Abstract
Vascular smooth muscle tone is controlled by a balance between the cellular signaling pathways that mediate the generation of force (vasoconstriction) and release of force (vasodilation). The initiation of force is associated with increases in intracellular calcium concentrations, activation of myosin light-chain kinase, increases in the phosphorylation of the regulatory myosin light chains, and actin-myosin crossbridge cycling. There are, however, several signaling pathways modulating Ca2+ mobilization and Ca2+ sensitivity of the contractile machinery that secondarily regulate the contractile response of vascular smooth muscle to receptor agonists. Among these regulatory mechanisms involved in the physiological regulation of vascular tone are the cyclic nucleotides (cAMP and cGMP), which are considered the main messengers that mediate vasodilation under physiological conditions. At least four distinct mechanisms are currently thought to be involved in the vasodilator effect of cyclic nucleotides and their dependent protein kinases: (1) the decrease in cytosolic calcium concentration ([Ca2+]c), (2) the hyperpolarization of the smooth muscle cell membrane potential, (3) the reduction in the sensitivity of the contractile machinery by decreasing the [Ca2+]c sensitivity of myosin light-chain phosphorylation, and (4) the reduction in the sensitivity of the contractile machinery by uncoupling contraction from myosin light-chain phosphorylation. This review focuses on each of these mechanisms involved in cyclic nucleotide-dependent relaxation of vascular smooth muscle under physiological conditions.
Keywords: Cyclic AMP, Cyclic GMP, PKA, PKG, Vascular smooth muscle, Calcium sensitivity, Myosin light chain, Vasorelaxation
Introduction
The impaired regulation of the vascular tone can play an important role in the pathophysiology of vascular diseases such as hypertension and vasospasm. Understanding the molecular mechanisms regulating the vascular tone, which is the result of the existing balance between the vasoconstrictor and the vasodilator signaling pathways, is thus essential for establishing new strategies for the prevention and treatment of vascular diseases [1, 2]. The intracellular signaling events that initiate contraction involve Ca2+-calmodulin interaction to stimulate phosphorylation of the 20-kDa regulatory light chain of myosin (MLC20). Phosphorylation of the MLC20 is the main event involved in the regulation of the smooth muscle contractility and is controlled by the opposing activities of myosin light-chain kinase (MLCK) and myosin light-chain phosphatase (MLCP) [3]. A complex network of protein kinases including cyclic cAMP-dependent protein kinase (PKA) and cyclic GMP-dependent protein kinase (PKG) are involved in the regulation of MLC20 phosphorylation.
Thus, changes in [Ca2+]c are the main events that regulate the contractile state of vascular smooth muscle (VSM) cells and, usually, an increase in [Ca2+]c results in VSM contraction and a decrease in [Ca2+]c results in VSM relaxation. In response to vasoconstrictor stimuli, increases in [Ca2+]c may arise from the opening of calcium channels in the plasma membrane allowing influx of extracellular Ca2+ or from the release of stores, such as sarcoplasmic reticulum (SR). The relative contribution of these two Ca2+ pools varies with different VSM cells and stimuli. On the other hand, in response to vasodilator stimuli or the removal of vasoconstrictor stimuli, the cytosolic [Ca2+]c decreases, mainly as a result of plasmalemmal Ca2+ extrusion and/or Ca2+ uptake into the SR, leading to the inactivation of MLCK and, hence, relaxation.
Furthermore, some studies suggested the existence of secondary mechanisms that regulate the Ca2+ sensitivity of MLC20 phosphorylation or contractile myofilaments [4]. The increase or decrease in the sensitivity of smooth muscle contractile response to [Ca2+]c is commonly referred to as “Ca2+ sensitization” or “Ca2+ desensitization”, respectively [3].
Numerous regulatory mechanisms modulate Ca2+ mobilization and Ca2+ sensitivity of MLC20 phosphorylation or contractile myofilaments in VSM cells. This review focuses on the pivotal role of cyclic nucleotides in mediating vasodilation under physiological conditions, reviewing the cyclic nucleotide-mediated vasodilation that occurs through the inhibition of both Ca2+ mobilization and Ca2+ sensitization in VSM cells.
Vascular smooth muscle contraction
A number of extracellular signals, including neural, humoral, ionic, and mechanical forces (stretch) induce contraction or relaxation of VSM. The balance between contraction and relaxation signals determines the intrinsic tone of VSM, which in turn regulates the blood vessel dynamics [1].
Under physiological conditions, the increases in [Ca2+]c leading to contraction are caused by either membrane depolarization (i.e., electromechanical coupling, attained for example, by blockade of outward potassium currents or activation of stretch-dependent channels in the plasma membrane) or by the binding of contractile agonists to specific cell surface receptors (i.e., pharmacomechanical coupling) [5]. However, it is important to note that changes in membrane potential may occur secondary in the pharmacomechanical coupling. These two mechanisms appear to occur simultaneously in a given VSM cell, and excitation caused by a single agent may be the result of either electromechanical or pharmacomechanical coupling alone, or a combination of the two mechanisms [6].
One of the most important mechanisms of pharmacomechanical coupling involves the activation of the phosphatidylinositol pathway as a result of interaction between a vasoconstrictor agonist (e.g., norepinephrine, angiotensin II, serotonin, endothelin) and a membrane receptor coupled to phospholipase C (PLC) via a heterotrimeric G-protein. This interaction induces formation of inositol 1,4,5-triphosphate (IP3) and 1,2-diacylglycerol (DAG) [7]. The IP3 diffuses from the cell membrane into the cytosol, while DAG remains in the cell membrane, being the physiological activator of PKC. In the cytosol, IP3 stimulates the SR IP3-sensitive Ca2+ channels to release Ca2+, which activates contractile proteins and initiate contraction. On the other hand, DAG, along with Ca2+, activates PKC, which acts on various cytoplasmic and membrane proteins, thereby modulating a number of cellular processes, such as the regulation of transmembrane Ca2+ transport and myofilament Ca2+ sensitivity [6, 8].
Following the initial increase in [Ca2+]c caused by PLC-mediated Ca2+ release from SR, a tonic increase in [Ca2+]c, which is dependent on extracellular Ca2+, is normally observed in contractile response to receptor agonists. The agonists may activate plasmalemmal Ca2+ channels without prior membrane depolarization, although contractile response to receptor agonists is normally associated with membrane depolarization and an increase in membrane conductance. However, the precise mechanism whereby the agonists cause membrane depolarization sufficient to activate voltage-operated Ca2+ channels (VOCCs) are not fully understood [9].
On the other hand, the endothelium regulates vascular tone via release of endothelium-derived factors and plays a fundamental role in the basal and dynamic regulation of the circulation. Besides, endothelial and VSM cells communicate via gap junctions, which can spread some signals among these cells and cause remote or conducted dilatation [10]. Myoendothelial gap junctions allow transfer of charge and small molecules such as IP3 and Ca2+ [11]. In this sense, transmission of hyperpolarization was described [11] and also a role of cAMP in this transmission was suggested by some authors [12, 13]. The ability of other small molecules (<1 kDa) to diffuse through these junctions could involve non-electrotonic mechanisms to contribute to endothelium-dependent relaxation. However, further studies are necessary to determine the relevance of these processes.
Among the mechanisms that play pivotal roles in the regulation of vascular tone, an increase in the cytosolic level of cAMP or cGMP in VSM cells is one of the major signaling pathways that mediate vasodilation under physiological conditions.
Mechanisms of cyclic nucleotide-mediated vasorelaxation
Cyclic nucleotides (cAMP and cGMP) are the main second messengers linked to vasodilation and the elevation of its intracellular levels represents a useful strategy for eliciting a variety of beneficial pharmacological effects on several cardiovascular pathological conditions. The intracellular levels of these two second messengers are the result of the balance between the rate of their synthesis and the rate of their degradation.
Cyclic AMP is synthesized from intracellular ATP by adenylate cyclases, which are usually activated by an external first message (neurotransmitter, hormone, drug) that binds to a G-protein-coupled receptor (Fig. 1). In mammals, there are at least nine closely related isoforms of adenylate cyclases (AC1–AC9) that can be regulated in several different ways [14, 15]. Depending on the properties and on the relative levels of the isoforms expressed in a done tissue or a cell type, extracellular signals received through the receptors can be differentially integrated [15].
Fig. 1.
Mechanisms involved in the regulation of cyclic nucleotides levels and activation of kinases by these nucleotides. Green arrows stimulation, AC adenylate cyclase. pGC particulate guanylate cyclase, sGC soluble guanylate cyclase, R receptor, PDE phosphodiesterase, G G-protein, cAMP cyclic adenosine 3′,5′-monophosphate, cGMP cyclic guanosine 3′,5′-monophosphate, 5′AMP adenosine 5′-monophosphate, 5′GMP guanosine 5′-monophosphate, ATP adenosine 5′-triphosphate, GTP guanosine 5′-triphosphate, PKA cAMP-dependent protein kinase, PKG cGMP-dependent protein kinase
Cyclic GMP may be synthesized in VSM cells by two main types of guanylate cyclases that differ in their cellular location and in their activation by specific compounds: (1) a particulate guanylyl cyclase (pGC), present at the plasma membrane, which is activated by natriuretic peptides such as atrial (ANP), brain (BNP), and C-type natriuretic peptide (CNP) [16]; and (2) a soluble guanylyl cyclase (sGC) that can be activated by nitric oxide (NO) and by NO donors [17] (Fig. 1).
Despite these classical activation pathways for guanylate cyclase, other regulatory pathways were described. Like NO, carbon monoxide (CO) is an endogenously produced gas molecule that activates sGC. This product of heme oxygenase (HO) was shown to elevate cGMP levels in smooth muscle cells, leading to relaxation of the vessels [18, 19]. Some authors showed that CO may regulate vascular tone under physiologic and pathophysiologic conditions such as hypoxia [18] and hypertension [19]. Also, among other functions, overexpression of HO has been shown to be cardioprotective [20]. Other authors also suggested that HO regulates blood pressure through CO only when the NOS pathway is fully operative, establishing a link between the role of NO and CO [21]. On the other hand, it was also recently shown that in VSM cells, thrombospondin-1, a matrix protein, can inhibit NO-stimulated sGC activation through binding to the cell surface receptor CD47 [22, 23]. Thus, thrombospondin-1 can, as a regulator of the canonical NO pathway at multiple levels, control acute vascular tone, blood flow, and angiogenic remodeling [24]. The cGMP and cAMP levels are elevated in tissues from thrombospondin-1 and CD47 null mice compared to wild-type controls [22]. In human VSM thrombospondin-1 modulates cellular cAMP levels directly via inhibition of adenylyl cyclase and indirectly via cGMP-dependent cross talk [23]. Concerning sGC regulation, some years ago it was also observed that hypoxia usually elicits pulmonary vasodilation through cGMP. The theories of how oxygen is sensed by pulmonary arterial smooth muscle focused on the modulation of reactive oxygen species biosynthesis and/or changes in the oxidation-reduction or redox status, which are also closely associated with the reactive oxygen species (ROS) generation [25]. In this sense, the peroxide, a ROS, was already recognized as an activator of sGC [26]. In recent studies, a cGMP-independent activation of PKG by peroxide was reported to operate as a vasodilator mechanism [27, 28]. On the other hand, crosstalk seems to occur between the sGC and pGC pathways to regulate cGMP-dependent vasodilatation in mice and humans. Some authors reported that cGMP derived from pGC desensitizes blood vessels to the effects of the NO donors spermine-NONOate and N(G)-nitro-l-arginine [29, 30]. This heterologous regulation provides a physiological mechanism linking the paracrine and endocrine functions of NO and natriuretic peptides [31]. Recently, a direct activation of pGC by testosterone was suggested to modulate human umbilical artery (HUA) vasodilatation through PKG activation [32].
The intracellular levels of cAMP and cGMP can be degraded by phosphodiesterases (PDE) to their biologically inactive metabolites 5′-AMP and 5′-GMP, respectively (Fig. 1) [33, 34]. These enzymes selectively catalyze the hydrolysis of the 3’ cyclic phosphate bonds of adenosine and/or guanosine 3’,5’ cyclic monophosphate, thereby limiting the activity of these molecules on their substrates, such as PKA and PKG (Fig. 1). There are 11 different PDE families that can be found in a variety of cells and, in general, each family has different isoforms and splice variants [33, 34].
