Abstract
Primates have evolved diverse cognitive capabilities to navigate their complex social world. To understand how the brain implements critical social cognitive abilities, we describe functional specialization in the domains of face processing, social interaction understanding, and mental state attribution. Systems for face processing are specialized from the level of single cells to populations of neurons within brain regions to hierarchically organized networks that extract and represent abstract social information. Such functional specialization is not confined to the sensorimotor periphery but appears to be a pervasive theme of primate brain organization all the way to the apex regions of cortical hierarchies. Circuits processing social information are juxtaposed with parallel systems involved in processing nonsocial information, suggesting common computations applied to different domains. The emerging picture of the neural basis of social cognition is a set of distinct but interacting subnetworks involved in component processes such as face perception and social reasoning, traversing large parts of the primate brain.
Keywords: functional brain organization, social brain, face processing, social interaction analysis, social cognition, social knowledge
INTRODUCTION
Social life in complex societies—in which members recognize one another and interpret each other’s actions and interactions—comprises a wide spectrum of behaviors, ranging from innate survival mechanisms to abilities relying on sophisticated cognitive processes. Primates exhibit elaborate social behaviors that are indicative of a rich cognitive repertoire: They recognize other conspecifics by their faces and voices (Cheney & Seyfarth 1980, Parr et al. 2000, Sliwa et al. 2011), classify individuals based on their social status and group affiliation (Bergman et al. 2003, Shutts et al. 2013, Silk 1999), rapidly detect and analyze social interactions between conspecifics (Grahe & Bernieri 1999, Isik et al. 2020), and understand others’ actions and interactions in terms of underlying mental states—such as intentions or knowledge—that drive them (Call & Tomasello 2008).
Primate brains have evolved in response to selective pressures exerted by the need to operate in complex societies (Dunbar 1998). Such pressures may explain the presence of brain mechanisms specialized to processing social information. These mechanisms are not limited to systems for high-level cognition but extend all the way to the sensory periphery—with receptors in the skin specialized for social touch (Elias et al. 2023)—as well as systems for motor output, such as vocalization control (Gavrilov & Nieder 2021). Are these rather diverse specializations related to each other? In other words, does the social brain constitute a coherent entity? Alternatively, do these mechanisms constitute a collection of isolated systems that evolved to perform particular social functions? Furthermore, does the presence of neural systems specialized for social information processing indicate that these systems perform distinct computations from the rest of the brain (Lockwood et al. 2020)?
Here we focus on how social cognitive functions are implemented from the level of neurons to areas and networks across the brain. We argue that (a) there is a high degree of specialization for social cognitive function throughout the primate brain; (b) this set of specialized mechanisms, collectively termed the social brain, comprises multiple distinct but interacting subsystems; (c) common networks within the social brain are found across humans and nonhuman primates; (d) similarities in the anatomical organization of the social brain and adjacent nonsocial systems suggest common computational operations performed by each; and (e) this specialization suggests unique opportunities for a mechanistic understanding of the neural mechanisms of intelligence within the domain of social cognition.
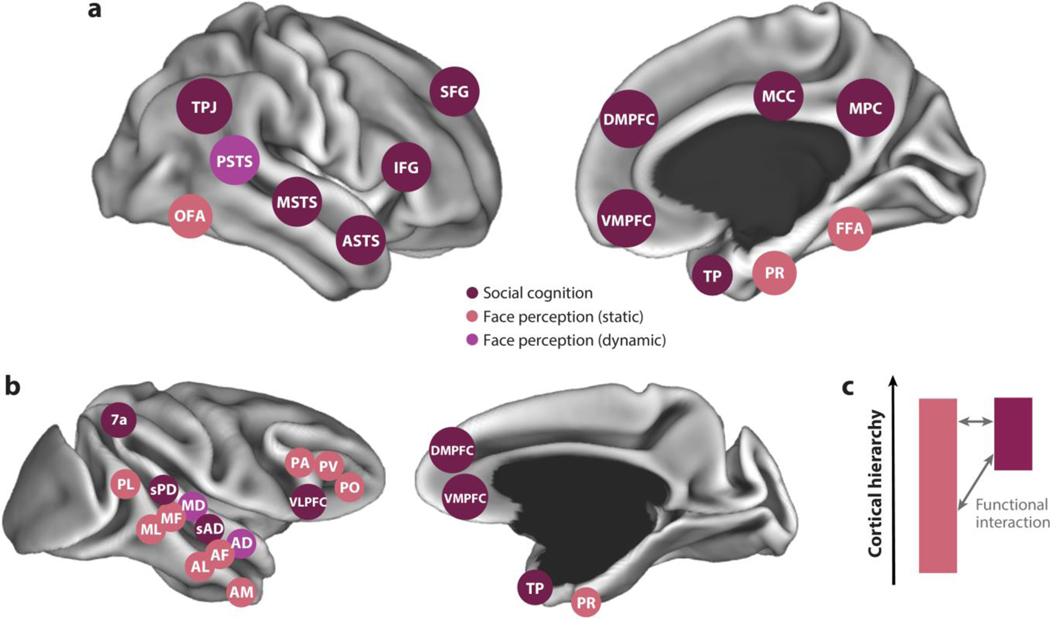
To elucidate the functional organization of the social brain, we focus on social cognitive functions that build on each other, allowing for increasingly deep inferences: face processing, social interaction understanding, and theory of mind. We review the computational challenges that primates face in each domain and the organization of the neural systems that solve them. In describing the functional organization of systems within the social brain, we highlight how they are positioned within an overall hierarchical organization. Hierarchy is one of the main organizational principles of the primate brain: Some brain areas are positioned closer to the sensory or motor periphery, while others are positioned farther from the periphery and closer to the limbic system and hippocampal formation (Felleman & Van Essen 1991). Studies in humans and nonhuman primates have identified areas of cortex that are specialized for distinct components of social understanding (Figure 1) throughout this hierarchy. Understanding how components of the social brain are situated within a hierarchy can provide insight into the nature of information processing (e.g., the level of representational abstraction), as well as relationships between different subsystems and potential homologies across species.
Figure 1.
Areas of functional specialization for social cognition in (a) humans and (b) macaques shown from a lateral view (left) and medial view (right). Areas specialized for static and dynamic face perception (salmon and purple, respectively) and high-level social cognition (burgundy), including theory of mind, are indicated. Areas are shown schematically on computer-rendered cortical surfaces. (c) Systems for face perception and social cognition occupy distinct regions of the primate anatomical connectome, but they interact functionally. Abbreviations: AD, anterior dorsal; AF, anterior fundus; AL, anterior lateral; AM, anterior medial; ASTS, anterior STS; DMPFC, dorsomedial prefrontal cortex; FFA, fusiform face area; IFG, inferior frontal gyrus; MCC, middle cingulate cortex; MD, middle dorsal; MF, middle fundus; ML, middle lateral; MPC, medial parietal cortex; MSTS, middle STS; OFA, occipital face area; PA, prefrontal arcuate; PL, posterior lateral; PO, prefrontal orbital; PR, perirhinal cortex; PSTS, posterior superior temporal sulcus; PV, prefrontal ventral; sAD, social anterior dorsal; SFG, superior frontal gyrus; sPD, social posterior dorsal; STS, superior temporal sulcus; TP, temporal pole; TPJ, temporo-parietal junction; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex.
FACE PROCESSING SYSTEMS AS A FRONT END OF THE SOCIAL BRAIN
The deep inferences primates make about the actions and interactions of others are rooted in the interpretation of perceptual information. One particularly rich source of socially relevant perceptual information is the face. Information from faces largely determines our first impressions of other people (Vernon et al. 2014) and influences behaviorally significant social behaviors such as mate choice (Currie & Little 2009). Faces provide diverse information about others, including their identity, gaze direction, head orientation, and movement direction. They enable inferences about unobservable mental states, including intentions, emotions, and direction of attention.
Given the importance of faces and the computational challenge of extracting facial information from the retinal image (Leibo et al. 2017), it comes as little surprise that primates dedicate large amounts of cortical infrastructure to this task. In the macaque monkey brain, six areas are dedicated to the analysis of faces in each cortical hemisphere and are organized in a hierarchical fashion along the superior temporal sulcus (STS) (Figure 2a): the posterior lateral (PL), middle lateral (ML), middle fundus (MF), anterior lateral (AL), anterior fundus (AF), and anterior medial (AM) face patches (Tsao et al. 2008). These areas were discovered using functional magnetic resonance imaging (fMRI) (Tsao et al. 2003, 2008). Face-selective cortical responses in humans share a similar anatomical organization, following a posterior-to-anterior axis along the ventral visual pathway in inferior occipitotemporal cortex (Haxby et al. 2000, Kanwisher et al. 1997, Tsao et al. 2008). Regions that respond specifically to dynamic faces—that is, videos of moving faces—have also been identified in macaques (Fisher & Freiwald 2015) and humans (Pitcher et al. 2011) and have been argued to constitute a distinct subsystem specialized for processing face motion (Bernstein et al. 2018, Pitcher & Ungerleider 2021).
