Abstract
Natural killer (NK) cells are a part of the innate immune system that functions mainly to kill transformed and infected cells. Their activity is controlled by signals derived from a panel of activating and inhibitory receptors. The natural cytotoxicity receptors (NCRs): NKp30, NKp44, and NKp46 (NCR1 in mice) are prominent among the activating NK cell receptors and they are, notably, the only NK-activating receptors that are able to recognize pathogen-derived ligands. In addition, the NCRs also recognize cellular ligands, the identity of which remains largely unknown. In this review, we summarize the current knowledge regarding viruses that are recognized by the NCRs, focusing on the diverse immune-evasion mechanisms employed by viruses to escape this detection. We also discuss the unique role the NCRs have in regulating NK cell activity with particular emphasis on the in vivo function of NKp46/NCR1.
Keywords: Natural cytotoxicity receptors, Natural cytotoxicity receptors ligands, NK cells, Viruses, Immune evasion
NK cells, NCRs, and their known ligands
Natural killer (NK) cells are a part of the innate immune system. They make up 5–15 % of peripheral blood leukocytes (PBLs) and a greater percent of resident leukocytes in certain tissues such as the liver and the decidua [1, 2]. Unlike B and T lymphocytes, NK cells do not express somatically rearranged receptors, but rather possess a variety of germline-encoded receptors [3]. NK cells are capable of recognizing cells that are transformed or infected with pathogens and kill these cells via the release of cytotoxic granules or through death receptors [2, 4, 5]. Consequently, NK cells have been studied as potential tools for immunotherapy, especially in the treatment of leukemia [6]. In addition, NK cells secrete cytokines such as IFN-γ, which is pivotal in fighting infection and in shaping the developing immune response [7].
NK cell activity is controlled by signals derived from activating and inhibitory receptors. In general, activating receptors recruit kinases through associated immunoreceptor tyrosine-based activating motif (ITAM)-containing adaptor molecules, whereas the inhibitory receptors recruit phosphatases through the immunoreceptor tyrosine-based inhibitory motifs (ITIMs) located in their long cytoplasmic tails [8]. Integration of all the inhibitory and activating signals determines whether the NK cells will kill or spare the target cells. The inhibitory receptors mostly interact with major histocompatibility complex (MHC) class I ligands [9]. Thus, cells evading recognition by CD8+ T cells through down-regulation of MHC class I proteins might become susceptible to NK cell-induced cytolysis. This is known as the “missing-self hypothesis” [10]. However, a lack of inhibitory signals is insufficient in itself to trigger NK cell activation and killing, and a parallel activating signal, delivered by activating receptors, is required [9].
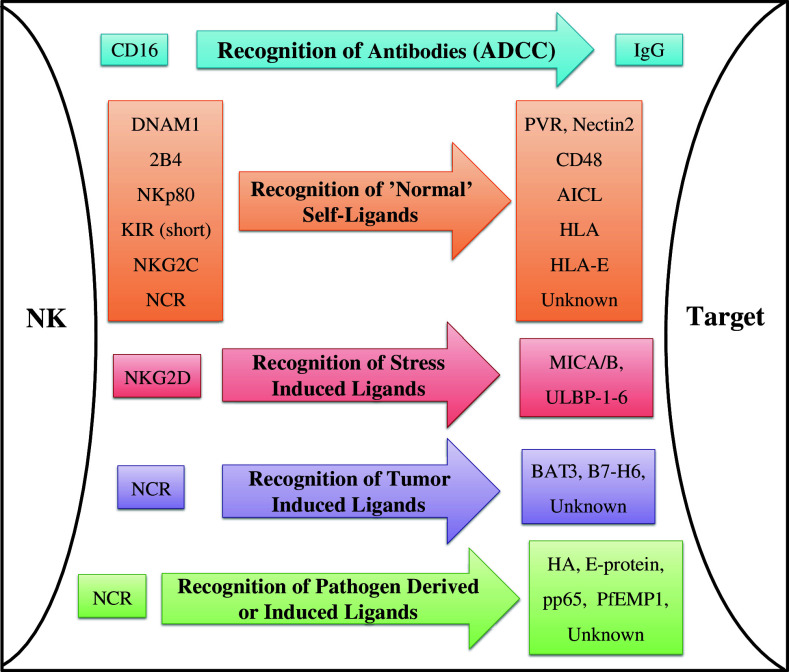
The activating NK receptors can be roughly classified into categories based upon the type of ligand they recognize (illustrated in Fig. 1): CD16, which mediates antibody-dependent cell-mediated cytotoxicity (ADCC); receptors recognizing “normal” self-molecules, such as NKG2C, 2B4, DNAM1, and others; NKG2D, which recognizes stress-induced self-molecules; and receptors recognizing pathogen-derived molecules, which to date include only the NCRs [1, 3, 4, 8, 11]. Certain NK receptors, mostly inhibitory ones such as the killer Immunoglobulin-like receptor (KIR) family, are characterized by genomic diversity, and viral susceptibility or resistance may be related to specific receptor haplotypes [6]. Moreover, different subsets of NK cells express different combinations of receptors (variations are mostly observed with regard to inhibitory receptors), enabling a differential response to stimuli [12]. Whether viruses develop mechanisms allowing them to efficiently escape from a particular haplotype is largely unknown.
Fig. 1.
