Abstract
Nanoparticles (NPs) comprised of nanoengineered complexes are providing new opportunities for enabling targeted delivery of a range of therapeutics and combinations. A range of functionalities can be included within a nanoparticle complex, including surface chemistry that allows attachment of cell-specific ligands for targeted delivery, surface coatings to increase circulation times for enhanced bioavailability, specific materials on the surface or in the nanoparticle core that enable storage of a therapeutic cargo until the target site is reached, and materials sensitive to local or remote actuation cues that allow controlled delivery of therapeutics to the target cells. However, despite the potential benefits of NPs as smart drug delivery and diagnostic systems, much research is still required to evaluate potential toxicity issues related to the chemical properties of NP materials, as well as their size and shape. The need to validate each NP for safety and efficacy with each therapeutic compound or combination of therapeutics is an enormous challenge, which forces industry to focus mainly on those nanoparticle materials where data on safety and efficacy already exists, i.e., predominantly polymer NPs. However, the enhanced functionality affordable by inclusion of metallic materials as part of nanoengineered particles provides a wealth of new opportunity for innovation and new, more effective, and safer therapeutics for applications such as cancer and cardiovascular diseases, which require selective targeting of the therapeutic to maximize effectiveness while avoiding adverse effects on non-target tissues.
Keywords: Nanoparticles, Drug delivery, Controlled drug release, Cardiovascular, Cancer
Introduction
Nanoparticles (NPs), as used for drug delivery, are generally less than 200 nm in size. The drug is usually encapsulated in a polymeric carrier, or adsorbed or conjugated onto the surface. To enhance uptake to the target tissue site, they can be administered locally, or actively targeted using some combination of cell-specific ligands, magnetic localization, or size-based selectivity. NPs as vehicles of drug delivery offer many advantages compared to conventional drug delivery approaches. It has been observed that NPs result in a higher intracellular uptake than microparticles [1]. Crucially, localized delivery with NPs can allow lower doses of drug to be used, and thus lead to reduced systemic side-effects, while drugs with low bioavailability can be targeted directly to the site required.
The chemical composition at the surface of NPs will largely define their chemical interactions, due to the high surface-to-volume ratio. Therefore, NPs behave quite differently than bulk materials. Many NPs are functionalized on the surface to increase their blood circulation, make them more biocompatible, and for targeted therapy. As such, it is appropriate to consider NPs for drug delivery as nano- or micro-particle complexes, engineered at the nanoscale to incorporate several functionalities within the nanoparticle, such as:
Ligands for specific attachment of the particles to target cells/tissues,
one or more therapeutic cargoes (small molecule or biologic), which can be encapsulated as part of the core of the particle, or decorated on its surface,
a linker molecule that can be triggered to release the therapeutic at the target site, based on specific local characteristics of the target tissue (e.g., pH, ionic strength or temperature) or compatible with remote triggering by optical or magnetic actuation,
a core or coating with magnetic or optical properties to facilitate localization of the particle at the target site by remote actuation (e.g., magnetic NPs), imaging of the location of the particles in the body (e.g., magnetite or gold), or the release of the therapeutic(s) from the particles by remote actuation,
coatings such as poly(ethyleneglycol)(PEG) on the particle to increase biocompatibility and/or slow clearance from the body.
There are currently many different materials reported in the literature that have been evaluated for use in micro- and nano-particle based drug delivery. Materials that can be used for NPs can be classified based on their character and origin, e.g., natural or synthetic, organic, or inorganic. Some of these include:
Natural polymers such as: gums (e.g., acacia, guar, etc.), chitosan, gelatin, lectin, sodium alginate, albumin;
synthetic polymers such as: cellulosics, poly (2-hydroxy ethyl methacrylate), poly (N-vinyl pyrrolidone), poly (methyl methacrylate), poly (vinyl alcohol) (PVA), poly (acrylic acid), polyacrylamide, poly (ethylene-co-vinyl acetate), PEG, poly (methacrylic acid);
biodegradable polymers including polyesters such as polylactides (PLA), polyglycolides (PGA), poly (lactide-co-glycolides) (PLGA) and polycaprolactone. Others include polyanhydrides, polyorthoesters, and polycyanoacrylates;
cyclic oligosaccharides such as functionalized cyclodextrin;
magnetic oxides such as Fe3O4, γ-Fe2O3; magnetic metals such as Iron (Fe) and cobalt (Co) and alloys such as FeCo;
metal oxides such as TiO2 and ZnO;
gold (Au);
silicon (including porous Si).
Polymer particles can be fabricated in a wide range of sizes and varieties and include some biodegradable NPs that do not accumulate in the body and can facilitate steady drug release for weeks. As such, polymeric NPs can be possible carriers of pharmacological therapies for a wide range of applications ranging from cancer therapy to diabetes, bone healing, and vaccination. Biodegradable NPs have the ability to carry various therapeutic agents including siRNA, DNA, proteins, peptides, and low-molecular-weight compounds. Many polymer NPs are already approved by the US Food and Drug Administration (FDA). While some, such as polylysine, are used as a natural preservative in food products, the guidelines for using NPs for drug administration are more stringent. Table 1 (taken from Farajhi et al. [2]) shows a list of FDA-approved nanoparticle drug delivery systems in clinical trials and/or use.
Table 1.
A list of FDA-approved nanoparticle drug delivery systems in clinical trials and/or in use [2]
| Therapeutic agent (trade name) | Indication |
|---|---|
| Liposomal amphotericin B (Ambisome, Ablecet, Amphotec) | Fungal infections, Leishmaniasis |
| PEG-adenosine deaminase (Pegadamase) | Severe combined immunodeficiency disease |
| PEG-stabilized liposomal doxorubicin (Doxil, Evacet) | Kaposi’s sarcoma, refractory ovarian cancer |
| Liposomal cytosine arabinoside (DepoCyt) | Lymphomatous meningitis, neoplastic meningitis |
| Interleukin 2-diptheria toxin fusion protein (Deniliekin, Diffitox) | Cutaneous T-cell lymphoma |
| Liposomal verteporfin (Visudyne) | Wet macular degeneration |
| PEG-interferon α-2b (Pegasys) | Hepatitis C |
| PEG-granulocyte colony stimulating factor (Neulasta) | Chemotherapy associated neutropenia |
| Protein bound paclitaxel (Abraxane) | Metastatic breast cancer |
| PEG L-asparaginase (Oncaspar) | Acute lymphocytic leukemia |
| PEG aptanib (Macugen) | Wet macular degeneration |
| Pemetrexid (Alimta) | Malignant pleural mesothelolioma |
The potential of several different types of nanosystems have been considered in the literature [3–20]. Despite a variation in their properties, they will be broadly referred to as NPs throughout this review. Some examples include liposomes and niosomes, magnetic NPs, nanoshells, quantum dots, carbon nanotubes, carbon nanohorns, nanodiamonds, colloidal gold, ceramics, dendrimers, solidlipid NPs (SLNs), micelles (including polymeric micelles) and nanoemulsions. It is beyond the scope of this review to describe the general characteristics of each of the above NPs with their merits and limitations. Rather, the focus of this review is to consider the potential of a range of different types of nanoparticle complexes (i.e., typically including several of the materials and functionalities already mentioned), to address the challenges of drug delivery for cancer and cardiovascular disease. A key challenge for NP-based drug delivery is to enable targeted and controlled delivery of therapeutics to specific cell types, while avoiding any release of the therapeutic to non-target tissues. Within these application domains, the article is focused on issues such as nanoparticle toxicity, stability and manufacturability, potential for controlled delivery and local targeted release, and the potential for enabling more effective combination treatments through delivery of two or more therapeutics.
One key benefit is that NPs can enable increased circulation time due to their small particle size. It has been shown that particles under 200 nm had longer circulation times than particles over 200 nm, irrespective of any surface modifications present [21]. NPs are generally cleared from the circulation by proteins of the immune system called opsonins, which activate the complement system and mark the NPs for destruction by macrophages and other phagocytic cells. Neutral particles are opsonized less than charged particles, and it has been shown that hydrophobic particles are cleared faster from the circulation than hydrophilic particles [21]. Thus, the particles can be conjugated with hydrophilic polymers such as PEG to prolong retention in the circulation and reduce uptake by the reticulo-endothelial system (RES) in sites such as the Kupffer cells in the liver.
Nanoparticle toxicity considerations
The main molecular mechanism of in vivo nanotoxicity is the induction of oxidative stress by free-radical formation. In excess, free radicals cause damage to biological components through oxidation of lipids, proteins, and DNA. Oxidative stress may have a role in the induction or the enhancement of inflammation through up-regulation of redox-sensitive transcription factors (e.g., NF-κB), activator protein-1, and kinases involved in inflammation. Free radicals can originate from several sources including phagocytic cell response to foreign material, insufficient amounts of anti-oxidants, the presence of transition metals, environmental factors, and the physicochemical properties of some nanomaterials. Slow clearance and tissue accumulation (storage) of potential free-radical-producing nanomaterials, as well as the prevalence of numerous phagocytic cells in the organs of RES make organs such as the liver and spleen the main targets of oxidative stress. The cytotoxicity is size- and/or shape-dependent, as well as NP chemistry-dependent. For example, smaller silica nanoparticles have shown higher cytotoxicity while silica nanoparticles with diameters of 104 and 335 nm showed very low cytotoxicity [22]. Similar size-dependence was shown for other particles including silver particles [23], gold particles [24, 25], and carbon-based particles, e.g., carbon nanotubes [26] and fullerenes [27]. The cytotoxicity of NPs such as silica can be reduced by functionalization with various organosilanes or treatment with polymers such as PEG [28].
Gold has been used as an anti-inflammatory and anti-rheumatic agent (Auranofin® and Tauredon®) in the treatment of rheumatoid arthritis. While many studies have suggested that bare gold nanomaterials, like bulk gold, can be used safely at the nanoscale particle size, surface charge and shape are key factors (see Fig. 1) determining the potential toxicity of medicinal gold complexes [29]. Some research has found gold to be toxic in the body, where elemental gold can undergo oxidation or become soluble by cyanidation. Studies have shown that gold is heavily taken up by the kidneys, causing nephrotoxicity, and can also initiate eryptosis (erythrocyte suicidal death). Several studies have examined the effect of gold nanoparticle size on toxicity. Specifically, gold nanoclusters of 1.4 nm have been shown to selectively and irreversibly bind to the major grooves of B-DNA and cause increased cytotoxicity compared to larger particles (18 nm). The lack of interaction of larger particles with DNA is suggested to be due to steric hindrance [30, 31].
Fig. 1.
