < 0.001 ouabain versus ouabain + BAPTA/AM. c Ouabain-induced COX-2 transcriptional activation was abrogated by BAPTA/AM. A549 cells were transfected with 1 μg full-length COX-2 promoter and 50 ng pRL-TK. The luciferase activity of control samples was arbitrarily set at 1.0. ***p < 0.001, ouabain versus ouabain + BAPTA/AM. d BAPTA/AM failed to decrease the ouabain-induced human COX-2 3′-UTR stabilization. A549 cells were transfected with 1 μg human COX-2 3′-UTR reporter plasmid and 50 ng pRL-TK. The luciferase activity of control samples was arbitrarily set at 1.0. e BAPTA/AM failed to block ouabain-induced HuR nuclear export in A549 cells. HuR cellular distribution was examined by indirect immunofluorescence analysis, as described in the “Materials and methods” section. Scale bars: 20 μm. Representative fluorescent images of three independent experiments with similar results are shown. In the above experimental settings, cells were pre-incubated with BAPTA/AM at 20 μM for 1 h (a–e), further incubated with ouabain at 100 nM for 6 h (a), or 12 h (b–e)
Discussion
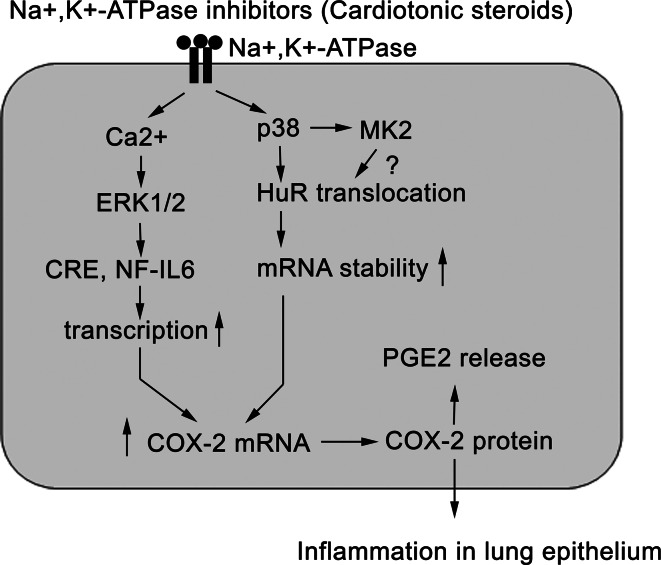
In the present study, we performed a series of experiments to show that inhaled ouabain could induce acute lung inflammation in mice, which was accompanied by COX-2 overexpression in lung epithelial cells. Further analysis revealed that after binding with Na+, K+-ATPase, CTS such as ouabain were able to activate Ca2+-dependent ERK1/2 phosphorylation leading to COX-2 gene transcription. However, more importantly, CTS were also able to trigger p38 MAPK-mediated HuR nuclear export, which stabilized COX-2 mRNA after binding with ARE site I within COX-2 3′-UTR (Fig. 11). To the best of our knowledge, CTS regulate cytokine production by HuR-mediated mRNA stabilization is first reported here.
Fig. 11.
The signaling pathway underlying Na+, K+-ATPase inhibitors-induced inflammation in lung epithelial cells
Na+, K+-ATPase and its inhibitors in pulmonary epithelial inflammation
Pulmonary inflammation is one of the common characteristics of a variety of respiratory disorders [42]. In this study, we found that Na+, K+-ATPase and its inhibitors were involved in lung epithelial inflammation. The reasons are as follows. First, ouabain induced acute lung epithelial inflammation in vivo. Second, multiple Na+, K+-ATPase inhibitors (but not ouabain alone) strongly induced COX-2 expression in lung epithelial cell line of human or murine origin. Third, the stimulatory effect of ouabain on COX-2 expression was critically dependent on Na+, K+-ATPase α1 expression. To conclude, we describe here a novel role of Na+, K+-ATPase, as well as its inhibitors in pulmonary disease. However, it is noteworthy to mention that although the rofecoxib used in this study was able to suppress ouabain-induced lung inflammation in mice, the additional or indirect effect of this chemical inhibitor in mice model can not yet be ruled out. As such, to further confirm the requirement of COX-2 in ouabain-induced lung inflammation, other in vivo evidence by using COX-2 knockout mouse model is absolutely needed in future studies.
