Abstract
Spindle elongation is crucial to normal chromosome separation in eukaryotes; in particular, it is required for or associated with the extension of distance between spindle poles and the further moving apart of the already separated chromosomes. However, little is known about the relationship between spindle elongation and the status of chromosome separation, and it is unknown whether spindle elongation in different organisms shares any quantitative feature. The Arabidopsis ask1-1 mutant might be a unique material for addressing these questions because it appears to have functional spindles, but a severe defect in homolog separation at male anaphase I (M. Yang, Y. Hu, M. Lodhi, W.R. McCombie, H Ma [1999] Proc Natl Acad Sci USA 96: 11416–11421). We have characterized male meiotic spindle lengths in wild-type and the ask1-1 mutant plants. We observed that during meiosis I some ask1-1 cells had spindles that were similar in length to fully elongated normal spindles, but the chromosomes in these cells did not show appreciable movement from the equator. Furthermore, greater movement of chromosomes from the equator was usually found in the ask1-1 cells that had longer than normal spindles. These results suggest that additional elongation of ask1-1 spindles occurred; one possible reason for the extra-long spindles may be that it is a consequence of chromosome non-separation. We also found that normal and ask1-1 spindle lengths are clustered at discrete values, and their differences are of multiples of 0.7 μm. A search of the literature revealed that in each of several organisms, spindle lengths also differ by multiples of 0.7 μm. These findings strongly suggest that the spindle elongates in response to status of chromosome separation, and perhaps there are conserved mechanisms controlling the extent of spindle elongation.
The mitotic spindle is essential for normal chromosome segregation. Sister chromatids separate at the onset of anaphase, and then move toward the spindle poles in a process that is defined as anaphase A and relies on kinetochore microtubules. In addition, spindle elongation is referred to as anaphase B and is correlated with the increase in the distance between the two spindle poles, possibly contributing to further separation of sister chromatids. In mitosis, anaphase A occurs before or concurrently with anaphase B in a number of organisms (Armstrong and Snyder, 1989; Hoyt and Geiser, 1996).
One mechanism that mitotic cells employ to ensure proper chromosome segregation at anaphase is the spindle assembly checkpoint. This checkpoint monitors the attachment of kinetochores to spindle microtubules and prevents the onset of anaphase when kinetochore attachment is not normal (Wells and Murray, 1996; Aaron, 1997; Kim et al., 1998; Goshima et al., 1999). In addition, mitotic spindles in certain cell types have characteristic lengths at metaphase and anaphase (Moens, 1976; Tippit et al., 1978; Snyder et al., 1986; Armstrong and Snyder, 1989; Hoyt and Geiser, 1996; Goshima et al., 1999), suggesting that the extent of spindle elongation is highly regulated. However, it is not known whether the status of sister chromatid separation has any effect on the extent of spindle elongation during anaphase. For example, it is not known whether a failure of sister chromatids to separate would prevent, interrupt, or prematurely terminate spindle elongation. In an alternate manner, tension generated due to the non-separation of chromosomes might trigger additional spindle elongation to compensate for the extra force.
The mechanisms of spindle elongation have been studied extensively. There are at least three mechanisms proposed for different organisms. In yeast and diatoms, it is thought that spindle elongation relies mostly on sliding of the interzonal microtubules that extend from both poles and interdigitate with each other near the equator (Baskin and Cande, 1990; Shelden and Wadsworth, 1990). As the microtubules slide and the spindle elongates, the overlap of existing microtubules is, at least transiently, reduced and it is thought that further microtubule growth is needed for maintaining such an overlap. In higher animals, it was proposed that spindle elongation is achieved by the pulling force on the two opposite poles without significant sliding of interzonal microtubules (Mastronarde et al., 1993). It was also proposed that microtubule assembly may contribute mostly to spindle elongation (Bajer, 1990) in higher animals. The mechanism of spindle elongation in higher plants is not yet clear.
It is known that microtubules are dynamic, with tubulins polymerizing and depolymerizing in “spurts,” allowing the microtubules to grow in discrete pulses. It is also known that motor proteins move by discrete steps (Lockhart et al., 1995; Coppin et al., 1996; Schnitzer and Block, 1997). Thus, both the pulses in microtubule assembly and discrete steps in motor protein movement might allow spindle elongation to occur in discrete increments. It is technically difficult to detect such discrete steps in spindle elongation because it occurs fast in a short period of time (Lockhart et al., 1995; Coppin et al., 1996; Yamasaki and Nakayama, 1996; Kitamura et al., 1999). Furthermore, if spindle elongation occurs in discrete steps, it would be interesting to know whether spindle elongation in different organisms shares any quantitative properties, since microtubule and motor protein are conserved in eukaryotic species.
