Abstract
Several reviews devoted to various aspects of ecdysone research have been published during the last few years. Therefore, this article concentrates mainly on the considerable progress in ecdysone research observed recently, and will cover the results obtained during the last 2 years. The main emphasis is put on the molecular mode of ecdysteroid receptor-mediated hormone action. Two examples of interaction with other hormonal signalling pathways are described, namely crosstalk with juvenile hormone and insulin. Some selected, recently investigated examples of the multitude of hormonal responses are described. Finally, ecological aspects and some practical applications are discussed.
Keywords: Ecdysone, Insulin, Juvenile hormone, Nuclear receptor, Ultraspiracle
Introduction
Ecdysteroids is a generic name for a class of steroid hormones involved in moulting, and in a variety of other processes [1–3] in arthropods. They are present in animals (zooecdysteroids), plants (phytoecdysteroids) and fungi (mycoecdysteroids). In plants, more than 300 ecdysteroids have been described so far, and the number of newly detected ecdysteroid variants is about 10–20 analogues per year with an expectation of about 1,000 compounds in total [4, 5]. The enormous number of analogues makes this class of steroids interesting for chemists. In addition, ecdysteroids are also mandatory regulators in the life of arthropods which have existed for at least 550 million years and belong to the animal class with the highest number of species and individuals. The ecdysteroid regulatory circuit is thus an evolutionary successful system, and basic principles of steroid hormone action and sequential gene expression were first described in studies with dipteran flies. This is still of special importance since Drosophila is becoming an “inclusive model for human diseases, growth and development” [6] and “diabetic larvae and obese flies” are considered to provide new insights into the causes and treatments of human metabolic disorders [7].
Besides basic biological questions like hormonal regulation of metabolism and development, applied aspects of ecdysteroid research are becoming increasingly important. Interference with ecdysteroid-controlled processes like moulting and metamorphosis is a successfully used target for the development of insecticides with low vertebrate toxicity [8]. The ecdysteroid receptor is used as a gene switch in mammals and plants [9] and for gene knockdown by ecdysone-based inducible RNAi in stably transfected mammalian cell lines [10]. Ecdysteroids or ecdysteroid receptor agonists have also been used in humans, because of their pharmacological actions combined with low toxicity [4, 11].
Since ecdysteroid-controlled development in insects and the mode of action of ecdysteroids have been treated in detail in recent books [1, 2], we will focus in this review on recent developments, and point out which questions we consider to be important in the near future for ecdysteroid research.
Hormone system
Hormones are typically synthesized in a hormone gland, and transported in the blood or hemolymph to the target tissue, where the biological response is elicited. Various positive and negative feedback loops either reinforce the hormonal signal or prevent overstimulation and cause termination of the hormone response. Crosstalk with other signalling pathways allows adaptation of the hormone response to the physiological requirements of the target tissues.
Biosynthesis
Arthropods cannot synthesize cholesterol de novo from acetate and are thus dependent on cholesterol or several other side-chain alkylated plant sterols as precursors for their ecdysteroid synthesis. During the last 5 years, enormous progress has been made in elucidating the scheme of biosynthesis of ecdysteroids in Drosophila by detailed investigation of the long-known mid-embryonic mutants of Drosophila. These mutants, belonging to the “Halloween-Family”, were all characterized by disturbances in the formation of the first instar cuticle. At least one of these mutants also exhibits low ecdysteroid titre, suggesting that these genes might be involved in insect ecdysteroidogenesis. It finally turned out that all five “Halloween” genes code for cytochrome P450 enzymes [12], indicating that modification of steroidal compounds is exerted by the same class of enzymes in insects and vertebrates. Another similarity to vertebrates is the transport of cholesterol to the hormone-producing cells by a lipoprotein comparable to LDL [13]. The intracellular sterol trafficking system is also similar in both phyla: Drosophila-like, the vertebrate system contains two NPC genes, which are involved in the Niemann Pick type C disease in humans due to an insufficient transport of cholesterol out of the endosomal compartment, leading to an intracellular enrichment of cholesterol and finally to a neurodegenerative disease. In Drosophila, NPC mutations cause intracellular enrichment of cholesterol, reduced ecdysteroidogenesis and finally death in the first larval instar. If an excess of cholesterol is given to these mutants, the phenotype can be fully rescued, which indicates that the biosynthetic pathway leading to ecdysone is intact [12].
The significance of the neurosecretory prothoracicotropic hormone (PTTH) for regulation of ecdysteroid synthesis is well established, but the PTTH signal transduction pathway is not yet fully known. Many components were shown to be involved like phospholipase C, protein kinases A and C, adenylyl cyclase, the ribosomal protein S6 and the small G protein Ras and calcium, but the exact interplay between these intracellular substances is not known with certainty [12].
Transport of ecdysteroids
In vertebrates, binding of steroid and thyroid hormones to specific and non-specific binding proteins in the blood is an important step for regulation of hormone availability. It allows the transport of weakly water soluble hormones, serves as a reservoir, increases half-life of the hormone and prevents unspecific adsorption to non-target tissues. Up to 99% of the totally circulating hormone is bound to carrier proteins in case of testosterone or T3/4. In arthropods, the situation is less well investigated. Binding of ecdysteroids to hemolymph proteins has been demonstrated in crustaceans and insects [14]. In the tarantula Eurypelma californicum, ecdysteroids are bound to hemocyanin [15]. Up to now, nothing is known about the biological relevance of carrier-bound ecdysteroids. Since ecdysteroids are much more water soluble than vertebrate steroid hormones due to the greater number of hydroxy groups, binding of ecdysteroids to hemolymph proteins might be less important.
Target cells
Target cells for a hormone are characterized by the presence of their corresponding receptors. Since ecdysteroids affect arthropod life from early embryogenesis to reproduction and adult life, it is not surprising that practically every organ is a target organ for ecdysteroids [16].
The ecdysteroid signalling cascade
Insect ecdysis is controlled by an endocrine cascade, which coordinates behaviour and physiological changes as described by [17], initiated by two steps: ecdysteroid-induced expression of receptors and transcription factors in the CNS, and also in Inka cells. Subsequently, peptide hormones and multiple central neuropeptides are released to control consecutive phases of the ecdysis process [18]. The importance of peptide hormone in insect development was pointed out recently [19]. Therefore, we will concentrate on the ecdysone receptor-mediated signal transduction during insect development.
The ecdysteroid receptor, a heterodimer of EcR and ultraspiracle
Generally, the heterodimer of the nuclear receptors ecdysteroid receptor (EcR) and ultraspiracle (Usp), an orthologue of the vertebrate retinoid X receptor (RXR), is considered as the functional ecdysteroid receptor [16, 20, 21], which mediates the hormonal response to coordinate the transcription of a multitude of genes during moulting and development, although there is increasing evidence that each partner also mediates additional functions on its own or with different partners.
The ecdysteroid receptor
Since ligand affinity to cell extracts containing only Drosophila EcR is about 90-fold lower compared to heterodimers with Usp, the biological significance of hormone binding of unpartnered EcR was often considered to be negligible, even though ligand induced changes in receptor function in the absence of a heterodimerization partner had been demonstrated, e.g. enhanced interaction with DNA [22] and chromatin [23] or shuttling of the ecdysteroid receptor into the nucleus [24]. Muristerone A stimulates transcriptional activity in vertebrate cells devoid of endogenous EcR and Usp, which are transfected with dmEcR to different degrees depending on the isoform used [25, 26]. This is not due to interaction with endogenous RXR, since the subclone of CHO-K1 used expressed only negligible amounts of RXR [27]. Recently [28], an ecdysone-mediated induction of genes of the broad complex (BRC) was shown, necessary for the expression of glue genes midway through the third instar, occurring without the participation of Usp or any other RXR like molecule.
In some species like Drosophila melanogaster [29] and Leptinotarsa decemlineata [30], EcR in the absence of Usp binds the ecdysteroid ponasterone A specifically, although with lower ligand affinity than the heterodimeric EcR [29]. Ligand binding of Liocheles australasiae EcR is not enhanced further by RXR, although RXR is necessary for binding of the receptor complex to ecdysone response elements [31].