The cAMP and cGMP actions were usually attributed to protein kinase A (PKA) and protein kinase G (PKG) activation, respectively. Structurally, PKA is a heterotrimeric serine/threonine protein kinase composed by a catalytic α subunit and regulatory β and γ subunits [35]. Its catalytic domain is located in the N-terminus of the α subunit and phosphorylation at Thr172 is vital for its activation [36], although other phosphorylation sites in the α and β subunit have been identified [37]. The β subunit is thought to act as a scaffold, possesses a glycogen-binding domain and is myristoylated, a process that may localize the kinase to the cell membrane. The γ subunit contains the AMP-binding site where the binding of AMP induces a conformational change in a subunit to lead to PKA activation [38]. The PKG are serine/threonine kinases that can be activated by cGMP. They are composed of an NH2-terminal domain, a regulatory domain, and a catalytic domain [39]. The regulatory domain contains cGMP-binding sites and occupation of both binding sites releases the inhibition of the catalytic center and allows the phosphorylation of serine/threonine residues in target proteins and in the aminoterminal autophosphorylation site. Activation of heterophosphorylation and autophosphorylation increases the basal activity of PKG [40]. In addition to controlling activation and inhibition of the catalytic center, the NH2 terminus serves two other functions [39]: (1) dimerization of PKG homodimers that are held together by a leucine zipper; and (2) targeting of PKG to different subcellular localizations.
As mentioned, cyclic nucleotides can activate PKA and PKG, which phosphorylate target effectors, although direct binding of cyclic nucleotides to proteins, such as ion channels or guanine-nucleotide-exchange factors were also described [41]. Furthermore, it is worth mentioning that each cyclic nucleotide can activate both protein kinases, PKA and PKG (Fig. 1). In fact, PKG can also be activated by cAMP, although it requires nearly a 10-fold-higher cAMP concentration than cGMP concentration to activate this kinase. Similarly, PKA can also be activated by cGMP, although it requires nearly a 10-fold-higher cGMP concentration than cAMP to activate this kinase. In smooth muscle, the cytosolic level of cAMP is typically nearly 10-fold greater than the cytosolic level of cGMP. Therefore, elevations in the cytosolic level of cAMP potentially could activate both PKA and PKG, whereas cGMP should only activate PKG [4]. In studies using rat aortic smooth cells, Lincoln and Cornwell observed that activation of PKG by cAMP leads to the reduction in intracellular Ca2+, whereas activation of PKA may only mediate the uptake of Ca2+ from extracellular sources [42]. On the other hand, it has recently been shown that in rat aortic cells, cGMP synthesized by sGC induces a decrease of Gi protein expression and then increases cAMP levels through a mitogen-activated protein kinase (MAPK)-mediated mechanism. This may be an additional mechanism responsible for the vasorelaxant effect of sGC activators [43]. Concerning the genetically modified mice, a null mutation in the major catalytic subunit of PKA results in early frequent postnatal lethality and infertility, but cardiovascular problems do not seem to be significant [44]. Conventional deletion of the PKG-I gene in mice leads to multiple phenotypes, including severe gastrointestinal disturbances and elevated blood pressure, leading to premature death of the animals [45, 46]. Also, these models revealed that reduction of the intracellular Ca2+ level by PKG-I may be one component of the signaling pathways regulating vascular tone in vivo [47].
On the other hand, an alternative cAMP target Epac (exchange protein directly activated by cAMP) for cAMP-mediated effects was revealed and its role in regulation emerged in the last years. Epac was identified to explain the PKA-independent activation of the small G-protein Rap by cAMP. Epac proteins function as guanine nucleotide exchange factors (GEFs) for Rap, a member of the Ras family of small G-proteins. Rap is inactive when bound to guanosine diphosphate (GDP) and activated when bound to guanosine triphosphate (GTP) [48]. These GEFs connect different inputs to Rap activation and are linked to distinct functions of Rap. Epac are multidomain proteins involved in VSM cell migration, vascular permeability, angiogenesis, and cardiacac contractility, but effects on vascular contractility have not yet been reported [49, 50].
Although an increase in the cytosolic level of cAMP or cGMP in VSM cells is one of the major mechanisms that mediate vasodilation under physiological conditions, its vasodilator mechanism is not yet fully understood and has been shown to vary between different blood vessels, between species, and also to be dependent on the stimulus leading to a previous vasoconstricted state.
Four main pathways are currently thought to be involved in the vasodilator effect of cyclic nucleotides [1, 3, 4, 6, 51–55]:
The decrease in intracellular calcium levels that can be achieved through activation of Ca2+ uptake by the SR, increased intracellular Ca2+ efflux, inhibition of Ca2+ release from the SR and/or decreased extracellular Ca2+ influx;
The hyperpolarization of the smooth muscle cell membrane potential through activation of outward potassium channels, inactivation of Na+ channels and/or inactivation of multiple channels;
The reduction in the sensitivity of the contractile machinery by decreasing the [Ca2+]c sensitivity of MLC20 phosphorylation due to a decrease of the myosin light-chain kinase activity and/or to an increase of the MLCP activity
The reduction in the sensitivity of the contractile machinery by uncoupling contraction from MLC20 phosphorylation via a thin-filament regulatory process.
The contribution of each of these mechanisms was reported to be dependent on VSM type, species and contractile stimulus and was suggested to be determined by the relative expression and/or colocalization of different proteins in each vascular bed [56, 57]. It is likely that no single pathway acts exclusively or independently in any one type of smooth muscle cell. However, the relative importance of the various pathways leading to cyclic nucleotide-induced relaxation is likely to be different in phasic compared to tonic contractile cells and in cells from large arteries compared to those from microvessels [52]. For example, the vasodilator agent nitric oxide (NO) activates sGC, elevates intracellular cGMP, and enhances SR Ca2+ uptake [58]. However, NO has also been shown to enhance the activity of large conductance voltage-dependent and Ca2+-activated K+ channels either directly [59] or indirectly via cGMP and PKA [60, 61]. However, Tanaka et al. showed that the contribution of KV channel is irrelevant for the relaxations induced by NO donors (nitroglycerin and NOR 3) and ANP in thoracic aorta but is significant in mesenteric artery isolated from the rat. These authors concluded that cGMP signaling concerning potassium channels could largely differ in large conduit arteries compared to small arteries [57]. On the other hand, a different mechanism described in the literature to justify the cyclic nucleotide effects can be conditioned by its diffusion along different cell compartments. The different localization, properties, and selectivity of the enzymes involved in the cyclic nucleotide pathways seem to play a role in this phenomenon that was named “compartmentalization of cyclic nucleotides”. This compartmentalization can limit the signals to specific subcellular regions due to local increases of cyclic nucleotides or to the existence of specific clusters of proteins in these regions [62, 63]. Thus, subcellular cyclic nucleotide localizations will be responsible for specific compartmentalized regulation of precise cellular functions so that cAMP and cGMP, in response to different stimuli, orchestrate a wide variety of cellular responses.
Decrease in intracellular calcium levels induced by cyclic nucleotides
The first mechanism proposed for cyclic nucleotide-dependent relaxation of vascular smooth muscle is the reduction of free intracellular cytosolic calcium concentration. As increases in [Ca2+]c are required for MLC20 phosphorylation and contraction, the reduction of [Ca2+]c by cyclic nucleotides and the subsequent dephosphorylation of MLC20 are seen primarily as a reversal of the contractile mechanism.
The decrease in intracellular calcium levels may be achieved by the following pathways:
Decreased release from SR, via phosphorylation of the SR IP3 receptor and/or inhibition of IP3 synthesis;
Increased sequestration in the SR, via phospholamban phosphorylation and activation of SR Ca2+-ATPase;
Decreased influx of extracellular calcium, via L-type Ca2+ channels (LTCC);
Increased efflux of intracellular calcium, via the stimulation of plasma membrane Ca2+-ATPase.
Decreased release from SR
In VSM, Ca2+ is stored intracellularly in the SR, which is an endomembrane system that plays a main role in the regulation of [Ca2+]c and vascular tone, not only as a supplier of the activator Ca2+ but also as a buffer against VSM activation by Ca2+. The SR in VSM cells contains at least two types of Ca2+-release channels, i.e., those activated by IP3 (IP3 receptor) and those sensitive to or activated by the plant alkaloid ryanodine and caffeine (ryanodine receptor). The IP3R channel is capable of releasing a large quantity of Ca2+ to the cytosol, and is believed to play a primary role in Ca2+ mobilization, which mediates the initial phase of agonist-induced contraction. The IP3R can be phosphorylated by various protein kinases, such as PKG, PKA, and PKC, and thus its function may be modulated by these kinases. Phosphorylation of the IP3R in intact VSM in response to cGMP-generating vasodilators or metabolically resistant cGMP analogs has been demonstrated [64, 65]. The IP3R was similarly phosphorylated by stimulation with forskolin, an agent that directly activates adenylate cyclase and increases cAMP level. However, the sensitivities of these phosphorylations to various protein kinase inhibitors suggest that PKG mediates phosphorylation of IP3R in response to increases of both cAMP and cGMP in intact cells [65]. Thus, in some cell types such as the vascular smooth muscle cell, it is conceivable that both cAMP and cGMP signaling pathways regulate IP3R activity through activation of PKG and the resultant phosphorylation of the receptor (Fig. 2). In this sense, one established and in vivo target for PKG is IRAG, which has been identified in a complex with the smooth muscle IP3 receptor and PKG [66, 67]. Phosphorylation of IRAG by PKG inhibits IP3-induced Ca2+ release from intracellular stores in smooth muscle cells [66, 68, 69]. On the other hand, it has also been reported that the differential interaction of PKG isozymes with their substrate proteins determines their intracellular localization and that IRAG restricts the intracellular localization of PKG [70, 71]. Using IRAG-deficient murine mutants, Desch et al. [72] showed that IRAG signaling does not modulate basal tone, but might be important for blood pressure regulation under pathophysiological conditions.
Fig. 2.
Mechanisms involved in the decrease in intracellular calcium levels induced by cyclic nucleotide-dependent protein kinases. Green arrows stimulation, red arrows inhibition, SR sarcoplasmic reticulum, G guanosine-5′-triphosphate-binding protein, R G-protein coupled receptor, IP3 inositol 1, 4, 5-triphosphate, PIP2 phospholipid phosphatidylinositol 4,5-bisphosphate, PLC phospholipase C, PKA cAMP-dependent protein kinase, PKG cGMP-dependent protein kinase, SERCA sarcoplasmic reticulum Ca2+-ATPase, NCX Na+/Ca2+ exchanger, PMCA plasma membrane Ca2+-ATPase, VOCC voltage-operated Ca2+ channels
Agonist-induced activation of phospholipase C was shown to be inhibited by cGMP and cAMP, presumably through activation of PKG and PKA, respectively [53, 73]. The inhibition of phosphoinositide hydrolysis and the subsequent IP3 synthesis by PKG was attributed to inhibition of the interaction between Gq protein and PLC [74] (Fig. 2).
Increased sequestration in the SR
Phospholamban (PLB) is a phosphorylatable protein component of smooth muscle SR that reversibly inhibits the activity of the sarcoplasmic reticulum Ca2+-ATPase (SERCA) [9] and SR Ca2+ transport. Phosphorylation of PLB by PKA or PKG at the Ser16 residue relieve this inhibition, thus increasing ATPase activity and the rate of Ca2+ uptake by the SR [75, 76] (Fig. 2). The stimulation of Ca2+ uptake into the SR via phospholamban phosphorylation and activation of SR Ca2+-ATPase, with the resultant relaxant effect may be mediated by cGMP- and cAMP-elevating agents, such as sodium nitroprusside and forskolin, as revealed in feline aorta, where this mechanism represents approximately one-third of the total relaxant effect of both compounds, which is consistent with the multiple mechanisms responsible for relaxation in smooth muscle [77].
Decreased influx of extracellular calcium
Although Ca2+ release from the SR is the main event to induce the initial rapid phasic component of VSM contraction, extracellular Ca2+ influx across plasmalemmal Ca2+ channels plays an important role for sustained Ca2+ entry and contractility. In the last years, two types of plasmalemmal Ca2+ channels were studied as relevant entry routes of extracellular Ca2+ in VSM cells: voltage-operated Ca2+ channels and store-operated calcium channels (SOCCs) [78–80].
The calcium entry by SOCCs is activated in response to the emptying of intracellular Ca2+ stores and act to replenish these stores [81]. Basically, two mechanisms have been proposed to explain extracellular Ca2+ influx triggered by discharge of endoplasmic reticulum stores [82]. The first mechanism involves the diffusible “calcium influx factor” (CIF) released from intracellular organelles, which has not yet been identified [83–85]. CIF, in turn, activates calcium-independent phospholipase A (iPLA2), which generates lysophospholipids activating SOCCs at the plasma membrane by an unknown process. The second model involves the re-organization of the endoplasmic reticulum Ca2+ sensor STIM I, which then activate Orai I, a transmembrane protein of the plasma membrane. The STIM I–Orai I complexes form in a spatially restricted manner at ER-plasma membrane junctions and SOCCs are activated in the vicinity of this nexus [86]. Recently, the SOCC have been identified as being transient receptor potential (TRP) channels [87]. Notably, STIM I also appears to activate Ca2+ entry TRPC I [88, 89]. In VSM, different TRP channels were linked with Ca2+ store depletion, G-protein-coupled receptor activation, membrane stretch, phospholipid signals, and other factors. However, the role of these channels has not yet been clarified, probably due to the lack of specific pharmacological tools, such as specific activators and/or inhibitors.