Mutants that exhibit abnormal spindle lengths might reveal features of spindles that are not obvious in the wild-type (WT) cells. The Arabidopsis ask1-1 mutant is defective in homolog separation in male meiosis I; furthermore, sister chromatid separation at meiosis II in ask1-1 cells seems to be abnormal (Yang et al., 1999). The ASK1 gene is a homolog (Yang et al., 1999) of the yeast SKP1 gene, which encodes an essential component of the SKP1, cullin, and an F-box protein ubiquitin ligase complex that mediates the degradation of critical mitotic cell cycle regulators by the proteosome (Bai et al., 1996; Connelly and Hieter, 1996). Mitotic and meiotic chromosome separation requires the removal of sister chromatid cohesin from the chromosomes (Nasmyth, 1999; Orr-Weaver, 1999). In particular, sister chromatid cohesin along chromosome arms needs to be removed proteolytically before homologs can separate at meiosis I (Nasmyth, 1999; Orr-Weaver, 1999). Although the anaphase promoting complex is involved in yeast cohesin removal, evidence from Xenopus has suggested that cohesin removal can be anaphase promoting complex-independent (Peter et al., 2001).
Therefore, a reasonable hypothesis is that ASK1 mediates a proteolytic event required for the removal of sister chromatid cohesin at anaphase I in Arabidopsis male meiosis. Consistent with this hypothesis, the ask1-1 male meiotic cells do not seem to have a structural defect in the spindle. The ask1-1 male meiotic cells exhibit chromosome stretching and spindle lengthening during anaphase I, indicating that the spindle was functional. These mutant properties make ask1-1 meiotic cells an excellent system to study the effect of homolog non-separation on spindle elongation. To quantify meiotic spindle elongation we measured spindle lengths at metaphase and anaphase during male meiosis I and II in WT and ask1-1 plants. Our results indicate that stable spindle lengths fall into few classes, suggesting that spindle elongation is discrete and spindle can elongate more in ask1-1 than in the WT. We also describe the extent of spindle elongation in relationship with status of chromosome separation, and a possible quantitative feature in spindle elongation.
RESULTS
WT Spindle Elongation: Uniform Metaphase and Anaphase Lengths in Meiosis I and II
In Arabidopsis during late meiotic prophase I at the diplotene stage, microtubules are found in the perinuclear region; they subsequently form bundles around the condensed chromosomes at the diakinesis stage (Peirson et al., 1997) similar to microtubule behaviors seen in maize meiocytes (Chan and Cande, 1998). There is no clear indication of a prometaphase I spindle in these plant cells, whereas the metaphase I spindle is easily detected. Our observations suggest that microtubule distributions at prophase I in WT and ask1-1 cells are similar (not shown).
To investigate the extent of spindle elongation in WT we measured the spindle lengths at metaphase and anaphase in meiosis I and II. We found that in the WT male meiosis, metaphase I and metaphase II spindles had the same approximate length of 7.0 μm (Figs. 1A, B, G, and H, and 2, A and B; Table I). On the other hand, anaphase I and anaphase II spindles had different lengths, respectively, of approximately 9.7 μm (Figs. 1, C–F and 2A; Table I) and 8.3 μm (Figs. 1, I–L and 2B; Table I). In WT, no spindle was observed with an intermediate length between those of the metaphase and anaphase spindles. In addition, all WT cells during anaphase I had spindles of approximately 9.7 μm, regardless of how far apart the two groups of chromosomes were (Fig. 2A). In a similar manner, all anaphase II spindles were about 8.3 μm (Fig. 2B). These results indicate that spindle lengths before and after elongation were highly uniform at each stage in meiosis I and II, suggesting that spindle elongation is tightly regulated.
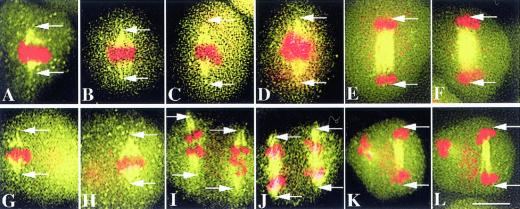
Figure 1.
WT spindles and microtubule structures during meiosis I and meiosis II. Arrows indicate the spindle poles. All panels have the same magnification. Bar = 5 μm. A and B, Metaphase I spindles (yellow) and chromosomes (red), which form a tight band at the equator. The pole-to-pole distance (between the arrow points) is 6.9 μm. C and D, Early anaphase I spindles of about 9.6 and 9.8 μm, respectively, with the chromosomes still near the equator. E and F, Late anaphase I spindles (9.7 and 9.8 μm, respectively) and chromosomes. The homologs have separated and form two groups of chromosomes near the poles. G and H, Metaphase II spindles of 6.8 and 6.9 μm, respectively, and congressed chromosomes; only one of the two spindles was in the focal plane in each panel. I, Early Anaphase II spindles (8.4 μm) and clearly separated sister chromatids. J, Mid-anaphase II showing both spindles (8.4 μm) with well-separated sister chromatids. K and L, Late anaphase II showing one of the two sets of microtubule structure and chromosomes at the end of anaphase II. For the other set, only the sister chromatids at one pole are in focus. The pole-to-pole distance is 8.3 μm in K and L. In addition to the major 4,6-diamino-2-phenylindole dihydrochloride (DAPI) staining areas representing chromosomes, other numerous small red dots, such as those in D, H, K, and L, represent DAPI staining in organelles.