Because of ROS generation and inadequate ATP supply, Na+, K+-ATPase subunits in lung epithelial cells are prone to internalization, and degradation under the condition of hypoxia [28]. However, other in vivo evidence revealed that hypoxia or hypoxemia could stimulate the release of EO from the midbrain and adrenal into plasma [43], the outcome of which is relevant to pulmonary hypertension [43, 44]. Based on our study findings, it seems reasonable to raise the possibility that hypoxia-induced EO release may also aggravate the lung injury by inflammatory cytokine production from lung epithelial cells.
The synergy between Na+, K+-ATPase ionic and receptor-mediated signaling mechanisms in COX-2 upregulation
Recently, the understanding of Na+, K+-ATPase has shifted from its classical pump and enzyme activities to the receptor for CTS. The Na+, K+-ATPase-mediated signaling pathways, or alternative pathways, as they are called, offer a reasonable explanation to many, but not all the effects of CTS [7]. In the present study, we found that Ca2+ signaling controlled the ouabain-induced COX-2 transcriptional upregulation. Moreover, the stimulatory effect of ouabain on COX-2 mRNA was mimickable by the sodium ionophore, or calcium ionophore (data not shown). These results suggest the classical ionic mechanism of Na+, K+-ATPase contributes to inflammatory cytokine production. By contrast, HuR nuclear export, as well as the resultant COX-2 mRNA stabilization by ouabain, was Ca2+ irrelevant, suggesting that the signaling role of Na+, K+-ATPase may also be involved in CTS-induced cytokine production. Collectively, we propose that the classical and the alternative pathways may act in cooperation to affect COX-2 expression by CTS [7]. In support of this presumption, recent reports have displayed that there is a “pool” of plasmalemmal Na+, K+-ATPases residing in the caveolae of cells that do not actively “pump” sodium, but function as noncanonical ouabain-binding receptors [45].
Pathological significance of HuR-mediated cytokine mRNA stabilization in diseases involving Na+, K+-ATPase and its inhibitors
Hu antigen R is originally identified as a member of the embryonic lethal abnormal vision (ELAV) family proteins, but increasing evidence demonstrates that it can also act as a trans-acting factor that stabilizes mRNA containing AREs [39, 40, 46–49]. In this study, we found ouabain stabilized COX-2 mRNA by enhancing binding of HuR to a proximal ARE (site I) within COX-2 3′-UTR. HuR can stabilize mRNAs encoding various other proteins implicated in inflammation, such as c-fos, TNF-α and vascular endothelial growth factor (VEGF) [50, 51]. The results of the current experiments suggest that it will be worthwhile to determine whether CTS regulate the expressions of these or other AREs containing messages. In addition, what is the relevant biological significance? Indeed, previous study has demonstrated that TNF-α secretion was dramatically upregulated in mononuclear cells after ouabain treatment, which contributed to the chronic inflammation in patients with rheumatoid arthritis [14]. Consistent with this finding, we found that ouabain was also able to increase TNF-α expression in lung epithelial cells (data not shown), which was suppressed by HuR silencing. We presume that this increase in TNF-α production by ouabain may have the similar pathological effect on lung epithelium as that of COX-2, because rofecoxib failed to completely suppress the inflammatory reactions in mice lung by inhaled ouabain (Fig. 1b).