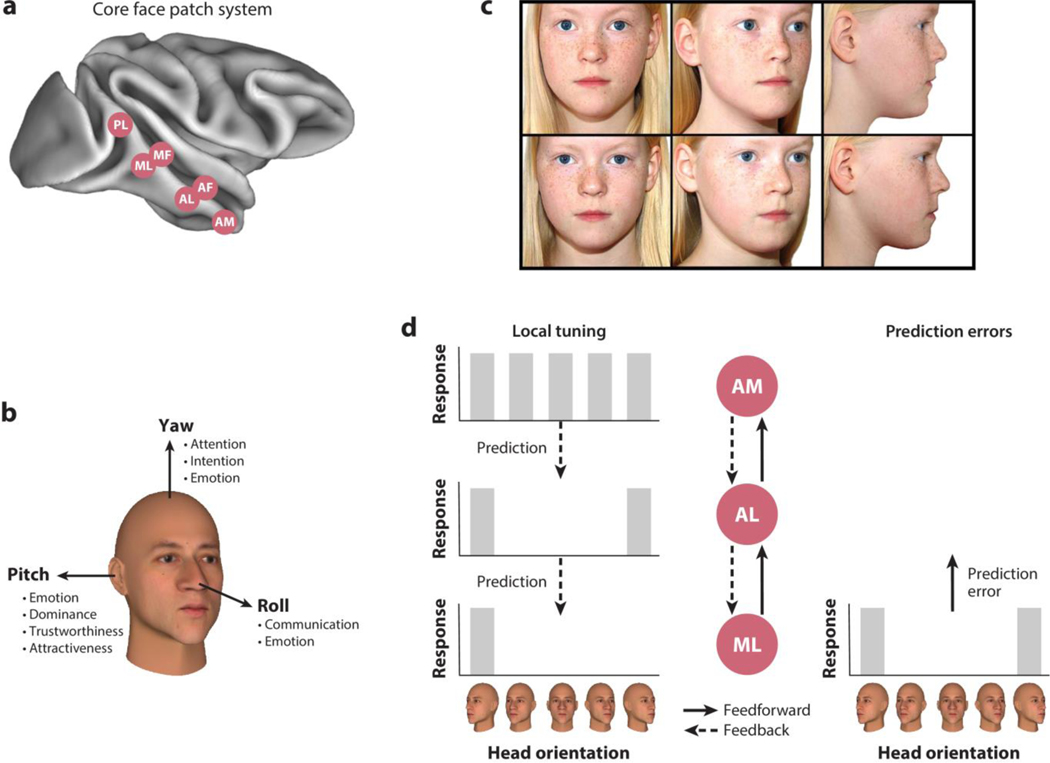
Figure 2.
(a) The core face patch system in the superior temporal sulcus of the macaque monkey consists of the face patches (in posterior-anterior direction) posterior lateral (PL), middle lateral (ML), middle fundus (MF), anterior lateral (AL), anterior fundus (AF), and anterior medial (AM). (b) Head orientation contains socially relevant information. For example, yaw is relevant for inferring attention, intention, and emotion; pitch is relevant for the perception of emotion, dominance, trustworthiness, and attractiveness; and roll is important for communicative and emotional signals. (c) Identity needs to be extracted irrespective of changes in the visual input, such as different head orientations. Parents of monozygotic twins are highly skilled in distinguishing their children accurately despite the similarity of visual input and outperform their children’s teachers, neighbors, and external family members. Panel c adapted with permission from Saether & Laeng (2008); copyright 2008 SAGE Publications. (d) Head orientation tuning (left) of three areas in the macaque face processing hierarchy: Neurons in ML are tuned to specific head orientations, neurons in AL are tuned to mirror symmetric ones, and AM neurons are only broadly, if at all, tuned to head orientation. Predictive coding suggests that higher brain areas such as the face patches AM and AL send predictions to lower brain areas such as face patch ML through feedback connections. This process should endow ML prediction error signals with the tuning properties of AM/AL. Indeed, prediction errors, that is, the difference between the prediction and the actual sensory inputs, show mirror-symmetric head orientation tuning and identity selectivity, which are tuning properties of AL and AM, respectively (Schwiedrzik & Freiwald 2017).
Electrophysiological recordings in macaque face areas showed that almost all of the visually responsive cells in these fMRI-identified areas are strongly face selective—that is, they respond at least twice as much to faces than to other objects (Freiwald & Tsao 2010, Tsao et al. 2006). While spatially disjunct, face patches form a tightly interconnected network spanning the anterior-to-posterior axis of the ventral visual processing hierarchy (Grimaldi et al. 2016, Moeller et al. 2008). Most monosynaptic connections of face areas originate or terminate in other face areas (Grimaldi et al. 2016). This network encodes various facial dimensions relevant to social information processing (Freiwald & Tsao 2010), including head orientation and facial identity.
Head Orientation
Head orientation is a highly relevant social signal (Figure 2b). Primates are sensitive to yaw (Emery et al. 1997, Itakura & Anderson 1996)—orientation to the left or right of the observer. Yaw provides information about others’ direction of attention (Langton & Bruce 2000) and intentions and can trigger automatic shifts of the viewer’s attention (Langton & Bruce 1999, 2000). Pitch (or head tilt) is informative about emotional states. Pitch can also contribute to dominance displays (Mignault & Chaudhuri 2003, Witkower & Tracy 2019) and influences judgments of trustworthiness (Torrance et al. 2020) and attractiveness (Marshall et al. 2020).
The perception of head orientation is mainly driven by the head’s contour and the orientation of the nose (Wilson et al. 2000) (Figure 2c). Relative to the possible range of rigid head motion (up to ~160° in head-neck rotation) (Ferrario et al. 2002), humans are sensitive to small differences in head orientation (~1.5°), especially in frontal views and at distances that are relevant for social interactions (Troje & Siebeck 1998, Wilson et al. 2000).
Neurons responsive to head orientation are found across the temporal lobe (Oram & Perrett 1992; Perrett et al. 1985, 1991), especially in more posterior face areas such as ML (Freiwald & Tsao 2010). Estimates of neural tuning width for head orientation vary between 20° and 100° (Eifuku et al. 2004, Freiwald & Tsao 2010, Perrett et al. 1991). Frontal views of faces, which are arguably the views with which interacting agents have the most experience, seem to be overrepresented (Hasselmo et al. 1989; Perrett et al. 1991, 1998). Many of these neurons are species (Murphy & Leopold 2019) and identity invariant (Freiwald & Tsao 2010, Perrett et al. 1991), suggesting that the face processing system extracts this property with both sensitivity and robustness.
Facial Identity
Identity is a major socially relevant piece of information that faces provide. Human cultural adaptations to track and verify identity, such as identification cards, point to the primacy of faces for successful identification. Identification is a key precursor to recalling social knowledge. Confusion of identity, in turn, can be costly, misdirecting behaviors like aggression or parental care (Tibbetts & Dale 2007).
Humans and other primates show variability in facial morphology that can be used for individual recognition (Santana et al. 2012, Sheehan & Nachman 2014). In humans, genetic loci associated with variation in normal facial morphology show elevated nucleotide diversity (compared with loci associated with variation in height), which suggests selection for sufficiently distinct faces (Sheehan & Nachman 2014). Humans excel in recognizing familiar individuals by the face, most evident in the ability of parents to distinguish monozygotic twins by their faces (Saether & Laeng 2008, Stevenage 1998) (Figure 2c). Other primates are also able to recognize others by their faces (Moeller et al. 2017, Rosenfeld & Van Hoesen 1979). Recognizing facial identity needs to be invariant to identity-preserving transformations such as changes in size, head orientation, lighting conditions, or emotional expression, even though they can alter visual input much more than changes in identity. Behavioral evidence shows that matching identity across these transformations holds, particularly for familiar faces (Etchells et al. 2017).
Neurons that respond selectively to the physical differences among faces that carry identity information have been found in the temporal lobe of macaque monkeys (Perrett et al. 1984), especially in anterior parts such as the face area AM (Freiwald & Tsao 2010). Neurons in AM respond selectively to facial identity irrespective of head orientation, position, or size (Freiwald & Tsao 2010) and are causally relevant for identity recognition: For example, when they are stimulated electrically, performance in an identity-matching task is strongly reduced (Moeller et al. 2017). AM neurons are thought to span a face space that encodes structural features of faces (Chang & Tsao 2017). Availability of identity information may arise from a sensitivity of these neurons to texture or shape differences between faces (Chang & Tsao 2017), paralleling human behavioral sensitivity to these stimulus dimensions (Jozwik et al. 2022). Such physical differences between faces form the basis on which familiar faces can be recognized. Two areas in the macaque monkey brain, outside the core face processing network, are selective for facial familiarity: one in the perirhinal cortex, and the other one in the temporal pole (TP) (Landi & Freiwald 2017). In the latter area, neurons with high identity selectivity for specific, personally familiar faces have been found (Landi et al. 2021), suggesting they form a linkage between generic face-space representations and person memory.