Table I.
Spindle lengths in Arabidopsis WT and ask1-1 male meiosis
| Length | Length Difference | |
|---|---|---|
| μm | ||
| WT | L1 7.0 ± 0.1a (n = 45) | – |
| L2 9.7 ± 0.1b (n = 26) | L2 − L1 = 2.7 | |
| L3 8.3 ± 0.1c (n = 16) | L3 − L1 = 1.3 | |
| ask1-1 | L1 7.0 ± 0.1d (n = 12) | – |
| L2 8.4 ± 0.1e (n = 23) | L2 − L1 = 1.4 | |
| L3 9.8 ± 0.1e (n = 25) | L3 − L2 = 1.4 | |
| L4 11.8 ± 0.1e (n = 11) | L4 − L3 = 2.0 | |
| L5 4.9f (n = 1) | L6 − L5 = 0.7 | |
| L6 5.6 ± 0.1f (n = 5) | L1 − L6 = 1.4 | |
Data are presented as means ± SEM, except for L5 of ask1-1. (n = no. of spindles measured). When similar spindle lengths were observed for meiosis I and II, the means were calculated with values from meiosis I and II.
WT metaphase I and II.
WT anaphase I.
WT anaphase II.
ask1-1 metaphase I and meiosis II.
ask1-1 anaphase I and meiosis II.
ask1-1 meiosis II.
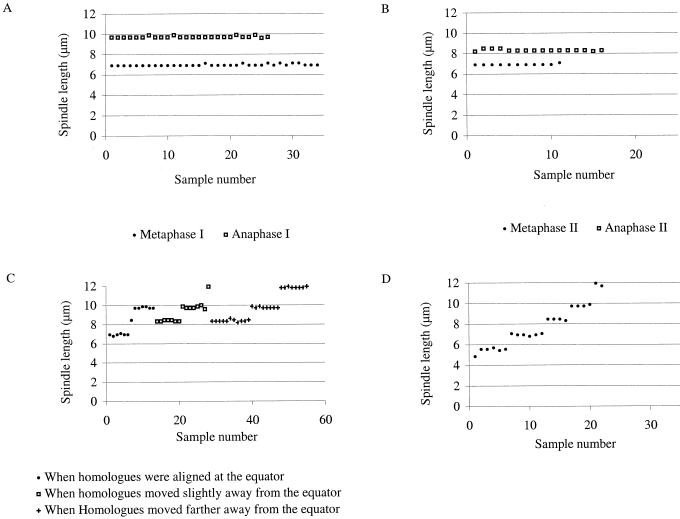
Figure 2.
Distribution of WT and ask1-1 spindle lengths in meiosis I and meiosis II. A, WT metaphase I and anaphase I. The lengths are essentially constant. B, WT metaphase II and anaphase II. The spindle lengths at metaphase II are about the same as those at metaphase I, but the spindle lengths at anaphase II are shorter than those at anaphase I. C, ask1-1 spindle lengths and their corresponding status of homolog separation during metaphase I and anaphase I. Four classes of spindle lengths are present. When homologs are aligned at the equator, some ask1-1 spindles have the same lengths as those in WT metaphase I and II, but others have lengths of WT spindles at anaphase I and anaphase II. When homologs show slight dislocation from the equator, ask1-1 spindles no longer have the WT spindle lengths at metaphase I and II, but have the lengths at WT anaphase I and II, and longer lengths not found in the WT. The same three classes of lengths are present when homologs show more obvious dislocation from the equator, but the fraction of the longest class was greater. D, Spindle lengths in meiosis II of ask1-1. One or two new classes of shorter spindles are present in addition to the ones present in meiosis I.
When the chromosomes had reached the poles at the end of anaphase I, spindle lengths (Fig. 1, E and F; the distances between the outer edges of chromosomes at either poles) remained the same as that at early anaphase I (Fig. 1, C and D). The lack of spindles with intermediate lengths and the fact that spindle lengths remained unchanged (approximately 9.7 μm) during anaphase I strongly supports the hypothesis that spindle elongation occurred very quickly and was completed at early anaphase I. In a similar manner, spindle lengths (approximately 8.3 μm) remained unchanged from early anaphase II to late anaphase II (compare Fig. 1, I and J with K and L), again suggesting that anaphase II spindle elongation was also rapid and completed in early anaphase II. As mentioned before, mitotic anaphase A usually occurs before or concurrent with anaphase B. Our observation that spindles have reached the final length before the chromosomes have reached the poles supports the idea that in Arabidopsis male meiosis anaphase B is completed before anaphase A.