On the other hand, ouabain at 100 nM time-dependently increased p21cip1 expression in breast cancer cells that resulted in cell growth arrest [52]. p21cip1 is a short-lived protein as that of COX-2, the 3′-UTR of p21 cip1 mRNA contains many AREs that can be recognized by HuR [53]. We did not further examine whether ouabain-induced p21cip1 upregulation was also relevant to HuR-mediated mRNA stabilization, because similar results have been obtained in a recent investigation [54]. Taken together, these results suggest the stabilization of ARE-containing mRNAs by HuR is likely a general mechanism for CTS in regulating cytokine production, at least in lung epithelial cells.
p38 MAPK phosphorylation induced by ouabain in lung epithelial cells
Several lines of evidence have demonstrated that the effect of ouabain on p38 MAPK phosphorylation is cell-type specific [36, 55]. We observed here that ouabain was able to induce p38 MAPK activation in lung epithelial cells. Further, p38 MAPK-MK2 signaling regulated the COX-2 mRNA stabilization induced by ouabain, probably through modulating HuR nuclear export. Concerning p38 MAPK and HuR translocation, a recent report has suggested that p38 MAPK may phosphorylate HuR on Thr118 [54], and lead to cytoplasmic build-up of HuR. Other reports suggest that MK2 is able to promote the interaction between TTP and 14-3-3 complexes, and thus inhibits TTP-dependent degradation of ARE-containing transcript [56]. Whether these mechanisms are also applicable to CTS-induced COX-2 mRNA stabilization warrants further investigation.
Of note, p38 MAPK phosphorylation occurred independent of Ca2+ signaling in this study, suggesting that the signaling function of Na+, K+-ATPase may account for this effect. This phenomena is indeed not uniquely happening here, because ouabain-induced p38 MAPK phosphorylation in renal epithelial cells was also irrelevant to the inhibition of Na+, K+-ATPase enzyme activity [36]. To understand the signaling mechanism, reports have suggested that the interaction between CTS with Na+, K+-ATPase may lead to the formation of a signaling complex in a manner analogous to that for classical receptor tyrosine kinases [57, 58]. As a result, Src kinase activity is activated. Several lines of evidence have implicated p38 MAPK as a downstream molecule of Src activation in tyrosine kinases-mediated signaling pathway [59]. Therefore, CTS may stimulate p38 MAPK phosphorylation by Na+, K+-ATPase-mediated Src activation, obviously, more experiments are needed to address this hypothesis.
Finally, we have to reiterate that under conditions when COX-2 mRNA expression was initiated by CTS, neither cell apoptosis nor necrosis was detectable. Further, no significant cell death was observed in the lung section of mice after ouabain inhalation, as examined by TUNEL staining (data not shown). Therefore, COX-2 upregulation in lung epithelial cells in vitro, as well as the inflammatory reaction in vivo is obviously irrelevant to the cytotoxic effects by CTS.
On the other hand, ouabain-induced COX-2 upregulation, at least in this study, is lung epithelial cell-specific, because ouabain was able to induce COX-2 upregulation in lung epithelial cells both in vivo and in vitro. Further, ouabain failed to trigger a significant increase of COX-2 protein expression in a variety of transformed, or non-transformed cells including primary T cells, Jurkat T cells, breast carcinoma cells MCF-7 and MDA231, hepatocellular carcinoma cells HepG2 and Bel7402 (data not shown). In HEK293 cells, ouabain even decreased the basal level of COX-2, the result of which was probably due to the lack of HuR nuclear export (Fig. 6c-1, c-2). Therefore, HuR nuclear export is indeed a critical cellular event to determine the cells' sensitivity towards ouabain-induced COX-2 production. CTS-Na+, K+-ATPase-mediated signaling in lung epithelial cells may specifically favor the HuR nuclear export, thereby rendering lung epithelial cells more sensitive to CTS treatment for COX-2 production than other types of cells. Undoubtedly, further clarification of upstream signaling events in CTS-induced HuR nuclear export would be very helpful to support this hypothesis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Natural Science Fund of China (30470644, 30600502, 30973528 to Y. W., 30425009, 30330530 to H. Z.C., 30730107 to X. Q). Natural Science Fund of Jiangsu Province (BK2008272 to Y. W.). Natural Science Fund of Shanghai (10ZR1438400 to B.J.J.). Open project of Jiangsu Key Lab for Drug Efficiency and Safety Evaluation (P09008); and the Ministry of Education of China (TRAPOY 199028418).