The voltage-operated Ca2+ channels, which include L-type and T-type Ca2+ channels (LTCC and TTCC, respectively), are activated after increases in the membrane potential: hyperpolarization closes these channels and leads to vasodilatation, whereas depolarization opens them, which results in vasoconstriction [79, 90]. In most VSM cells, LTCCs are the most numerous channels and probably the most important route for calcium influx, although T-type Ca2+ channels have also been reported [6, 79, 80, 91]. Experiments in mice with smooth muscle-specific inactivation of the LTCC gene revealed that these channels are key players in the hormonal regulation of blood pressure and development of myogenic tone. These experiments also suggested that half of the phenylephrine-induced peripheral vessel resistance is due to Ca2+ influx through the LTCC [92].
Voltage-gated Ca2+ channels are modulated by several signaling systems, including cyclic nucleotides. Data on the modulation of LTCC in VSM cells by cyclic nucleotides are sometimes contradictory [93] but, in general, is accepted that cyclic nucleotides inhibit LTCC [94] (Fig. 2). Using a cell-attached patch-clamp, Liu et al. demonstrated that cAMP and cGMP inhibit L-type Ca2+ channel activities in VSM cells from rat portal vein [95]. However, Taguchi et al. [96] described that, in rat mesenteric artery, LTCC activity is stimulated by cAMP and inhibited by cGMP [96]. Ishikawa and coworkers [97] reported that low concentrations of intracellular cAMP produce modest increases in whole-cell LTCC current, whereas cGMP and higher cAMP concentrations result in inhibition of L-type channel activity in VSM cells from the rabbit portal vein. Additional studies from the same group led to the conclusion that LTCC activity in the rabbit portal vein is enhanced by cAMP/PKA stimulation and inhibited by cGMP/PKG stimulation and that each cyclic nucleotide has a primary action mediated by its own kinase as well as a secondary action mediated by the opposing kinase, an action that has been referred to as cross activation or “crossover” [98]. Sustained inhibition of LTCC was also shown in freshly isolated VSM cells from the rabbit portal vein treated with forskolin, 8-bromo-cAMP, and 8-bromo-cGMP [99]. Lorenz and coworkers demonstrated that both cyclic nucleotides inhibit LTCC in A7r5 cells and the obtained data were consistent with a channel phosphorylation-dependent mechanism. These authors concluded that inhibition of LTCC by the cyclic nucleotides presumably lowers calcium influx and cell excitability and therefore causes vasodilation [100]. Satoh and Sperelakis [101] also observed inhibition of LTCC by both nucleotides in A7r5 cells [101].
Increased efflux of intracellular calcium through stimulation of plasma membrane Ca2+-ATPase
Plasma membrane Ca2+-ATPase (PMCA) mediates extrusion of Ca2+ into the extracellular space and plays a crucial role in reducing excessive [Ca2+]c levels in the resting condition or in mediating the vasodilator action of various endogenous agents (Fig. 2). There are several PMCA isoforms, being the isoform PMCA 1b, with a molecular weight of around 140 kDa, the most frequently expressed in smooth muscle cells [7]. PMCA uses energy from ATP hydrolysis to produce Ca2+ efflux in exchange for 2H+ influx against a high electrochemical gradient across the plasma membrane. The C-terminal of the cytosolic domain of the PMCA pump contains both CaM-binding sites and substrates for several protein kinases such as PKA, PKG, and PKC. Conversely, when CaM is absent, the CaM-binding domain interacts with cytosolic regions of the enzyme, inhibiting its activity (i.e., autoinhibition). Phosphorylation of some sites in the CaM binding regions by several protein kinases—such as PKA, PKG, PKC, and Ca2+/CaM-dependent protein kinase—results in activation of the PMCA pump [7, 102]. Strong evidence in favor of a stimulation by PKG of the Ca2+ extrusion from smooth muscle cells via the ATP-dependent Ca2+ pump of the plasmalemma has been presented [103, 104]. Results obtained in cultured rat aortic smooth muscle cells suggested a possible mechanism of action for cGMP in mediating decreases in cytosolic Ca2+ through activation of a Ca2+-ATPase and the subsequent removal of Ca2+ from the cell [104] (Fig.2). In this study, the authors used 8-bromo-cGMP, a membrane-permeable analogue of cGMP, and it was observed that cAMP-dependent protein kinase catalytic subunit and PKC were either ineffective or less effective than PKG in stimulating the Ca2+-ATPase. Furthermore, a cGMP-dependent phosphorylation enhancement of the affinity for Ca2+ and the activity of the PMCA pump has been observed in crude membrane fractions [104], in plasma membrane-enriched fractions [105], and in the solubilized plasmalemmal Ca2+ pump purified by calmodulin affinity chromatography [106]. Also, Vrolix et al. [107] reported that PKG, but not PKA, stimulates the plasmalemmal Ca2+ pump of pig aorta smooth muscle indirectly by increasing phosphatidylinositol phosphate, probably via the phosphorylation of a phosphatidylinositol kinase that co-purified with the Ca2+-transport ATPase [107].
Increased efflux of intracellular calcium through stimulation of Na+/Ca2+ exchanger
Although VSM cells are known to express the Na+/Ca2+ exchanger (NCX), its functional role has remained unclear, mainly because of its relatively low expression. These cells possess an NCX in their plasmalemma with a high capacity to produce Ca2+ efflux [7]. The NCX is driven by the transmembrane Na+ gradient and maintained by the Na+ pump (Na+/K+ ATPase) and normally transports 1 Ca2+ out in exchange for 3 Na+ (Fig. 2). The NCX may contribute to either Ca2+ extrusion or Ca2+ influx, depending on the conditions [108, 109]. Because even large changes in the Na+ electrochemical gradient across the plasmalemma do not significantly alter the resting [Ca2+]c level in VSM cells, the physiological significance of the NCX in these cells has been questioned for many years. Although the importance of NCX in the regulation of cellular Ca2+ homeostasis seems to be high for some authors [6, 51, 110], others have reported a scarce role [111]. These controversial results may be due to differences between species and tissues used [51]. It has been reported that the plasmalemmal Ca2+ pump plays a more important role in Ca2+ extrusion than does NCX [51, 112]. For example, Furukawa et al. [112] have presented evidence that at least 90% of the extracellular Na+-independent Ca2+ extrusion from cultured rat aortic VSM cells occurs via the ATP-dependent Ca2+ pump, and that this Ca2+ pump is stimulated by intracellular cGMP but not by cAMP [112]. Then, to further elucidate the relaxation-promoting effect of cGMP, the same research team examined whether cGMP also regulates Na+/Ca2+ exchange and observed that 8-bromo-cGMP or atrial natriuretic peptide (ANP) enhanced Na+/Ca2+ exchange in primary cultures of VSM cells prepared from rat aorta [110]. In contrast, Karashima et al. [109] reported that NCX was involved in the forskolin-induced reduction of intracellular [Ca2+] and tension in the mouse thoracic aorta (Fig. 2).
Hyperpolarization of the smooth muscle cell membrane potential induced by cyclic nucleotides
The membrane potential is determined by membrane permeability to several ions, including K+, Ca2+, Na+ and Cl− ions and is a major determinant of vascular tone, particularly in systemic resistance vessels. In VSM cells, K+ channels situated in the plasmalemma play a fundamental role in maintaining the membrane potential [79]. Other ion transport systems such as the Na+/K+ pump or anion transporters can also contribute.
Changes in the activity of K+ channels play the most prominent role in regulating the membrane potential and, hence, vascular tone. Specifically, the blockade of K+ channels results in membrane depolarization and increased Ca2+ influx through VOCCs, leading to vasoconstriction. In contrast, the activation of K+ channels results in plasmalemmal K+ efflux, membrane hyperpolarization, and reduced Ca2+ influx through VOCCs, leading to vasodilation [113, 114]. By their dominance in setting membrane potential, K+ channels play a central role in the determination and regulation of vascular tone, and their opening is considered as one major mechanism that mediates vasodilation under physiological conditions.
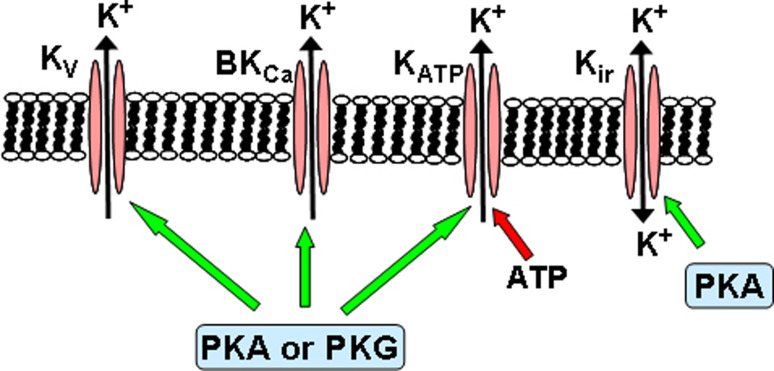
Multiple distinct K+ channels are present in most VSM cells and their distribution and regulation by multiple dilator and constrictor signals depends on the vascular bed and species examined:
Voltage-gated K+ (Kv) channels;
Large-conductance Ca2+-activated K+ (BKCa or MaxiK) channels;
ATP-sensitive K+ (KATP) channels;
Inward rectifier K+ (Kir) channels;
Voltage-gated K+ channels
The Kv channel activation may be involved in the vasodilator response to several endogenous and exogenous substances. β-adrenoreceptor stimulation and forskolin has been shown to activate Kv currents through cAMP/PKA in rabbit VSM cells [115, 116] (Fig. 3). Also, it has been suggested that activation of Kv channels by endogenous prostacyclin (PGI2), known to elevate intracellular levels of cAMP, may contribute to acetylcholine (ACh)-induced endothelium-dependent vasorelaxation in rabbit middle cerebral arteries, although activation of BKCa channels are also involved [61]. In endothelium-denuded rabbit middle cerebral arteries precontracted with histamine, exogenous PGI2- or forskolin-induced vasorelaxation was also mediated by Kv and BKCa channels, via an adenylate cyclase/cAMP pathway [61]. In VSM of HUA, testosterone-induced vasorelaxation is mediated by the pGC/cGMP pathway which stimulates Kv and BKCa channels [32]. Other authors attributed NO donors (nitroglycerin, NOR 3 and sodium nitroprusside) and/or ANP-induced relaxation of rat aorta to the activation of Kv channels in rat aorta [57] and in ovine pulmonary artery [117] (Fig. 3).
Fig. 3.
Regulation of K+ channels by cyclic nucleotide-dependent protein kinases in VSM cells. Green arrows stimulation, red arrows inhibition, PKA cAMP-dependent protein kinase, PKG cGMP-dependent protein kinase
On the other hand, Palen et al. showed that nitric oxide dephosphorylated Kv through cGMP and SHP-1 tyrosine phosphatase, suggesting that tyrosine dephosphorylation may be linked to increased channel activity. These authors suggested that this dephosphorylation involved a complex interaction between guanylate cyclases, Kv channels and the SHP-1 tyrosine phosphatase [118].
Large conductance Ca2+-activated K+ channels
The BKCa channels increase their activity with membrane depolarization and with the elevation of intracellular Ca2+ concentration. These channels have been recognized to be involved in the relaxant response induced by many endogenous vasodilators (e.g., adenosine, prostacyclin, NO) and to play an important role as a negative feedback mechanism to limit membrane depolarization and, hence, vasoconstriction. They are upregulated in hypertensive subjects, constituting an important homeostatic mechanism for buffering the increased arterial reactivity [119].
Activation of BKCa channels of different VSM cells by NO, exogenous nitrovasodilators and PGI2 is likely to involve phosphorylation of the channels or their regulatory subunits by PKG and PKA (Fig. 3), respectively, based on the use of selective inhibitors of these kinases and/or direct application of biochemically purified kinase to the cytoplasmic face of excised, inside-out membrane patches containing BKCa [60, 120–126]. For example, BKCa activation by cAMP/PKA was observed in murine renal artery [127] and rat pulmonary artery [128]. On the other hand, BKCa activation by cGMP/PKG was reported in several blood vessels [32, 129–131], and functional studies confirm that such activation can contribute to vasorelaxation. The phosphorylation of alpha-subunit of BKCa channels by PKG-I was proved in oocytes co-expressing both proteins as a mechanism regulating these channels [132], although NO has also been reported to activate BKCa channels directly [59]. White et al. reported that BKCa channels stimulation by natriuretic peptides through cGMP is due to the phosphorylation and activation of protein phosphatase 2A [133]. On the other hand, Natarajan et al. [134] suggested that in human coronary artery SMC, activation of receptors increasing cAMP mediates the stimulation of the BKCa channels via PKG [134]. These results imply that there may be cell- and tissue-specific BKCa channels that are activated by PKG, one type activated by phosphorylation and the second type activated by dephosphorylation. It should also be emphasized that the contribution of a given K+ channel type to the relaxant response depends on the vascular bed and species examined.
