Abstract
The aim of the present study was to report the feasibility of proton beam reirradiation for patients with locally recurrent rectal cancer (LRRC) with prior pelvic irradiation. The study population included patients who were treated with proton beam therapy (PBT) for LRRC between 2008 and December 2019 in our institution. Those who had a history of distant metastases of LRRC, with or without treatment, before reirradiation, were excluded. Overall survival (OS), progression-free survival (PFS) and local control (LC) were estimated using the Kaplan–Meier method. Ten patients were included in the present study. The median follow-up period was 28.7 months, and the median total dose of prior radiotherapy (RT) was 50 Gy (range, 30 Gy–74.8 Gy). The median time from prior RT to reirradiation was 31.5 months (range, 8.1–96.6 months), and the median reirradiation dose was 72 Gy (relative biological effectiveness) (range, 56–77 Gy). The 1-year/2-year OS, PFS and LC rates were 100%/60.0%, 20.0%/10.0% and 70.0%/58.3%, respectively, with a median survival time of 26.0 months. Seven patients developed a Grade 1 acute radiation dermatitis, and no Grade ≥ 2 acute toxicity was recorded. Grade ≥ 3 late toxicity was recorded in only one patient, who had developed a colostomy due to radiation-related intestinal bleeding. Reirradiation using PBT for LRRC patients who had previously undergone pelvic irradiation was feasible. However, the indications for PBT reirradiation for LRRC patients need to be considered carefully due to the risk of severe late GI toxicity.
Keywords: reirradiation, proton beam therapy, locally recurrent rectal cancer (LRRC), toxicity
INTRODUCTION
Locally recurrent rectal cancer (LRRC) is a life-threatening disease. Eighty-six percent of patients have uncomfortable symptoms such as pain, obstruction or bleeding [1]. Treatment for LRRC is still challenging. The standard of care for locally advanced rectal cancer is neoadjuvant chemoradiotherapy (nCRT), followed by total mesorectal excision (TME) and adjuvant chemotherapy [2]. Recently, a prospective, randomized Phase II trial reported a 3-year TME-free survival rate of 41–53% [3]. In this multimodality treatment era, organ preservation strategy with non-operable management plays an important role in the treatment of rectal cancer. However, despite strong nCRT with induction or consolidation chemotherapy followed by TME, the local recurrence rate remained at 6% [3]. Therefore, we will experience more and more LRRC patients who have a history of pelvic irradiation in the future. An optimal method of reirradiation for LRRC patients has not yet been established, although numerous studies of reirradiation with photon therapy for LRRC patients have been published [4–11]. Recently, a systematic review of reirradiation for LRRC was published [12]. In the systematic review, the study of carbon ion radiotherapy (CIRT), intensity-modulated radiotherapy and stereotactic ablative radiotherapy (SABR) were included. However, the available data on reirradiation with proton beam therapy (PBT) for LRRC are limited. We therefore retrospectively analyzed the outcome of reirradiation using PBT for LRRC patients at our institution.
MATERIALS AND METHODS
Patient selection
The study population included patients who were treated with PBT for LRRC between 2008 and December 2019 in our institution. Those who had a history of distant metastases of LRRC, with or without treatment, before reirradiation, were excluded. Patients who had undergone salvage surgery after initial radiotherapy (RT), such as total pelvic exenteration, as well as those who had a history of chemotherapy, were included. The present study was approved by the Institutional Review Board.
Proton beam therapy
The method of PBT in our institution was reported previously [13]. We routinely contour by simulation CT and magnetic resonance imaging. A proton-type particle therapy system (Hitachi, Kashiwa, Japan) and XiO-M (Hitachi, Kashiwa, Japan) were used. Reirradiation was performed 5 days a week, and PBT was administered using the passive scattering method. The standard treatment position for irradiation of sacral lesions was the prone position. However, many patients could not take a prone position due to a colostomy, and in such cases, the supine position was used. The radiation oncologist in charge decided the radiation dose and fractionation based on tumor location, prior radiation field and total dose, the distance between tumors and organs at risk (OARs) and the patient’s condition. A relative biological effectiveness (RBE) value of 1.1 was used in this study. Replanning, including a boost plan to reduce OARs dose, was adopted for most patients during PBT.
Statistical analysis
We estimated overall survival (OS), progression-free survival (PFS) and local control (LC) using the Kaplan–Meier method. The follow-up period started on the date of PBT reirradiation completion. All statistical analyses were performed using STATA version 18 (StataCorp LLC, Texas, USA).
