Abstract
The androgen receptor protein has specific domains involved in DNA binding, ligand binding, and transactivation, whose activities need to be integrated during transcription activation. The hinge region, more particular a 629RKLKK633 motif, seems to play a crucial role in this process. Indeed, although the motif is not part of the DNA-binding domain, its positive residues are involved in optimal DNA binding and nuclear translocation as shown by mutation analysis. When the mutated ARs are forced into the nucleus, however, the residues seem to play different roles in transactivation. Moreover, we show by FRAP analysis that during activation, the AR is distributed in the nucleus in a mobile and two immobile fractions, and that mutations in the 629RKLKK633 motif affect the distribution of the AR over these three intranuclear fractions. Taken together, the 629RKLKK633 motif is a multifunctional motif that integrates nuclear localization, receptor stability, DNA binding, transactivation potential and intranuclear mobility.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0302-1) contains supplementary material, which is available to authorized users.
Keywords: Androgen receptor, DNA binding, Hinge region, Intranuclear mobility, Transcriptional activity
Introduction
The androgen receptor (AR) belongs to the super family of the nuclear receptors. Upon ligand binding, the AR translocates to the nucleus, binds to specific DNA response elements and subsequently regulates gene expression by the recruitment of co-regulatory complexes. These complexes remodel chromatin, alter DNA topology and enhance different steps of the transcription process. Although the major steps involved in this general action mechanism of nuclear receptors have been documented abundantly by in vitro biochemical analyses, studies on the in vivo dynamics of the different players have been limited and sometimes difficult to reconcile [1].
As all nuclear receptors, the AR has a DNA-binding domain (DBD) flanked at its N-terminal site by a complex activation domain (NTD) and at its C-terminal site by the ligand-binding domain (LBD). Although each of these domains can act autonomously, their functions need to be integrated in a correct spatial–temporal sequence to enable the proper transactivation of the target genes.
The hinge that connects the DBD and LBD was initially considered just to be a flexible linker, but later on evidence suggested that it is functionally crucial for the control of the activity of nuclear receptors [2, 3]. A crystal structure of the ppARγ-RXRα heterodimer on DNA revealed that this region is implicated in DNA-binding and receptor-receptor interactions [4]. Bag-1M is a co-chaperone that interacts with the hinge of glucocorticoid receptor and down-regulates transactivation [5]. The activities of the retinoic acid receptor-α, the glucocorticoid receptor and the estrogen receptor-α, are regulated by posttranslational modifications such as methylation [6–9] and acetylation of their hinge regions [8, 10].
For the AR, the hinge has also been implicated in DNA binding [11], nuclear localization [12–14], and is a target for posttranslational modifications [15]. Moreover, many co-regulatory proteins depend on the hinge for proper interactions with the AR, and activating as well as inactivating hinge mutations have been described in prostate cancer biopsies and androgen insensitivity syndromes, respectively [16, 17].
Here, we identify a 629RKLKK633 motif that is involved in diverse features of the AR. We come to the surprising conclusion that the effect of mutations on transcriptional activity, DNA binding, nuclear translocation, and intranuclear immobility are completely unanticipated. Our data clearly challenge the current simplified concepts of transcriptional regulation by the nuclear receptors in general and the AR in particular.
Materials and methods
Plasmid constructs
The pSG5-flag-wtAR plasmid and the Δ629–636 deletion mutant (referred to as Δ11) have been previously described [19]. Flag-tagged full-length AR mutants containing either single, double, or quadruple amino acid substitutions were created by two-step PCR site-directed mutagenesis using two internal primers and two external primers. The resulting mutated human AR cDNA fragment was cloned as a HindIII/PsyI fragment into the pSG5-flag-wtAR construct, from which the corresponding fragment had been excised by the same restriction enzymes. pEGFP-(GA)6-wtAR and pEGFP-(GA)6-A573D, encoding the full-length wild-type human AR and the full-length human AR DNA-binding mutant (A573D) respectively, and both fused N-terminally to the enhanced green fluorescent protein (EGFP), were previously described in [20]. Additional EGFP-AR fusions (∆629–636, R629/K630V, K632/K633V, and R629/K630/K632/K633V) were created by subcloning the HindIII/EcoRI fragments from the pSG5 expression vector bearing the corresponding mutation into the pEGFP-(GA)6-wtAR vector from which the HindIII/EcoRI fragment had been removed. The deletion mutant, Δ629–636, has been previously described in [19] where it is referred to as Δ11. The pEGFP-NLS-(GA)6-wtAR expression plasmid was created by inserting an oligonucleotide encoding the SV40-NLS (PKKKRKVD) with BglII and BamHI sticky ends into the pEGFP-(GA)6-wtAR vector that had been digested with BglII. Similarly, EGFP-NLS-AR expression vectors were created for the A573D, ∆629–636, R629/K630V, K632/K633V, and RKKK/VVVV mutants. For simplicity, the R629/K630V, the K632/K633V, and R629/K630/K632/K633V mutants were renamed to RK, KK and RK + KK mutants, respectively.
Cell culture, transient transfections, and generation of stable cell lines
HeLa and COS-7 cells were maintained as previously described [19]. Hep3B cell lines expressing the various EGFP-NLS-AR proteins were established and cultured as described [20]. The stable MMTV reporter cell line was established by the integration of a pcDNA5/FRT/TO-derived vector into the HEK 293 FLP-In cell line according to Denayer et al. [18]. Transient transfections were preformed as described [19].
Immunocytochemistry and fluorescence microscopy
HeLa cells were seeded into chambered cover glass four-well Labtek II slides (Sanbio) at 6 × 104 cells/well. The following day, cells were transfected with 200 ng AR expression vector per well. Cells were stimulated for 1 h with 10 nM R1881 before fixation. Proteins were immunostained with the M2 anti-flag antibody (Stratagene), followed by an incubation with TRITC-conjugated goat anti-mouse antibody (Sigma-Aldrich). The subcellular distribution of the expressed proteins was analyzed by fluorescence microscopy using a Zeiss LSM510 confocal scanning microscope (Carl Zeiss).