Abbreviations
- Act D
Actinomycin D
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- ARE
AU-rich elements
- BALF
Bronchoalveolar lavage fluid
- COX-2
Cyclooxygenase-2
- CTS
Cardiotonic steroids
- DMEM
Dulbecco’s modified Eagle’s medium
- DN
Dominant negative
- EO
Endogenous ouabain
- ERK
Extracellular regulated protein kinases
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HA
Hemagglutinin
- H&E
Hematoxylin and eosin
- HRP
Horseradish peroxidase
- HuR
Hu antigen R
- IκB
Inhibitory subunit of nuclear factor kappa B
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MK2
MAPK-activated protein kinase 2
- MPO
Myeloperoxidase
- NO
Nitric oxide
- PGE2
Prostaglandin E2
- ROS
Reactive oxygen species
- TNF
Tumor necrosis factor
- UTR
Untranslated region
- VEGF
Vascular endothelial growth factor
Footnotes
S. Feng and W. Chen contributed equally to this paper.
Contributor Information
Zi-Chun Hua, Email: zchua@nju.edu.cn.
Wu Yin, Phone: +86-25-83593692, FAX: +86-25-83324605, Email: wyin@nju.edu.cn.
References
- 1.Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na, K-ATPase in acute lung injury. Intensive Care Med. 2007;33:1243–1251. doi: 10.1007/s00134-007-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na(+)-K(+)-ATPase. J Appl Physiol. 2002;93:1860–1866. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- 3.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder JI. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na, K-ATPase beta1 subunit gene. J Clin Invest. 1998;102:1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GR, Yeldandi AV, Sznajder JI, Dean DA. Gene transfer of the Na+, K+-ATPase beta1 subunit using electroporation increases lung liquid clearance. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, Dean DA. Electroporation-mediated gene transfer of the Na+, K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176:582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strieter RM, Lukacs NW, Standiford TJ, Kunkel SL. Cytokines. 2. Cytokines and lung inflammation: mechanisms of neutrophil recruitment to the lung. Thorax. 1993;48:765–769. doi: 10.1136/thx.48.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahimtoola SH, Tak T. The use of digitalis in heart failure. Curr Probl Cardiol. 1996;21:781–853. doi: 10.1016/S0146-2806(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 9.Mathews WR, DuCharme DW, Hamlyn JM, Harris DW, Mandel F, Clark MA, Ludens JH. Mass spectral characterization of an endogenous digitalislike factor from human plasma. Hypertension. 1991;17:930–935. doi: 10.1161/01.hyp.17.6.930. [DOI] [PubMed] [Google Scholar]
- 10.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues-Mascarenhas S, Silva Da, de Oliveira A, Amoedo ND, Affonso-Mitidieri OR, Rumjanek FD, Rumjanek VM. Modulation of the immune system by ouabain. Ann NY Acad Sci. 2009;1153:153–163. doi: 10.1111/j.1749-6632.2008.03969.x. [DOI] [PubMed] [Google Scholar]
- 12.Dornand J, Favero J, Bonnafous JC, Mani JC. Paradoxical production of mouse thymocyte activating factor by ouabain-treated human mononuclear cells. Cell Immunol. 1984;83:351–359. doi: 10.1016/0008-8749(84)90314-9. [DOI] [PubMed] [Google Scholar]
- 13.Matsumori A, Ono K, Nishio R, Igata H, Shioi T, Matsui S, Furukawa Y, Iwasaki A, Nose Y, Sasayama S. Modulation of cytokine production and protection against lethal endotoxemia by the cardiac glycoside ouabain. Circulation. 1997;96:1501–1506. doi: 10.1161/01.cir.96.5.1501. [DOI] [PubMed] [Google Scholar]
- 14.Foey AD, Crawford A, Hall ND. Modulation of cytokine production by human mononuclear cells following impairment of Na, K-ATPase activity. Biochim Biophys Acta. 1997;1355:43–49. doi: 10.1016/S0167-4889(96)00116-4. [DOI] [PubMed] [Google Scholar]
