Abstract
The scientific interest in the family of the so-called nervous vascular parallels has been growing steadily for the past 15 years, either by addition of new members to the group or, lately, by deepening the analysis of established concepts and mediators. Proteins governing both neurons and vascular cells are known to be involved in events such as cell fate determination and migration/guidance but not in the last and apparently most complex step of nervous system development, the formation and maturation of synapses. Hence, the recent addition to this family of the specific synaptic proteins, Neurexin and Neuroligin, is a double innovation. The two proteins, which were thought to be “simple” adhesive links between the pre- and post-synaptic sides of chemical synapses, are in fact extremely complex and modulate the most subtle synaptic activities. We will discuss the relevant data and the intriguing challenge of transferring synaptic activities to vascular functions.
Keywords: Nervous vascular parallels, Synapse, Neurexin, Neuroligin
Introduction
Neurexins and Neuroligins have been some of the most studied specific modulators of synaptic activity of recent years. The interest in these proteins has been raised constantly since their identification, first because of their key role as synaptic inducers and modulators and then for their involvement in autism. Neurexins and Neuroligins have hence attracted the most attention from the neurobiology field, and also from clinical neurobiology [1], genetics [2–4] and neuropsychiatry [5]. These various aspects have been recently and thoroughly reviewed [6–10]. We will cover the general properties of the two proteins with special attention to some basic molecular features that are possibly relevant to a widespread function. Next, we will address the nervous vascular parallels and our recent data on the expression and function of Neurexin and Neuroligin in the vascular system and propose a number of working hypotheses for future studies. Moreover, we will expose the idea that these proteins in blood vessels affect cellular processes rather distant from cell migration and guidance, which are to date the most thoroughly studied events that neurons and endothelial cells share on a molecular basis.
General features of neurexins and neuroligins
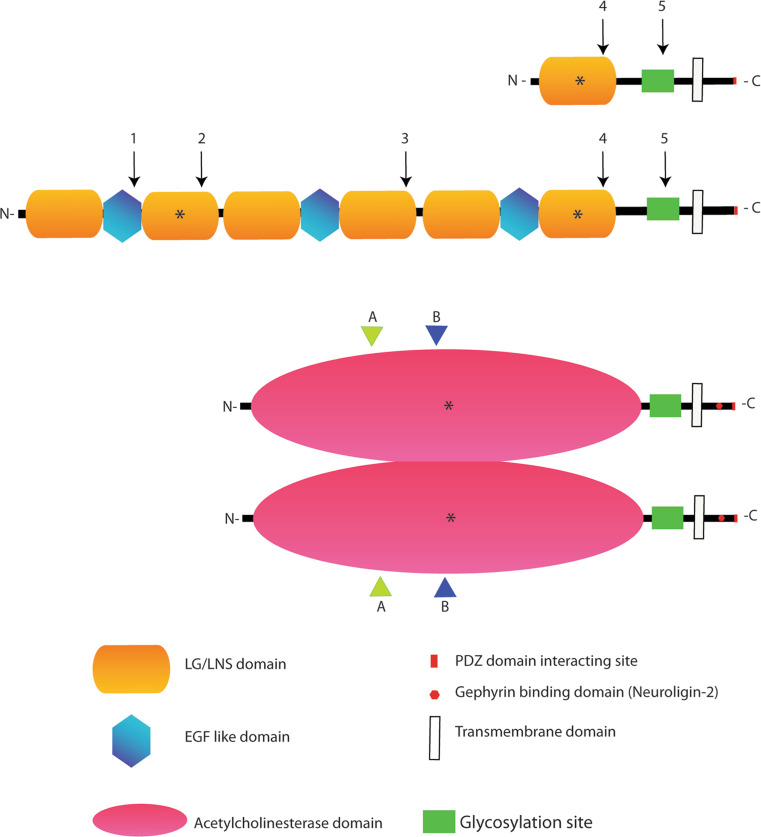
Neurexins were cloned in 1992 as pre-synaptic transmembrane receptors for α-latrotoxin, a toxin from a spider venom, and their most peculiar feature immediately appeared to be their large heterogeneity due to alternative splicing [11]. Intriguingly, the theoretical number of isoforms of Neurexin generated by alternative splicing nears that of the exceptional family of immunoglobulin proteins, Dscam, found in Drosophila [12]. Because of these features, Neurexins were proposed as candidate molecules coding for the neuron-to-neuron recognitions events which make up the complexity of the central nervous system [13, 14]. A few years later, Neuroligin, a transmembrane post-synaptic protein that interacts with Neurexin, was discovered [15]. Neurexins and Neuroligins are codified respectively by three and five genes in Homo sapiens. Neurexins are produced in a long (α) and a short (β) isoform from each gene. β-Neurexins contain a single LNS domain (laminin, Neurexin, sex-hormone binding protein domain; also called Laminin G domain), whereas α-Neurexins contain six LNS domains organized in three modules with EGF-like domains (Fig. 1). The major extracellular domain of Neuroligins is homologous to acetylcholinesterase (AChE), but is devoid of enzymatic activity and mediates binding to Neurexins. Neuroligins form homo-multimers through the AChE-homologous domain, a structural association that is important for Neuroligin function [16, 17]. Neurexin and Neuroligin are believed to form trans-synaptic complexes which are covered on each intracellular face by a scaffolding network of PDZ (postsynaptic density 95; discs large, Dlg; zonula occludens-1, ZO-1) domain containing proteins [18]. Alternative splicing of both proteins regulates this interaction and modulates their acitivity (Fig. 1 [10, 19–23]).
Fig. 1.
