Abstract
Insects mostly develop on decaying and contaminated organic matter and often serve as vectors of biologically transmitted diseases by transporting microorganisms to the plant and animal hosts. As such, insects are constantly ingesting microorganisms, a small fraction of which reach their epithelial surfaces, mainly their digestive tract, where they can establish relationships ranging from symbiosis to mutualism or even parasitism. Understanding the tight physical, genetic, and biochemical interactions that takes place between intestinal epithelia and either resident or infectious microbes has been a long-lasting objective of the immunologist. Research in this field has recently been re-vitalized with the development of deep sequencing techniques, which allow qualitative and quantitative characterization of gut microbiota. Interestingly, the recent identification of regenerative stem cells in the Drosophila gut together with the initial characterization of Drosophila gut microbiota have opened up new avenues of study aimed at understanding the mechanisms that regulate the dialog between the Drosophila gut epithelium and its microbiota of this insect model. The fact that some of the responses are conserved across species combined with the power of Drosophila genetics could make this organism model a useful tool to further elucidate some aspects of the interaction occurring between the microbiota and the human gut.
Keywords: Drosophila, Innate immune response, Immune mechanisms, Gut microbiota, Intestinal epithelium
Introduction
The insect gut carries out some of the most vital functions of nutrition and solute and water balance of the organism. It is the first line of defense against ingested pathogens and also the portal of entry of viruses and parasites for which insect species are major vectors. The diet of insects is of such wide variety in terms of texture—ranging in composition from liquid plant sap to solid bark, from blood to decomposing animals—that the digestive system of each insect species is specialized in structure and in biochemical machinery. However, anatomical and structural studies conducted over the years on different insect species have highlighted an evident conservation of global organization and function of the alimentary canal amongst insects. Here, I first describe this overall structure, focusing on data collected in the well-studied Drosophila melanogaster model system.
Drosophila gut architecture and function
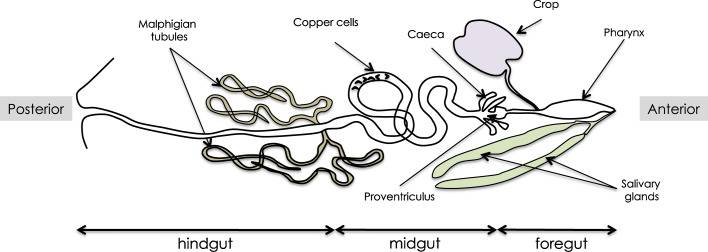
The intestinal tract of Drosophila can be divided into three different sections, each consisting of different cell types and exerting different physiological functions: the foregut, the midgut, and the hindgut (Fig. 1). The anterior-most domain, the foregut, forms a tube-like structure extending from the mouth to the proventriculus. It is connected to salivary glands and to a diverticulum (called the crop) that respectively function as saliva-producing glands useful to initiate digestion and as a food storage pouch. The foregut is followed by the midgut and the hindgut, which are respectively the equivalent of the mammalian intestine and colon. Ingested food passes through the foregut to be stored temporarily in the crop and then moves to the anterior midgut where nutrient absorption commences [1]. The anterior-most extremity of the midgut is a heavy-walled, bulb-like region called the proventriculus or cardium whose inner intima layer is chitinous; this region is used for mechanical breakdown of the food. In addition, the proventriculus synthesizes a semi-permeable chitinoproteinaceous membrane known as the peritrophic membrane, which lines the midgut and has important roles in the maintenance of insect gut structure, facilitation of digestion, and protection from invasion by microorganisms and parasites. In larvae, posterior to the proventriculus, is a ring of four blind-ending fingerlike diverticules called caeca that participate in digestion by secreting enzymes. The midgut lumen comprises large longitudinal gradients of pH that delimit three contiguous segments: an anterior near-neutral part, a short and narrow strongly acidic middle segment, and a wider third zone of increasing alkalinity that secretes base into the lumen [2]. The middle region of low pH contains iron and copper cells (Fe/Cu cells) whose function is essential to maintain acidity [3]. The boundary between the mid- and hindgut corresponds to the branching point of the Malpighian tubules. These renal-like evaginations absorb solutes, water, and waste from the surrounding hemolymph (insect blood) and release them into the gut lumen in the form of solid nitrogenous compounds [4]. The rest of the gut consists of the hindgut, which resorbs water and ions and expels undigested waste. The foregut, proventriculus, anterior end of the midgut, and hindgut are innervated by neurons emanating from the central nervous system [5]. Feeding and the ingestion of food into the midgut are mediated through interactions of the foregut with the nervous system via the stomatogastric nervous system [6]. Recent work has resulted in the identification of neuron subsets that innervate distinct regions of the gut and play an essential role in the control of gut physiological functions, such as pH regulation and diuresis control [7].
Fig. 1.