Moreover, because metaphase I and II spindles had the same length and anaphase I spindles were longer than anaphase II spindles, the meiosis I spindles would seem to elongate to a greater extent than meiosis II spindles.
Spindle Elongates to a Greater Extent in ask1-1 Meiosis I
We previously showed that the ask1-1 male meiotic cells have a normal morphology of the metaphase I spindle, but a severe defect in homolog separation during anaphase I in microspore mother cells (Yang et al., 1999), making it possible to study the effect of homolog non-separation on spindle elongation. It is obvious that homolog non-separation will impact anaphase A directly because even though homologs are pulled at the kinetochore by the kinetochore microtubules, the attachment at the chiasmata will prevent the homologs from moving toward the poles. At the same time, homolog non-separation might also affect anaphase B because their attachment to the kinetochore microtubules could inhibit the outward movement of the poles, even when the non-kinetochore overlapping microtubules are pushing the poles apart.
Our observations indicate that ask1-1 metaphase I spindles had an average length of 7.0 μm, essentially the same as that of the WT (Fig. 3A; Table I), consistent with our previous finding that spindle assembly seems normal in the ask1-1 mutant male meiotic cells (Yang et al., 1999). This suggests that the normal mechanism regulating spindle length at metaphase I is intact in the ask1-1 mutant. However, during anaphase I, the lengths of the ask1-1 mutant spindles clustered near three values: 8.4, 9.8, or 11.8 μm (Figs. 2C and 3, B–J). The fact that all of these spindles were clearly longer than the metaphase I spindles indicates that spindles can elongate in the ask1-1 mutant, despite the fact that homolog separation is defective. The first two length values were the same as that in WT anaphase II and anaphase I, respectively. The 11.8-μm length was not found in WT meiosis, suggesting that ask1-1 spindles can elongate to a greater extent than WT spindles.
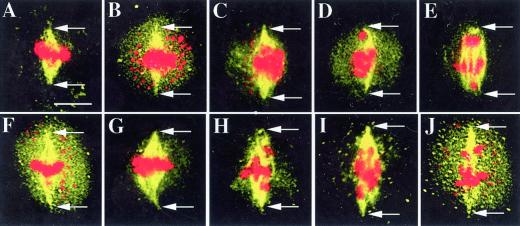
Figure 3.
Examples of spindles of different lengths in meiosis I of ask1-1. Arrows indicate the poles of the spindle. All figures have the same magnification. Bar = 5 μm. A, A metaphase I spindle with a length (7.0 μm) of the WT metaphase spindles. B, A spindle of 8.5 μm; the chromosomes do not appear to be separating. C, A spindle of 8.6 μm; the chromosomes have moved away from the equator slightly. D, A spindle of 8.4 μm; a chromosome or a portion of a chromosome has reached the pole region. E, A spindle of 8.5 μm appears to be at late anaphase I; some chromosomes or portions of chromosomes are at the poles and others are near the equator. F, A spindle of 9.8 μm; there is no obvious homolog separation. G, A spindle of 9.9 μm; chromosomes have moved slightly. H, A spindle of 9.7 μm; chromosomes have clearly moved. I and J, Images of cells with spindles of 11.9 and 11.8 μm, respectively, and obvious chromosome movement. The numerous small red dots in B, C, F, and J represent organelles.
Multiple Spindle Lengths in ask1-1 Meiosis I Suggest an Interaction of Spindle Elongation with Extent of Chromosome Dislocation
To understand the relationship between chromosome separation and the extent of spindle elongation we have examined patterns of chromosome distribution in the ask1-1 cells. We found three patterns: chromosomes were at the equator, similar to those at metaphase I (Fig. 3, B and F); chromosomes had moved slightly from the equator (Fig. 3, C and G); and one or more chromosomes were near or at the pole(s) (Fig. 3, D, E, H, and J). The fact that the chromosome distribution is often uneven and sometimes most chromosomes move to one pole (Yang et al., 1999) suggests that even when the chromosomes had moved away from the equator, the homologs remained attached. These chromosomes might be detached from microtubules from one of the poles. Figure 2C shows the summary of the relationship of chromosome positions and spindle lengths. Although there is not a strict correlation between pole-ward chromosome movement and spindle length, there is a clear tendency for the cells with shorter spindles to have less chromosome movement.
Spindles Have Specific Lengths during Meiosis II of ask1-1
To determine whether spindle elongation was affected during male meiosis II in the ask1-1 mutant cells we examined spindle lengths in ask1-1 meiosis II. In ask1-1 cells, largely as a consequence of chromosome missegregation during meiosis I, it is difficult to distinguish metaphase II from anaphase II. Nevertheless, meiotic II ask1-1 spindle lengths were observed to cluster near specific values. In addition to those found in ask1-1 meiosis I, two shorter spindle lengths of approximately 5.6 and 4.9 μm were observed (Figs. 2D and 4, A–F). Again, discrete spindle lengths rather than continuous distribution support the idea that spindle elongation is regulated in ask1-1. The shorter spindles appeared to be correlated with fewer chromosomes, suggesting that initial spindle length may be regulated in coordination with the number of chromosomes.
