Abstract
T cell activation requires the integration of signals that arise from various types of receptors. Although TCR triggering is a necessary condition, it is often not sufficient to induce full T-cell activation, as reflected in cell proliferation and cytokine secretion. This has been firmly demonstrated for conventional αβ T cells, for which a large panel of costimulatory receptors has been identified. By contrast, the area remains more obscure for unconventional, innate-like γδ T cells, as the literature has been scarce and at times contradictory. Here we review the current state of the art on the costimulatory requirements of γδ T cell activation. We highlight the roles of members of the immunoglobulin (like CD28 or JAML) or tumour necrosis factor receptor (like CD27) superfamilies of coreceptors, but also of more atypical costimulatory molecules, such as NKG2D or CD46. Finally, we identify various areas where our knowledge is still markedly insufficient, hoping to provoke future research on γδ T cell costimulation.
Keywords: γδ T cells, T cell activation, Costimulation, CD27, CD28, NKG2D
T cell costimulation in brief
T cells use their signature TCR to recognise antigens and initiate cellular immune responses whose magnitude depends on the integrated activation of a series of signalling pathways. These must ultimately lead to critical changes in gene expression, such as induction of pro-survival and cell cycle genes that control T cell expansion, or cytokine genes that orchestrate T cell effector function. Thus, in a nutshell, T cell activation typically requires signal transduction via the MAPK/ Erk and PI3 K/ Akt pathways, which relay information to Fos/Jun and NF-kB/NFAT transcription factors, respectively, and these control the expression of genes like IL-2, cyclins and Bcl2 family members (reviewed in [1]).
While the TCR makes a key contribution to the activation of these molecular pathways, extensive research on αβ T cell responses has convincingly established that TCR signalling alone is not sufficient to release αβ T cells from their quiescent state. In fact, TCRαβ stimulation (“signal 1”) in the absence of additional activating signals results in a non-responsive state (also called “anergy”) that is refractory to restimulation (reviewed in [1]). To avoid “anergy” and induce αβ T cell activation, co-ligation of other receptors, which provide “signal 2” (or costimulation), is usually required.
Much of what we know about the importance of “signal 2” has come from studies on the CD28 coreceptor. CD28 signalling has been shown to produce both qualitative and quantitative changes leading to lower activation threshold and enhanced T cell activity. This is critically reflected in the impaired immune responses mounted by CD28-deficient mice against a variety of infectious agents (reviewed in [2]).
The list of costimulatory receptors that, like CD28, affect T cell activation, division, survival and cytokine secretion, has been growing steadily over the past 20 years. Typical costimulatory receptors are type I transmembrane proteins that can be divided into two groups, based on their structural characteristics: Immunoglobulin (Ig) or tumour necrosis factor receptor (TNFR) superfamilies. Ig superfamily members are characterised by a variable Ig-like extracellular domain and a short cytoplasmic tail, whereas TNFR family members present extracellular domains rich in six cysteine repeats (which form disulphide bridges), and posess a more complex cytoplasmic tail (reviewed in [3]).
Ig superfamily costimulatory receptors are also collectively denominated the CD28 family. Although CD28 was the first costimulatory receptor to be identified, other Ig superfamily members were later shown to share structural and functional characteristics with CD28. These include CD2, CTLA-4, ICOS and PD-1, among others (reviewed in [4]). On the other hand, within the TNFR superfamily we find the costimulatory 4-1BB (CD137), OX40 (CD134), CD27 and HVEM, among others (reviewed in [5]).
These two main types of costimulatory receptors use distinct modes of intracellular signalling: whereas the CD28 family members associate directly with protein kinases (such as PI3 K), TNFR superfamily coreceptors require the adaptor proteins TRAF (TNFR-associated factor), namely TRAF2 and TRAF5, to link to downstream signalling events (Fig. 1). Of note, whereas many non-costimulatory members of the TNFR superfamily contain cytoplasmic death domains, the costimulatory members lack death domains, and instead contain TRAF-binding motifs (reviewed in [3]).
Fig. 1.

T cell costimulatory receptors activate distinct signalling pathways. Schematic of the first layer of signalling events downstream of three classes of costimulatory receptors: Immunoglobulin (CD28), tumour necrosis factor receptor (CD27) and c-type lectin-like (NKG2D). CD28 and NKG2D activate PI3 K/Akt either directly (CD28) or via Src-PTK (NKG2D). CD27 activates the MEKK/JNK and IKK/NFκB pathways through the adaptors TRAF2 and TRAF5. Signal transduction downstream of JNK, NFκB and Akt will then modify gene expression in the nucleus (not represented)
While the molecular mechanisms of costimulatory signal transduction are likely to be conserved, this review will focus on the particular functional outcomes of coreceptor triggering on the various subsets of human and murine γδ T cells [6]. In humans, the major (>60%) subset of peripheral blood γδ T cells expresses a Vγ9Vδ2 TCR, which enables the cells to (uniquely) respond to non-peptidic prenyl pyrophosphates (“phosphoantigens”) [7, 8]. Importantly, the presence of antigen presenting cells (APC) can enhance Vγ9Vδ2 T cell responses [9], suggesting that accessory molecules are also involved. The most promising costimulatory receptors thus far characterised on human and/or murine γδ T cells will be discussed in this review.
Ig superfamily coreceptors in γδ T cell activation
The complex role of CD28
CD28 ligation by its ligands B7.1 (CD80) or B7.2 (CD86) (Fig. 2) is known to promote proliferation, survival and cytokine production of CD4+ and CD8+ T cells. Thus, CD28 costimulation increases IL-2 transcription [10] and mRNA stability [11], and it enhances the expression of anti-apoptotic Bcl-xL [12]. As a result, αβ T cell responses are very frequently impaired when CD28 signaling is impeded in vitro (using blocking reagents) or in vivo (using CD28-deficient mice) (reviewed in [2]).
Fig. 2.