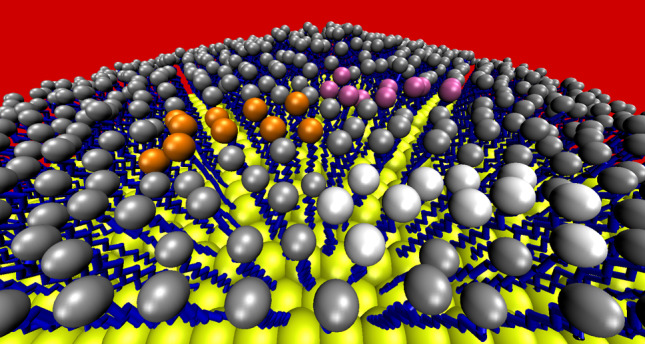
Computer model of a 30-nm-diameter gold nanoparticle coated with alkanethiol molecules. Nanoparticle gold atoms are shown as yellow spheres, alkyl chain carbon atoms are shown as blue sticks and terminal, and surface-exposed sites are shown as grey spheres. The red background represents the biological environment. The introduction of therapeutic drug molecules (by, e.g., ligand exchange and/or regioselective surface patterning) is depicted by the clusters of molecules terminating in white, orange, and pink spheres to represent different functional groups (D. Thompson, unpublished data)
Biocompatibility/tolerability/toxicity is not a problem for lipid NPs, unlike the hard materials discussed above or indeed some synthetic polymers, which may “become” toxic in NP form [32]. Furthermore, lipid coatings can improve biocompatibility of other particles. For example, a recent study [33] compared bare and lipid-coated silica NPs in mice. They used a silica quantum dot (QD) particle with a paramagnetic lipid coat [34] and used a wide variety of techniques [fluorescence imaging, inductively coupled plasma mass spectrometry, magnetic resonance imaging, confocal laser scanning microscopy (CLSM), and transmission electron microscopy (TEM)] to show that the lipid coating enables straightforward functionalization and introduction of multiple properties, while increasing bio-applicability and improving pharmacokinetics, as well as increased blood circulation half-life and prevention of aggregation in capillaries [33]. Similarly, solid lipid NPs (SLNs) loaded with CdSe/ZnS QDs were shown to be biocompatible [35]. Other NPs, such as high-density lipoproteins (HDL) end up mainly in liver and to a lesser extent in spleen, and are barely detectable in heart, lungs, and kidneys [36].
Stability and manufacturability
The stability of the NP-containing fluids is crucial to their performance for any application, thus the factors that determine stability have been a strong focus of research activity. This is especially important for magnetic NPs. For biological applications, the stability in aqueous suspension, or under approximate physiological conditions (e.g., in PBS buffer at 37°C), is also critical for assessing the shelf life of the suspensions, and is a reasonable indicator of their initial stability on introduction, usually by IV injection, into the bloodstream. The stability of the suspensions on the bench, i.e., the stability with respect to self-aggregation, is determined in the first instance by the nature of the surface of the NPs or nanoparticle clusters. The stability of magnetic nano-suspensions is therefore defined by the nature of surface capping stabilizers, which can be chemically linked or physically adsorbed on magnetic NPs, preventing their aggregation and precipitation. Magnetic NPs can be coated during (in situ) or after the synthesis. The selection of coating frequently depends on the final application of the particles [37–43]. Three main types of coatings are used to stabilize NPs in aqueous solutions: monomeric organic stabilizers, polymeric stabilizers, and inorganic coatings [44–46].
Surface modification with monomeric stabilizers
Organic surfactants are frequently employed for stabilization and coating of magnetic NPs. A common traditional approach is to use fatty acids (e.g., oleic or stearic) to stabilize aqueous magnetic fluids, by formation of a surface bilayer [47] with a chemisorbed fatty acid primary layer and an interpenetrating second layer, which is physi-adsorbed onto the primary layer, with the hydrophilic head-groups pointing outwards. The structure and stability of the resulting nanoparticle clusters (ca. 100 nm) formed from the particles (ca. 10 nm) in suspension have been studied by light scattering and cryo-TEM [48]. Fatty acid stabilized particles are interesting as model colloidal systems, which are easy to produce and might even have good bio-compatibility. Di- and tri-carboxylic acids are also frequently utilized for the surface functionalization and stabilization of magnetic iron-oxide-based nanoparticles in solution. In these acids, some of the functional groups can bind to the surface of metal oxide, while the remaining carboxylates provide negative charge, depending on the pH, and improve the hydrophilicity of the particle surface. Typical examples are citric, tartaric, and dimercaptosuccinic acids [49–51]. For particles stabilized with these types of monomeric anionic stabilizers, a critical factor influencing stability is the zeta-potential, the surface charge at the slipping plane. Thus, charged particles repel each other (double-layer repulsion) and produce stable suspensions [52].
Modification using polymeric stabilizers
Most of the biocompatible magnetic NPs are stabilized by polymers containing various functional groups such as carboxylic acids, hydroxyls, phosphates, and sulphates [46]. In this case, the stabilization of NPs can be achieved due to a group of interactions that are collectively termed steric forces [53, 54]. Computer simulations provide a valuable ally to empirical experiments for investigating the physicochemical properties of NPs and their aggregation in solution and on surfaces, both in terms of delivery materials (e.g., stents) and in vivo (e.g., in blood plasma, at cell membranes) [55, 56].
Due to their good solubility in water, biocompatibility and indeed permeability, polysaccharides such as dextran or carboxydextran are among the most popular polymer coatings used for stabilization of magnetic NPs. One of the commercially available dextran-stabilized magnetic fluids is Ferumoxtran-10 (also known as AMI 227, Sinerem® and Combidex®), which consists of superparamagnetic magnetite cores of about 5 nm in diameter that are coated with a dextran layer, resulting in a hydrodynamic diameter normally between 15 and 30 nm. These particles demonstrated prolonged blood residence time and excellent biocompatibility [44, 49]. Dextran-coated superparamagnetic iron-oxide particles can also form stable complexes with transfection agents. These complexes can be internalized by endosomes/lysosomes and have been utilized for cell labeling and in vivo magnetic resonance imaging (MRI) cell tracking [57].
Chitosan is a biocompatible and biodegradable polymer, which is of particular interest for coating of magnetic NPs [58, 59]. It was reported that oleic acid-coated superparamagnetic iron-oxide NPs can be easily dispersed in chitosan, producing stable ferrofluids with the hydrodynamic diameters of ~65 nm. Polylactic acid is another biodegradable polymer that has been used to prepare stable biocompatible ferrofluids with varying ferromagnetic particle sizes from 10 to 180 nm [60]. In addition, polylactic acid-coated NPs can be loaded with anticancer drugs (e.g., Tamoxifen), allowing their utilization for simultaneous tumor imaging, drug delivery, and real-time monitoring of therapeutic effects [61].
A very successful strategy for the preparation of stable and biocompatible NPs is to graft PEG onto the surface, i.e., to PEGylate. PEG is biocompatible and has favorable chemical properties and solubility. Stabilization is primarily due to steric interactions and PEGylation can also be used to further enhance the pharmacokinetic properties and improve blood circulation times [62, 63]. This so-called “stealth” technology is very widely investigated in drug-delivery applications. Various PEG-containing block copolymers have been developed and employed to coat magnetic NPs for various biomedical applications [63–72]. Aside from the extended blood half-life that it can provide, one of the great advantages of PEG coating, is that it can also be easily conjugated to antibodies or other biomolecules to achieve a specific targeted delivery. For example, in a recent report, biocompatible water-soluble magnetite nanocrystals were fabricated by thermal decomposition of ferric triacetylacetonate in 2-pyrrolidone in the presence of monocarboxyl-terminated poly(ethylene glycol) (MPEG-COOH) [68]. The carboxylic acid groups on the surface of the particles were conjugated with a cancer-targeting anti-carcinoembryonic antigen (CEA) monoclonal antibody, via carbodiimide coupling reaction [73]. PEG-coated iron-oxide NPs can also be conjugated to specific targeting peptides and receptors such as chlorotoxin [74], transactivator protein (Tat) of HIV-1 [75–77], and integrins [78, 79].
Coating of magnetic NPs with PEG-modified phospholipids, which are often introduced as micelles during the synthesis, produces highly biocompatible and water-stable “magnetoliposomes” [80–82]. The liposome encapsulation delays the natural dilution of the contrast agents and limits their interactions with biological media. In addition, this approach may enable the simultaneous combination of diagnosis and therapeutics by encapsulating an MRI contrast agent and a drug together [83]. It has been demonstrated that double- and single-stranded DNA can serve as a very good stabilizer for magnetic iron-oxide NPs, allowing preparation of very stable magnetic fluids which exhibit unprecedented high relaxivities and good potential for MRI and drug delivery [84].
Modification using inorganic coatings
Inorganic coatings for magnetic NPs include silica, carbon, precious metals (such as silver (Ag) and Au) or metal oxides [46]. Silica coating is one of the most frequently used inorganic coatings for several reasons. The silica significantly improves the stability of magnetic NPs, preventing them from oxidizing. In addition, a silica coating can reduce potential toxic effects of the NPs [85] and it also helps to prevent particle aggregation and increases their stability in solution. The isoelectric point of magnetite is at pH 7, so it is necessary to further coat the particles in order to make them stable in the pH region 6–10. Application of a thin layer of silica lowers this isoelectric point to approximately pH 3, which increases the stability near neutral pH [85]. Another important advantage of silica coating, over the traditional organic monomeric surfactants such as stearic or oleic acid considered above, is that there is no possibility of desorption of the strongly covalently bound silica shells. Finally, the silica surface can be easily functionalized, enabling chemical bonding of various biological molecular species to the surface for site-specific targeted delivery [44, 86, 87]. Silica coating on magnetic NPs can be prepared using several different approaches. One of the most popular approaches is sol–gel processing using tetraethoxysilane, TEOS (Stöber method) [85]. In this method, silica shell formation is achieved by hydrolysis of TEOS in the presence of ammonia and magnetic NPs. The thickness of the silica coating can be controlled by varying the concentration of ammonium and the ratio of TEOS to water.
Another approach to silica coating is the microemulsion method [88–90]. With this technique, micelles or inverse micelles are used to deposit and control the coating. In this case, the water nanodroplets present in the bulk oil phase serve as nanoreactors for the synthesis and coating of NPs. One of the advantages of the microemulsion method is that it also facilitates incorporation of biological macromolecules, as the nanocomposites formed are porous [91]. Interesting iron-oxide-based magnetic nanocomposites with silica-enriched surface layers have been prepared by a modified microemulsion technique, which involved aerosol pyrolysis of an iron ammonium citrate/TEOS solution. Thus, silica coating is a very convenient and widely used approach for protection and stabilization of magnetic NPs. However, among the drawbacks is the fact that silica is not stable under basic conditions and is usually porous. Therefore oxygen and other species can diffuse through the materials, which may result in oxidation and deterioration of the magnetic core [92].