Neurexin and Neuroligin protein structures, interactions domains and alternative splicing sites. The biochemical interaction sites for the two proteins are indicated by an asterisk. In the extracellular region of Neurexin, the second LG/LNS domain of the α isoforms binds α-distroglican, while the sixth LG/LNS domain of α-Neurexin and the only LG/LNs domain of β-Neurexin mediate the binding with various Neuroligin isoforms, α- distroglycan and a member of the leucine-rich repeat transmembrane neuronal proteins (LRRTM2). In the case of Neuroligin, the acetylcholinesterase domain is involved in Neuroligin dimerization and binding to all Neurexin isoforms. The intracellular C-terminal region of Neurexin binds to the PDZ domain containing protein CASK while the Neuroligin C-terminal domain binds to the PDZ containing proteins PSD-95 and S-SCAM. Finally, an intracellular stretch of 15 aminoacids in Neuroligin 2 binds Gephyrin. Neuroligin is displayed as a dimer since this is the accepted functional form of the protein [16, 17]. The position of the alternatively spliced sites are indicated by numbers (1–5) in the case of Neurexins and by letters (A–B) in the case of Neuroligins (with splice site B restricted to Neuroligin 1). Splicing variants in the site B of Neuroligin 1 and site splice 4 (SS4) in β Neurexin control the interaction between these proteins. Neurexin 1β SS4+, as Neurexin 1α, has less affinity for Neuroligin 1 with splice site B and binds Neuroligin 2, while Neurexin 1β SS4− binds with high affinity Neuroligin 1 in all the isoforms [10, 19–23]
The expression of Neurexins and Neuroligins has been described as only neuronal with a few very specific exceptions. Neuroligin 3 has been detected in pancreas, skeletal/cardiac muscle [24] and glia [25], Neurexin 3 is expressed as a heart-specific splice isoform [26], while Neurexin 1-2 and Neuroligin mRNAs are, respectively, present in human microvascular endothelial cells and endothelial cells from large vessels [27, 28]. Interestingly, it was recently described that pancreatic β-cells express Neurexins and Neuroligins and that Neuroligin affects insulin secretion in INS-1 β-cells and rat pancreatic islet cells [29].
In addition to α- and β-Neurexins, neurons express Neurexin-related proteins called CASPRs (contactin-associated proteins), which resemble α-Neurexins but contain an additional extracellular domain. CASPRs also function as cell-to-cell adhesion molecules, but are mainly involved in neuron–glia interactions outside synapses [30] and will not be considered further here.
Evolution and alternative splicing
Global analysis of the gene structure and in particular of the sequences regulating alternative splicing in the Neurexin and Neuroligin gene families suggests that their functions may have been conserved, but also differentiated during evolution. The two protein families indeed represent strong candidates to study mechanisms of genomic evolution. Invertebrates present five different Neuroligins [31] and just a unique Neurexin gene (encoding α-forms only) [32–34]. Protein sequences and major structural features of all these proteins are conserved between invertebrates and humans, suggesting a conservation of the function of these molecules during evolution. In fact, different studies demonstrated an involvement of Neurexin and Neuroligin 1 in Drosophila synaptic activity at neuromuscolar junctions [32, 34–36]. However, gene structure and alternative splicing mechanisms appear different, probably due to the high evolutive distance between vertebrates and the analyzed species (C. elegans, D. melanogaster, A. mellifera and B. mori) [31, 33, 37]. Intriguingly, in contrast to Drosophila in which alternative splicing of Neurexin has been supposed but never observed [33], the honeybee Neurexin 1 presents specific features of mammalian Neurexins. In particular, this protein presents a relative amount of different alternatively spliced isoforms, if compared to vertebrate heterogeneity; moreover, similarly to vertebrate Neurexins 3, some of them are putative soluble forms given the lack of a transmembrane portion [31]. It has been proposed that alternative splicing has developed before the duplication of the ancestral α-Neurexin gene into three different genes [38]. The presence of different alternatively spliced isoforms in A. mellifera partially confirm the proposed evolutionary model, but it indicates that the alternative splicing mechanism had already appeared in insects. Moreover, it is interesting to note that specific features of vertebrate Neurexins are present in some insects. Further analyses are required in order to understand whether they represent an old character or instead a recent acquisition due to a possible evolutionary convergence. Generally, vertebrates present three different Neurexin genes and at least four Neuroligins. Different studies indicated that vertebrate Neurexins and Neuroligins are strongly conserved from fish to human [6, 39–42]. The comparison of human Neurexin genes indicated a strong preservation of gene structure and a high degree of sequence conservation in the introns flanking alternative splicing sites, in particular between Neurexin 1 and 3 [33, 43]. Comparing human and zebrafish genes, we found similar levels of sequence conservation [41], in particular at the same intronic flanking regions highlighted in a previous study [43]. Our analyses indicated a strong selective pressure acting on exonic and intronic regions during vertebrate evolution and highlighted the presence of neo- and subfunctionalization events (respectively, the de novo acquisition of a function, or the partitioning of ancestral functions between gene duplicates [44]) in zebrafish homologs [41]. Notably, during evolution, the selective pressure did not act generically, but was more restricted to specific alternative splicing sites (splice sites 2–4). These differences remain to be explained and they could provide useful information about the evolution of alternative splicing mechanisms in vertebrates. From this point of view, given the features previously described, Neurexins might represent an excellent model for computational analyses on conserved intronic flanking regions involved in alternative splicing regulation. Although Neuroligins are highly conserved in all the vertebrates, in zebrafish duplicated genes underwent different evolutionary fates differentiating their alternative splicing regulation [42], thus a similar approach could be useful for this family as well. Indeed, in our analyses, we found some cases of sub-functionalization events within paralogous members of this gene family in zebrafish [42]. However, in vertebrate Neuroligin genes, the intronic sequence conservation around alternative splicing sites is less evident, probably because of the reduced extent of alternative splicing. Overall, these data indicate that alternative splicing patterns have diverged after gene and genome duplication during the evolutionary history of both Neurexins and Neuroligins, contributing to their functional evolution.
Functions in the nervous system
During the formation of new synapses, different and sequential events take place. Initially, axons grow toward the target dendrites and form contacts, then the establishment of synaptic junction with recruitment of pre- and post-synaptic machinery occurs, followed by maturation and specification of the synapses. All these key steps are regulated by cell adhesion proteins [45, 46] and most of the recent efforts have focused on understanding which of these events involves Neurexin and Neuroligin. In a first set of experiments, in vitro assays of synaptogenesis (the formation of new synaptic contacts) were employed, suggesting a role for Neurexin and Neuroligin in the establishment of nascent synapses. Indeed, when Neuroligin is over-expressed in non-neuronal cells co-cultured with neurons, it triggers the assembly of pre-synaptic structures in contacting axons [47]. Similarly, β-Neurexin over-expressed in non-neuronal cells induces the formation of GABA (inhibitory) and glutamate (excitatory) post-synaptic differentiations [48, 49]. The over-expression of Neuroligins also increases the total number of synapses and promotes post-synaptic apparatus assembly [50, 51], while their knock-down by RNAi has the opposite effect [51]. These results have sustained the original idea of Neurexin and Neuroligin as mediators of cell-to-cell contacts during synaptogenesis.