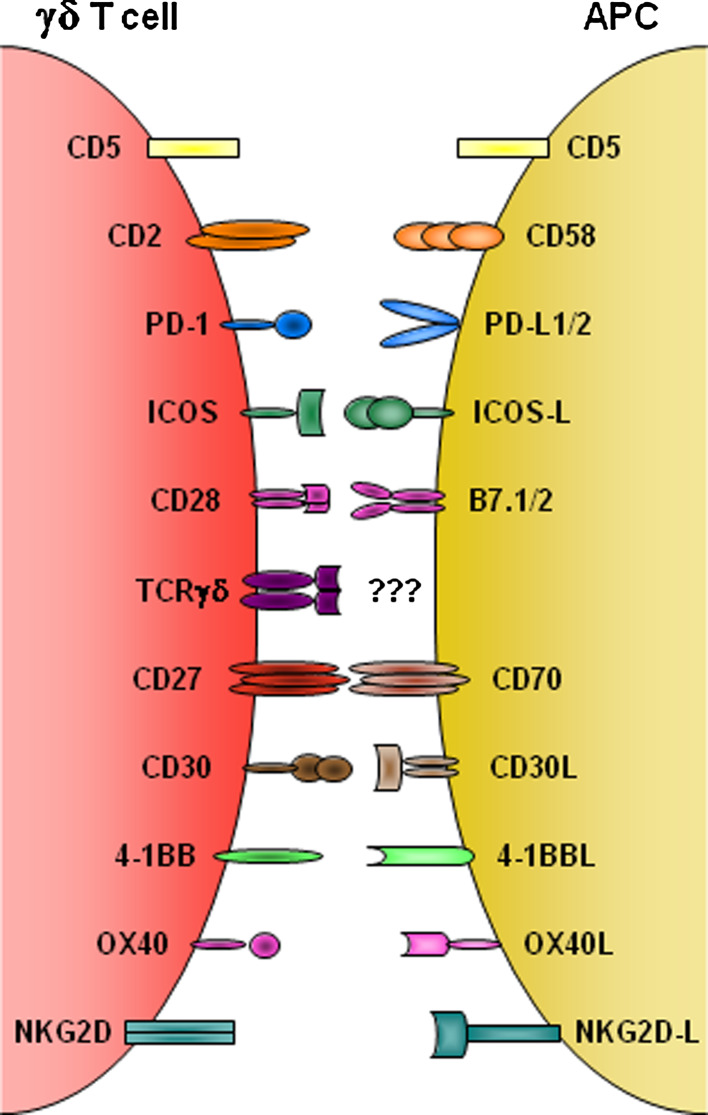
Costimulatory receptors on γδ T cells and corresponding ligands on antigen-presenting cells. Schematic of the main costimulatory receptors known to be expressed on human and/or mouse γδ T cells, which are discussed in this review (CD46 and JAML are not represented). With exceptions of the inhibitory molecules PD-1 and CD5, the coreceptors provide positive signals that enhance (TCR-driven) γδ T cell proliferation and/or cytokine production. This is contingent on receptor binding to cognate ligands expressed on a variety of possible “antigen presenting cells” (APC), such as dendritic cells, epithelial cells or activated lymphocytes
Taking into account the key role of CD28 in αβ T cell activation, it is maybe surprising that its relevance for γδ T cell responses remains controversial. This possibly stems from some discrepancies among reports mostly published in the 1990s and from the complex pattern of expression of CD28 in γδ T cell populations. Thus, whereas αβ T cells constitutively express high levels of CD28, resting murine γδ splenocytes [13] and various intraepithelial lymphocytes (IEL) subsets [14–17], as well as ruminant γδ T cells [18], were reported to mostly lack CD28. However, CD28 levels were shown to increase significantly upon activation of mouse [13] or avian [19] γδ T cells. This pattern contrasts with that observed with human peripheral blood γδ T cells: CD28 is expressed by 40–60% of freshly isolated cells [20–22], but very few (<10%) activated cells [22]. It is therefore difficult to predict the role of CD28 in γδ T cell physiology based on such atypical expression patterns.
Nonetheless, a series of functional assays performed by various groups in the 1990s suggested an active role for CD28 in γδ T cell activation. Thus, anti-CD28 agonist antibodies enhanced human γδ T cell proliferation [20], whereas blocking antibodies inhibited it significantly [21]. Moreover, the generation of TCRγδ transgenic mouse lines allowed the examination of CD28 function in molecularly well-defined models. Namely, proliferation of G8 transgenic γδ splenocytes and IELs was reduced upon treatment with an antagonistic fusion protein (CTLA-4Ig), but augmented by CD28 agonistic antibodies or exogenous IL-2 [13]. This has also been reproduced more recently using polyclonal “wild-type” splenocytes [17]. In another study, Tak Mak and collaborators showed that the alloreactivity of Vγ2+ transgenic lymph node cells was also severely impaired in the CD28-deficient background [23].
Although these reports suggested that CD28 costimulation promotes the proliferation of peripheral γδ T cells, it is important to note that other biological processes appear to be CD28-independent. Namely, the development and activation (alloreactivity) of murine Vγ2+ transgenic thymocytes was normal in the genetic absence of CD28 [23]. Moreover, TNF-α was shown to be produced by Vγ9Vδ2 T cells that mostly lacked CD28 [22].
An important limitation of the studies aimed at investigating the role of CD28 in γδ T cell activation is that functional responses, particularly to infectious agents, were not evaluated in CD28-deficient mice. While this was justifiable in the 1990s, when most of the studies reviewed here were performed, they could easily have been revisited more recently, as has been done for the TNFR superfamily receptor CD27 (see ahead).
A contribution by CD2 at the immunological synapse
CD2 is an Ig superfamily receptor that has been implicated in T cell activation for over 2 decades. CD2 is expressed on all T and NK lymphocytes, and also on a subset of human DCs [24]. CD2 is known to contribute to T and NK cell activation through its interaction with LFA-3 (CD58) expressed on antigen-presenting cells (APC) (Fig. 2). CD2-CD58 ligation optimises T cell recognition through stabilisation of cell-cell contact, “anchoring” cell membranes at a distance suitable for TCR-ligand interaction, while also inducing T cell polarisation [25]. CD2 signaling occurs in microdomains formed by actin-dependent coalescence of signaling molecules shared with TCR cascades, such as TCRξ, Lck or LAT [26]. Moreover, CD2 and TCR appear to synergise in recruiting and activating phospholipase Cγ1 (PLCγ1), a key component of the calcium mobilisation pathway, at the immunological synapse [27]. Specifically on γδ T cells, CD2 has been shown to be enriched at the cell surface contact point with tumour target cells [28].