Overall structure of the Drosophila adult gut. It should be noted that cecae are shown, although they only exist at the larval stage
Drosophila gut development
The various functions of the digestive system require its regionalization into distinct functional domains and morphogenesis of a characteristic sequence of gut accessory organs that arise during embryonic development at stereotype positions along the anterior–posterior axis of body. Our understanding of the developmental processes that pattern the gut and give rise to their identity as different parts of the gut is quite advanced in Drosophila and highlights striking similarities to its mammalian homolog. Similar to vertebrates in which the gut develops from an epithelial lining and a surrounding mesenchyme [8], the Drosophila larval gut develops from a primordia composed of a simple epithelium surrounded by visceral mesoderm [9, 10]. The gut parts all arise from an anterior and a posterior anlage specified early during embryogenesis. The epithelium of the larval alimentary tract originates from two embryonic germ layers, the endoderm and ectoderm. The endoderm gives rise to most of the midgut, whereas the ectoderm forms the foregut and hindgut [11]. Graded activities of maternal signals at the anterior and posterior terminal domains of the embryo turns on patterning gap genes (tailless and Huckebein) which in turn activate genes that specify gut primordia: GATA and Forkhead transcription factors are required for endodermal midgut anlagen specification and brachyenteron (Brachyury homolog) for the ectodermal hindgut primordium [12]. The midgut is derived from endodermal cells located in the primordial which will migrate to form the definitive larval midgut [12]. The signaling events leading to the regionalization of the gastrointestinal tract have been well studied in both vertebrates and Drosophila. In the chick and mouse, the activity of Hedgehog and transforming growth factor-beta (TGF-β) signaling molecules are required for the generation of boundaries from which foregut gut-associated organs, such as the lungs, liver, or pancreas, develop [13, 14]. Similarly, the regionalization of the Drosophila foregut and the development of the foregut-associated proventriculus organ require the activities of Hedgehog, Wingless (Wg), and the TGF-β homolog Dpp [15]. Subdivision of the midgut epithelium is regulated by inductive signals emanating from the visceral mesoderm in both the embryonic development of the Drosophila midgut and mammalian intestine, demonstrating the evolutionary conservation of some of the mechanisms driving gut formation [16, 17].
The larval midgut is an endothelial tube containing two differentiated cell types, namely, enterocytes and enteroendocrine cells, which arise from stem cell crypts located within the gut and differentiate into either cell type in a Notch signaling pathway-dependant manner [18, 19]. Depending on position cues within the gut tube, the enterocytes develop a wide variety of morphologies and functions and represent the majority of the cells within the midgut. While many of the enterocytes are involved in the absorption of nutrients at various stages of digestion, others, such as the acid-producing copper cells within the acid region of the midgut, are highly specialized [2, 20]. Comprising a smaller portion of the cell population of the gut are the enteroendocrine cells, which have been shown to play several roles, including the secretion of a number of peptide hormones [21].
In holometabolous insects (which undergo complete metamorphosis), the adult appendages and internal organs form anew from larval progenitor cells during metamorphosis. The entire gut is remodeled during metamorphosis from imaginal cells at the base of the salivary glands (foregut), at the junction of the foregut and midgut, in the hindgut, and around the anus. Malpighian tubules are an exception to this remodeling process since they persist unmodified throughout metamorphosis. Transformation of the larval anlagen into adult structures has been well documented for the midgut. The larval midgut comprises two major cell types: the large larval epithelial cells with decondensed polyploid nuclei and, embedded among them, small diploid adult midgut progenitors cells (AMPc) [22–24]. In response to ecdysone, the hormone driving metamorphosis, the larval epidermal cells initiate programmed cell death, while AMPc proliferate [25]. Dividing AMPc first disperse, but later proliferate within distinct islands, forming large cell clusters that eventually fuse during metamorphosis to form the adult midgut epithelium. Signaling through the EGFR/RAS/MAPK pathway is necessary and limiting for AMPc proliferation [26, 27]. Midgut visceral muscle produces a weak epidermal growth factor receptor (EGFR) ligand, Vein, which is required for early AMPc proliferation. Two stronger EGFR ligands, Spitz and Keren, are expressed by the AMPc themselves and provide an additional autocrine mitogenic stimulus to the AMPc during late larval stages [27]. At the adult stage, the final digestive tract is composed of epithelial cells with absorptive and secretory functions and a population of intestinal stem cells (ISCs) that are interspersed between them and required to maintain homeostasis.