Electrophoretic mobility shift assays
Electrophoretic mobility shift assays were performed as described [19]. For supershifts, 1 μl of a three-fold dilution of the M2 anti-flag antibody was added to the extracts prior to the probe.
FRAP and computer analysis of FRAP data
FRAP studies and computer analyses were performed as described [20]. FRAP curves were generated by Monte Carlo computer simulations. In simulations of two immobile fractions with different kinetics, two immobilization/mobilization probabilities were evaluated each unit time step. Data from FRAP experiments were fitted to simulated curves either assuming free diffusion with a diffusion coefficient of 5.76 μm2/s (as computed for the A573D mutant), or assuming the presence of an immobile fraction (wtAR) with a diffusion coefficient of 1.44 μm2/s.
Results
A putative α-helix in the hinge
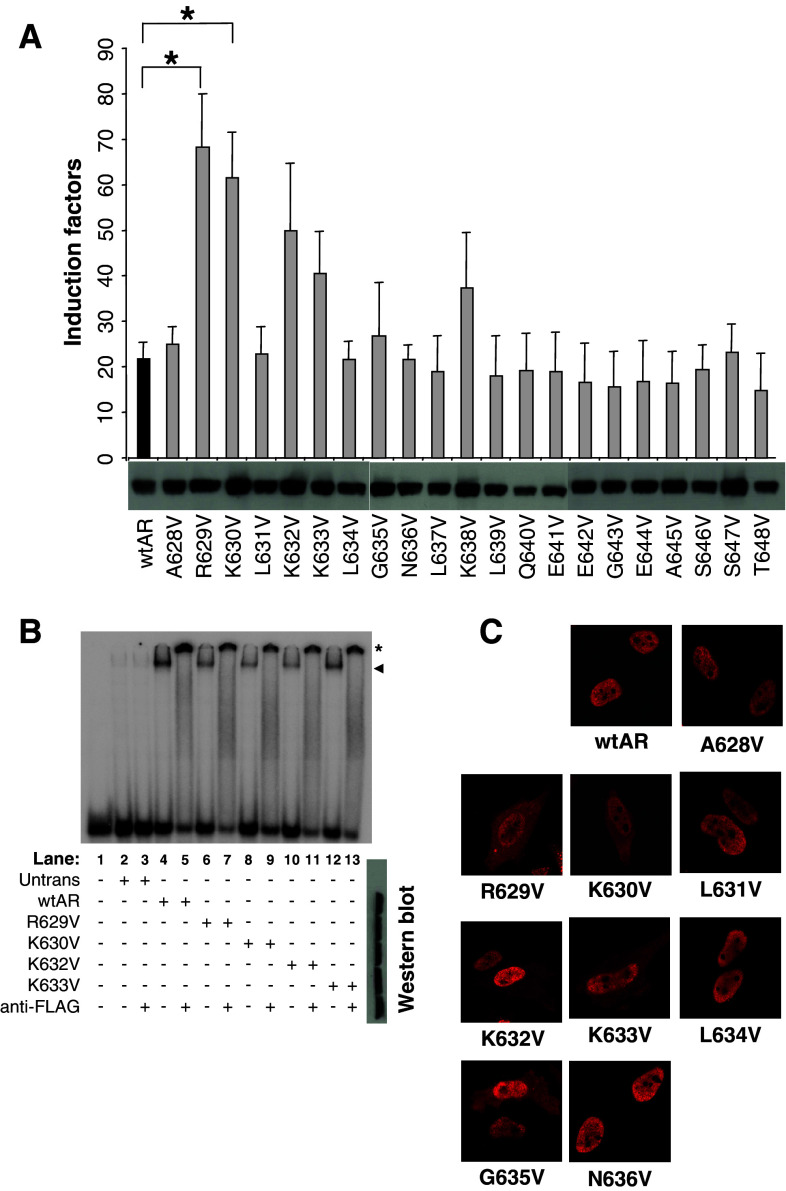
Some prostate cancer mutations and deletions in the AR hinge region are known to result in an increased transactivation potential [19]. Here, we set out to determine the underlying mechanisms and the amino acids involved in this phenomenon. Therefore, we set up a valine screening by substituting every single residue from A628 until T648 by a valine and analyzed the effect on transcriptional activity (Fig. 1a). From the 21 point mutations we introduced in the hinge region, only those affecting positive charges (R or K) resulted in a small increase of AR activity (Fig. 1a). Only the R629V and K630V mutants showed a statistically significant increase activity. Interestingly, these residues lie within the 629RKLKK633 motif described as a nuclear translocation signal [13]. We therefore investigated the effect of several mutations on the nuclear translocation of the AR. When compared to the wtAR, we see an impaired nuclear transport when the positively charged residues are affected (Fig. 1c). With regards to the other residues in this motif, only the G635V mutant demonstrated a reduced nuclear translocation.
Fig. 1.