The role of CD2 in γδ T cell activation was first addressed by Pawelec et al. [29] and by Kabelitz and coworkers [30]. These two studies showed that distinct panels of agonist antibodies against CD2 promoted human γδ T cell proliferation [29, 30] and IL-2 secretion [30]. Interestingly, they also demonstrated that γδ T cell activation was induced by a single anti-CD2 mAb (directed against the sheep erythrocyte-binding epitope, T11.1), by contrast to αβ T cells that required simultaneous engagement of a second mAb directed against a different (T11.2 or T11.3) CD2 epitope [29, 30].
Conversely, antagonist antibodies to CD2 were shown to inhibit γδ T cell proliferation [21]. Wang and Malkovksy reported that blockade of CD2 or CD58 led to reduced proliferation and impaired TNF-α and IL-2 secretion by Vγ9Vδ2 T cells, without affecting their cytotoxic activity [9]. Moreover, the impact of CD2/CD58 blockade on cytokine production could be reversed by adding exogenous IL-2 [9]. The interplay between CD2 signals and another key cytokine, IL-12, was addressed by Lopez et al. [31]. The authors showed that agonistic CD2 mAb and IL-12 acted synergistically to promote human γδ T cell expansion in vitro. Thus, while CD2 signals conferred resistance to activation-induced cell death, the presence of IL-12 was required for active cell proliferation [31].
More recently, Brenner and collaborators examined the role of CD2 in the activation of the Vδ1 subset of human γδ T cells [32], which is enriched at epithelial surfaces [6]. By blocking CD2 or CD58 in co-cultures between Vδ1 T cells and DCs, they obtained a significant, dose-dependent effect on Vδ1 T cell proliferation [32].
Somewhat surprisingly, very few studies have addressed the role of CD2 in murine γδ T cell activation. Nonetheless, it seems clear that for both lymph node [33] and IEL [34] γδ T cells, CD2 expression correlates with increased proliferative capacity. Additional functional studies are required to understand the importance of CD2 signals in murine γδ T cell responses, particularly in models of infection or autoimmunity.
Other Ig superfamily coreceptors
ICOS
After being induced by T cell activation, inducible costimulator (ICOS) binds to ICOS-L (B7 h) to provide positive signals that control αβ T cell proliferation and differentiation. Recently it was shown that ICOS signals can differentially impact on Th1/Th17/Tfh (follicular helper T) CD4+ cell responses, depending on the inflammatory milieu [35]. Moreover, ICOS deficiency results in impaired immune responses [36–38], while mice doubly deficient for ICOS and CD28 are even more immune-compromised [39].
Human peripheral blood Vγ9Vδ2 T cells have been shown to induce ICOS expression upon activation, with >50% of cells becoming ICOS+ after a few days in culture in the presence of phosphoantigens and IL-2 [40]. On the other hand, while freshly isolated blood Vγ9Vδ2 T cells lacked ICOS expression, a subpopulation of Vγ9Vδ2 T cells present in the tonsils readily exhibited high levels of ICOS [41]. This subset, originally defined as CXCR5+, also expressed various other activations (like CD25 and HLA-DR) and memory markers, and promoted IgG, IgA and IgM secretion in co-cultures with B cells. In fact, tonsil CXCR5+ ICOS+ Vγ9Vδ2 T cells displayed overt Tfh properties [41], unlike blood Vγ9Vδ2T cells that are strongly Th1-biased [42]. Despite these interesting findings associated with ICOS expression on activated human γδ T cells, it remains to be elucidated whether ICOS signals contribute to proliferation or cytokine secretion following TCRγδ stimulation.
PD-1
Unlike ICOS, programmed death-1 (PD-1 or CD279) provides negative signals that limit the activation and expansion of TCR-triggered T cells, thus contributing to the maintenance of tolerance. PD-1-deficient mice develop autoimmune syndromes that include cardiomyopathy and a lupus-like disease [43]. The expression of PD-1 is upregulated after αβ T cell activation to deliver, upon ligation by PD-L1 (B7-H1) or PD-L2 (B7-DC), inhibitory signals (often along the TCR signaling cascade) that suppress proliferation and cytokine secretion. More recently, PD-1 was shown to play a crucial role in tumour evasion of CD8+ T cell surveillance [44, 45].
Two sets of unpublished findings suggest that PD-1 may also control γδ T cell activation and anti-tumour responses. Resting human Vγ9Vδ2 T cells express PD-1 and further upregulate it upon phosphoantigen treatment. Moreover, PD-1 is recruited to the immunological synapse during Vγ9Vδ2 T cell activation, and PD-1 blockade enhanced proliferation and the secretion of Th1-type cytokines (Daniel Olive, personal communication).
Furthermore, data obtained in the mouse suggest that inhibitory PD-1 signals may play a key role in the exhaustion of tumour-reactive γδ-T cells in vivo. PD-1 is expressed to a greater extent on γδ-T cells isolated from tumour-bearing mice as compared to γδ-T cells from healthy controls (Richard Lopez, personal communication). In addition, a far greater proportion of the PD-1-positive γδ T cells failed to proliferate and underwent apoptosis, when compared to the PD-1-negative population. Most importantly, PD-L1 blockade substantially restored γδ-T cell proliferation in these assays (Richard Lopez, personal communication). This suggests that suppressing the PD-1 pathway may possibly “rescue” or “revive” γδ T-cell expansion in tumour-bearing hosts. Thus, the manipulation of PD-1 signals may prove to be of significant value for (γδ-)T cell-based immunotherapy.
JAML
Junctional adhesion molecule-like (JAML) was initially identified as a player in leukocyte transmigration, as its ectopic expression in myeloid leukemia cells resulted in enhanced cell adhesion to endothelial cells [46]. Recent data from the Havran group have demonstrated a key costimulatory function of JAML in murine dendritic epidermal T cells (DETC), a murine IEL subset that expresses an oligoclonal Vγ5 Vδ1 TCR [6]. JAML binds to Coxsackie and adenovirus receptor (CAR) expressed on keratinocytes [17]. This induces JAML clusters on the DETC surface and recruits PI3 K through a mechanism analogous to that employed by CD28 [47].
