Abstract
The non-coding microRNA (miRNA) is involved in the regulation of hepatitis C virus (HCV) infection and offers an alternative target for developing anti-HCV agent. In this study, we aim to identify novel cellular miRNAs that directly target the HCV genome with anti-HCV therapeutic potential. Bioinformatic analyses were performed to unveil liver-abundant miRNAs with predicted target sequences on HCV genome. Various cell-based systems confirmed that let-7b plays a negative role in HCV expression. In particular, let-7b suppressed HCV replicon activity and down-regulated HCV accumulation leading to reduced infectivity of HCVcc. Mutational analysis identified let-7b binding sites at the coding sequences of NS5B and 5′-UTR of HCV genome that were conserved among various HCV genotypes. We further demonstrated that the underlying mechanism for let-7b-mediated suppression of HCV RNA accumulation was not dependent on inhibition of HCV translation. Let-7b and IFNα-2a also elicited a synergistic inhibitory effect on HCV infection. Together, let-7b represents a novel cellular miRNA that targets the HCV genome and elicits anti-HCV activity. This study thereby sheds new insight into understanding the role of host miRNAs in HCV pathogenesis and to developing a potential anti-HCV therapeutic strategy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-0940-6) contains supplementary material, which is available to authorized users.
Keywords: microRNA, Let-7b, HCV
Introduction
Hepatitis C virus (HCV) frequently causes chronic infection, leading to hepatic fibrosis and hepatocellular carcinoma [1]. Due to the lack of viral vaccine, the population affected by HCV infection is increased substantially [2]. With the strong side-effects and the moderate successful rate associated with the first-line interferon (IFN)-based treatment [3], development of effective therapeutic regimens is still an emerging focus in the control of HCV infection.
Small molecules such as telaprevir have been developed to alleviate disease progression. However, the infidelity of HCV RNA polymerase constantly causes mutation and genome instability that result in the generation of drug-resistant viral strain [4, 5]. Targeting the host factors with important roles in viral infection offers an alternative strategy for development of anti-HCV regimen [6]. Apart from host proteins, a new class of small non-coding endogenous RNA molecule microRNA (miRNA) has been recently unveiled [7]. Although it is not yet fully clarified, miRNA is involved in various biological functions, including the response to HCV infection [7–10]. For example, miR-122 enhances whereas mir-199a* suppresses HCV replication and viral production [11–13]; interferon β (IFN-β)-mediated attenuation of viral replication is associated with an increase in miRNAs that have predicted target sequences within the HCV genome [14]. In addition, miRNA effectors including Argonaute 2 (Ago2) and DDX6 were found to positively regulate HCV replication [15, 16]. These findings suggest that cellular miRNAs regulate HCV gene expression and play roles in the host response against HCV infection.
In this study, bioinformatic analyses were performed to identify liver miRNAs targeting the HCV genome. Various cellular and viral systems were used to confirm bioinformatic prediction and to investigate the functional effects of the selected miRNAs. Our data reveal for the first time that let-7b is a negative regulator of HCV replication with the effective target sequences located on the 5′-untranslation region (UTR) and NS5B coding region of the HCV genome. The suppressive effect of let-7b on HCV RNA is not through translation inhibition. Besides, let-7b and Peginterferon alpha-2a (IFNα-2a) elicit a synergistic inhibitory effect on HCV infection. The roles of let-7b in the regulation of HCV pathogenesis and in the development of novel anti-HCV therapeutic strategy are discussed.
Materials and methods
Materials
The plasmid pRep-Feo and the replicon cells Huh7/Rep-Feo were obtained from Dr. Naoya Sakamoto (Tokyo Medical and Dental University). The plasmids pFL-J6/JFH, pJ6/JFH(p7-Rlu2A), and pJ6/JFH(p7-Rlu2A)GNN, the ConI replicon cells and Huh7.5 [17, 18], were kindly provided by Professor Charles Rice (The Rockefeller University, NY). The plasmid JC1-Luc2A, which replaced the Rluc gene of pJ6/JFH(p7-Rlu2A) to firefly luciferase (Luc) gene was kindly provided by Professor Robert T. Schooley (University of California San Diego, CA). Pre-miRNA, miRNA inhibitors and negative control for miRNA and miRNA inhibitors were purchased from Ambion (Austin, TX). The pLKO.1-shGFP control plasmid (clone ID: TRCN0000072197) and the two pLKO.1-shHMGA2 plasmids (clone ID: TRCN0000021965 and TRCN0000021968) were purchased from National RNAi Core Facility (Academia Sinica, Taiwan). The Lipofectamine 2000 (LF2000) and RNAiMAX transfection reagents were purchased from Invitrogen (Carlsbad, CA). The anti-HCV NS5A antibody was purchased from BioDesign (Carmel, NY). The anti-HCV Core antibody was purchased from Affinity BioReagents (Golden, CO). The anti-β-actin antibody was purchased from Sigma (St. Louis, MO). The IFNα-2a was purchased from Roche (Mannheim, Germany). The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)- 2H-tetrazolium (MTS) reduction assay and the luciferase assay reagents were purchased from Promega (Madison, WI).