Substitution analysis of the AR hinge region. a Functional analysis. A TAT-GRE-based reporter was co-transfected in HeLa cells with AR expression plasmids as indicated. Results are shown as 10 nM R1881 induction factors ± SEM. *p < 0.05 versus wtAR as determined by the Student’s t-test. Western-blot data for each mutant is given below the X-axis. b Electromobility shift assays. The labeled TAT-GRE probe was incubated with cell extracts containing the indicated mutant AR. The shifted complexes are indicated with an arrowhead. The supershifts, which are indicated with an asterisk, were obtained by adding an anti-flag antibody. Western blots of the extracts are shown at the right. c Nuclear translocation studies. HeLa cells were transfected with the indicated AR expression vectors, stimulated, fixed, and immunostained. Cellular localization of the expressed AR proteins was analyzed by fluorescence microscopy
The structural prediction program, Predictprotein [21], proposes that part of the hinge region between amino acids G627 and N636 forms an amphipatic α-helix (Supplementary Fig. 1a). To establish whether the increase in activity was a result of a disruption of this putative helical structure, we disrupted this helix by converting A628, L631, and L634 to asparagines (A628N, L631N, L634 N), but we did not observe any significant changes in AR activity (Supplementary Fig. 1b). In addition, L631P and L634P mutations also had no effect on activity. By contrast, the K632P and K633P mutations showed an increased activity (Supplementary Fig. 1c) very similar to that of K632V and K633V seen in Fig. 1a. It thus appears that it is the charged nature of the side chains, rather than the secondary structure, that is of importance to the inhibitory function of this region. Western blots for each set of mutants indicate that they are expressed to similar levels as wtAR (Fig. 1a and Supplementary Fig. 1d).
Multiple functions of the 629RKLKK633 hinge motif
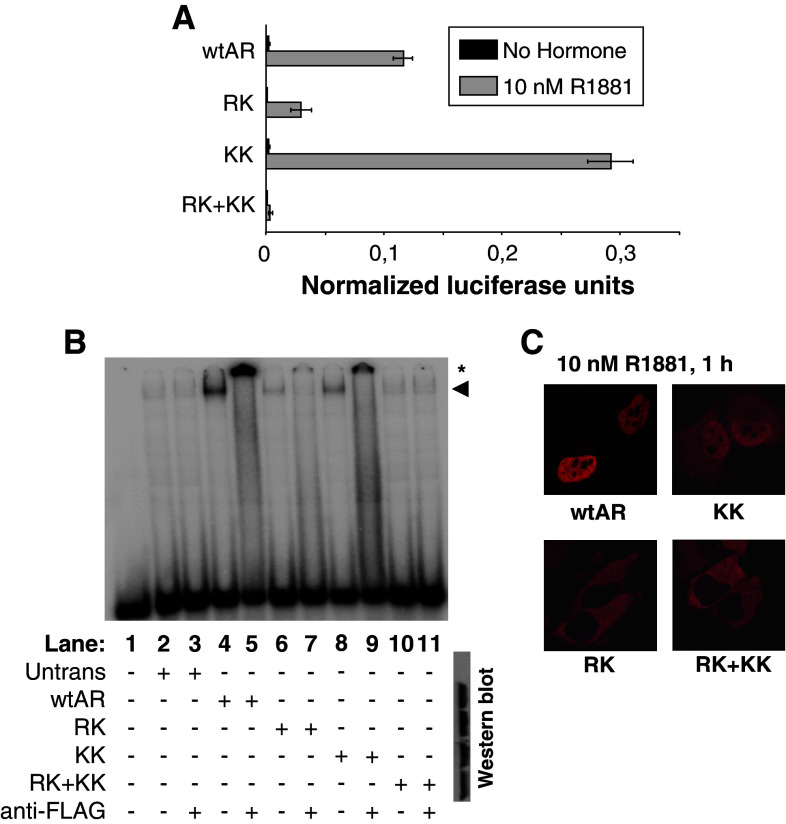
Some prostate cancer mutations as well as experimental deletions in the hinge region result in an increased transactivation potential of the AR [10, 19]. A mutation analysis of the hinge region revealed that substitutions affecting positively charged residues result in an increase of AR activity (Fig. 1 and Supplementary Fig. 1). Only when double mutants were analyzed did we observe an effect on DNA binding (Fig. 2b). However, the double RK and KK mutants are affected differently. The KK double mutant binds DNA well and undergoes nuclear translocation but displays a dramatic increase in potency, while the RK and quadruple RK + KK mutants have a lowered activity, strongly reduced nuclear import and low affinity for DNA (Fig. 2).
Fig. 2.