While JAML is present at low levels in resting DETC, as well as in intestinal (but not in thymic or splenic) γδ T cells, its expression is significantly upregulated after stimulation with a mitogen [17]. JAML costimulation was shown to induce DETC proliferation and the secretion of IFN-γ, TNF-α and IL-2. Furthermore, JAML blockade after wounding decreased local DETC activation and impaired the healing process [17]. These data established JAML as a key coreceptor in mouse DETC activation. It is still unknown whether JAML plays any role in the costimulation of other (including human) γδ T cell subsets.
TNFR superfamily coreceptors in γδ T cell activation
CD27 costimulation of IFN-γ-producing γδ T cells
Extensive work by the Borst and van Lier groups, among others, have clearly shown that CD27 (TNFRSF7) plays critical roles in αβ T cell activation [48, 49]. For example, mice lacking CD27 showed reduced numbers of virus-specific CD8+ T cells in the lung and spleen following primary and secondary infection with Influenza [48, 50]. Conversely, CD70 (CD27-ligand) transgenic mice displayed increased αβ T-cell responses to viral or to tumour challenge [51, 52], and chronic immune activation resulted in immunodeficiency [51, 53]. Recent work has demonstrated that CD70–CD27 interactions promote the survival of virus-specific CD8+ T cells (particularly in the infected tissue) and has identified IL-2 as a key positive target of CD27 signalling [54].
We have shown that CD27 levels define two distinct and stable subsets of γδ T cells in naïve C57Bl/6 mice [55]. In the spleen, lymph nodes and various other tissues (such as lung or gut) of adult mice, approximately 75–90% of γδ T cells are CD27+. Upon activation, these cells make IFN-γ, whereas IL-17 is only produced by their CD27− counterparts. Interestingly, these distinct phenotypes are established in the thymus, where CD27+ γδ cells express genes associated with a Th1 differentiation programme, while CD27− thymocytes constitutively express IL-17 [55, 56]. In fact, the “developmental pre-programming” of γδ T cells [57] could be observed already in the foetal thymus [55, 58]. In brief, the current model of γδ T cell development proposes that ligation of both TCRγδ [59] and CD27 [55] are required for the differentiation of IFN-γ-producing γδ T cells, whereas the Th17-like pathway is preferentially driven by cytokines such as TGF-β [56].
Having identified CD27 as a thymic determinant of γδ T cell differentiation, we went on to explore its role in the peripheral activation of “pre-programmed” IFN-γ-producing γδ T cells. We observed that CD27 signals are absolutely required for the expansion of these cells upon infection with herpes viruses or malaria parasites in mice [60]. We further showed that CD70-CD27 interactions provide survival and proliferative signals that control TCRγδ-driven activation. Thus, CD27 signalling activated the non-canonical NF-kB pathway and enhanced the expression of anti-apoptotic and cell cycle-related genes [60].
While the data above were obtained with murine γδ T cells, we have more recently addressed the role of CD27 costimulation in human γδ T cell activation. An average of 80% of Vγ9Vδ2 T cells express CD27 [61] and are considered to include both naive and central memory cells [62]. Upon activation with PMA and ionomycin, the vast majority of CD27+ Vγ9Vδ2 T cells produced IFN-γ, whereas less than 1% made IL-17 [61]. Importantly, the proliferation of these cells was sensitive to CD70-CD27 modulation: administration of soluble recombinant CD70 enhanced, whereas anti-CD27 (or anti-CD70) mAbs reduced, Vγ9Vδ2 T cell expansion in vitro. Moreover, CD27 signals induced calcium flux and upregulated the expression of cyclin D2 and the anti-apoptotic gene Bcl2a1. In fact, a major role of CD27 costimulation appeared to be the protection from AICD following phosphoantigen stimulation [61]. These data suggest that the modulation of CD70-CD27 signals may be of great value for T cell-based immunotherapy. Consistent with this, work on CD8+ T cells has demonstrated that the expansion of tumour-specific cytotoxic T lymphocytes (CTLs) is critically dependent on CD27 costimulation [63, 64].
Regulation of cytokine production by CD30 costimulation
CD30 is a lymphoid-restricted receptor, widely used as a Hodgkin’s lymphoma marker, which is physiologically involved in T cell differentiation. Although initially associated with Th2 cells and clones [65], recent work has proposed a role for CD30 in both Th1 [66] and Th17 [67] differentiation of CD4+ T cells.
In human γδ T cells, CD30 is expressed upon activation, usually declining in long-term (>2 weeks) cultures [68]. Moreover, CD30 can be shed (as soluble CD30, sCD30) from the surface of γδ T cells stimulated with anti-CD3 and anti-CD30 mAbs [69]. Of note, sCD30 is regarded as a marker of chronic B or T cell activation, and it has significant predictive value for transplant rejection [70].
Consistent with earlier studies on αβ T cells [65], high levels of CD30 expression in human γδ T cells were linked to a Th2-like cytokine profile [71–73]. However, CD30 costimulation has been shown to enhance transcription and cytokine production of both IL-4 and IFN-γ in TCR-activated γδ T cells [72]. Moreover, CD30 signalling, which prolonged the calcium flux induced by TCR activation, appeared to have a selective effect on cytokine production, but not on γδ T cell survival or proliferation [72].
Although CD30 function is clearly important in various infection and inflammation models, such as mycobacterial infection [66] and inflammatory colitis [74], its specific relevance to the γδ T cell response in vivo remains to be elucidated.
Other TNFR superfamily coreceptors
Based on their important roles in the activation of other lymphocyte lineages, the TNFR superfamily members OX40 (CD134) [75] and 4-1BB (CD137) [76] would be strong candidates to impact on γδ T cell costimulation. 4-1BB is expressed on a variety of murine and human γδ T cell subsets [72] (our unpublished data), whereas OX40 is upregulated following phosphoantigen activation of Vγ9Vδ2 T cells, with up to 90% of cells expressing OX40 after a few days in culture [40]. However, the functional implication of these coreceptors in γδ T cell activation is still unknown.