Diagram representing the functions of NK-activating receptors. The receptors (left) are grouped according to the type of ligand which they recognize (stated on the arrows in the center), and are matched with their respective ligands (right) [1–4, 8, 11, 17]
The NCR family includes three members: NKp46, NKp44, and NKp30 [13–16]. The NKp46-encoding gene, similar to the genes encoding KIR, maps on human chromosome 19 within the leukocyte receptor complex. NKp30 and NKp44 are found in chromosome 6 [8]. NKp46 has two extra-cellular Ig-like domains while the other two receptors have a single, Ig-like, extra-cellular domain [17]. All three receptors contain a short intra-cytoplasmic tail and are associated with an ITAM-containing adaptor molecule via a positively charged residue located in their transmembrane portion. NKp46 associates with both CD3ζ chain and FcεRIγ (as homodimers or heterodimers), while NKp30 was shown to associate with CD3ζ chain and perhaps also with FcεRIγ (to the best of our knowledge, there is no published experimental evidence regarding an association between NKp30 and FcεRIγ). NK cells do not express the entire CD3 complex, but rather express only the CD3ζ chain that functions in signal transduction. NKp44 is the only NCR associated with DAP12 [17] and is unique in its selective expression, which is primarily limited to activated NK cells [15]. NKp46 is the only NCR that has a murine orthologue, termed NCR1 [3].
While some of the NCR ligands have yet to be identified, the known NCR ligands include cellular as well as pathogen-derived molecules ([1]; Table 1). Specifically, all the NCRs recognize viral ligands ([18–22]; Table 1) and these will be detailed separately in a subsequent section. Following is a brief description of other known NCR ligands:
Table 1.
A summary of known natural cytotoxicity receptors ligands, their source, and their effect [11, 18–20, 22, 24, 29, 30, 38–41, 45, 47–49]
| Receptor | Ligand | Source | Effect |
|---|---|---|---|
| NKp46 | Viral HA and HN | Influenza virus, Poxvirus, Sendai virus, Newcastle disease virus | Activating |
| Bacterial ligand | Fusobacterium nucleatum | Activating | |
| Sialylated and sulfated glycans | Cellular | Activating co-ligand | |
| NKp44 | Viral HA and HN | Influenza virus, Sendai virus, Newcastle disease virus | Activating |
| E-protein | Dengue virus, West Nile virus | Activating | |
| PCNA | Cellular | Inhibitory | |
| Bacterial cell wall elements | Pseudomonas aeruginosa, Nocardia spp., Mycobacteria spp. | Activating | |
| Sialylated and sulfated glycans | Cellular | Activating co-ligand | |
| NKp30 | Viral HA | Poxvirus | Inhibitory |
| pp65 | HCMV | Inhibitory | |
| PfEMP-1 | Plasmodium falciparum | Activating | |
| BAT3 | Stressed cells | Activating | |
| B7-H6 | Transformed cells | Activating | |
| Sialylated and sulfated glycans | Cellular | Activating co-ligand |
NKp46 has been implicated in the killing of cells infected with Mycobacterium tuberculosis [23] and we have recently shown that it also recognizes an unknown ligand expressed by Fusobacterium nucleatum ([24]; Table 1). The identity of the cellular/tumor ligands of NKp46 is unknown, but experimental evidence suggests that NKp46 interacts with several distinct ligands [11, 25, 26]. Through the use of fusion proteins containing the extracellular portion of NKp46 fused to the Fc portion of IgG1, it was shown that the NKp46 ligands are expressed in very few normal tissues, mainly on pancreatic beta-cells where the NKp46 ligand has been found to play a role in the pathogenesis of type I diabetes [26, 27]. The beta cell ligand for NKp46 is expressed ubiquitously on all beta cells derived from both normal and diseased individuals. It was suggested that diabetes does not develop in healthy individuals because under normal conditions NK cells are not found in the pancreas, and they are only able to penetrate this organ following a pathological process [27]. NKp46 also recognizes ligands on liver stellate cells, and in contrast to its destructive role in diabetes pathogenesis, the recognition of stellate cell ligands by NKp46 results in the attenuation of liver fibrosis [28]. Additionally, NKp46 ligands are expressed on a wide range of cancer cells [4, 11, 25].
NKp44 binds unknown elements in bacterial cell walls ([29]; Table 1). Additionally, like NKp46, the cellular ligands of this receptor remain largely unknown, with the exception of the nucleoprotein PCNA, which was recently shown to interact with NKp44 and induce an inhibitory effect ([30]; Table 1). In vitro assays using PCNA fused to GFP (PCNA-GFP) showed that PCNA-GFP was recruited to the immune synapse formed between tumor cells and NK cells. It is not yet clear how this intracellular ligand reaches the cell surface. Therefore, the functional significance of this interaction has yet to be ascertained.
As mentioned above, tumors express unknown ligands for NKp44 and NKp46. Indeed, both receptors are pivotal for the killing of tumor cells and it was demonstrated that tumor cell killing by NK cells correlates with the expression of NKp44 and of NKp46 [31, 32]. While the NCR ligands are not found on most normal cells (apart from beta and activated hepatic stellate cells [27, 28]), they are constitutively expressed by several types of hematopoietic system cells [granulocytes, monocytes, and dendritic cells (DCs)] [33–35]. The reciprocal interaction between these cells and NK cells can result in their mutual activation [36, 37]. Alternately, this interaction can sometimes lead to NK cell-mediated killing of immature DCs [36].
NKp30 has been linked to the recognition of Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1) ([38]; Table 1) and two cellular ligands were identified for this receptor: BAT3, a nuclear factor thought to be released by exosomes in response to stress ([39, 40]; Table 1) and B7-H6, a transmembrane protein expressed on tumor cells ([41, 42]; Table 1). However, because the identified ligands are not present on normal cells and since NKp30 recognizes several normal cell types such as DCs, it is conceivable that NKp30 also binds as-yet unidentified ligands [43]. Additionally, experimental evidence indicates that sialylated and sulfated glycans, and in particular heparin/heparan sulfate, serve as co-ligands to all three NCRs ([44–48]; Table 1).