Coating with inert precious metals is another good route to protect the magnetic cores against oxidation and stabilize the aqueous solutions, and several methods have been reported [93]. Reverse micelle (microemulsion) methods can be utilized to deposit a gold coating on iron NPs [28]. Redox transmetallation is another approach that has been used to fabricate “core–shell” types of Co–Pt nanoalloys with a particle size of less than 10 nm [94]. A redox approach has also been used for the synthesis of Au-coated magnetic Fe NPs [95]. In another study, multifunctional magnetic nanocomposites have been prepared by coating silica spheres with gold nanoshells embedded with Fe3O4 NPs [96]. These superparamagnetic gold nanoshells demonstrated a good potential as agents for both MRI and photothermal therapy [97]. Finally, coating/protection of magnetic NPs with carbon is fast becoming a popular approach. Carbon offers very high chemical and thermal stability, as well as improved biocompatibility. In addition, carbon-coated NPs are usually in the metallic state, and therefore have a higher magnetic moment than the corresponding oxides [46, 98, 99]. Micelles are useful for encapsulating non-water-soluble drugs and can be administered intravenously [98, 99].
Potential for controlled delivery and local targeted release
It is well recognized that an efficient delivery system should have the capability to transport the desired guest molecules without any loss before reaching the targeted location. Upon reaching the destination, the system needs to be able to release the cargo in a controlled manner. Any premature release of guest molecules could be catastrophic. This is particularly important in the case of toxic anti-tumor drugs, where release at non-target healthy tissues can lead to serious side-effects for patients. The release mechanism of many current biodegradable, polymer-based drug delivery systems relies on hydrolysis-induced erosion of the carrier structure. The release of matrix encapsulated drug cargoes usually takes place immediately upon dispersion of these composites in water. Also, such systems typically require the use of organic solvents for drug loading that can trigger undesirable modifications of the structure and/or function of the encapsulated molecules, such as protein denaturation and aggregation.
SLNs have been investigated as drug-delivery vehicles that can be used to overcome the rapid removal of drug from the administration site associated with fat emulsions, and so enhance targeting [100]. Modification of the SLN core through surface functionalization with pH-titratable peptides/polymers allows for selective release at target sites [101]. Interestingly, dual drug (doxorubicin/verapamil or quinidine/verapamil)-loaded dextran sulphate (DS) SLNs (DS-SLNs), released both drugs without noticeable interference with each other. Most polymer-loaded SLNs released half of the drug in the first few hours and the remaining drug in 15 h or more. The presence of counterions in the medium, especially divalent ions, promoted drug release [102]. SLNs with Chol-but (longer-lasting prodrug of butyric acid) lipid matrix are biocompatible, display spherical shape and favorable zeta potential (good drug-lipid electrostatic interaction). They can be rapidly, consistently, and persistently entrapped in intracellular compartments, so allowing a possible prolonged drug release, and do not substantially modify the specific effect of the active drug [103]. Proteins and antigens intended for therapeutic purposes may be incorporated or adsorbed onto SLNs. Formulation in SLNs confers improved protein stability, avoids proteolytic degradation, as well as sustained release of the incorporated molecules. Important peptides such as cyclosporine A, insulin, calcitonin, and somatostatin have been incorporated into SLNs [104], while SLNs have been functionalized with a homing peptide for gene delivery [105]. Membrane-targeted lipid NPs have recently shown the possibility of using lower drug doses due to direct absorption at target sites on cell surfaces, without the need for full internalization of the NP within the cell. So it is a potentially better option than, e.g., liposome-facilitated delivery (Doxil®) or albumin-facilitated delivery (Abraxane®) which involves diffusion inside the cell. A lipid-coated perfluorocarbon (PFC) core is used as the NP, which binds to specific target sites on the cell for tumor treatment [106].
EPR-based NP selectivity
It is readily acknowledged that liposomes may be employed for passive targeting of tumor tissue through the EPR effect; however, the formulation of the vesicles has important consequences for their targeting, uptake, and efficacy. Neutral lipids have been shown to exhibit preferential localization in solid tumors based on the EPR effect, which relies on gradual passive accumulation of liposomes in the tumor [107]. In contrast, cationic liposomes which have been primarily investigated in gene delivery applications, are rapidly cleared from the circulation by the liver, spleen and the lung, but have been shown to selectively target tumor endothelial cells [108]. The same study demonstrated enhanced cellular uptake and in vitro cytotoxicity using cationic liposomes composed of dimethyl dioctadecyl ammonium bromide (DDAB) for vascular targeting of doxorubicin. Preferential targeting of tumor vessels compared to normal tissue using paclitaxel-containing cationic liposomes has also been demonstrated [109, 110]. Modification of the surface of PEGylated liposomes with the addition of recombinant human serum albumin (rHSA) [111], has been shown to reduce the association of serum proteins including some serum opsonins onto the surface, resulting in a more prolonged circulation time of the complex. It was also observed [112] that the coupling of rHSA to PEGylated liposomes containing doxorubicin (DXR) increased the efficacy and potential safety by significantly prolonging the blood circulation time, leading to higher DXR in the tumor, but a lower level of DXR in heart after intravenous administration thus minimizing drug-related cardio-toxicity.
Ligand-enabled selective NP selectivity
Liposomal research is focusing on enhancing delivery utilizing selective targeting mechanisms. Active targeting of the drug containing liposome to the tissue of interest may be achieved using a ligand coupled to the vesicle surface, which recognizes specific marker molecules on tumor cells [113]. Most tumors over-express receptors for growth factors and peptide hormones, which are being extensively studied as a means of selective delivery of cytotoxic compounds to the target tissue [114]. Molecules that have been identified as potentially successful targets include folate and transferrin. An investigation of the transferrin-conjugated liposomes as drug-delivery systems for inhalation therapy of lung cancer has been reported [115]. The authors concluded that the conjugation of transferrin to liposome vesicles as a homing device increased uptake into cancer cells by utilizing transferrin-receptor-mediated endocytosis to internalize the delivery complex, but further studies to examine liposomal systems in vivo were required. Liposomal vesicles have also been formulated to release their drug cargo in response to trigger mechanisms [116]. The presence of secretory phospholipase A2 (sPLA2), which is over-expressed in inflammatory and tumor tissue as a site-specific trigger of long circulating liposomes is one such approach [117]. In addition, the liposome vesicles may also be composed of masked anti-cancer ether lipids (AELs) that become toxic to tumor cells when activated by sPLA2. In this way, liposomes composed of proAELs can entrap and transport conventional chemotherapeutics such as doxorubicin and cisplatin to the target tumor tissue [118, 119].
Nanocomplexes based on antisense oligo deoxynucleotide or siRNA together with carrier DNA and the cationic polypeptide protamine have been developed for the treatment of lung cancer [120]. The nanocomplexes were then coated with cationic liposomes containing PEG surface chains. Targeting delivery of the nanocomplexes with anisamide, a ligand for targeting nanocomplexes to human lung cancer cells over-expressing sigma transmembrane, was evaluated in vitro in H1299 lung cancer cells. A number of techniques including RT-PCR, immunohistochemistry, and ELISA techniques were used to confirm that the nanocomplex significantly improved delivery and down-regulation of the targeted gene surviving at the RNA and protein levels. Additionally, targeted therapy resulted in enhanced cell cytotoxicity and susceptibility to the chemotherapeutic drug, cisplatin. In vivo studies with xenograft models also demonstrated the targeted nanocomplex preferentially localized at the tumor site, highlighting its potential benefit in anti-cancer applications [121].
The first patented nanodrug developed (designated CYT-6091) actively targets and sequesters recombinant human tumor necrosis factor-alpha (TNF-α) in solid tumors [122], while avoiding uptake by healthy tissues and clearance by the RES. The drug is comprised of TNF and thiolated polyethylene glycol (PEG-Thiol, an RES-avoidance molecule) each of which is individually and covalently bound to the surface of 26-nm gold NPs. Data shows that the binding of PEG-thiol to the surface of the nanoparticle prevents uptake by the liver and spleen, which is hypothesized to be due to the ability of each molecule of PEG to become hydrated once in the bloodstream [123]. By creating a hydrophilic shield surrounding each particle, the particles do not get recognized by the RES, and traffic freely through the circulation. In vivo, the data demonstrated that CYT-6091 accumulates only in solid tumors. It was hypothesized that this is due to the inherent leakiness of the tumor neovasculature and the presence of TNF-binding molecules in and around the tumor. The data also support the hypothesis that, once the NPs exit the tumor neovasculature, each molecule of TNF bound to the surface of the PEGylated cAu NPs serves one of two functions. First, as expected from TNF’s known biological action, CYT-6091 serves as an anti-cancer therapeutic. Second (and more importantly), TNF serves as a tumor-targeting ligand, bringing ten times more TNF to the tumor. Building on this discovery, CytImmune Sciences (Rockville, MD, USA) is expanding its repertoire of nanotherapies built on the PEGylated colloidal gold platform with TNF as a tumor-targeting ligand [124], by developing new multifunctional therapeutics that may synergize with TNF’s anti-cancer action. The first of these new nanodrugs, termed CYT-21001, is comprised of both TNF and an analog of paclitaxel (Taxol®) bound to the surface of PEGylated colloidal gold NPs. CytImmune has demonstrated that this nanodrug delivers ten times more paclitaxel to solid tumors compared to the paclitaxel analog administered alone, and that the nanodrug causes tumor regression in a TNF-insensitive tumor model. CYT-6091 is awaiting phase II results, while CYT-21001, the combination of TNF and paclitaxel on a single particle of PEGylated colloidal gold, could be a prototype for a pipeline of new, patentable cancer nanotherapeutics.
Selective targeting using magnetic fields
A good example of targeting in tumor models is a study [125] where MRI was used to confirm the migration of NPs towards NdFeB magnets placed outside the peritoneal cavity, above grafts of a human ovarian carcinoma. A recent example of the demonstration of targeting in culture came from the group of Hyeon [126], where the uptake of polymer NPs was enhanced by application of a magnetic field, when clusters of Fe3O4 NPs were loaded in doxorubicin-loaded polymer NPs. The polymer particles composed of biodegradable PLGA were PEGylated and surface-coated with folate for active targeting to cancer cells. Interest in these targeting applications also arises from the possibility of detecting the particles after treatment, by MRI, and correlating the results with histology [127]. In fact, polymer/iron-oxide composites are the most commonly reported magnetic theranostic NPs. Another example [128] is the development of functionalized linkers to couple to aminoPVA, by amide linkages, to produce drug-functionalized-aminoPVA-iron-oxide nanocomposites, as vectors for drug delivery. Linkers were developed to which the anti-cancer drugs 5-fluorouridine and doxorubicin were attached as biologically labile esters or peptides, respectively. The former proved to be viable delivery vehicles when tested using human melanoma cells in culture—they were taken up by the cells and proved to be efficient anti-tumor agents.