Control of midgut epithelium homeostasis
Intestinal stem cells in the adult Drosophila midgut proliferate and self renew to produce differentiating daughter cells that replace those lost as part of normal gut function. It is estimated that under normal physiological conditions, Drosophila intestinal cells turn over approximately weekly. Using cell lineage labeling methods relying on the production of marked clones after mitotic recombination, several laboratories have identified and characterized midgut intestinal stem cells [23, 28, 29]. ISCs are distributed evenly along the midgut and are the only cells capable of cell division in the adult midgut. These basally located cells, which can give rise to both enterocytes and small secretory enteroendocrine cells, can be identified by their small nuclear size and expression of the Notch ligand Delta (Fig. 2). ISC self-renewal produces an identical daughter ISC along with an immature diploid daughter (progenitor) cell, termed the enteroblast (EB). It seems that the hindgut is lacking ISCs, although this issue remains controversial [28, 30, 31]. The Delta–Notch signaling pathway plays a critical role in ISC fate determination [23, 29]. Drosophila midgut ISC division is morphologically symmetrical, giving rise to two daughter cells that are initially similar. However, soon after division, one cell retains high levels of Delta and remains an ISC, while the other cell quickly loses Delta expression and becomes an EB. Enteroblast differentiation coincides with a reduction of contact with the basement membrane and the underlying visceral muscle cells that produce Wg, an important ISC survival factor that promotes stemness and suppresses differentiation. Clonal analysis indicates that downstream components of the Wg pathway, including Frizzled, Dishevelled, and Armadillo, act autonomously within ISCs to ensure self-renewal [32, 33].
Fig. 2.
Pathways regulating gut epithelial homeostasis. See the text for details. EC Enterocytes, EB enteroblasts, ISC intestinal stem cells
In response to damage, adult ISCs switch on a rapid proliferative state that facilitates the replacement of damaged tissue and contributes to epithelial homeostasis maintenance [27, 34–37]. The Jak/Stat signaling pathway that has been implicated in the regulation of stem cells in multiple tissues also promotes gut ISC proliferation [38]. In the adult midgut, the Jun N-Terminal Kinase (JNK) pathway is activated in damaged enterocyte cells (ECs) following injury. This leads to the production of secreted Unpaired (Upd) cytokines from ECs, which in turn activate the Janus kinase signal transducer (JAK) and activator of transcription STAT in intestinal SCs, stimulating their proliferation. In addition, the Hippo pathway has been recently implicated in the regulation of Upd cytokine production from the ECs [39–41] (Fig. 2).
If it is clear that gut epithelial homeostasis is constantly controlled throughout the fly’s life to repair aging-associated damage, recent reports identify mechanisms at work to maintain epithelial homeostasis in response to gut flora and bacterial infection. Before going into these aspects, our current view on Drosophila gut flora composition and function is presented in the following section.
Gut flora: composition and role
Despite its widespread use as a model system for dissecting innate immune response, relatively little was known until recently on the interactions between the Drosophila gut epithelium and its associated microbial communities. This situation is progressively evolving with the recent publication of relatively comprehensive studies on the bacterial species that associate with natural and laboratory Drosophila strains and of studies that aim at unraveling microbiota evolution and function in flies [42–44]. In contrast to the high complexity of the human microbiota, which contains more than 500 species [45], Drosophila’s bacterial communities are relatively simple, composed of about 20 different species, including four or five main species of the Acetobacter and Lactobacillus genus, the latter also being found in human microbiota. However, unlike the human microbiota, Drosophila harbors very few anaerobes and in particular lacks Bacteroidetes species, the major constituents of the human gut microbiota.
More than 100 years ago, Ilya Metchnikov and Louis Pasteur suggested that some microflora species could exert beneficial roles in the physiology of gut epithelia and in host fitness. Several studies in mammals have now shown that commensal microbes profoundly modulate the expression of many host genes involved in diverse functions, thereby actively shaping host biology [46, 47]. Although it is now accepted that Drosophila is also carrying gut flora, the precise roles and functions of these flora have only recently been studied. The observation that Drosophila embryos raised under axenic conditions give rise to perfectly well-formed and fully fertile adults tends to indicate that bacteria are largely dispensable for the various developmental processes that are taking place, under laboratory conditions, during embryogenesis, the larval stage, and metamorphosis. However, recent reports suggest that these gut bacterial communities are important for some other aspects of insect physiology and that modifying the relative proportion of different bacterial species can have deleterious effects on the gastro-intestinal (GI) tract [47]. Firstly, gut microbiota have been associated with life span. In a recent report, Brummel et al. [48] show that the longevity of adult flies reared under axenic conditions is reduced and that reintroducing bacteria during the first week of adult life can restore wild-type longevity. These results suggest a positive impact of bacterial community on fly longevity; however, they are at odds with those published by Ren et al. who did not observe any difference in life span of axenic and control flies for the Oregon-R strain or the shorter-lived Canton-S strain [49]. Further work will be needed to solve this issue. Another emerging role of commensal bacteria relies on its putative influence on mating preference. Sharon et al. [50] showed that when an individual fly issued from two genetically identical Drosophila populations, one reared on a molasses medium and the other on a starch medium, are mixed, “molasses flies” preferred to mate with other molasses flies while “starch flies” preferred to mate with other starch flies. Since this mating preference is abolished after antibiotic treatment and can be induced by infection with microbiota obtained from the fly media (before antibiotic treatment), the results suggest that symbiotic bacteria can influence mating preference. Chemical analyses suggest that bacteria mediate their effect on mating preference by changing the levels of cuticular hydrocarbon sex pheromones. The guts of ruminants and termites are well-studied examples of bioreactors “programmed” with anaerobic bacteria charged with the task of breaking down ingested polysaccharide into short-chain fatty acids. Although, no anaerobic bacteria have been found in the fly alimentary canal (oxygen can freely access the Drosophila GI tract), one can speculate whether gut bacteria can also participate in food assimilation and digestion in Drosophila. Our unpublished results indicate that some commensal bacteria species favor developmental growth and accelerate developmental timing in flies raised under poor nutrient conditions (J Royet and F Leulier, unpublished resutls). These data, although related to processes not totally dissected at the molecular level, point to an importance of the microbiota in Drosophila physiology. The existence of a gut flora in the fly and its recently revealed functional importance imply that Drosophila has developed mechanisms aimed firstly to control its size and shape and, secondly, to tolerate it, a process involving the Drosophila gut immune response.