Interestingly, activated Vγ9Vδ2 T cells also express high levels of 4-1BBL (CD137L) [77], which besides acting as a ligand for 4-1BB on T and NK cells, may also participate in γδ T cell activation because of its known reverse signalling ability [78]. This in fact also applies to CD70 (CD27-ligand), which is highly induced upon phosphoantigen-mediated stimulation of Vγ9Vδ2 T cells [40, 61]. These issues deserve further investigation.
NKG2D as “signal 2” in γδ T cell activation
While costimulation is traditionally attributed to members of the Ig or TNFR superfamilies, unrelated proteins can also make important contributions to T cell activation. For γδ T cells, the most notable case is the C-type lectin-like NKG2D (natural killer group 2, member D) receptor (Fig. 1). This protein is highly expressed on NK, γδ and CD8+ T cells, and provides activating signals upon ligation to one of its multiple ligands. In humans, these belong to the MIC (A-B) and ULBP (1-6) families, whereas in the mouse there are H60, MULT1 and various RAE1 molecules [79]. Interestingly, and although NKG2D is 70% identical between the two species, its ligands are not necessarily orthologous. NKG2D ligands are induced in response to cellular stress, for example, downstream of the DNA-damage response pathway in tumour cells [80, 81]. Consequently, NKG2D-expressing lymphocytes are activated to kill tumour [82] or pathogen-infected cells [83]. The biological significance of this recognition system is testified to by the increased susceptibility of NKG2D-deficient mice to tumour development [84].
NKG2D can either directly activate lymphocytes, as happens for NK cells, or “play second fiddle” (coreceptor) to the TCR, as described for CD8+ T cells [85]. A costimulatory function of NKG2D in γδ T cells was first reported by Das et al. [86]. They showed that MICA-NKG2D interactions enhanced the response (i.e., cytokine production) of Vγ9Vδ2 T cells upon TCR activation, and proposed that NKG2D could be a functional equivalent of CD28, which was absent in many of these cells [86]. More recently, Scotet and coworkers have performed a detailed study on intracellular calcium mobilisation following Vγ9Vδ2 T cell activation [87]. The authors observed that while NKG2D per se could not induce calcium flux, its co-engagement significantly augmented the intensity of TCR/CD3-mediated responses, which also translated into enhanced cytotoxic activity. By contrast, the production of IFN-γ was unaffected by NKG2D costimulation [87].
Other researchers have defended that NKG2D signals can activate γδ T cells in the absence of TCR engagement. Thus, NKG2D ligation in Vγ9Vδ2 T cells upregulated the activation marker CD69 independently of (and to similar extent as) TCR stimulation [88]. Moreover, mouse DETCs, which are very sensitive to NKG2D ligand expression [89, 90], were suggested to kill some tumours solely based on NKG2D engagement [91].
To complicate matters further, some studies have suggested that NKG2D ligands can also bind to TCRγδ. Thus, MIC proteins were shown to bind to Vδ1+ TCR [92], whereas ULBP4 was reported to bind to the Vγ9Vδ2 TCR [93]. In this scenario, NKG2D ligands could provide both “signal 1” and “signal 2” in γδ T cell activation.
Our own data have dissociated the roles of NKG2D and TCRγδ in the context of anti-tumour Vγ9Vδ2 T cell responses. We thus favour a two-step model where the two receptors act independently (unlike costimulation): TCRγδ mediates bona fide activation, i.e., proliferation and cytokine production, for which the contribution of NKG2D is negligible; however, the recognition of “stressed self” is critically mediated by NKG2D rather than TCRγδ [94]. This view is supported by our findings that although TCR-mediated activation is a prerequisite for effector Vγ9Vδ2 T cell function [42], the definition of killing targets segregates with ULBP1 expression in haematopoietic tumours [95] and is selectively abrogated upon NKG2D, but not TCRγδ, antibody-mediated blockade [96].
While future research will undoubtedly clarify how much and what type of “signal 2” is provided by NKG2D engagement on γδ T cells, its positive impact on functional properties should be promptly applied in the clinic [94].
Other receptors (potentially) involved in γδ T cell costimulation
CD5
CD5 is a type-I transmembrane glycoprotein found on essentially all T cells; it was actually used as a T cell marker before the discovery of CD3. The extracellular region of CD5 consists of three domains belonging to the scavenger receptor cysteine-rich (SRCR) superfamily. The SRCR domain is an ancient and highly conserved protein module of approximately 100-110 amino acids, present in a series of soluble or membrane-bound receptors found on hematopoietic and non-hematopoietic cells [97].
CD5 is known to inhibit αβ T cell activation, presumably by interfering with the signalling machinery used by the positive regulators TCRαβ, CD28 and CD2. Thus, CD5-deficient T cells are hyper-responsive to TCR/CD3 stimulation, which has inspired the concept of CD5 signalling being protective against autoimmunity [98].
Although almost all human γδ T cells express CD5, specific antibody blockade had no effect on their proliferation [21]. In mice, whereas most splenic γδ T cells express CD5, only small subsets do so in the gut intraepithelial and lamina propria compartments. Intestinal CD5+ γδ T cells were proposed to exert a regulatory role in colitis [99], but this, and more generally CD5 function on γδ T cells, requires further investigation. A novel piece of data that should be taken into account is that CD5 is involved in species-specific homophilic interactions, i.e., CD5 binds to CD5 [100] (Fig. 2).
CD46
CD46 is an ubiquitously expressed type I glycoprotein that regulates complement activity by binding to C3b and C4b, and acting as a cofactor for their proteolytic degradation by serine protease factor I [101]. On the other hand, CD46 is known to serve as a receptor for seven pathogens (viral and bacterial) that infect humans. More recently, CD46 was shown to act as a costimulatory receptor for αβ T cells. Co-engagement of TCR and CD46 on human CD4+ T cells induces substantial IL-10 production, at the expense of IFN-γ secretion [102, 103]. In fact, CD46 signalling attenuates IL-2 production and upregulates IL-10 expression in polarised Th1 cultures [103]. However, unlike CD4+ T cells, Vγ9Vδ2 T cells do not express the BC1 isoform of CD46 (linked to Th1 switch to IL-10 producers), but an alternative BC2 version of the protein, which contains a distinct cytoplasmic domain. Signalling through this isoform fails to induce IL-10 expression, but reduces IFN-γ and TNF-α secretion in HMB-PP-stimulated Vγ9Vδ2 T cell cultures [103]. These data suggest a novel and unique role for CD46 in regulating the production of (Th1-like) pro-inflammatory cytokines in human γδ T cells.