NCR recognition of virus-infected cells and viral-evasion mechanisms
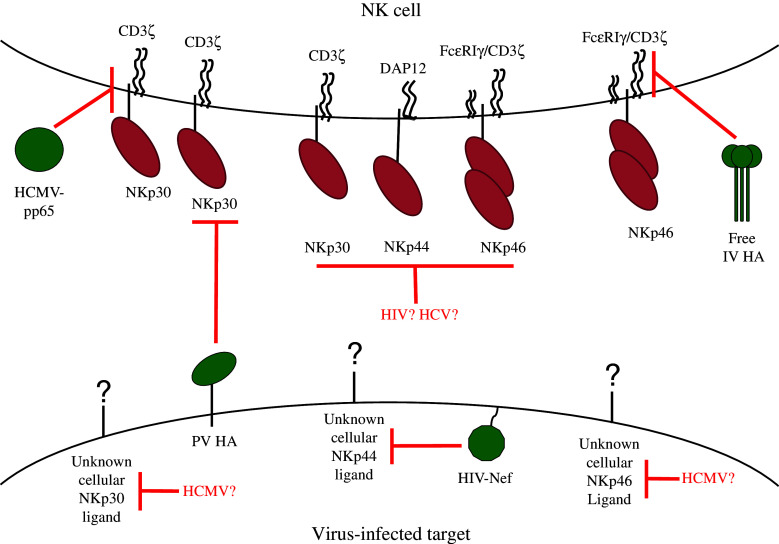
The NCRs have been implicated in the recognition and killing of cells infected by a wide variety of viruses. However, many of the ligands have not yet been identified, and among the unknown ligands many are not of viral origin. This naturally renders the discovery of NCR-dependent viral immune-evasion mechanisms quite challenging. Nevertheless, because the NCRs play such a major role in recognizing and eliminating virus-infected cells, it is reasonable to assume that viruses would have evolved a range of mechanisms to thwart their activity. Indeed, in the past few years, a few interesting examples have come to light (illustrated in Fig. 2).
Fig. 2.
Diagram representing viral strategies of evading NCR-mediated recognition. The NK cell (top) expresses NCRs, which would normally recognize NCR ligands (cellular and pathogen-derived) on the infected cell (bottom). However, viruses evade this detection through the downregulation of cellular NCR ligands; through expression of inhibitory NCR ligands; through degradation of NCR-associated adaptor molecules; and possibly through the downregulation of the NCRs themselves. Viral proteins are depicted in green. Speculative viral immune-evasion mechanisms are indicated by a question mark next to the virus name [20, 22, 34, 57, 64, 65, 68]
Influenza virus and paramyxoviruses
The first NCR ligands identified were the influenza virus (IV) hemagglutinin (HA) and the Sendai virus hemagglutinin–neuraminidase (HN) [18]. The interaction of NKp46 and NKp44 with HA is mediated via binding of specific sialylated residues, which are mainly located at the receptors’ stalk region [18, 49–51]. The HN of Newcastle disease virus (which along with the Sendai virus belongs to the Paramyxovirus family) is recognized by NKp46 and NKp44 in a similar fashion [19]. The importance of HA–NKp46 interactions was demonstrated in vivo using mice deficient in the NKp46 orthologue NCR1 (Ncr1 gfp/gfp) [52].
Surprisingly, it was recently demonstrated by using Ncr1 mutant mice (Ncr1 Noé/Noé) [53] that the NK cells of these mice manifest receptor-independent, hyper-reactivity properties [53]. This is in contrast to NK cells derived from the Ncr1 gfp/gfp mice, which exhibit NCR1-mediated deficiencies ([24, 26–28, 52, 54–56]; Table 2). Some of these findings were also reproduced in another mouse in which iCre was inserted in the 3′ UTR of Ncr1 (Ncr1 iCre/iCre) [53]. Furthermore, the hyper-reactive phenotype endowed the Ncr1 Noé/Noé and the Ncr1 iCre/iCre mice with increased resistance to pathogens, including to IV.
Table 2.
| Attribute | Ncr1 gfp/gfp | Ncr1 Noé/Noé | Ncr1 iCre/iCre |
|---|---|---|---|
| Phenotype | NCR1-dependent deficiencies | Receptor-independent hyperactivation | Receptor-independent hyperactivation |
| Full-length Ncr1 DNA | No; the DNA containing Exons 5-7 was replaced by IRES-EGFP | Yes; point mutation (W32R) | Yes; insertion of IRES-iCre into the 3′UTR region |
| Full-length Ncr1 mRNA | No | Yes; normal expression levels | Yes; disrupted 3′ UTR and reduced expression levels |
| Full-length NCR1 protein | No | Yes; intracellular expression | No surface expression; unknown if the protein is present intracellularly |
| Additional differences | EGFP activity | Additional mutations | iCre activity |
Substantial differences exist between the various mice (see Table 2), which might explain how changes made to the same gene can give rise to such radically different phenotypes. At the DNA level, while the Ncr1 gfp/gfp mice only retain a portion of the Ncr1 extracellular domain ([49]; Table 2), the other mice (Ncr1 Noé/Noé and Ncr1 iCre/iCre) harbor alterations that preserve the complete Ncr1 gene ([53]; Table 2). The Ncr1 Noé/Noé mice contain a single conformation-changing point mutation within the CDS (W32R), and in the Ncr1 iCre/iCre knock-in mice, the 3’ UTR of the gene is disrupted, ostensibly destabilizing the Ncr1 mRNA ([50]; Table 2). At the mRNA level, while the complete mRNA of Ncr1 is still transcribed in the Ncr1 Noé/Noé and in the Ncr1 iCre/iCre mice [53], in the Ncr1 gfp/gfp it is absent ([52]; Table 2). Finally, at the protein level, the full-length NCR1 protein is missing in the Ncr1 gfp/gfp mice, while it is retained intracellularly in the Ncr1 Noé/Noé mice ([52, 53]; Table 2). The intracellular NCR1 protein may also be found inside the NK cells in the Ncr1 iCre/iCre mice, as the Ncr1 mRNA is fully transcribed in these mice (albeit at reduced levels), while the protein is entirely missing from the cell surface ([53]; Table 2).
