Abstract
The use of plants as integral components of life support systems remains a cornerstone of strategies for long-term human habitation of space and extraterrestrial colonization. Spaceflight experiments over the past few decades have refined the hardware required to grow plants in low-earth orbit and have illuminated fundamental issues regarding spaceflight effects on plant growth and development. Potential incipient hypoxia, resulting from the lack of convection-driven gas movement, has emerged as a possible major impact of microgravity. We developed transgenic Arabidopsis containing the alcohol dehydrogenase (Adh) gene promoter linked to the β-glucuronidase (GUS) reporter gene to address specifically the possibility that spaceflight induces the plant hypoxia response and to assess whether any spaceflight response was similar to control terrestrial hypoxia-induced gene expression patterns. The staining patterns resulting from a 5-d mission on the orbiter Columbia during mission STS-93 indicate that the Adh/GUS reporter gene was activated in roots during the flight. However, the patterns of expression were not identical to terrestrial control inductions. Moreover, although terrestrial hypoxia induces Adh/GUS expression in the shoot apex, no apex staining was observed in the spaceflight plants. This indicates that either the normal hypoxia response signaling is impaired in spaceflight or that spaceflight inappropriately induces Adh/GUS activity for reasons other than hypoxia.
Plants grown in the low-Earth orbital environments experienced during shuttle flight or space-station experiments often display an altered physiology compared with plants in ground-based controls. At the cellular level, spaceflight has been associated with disruptions of microtubular self-organization (Papaseit et al., 2000), changes in amyloplast distribution (Perbal et al., 1997; Kiss et al., 1999; Driss-Ecole et al., 2000) and energy metabolism (Hampp et al., 1997), and alterations in the distribution and partitioning of calcium ions (Merkys and Darginaviciene, 1997). At the organismal level, plants have responded to spaceflight with variations in basic physiological processes such as electron transport rates in photosynthetic processes (Tripathy et al., 1996) and stress metabolism responses related to hypoxia (Porterfield et al., 1997b).
A variety of factors in addition to microgravity have been implicated in the differential metabolisms associated with spaceflight. Elevated levels of ethylene or CO2, reduced levels of available oxygen, and fungal pathogens all contribute to metabolic stress in plants, and all are common in closed environments such as those experienced in current orbital vehicles (Tripathy et al., 1996; Bishop et al., 1997; Viktorov et al., 1998; Guisinger and Kiss, 1999; Salisbury, 1999). Hypoxia is of particular concern in space-grown plants as many of the features in plants returning from space flight environments resemble those of hypoxically stressed plants, even though the plants were ostensibly grown with adequate levels of oxygen. There are several physiological and metabolic indicators of hypoxia in plants; central among them is an increase in the expression of alcohol dehydrogenase (ADH). ADH is a crucial enzyme for plant fermentative metabolism, which functions in the regeneration of the NAD+ needed to sustain glycolysis and maintain basal production of ATP when the cytochrome chain is arrested under oxygen-limiting conditions (Crawford, 1982; Jackson and Drew, 1984; Daugherty et al., 1994; Vartapetian and Jackson, 1997). Initial analyses of plants grown in spaceflight revealed elevated levels of ADH activity and Adh mRNA compared with ground-control plants (Porterfield et al., 1997a, 1997b). These observations suggest that hypoxic stress, perhaps caused by the lack of convective gas exchange in microgravity, may play a major role in the effects of spaceflight on plant growth and development.
To develop a robust biological sensor for detecting hypoxia-related plant responses in spaceflight environments, Arabidopsis plants were engineered with the GUS reporter gene driven by the Arabidopsis Adh promoter (Chung and Ferl, 1999). The regulatory portion of the Adh gene is exquisitely sensitive to exogenous hypoxic stress, and the important cis-acting elements and transcription factors responsible for Adh regulation are known (Ferl and Laughner, 1989; Ferl, 1990; McKendree et al., 1990; Paul and Ferl, 1991, 1997; McKendree and Ferl, 1992; Dolferus et al., 1994; Lu et al., 1996; Hoeren et al., 1998; Dennis et al., 2000). Further, the Adh promoter responds to stresses other than hypoxia with well-characterized responses to cold, salt, Glc, and abcissic acid (Dolferus et al., 1994; de Bruxelles et al., 1996; Ishitani et al., 1998; Conley et al., 1999; Ellis et al., 1999; Koch et al., 2000). In transformed Arabidopsis plants, the chimeric Adh/GUS reporter transgene responds to exogenous stress in transgenic plants with a similar profile as the native Adh gene (Dolferus et al., 1990, 1994; Chung and Ferl, 1999; Ellis et al., 1999).
Arabidopsis bearing the Adh/GUS transgene were flown as part of the PGIM-01 (Plant Growth Investigations in Microgravity) experiment, conducted on the STS-93 mission aboard the orbiter Columbia. The diagnostic patterns of Adh/GUS reporter gene expression under controlled inductions with hypoxia and other stresses were evaluated as a framework for evaluation of the patterns of reporter gene expression in plants exposed to spaceflight conditions.