The effect of duplicate or quadruplicate mutations in the 629RKLKK633 motif. a Functional analysis. A TAT-GRE-based reporter was co-transfected in HeLa cells with AR expression plasmids as indicated. Results are shown as normalized luciferase units. Western-blot data for each mutant is given below the X-axis. b Electrophoretic mobility shift assays. The labeled TAT-GRE probe was incubated with cell extracts in which the mutated ARs have been over-expressed. The shifted complexes are indicated with an arrowhead. The supershifts, which are indicated with an asterisk, were obtained by adding an anti-flag antibody. Western blots of the extracts are given at the right. c Nuclear translocation studies. HeLa cells were transfected with the indicated AR expression vectors, stimulated, fixed, and immunostained. Cellular localization of the expressed AR proteins was analyzed by fluorescence microscopy
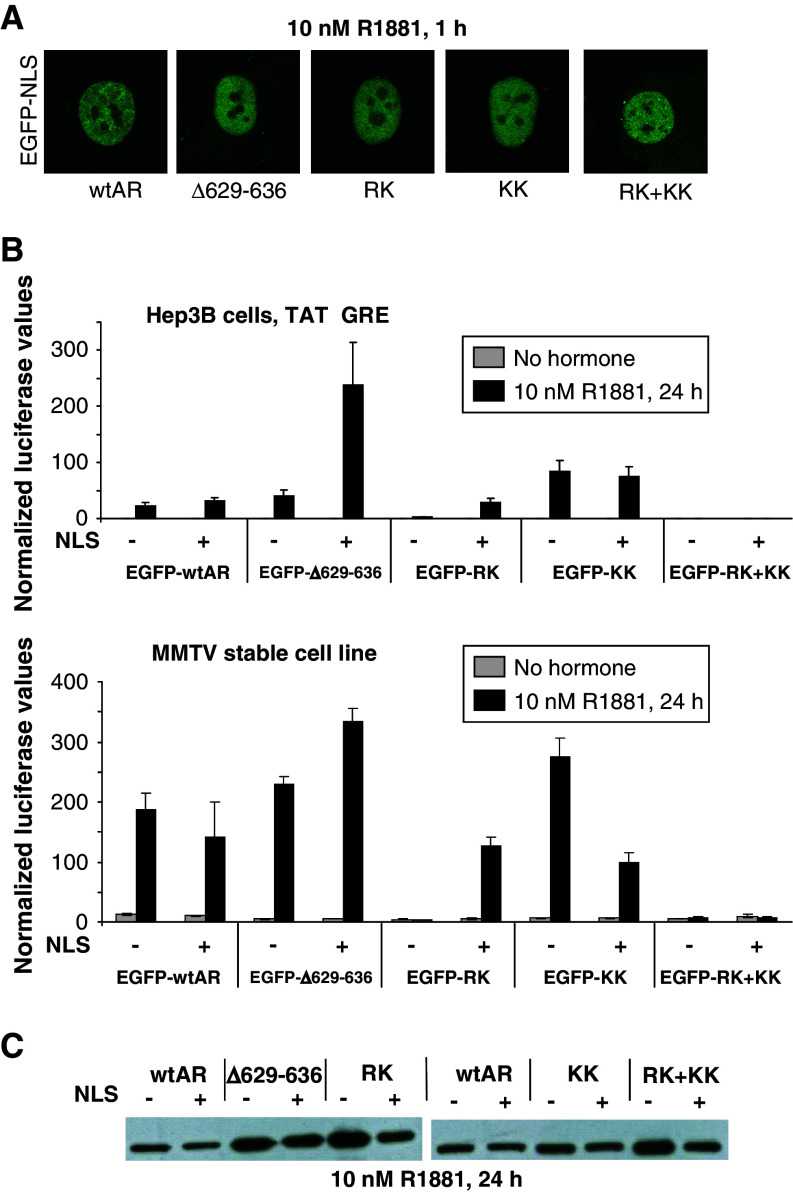
These four charged residues form what we here call the 629RKLKK633 motif which overlaps with the nuclear translocation signal [12]. To exclude that the changes in activity of the ∆629–636, RK, KK, and RK + KK mutants are a direct effect of their impaired nuclear translocation, the AR mutants were fused to EGFP plus or minus the SV40-NLS. These fusion proteins all display nuclear localization after hormone stimulation. The SV40-NLS fusion does not change the ability of wtAR and KK mutant to transactivate transcription on plasmid-based or chromatin-based templates. Despite its reduced DNA binding (Fig. 2b) the RK mutant was rescued by the NLS fusion, while the quadruple RK + KK mutant was not (Fig. 3).
Fig. 3.
Activity of hinge region mutants when localized in the nucleus. a Nuclear localization study. Confocal images of Hep3B cells stably expressing EGFP-NLS-AR proteins. b Functional analysis. Hep3B cells or the MMTV stable cell line were transiently transfected with the indicated expression vector. For the Hep3B cells, the TAT-GRE-luc reporter was cotransfected. Results are shown as normalized luciferase values ± SEM. c Western blot. The indicated receptor proteins fused to EGFP plus or minus the SV40-NLS were expressed in HeLa cells and detected with an anti-flag antibody
The 629RKLKK633 motif regulates intranuclear mobility
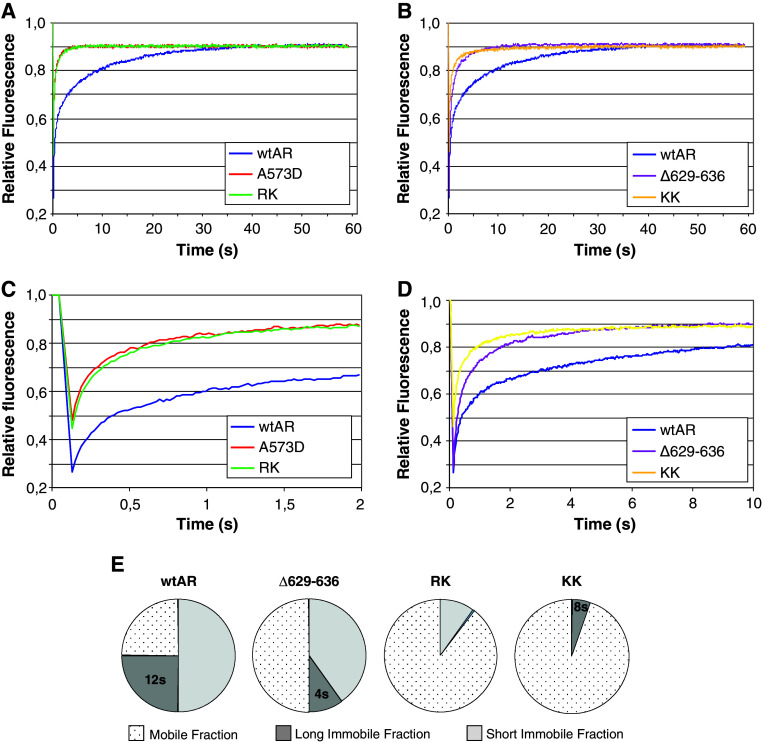
The fusion of EGFP also enabled studies of the effects of the 629RKLKK633 motif mutations on the in vivo intranuclear mobility by fluorescence recovery after photobleaching (FRAP) assays. For the inactive AR mutant that lost DNA binding (A573D), only a mobile fraction could be computed. This highly mobile DNA-binding mutant was included as a control in our FRAP experiments. These data are consistent with previous reports [20].
In Fig. 4a, the FRAP curve for wtAR shows a relatively slow diffusion and secondary redistribution that is typical for a protein distributed over both a mobile and a transiently immobile fraction. In parallel FRAP experiments, all the AR hinge mutants have a faster recovery compared to wtAR (Fig. 4a and b). Closer inspection of the curves over the first seconds of recovery revealed significant differences in mobility (Fig. 4c and d).