Controlled release
Drugs can passively target tumors by conjugation to polymers and exploiting the EPR effect that occurs in tumors, areas of inflammation and sites of infection. Solid tumors may have leaky vasculature due to uncontrolled proliferation and angiogenesis, which may be permeable to drug carriers below a specific size (200 nm). This polymer conjugate approach offers the possibility of incorporating two cancer drugs on the polymer backbone allowing release of both drugs at the tumor site, and thus allowing the drugs to act together synergistically and with potentially higher efficacy. N-(2-hydroxypropyl)methacrylamide (HPMA) is a polymer that has been used for the conjugation and delivery of different anticancer drugs. HPMA is hydrophilic, biocompatible, non-immunogenic and can accumulate in tumors [129]. A peptide linker is sometimes attached to the HPMA and the drug, which is cleaved from the polymer by enzymes only present in the cancer cells, thereby releasing the drug slowly to allow a localized action. TNP-470 (Caplostatin), an anticancer drug, was attached via a peptide linker to HPMA, along with the bisphosphonate alendronate. In this case, alendronate has an affinity for bone mineral, while the linker used was only cleavable at a site in bone tissues. In vivo evaluation of the drug-polymer conjugate in mice infected with human osteosarcoma cells showed an inhibition of osteosarcoma growth by 96% [129]. A similar alendronate and HPMA conjugate has been developed to deliver paclitaxel. It inhibited the growth of prostate carcinoma cells and also appeared to be anti-angiogenic in vitro [130].
One way to overcome the challenge of non-specific drug targeting is to use carriers that become activated by some trigger, either one that is specific to the target site or that responds to a remote trigger. An example of the former approach includes pH-responsive materials, which are applicable for drug delivery in cases where a change in pH can act as a trigger for drug release. Potential applications include exploiting the presence of a mildly acidic environment inside inflammatory and tumor tissues (pH 6.8), or cellular vesicles like endosomes (pH 5.5–6) and lysosomes (pH 4.5–5.0). This approach has been investigated in the case of nitric oxide (NO), which plays a role in the regulation of multiple cellular processes including angiogenesis, vasodilation, and the immune response. Controlled release of NO could be an effective therapy for hypoxic respiratory failure associated with pulmonary hypertension. It has been demonstrated [108] that NO can be efficiently stored by covalent linking with polyamine stabilized gold NPs giving effective release of NO from the water-soluble nanocontainers in acidic conditions (pH = 3). A method for the controlled release of pharmaceutical cargoes by remotely actuating drug delivery has also been reported [128]. One or more agents to be delivered (e.g., drugs, therapeutic agents, prophylactic agents, diagnostic agents, etc.) are associated with thermally modulated surfaces (e.g., gold NPs) via thermally responsive linkers (groups consisting of nucleic acids, peptides, proteins, lipids, carbohydrates, and polymers), thereby yielding thermally responsive conjugates. When the thermally responsive linker is exposed to a characteristic temperature and/or characteristic temperature range (i.e., a “trigger temperature”), the linker is disrupted and the agent is released. Thermally responsive linkers can be designed to be disrupted at different temperatures, enabling delivery of complex drug profiles, in a specific order, over variable periods of time. One of the administration routes of particle conjugates is via a portal vein by a catheter [131].
Drug-loaded magnetic-polymer nanocomposites and magnetoliposomes or NPs grafted with drug molecules have a great potential for targeted drug delivery. These have potentially favorable biodistribution and pharmacokinetics, which could be enhanced by magnetic positioning at the site of action on application of a static magnetic field. An integrated concept of biological and physical drug targeting using liposomes with multifunctional properties has been considered, these temperature sensitive magnetic liposomes with folate-targeting ligands have been investigated to optimize targeting, uptake, and release of the anti-cancer agent doxorubicin. The targeted liposomes, which co-encapsulated magnetic NPs and doxorubicin, enabled improved tumor cell killing in comparison to non-magnetic folate-targeted liposomes and Caelyx® [132].
Lung cancer treatment by nebulized delivery of nanocarriers
There is currently no “gold standard” therapy for advanced lung cancer, although platinum-based chemotherapies are the most widely used in the first-line setting for both non-small cell and small cell lung cancers. The median survival with the most commonly used chemotherapeutic regimens in advanced non-small cell lung cancer is only 8 months. The above facts illustrate the necessity of novel strategies to improve the therapeutic outcome for lung cancer treatment. Among the major reasons of treatment failure are the devastating side-effects caused by systemically applied chemotherapeutic agents. Indeed, pharmacologically effective drug concentrations have to be achieved in the diseased lung tissue to lead to tumor regression, but, simultaneously, hazardous drug levels have to be avoided in non-diseased tissue. Although conventional chemotherapeutic agents are principally effective in killing cancer cells, one of their major limitations is the high dosage needed to achieve effective drug concentrations in the lung tissue when applied intravenously, which often results in unwanted dose-limiting side-effects. Chemotherapy agents currently administered for the treatment of lung cancer are predominantly administered by the injectable route [133–135]. However, recent research in targeted therapeutics for lung cancer involving inhalable systems [121] as pharmaceutical aerosols demonstrates this as an excellent mode of delivering drugs directly to the lungs for treating disease. It represents a straightforward strategy to target the lung tumor tissue and allows delivery of a high dosage of the chemotherapeutic agent directly to the lung.
Liposomal drug delivery systems have been studied in detail to increase the therapeutic index of chemotherapy [136]. It has been observed [137] that efficient lung deposition and prolonged presence in the human respiratory tract could be accomplished; however, one study observed that the location of the delivery was dependent on the size of the aerosol droplets produced by the nebulizers used in their delivery rather than on the properties of the vesicles. The impact of vesicle size on rate of liposome clearance has been investigated [138]; irrespective of size, similar clearance patterns were observed with approximately 60% of activity remaining after 20 h, which was attributed to alveolar deposition.
Arguably the most relevant reported research for the current review is the use of novel nebulizer-compatible SLNs for pulmonary delivery of insulin. Fluorescent labeling showed homogenous distribution in lung alveoli, while NP delivery increases in vitro and in vivo stability and enhanced bioavailability (prolonged hypoglycemic effect). Crucially, no cell toxicity was detected [139]. Recent model studies revealed how charged drugs interact with the lung monolayer; NP-lung monolayer interaction can affect monolayer potential, although the structural integrity of the monolayer is well preserved. This highlights the importance of designing materials to give desired interactions between drug and cell and other biological molecules and the cell, as well as drug–NP and NP–cell interactions [140].
Cardiovascular disease treatment with NP carriers
Mechanical interventions such as percutaneous transluminal coronary balloon angioplasty (PTCA) are used to treat narrowing (stenosis) and occlusion of the lumen of coronary arteries that occurs in atherosclerotic coronary artery disease. Such a process is caused by the build-up of fatty deposits called plaques on the inside of the vessel wall, leading to blockage of blood flow to tissues, thrombosis, and to cardiovascular events. In PTCA, a balloon is inserted into the artery, inflated, and the pressure compresses the plaque against the vessel wall. The interventional procedure inevitably results in damage to the blood vessel wall, triggering a process called restenosis. Restenosis, which results in vessel recoil and neointima formation due to a complex thrombotic and inflammatory response to injury [141], leads 30–50% of patients to develop reocclusion within 3–6 months [1]. Systemic therapy, using anti-proliferative and anti-thrombotic drugs, has proven ineffective [141]. To overcome this problem, the insertion of a stent at the time of the intervention has been undertaken to prevent elastic recoil and reduce the incidence of restenosis [1]. However, 20–30% of patients still require repeat procedures [142]. In-stent restenosis occurs due to stretching of the lumen and damage to the lining of the inner vessel wall, also resulting in neointimal hyperplasia. Sufficiently high drug concentrations at the site of injury may not be achieved using systemic therapy without serious side-effects at non-target tissues [143]. Thus, efforts have been directed towards achieving local drug delivery to the injured blood vessel at the time of intervention using drug-eluting stents (DES) in which a drug is released from a polymer coating or else loaded directly onto the stent (on-strut approach) [144, 145]. Both strategies have been investigated for the delivery of paclitaxel, an anti-proliferative drug that arrests cell division. Both paclitaxel and sirolimus have been used in DES devices that are currently marketed [146, 147]. These first-generation DES have proven effective in the delivery of sirolimus and paclitaxel, improving results of percutaneous coronary intervention (PCI) by reducing the risk of restenosis [148]. However, DES are not without their problems [149], hence research focus has increased in the areas of biodegradable polymer stents and nanocarrier systems for cardiovascular drug delivery [150]. The ideal solution would be a targeting system that could facilitate systemic delivery of the NP-therapeutic complex. However, this would require a specific targeting of the plaque cells. Alternatively, the therapeutic-NP complex can be locally delivered using a guidewire or catheter, which significantly simplifies the challenge. Advances relating to both these options are reviewed below.
Targeting of cardiovascular plaques
Hydrophobically modified glycol chitosan NPs functionalized with the plaque-targeting peptide bind specifically to plaques that contain signaling molecules indicative of plaque rupture [151, 152]. Macrophages in plaques can be specifically detected by molecular MRI and optical methods using a PEGylated micellar contrast agent [153, 154]. A key requirement for facilitating cardiovascular treatments therefore involves local delivery of anti-proliferative drugs, which have already been reported in other contexts. A self-assembled mixed micelle of labeled and tumor-homing peptides has been used to deliver Abraxane®, clinically approved paclitaxel-albumin NP (Abraxane® FDA approved in 2005 for breast cancer [155]). The micelle delivered Abraxane to tumors with approximately three times stronger accumulation in the tumor compared to non-targeted tissues [156]. Very recently, a modular multi-functional micelle that contains a targeting element, a fluorophore, and a drug component all in the same particle, 17-nm-diameter NPs with a half-life of 100–130 min, allowed the construction of probes for more sensitive and penetrating imaging techniques, such as positron emission tomography (PET) or MRI. The micelles bind to fibrin on the plaques and tend to congregate at the plaque regions most vulnerable to rupture (shoulders) and deliver an increased concentration of the anticoagulant drug, Hirulog, to the plaque (Hirulog is a direct thrombin inhibitor, so results in fewer side-effects than heparin), so reducing the drug dosage required to prevent clot formation. Unlike iron-oxide NPs, these micelle-based NPs do not induce clotting [155].