Identification of liver miRNAs targeting the HCV genome
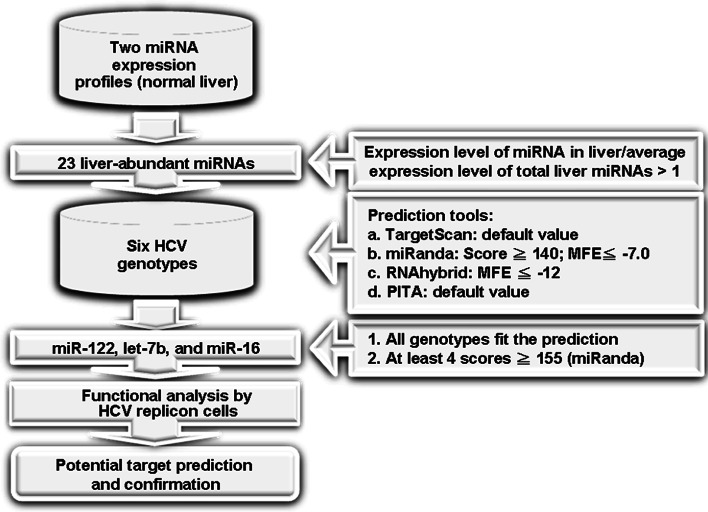
Bioinformatic strategy for the identification of liver miRNAs targeting the HCV genome is presented in Fig. 1. Briefly, two published miRNAs expression profiles [19, 20] were used to select for liver-abundant miRNAs. The miRNA is defined as liver abundance when the expression level of a specific liver miRNA divided by the average expression level of the total liver miRNAs is greater than one. The 23 miRNAs (Supplementary Table 1) that were identified as liver abundant in the two profiling databases were subject to bioinformatic analyses using miRanda, RNAhybrid, TargetScan, and PITA [21–24] to predict their target sequences on all six HCV genotypes (Supplementary Table 2). According to the calculation of miRanda, a filter was set to select for miRNAs of which the prediction scores for at least four genotypes were higher than 155. These miRNAs were considered as the candidate liver miRNAs targeting the HCV genome.
Fig. 1.
Bioinformatic strategy for identifying liver miRNAs with target sites on HCV genome. Two published normal liver tissue miRNA expression profiles were used to select liver-abundant miRNAs for bioinformatics analyses as described in “Materials and methods”
Cell culture and viability assay
Human hepatoma Huh7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). The Huh7.5 cells that represent a Huh7 subline and are highly permissive for HCV replication were maintained in DMEM with 1% non-essential amino acid (NEAA). The ConI cells were cultured in DMEM supplemented with 10% FBS and 750 μg/ml G418. The Huh7/Rep-Feo subgenome replicon cells were maintained in the same medium except that 1% NEAA was added and only 250 μg/ml G418 was used [25]. Viable cells were determined by the CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit.
Plasmid construction
For luciferase (luc) reporter plasmids, the predicted miRNA responsive element (MRE) for let-7b, as listed in Table 1, was inserted into the EcoRI/XbaI site downstream of the luciferase gene in phDab2-luc [26] to generate pluc-c-let-7b, pluc-MRE1, pluc-MRE2, pluc-MRE3, and pluc-MRE4. For generation of HCV subgenome mutants with nucleotide mismatch at the ‘‘seed region’’ of let-7b or “S1 binding site” of miR-122, site-directed mutagenesis was performed by QuickChange (Stratagene, CA) using pRep-Feo as the template [25] and the primer sets for mMRE1, mMRE2, mMRE3, and miR-122-mut as listed in Table 1. To generate a capped RNA transcript encoding firefly luciferase (FLuc) for use as an internal control in the transient translation assay, the FLuc gene from the plasmid pGL3-Promoter (Madison, WI) was first digested by the restriction enzyme NcoI. The nucleotides at the sticky end were filled up as blunt end by Klenow DNA polymerase and the FLuc gene was then excised by the restriction enzyme XbaI. On the other hand, pRL-TK plasmid was digested by NheI and the sticky end was filled up by Klenow DNA polymerase followed by XbaI restriction enzyme digestion to remove the Renilla luciferase gene (RLuc). The Fluc gene fragment was then cloned into pRL-TK to replace RLuc to generate pLUC-TK. This plasmid contains a T7 promoter and can transcribe mRNA after linearization by BamHI.
Table 1.
The sequences for the primers used in this study
| Primer name | Primer sequences | Size (mer) |
|---|---|---|
| For luciferase reporter constructs a | ||
| c-let-7b | S:5′-CTAGAAACCACACAACCTACTACCTCAG-3′ | 22 |
| AS:5′-AATTCTGAGGTAGTAGGTTGTGTGGTTT-3′ | ||
| MRE1 | S:5′-CTAGACACCATGAGCACGAATCCTAAACCTCAG-3′ | 27 |
| AS: 5′-AATTCTGAGGTTTAGGATTCGTGCTCATGGTGT-3′ | ||
| MRE2 | S: 5′-CTAGAGGCAAAAGGGTGTACTACCTCAG-3′ | 22 |
| AS: 5′-AATTCTGAGGTAGTACACCCTTTTGCCT-3′ | ||
| MRE3 | S: 5′-CTAGAAGCCACTTGACCTACCTCAG-3′ | 19 |
| AS: 5′-AATTCTGAGGTAGGTCAAGTGGCTT-3′ | ||
| MRE4 | S:5′-CTAGAGCCGCATGACTGCAGAGAGTGCTGATACTGGCCTCTG-3′ | 38 |
| AS: 5′-AATTCAGAGGCCAGTATCAGCACTCTCTGCAGTCATGCGGCT-3′ | ||
| For in vitro mutagenesis b | ||
| mMRE1 | F: 5′-GAGCACGAATCCTAATGGAGTAAGAAAAACCAAAGG-3′ | 36 |
| R: 5′-CCTTTGGTTTTTCTTACTCCATTAGGATTCGTGCTC-3′ | ||
| mMRE2 | F: 5′-CTGGCAAAAGGGTGTATTATCTCACTCGCGATCCCAC-3′ | 47 |
| R: 5′-GTGGGATCGCGAGUGAGATAATACACCCTTTTGCCAG-3′ | ||
| mMRE3 | F: 5′-CATTGAGCCACTTGACCTTCCGCAGATCATTGAACGACTC-3′ | 40 |
| R: 5′-GAGTCGTTCAATGATCTGCGGAAGGTCAAGTGGCTCAATG-3′ | ||
| miR-122 mut | F: 5′-CCCGATTGGGGGCGACACAGCACCATAGATCACTCCCC-3′ | 38 |
| R: 5′-GGGGAGTGATCTATGGTGCTGTGTCGCCCCCAATCGGG-3′ | ||
| For synthesis of mature miRNA c | ||
| 7b | S: 5′UGAGGUAGUAGGUUGUGUGGUU 3′ AS: | 22 |
| 5′UUCCACACAACCUACUACCUCA 3′ | ||
| m7b | S: 5′UGAACUAAUAGGUUGUGUGGUU 3′ AS: | 22 |
| 5′UUCCACACAACCUAUUAGUUCA 3′ | ||
S sense strand, AS antisense strand F forward primer, R reverse primer
aThe bold letters indicate the predicted sequence while the other sequence was generated for cloning into EcoRI/XbaI restriction enzyme site
b,cThe bold letters indicate the mutated nucleotides
Transient transfection and luciferase activity assay
For transient transfection, Huh7/Rep-Feo cells were seeded at a density of 1 × 104 cells/well for 24 h and the miRNA precursor or inhibitor was transfected into cells by LF2000. At 72 h after transfection, the luciferase activity was quantified using the Bright-Glo luciferase assay reagent. On the other hand, 293T cells were seeded at a density of 2 × 104 cells/well and the reporter plasmid, pRL-TK and miRNA precursor (100 nM) were cotransfected into cells by LF2000. At 24 h after transfection, the firefly and Renilla luciferase activities were quantified using the Dual-Glo luciferase assay reagent.