RESULTS
Plant Growth on Vertical Plates
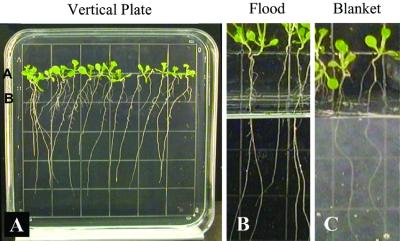
The growth of plants on vertical media plates encourages a surface root-growth habit that provides adequate gas exchange to support fully aerobic metabolism and results in no background Adh/GUS expression. Figure 1 illustrates the growing system that was used for both the flight and ground-control experiments and provides a view of the spaceflight plants before fixing and staining. Age-matched plates were chosen for flight (Fig. 1A) and loaded into a plant growth chamber (PGC) in a vertical orientation (Fig. 1B), which allowed the roots to grow along the surface of the agar rather than into the media (Fig. 1C). The vertical orientation was maintained as the PGCs were loaded in the plant growth facility (PGF), transported to the launch pad, and for the remaining pre-launch period. The orientation was maintained during the brief high gravity load of launch and then became irrelevant during the orbital phase of the mission.
Figure 1.
The vertical plate system of Arabidopsis culture used for shuttle flight STS-93. Transgenic Arabidopsis seeds were planted along the upper portion of a square petri plate containing nutrient agar and grown in a vertical position (A). The vertical plate was transferred to a PGC the day before launch when the seedlings were 6 d old (B). The plate was maintained in a vertical orientation in the PGC, in front of a set of tubes that contained older plants for a different experiment. The day before launch the seedlings were growing vertically along the surface of the plate and had cotyledons and the beginnings of the first set of true leaves (C). The plants were 12 d old at landing with six to eight leaves (D) and exhibited a random orientation of root growth along the surface of the agar during the flight (E). Each square of the grid visible on the petri plate measures 13 mm per side.
Plants grown on vertical plates do not typically express the Adh/GUS transgene in normal growing conditions (Chung and Ferl, 1999) nor is the Adh/GUS transgene induced in similar plants subjected to hypergravity simulations of shuttle launches (data not shown). Staining plants selected from the pool of plates prepared for the launch (but not chosen for the actual flight) indicated that these plants did not express Adh/GUS before the flight experiment. The plates for the ground control were also chosen from this same pool.
Adh/GUS Transgene Expression in Response to Spaceflight Conditions
When the experiment was turned over to Kennedy Space Center (KSC) personnel 27 h prior to launch, the 6-d-old plants had four leaves (cotyledons and a pair of true leaves) and roots that were 4 to 5 cm in length (Fig. 1, A and C). The plants maintained a root growth habit along the surface of the medium throughout the orbital flight as shown by the mass of surface roots on the post-flight plate (Fig. 1D). At landing, the plants were 12 d old, had extensive root systems, and had 6 to 8 leaves. The ground-control plants were grown in PGCs and a PGF homologous to the flight hardware and maintained under the flight environmental profile in the orbiter environmental simulator (OES). The ground-control plants exhibited a surface growth profile similar to the flight plants except that the root growth was directed by gravity.
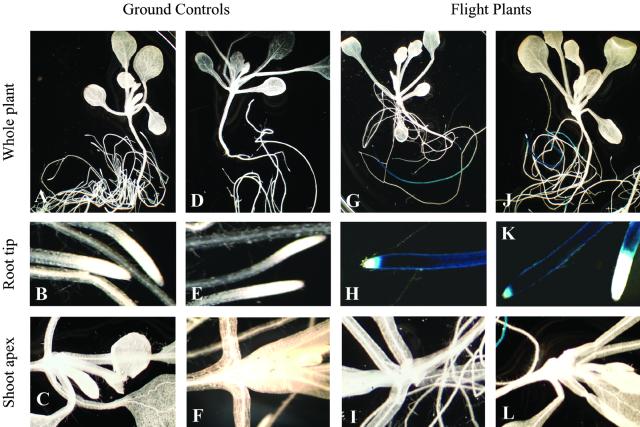
The ground-control plants show virtually no evidence of Adh/GUS expression in the roots or the shoots (Fig. 2, A–F). However, both sets of flight plants (one from PGC no. 5 and one from PGC no. 6; Fig. 1B) show Adh/GUS expression in the distal regions of primary roots (Fig. 2, G, H, J, and K) with many of the flight roots showing a dramatic accumulation of Adh/GUS activity (Fig. 2, H and K). The activity was usually extended several centimeters up the root length but was often absent from the very end of the root. However, the shoot apices from all of the flight plants lack Adh/GUS expression (Fig. 2, I and L).
Figure 2.
Adh/GUS expression in flight and ground-control vertical plate plants of PGIM-01. Histochemical staining of the ground controls shows that Adh/GUS was not expressed in these plants (A–F). In the flight plants, Adh/GUS was expressed in the distal portion of the roots (G–L). The pattern of expression consisted of a dark-blue root with a white tip (H and K). There is no evidence of Adh/GUS expression in the shoots of the flight plants (I and L).