Functional Organization of Face Processing Systems
The face processing system in the macaque monkey brain, as we have reviewed thus far, is characterized by five main organizational principles: (a) the clustering of face-selective cells into face areas, (b) consisting of not one but multiple areas, (c) each with a unique functional specialization, (d) all specifically and tightly interconnected into a network, and (e) displaying network organization along a hierarchy.
Why are face-selective cells clustered into brain areas? Functional clustering occurs spontaneously in deep convolutional neural networks trained on face and object recognition (Dobs et al. 2022), suggesting that this organizational principle may result from the nature of the computational problem posed by face recognition, rather than details unique to the system that primates have evolved to solve it. The computational effectiveness of functional clustering, combined with biophysical constraints on the size of axonal and dendritic processes, may explain spatial clustering of face-selective neurons within the brain.
While biophysical constraints might suggest the evolution of a single face area, primates have instead evolved a network of multiple areas. Face regions may thus have evolved at repeated locations along and within larger visual maps for object and color recognition (Conway 2018, Arcaro & Livingstone 2021). This parallel organization between face and object-processing extends along the ventral visual processing hierarchy in the temporal lobe (Bao et al. 2021), suggesting that the computations within each face area and the transformations between them are not unique for faces.
Division of labor between face areas (e.g., McMahon et al. 2015) might then be a consequence of this large-scale organization. This organization follows a hierarchical scheme, from posterior areas connected to early visual cortex to anterior areas connected to the hippocampal formation via perirhinal cortex (Felleman & Van Essen 1991). In the face processing system, this hierarchy implements two major transformations to solve the invariant face recognition problem (Freiwald & Tsao 2010). Neurons in posterior face areas, ML and MF, are primarily sensitive to head orientation (Figure 2d). Neurons in anteriorly located face area AL display partial view invariance, exhibiting mirror-symmetric yaw tuning. And neurons in the most anterior face patch AM exhibit view invariance, differentiating between identity-specific physical properties of faces. This, together with increasing response latencies from ML to AL to AM and increasing receptive field sizes, suggests that the brain takes a series of computational steps from relatively image-bound representations in ML toward an abstract, view-invariant, identity-specific representation in AM (Leibo et al. 2017).
How might hierarchical organization help solve the computational challenge of face recognition? Most thinking about hierarchies has focused on feedforward processing and its ability to generate increasingly more sophisticated representations (DiCarlo & Cox 2007). But face areas are connected by a similar density of feedforward and feedback connections (Grimaldi et al. 2016), indicating the importance of reverse information flow from high-level to low-level areas. In a predictive coding framework (Huang & Rao 2011, Koster-Hale & Saxe 2013, Mumford 1992), feedback connections allow higher brain areas to send predictions through to lower brain areas. There they are compared to inputs, allowing for deviations from expectations—that is, prediction errors—to be computed. Prediction errors are in turn propagated downstream as learning signals.
In support of this idea, there is evidence for predictive coding in the face processing hierarchy (Issa et al. 2018, Schwiedrzik & Freiwald 2017). In one study (Schwiedrzik & Freiwald 2017) (Figure 2d), after monkeys had formed expectations about temporal sequences of faces, the occurrence of an unexpected face led to a prediction error signal in the response of individual ML neurons. Importantly, the prediction error in ML was identity specific—that is, tuned similar to cells in higher-order face area AM—and showed mirror-symmetric head orientation tuning like AL, suggesting an origin of the prediction signal in these higher face areas. Another study (Issa et al. 2018) found prediction errors in lower-level areas for atypical and thus unpredictable spatial face configurations, while higher areas exhibited the preference for typical configurations expected for areas encoding higher-level predictions about face structure. While the clear functional organization of the face processing hierarchy facilitated the discovery of these properties in support of predictive coding, similar operations might exist in neighboring non-face-selective regions (Esmailpour et al. 2023, Meyer & Olson 2011). Thus, the current understanding of the face processing system is consistent with similar computations performed by face-preferring and non-preferring portions of the visual hierarchy.
Connections Between Face Processing Circuits and Other Social Brain Areas
Are systems for face processing integrated with broader regions involved in social cognition in the primate brain? Or, alternatively, do these constitute separate, functionally isolated components of the social brain? As discussed below, areas implicated in high-level social cognition fall within a zone of cortex termed the default mode network (DMN). The DMN constitutes a network of interconnected association areas across the frontal, temporal, and parietal lobes (Buckner & Margulies 2019, Margulies et al. 2016). Originally identified in humans, a DMN is also found in macaques and other primate species (Buckner & Margulies 2019, Garin et al. 2022, Liu et al. 2019, Mantini et al. 2011, Vincent et al. 2007) and is argued to be positioned at the apex of the primate cortical hierarchy (Margulies et al. 2016). Recent work has suggested the presence of social cognitive processing in several candidate homologs to human DMN areas, including the medial prefrontal cortex (MPFC), the dorsal STS, and area 7a in the inferior parietal lobule (Cléry et al. 2021, Roumazeilles et al. 2021, Sliwa & Freiwald 2017). This suggests a more specific framing of our question: Does the macaque face patch system interact with social cognition areas of the DMN?
Studies of structural connectivity of face areas, surprisingly, have found that most connections are with other face areas, with only a few specific connections to outside regions, which include the medial temporal lobes (perirhinal and parahippocampal cortex), amygdala, pulvinar, and claustrum (Grimaldi et al. 2016, Moeller et al. 2008). The connection to the amygdala, which contains face-selective neurons (Gothard et al. 2007), could plausibly provide a domain-specific link between face processing and social cognition systems. However, direct monosynaptic connections between face areas and parts of the DMN have not been observed. This conclusion is consistent with the broader literature on anatomical connectivity in the macaque, which suggests that the ventral bank of the STS (containing face areas) and the dorsal bank (associated with the DMN) are largely separate in anatomical connectivity (Seltzer & Pandya 1989). This pattern of anatomical connectivity indicates that systems for face perception and social cognition constitute separate streams within a complex hierarchical structure (Figure 1c).
In contrast, functional connectivity reveals a broad pattern of interactions (Schwiedrzik et al. 2015). While some differences are observed in the specific connectivity profiles of individual face areas, all areas showed consistent connectivity with a distributed set of areas termed the face patch functional connectome. Remarkably, the face patch functional connectome intersected substantially with the DMN. This intersection was confined to three regions: the MPFC, medial parietal cortex, and a region within the upper bank of the posterior STS (Schwiedrzik et al. 2015). In humans, face-responsive areas in ventral temporal cortex have stronger functional connectivity with parts of medial parietal cortex responsive to person memory tasks (Silson et al. 2019). Thus, while systems for face perception and social cognition in primates are situated within separate regimes of anatomical connectivity, initial evidence argues for a specific functional interaction between these systems. This interaction is likely mediated by indirect anatomical connections through intermediary areas. These results suggest that distinct components of the social brain are not entirely isolated, but function in concert.
FROM SOCIAL PERCEPTION TO SOCIAL COGNITION
Social Interaction Understanding
Much of what a primate learns about others—their personalities, internal mental states, and group associations—comes from observing their interactions. The visual analysis of interactions is a computationally daunting problem: It concerns multiple players, such as their face, body shape, and posture; their configuration relative to one another; and the dynamics of all of these properties, and even subtle details (a cold versus warm embrace) matter (Baldassano et al. 2017, Zhou et al. 2019). Yet primates readily detect interactions and understand their meaning (Su et al. 2016).
To meet these computational challenges, which are even more demanding than those of face recognition, a neural machinery that is at least as extensive appears necessary, and one that builds on face and body processing systems. In both monkeys and humans, this machinery originates within visual cortex (Wurm et al. 2017), specifically face and body areas (Abassi & Papeo 2020, Georgescu et al. 2014, Quadflieg & Koldewyn 2017, Sliwa & Freiwald 2017). An fMRI study in macaque monkeys localized face and body areas and found that body areas, but not immediately adjacent face areas, were substantially more active during the observation of agents engaged in goal-directed, nonsocial tasks and that both face and body areas were even more active during the observation of social interactions (Sliwa & Freiwald 2017) (Figure 3a,b). Thus, neural machinery engaged by face and body shape might exhibit much more complex selectivity supporting the understanding of whole agents and their interactions.