Thus, the various mutated Ncr1 mice are markedly different. Accordingly, the explanation we favor is that the existence of the NCR1 protein within the cytoplasm (see Table 2) of the Ncr1 Noé/Noé mice and possibly also in the Ncr1 iCre/iCre mice might lead to aberrant signaling (perhaps during NK cell development), resulting in the hyper-reactive phenotype of these mice. Alternately, or in addition, it is possible that the lack of the full Ncr1 gene in the Ncr1 gfp/gfp mice (see Table 2) is responsible for the substantial differences observed among the various mice. In particular, the absent Ncr1 gene segments in the Ncr1 gfp/gfp mice may encode regulatory elements which function in the DNA, RNA, and/or protein levels. If such elements exist, their absence can contribute to the observed phenotype of these mice. A final possibility, which we consider to be less likely, is that the functional differences between the mice might be caused by other mutations that exist in the Ncr1 Noé/Noé mice, or by the iCre activity in the Ncr1 iCre/iCre mice. Regardless of the reason for these differences, it is clear that the Ncr1 gfp/gfp mice are the most suitable model for studying NKp46-dependent deficiencies.
IV employs an intriguing immune-evasion mechanism based on the dual nature of its HA: on the one hand, it is an activating ligand of NKp46 and NKp44 when expressed on the infected cells [18, 49]. On the other hand, influenza-infected cells release HA along with new virions, and this free HA is taken up by NK cells. Once inside NK cells, HA induces the lysosomal degradation of CD3ζ chain, thereby preventing the transduction of activating signals from NKp46 and NKp30 [57].
Poxviruses (PV)
Poxviral HA, which has no lectin-binding properties, was recently shown to be an NCR ligand [20]. In contrast to the other viral HA, it binds both NKp46 and NKp30, and interacts weakly with NKp44. Surprisingly, the poxviral HAs binding to NKp30 results in NK cell inhibition, a mechanism which seemingly parallels the activity of the free IV HA and of pp65 (discussed below). The exact nature of this inhibitory mechanism has yet to be elucidated, but unlike IV HA and pp65, it does not appear to be related to CD3ζ chain [20]. The net effect of poxviral HA on NK cell activity depends upon its levels of expression, which change in the course of the infection. Hence, HA can either reduce or slightly increase the killing of PV-infected cells [20]. A previous study also reported that PV-infected cells were killed in an NCR-dependent manner, but did not find an inhibitory effect [58]. Despite these inconsistencies, all studies performed to date point to NCR involvement in the killing of PV-infected cells.
Herpesviruses
NK cells are particularly instrumental in the control of herpesviruses, as recurrent herpesvirus infections were noted in individuals suffering from NK cell deficiencies [4]. The NCRs were shown to play an important role in the recognition of cells infected mainly by two herpesvirus family members: Herpes Simplex virus-1 (HSV-1) and Human Cytomegalovirus (HCMV). Specifically, cells infected with HSV-1 are killed more efficiently by NK cells, in part due to an increase in the expression of unknown ligands to all three NCRs (detected by the use of NCR-Ig fusion proteins) [59]. The expression of the immediate–early protein ICP0 is both necessary and sufficient for this upregulation [59]. The mechanism whereby ICP0 causes the upregulation of NCR ligands is not known. It is unlikely that ICP0 itself is the ligand, as it is not considered to be a transmembrane protein. A more plausible explanation is that ICP0 triggers a cellular pathway resulting in the upregulation of NCR ligands.
Interestingly, several cells that are permissive to HCMV infection constitutively express ligands for the NCRs: NKp30 ligand(s) are expressed on fibroblasts [22] and ligands for both NKp46 and NKp30 are expressed on monocyte-derived DCs [34] and on macrophages [34]. The latter two are considered to be important targets of HCMV in vivo. These cells continue to express activating NCR ligands at early and intermediate times post-infection, and they participate to varying degrees in NK cell activation: NKp30 chiefly mediates the killing of infected fibroblasts [22], whereas NKp46 mainly mediates the killing of infected DCs [34] and macrophages [35]. While it is possible that at least some of these activating NCR ligands are virus-derived/induced, the simpler explanation is that the same constitutively expressed cellular ligands also mediate the killing of infected cells. Indeed, infected DCs and macrophages are killed more efficiently than uninfected cells, despite the fact that the surface expression levels of the activating ligands remain unchanged. This is presumably due to a shift in inhibitory versus activating signals, as HCMV also impairs the surface expression of MHC class I molecules [34, 35].
An immune-evasion mechanism reminiscent of both IV and PV HA is utilized by HCMV as its tegument protein, pp65, acts as an inhibitory ligand of NKp30. The viral protein binds to this NCR and causes it to dissociate from CD3ζ chain, thus preventing transduction of activating signals [22]. Another presumptive HCMV immune-evasion mechanism reported in HCMV-infected macrophages and DCs relates to the finding that at late time points post-infection (48–72 h), both infected and non-infected cell fractions downregulate NKp30 and NKp46 ligands, and consequently the NCR-mediated killing of infected cells is decreased. It is possible that a soluble mediator, released from the infected cells, induces the downregulation of NCR ligands [34].