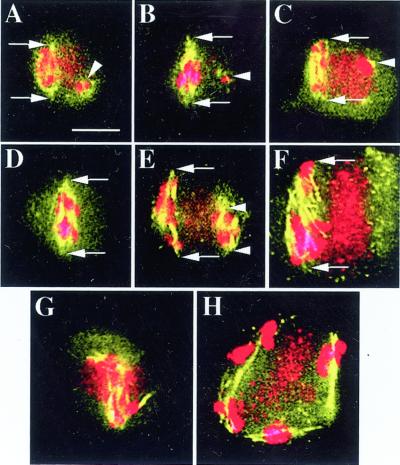
Figure 4.
Examples of meiosis II spindles and other microtubule structures in ask1-1. Arrows and arrowheads in E indicate the poles of the spindle. All figures have the same magnification. Bar = 5 μm. A, Spindle with an approximate length of 5.6 μm; also shown is a small amount of microtubules associated with a small amount of chromosomes (arrowhead). B and C, Images with features similar to those in A, except that the spindles are 7.0 and 6.7 μm, respectively. D, Single spindle in meiosis II with an length of 8.3 μm. E, Shown are two spindles with lengths of 9.8 μm and 4.9 μm, respectively. F, Single spindle with a length of 11.7 μm. G and H, Spindle-like structures are colocalized with chromosomes. In H, five bivalents still appear to be intact, suggesting that the association of homologs is unusually stable in ask1-1. In meiosis II, there is a strong DAPI staining organellar bands, clearly visible in C, F, and H, and also detectable in A, E, and G. The organellar band in G is superimposed onto the spindle.
Microtubules Are Colocalized with Chromosomes in Meiosis II of ask1-1
Another feature frequently observed in meiosis II of ask1-1 is that spindle-like structures (Fig. 4, G and H) and small microtubule aggregates (Fig. 4, A–C, arrowheads) formed around chromosomes. In addition, the amounts of microtubules in these small spindle-like structures appeared to correlate well with the amounts of chromosomal materials. Figure 4, D and F, represents an extreme situation in which all the chromosomes were congregated in one region of the cell, so that only one spindle was formed instead of the normal two spindles. Taken together, these results support the idea that chromosomes probably play a key role in spindle assembly or microtubule nucleation. Similar conclusions about the role of chromosomes in spindle assembly have been made previously (Zhang and Nicklas, 1995a, 1995b; Chan and Cande, 1998).
DISCUSSION
Extent of Spindle Elongation Differs between Meiosis I and II
Our data strongly suggest that WT spindles in meiosis I elongate to a greater extent than that in meiosis II. One possible explanation for this phenomenon is that the extent of spindle elongation is influenced by the position of chromosome attachment points. During meiosis I, the homologs are attached at the chiasmata, which are localized along the chromosome arms; in contrast, during meiosis II, sister chromatids are held together at the kinetochores. It is reasonable to postulate that, in meiosis I, generation of tension at the chiasmata and kinetochores requires that the kinetochores move toward opposite poles by some distance, which is dependent on the distance between the chiasma and the kinetochore of each pair of homologs. On the other hand, for separation of chromatids, attached kinetochores will be immediately under tension when the spindle starts to pull on the kinetochores. Therefore, a greater extent of spindle elongation might be needed to generate sufficient tension (not necessarily greater tension) to separate homologs than sister chromatids. A corollary of this idea is that the extent of spindle elongation may be regulated in response to how chromosomes are attached.
An alternative explanation for a greater spindle length at anaphase I than anaphase II might be that kinetochores at metaphase I are larger than those at metaphase II. However, there is no evidence for a correlation between final spindle lengths and kinetochore sizes. Another possible explanation is that the different final spindle lengths could be simply due to the difference in space constraint at meiosis I and meiosis II. Microspore mother cells at meiosis II contains two spindles, which might make the space more limited for spindle elongation than at meiosis I. It is worth pointing out that our measurement of spindle lengths could be affected by the extent of cell flattening in our experiments and by the angle of spindles to the focal planes when the images were recorded. However, the fact that we observed clusters of spindle lengths within narrow ranges suggests that variation due to the spindle angle is not substantial.
Spindle Is Functional in ask1-1 Male Meiosis I
Because ask1-1 is defective in homolog separation, one may ask whether such a defect will compromise its spindle function by interfering with spindle elongation. The observation that ask1-1 spindles increased in lengths from metaphase I to anaphase I suggests that the forces that cause spindle elongation are functional. Moreover, because the spindle lengths in ask1-1 at anaphase I were the same as or longer than those of WT spindles, the extent of spindle elongation during meiosis I in ask1-1 cells does not seem to be diminished. This suggests that the chromosomal separation defects of the mutant do not prevent spindle elongation (see below for more discussion).