For RNA transfection, the XbaI-digested wild-type pRep-Feo or the mutant subgenome plasmid was subject to in vitro transcription for RNA synthesis. The Huh7.5 cells were transfected with 10 μg HCV RNA, 100 pmol miRNA, and 10 μg pRL-TK by electroporation using Gene Pulser II (Bio-Rad) at 260 V and 950 μF.
For permissive assay, Huh7 cells were seeded at a density of 2 × 105 cells/well and were transfected with the miRNA precursor (100 pmol) by RNAiMAX (Invitrogen) for 24 h. The transfected cells were subsequently infected with HCVcc (6 × 106 copies/ml) for 4 h. After washing away the virus, the cells were cultured for 72 h and the HCV RNA was detected from the infected cells by real-time reverse transcription-PCR (RT-PCR).
Ribonucleoprotein immunoprecipitation (RIP) assay
The RIP assay was performed using the miRNA isolation kit (Wako Laboratory Chemicals, Osaka, Japan) according to the manufacturer’s instruction. Briefly, 10 μg of Rep-Feo subgenomic RNA was obtained by in vitro transcription and was co-transfected with 100 pmol of the indicated miRNA into Huh7.5 cells by electroporation. At 6 h after transfection, the cells were lysed in 1 ml of cell lysis solution (20 mM Tris–HCl, pH 7.4, 2.5 mM MgCl2, 200 mM NaCl, and 0.05% NP40). After centrifugation, the supernatant was collected and mixed with anti-human Ago2 monoclonal antibody-conjugated agarose beads for 2 h at 4°C. After several washes with cell lysis solution, the HCV RNAs associated with Ago2-containing miRNA ribonucleoprotein (miRNP) complexes were eluted and were quantified by real-time RT-PCR. For knockdown of endogenous let-7b, the let-7b inhibitor (100 pmol) was transfected into Huh7.5 cells for 24 h followed by electroporation of the cells with the Rep-Feo HCV subgenomic RNA mutated at the miR-122 binding site (10 μg) and 100 pmol of let-7b inhibitor.
Western-blot analysis
The cell lysates were harvested and separated by 10% SDS-PAGE. The expression of HCV viral protein was detected using ECL kit (Perkin-Elmer) as described previously [27].
Production of HCVcc infectious particles and infectivity inhibition assay
The HCVcc infectious particle was produced as described previously [28]. Briefly, in vitro transcribed J6/JFH-based HCV genomic RNA was electroporated into Huh7.5 cells. The virus-containing supernatant was clarified by low-speed centrifugation, passed through a 0.45-μm filter, and concentrated by ultracentrifugation.
For infectivity inhibition assay, Huh7.5 cells were seeded in a six-swell plate at a density of 2 × 105 cells/well. At 24 h after plating, 100 nM miRNA was transfected into the cells using LF2000. HCVcc (0.1 MOI) was then added to each well for 4 h and the transfection complex was replaced with 2% FBS-containing medium for 72 h. The cells were fixed and stained by anti-Core antibody following by FITC-conjugated second antibody, counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and the infectious foci were counted using fluorescence microscopy.
For JC1-Luc2A HCV reporter virus, Huh7.5 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well. At 24 h after plating, HCV reporter virus (0.01 MOI) was added to each well for 4 h. Then 100 nM of the indicated miRNA was transfected into the infected cells using RNAiMax and the transfection complex was replaced with 2% FBS-containing medium for 72 h. The cell lysates were collected for luciferase activity and MTS assay.
RNA isolation and real-time quantitative RT-PCR
Total RNAs were extracted using ReZol method and were quantified using a NanoDrop spectrophotometer. For quantification of HCV RNA expression, total cellular RNA (100 ng) was subject to one-step RT-PCR (25 μl) containing 2× TaqMan master mix and the primer/probe set for HCV (HCV-F: 5′-TGCGGAACCGGTGAGTACA-3′, HCV-R: 5′-CTTAAGGTTTAGGATTCGTGCTCAT-3′, and probe: 5′-CACCCTATCAGGCAGTACCACAAGGCC-3′). The reaction condition was one cycle of 48°C for 30 min, one cycle of 95°C for 10 min, and 40 cycles of 95°C for 15 s followed by 60°C for 1 min using the ABI Prism 7000 Sequence Detection System. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a normalization control. HCV RNA expression was quantified by the ∆∆C t method, where C t represented the threshold cycle.