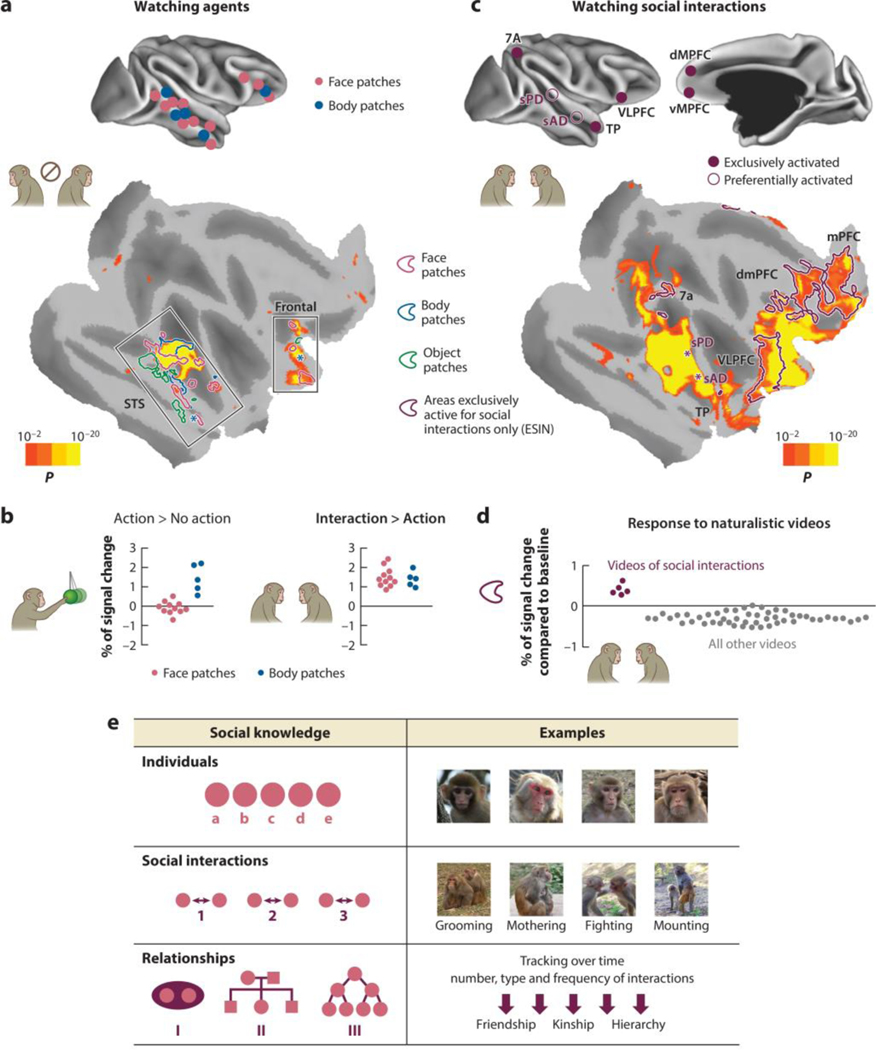
Figure 3.
(a) Watching agents in naturalistic videos enhances functional MRI (fMRI) signal in the core face patch system and the core body patch system in the superior temporal sulcus (STS) and frontal cortex. A statistical map of enhanced activation is shown on a flattened F99 cortical model of the right hemisphere, with dark gray regions representing sulci and light gray regions representing gyri [P< 0.01, false discovery rate (FDR)-corrected for multiple comparisons, asterisks indicate body patches for which location varied in between subjects (bottom)]. Panel a adapted from Sliwa & Freiwald (2017). (b) When agents are acting, fMRI activation is enhanced in the body but not face patches. When agents are interacting, fMRI activation is enhanced in both body and face patches. Scatter plots are normalized signal changes for the contrast for all face and body patches. (c) Watching social interactions in naturalistic videos shows an extended network of regions that are significantly activated, including in the STS social posterior dorsal (sPD) and social anterior dorsal (sAD) areas. Burgundy lines outline areas that are exclusively activated by videos of social interactions only and either deactivated or not activated by any other visual stimulation [exclusively social interaction network (ESIN)], and these areas include the medial and dorsomedial prefrontal cortices (mPFC and dmPFC), the ventrolateral prefrontal cortex (VLPFC), the temporal pole (TP), and parietal area 7a (bottom). Asterisks indicate location of sPD and sAD. Panel c adapted from Sliwa & Freiwald (2017). (d) Scatter plots represent percent of fMRI signal change in regions of interest of the ESIN for videos of social interactions and any other naturalistic videos. Data for panel d from Sliwa & Freiwald (2017). (e) Schematic of primates’ three levels of social understanding of their societies based on Cheney et al. (1986). Macaque photos by V. Martignac and F. Wu.
Dynamic social interactions engage not only face and body areas but also additional areas in the macaque dorsal STS [social posterior dorsal (sPD) and social anterior dorsal (sAD), located dorsally to face patches PL and AF; see Figures 1 and 3c] (Sliwa & Freiwald 2017). Similarly, in humans, pairwise social interactions depicted in point-light displays elicit responses in the STS (Isik et al. 2017, Landsiedel et al. 2022, Lee Masson & Isik 2021, Walbrin et al. 2018), including an area just posterior to the dynamic face area (Isik et al. 2017), consistent in location with previously reported body motion responses (Deen et al. 2015). Macaque and human STS thus comprise complex functional landscapes, with neural responses to social interactions found in regions selective for dynamic faces and bodies, and beyond.
Furthermore, when macaque monkeys view social interactions, large swaths of cortex outside of the temporal lobe are engaged (Figure 3c). These regions include areas associated with the DMN, including the MPFC (areas 10mr and 9m), temporo-parietal cortex (areas TPOc and 7a), ventrolateral prefrontal cortex (including parts of areas F5, 44, 47, 12, and OPro), orbitofrontal cortex (parts of areas 10o, 11l, 14r), and a region of TP (Sliwa & Freiwald 2017). Some of these regions are so highly selective that they respond above baseline only during the observation of social interactions and not during any other control condition (Figure 3d). A similar set of activations during the observation of social interactions is found in the evolutionarily more distant New World marmoset monkey (Cléry et al. 2021), highlighting a common evolutionary origin of the neural circuitry supporting complex social cognitive functions.
Levels of Primate Social Understanding
It has been argued that primates understand their social world at three levels (Cheney et al. 1986) (Figure 3e). At the first level, there is knowledge of the individual. At the second level, primates analyze social interactions, as we have reviewed above. And at the third level, these two sets of information are combined into structured knowledge of the relations between specific individuals. For instance, monkeys infer their peers’ rank by watching them fight and then use this information to recruit allies during later disputes (Schino et al. 2006, Silk 1999, Zumpe & Michael 1970). By tracking the nature and frequency of social interactions over time, primates infer the relationships of others and classify them according to their rank (Bergman et al. 2003, Schino et al. 2006), kin (Cheney & Seyfarth 1980, Dasser 1988), friendships (Whitehouse & Meunier 2020), partners (Pfefferle et al. 2008), and group membership (Schell et al. 2011). This process involves not only recognizing the social encounter and individual identities but also comparing the interacting individuals to each other. Little is known about where this knowledge is stored and how it is used, particularly at the level of neural coding. That said, the computational demands of these processes—requiring abstract representations of individuals and their relationships—may require neural machinery positioned relatively high in the cortical hierarchy. Indeed, studies in humans suggest that abstract knowledge about individuals is encoded at the apex of the cortical hierarchy, within the DMN (Koski et al. 2017, Mason et al. 2014).
SOCIAL COGNITION: UNDERSTANDING THE ABSTRACT CAUSAL BASIS OF BEHAVIOR
Theory of Mind in Humans and Nonhuman Primates
Social cognition is not confined to the processing of sensory information and inferences about observable physical entities, such as recognizing their identity. We understand others’ actions in terms of inferred underlying causes—unobservable mental states like beliefs, intentions, and emotions. We use theory of mind (ToM): an internal causal model of how states interact and give rise to behavior (Baker et al. 2017). This process is refined by our experience with familiar individuals, which enables us to predict their behavior using social memory—information about familiar conspecifics stored in long-term memory (Srull & Wyer 1989). ToM is computationally challenging: It requires representing knowledge about the world—complex propositional states such as “Emily hopes to pursue a career as a physician”—and understanding how causal relationships between propositional states influence behavior (Anzellotti & Young 2020).
While social cognition has been studied most extensively in humans, behavioral research with nonhuman primates demonstrates an understanding of conspecifics’ actions. A range of species, including macaques and nonhuman great apes, can predict another animal’s actions based on what the other animal has observed in the past (Hare et al. 2001, Kano et al. 2019, Santos et al. 2006), which has been argued to constitute an understanding of others’ knowledge (Call & Tomasello 2008, Phillips et al. 2021). Monkeys and apes are also sensitive to others’ goals and intentions, further components of a causal understanding of behavior (Call et al. 2004, Phillips et al. 2009). Debate continues as to how rich and human-like theories of mind are in nonverbal animals (Heyes 2015, Penn & Povinelli 2007). In particular, the ability of nonhuman primates to represent full, belief-like propositional states, as opposed to distinguishing between knowledge and ignorance, remains contested (Phillips et al. 2021). Nevertheless, positing some form of internal causal model or proto-ToM provides a parsimonious explanation for a diverse array of behavioral phenomena in primates (Call & Tomasello 2008).
Neuroimaging studies of ToM in humans have reliably found that a set of brain regions across high-level association cortex in the frontal, parietal, and temporal lobes are engaged when we reason about other people’s behavior, relative to a range of control conditions (Fletcher et al. 1995, Saxe & Kanwisher 2003) (Figure 1). These areas fall within the DMN (Buckner et al. 2008, Raichle 2015), including the MPFC, medial parietal cortex (MPC, including the posterior cingulate cortex and precuneus), TPJ (centered on the angular gyrus), STS, TP, and superior frontal gyrus. Given their anatomical positioning at the tip of associative cortices near the apex of the cortical hierarchy, these areas are well placed to generate high-level predictions about others’ behavior based on abstract mental states (Koster-Hale & Saxe 2013).