Gut immune response
As mentioned earlier in this review, the first barrier against bacteria is the peritrophic membrane, a chitinous matrix secreted by the proventriculus that lines the mid- and foregut. This semi-permeable membrane allows the passage of digestive enzymes and defense molecules secreted by the intestinal epithelium while at the same time permitting the intake of nutrients from the lumen by the epithelium. This matrix prevents direct contact between the gut flora and the intestinal epithelium, a function that is to some extent is fulfilled in vertebrates by the mucus secreted by intestinal goblet cells. Bacteria are also probably eliminated—or at least their proliferation controlled—by their journey through the gut acidic region and by their interaction with bacterial cell wall-modifying proteins, such as lysozymes and peptidoglycan-hydrolyzing enzymes present in the gut lumen [51]. These constitutive physical and chemical antibacterial agents are complemented by two largely inducible chemical defenses, an oxidative shock and the production of antimicrobial peptides (AMPs) by the gut epithelium [37, 52–54].
AMPs production in the Drosophila gut
Upon bacterial infection, Drosophila immune-competent tissues, such as the fat body, hemocyte, trachea, or gut epithelium, produce antimicrobial peptides that are released into the circulating hemolymph or into the trachea and gut lumen to control microbial proliferation. Integrated analysis of the results generated by large genetic screens conducted in flies or in cell cultures and of microarray experiments have put together a very detailed picture of the gene cascades that regulate AMP production upon bacterial infection in Drosophila (for a review see [55]). The main conclusion of these studies is that the transcription of most immune-induced genes is under the control of two signaling pathways known as Toll and IMD which show high similarities with the mammalian IL-1/TLR and TNF-α pathways. However, whereas AMP gene transcription is under the control of both the Toll and IMD signaling pathways during the systemic response that take place in the fat body, it is largely dependent on the IMD, and to a lesser extent to the JAK/STAT cascade in gut epithelial cells [35, 56, 57]. Indeed, most genes whose transcription is upregulated in the gut following oral bacterial infection are no longer induced in an IMD mutant background [35]. The results of recent studies consistently point towards the important role of IMD-dependent AMP production in providing protection against orally infectious Gram-negative bacteria, such as Pseudomonas entomophila and Seratia marscesens, thereby emphasizing the critical role of local antimicrobial peptide expression against food-borne pathogens [52, 58]. The IMD pathway is composed of multiple intracellular components (IMD, Dredd, FADD, among others) whose successive activation leads to nuclear translocation of the NF-κB transcription factor Relish (for a review, see [59]. Upstream of this pathway, bacteria are recognized by two receptors belonging to the Peptidoglycan recognition protein family (PGRP). The transmembrane receptor PGRP-LC serves as a pattern recognition receptor that combines the function of a microbial binding protein (through its PGRP domain) and of a signaling receptor (through its intracellular tail) [60–62]. Another receptor, PGRP-LE has been shown to act as an intracellular receptor for diaminopimelic acid (DAP)-type polymeric peptidoglycan (PGN) in blood cells, but its function in the gut remains unknown [63]. In the absence of infection, but in the presence of indigenous flora, AMPs are only produced in very restricted domains of the gut. The fact that gut commensal bacteria secrete PGN derivatives (muropeptides) which are very potent activators of the IMD pathway and that all gut cells are competent to activate IMD-dependent AMPs suggest that gut epithelial cells are well equipped with tools aimed at preventing constitutive and permanent activation of the IMD pathway.