The TaqMan® microRNA Assay System was used for miRNA detection and quantification. Briefly, the RT reaction was performed in a final volume of 15 μl containing 1.5 μl of 10× RT buffer, 2.5 μl of total RNA (25 ng), 3 μl of 5× miRNA-specific RT primer, 0.15 μl of 100 mM dNTP, 0.2 μl of 40 U/μl RNase inhibitor, and 1 μl of MultiScribe reverse transcriptase (50 U/μl). The reaction condition was 30 min at 16°C, 30 min at 42°C, and 5 min at 85°C. Real-time PCR was then performed in a 20-μl PCR containing 1.33 μl of RT product, 10 μl of 10× TaqMan Universal PCR master mix, and 1 μl of the primer and probe mix from the TaqMan® MicroRNA Assay Kit. The reaction condition was 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. The expression of RNU6B gene was used as the internal control.
HCV translation assay
Huh7.5 cells were seeded into a six-well plate at a density of 4 × 105 cells/well. At 24 h after transfection of let-7b miRNA (100 nM), the replication-deficient J6/JFH (p7-Rlu2A) GNN mutant RNA (1.25 μg/well) was transfected together with the capped and polyadenylated FLuc mRNA (125 ng/well) by LF2000. After 4 h, cells were harvested and dual luciferase activity assays were performed.
Statistical analysis
Statistical analysis was performed by Student’s t test. p < 0.05 was considered as statistically significant.
Results
Identification and functional characterization of liver miRNAs with potential recognition sequences on HCV genome
A bioinformatic strategy as described in the “Materials and methods” section was developed to search for novel miRNAs with potential recognition sequences on HCV genome (Fig. 1). Three miRNAs including miR-122, let-7b, and miR-16 were uncovered. To elucidate whether these miRNAs have any functional effect on HCV infection, Huh7/Rep-Feo replicon cells (genotype 1b) were transfected with the indicated miRNAs and the luciferase activity was determined (Fig. 2a, b). In accord with a previous report [11], miR-122 enhanced HCV expression (p < 0.05). Notably, let-7b significantly suppressed HCV expression (p < 0.01) while miR-16 had only a moderate effect (p = 0.343).
Fig. 2.
Characterization of miRNAs with putative target sites on HCV genome. a Genomic structures of HCV maintaining in Huh7/Rep-Feo and ConI cells. b The miRNA precursors or mutant let-7b (m7b) were transfected into Huh7/Rep-Feo cells. MTS and luciferase activity assays were then performed at 72 h post-transfection. c The miRNA precursors (left panel) or the mutant form of let-7b (right panel) were transfected into the ConI cells. Western-blot analysis was then performed using the anti-NS5A and anti-β-actin antibody at 72 h post-transfection. The ratios for the relative band intensities of NS5A after normalization with β-actin were shown. NC negative control miRNA. d The precursors of let-7b (left panel) or shHMGA2 (right panel) were transfected into ConI replicon cells. Western-blot analysis was then performed using the anti-NS5A and anti-β-actin antibody at 72 h post-transfection. NC negative control miRNA. e Real-time RT-PCR of let-7b was performed using the total RNAs from the indicated cells. RNU6B was used as an internal control for normalization. The data represented the mean ± SD (n = 3; *p < 0.05, **p < 0.01, ***p < 0.001). f The let-7b inhibitor (Anti-let-7b) or control inhibitor (Anti-NC) was transfected into Huh7 cells, respectively. HCV RNA expression was quantified by real-time RT-PCR using the total RNAs from the indicated transfected cells. The expression of GAPDH was used as a control for normalization. The data represented the mean ± SD (n = 3; *p < 0.05)
To further confirm that let-7b can regulate HCV RNA accumulation, mutated let-7b (m7b) was designed to change three nucleotides on the wild-type let-7b sequences (Fig. 2b; Table 1). As calculated and predicted by miRanda, no m7b target sequence was found on HCV genome (data not shown). After transfection into Huh7/Rep-Feo replicon cells, m7b abrogated the inhibitory effect of let-7b on the luciferase activity (Fig. 2b) thereby demonstrating that let-7b is a negative regulator of HCV expression.
The effects of the three selected miRNAs on HCV expression were also evaluated using ConI replicon cells (Fig. 2c). These miRNAs were transfected into the replicon cells and the expression of viral proteins was determined at 72 h after transfection. Western-blot analysis revealed that miR-122 increased NS5A expression, while let-7b but not miR-16 caused a decrease in NS5A (Fig. 2c, left panel). Furthermore, the let-7b mutant form m7b lost its inhibitory effect on HCV and did not alter NS5A expression (Fig. 2c, right panel). These data thereby implicate that let-7b elicits suppressive activity in HCV protein expression.
HMGA2 is one of the major let-7b target genes and is down-regulated in let-7b-transfected ConI cells as previous reported (Fig. 2d, left panel) [29]. To rule out down-regulation of host transcripts accounts for the inhibitory effect of let-7b on HCV expression, HMGA2 was knockdown by two independent shHMGA2 plasmids. Although HMGA2 was significantly down-regulated in the shHMGA2 expressing cells, no effect was observed for the expression of the viral protein NS5A (Fig. 2d, right panel). These data indicate that down-regulation of HMGA2 does not contribute to the effect of let-7b on HCV expression.
To further delineate the association between let-7b and HCV infectivity, let-7b expression in various HCV-associated cell lines were determined. As shown in Fig. 2e, let-7b expression in the HCV permissive Huh7.5 cells was less than its expression in the parental Huh7 cells (p < 0.01). Consistent with these observations, ConI cells bearing replicated HCV genome also had much lower let-7b (Fig. 2e, p < 0.01). Furthermore, Huh7 cells were more permissive for HCVcc infection when let-7b was inactivated by the let-7b inhibitor (Fig. 2f, p < 0.05). These data indicate that the cells capable of persistent HCV replication are usually associated with a low level of let-7b expression.