RESULTS
Ten patients were included in the analysis. The patient and treatment characteristics are summarized in Table 1. The median follow-up time was 28.7 months, and the median age of the patients was 58 years (range, 40–70 years). All of the patients had adenocarcinoma. The indication of prior RT was the following; preoperative RT by photon in three patients, postoperative adjuvant RT by photon in one patient, postoperative salvage RT by photon in four patients and postoperative salvage PBT in two patients. Most of the prior RT was performed in other institutions. The median total dose of prior RT was 50 Gy (range, 30 Gy–74.8 Gy), and the median time from prior RT to reirradiation was 31.5 months (range, 8.1–96.6 months). All of the patients had a history of chemotherapy before reirradiation (median: three regimens, range, 1–5). Seven patients had a history of using Bevacizumab. Before reirradiation, two patients had undergone salvage surgery with omentum spacer placement, and one patient had undergone omentum spacer placement surgery alone. The postoperative recurrence sites at the time of reirradiation were as follows: five patients had a recurrence in the presacral region, three patients in the pelvic sidewall and two patients in anastomoses. The median maximum size of the recurrent tumor at the time of reirradiation was 34 mm (range, 20–50 mm). In the reirradiation setting, seven patients underwent PBT alone, and two patients were administered concurrent chemotherapy (one patient received S-1 alone, and another patient received irinotecan plus S-1). The remaining patient had received hyperthermia three times during reirradiation. Gross tumor volume (GTV) was defined as a recurrent tumor, and the median clinical target volume (CTV) margin around it was 5 mm (range, 3–10 mm). The median planning target volume (PTV) margin around the CTV was also 5 mm (range, 3–5 mm). Nine patients were in the supine position as the treatment position, and the remaining patient was in the prone position. The median reirradiation dose was 72 Gy (RBE) (range, 56–77 Gy). The dose-fractionation regimens of prior RT and reirradiation of all patients are listed in Table 2. The median cumulative equivalent dose in 2 Gy fraction (EQD2) (α/β = 10) for the tumor was 121 Gy (range, 98.2–151.2 Gy), respectively. The radiation field and dose distribution of Patient #6 are shown in Fig. 1. The most concerned OARs at the time of reirradiation varied from patient to patient due to the tumor location and reirradiation field. We estimated the cumulative EQD2 (α/β = 3) of OARs in prior RT and reirradiation as possible as we could. The estimated dose of the most-concerned OARs for each patient and late toxicities is summarized in Table 3. The median cumulative EQD2 (α/β = 3) for the OARs was 101.7 Gy (range, 54.7–143.4 Gy).
Table 1.
Patient and treatment characteristics
| Number of patients | 10 |
| Median age (range) | 58 (40–70) |
| Gender | |
| Male/Female | 8/2 |
| PS (ECOG) | |
| 0/1 | 9/1 |
| Median Prior RT dose (Gy, RBE) (range) | 50.0 (30–74.8) |
| Indication of prior RT | |
| Preoperative RT (photon) | 3 |
| Postoperative adjuvant RT (photon) | 1 |
| Postoperative salvage RT (photon) | 4 |
| Postoperative salvage PBT | 2 |
| Histology | |
| Adenocarcinoma | 10 |
| Primary tumor stage (UICC 7th) | |
| I/II/III/unknown | 2/3/4/1 |
| Recurrent site at the time of Re-RT | |
| Presacral | 5 |
| Pelvic side wall | 3 |
| Anastomosis | 2 |
| Median recurrent tumor size (mm) (range) | 34 (20–50) |
| Median GTV (cm3) (range) | 26.1 (6.3–151.7) |
| Concurrent CTx with Re-RT | 2 |
| Median Re-RT dose (Gy, RBE) (range) | 72.0 (56–77) |
| Time from prior RT to Re-RT (month) (range) | 31.5 (8.1–96.6) |
| History of CTx before Re-RT | 10 |
| Median CTx regimen before Re-RT (range) | 3 (1–5) |
| History of using bevacizumab | 7 |
| Omentum spacer placement before Re-RT | 3 |
| CTx = chemotherapy; ECOG = Eastern Cooperative Oncology Group; PS = performance status; Re-RT = reirradiation. | |
Table 2.