Spindle Elongation May Occur in Discrete Steps
The fact that there were only discrete spindle lengths instead of a continuous length distribution in WT and ask1-1 cells suggests that the spindle elongation is rapid and may occur in discrete steps. The fact that ask1-1 spindles also had discrete lengths, some of which are the same as those in WT cells, suggests that the regulation of spindle elongation is functioning properly in the ask1-1 cells. Multiple spindle lengths were observed at anaphase I in the ask1-1 cells. In particular, some of the ask1-1 anaphase I spindles had lengths of approximately 8.4 μm; these might represent intermediates that stalled before further elongation. If that was the case, such spindles should also exist in the WT anaphase I cells, but were not observed most likely because spindle elongation occurs quickly. In contrast, the ask1-1 spindle elongation might be slowed down enough to reveal the intermediate length presumably because of the tension from unseparated homologs. Many cells with this spindle length still had chromosomes that were at the equator or moved slightly toward the poles (Fig. 2C). A slowdown of spindle elongation rate was recently observed in the fission yeast Schizosaccharomyces pombe mutant with lagging chromosomes that fail to reach the poles properly (Pidoux et al., 2000).
Spindle Elongation May Respond to the Status of Chromosome Separation
The spindles of approximately 11.8 μm in length were observed only in ask1-1 cells, not in the WT, suggesting that they might be due to additional spindle elongation. The presence of the extra long spindles suggests that spindle elongation may be regulated in response to the status of chromosome attachments; prolonged tension due to chromosome non-separation and kinetochore microtubule attachment might cause additional elongation of the spindles. To our knowledge, if true, this would be the first description of such a phenomenon. It is possible that additional spindle elongation is activated by a mechanism that can sense the status of chromosome/microtubule stress. It has been found that tension exerted on chromosomes induces the increase of the number of kinetochore microtubules in the grasshopper spermatocyte, suggesting spindle structure can change in response to increase in tension on the chromosomes (King and Nicklas, 2000).
Our results that cells with elongated spindles can still have equatorially located chromosomes suggest that anaphase A and B can be separated, although there does seem to be some coordination between the two components, as discussed above. The fact that cells with the same length of elongated spindles can have chromosomes of different degree of movement away from the equator further supports the idea that anaphase A and B are separable. It is possible that the variation of chromosome movement depends in part on the position of chiasmata relative to the kinetochore. A more kinetochore-distal chiasma would allow the attached homologs to be stretched more than a more proximal chiasma. In some cases, a pair of homologs might detach from microtubules extending from one pole and move to the other pole as a pair. The number or timing of such detachments also could contribute to the variability of pole-ward chromosome movement. Another possibility is that kinetochore microtubules might be extended to allow spindle elongation in the absence of homolog separation and kinetochore detachment.
Mutant Cells May Have Different Terminal Spindle Lengths
An alternative explanation for the observed multiple spindle lengths in the ask1-1 meiosis I cells might be that they are all terminal spindles. The different lengths might be related to chromosome separation status. For example, for cells with largely unseparated chromosomes that remain attached to microtubules from both poles, the spindle elongation was reduced and a shorter final spindle length was reached. For cells that had most of the chromosomes separated by force or detached from microtubules extending from one or the other of the poles, the reduction in opposing force allowed a great extent of spindle elongation. These explanations invoke different mechanisms from those discussed above. Nevertheless, they are still consistent with the ideas that forces causing elongation of the spindle are intact in the ask1-1 cells, that spindle elongation occurs with discrete values, and that spindle elongation is regulated by the status of chromosome separation.
Spindle Length Differences in Arabidopsis and Other Organisms
As mentioned earlier, in WT and ask1-1 cells, spindle lengths clustered near specific values, as summarized in Table I. We suggest that the longer spindles are the result of elongation from the shorter ones. If so, one can deduce the extent of spindle elongation by calculating the differences between the lengths of spindles. Although this difference for WT spindles alone is not noticeably striking, the multiple values of spindle lengths in the ask1-1 mutant, as well as the WT, revealed the following intriguing phenomenon. As shown in Table I, the differences between any of the ask1-1 spindle lengths, or between the WT ones, are approximately multiples of 0.7 μm. This is highly unlikely to be due to coincidence. Thus, it is possible that spindles elongate by multiples of 0.7 μm in Arabidopsis male meiosis.
To learn whether this phenomenon of spindle elongation is unique to Arabidopsis or is shared by other organisms, we searched the literature for relevant information. We found data on quantitative measurement of spindle lengths from four other organisms, the budding yeast Saccharomyces cerevisiae, slime mold, the diatom Fragilaria capucina, and the PtK1 cells from rat kangaroo. These data suggest that the spindles also elongate by multiples of 0.7 μm (Table II). It is not known what the mechanisms controlling the proposed discrete elongation of spindles in any organisms might be, and it is not known whether there is conservation between different organisms in such mechanisms.