Let-7b reduces HCVcc infectivity
The HCVcc system was used to elucidate the role of let-7b in HCV infectivity. Let-7b was transfected into Huh7.5 cells followed by infection with HCVcc derived from J6/JFH-1 (genotype 2a). Hepatitis C virus expression was then monitored by fluorescent staining using the anti-HCV Core antibody (Fig. 3a). Our data revealed that let-7b reduced HCV infectivity for 42% (p < 0.05) while miR-122 enhanced the infectivity for 63% (p < 0.05) when compared to the cells expressing negative control miRNA (Fig. 3b). The HCV RNA was also decreased in let-7b-transfected cells (Fig. 3c, p < 0.05) indicating that let-7b suppresses HCV RNA level leading to a decrease in viral production.
Fig. 3.
Let-7b reduces HCVcc infectivity. a The indicated miRNAs were transfected into Huh7.5 cells for 24 h followed by infection with J6/JFH-based HCVcc. After 72 h, the cells were stained by anti-Core antibody. Nuclei were visualized by DAPI staining. NC negative control. b The infectious foci were counted by fluorescence microscopy. The infectivity for the cells transfected with negative control miRNA (NC) was set as one. The data represented the mean ± SD (n = 3; *p < 0.05). c HCV RNA expression was quantified by real-time RT-PCR using the total RNAs from the indicated transfected cells. The expression of GAPDH was used as a control for normalization. The data represented the mean ± SD (n = 3; *p < 0.05). d The miRNA precursors or the mutant let-7b (m7b) were transfected into Huh7.5 cells for 24 h followed by infection with JC1-luc2A HCV reporter virus. After 72 h, cell viability was determined by MTS assay and the cell extracts were collected for luciferase activity assay. The relative firefly luciferase versus MTS activity was shown and the negative control miRNA was arbitrarily denoted as one. The data represented the mean ± SD (n = 3; *p < 0.05; ***p < 0.001)
To further confirm the negative regulatory effect of let-7b on HCVcc production, Huh7.5 cells were infected with the JC1-Luc2A HCV reporter virus and the luciferase activity was used to evaluate HCV viral production. Our data revealed that let-7b reduced 75% of the HCV reporter virus luciferase activity (Fig. 3d, p < 0.001) when compared to the cells expressing negative control miRNA. As a control, miR-122 increased 87% of the HCV reporter virus luciferase activity (p < 0.05). In contrast, mutation of let-7b (m7b) diminished its inhibitory effect on HCV expression and resulted in a slight inhibition of HCV reporter virus luciferase activity (p < 0.05). Together, these data implicate that inhibition of HCV RNA expression accounts for the suppressive effect of let-7b on HCV infection.
Let-7b physically interacts with the HCV genome
Argonaute 2 is the core component of miRNA-induced silencing complex (miRISC), which binds the guide miRNA to silence target mRNAs [30]. To determine whether Ago2 together with let-7b and HCV RNA form a miRISC complex, HCV RNA was co-transfected with let-7b into Huh7.5 cells followed by immunoprecipitation using the anti-Ago2 antibody (Ago2-IP). Because the “site 1” sequence (Table 1) is the most important HCV genome sequence for miR-122 binding and for regulation of HCV by miR-122, “site 1” mutation S1-p34 m (miR-122-mut) [11, 12] was introduced into the HCV subgenome to minimize the interference from the endogenous miR-122. The amount of Ago2-IP-associated HCV RNA was quantified by real-time RT-PCR. As shown in Fig. 4a, both HCV RNA subgenomes with wild-type or mutant miR-122 binding site were found to associate with the miRNP complex, while let-7b promoted HCV-miRNP interactions when miR-122 binding site was mutated. The total amount of let-7b associating with the Ago2-IP fraction was unchanged (Fig. 4b). Furthermore, knockdown of endogenous let-7b in Huh7.5 cells followed by Ago2-IP revealed that both the amounts of HCV RNA (Fig. 4c) and let-7b (Fig. 4d) in the Ago2-IP fraction were dramatically decreased. These data thereby indicate that let-7b physically interacts with HCV RNA in the Ago2-containing miRNP complex.
Fig. 4.
Let-7b is associated with HCV genome in miRNP complex. a, b Huh7.5 cells were transfected with 100 pmol of miRNA along with 10 μg of either wild-type (miR-122-wt) or mutant (miR-122-mut) HCV subgenome RNA. The cell extracts were collected to perform co-immunoprecipitation with the anti-Ago2 antibody (Ago2-IP). HCV replicon RNA (panel a) and let-7b (panel b) were measured by real-time RT-PCR using total RNA sample from the Ago2-IP fraction. c ,d For knockdown of endogenous let-7b, Huh7.5 cells were transfected with 100 pmol of the indicated miRNA inhibitors (Anti-NC and Anti-let-7b) for 24 h followed by electroporation of the cells with 10 μg of miR-122-mut and 100 pmol of the indicated miRNA inhibitors. The cell extracts were collected to perform Ago2-IP and the HCV replicon RNA (panel c) and let-7b (panel d) were measured by real-time RT-PCR. The relative levels for HCV RNA and let-7b in the Ago2-IP complexes were shown. The data represented the mean ± SD (n = 3; *p < 0.05, **p < 0.01)
Identification of let-7b-responsive elements on the HCV genome
To identify MRE for let-7b, HCV-N genomic sequences (genotype 1b) were subject to bioinformatic prediction using miRanda and RNAhybrid. Several putative MREs for let-7b were revealed (Fig. 5a; Supplementary Tables 3, 4). It is noted that MRE2 (nt 8,745–8,766) and MRE3 (nt 8,977–8,995) that located within the HCV NS5B coding region had the highest prediction score and were selected for analysis. Moreover, the MRE1 (nt 338–365) and MRE4 (nt 9,566–9,603) at the non-coding region that had the lowest minimum free energies (MFE) in NCR region (Table 2) were also subject to further analysis.
Fig. 5.