Characteristics of dose fractionation regimens for prior RT, Re-RT and outcome
| Patient | Prior RT dose (Gy, RBE) |
Prior RT Fr |
Prior RT modality |
Re-RT dose (Gy, RBE) |
Re-RT Fr |
Total EQD2 (α/β = 10) |
Recurrence type after Re-RT |
Recurrence time after Re-RT (months) | Survival time after Re-RT (months) | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 45.0 | 25 | Photon | 70.0 | 35 | 114.2 | 42.1 | NED | ||
| #2 | 30.0 | 20 | Photon | 67.2 | 28 | 98.2 | Distant (lung) | 3.1 | 31.4 | DOD |
| #3 | 40.0 | 20 | Photon | 75.0 | 30 | 118.1 | Distant (lung, bone) | 7.9 | 22.1 | DOD |
| #4 | 72.0 | 30 | PBT | 66.0 | 30 | 141.5 | Distant (lung, paraaortic LN) |
2.9 | 14.7 | DOD |
| #5 | 50.0 | 25 | Photon | 74.0 | 37 | 124 | Distant (liver) | 9.0 | 20.3 | DOD |
| #6 | 56.0 | 28 | Photon | 60.0 | 24 | 118.5 | Local and Distant (peritoneal dissemination) | 3.4 | 16.1 | DOD |
| #7 | 60.0 | 30 | Photon | 77.0 | 35 | 138.3 | Local | 6.5 | 26.0 | DOD |
| #8 | 74.8 | 34 | PBT | 74.8 | 34 | 152.1 | Distant (lung) | 2.2 | 59.8 | DOD |
| #9 | 50.0 | 25 | Photon | 56.0 | 28 | 106 | Local | 9.0 | 36.3 | DOD |
| #10 | 50.4 | 28 | Photon | 74.8 | 34 | 125.6 | Local | 15.5 | 41.1 | DOD |
DOD = died of disease; EQD2 = equivalent dose in 2 Gy fraction; Fr = fractionation; LN = lymph node; NED = no evidence of disease; PBT = proton beam therapy; Re-RT = reirradiation; RT = radiation therapy.
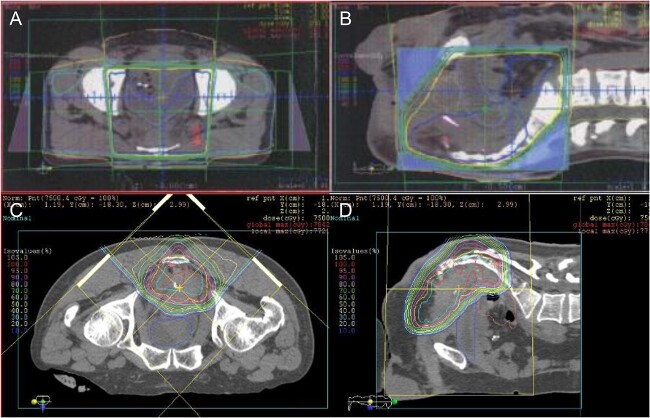
Fig. 1.
Radiation field and dose distribution of Patient #6. Prior photon pelvic irradiation (A and B). Fifty Gy/ 25 fractions for whole pelvis followed by 6 Gy/ 3 fractions boost. Proton beam re-irradiation (C and D). Sixty Gy (RBE)/ 24 fractions for presacral recurrent tumor.
Table 3.