ATP-sensitive K+ channels
The KATP channels are thought to play an important physiological role as mediators of the response of the VSM to a variety of pharmacological and endogenous vasodilators as well as to changes in metabolic activity. A hallmark and defining feature of KATP channels is their inhibition by micromolar concentrations of intracellular ATP (Fig. 3). Presumably, any pathophysiological condition that reduces ATP mitochondrial generation would activate the KATP channels and thereby cause vasodilation. Thus, the activation of these channels has been proposed to mediate vasodilation in the coronary and cerebral circulations during hypoxia [135], ischemia [136], acidosis [137], and endotoxemia and the associated septic shock [138] and, in these disease states, may play an important role in the regulation of tissue perfusion. In contrast, the inhibition of KATP channels via the PKC pathway seems to be involved in vasoconstrictor responses to endothelin [139], vasopressin [140], and angiotensin II [141, 142]. Also, calcineurin activation seems to inhibit KATP channels through the inhibition of PKA-dependent phosphorylation of the channel [143].
Another key distinguishing feature of KATP channels is the selective inhibition of channel activity by sulphonylurea agents such as glibenclamide (Ki ≈ 20–100 nM) or tolbutamide (Ki ≈ 350 μM). The KATP channels in VSM are also responsive to many pharmacological and endogenous vasodilators that activate these channels by stimulating the formation of cAMP and increasing the activity of PKA (e.g., calcitonin gene-related peptide, vasoactive intestinal peptide, prostacyclin, adenosine, isoprenaline, forskolin) [114, 144, 145] (Fig. 3). Thus, activation of KATP channels has been shown to occur in response to calcitonin gene-related peptide (CGRP), adenosine and isoprenaline in channels of mesenteric and coronary arteries [144–148], and a variety of experimental manipulations have provided good evidence for the involvement of the cAMP/PKA pathway. KATP channel activation through cAMP/PKA has also been implicated in vasodilation induced by vasoactive intestinal peptide, prostacyclin, and pituitary adenylate cyclase-activating peptide [149, 150]. KATP currents were also activated by stimulation of adenylate cyclase with forskolin or by intracellular perfusion with cAMP, while glibenclamide-sensitive currents in response to receptor stimulation (e.g., with CGRP or adenosine) were reduced by the inhibitors of PKA Rp-cAMPS, H8 or H89, and abolished by PKA-inhibitor peptide (PKI) [150]. Further, the catalytic subunit of PKA activated KATP channels, either in whole cells or excised membrane patches [150].
There is also some evidence that activators of guanylate cyclases (NO, ANP, isosorbide dinitrate) can activate KATP channels in certain types of VSM [151–153] (Fig. 3), and glibenclamide-sensitive hyperpolarizations by the NO donor 3-morpholinosydnonimine, via cGMP/PKG pathway, have been reported in some cases [153], although it has also been suggested that it is possible that these effects may result from cross-activation of PKA by cGMP [154, 155].
In the last decade, hydrogen sulfide (H2S) generated from l-cysteine in mammalian tissues was shown to induce vessel relaxation [156]. Some studies demonstrated that H2S activates KATP channels and induce the membrane hyperpolarization in rat mesenteric artery VSM cells [157]. Other studies have demonstrated that H2S produced within the vessel wall inhibits both cGMP and cAMP breakdown by phosphodiesterases [158].
Inward rectifier K+ channels
The Kir channels regulate membrane potential in smooth muscle cells from several types of resistance arteries and may be responsible for external K+-induced dilations. The existence of currents through these channels has been reported in a variety of vascular smooth muscles, including coronary, cerebral, and mesenteric arteries, and may be preferentially expressed in small rather than large arteries [113, 150]. These Kir channels conduct K+ current into cells much more readily than they conduct outward K+ current, due to its activation by hyperpolarization rather than depolarization [113, 159]. Importantly though, Kir channels are activated by slight increases in extracellular K+. At physiological membrane potential, a small increase above the normal extracellular K+ concentration of 3–5 mM leads to an increase in the resting outward K+ current through Kir channels. Thus, a modest increase in extracellular K+, from normal levels to only 7–10 mM, as may occur during neuronal or muscle activation, can paradoxically lead to substantial vascular hyperpolarization and vasodilation due to K+ efflux through Kir channels. Selective block of Kir channels can be achieved pharmacologically by using 30–100 μM of Ba2+ and because this inhibitor causes vascular depolarization and constriction, it is thought that vascular Kir channels may be active under resting conditions and contribute to the maintenance of the resting membrane potential in resistance vessels [159]. Findings in gene-targeted mice indicate that Ba2+-sensitive K+-induced vasorelaxation is mediated by activation of the Kir2.1 channel [160]. Kir channels, like other K+ channels, may also be modulated by several vasodilators (e.g., adenosine, prostacyclin analogue cicaprost, Dendroaspis natriuretic peptide, and sodium nitroprusside) [161–165] and cAMP/PKA pathway has been reported to be involved in some Kir-mediated vasodilations [163, 164]. For example, hypoxia augments the Kir currents in rabbit coronary arterial smooth muscle cells via cAMP- and PKA-dependent signaling cascades, which might, at least partly, explain the hypoxia-induced coronary vasodilation [163]. Also, the adenosine-induced activation of Kir channels is important in coronary blood flow in vivo on rabbit coronary arterial smooth muscle and occurs via the increase in the intracellular cAMP level and the activation of PKA [164] (Fig. 3). It should, however, be noted that several Kir-mediated vasodilations seems not to be mediated by cyclic nucleotides and their dependent kinases [165] or were not yet demonstrated to be mediated by cyclic nucleotides and their dependent kinases [161, 162].
Inhibition of MLC20 phosphorylation Ca2+ sensitivity induced by cyclic nucleotides
Following increases in [Ca2+]c in response to vasoconstrictor stimuli with receptor agonists and/or membrane potential depolarization, the Ca2+-dependent activation of MLCK and its phosphorylation of MLC20 is generally considered the primary mechanism leading to myosin–actin interactions responsible for the initial development of contractile force in VSM cells (Fig. 4). However, the relation between [Ca2+]c and MLC20 phosphorylation or the sensitivity of contractile myofilaments to [Ca2+]c might vary during the subsequent development and maintenance of the contractile force, indicating that Ca2+ sensitivity of MLC20 phosphorylation or Ca2+ sensitivity of contractile myofilaments can be secondarily modulated by other signaling pathways [4].
Fig. 4.
Role of calcium in the regulation of the contractile state of VSM cells. CaM calmodulin, Ca-CaM calcium-calmodulin complex, MLCK myosin light-chain kinase, MLC 20 20-kDa regulatory light chain of myosin, P-MLC 20 phosphorylated MLC20, ROCK rho-activated protein kinase, MLCP myosin light-chain phosphatase, PKA cAMP-dependent protein kinase, PKG cGMP-dependent protein kinase, HSP20 20 kDa heat shock protein, P-HSP20 phosphorylated HSP20
MLC20 phosphorylation at serine-19 is necessary for actin activation of myosin ATPase and subsequent crossbridge cycling. Two key enzymes involved in the control of MLC20 phosphorylation are MLCK, a Ca2+/CaM-activated kinase, and MLC phosphatase (MLCP), a serine/threonine protein phosphatase type I [52]. Either Ca2+-independent activation of MLC20 phosphorylation or inhibition of MLC20 dephosphorylation causes an increase in the level of MLC20 phosphorylation for a given increase in Ca2+ signal, and thereby increases the Ca2+ sensitivity of MLC20 phosphorylation. Cyclic nucleotides can reduce the Ca2+ sensitivity of MLC20 phosphorylation, causing vasorelaxation, via the two mentioned pathways: (1) MLCK phosphorylation and thereby decreasing its affinity for the Ca2+–CaM complex, resulting in a decrease of MLC20 phosphorylation, and (2) upregulation of myosin phosphatase and the resultant accelerated MLC20 dephosphorylation.
Inhibition of myosin light-chain kinase activity
The activity of MLCK is primarily regulated by the Ca2+–CaM complex. However, the phosphorylation of the MLCK at a specific serine residue in the region of its CaM-binding domain (i.e., site A) decreases its affinity for the Ca2+–CaM complex and, hence, its phosphorylating activity [166]. It has been proposed that the increase in cAMP levels reduces myofilament Ca2+ sensitivity by phosphorylating MLCK and thereby decreasing its affinity for the Ca2+–CaM complex [4, 167]. In fact, in vitro experiments has demonstrated that PKA phosphorylates and markedly decreases the activity of MLCK, leading to the supposition that cAMP-dependent phosphorylation of MLCK could potentially decrease the [Ca2+]c sensitivity of MLC20 phosphorylation in vivo [4, 167] (Fig. 4). Although cross-activation of PKA by cGMP may also be involved in vasodilation by this cyclic nucleotide, through inhibition of MLCK activity, there has been no firm evidence that PKG-dependent phosphorylation of MLCK inhibits its activity [52, 168]. It should, however, be noted that although PKA can phosphorylate MLCK and reduce its activity in vitro [167], phosphorylation of MLCK in vivo appears to be primarily due to CaMKII, which may play a physiological role in downregulating the Ca2+ signal by decreasing the Ca2+ sensitivity of MLC20 phosphorylation [3, 166].
Increase of the myosin light-chain phosphatase (MLCP) activity
Most of the recent work suggests that PKG activates MLCP, thereby inhibiting MLC20 phosphorylation and contraction [52, 169–171] (Fig. 4). The mechanism of cGMP-dependent activation of MLCP may involve direct phosphorylation of the regulatory subunit (MYPT-1) of MLCP at Ser 695 (numbering of the human isoform) [3, 170]. Several recent studies demonstrated that phosphorylation of Ser-695 by PKA/PKG prevents phosphorylation of MYPT-1 at Thr-696 by several kinases and therefore inhibition of MLCP [170, 172, 173]. Thus, phosphorylation of MYPT-1 by PKA/PKG increases MLCP activity resulting in the decrease in MLC20 phosphorylation and smooth muscle relaxation [3, 170–172]. It is worth mentioning that Nakamura et al. reported that Thr-696 phosphorylation decreased by only 50% with 8-Br-cGMP stimulation, whereas the tension of phenylephrine-precontracted arteries and regulatory light-chain phosphorylation fell to baseline, presumably indicating that Ser-695 phosphorylation is not the sole explanation of PKG-induced Ca2+ desensitization. In fact, activation of PKG can mediate other processes resulting in changes in contractility, such as PKG phosphorylation of telokin (or other similar mediator proteins possibly present in VSM, like the calponin homology-associated smooth muscle protein [CHASM]), which activates MLCP activity, independently of changes in MYPT1 phosphorylation, through an unknown mechanism [3, 174–176]. On the other hand, using yeast two-hybrid system screening, it was also shown that PKG is targeted to the smooth muscle cell contractile apparatus by a leucine zipper interaction with the myosin-binding subunit (MBS) of MLCP. MBS is the regulatory subunit of MLCP that confers the specificity for MLC and is the site of regulation by Rho-kinase. Uncoupling of the PKG–MBS interaction prevents cGMP-dependent dephosphorylation of MLC, demonstrating that this interaction is essential to the regulation of vascular smooth muscle cell tone [169].
It has also been reported that cGMP-induced inhibition of Ca2+ sensitization can be directly mediated by RhoA/Rho-kinase signaling [54, 176, 177]. Rho-Kinase is essentially distributed in the cytoplasm but is partially translocated to the membrane following activation by RhoA, a member of the Ras family of small GTP-binding proteins. Myosin light-chain phosphatase activity is controlled by the small GTPase RhoA and its target Rho kinase (ROCK). ROCK is believed to be the most important modulator of Ca2+ sensitivity in smooth muscle [178–180]. Phosphorylation of myosin-binding subunit by ROCKs leads to the inhibition of MLCP, which prevents MLC dephosphorylation and hence increases Ca2+ sensitivity and smooth muscle contractility [178]. In addition, ROCK targets other substrates that are important for smooth muscle contraction such as CPI-17, calponin, or MLC [179, 181]. It was shown that PKG-mediated phosphorylation of RhoA at Ser-188 prevents its binding to ROCK and this phosphorylation inhibits RhoA-induced Ca2+ sensitization and actin cytoskeleton organization contributing to the vasodilator action [177, 181, 182].