Adh/GUS Transgene Expression in Controlled Oxygen Concentrations
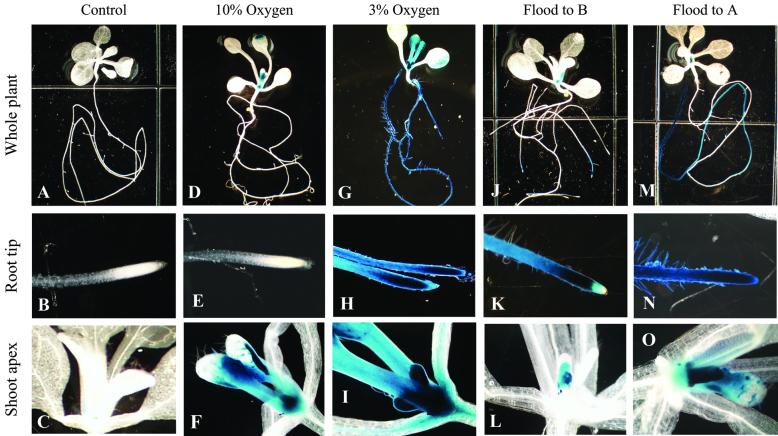
As a baseline calibration of the Adh/GUS sensitivity to hypoxic conditions, plants were grown on vertical plates for 8 d and subjected to controlled O2/N2 gas mixtures within sealed chambers for 2 d. Figure 3 illustrates the physical setup for all of the controlled induction experiments. No Adh/GUS activity was observed in plants from plates maintained in room air (Fig. 4, A–C). At 10% O2, Adh/GUS transgene activity was sporadically seen in cotyledons, was clearly evident in the shoot apex and in the trichomes of the shoot apex area, but was entirely absent in the roots (Fig. 4, D–F). At 3% O2, Adh/GUS transgene activity was quite strong in the shoot apex and in the trichomes of the shoot apex area. In addition, the root tips show strong Adh/GUS transgene activity (Fig. 4, G–I). These laboratory control inductions of whole plants in reduced oxygen atmospheres did not produce the root-only Adh/GUS staining patterns consistent with those observed in the spaceflight samples (Fig. 2, G–L).
Figure 3.
Vertical plate set up for controlled inductions. Controlled inductions were set up in vertical plates to mimic various degrees of hypoxia. Seeds were planted on grid-line A of square petri plates and allowed to grow normally for 8 d (A). Whole plant hypoxia was achieved by placing ventilated plates into continuous flow chambers with atmospheres of 10% or 3% oxygen (A). Root zone hypoxia was mimicked by flooding vertical plates to grid B on the plate, 14 mm below the root/shoot junction (A and B). Root zone stress and signal transduction experiments were conducted by over-layering vertical plates with solid agar “blankets” that cover the plant roots to grid B on the plate (A and C).
Figure 4.
Adh/GUS expression in controlled induction experiments. Histochemical staining of normally aerated vertical plate plants shows that Adh/GUS was not expressed in these plants (A–C). Plants grown in an atmosphere of 10% O2/90% N2 express Adh/GUS in the shoot apex and trichomes of newly emerged leaves (D and F) but not in the roots (E). Plants grown in an atmosphere of 3% O2/97% N2 express Adh/GUS throughout most of the plant (G–I) and Adh/GUS expression extends through the root tip (H). Plates that were flooded with water to grid B show Adh/GUS expression in the distal portion of the primary roots, but not at the root tip (J and K), and also express Adh/GUS in the shoot apex (L). Plants that were flooded to grid A show Adh/GUS expression in along most of the length of the primary roots (M and N) and in the shoot apex and trichomes of newly emerged leaves (O).
Adh/GUS Transgene Expression with Controlled Root-Zone Hypoxia in a Flight Environmental Background
In an attempt to recreate the root-only pattern of Adh/GUS expression observed in the flight plants, an additional set of ground-control inductions was conducted within the PGC/PGF flight hardware in the OES programmed with the environmental conditions of the STS-93 flight. This first set of controlled inductions exposed Adh/GUS plants to varying degrees of flooding intended to simulate root-zone hypoxia. Plants on vertical plates were allowed to grow normally until loaded into the PGCs and PGF at ages that correlated directly with the flight experiment (Fig. 3A). Shortly after the simulated launch of the experiment, the plates within the PGCs were recovered and placed in plastic boxes such that the plates could be flooded with water to various levels (Fig. 3B). One set of plates was flooded up to grid-line B, approximately 14 mm below the root shoot junction, to simulate lower root-zone hypoxia. A separate set of plates was flooded to the root/shoot junction at grid line A to simulate whole root hypoxia. The flooded vertical plates were returned to the PGC/PGF hardware in the OES and the effects of these treatments on Adh/GUS expression were evaluated after 48 h.
Plants from plates flooded to grid-line B, 14 mm below the shoot/root junction, showed limited Adh/GUS expression in the roots and in the shoot apex (Fig. 4, J–L). The only intensely staining region of the roots in these plants was the distal region of the primary root, and staining was absent at the very tip (Fig. 4K). Adh/GUS expression is evident in the shoot apex of these plants but is limited to the central region (Fig. 4L). Plants from plates flooded to grid-line A, the shoot/root junction, demonstrated a more extensive Adh/GUS expression in the roots and shoot apex (Fig. 4, M–O). The plants with partially flooded roots (Fig. 4, J and K) expressed Adh/GUS in a root pattern similar to that seen in the stress response related to space flight (Fig. 2, H and K). However, in all cases, flooding to a degree sufficient for expression of Adh/GUS in the roots resulted in concomitant expression in the shoot apex (Fig. 4, L and O).