- 15.Sowa G, Przewlocki R. Ouabain enhances the lipopolysaccharide-induced nitric oxide production by rat peritoneal macrophages. Immunopharmacology. 1997;36:95–100. doi: 10.1016/S0162-3109(96)00159-2. [DOI] [PubMed] [Google Scholar]
- 16.Padilha AS, Pecanha FM, Vassallo DV, Alonso MJ, Salaices M. Ouabain treatment changes the role of endothelial factors in rat resistance arteries. Eur J Pharmacol. 2008;600:110–116. doi: 10.1016/j.ejphar.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Cook-Johnson RJ, Demasi M, Cleland LG, Gamble JR, Saint DA, James MJ. Endothelial cell COX-2 expression and activity in hypoxia. Biochim Biophys Acta. 2006;1761:1443–1449. doi: 10.1016/j.bbalip.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–1057. doi: 10.1016/S0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 20.Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem. 2000;275:11750–11757. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- 21.Kitz R, Rose MA, Placzek K, Schulze J, Zielen S, Schubert R. LPS inhalation challenge: a new tool to characterize the inflammatory response in humans. Med Microbiol Immunol. 2008;197:13–19. doi: 10.1007/s00430-007-0053-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuebler WM, Abels C, Schuerer L, Goetz AE. Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. Int J Microcirc Clin Exp. 1996;16:89–97. doi: 10.1159/000179155. [DOI] [PubMed] [Google Scholar]
- 23.Xu YZ, Di Marco S, Gallouzi I, Rola-Pleszczynski M, Radzioch D. RNA-binding protein HuR is required for stabilization of SLC11A1 mRNA and SLC11A1 protein expression. Mol Cell Biol. 2005;25:8139–8149. doi: 10.1128/MCB.25.18.8139-8149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doller A, Huwiler A, Muller R, Radeke HH, Pfeilschifter J, Eberhardt W. Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: implications for posttranscriptional regulation of cyclooxygenase-2. Mol Biol Cell. 2007;18:2137–2148. doi: 10.1091/mbc.E06-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vielkind U, Swierenga SH. A simple fixation procedure for immunofluorescent detection of different cytoskeletal components within the same cell. Histochemistry. 1989;91:81–88. doi: 10.1007/BF00501916. [DOI] [PubMed] [Google Scholar]
- 26.Yin W, Cheng W, Shen W, Shu L, Zhao J, Zhang J, Hua ZC. Impairment of Na(+), K(+)-ATPase in CD95(APO-1)-induced human T-cell leukemia cell apoptosis mediated by glutathione depletion and generation of hydrogen peroxide. Leukemia. 2007;21:1669–1678. doi: 10.1038/sj.leu.2404791. [DOI] [PubMed] [Google Scholar]
- 27.Comellas AP, Briva A, Dada LA, Butti ML, Trejo HE, Yshii C, Azzam ZS, Litvan J, Chen J, Lecuona E, Pesce LM, Yanagisawa M, Sznajder JI. Endothelin-1 impairs alveolar epithelial function via endothelial ETB receptor. Am J Respir Crit Care Med. 2009;179:113–122. doi: 10.1164/rccm.200804-540OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na, K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na, K-ATPase endocytosis. J Clin Invest. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na, K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dada LA, Novoa E, Lecuona E, Sun H, Sznajder JI. Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na, K-ATPase in A549 cells. J Cell Sci. 2007;120:2214–2222. doi: 10.1242/jcs.003038. [DOI] [PubMed] [Google Scholar]
- 32.Vadasz I, Morty RE, Kohstall MG, Olschewski A, Grimminger F, Seeger W, Ghofrani HA. Oleic acid inhibits alveolar fluid reabsorption: a role in acute respiratory distress syndrome? Am J Respir Crit Care Med. 2005;171:469–479. doi: 10.1164/rccm.200407-954OC. [DOI] [PubMed] [Google Scholar]
- 33.Abonyo BO, Alexander MS, Heiman AS. Autoregulation of CCL26 synthesis and secretion in A549 cells: a possible mechanism by which alveolar epithelial cells modulate airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L478–L488. doi: 10.1152/ajplung.00032.2005. [DOI] [PubMed] [Google Scholar]