Homologs of the human DMN, as we have seen above, also exist in nonhuman primates. A DMN has been identified in terms of functional and anatomical connectivity in macaques (Mantini et al. 2011, Vincent et al. 2007) and marmosets (Buckner & Margulies 2019, Liu et al. 2019). fMRI studies on social interaction described above have provided evidence for evolutionary precursors to human social cognition responses within the DMN (Cléry et al. 2021, Roumazeilles et al. 2021, Sliwa & Freiwald 2017). While social responses within MPFC (areas 9 and 10) afford a straightforward potential homology across species, homologies within the parietal and temporal lobe remain debated. Some have argued that the socially responsive macaque mid-STS is a homolog to the human TPJ (Mars et al. 2013, Ninomiya et al. 2021, Roumazeilles et al. 2021). An alternative view is that the macaque homolog of TPJ falls within area 7a, also known as PG/Opt. Just inferior to the intraparietal sulcus, macaque 7a shares gross anatomical and cytoarchitectonic properties with the human angular gyrus or area PG (Niu & Palomero-Gallagher 2023). This area forms the lateral parietal node of the DMN in macaques and has a strongly selective response to social interaction over other visual inputs (Sliwa & Freiwald 2017). Based on this hypothesis, social responses in the macaque mid-STS could instead form precursors to human ToM areas in middle or anterior STS.
Domain Specificity of Theory of Mind Responses
How do neural systems for social cognition relate to the machinery for other high-level cognitive functions, such as long-term memory and domain-general reasoning? In addition to ToM tasks, brain areas within the DMN have been found to respond during tasks that involve internal thought processes, such as recalling episodic memories or imagining future events (Addis et al. 2007, Szpunar et al. 2007). This coarse anatomical similarity between patterns of response to ToM and other tasks has been taken as evidence that the DMN is a domain-general system for abstract reasoning and memory (Barrett & Satpute 2013, Buckner & Carroll 2007, Margulies et al. 2016, Spreng et al. 2009). However, this argument relies on a comparison of fMRI responses at the group level—after combining responses from multiple individual brains in a standard template space—and this method entails spatial blurring that can introduce spurious overlap between nearby but distinct functional responses (Fedorenko 2021, Saxe et al. 2006). In contrast, studies of responses from functionally defined ToM-sensitive regions within the DMN in individual brains found response profiles that were strongly selective for social content, arguing in favor of a domain-specific system (Saxe & Kanwisher 2003, Saxe & Powell 2006).
Recent work has advanced this debate by characterizing precise functional architecture within the DMN. Silson et al. (2019) identified a distinct set of regions within the MPC that are engaged during recall of familiar people and places, showing an alternating pattern of response along the axis of the cingulate sulcus (Figure 4a). Braga & Buckner (2017), analyzing functional connectivity in densely sampled individual participants, found that the DMN constitutes two distinct, spatially interdigitated subnetworks, with resting-state correlations observed within each network but not between the two (Figure 4b). DiNicola et al. (2020) provided an initial functional characterization of these two subnetworks, establishing a double dissociation in which one network responded during a ToM task, while the other responded during an episodic recall task. These findings suggest that previously observed functional overlap within the DMN may have resulted from the blurring of two finely interdigitated networks as a result of group averaging.
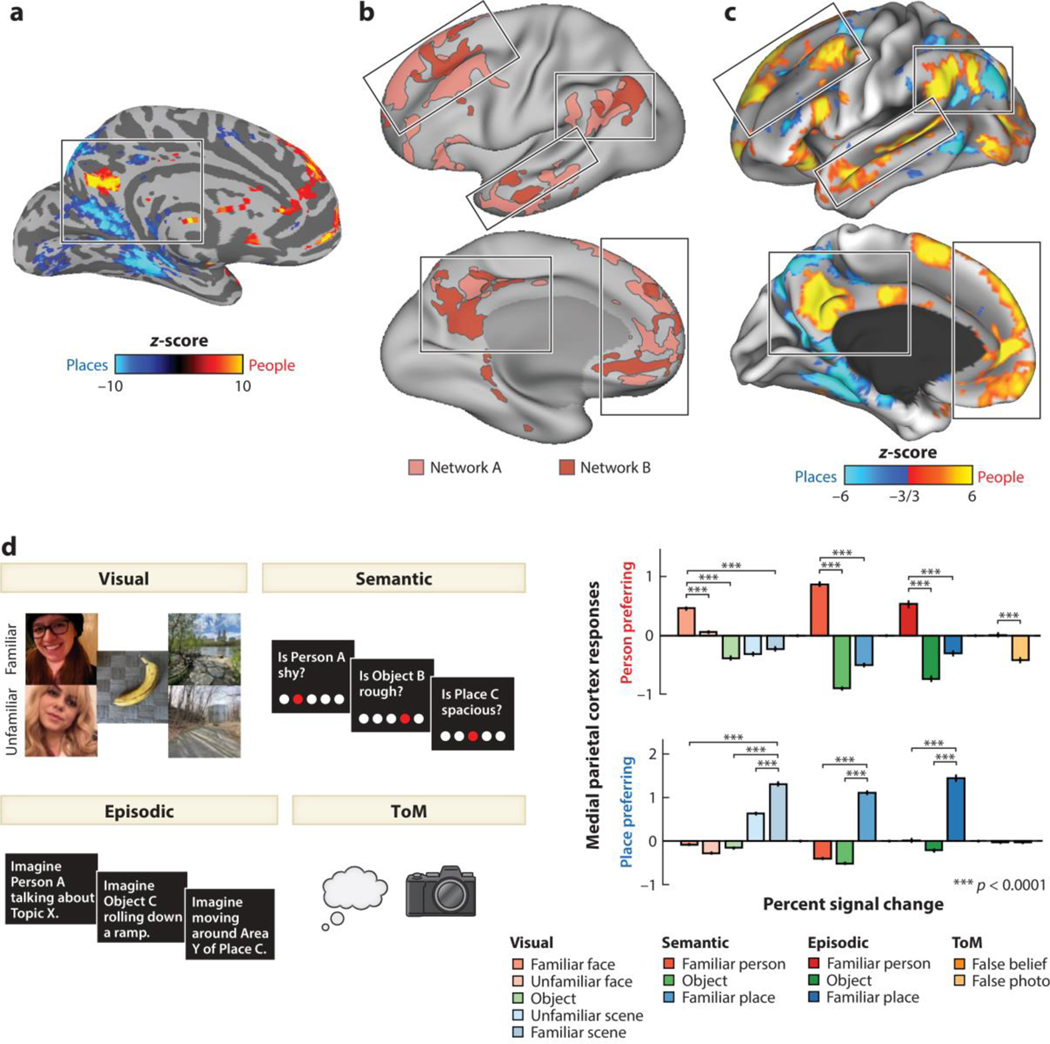
Figure 4.
Domain-specific functional organization within the default mode network (DMN). (a) Different areas within the medial parietal cortex respond during the recollection of familiar faces and scenes. These areas show an alternating pattern of domain preferences along the axis of the cingulate sulcus. Panel a adapted from Silson et al. (2019) (CC0 1.0). (b) Resting-state functional connectivity identifies two parallel, interdigitated networks within the DMN. Signals from within network are spontaneously correlated across different lobes and hemispheres but are not correlated across networks. Panel b adapted from Braga & Buckner (2017) (CC BY 4.0). (c) Distinct subnetworks within the DMN respond when imagining events involving familiar people and places (responses from a representative individual subject). Data for panel c from Deen & Freiwald (2021). (d) Responses to multiple tasks that involve thinking about familiar people and places, extracted from person- and place-preferring regions within the DMN (medial parietal cortex is shown as an example; similar response profiles are observed in other areas). These results demonstrate the domain specificity of functional responses within the DMN. Furthermore, they show that similar regions respond to tasks that involve thinking about familiar people, and a standard theory of mind (ToM) localizer. Data for panel d from Deen & Freiwald (2021).
How then should we understand the functional role of DMN subnetworks? While neuroimaging studies typically contrast two conditions to isolate a specific cognitive or perceptual process, individual comparisons generally contain confounds that allow for alternative explanations. One way to overcome this limitation is to study responses in individual humans performing a whole range of tasks, thus providing a rich functional characterization across conditions with differing confounds (Deen et al. 2015).
Using this approach, a recent study has argued that parallel DMN subnetworks can be understood as implementing a common cognitive process across distinct content domains rather than separate processes such as ToM and episodic recall (Deen & Freiwald 2021). Specifically, the authors hypothesized that these two networks support internal models of familiar people and familiar places, respectively. To test for domain specificity within the DMN, individual participants were scanned several times while performing a range of tasks eliciting representations of familiar people and places: visual perception, semantic judgment, and episodic projection. One DMN subnetwork specifically responded to familiar people across different tasks, while the other subnetwork specifically responded to familiar places across tasks (Deen & Freiwald 2021) (Figure 4c,d). While a difference in response to people versus places in one task could be driven by a confound (e.g., images of people and places have different visual features), observing a consistent category preference across multiple tasks cannot be explained by a confound unique to any one task and is more easily explained as an effect of content domain. These results provide strong evidence against the notion of a domain-general DMN, instead arguing that subsystems within the DMN process information from distinct domains. That said, similar to the organization of face processing areas in the ventral visual stream, areas within the DMN involved in high-level social cognition have a parallel organization to areas involved in nonsocial processing, indicating a parallel computational role.