Adh/GUS Transgene Expression in the Presence of Inhibitors of Calcium-Mediated Signal Transduction
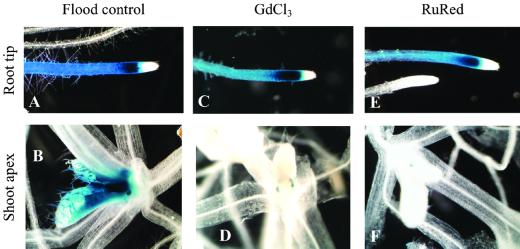
Additional ground-control experiments were conducted to address the transduction of the hypoxic signal from the roots to the shoot, again within the PGC/PGF flight hardware and in the OES programmed to the environmental conditions of the STS-93 orbiter. Plants were allowed to grow on vertical plates normally until loaded into the PGCs and PGF at ages that correlated directly with the flight experiment. Shortly after the simulated launch of the experiment, the plates within the PGCs were recovered and the vertical plates were layered with a “blanket” of agar media to cover the roots to grid B (e.g. Fig. 3D). The blanket of agar eliminates airflow around the roots. This creates a short-term state of hypoxia similar to roots growing through agar (Chung and Ferl, 1999). One set of blankets consisted of standard agar media and comprised the positive controls (Fig. 5, A and B). For the second set, blankets were made of standard agar media containing 1 mm GdCl3, (Fig. 5, C and D) or 20 μm Ruthenium Red (Fig. 5, E and F). In all three cases, the blankets induced limited Adh/GUS expression chiefly in the distal portion of the primary root. The pattern of Adh/GUS expression in the roots is virtually identical for all three treatments (Fig. 5, A, C, and E). However, the presence of GdCl3 or Ruthenium Red in the blankets (Fig. 5, D and F) inhibited the Adh/GUS expression in the shoot apex that accompanied root expression in the blanket positive control (Fig. 5B).
Figure 5.
Inhibitors of calcium-mediated signal transduction. Plants from plates that have been covered with a blanket of plain Murashige and Skoog media agar to grid line B show Adh/GUS expression is in the distal portion of the primary roots but not at the root tip (A). The shoot apex of plants blanketed to grid B also express Adh/GUS the distal portion of the primary root (B). When 1 mm GdCl3 is added to the blanket material, Adh/GUS expression is seen in the distal portion of the primary root (C) but is absent from the shoot apex (D). When 20 μm Ruthenium Red is added to the blanket material, Adh/GUS expression is again seen in the distal portion of the primary root (E) but is also absent from the shoot apex (F).
DISCUSSION
It is generally agreed that a spaceflight environment influences plant physiology (Tripathy et al., 1996; Hampp et al., 1997; Merkys and Darginaviciene, 1997; Perbal et al., 1997; Porterfield et al., 1997b; Kiss et al., 1999; Driss-Ecole et al., 2000). However, the causes of these influences are not easily understood, and it is difficult to dissect the intrinsic biological effects of microgravity or spaceflight away from the secondary and synergistic effects generated by the systems designed for life support. One stressful feature of spaceflight that may reflect this type of synergism is the apparent hypoxia-like effects that have been observed in orbital plant experiments. Plants demonstrate increased aerenchyma formation and an increase in ADH mRNA and enzyme activity, all symptoms consistent with root zone hypoxia (Musgrave et al., 1997; Porterfield et al., 1997b). In these cases, the plants were grown such that the roots were encased within an agar growth medium and the ground controls exhibited elevated levels of Adh mRNA and enzyme activity, as well.
Growing plants on the surface of a nutrient media looked to provide a means to remove growth media considerations from impacting potential root zone health and present plants with essentially zero levels of Adh/GUS expression at launch. Under normal gravity, in standard culture environments, plants growing on vertical plates receive sufficient aeration to support aerobic metabolism and prevent background Adh/GUS expression (Fig. 4, A–C; Chung and Ferl, 1999). The ground-control plants for STS-93 growing on vertical plates under the flight environmental profile were also free of Adh/GUS expression (Fig. 2, A–F). Taken together, these results indicate that the vertical plate growth system prevents root zone hypoxia under situations of normal gravity and should provide a biologically direct evaluation of spaceflight effects on the initiation of Adh/GUS expression from a completely uninduced state. Control inductions indicate that whole plant hypoxia induced by 3% oxygen initiates strong Adh/GUS expression in both the roots and shoots (Fig. 4, G–I). The relatively mild hypoxic condition of 10% O2 results in the expression of Adh/GUS in the shoot apex, but no expression is seen in the roots (Fig. 4, D–F). Thus, the shoot apex appears to be the most sensitive region of the plant to respond to whole plant hypoxia stress with the root responding only as the hypoxia becomes more severe. This conclusion is consistent with observations that Arabidopsis roots and shoots possess different mechanisms for responding to hypoxia (Ellis et al., 1999).