Preclinical research on the use of Abraxane with coronary stents has been reported [157]. Polymer nanoencapsulates used to co-precipitate hydrophobic polymers and drugs can feature low drug loadings, uncontrolled encapsulation, and burst release effects. A promising alternative is drug-initiated controlled preparation using metal-paclitaxel-mediated polymerization (quantitative formation of the metal complex) as an alternative to polyester-drug conjugation (coupling chemistry). From 70 to 100-nm micelle-type NPs were synthesized, with metal and chelating ligands removed by solvent extraction, giving salt-stable NPs that can be prepared in a few hours on a gram or larger scale [158].
Localized delivery using catheters
Polymeric NPs could provide an ideal mechanism for local drug delivery. Smaller particles achieve a higher uptake in the arterial wall [159] and the potential exists for sustained delivery of the drug by varying factors such as polymer characteristics. It was shown that release of sirolimus from PLGA NPs could be sustained for 50 days by treating the particles with gelatin [157]. By delivery through a catheter device to the site of injury, the NPs will penetrate the arterial wall under pressure. Once the pressure is released, they become trapped in the arterial wall, providing a drug reservoir. Because PLGA NPs are biodegradable, no polymer remains once the drug has been delivered and the matrix degrades. Compared to DES this is an advantage, as in their case, the remaining scaffold and polymer has the potential to result in an inflammatory response once the drug has been depleted [142]. The small size of NPs results in higher drug uptake into the arterial wall, and the potential exists for sustained delivery of the drug by varying factors such as polymer characteristics (molecular weight, cross-linking, and monomer ratio in the case of copolymers).
A study involving the delivery of paclitaxel by PVA-PLGA NPs in a New Zealand white rabbit ileac arterial model showed a 50% reduction in neointimal area [160]. Biodegradable polymers have also been used to deliver heparin in rat arterial models and have been shown to reduce thrombosis [161]. PLGA NPs have also been investigated for the delivery of alendronate, resulting in reduced neointimal growth and restenosis in a balloon-injured rabbit model [162]. These PLGA NPs were negatively charged, had an average size of 223 nm, and a high entrapment efficiency of 55.1% [162]. Other animal models have also shown that PLGA NPs as drug carriers can inhibit restenosis [163]. PVA-PLGA NPs used to contain paclitaxel have shown good biocompatibility in rabbit vascular smooth muscle cells [160].
Another advancement in the design of NPs for local delivery of sirolimus was achieved recently when NPs were manufactured using carbopol 940 as a bio-adhesive and stabilizer [164]. Carbopol has low toxicity and is biocompatible. Bio-adhesion to the endothelium of the vessel walls would prolong retention time at the local site and allow increased uptake. The study concluded that carbopol could be a better stabilizer than PVA. Polyanhydride polymers have also been studied in localized delivery to the arteries. The polyanhydride poly(fatty acid dimer-sebacic acid) P(FAD:SA) was used to deliver heparin transmurally to prevent venous thrombosis [165].
There have as yet been no restenosis studies that have demonstrated that NPs could be targeted to areas of arterial injury via systemic delivery. Thus, localized delivery via catheters appears to be the most promising at present. NPs have been shown to deposit into the arterial vessel by using porous balloon catheters. In this study, the local administration of polystyrene NPs using a perforated balloon catheter could be successfully visualized by CLSM and TEM. It was demonstrated that NPs can penetrate the non-atherosclerotic arterial vessel wall and that the penetration depth can be adjusted by varying the particle size [166]. The same group concluded that local delivery efficiency of nanoparticulate carriers is critically affected by infusion pressure, and concentration of carrier suspensions. These factors need to be taken into consideration for the design of in vivo experiments [167]. Catheter-based local delivery of biodegradable NPs with sustained release characteristics represents a therapeutic approach to reduce restenosis. Paclitaxel-loaded NPs consisting of PVA grafted to PLGA (PVA-g-PLGA) with varying PLGA chain length were prepared by a solvent evaporation technique. The modification of PLGA side-chain length and PVA backbone composition leads to a more versatile polymer platform compared to commonly used PLA and PLGA. The paclitaxel-loaded NPs showed an increased anti-proliferative effect on cells in comparison to free drug [167].
Conclusions
Research to date has demonstrated that it is possible to effectively enable targeting of plaque and cancer cells, using a combination of cell-specific ligands and custom-engineered nanoparticle complexes. While much of the published literature has focused on polymeric NPs, the enhanced functionality possible by integrating metallic compounds needs to be balanced against the greater risks associated with potential toxicity issues. This balance is likely to favor NP complexes that include metallic layers that confer magnetic or optical properties in cases such as controlled and targeted delivery of therapeutics for cancer and cardiovascular disease, where there is a need to ensure that potent cytotoxic drugs are delivered at relatively high concentrations to the target cells, while avoiding adverse effects on non-target tissues. The need for a comprehensive understanding of NP: bio atom-scale interaction mechanisms is emphasized by recent investigations that point to extensive potential applications for functionalized NPs in biomedical sensing and therapeutic devices [168, 169] as well as in regenerative medicine where they can promote, and even direct, cell interactions and control tissue developments [170, 171]. This new generation of combination therapeutics promises to facilitate more effective and safer treatments, but still requires extensive development and validation. This means that the mainstream uptake for the technology is likely to be in the medium to long term.
Acknowledgments
Funding through the Competence Centre for Applied Nanotechnology (CCAN) from Enterprise Ireland and IDA Ireland (Industrial Development Agency) is gratefully acknowledged.
References
- 1.Panyam J, Labbhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/S0169-409X(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 2.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17:2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 3.Loomis K, McNeeley K, Bellamkonda RV. Nanoparticles with targeting, triggered release, and imaging functionality for cancer applications. Soft Matter. 2011;7(3):839–856. doi: 10.1039/c0sm00534g. [DOI] [Google Scholar]
- 4.Su X, Zhan X, Tang F, Yao JY, Wu J. Magnetic nanoparticles in brain disease diagnosis and targeting drug delivery. Curr Nanosci. 2011;7(1):37–46. doi: 10.2174/157341311794480363. [DOI] [Google Scholar]
- 5.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40(3):1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald KT, Holladay CA, McCarthy C, Power KA, Pandit A, Gallagher WM. Standardization of models and methods used to assess nanoparticles in cardiovascular applications. SMALL. 2011;7(6):705–717. doi: 10.1002/smll.201001347. [DOI] [PubMed] [Google Scholar]
- 7.Petkar KC, Chavhan SS, Agatonovik-Kustrin S, Sawant KK. Nanostructured materials in drug and gene delivery: a review of the state of the art. Cri Rev Ther Drug Carr Sys. 2011;28(2):101–164. doi: 10.1615/critrevtherdrugcarriersyst.v28.i2.10. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharmscitech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng YY, Zhao LB, Li YW, Xu TW. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev. 2011;40(5):2673–2703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267(1):9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaasgaard T, Andresen TL. Liposomal cancer therapy: exploiting tumor characteristics. Expert Opin Drug Deliv. 2010;7(2):225–243. doi: 10.1517/17425240903427940. [DOI] [PubMed] [Google Scholar]
- 12.Slevin M, Badimon L, Grau-Olivares M, Ramis M, Sendra J, Morrison M, Krupinski J. Combining nanotechnology with current biomedical knowledge for the vascular imaging and treatment of atherosclerosis. Mol Biosys. 2010;6(3):444–450. doi: 10.1039/b916175a. [DOI] [PubMed] [Google Scholar]
- 13.Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010;141(3):265–276. doi: 10.1016/j.jconrel.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 15.Duceppe N, Tabrizian M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opin Drug Deliv. 2010;7(10):1191–1207. doi: 10.1517/17425247.2010.514604. [DOI] [PubMed] [Google Scholar]
- 16.Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther. 2010;128(2):324–335. doi: 10.1016/j.pharmthera.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Tran N, Webster TJ. Magnetic nanoparticles: biomedical applications and challenges. J Mater Chem. 2010;20(40):8760–8767. doi: 10.1039/c0jm00994f. [DOI] [Google Scholar]
- 18.Zrazhevskiy P, Sena M, Gao XH. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem Soc Rev. 2010;39(11):4326–4354. doi: 10.1039/b915139g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carr Sys. 2009;26(6):523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devalapally H, Chakilam A, Amiji M. Role of nanotechnology in pharmaceutical product development. J Pharm Sci. 2007;96:2547–2565. doi: 10.1002/jps.20875. [DOI] [PubMed] [Google Scholar]
- 22.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. Biomaterials. 2009;30:6065–6075. doi: 10.1016/j.biomaterials.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 23.Choi O, Hu ZQ. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- 24.Bar-Ilan O, Albrecht RM, Fako VE, et al. Toxicity assessments of multisized gold and silver nanoparticles in Zebrafish embryos. SMALL. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- 25.Tarantola M, Pietuch A, Schneider D, et al. Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micromotility of epithelial cells Nanotoxicology. 2011;5(2):254–268. doi: 10.3109/17435390.2010.528847. [DOI] [PubMed] [Google Scholar]
- 26.Jin H, Heller DA, Sharma R, et al. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS NANO. 2009;3(1):149–158. doi: 10.1021/nn800532m. [DOI] [PubMed] [Google Scholar]