Surprisingly, however, neither Neurexin nor Neuroligin null mice display defects in the number of synapses or in their anatomical structure [52, 53]. Knock-out mice have been generated only for the α isoforms of Neurexin, while no data are yet available for β-Neurexins. α-Neurexins knock-out mice are viable, but die perinatally from respiratory troubles. The elimination of the three α-Neurexins induces the highest penetrance of defects which, however, do not include significant morphological synaptic alterations. In particular, these mice only exhibit a decrease in the number GABAergic, but not glutamatergic terminals. Since Neurexins are expressed at both excitatory and inhibitory synapses, it is possible that the function of α-Neurexin is redundant at excitatory synapses, perhaps because of β-Neurexins expression. The most relevant functional phenotype in α-Neurexin null mice is the impairment of neurotransmitters release, due to a decrease of Ca2+ channels function, in particular of the N-type [52]. The general idea is hence that Neurexins are important in Ca2+-triggered neurotransmitters release. This is in line with the molecular interaction of Neurexin with the synaptic vesicle exocytotic apparatus. Indeed, at the intracellular side, Neurexins bind CASK, a component of a tripartite complex that, with Mint and Veli, directly couples synaptic vesicles to adhesion molecules [54]. Analogous to α-Neurexin null mice, Neuroligin 1-3 triple knock-out mice lack any significant defect in the synaptic ultrastructure [53]. These mice are born normally, but die postnatally due to respiratory problems and show a dramatic decrease in spontaneous inhibitory synaptic activity.
Globally, the phenotypes observed in the genetic mouse models demonstrate that Neurexin and Neuroligin have a fundamental role in synaptic transmission rather than in the early adhesive steps of synapses formation. These results seem to contradict the synapse-inducing capacity of Neurexin and Neuroligin in cultured cells. However, as previously proposed [6], this discrepancy can be justified by the fact that, in vitro, an increase in signal transmission due to Neurexin–Neuroligin interaction can stabilize transient synaptic contacts, thus increasing their number [50]. The in vivo studies also highlight the existence of a functional redundancy within the Neurexin and Neuroligin gene families, at least in mice. Indeed, for both proteins, only deletion of two or more isoforms leads to a severe phenotype and causes the perinatal death of the mice.
During the years, the idea that Neurexins and Neuroligins may regulate the validation of synapses and the maturation of excitatory and inhibitory specializations [50] has been raised. Neuroligin 1 and 2 localize, respectively, at the excitatory and inhibitory synapses, while Neuroligin 3 appears to be present at both [50, 51, 55, 56]. The different isoforms of Neuroligin may act by nucleating different post-synaptic scaffold molecules. Neuroligin 1 binds to PSD95, an intracellular protein required for the differentiation of excitatory synapses, whose amount also dictates the balance between excitatory and inhibitory contacts [57]. On the other hand, Neuroligin 2 contributes to the assembly of inhibitory synapses by interacting with the specific inhibitory post-synaptic scaffold molecule gephyrin [58, 59]. Coupling of scaffold molecules at the post-synaptic density allows, moreover, the recruitment of transmembrane receptors to the post-synaptic side such as NMDA-type and AMPA-type glutamate receptors [60, 61]. The role of Neurexins in the specification of synapses is less clear, even though, apparently, a hierarchical code based on alternative splicing governs its interaction with different Neuroligins at excitatory and inhibitory sites [10, 19–21]. Moreover, α- and β- Neurexin 1 interact with the post-synaptic leucine-rich repeat trans membrane protein LRRTM2 and promote excitatory synapse formation [62, 63].
Recent reports indicate that the trans-synaptic interaction between pre- and post-synaptic adhesion molecules controls vesicular clustering and maturation at the pre-synaptic side, and Neurexin and Neuroligin are appealing candidates in mediating this mechanism. In this respect, over-expression of Neuroligin promotes pre-synaptic maturation, synaptic zone stability and increase in pre-synaptic vesicle clustering in collaboration with N-Cadherin and the post-synaptic scaffolding molecules S-SCAM [64, 65].
Another recently reported process is that the synaptic localization of Neurexin and Neuroligin is dynamically regulated through membrane turnover. Synaptic activity affects membrane exposure of both proteins by increasing Neurexin and Neuroligin membrane insertion and decreasing their internalization [66, 67]. These data support the idea that Neurexin and Neuroligin interaction increases after the formation of synaptic contacts and that they are essential in the maintenance of proper neural-circuits in pre-formed synapses.
Finally, the discussion on the synaptic functions of Neurexin and Neuroligin must include a comment on their involvement in human pathology. Indeed, notwithstanding a number of important open questions [6, 68, 69], the role of Neurexin and Neuroligin in the pathogenesis of autism spectrum disorders (ASDs) and other cognitive diseases is generally accepted. Different genetic alterations (point mutations, translocations, frameshifts, large-scale deletions, missense mutations, internal deletions) have been detected in the Neurexin and Neuroligin genes in patients with autism [70–73]. Of general interest is the fact that a point mutation in the Neuroligin 3 gene (R451C) causes defective protein trafficking and leads to retention of the protein in the endoplasmic reticulum. As a direct consequence, the delivery of Neuroligin 3 to the cell surface is decreased as well as its binding activity to Neurexin [74]. Furthermore, the R451C mutation induces a misfolding problem in the α/β-hydrolase fold that is common to other disease-inducing point mutations [75].
Functions in the vascular system
Biochemistry and cell biology teach us how nature economizes its resources. Once a molecule or a molecular module has been engineered at significant cost by evolution, it will be used in many different frameworks and, if specific functions are needed, they will be achieved by combining these elementary units. The nervous and vascular systems are not atypical in this context, rather, they share anatomical and functional features in addition to many molecular cues. Indeed, in the last decade, there has been an exponential surge of interest on these relationships. Three paradigmatic phases of nervous system development can be broadly identified: genesis of neurons, outgrowth/guidance of axons and synapses formation. The first two of these steps share a number of molecular determinants and cellular events with the vascular system. For example, the Delta/Notch and the gridlock proteins are involved in both neural and vascular cell fate specification (reviewed in [76, 77]), while the Semaphorins/Plexins-Neuropilins, the EphrinB2/EphB4, the Netrin/DCC/Unc5 and the Slit/Roundabout families are involved in axon guidance and vascular patterning [78, 79].