Detailed analysis of OARs and summary of the late toxicity
| Patient | Prior RT field | High-dose overlap | Most concerned OARs at Re-RT | Prior RT EQD2 (α/β = 3) |
Re-RT EQD2 (α/β = 3) |
Total EQD2 (α/β = 3) |
Bev use |
Late toxicity (CTCAE Ver.5.0) |
Time to toxicity (months) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | WP | Complete | Bladder Small bowel Urethra |
43.2 43.2 43.2 |
26.9 11.9 21.2 |
70.1 55.1 64.4 |
Yes | Urinary frequency (Gr 1) lower leg edema (Gr 2) |
19 23 |
| #2 | SP | Complete | Small bowel | 27 | 54.1 | 81.1 | Yes | ||
| #3 | WP | Complete | Small bowel | 40 | 14.7 | 54.7 | Yes | ||
| #4 | Anastomosis presacrum |
Complete | Bladder Small bowel |
79.3 37.4 |
45.2 33.3 |
124.5 70.7 |
Yes | Dysuria (Gr 1) | 7 |
| #5 | HP | Partial | Bladder Sigmoid/rectum |
50 50 |
68.3 58.6 |
118.3 108.6 |
No | ||
| #6 | WP | Complete | Bladder Small bowel |
56 56 |
58.8 45.7 |
114.8 101.7 |
Yes | ||
| #7 | WP | Complete | Bladder Sigmoid/rectum Uterus |
60 60 60 |
74.4 83.4 82.2 |
134.4 143.4 142.2 |
Yes | ||
| #8 | Presacrum | Partial | Bladder Small bowel Sigmoid/rectum |
4.2 0 79.5 |
63.5 66.8 8.4 |
67.7 66.8 87.9 |
No | Colonic hemorrhage (colostomy) (Gr 4) | 15 |
| #9 | WP | Complete | Bladder Sigmoid/rectum |
50 50 |
52.1 57.1 |
102.1 107.1 |
Yes | ||
| #10 | HP | Complete | Sigmoid Pelvic bone |
48.4 48.4 |
23 68.5 |
71.4 116.9 |
No |
Bev = bevacizumab; CTCAE = Common Terminology Criteria for Adverse Events; EQD2 = equivalent dose in 2 Gy fraction; HP = hemi pelvis; Re-RT = reirradiation; RT = radiation therapy; SP = small pelvis; WP = whole pelvis.
The 1-year/2-year OS, PFS and LC rates were 100%/60.0%, 20.0%/10.0% and 70.0%/58.3%, respectively (Fig. 2), and the median survival time was 26 months. Four patients had further local recurrence in the reirradiation field and six patients had distant metastasis after reirradiation (Table 2). Of the 10 patients, nine died from cancer progression after reirradiation; the remaining patient (Patient #1) had no local recurrence or distant metastasis during the follow-up period. In the acute toxicity, seven patients developed Grade 1 acute radiation dermatitis. In the late toxicity, Grade 1 urinary frequency and Grade 1 dysuria were observed in one patient each, and a late toxicity Grade of ≥3 was only recorded in one patient (Patient #8), who had undergone radical surgery in 2005. The first local recurrence was detected in the presacral region in 2011 (Fig. 3A), and the initial salvage RT by PBT was performed with 74.8 Gy (RBE) in 34 fractions (Fig. 3B). Thirteen months after the initial PBT, right iliac lymph node metastasis appeared (Fig. 3C). There was no indication for a surgical approach and no evidence of distant metastasis. The initial recurrent tumor, which was located in the presacral region, was under control; therefore, another salvage reirradiation with PBT was planned; the total dose of reirradiation was 74.8 Gy (RBE) in 34 fractions (Fig. 3D). The estimated cumulative EQD2 (α/β = 3) for the bladder, small bowel, and sigmoid/ rectum was 67.7 Gy, 66.8 Gy and 87.9 Gy, respectively (Table 3). Unfortunately, multiple lung metastases occurred during the 2 months after reirradiation. Fifteen months after reirradiation, colostomy was performed due to severe bleeding of the colon. Unfortunately, the location of bleeding of the colon was unknown. This patient had no history of bevacizumab use before reirradiation.
Fig. 2.
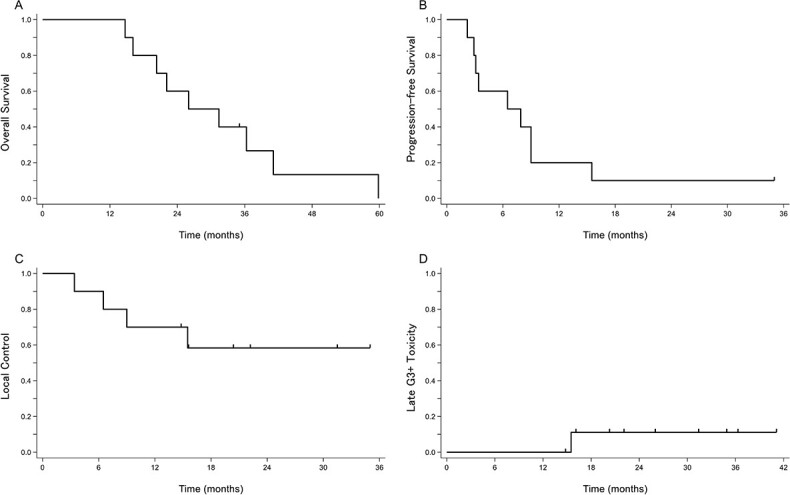
Kaplan–Meier curves for (A) OS, (B) PFS, (C) LC and (D) late Grade ≥ 3 toxicities for all patients (n = 10).