Ultraspiracle
Ultraspiracle plays a central role as ubiquitous heterodimerization partner of many nuclear receptors. In heterometabolous insects like Locusta migratoria, this transcription factor is more similar to human RXR. LmRXR was detected in early embryos, when EcR transcripts were absent, suggesting an additional role apart from ecdysone signalling [32]. Phylogenetic analysis suggests that RXR–USPs have undergone remarkable functional shifts during evolution especially in the ligand binding domain of Usp–RXR [33]. Whereas the ability of Usp to bind ligands has changed considerably, the role of Usps and RXRs as heterodimerization partner has been retained throughout evolution.
Ligand binding
The X-ray structures of the ligand binding pockets of EcR and Usp from various species are elucidated as summarized in [34]. As shown for vertebrate nuclear receptors, the ligand binding pocket consists of 11–12 helices arranged as an anti-parallel α-helical sandwich. Helix 12 of EcR, which harbours a ligand-dependent activation function stimulating transcriptional activity of the receptor complex, changes its position upon ligand binding. This altered (agonistic) position is a stabilized salt bridge between helix 4 and helix 12, thus allowing interaction with coactivators. Whereas these general features are common to all ecdysteroid receptors analyzed so far, there is a pronounced difference in binding affinities to various ecdysteroids besides 20-OH-ecdysone in the receptors of different origin [34]. EcR is described as a “remarkable protein which can adapt its binding pocket to very different ligand chemistries” [34]. This is not so surprising since some insects use moulting hormones other than 20-OH-ecdysone, mainly due to the fact that different precursors are used [35].
In the absence of DNA, ecdysteroid binding is essentially the same for heterodimers with all EcR isoforms, but in the presence of hormone response elements, ligand ecdysteroid binding is increased [36]. Whereas the impact of the ligand binding domain (LBD) especially in the presence of hormone on DNA binding of EcR and EcR/Usp is reported several times ([25, 37]; Braun and Spindler-Barth, unpublished results), these data support the hypothesis that DNA binding also modulates the functionality of the ligand binding domain. Recently, X-ray data of intact PPAR and RXR bound as heterodimer to DNA revealed that the PPAR–LBD is tightly coupled to other receptor segments, e.g. the DBDs, thus cooperating with the DNA binding domains of both receptor molecules to enhance response element binding. Whereas the RXR–LBD forms no contacts within the receptor complex outside the PPAR–LBD, the PPAR–LBD also contacts the DBD’s of both receptors, thus allowing modulation of ligand binding activity. In addition, Chandra et al. [38] report that the PPAR–LBD directly contacts DNA. Since the 3D-architecture of nuclear receptors is rather similar despite considerable differences in the amino acid sequence, these structural data support the hypothesis that ligand binding properties of EcR/Usp can be modulated by hormone response elements and explain the intense interdomain signalling.
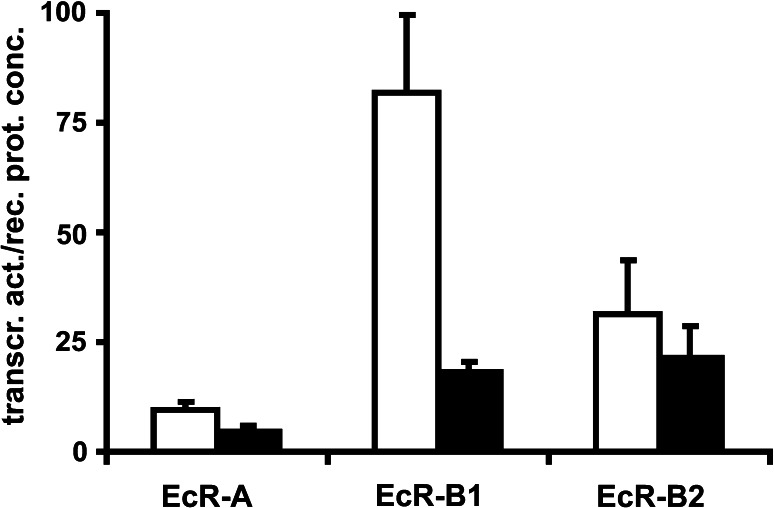
The existence and necessity of specific endogenous ligands for RXR–USP is still a matter of debate. In vertebrates, a wide range of ligands can activate RXRs. The presence of retinoic acid in sufficiently high amounts in embryos and its high affinity (3 nM) to LmRXR argues in favour of a functional role in locusts [32]. Jones et al. [39] report that Usp binds with nanomolar affinity the endogenous ligand methyl farnesoate (K D = 40 nM) in D. melanogaster. However, the crystal structure of the ligand-binding domain of Usp from Tribolium castaneum (TcUSP) revealed a ligand-independent functional conformation [40], if partnered with EcR. This might indicate either that in this species Usp has lost its ligand binding capability or that EcR negatively affects ligand binding to Usp. The ligand binding pocket of TcUSP is representative of most arthropod RXR–Usps, with high sequence homology to vertebrate/mollusc RXRs. It has a ligand-independent functional conformation adopting an apo structure. Functional data demonstrate that TcUsp is a constitutively silent partner of EcR, and that none of the RXR ligands binds or activates TcUsp [40]. In contrast, helix 12 of dmUsp [41] and Heliothis virescens Usp [42] are fixed in an antagonistic position, even in the absence of a specific ligand. This unusual position is of functional importance, since mutation of amino acids, e.g. L259, involved in fixation of helix 12 by interacting with the loop between helix 1 and 3 [41] impairs basal transcriptional activity of the EcR/Usp expressed in a heterlogeous system (Fig. 1) in an EcR-isoform-specific manner, whereas ecdysone induced stimulation of transcriptional activity is not affected (data not shown).
Fig. 1.
Transcriptional activity of EcR/Usp in CHO-K1 cells. Heterodimers of EcR isoforms with wild-type and mutated Usps are compared. L259 is located in the loop between helix 1 and helix 3. This amino acid is involved in fixation of helix 12 in the antagonistic position according to Clayton et al. [40]
The ligand binding pocket of Usp from Heliothis and Drosophila is filled by a phospholipid. Mutational analysis revealed that amino acids involved in phospholipid binding and amino acids considered as putative juvenile hormone binding sites are important for receptor function [43].
The Heterodimer EcR/Usp
The heterodimer EcR/Usp is still considered as the main functional ecdysteroid receptor. Expression of EcR and Usp can be regulated by the cognate ligand itself, an observation also known from vertebrate steroid receptors. This is achieved by downregulation of miR-14, which is involved in expressing EcR [44]. The importance of limiting EcR levels is demonstrated by many defects observed in miR-14 mutants. Concentration of different isoforms may deviate due to differences in synthesis, different promotors or altered stability of isoforms [26, 45], leading to variation in receptor protein concentrations of individual isoforms. Therefore, a comparison of isoform-specific activities like DNA binding or transcriptional activity based on determination of transfection efficiency by transfection of a constitutively expressed reporter is not suitable, but instead a careful quantification of functional receptor proteins by ligand binding or western blots is required [46].
In many insect species, EcR and Usp are present in various isoforms differing in their AB-domains, which harbour a ligand-independent transactivation domain AF-1, although in D. melanogaster only one Usp type is found. The isoforms are expressed in a tissue- and developmental stage-specific manner. They are involved in different pathways and cannot replace each other, although some functional redundancy is also observed [47].
Dimerization of EcR and Usp is mediated by at least two different dimerization interfaces: one in the C-domain, which is dependent on the presence of DNA, and a second one in the ligand binding domain, which is reinforced by the presence of hormone. Graham et al. [48] showed that the DE/F segment pairs of EcR and Usp in four insect species (Lucilia cuprina, Myzus persicae, Bemisia tabaci, Helicoverpa armigera) associated spontaneously with high affinity to form heterodimers already in the absence of ligand, which subsequently avidly bind an ecdysteroid ligand. This demonstrates that ligands are not essential for the formation of tightly associated and functional LBD heterodimers in these species [48, 49].