In the flight plants of PGIM-01 on STS-93, Adh/GUS expression was evident in the distal portions of the primary root but absent from the shoot apex (Fig. 2, G–L). This pattern of expression is in direct contrast to the ground-based, whole-plant hypoxia control inductions and suggests that spaceflight induces a unique response pattern that is it independent of oxygen concentrations in the air. The pattern of root Adh/GUS expression in the flight plants is, however, similar to ground-control plants that have been treated to mimic hypoxia that is limited to the root-zone. Plants that had only a portion of their roots subjected to flooding (Fig. 4, J–L) or treated to a localized diminished airflow by means of an agar blanket (Fig. 5, A and B) had the same pattern of expression in their primary roots as did the flight plants. However, unlike the flight plants, flooded and blanketed ground-control plants also show a concomitant expression of Adh/GUS in the shoot apex, even though the apex is maintained in an aerated environment. This transmission of a hypoxia signal from the root to the shoot is consistent with earlier observations of root zone hypoxia in Arabidopsis (Chung and Ferl, 1999) and with general concepts in root zone flooding signals in plants (Jackson and Drew, 1984).
The conclusion drawn from these data is that the Adh/GUS induction observed during spaceflight is not consistent with simple terrestrial induction patterns for either root-zone hypoxia or whole plant hypoxia. Does the lack of convective gas mixing create hypoxia conditions during spaceflight? If so, then the whole plant does not experience hypoxia, as the Adh/GUS transgene responds to mild, whole plant hypoxia of 10% O2 with an expression pattern limited to the shoot apex and trichomes. This region was completely devoid of Adh/GUS expression in the spaceflight plants, hence it appears unlikely that the shoot apex perceives even mild hypoxia during spaceflight. Do only root zones experience hypoxia in spaceflight? Possibly, although this would seem unlikely because the entire root system is on the surface of the medium and ostensibly exposed to the same gaseous environment as the shoot. Nonetheless, the staining pattern of roots from ground-control plants that were partially flooded, or partially blanketed with agar, is similar to the staining pattern of roots from the spaceflight plants. However, ground-control root zone hypoxia plants always showed concomitant Adh/GUS expression in the shoot apex, whereas all of the flight plants that displayed localized Adh/GUS expression in their roots lacked expression in their shoots. If root-zone specific hypoxia exists, or is perceived by spaceflight plants, then the normal root-to-shoot signaling is impaired.
Signal transduction is a vital aspect of virtually all metabolic processes, and the calcium ion is among the most widely used mediators of signal transduction in plants (Sheen, 1996; Sinclair and Trewavas, 1997; Roos, 2000). In both maize and Arabidopsis, calcium has a key role in sustaining an appropriate response to hypoxia (Subbaiah et al., 1994a, 1994b) and is involved in regulating secondary effects of hypoxia such as aerenchyma formation and tissue necrosis (Drew et al., 2000; Subbaiah et al., 2000). Calcium has also been implicated in the signal transduction associated with gravity orientation (Reddy et al., 1987; Merkys and Darginaviciene, 1997; Kiss, 2000). Spaceflight can disrupt the ability of fern spores to establish proper polarity, and ground-based treatments with a calcium-channel blocker that reduced the calcium current similarly disabled the polarity orienting influence of gravity (Chatterjee et al., 2000). Calcium has also been identified as a possible factor in the metabolic signaling required for normal T-cell culture proliferation in situations where gravitational cues are disrupted, such as in spaceflight and clinorotation (Hashemi et al., 1999). The ground-control experiments described herein also suggest that calcium signaling is potentially disrupted during spaceflight, as the calcium-blocking agents gadolinium chloride and Ruthenium Red inhibited Adh/GUS expression in the shoots of plants that were expressing Adh/GUS in their roots (Fig. 5, C–F). The best terrestrial mimic of spaceflight Adh/GUS expression patterns was produced by partially overlaying roots with blankets that induced limited hypoxia and inhibited calcium signaling.
Microgravity is certainly a compelling source of potential plant stress during spaceflight and is a potential source of hypoxia if temperature differential convection is necessary for gas exchange at plant surfaces. If hypoxia occurs during spaceflight, current results suggest that either the hypoxia is somehow limited to the root zone and the normal root-to-shoot calcium signaling mechanism is handicapped, or the hypoxia is not limited to the root zone and Adh/GUS expression in shoots is directly inhibited by spaceflight. We submit, however, that there are other Adh activation pathways that are potentially impacted by spaceflight. Trace gases in the atmosphere, including ethylene, are variable and difficult to control, as are acceleration, radiation, air movement, and noise. Therefore, it is possible that the Adh/GUS transgene is not activated by spaceflight hypoxia, but rather it is induced through one of the other signaling pathways that intersect with the Adh promoter. Flight experiments with dissected Adh promoters and promoters from other pathways should refine the detection of spaceflight stress from the biological perspective. It is likely that successful extraterrestrial plant growth will require mechanical and electrical engineering to improve the space-based plant growth habitats to remove extrinsic stress factors and biological engineering to enhance specific stress tolerances.
MATERIALS AND METHODS
Plants
Seedlings from the transgenic Arabidopsis line WS/-846 Adh/226-257-1 were used throughout. Plants were grown on the vertical surface of petri plates containing solid agar media (Chung and Ferl, 1999). The shuttle flight plants were 7 d old at launch and 12 d old when harvested after landing. The plants used in the controlled induction experiments were 8 d old at the beginning of treatment and 10 d old at harvest.