- 34.Woo CH, Lim JH, Kim JH. VCAM-1 upregulation via PKCdelta-p38 kinase-linked cascade mediates the TNF-alpha-induced leukocyte adhesion and emigration in the lung airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2005;288:L307–L316. doi: 10.1152/ajplung.00105.2004. [DOI] [PubMed] [Google Scholar]
- 35.Fujii T, Hogg JC, Keicho N, Vincent R, Van Eeden SF, Hayashi S. Adenoviral E1A modulates inflammatory mediator expression by lung epithelial cells exposed to PM10. Am J Physiol Lung Cell Mol Physiol. 2003;284:L290–L297. doi: 10.1152/ajplung.00197.2002. [DOI] [PubMed] [Google Scholar]
- 36.Akimova OA, Lopina OD, Rubtsov AM, Gekle M, Tremblay J, Hamet P, Orlov SN. Death of ouabain-treated renal epithelial cells: evidence for p38 MAPK-mediated Na (i) (+)/K (i) (+) -independent signaling. Apoptosis. 2009;14:1266–1273. doi: 10.1007/s10495-009-0404-0. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Cai T, Yang C, Turner DA, Giovannucci DR, Xie Z. Regulation of inositol 1, 4, 5-trisphosphate receptor-mediated calcium release by the Na/K-ATPase in cultured renal epithelial cells. J Biol Chem. 2008;283:1128–1136. doi: 10.1074/jbc.M708025200. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta S, Jang BC, Wu MT, Paik JH, Furneaux H, Hla T. The RNA-binding protein HuR regulates the expression of cyclooxygenase-2. J Biol Chem. 2003;278:25227–25233. doi: 10.1074/jbc.M301813200. [DOI] [PubMed] [Google Scholar]
- 39.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan XC, Steitz JA. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA. 1998;95:15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy MJ. Inflammation and cystic fibrosis pulmonary disease. Pharmacotherapy. 2001;21:593–603. doi: 10.1592/phco.21.6.593.34546. [DOI] [PubMed] [Google Scholar]
- 43.De Angelis C, Haupert GT., Jr Hypoxia triggers release of an endogenous inhibitor of Na(+)-K(+)-ATPase from midbrain and adrenal. Am J Physiol. 1998;274:F182–F188. doi: 10.1152/ajprenal.1998.274.1.F182. [DOI] [PubMed] [Google Scholar]
- 44.Anand IS, Chandrashekhar Y, Rao SK, Malhotra RM, Ferrari R, Chandana J, Ramesh B, Shetty KJ, Boparai MS. Body fluid compartments, renal blood flow, and hormones at 6,000 m in normal subjects. J Appl Physiol. 1993;74:1234–1239. doi: 10.1152/jappl.1993.74.3.1234. [DOI] [PubMed] [Google Scholar]
- 45.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 46.Myer VE, Fan XC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997;16:2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng SS, Chen CY, Xu N, Shyu AB. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Yang X, Cristofalo VJ, Holbrook NJ, Gorospe M. Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol Cell Biol. 2001;21:5889–5898. doi: 10.1128/MCB.21.17.5889-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 2001;61:2154–2161. [PubMed] [Google Scholar]
- 52.Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67:929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 53.Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000;20:760–769. doi: 10.1128/MCB.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol. 2009;29:4341–4351. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues-Mascarenhas S, Bloise FF, Moscat J, Rumjanek VM. Ouabain inhibits p38 activation in thymocytes. Cell Biol Int. 2008;32:1323–1328. doi: 10.1016/j.cellbi.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14–3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 58.Pierre SV, Xie Z. The Na, K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys. 2006;46:303–316. doi: 10.1385/CBB:46:3:303. [DOI] [PubMed] [Google Scholar]
- 59.Mocsai A, Jakus Z, Vantus T, Berton G, Lowell CA, Ligeti E. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J Immunol. 2000;164:4321–4331. doi: 10.4049/jimmunol.164.8.4321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.