Human immunodeficiency virus-1 (HIV-1)
Like herpesviruses, HIV-1 causes latent infections and has developed multiple immune-evasion strategies [60]. NK cells inefficiently kill HIV-1-infected cells, despite the downregulation of MHC class I molecules and the upregulation of NKG2D ligands. Several mechanisms have been proposed to explain these contradicting observations, including the downregulation of ligands for other activating receptors and co-receptors [60, 61]. With regard to NCRs, the viral envelope protein gp41 has been shown to induce the upregulation of a cellular NKp44 ligand on CD4+ T cells [62]. The viral protein binds to gC1qR, the receptor for the globular domain of the complement component C1q, and leads to the translocation of NKp44 ligand to the plasma membrane [63]. However, this ligand is only expressed on uninfected CD4+ T cells, resulting in their killing by autologous NK cells, which are activated due to the infection and therefore express NKp44 [61, 64]. The infected cells are protected from NKp44-mediated killing due to the action of the viral protein Nef, which causes the intracellular retention of NKp44 ligand [64]. It is also noteworthy that the expression of NCRs on NK cells is reduced during HIV-infection even before profound immunosuppression develops, and as a result, NK cell function is impaired [65]. However, the mechanism underlying this effect is unknown.
Hepatitis C virus (HCV)
The interaction between HCV and NK cells, which comprise a relatively high percentage (30–50 %) of liver lymphocytes [66], is intensely studied. Several studies have implicated NK cells in determining the outcome of primary infection: viral clearance (which is only attained in 20 % of cases); or viral persistence [66, 67]. However, it is difficult to draw any conclusions regarding the interaction between NK cells and HCV-infected cells because of the contradictory nature of the experimental results [66, 68]. For example, ex vivo studies performed with NK cells taken from patients chronically infected with HCV reported either upregulation [69–71] or downregulation [72–74] of the NCRs. Moreover, in vitro studies also yielded differing results. On the one hand, several studies indicated that NK cells can be stimulated by HCV and attenuate its spread, in an NCR-dependent manner [75, 76]. On the other hand, cell-to-cell contact of NK cells with HCV-infected cells caused a reduction in their cytotoxic capacity and in NCR expression [68]. Therefore, although the effect is disputed, it is possible that HCV evades NCR-mediated killing by causing a reduction in NCR levels, through as-yet-undiscovered mechanisms.
Several prospective studies were also performed, but failed to provide incisive results. Correlations were found between increased NCR expression and protection against HCV infection [75], but also between reduced NCR expression and positive outcome in primary and chronic infection [72, 73]. Interestingly, one of the studies [73] indicated a differential response to antiviral treatment in patient subsets: the patients who responded to antiviral treatment increased their NCR expression levels during the treatment, while the non-responders did not. The observation that patients respond in opposed ways may help to explain the contradictions between studies. In any case, there are multiple indications that NCRs play a role in HCV infection, but its exact nature still awaits clarification.
Filoviruses
Killing of immature DCs infected with Ebola and Marburg viruses in vitro was shown to be mediated by NKp30 [77]. Furthermore, virus-like particles of the Ebola virus, which lack viral membrane proteins known to exert an inhibitory effect on the immune system, cause NK cell activation and increased surface expression of NKp30 [77]. However, authentic virions do not have this activating effect. This may be due to the action of the inhibitory proteins, or because their absence leads to conformational changes in the viral proteins of the virus-like particles [77].
Flaviviruses
NKp44 mediates the recognition and killing of cells infected by Dengue and West Nile viruses, via protein–protein interactions between this NCR and the viral E-protein [21]. The functional significance of these results in vivo is not clear, since these viruses also induce the upregulation of MHC class I molecules, resulting in a strong inhibition of NK cells [21]. The lack of a murine orthologue for NKp44 renders this question difficult to resolve.
General discussion, conclusions, and future directions
NCRs are the only NK-activating receptors shown to directly recognize pathogen-derived ligands (Figs. 1, 2; Table 1). Surprisingly, despite their unique ability to bind viral ligands, in many cases it seems that NCRs recognize virus-infected cells through cellular ligands. Of particular interest is the possibility that some of these cellular ligands are not constitutively expressed, but are induced in response to viral infection [59, 61, 62], though some virus-infected cells are detected by cellular ligands constitutively expressed on these particular cell types [34, 35]. Two points are striking with regards to NCR-recognized ligands: first, it seems that the pathogen-derived ligands that are recognized by the NCRs are quite specific to a pathogen genus/family and are not broadly expressed by many different types of pathogens. Second, it seems as though the NCRs are capable of binding many disparate, structurally unrelated ligands through different types of interactions. These include protein–protein interactions such as those formed between NKp44 and flavivirus E-protein [21] or between NKp30 and B7-H6 [41, 42], as well as interactions mediated via the glycosylated residues on the NCRs, such as the ones between NKp46 and IV HA or between NKp46 and pancreatic beta cells [18, 26, 27, 50, 51]. Finally, the NCRs can also form ionic interactions with sulfated or sialylated glycans [44–48].
NKG2D is another major NK-activating receptor, which binds multiple ligands, but in contrast to the NCR ligands, these are all structurally related. NKG2D ligands are induced following diverse types of stress including heat shock, genotoxic shock, viral infection [78], and p53 activation [79, 80]. Furthermore, pathogen-detection pathways (TLR activation) were also shown to induce NKG2D ligand expression [81, 82]. A comparative analysis of the two receptor types is useful for identifying the unique aspects of NCR function. In this context, while the ligands of the NCRs and of NKG2D can be induced by viral infection and cancerous transformation, there are indications that the mechanisms governing their expression are distinct.
Firstly, some stresses that induce NKG2D ligand expression do not induce NCR ligand expression; for example, p53 activation induces ULBP1 and ULBP2, but not NCR ligand expression [80]. Furthermore, a pilot study testing a panel of DNA-damaging agents demonstrated no effect on B7-H6 expression [43]. Finally, while most of the regulation of NKG2D ligands is on the transcriptional and post-transcriptional levels [78], two examples of NCR ligands regulation seem to be post-translational: NKp44 ligand expression on CD4+ T cells is controlled via its translocation to the plasma membrane [63], and BAT3, a widely present nuclear protein, is released by exosomes only from transformed and stressed cells, and only then is it capable of interacting with NKp30 [39, 40].