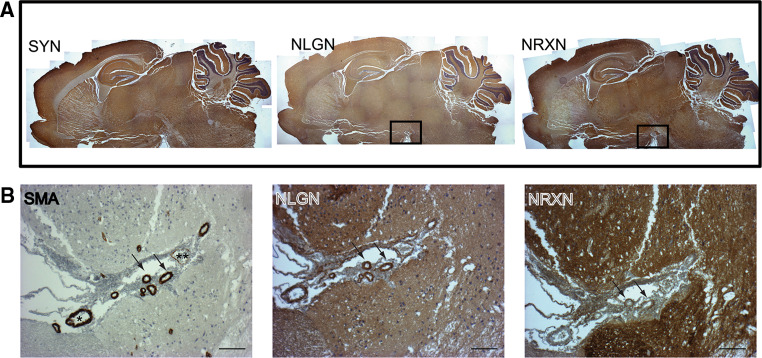
The molecular pathways and cellular activities of the guidance proteins have been the focus of much interest in the last few years [78, 79]. On the other hand, even though some of the same proteins can be expressed at the synapses, none of the specific components of the synaptic machinery has been shown to participate to the vascular function until recently. In fact, we discovered that α/βNeurexins and various isoforms of Neuroligins are expressed by cells of the vascular wall (endothelial and smooth muscle cells), in which they are alternatively spliced and form endogenous complexes in analogy to their behavior in the brain [80]. Next, we targeted β-Neurexin with a recombinant antibody that specifically recognizes the native protein and demonstrated that this reagent affects an important property of vascular smooth muscle cells (VSMC), i.e. vessel tone control, by reducing the tension induced by noradrenalin in whole chick embryo arteries. This is not surprising considering the role of Neurexins as calcium ion channels modulators [52]. Furthermore, as supported by the links between vascular tone, hemodynamics, and vascular remodeling [81], we demonstrated that the anti-β-Neurexin antibody significantly reduces the FGF-2-induced angiogenesis in the chicken chorioallantoic membrane (CAM) assay. More of our data are discussed below, while detailed information can be found in ref. [80]. A representative image of Neurexin/Neuroligin expression in whole mouse brain and blood vessels is presented in Fig. 2 (modified from [80]).
Fig. 2.
Neurexin and Neuroligin expression in blood vessels of the adult mouse brain. a The three panels were produced by merging several low magnification images taken from a sagittal section of a mouse brain stained with antibodies to Synaptophysin, Neurexin and Neuroligin, which globally produce a similar expression pattern. Enriched areas for Neurexin and Neuroligin expression are located in the cortex, hippocampus, hypothalamus and cerebellum. b Magnifications of an area proximal to the hypothalamus (boxed area in a), which presents many large vessels, including arteries and veins. An artery (*) and a vein (**) are highlighted. Arrows indicate blood vessels in which Neurexin expression is limited to the outer layer of the vessel wall. Scale bar 100 μm. This figure was partially modified from ref. [80]
Why synaptic proteins on blood vessels?
Probably the most appealing and certainly challenging issue of the novel findings described above does not lie merely in the two proteins being expressed and functioning in the vascular system, but in the fact that synaptic activity appears distant from the vascular functions. This is an important issue that distinguishes Neurexins and Neuroligins from the proteins that mediate axon or vessel patterning, whose neuronal role can easily be linked to many cellular events of angiogenesis (especially migration and adhesion, but also proliferation and cell survival). This fact, along with the heterogeneity of the Neurexin and Neuroligin protein families and their multifaceted functions, complicates the anticipation of their role in the vascular context. Moreover, although β-Neurexin null mice are not yet available, no vascular defects have been described for mice carrying null mutations within the three α-Neurexins [52] or the three Neuroligin [53] genes, so that the most direct approaches aimed at illustrating the functions of these proteins have not been informative in this context. We cannot exclude, however, that dedicated studies could reveal vascular defects in Neurexin and Neuroligin null mice. In particular, since these animals die of respiratory failure [52, 53], the lung, the organ with the highest proportion of endothelial cells, should be one of the first targets of analysis.
On this background, we can hypothesize the role and mechanism of action of Neurexin and Neuroligin in the vascular system taking into account two points of view: (1) the role that these two proteins may have during the growth and remodeling of the vascular system itself, and (2) the possibility that they mediate physical neurovascular connections.
In the first instance, we should consider that Neurexin and Neuroligin have been originally described as cell-to-cell adhesive proteins and that, in principle, they could promote adhesion in the vascular context. Cell adhesion is never a purely mechanical phenomenon [82], so that adhesive molecules can modulate different cellular events including the paradigmatic steps of new vessels formation (proliferation, migration, matrix adhesion, cell survival). Nevertheless, as explained above, the mouse genetic models indicate that the two proteins are not heavily involved in the early adhesive events at the synapse and, at least in the case of β-Neurexin, our data do not point to such roles: neither overexpression of the protein, nor the treatment of β-Neurexin expressing cells with the anti-β-Neurexin antibody, that reduces angiogenesis, alters these parameters significantly [80]. Reasonably, Neurexin and Neuroligin could modulate phenotypes that have received little interest in relation to angiogenesis, such as exocytosis and smooth muscle cells tone. The involvement in exocytosis is well supported for both proteins. Neurexin mediates calcium-dependent exocytosis [52] and forms intracellular complexes with modulators of this process [54], while Neuroligin 2 affects insulin secretion [29] and binds to a modulator of insulin release (Epac2) [83, 84]. Exocytosis in endothelial cells plays an important role by allowing the control of vascular tone, inflammation and angiogenesis. One of the exocytotic mechanisms typical of endothelial cells involves specific organelles called Weibel−Palade bodies, which can quickly release their contents in a rapid response to a stimulus [85]. The role of Neurexins as VSMC tone modulators is directly supported by our data [80] and again by their role as calcium ion channels modulators [52].