LFA-1
Lymphocyte function-associated antigen (LFA-1) is an integrin expressed on T and B cells, and granulocytes/macrophages. LFA-1 is critical for cell-cell adhesion and thus affects many aspects of T cell physiology. One study has suggested that LFA-1 blockade partially inhibits Vδ1 T cell proliferation in co-cultures with DCs [32]. By contrast, work on Vγ9Vδ2 T cells showed that blocking LFA-1 had no effect on proliferation or cytokine secretion, but inhibited cytolytic activity against lymphoma cells [9]. By analogy to αβ CD8+ T cells, it is very plausible that LFA-1 is important to stabilise lytic synapses between γδ T cells and their targets, without directly affecting γδ T cell activation.
Concluding remarks and future directions
We have made significant progress over the past 2 decades towards understanding the roles of γδ T cells in immunity to pathogens and tumours. However, some key aspects of the basic biology of the cells still elude us. Thus, most TCRγδ ligands are yet to be identified, and the functional relevance of many costimulatory molecules expressed on the surface of γδ T cells remains unclear (or even controversial).
We believe it will be critical to clarify the individual roles of costimulatory receptors during distinct phases of γδ T cell responses, both in murine models and in human diseases. Some of these studies are underway. However, while some receptors, such as NKG2D, CD27 and the newly implicated JAML and CD46, are under the spotlight, many others have been forgotten over the last 10 years. These include proteins such as ICOS, OX40 or 4-1BB, known to play crucial roles in αβ T cell activation. Therefore, it would be important to (re)visit the function of these molecules using experimental tools such as antagonist/agonist monoclonal antibodies or fusion proteins, and genetically modified mice. These could provide novel functional insight based on infection [60] or autoimmune models [104, 105] that illicit γδ T cell responses.
From a fundamental point of view, we still need to understand exactly which costimulatory molecules impact on survival, proliferation or cytokine production (considering that different rules may apply to IFN-γ-producing versus IL-17-producing γδ T cells); and to what extent coreceptors that provide negative signals (like PD-1) contribute to the regulation of γδ T cell function. On the other hand, aiming at translational benefit, it will be key to manipulate the activity of costimulatory receptors in pre-clinical models and in clinical trials. We think the success of γδ T cell-based immunotherapy could be currently limited by the lack of a costimulation component and hope this caveat can be overcome by future research.
Acknowledgments
We thank Richard Lopez and Daniel Olive for their personal communications, and the European Molecular Biology Organisation (Installation Grant, project no. 1440) and Fundação para a Ciência e Tecnologia (PTDC/SAU-MII/ 104158/ 2008) for funding.
Conflict of interest
None.
Footnotes
J. C. Ribot and A. de Barros contributed equally to this work.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 3.Duttagupta PA, Boesteanu AC, Katsikis PD. Costimulation signals for memory CD8+ T cells during viral infections. Crit Rev Immunol. 2009;29:469–486. doi: 10.1615/critrevimmunol.v29.i6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 7.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 8.Nedellec S, Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: from signals to functions. Semin Immunol. 2010;22:199–206. doi: 10.1016/j.smim.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Malkovsky M. Different roles of the CD2 and LFA-1 T-cell co-receptors for regulating cytotoxic, proliferative, and cytokine responses of human V gamma 9/V delta 2 T cells. Mol Med. 2000;6:196–207. [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 11.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 12.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 13.Sperling AI, Linsley PS, Barrett TA, Bluestone JA. CD28-mediated costimulation is necessary for the activation of T cell receptor-gamma delta+ T lymphocytes. J Immunol. 1993;151:6043–6050. [PubMed] [Google Scholar]
- 14.Ohteki T, MacDonald HR. Expression of the CD28 costimulatory molecule on subsets of murine intestinal intraepithelial lymphocytes correlates with lineage and responsiveness. Eur J Immunol. 1993;23:1251–1255. doi: 10.1002/eji.1830230609. [DOI] [PubMed] [Google Scholar]
- 15.Rakasz E, Hagen M, Sandor M, Lynch RG. Gamma delta T cells of the murine vagina: T cell response in vivo in the absence of the expression of CD2 and CD28 molecules. Int Immunol. 1997;9:161–167. doi: 10.1093/intimm/9.1.161. [DOI] [PubMed] [Google Scholar]
- 16.Rakasz E, Sandor M, Hagen M, Lynch RG. Activation features of intraepithelial gamma delta Tcells of the murine vagina. Immunol Lett. 1996;54:129–134. doi: 10.1016/S0165-2478(96)02662-4. [DOI] [PubMed] [Google Scholar]
- 17.Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanrahan CF, Kimpton WG, Howard CJ, Parsons KR, Brandon MR, Andrews AE, Nash AD. Cellular requirements for the activation and proliferation of ruminant gammadelta T cells. J Immunol. 1997;159:4287–4294. [PubMed] [Google Scholar]
- 19.Koskela K, Arstila TP, Lassila O. Costimulatory function of CD28 in avian gammadelta T cells is evolutionarily conserved. Scand J Immunol. 1998;48:635–641. doi: 10.1046/j.1365-3083.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- 20.Testi R, Lanier LL. Functional expression of CD28 on T cell antigen receptor gamma/delta-bearing T lymphocytes. Eur J Immunol. 1989;19:185–188. doi: 10.1002/eji.1830190129. [DOI] [PubMed] [Google Scholar]
- 21.Takamizawa M, Fagnoni F, Mehta-Damani A, Rivas A, Engleman EG. Cellular and molecular basis of human gamma delta T cell activation. Role of accessory molecules in alloactivation. J Clin Invest. 1995;95:296–303. doi: 10.1172/JCI117654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafont V, Liautard J, Gross A, Liautard JP, Favero J. Tumor necrosis factor-alpha production is differently regulated in gamma delta and alpha beta human T lymphocytes. J Biol Chem. 2000;275:19282–19287. doi: 10.1074/jbc.M910487199. [DOI] [PubMed] [Google Scholar]