Intracellular localization of EcR and Usp
Intracellular localization and its regulation have been reviewed recently [50]. If expressed separately, Usp and EcR are able to form nuclear complexes in the absence of the cognate dimerization partner [51]. Nuclear import of separately expressed EcR is increased in the presence of hormone [24], although sub-cellular localisation is not significantly changed [51]. This is presumably due to the efficient export mechanism [52]. Both import and export are energy dependent and are mediated by Ran and CRM-1, respectively. Intracellular localisation of EcR is regulated mainly by leptomycine-sensitive export, which is impaired by dimerisation, rendering the nuclear export signal located in the ligand binding domain of EcR inaccessible [52].
Interaction of the receptor complex with DNA
The structure of the DNA-binding heterodimeric complex with the hormone, response element hsp27 has been elucidated in great detail [53] and the dissociation constants (nMolar range) determined [54, 55]. In addition to the C-domain, the extension at the C-terminus of the DNA-binding domain is also involved in the interaction with DNA [56]. A remarkable mutational tolerance between the EcR–DBD mutants and the wild-type Usp DNA-binding domain is observed. The EcR–DBD, characterised by a low alpha-helix content and low stability, shows a high degree of intramolecular plasticity [57], which may represent the molecular basis for interaction with a variety of hormone response elements. Ultraspiracle seems to play a dominant role in the interaction of the receptor complex with DNA. It determines the polarity of the DNA–receptor complex directing the Usp–DBD to the 5′ half site of the hormone response element hsp27 [58]. The DNA-binding domain of ultraspiracle causes a deformation of the response element, whereas the C-domain of the ecdysone receptor adds only a slight change to the preformed structure [59]. Nuclear localisation is not necessarily associated with DNA binding [23], which is stimulated by hormone [24]. This is confirmed by EMSA experiments: they revealed weak interaction with DNA, which is reinforced by addition of hormone. EcR isoforms already interact with DNA to various extents in the absence of ultraspiracle [22]. This interaction is enhanced in the presence of the Usp–DBD [22, 25]. The intensity of DNA binding does not parallel with transcriptional activity, an indication that transactivation potency is regulated by interaction with comodulators.
Transcriptional activity of the ecdysteroid receptor
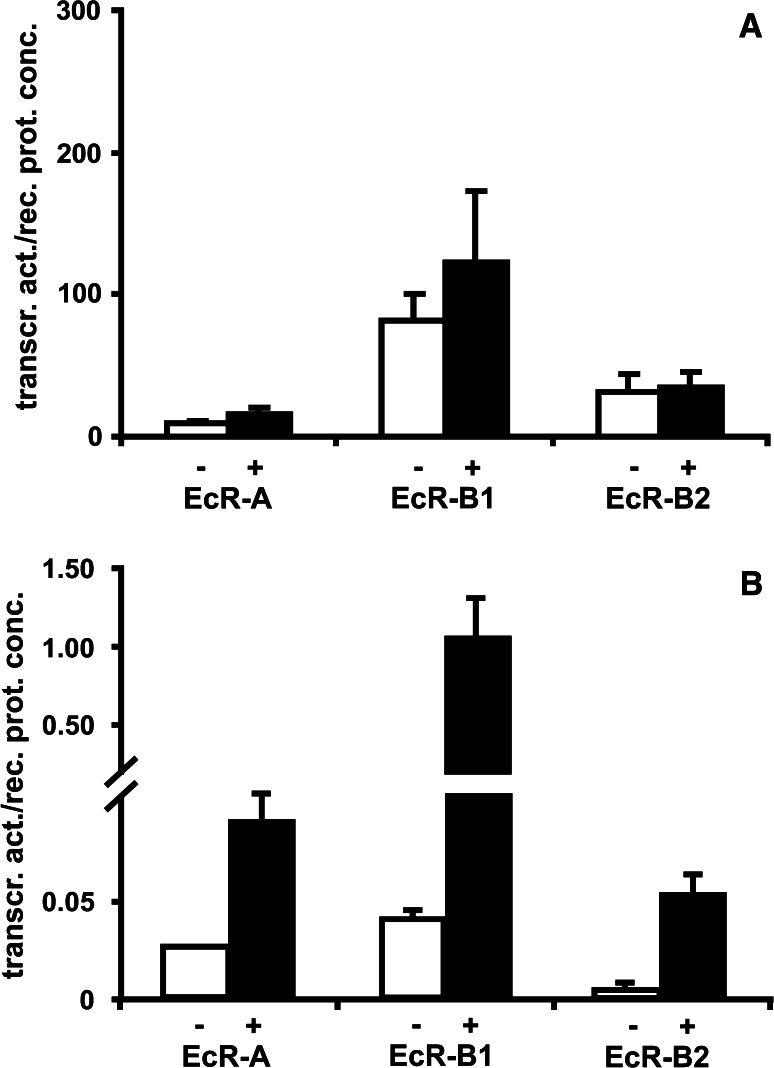
Heterologeous expression of EcR and Usp in vertebrate cells is considered a suitable model to study transcriptional activity of the receptor complex [43]. Since vertebrate cells are devoid of endogenous EcR and some subclones of cell lines, e.g. CHO-K1 cells, express only negligible amounts of RXR, this model allows one to study transcriptional activity of EcR and Usp separately [26, 46]. In this experimental setting, constitutive as well as hormone-induced transcriptional activity of EcR isoforms are different and are considerably enhanced in the presence of Usp. In vertebrate cells, transcriptional activity of heterodimers with wild-type Usp is rather low. Therefore, the original AB-domain is replaced routinely by the AD-domain of the transcription factor Vp16 [20]. As shown in Fig. 2, the inhibitory action of the original AB-domain of wild-type Usp in combination with hormone and EcR is restricted to vertebrate cells and is not observed in Drosophila cell cultures (S2). Moreover, the hormone-induced stimulation of transcriptional activity is much more pronounced and differs considerably for each isoform (A:B1:B2 = 8:26:9). For these experiments, a S2-sublcone devoid of endogenously expressed EcR, but with a high endogenous Usp level (Hönl, unpublished), was used. This way, the transcriptional activity of the heterodimers comprising the various EcR isoforms could be compared in an insect-specific environment. In vertebrate cells, basal transcriptional activity of the heterodimer is above background already in the absence of hormone [26]. However, repression by the non-liganded heterodimer is observed in insect cells [60].
Fig. 2.
Transcriptional activity of EcR isoforms partnered with wild-type Usp. a CHO-K1 cells. b Insect (S2, Drosophila) cells. Transcriptional activity was determined by a luciferase reporter and normalised on EcR concentration as described in Ruff et al. [26]. White bars without hormone, black bars with 1μM muristerone A
These data show that the cellular context plays an important role in regulating the transcriptional activity. Hence, the results obtained in a given experimental setting must be interpretated cautiously, and the modulatory action of cell specific comodulators has also to be taken into account.
EcR isoforms and interdomain signalling
Independent of the cellular context, several conclusions concerning the functionality of receptor complexes are evident. The differences in DNA-binding of EcR isoforms and the corresponding EcR/Usp receptor complexes [22, 25] clearly indicate an interaction between the DNA-binding domain and the AB-domains of EcR. From vertebrate nuclear receptors, it is known that the functional 3-D structure of the AB-domain is formed only in contact with the DNA binding domain [61].
Since not only basal but also hormone-induced stimulation of transcriptional activity varies between different EcR isoforms, an interaction between the N-terminus and the ligand binding domain of EcR seems mandatory. This is confirmed by FRET analysis of EcR domains (Tremmel and Schäfer, unpublished results). A mutual influence of the C- and E-domains of EcR is also observed. A close contact of LBD and DBD is documented for the vertebrate nuclear receptor PPAR [38]. As mentioned above, ecdysteroids increase DNA-binding [22, 25], but in the presence of hormone response elements ligand binding is also enhanced. This is certainly partially due to improved dimerisation, but ligand affinity is additionally altered as mentioned above [36].