Fig. 4.
Intranuclear distribution and mobility of EGFP-NLS-AR proteins as measured by FRAP. a–d Fitted curves of experimental FRAP data. FRAP data of wtAR is plotted on all curves for comparative purposes. Three independent experiments were performed and data represent the mean of at least 45 cells. e Schematic representation of analysis of FRAP data. FRAP data fitted best with simulated curves with indicated parameters for immobile fractions and residence times. The percentage of molecules residing within the long immobile fraction (LIF) and short immobile fraction (SIF) are quantified in the pie charts. The remaining molecules were assumed to all reside in the freely diffusing mobile fraction. Residence time characterizes the mean immobilization of individual ARs in seconds. The calculated residence time for the various LIFs is indicated on the pie charts
Computational analysis of fitted FRAP data
FRAP data analysis using computer simulations gives rise to three quantitative mobility parameters, i.e.: diffusion coefficient, immobilized fractions, and retention times. These best-fitting simulations suggest the possibility that wtAR is distributed between at least three different fractions (Fig. 4e). Approximately one in four wtAR molecules are engaged in a long immobile fraction (LIF), with a retention time of approximately 12 s. Secondly, half of the AR molecules are involved in short-lived types of binding, which is here referred to as the short immobile fraction (SIF) with an estimated retention time of 0.1 s. The remaining AR molecules are referred to as the mobile fraction and are freely diffusing similar to the DBD mutant. For the Δ629–636 mutant, a similar dual-binding behavior was observed although with different distribution of the molecules across the various fractions (Fig. 4e). For the RK mutant, there appears to be no LIF and only 10% of these receptors are in the SIF. By contrast, the KK mutant curve suggests a complete loss of the SIF and the presence of only 5% of receptors in a LIF. Conversely, mutating the 629RKLKK633 motif results in a dramatic increase of the immobile AR fraction.
Discussion
A role of the hinge region of the steroid receptors in nuclear translocation and DNA binding has been well documented, but its possible implication in the transactivation properties is unclear [11, 22–25]. Deletion of the 629RKLKK633 motif from the AR hinge resulted in a receptor with strongly reduced DNA binding and virtual exclusion from the nucleus, but with an increased transactivation potential [19]. In an effort to explain the apparent contradictions between these data, we dissected this motif and were able to ascribe different functions to different residues.
Nuclear translocation and transactivation
First, we confirmed that the positive residues in the 629RKLKK633 motif are involved in the hormone-induced nuclear translocation and transcriptional activity of the AR (Fig. 1 and Supplementary Fig. 1). Since the deletion of the RKLKKLGN fragment was shown earlier to be sufficient to induce a higher AR activity, we further focused our attention on the positive residues in this part. Mutation of lysine K638 also appears to have a slightly increased activity (Fig. 1a). Since this increase is not statistically significant, the K638 residue was left out of further studies.
R629 and K630 are imperative for the ligand-induced nuclear translocation (Fig. 2c and Table 1), and this is most likely the explanation for the reduced activity displayed by these mutants (Fig. 2a). K632 and K633 act as secondary regulators for nuclear translocation (Fig. 2c). Our data fit with the recently described crystal structure of the AR NLS–importin α complex [13]. Single point mutations within this NLS lower the binding affinity for the importin-α and consequently affect the nuclear translocation.
Table 1.
Summary of the results obtained with wtAR and mutant proteins
| Without SV40-NLS | EGFP-SV40-NLS | ||||
|---|---|---|---|---|---|
| Activity | Localization | DNA binding | Long immobile fraction | Short immobile fraction | |
| wtAR | ↔ | N ≫ C | ↔ | 25% | 50% |
| Δ629–636 | ↑↑↑ | N > C | ↓↓ | ↓ | ↓ |
| RK | ↓ | N < C | ↓↓ | – | ↓↓ |
| KK | ↑↑ | N > C | ↓ | ↓ | – |
| RK + KK | ↓ | N < C | – | ND | ND |
Data for wtAR, Δ629–636, RK, KK, and RK + KK represented in Figs. 2, 3, and 4 and in a previous study [19] are summarized. Transcriptional activity, cellular localization and DNA binding affinity are shown as observed in the absence of the SV40-NLS. Data on the long and short immobile fractions are derived from the FRAP experiments with the EGFP-NLS-fusion proteins
Arrowheadsfacingupwards represent increases, whereas arrowheads facing downwards represent decreases. The number of arrows depicts the relative degree of increase or decrease with regards to wtAR. Horizontal arrows represent behavior similar to wtAR, whereas dashes indicate a loss of the function
LIF long immobile fraction, SIF short immobile fraction, ND not determined
The importance of the positively charged residues in regulating transcriptional activity (Fig. 1a) was confirmed when using double or quadruple substitutions (Fig. 3a). To eliminate the effect of changes in nuclear translocation on the activity of the various AR mutants, they were fused to the SV40-NLS (Fig. 3b). This did not affect the activity of the wtAR and KK proteins, which were already translocated to the nucleus in the absence of the SV40-NLS (Fig. 2c). The Δ629–636 and the RK proteins have an almost exclusive cytoplasmic fraction in the absence of the SV40-NLS (Fig. 2c) [19]. Addition of the NLS facilitates the nuclear translocation of both mutants (Fig. 3a) and the activity of the RK mutant increased substantially (Fig. 3b). On the chromatinized template, but not in transient transfections the fusion of an NLS to the KK mutant lowers the activity. The reason for this remains unknown.
Experiments with EGFP-NLS-RK show that residues R629 and K630 are essential for translocation to the nucleus, but are not necessary for the transactivation properties of the AR. This means that nuclear localization of the AR is required for a full androgen-induced response, but it also means that the 629RKLKK633 motif has an additional separate role in the transactivation mechanism of the AR.