- 23.Penninger JM, Timms E, Shahinian A, Jezo-Bremond A, Nishina H, Ionescu J, Hedrick SM, Mak TW. Alloreactive gamma delta thymocytes utilize distinct costimulatory signals from peripheral T cells. J Immunol. 1995;155:3847–3855. [PubMed] [Google Scholar]
- 24.Crawford K, Stark A, Kitchens B, Sternheim K, Pantazopoulos V, Triantafellow E, Wang Z, Vasir B, Larsen CE, Gabuzda D, et al. CD2 engagement induces dendritic cell activation: implications for immune surveillance and T-cell activation. Blood. 2003;102:1745–1752. doi: 10.1182/blood-2002-07-2206. [DOI] [PubMed] [Google Scholar]
- 25.Tibaldi EV, Salgia R, Reinherz EL. CD2 molecules redistribute to the uropod during T cell scanning: implications for cellular activation and immune surveillance. Proc Natl Acad Sci USA. 2002;99:7582–7587. doi: 10.1073/pnas.112212699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaizuka Y, Douglass AD, Vardhana S, Dustin ML, Vale RD. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J Cell Biol. 2009;185:521–534. doi: 10.1083/jcb.200809136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espagnolle N, Depoil D, Zaru R, Demeur C, Champagne E, Guiraud M, Valitutti S. CD2 and TCR synergize for the activation of phospholipase Cgamma1/calcium pathway at the immunological synapse. Int Immunol. 2007;19:239–248. doi: 10.1093/intimm/dxl141. [DOI] [PubMed] [Google Scholar]
- 28.Favier B, Espinosa E, Tabiasco J, Dos Santos C, Bonneville M, Valitutti S, Fournie JJ. Uncoupling between immunological synapse formation and functional outcome in human gamma delta T lymphocytes. J Immunol. 2003;171:5027–5033. doi: 10.4049/jimmunol.171.10.5027. [DOI] [PubMed] [Google Scholar]
- 29.Pawelec G, Schaudt K, Rehbein A, Olive D, Buhring HJ. Human T cell clones with gamma/delta and alpha/beta receptors are differently stimulated by monoclonal antibodies to CD2. Cell Immunol. 1990;129:385–393. doi: 10.1016/0008-8749(90)90214-C. [DOI] [PubMed] [Google Scholar]
- 30.Wesselborg S, Janssen O, Pechhold K, Kabelitz D. Selective activation of gamma/delta + T cell clones by single anti-CD2 antibodies. J Exp Med. 1991;173:297–304. doi: 10.1084/jem.173.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez RD, Xu S, Guo B, Negrin RS, Waller EK. CD2-mediated IL-12-dependent signals render human gamma delta-T cells resistant to mitogen-induced apoptosis, permitting the large-scale ex vivo expansion of functionally distinct lymphocytes: implications for the development of adoptive immunotherapy strategies. Blood. 2000;96:3827–3837. [PubMed] [Google Scholar]
- 32.Das H, Sugita M, Brenner MB. Mechanisms of Vdelta1 gammadelta T cell activation by microbial components. J Immunol. 2004;172:6578–6586. doi: 10.4049/jimmunol.172.11.6578. [DOI] [PubMed] [Google Scholar]
- 33.Budd RC, Russell JQ, van Houten N, Cooper SM, Yagita H, Wolfe J. CD2 expression correlates with proliferative capacity of alpha beta + or gamma delta + CD4-CD8- T cells in lpr mice. J Immunol. 1992;148:1055–1064. [PubMed] [Google Scholar]
- 34.Van Houten N, Mixter PF, Wolfe J, Budd RC. CD2 expression on murine intestinal intraepithelial lymphocytes is bimodal and defines proliferative capacity. Int Immunol. 1993;5:665–672. doi: 10.1093/intimm/5.6.665. [DOI] [PubMed] [Google Scholar]
- 35.Simpson TR, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Curr Opin Immunol. 2010;22:326–332. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/S1074-7613(00)00011-X. [DOI] [PubMed] [Google Scholar]
- 37.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 38.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 39.Suh WK, Tafuri A, Berg-Brown NN, Shahinian A, Plyte S, Duncan GS, Okada H, Wakeham A, Odermatt B, Ohashi PS, et al. The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J Immunol. 2004;172:5917–5923. doi: 10.4049/jimmunol.172.10.5917. [DOI] [PubMed] [Google Scholar]
- 40.Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- 41.Caccamo N, Battistini L, Bonneville M, Poccia F, Fournie JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 42.Correia DV, d’Orey F, Cardoso BA, Lanca T, Grosso AR, deBarros A, Martins LR, Barata JT, Silva-Santos B. Highly active microbial phosphoantigen induces rapid yet sustained MEK/Erk- and PI-3 K/Akt-mediated signal transduction in anti-tumor human gammadelta T-cells. PLoS One. 2009;4:e5657. doi: 10.1371/journal.pone.0005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 44.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moog-Lutz C, Cave-Riant F, Guibal FC, Breau MA, Di Gioia Y, Couraud PO, Cayre YE, Bourdoulous S, Lutz PG. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood. 2003;102:3371–3378. doi: 10.1182/blood-2002-11-3462. [DOI] [PubMed] [Google Scholar]
- 47.Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3 K. Science. 2010;329:1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 49.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol. 2011;89:195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 50.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/S1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 52.Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RA, Schumacher TN, Schumacher TN, van Oers MH. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199:1595–1605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 54.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120:168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 59.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribot JC, Chaves-Ferreira M, d’Orey F, Wencker M, Goncalves-Sousa N, Decalf J, Simas JP, Hayday AC, Silva-Santos B. Cutting Edge: Adaptive Versus Innate Receptor Signals Selectively Control the Pool Sizes of Murine IFN-{gamma}- or IL-17-Producing gamma}{delta T Cells upon Infection. J Immunol. 2010;185:6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.deBarros A, Chaves-Ferreira M, d’Orey F, Ribot JC, Silva-Santos B. CD70–CD27 interactions provide survival and proliferative signals that regulate T-cell receptor-driven activation of human γδ peripheral blood lymphocytes. Eur J Immunol. 2011;41:195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 62.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Johnson PW, Tutt AL, Al-Shamkhani A, Glennie MJ. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 64.Glouchkova L, Ackermann B, Zibert A, Meisel R, Siepermann M, Janka-Schaub GE, Goebel U, Troeger A, Dilloo D. The CD70/CD27 pathway is critical for stimulation of an effective cytotoxic T cell response against B cell precursor acute lymphoblastic leukemia. J Immunol. 2009;182:718–725. doi: 10.4049/jimmunol.182.1.718. [DOI] [PubMed] [Google Scholar]