Dissociation of contractile force from MLC20 phosphorylation or thin filament regulation by cyclic nucleotides
A number of studies have shown that cyclic nucleotides signaling pathways uncouple contractile force from MLC phosphorylation. The dephosphorylation of MLC20 is slower than the decrease in active tension in forskolin-treated muscle [1]. There are data indicating that increases in intracellular cyclic nucleotides concentrations can lead to relaxation of tonic smooth muscle by mechanisms independent of changes in intracellular calcium concentrations or in the state of MLC20 phosphorylation. In carotid artery, activation of cyclic nucleotides-dependent signaling pathways inhibited contractile responses to serotonin but did not inhibit myosin light-chain phosphorylation or oxygen consumption [183]. This cyclic nucleotide-dependent inhibition of contraction was associated with increases in the small 20-kDa heat shock protein (HSP20) phosphorylation [183]. The hypothesized mechanisms for smooth muscle relaxation by HSP20 are related with agents that inhibit contraction by cyclic nucleotides which, through activation of PKA and/or PKG, phosphorylate HSP20 (Fig. 4). This phosphorylation induces relaxation of smooth muscle which, in some cases, is independent of changes in MLC phosphorylation. Two proposed mechanisms for relaxation are [1, 183–185]:
(a) Depolymerization of actin via an indirect activation of cofilin involving activation of slingshot phosphatase. Phosphorylated HSP20 is thought to compete with slingshot for binding to 14-3-3 proteins. Unbound slingshot keeps cofilin active and favors actin depolymerization;
(b) HSP20 has also been proposed to interact with and inhibit actomyosin by virtue of a troponin I motif.
In fact, there are several studies indicating that relaxation and inhibition of contraction by cyclic nucleotides are associated with increases in the phosphorylation of the small heat shock-related protein HSP20 at Ser16 by PKA and PKG [183, 184, 186–189]. For example, examination of the extent of HSP20 phosphorylation compared with the percent relaxation in bovine carotid and human umbilical artery (HUA) smooth muscle reveal a linear correlation between HSP20 phosphorylation and relaxation [1]. The slower phosphorylation of HSP20 in HUA smooth muscle cells corresponds with a much slower relaxation response. The data suggests that HSP20 may be a downstream signaling molecule that modulates relaxation due to cyclic nucleotides [1]. Although the precise mechanisms of HSP20-induced relaxation are not known, some authors hypothesized that when HSP20 becomes phosphorylated, it interacts with F-actin or F-actin-associated proteins preventing phosphorylated myosin from interacting with actin [1, 190]. Thus HSP20 may mediate relaxation via a thin-filament regulatory process (Fig. 4) [191]. In fact, studies using inhibitory peptides for HSP20 indicate that this protein mediates relaxation primarily through interactions with thin filaments [52, 192].
A second possibility is that activation of cyclic nucleotides signaling pathways and the resulting HSP20 phosphorylation dissociates the contractile apparatus from specific focal contacts, such as dense-bodies and/or dense-plaques, which provide a framework for the attachment of contractile structures to the cytoskeleton [193, 194]. Such a mechanism would result in normal crossbridge phosphorylation, crossbridge cycling, and oxygen consumption. Indeed, activation of cyclic nucleotides signaling pathways have been associated with disruption of focal adhesion complexes in rat aortic smooth muscle cells [195].
Compartmentalization of cyclic nucleotide signals
The different localization, properties, and selectivity of the enzymes involved in the cyclic nucleotide pathways can lead to the existence of clusters or subcellular compartments of these nucleotides [62, 63]. These clusters can be localized close to different effectors, leading to different and specific functional responses depending on the compartment [196, 197]. Thus, the multiple components of the cyclic nucleotide pathways, such as cyclases, PDE, PKA, and PKG, co-localized to discrete regions of the plasma membrane or to specific locations in the cytosol and make it possible to limit these signals to specific subcellular regions [33, 198]. These localized cyclic nucleotide signals are important for the speed and specificity of cAMP and cGMP-mediated events, allowing the cell to distinguish between different external stimuli acting on a common signaling pathway. Thus, subcellular cyclic nucleotides localizations will be responsible for specific compartmentalized regulation of precise cellular functions and cAMP and cGMP, in response to different stimuli, could orchestrate a wide variety of cellular responses. This is a crucial point because, in distinct cell types or tissues, the compartmentalization can be different, depending on the expression and localization of proteins involved in the cyclic nucleotide pathways and this could explain the different results obtained that seems to depend on the species and artery types observed in the literature.
The first propositions suggesting a heart compartmentalization in the cAMP signaling were made more than 25 years ago [199] and some posterior studies tried to shed some light on this phenomenon [197]. At the cardiovascular level, most evidence on cyclic nucleotide compartmentalization was obtained in cardiomyocytes [196, 197, 200], although some facts concerning this compartmentalization were also observed in VSM cells.
The existence of multiple PDE isoforms was associated with the regulation by cyclic nucleotide pools of multiple cellular processes. Several studies associated PDE families (PDE1, PDE2-5) with the regulation of VSM contractility [33], although the relative contribution of each PDE may vary depending upon the species, vascular bed, and the status of the cells [62, 201]. In rat aorta, PDE1A is expressed predominantly in the cytoplasm of VSM cells and regulates the vascular tone [202, 203]. However, in VSM cells, there is also a nuclear PDE1A with an important role in growth and survival [203]. On the other hand, some authors have suggested that PDE5 differentially regulates the effects of NO donors (DEA/NO, DETA/NO) and natriuretic peptides in VSM cells of rat aorta in VSM cells of rat aorta [204, 205]. These authors observed the existence of subcellular compartments of cGMP and that NO donors induce transitory increase of cGMP, which are dependent on the PDE5 activity.
Concerning the localization of cyclases, Piggott et al. [206] demonstrated that cGMP signals elicited by ANP and NO donors (S-nitroso-n-acetylpenicillamine; SNAP) are spatially segregated, and this may underlie the regulation of different cellular targets by those compounds. Gros et al. [207] also suggested that an association between ERK and adenylate cyclase 1 in discrete “functional compartments” result in isoform-selective regulation of rat VSM cell growth versus cytoskeletal organization. In human umbilical artery VSM, vasorelaxation occurs after pGC or sGC activation, but only the activation of pGC induces stimulation of Kv and BKCa channels [32].
The PKA anchoring proteins (AKAPs, A-kinase anchoring proteins) provided a molecular mechanism for cAMP compartmentalization, allowing for the precise control of PKA-mediated phosphorylation events because different PKA isoforms are anchored by AKAPs in specific cellular locations [208]. AKAPs can be also clustering elements of other proteins such as PDE, phosphatases, substrates of PKA, and others [208, 209]. In VSM cells from rabbit portal vein, Zhong and coworkers [210] have shown that cAMP-dependent stimulation of L-type Ca2+ channels requires localization of PKA through binding to anchoring proteins, although the specific subtypes of AKAPs involved in this process remain unclear. Likewise, evidence for the involvement of an AKAP in activation of rat mesenteric artery KATP channels by PKA has also been provided [211]. On the other hand, AKAP75 (a prototype AKAP that targets PKA to the membrane) has been identified in VSM cells and its expression amplifies and stimulates cAMP-PKA signaling, resulting in activation of cAMP-induced transcription, increased levels of the cyclin-dependent kinase-2 inhibitor p27kip1, and suppression both of VSM cells growth in vitro and of neointimal hyperplasia after balloon injury [212].
Another aspect of compartmentalization involves caveolae, which are flask-like invaginated structures of the plasma membrane. These cellular micro-domains constitute a subset of lipid rafts and are enriched in cholesterol and sphingolipids as well as in specific proteins such as the caveolae coat proteins, caveolins. The caveolin proteins serve as the structural components of caveolae, while also functioning as scaffolding proteins capable of recruiting numerous signaling molecules to caveolae, as well as regulating their activity [213]. Examples of signal transduction proteins involved in the cyclic nucleotide pathways that localize in caveolae and/or interact with caveolin in VSM cells are PKA [214] and several types of adenylate cyclase (AC3, AC5, and AC6) [63]. The localization of these proteins to restricted plasmalemmal microdomains probably facilitates rapid and specific signal transduction [63, 215]. Sampson et al. revealed that the co-localization of KATP channels and adenylate cyclase in rat aorta smooth muscle caveolae has functional significance, demonstrating that the integrity of caveolae is important for adenylate cyclase-mediated KATP channels modulation [214]. In VSM cells, evidence of a role of caveolin-1 in artery contractility comes from caveolin-1 null mice that display reduced myogenic tone [216]. Mutant animals displayed profound dysfunction of the vascular system, pulmonary hypertension, and increased systemic NO levels, which can induce adverse effects to the myocardium [217, 218]. Caveolae integrity also seems to be necessary for the efficient Ca2+ signaling in VSM cells [219]. Concerning the endothelium, in mice deficient in caveolin-1, some cGMP-dependent responses are largely enhanced and not reduced, illustrating a lack of relevance of caveolae for eNOS localization and activation [216, 218]. Caveolae thus appear to constitute an important signaling domain that plays a role not only in regulation of smooth muscle tone but also in proliferation, such as seen in neointima formation and atherosclerosis [220].
Summary
Myoplasmatic calcium concentration changes are among the principal mechanisms involved in the regulation of the contractile state of VSM cells. In general, an increase or decrease in [Ca2+]c result in VSM contraction and relaxation, respectively, although changes in the vascular tone can occur independently of changes in [Ca2+]c. Most contractile stimuli induce vasoconstriction by increasing [Ca2+]c, through Ca2+ mobilization from either intracellular stores (i.e., SR) and/or extracellular space. The relative contribution from these two Ca2+ pools varies with different VSM cells and stimuli. The increase in [Ca2+]c, in turn, activates the Ca2+–CaM–MLCK pathway and stimulates MLC20 phosphorylation on serine 19 and activates ATPase activity of myosin, leading to myosin–actin interactions and, hence, the development of contractile force. This is the most widely accepted primary mechanism for the development of contractile force in VSM cells. There are, however, several regulatory mechanisms modulating Ca2+ mobilization, Ca2+ sensitivity of MLC20 phosphorylation, and Ca2+ sensitivity of contractile myofilaments that secondarily determine the contractile response to receptor agonists. Among these regulatory mechanisms involved in the physiological regulation of vascular tone are the cyclic nucleotides (i.e., cAMP and cGMP). An increase in the cytosolic level of cAMP or cGMP in VSM cells is considered one of the major mechanisms that mediate vasodilation under physiological conditions. Several (at least four distinct) mechanisms are currently thought to be involved in the vasodilator effect of cyclic nucleotides: (1) the decrease in intracellular calcium levels (e.g., through activation of Ca2+ uptake by the SR, increased intracellular Ca2+ efflux, inhibition of Ca2+ release from the SR and/or decreased extracellular Ca2+ influx), (2) the hyperpolarization of the smooth muscle cell membrane potential (e.g., through activation of outward potassium channels, inactivation of Na+ channels and/or inactivation of multiple channels), (3) the reduction in the sensitivity of the contractile machinery by decreasing the [Ca2+]c sensitivity of MLC20 phosphorylation (due to a decrease of the myosin light-chain kinase activity and/or to an increase of the MLCP activity), and (4) the reduction in the sensitivity of the contractile machinery by uncoupling contraction from MLC20 phosphorylation (e.g., via a thin-filament regulatory process). The contribution of each of these mechanisms and of each cyclic nucleotide depends on VSM type, species, and contractile stimulus, and was suggested to be determined by the relative expression and/or co-localization of different proteins in each vascular bed. It is likely that no single pathway acts exclusively or independently in any one type of smooth muscle cell. On the other hand, the spatial organization of the multiple components of the cyclic nucleotide pathways restricts cyclic nucleotide signals to discrete regions of the plasma membrane or to specific locations in the cytosol and make it possible to limit these signals to specific subcellular regions. This compartmentalization of cyclic nucleotide signals will be responsible for the regulation of specific cellular functions so that cAMP and cGMP, in response to different stimuli, can activate a wide variety of cellular responses.
A better understanding of the cellular and molecular mechanisms involved in cyclic nucleotide-dependent vasorelaxation would help the development of strategies for eliciting a variety of beneficial pharmacological effects on several cardiovascular pathological conditions, given that the cytosolic levels of cAMP and cGMP may be pharmacologically increased with PDE inhibitors and/or adenylate/guanylate cyclase activators.
Acknowledgements
We thank the FCT (Fundação para a Ciência e a Tecnologia) for supporting the fellowship grant SFRH/BD/36756/2007.