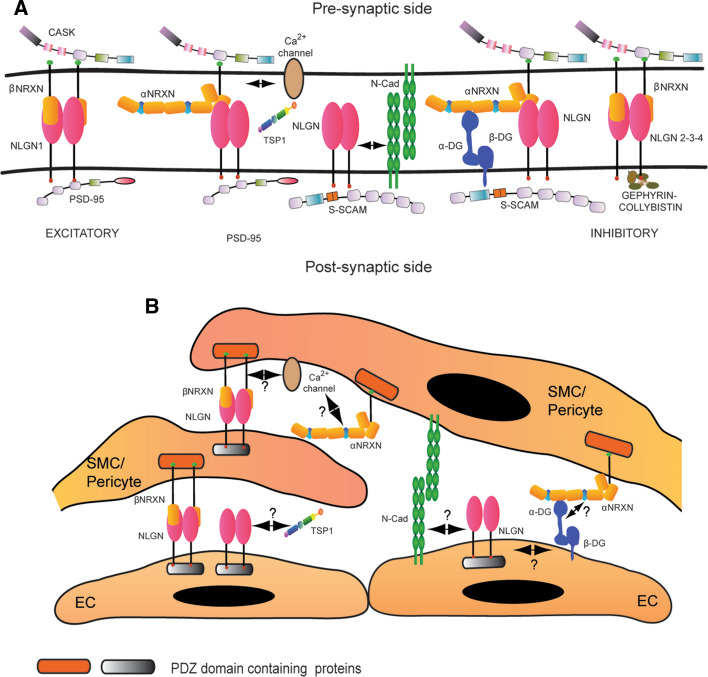
Regarding the mechanism of action of Neurexins and Neuroligins, we should first of all address the potential molecular components of their vascular pathways (Fig. 3). Synaptic protein partners of the two proteins that are identical or have analogous counterparts in the vascular system should be obviously considered. Representative examples are: dystroglycan, a widely distributed protein connecting the extracellular matrix and cytoskeleton [86, 87], calcium channels [52, 88], thrombospondin-1, a well-known angiogenesis modulator [89, 90], and N-Cadherin, a member of a family of proteins that mediate Ca2+-dependent cell-to-cell adhesion [64, 91]. Moreover, good candidates can be found among intracellular PDZ domain-rich proteins such as the homologs of PSD-95, an intracellular “scaffold” protein that binds Neuroligin [92], and CASK, the interactor of Neurexins [80, 92, 93]. On a wider perspective, a literature analysis indicates that one of the most plausible downstream mediators for vascular Neuroligin is nitric oxide, a mediator involved in all aspects of blood vessel functions [90, 94–97]. This is sustained by two facts: (1) PSD-95 modulates its synaptic activities through nitric oxide [98] and binds to neuronal nitric oxide synthase [99], which is structurally and functionally similar to endothelial nitric oxide synthase, and (2) thrombospondin-1, that is known to inhibit nitric oxide activity [90], binds to Neuroligin and accelerates synaptogenesis in the nervous system [89]. Finally, more vascular protein partners of Neurexins and Neuroligins should be searched among modulators of membrane trafficking. Indeed, as mentioned above, the two proteins are dynamically exchanged at the membrane [66, 67], and receptor trafficking is recognized as a key mechanism for altering the strength of synapses during synaptic plasticity [100]. One last note on the mechanism of action of Neurexins and Neuroligins concerns their functional interdependence. Even though we have found that the two proteins physically associate within blood vessels as well as in brain, the possibility that they perform autonomous separate functions cannot be ruled out, as recently shown in neurons [101]. This idea is further suggested by the much higher amount of Neuroligin that is expressed in blood vessels and by the fact that vascular Neuroligin co-precipitates specifically with β-Neurexins and not with α-Neurexins [80], which can remain orphan.
Fig. 3.
Molecular interactions of Neurexin and Neuroligin. a General summary of the main biochemical interactions described for Neurexin and Neuroligin in the central nervous system. Neurexin and Neuroligin localize at the pre- and post-synaptic side, respectively, and two β-Neurexin molecules bind a Neuroligin dimer. Neuroligin 1 is enriched at the excitatory synapses, where it binds PSD-95. Neuroligin 2 and 3 are preferentially expressed at inhibitory synapses, where the post-synaptic scaffold is nucleated by Gephyrin and Collybistin. Another PDZ-domain protein that interacts with Neuroligin is S-SCAM, which mediates the binding with N-Cadherin (N-Cad) and Distroglycan (DG). Thrombospondin 1 (TSP1) has been recently reported as a new interactor for Neuroligin 1. CASK is the only intracellular binding partner identified for Neurexin and, through the recruitment of other adaptor proteins, it creates a bridge with Ca2+channels. The three-dimensional molecular arrangement of Neurexins and Neuroligins as well as the identity of the other interacting partners at the synapse were derived from references [58, 120–122]. The double arrow represents the functional interaction between Neuroligin 1 and N-cadherin [64] and between α-Neurexins and voltage dependent calcium channels (VDCC) [52]. b Hypothetical reconstruction of the Neurexin and Neuroligin molecular partners in blood vessels. Very little is known about the biochemical network around Neurexin and Neuroligin in the vascular system. We demonstrated that Neuroligin is widely expressed in blood vessels, while Neurexin is preferentially restricted to mural cells. In the vascular system, the β isoform of Neurexin preferentially binds Neuroligin. Moreover, a link between Neurexin and Ca2+channels might also exist in blood vessels. Considering the vascular expression of several described Neurexin and Neuroligin partners, we propose a potential scenario of molecular interactions that may involve these proteins in blood vessels. Question marks indicate the hypothetical interactions. Finally, since their identity needs to be confirmed, the intracellular partners of Neurexin and Neuroligin are generally indicated as PDZ domain containing proteins and labeled with a different color code because of their are likely part of respectively “pre- and post-synaptic” families
The second point of view concerns the possible existence of a physical cross-talk between neurons and blood vessels mediated by Neurexins and Neuroligins. This event could be part of very different scenarios. One is the recognition/adhesion between the axon terminals (varicosities) and the target region of the blood vessels that takes place in the final phase of the autonomic innervation of the vascular system [102]. These cell-to-cell adhesive events would be temporary, since normally the varicosities are located at a certain distance from the smooth muscle layer [103]. Another intriguing possibility is that Neurexin and Neuroligin mediate cross-talk between the nervous and vascular system during angiogenesis/synaptogenesis/neurogenesis events that take place in the cerebellum and hippocampus upon repetitive physical activities and/or motor skill learning [104, 105]. It is well known that Neuroligin and Neurexin produced by non-neural cells can induce, respectively, pre- and post-synaptic specialization in neurons [47, 49]. Hence, in the right environment, Neurexins and Neuroligins produced by vascular cells could stimulate synaptic plasticity. In this respect, it is very intriguing that spatial motor learning in the rat results in the induction of Neuroligin 1 in the hippocampus [106]. Neurexin–Neuroligin-mediated cross-talk between blood vessels and neuronal cells could also be at play in the so-called “vascular niche” of the subgranular zone (SGZ) of the hippocampus where neuronal precursors are in intimate contact with the blood vessels (reviewed in [107]). Finally, as suggested by the expression of Neurexin and Neuroligin in the vessels of the brain parenchyma [80], these two proteins could mediate a pervasive interaction between the vasculature and the surrounding neural tissue. If this is the case, they would be ideally positioned within the so-called “neurovascular unit” to rapidly match the cerebral blood flow to the metabolic demands of neurons [108]. Apposition of neuronal processes or perikaria to the basal lamina of blood vessels is anatomically possible [109–112], but has never been thoroughly investigated. Since Neurexin and Neuroligin normally interact in an extracellular matrix-free environment, discontinuities in the basal lamina and/or synapse-like junctions such as those present at the boundary between endothelial cells and pericytes (peg-sockets) [110, 113] would also need to be present at the neuron/blood vessel interface. In the circumstance of Neurexin/Neuroligin being part of the neurovascular unit, their bond with autism could be double. Indeed, two independent studies [114, 115] have described bilateral temporal hypoperfusion in autistic children. Temporal regions are implicated in social perception, language, and theory-of-mind, abilities that are impaired in autism. Moreover, a significant negative correlation has been observed between rCBF (regional cerebral blood flow) in the left superior temporal gyrus and the diagnostic score for autism ADI-R. The more severe the autistic syndrome, the more rCBF is low in this region, suggesting that left superior temporal hypoperfusion is related to autistic behavior severity [116]. These last observations are particularly intriguing when related to the involvement of β-Neurexin in vessel tone maintenance [80].