The immune-evasion mechanisms discovered so far, which viruses employ to escape NCR recognition and killing, are diverse, affecting both NCR ligands and NCRs themselves. The NCRs are targeted via inhibitory interactions with functional antagonists [20, 22]; through a reduction of their expression [65, 68]; or indirectly, through disrupting their associated adaptor molecules [57]. Remarkably, while different pathogens target the NCRs, no indication has been found to date of NKG2D being similarly targeted. This may be because the NCRs recognize essential pathogen components, and therefore these essential components cannot be downregulated by the pathogen without severely hampering its fitness. Direct inhibition of the NCRs would bypass this obstacle. Disruption of NCR association with adaptor molecules seems to be a common means of impeding their function, utilized by at least two different viruses (IV, HCMV), via different mechanisms [22, 57]. Indeed, targeting the CD3ζ chain would “kill three birds with one stone” as it will inhibit the activity of NKp46, NKp30, and CD16.
These findings likely represent only the proverbial “tip of the iceberg”. Future directions for research include elucidating the identity of the cellular NCR ligands, discovering why the various Ncr1-manipulated mice demonstrate opposite phenotypes, and obtaining a more comprehensive characterization of the pathogen-derived NCR ligands. In this regard, viral microRNAs (miRNAs) seem to be a promising lead: these 22-bp-long molecules have been shown to play a major role in viral immune-evasion strategies [83], in particular mediating the downmodulation of other activating receptor ligands [84–86]. Moreover, several of the viruses recognized by NCRs encode miRNAs, most notably the herpesviruses [83]. It is therefore plausible that viral miRNAs targeting NCR ligands exist. Should such a viral miRNA be found, this will not only promote a deeper understanding of the ways in which viruses circumvent NK cell recognition, but may also provide the key to discovering the identity of these elusive ligands.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- DC
Dendritic cell
- HA
Hemagglutinin
- HCMV
Human cytomegalovirus
- HCV
Hepatitis C virus
- HIV-1
Human immunodeficiency virus 1
- HN
Hemagglutinin-neuraminidase
- HSV-1
Herpes simplex virus type 1
- ITAM
Immunoreceptor tyrosine-based activating motif
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- IV
Influenza virus
- KIR
Killer immunoglobulin-like receptor
- MHC
Major histocompatibility complex
- NCR
Natural cytotoxicity receptor
- NK
Natural killer
- PBL
Peripheral blood leukocytes
- PV
Poxvirus
References
- 1.Cheent K, Khakoo SI. Natural killer cells: integrating diversity with function. Immunology. 2009;126(4):449–457. doi: 10.1111/j.1365-2567.2009.03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065X.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 4.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16(5):348–358. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42(4):501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 11.Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26(4):221–226. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- 13.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190(10):1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188(5):953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187(12):2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189(5):787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce MG, Sun PD (2011) The structural basis of ligand recognition by natural killer cell receptors. J Biomed Biotechnol 2011, art ID 203628 [DOI] [PMC free article] [PubMed]
- 18.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 19.Jarahian M, Watzl C, Fournier P, Arnold A, Djandji D, Zahedi S, Cerwenka A, Paschen A, Schirrmacher V, Momburg F. Activation of natural killer cells by Newcastle disease virus hemagglutinin-neuraminidase. J Virol. 2009;83(16):8108–8121. doi: 10.1128/JVI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarahian M, Fiedler M, Cohnen A, Djandji D, Hammerling GJ, Gati C, Cerwenka A, Turner PC, Moyer RW, Watzl C, Hengel H, Momburg F. Modulation of NKp30- and NKp46-mediated natural killer cell responses by poxviral hemagglutinin. PLoS Pathog. 2011;7(8):e1002195. doi: 10.1371/journal.ppat.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershkovitz O, Rosental B, Rosenberg LA, Navarro-Sanchez ME, Jivov S, Zilka A, Gershoni-Yahalom O, Brient-Litzler E, Bedouelle H, Ho JW, Campbell KS, Rager-Zisman B, Despres P, Porgador A. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183(4):2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, Gazit R, Gonen-Gross T, Hanna J, Nahari E, Porgador A, Honigman A, Plachter B, Mevorach D, Wolf DG, Mandelboim O. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6(5):515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 23.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, Samten B, Porgador A, Barnes PF. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168(7):3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 24.Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, Polak D, Achdout H, Bachrach G, Mandelboim O. Direct Recognition of Fusobacterium nucleatum by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease. PLoS Pathog. 2012;8(3):e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, Bar-Ilan A, Bloushtain N, Lev M, Joseph A, Kedar E, Porgador A, Mandelboim O. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103(2):664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 26.Gur C, Enk J, Kassem SA, Suissa Y, Magenheim J, Stolovich-Rain M, Nir T, Achdout H, Glaser B, Shapiro J, Naparstek Y, Porgador A, Dor Y, Mandelboim O. Recognition and killing of human and murine pancreatic beta cells by the NK receptor NKp46. J Immunol. 2011;187(6):3096–3103. doi: 10.4049/jimmunol.1101269. [DOI] [PubMed] [Google Scholar]