DNA binding and intranuclear mobility
An obvious necessary step in transcriptional activation by the AR via androgen-responsive reporter genes is the requirement for direct binding to DNA. The 629RKLKK633 motif is clearly involved in AR binding to androgen-response elements (AREs) in vitro. It is surprising that all mutant proteins that have a decreased in vitro DNA affinity possess an increased activity compared to wtAR (Table 1). However, when the positively charged residues in the 629RKLKK633 motif are all substituted, the protein is no longer transcriptionally active, even when forced into the nucleus. This is most likely the result of the inability of the RK + KK mutant to bind to AREs (Fig 2b).
To reconcile the strongly reduced in vitro affinity for AREs with the increased transactivation, we propose that the mutant ARs are cycling faster at the androgen-regulated enhancers. The ‘hit-and-run” mechanism is well established now for nuclear receptors such as the glucocorticoid receptor and the estrogen receptor [1, 26, 27]. In this model, the receptor only transiently interacts with the promoter (‘hit’). Other factors are recruited such as chromatin remodeling complexes, histone modifying complexes, and components of the transcription machinery in a time-regulated sequential way [1, 26, 27]. The receptor itself is displaced from the hormone response elements (‘run’). Such faster cycling of ‘hits-and-runs’ might result in a more rapid recruitment and hence cycling of the co-activator complexes. Faster cycling should then lead to an intensified RNA polymerase II recruitment/initiation at the AREs.
In order to study the effects of 629RKLKK633 motif mutations on the in vivo intranuclear mobility, we made use of the FRAP technique (Fig. 4a–d). For this purpose, we developed cell lines (Hep3B) that stably express the EGFP-NLS-AR protein described above. After 1 h of hormone stimulation, all the stably expressed proteins were predominantly localized in the nucleus. In Fig. 4, the FRAP curve for wtAR shows the relatively slow diffusion and secondary redistribution that is indicative for the fact that this protein possesses both a mobile and a transiently immobile fraction. For the inactive AR mutant that lost DNA binding (A573D), only a mobile fraction could be computed. This highly mobile DNA-binding mutant was included as a control in our FRAP experiments. These data are consistent with previous reports [20].
Computational analysis of the experimental data allowed us to demonstrate that the wtAR is distributed between at least three different fractions: LIF, SIF, and freely diffusing fraction (Fig. 4e). The distribution between two immobile fractions has previously been demonstrated for other transcription factors [29]. Also, for the AR Klokk et al. [26] showed two transiently immobile fractions on an array of chromatinized MMTV-LTR promoter. By means of specific AR mutants, we provide the first lines of evidence that AR action is regulated at the subnuclear level by alternate interaction times with subnuclear structures, and that this regulation is maintained by the positively charged residues at the N-terminus of the hinge region (Table 1).
Based on the correlations between DNA binding en FRAP data of the 629RKLKK633 motif mutants, it is very likely that the differences in immobile fractions are explained by changed DNA-binding properties. Loss of DNA binding results in a freely migrating AR. A lower in vitro DNA binding affinity is correlated with a decrease of both types of mobile fractions. We propose a link between the LIF and high affinity for DNA in vitro since the residues R629 and K630 that are involved in strong DNA binding are also essential for the LIF. With the presented data, we cannot exclude which mechanisms are behind the long and short immobile fractions of the AR.
The correlation between increased transcriptional activity and lower DNA binding affinity with shorter residence time in the LIF supports our theory of a faster cycling at the response elements resulting in higher activity (Table 1). One possible hypothesis to explain these apparent contradictions would be what we refer to as the “anchor theory”. In a recently proposed model, a sequence-specific binding protein can diffuse rapidly in the nucleus and bind very shortly non-specifically to chromatin [30]. Alternatively, it can slide along the chromatin fiber until it encounters a specific site, resulting in a more stable binding. In our anchor theory, we propose that the 629RKLKK633 motif functions as a stabilizer for DNA interactions in chromatin. This would explain why the RK and KK mutants have reduced affinity for AREs and display reduced LIF and SIF. One of these fractions could contain for example receptors scanning the chromatin for AREs.
It is indeed tempting to speculate that the immobile fractions represent DNA- or chromatin-binding receptors. However, the AR distribution between, and the composition of, the different immobile fractions might not only be determined by DNA binding. It would be interesting to see what the role of different co-activators in the different immobile fractions is.
Transcriptional productive complexes are rarely formed on promoters [1, 30]. Transcription initiation requires the occurrence of a specific sequence of events. Each such event is the result of many rapid stochastic and transient collisions of factors that are unproductive [1, 30]. Indeed, differences between the two immobile fractions LIF and SIF could be explained by interactions between the AR and co-regulators. The mutations we introduced might have differential effects on hinge-interacting coregulators. Indeed, a large number of co-regulators have been shown to interact with the AR at sites overlapping with the hinge, including coactivators [10, 31–38], but also corepressors [10, 31, 39]. In addition, mutation within the 630KLKK633 motif can alter the binding of the co-regulators p300, HDAC1, and NCoR [10, 31]. The hinge region of the AR can be modified by phosphorylation, acetylation, SUMOylation, neddylation, and ubiquitylation, and these covalent changes affect receptor stability, subcellular localization, and interaction with other proteins [40, 41]. The multitude of possible interaction partners can result in different complexes, each having their own mobility and transactivation potential.