Our final comments are dedicated to the broadest significance of the synaptic–vascular parallels. On the one hand, the capacity to form billions of specific and plastic cell-to-cell contacts through the synapses represents the most fascinating anatomical and biochemical features of the nervous system. Indeed, the trans-synaptic signaling specializations include the differentiation and coordination of the pre- and post-synaptic membranes, including the formation of excitatory and inhibitory contacts, the directionality of the signal, the selective formation or stabilization of the contacts and the destabilization of the inappropriate ones [17]. Neurexin and Neuroligin participate in many of these activities and are likely to regulate more of them as the investigation goes on. While the task of forming and operating synapses is enormously complex, blood vessels do not appear to have the same needs, so that proteins engineered to support synaptic functions may appear “wasted” in a simpler context such as the vascular system. However, we may consider this situation as another example of the already cited “economy of nature”, or the use of efficient solutions in different frameworks. For example, an intriguing parallel can be drawn between the scaffolding proteins that are positioned at the intracellular side of tight junctions (which are typical of endothelial and epithelial linings) and synapses [54, 117]. Moreover, our data show that some of these intracellular proteins are modulated during endothelial–mural cells interaction [80]. From the opposite point of view, we should not dismiss the idea that the vascular system requires processes previously unknown or underestimated in their complexity. In particular, we should remember that blood vessels are highly heterogeneous [118] and perform a multitude of tasks, some of which require rather accurate cell-to-cell recognition events (e.g., leukocyte extravasation [119]). The existence of alternatively spliced transcripts of Neurexins and Neuroligins in vascular cells [80] is in favor of not just a mechanical/adhesive function, and we believe that this discovery will be the ground for the introduction of novel paradigms in vascular biology.
Acknowledgments
We thank Dr. Anna Gualandris for carefully reading the manuscript. This work was supported in part by grant of the Italian Association for Cancer Research (AIRC); Regione Piemonte (Finalized Health Research 2006, 2008 and 2009; Industrial Research and Precompetitive Development 2006: grants PRESTO and SPLASERBA; Technological Platforms for Biotechnology: grant DRUIDI; Converging Technologies: grant PHOENICS; Industrial Research 2009: grants BANP and eLab) CRT Foundation, and Italian Ministry of Health (Oncological Research Program 2006; Finalized Research 2006).
Footnotes
F. Bussolino and M. Arese equally contributed in the work.
Contributor Information
Federico Bussolino, FAX: +39-011-9933524, Email: federico.bussolino@unito.it.
Marco Arese, FAX: +39-011-9933524, Email: marco.arese@unito.it.
References
- 1.Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, Murray R, Ruggeri M, Tosato S, Bonetto C, Steinberg S, Sigurdsson E, Sigmundsson T, Petursson H, Gylfason A, Olason PI, Hardarsson G, Jonsdottir GA, Gustafsson O, Fossdal R, Giegling I, Moller HJ, Hartmann AM, Hoffmann P, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Djurovic S, Melle I, Andreassen OA, Hansen T, Werge T, Kiemeney LA, Franke B, Veltman J, Buizer-Voskamp JE, Sabatti C, Ophoff RA, Rietschel M, Nothen MM, Stefansson K, Peltonen L, St Clair D, Stefansson H, Collier DA. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 2009;18(5):988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lintas C, Persico AM. Autistic phenotypes and genetic testing: state-of-the-art for the clinical geneticist. J Med Genet. 2009;46(1):1–8. doi: 10.1136/jmg.2008.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wermter AK, Kamp-Becker I, Strauch K, Schulte-Korne G, Remschmidt H. No evidence for involvement of genetic variants in the X-linked neuroligin genes NLGN3 and NLGN4X in probands with autism spectrum disorder on high functioning level. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):535–537. doi: 10.1002/ajmg.b.30618. [DOI] [PubMed] [Google Scholar]
- 4.Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, Austin C, Becker RE, Berry GT, Driscoll K, Engle EC, Friedman S, Gusella JF, Hisama FM, Irons MB, Lafiosca T, LeClair E, Miller DT, Neessen M, Picker JD, Rappaport L, Rooney CM, Sarco DP, Stoler JM, Walsh CA, Wolff RR, Zhang T, Nasir RH, Wu BL. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(4):937–947. doi: 10.1002/ajmg.b.31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill M, Donohoe G, Corvin A. What have the genomics ever done for the psychoses? Psychol Med. 2010;40(4):529–540. doi: 10.1017/S0033291709991139. [DOI] [PubMed] [Google Scholar]
- 6.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18(1):77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourgeron T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb Symp Quant Biol. 2007;72:645–654. doi: 10.1101/sqb.2007.72.020. [DOI] [PubMed] [Google Scholar]
- 9.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17(1):43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 12.Schmucker D, Chen B. Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009;23(2):147–156. doi: 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]
- 13.Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14(3):497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 14.Missler M, Sudhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14(1):20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- 15.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81(3):435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 16.Comoletti D, Flynn R, Jennings LL, Chubykin A, Matsumura T, Hasegawa H, Sudhof TC, Taylor P. Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1beta. J Biol Chem. 2003;278(50):50497–50505. doi: 10.1074/jbc.M306803200. [DOI] [PubMed] [Google Scholar]
- 17.Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6(7):708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baudouin S, Scheiffele P SnapShot: neuroligin-neurexin complexes. Cell 141 (5):908–908.e1 [DOI] [PubMed]
- 19.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48(2):229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51(2):171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26(16):4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Sudhof TC, Taylor P. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry. 2006;45(42):12816–12827. doi: 10.1021/bi0614131. [DOI] [PubMed] [Google Scholar]
- 23.Koehnke J, Katsamba PS, Ahlsen G, Bahna F, Vendome J, Honig B, Shapiro L, Jin X. Splice form dependence of beta-neurexin/neuroligin binding interactions. Neuron. 2010;67(1):61–74. doi: 10.1016/j.neuron.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philibert RA, Winfield SL, Sandhu HK, Martin BM, Ginns EI. The structure and expression of the human neuroligin-3 gene. Gene. 2000;246(1–2):303–310. doi: 10.1016/s0378-1119(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert M, Smith J, Roskams AJ, Auld VJ. Neuroligin 3 is a vertebrate gliotactin expressed in the olfactory ensheathing glia, a growth-promoting class of macroglia. Glia. 2001;34(3):151–164. doi: 10.1002/glia.1050. [DOI] [PubMed] [Google Scholar]