References
- 1.Woodrum DA, Brophy CM. The paradox of smooth muscle physiology. Mol Cell Endocrinol. 2001;177(1–2):135–143. doi: 10.1016/S0303-7207(01)00407-5. [DOI] [PubMed] [Google Scholar]
- 2.Hirano K, Hirano M, Kanaide H. Regulation of myosin phosphorylation and myofilament Ca2+ sensitivity in vascular smooth muscle. J Smooth Muscle Res. 2004;40(6):219–236. doi: 10.1540/jsmr.40.219. [DOI] [PubMed] [Google Scholar]
- 3.Ihara E, MacDonald JA. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can J Physiol Pharmacol. 2007;85(1):79–87. doi: 10.1139/y06-103. [DOI] [PubMed] [Google Scholar]
- 4.Rembold CM. Regulation of contraction and relaxation in arterial smooth muscle. Hypertension. 1992;20(2):129–137. doi: 10.1161/01.hyp.20.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998;164(4):437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 6.Orallo F. Regulation of cytosolic calcium levels in vascular smooth muscle. Pharmacol Ther. 1996;69:153–171. doi: 10.1016/0163-7258(95)02042-X. [DOI] [PubMed] [Google Scholar]
- 7.Marin J, Encabo A, Briones A, Garcia-Cohen EC, Alonso MJ. Mechanisms involved in the cellular calcium homeostasis in vascular smooth muscle: calcium pumps. Life Sci. 1999;64(5):279–303. doi: 10.1016/S0024-3205(98)00393-2. [DOI] [PubMed] [Google Scholar]
- 8.Hirano K, Derkach DN, Hirano M, Nishimura J, Kanaide H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol Cell Biochem. 2003;248(1–2):105–114. doi: 10.1023/A:1024180101032. [DOI] [PubMed] [Google Scholar]
- 9.Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 1: basic mechanisms controlling cytosolic Ca2+ concentration and the Ca2+-dependent regulation of vascular tone. J Anesth. 2007;21(2):220–231. doi: 10.1007/s00540-006-0487-5. [DOI] [PubMed] [Google Scholar]
- 10.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74(2):226–232. doi: 10.1253/circj.CJ-09-0879. [DOI] [PubMed] [Google Scholar]
- 11.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459(6):897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 12.Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci USA. 2002;99(9):6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaytor AT, Taylor HJ, Griffith TM. Gap junction-dependent and -independent EDHF-type relaxations may involve smooth muscle cAMP accumulation. Am J Physiol Heart Circ Physiol. 2002;282(4):H1548–H1555. doi: 10.1152/ajpheart.00903.2001. [DOI] [PubMed] [Google Scholar]
- 14.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87(3):965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 16.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 17.Cary SP, Winger JA, Derbyshire ER, Marletta MA. Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci. 2006;31(4):231–239. doi: 10.1016/j.tibs.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92(5):1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ushiyama M, Morita T, Katayama S. Carbon monoxide regulates blood pressure cooperatively with nitric oxide in hypertensive rats. Heart Vessels. 2002;16(5):189–195. doi: 10.1007/s003800200020. [DOI] [PubMed] [Google Scholar]
- 20.Peers C, Steele DS (2011) Carbon monoxide: a vital signalling molecule and potent toxin in the myocardium. J Mol Cell Cardiol (in press) [DOI] [PubMed]
- 21.Polizio AH, Santa-Cruz DM, Balestrasse KB, Gironacci MM, Bertera FM, Hocht C, Taira CA, Tomaro ML, Gorzalczany SB. Heme oxygenase-1 overexpression fails to attenuate hypertension when the nitric oxide synthase system is not fully operative. Pharmacology. 2011;87(5–6):341–349. doi: 10.1159/000327939. [DOI] [PubMed] [Google Scholar]
- 22.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28(2):110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res. 2011;63(1):13–22. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109(5):1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98(1):390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 26.Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation—role of guanosine 3’, 5’-cyclic monophosphate. Am J Physiol. 1991;261:L393–L398. doi: 10.1152/ajplung.1991.261.6.L393. [DOI] [PubMed] [Google Scholar]
- 27.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317(5843):1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 28.Neo BH, Kandhi S, Wolin MS. Roles for soluble guanylate cyclase and a thiol oxidation-elicited subunit dimerization of protein kinase G in pulmonary artery relaxation to hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2010;299(4):H1235–H1241. doi: 10.1152/ajpheart.00513.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain MB, MacAllister RJ, Hobbs AJ. Reciprocal regulation of cGMP-mediated vasorelaxation by soluble and particulate guanylate cyclases. Am J Physiol Heart Circ Physiol. 2001;280(3):H1151–H1159. doi: 10.1152/ajpheart.2001.280.3.H1151. [DOI] [PubMed] [Google Scholar]
- 30.Madhani M, Scotland RS, MacAllister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol. 2003;139(7):1289–1296. doi: 10.1038/sj.bjp.0705365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madhani M, Okorie M, Hobbs AJ, MacAllister RJ. Reciprocal regulation of human soluble and particulate guanylate cyclases in vivo. Br J Pharmacol. 2006;149(6):797–801. doi: 10.1038/sj.bjp.0706920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cairrao E, Santos-Silva AJ, Verde I. PKG is involved in testosterone-induced vasorelaxation of human umbilical artery. Eur J Pharmacol. 2010;640:94–101. doi: 10.1016/j.ejphar.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109(3):366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100(3):309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 35.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332(6036):1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 36.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(Pt 3):437–443. doi: 10.1042/0264-6021:3450437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278(31):28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson DS, Summers RJ, Bengtsson T. Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol Ther. 2008;119(3):291–310. doi: 10.1016/j.pharmthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86(1):1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA, Reed RB, Francis SH, Grimes K, Corbin JD. Distinguishing the roles of the two different cGMP-binding sites for modulating phosphorylation of exogenous substrate (heterophosphorylation) and autophosphorylation of cGMP-dependent protein kinase. J Biol Chem. 2000;275(1):154–158. doi: 10.1074/jbc.275.1.154. [DOI] [PubMed] [Google Scholar]
- 41.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99(8):816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 42.Lincoln TM, Cornwell TL, Taylor AE. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am J Physiol. 1990;258((3 Pt 1)):C399–C407. doi: 10.1152/ajpcell.1990.258.3.C399. [DOI] [PubMed] [Google Scholar]
- 43.Arejian M, Li Y, Anand-Srivastava MB. Nitric oxide attenuates the expression of natriuretic peptide receptor C and associated adenylyl cyclase signaling in aortic vascular smooth muscle cells: role of MAPK. Am J Physiol Heart Circ Physiol. 2009;296(6):H1859–H1867. doi: 10.1152/ajpheart.01108.2008. [DOI] [PubMed] [Google Scholar]
- 44.Skalhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, Burton KA. Mutation of the Calpha subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol. 2002;16(3):630–639. doi: 10.1210/me.16.3.630. [DOI] [PubMed] [Google Scholar]
- 45.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17(11):3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F, Feil R. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res. 2007;101(11):1096–1103. doi: 10.1161/CIRCRESAHA.107.154351. [DOI] [PubMed] [Google Scholar]
- 47.Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, Brummer S, Kuhbandner S, Hofmann F. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res. 2002;90(10):1080–1086. doi: 10.1161/01.RES.0000019586.95768.40. [DOI] [PubMed] [Google Scholar]
- 48.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 49.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4-6):345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 50.Metrich M, Berthouze M, Morel E, Crozatier B, Gomez AM, Lezoualc’h F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 2010;459(4):535–546. doi: 10.1007/s00424-009-0747-y. [DOI] [PubMed] [Google Scholar]
- 51.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49(2):157–230. [PubMed] [Google Scholar]
- 52.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91(3):1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 53.Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J Anesth. 2007;21(2):232–242. doi: 10.1007/s00540-006-0488-4. [DOI] [PubMed] [Google Scholar]
- 54.Cogolludo A, Moreno L, Villamor E. Mechanisms controlling vascular tone in pulmonary arterial hypertension: implications for vasodilator therapy. Pharmacology. 2007;79(2):65–75. doi: 10.1159/000097754. [DOI] [PubMed] [Google Scholar]
- 55.Martel G, Hamet P, Tremblay J. Central role of guanylyl cyclase in natriuretic peptide signaling in hypertension and metabolic syndrome. Mol Cell Biochem. 2010;334(1–2):53–65. doi: 10.1007/s11010-009-0326-8. [DOI] [PubMed] [Google Scholar]
- 56.Vaandrager AB, de Jonge HR. Signalling by cGMP-dependent protein kinases. Mol Cell Biochem. 1996;157(1–2):23–30. doi: 10.1007/BF00227877. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka Y, Tang G, Takizawa K, Otsuka K, Eghbali M, Song M, Nishimaru K, Shigenobu K, Koike K, Stefani E, Toro L. Kv channels contribute to nitric oxide- and atrial natriuretic peptide-induced relaxation of a rat conduit artery. J Pharmacol Exp Ther. 2006;317(1):341–354. doi: 10.1124/jpet.105.096115. [DOI] [PubMed] [Google Scholar]
- 58.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res. 1999;84(2):210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- 59.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 60.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265(1 Pt 1):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 61.Dong H, Waldron GJ, Cole WC, Triggle CR. Roles of calcium-activated and voltage-gated delayed rectifier potassium channels in endothelium-dependent vasorelaxation of the rabbit middle cerebral artery. Br J Pharmacol. 1998;123(5):821–832. doi: 10.1038/sj.bjp.0701680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93(4):280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 63.Ostrom RS, Liu X, Head BP, Gregorian C, Seasholtz TM, Insel PA. Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol Pharmacol. 2002;62(5):983–992. doi: 10.1124/mol.62.5.983. [DOI] [PubMed] [Google Scholar]
- 64.Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1, 4, 5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem. 1994;269(12):8701–8707. [PubMed] [Google Scholar]
- 65.Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1, 4, 5-trisphosphate receptor—cyclic GMP-dependent protein kinase mediates cAMP and cGMP dependent phosphorylation in the intact rat aorta. J Biol Chem. 1996;271:21933–21938. doi: 10.1074/jbc.271.36.21933. [DOI] [PubMed] [Google Scholar]
- 66.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404(6774):197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- 67.Casteel DE, Boss GR, Pilz RB. Identification of the interface between cGMP-dependent protein kinase Ibeta and its interaction partners TFII-I and IRAG reveals a common interaction motif. J Biol Chem. 2005;280(46):38211–38218. doi: 10.1074/jbc.M507021200. [DOI] [PubMed] [Google Scholar]
- 68.Geiselhoringer A, Werner M, Sigl K, Smital P, Worner R, Acheo L, Stieber J, Weinmeister P, Feil R, Feil S, Wegener J, Hofmann F, Schlossmann J. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J. 2004;23(21):4222–4231. doi: 10.1038/sj.emboj.7600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fritsch RM, Saur D, Kurjak M, Oesterle D, Schlossmann J, Geiselhoringer A, Hofmann F, Allescher HD. InsP3R-associated cGMP kinase substrate (IRAG) is essential for nitric oxide-induced inhibition of calcium signaling in human colonic smooth muscle. J Biol Chem. 2004;279(13):12551–12559. doi: 10.1074/jbc.M313365200. [DOI] [PubMed] [Google Scholar]
- 70.Geiselhoringer A, Gaisa M, Hofmann F, Schlossmann J. Distribution of IRAG and cGKI-isoforms in murine tissues. FEBS Lett. 2004;575(1–3):19–22. doi: 10.1016/j.febslet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 71.Casteel DE, Zhang T, Zhuang S, Pilz RB. cGMP-dependent protein kinase anchoring by IRAG regulates its nuclear translocation and transcriptional activity. Cell Signal. 2008;20(7):1392–1399. doi: 10.1016/j.cellsig.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desch M, Sigl K, Hieke B, Salb K, Kees F, Bernhard D, Jochim A, Spiessberger B, Hocherl K, Feil R, Feil S, Lukowski R, Wegener JW, Hofmann F, Schlossmann J. IRAG determines nitric oxide- and atrial natriuretic peptide-mediated smooth muscle relaxation. Cardiovasc Res. 2011;86(3):496–505. doi: 10.1093/cvr/cvq008. [DOI] [PubMed] [Google Scholar]
- 73.Rapoport RM. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- 74.Hirata M, Kohse KP, Chang CH, Ikebe T, Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990;265(3):1268–1273. [PubMed] [Google Scholar]
- 75.Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol. 1991;40(6):923–931. [PubMed] [Google Scholar]
- 76.Lincoln TM, Cornwell TL. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991;28(1–3):129–137. doi: 10.1159/000158852. [DOI] [PubMed] [Google Scholar]