Neural Basis of Person Knowledge
In addition to mental states, primates use information about other specific animals to make inferences about their behavior. In humans, research in social psychology has argued that personalities are modeled in terms of low-dimensional feature spaces, with continuous values for traits like valence—whether someone is good or bad—and competence or causal efficacy (Anzellotti & Young 2020, Fiske et al. 2007).
In the macaque brain, Báez-Mendoza et al. (2021) identified neurons within the dorsal MPFC (area 24) with responses tuned to the identity of other animals during a three-animal economic game. These cells were only active during interactions and not when simply looking at another monkey. This finding contrasts with the aforementioned neurons in the TP that represent familiar faces in macaque temporal cortex (Landi et al. 2021), as well as fMRI evidence for representations of familiar faces in human MPFC (di Oleggio Castello et al. 2017). Rather than providing a generic identity representation, neurons in area 24 track other monkeys’ reward history, monitor the social outcomes of others’ choices, and predict future reciprocation and retaliation (Báez-Mendoza et al. 2021).
These findings motivate the question whether social knowledge and ToM are supported by separate systems. Prior studies in humans have identified responses within the DMN to both ToM and familiar person tasks (Gobbini & Haxby 2007, Mitchell 2009, Tamir et al. 2016, Thornton & Mitchell 2017), as well as tasks involving judgments about the relative status of multiple individuals (Koski et al. 2017, Mason et al. 2014). However, these comparisons have only been made at the group level and not within individual brains. A recent study found that the social subnetwork of the DMN responded strongly to both a previously established ToM localizer task and various familiar person tasks (Deen & Freiwald 2021) (Figure 4d). Areas defined by a ToM contrast also responded strongly to social memory manipulations, and vice versa. These data argue for a common system for reasoning about mental states and storing information about familiar people in long-term memory. This system may support the formation of internal causal models of specific familiar people using information from experience to support refined behavioral predictions for specific familiar others. Such models make high-level predictions about others’ behavior, which may in turn inform processing in lower-level areas representing information about social interactions, actions, bodies, and faces.
CONCLUSION
Functionally specific areas for social information processing can be found across primate cortex (Figure 1). These areas interact with one another, forming a social brain—a neural system specialized for social cognition. In face processing circuits (Figure 2), specialized face cells are grouped into face areas at highly reproducible locations and with functional properties that are highly homogenous locally, yet qualitatively different across areas. Face regions are bound together through highly specific connections into a higher level of organization: a face processing network, in which information is transformed from one node to the next. Interspersed in this face processing network are visual regions that process non-facial information, revealing a juxtaposition of social and nonsocial information processing circuits in the primate.
The face processing network forms one of multiple subcircuits of the social brain, along with a system for social cognition positioned within the DMN. These systems fall within distinct regimes of anatomical connectivity but appear to interact functionally, consistent with the idea that the social brain constitutes a coherent entity. Though our mechanistic understanding of social cognition systems in the brain is much less advanced than that of the face processing system, similar organizational motifs between systems and homologies across species suggest promise for a systems-level understanding of high-level processes. The high level of specialization and the juxtaposition of social and nonsocial information processing circuits are not limited to the sensory periphery. This organization is maintained throughout the cortical hierarchy (Figure 4). This picture of the social brain suggests that processes such as the construction of ToM-like internal causal models might become just as mechanistically tractable as face processing already has. The homologies that exist between different primate species will be important in transferring such detailed mechanistic knowledge to understand the human social brain and to uncover general principles of social brain function. They will also help elucidate just how much detailed organization—from single cells and local feature maps to areas, networks, and subcircuits—exists within the social brain.
ACKNOWLEDGMENTS
The following funding support made this work possible: a Helen Hay Whitney fellowship and a Leon Levy postdoctoral fellowship, both at The Rockefeller University, to B.D.; a Human Frontier Science Program long-term fellowship (LT001118/2012-L) at The Rockefeller University, the Leibniz Collaborative Excellence Program (K265/2019), German Research Foundation (454648639)—SFB 1528—Cognition of Interaction (project A04), and the Emmy Noether Programme of the German Research Foundation (SCHW1683/2-1) to C.M.S.; a Women & Science postdoctoral fellowship and a Kavli Neural Systems Institute postdoctoral fellowship (both at The Rockefeller University), the Agence National de la Recherche (ANR-21-CE37-0016-02, ANR-19-CE37-0002-01, ANR-10-IAIHU-06), and a European Research Council (101042884-NEURO-SOCIETY) grant to J.S.; and the National Science Foundation Center for Brains, Minds and Machines (CCF-1231216/5710003506), the National Institute of Mental Health (R01MH105397), and the Department of the Navy’s Office of Naval Research (N00014-20-1-2292) to W.A.F. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Science Foundation, the European Union, or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abassi E, Papeo L. 2020. The representation of two-body shapes in the human visual cortex. J. Neurosci. 40:852–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. 2007. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45:1363–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzellotti S, Young LL. 2020. The acquisition of person knowledge. Annu. Rev. Psychol. 71:613–34 [DOI] [PubMed] [Google Scholar]
- Arcaro MJ, Livingstone MS. 2021. On the relationship between maps and domains in inferotemporal cortex. Nat. Rev. Neurosci. 22:573–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Mastrobattista EP, Wang AJ, Williams ZM. 2021. Social agent identity cells in the prefrontal cortex of interacting groups of primates. Science 374:eabb4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Jara-Ettinger J, Saxe R, Tenenbaum JB. 2017. Rational quantitative attribution of beliefs, desires and percepts in human mentalizing. Nat. Hum. Behav. 1:0064 [Google Scholar]
- Baldassano C, Beck DM, Li F-F. 2017. Human–object interactions are more than the sum of their parts. Cereb. Cortex 27:2276–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao P, She L, McGill M, Tsao DY. 2020. A map of object space in primate inferotemporal cortex. Nature 583:103–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 23:361–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. 2003. Hierarchical classification by rank and kinship in baboons. Science 302:1234–36 [DOI] [PubMed] [Google Scholar]
- Bernstein M, Erez Y, Blank I, Yovel G. 2018. An integrated neural framework for dynamic and static face processing. Sci. Rep 8:7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Buckner RL. 2017. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron 95:457–71.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. 2007. Self-projection and the brain. Trends Cogn. Sci. 11:49–57 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Margulies DS. 2019. Macroscale cortical organization and a default-like apex transmodal network in the marmoset monkey. Nat. Commun. 10:1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call J, Hare B, Carpenter M, Tomasello M. 2004. ‘Unwilling’ versus ‘unable’: chimpanzees’ understanding of human intentional action. Dev. Sci. 7:488–98 [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. 2008. Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn. Sci. 12:187–92 [DOI] [PubMed] [Google Scholar]
- Chang L, Tsao DY. 2017. The code for facial identity in the primate brain. Cell 169:1013–28.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D, Seyfarth RM. 1980. Vocal recognition in free-ranging vervet monkeys. Anim. Behav. 28:362–67 [Google Scholar]
- Cheney D, Seyfarth R, Smuts B. 1986. Social relationships and social cognition in nonhuman primates. Science 234:1361–66 [DOI] [PubMed] [Google Scholar]
- Cléry JC, Hori Y, Schaeffer DJ, Menon RS, Everling S. 2021. Neural network of social interaction observation in marmosets. eLife 10:e65012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR. 2018. The organization and operation of inferior temporal cortex. Annu. Rev. Vis. Sci. 4:381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie TE, Little AC. 2009. The relative importance of the face and body in judgments of human physical attractiveness. Evol. Hum. Behav. 30:409–16 [Google Scholar]
- Dasser V. 1988. A social concept in Java monkeys. Anim. Behav. 36:225–30 [Google Scholar]
- Deen B, Freiwald W. 2021. Parallel systems for social and spatial reasoning within the cortical apex. bioRxiv 2021.09.23.461550. 10.1101/2021.09.23.461550 [DOI] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. 2015. Functional organization of social perception and cognition in the superior temporal sulcus. Cereb. Cortex 25:4596–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Oleggio Castello MV, Halchenko YO, Guntupalli JS, Gors JD, Gobbini MI. 2017. The neural representation of personally familiar and unfamiliar faces in the distributed system for face perception. Sci. Rep. 7:12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JJ, Cox DD. 2007. Untangling invariant object recognition. Trends Cogn. Sci. 11:333–41 [DOI] [PubMed] [Google Scholar]