Media
The solid media was composed of 2.2 g of Murashige and Skoog salts (Murashige and Skoog, 1962), 0.5 g of MES buffer, 5 g of Suc, and 1 mL of 1,000× Gamborg vitamins (Sigma, St. Louis) per liter at pH of 5.75. Phytagel (Sigma) was added to a concentration of 0.4% (w/v) and autoclaved. After autoclaving, the fungicide benomyl was added to a concentration of 3 ppm (Paul et al., 2001), and the media was aliquoted into sterile square petri plates (100 mm2).
Seed Sterilization and Planting
Dry, vernalized seeds were sterilized in microcentrifuge tubes with a 70% (v/v) ethanol wash followed by treatment in a solution of 50% (v/v) bleach and approximately 0.5% (v/v) Tween 20 for 10 min. The bleach solution was removed in a laminar flow hood with a sterile transfer pipette, and then the seeds were rinsed 8 to 10 times with sterile water. Approximately 12 to 15 seeds were planted individually onto vertical plates 13 to 18 mm from the top edge of the plate. Plates were taped with breathable surgical tape (Micropore, 3M, St. Paul) then placed in racks to hold them in the vertical position during growth. Plants were grown in continuous light (70–80 μmol m−2 s−1) at 24°C to 26°C prior to flight or control experiments.
STS-93 Flight and Ground-Control Preparations
The major hardware used for flight experiment PGIM-01 was the PGF, which houses PGCs for cultivation on orbit as a mid-deck locker experiment (Chapman et al., 1995; Chapman and Wells, 1996). The PGF provides lighting and monitoring of temperature, relative humidity, and CO2. During flight, the temperatures ranged from 15°C to 25°C, the relative humidity averaged 70%, and the CO2 concentration fluctuated between 500 and 1,500 ppm. Vertical plates were flown in PGCs #5 and #6. The remaining space in the PGF was occupied by Arabidopsis plants that were subsequently used for a separate experiment.
STS-93 was in orbit for just under 5 d (launch, 12:31 am July 23; landing, 11:20 pm July 27). The experimental plants were loaded into PGCs and the turned over to KSC personnel 27 h prior to launch for loading into the PGF and installation into the shuttle orbiter. The plants were 6 d old when turned over to KSC personnel. Hardware environmental profile monitoring began as soon as the PGCs became resident in the PGF.
After return to Earth, the PGF was unloaded and the PGC-grown vertical plate plants were harvested approximately 3 h after landing. Plants were immediately fixed in histochemical stain containing the β-glucuronidase substrate X-Gluc (2 mm 5-bromo-4-chloro-3-indolylglucuronide, 1% [w/v] dimethylformamide, 0.1 mm K3[Fe(CN)6], 0.1 mm, K4[Fe(CN6)] · 3H2O, 1 mm EDTA, and 50 mm NaPO4, pH 7.0).
Ground-control plants were organized as described above for the flight plants. The ground-control PGF was maintained in the OES for the duration of the shuttle mission. The OES provides an environmental profile that tracks the profile of the orbiter with regard to temperature, humidity, and CO2 concentrations. The OES profile reflects a 24-h delay, thus the ground-control experiment was initiated 24 h after the flight experiment. The plants in the flight experiment and the ground control were of the same chronological age, as the ground-control plants were planted 1 d later than those of the flight experiment.
Controlled Induction Experiments
Plants were grown on vertical plates for 8 d under normal conditions and then subjected to exogenous stresses intended to induce gene expression. There were three sets of experiments designed to influence Adh/GUS expression. The first set of plants was subjected to controlled hypoxia by placing vertically held plates of plants in chambers provided with continuous flow of 3% O2 in nitrogen or 10% O2 in nitrogen. In the second set, plants were subjected to two different levels of flooding. Flooding was accomplished by placing vertically held plates into chambers filled with water to submerge the roots to 14 mm below the root/shoot junction or all the way up to the root/shoot junction. The third set of plants consisted of vertical plate plants whose roots were covered with a precast “blanket” of agar media to 14 mm below the root/shoot junction. The positive control blankets were composed of standard media with 0.3% (w/v) phytagel. Additional blankets were cast with either 1 mm gadolinium chloride (GdCl3) or 20 μm Ruthenium Red. The controlled oxygen atmosphere inductions were conducted in the laboratory, whereas the flooded and blanketed plants were placed in the PGC/PGF within the OES under STS-93 flight conditions. The plants were subjected to control inductions for 48 h.
Photographic Data Collection and Histochemical Assays
Plants from the various treatments were evaluated for transgene activity by histochemical staining in X-Gluc followed by decoloration with repeated changes of 70% (v/v) ethanol. Histochemical data and features of plant growth and morphology were recorded photographically using a model SZH10 stereo dissecting microscope and model DP10 digital camera (Olympus, Tokyo).