Mechanisms controlling immune tolerance and immune response intensity
Although most mechanisms regulating immune tolerance to resident bacteria in Drosophila have probably yet to be discovered, recent evidence points to the importance of regulation of activation of the IMD pathway in this process (Fig. 3). Recently conducted genetic screens have surprisingly shown that the IMD pathway contains more negative regulators than proteins required to convey the signal from the upstream PGRP-LC receptor to the nucleus [64]. Although the function of each of these has not yet been studied at the resolution of the tissue, some of these are clearly and directly implicated in gut immune tolerance. Some regulators are required to prevent IMD pathway activation in the absence of an immune challenge. These are often ubiquitous factors that regulate the protein stability of defined intracellular IMD pathway members, often by regulating their ubiquitination status (SKPA/SLMB/DCUL1, dUSP36, CYLD, POSH, DNR-1, Caspar). While it is evident that these genes do regulate basal IMD pathway activation in the fat body and blood cells, their function in the gut has not yet been determined. The inhibition provided by basal regulators appears to be constitutive, but the negative regulation imposed by other IMD repressors, such as PGRP-SC/LB/LF and PIRK, is the result of a negative feedback loop of the IMD pathway. By cleaving PGN into non-immunostimulatory fragments, PGRP with amidase enzymatic activity (such as PGRP-SC and PGRP-LB) reduce the amount of immune elicitor and, in turn, tune-down the gut response to endogenous flora [65, 66]. This extracellular response is associated with mechanisms that act intracellularly or at the membrane plasma membrane to attenuate the immune response. By forming inactive heterodimers with PGRP-LCx isoforms, PGRP-LF reduces IMD pathway activation [67, 68]. At the other end of the IMD pathway, in the nucleus, the Caudal transcription function directly suppresses IMD-dependent NF-κB-mediated AMP expression in the posterior midgut [69]. This constant transcriptional repression is essential to maintain the integrity of the gut epithelium and the homeostasis of its microbiota. The absence of such control, as observed upon Caudal silencing, leads to constant Relish-dependent AMP production, which causes modifications in the commensal bacteria population, apoptosis of epithelial cells and, eventually, a reduced lifespan. This finding indicates that controlling the quantity and the quality of the intestinal microbial burden is a vital process for the fly. It should, however, be emphasized that IMD-mediated immune tolerance in the gut epithelium is compartimentalized, as exemplified by the regional requirement for Caudal in this process. Another IMD pathway regulator is the protein Pirk which, by reducing PGRP-LC plasma membrane localization, renders the microbiota invisible for the gut epithelia as far as IMD pathway activation is concerned [70–72]. It is hypothesized that in the absence of Pirk, PGRP-LC is no longer maintained in the cytoplasm and passes into the membrane where it can be inappropriately activated by the microbiota, thus leading to ectopic AMP expression in gut epithelial cells. Pirk and PGRP-LB, both targets and regulators of the IMD signaling pathway, establish a negative feedback loop in the IMD signaling pathway to avoid the hyper-activation of target genes and impose a brake on the PGN-driven signaling due to commensal bacteria. In the presence of high bacterial load, i.e., in an infectious context, high levels of PGN are released by proliferating bacteria, thereby transiently increasing the signal strength so as to overwhelm the basic inhibition of the pathway and to trigger AMP production. As the inhibitory circuitry is under transcriptional control of the IMD pathway, the system quickly returns to homeostasis upon elimination of the bacteria by AMPs.
Fig. 3.
Signaling networks controlling gut antimicrobial peptides (AMPs) and reactive oxygen species (ROS) production. See text for details
If we accept that these data clearly demonstrate that regulation of immune tolerance and of the intensity of the immune response in the gut is largely dependent on negative regulators of the IMD pathway, it is likely that some functional redundancy does exist in between these regulators. To appreciate the real consequences associated with a IMD pathway deregulation on gut homeostasis, microbiota composition, and even on fly integrity, analysis should be performed on individuals in which multiple regulators have been simultaneously inactivated.
Reactive oxygen species
The second major bacterial proliferation attenuator in the gut epithelia are reactive oxygen species (ROS). Natural infections by bacteria induce in the fly gut, as in that of mammals, a rapid ROS burst, a reaction that is compulsory to keep bacterial growth under control. Although directed towards the same microbial targets, the regulation of ROS production in the Drosophila gut is quite distinct from that of AMPs (Fig. 3). ROS are constitutively produced in the gut at a basal level by the membrane-associated dual oxidase (DUOX), a member of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family [54] (Fig. 3). Interaction between the gut microbiota and epithelial cells triggers the phospholipase C-β (PLCβ)-dependent production of 1,4,5-triphosphate (IP3) and subsequent mobilization of intracellular calcium, which modulates DUOX enzymatic activity [37]. This activation, triggered by soluble microbial extracts distinct from PGN, requires a functional Gα protein (Gαq), suggesting that a G-coupled receptor acts upstream of the pathway. The observation that fly mutants for PLCβ have a reduced life span due to the uncontrolled proliferation of dietary microbes demonstrates the importance of basal ROS production in routine microbe control. The molecular nature of the bacterial elicitor of the DUOX activation pathway remains elusive but is clearly different from that of bacterial PGN.