Influence of comodulators
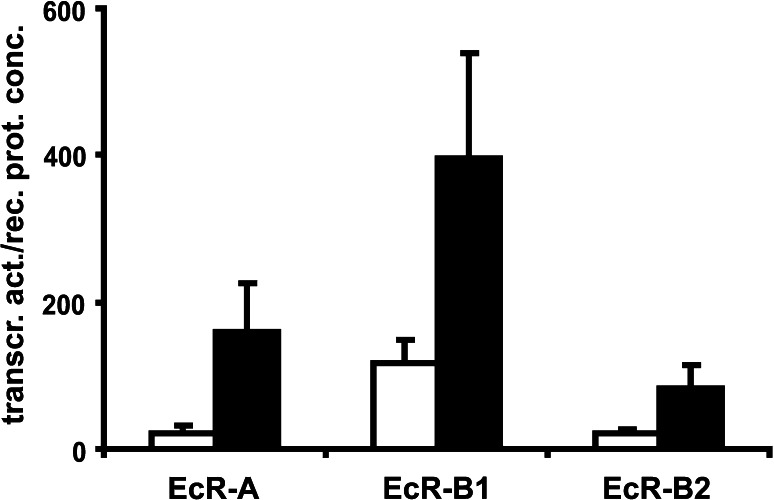
Hormone agonists induce a conformational change in the carboxy-terminal transcriptional activation domain, AF-2, that creates a new interaction site on the surface of the LBD that is recognised by LXXLL motifs in the p160 family of coactivators. Thus, the cellular availability of coactivators and corepressors is an important determinant in the biological response to steroid hormones. As shown in Fig. 2, the transcriptional activity especially the hormone-induced stimulation differs considerably depending on the cell type. Since transcriptional activity is normalised on receptor concentration, it is reasonable to assume that differences in the cellular milieu are responsible for the variation in transcriptional activity between insect and vertebrate cells. Interaction with copressors seems likely since mutation of the amino acid lys497 [25] localised in a conserved comodulator binding site of the ligand binding domain [62, 63] increases transcriptional activity in an isoform-specific manner as also shown in Fig. 3.
Fig. 3.
Basal transcriptional activity of EcRK497E isoforms in the absence of Usp in CHO-K1 cells. Transcriptional activity was determined by a luciferase reporter and normalised on EcR concentration as described in [26]. White bars without hormone, black bars with 1μM muristerone A
Dressel et al. [64] described specific interaction of the corepressor Alien with Drosophila nuclear hormone receptors, such as the ecdysone receptor and Seven-up, the Drosophila homologue of COUP-TF1, but not with RXR, USP, DHR 3, DHR 38, DHR 78, or DHR 96. Meanwhile, additional comodulators of EcR are described, e.g. the Drosophila JIL-1 kinase, known to phosphorylate histone H3 at Ser10, which is associated with transcriptional activation. JIL-1 is involved in early elongation of a broad range of genes, and is considered as a hallmark of early transcription elongation in Drosophila [65]. A Drosophila arginine methyltransferase1 (DART1) acts as a co-repressor of EcR [66], and the expression of FTZ-F1 is controlled by the corepressor B lymphocyte-induced maturation protein 1 (dBlimp-1) [67]. β-catenin reduces basal and hormone-induced transcriptional activity of all ecdysone receptor isoforms (Ruff, unpublished observation). Currently, it is not known whether this is due to direct physical contact with the ligand binding domain as reported for AR [68] or whether this is caused by an alternative crosstalk with the WNT-pathway.
Several coactivators are described in Drosophila: p160 class coactivators associate with histone acetyltransferases and arginine histone methyltransferases. For example, TRR is a histone methyltransferase capable of trimethylating lysine 4 of histone H3, is required for retinal differentiation. EcR and TRR can be co-immunoprecipitated upon ecdysone treatment and alter the chromatin structure at ecdysone-responsive promoters [69]. Interaction between betaFtz-F1 and the p160/SRC coactivator of the ecdysone receptor, FISC, is crucial for the stage-specific expression of the 20E effector genes and causes enhanced local histone H4 acetylation activation of target genes [70]. The multi-catalytic histone methyltransferase dG9a with specificity for lysines 9 and 27 on H3 and H4 is also involved in ecdysone regulatory pathways [71]. The importance of the chromatin architecture and its remodelling is demonstrated by the influence of the nucleosome remodelling factor (NURF), interacting with EcR in an ecdysteroid-dependent manner [72], and also by HmgD, a homologue of high mobility group proteins [73]. Both are essential for normal Drosophila development by affecting the ecdysone-induced signalling cascade during metamorphosis.
Membrane-bound ecdysteroid receptor
In addition to the “classical” genomic action of steroid hormones, they can also exert rapid effects within seconds. These so-called non-genomic actions are also reported for ecdysteroids [74–76]. Non-genomic action of ecdysteroids mediated by a membrane-bound receptor [77] has been implicated in several 20E-dependent events including the programmed cell death of Bombyx anterior silk glands. The level of cAMP increases within 30 s after hormonal stimulation [78], which rules out signal transduction by a nuclear receptor. A membrane-bound G-protein-coupled receptor, responsible for ecdysteroid- and catecholamine-induced effects in Drosophila, was described recently [79].
Ecdysone-induced effects
Amongst the multitude of ecdysteroid-induced effects during development only some examples can be given.
Influence of NFκB on immune response and differentiation
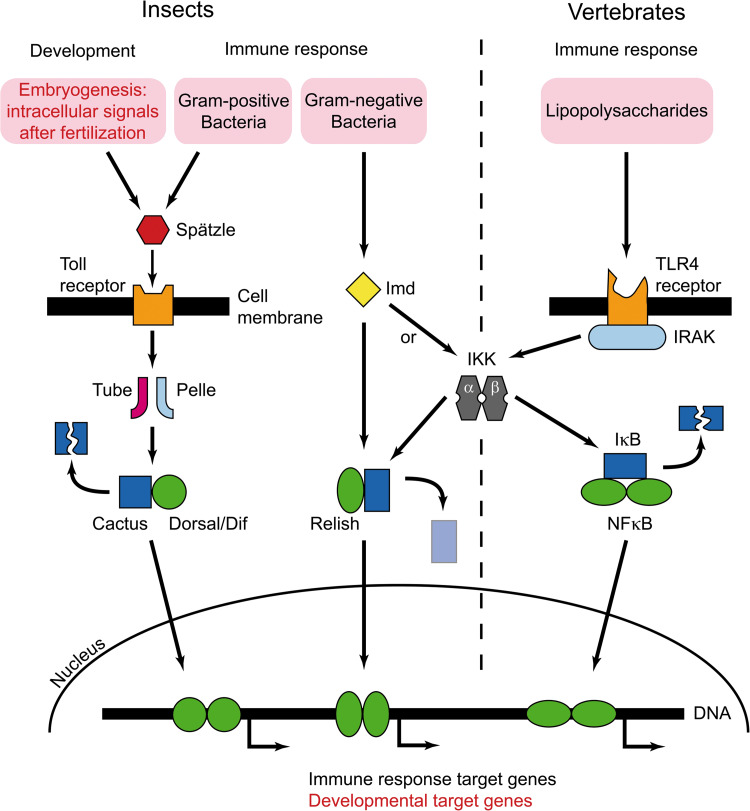
In contrast to mammals, the Toll signalling cascade is involved not only in innate immunity but also in regulation of developmental processes in insects (Fig. 4). Dorsal, the insect homologue of NFκB, mediates defence mechanisms against pathogens in insects [80] but also participates in morphogenetic events, e.g. in establishing the dorsal–ventral axis during embryonic development [81]. Therefore, it was hypothesised that ecdysone may also regulate the Toll pathway. Interaction of NFκB with vertebrate nuclear receptors is well characterised. In fact, intracellular localisation and the level of expressed dorsal protein are modulated by ecdysone (Hönl, unpublished observation).
Fig. 4.
Scheme of Toll signalling pathways involved in regulation of insect development and innate immunity in insects and vertebrates (modified according to Kimbrell and Beutler [146]). Dif Dorsal-related immunity factor, Imd immune deficiency, TLR4 Toll-like receptor 4, IRAK interleukin-1 receptor-associated kinase, homologue to Drosophila Pelle, IκB inhibitor of κB, IKK IκB kinase
According to [80], JH and 20E have antagonistic effects on the induction of antimicrobial peptide (AMP) by NFκB homologues. JH III and JH analogs interfere with 20E-dependent immune potentiation, although these hormones did not inhibit other 20E-induced cellular changes. EcR and Usp are both necessary to mediate this ecdysone-triggered response, whereas silencing methoprene-tolerant (Met) did not impair immuno-suppression by JH III indicating that in this context MET is not a necessary JH receptor.