The MREs of let-7b are located at the NS5B coding sequences and 5′-UTR of HCV genome. a Schematic representation for the predicted MREs of let-7b on HCV genome. The number corresponds to the first nucleotide of the predictive seed region. b The precursor of let-7b or negative control miRNA (NC) was co-transfected with the indicated luciferase reporter plasmid and pRL-TK into 293T cells for 24 h. The luciferase activities were measured and the relative firefly versus Renilla luciferase activity was shown. The plasmid containing perfect complementary sequence of let-7b (c-let-7b) and the vector control reporter plasmid (luciferase activity arbitrarily denoted as one) was used as the positive and negative control, respectively. The data represented the mean ± SD (n = 3; *p < 0.05; **p < 0.01; ***p < 0.001; NS no significance). c The RNAs for wild-type (wt) HCV genome and the genome with mutations at the indicated MREs regions were obtained by in vitro transcription and were transfected individually into Huh7.5 cells along with let-7b precursor or negative control miRNA (NC) by electroporation. The luciferase activity was determined at 96 h post-transfection. The luciferase activity generated by wild-type HCV genome was arbitrarily denoted as 1 and the luciferase activity derived from each mutant HCV genome normalized by luciferase activity from wild-type was shown. The data represented the mean ± SD (n = 3; **p < 0.01)
Table 2.
Characterization of let-7b predicted binding sites on HCV genome

aMFE was calculated by RNAhybrid while Score was calculated by miRanda
To determine whether any of these sequences is the authentic MREs for let-7b, luciferase reporter plasmids with the reported let-7b target sequence (pluc-let-7b) and the putative MREs in HCV genome were constructed (pluc-MRE1, pluc-MRE2, pluc-MRE3, and pluc-MRE4). After co-transfection with let-7b or a negative control miRNA into 293T cells, the luciferase activities for each individual reporter plasmid were measured. Our data revealed that let-7b decreased pluc-MRE1, pluc-MRE2, and pluc-MRE3 luciferase activity by 28, 31, and 37%, respectively (Fig. 5b). No effect was found for pluc-MRE4. These data indicate that the MRE1, MRE2, and MRE3 are the potential let-7b binding sites on HCV genome.
Mutations of the putative let-7b MREs were introduced into the HCV subgenome to exam whether these MREs are responsible for the suppressive effect of let-7b. Silent mutations of MRE2 and MRE3 were designed to avoid amino acid changes while a six-nucleotide substitution mutation was introduced into HCV Rep-Feo subgenome (wild-type) to generate mMRE1, mMRE2, mMRE3, and mMRE2,3, respectively. Structural analysis of these mutations revealed that most of the HCV RNA genome structures were maintained except that mMRE1 appeared to generate a small stem-loop structure (Supplementary Fig. S1). These mutated HCV RNAs were obtained by in vitro transcription and, together with let-7b, electroporated into Huh7.5 followed by analysis of luciferase activity. Our data revealed that the luciferase activities for HCV subgenome with mMRE1, mMRE2, and mMRE3 were 87, 40, and 86% higher than the wild-type HCV subgenome, respectively (Fig. 5c), implicating that let-7b-mediated suppression of HCV replicon activity is abrogated by mutating the target sequences on HCV genome. Moreover, HCV subgenome with double mutations of MRE2 and MRE3 synergistically enhanced luciferase activity when compared to the single mutant for these two MREs. The RNA structure did not contribute to the loss of let-7b responsiveness because Mfold analysis demonstrated that the wild-type and mutant HCV subgenome had similar RNA structure (Supplement Fig. S1). These data thereby indicate that 5′-UTR and NS5B coding sequences contain let-7b binding sites.
The antiviral effect of let-7b is independent of inhibition of HCV translation
It has been reported that miR-122 enhances HCV replication by stimulating internal ribosome entry site (IRES)-mediated translation in cultured cells [12]. Because MRE1 is located at domain IV of IRES [31], the possibility of let-7b modulating HCV replication through translation was also examined. The replication-deficient J6/JFH (p7-Rlu2A) GNN HCV mutant RNA was transfected along with a capped and polyadenylated FLuc mRNA as an internal control for transfection and translation. The ratio of Renilla luciferase (RLuc) to firefly luciferase (FLuc) activity was used to measure the IRES-directed translation activity. As shown in Fig. 6, miR-122 enhanced HCV IRES activity for approximate threefold, while miR-122 inhibitor resulted in approximate 50% decrease of the activity. However, let-7b or its inhibitor had no effects on HCV translation (Fig. 6). These data indicate that let-7b regulates HCV RNA replication through a mechanism independent of HCV translational regulation.
Fig. 6.
Let-7b decreases HCV RNA expression independent on translation inhibition. a, b Huh7.5 cells were transfected with the indicated miRNAs (panel a) or miRNA inhibitors (panel b). Twenty-four hour later, HCV RNAs carrying GND mutation and Renilla luciferase coding sequence were transfected with a capped and polyadenylated firefly luciferase mRNA. At 4 h after transfection, the cell lysates were subject to dual luciferase activity assays. The relative firefly versus Renilla luciferase activity is shown. The data represented the mean ± SD (n = 3; **p < 0.01; ***p < 0.001; NS no significance)
Let-7b and INFα-2a elicit synergistic anti-HCV activity
We examined further whether there is a synergistic effect between let-7b and IFNα-2a. The Huh7/Rep-Feo cells were treated with different concentrations of IFNα-2a and the luciferase activity for HCV subgenome was measured to determine the optimized dosage of IFNα-2a for synergistic study. As shown in Fig. 7a, the luciferase activity was suppressed by IFNα-2a in a dose-dependent manner with the IC50 equivalent to 1.39 ng/ml. When Huh7/Rep-Feo cells were transfected with let-7b followed by treatment with IFNα-2a, a 60 and 70% decrease in luciferase activity was observed in relative to let-7b or IFNα-2a alone, respectively (Fig. 7b, p < 0.01). These data thereby indicate that let-7b and IFNα-2a elicit synergistic inhibitory effect on HCV expression.
Fig. 7.