- 27.Gur C, Porgador A, Elboim M, Gazit R, Mizrahi S, Stern-Ginossar N, Achdout H, Ghadially H, Dor Y, Nir T, Doviner V, Hershkovitz O, Mendelson M, Naparstek Y, Mandelboim O. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol. 2010;11(2):121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 28.Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, Mandelboim O. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2011 doi: 10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
- 29.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76(4):1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosental B, Brusilovsky M, Hadad U, Oz D, Appel MY, Afergan F, Yossef R, Rosenberg LA, Aharoni A, Cerwenka A, Campbell KS, Braiman A, Porgador A. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J Immunol. 2011;187(11):5693–5702. doi: 10.4049/jimmunol.1102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 32.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29(5):1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, Cosman D, De Libero G, Wodnar-Filipowicz A. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105(9):3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 34.Magri G, Muntasell A, Romo N, Saez-Borderias A, Pende D, Geraghty DE, Hengel H, Angulo A, Moretta A, Lopez-Botet M. NKp46 and DNAM-1 NK-cell receptors drive the response to human cytomegalovirus-infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood. 2011;117(3):848–856. doi: 10.1182/blood-2010-08-301374. [DOI] [PubMed] [Google Scholar]
- 35.Romo N, Magri G, Muntasell A, Heredia G, Baia D, Angulo A, Guma M, Lopez-Botet M. Natural killer cell-mediated response to human cytomegalovirus-infected macrophages is modulated by their functional polarization. J Leukoc Biol. 2011;90(4):717–726. doi: 10.1189/jlb.0311171. [DOI] [PubMed] [Google Scholar]
- 36.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195(3):343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elhaik-Goldman S, Kafka D, Yossef R, Hadad U, Elkabets M, Vallon-Eberhard A, Hulihel L, Jung S, Ghadially H, Braiman A, Apte RN, Mandelboim O, Dagan R, Mizrachi-Nebenzahl Y, Porgador A. The natural cytotoxicity receptor 1 contribution to early clearance of Streptococcus pneumoniae and to natural killer-macrophage cross talk. PLoS ONE. 2011;6(8):e23472. doi: 10.1371/journal.pone.0023472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mavoungou E, Held J, Mewono L, Kremsner PG. A Duffy binding-like domain is involved in the NKp30-mediated recognition of Plasmodium falciparum-parasitized erythrocytes by natural killer cells. J Infect Dis. 2007;195(10):1521–1531. doi: 10.1086/515579. [DOI] [PubMed] [Google Scholar]
- 39.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27(6):965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS ONE. 2008;3(10):e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med. 2011;208(4):703–714. doi: 10.1084/jem.20102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaifu T, Escaliere B, Gastinel LN, Vivier E, Baratin M. B7-H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. Cell Mol Life Sci. 2011;68(21):3531–3539. doi: 10.1007/s00018-011-0802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloushtain N, Qimron U, Bar-Ilan A, Hershkovitz O, Gazit R, Fima E, Korc M, Vlodavsky I, Bovin NV, Porgador A. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173(4):2392–2401. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 45.Hecht ML, Rosental B, Horlacher T, Hershkovitz O, De Paz JL, Noti C, Schauer S, Porgador A, Seeberger PH. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8(2):712–720. doi: 10.1021/pr800747c. [DOI] [PubMed] [Google Scholar]
- 46.Hershkovitz O, Jarahian M, Zilka A, Bar-Ilan A, Landau G, Jivov S, Tekoah Y, Glicklis R, Gallagher JT, Hoffmann SC, Zer H, Mandelboim O, Watzl C, Momburg F, Porgador A. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immunoreceptors as an immunological tool. Glycobiology. 2008;18(1):28–41. doi: 10.1093/glycob/cwm125. [DOI] [PubMed] [Google Scholar]
- 47.Ito K, Higai K, Sakurai M, Shinoda C, Yanai K, Azuma Y, Matsumoto K. Binding of natural cytotoxicity receptor NKp46 to sulfate- and alpha2,3-NeuAc-containing glycans and its mutagenesis. Biochem Biophys Res Commun. 2011;406(3):377–382. doi: 10.1016/j.bbrc.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 48.Ito K, Higai K, Shinoda C, Sakurai M, Yanai K, Azuma Y, Matsumoto K. Unlike Natural Killer (NK) p30, Natural Cytotoxicity Receptor NKp44 Binds to Multimeric alpha2,3-NeuNAc-Containing N-Glycans. Biol Pharm Bull. 2012;35(4):594–600. doi: 10.1248/bpb.35.594. [DOI] [PubMed] [Google Scholar]
- 49.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31(9):2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::AID-IMMU2680>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 50.Achdout H, Meningher T, Hirsh S, Glasner A, Bar-On Y, Gur C, Porgador A, Mendelson M, Mandelboim M, Mandelboim O. Killing of avian and Swine influenza virus by natural killer cells. J Virol. 2010;84(8):3993–4001. doi: 10.1128/JVI.02289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendelson M, Tekoah Y, Zilka A, Gershoni-Yahalom O, Gazit R, Achdout H, Bovin NV, Meningher T, Mandelboim M, Mandelboim O, David A, Porgador A. NKp46 O-glycan sequences that are involved in the interaction with hemagglutinin type 1 of influenza virus. J Virol. 2010;84(8):3789–3797. doi: 10.1128/JVI.01815-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7(5):517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 53.Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A, Mahmood S, Gut M, Heath SC, Estelle J, Bertosio E, Vely F, Gastinel LN, Beutler B, Malissen B, Malissen M, Gut IG, Vivier E, Ugolini S. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science. 2012;335(6066):344–348. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- 54.Babic M, Pyzik M, Zafirova B, Mitrovic M, Butorac V, Lanier LL, Krmpotic A, Vidal SM, Jonjic S. Cytomegalovirus immunoevasin reveals the physiological role of “missing self” recognition in natural killer cell dependent virus control in vivo. J Exp Med. 2010;207(12):2663–2673. doi: 10.1084/jem.20100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, Pende D, Groh V, Biassoni R, Hoglund P, Kato M, Shibuya K, Schadendorf D, Anichini A, Ferrone S, Velardi A, Karre K, Shibuya A, Carbone E, Colucci F. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119(5):1251–1263. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, Mandelboim O. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J Immunol. 2012;188(6):2509–2515. doi: 10.4049/jimmunol.1102461. [DOI] [PubMed] [Google Scholar]