- 27.Chae SR, Badireddy AR, Budarz JF, et al. Heterogeneities in fullerene nanoparticle aggregates affecting reactivity, bioactivity, and transport. ACS NANO. 2010;4(9):5011–5018. doi: 10.1021/nn100620d. [DOI] [PubMed] [Google Scholar]
- 28.Gillies ER, Frechet JMJ. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconj Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 29.AillonKL Xie Y, El-Gendy N, BerklandCJ Laird, Forrest M. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev. 2009;61:457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alivisatos AP, Johnsson KP, Peng X, et al. Organization of ‘nanocrystal molecules’ using DNA. Nature. 1996;382:609–611. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- 31.Chhabra R, Sharma J, Liu Y, et al. DNA self-assembly for nanomedicine. Adv Drug Deliv Rev. 2010;62(6):617–625. doi: 10.1016/j.addr.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Muller RH, Mader K, Gohla ÈS. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharma Biopharma. 2000;50:161–177. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 33.van Schooneveld MM, Vucic E, Koole R, et al. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: a multimodality investigation. Nanoletters. 2008;8(8):2517–2525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 34.Stevens PJ, Sekido M, Lee RJ. A folate receptor-targeted lipid nanoparticle formulation for a lipophilic paclitaxel prodrug. Pharm Res. 2004;21(12):2153–2157. doi: 10.1007/s11095-004-7667-5. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, He Z, Liang J, Zhu Y, Xu H, Yang X. Preparation and characterization of novel fluorescent nanocomposite particles: CdSe/ZnS core-shell quantum dots loaded solid lipid nanoparticles. J Biomed Mater Res Part A. 2008;84A:1018. doi: 10.1002/jbm.a.31205. [DOI] [PubMed] [Google Scholar]
- 36.Cormode DP, Chandrasekar R, Delshad A, et al. Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconj Chem. 2009;20(5):937–943. doi: 10.1021/bc800520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzen S. A comparison of peptide and folate receptor targeting of cancer cells: from single agent to nanoparticle. Expert Opin Drug Deliv. 2011;8(3):281–298. doi: 10.1517/17425247.2011.554816. [DOI] [PubMed] [Google Scholar]
- 38.Chen B, Wu W, Wang X. Magnetic iron oxide nanoparticles for tumor-targeted therapy. Curr Cancer Drug Targets. 2011;11(2):184–189. doi: 10.2174/156800911794328475. [DOI] [PubMed] [Google Scholar]
- 39.Tan ML, Choong PFM, Dass CR. Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides. 2010;31(1):184–193. doi: 10.1016/j.peptides.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Veiseh O, Gunn JW, Zhang MQ. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62(3):284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundar S, Kundu J, Kundu SC (2010) Biopolymeric nanoparticles. Sci Technol Adv Mater 11(1): Art No. 014104 2010 [DOI] [PMC free article] [PubMed]
- 42.Schartl W. Current directions in core-shell nanoparticle design. Nanoscale. 2010;2(6):829–843. doi: 10.1039/c0nr00028k. [DOI] [PubMed] [Google Scholar]
- 43.Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull. 2010;58(11):1423–1430. doi: 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- 44.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108(6):2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalisation, and application. Angewandte Chemie-Int Ed . 2007;46(8):1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 47.Shen L, Laibinis PE, Hatton TA. Bilayer surfactant stabilized magnetic fluids: synthesis and interactions at interfaces. Langmuir. 1999;15:447–453. doi: 10.1021/la9807661. [DOI] [Google Scholar]
- 48.Shen L, Stachowiak A, Fateen SEK, Laibinis PE, Hatton TA. Structure of alkanoic acid stabilized magnetic fluids. A small-angle neutron and light scattering analysis. Langmuir. 2001;17(2):288–299. doi: 10.1021/la9916732. [DOI] [Google Scholar]
- 49.Racuciu M, Creanga DE, Calugaru G. Synthesis and rheological properties of an aqueous ferrofluid. J Optoelectron Adv Mater. 2005;7(6):2859–2864. [Google Scholar]
- 50.Racuciu M, Creanga DE, Airinei A. Citric-acid-coated magnetite nanoparticles for biological applications. Eur Phy J E. 2006;21(2):117–121. doi: 10.1140/epje/i2006-10051-y. [DOI] [PubMed] [Google Scholar]
- 51.Hodenius MAJ, Niendorf T, Krombach GA, Richtering W, Eckert T, Lueken H, Speldrich M, Gunther RW, Baumann M, Soenen SJH, De Cuyper M, Schmitz-Rode T. Synthesis, physicochemical characterization and MR relaxometry of aqueous ferrofluids. J Nanosci Nanotechnol. 2008;8(5):2399–2409. doi: 10.1166/jnn.2008.312. [DOI] [PubMed] [Google Scholar]
- 52.Prow TW, Rose WA, Wang N, Reece LM, Lvov Y, Leary JF. Biosensor-controlled gene therapy/drug delivery with nanoparticles for nanomedicine. Proc SPIE. 2005;5692:199–208. doi: 10.1117/12.589422. [DOI] [Google Scholar]
- 53.Smitham JB, Evans R, Napper DH. Analytical theories of steric stabilization of colloidal dispersions. J Chem Soc—Faraday Transac I. 1975;71(2):285–297. doi: 10.1039/f19757100285. [DOI] [Google Scholar]
- 54.Evans R, Napper DH. Perturbation method for incorporating concentration-dependence of Flory–Huggins parameter in the theory of steric stabilization. J Chem Soc—Faraday Transac I. 1977;73:1377–1385. doi: 10.1039/f19777301377. [DOI] [Google Scholar]
- 55.Barnard AS. Modelling of nanoparticles: approaches to morphology and evolution. Rep Prog Phys. 2010;73:086502. doi: 10.1088/0034-4885/73/8/086502. [DOI] [Google Scholar]
- 56.Lin J, Zhang H, Chen Z, Zheng Y. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 2010;284(9):5421–5429. doi: 10.1021/nn1010792. [DOI] [PubMed] [Google Scholar]
- 57.Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: implications for cellular magnetic resonance imaging. NMR Biomed. 2005;18(6):383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- 58.Lee HS, Kim EH, Shao HP, Kwak BK. Synthesis of SPIO-chitosan microspheres for MRI-detectable embolotherapy. J Magn Magn Mater. 2005;293(1):102–105. doi: 10.1016/j.jmmm.2005.01.049. [DOI] [Google Scholar]
- 59.Lee HS, Shao HP, Huang YQ, Kwak BK. Synthesis of MRI contrast agent by coating superparamagnetic iron oxide with chitosan. IEEE Transac Magn. 2005;41(10):4102–4104. doi: 10.1109/TMAG.2005.855338. [DOI] [Google Scholar]
- 60.Lee SJ, Jeong JR, Shin SC, Kim JC, Chang YH, Chang YM, Kim JD. Nanoparticles of magnetic ferric oxides encapsulated with poly(d, l latide-co-glycolide) and their applications to magnetic resonance imaging contrast agent. J Magn Magn Mater. 2004;272(76):2432–2433. doi: 10.1016/j.jmmm.2003.12.416. [DOI] [Google Scholar]
- 61.Hu FX, Neoh KG, Kang ET. Synthesis and in vitro anti-cancer evaluation of tamoxifen-loaded magnetite/PLLA composite nanoparticles. Biomaterials. 2006;27(33):5725–5733. doi: 10.1016/j.biomaterials.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Bulte JWM, de Cuyper M, Despres D, Frank JA. Preparation, relaxometry, and biokinetics of PEGylated magnetoliposomes as MR contrast agent. J Magn Magn Mater. 1999;194:204–209. doi: 10.1016/S0304-8853(98)00556-3. [DOI] [Google Scholar]
- 63.Shultz MD, Calvin S, Fatouros PP, Morrison SA, Carpenter EE. Enhanced ferrite nanoparticles as MRI contrast agents. J Magn Magn Mater. 2007;311(1):464–468. doi: 10.1016/j.jmmm.2006.10.1188. [DOI] [Google Scholar]
- 64.Martina M-S, Fortin J-P, Ménager C, Clément O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J Am Chem Soc. 2005;127:10676–10685. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 65.Nitin N, LaConte LEW, Zurkiya O, Hu X, Bao G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J Biol Inorg Chem. 2004;9(6):706–712. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 66.Kohler N, Fryxell GE, Zhang MQ. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J Am Chem Soc. 2004;126(23):7206–7211. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- 67.Ai H, Flask C, Weinberg B, Shuai X, Pagel MD, Farrell D, Duerk J, Gao JM. Magnetite-loaded polymeric micelles as ultrasensitive magnetic-resonance probes. Adv Mater. 2005;17(16):1949. doi: 10.1002/adma.200401904. [DOI] [Google Scholar]
- 68.Li Z, Wei L, Gao MY, Lei H. One-pot reaction to synthesize biocompatible magnetite nanoparticles. Adv Mater. 2005;17(8):1001. doi: 10.1002/adma.200401545. [DOI] [Google Scholar]
- 69.Sun C, Sze R, Zhang MQ. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J Biomed Mater Res Part A. 2006;78A(3):550–557. doi: 10.1002/jbm.a.30781. [DOI] [PubMed] [Google Scholar]
- 70.Xie J, Xu C, Kohler N, Hou Y, Sun S. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells. Adv Mater. 2007;19(20):3163. doi: 10.1002/adma.200701975. [DOI] [Google Scholar]
- 71.Fan QL, Neoh KG, Kang ET, Shuter B, Wang SC. Solvent-free atom transfer radical polymerization for the preparation of poly(poly(ethyleneglycol) monomethacrylate)-grafted Fe3O4 nanoparticles: synthesis, characterization and cellular uptake. Biomaterials. 2007;28(36):5426–5436. doi: 10.1016/j.biomaterials.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Ng YW, Chen Y, Shuter B, Yi J, Ding J, Wang SC, Feng SS. Formulation of superparamagnetic iron oxides by nanoparticles of biodegradable polymers for magnetic resonance imaging. Adv Func Mater. 2008;18(2):308–318. doi: 10.1002/adfm.200700456. [DOI] [Google Scholar]
- 73.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv Mater. 2006;18(19):2553. doi: 10.1002/adma.200600385. [DOI] [Google Scholar]