Fig. 3.
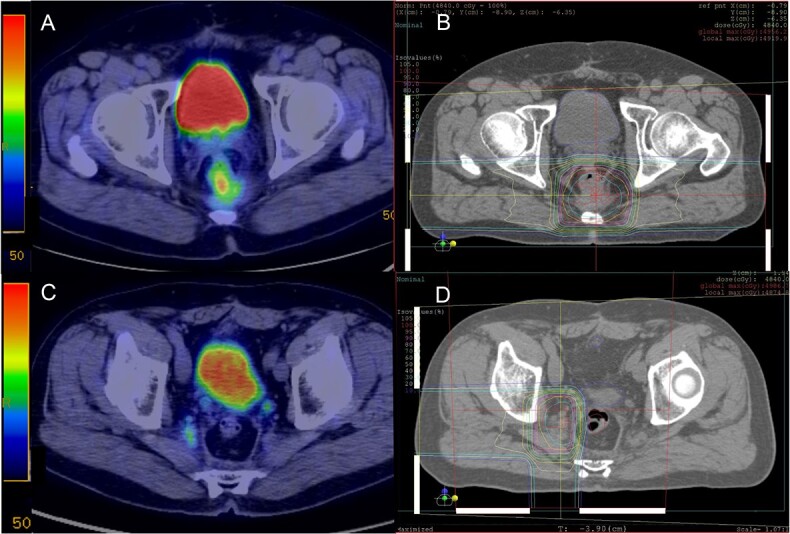
Radiation field and dose distribution of Patient #8. Initial PBT with 74.8 Gy (RBE)/34 fractions for presacral local recurrence after surgery (A and B). Proton beam reirradiation with 74.8 Gy (RBE)/34 fractions for right iliac lymph node metastasis at 13 months after initial PBT (C and D).
DISCUSSION
We reported in PBT reirradiation with acceptable toxicity for patients with LRRC treated with prior pelvic RT. Even in this multi-modal treatment era, treating LRRC is still challenging. Surgical resection with negative margins has the only chance to achieve long-term LC and survival [14–16]. However, only 31–40% of the patients with LRRC are considered as resectable [17, 18]. Therefore, reirradiation could be a treatment option if complete surgical resection is not feasible or the patient denies salvage surgery for LRRC. The results of an Italian national survey showed that reirradiation was an option for neoadjuvant treatment in resectable and unresectable LRRC in 55 and 75% of cases, respectively [19].
In general, reirradiation is performed as palliative treatment because of the high risk of severe radiation-related toxicity. A previous study showed more than 80% of symptomatic relief for LRRC patients treated with reirradiation [20]. Historically, photon reirradiation using hyperfractionated accelerated RT has been administered [4,6–11]. However, previous studies have reported median total reirradiation doses of 30–40 Gy and the outcomes were not satisfied. In another approach to photon reirradiation, Johnstone et al. published the multicenter retrospective data of SABR reirradiation in LRRC patients [5]. They used a linear accelerator with volumetric modulated arc therapy (VMAT) or a Cyberknife with 30 Gy in five fractions. They reported a 2-year OS rate of 77% and a median survival time of 38.7 months without a surgical approach. Due to the improvement in radiation technology, a higher dose could be given for a recurrent lesion without increasing the dose for the OARs. However, they also reported a 2-year PFS rate of 28%. In addition, 42.6% of patients developed posttreatment local relapses. This indicates that even reirradiation with SABR might not be enough to prevent further LC or distant metastases.