- 26.Occhi G, Rampazzo A, Beffagna G, Antonio Danieli G. Identification and characterization of heart-specific splicing of human neurexin 3 mRNA (NRXN3) Biochem Biophys Res Commun. 2002;298(1):151–155. doi: 10.1016/s0006-291x(02)02403-8. [DOI] [PubMed] [Google Scholar]
- 27.Nelson GM, Padera TP, Garkavtsev I, Shioda T, Jain RK. Differential gene expression of primary cultured lymphatic and blood vascular endothelial cells. Neoplasia. 2007;9(12):1038–1045. doi: 10.1593/neo.07643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100(19):10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suckow AT, Comoletti D, Waldrop MA, Mosedale M, Egodage S, Taylor P, Chessler SD. Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic beta-cells and the involvement of neuroligin in insulin secretion. Endocrinology. 2008;149(12):6006–6017. doi: 10.1210/en.2008-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin–novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21(10):444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- 31.Biswas S, Russell RJ, Jackson CJ, Vidovic M, Ganeshina O, Oakeshott JG, Claudianos C. Bridging the synaptic gap: neuroligins and neurexin I in Apis mellifera. PLoS One. 2008;3(10):e3542. doi: 10.1371/journal.pone.0003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55(5):741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79(6):849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 34.Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett. 2007;581(13):2509–2516. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 35.Banovic D, Khorramshahi O, Owald D, Wichmann C, Riedt T, Fouquet W, Tian R, Sigrist SJ, Aberle H. Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron. 2010;66(5):724–738. doi: 10.1016/j.neuron.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, Liu L, Zeng X, Xu M, Fang M, Xie W. Genetic interaction between Neurexin and CAKI/CMG is important for synaptic function in Drosophila neuromuscular junction. Neurosci Res. 2009;64(4):362–371. doi: 10.1016/j.neures.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Tsubota T, Shiotsuki T. Genomic analysis of carboxyl/cholinesterase genes in the silkworm Bombyx mori . BMC Genomics. 2010;11:377. doi: 10.1186/1471-2164-11-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Missler M, Fernandez-Chacon R, Sudhof TC. The making of neurexins. J Neurochem. 1998;71(4):1339–1347. doi: 10.1046/j.1471-4159.1998.71041339.x. [DOI] [PubMed] [Google Scholar]
- 39.Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, Sudhof TC. Unusually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci USA. 2008;105(17):6421–6426. doi: 10.1073/pnas.0801383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davey C, Tallafuss A, Washbourne P. Differential expression of neuroligin genes in the nervous system of zebrafish. Dev Dyn. 2010;239(2):703–714. doi: 10.1002/dvdy.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rissone A, Monopoli M, Beltrame M, Bussolino F, Cotelli F, Arese M. Comparative genome analysis of the neurexin gene family in Danio rerio: insights into their functions and evolution. Mol Biol Evol. 2007;24(1):236–252. doi: 10.1093/molbev/msl147. [DOI] [PubMed] [Google Scholar]
- 42.Rissone A, Sangiorgio L, Monopoli M, Beltrame M, Zucchi I, Bussolino F, Arese M, Cotelli F. Characterization of the neuroligin gene family expression and evolution in zebrafish. Dev Dyn. 2010;239(2):688–702. doi: 10.1002/dvdy.22196. [DOI] [PubMed] [Google Scholar]
- 43.Rowen L, Young J, Birditt B, Kaur A, Madan A, Philipps DL, Qin S, Minx P, Wilson RK, Hood L, Graveley BR. Analysis of the human neurexin genes: alternative splicing and the generation of protein diversity. Genomics. 2002;79(4):587–597. doi: 10.1006/geno.2002.6734. [DOI] [PubMed] [Google Scholar]
- 44.Ohno S. Evolution by gene duplication. New York: Springer; 1970. [Google Scholar]
- 45.Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- 46.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8(3):206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101(6):657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 48.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci USA. 2005;102(17):6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54(6):919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307(5713):1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 52.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2 + channels to synaptic vesicle exocytosis. Nature. 2003;423(6943):939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 53.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94(6):773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 55.Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26(7):1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- 56.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96(3):1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci USA. 2004;101(38):13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63(5):628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2(4):240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 60.Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci USA. 2008;105(52):20947–20952. doi: 10.1073/pnas.0804007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24(4):916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64(6):799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64(6):791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, Lessmann V, Dresbach T, Gottmann K. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc Natl Acad Sci USA. 2010;107(24):11116–11121. doi: 10.1073/pnas.0914233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wittenmayer N, Korber C, Liu H, Kremer T, Varoqueaux F, Chapman ER, Brose N, Kuner T, Dresbach T. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc Natl Acad Sci USA. 2009;106(32):13564–13569. doi: 10.1073/pnas.0905819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schapitz IU, Behrend B, Pechmann Y, Lappe-Siefke C, Kneussel SJ, Wallace KE, Stempel AV, Buck F, Grant SG, Schweizer M, Schmitz D, Schwarz JR, Holzbaur EL, Kneussel M Neuroligin 1 is dynamically exchanged at postsynaptic sites. J Neurosci 30(38):12733–12744 [DOI] [PMC free article] [PubMed]
- 67.Thyagarajan A, Ting AY. Imaging activity-dependent regulation of neurexin-neuroligin interactions using trans-synaptic enzymatic biotinylation. Cell. 2010;143(3):456–469. doi: 10.1016/j.cell.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29(1):21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Geschwind DH. Autism: the ups and downs of neuroligin. Biol Psychiatry. 2009;66(10):904–905. doi: 10.1016/j.biopsych.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng J. High frequency of neurexin 1[beta] signal peptide structural variants in patients with autism. Neurosci Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M, Hijmans C, Staal WG, Baird G, Bolton PF, Rutter ML, Weisblatt E, Green J, Aldred C, Wilkinson JA, Pickles A, Le Couteur A, Berney T, McConachie H, Bailey AJ, Francis K, Honeyman G, Hutchinson A, Parr JR, Wallace S, Monaco AP, Barnby G, Kobayashi K, Lamb JA, Sousa I, Sykes N, Cook EH, Guter SJ, Leventhal BL, Salt J, Lord C, Corsello C, Hus V, Weeks DE, Volkmar F, Tauber M, Fombonne E, Shih A, Meyer KJ. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82(1):199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24(20):4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radic Z, Lockridge O, Ellisman MH, Taylor P. A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the alpha, beta-hydrolase fold protein family. J Biol Chem. 2006;281(14):9667–9676. doi: 10.1074/jbc.M510262200. [DOI] [PubMed] [Google Scholar]