- 77.Mundina-Weilenmann C, Vittone L, Rinaldi G, Said M, de Cingolani GC, Mattiazzi A. Endoplasmic reticulum contribution to the relaxant effect of cGMP- and cAMP-elevating agents in feline aorta. Am J Physiol Heart Circ Physiol. 2000;278(6):H1856–H1865. doi: 10.1152/ajpheart.2000.278.6.H1856. [DOI] [PubMed] [Google Scholar]
- 78.Gibson A, McFadzean I, Wallace P, Wayman CP. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol Sci. 1998;19(7):266–269. doi: 10.1016/S0165-6147(98)01222-X. [DOI] [PubMed] [Google Scholar]
- 79.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35(1 Pt 2):173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brueggemann LI, Martin BL, Barakat J, Byron KL, Cribbs LL. Low voltage-activated calcium channels in vascular smooth muscle: T-type channels and AVP-stimulated calcium spiking. Am J Physiol Heart Circ Physiol. 2005;288(2):H923–H935. doi: 10.1152/ajpheart.01126.2003. [DOI] [PubMed] [Google Scholar]
- 81.Putney JW, Jr, McKay RR. Capacitative calcium entry channels. BioEssays. 1999;21(1):38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 82.Ross K, Whitaker M, Reynolds NJ. Agonist-induced calcium entry correlates with STIM1 translocation. J Cell Physiol. 2007;211(3):569–576. doi: 10.1002/jcp.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 84.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6(2):113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 85.Bolotina VM, Csutora P. CIF and other mysteries of the store-operated Ca2+ -entry pathway. Trends Biochem Sci. 2005;30(7):378–387. doi: 10.1016/j.tibs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 86.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174(6):815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006;112(3):744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 88.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8(9):1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 89.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281(38):28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 90.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C18–C33. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 91.Hughes AD. Calcium channels in vascular smooth muscle cells. J Vasc Res. 1995;32(6):353–370. doi: 10.1159/000159111. [DOI] [PubMed] [Google Scholar]
- 92.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003;22(22):6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 94.Xiong Z, Sperelakis N. Regulation of L-type calcium channels of vascular smooth muscle cells. J Mol Cell Cardiol. 1995;27(1):75–91. doi: 10.1016/S0022-2828(08)80009-0. [DOI] [PubMed] [Google Scholar]
- 95.Liu H, Xiong Z, Sperelakis N. Cyclic nucleotides regulate the activity of L-type calcium channels in smooth muscle cells from rat portal vein. J Mol Cell Cardiol. 1997;29(5):1411–1421. doi: 10.1006/jmcc.1997.0379. [DOI] [PubMed] [Google Scholar]
- 96.Taguchi K, Ueda M, Kubo T. Effects of cAMP and cGMP on L-type calcium channel currents in rat mesenteric artery cells. Jpn J Pharmacol. 1997;74(2):179–186. doi: 10.1254/jjp.74.179. [DOI] [PubMed] [Google Scholar]
- 97.Ishikawa T, Hume JR, Keef KD. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res. 1993;73(6):1128–1137. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- 98.Ruiz-Velasco V, Zhong J, Hume JR, Keef KD. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ Res. 1998;82(5):557–565. doi: 10.1161/01.res.82.5.557. [DOI] [PubMed] [Google Scholar]
- 99.Xiong Z, Sperelakis N, Fenoglio-Preiser C. Regulation of L-type calcium channels by cyclic nucleotides and phosphorylation in smooth muscle cells from rabbit portal vein. J Vasc Res. 1994;31(5):271–279. doi: 10.1159/000159053. [DOI] [PubMed] [Google Scholar]
- 100.Lorenz JN, Bielefeld DR, Sperelakis N. Regulation of calcium channel current in A7r5 vascular smooth muscle cells by cyclic nucleotides. Am J Physiol. 1994;266(6 Pt 1):C1656–C1663. doi: 10.1152/ajpcell.1994.266.6.C1656. [DOI] [PubMed] [Google Scholar]
- 101.Satoh H, Sperelakis N. Modulation of L-type Ca2+ current by isoprenaline, carbachol and phorbol ester in cultured rat aortic vascular smooth muscle (A7r5) cells. Gen Pharmacol. 1995;26(2):369–379. doi: 10.1016/0306-3623(94)00193-Q. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez JM, Jost LJ, Rouse D, Suki WN. Plasma membrane and sarcoplasmic reticulum Calcium-ATPase and smooth muscle. Miner Electrolyte Metab. 1996;22:345–348. [PubMed] [Google Scholar]
- 103.Kobayashi S, Kanaide H, Nakamura M. Cytosolic-free calcium transients in cultured vascular smooth muscle cells: microfluorometric measurements. Science. 1985;229(4713):553–556. doi: 10.1126/science.3927484. [DOI] [PubMed] [Google Scholar]
- 104.Rashatwar SS, Cornwell TL, Lincoln TM. Effects of 8-bromo-cGMP on Ca2+ levels in vascular smooth muscle cells: possible regulation of Ca2+-ATPase by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1987;84(16):5685–5689. doi: 10.1073/pnas.84.16.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Popescu LM, Panoiu C, Hinescu M, Nutu O. The mechanism of cGMP-induced relaxation in vascular smooth muscle. Eur J Pharmacol. 1985;107(3):393–394. doi: 10.1016/0014-2999(85)90269-9. [DOI] [PubMed] [Google Scholar]
- 106.Furukawa K, Nakamura H. Cyclic GMP regulation of the plasma membrane (Ca2+-Mg2 +)ATPase in vascular smooth muscle. J Biochem. 1987;101(1):287–290. doi: 10.1093/oxfordjournals.jbchem.a121904. [DOI] [PubMed] [Google Scholar]
- 107.Vrolix M, Raeymaekers L, Wuytack F, Hofmann F, Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matthew A, Shmygol A, Wray S. Ca2+ entry, efflux and release in smooth muscle. Biol Res. 2004;37(4):617–624. doi: 10.4067/S0716-97602004000400017. [DOI] [PubMed] [Google Scholar]
- 109.Karashima E, Nishimura J, Iwamoto T, Hirano K, Hirano M, Kita S, Harada M, Kanaide H. Involvement of Na + -Ca2+ exchanger in cAMP-mediated relaxation in mice aorta: evaluation using transgenic mice. Br J Pharmacol. 2007;150(4):434–444. doi: 10.1038/sj.bjp.0707119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furukawa K, Ohshima N, Tawada-Iwata Y, Shigekawa M. Cyclic GMP stimulates Na +/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem. 1991;266(19):12337–12341. [PubMed] [Google Scholar]
- 111.Aaronson PI, Benham CD. Alterations in [Ca2+]i mediated by sodium-calcium exchange in smooth muscle cells isolated from the guinea-pig ureter. J Physiol. 1989;416:1–18. doi: 10.1113/jphysiol.1989.sp017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Furukawa K, Tawada Y, Shigekawa M. Regulation of the plasma membrane Ca2+ pump by cyclic nucleotides in cultured vascular smooth muscle cells. J Biol Chem. 1988;263(17):8058–8065. [PubMed] [Google Scholar]
- 113.Brayden JE. Potassium channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 1996;23:1069–1076. doi: 10.1111/j.1440-1681.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 114.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44(2):65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 115.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268(2 Pt 2):H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 116.Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol. 1998;275(2 Pt 2):H448–H459. doi: 10.1152/ajpheart.1998.275.2.H448. [DOI] [PubMed] [Google Scholar]
- 117.Sathishkumar K, Ross RG, Bawankule DU, Sardar KK, Prakash VR, Mishra SK. Segmental heterogeneity in the mechanism of sodium nitroprusside-induced relaxation in ovine pulmonary artery. J Cardiovasc Pharmacol. 2005;45(6):491–498. doi: 10.1097/01.fjc.0000159043.50488.ac. [DOI] [PubMed] [Google Scholar]
- 118.Palen DI, Belmadani S, Lucchesi PA, Matrougui K. Role of SHP-1, Kv.1.2, and cGMP in nitric oxide-induced ERK1/2 MAP kinase dephosphorylation in rat vascular smooth muscle cells. Cardiovasc Res. 2005;68(2):268–277. doi: 10.1016/j.cardiores.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 119.Rusch NJ, Liu Y, Pleyte KA. Mechanisms for regulation of arterial tone by Ca2+ -dependent K + channels in hypertension. Clin Exp Pharmacol Physiol. 1996;23(12):1077–1081. doi: 10.1111/j.1440-1681.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 120.Taniguchi J, Furukawa KI, Shigekawa M. Maxi K+ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflugers Arch. 1993;423(3–4):167–172. doi: 10.1007/BF00374390. [DOI] [PubMed] [Google Scholar]
- 121.Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca(2+)-dependent K+ channels. Circ Res. 1995;76(6):1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- 122.Schubert R, Serebryakov VN, Engel H, Hopp HH. Iloprost activates KCa channels of vascular smooth muscle cells: role of cAMP-dependent protein kinase. Am J Physiol. 1996;271:C1203–C1211. doi: 10.1152/ajpcell.1996.271.4.C1203. [DOI] [PubMed] [Google Scholar]
- 123.Schubert R, Serebryakov VN, Mewes H, Hopp HH. Iloprost dilates rat small arteries: role of K(ATP)- and K(Ca)-channel activation by cAMP-dependent protein kinase. Am J Physiol. 1997;272(3 Pt 2):H1147–H1156. doi: 10.1152/ajpheart.1997.272.3.H1147. [DOI] [PubMed] [Google Scholar]
- 124.Wu BN, Chen CF, Hong YR, Howng SL, Lin YL, Chen IJ. Activation of BKCa channels via cyclic AMP- and cyclic GMP-dependent protein kinases by eugenosedin-A in rat basilar artery myocytes. Br J Pharmacol. 2007;152(3):374–385. doi: 10.1038/sj.bjp.0707406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fellner SK, Arendshorst WJ. Complex interactions of NO/cGMP/PKG systems on Ca2+ signaling in afferent arteriolar vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2010;298(1):H144–H151. doi: 10.1152/ajpheart.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang CF, Au AL, Leung SW, Ng KF, Feletou M, Kwan YW, Man RY, Vanhoutte PM. Endothelium-derived nitric oxide inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing big conductance calcium-activated potassium channels of vascular smooth muscle. J Pharmacol Exp Ther. 2010;334(1):223–231. doi: 10.1124/jpet.110.166652. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Pertens E, Janssen LJ. 8-isoprostaglandin E2 activates Ca2+-dependent K+ current via cyclic AMP signaling pathway in murine renal artery. Eur J Pharmacol. 2005;520(1–3):22–28. doi: 10.1016/j.ejphar.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Barman SA, Zhu S, Han G, White RE. cAMP activates BKCa channels in pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L1004–L1011. doi: 10.1152/ajplung.00295.2002. [DOI] [PubMed] [Google Scholar]
- 129.Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol. 2004;286(6):13. doi: 10.1152/ajplung.00259.2003. [DOI] [PubMed] [Google Scholar]
- 130.Prieto D, Rivera L, Benedito S, Recio P, Villalba N, Hernandez M, Garcia-Sacristan A. Ca2+-activated K+ (KCa) channels are involved in the relaxations elicited by sildenafil in penile resistance arteries. Eur J Pharmacol. 2006;531(1–3):232–237. doi: 10.1016/j.ejphar.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Tazzeo T, Chu V, Janssen LJ. Membrane potassium currents in human radial artery and their regulation by nitric oxide donor. Cardiovasc Res. 2006;71(2):383–392. doi: 10.1016/j.cardiores.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem. 1998;273(49):32950–32956. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- 133.White RE, Lee AB, Shcherbatko AD, Lincoln TM, Schonbrunn A, Armstrong DL. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993;361(6409):263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- 134.Natarajan A, Han G, Chen SY, Yu P, White R, Jose P. The D5 dopamine receptor mediates large-conductance, calcium- and voltage-activated potassium channel activation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther. 2010;332(2):640–649. doi: 10.1124/jpet.109.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- 136.Kanatsuka H, Sekiguchi N, Sato K, Akai K, Wang Y, Komaru T, Ashikawa K, Takishima T. Microvascular sites and mechanisms responsible for reactive hyperemia in the coronary circulation of the beating canine heart. Circ Res. 1992;71(4):912–922. doi: 10.1161/01.res.71.4.912. [DOI] [PubMed] [Google Scholar]
- 137.Ishizaka H, Gudi SR, Frangos JA, Kuo L. Coronary arteriolar dilation to acidosis: role of ATP-sensitive potassium channels and pertussis toxin-sensitive G proteins. Circulation. 1999;99(4):558–563. doi: 10.1161/01.cir.99.4.558. [DOI] [PubMed] [Google Scholar]
- 138.Landry DW, Oliver JA. The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest. 1992;89(6):2071–2074. doi: 10.1172/JCI115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992;70(3):612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- 140.Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992;263(2 Pt 2):H491–H496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- 141.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29(4):312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- 142.Miyoshi Y, Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991;181(2):700–706. doi: 10.1016/0006-291X(91)91247-A. [DOI] [PubMed] [Google Scholar]
- 143.Orie NN, Thomas AM, Perrino BA, Tinker A, Clapp LH. Ca2+/calcineurin regulation of cloned vascular K ATP channels: crosstalk with the protein kinase A pathway. Br J Pharmacol. 2009;157(4):554–564. doi: 10.1111/j.1476-5381.2009.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(Pt 1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1995;92(26):12441–12445. doi: 10.1073/pnas.92.26.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miyoshi H, Nakaya Y. Activation of ATP-sensitive K+ channels by cyclic AMP-dependent protein kinase in cultured smooth muscle cells of porcine coronary artery. Biochem Biophys Res Commun. 1993;193(1):240–247. doi: 10.1006/bbrc.1993.1615. [DOI] [PubMed] [Google Scholar]