On the other hand, PBT and CIRT have unique physical characteristics, such as the Bragg peak. Therefore, PBT and CIRT enable a higher dose of LRRC without severe toxicity compared to conventional photon RT. A dosimetric study described proton pencil beam scanning and carbon-ion pencil beam scanning achieved both better coverage for mean PTV-D95% and lower doses for the rectum and bladder than VMAT [21]. PBT reirradiation with passive scattering method could reduce bowel and bone marrow dose more than photon therapy can [22, 23]. However, the short follow-up periods in these studies (14 months in both) may not be sufficient to assess clinical efficacy. Koroulakis et al. reported on reirradiation using pencil beam scanning PBT for 28 patients with rectal cancer (18 patients were recurrent rectal cancer and ten patients were de novo rectal cancer) [24]. In their study, with a median follow-up time of 28.6 months, 1-year OS, PFS and LC were 81.8, 45.0 and 66.3%, respectively. Regarding clinical outcomes, the results of the passive scattering method in the present study were comparable with those in Koroulakis et al.’s study. Shiba et al. demonstrated reirradiation with CIRT for seven patients with LRRC after preoperative chemoradiotherapy [25]. They stated that 2-year OS, LC and PFS rates were 100, 83.3 and 28.6%, respectively (the median follow-up period was 30.9 months). Considering previous reports, the clinical outcomes of reirradiation for LRRC patients using CIRT are superior to those of photon and PBT. C-ion beam has not only a Bragg peak but also high linear energy transfer, this biological advantage leads to a higher cell-killing effect even in radioresistant and hypoxic cells. However, it is still difficult to compare the clinical outcomes of reirradiation between SABR, PBT and CIRT because the LRRC patient’s characteristics and background including treatment history had many variations. Moreover, our patients had several chemotherapy regimens before reirradiation. In fact, 80% of our patients developed further local recurrence and/or distant metastasis within 1 year after reirradiation (Table 2). Therefore, our cohort might have more treatment-resistant, aggressive biological recurrent tumors than previous reports. In addition, the median GTV (26.1 cm3) of ours was slightly larger than those of SABR (13.4 cm3) and CIRT (15.6 cm3) studies [5, 25] (Table 1). As a result, we considered that the clinical outcomes in our study were slightly lower than those of photon SABR and CIRT.
On the other hand, the outcome of second-time surgery for the patients with LRRC was superior to those of reirradiation [12,14–16]. However, there might have been a selection bias of the patients with LRRC in treatment decisions. That is, small and resectable tumors with the LRRC patients are likely to be treated by salvage surgery including pelvic exenteration. While, bulky, unresectable and chemo-resistant tumors are likely to refer to non-surgical treatment such as reirradiation.
In an Italian national survey, the irradiation of the cumulative dose of EQD2 (equivalent dose in 2 Gy) was 90–100 Gy considering the previous RT dose [19]. In the present study, the dose with the median cumulative EQD2 (α/β = 10) was high, at 121 Gy (Table 2); unfortunately, four patients (40%) had further local recurrence in the reirradiation field. Due to the aggressive biology, many LRRC patients develop further local recurrence or distant metastasis even after reirradiation. Similar to several previous reirradiation studies, there were poor PFS rates in the present study. Recent advances in systemic chemotherapy including immunotherapy could improve the PFS rate for patients with LRRC after reirradiation.
The data of systemic chemotherapy for the patients with LRRC who had previous pelvic RT history are limited. In such a situation, most of the chemotherapy is administered as only palliation. However, previously irradiated patients with LRRC had a lower response rate to conventional chemotherapy of the in-field local recurrence than those of out-field distant metastasis (10 vs 41%, P = 0.034) [26]. Meanwhile, more than half of the patients achieved LC after reirradiation in our study. Therefore, reirradiation for LRRC could be a better treatment option than palliative chemotherapy in well-selected patients.
Regarding toxicity, only one patient (10%) had Grade ≥ 3 late toxicity in the current study. Although we estimated the cumulative dose for the most concerned OARs for each patient (Table 3), we could not find a correlation between cumulative dose for OARs and late toxicity. Therefore, the indication for reirradiation should be carefully determined after simulating the initial RT field and examining the dose–volume histogram of the overlap with the reirradiation field for each patient. This is crucial because the incidence of late toxicity may be underestimated due to the poor prognosis of patients with LRRC.
A combination with some systemic drugs may increase the risk of radiation-induced gastrointestinal perforation [27]. Although bevacizumab plays an important role in chemotherapy for patients of recurrent and/or inoperable rectal cancer, it is well known for developing a risk of gastrointestinal perforation [28]. In the present study, there was a high rate of bevacizumab use (70%) before reirradiation among the patients. Furthermore, in this study, three of the 10 patients had received omentum spacer placement surgery before reirradiation in the present study. Recently, a nonwoven fabric bioabsorbable spacer was developed for particle therapy [29]; this spacer will enable administration of reirradiation more safely in the future.