Weight and growth control in insects
In insects, growth occurs during nymphal or larval life. The timing of moulting and metamorphosis is coordinated by a rise in the titre of ecdysteroids. With the last moult, the adult size is reached. Body size is intimately linked to nutritional, environmental and genetic cues. Three essential checkpoints must be passed: the “threshold size for metamorphosis” (in Drosophila the late second instar), the “minimal viable weight” necessary for a larva to survive metamorphosis, and finally the “critical weight”, where starvation no longer affects the time to pupation. These phases of weight and size control have been elucidated by feeding experiments and variation of environmental factors [82], which revealed a combination of nutritional and environmental determinants guiding insect development.
In the last few years, mechanisms of these regulatory steps have been found, and at least two players are of utmost importance, the prothoracic gland (PG) and its stimulating hormone (PTTH) and the insulin signalling pathway. There is an intense crosstalk between the ecdysteroid and insulin signalling pathways [83]. If the growth of the Drosophila PG was suppressed by GAL4 lines targeted to the ring gland, non-viable larvae were attained. Surprisingly, larger PGs result in smaller larvae and adults. Cell growth of the PG is stimulated by insulin [84]). Ablation of PTTH-producing neurons, which innervate the PG, results in a delayed larval development and eclosion of larger flies with more cells as measured in the wing gland. Although PTTH controls the assessment of a critical weight in Drosophila, it is not essential for viability [85]. Insulin stimulates insect growth by a signalling cascade very similar to that of mammals. The seven Drosophila insulin-like peptides (DILPs) are synthesized in various organs including the brain. Expression of two DILPs is downregulated by starvation. Ablation of DILP-producing neurosecretory cells leads to a severe reduction in size [82]. Negative regulators of growth are ecdysteroids [80] and under certain circumstances also juvenile hormones [86], which usually tend to be a “status quo hormone” [1]. The exact interplay between the insulin-signalling pathway and ecdysteroids is not yet known, one possibility being a direct effect of insulin on ecdysteroidogenesis [82]. The complexity of growth regulation in insects is underlined by the recent finding that serotonergic neurons control adult Drosophila body size by regulating the insulin/IGF pathway in peripheral tissues [87]. An antagonistic action has also been demonstrated in a lepidopteran cell line where insulin promotes cell growth and ecdysteroids inhibit it [88].
Ecdysteroids and cell cycle
An influence of the cell cycle on nuclear receptors is well documented in vertebrates and seems to be different for individual receptor proteins. Variation of receptor concentration during the cell cycle as well as cell cycle-dependent regulation of transcriptional activity has been demonstrated for many vertebrate nuclear receptors [89–91]. Cyclin D1, a key regulator of the cell cycle, has quite different effects on nuclear receptors. For example, cyclin D1 activates transcriptional activity of ER by coactivator recruitment [92], whereas in the case of the androgen receptor, cyclin D1 acts as a strong corepressor [93]. In arthropods, ecdysteroids can either stimulate cell proliferation or initiate cell differentiation [94–96] depending on the level of moulting hormones. This is accompanied by an arrest either at G2 [95, 97, 98] or at G1/S [99]. Periodic expression of EcR and Usp during the cell cycle and their impact on regulation of cell proliferation was reported recently [100–102]. EcR, but not Usp, reduces cyclin D1 expression. Co-immunoprecipitation studies also revealed a direct physical interaction of EcR and cyclin D1 [102]. In Plodia interpunctella, it was clearly shown by RNAi techniques that the ecdysteroid-regulated cellular differentiation and proliferation is mediated by a genomic signalling pathway involving EcR, Usp and HR3 [103].
Interaction of moulting hormone and juvenile hormone
Ecdysone and JH are involved in regulating insect development and metamorphosis. We will describe only one example, namely remodelling of the gut during metamorphosis, to demonstrate the mutual interaction between both hormones. Mid-gut remodelling involves two processes: programmed cell death of larval cells, and proliferation and differentiation of imaginal cells in the formation of the pupal/adult mid-gut. As described in Aedes aegypti, 20-OH-ecdysone (20E) coordinates this process through stage- and cell-specific expression of nuclear receptor isoforms. EcR-B/USP-A is important for programmed cell death of larval cells whereas EcR-A/USP-B is involved in formation of pupal/adult mid-gut [104]. In Aedes, JH agonists seem to uncouple mid-gut remodelling from metamorphosis, whereas in Heliothis, JH affects both mid-gut remodelling and larval–pupal metamorphosis [105]. Methoprene treatment of Aedes blocks ecdysone-induced proliferation and differentiation of imaginal cells and programmed cell death by modulating the expression of ecdysone receptor B, ultraspiracle A, broad complex, E93, ftz-f1, dronc and drice, genes known to be involved in 20-OH-ecdysone action [106].
In T. castaneum, 20-OH-ecdysone induces cell proliferation of intestinal stem cells in the absence of JH, this being selectively mediated by one isoform, in this case EcR-A/Usp but not EcR-B [94]. The transcription factor Broad mediates 20-OH-ecdysone action and prevents an increase in the mRNA levels of JH-response genes (JHE and Kr-h1b) [107]. On the other hand, JH prevents premature development of adult structures during larval–pupal metamorphosis and this JH action seems to be meditated by the bHLH-PAS family transcription factor methoprene-tolerant [108]. These examples revealed considerable species-specific differences in the molecular mode of interaction of these two hormones, demonstrating that generalisations about the mode of action of insect hormones should be made only with caution. There are also some indications showing that JH action may be mediated by diverse molecular mechanisms.
There is some experimental evidence that Usp may be involved in mediating JH action, thus integrating the signaling pathways of the two morphogenetic hormones [109]. Usp participates in regulation of pupal development in Apis mellifera, e.g. the expression of regulatory ftz transcription factor 1 (ftz-f1) and juvenile hormone esterase. In contrast, vitellogenin expression is not controlled by Usp in the honey bee, although it is regulated by JH during the last stages of pupal development, indicating that in this case JH hormone action is mediated by different signalling pathways independent of Usp [110]. In T. castaneum, JH action seems to be mediated by met protein [111, 112]. In Drosophila, the situation is more complex, because larval–pupal transition does not require the absence of JH. Li et al. [113] recently identified two proteins (FKB 39 and Chd64) which interact with JH response elements EcR, Usp and met protein, suggesting participation of these proteins in crosstalk between JH and ecdysteroids in Drosophila. Jones et al. [39] claim that nMolar concentrations of JH bind to Usp of Drosophila and locusts [32]. The data presented by [114] also suggest that juvenile hormones serve as Usp ligands that antagonise EcR-mediated ecdysone actions through the recruitment of histone deacetylase complexes. Henrich et al. [115] report that juvenile hormone potentiates ecdysone mediated transcriptional activity in vertebrate cells by an as yet unknown mechanism.
Ecological aspects
Endocrine disruptors of vertebrate steroid hormone action seem to also interfere with moulting hormone signalling; e.g. bisphenol A (BPA) increases the mRNA level of the EcR in Chironomus riparius suggesting a common way of BPA action, shared by vertebrates and invertebrates [116]. Exposure of the crab Carcinus maenas to 4-nonylphenol (L(-1) 4-NP) resulted in a reduced testis weight, increased liver weight and altered levels of ecdysone equivalents [117]. Xenobiotics can also modify the expression of Drosophila CYP genes encoding cytochromes P450, which are associated with detoxification. Xenobiotic-induced changes in P450 levels can affect insect fitness by interfering with hormonally regulated networks. Moreover, xenobiotic inducibility of some CYP genes is associated with insecticide resistance in laboratory-selected strains [118].