- 57.Mao H, Tu W, Liu Y, Qin G, Zheng J, Chan PL, Lam KT, Peiris JS, Lau YL. Inhibition of human natural killer cell activity by influenza virions and hemagglutinin. J Virol. 2010;84(9):4148–4157. doi: 10.1128/JVI.02340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chisholm SE, Reyburn HT. Recognition of vaccinia virus-infected cells by human natural killer cells depends on natural cytotoxicity receptors. J Virol. 2006;80(5):2225–2233. doi: 10.1128/JVI.80.5.2225-2233.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chisholm SE, Howard K, Gomez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195(8):1160–1168. doi: 10.1086/512862. [DOI] [PubMed] [Google Scholar]
- 60.Sowrirajan B, Barker E. The natural killer cell cytotoxic function is modulated by HIV-1 accessory proteins. Viruses. 2011;3(7):1091–1111. doi: 10.3390/v3071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110(4):1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Nat Acad Sci USA. 2005;102(31):10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fausther-Bovendo H, Vieillard V, Sagan S, Bismuth G, Debre P. HIV gp41 engages gC1qR on CD4+ T cells to induce the expression of an NK ligand through the PIP3/H2O2 pathway. PLoS Pathog. 2010;6:e1000975. doi: 10.1371/journal.ppat.1000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fausther-Bovendo H, Sol-Foulon N, Candotti D, Agut H, Schwartz O, Debre P, Vieillard V. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS (London, England) 2009;23(9):1077–1087. doi: 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 65.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33(9):2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 66.Cheent K, Khakoo SI. Natural killer cells and hepatitis C: action and reaction. Gut. 2011;60(2):268–278. doi: 10.1136/gut.2010.212555. [DOI] [PubMed] [Google Scholar]
- 67.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O’Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 68.Yoon JC, Lim JB, Park JH, Lee JM. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J Virol. 2011;85(23):12557–12569. doi: 10.1128/JVI.00838-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138(1):325–322. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37(2):445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 71.Harrison RJ, Ettorre A, Little AM, Khakoo SI. Association of NKG2A with treatment for chronic hepatitis C virus infection. Clin Exp Immunol. 2010;161(2):306–314. doi: 10.1111/j.1365-2249.2010.04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alter G, Jost S, Rihn S, Reyor LL, Nolan BE, Ghebremichael M, Bosch R, Altfeld M, Lauer GM. Reduced frequencies of NKp30+NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. J Hepatol. 2011;55(2):278–288. doi: 10.1016/j.jhep.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bozzano F, Picciotto A, Costa P, Marras F, Fazio V, Hirsch I, Olive D, Moretta L, De Maria A. Activating NK cell receptor expression/function (NKp30, NKp46, DNAM-1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol. 2011;41(10):2905–2914. doi: 10.1002/eji.201041361. [DOI] [PubMed] [Google Scholar]
- 74.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55(6):869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR (2010) Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology (Baltimore, MD) 52(5):1581–1589 [DOI] [PMC free article] [PubMed]
- 76.Farag MM, Weigand K, Encke J, Momburg F. Activation of natural killer cells by hepatitis C virus particles in vitro. Clin Exp Immunol. 2011;165(3):352–362. doi: 10.1111/j.1365-2249.2011.04431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuller CL, Ruthel G, Warfield KL, Swenson DL, Bosio CM, Aman MJ, Bavari S. NKp30-dependent cytolysis of filovirus-infected human dendritic cells. Cell Microbiol. 2007;9(4):962–976. doi: 10.1111/j.1462-5822.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 78.Stern-Ginossar N, Mandelboim O. An integrated view of the regulation of NKG2D ligands. Immunology. 2009;128(1):1–6. doi: 10.1111/j.1365-2567.2009.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H, Lakshmikanth T, Garofalo C, Enge M, Spinnler C, Anichini A, Szekely L, Karre K, Carbone E, Selivanova G. Pharmacological activation of p53 triggers anticancer innate immune response through induction of ULBP2. Cell Cycle (Georgetown, Tex) 2011;10(19):3346–3358. doi: 10.4161/cc.10.19.17630. [DOI] [PubMed] [Google Scholar]
- 80.Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011;71(18):5998–6009. doi: 10.1158/0008-5472.CAN-10-3211. [DOI] [PubMed] [Google Scholar]
- 81.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol. 2010;184(12):6901–6909. doi: 10.4049/jimmunol.0903985. [DOI] [PubMed] [Google Scholar]
- 82.Ebihara T, Masuda H, Akazawa T, Shingai M, Kikuta H, Ariga T, Matsumoto M, Seya T. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection. Int Immunol. 2007;19(10):1145–1155. doi: 10.1093/intimm/dxm073. [DOI] [PubMed] [Google Scholar]
- 83.Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annu Rev Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stern-Ginossar N, Elefant N, Zimmermann A, Wolf DG, Saleh N, Biton M, Horwitz E, Prokocimer Z, Prichard M, Hahn G, Goldman-Wohl D, Greenfield C, Yagel S, Hengel H, Altuvia Y, Margalit H, Mandelboim O. Host immune system gene targeting by a viral miRNA. Science. 2007;317(5836):376–381. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5(4):376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Bauman Y, Nachmani D, Vitenshtein A, Tsukerman P, Drayman N, Stern-Ginossar N, Lankry D, Gruda R, Mandelboim O. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9(2):93–102. doi: 10.1016/j.chom.2011.01.008. [DOI] [PubMed] [Google Scholar]