ACKNOWLEDGMENTS
We thank Beth Laughner, Paul Sehnke, Hwa-Jee Chung, and especially Carla Lyerly for laboratory-based support during the preparations for these experiments. We thank William Piastuch, Howard Levine, Billy Wells, Chung Wang, Joseph Velasquez, Charles McFarland, William McLamb, Roberteen McCray, and Deborah Wells for providing invaluable assistance at Kennedy Space Center in the areas of experiment development, hardware development, and payload management.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAG 10–0145). This manuscript is no. R–07983 of the Florida Agricultural Experiment Station.
LITERATURE CITED
- Bishop DL, Levine HG, Kropp BR, Anderson AJ. Seedborne fungal contamination: consequences of space-grown wheat. Phytopathology. 1997;87:1125–1133. doi: 10.1094/phyto.1997.87.11.1125. [DOI] [PubMed] [Google Scholar]
- Chapman DK, Wells HW. Development of the plant growth facility for the use in the shuttle middeck and test units for ground-based experiments. In: Kaldeich-Schurmann B, editor. Proceedings of the Sixth European Symposium on Life Science Research in Space. Trondheim, Norway: European Space Agency Publishing Division; 1996. pp. 37–41. [Google Scholar]
- Chapman DK, Wells HW, Levine HG. Plant Growth Facility: A Recent Design Provides Improved Capabilities for Use in the Shuttle Middeck. Warrendale, PA: SAE International; 1995. [Google Scholar]
- Chatterjee A, Porterfield DM, Smith PS, Roux SJ. Gravity-directed calcium current in germinating spores of Ceratopteris richardii. Planta. 2000;210:607–610. doi: 10.1007/s004250050050. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Ferl RJ. Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol. 1999;121:429–436. doi: 10.1104/pp.121.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley TR, Peng HP, Shih MC. Mutations affecting induction of glycolytic and fermentative genes during germination and environmental stresses in Arabidopsis. Plant Physiol. 1999;119:599–608. doi: 10.1104/pp.119.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RMM. Physiological responses to flooding. Encycl Plant Physiol. 1982;12B:453–441. [Google Scholar]
- Daugherty CJ, Rooney M, Paul A-L, DeVetten NC, Vega-Palas MA, Lu GL, Gurley WB, Ferl RJ. Environmental stress and gene regulation. In: Meyerowitz E, Somerville C, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1994. pp. 769–806. [Google Scholar]
- de Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R. Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 1996;111:381–391. doi: 10.1104/pp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ES, Dolferus R, Ellis M, Rahman M, Wu Y, Hoeren FU, Grover A, Ismond KP, Good AG, Peacock WJ. Molecular strategies for improving waterlogging tolerance in plants. J Exp Bot. 2000;51:89–97. [PubMed] [Google Scholar]
- Dolferus R, Jacobs M, Peacock WJ, Dennis ES. Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol. 1994;105:1075–1087. doi: 10.1104/pp.105.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R, Van den Bossche D, Jacobs M. Sequence analysis of two null-mutant alleles of the single Arabidopsis Adh locus. Mol Gen Genet. 1990;224:297–302. doi: 10.1007/BF00271565. [DOI] [PubMed] [Google Scholar]
- Drew MC, He I, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Driss-Ecole D, Jeune B, Prouteau M, Julianus P, Perbal G. Lentil root statoliths reach a stable state in microgravity. Planta. 2000;211:396–405. doi: 10.1007/s004250000298. [DOI] [PubMed] [Google Scholar]
- Ellis MH, Dennis ES, Peacock WJ. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999;119:57–64. doi: 10.1104/pp.119.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferl R. ARF-B2: a protein complex that specifically binds to part of the anaerobic response element of maize Adh1. Plant Physiol. 1990;93:1094–1101. doi: 10.1104/pp.93.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferl RJ, Laughner BH. In vivo detection of regulatory factor binding sites of Arabidopsis thaliana Adh. Plant Mol Biol. 1989;12:257–266. doi: 10.1007/BF00017576. [DOI] [PubMed] [Google Scholar]
- Guisinger MM, Kiss JZ. The influence of microgravity and spaceflight on columella cell ultrastructure in starch-deficient mutants of Arabidopsis. Am J Bot. 1999;86:1357–1366. [PubMed] [Google Scholar]
- Hampp R, Hoffmann E, Schonherr K, Johann P, De Filippis L. Fusion and metabolism of plant cells as affected by microgravity. Planta. 1997;203:S42–S53. doi: 10.1007/pl00008114. [DOI] [PubMed] [Google Scholar]
- Hashemi BB, Penkala JE, Vens C, Huls H, Cubbage M, Sams CF. T cell activation responses are differentially regulated during clinorotation and in spaceflight. FASEB J. 1999;13:2071–2082. doi: 10.1096/fasebj.13.14.2071. [DOI] [PubMed] [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics. 1998;149:479–490. doi: 10.1093/genetics/149.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Xiong L, Lee H, Stevenson B, Zhu JK. HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell. 1998;10:1151–1161. doi: 10.1105/tpc.10.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Drew MC. Effects of flooding on growth and metabolism of herbacious plants. In: Kozlowski TT, editor. Flooding and Plant Growth. New York: Academic Press; 1984. pp. 47–128. [Google Scholar]