Table II.
Spindle length differences in four other organisms
| Organism | Length | Length Difference |
|---|---|---|
| S. cerevisiae (Winey et al., 1995) | L1 0.7 ± 0.1 (n = 4) | L2 − L1 = 0.7 |
| L2 1.4 ± 0.1 (n = 6) | ||
| F. capucina (Tippit et al., 1978) | L1 1.3 ± 0.1 (n = 2) | L2 − L1 = 1.3 |
| L2 2.6 ± 0.1 (n = 25) | ||
| Slime mold (Moens, 1976) | L1 2.1 ± 0.1 (n = 3) | L2 − L1 = 2.8 |
| L2 4.9 ± 0.3 (n = 6) | ||
| Rat kangaroo (PtK1 cells; Armstrong and Snyder, 1989) | L1 13.2 (n = 5) | L2 − L1 = 4.2 |
| L2 17.4 (n = 5) | ||
| Rat kangaroo (PtK1 cells; Snyder et al., 1986) | L1 12.2 (n ≥ 6) | L2 − L1 = 4.2 |
| L2 16.4 (n ≥ 6) |
Data presented as means ± se, which were calculated based on the cited publications for spindles with similar lengths, or as mean only according to the publications. (n = no. of spindles examined). In the case of S. cerevisiae, values of the extra-long spindles at late stages of cell division (telophase/cytokinesis) were not included here.
MATERIALS AND METHODS
Plant Materials and Fluorescence Microscopy
The Arabidopsis WT and ask1-1 plants are in the Landsberg erecta ecotype background and were grown to maturity using the same type of soil and under the same greenhouse conditions. Inflorescences of the WT and ask1-1 plants were harvested and fixed in 3.7% (w/v) paraformaldehyde as previously described (Peirson et al., 1997). Microspore mother cells (male meiotic cells) were manually dissected from the anthers of the fixed inflorescence and were processed for immunostaining as previously described (Yang et al., 1999). Microtubule structures in these cells were visualized using a primary monoclonal antibody against β-tubulin (2–5 μg mL−1, Roche Molecular Biochemicals, Indianapolis) and secondary antibodies (2–5 μg mL−1, Roche Molecular Biochemicals) conjugated with fluorescein. Samples were also stained with DAPI or propidium iodide in Vectashield mounting medium (1 μg mL−1, Vector Laboratories, Burlingame, CA) to reveal chromosomes. The fluorescence images were captured and recorded using a digital camera that was connected with a conventional fluorescence microscope (Nikon, Tokyo) and a computer.
Manipulation of Digital Image Files
Adobe Photoshop 5.5 (Adobe Systems, San Jose, CA) was used for processing images. All of the fluorescence images shown are superimposed spindle and DAPI images of the same cells. The original spindle and DAPI fluorescence images were green and blue, respectively. To better contrast the two types of images after superimposition, DAPI images were first altered in color using Photoshop. Thus, the spindles appear yellow-orange and the chromosomes appear red after superimposition. The contrast and brightness of the images were also adjusted before and after superimposition in Photoshop.
Measurement of Spindle Lengths
Spindle lengths were determined by measuring spindle pole-to-pole distances with a ruler on a computer monitor screen after the spindle images were magnified 7,200 times. Spindle poles are defined as where spindle microtubules are focused. Meiotic stages were determined based on DAPI/propidium iodide images and the spindle images.
ACKNOWLEDGMENTS
We thank Z. Cande, M.A. Hoyt, and F. Solomon for helpful discussions, and A. Bajer, R. Cyr, and S. McCormick for critical reading of this manuscript. We also thank A. Omeis for help with plant care and A. Hemling for technical assistance.
Footnotes
This work was supported by the Biology Department and the Life Science Consortium at Pennsylvania State University and by the National Science Foundation (grant no. MCB-9728772 to H.M.). M.Y. was a recipient of a National Institutes of Health/National Research Service Award postdoctoral fellowship.