- 74.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4(3):372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dodd CH, Hsu HC, Chu WJ, Yang PG, Zhang HG, Mountz JD, Zinn K, Forder J, Josephson L, Weissleder R, Mountz JM, Mountz JD. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J Immunol Methods. 2001;256(1–2):89–105. doi: 10.1016/S0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 76.Dodd C, Mountz J, Chu WJ, Josephson L, Zhang HG, Weissleder R, Mountz JM, Zinn K, Mountz JD, Hsu HC. In vivo magnetic resonance imaging (MRI) of T cells loaded with HIV transactivator (tat) peptide-derived super-paramagnetic nanoparticles. Faseb J. 2001;15(5):A744–A744. doi: 10.1016/s0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 77.Hsu HC, Dodd C, Chu WJ, Yang PA, Josephson L, Sun SH, Zhang HG, Weissleder R, Mountz JD. Normal response of T cells loaded with HIV transactivator (tat) peptide-derived super-paramagnetic nanoparticles for magnetic resonance imaging (MRI) Faseb J. 2001;15(4):A330–A330. doi: 10.1016/s0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 78.Asongkla N, Bey E, Ren JM, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao JM. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6(11):2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 79.von zur Muhlen C, von Elverfeldt D, Bassler N, Neudorfer I, Steitz B, Petri-Fink A, Hofmann H, Bode CPK. Superparamagnetic iron oxide binding and uptake as imaged by magnetic resonance is mediated by the integrin receptor Mac-1 (CD11b/CD18): implications on imaging of atherosclerotic plaques. Atherosclerosis. 2007;193(1):102–111. doi: 10.1016/j.atherosclerosis.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 80.Martina MS, FortinJP Menager C, Clement O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J Am Chem Soc. 2005;127(30):10676–10685. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 81.Larsen BA, Haag MA, Serkova NJ, Shroyer KR, Stoldt CR (2008) Controlled aggregation of superparamagnetic iron oxide nanoparticles for the development of molecular magnetic resonance imaging probes. Nanotechnology 19(26): 265102. doi:10.1088/0957-4484/19/26/265102 [DOI] [PubMed]
- 82.Meincke M, Schlorf T, Kossel E, Jansen O, Glueer CC, Mentlein R. Iron oxide—loaded liposomes for MR imaging. Frontiers Biosci. 2008;13:4002–4008. doi: 10.2741/2987. [DOI] [PubMed] [Google Scholar]
- 83.Hultman KL, Raffo AJ, Grzenda AL, Harris PE, Brown TR, O’Brien S. Magnetic resonance imaging of major histocompatibility class II expression in the renal medulla using immunotargeted superparamagnetic iron oxide nanoparticles. Acs Nano. 2008;2(3):477–484. doi: 10.1021/nn700400h. [DOI] [PubMed] [Google Scholar]
- 84.Byrne SJ, Corr SA, Gun’ko YK, Kelly JM, Brougham DF, Ghosh S. Magnetic nanoparticle assemblies on denatured DNA show unusual magnetic relaxivity and potential applications for MRI. Chem Commun. 2004;22:2560–2561. doi: 10.1039/b409603g. [DOI] [PubMed] [Google Scholar]
- 85.Philipse AP, Vanbruggen MPB, Pathmamanoharan C. Magnetic silica dispersions—preparation and stability of surface-modified silica particles with a magnetic core. Langmuir. 1994;10(1):92–99. doi: 10.1021/la00013a014. [DOI] [Google Scholar]
- 86.Yan F, Xu H, Anker J, Kopelman R, Ross B, Rehemtulla A, Reddy R. Synthesis and characterization of silica-embedded iron oxide nanoparticles for magnetic resonance imaging. J Nanosci Nanotechnol. 2004;4(1–2):72–76. doi: 10.1166/jnn.2004.074. [DOI] [PubMed] [Google Scholar]
- 87.Liang S, Wang YX, Zhang CF, Liu XQ, Liu ZF, Xu RH, Yin DZ. Synthesis of amino-modified magnetite nanoparticles coated with Hepama-1 and radiolabeled with Re-188 for bio-magnetically targeted radiotherapy. J Radioanal Nucl Chem. 2006;269(1):3–7. doi: 10.1007/s10967-006-0223-5. [DOI] [Google Scholar]
- 88.Santra S, Tapec R, Theodoropoulou N, Dobson J, Hebard A, Tan WH. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: the effect of non-ionic surfactants. Langmuir. 2001;17(10):2900–2906. doi: 10.1021/la0008636. [DOI] [Google Scholar]
- 89.Tartaj P, Serna CJ. Microemulsion-assisted synthesis of tuneable superparamagnetic composites. Chem Mater. 2002;14(10):4396–4402. doi: 10.1021/cm021214d. [DOI] [Google Scholar]
- 90.Tartaj P, Serna CJ. Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites. J Am Chem Soc. 2003;125(51):15754–15755. doi: 10.1021/ja0380594. [DOI] [PubMed] [Google Scholar]
- 91.Yang HH, Zhang SQ, Chen XL, Zhuang ZX, Xu JG, Wang XR. Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal Chem. 2004;76(5):1316–1321. doi: 10.1021/ac034920m. [DOI] [PubMed] [Google Scholar]
- 92.Carpenter EE, Sangregorio C, O’Connor CJ. Effects of shell thickness on blocking temperature of nanocomposites of metal particles with gold shells. IEEE Transac Magn. 1999;35(5):3496–3498. doi: 10.1109/20.800568. [DOI] [Google Scholar]
- 93.Cho SJ, Idrobo JC, Olamit J, Liu K, Browning ND, Kauzlarich SM. Growth mechanisms and oxidation resistance of gold-coated iron nanoparticles. Chem Mater. 2005;17(12):3181–3186. doi: 10.1021/cm0500713. [DOI] [Google Scholar]
- 94.Park JI, Cheon J. Synthesis of “solid solution” and “core-shell” type cobalt-platinum magnetic nanoparticles via transmetalation reactions. J Am Chem Soc. 2001;123(24):5743–5746. doi: 10.1021/ja0156340. [DOI] [PubMed] [Google Scholar]
- 95.Ban ZH, Barnakov YA, Golub VO, O’Connor CJ. The synthesis of core-shell iron@gold nanoparticles and their characterization. J Mater Chem. 2005;15(43):4660–4662. doi: 10.1039/b504304b. [DOI] [Google Scholar]
- 96.Lyon JL, Fleming DA, Stone MB, Schiffer P, Williams ME. Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett. 2004;4(4):719–723. doi: 10.1021/nl035253f. [DOI] [Google Scholar]
- 97.Kim J, Park S, Lee JE, Jin SM, Lee JH, Lee IS, Yang I, Kim JS, Kim SK, Cho MH, Hyeon T. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angewandte Chemie-Int Ed. 2006;45(46):7754–7758. doi: 10.1002/anie.200602471. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi T, Hirono S, Tomita M, Umemura S. Magnetic thin films of cobalt nanocrystals encapsulated in graphite-like carbon. Nature. 1996;381(6585):772–774. doi: 10.1038/381772a0. [DOI] [Google Scholar]
- 99.Nikitenko SI, Koltypin Y, PalchikO Felner I, Xu XN, Gedanken A. Synthesis of highly magnetic, air-stable iron iron carbide nanocrystalline particles by using power ultrasound. Angewandte Chemie-Int Ed. 2001;40(23):4447. doi: 10.1002/1521-3773(20011203)40:23<4447::AID-ANIE4447>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 100.Davis SS. Coming of age of lipid-based drug delivery systems. Adv Drug Deliv Rev. 2004;56:1241–1242. doi: 10.1016/j.addr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 101.UlrichAS AS. Biophysical aspects of using liposomes as delivery vehicles. Biosci Rep. 2002;22(2):129–150. doi: 10.1023/A:1020178304031. [DOI] [PubMed] [Google Scholar]
- 102.Wong HL, Bendayan R, Rauth AM, Wu XY. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J Pharma Sci. 2004;93(8):1993. doi: 10.1002/jps.20100. [DOI] [PubMed] [Google Scholar]
- 103.Brioschi A, Zara GP, Calderoni S, Gasco MR, Mauro A. Cholesterylbutyrate solid lipid nanoparticles as a butyric acid prodrug. Molecules. 2008;13:230–254. doi: 10.3390/molecules13020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 105.del Pozo-Rodríguez A, Pujals S, Delgado D, Solinís MA, Gascón AR, Giralt E, Pedraz JL. A proline-rich peptide improves cell transfection of solid lipid nanoparticle-based non-viral vectors. J Control Release. 2009;133:52–59. doi: 10.1016/j.jconrel.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Partlow KC, Lanza GM, Wickline SA. Exploiting lipid raft transport with membrane targeted nanoparticles: a strategy for cytosolic drug delivery. Biomaterials. 2008;29:3367–3375. doi: 10.1016/j.biomaterials.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang SK, Mayhew E, Gilani S, Lasic D, Martin FJ, Papahadjopoulos D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res. 1992;52:6774–6781. [PubMed] [Google Scholar]
- 108.Wu X, Deng Y, Wang G, Tao K. Combining siRNAs at two different sites in the EGFR to suppress its expression, induce apoptosis, and enhance 5-fluorouracil sensitivity of colon cancer cells. J Surg Res. 2007;138(1):56–63. doi: 10.1016/j.jss.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 109.Schmitt-Sody M, Strieth S, Krasnici S, Sauer B, Schulze B, Teifel M. Neovascular targeting therapy: paclitaxel encapsulated in cationic liposomes improves antitumoral efficacy. Clin Cancer Res. 2003;9:2335–2341. [PubMed] [Google Scholar]
- 110.Sebastian S, Martin EE, Birgitta S, Brita S, Michael T, Uwe M, Marc D. Neovascular targeting chemotherapy: encapsulation of paclitaxel in cationic liposomes impairs functional tumor microvasculature. Int J Cancer. 2004;110:117–124. doi: 10.1002/ijc.20083. [DOI] [PubMed] [Google Scholar]
- 111.Furumoto K, Yokoe J-I, Ogawara K-I, Amano S, Takaguchi M, Higaki K, Kai T, Kimura T. Effect of coupling of albumin onto surface of PEG liposome on its in vivo disposition. Int J Pharma. 2007;329:110–116. doi: 10.1016/j.ijpharm.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 112.Yokoe J-I, Sakuragi S, Yamatoto K, Teragaki T, Ogowara K-I, Higaki K, Katayama N, Kai T, Sato M, Kimura T. Albumin-conjugated PEG liposome enhances tumor distribution of liposomal doxorubicin in rats. Int J Pharma. 2008;353:28–34. doi: 10.1016/j.ijpharm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Fonseca C, Moreira JN, Ciudad CJ, Pedroso de Lima MC, Simoes S. Targeting of sterically stabilised pH-sensitive liposomes to human T-leukaemia cells. Eur J Pharma Biopharma. 2005;59:359–366. doi: 10.1016/j.ejpb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 114.Anabousi S, Bakowski U, Schneider M, Huwer H, Lehr C-M, Ehrhardt C. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur J Pharma Sci. 2006;29:367–374. doi: 10.1016/j.ejps.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 115.Anabousi S, Kleeman E, Bakowski U, Kissel T, Schneider T, Gessler T, Seeger W, Lehr C-M, Ehrhardt C. Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J Nanosci Nanotechnol. 2006;6:3010–3016. doi: 10.1166/jnn.2006.461. [DOI] [PubMed] [Google Scholar]
- 116.Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci. 2008;97(11):4696–4740. doi: 10.1002/jps.21358. [DOI] [PubMed] [Google Scholar]
- 117.Zhu G, Mock JN, Aljuffali I, Cummings BS, Arnold RD (2011) Secretory phospholipase A2 responsive liposomes. J Pharm Sci. Article first published online: 31 MAR 2011 [DOI] [PMC free article] [PubMed]
- 118.Andresen TL, Jensen SS, Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44(1):68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 119.Jensen SS, Andresen TL, Davidsen J, Høyrup P, Shnyder SD, Bibby MC, Gill JH, Jørgensen K. Secretory phospholipase A2 as a tumor-specific trigger for targeted delivery of a novel class of liposomal prodrug anticancer etherlipids. Mol Cancer Ther. 2004;3(11):1451–1458. [PubMed] [Google Scholar]
- 120.LiS-D Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharm. 2006;3:579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- 121.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 122.Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischo JC. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-A delivery. Mol Cancer Ther. 2006;5(4):1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 123.Farma JM, Puhlmann M, Soriano PA, Cox D, Paciotti GF, Tamarkin L, Alexander HR. Direct evidence for rapid and selective induction of tumor neovascular permeability by tumor necrosis factor and a novel derivative, colloidal gold bound tumor necrosis factor Int. J Cancer. 2007;120:2474–2480. doi: 10.1002/ijc.22270. [DOI] [PubMed] [Google Scholar]