- DiNicola LM, Braga RM, Buckner RL. 2020. Parallel distributed networks dissociate episodic and social functions within the individual. J. Neurophysiol. 123:1144–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobs K, Martinez J, Kell AJE, Kanwisher N. 2022. Brain-like functional specialization emerges spontaneously in deep neural networks. Sci. Adv. 8:eabl8913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI. 1998. The social brain hypothesis. Evol. Anthropol. 6:178–90 [Google Scholar]
- Eifuku S, De Souza WC, Tamura R, Nishijo H, Ono T. 2004. Neuronal correlates of face identification in the monkey anterior temporal cortical areas. J. Neurophysiol. 91:358–71 [DOI] [PubMed] [Google Scholar]
- Elias LJ, Succi IK, Schaffler MD, Foster W, Gradwell MA, et al. 2023. Touch neurons underlying dopaminergic pleasurable touch and sexual receptivity. Cell 186:577–90.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. 1997. Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J. Comp. Psychol. 111:286–93 [DOI] [PubMed] [Google Scholar]
- Esmailpour H, Raman R, Vogels R. 2023. Inferior temporal cortex leads prefrontal cortex in response to a violation of a learned sequence. Cereb. Cortex 33:3124–41 [DOI] [PubMed] [Google Scholar]
- Etchells DB, Brooks JL, Johnston RA. 2017. Evidence for view-invariant face recognition units in unfamiliar face learning. Q. J. Exp. Psychol. 70:874–89 [DOI] [PubMed] [Google Scholar]
- Fedorenko E. 2021. The early origins and the growing popularity of the individual-subject analytic approach in human neuroscience. Curr. Opin. Behav. Sci. 40:105–12 [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1:1–47 [DOI] [PubMed] [Google Scholar]
- Ferrario VF, Sforza C, Serrao G, Grassi G, Mossi E. 2002. Active range of motion of the head and cervical spine: a three-dimensional investigation in healthy young adults. J. Orthop. Res. 20:122–29 [DOI] [PubMed] [Google Scholar]
- Fisher C, Freiwald WA. 2015. Contrasting specializations for facial motion within the macaque face-processing system. Curr. Biol 25:261–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJ, Glick P. 2007. Universal dimensions of social cognition: warmth and competence. Trends Cogn. Sci. 11:77–83 [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, et al. 1995. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 57:109–28 [DOI] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY. 2010. Functional compartmentalization and viewpoint generalization within the macaque face processing system. Science 330:845–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin CM, Hori Y, Everling S, Whitlow CT, Calabro FJ, et al. 2022. An evolutionary gap in primate default mode network organization. Cell Rep. 39:110669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov N, Nieder A. 2021. Distinct neural networks for the volitional control of vocal and manual actions in the monkey homologue of Broca’s area. eLife 10:e62797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu AL, Kuzmanovic B, Santos NS, Tepest R, Bente G, et al. 2014. Perceiving nonverbal behavior: neural correlates of processing movement fluency and contingency in dyadic interactions. Hum. Brain Mapp. 35:1362–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. 2007. Neural systems for recognition of familiar faces. Neuropsychologia 45:32–41 [DOI] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. 2007. Neural responses to facial expression and face identity in the monkey amygdala. J. Neurophysiol. 97:1671–83 [DOI] [PubMed] [Google Scholar]
- Grahe JE, Bernieri FJ. 1999. The importance of nonverbal cues in judging rapport. J. Nonverbal Behav. 23:253–69 [Google Scholar]
- Grimaldi P, Saleem KS, Tsao D. 2016. Anatomical connections of the functionally defined “face patches” in the macaque monkey. Neuron 90:1325–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. 2001. Do chimpanzees know what conspecifics know? Anim. Behav. 61:139–51 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Rolls ET, Baylis GC, Nalwa V. 1989. Object-centered encoding by face-selective neurons in the cortex in the superior temporal sulcus of the monkey. Exp. Brain Res. 75:417–29 [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. 2000. The distributed human neural system for face perception. Trends Cogn. Sci. 4:223–33 [DOI] [PubMed] [Google Scholar]
- Heyes C. 2015. Animal mindreading: What’s the problem? Psychon. Bull. Rev. 22:313–27 [DOI] [PubMed] [Google Scholar]
- Huang Y, Rao RPN. 2011. Predictive coding. Wiley Interdiscip. Rev. Cogn. Sci. 2:580–93 [DOI] [PubMed] [Google Scholar]
- Isik L, Koldewyn K, Beeler D, Kanwisher N. 2017. Perceiving social interactions in the posterior superior temporal sulcus. PNAS 114:E9145–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik L, Mynick A, Pantazis D, Kanwisher N. 2020. The speed of human social interaction perception. NeuroImage 215:116844 [DOI] [PubMed] [Google Scholar]
- Issa EB, Cadieu CF, DiCarlo JJ. 2018. Neural dynamics at successive stages of the ventral visual stream are consistent with hierarchical error signals. eLife 7:e42870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura S, Anderson JR. 1996. Learning to use experimenter-given cues during an object-choice task by a capuchin monkey. Curr. Psychol. Cogn. 15:103–12 [Google Scholar]
- Jozwik KM, O’Keeffe J, Storrs KR, Guo W, Golan T, Kriegeskorte N. 2022. Face dissimilarity judgments are predicted by representational distance in morphable and image-computable models. PNAS 119:e2115047119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano F, Krupenye C, Hirata S, Tomonaga M, Call J. 2019. Great apes use self-experience to anticipate an agent’s action in a false-belief test. PNAS 116:20904–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. 1997. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17:4302–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski JE, Collins JA, Olson IR. 2017. The neural representation of social status in the extended face processing network. Eur. J. Neurosci. 46:2795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J, Saxe R. 2013. Theory of mind: a neural prediction problem. Neuron 79:836–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi SM, Freiwald WA. 2017. Two areas for familiar face recognition in the primate brain. Science 357:591–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi SM, Viswanathan P, Serene S, Freiwald WA. 2021. A fast link between face perception and memory in the temporal pole. Science 373:581–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsiedel J, Daughters K, Downing PE, Koldewyn K. 2022. The role of motion in the neural representation of social interactions in the posterior temporal cortex. NeuroImage 262:119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton SRH, Bruce V. 1999. Reflexive visual orienting in response to the social attention of others. Vis. Cogn. 6:541–67 [Google Scholar]
- Langton SRH, Bruce V. 2000. You must see the point: automatic processing of cues to the direction of social attention. J. Exp. Psychol. Hum. Percept. Perform. 26:747–57 [DOI] [PubMed] [Google Scholar]
- Lee Masson H, Isik L. 2021. Functional selectivity for social interaction perception in the human superior temporal sulcus during natural viewing. NeuroImage 245:118741. [DOI] [PubMed] [Google Scholar]
- Leibo JZ, Liao Q, Anselmi F, Freiwald WA, Poggio T. 2017. View-tolerant face recognition and Hebbian learning imply mirror-symmetric neural tuning to head orientation. Curr. Biol. 27:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yen CC-C, Szczupak D, Ye FQ, Leopold DA, Silva AC. 2019. Anatomical and functional investigation of the marmoset default mode network. Nat. Commun. 10:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MA, Chang SW. 2020. Is there a ‘social’ brain? Implementations and algorithms. Trends Cogn. Sci. 24:802–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand J-B, Joly O, et al. 2011. Default mode of brain function in monkeys. J. Neurosci. 31:12954–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, et al. 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. PNAS 113:12574–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Neubert F-X, Rushworth MFS. 2013. Connectivity profiles reveal the relationship between brain areas for social cognition in human and monkey temporoparietal cortex. PNAS 110:10806–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P, Bartolacci A, Burke D. 2020. Human face tilt is a dynamic social signal that affects perceptions of dimorphism, attractiveness, and dominance. Evol. Psychol. 18:147470492091040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M, Magee JC, Fiske ST. 2014. Neural substrates of social status inference: roles of medial prefrontal cortex and superior temporal sulcus. J. Cogn. Neurosci. 26:1131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DB, Russ BE, Elnaiem HD, Kurnikova AI, Leopold DA. 2015. Single-unit activity during natural vision: diversity, consistency, and spatial sensitivity among AF face patch neurons. J. Neurosci. 35:5537–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Olson CR. 2011. Statistical learning of visual transitions in monkey inferotemporal cortex. Proc. Natl. Acad. Sci. Unit. States. Am. 108(48):19401–19406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignault A, Chaudhuri A. 2003. The many faces of a neutral face: head tilt and perception of dominance and emotion. J. Nonverbal Behav. 27:111–32 [Google Scholar]
- Mitchell JP. 2009. Social psychology as a natural kind. Trends Cogn. Sci. 13:246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Crapse T, Chang L, Tsao DY. 2017. The effect of face patch microstimulation on perception of faces and objects. Nat. Neurosci. 20:743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Freiwald WA, Tsao DY. 2008. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science 320:1355–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford D. 1992. On the computational architecture of the neocortex. Biol. Cybern. 66:241–51 [DOI] [PubMed] [Google Scholar]