As a result, with regard to gastrointestinal late toxicity, our results suggest that even if the patient had pelvic RT history, PBT could be administered safely. However, Koroulakis et al. reported that 14.7% of their patients had developed Grade ≥ 3 late toxicities, including in one patient with Grade 5 toxicity who had a prior RT-related injury [24]. Therefore, in the reirradiation setting, we should pay more attention to the late toxicity of prior RT, because the toxicity in the patient who had experienced severe late toxicity in the prior RT has the potential to worsen during reirradiation.
There are some limitations to the present study. First, this study analyzed retrospectively a small number of cases in a single institution. Second, the PBT reirradiation dose and fractionation regimen differed depending on the doctor in charge and the patient’s condition. Thus, it is difficult to discuss the treatment efficacy of PBT reirradiation. Although a multi-center retrospective study is warranted, the indication of reirradiation using PBT for the patients with LRRC has a lot of variation between each institution. We believe that PBT reirradiation enables to give curative dose for the recurrent tumor with less toxicity for OARs and it has the potential to improve the outcomes in well-selected LRRC patients. Therefore, retrospective studies with small cases in a single institution including our study would be valuable for the reirradiation setting.
CONCLUSIONS
Reirradiation using PBT for LRRC patients with prior pelvic irradiation was feasible. However, the indication of PBT reirradiation for LRRC patients’ needs to be considered carefully in terms of the risk of severe late GI toxicity.
CONFLICT OF INTEREST
None.
FUNDING
There was no funding support.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from the patient for publication of this study and accompanying images. The present study was approved by the Institutional Review Board at our institution.
Contributor Information
Yoshiaki Takagawa, Department of Minimally Invasive Surgical and Medical Oncology, Fukushima Medical University, Fukushima, Japan; Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Motohisa Suzuki, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Ichiro Seto, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Yusuke Azami, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Masanori Machida, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Kanako Takayama, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Nor Shazrina Sulaiman, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Tatsuhiko Nakasato, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Yasuhiro Kikuchi, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Masao Murakami, Department of Radiation Oncology, Southern TOHOKU Proton Therapy Center, Fukushima, Japan.
Michitaka Honda, Department of Minimally Invasive Surgical and Medical Oncology, Fukushima Medical University, Fukushima, Japan; Department of Surgery, Southern TOHOKU General Hospital, Fukushima, Japan.
Yasushi Teranishi, Department of Surgery, Southern TOHOKU General Hospital, Fukushima, Japan.
Koji Kono, Department of Gastrointestinal Tract Surgery, Fukushima Medical University School of Medicine, Fukushima, Japan.
REFERENCES
- 1. Kodeda K, Derwinger K, Gustavsson B, Nordgren S. Local recurrence of rectal cancer: a population-based cohort study of diagnosis, treatment and outcome. Color Dis 2012;14:e230–7. 10.1111/j.1463-1318.2011.02895.x. [DOI] [PubMed] [Google Scholar]
- 2. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Cancer Netw 2020;18:806–15. 10.6004/jnccn.2020.0032. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol 2022;40:2546–56. 10.1200/JCO.22.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Das P, Delclos ME, Skibber JM, et al. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys 2010;77:60–5. 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 5. Johnstone P, Okonta L, Aitken K, et al. A multicentre retrospective review of SABR reirradiation in rectal cancer recurrence. Radiother Oncol 2021;162:1–6. 10.1016/j.radonc.2021.06.030. [DOI] [PubMed] [Google Scholar]
- 6. Mohiuddin M, Lingareddy V, Rakinic J, Marks G. Reirradiation for rectal cancer and surgical resection after ultra high doses. Int J Radiat Oncol Biol Phys 1993;27:1159–63. 10.1016/0360-3016(93)90538-7. [DOI] [PubMed] [Google Scholar]
- 7. Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 2002;95:1144–50. 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 8. Mohiuddin M, Marks GM, Lingareddy V, et al. Curative surgical resection following reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys 1997;39:643–9. 10.1016/S0360-3016(97)00340-4. [DOI] [PubMed] [Google Scholar]