- 124.Bahtia NS et al. (2009) US Patent Application 2009/0093551
- 125.Klostergaard J, Bankson J, Auzenne E, Gibson D, Yuill W, Seeney CE. Magnetic vectoring of magnetically responsive nanoparticles within the murine peritoneum. J Magn Magn Mater. 2007;311(1):330–335. doi: 10.1016/j.jmmm.2006.10.1163. [DOI] [Google Scholar]
- 126.Kim J, Lee JE, Lee SH, Yu JH, Lee JH, Park TG, Hyeon T. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Adv Mater. 2008;20(3):478. doi: 10.1002/adma.200701726. [DOI] [Google Scholar]
- 127.Jurgons R, Seliger C, Hilpert A, Trahms L, Odenbach S, Alexiou C. Drug loaded magnetic nanoparticles for cancer therapy. J Phys Condens Matter. 2006;18(38):S2893–S2902. doi: 10.1088/0953-8984/18/38/S24. [DOI] [Google Scholar]
- 128.Hanessian S, Grzyb JA, Cengelli F, Juillerat-Jeanneret L. Synthesis of chemically functionalized superparamagnetic nanoparticles as delivery vectors for chemotherapeutic drugs. Bioorg Med Chem. 2008;16(6):2921–2931. doi: 10.1016/j.bmc.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 129.Segal E, Satchi-Fainaro R. Design and development of polymer conjugates as anti-angiogenic agents. Adv Drug Deliv Rev. 2009;61:1159–1176. doi: 10.1016/j.addr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 130.Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugate. Angew Chem Int Ed Engl. 2009;48:2949–2954. doi: 10.1002/anie.200805133. [DOI] [PubMed] [Google Scholar]
- 131.Dai H, Jiang X, Tan GC, Chen Y, Torbenson M, Leong KW, Mao HQ. Chitosan-DNA nanoparticles delivered by intrabiliary infusion enhance liver-targeted gene delivery. Int J Nanomed. 2006;1(4):507–522. doi: 10.2147/nano.2006.1.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pradhana P. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172(5):523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 134.Celikoglu F, Celikoglu SI, Goldberg EP. Techniques for intratumoral chemotherapy of lung cancer by bronchoscopic drug delivery. Cancer Ther. 2008;6:545–552. doi: 10.1016/j.lungcan.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 135.Celikoglu F, Celikoglu SI, Goldberg EP. Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J Pharm Pharmacol. 2010;62(3):287–295. doi: 10.1211/jpp.62.03.0001. [DOI] [PubMed] [Google Scholar]
- 136.Andresen TL, Jensen SS, Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Taylor K, Newton JM. Liposomes for controlled delivery of drugs to the lung. Thorax. 1992;47:257–259. doi: 10.1136/thx.47.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Farr SJ, Kellaway IW, Parry-Jones DR, Woolfrey SG. 99 m-Technetium as a marker of liposomal deposition and clearance in the human lung. Int J Pharma. 1985;26:303–316. doi: 10.1016/0378-5173(85)90239-X. [DOI] [Google Scholar]
- 139.Liu J, et al. Solid lipid nanoparticles for pulmonary delivery of insulin. Int J Pharma. 2008;356:333–344. doi: 10.1016/j.ijpharm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 140.Ku T, et al. Size dependent interactions of nanoparticles with lung surfactant model systems and the significant impact on surface potential. J Nanosci Nanotechnol. 2008;8:2971–2978. doi: 10.1166/jnn.2008.171. [DOI] [PubMed] [Google Scholar]
- 141.Kavanagh CA, Rochev YA, Gallagher WM, Dawson KA, Keenan AK. Local drug delivery in restenosis injury: thermoresponsive co-polymers as potential drug delivery systems. Pharmacol Ther. 2004;102:1–15. doi: 10.1016/j.pharmthera.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 142.Bennett MJ, O’Sullivan M. Mechanisms of angioplasty and stent restenosis: implications for design of rational therapy. Pharmacol Ther. 2001;91:149–166. doi: 10.1016/S0163-7258(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 143.Burta HM, Hunter WL. Drug-eluting stents: a multidisciplinary success story. Adv Drug Deliv Rev. 2006;58(3):350–357. doi: 10.1016/j.addr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 144.van der Hoeven BL, Pires NM, Warda HM, Oemrawsingh PV, van Vlijmen BJ, Quax PH, Schalij MJ, van der Wall EE, Jukema JW. Drug-eluting stents: results, promises and problems. Int J Cardiol. 2005;99(1):9–17. doi: 10.1016/j.ijcard.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 145.Acharyab G, Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv Drug Deliv Rev. 2006;58(3):387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 146.Kamath KR, Barrya JJ, Millera KM. The Taxus™ drug-eluting stent: a new paradigm in controlled drug delivery. Adv Drug Deliv Rev. 2006;58(3):412–436. doi: 10.1016/j.addr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 147.Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113:1108–1113. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- 148.Sheiban I, Villata G, Bollati M, Sillano D, Lotrionte M, Biondi-Zoccai G. Next-generation drug-eluting stents in coronary artery disease: focus on everolimus-eluting stent (Xience V) Vasc Health Risk Manag. 2008;4(1):31–38. doi: 10.2147/vhrm.2008.04.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA-J Am Med Assoc. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 150.Lee SH, Szinai I, Carpenter K, Katsarava R, Jokhadze G, Chu CC, et al. In vivo biocompatibility evaluation of stents coated with a new biodegradable elastomeric and functional polymer. Coron Artery Dis. 2002;13:237–241. doi: 10.1097/00019501-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 151.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of Atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 152.Park K, Hong H-Y, Moon HJ, Lee B-H, Kim I-S, Kwon IC, Rhee K. A new atherosclerotic lesion probe based on hydrophobically modified chitosan nanoparticles functionalized by the atherosclerotic plaque targeted peptides. J Control Release. 2008;128:217–223. doi: 10.1016/j.jconrel.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 153.Mulder WJM, Strijkers GJ, Briley-Saboe KC, et al. Molecular imaging of macrophages in antherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58(6):1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 154.Broz P, Marsch S, Hunzikel P. Targeting of vulnerable plaque macrophages with polymer-based nanostructures. Trends in Cardiovasc Medic. 2007;17(6):190. doi: 10.1016/j.tcm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Peters D, et al. Targeting atherosclerosis by using modular, multifunctional micelles. PNAS. 2009;106(24):9815–9819. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Karmali PP, et al. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine. 2008;5:73–82. doi: 10.1016/j.nano.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zweers MLT, Engbers GHM, Grijpma DW, Feijen J. Release of anti-restenosis drugs from poly(ethylene oxide)-poly(dl-lactic-co-glycolic acid) nanoparticles. J Control Release. 2006;114:317–324. doi: 10.1016/j.jconrel.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 158.Tong R, Cheng J. Paclitaxel-initiated, controlled polymerization of lactide for the formulation of polymeric nanoparticulate delivery vehicles. Angew Chem. 2008;47:4830–4834. doi: 10.1002/anie.200800491. [DOI] [PubMed] [Google Scholar]
- 159.Westedt UB-TL, Schaper AK. Deposition of nanoparticles in the arterial vessel by porous balloon catheters: localization by confocal laser scanning microscopy and transmission electron microscopy. AAPS PharmSci. 2002;44:1–6. doi: 10.1208/ps040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Westedt U, Kalinowski M, Wittmar M, Merdan T, Unger F, Fuchs J, Schaller S, Bakowsky U, Kissel T. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J Control Release. 2007;119:41–51. doi: 10.1016/j.jconrel.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 161.Orloff LA, Domb AJ, Teomim D, Fishbein I, Golomb G. Biodegradable implant strategies for inhibition of restenosis. Adv Drug Deliv Rev. 1997;24:3–9. doi: 10.1016/S0169-409X(96)00478-4. [DOI] [Google Scholar]
- 162.Cohen-Sela E, Rosenweig O, Gao J, Epstein H, Gati I, Reich R, Dananberg HD, Golomb G. Alendronate-loaded nanoparticles deplete monocytes and attenuate restenosis. J Control Release. 2006;113:23–30. doi: 10.1016/j.jconrel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 163.Brito L, Amiji M. Nanoparticulate carriers for the treatment of coronary restenosis. Int J Nanomed. 2007;2:143–161. doi: 10.2217/17435889.2.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zou WCG XIY, Zhang N. New approach for local delivery of rapamycin by bioadhesive PLGA-carbopol nanoparticles. Drug Deliv. 2009;16:15–23. doi: 10.1080/10717540802481307. [DOI] [PubMed] [Google Scholar]
- 165.Orloff LA, Glenn MG, Domb AJ, Esclamado RA. Prevention of venous thrombosis in microvascular surgery by transmural release of heparin from a polyanhydride polymer. Surgery. 1995;117:554–559. doi: 10.1016/S0039-6060(05)80255-7. [DOI] [PubMed] [Google Scholar]
- 166.Westedt U, Barbu-TudoranL Schaper AK, Kalinowski M, Alfke H, Kissel T. Effects of different application parameters on penetration characteristics and arterial vessel wall integrity after local nanoparticle delivery using a porous balloon catheter. Eur J Pharma Biopharma. 2004;58:161–168. doi: 10.1016/j.ejpb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 167.McCarthy JR, Perez JM, Bruckner C, Weissleder R. Polymeric nanoparticle preparation that eradicate tumors. Nano Lett. 2005;5:2552–2556. doi: 10.1021/nl0519229. [DOI] [PubMed] [Google Scholar]
- 168.Lacroix L-M, Ho D, Sun S. Magnetic nanoparticles as both imaging probes and therapeutic agents. Curr Top Med Chem. 2010;10:1184–1197. doi: 10.2174/156802610791384207. [DOI] [PubMed] [Google Scholar]
- 169.MaherRC MaierSA, Cohen LF, Koh L, Laromaine A, Dick JAG, Stevens MM. Exploiting SERS hot spots for disease-specific enzyme detection. J Phys Chem. 2010;C114(16):7231–7235. [Google Scholar]
- 170.Khang D, Carpenter J, Chun YW, Pareta R, Webster TJ. Nanotechnology for regenerative medicine. Biomed Microdevices. 2010;12(4):575–587. doi: 10.1007/s10544-008-9264-6. [DOI] [PubMed] [Google Scholar]
- 171.Salgado AJ, Oliveira JM, Pirraco RP, Pereira VH, Fraga JS, Marques AP, Neves NM, Mano JF, Reis RL, Sousa N. Carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles in central nervous systems-regenerative medicine: effects on neuron/glial cell viability and internalization efficiency. Macromol Biosci. 2010;10(10):1130–1140. doi: 10.1002/mabi.201000005. [DOI] [PubMed] [Google Scholar]