- 149.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 150.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 1998;164(4):549–557. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 151.Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994;74(3):471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- 152.Miyoshi H, Nakaya Y, Moritoki H. Nonendothelial-derived nitric oxide activates the ATP-sensitive K+ channel of vascular smooth muscle cells. FEBS Lett. 1994;345(1):47–49. doi: 10.1016/0014-5793(94)00417-X. [DOI] [PubMed] [Google Scholar]
- 153.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486(Pt 1):47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eckly-Michel A, Martin V, Lugnier C. Involvement of cyclic nucleotide-dependent protein kinases in cyclic AMP-mediated vasorelaxation. Br J Pharmacol. 1997;122:158–164. doi: 10.1038/sj.bjp.0701339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence of cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem. 1992;267:1015–1019. [PubMed] [Google Scholar]
- 156.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tang G, Wu L, Liang W, Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol. 2005;68(6):1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 158.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2011;30(10):1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 159.Sobey CG. Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol. 2001;21(1):28–38. doi: 10.1161/01.ATV.21.1.28. [DOI] [PubMed] [Google Scholar]
- 160.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87(2):160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 161.Best PJ, Burnett JC, Wilson SH, Holmes DR, Jr, Lerman A. Dendroaspis natriuretic peptide relaxes isolated human arteries and veins. Cardiovasc Res. 2002;55(2):375–384. doi: 10.1016/S0008-6363(02)00402-9. [DOI] [PubMed] [Google Scholar]
- 162.Schubert R, Krien U, Wulfsen I, Schiemann D, Lehmann G, Ulfig N, Veh RW, Schwarz JR, Gago H. Nitric oxide donor sodium nitroprusside dilates rat small arteries by activation of inward rectifier potassium channels. Hypertension. 2004;43(4):891–896. doi: 10.1161/01.HYP.0000121882.42731.6b. [DOI] [PubMed] [Google Scholar]
- 163.Park WS, Han J, Kim N, Ko JH, Kim SJ, Earm YE. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289(6):H2461–H2467. doi: 10.1152/ajpheart.00331.2005. [DOI] [PubMed] [Google Scholar]
- 164.Son YK, Park WS, Ko JH, Han J, Kim N, Earm YE. Protein kinase A-dependent activation of inward rectifier potassium channels by adenosine in rabbit coronary smooth muscle cells. Biochem Biophys Res Commun. 2005;337(4):1145–1152. doi: 10.1016/j.bbrc.2005.09.176. [DOI] [PubMed] [Google Scholar]
- 165.Orie NN, Fry CH, Clapp LH. Evidence that inward rectifier K+ channels mediate relaxation by the PGI2 receptor agonist cicaprost via a cyclic AMP-independent mechanism. Cardiovasc Res. 2006;69(1):107–115. doi: 10.1016/j.cardiores.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 166.Stull JT, Tansey MG, Tang DC, Word RA, Kamm KE. Phosphorylation of myosin light chain kinase: a cellular mechanism for Ca2+ desensitization. Mol Cell Biochem. 1993;127–128:229–237. doi: 10.1007/BF01076774. [DOI] [PubMed] [Google Scholar]
- 167.Conti MA, Adelstein RS. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3’:5’ cAMP-dependent protein kinase. J Biol Chem. 1981;256(7):3178–3181. [PubMed] [Google Scholar]
- 168.van Riper DA, McDaniel NL, Rembold CM. Myosin light chain kinase phosphorylation in nitrovasodilator induced swine carotid artery relaxation. Biochim Biophys Acta. 1997;1355(3):323–330. doi: 10.1016/S0167-4889(96)00144-9. [DOI] [PubMed] [Google Scholar]
- 169.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science. 1999;286(5444):1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 170.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res. 2007;101(7):712–722. doi: 10.1161/CIRCRESAHA.107.153981. [DOI] [PubMed] [Google Scholar]
- 171.Kitazawa T, Semba S, Huh YH, Kitazawa K, Eto M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol. 2009;587(Pt 14):3587–3603. doi: 10.1113/jphysiol.2009.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004;279(33):34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 173.Somlyo AV. Cyclic GMP regulation of myosin phosphatase: a new piece for the puzzle? Circ Res. 2007;101(7):645–647. doi: 10.1161/CIRCRESAHA.107.161893. [DOI] [PubMed] [Google Scholar]
- 174.Wu X, Haystead TA, Nakamoto RK, Somlyo AV, Somlyo AP. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. Synergism with cyclic nucleotide-activated kinase. J Biol Chem. 1998;273(18):11362–11369. doi: 10.1074/jbc.273.18.11362. [DOI] [PubMed] [Google Scholar]
- 175.Borman MA, MacDonald JA, Haystead TA. Modulation of smooth muscle contractility by CHASM, a novel member of the smoothelin family of proteins. FEBS Lett. 2004;573(1–3):207–213. doi: 10.1016/j.febslet.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 176.Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci USA. 2006;103(7):2440–2445. doi: 10.1073/pnas.0508566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275(28):21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 178.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+ -dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93(6):548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 179.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98(3):322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 180.Santos-Silva AJ, Cairrão E, Verde I. Study of the mechanisms regulating human umbilical artery contractility. Health (N. Y). 2010;2(4):321–331. [Google Scholar]
- 181.Bonnevier J, Arner A. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca2+ sensitization in smooth muscle. J Biol Chem. 2004;279(28):28998–29003. doi: 10.1074/jbc.M404259200. [DOI] [PubMed] [Google Scholar]
- 182.Bolz SS, Vogel L, Sollinger D, Derwand R, De Wit C, Loirand G, Pohl U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation. 2003;107(24):3081–3087. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- 183.Woodrum DA, Brophy CM, Wingard CJ, Beall A, Rasmussen H. Phosphorylation events associated with cyclic nucleotide-dependent inhibition of smooth muscle contraction. Am J Physiol. 1999;277(3 Pt 2):H931–H939. doi: 10.1152/ajpheart.1999.277.3.H931. [DOI] [PubMed] [Google Scholar]
- 184.Beall AC, Kato K, Goldenring JR, Rasmussen H, Brophy CM. Cyclic nucleotide-dependent vasorelaxation is associated with the phosphorylation of a small heat shock-related protein. J Biol Chem. 1997;272(17):11283–11287. doi: 10.1074/jbc.272.17.11283. [DOI] [PubMed] [Google Scholar]
- 185.Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008;119(1):44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Beall A, Bagwell D, Woodrum D, Stoming TA, Kato K, Suzuki A, Rasmussen H, Brophy CM. The small heat shock-related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J Biol Chem. 1999;274(16):11344–11351. doi: 10.1074/jbc.274.16.11344. [DOI] [PubMed] [Google Scholar]
- 187.Rembold CM, Foster DB, Strauss JD, Wingard CJ, Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000;524(Pt 3):865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Woodrum D, Pipkin W, Tessier D, Komalavilas P, Brophy CM. Phosphorylation of the heat shock-related protein, HSP20, mediates cyclic nucleotide-dependent relaxation. J Vasc Surg. 2003;37(4):874–881. doi: 10.1067/mva.2003.153. [DOI] [PubMed] [Google Scholar]
- 189.Fan GC, Kranias EG (2010) Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J Mol Cell Cardiol [DOI] [PMC free article] [PubMed]
- 190.Brophy CM, Lamb S, Graham A. The small heat shock-related protein-20 is an actin-associated protein. J Vasc Surg. 1999;29(2):326–333. doi: 10.1016/S0741-5214(99)70385-X. [DOI] [PubMed] [Google Scholar]
- 191.Flynn CR, Brophy CM, Furnish EJ, Komalavilas P, Tessier D, Thresher J, Joshi L. Transduction of phosphorylated heat shock-related protein 20, HSP20, prevents vasospasm of human umbilical artery smooth muscle. J Appl Physiol. 2005;98(5):1836–1845. doi: 10.1152/japplphysiol.01043.2004. [DOI] [PubMed] [Google Scholar]
- 192.Brophy CM, Dickinson M, Woodrum D. Phosphorylation of the small heat shock-related protein, HSP20, in vascular smooth muscles is associated with changes in the macromolecular associations of HSP20. J Biol Chem. 1999;274(10):6324–6329. doi: 10.1074/jbc.274.10.6324. [DOI] [PubMed] [Google Scholar]
- 193.Kargacin GJ, Cooke PH, Abramson SB, Fay FS. Periodic organization of the contractile apparatus in smooth muscle revealed by the motion of dense bodies in single cells. J Cell Biol. 1989;108(4):1465–1475. doi: 10.1083/jcb.108.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dreiza CM, Brophy CM, Komalavilas P, Furnish EJ, Joshi L, Pallero MA, Murphy-Ullrich JE, von Rechenberg M, Ho YS, Richardson B, Xu N, Zhen Y, Peltier JM, Panitch A. Transducible heat shock protein 20 (HSP20) phosphopeptide alters cytoskeletal dynamics. FASEB J. 2005;19(2):261–263. doi: 10.1096/fj.04-2911fje. [DOI] [PubMed] [Google Scholar]
- 195.Murphy-Ullrich JE, Pallero MA, Boerth N, Greenwood JA, Lincoln TM, Cornwell TL. Cyclic GMP-dependent protein kinase is required for thrombospondin and tenascin mediated focal adhesion disassembly. J Cell Sci. 1996;109(Pt 10):2499–2508. doi: 10.1242/jcs.109.10.2499. [DOI] [PubMed] [Google Scholar]
- 196.Castro LRV, Verde I, Cooper DMF, Fischmeister R. Cyclic GMP compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 198.Vo NK, Gettemy JM, Coghlan VM. Identification of cGMP-dependent protein kinase anchoring proteins (GKAPs) Biochem Biophys Res Commun. 1998;246(3):831–835. doi: 10.1006/bbrc.1998.8722. [DOI] [PubMed] [Google Scholar]
- 199.Corbin JD, Sugden PH, Lincoln TM, Keely SL. Compartmentalization of adenosine 3’:5’-monophosphate and adenosine 3’:5’-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977;252(11):3854–3861. [PubMed] [Google Scholar]
- 200.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295(5560):1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 201.Yan C, Nagel DJ, Jeon K. In: Cyclic nucleotide phosphodiesterases in health and disease. Beavo J, Francis S, Houslay M, editors. Boca Raton: CRC Press, Taylor & Francis Group; 2007. pp. 465–484. [Google Scholar]
- 202.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104(19):2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 203.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98(6):777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cawley SM, Sawyer CL, Brunelle KF, van der Vliet A, Dostmann WR. Nitric oxide-evoked transient kinetics of cyclic GMP in vascular smooth muscle cells. Cell Signal. 2007;19(5):1023–1033. doi: 10.1016/j.cellsig.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 205.Nausch LW, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc Natl Acad Sci USA. 2008;105(1):365–370. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol. 2006;128(1):3–14. doi: 10.1085/jgp.200509403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res. 2006;99(8):845–852. doi: 10.1161/01.RES.0000245189.21703.c0. [DOI] [PubMed] [Google Scholar]
- 208.Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3(9):710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 209.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5(12):959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 210.Zhong J, Hume JR, Keef KD. Anchoring protein is required for cAMP-dependent stimulation of L-type Ca2+ channels in rabbit portal vein. Am J Physiol. 1999;277(4 Pt 1):C840–C844. doi: 10.1152/ajpcell.1999.277.4.C840. [DOI] [PubMed] [Google Scholar]
- 211.Hayabuchi Y, Dart C, Standen NB. Evidence for involvement of A-kinase anchoring protein in activation of rat arterial K(ATP) channels by protein kinase A. J Physiol. 2001;536(Pt 2):421–427. doi: 10.1111/j.1469-7793.2001.0421c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Indolfi C, Stabile E, Coppola C, Gallo A, Perrino C, Allevato G, Cavuto L, Torella D, Di Lorenzo E, Troncone G, Feliciello A, Avvedimento E, Chiariello M. Membrane-bound protein kinase A inhibits smooth muscle cell proliferation in vitro and in vivo by amplifying cAMP-protein kinase A signals. Circ Res. 2001;88(3):319–324. doi: 10.1161/01.res.88.3.319. [DOI] [PubMed] [Google Scholar]
- 213.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99(3):129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 214.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95(10):1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 215.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 216.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 217.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99(17):11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276(41):38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 219.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94(11):1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 220.Bergdahl A, Sward K. Caveolae-associated signalling in smooth muscle. Can J Physiol Pharmacol. 2004;82(5):289–299. doi: 10.1139/y04-033. [DOI] [PubMed] [Google Scholar]