Let-7b and IFNα-2a elicit synergistic inhibitory effects on HCV RNA accumulation. a Huh7/Rep-Feo cells (3 × 104) were treated with the indicated doses of IFN-2α for 72 h and the luciferase activity and cell viability were determined. b Huh7/Rep-Feo cells were transfected with 100 nM of let-7b or negative control miRNA (NC) by RNAiMAX for 4 h followed by treatment with INF-2α or medium control for additional 72 h. The luciferase activity and cell viability were determined. The data represented the mean ± SD (n = 3) with the luciferase activity normalized by the cell viability. (*p < 0.05; **p < 0.01)
Discussion
The interplays between viral infection and miRNA have been demonstrated since the first report unfolding miR-32 as the negative regulator of primate foamy virus RNA accumulation [32]. Subsequently a number of miRNAs were found to elicit anti-HCV activity [11, 13, 14]. In this study, bioinformatic tools and virological analyses are employed to unveil novel cellular miRNAs associated with HCV infection. We demonstrate that, in addition to miR-122 that has been reported to augment HCV infection, let-7b targets the HCV genome leading to a decrease in HCV RNA accumulation and viral production. This study thereby represents the first report to identify let-7b as a negative regulator of HCV infection.
Let-7b is the first known human miRNA [33] that is closely associated with the status of cellular differentiation and is usually down-regulated in cancers [34, 35]. Experimental evidence we present in this study unveils the role of let-7b in the control of HCV pathogenesis. Let-7b expression is irreversibly correlated with HCV infectivity in the cell-based systems; the cell lines bearing replicated HCV genome that are permissive for HCV replication (such as Huh7.5 and Con1) usually have low levels of let-7b expression. Moreover, let-7b is associated with HCV genome in Ago2 miRNP complex. Cellular study further reveals that let-7b diminishes luciferase reporter gene expression in Huh7/Rep-Feo subgenome replicon (1b genotype), the viral protein expression in ConI replicon (1b genotype) and HCV RNA accumulation and viral production upon HCVcc infection (2a genotype). These findings not only indicate that let-7b plays a role in the host antiviral response, but also reveal the universal effects of let-7b on different HCV genotypes.
The molecular basis for the anti-HCV effect of let-7b is also elucidated in this study. Our data support the notion that let-7b directly interacts with the HCV genome and modulates virus production. Although a number of miRNAs have been reported to regulate HCV replication and pathogenesis, only miR-122 and miR199a* were demonstrated to directly target on HCV genome [11, 13]. Let-7b thereby represents the third cellular miRNA that elicits a direct effect on HCV genome and modulates HCV replication. In contrast to miR-122 and miR-199a*, of which the target sequences mapped to 5′-UTR [11, 13], one of the unique features for let-7b is that two of the let-7b target sequences, MRE2 and MRE3, are located within the coding region of NS5B. Although it is not common, miRNA has been shown to affect gene expression by interacting with mRNA coding regions [36, 37]. For example, miR-148 and miR-24 repress DNA methyltransferase 3b and p16 expression, respectively, primarily through the coding region recognition site [38, 39]. Let-7b targets the coding sequence of Dicer and establishes a miRNA/Dicer autoregulatory negative feedback loop. While the advantages for let-7b targeting the HCV coding sequence remain to be elucidated, let-7b symbolizes the first cellular miRNA with recognition sequences in the coding region of the HCV genome.
Although our data indicate that let-7b acts on the HCV genome leading to a decrease in HCV expression, we cannot rule out that the host factors regulated by let-7b may also play a role in the regulation of HCV expression. Several host factors are down-regulated by let-7b, including HMGA2 [40–42]. Moreover, TargetScan prediction reveals at least 79 cellular target genes regulated by let-7b have some associations with HCV infection. Despite that knockdown of HMGA2 does not have any effect of HCV protein expression, whether the other host factors mediating let-7b effects on HCV expression remains to be investigated.
While only let-7b meets our preset selection criteria, bioinformatic prediction data reveal that three other family members of let-7, including let-7a, let-7c, and let-7f, are also liver-abundant (Supplementary Table 1). Because let-7 family members differ by only one to a few nucleotides [43], let-7a, let-7c, and let-7f were also tested for their potential effects on HCV expression. As shown in Supplementary Fig. S2, let-7a and let-7f were expressed at a lower level in HCV-sensitive cell lines while let-7c was expressed at a higher level. In addition, these three miRNAs can also reduce HCV activity on subgenome replicon cells and reporter virus. However, let-7b exhibits more prominent suppressive effects than the others. Hence, it is likely that let-7 family members may act on HCV in a similar way to let-7b with various regulations.
In addition to controlling multiple cellular events, miRNA has been proposed as a therapeutic regimen for various diseases [44, 45]. Recently, a locked nucleic acid-modified oligonucleotide complementary to miR-122 exhibits a long-lasting suppression of HCV viremia in chronically infected chimpanzees [46]. Small-molecule inhibitors and activators of miR-122 have been developed to reduce HCV viral replication [47]. In this study, we found that let-7b plays a role in host defense to combat HCV infection and reduces HCV infectivity. The synergistically inhibitory effect of let-7b and IFNα-2a on HCV replication further implies that let-7b is a good candidate for developing an adjuvant regimen for IFNα-2a in a clinical setting.
In conclusion, we demonstrate for the first time that let-7b inhibits HCV expression and replication by targeting the conserved HCV 5′UTR and coding region. Furthermore, let-7b and IFNα-2a elicit synergistically inhibitory effect on HCV infection. This study thereby contributes to our understanding for let-7b on the control of HCV pathogenesis and offers new insight for developing novel anti-HCV therapeutic approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Dr. Naoya Sakamoto for providing HCV subgenome replicon cells, Professor Charles Rice (The Rockefeller University, USA) for providing ConI replicon, Huh7.5 cells, and plasmids pFL-J6/JFH, pJ6/JFH(p7-Rluc2A), Professor Robert T. Schooley (University of California-San Diego) for providing plasmid pJC1-Luc2A (with the permission of Apath). This work was supported in part by grants NSC 97-2320-B-039-026-MY3 and NSC 100-2320-B-039-007-MY3 (J.C.C.) and part by NSC-100-2911-I-009-101 (H.D.H.) from the National Science Council and part by Chang Gung Molecular Medicine Research Center Grant (C.P.T.).