Although capable of controlling normal gut microbiota, this system of basal ROS production is not potent enough to eliminate food-borne bacterial infection. When the gut bacterial burden increases, not only DUOX activity by also DUOX protein expression levels and, consequently ROS levels, are consistently dramatically upregulated [37]. This DUOX gene transcriptional upregulation is under the control of the P38 pathway in the gut. However, as opposed to the DUOX activation pathway itself, the P38-dependent transcriptional regulation of the Duox gene is under the control of both PGN-dependent and -independent pathways and is not required to mediate the basal level production of ROS in the alimentary duct. Whereas MEKK1 and P38 mutant flies are highly susceptible to gut bacterial infection, they consistently show normal resistance to systemic infection by microorganisms. Importantly, recent reports indicate that ROS, in addition to its direct microbicidal role, could be implicated in maintaining gut homeostasis after infection by regulating intestinal stem cell proliferation (see below) [73].
Maintenance of gut homeostasis after bacterial infection
As in most animals, the integrity of the Drosophila gut relies on the homeostasis of the intestinal epithelium, which is based on a strict balance between intestinal epithelial cell death and their renewal from a pool of intestinal stem cells. Several recent reports demonstrate that intestinal infections trigger epithelial cell death associated with the proliferation and differentiation of intestinal stem cells in order to maintain tissue integrity. Thus, the reaction of the Drosophila gut to microorganisms is not restricted to activation of the immune system but extends to integrated responses aimed at maintaining gut tissue integrity. ISC division and intestinal renewal can be stimulated by feeding flies damaging agents or expressing apoptotic genes in the gut (Fig. 2) [27, 74, 75]. For example, the ingestion of the DNA-damaging agent Bleomycin and dextran sulfate sodium causes cell death and stimulates both ISC division and EB differentiation. Targeted expression of the anti-apoptotic protein P35 specifically in ECs can partially suppress Bleomycin-dependent ISC division [37]. Dying ECs are thus able to induce ISCs to divide and to differentiate into EBs in order to compensate for gut cell loss and, eventually, to maintain gut homeostasis. A functional insulin signaling pathway is required for this process since loss-of-function mutations in either the insulin receptor (InR), the InR substrate Chico, or the downstream transcription factor FOXO, abolish Bleomycin-dependent ISC stimulation [37].
The ingestion of invasive bacteria, such as Erwinia carotovora (Ecc15) or Pseudomonas entomophila, is also able to efficiently stimulate ISC division [37, 75]. Experiments using anti-phospho histone H3 staining have shown that ISC division is increased tenfold after oral bacterial infection. Large mitotic clones can be consistently recovered in adult midguts 3 days following Ecc15 infection, while only small clones are visible in control guts. The escargot-GFP marker, which strongly labels ISCs, EBs, and newly synthesized ECs, stains an increased number of cells after Ecc15 ingestion, indicating a recent and extensive increase in the quantity of stem cell-derived cells. Both bacterial recognition by PGRP-LC and activation of the IMD pathway are dispensable for ISC division and EB differentiation induced by Ecc15 ingestion [75]. These surprising observations indicate that invasive bacteria are not recognized per se and that PGN is not the bacterial elicitor that triggers ISC activation. Rather, they suggest that it is the bacteria-induced damage to gut cells that stimulates ISC division. This hypothesis is in good agreement with the observation that bacterial ingestion correlates with increased EC apoptosis. Importantly, the induction of the oxidative burst following Ecc15 bacterial infection appears to be a major inducer of cell damage and epithelium renewal since ISC activation and intestinal renewal do not occur in infected flies fed with the antioxidants N-acetyl cysteine or glutathione or when the Duox gene is silenced in the gut by RNAi [75]. What are then the signaling pathway(s) that are activated in the gut cells following bacterial infection and required to trigger ISC proliferation? Recent studies show that these pathways are the same as those previously described to control gut homeostasis in the absence of immune challenge (JAK/STAT; EGF) (Fig. 2) [34, 37, 75]. Consistent with this findings, one of the JAK/STAT ligands, Upd3, is strongly induced in ECs after bacterial ingestion or by EC-induced apoptosis [34, 37, 75], and the reduction of JAK/STAT signaling specifically in ISCs decreases epithelial renewal induced upon bacterial infection [34, 75]. The cellular and molecular mechanisms responsible for Upd3 expression by ECs remain unknown, although cell damage is a likely key factor. Indeed, physical injury of the gut with forceps is sufficient to induce localized expression of Upd3 in the vicinity of damaged ECs. Moreover, the DUOX-dependent oxidative burst is essential for Upd3 production and JAK/STAT signaling activation [75]. In addition, bacteria-dependent ISC activation requires the cytoprotective JNK pathway that is strongly activated in both ISCs and ECs. Inhibition of this pathway specifically in ISCs dramatically affects their number and their subsequent progeny [36, 75]. Intestinal renewal and maintenance of gut homeostasis are essential in the Drosophila defense against Ecc15 infection. Flies with reduced JAK/STAT or JNK signaling in ISCs, or missing Upd3 in ECs, succumbed much earlier than control flies after Ecc15 infection [75]. Such mutant flies lack epithelial renewal and display morphologically altered guts with abnormal and missing ECs. It has recently been shown that the EGFR pathway contributes to gut morphogenesis through its activity in enterocytes and is required to properly coordinate the delamination and anoikis of damaged cells [76, 77].