It has recently been shown that in Drosophila about 1,000 transcripts are significantly affected by phenobarbital treatment. This response is mediated by the Drosophila ortholog of the human SXR and CAR xenobiotic receptors, DHR96. It was also demonstrated that DHR96 null mutants displayed increased sensitivity to the sedative effects of phenobarbital and the pesticide DDT as well as defects in the expression of many phenobarbital-regulated genes. Metabolic and stress-response genes are also controlled by DHR96, implicating its role in coordinating multiple response pathways [119].
Practical applications of ecdysteroids
The importance of the ecdysteroid receptor as a target for application in agriculture and medicine has been previously summarised [8]. In addition, in two recent reviews, the impact of phytoecdysteroids on humans is summarised [120, 121]. We therefore concentrate on recent developments.
Ecdysteroid mimicks as pesticides
Diacylhydrazines have been used as pesticides for about 20 years and are considered as environmentally friendly compounds with low vertebrate toxicity [122]. Ecdysone agonists can even be used if beneficial insects like bumblebees are present simultaneously [123]. The search for suitable new diacylhadrazine derivatives to increase the toxic potential and the selectivity for certain pest organisms important for agriculture gave rise to the development of tests for mass screening [124]. This led to improvements of analytical tools for identifying additional pytoecdysones, some of which may represent interesting leads for the development of novel pesticides [125]. X-ray structures of the ligand-binding domain of EcR from various insect pests were elucidated [126] in order to identify differences in the ligand-binding pocket, providing a molecular basis for the selectivity of diacylhydrazine insecticides, while computational modelling of receptor ligand interactions was developed [127–130]. Considerable efforts were made to characterise the ligand binding properties of EcR from various insect pests [48]. In addition, photoaffinity labelling of the ligand binding pocket with a diacylhydrazine compound [131] was used for characterising the ligand binding properties. Since it is assumed that ligand binding reflects to a considerable extent the efficacy of a putative pesticide, tests for high-throughput screening using a fluorescein-labelled ecdysteroid were established [48]. Alternative test systems, e.g. yeast cells [132], and additional tests for interference with the ecdysone signalling cascade by JH were established [133].
Meanwhile, resistance to ecdysone agonists was observed in insect cells due to receptor defects [134] or altered metabolism [135]. Another resistance mechanism, which resides in the coupling between the conserved hierarchical cascade of early and early–late gene expression, was described recently in Spodoptera cells [136].
Resistance due to exclusion of ecdysone agonists by increased export from the target cells was reported from Dm-2 cells [137] and was suggested to also occur under field conditions [138].
Gene switch
Chemically inducible gene switches that regulate expression of endogenous genes have multiple applications in basic gene expression and gene therapy. Ecdysteroids showed no significant toxicity in vertebrate cells [139]. Consequently, due to this benign pharmacology, ecdysteroids and nonsteroidal agonists are attractive tools for application in medicine [140]. The retinoid X receptor-alpha/ecdysone receptor system can also be used in conjunction with other receptors with no detectable overlap of activity, thus enabling concurrent and temporal regulation of multiple genes within the same cell [123]. The receptor proteins showed negligible basal regulation in the absence of ligand [141, 142]. The available single-vector format facilitates the production of stable cell lines [143]. The ecdysone-inducible system can also be applied for RNA interference (RNAi) techniques, which allow high induction and adjustable control of short hairpin RNA (shRNA) expression for silencing gene expression in a wide range of mammalian cells. Meanwhile, ecdysone receptor-based gene switches have been developed for application in plants [144, 145], and their usefulness in regulation of transgene expression has been demonstrated even under large-scale field conditions. Genetic engineering of plants using transgenic technologies to enhance agronomic performance or improved quality traits can be applied to a wide variety of plant species.
Summary and outlook
The complexity of hormonal regulation triggered by ecdysteroids becomes more and more apparent. Multiple possibilities for diversification of ecdysteroid receptor activity have been characterised. Data obtained with ecdysteroid receptors expressed in heterologeous systems, preferentially in vertebrate cells, demonstrate the properties of receptor-mediated hormone action on a molecular level. In future, experiments with transgenic flies will show whether these capabilities characterised in cell cultures will actually be used in animals under certain physiological conditions.
Increasing evidence shows the importance of crosstalk with other hormone signalling pathways. Especially the multifaceted interaction of ecdysone and juvenile hormone needs to be further unravelled. The investigation of this regulator network is complicated by the fact that several signal transduction pathways may be used in different species or even within the same animal. The elucidation of the molecular mode of action is additionally hindered, because different solutions for the same problem have evolved in the insect phylum. Therefore, general conclusions about the mode of action of ecdysteroids should be drawn only with great caution.
References
- 1.Litwack G. Insect hormones. Vitam Horm. 2005;73:1–288. [Google Scholar]
- 2.Smagghe G. Ecdysones, structures and functions. Berlin: Springer; 2009. [Google Scholar]
- 3.Spindler K-D, Przibilla S, Spindler-Barth M. Moulting hormones of arthropods: molecular mechanisms. Zoology. 2001;103:189–201. [Google Scholar]
- 4.Dinan L (2009) Phytoecdysteroids: what use are they? Arch. Insect Biochem Physiol (in press) [DOI] [PubMed]
- 5.Dinan L, Harmatha J, Volodin V, Lafont R. Phytoecdysteroids: diversity, biosynthesis and distribution. In: Smagghe G, editor. Ecdysteroids, structures and functions. Berlin: Springer; 2009. pp. 3–45. [Google Scholar]
- 6.Gilbert LI. Drosophila is an inclusive model for human diseases, growth and development. Mol Cell Endocrinol. 2008;293:25–31. doi: 10.1016/j.mce.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Baker KD, Thummel CS. Diabetic larvae and obese flies—emerging studies of metabolism in Drosophila . Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palli SR, Hormann RE, Schlattner U, Lezzi M. Ecdysteroid receptors and their applications in agriculture and medicine. Vitam Horm. 2005;73:60–100. doi: 10.1016/S0083-6729(05)73003-X. [DOI] [PubMed] [Google Scholar]
- 9.Palli SR, Taava VS. Ecdysone receptor gene switches for applications in medicine and agriculture. In: Smagghe G, editor. Ecdysone: structures and functions. Berlin: Springer; 2009. pp. 511–538. [Google Scholar]
- 10.Rangasamy D, Tremethick DJ, Greaves IK. Gene knockdown by ecdysone-based inducible RNAi in stable mammalian cell lines. Nat Protoc. 2008;3:79–88. doi: 10.1038/nprot.2007.456. [DOI] [PubMed] [Google Scholar]
- 11.Lafont R, Dinan L. Practical uses for ecdsteroids in mammals including humans: an update. J Insect Sci. 2003;3.7:1–30. doi: 10.1093/jis/3.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. J Genet Genomics. 2008;35:1–10. doi: 10.1016/S1673-8527(08)60001-6. [DOI] [PubMed] [Google Scholar]