Conflict of interest
None.
Abbreviations
- miRNA
microRNA
- HCV
Hepatitis C virus
- MRE
MicroRNA responsive element
- IFNα-2a
Peginterferon alpha-2a
- IFN
Interferon
- LF2000
Lipofectamine 2000
- DMEM
Dulbecco’s modified Eagle’s medium
- FITC
Fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Ju-Chien Cheng and Yung-Ju Yeh contributed equally to this work.
Contributor Information
Ju-Chien Cheng, Phone: +88-64-22053366, FAX: +88-64-22022073, Email: jccheng@mail.cmu.edu.tw.
Hsien-Da Huang, Phone: +88-63-5712121, FAX: +88-63-5739320, Email: bryan@mail.nctu.edu.tw.
References
- 1.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. doi: 10.1016/S0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Muh U, Welker M, Wincheringer D, Zhou Y, Chu HM, Lin C, Weegink C, Reesink H, Zeuzem S, Kwong AD. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767–1777. doi: 10.1053/j.gastro.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 5.De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 6.Ng TI, Mo H, Pilot-Matias T, He Y, Koev G, Krishnan P, Mondal R, Pithawalla R, He W, Dekhtyar T, Packer J, Schurdak M, Molla A. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology. 2007;45:1413–1421. doi: 10.1002/hep.21608. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe. 2008;3:375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 12.Jangra RK, Yi M, Lemon SM. Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J Virol. 2010;84:6615–6625. doi: 10.1128/JVI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami Y, Aly HH, Tajima A, Inoue I, Shimotohno K. Regulation of the hepatitis C virus genome replication by miR-199a. J Hepatol. 2009;50:453–460. doi: 10.1016/j.jhep.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JA, Zhang C, Huys A, Richardson CD. Human Ago2 is required for efficient microRNA 122 regulation of hepatitis C virus RNA accumulation and translation. J Virol. 2011;85:2342–2350. doi: 10.1128/JVI.02046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jangra RK, Yi M, Lemon SM. DDX6 (Rck/p54) is required for efficient hepatitis C virus replication but not for internal ribosome entry site-directed translation. J Virol. 2010;84:6810–6824. doi: 10.1128/JVI.00397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones CT, Murray CL, Eastman DK, Tassello J, Rice CM. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J Virol. 2007;81:8374–8383. doi: 10.1128/JVI.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu SD, Chu CH, Tsou AP, Chen SJ, Chen HC, Hsu PW, Wong YH, Chen YH, Chen GH, Huang HD. miRNAMap 2.0: genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res. 2008;36:D165–D169. doi: 10.1093/nar/gkm1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166–187. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 24.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 25.Tanabe Y, Sakamoto N, Enomoto N, Kurosaki M, Ueda E, Maekawa S, Yamashiro T, Nakagawa M, Chen CH, Kanazawa N, Kakinuma S, Watanabe M. Synergistic inhibition of intracellular hepatitis C virus replication by combination of ribavirin and interferon-alpha. J Infect Dis. 2004;189:1129–1139. doi: 10.1086/382595. [DOI] [PubMed] [Google Scholar]
- 26.Tseng CP, Huang CH, Tseng CC, Lin MH, Hsieh JT, Tseng CH. Induction of disabled-2 gene during megakaryocyte differentiation of K562 cells. Biochem Biophys Res Commun. 2001;285:129–135. doi: 10.1006/bbrc.2001.5133. [DOI] [PubMed] [Google Scholar]
- 27.Cheng JC, Chang MF, Chang SC. Specific interaction between the hepatitis C virus NS5B RNA polymerase and the 3′ end of the viral RNA. J Virol. 1999;73:7044–7049. doi: 10.1128/jvi.73.8.7044-7049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry KE, Waghray S, Doudna JA. The HCV IRES pseudoknot positions the initiation codon on the 40S ribosomal subunit. RNA. 2010;16:1559–1569. doi: 10.1261/rna.2197210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- 33.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 34.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 35.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 36.Forman JJ, Coller HA. The code within the code: MicroRNAs target coding regions. Cell Cycle. 2010;9:1533–1541. doi: 10.4161/cc.9.8.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S, Wu S, Ding J, Lin J, Wei L, Gu J, He X. MicroRNA-181a modulates gene expression of zinc finger family members by directly targeting their coding regions. Nucleic Acids Res. 2010;38:7211–7218. doi: 10.1093/nar/gkq564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14:872–877. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R, Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X, Ahmed F, Navarro F, Dykxhoorn D, Lieberman J, Gorospe M. p16(INK4a) translation suppressed by miR-24. PLoS One. 2008;3:e1864. doi: 10.1371/journal.pone.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci USA. 2008;105:14879–14884. doi: 10.1073/pnas.0803230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahajan A, Liu Z, Gellert L, Zou X, Yang G, Lee P, Yang X, Wei JJ. HMGA2: a biomarker significantly overexpressed in high-grade ovarian serous carcinoma. Mod Pathol. 2010;23:673–681. doi: 10.1038/modpathol.2010.49. [DOI] [PubMed] [Google Scholar]
- 42.Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA. 2010;107:1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Branch AD, Rice CM. Antisense gets a grip on miR-122 in chimpanzees. Sci Transl Med. 2010;2:13ps11. doi: 10.1126/scitranslmed.3000605. [DOI] [PubMed] [Google Scholar]
- 45.Jackson A, Linsley PS. The therapeutic potential of microRNA modulation. Discov Med. 2010;9:311–318. [PubMed] [Google Scholar]
- 46.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young DD, Connelly CM, Grohmann C, Deiters A. Small molecule modifiers of microRNA miR-122 function for the treatment of hepatitis C virus infection and hepatocellular carcinoma. J Am Chem Soc. 2010;132:7976–7981. doi: 10.1021/ja910275u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.