Taken together, the 629RKLKK633 motif in the hinge region of the AR is a multifunctional domain that integrates nuclear localization, receptor stability, DNA binding, transactivating potential, and intranuclear mobility (Table 1). In this study, we define the role of the 629RKLKK633 motif in these processes. Mutations that decrease DNA binding, but conserve activity, show an increased intranuclear mobility. We provide the first lines of evidence that indicate that AR action is regulated at the subnuclear level by alternate interaction times with subnuclear structures, and that the 629RKLKK633 motif in the hinge region plays a crucial role in these processes. At this moment, the characteristics of these different fractions are unknown, but DNA/chromatin interactions rather than coactivator recruitment seem to be a main player.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Functional study of the putative amphipatic helix in the hinge region. (A) A schematic representation of the putative helical structure of the N-terminal part of the hinge region as determined by the structural prediction programme, PredictProtein. (B) Functional analysis of the single asparagine mutants that should disrupt the putative helix. HeLa cells were transfected with the TAT-GRE-luc reporter together with the appropriate AR expression vector. Cells were stimulated for 24 hours with vehicle or 10 nM R1881. Results are shown as induction factors and represent three experiments performed in triplicate, where error bars indicate the SEM. (C) Functional analysis of the proline mutants at the TAT-GRE-luc reporter in HeLa cells (experiments executed as in B above). (D) Expression of the single asparagine mutants (left) and proline point-mutants (right), in HeLa cells after 24 hours stimulation with 10 nM R1881. The expressed proteins were detected using the M2 anti-flag antibody. (PPT 176 kb)
Acknowledgments
This work was supported by grants from the “Bijzonder Onderzoeksfonds K.U.Leuven” (Onderzoekstoelage and CREA/08/031); the Flanders Research Foundation (F.W.O. grant 1.5.065.05); and the Congressionally Directed Medical Research Program (Prostate Cancer Research Program award DAMD17-02-1-0082). C.H. is holder of a F.W.O. Ph.D. fellowship, A.H. is a postdoctoral F.W.O. fellow.
References
- 1.Métivier R, Reid G, Gannon F. Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO reports. 2006;7:161–167. doi: 10.1038/sj.embor.7400626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu P, Liu Y, Shan S, Kong Y, Zhou Q, Li M, Ding J, Xie Y, Wang Y. Molecular mechanism for the potentiation of the transcriptional activity of human liver receptor homolog 1 by steroid receptor coactivator-1. Mol Endocrinol. 2004;18:1887–1905. doi: 10.1210/me.2003-0334. [DOI] [PubMed] [Google Scholar]
- 3.Iordanidou P, Aggelidou E, Demetriades C, Hadzopoulou-Cladaras M. Distinct amino acid residues may be involved in coactivator and ligand interactions in hepatocyte nuclear factor-4α. J Biol Chem. 2005;280:21810–21819. doi: 10.1074/jbc.M501221200. [DOI] [PubMed] [Google Scholar]
- 4.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR-γ-RXR-α nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong W, Baniahmad A, Liu Y, Li H. Bag-1 M is a component of the in vivo DNA-glucocorticoid receptor complex at hormone-regulated promoter. J Mol Biol. 2008;384:22–30. doi: 10.1016/j.jmb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylation its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huq MD, Ha SG, Wei LN. Modulation of retinoic acid receptor alpha activity by lysine methylation in the DNA binding domain. J Proteome Res. 2008;7:4538–4545. doi: 10.1021/pr800375z. [DOI] [PubMed] [Google Scholar]
- 8.Berry NB, Fan M, Nephew KP. Estrogen receptor-α hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol. 2008;22:1535–1551. doi: 10.1210/me.2007-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian K, Jia D, Kapoor-Vazirani P, Powell DR, Collins RE, Sharma D, Peng J, Cheng X, Vertino PM. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Mol Cell. 2008;30:336–347. doi: 10.1016/j.molcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu M, Wang C, Reutens AT, Wang J, Angeletti RH, Siconolfi-Baez L, Ogryzko V, Avantaggiati ML, Pestell RG. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000;275:20853–20860. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- 11.Haelens A, Verrijdt G, Callewaert L, Christiaens V, Schauwaers K, Peeters B, Rombauts W, Claessens F. DNA recognition by the androgen receptor: evidence for an alternative DNA-dependent dimerization, and an active role of sequences flanking the response element on transactivation. Biochem J. 2003;369:141–151. doi: 10.1042/BJ20020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenster G, Trapman J, Brinkmann AO. Nuclear import of the androgen receptor. Biochem J. 1993;293:761–768. doi: 10.1042/bj2930761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutress ML, Withaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–968. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009;284:25576–25584. doi: 10.1074/jbc.M109.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faus H, Haendler B. Androgen receptor acetylation sites differentially regulate gene control. J Cell Biochem. 2008;104:511–524. doi: 10.1002/jcb.21640. [DOI] [PubMed] [Google Scholar]
- 16.Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor domains in AR-mediated signalling. N.R.S. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeb A, Jääskeläinen J, Dattani M, Whitaker C, Costigan C, Hughes IA. A novel mutation in the human androgen receptor suggests a regulatory role for the hinge region in amino-terminal and carboxy-terminal interactions. J Clin Endocrinol Metab. 2008;93:3691–3696. doi: 10.1210/jc.2008-0737. [DOI] [PubMed] [Google Scholar]
- 18.Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F (2010) The rules of DNA recognition by the androgen receptor. Mol Endocrinol (in press) [DOI] [PMC free article] [PubMed]
- 19.Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation and transactivation of the androgen receptor. Cancer Res. 2007;67:4514–4523. doi: 10.1158/0008-5472.CAN-06-1701. [DOI] [PubMed] [Google Scholar]