LITERATURE CITED
- Aaron FS. Cell cycle: checkpoint proteins and kinetochores. Curr Biol. 1997;7:613–616. doi: 10.1016/s0960-9822(06)00315-0. [DOI] [PubMed] [Google Scholar]
- Armstrong L, Snyder JA. Selective reduction of anaphase B in quinacrine-treated PtK1 cells. Cell Motil Cytoskel. 1989;14:220–229. doi: 10.1002/cm.970140208. [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Bajer AS. The elusive organization of the spindle and the kinetochore fiber: a conceptual retrospect. Adv Cell Biol. 1990;3:65–93. [Google Scholar]
- Baskin TI, Cande WZ. Kinetic analysis of mitotic spindle elongation in vitro. J Cell Sci. 1990;97:79–89. doi: 10.1242/jcs.97.1.79. [DOI] [PubMed] [Google Scholar]
- Chan A, Cande WZ. Maize meiotic spindles assemble around chromatin and do not require paired chromosomes. J Cell Sci. 1998;111:3507–3515. doi: 10.1242/jcs.111.23.3507. [DOI] [PubMed] [Google Scholar]
- Connelly C, Hieter P. Budding yeast SKP1 encodes an evolutionarily conserved kinetochore protein required for cell cycle progression. Cell. 1996;86:275–285. doi: 10.1016/S0092-8674(00)80099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin CM, Finer JT, Spudich JA, V. RD. Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc Natl Acad Sci USA. 1996;93:1913–1917. doi: 10.1073/pnas.93.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Saitoh S, Yanagida M. Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev. 1999;13:1664–1677. doi: 10.1101/gad.13.13.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA, Geiser JR. Genetic analysis of the mitotic spindle. Annu Rev Genet. 1996;30:7–33. doi: 10.1146/annurev.genet.30.1.7. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lin DP, Matsumoto S, Kitazono A, Tomohiro M. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- King JM, Nicklas RB. Tension on chromosomes increases the number of kinetochore microtubules but only with limits. J Cell Sci. 2000;113:3815–3823. doi: 10.1242/jcs.113.21.3815. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Tokunaga M, Iwane AH, Yanagida T. A single myosin head moves along an actin filament with regular steps of 5.3 nanometers. Nature. 1999;397:129–134. doi: 10.1038/16403. [DOI] [PubMed] [Google Scholar]
- Lockhart A, Crevel IM, Cross RA. Kinesin and ncd bind through a single head to microtubules and compete for a shared MT binding site. J Mol Biol. 1995;249:763–771. doi: 10.1006/jmbi.1995.0335. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB. Spindle and kinetochore morphology of Dictyostelium discoideum. J Cell Biol. 1976;68:113–122. doi: 10.1083/jcb.68.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. Separating sister chromatids. Trends Biochem Sci. 1999;24:98–104. doi: 10.1016/s0968-0004(99)01358-4. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver TL. The ties that bind: localization of the sister-chromatid cohesin complex on yeast chromosomes. Cell. 1999;99:1–4. doi: 10.1016/s0092-8674(00)80055-0. [DOI] [PubMed] [Google Scholar]
- Peirson BN, Bowling SE, Makaroff CA. A defect in synapsis causes male sterility in a T-DNA-tagged Arabidopsis thaliana mutant. Plant J. 1997;11:659–669. doi: 10.1046/j.1365-313x.1997.11040659.x. [DOI] [PubMed] [Google Scholar]
- Peter M, Castro A, Lorca T, Peuch CL, Magnaghi-Jaulin L, Dorée M, Labbé J-C. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat Cell Biol. 2001;3:83–87. doi: 10.1038/35050607. [DOI] [PubMed] [Google Scholar]
- Pidoux A, Uzawa S, Perry PE, Cande WZ, Allshire RC. Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J Cell Sci. 2000;113:4177–4191. doi: 10.1242/jcs.113.23.4177. [DOI] [PubMed] [Google Scholar]
- Schnitzer MJ, Block SM. Kinesin hydrolyses one ATP per 8-nm step. Nature. 1997;388:386–390. doi: 10.1038/41111. [DOI] [PubMed] [Google Scholar]
- Shelden E, Wadsworth P. Interzonal microtubules are dynamic during spindle elongation. J Cell Sci. 1990;97:273–281. doi: 10.1242/jcs.97.2.273. [DOI] [PubMed] [Google Scholar]
- Snyder JA, Golub RJ, Berg SP. Role of non-kinetochore microtubules in spindle elongation in mitotic PtK1 cells. Eur J Cell Biol. 1986;39:373–379. [PubMed] [Google Scholar]
- Tippit DH, Schultz D, Pickett-Heaps JD. Analysis of the distribution of spindle microtubules in the diatom Fragilaria. J Cell Biol. 1978;79:737–763. doi: 10.1083/jcb.79.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WA, Murray AW. Aberrantly segregating centromeres activate the spindle checkpoint in budding yeast. J Cell Biol. 1996;133:75–84. doi: 10.1083/jcb.133.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Mamay CL, O'Toole ET, Mastronarde DN, Giddings THJ, McDonald KL, McIntosh JR. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Nakayama H. Fluctuation analysis of myosin-coated bead along actin bundles of Nitella. Biochem Biophys Res Commun. 1996;221:831–836. doi: 10.1006/bbrc.1996.0682. [DOI] [PubMed] [Google Scholar]
- Yang M, Hu Y, Lodhi M, McCombie WR, Ma H. The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc Natl Acad Sci USA. 1999;96:11416–11421. doi: 10.1073/pnas.96.20.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. Chromosomes initiate spindle assembly upon experimental dissolution of the nuclear envelope in grasshopper spermatocytes. J Cell Biol. 1995a;131:1125–1131. doi: 10.1083/jcb.131.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J Cell Biol. 1995b;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]