In addition to their role in controlling ISC proliferation and differentiation in infected intestines, basal levels of the JAK/STAT and JNK signaling pathways are essential to maintain gut homeostasis in uninfected guts, ensuring the proper differentiation of ISC progeny and ISC maintenance [75]. This function illustrates well that bacterial infection stimulates—rather than initiates—a molecular and cellular response (i.e., ISC activation) that occurs naturally in uninfected flies. Interestingly, flies raised under axenic conditions display reduced ISC division and intestinal epithelial renewal. Thus, in addition to constantly triggering basal levels of IMD (but without AMP production) and DUOX pathway activation, indigenous microbiota maintain a basal level of epithelium regeneration probably by a mild activation of the JAK/STAT and JNK signaling pathways.
Conclusions
The contribution of Drosophila melanogaster to our understanding of the mechanisms governing the innate immune response, although relatively recent, has been remarkable. Recent findings showing that fruit flies carry commensal gut bacteria and that they have developed means to tolerate these bacteria have led researchers to embark on an array of totally new and exciting fields of research. Given the remarkable evolutionary conservation in most immune mechanisms, it is very likely that the results obtained in Drosophila will be of significant relevance to an in-depth understanding of antimicrobial defense strategies in other organisms, including humans.
Acknowledgments
I would like to thank François Leulier and Bernard Charroux for comments on the manuscript. Julien Royet and his research group are supported by CNRS, ANR, FRM, AFM, and IUF.
References
- 1.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 2.Dubreuil RR. Copper cells and stomach acid secretion in the Drosophila midgut. Int J Biochem Cell Biol. 2004;36:745–752. doi: 10.1016/j.biocel.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exp Biol. 2009;212:1731–1744. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- 4.Abdelsadik A, Roeder T. Chronic activation of the epithelial immune system of the fruit fly’s salivary glands has a negative effect on organismal growth and induces a peculiar set of target genes. BMC Genomics. 2010;11:265. doi: 10.1186/1471-2164-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiess R, Schoofs A, Heinzel HG. Anatomy of the stomatogastric nervous system associated with the foregut in Drosophila melanogaster and Calliphora vicina third instar larvae. J Morphol. 2008;269:272–282. doi: 10.1002/jmor.10581. [DOI] [PubMed] [Google Scholar]
- 6.Schoofs A, Niederegger S, Spiess R. From behavior to fictive feeding: anatomy, innervation and activation pattern of pharyngeal muscles of Calliphora vicina 3rd instar larvae. J Insect Physiol. 2009;55:218–230. doi: 10.1016/j.jinsphys.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16:124–130. doi: 10.1016/S0168-9525(99)01957-5. [DOI] [PubMed] [Google Scholar]
- 9.Bienz M. Homeotic genes and positional signalling in the Drosophila viscera . Trends Genet. 1994;10:22–26. doi: 10.1016/0168-9525(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Lengyel JA, Liu XJ. Posterior gut development in Drosophila: a model system for identifying genes controlling epithelial morphogenesis. Cell Res. 1998;8:273–284. doi: 10.1038/cr.1998.27. [DOI] [PubMed] [Google Scholar]
- 11.Technau G, Campos-Ortega JA. Fate mapping in wild type Drosophila melanogaster . Wilhelm Roux’s Arch Dev Biol. 1985;194:196–212. doi: 10.1007/BF00848247. [DOI] [Google Scholar]
- 12.Murakami R, Takashima S, Hamaguchi T. Developmental genetics of the Drosophila gut: specification of primordia, subdivision and overt-differentiation. Cell Mol Biol (Noisy-le-grand) 1999;45:661–676. [PubMed] [Google Scholar]
- 13.Kim SK, Melton DA. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc Natl Acad Sci USA. 1998;95:13036–13041. doi: 10.1073/pnas.95.22.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 15.Pankratz MJ, Hoch M. Control of epithelial morphogenesis by cell signaling and integrin molecules in the Drosophila foregut. Development. 1995;121:1885–1898. doi: 10.1242/dev.121.6.1885. [DOI] [PubMed] [Google Scholar]
- 16.Stainier DY. No organ left behind: tales of gut development and evolution. Science. 2005;307:1902–1904. doi: 10.1126/science.1108709. [DOI] [PubMed] [Google Scholar]
- 17.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 18.Nakagoshi H. Functional specification in the Drosophila endoderm . Dev Growth Differ. 2005;47:383–392. doi: 10.1111/j.1440-169X.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 19.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubreuil RR, Grushko T, Baumann O. Differential effects of a labial mutation on the development, structure, and function of stomach acid-secreting cells in Drosophila melanogaster larvae and adults. Cell Tissue Res. 2001;306:167–178. doi: 10.1007/s004410100422. [DOI] [PubMed] [Google Scholar]