- Murphy AP, Leopold DA. 2019. A parameterized digital 3D model of the Rhesus macaque face for investigating the visual processing of social cues. J. Neurosci. Methods 324:108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya T, Noritake A, Isoda M. 2021. Live agent preference and social action monitoring in the macaque mid-superior temporal sulcus region. PNAS 118:e2109653118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M, Palomero-Gallagher N. 2023. Architecture and connectivity of the human angular gyrus and of its homolog region in the macaque brain. Brain Struct. Funct 228:47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram MW, Perrett DI. 1992. Time course of neural responses discriminating different views of the face and head. J. Neurophysiol. 68:70–84 [DOI] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD, de Waal FBM. 2000. Recognizing facial cues: Individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta). J. Comp. Psychol. 114:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DC, Povinelli DJ. 2007. On the lack of evidence that non-human animals possess anything remotely resembling a ‘theory of mind’. Philos. Trans. R. Soc. B 362:731–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Oram MW, Ashbridge E. 1998. Evidence accumulation in cell populations responsive to faces: an account of generalisation of recognition without mental transformations. Cognition 67:111–45 [DOI] [PubMed] [Google Scholar]
- Perrett DI, Oram MW, Harries MH, Bevan R, Hietanen JK, et al. 1991. Viewer-centred and object-centred coding of heads in the macaque temporal cortex. Exp. Brain Res. 86:159–73 [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, et al. 1984. Neurones responsive to faces in the temporal cortex: studies of functional organization, sensitivity to identity and relation to perception. Hum. Neurobiol. 3:197–208 [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, et al. 1985. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc. R. Soc. B 223:293–317 [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Heistermann M, Hodges JK, Fischer J. 2008. Male Barbary macaques eavesdrop on mating outcome: a playback study. Anim. Behav. 75:1885–91 [Google Scholar]
- Phillips J, Buckwalter W, Cushman F, Friedman O, Martin A, et al. 2021. Knowledge before belief. Behav. Brain Sci. 44:e140. [DOI] [PubMed] [Google Scholar]
- Phillips W, Barnes JL, Mahajan N, Yamaguchi M, Santos LR. 2009. ‘Unwilling’ versus ‘unable’: capuchin monkeys’ (Cebus apella) understanding of human intentional action. Dev. Sci. 12:938–45 [DOI] [PubMed] [Google Scholar]
- Pitcher D, Dilks DD, Saxe RR, Triantafyllou C, Kanwisher N. 2011. Differential selectivity for dynamic versus static information in face-selective cortical regions. Neuroimage 56:2356–63 [DOI] [PubMed] [Google Scholar]
- Pitcher D, Ungerleider LG. 2021. Evidence for a third visual pathway specialized for social perception. Trends Cogn. Sci. 25:100–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadflieg S, Koldewyn K. 2017. The neuroscience of people watching: how the human brain makes sense of other people’s encounters. Ann. N. Y. Acad. Sci. 1396:166–82 [DOI] [PubMed] [Google Scholar]
- Raichle ME. 2015. The brain’s default mode network. Annu. Rev. Neurosci. 38:433–47 [DOI] [PubMed] [Google Scholar]
- Rosenfeld SA, Van Hoesen GW. 1979. Face recognition in the rhesus monkey. Neuropsychologia 17:503–9 [DOI] [PubMed] [Google Scholar]
- Roumazeilles L, Schurz M, Lojkiewiez M, Verhagen L, Schüffelgen U, et al. 2021. Social prediction modulates activity of macaque superior temporal cortex. Sci. Adv. 7:eabh2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saether L, Laeng B. 2008. On facial expertise: processing strategies of twins’ parents. Perception 37:1227–40 [DOI] [PubMed] [Google Scholar]
- Santana SE, Lynch Alfaro J, Alfaro ME. 2012. Adaptive evolution of facial colour patterns in Neotropical primates. Proc. Biol. Sci. 279:2204–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos LR, Nissen AG, Ferrugia JA. 2006. Rhesus monkeys, Macaca mulatta, know what others can and cannot hear. Anim. Behav. 71:1175–81 [Google Scholar]
- Saxe R, Brett M, Kanwisher N. 2006. Divide and conquer: a defense of functional localizers. Neuroimage 30:1088–96 [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. 2003. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind.” NeuroImage 19:1835–42 [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. 2006. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 17:692–99 [DOI] [PubMed] [Google Scholar]
- Schell A, Rieck K, Schell K, Hammerschmidt K, Fischer J. 2011. Adult but not juvenile Barbary macaques spontaneously recognize group members from pictures. Anim. Cogn. 14:503–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Tiddi B, Di Sorrentino EP. 2006. Simultaneous classification by rank and kinship in Japanese macaques. Anim. Behav. 71:1069–74 [Google Scholar]
- Schwiedrzik CM, Freiwald WA. 2017. High-level prediction signals in a low-level area of the macaque face processing hierarchy. Neuron 96:89–97.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiedrzik CM, Zarco W, Everling S, Freiwald WA. 2015. Face patch resting state networks link face processing to social cognition. PLOS Biol. 13:e1002245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1989. Intrinsic connections and architectonics of the superior temporal sulcus in the rhesus monkey. J. Comp. Neurol. 290:451–71 [DOI] [PubMed] [Google Scholar]
- Sheehan MJ, Nachman MW. 2014. Morphological and population genomic evidence that human faces have evolved to signal individual identity. Nat. Commun. 5:4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutts K, Pemberton CK, Spelke ES. 2013. Children’s use of social categories in thinking about people and social relationships. J. Cogn. Dev. 14:35–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB. 1999. Male bonnet macaques use information about third-party rank relationships to recruit allies. Anim. Behav. 58:45–51 [DOI] [PubMed] [Google Scholar]
- Silson EH, Steel A, Kidder A, Gilmore AW, Baker CI. 2019. Distinct subdivisions of human medial parietal cortex support recollection of people and places. eLife 8:e47391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa J, Duhamel JR, Pascalis O, Wirth S. 2011. Spontaneous voice-face identity matching by rhesus monkeys for familiar conspecifics and humans. PNAS 108:1735–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa J, Freiwald WA. 2017. A dedicated network for social interaction processing in the primate brain. Science 356:745–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21:489–510 [DOI] [PubMed] [Google Scholar]
- Srull TK, Wyer RS. 1989. Person memory and judgment. Psychol. Rev. 96:58. [DOI] [PubMed] [Google Scholar]
- Stevenage SV. 1998. Which twin are you? A demonstration of induced categorical perception of identical twin faces. Br. J. Psychol. 89:39–57 [Google Scholar]
- Su J, van Boxtel JJA, Lu H. 2016. Social interactions receive priority to conscious perception. PLOS ONE 11:e0160468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. 2007. Neural substrates of envisioning the future. PNAS 104:642–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Thornton MA, Contreras JM, Mitchell JP. 2016. Neural evidence that three dimensions organize mental state representation: rationality, social impact, and valence. PNAS 113:194–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton MA, Mitchell JP. 2017. Consistent neural activity patterns represent personally familiar people. J. Cogn. Neurosci. 29:1583–94 [DOI] [PubMed] [Google Scholar]
- Tibbetts EA, Dale J. 2007. Individual recognition: It is good to be different. Trends Ecol. Evol. 22:529–37 [DOI] [PubMed] [Google Scholar]
- Torrance JS, Holzleitner IJ, Lee AJ, DeBruine LM, Jones BC. 2020. Evidence head tilt has dissociable effects on dominance and trustworthiness judgments, but does not have category-contingent effects on hypothetical leadership judgments. Perception 49:199–209 [DOI] [PubMed] [Google Scholar]
- Troje NF, Siebeck U. 1998. Illumination-induced apparent shift in orientation of human heads. Perception 27:671–80 [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. 2003. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 6:989–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. 2006. A cortical region consisting entirely of face-selective cells. Science 311:670–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. 2008. Comparing face patch systems in macaques and humans. PNAS 105:19514–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RJ, Sutherland CA, Young AW, Hartley T. 2014. Modeling first impressions from highly variable facial images. PNAS 111:E3353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Patel G, Fox M, Snyder A, Baker J, et al. 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86 [DOI] [PubMed] [Google Scholar]
- Walbrin J, Downing P, Koldewyn K. 2018. Neural responses to visually observed social interactions. Neuropsychologia 112:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse J, Meunier H. 2020. An understanding of third-party friendships in a tolerant macaque. Sci. Rep. 10:9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Wilkinson F, Lin L-M, Castillo M. 2000. Perception of head orientation. Vision Res. 40:459–72 [DOI] [PubMed] [Google Scholar]
- Witkower Z, Tracy JL. 2019. A facial-action imposter: How head tilt influences perceptions of dominance from a neutral face. Psychol. Sci. 30:893–906 [DOI] [PubMed] [Google Scholar]
- Wurm MF, Caramazza A, Lingnau A. 2017. Action categories in lateral occipitotemporal cortex are organized along sociality and transitivity. J. Neurosci. 37:562–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Han M, Liang Q, Hu YF, Kuai SG. 2019. A social interaction field model accurately identifies static and dynamic social groupings. Nat. Hum. Behav. 3:847–55 [DOI] [PubMed] [Google Scholar]
- Zumpe D, Michael RP. 1970. Redirected aggression and gonadal hormones in captive rhesus monkeys (Macaca mulatta). Anim. Behav. 18:11–19 [DOI] [PubMed] [Google Scholar]