- 9. Tao R, Tsai CJ, Jensen G, et al. Hyperfractionated accelerated reirradiation for rectal cancer: an analysis of outcomes and toxicity. Radiother Oncol 2017;122:146–51. 10.1016/j.radonc.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 10. Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys 2006;64:1129–39. 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 11. Youssef FF, Parikh PJ, DeWees TA, et al. Efficacy and toxicity of rectal cancer reirradiation using IMRT for patients who have received prior pelvic radiation therapy. Adv Radiat Oncol 2016;1:94–100. 10.1016/j.adro.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mantello G, Galofaro E, Bisello S, et al. Modern techniques in Re-irradiation for locally recurrent rectal cancer: a systematic review. Cancers (Basel) 2023;15:4838. 10.3390/cancers15194838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takagawa Y, Suzuki M, Yamaguchi H, et al. Outcomes and prognostic factors for locally recurrent rectal cancer treated with proton beam therapy. Adv Radiat Oncol 2023;8:101192. 10.1016/j.adro.2023.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der MeijW, Rombouts AJ, Rütten H, et al. Treatment of locally recurrent rectal carcinoma in previously (chemo)irradiated patients: a review. Dis Colon Rectum 2016;59:148–56. 10.1097/DCR.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 15. Alberda WJ, Verhoef C, Nuyttens JJ, et al. Outcome in patients with resectable locally recurrent rectal cancer after total mesorectal excision with and without previous neoadjuvant radiotherapy for the primary rectal tumor. Ann Surg Oncol 2014;21:520–6. 10.1245/s10434-013-3306-x. [DOI] [PubMed] [Google Scholar]
- 16. Bhangu A, Ali SM, Darzi A, et al. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Color Dis 2012;14:1457–66. 10.1111/j.1463-1318.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 17. Bakx R, Visser O, Josso J, et al. Management of recurrent rectal cancer: a population based study in greater Amsterdam. World J Gastroenterol 2008;14:6018–23. 10.3748/wjg.14.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007;14:447–54. 10.1245/s10434-006-9256-9. [DOI] [PubMed] [Google Scholar]
- 19. Mantello G, Galofaro E, Caravatta L, et al. Pattern of care for re-irradiation in locally recurrent rectal cancer: a national survey on behalf of the AIRO gastrointestinal tumors study group. Radiol Med 2023;128:869–76. 10.1007/s11547-023-01652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guren MG, Undseth C, Rekstad BL, et al. Reirradiation of locally recurrent rectal cancer: a systematic review. Radiother Oncol 2014;113:151–7. 10.1016/j.radonc.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 21. Mori S, Bhattacharyya T, Furuichi W, et al. Comparison of dosimetries of carbon-ion pencil beam scanning, proton pencil beam scanning and volumetric modulated arc therapy for locally recurrent rectal cancer. J Radiat Res 2023;64:162–70. 10.1093/jrr/rrac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berman AT, Both S, Sharkoski T, et al. Proton reirradiation of recurrent rectal cancer: dosimetric comparison, toxicities, and preliminary outcomes. Int J Particle Ther 2014;1:2–13. 10.14338/IJPT.13-00002.1. [DOI] [Google Scholar]
- 23. Moningi S, Ludmir EB, Polamraju P, et al. Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin Transl Radiat Oncol 2019;19:59–65. 10.1016/j.ctro.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koroulakis A, Molitoris J, Kaiser A, et al. Reirradiation for rectal cancer using pencil beam scanning proton therapy: a single institutional experience. Adv Radiat Oncol 2021;6:100595. 10.1016/j.adro.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shiba S, Okamoto M, Shibuya K, et al. Safety and efficacy of Re-irradiation with carbon-ion radiotherapy for pelvic recurrence of rectal cancer after preoperative chemoradiotherapy: a retrospective analysis. In Vivo 2022;36:2473–80. 10.21873/invivo.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alberda WJ, Haberkorn BC, Morshuis WG, et al. Response to chemotherapy in patients with recurrent rectal cancer in previously irradiated area. Int J Color Dis 2015;30:1075–80. 10.1007/s00384-015-2270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng Y, Gao W, Spratt DE, et al. Management of gastrointestinal perforation related to radiation. Int J Clin Oncol 2020;25:1010–5. 10.1007/s10147-020-01662-5. [DOI] [PubMed] [Google Scholar]
- 28. Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 2007;14:1860–9. 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 29. Sasaki R, Demizu Y, Yamashita T, et al. First-in-human phase 1 study of a nonwoven fabric bioabsorbable spacer for particle therapy: space-making particle therapy (SMPT). Adv Radiat Oncol 2019;4:729–37. 10.1016/j.adro.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]