- 65.Romagnani S, Del Prete G, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leukoc Biol. 1995;57:726–730. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- 66.Tang C, Yamada H, Shibata K, Muta H, Wajjwalku W, Podack ER, Yoshikai Y. A novel role of CD30L/CD30 signaling by T-T cell interaction in Th1 response against mycobacterial infection. J Immunol. 2008;181:6316–6327. doi: 10.4049/jimmunol.181.9.6316. [DOI] [PubMed] [Google Scholar]
- 67.Sun X, Yamada H, Shibata K, Muta H, Tani K, Podack ER, Yoshikai Y. CD30 ligand/CD30 plays a critical role in Th17 differentiation in mice. J Immunol. 2010;185:2222–2230. doi: 10.4049/jimmunol.1000024. [DOI] [PubMed] [Google Scholar]
- 68.Ferrarini M, Delfanti F, Gianolini M, Rizzi C, Alfano M, Lazzarin A, Biswas P. NF-kappa B modulates sensitivity to apoptosis, proinflammatory and migratory potential in short- versus long-term cultured human gamma delta lymphocytes. J Immunol. 2008;181:5857–5864. doi: 10.4049/jimmunol.181.9.5857. [DOI] [PubMed] [Google Scholar]
- 69.Biswas P, Mantelli B, Delfanti F, Ferrarini M, Poli G, Lazzarin A. CD30 ligation differentially affects CXCR4-dependent HIV-1 replication and soluble CD30 secretion in non-Hodgkin cell lines and in gamma delta T lymphocytes. Eur J Immunol. 2003;33:3136–3145. doi: 10.1002/eji.200324344. [DOI] [PubMed] [Google Scholar]
- 70.Platt RE, Wu KS, Poole K, Newstead CG, Clark B. Soluble CD30 as a prognostic factor for outcome following renal transplantation. J Clin Pathol. 2009;62:662–663. doi: 10.1136/jcp.2008.060665. [DOI] [PubMed] [Google Scholar]
- 71.Spinozzi F, Agea E, Bistoni O, Forenza N, Monaco A, Falini B, Bassotti G, De Benedictis F, Grignani F, Bertotto A. Local expansion of allergen-specific CD30+ Th2-type gamma delta T cells in bronchial asthma. Mol Med. 1995;1:821–826. [PMC free article] [PubMed] [Google Scholar]
- 72.Biswas P, Rovere P, De Filippi C, Heltai S, Smith C, Dagna L, Poli G, Manfredi AA, Ferrarini M. Engagement of CD30 shapes the secretion of cytokines by human gamma delta T cells. Eur J Immunol. 2000;30:2172–2180. doi: 10.1002/1521-4141(2000)30:8<2172::AID-IMMU2172>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 73.Dagna L, Iellem A, Biswas P, Resta D, Tantardini F, Fortis C, Sabbadini MG, D’Ambrosio D, Manfredi AA, Ferrarini M. Skewing of cytotoxic activity and chemokine production, but not of chemokine receptor expression, in human type-1/-2 gamma delta T lymphocytes. Eur J Immunol. 2002;32:2934–2943. doi: 10.1002/1521-4141(2002010)32:10<2934::AID-IMMU2934>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 74.Sun X, Yamada H, Shibata K, Muta H, Tani K, Podack ER, Iwakura Y, Yoshikai Y. CD30 ligand is a target for a novel biological therapy against colitis associated with Th17 responses. J Immunol. 2010;185:7671–80. doi: 10.4049/jimmunol.1002229. [DOI] [PubMed] [Google Scholar]
- 75.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Lin GH, McPherson AJ, Watts TH. **Immune regulation by 4–1BB and 4–1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 77.Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89:21–9. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 79.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–744. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 80.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 86.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/S1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 87.Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human V gamma 9 V delta 2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185:55–63. doi: 10.4049/jimmunol.1000373. [DOI] [PubMed] [Google Scholar]
- 88.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9 V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 89.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 90.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 91.Nitahara A, Shimura H, Ito A, Tomiyama K, Ito M, Kawai K. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol. 2006;126:1052–1058. doi: 10.1038/sj.jid.5700112. [DOI] [PubMed] [Google Scholar]
- 92.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 93.Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood. 2009;114:310–317. doi: 10.1182/blood-2008-12-196287. [DOI] [PubMed] [Google Scholar]
- 94.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 95.Gomes AQ, Correia DV, Grosso AR, Lanca T, Ferreira C, Lacerda JF, Barata JT, Silva MG, Silva-Santos B. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood gammadelta T cells. Haematologica. 2010;95:1397–1404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lanca T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, Ramalho JS, Barata JT, Moita LF, Gomes AQ, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115:2407–2411. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 97.Sarrias MR, Gronlund J, Padilla O, Madsen J, Holmskov U, Lozano F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: an ancient and highly conserved protein module of the innate immune system. Crit Rev Immunol. 2004;24:1–37. doi: 10.1615/CritRevImmunol.v24.i1.10. [DOI] [PubMed] [Google Scholar]
- 98.Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8:349–353. doi: 10.1016/j.autrev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Mizoguchi A, Mizoguchi E, de Jong YP, Takedatsu H, Preffer FI, Terhorst C, Bhan AK. Role of the CD5 molecule on TCR gammadelta T cell-mediated immune functions: development of germinal centers and chronic intestinal inflammation. Int Immunol. 2003;15:97–108. doi: 10.1093/intimm/dxg006. [DOI] [PubMed] [Google Scholar]
- 100.Brown MH, Lacey E. A ligand for CD5 is CD5. J Immunol. 2010;185:6068–6074. doi: 10.4049/jimmunol.0903823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liszewski MK, Kemper C, Price JD, Atkinson JP. Emerging roles and new functions of CD46. Springer Semin Immunopathol. 2005;27:345–358. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 102.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 103.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]