- 21.Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336:309–323. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- 22.Hartenstein AY, Rugendorff A, Tepass U, Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–1220. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- 23.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 24.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 25.Li TR, White KP. Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev Cell. 2003;5:59–72. doi: 10.1016/S1534-5807(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 26.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 29.Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5:290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie T. Stem cell in the adult Drosophila hindgut: just a sleeping beauty. Cell Stem Cell. 2009;5:227–228. doi: 10.1016/j.stem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Xu N, Wang SQ, Tan D, Gao Y, Lin G, et al. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2011;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 34.Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory L, Came PJ, Brown S. Stem cell regulation by JAK/STAT signaling in Drosophila. Semin Cell Dev Biol. 2008;19:407–413. doi: 10.1016/j.semcdb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2011;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DEL. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol. 2007;73:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggrawal K, Silverman N. Peptidoglycan recognition in Drosophila. Biochem Soc Trans. 2007;35:1496–1500. doi: 10.1042/BST0351496. [DOI] [PubMed] [Google Scholar]
- 44.Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 2007;75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuczynski J, Costello EK, Nemergut DR, Zaneveld J, Lauber CL, et al. Direct sequencing of the human microbiome readily reveals community differences. Genome Biol. 2010;11:210. doi: 10.1186/gb-2010-11-5-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–274. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brummel T, Ching A, Seroude L, Simon AF, Benzer S. Drosophila lifespan enhancement by exogenous bacteria. Proc Natl Acad Sci USA. 2004;101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daffre S, Kylsten P, Samakovlis C, Hultmark D. The lysozyme locus in Drosophila melanogaster: an expanded gene family adapted for expression in the digestive tract. Mol Gen Genet. 1994;242:152–162. doi: 10.1007/BF00391008. [DOI] [PubMed] [Google Scholar]
- 52.Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, et al. A model of bacterial intestinal infections in Drosophila melanogaster . PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kambris Z, Brun S, Jang IH, Nam HJ, Romeo Y, et al. Drosophila immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol. 2006;16:808–813. doi: 10.1016/j.cub.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 54.Chang CI, Ihara K, Chelliah Y, Mengin-Lecreulx D, Wakatsuki S, et al. Structure of the ectodomain of Drosophila peptidoglycan-recognition protein LCa suggests a molecular mechanism for pattern recognition. Proc Natl Acad Sci USA. 2005;102:10279–10284. doi: 10.1073/pnas.0504547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 56.Basset A, Khush RS, Braun A, Gardan L, Boccard F, et al. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc Natl Acad Sci USA. 2000;97:3376–3381. doi: 10.1073/pnas.070357597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/S1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 58.Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2006;2:e56. doi: 10.1371/journal.ppat.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aggarwal K, Silverman N. Positive and negative regulation of the Drosophila immune response. BMB Rep. 2008;41:267–277. doi: 10.5483/BMBRep.2008.41.4.267. [DOI] [PubMed] [Google Scholar]
- 60.Gottar M, Gobert V, Michel T, Belvin M, Duyk G, et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:640–644. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- 61.Choe KM, Lee H, Anderson KV. Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proc Natl Acad Sci USA. 2005;102:1122–1126. doi: 10.1073/pnas.0404952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellroth P, Karlsson J, Hakansson J, Schultz N, Goldman WE, et al. Ligand-induced dimerization of Drosophila peptidoglycan recognition proteins in vitro. Proc Natl Acad Sci USA. 2005;102:6455–6460. doi: 10.1073/pnas.0407559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee KZ, Ferrandon D. Negative regulation of immune responses on the fly. EMBO J. 2011;30:988–990. doi: 10.1038/emboj.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaidman-Remy A, Herve M, Poidevin M, Pili-Floury S, Kim MS, et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, et al. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basbous N, Coste F, Leone P, Vincentelli R, Royet J, et al. The Drosophila peptidoglycan-recognition protein LF interacts with peptidoglycan-recognition protein LC to downregulate the Imd pathway. EMBO Rep. 2011;12:327–333. doi: 10.1038/embor.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J. The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe. 2008;3:293–303. doi: 10.1016/j.chom.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 70.Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, et al. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe. 2008;4:147–158. doi: 10.1016/j.chom.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Aggarwal K, Rus F, Vriesema-Magnuson C, Erturk-Hasdemir D, Paquette N, Silverman N. Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS Pathog. 2008;4:e1000120. doi: 10.1371/journal.ppat.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kleino A, Myllymaki H, Kallio J, Vanha-aho LM, Oksanen K, et al. Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol. 2008;180:5413–5422. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 73.Lee WJ. Bacterial-modulated host immunity and stem cell activation for gut homeostasis. Genes Dev. 2009;23:2260–2265. doi: 10.1101/gad.1858709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]