- Kiss JZ. Mechanisms of early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Edelmann RE, Wood PC. Gravitropism of hypocotyls of wild-type and starch-deficient Arabidopsis seedlings in spaceflight studies. Planta. 1999;209:96–103. doi: 10.1007/s004250050610. [DOI] [PubMed] [Google Scholar]
- Koch KE, Ying Z, Wu Y, Avigne WT. Multiple paths of sugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolism. J Exp Bot. 2000;51:417–427. doi: 10.1093/jexbot/51.suppl_1.417. [DOI] [PubMed] [Google Scholar]
- Lu G, Paul AL, McCarty DR, Ferl RJ. Transcription factor veracity: is GBF3 responsible for ABA-regulated expression of Arabidopsis Adh? Plant Cell. 1996;8:847–857. doi: 10.1105/tpc.8.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendree WL, Jr, Ferl RJ. Functional elements of the Arabidopsis Adh promoter include the G-box. Plant Mol Biol. 1992;19:859–862. doi: 10.1007/BF00027081. [DOI] [PubMed] [Google Scholar]
- McKendree WL, Paul AL, DeLisle AJ, Ferl RJ. In vivo and in vitro characterization of protein interactions with the dyad G-box of the Arabidopsis Adh gene. Plant Cell. 1990;2:207–214. doi: 10.1105/tpc.2.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkys A, Darginaviciene J. Plant gravitropic response. Adv Space Biol Med. 1997;6:213–230. [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–479. [Google Scholar]
- Musgrave ME, Kuang A, Matthews SW. Plant reproduction during spaceflight: importance of the gaseous environment. Planta. 1997;203:S177–S184. doi: 10.1007/pl00008107. [DOI] [PubMed] [Google Scholar]
- Papaseit C, Pochon N, Tabony J. Microtubule self-organization is gravity-dependent. Proc Natl Acad Sci USA. 2000;97:8364–8368. doi: 10.1073/pnas.140029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AL, Ferl RJ. The hypoxic response of three alcohol dehydrogenase genes: in vivo and in vitro footprinting of DNA/protein interactions describes multiple signaling connections. Ann Bot. 1997;79:33–37. [Google Scholar]
- Paul A-L, Ferl RJ. Adh1 and Adh2 regulation. Maydica. 1991;36:129–134. [Google Scholar]
- Paul A-L, Semer C, Kucharek T, Ferl RJ. The fungicidal and phytotoxic properties of benomyl and PPM in supplemented agar media supporting transgenic arabidopsis plants for a Space Shuttle flight experiment. Appl Microbiol Biotechnol. 2001;55:480–485. doi: 10.1007/s002530000521. [DOI] [PubMed] [Google Scholar]
- Perbal G, Driss-Ecole D, Tewinkel M, Volkman D. Statocyte polarity and gravisensitivity in seedling roots grown in microgravity. Planta. 1997;203:S57–S62. doi: 10.1007/pl00008115. [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Crispi ML, Musgrave ME. Changes in soluble sugar, starch, and alcohol dehydrogenase in Arabidopsis thaliana exposed to N2 diluted atmospheres. Plant Cell Physiol. 1997a;38:1354–1358. doi: 10.1093/oxfordjournals.pcp.a029129. [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Matthews SW, Daugherty CJ, Musgrave ME. Spaceflight exposure effects on transcription, activity, and localization of alcohol dehydrogenase in the roots of Arabidopsis thaliana. Plant Physiol. 1997b;113:685–693. doi: 10.1104/pp.113.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, McFadden JJ, Friedmann M, Poovaiah BW. Signal transduction in plants: evidence for the involvement of calcium and turnover of inositol phospholipids. Biochem Biophys Res Commun. 1987;149:334–339. doi: 10.1016/0006-291x(87)90371-8. [DOI] [PubMed] [Google Scholar]
- Roos W. Ion mapping in plant cells: methods and applications in signal transduction research. Planta. 2000;210:347–370. doi: 10.1007/PL00008144. [DOI] [PubMed] [Google Scholar]
- Salisbury FB. Growing crops for space explorers on the moon, Mars, or in space. Adv Space Biol Med. 1999;7:131–162. doi: 10.1016/s1569-2574(08)60009-x. [DOI] [PubMed] [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas A. Calcium in gravitropism: a re-examination. Planta. 1997;203:S85–S90. doi: 10.1007/pl00008120. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-cultured cells. Plant Cell. 1994a;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Kollipara KP, Sachs MM. A Ca(2+)-dependent cysteine protease is associated with anoxia-induced root tip death in maize. J Exp Bot. 2000;51:721–730. [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol. 1994b;105:369–376. doi: 10.1104/pp.105.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy BC, Brown CS, Levine HG, Krikorian AD. Growth and photosynthetic responses of wheat plants grown in space. Plant Physiol. 1996;110:801–806. doi: 10.1104/pp.110.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot. 1997;79:3–20. [Google Scholar]
- Viktorov AN, Novikova ND, Deshevaia EA, Bragina MP, Shnyreva AV, Sizova TP, D'Iakov Iu T. Residential colonization of orbital complex “Mir” environment by penicillium chrysogenum and problem of ecological safety in long-term space flight. Aviakosm Ekolog Med. 1998;32:57–62. [PubMed] [Google Scholar]