- 20.van Royen ME, Farla P, Mattern KA, Geverts B, Trapman J, Houtsmuller AB. Fluorescence recovery after photobleaching (FRAP) to study nuclear protein dynamics in living cells. Methods Mol Biol. 2009;464:365–385. doi: 10.1007/978-1-60327-461-6_20. [DOI] [PubMed] [Google Scholar]
- 21.Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guiochon-Mantel A, Lescop P, Christin-Maitre S, Loosfelt H, Perrot-Applanat M, Milgrom E. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO J. 1991;12:3851–3859. doi: 10.1002/j.1460-2075.1991.tb04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mader S, Chambon P, White JH. Defining a minimal estrogen receptor DNA binding domain. Nucleic Acids Res. 1993;21:1125–1132. doi: 10.1093/nar/21.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SA, Lopez GN, Kushner PJ, Pestell RG. Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem. 2001;276:18375–18383. doi: 10.1074/jbc.M100800200. [DOI] [PubMed] [Google Scholar]
- 25.Roemer SC, Donham DC, Sherman L, Pon VH, Edwards DP, Churchill MEA. Structure of the progesterone receptor-DNA complex: novel interactions required for binding to half-site response elements. Mol Endocrinol. 2006;20:3042–3052. doi: 10.1210/me.2005-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klokk TI, Kurys P, Elbi C, Nagaich AK, Hendarwanto A, Slagsvold T, Chang C, Hager GL, Saatcioglu F. Ligand-specific dynamics of the androgen receptor at its response element in living cells. Mol Cell Biol. 2007;27:1823–1843. doi: 10.1128/MCB.01297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George AA, Schiltz RL, Hager GL. Dynamic access of the glucocorticoid receptor to response elements in chromatin. Int J Biochem Cell Biol. 2009;41:214–224. doi: 10.1016/j.biocel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid G, Gallais R, Métivier R. Marking time: the dynamic role of chromatin and covalent modification in transcription. Int J Biochem Cell Biol. 2009;41:155–163. doi: 10.1016/j.biocel.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Phair RD, Scaffidi P, Elbi C, Vecerova J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol Cell Biol. 2004;24:6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinant C, Luijsterburg MS, Höfer T, von Bornstaedt G, Vermeulen W, Houtsmuller AB, van Driel R. Assembly of multiprotein complexes that control genome function. Cell Biol. 2009;185:21–26. doi: 10.1083/jcb.200811080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaughan L, Logan IR, Cook S, Neal DE, Robsonn CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–25913. doi: 10.1074/jbc.M203423200. [DOI] [PubMed] [Google Scholar]
- 32.Poukka H, Aarnisalo P, Karvonen U, Palvimo JJ, Jänne OA. Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription. J Biol Chem. 1999;274:19441–19446. doi: 10.1074/jbc.274.27.19441. [DOI] [PubMed] [Google Scholar]
- 33.Poukka H, Karvonen U, Yoshikawa N, Tanaka H, Palvimo JJ, Jänne OA. The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J Cell Sci. 2000;113:2991–3001. doi: 10.1242/jcs.113.17.2991. [DOI] [PubMed] [Google Scholar]
- 34.Moilanen AM, Karvonen U, Poukka H, Yan W, Toppari J, Jänne OA, Palvimo JJ. A testis-specific androgen receptor coregulator that belongs to a novel family of nuclear proteins. J Biol Chem. 1999;274:3700–3704. doi: 10.1074/jbc.274.6.3700. [DOI] [PubMed] [Google Scholar]
- 35.Link KA, Balasubramaniam S, Sharma A, Comstock CES, Godoy-Tundidor S, Powers N, Cao KH, Haelens A, Claessens F, Revelo MP, Knudsen KE. Targeting the BAF57 SWI/SNF subunit in prostate cancer: a novel platform to control androgen receptor activity. Cancer Res. 2008;68:4551–4558. doi: 10.1158/0008-5472.CAN-07-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reutens AT, Fu M, Wang C, Albanese C, McPhaul MJ, Sun Z, Balk SP, Jänne OA, Palvimo JJ, Pestell RG. Cyclin D1 binds the androgen receptor and regulates hormone-dependent signaling in a p300/CBP-associated factor (P/CAF)-dependent manner. Mol Endocrinol. 2001;15:797–811. doi: 10.1210/me.15.5.797. [DOI] [PubMed] [Google Scholar]
- 37.Verrijdt G, Haelens A, Schoenmakers E, Rombauts W, Claessens F. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocortocoid receptors. Biochem J. 2002;361:97–103. doi: 10.1042/0264-6021:3610097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Léotoing L, Meunier L, Manin M, Mauduit C, Decaussin M, Verrijdt G, Claessens F, Benahmed M, Veyssière G, Morel L, Beaudoin C. Influence of nucleophosmin/B23 on DNA binding and transcriptional activity of the androgen receptor in prostate cancer cell. Oncogene. 2008;27:2858–2867. doi: 10.1038/sj.onc.1210942. [DOI] [PubMed] [Google Scholar]
- 39.Cheng S, Brzostek S, Lee SR, Hollenberg AN, Balk SP. Inhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressor. Mol Endocrinol. 2002;16:1492–1501. doi: 10.1210/me.16.7.1492. [DOI] [PubMed] [Google Scholar]
- 40.Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–528. doi: 10.1016/j.biopha.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 41.Jaworski T. Degradation and beyond: control of androgen receptor activity by the proteasome system. Cell Mol Biol Lett. 2006;11:109–131. doi: 10.2478/s11658-006-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Functional study of the putative amphipatic helix in the hinge region. (A) A schematic representation of the putative helical structure of the N-terminal part of the hinge region as determined by the structural prediction programme, PredictProtein. (B) Functional analysis of the single asparagine mutants that should disrupt the putative helix. HeLa cells were transfected with the TAT-GRE-luc reporter together with the appropriate AR expression vector. Cells were stimulated for 24 hours with vehicle or 10 nM R1881. Results are shown as induction factors and represent three experiments performed in triplicate, where error bars indicate the SEM. (C) Functional analysis of the proline mutants at the TAT-GRE-luc reporter in HeLa cells (experiments executed as in B above). (D) Expression of the single asparagine mutants (left) and proline point-mutants (right), in HeLa cells after 24 hours stimulation with 10 nM R1881. The expressed proteins were detected using the M2 anti-flag antibody. (PPT 176 kb)