- 76.Shima DT, Mailhos C. Vascular developmental biology: getting nervous. Curr Opin Genet Dev. 2000;10(5):536–542. doi: 10.1016/s0959-437x(00)00124-6. [DOI] [PubMed] [Google Scholar]
- 77.Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4(9):710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 78.Segura I, De Smet F, Hohensinner PJ, Ruiz de Almodovar C, Carmeliet P. The neurovascular link in health and disease: an update. Trends Mol Med. 2009;15(10):439–451. doi: 10.1016/j.molmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Melani M, Weinstein BM. Common factors regulating patterning of the nervous and vascular systems. Annu Rev Cell Dev Biol. 2010;26:639–665. doi: 10.1146/annurev.cellbio.093008.093324. [DOI] [PubMed] [Google Scholar]
- 80.Bottos A, Destro E, Rissone A, Graziano S, Cordara G, Assenzio B, Cera MR, Mascia L, Bussolino F, Arese M. The synaptic proteins neurexins and neuroligins are widely expressed in the vascular system and contribute to its functions. Proc Natl Acad Sci USA. 2009;106(49):20782–20787. doi: 10.1073/pnas.0809510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Girard H. Arterial pressure in the chick embryo. Am J Physiol. 1973;224(2):454–460. doi: 10.1152/ajplegacy.1973.224.2.454. [DOI] [PubMed] [Google Scholar]
- 82.Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9(12):M33–M37. [PubMed] [Google Scholar]
- 83.Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, Penzes P. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12(10):1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104(49):19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15(8):302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Sugita S. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52(3):372–386. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 88.Munaron L, Fiorio Pla A. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem. 2009;16(35):4691–4703. doi: 10.2174/092986709789878210. [DOI] [PubMed] [Google Scholar]
- 89.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13(1):22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- 90.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9(3):182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 92.Irie M. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 93.Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16(8):2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115(7):1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kashiwagi S, Tsukada K, Xu L, Miyazaki J, Kozin SV, Tyrrell JA, Sessa WC, Gerweck LE, Jain RK, Fukumura D. Perivascular nitric oxide gradients normalize tumor vasculature. Nat Med. 2008;14(3):255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- 96.Jing-Ping Z, Tian QB, Sakagami H, Kondo H, Endo S, Suzuki T. p55 protein is a member of PSD scaffold proteins in the rat brain and interacts with various PSD proteins. Brain Res Mol Brain Res. 2005;135(1–2):204–216. doi: 10.1016/j.molbrainres.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 97.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nature Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 98.Nikonenko I, Boda B, Steen S, Knott G, Welker E, Muller D. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183(6):1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and [alpha]1-syntrophin mediated by PDZ domains. Cell. 1996;84(5):757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 100.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 101.Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, Sudhof TC. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J. 2009;28(20):3244–3255. doi: 10.1038/emboj.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 103.Burnstock G. Non-synaptic transmission at autonomic neuroeffector junctions. Neurochem Int. 2008;52(1–2):14–25. doi: 10.1016/j.neuint.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 105.Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- 106.Haberman RP, Lee HJ, Colantuoni C, Koh MT, Gallagher M. Rapid encoding of new information alters the profile of plasticity-related mRNA transcripts in the hippocampal CA3 region. Proc Natl Acad Sci USA. 2008;105(30):10601–10606. doi: 10.1073/pnas.0804292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13(5):543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 108.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102(2):141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 109.Maynard EA, Schultz RL, Pease DC. Electron microscopy of the vascular bed of rat cerebral cortex. Am J Anat. 1957;100(3):409–433. doi: 10.1002/aja.1001000306. [DOI] [PubMed] [Google Scholar]
- 110.Allsopp G, Gamble HJ. An electron microscopic study of the pericytes of the developing capillaries in human fetal brain and muscle. J Anat. 1979;128(Pt 1):155–168. [PMC free article] [PubMed] [Google Scholar]
- 111.Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421(2):161–171. [PubMed] [Google Scholar]
- 112.Jones EG. On the mode of entry of blood vessels into the cerebral cortex. J Anat. 1970;106(Pt 3):507–520. [PMC free article] [PubMed] [Google Scholar]
- 113.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 114.Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123(Pt 9):1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- 115.Zilbovicius M, Boddaert N, Belin P, Poline JB, Remy P, Mangin JF, Thivard L, Barthelemy C, Samson Y. Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. Am J Psychiatry. 2000;157(12):1988–1993. doi: 10.1176/appi.ajp.157.12.1988. [DOI] [PubMed] [Google Scholar]
- 116.Gendry Meresse I, Zilbovicius M, Boddaert N, Robel L, Philippe A, Sfaello I, Laurier L, Brunelle F, Samson Y, Mouren MC, Chabane N. Autism severity and temporal lobe functional abnormalities. Ann Neurol. 2005;58(3):466–469. doi: 10.1002/ana.20597. [DOI] [PubMed] [Google Scholar]
- 117.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 118.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98(2):159–162. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 119.Vestweber D. Regulation of endothelial cell contacts during leukocyte extravasation. Curr Opin Cell Biol. 2002;14(5):587–593. doi: 10.1016/s0955-0674(02)00372-1. [DOI] [PubMed] [Google Scholar]
- 120.Sumita K, Sato Y, Iida J, Kawata A, Hamano M, Hirabayashi S, Ohno K, Peles E, Hata Y. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J Neurochem. 2007;100(1):154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- 121.Comoletti D, Miller MT, Jeffries CM, Wilson J, Demeler B, Taylor P, Trewhella J, Nakagawa T. The macromolecular architecture of extracellular domain of alphaNRXN1: domain organization, flexibility, and insights into trans-synaptic disposition. Structure. 2010;18(8):1044–1053. doi: 10.1016/j.str.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Comoletti D, Grishaev A, Whitten AE, Tsigelny I, Taylor P, Trewhella J. Synaptic arrangement of the neuroligin/beta-neurexin complex revealed by X-ray and neutron scattering. Structure. 2007;15(6):693–705. doi: 10.1016/j.str.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]