- 13.Rodenburg KW, van der Horst DJ. Lipoprotein-mediated lipid transport in insects: analogy to mammalian lipid carrier system and novel concepts for the functioning of LDL receptor family members. Biochim Biophys Acta. 2005;1736:10–29. doi: 10.1016/j.bbalip.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead L. Ecdysteroid carrier proteins. In: Koolman J, editor. Ecdysone—from chemistry to mode of action. Stuttgart: Thieme; 1989. pp. 232–244. [Google Scholar]
- 15.Jänicke E, Föll R, Decker H. Spider hemocyanin binds ecdysone and 20-OH-ecdysone. J Biol Chem. 1999;274:34267–34271. doi: 10.1074/jbc.274.48.34267. [DOI] [PubMed] [Google Scholar]
- 16.Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam Horm. 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- 17.Truman JW. Hormonal control of insect ecdysis; endocrine cascades for coordinating behavior with physiology. Vitam Horm. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- 18.Zitnan D, Kim YJ, Zitnanová I, Roller L, Adams ME. Complex steroid–peptide–receptor cascade controls insect ecdysis. Gen Comp Endocrinol. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Loof A. Ecdysteroids, juvenile hormone and insect neuropeptides: recent success and remaining major challenges. Gen Comp Endocrinol. 2008;155:3–13. doi: 10.1016/j.ygcen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Henrich VC (2005) The ecdysteroid receptor In: Iatrou K, Gill SS (eds) Comprehensive insect physiology, biochemistry, and molecular biology series, vol 3. Elsevier, Oxford, pp 243–286
- 21.Spindler-Barth M, Spindler K-D (2003) Ecdysteroid receptors (EcR/USP). In: Henry HL, Norman AW (eds) Encyclopedia of hormones and related cell regulators. Academic Press, London, pp 466–470
- 22.Braun S, Azoitei A, Spindler-Barth M (2009) DNA binding properties of Drosophila ecdysone receptor isoforms and its modification by the heterodimerization partner ultraspiracle. Arch Insect Biochem Physiol (in press) [DOI] [PubMed]
- 23.Cronauer MV, Braun S, Tremmel Ch, Kröncke KD, Spindler-Barth M. Nuclear localization and DNA binding of ecdysone receptor and ultraspiracle. Arch Insect Biochem Physiol. 2007;65:125–133. doi: 10.1002/arch.20184. [DOI] [PubMed] [Google Scholar]
- 24.Nieva C, Spindler-Barth M, Spindler K-D. Influence of hormone on intracellular localization of the Drosophila melanogaster ecdysteroid receptor (EcR) Cell Signal. 2007;19:2582–2587. doi: 10.1016/j.cellsig.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Beatty J, Fauth T, Callender JL, Spindler-Barth M, Henrich VC. Analysis of transcriptional activity mediated by the Drosophila melanogaster ecdysone receptor isoforms in a heterologous cell culture system. Insect Mol Biol. 2006;15:785–795. doi: 10.1111/j.1365-2583.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 26.Ruff H, Tremmel Ch, Spindler-Barth M (2009) Transcriptional activity of ecdysone receptor isoforms is regulated by modulation of receptor stability and interaction with AB- and C-domains of the heterodimerization partner Ultraspiracle. Arch Insect Biochem Physiol (in press) [DOI] [PubMed]
- 27.Nieva C, Spindler-Barth M, Spindler KD. Impact of heterodimerization on intracellular localization of the ecdysteroid receptor (EcR) Arch Insect Biochem Physiol. 2008;68:40–48. doi: 10.1002/arch.20234. [DOI] [PubMed] [Google Scholar]
- 28.Costantino BF, Bricker DK, Alexandre K, Shen K, Merriam JR, Antoniewski C, Callender JL, Henrich VC, Presente A, Andres AJ. A novel ecdysone receptor mediates steroid-regulated developmental events during the mid-third instar of Drosophila . PLoS. 2008;4:e1000102. doi: 10.1371/journal.pgen.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grebe M, Rauch P, Spindler-Barth M. Characterization of subclones of the epithelial cell line from Chironomus tentans resistant to the insecticide RH 5992, a non-steroidal moulting hormone agonist. Insect Biochem Mol Biol. 2000;30:591–600. doi: 10.1016/s0965-1748(00)00032-1. [DOI] [PubMed] [Google Scholar]
- 30.Ogura T, Minakuchi C, Nakagawa Y, Smagghe G, Miyagawa H. Molecular cloning, expression analysis and functional confirmation of ecdysone receptor and ultraspiracle from the Colorado potato beetle Leptinotarsa decemlineata . FEBS J. 2005;272:4114–4128. doi: 10.1111/j.1742-4658.2005.04823.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa Y, Sakai A, Magata F, Ogura T, Miyashita M, Miyagawa H. Molecular cloning of the ecdysone receptor and the retinoid X receptor from the scorpion Liocheles australasiae . FEBS J. 2007;274:6191–6203. doi: 10.1111/j.1742-4658.2007.06139.x. [DOI] [PubMed] [Google Scholar]
- 32.Nowickyj SM, Chithalen JV, Cameron D, Tyshenko MG, Petkovich M, Wyatt GR, Jone G, Walker VK. Locust retinoid X receptors: 9-cis-retinoic acid in embryos from a primitive insect. Proc Natl Acad Sci USA. 2008;105:9540–9545. doi: 10.1073/pnas.0712132105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laudet V, Bonneton F (2005) Evolution of nuclear hormone receptors in insects. In: Iatrou K, Gill SS (eds) Comprehensive insect physiology, biochemistry, and molecular biology series, vol 3. Elsevier, Oxford, pp 287–318
- 34.Billas I, Browning C, Lawrence MC, Graham LD, Moras D, Hill RJ. The structure and function of ecdysone receptors. In: Smagghe G, editor. Ecdysteroids, structures and functions. Berlin: Springer; 2009. pp. 335–375. [Google Scholar]
- 35.Lafont R, Koolman J. Diversity of ecdysteroids in animal species. In: Smagghe G, editor. Ecdysteroids, structures and functions. Berlin: Springer; 2009. pp. 47–71. [Google Scholar]
- 36.Azoitei A, Spindler-Barth M. DNA affects ligand binding of the ecdysone receptor of Drosophila melanogaster . Mol Cell Endocrinol. 2009;303:91–99. doi: 10.1016/j.mce.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Elke C, Rauch P, Spindler-Barth M, And Spindler K-D. DNA-binding properties of the ecdysteroid receptor-complex (EcR/USP) of the epithelial cell line from Chironomus tentans . Arch Insect Biochem Physiol. 1999;41:124–133. doi: 10.1002/(SICI)1520-6327(1999)41:3<124::AID-ARCH3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 38.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR–gamma-RXR-nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones G, Jones D, Teal P, Sapa A, Wozniak M. The retinoid-X receptor ortholog, ultraspiracle, binds with nanomolar affinity to an endogenous morphogenetic ligand. FEBS J. 2006;273:4983–4996. doi: 10.1111/j.1742-4658.2006.05498.x. [DOI] [PubMed] [Google Scholar]
- 40.Iwema T, Billas IM, Beck Y, Bonneton F, Nierengarten H, Chaumot A, Richards G, Laudet V, Moras D. Structural and functional characterization of a novel type of ligand-independent RXR–USP receptor. EMBO J. 2007;26:3770–3782. doi: 10.1038/sj.emboj.7601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton GM, Peak-Chew SY, Evans RM, Schwabe JWR. The structure of the ultraspiracle ligand-binding domain reveals a nuclear receptor locked in an inactive conformation. Proc Natl Acad Sci USA. 2001;98:1549–1554. doi: 10.1073/pnas.041611298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billas IML, Moulinier L, Rochel N, Moras D. Crystal structure of the ligand binding domain of the ultraspiracle protein USP, the ortholog of RXRs in insects. J Biol Chem. 2001;276:7465–7474. doi: 10.1074/jbc.M008926200. [DOI] [PubMed] [Google Scholar]
- 43.Henrich VC, Beatty JM, Ruff H, Callender J, Grebe M, Spindler-Barth M. The multidimensional partnership of EcR and USP. In: Smagghe G, editor. Ecdysones, structures and functions. Berlin: Springer; 2009. [Google Scholar]
- 44.Varghese J, Cohen SM. MicroRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila . Genes Dev. 2007;21:2277–2282. doi: 10.1101/gad.439807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirai H, Kamimura M, Fujiwara H. Characterization of core promoter elements for ecdysone receptor isoforms of the silkworm, Bombyx mori . Insect Mol Biol. 2007;16:253–264. doi: 10.1111/j.1365-2583.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 46.Azoitei A, Ruff H, Tremmel Ch, Braun S, Spindler-Barth M. Functional analysis of ecdysteroid receptor from Drosophila melangoaster “In Vitro”. In: Smagghe G, editor. Ecdysones, structures and functions. Berlin: Springer; 2009. pp. 377–388. [Google Scholar]
- 47.Cherbas L, Hu X, Zhimulyaeva J, Cherbas P. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 2003;130:271–284. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- 48.Graham LD, Pilling PA, Eaton RE, Gorman JJ, Braybrook C, Hannan GN, Pawlak-Skrzecz A, Noyce L, Lovrecz GO, Lu L, Hill RJ. Purification and characterization of recombinant ligand-binding domains from the ecdysone receptors of four pest insects. Protein Exp Purif. 2007;53:309–324. doi: 10.1016/j.pep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Graham LD, Johnson WM, Pawlak-Skrzeca A, Eaton RE, Bliese M, Howell L, Hannan GN, Hill RJ. Ligand binding by recombinant domains from insect ecdysone receptors. Insect Biochem Mol Biol. 2007;37:611–626. doi: 10.1016/j.ibmb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Spindler K-D, Betanska K, Nieva C, Gwozdz T, Dutko-Gwozdz J, Ozyhar A, Spindler-Barth M. Intracellular localization of the ecdysteroid receptor. In: Smagghe G, editor. Ecdysteroids, structures and functions. Berlin: Springer; 2009. pp. 389–409. [Google Scholar]
- 51.Dutko-Gwóźdź J, Gwóźdź T, Orłowski M, Greb-Markiewicz B, Duś D, Dobrucki J, Ozyhar A. The variety of complexes formed by EcR and Usp nuclear receptors in the nuclei of living cells. Mol Cell Endocrinol. 2008;294:45–51. doi: 10.1016/j.mce.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 52.Betanska K, Nieva C, Spindler-Barth M, Spindler K-D. Nucleocytoplasmic shuttling of the ecdysteroid receptor (EcR) and of ultraspiracle (Usp) from Drosophila melanogaster in mammalian cells: energy requirement and interaction with exportin. Arch Insect Biochem Physiol. 2007;65:134–142. doi: 10.1002/arch.20185. [DOI] [PubMed] [Google Scholar]
- 53.Devarakonda S, Harp JM, Kim Y, Ozyhar A, Rastinejad F. Structure of the heterodimeric ecdysone receptor DNA-binding complex. EMBO J. 2003;22:5827–5840. doi: 10.1093/emboj/cdg569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krusinski T, Laskowska A, Ozyhar A, Dobryszycki P. The application of an immobilized molecular beacon for the analysis of the DNA binding domains from the ecdysteroid receptor proteins Usp and EcR’s interaction with the hsp27 response element. J Biomol Screen. 2008;13:899–905. doi: 10.1177/1087057108324496. [DOI] [PubMed] [Google Scholar]
- 55.Krusiński T, Wietrzych M, Grad I, Ozyhar A, Dobryszycki P. Equilibrium analysis of the DNA binding domain of the ultraspiracle protein interaction with the response element from the hsp27 gene promoter—the application of molecular beacon technology. J Fluoresc. 2008;18:1–10. doi: 10.1007/s10895-007-0285-y. [DOI] [PubMed] [Google Scholar]
- 56.Jakób M, Kołodziejczyk R, Orłowski M, Krzywda S, Kowalska A, Dutko-Gwóźdź J, Gwóźdź T, Kochman M, Jaskólski M, Ozyhar A. Novel DNA-binding element within the C-terminal extension of the nuclear receptor DNA-binding domain. Nucleic Acids Res. 2007;35:2705–2718. doi: 10.1093/nar/gkm162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orlowski M, Szyszka M, Kowalska A, Grad I, Zoglowek A, Rymarczyk G, Dobryszycki P, Krowarsch D, Rastinejad F, Kochman M, Ozyhar A. Plasticity of the ecdysone receptor DNA binding domain. Mol Endocrinol. 2004;18:2166–2184. doi: 10.1210/me.2004-0154. [DOI] [PubMed] [Google Scholar]
- 58.Niedziela-Majka A, Kochman M, Ozyhar A. Polarity of the ecdysone receptor complex interaction with the palindromic response element from the hsp27 gene promoter. Eur J Biochem. 2000;267:507–519. doi: 10.1046/j.1432-1327.2000.01027.x. [DOI] [PubMed] [Google Scholar]
- 59.Dobryszycki P, Grad I, Krusiński T, Michaluk P, Sawicka D, Kowalska A, Orłowski M, Jakób M, Rymarczyk G, Kochman M, Ozyhar A. The DNA-binding domain of the ultraspiracle drives deformation of the response element whereas the DNA-binding domain of the ecdysone receptor is responsible for a slight additional change of the preformed structure. Biochemistry. 2006;45:668–675. doi: 10.1021/bi051354b. [DOI] [PubMed] [Google Scholar]
- 60.Schubiger M, Carré C, Antoniewski C, Truman JW. Ligand-dependent de-repression via EcR/USP acts as a gate to coordinate the differentiation of sensory neurons in the Drosophila wing. Development. 2005;132:5239–5248. doi: 10.1242/dev.02093. [DOI] [PubMed] [Google Scholar]
- 61.Kumar R, Thompson EB. Transactivation of the N-terminal domains of nuclear receptors: protein folding and coactivator interactions. Mol Endocrinol. 2003;17:1–10. doi: 10.1210/me.2002-0258. [DOI] [PubMed] [Google Scholar]
- 62.Wurtz J-M, Bourguet W, Renaud J-P, Vivat V, Cambon P, Moras D, Gronemeyer H. A canonical structure for the ligand-binding domain of nuclear receptors. Nat Struct Biol. 1995;3:87–94. doi: 10.1038/nsb0196-87. [DOI] [PubMed] [Google Scholar]
- 63.Wurtz J-M, Bourguet W, Renaud J-P, Vivat V, Cambon P, Moras D, Gronemeyer H. Erratum. Nat Struct Biol. 1995;3:206. doi: 10.1038/nsb0296-206. [DOI] [PubMed] [Google Scholar]
- 64.Dressel U, Thormeyer D, Altincicek B, Paululat A, Eggert M, Schneider S, Tenbaum SP, Renkawitz R, Baniahmad A. Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:3383–3394. doi: 10.1128/mcb.19.5.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivaldi MS, Karam CS, Corce VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila . Genes Dev. 2007;21:2818–2831. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura S, Sawatsubashi S, Ito S, Kouzmenko A, Suzuki E, Zhao Y, Yamagata K, Tanabe M, Ueda T, Fujiyama S, Murata T, Matsukawa H, Takeyama K, Yaegashi N, Kato S. Drosophila arginine methyltransferase 1 (DART1) is an ecdysone receptor co-repressor. Biochem Biophys Res Commun. 2008;371:889–893. doi: 10.1016/j.bbrc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Agawa Y, Sarhan M, Kageyama Y, Akagi K, Takai M, Hashiyama K, Wada T, Handa H, Iwamatsu A, Hirose S, Ueda H. Drosophila Blimp-1 is a transient transcriptional repressor that controls timing of the ecdysone-induced developmental pathway. Mol Cell Biol. 2007;27:8739–8747. doi: 10.1128/MCB.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol Cell Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sedkov Y, Cho E, Petruk S, Cherbas L, Smith ST, Jones RS, Cherbas P, Canaani E, Jaynes JB, Mazo A. Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila . Nature. 2003;426:78–83. doi: 10.1038/nature02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu J, Chen L, Sun G, Raikhel AS. The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol Cell Biol. 2006;26:9402–9412. doi: 10.1128/MCB.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stabell M, Eskeland R, Bjørkmo M, Larsson J, Aalen RB, Imhof A, Lambertsson A. The Drosophila G9a gene encodes a multi-catalytic histone methyltransferase required for normal development. Nucleic Acids Res. 2006;200634:4609–4621. doi: 10.1093/nar/gkl640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badenhorst P, Xiao H, Cherbas L, Kwon SY, Voas M, Rebay I, Cherbas P, Wu C. The Drosophila nucleosome remodeling factor NURF is required for ecdysteroid signaling and metamorphosis. Genes Dev. 2005;19:2540–2545. doi: 10.1101/gad.1342605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Wang H, Wang YF. Overexpression of HmgD causes the failure of pupariation in Drosophila by affecting ecdysone receptor pathway. Arch Insect Biochem Physiol. 2008;68:123–133. doi: 10.1002/arch.20237. [DOI] [PubMed] [Google Scholar]
- 74.Koolman J, Spindler K-D. Mechanism of action of ecdysteroids. In: Downer RGH, Laufer H, editors. Invertebrate endocrinology—endocrinology of insects. New York: Alan Liss; 1983. pp. 179–201. [Google Scholar]
- 75.Tomaschko K-H. Nongenomic effects of ecdysteroids. Arch Insect Biochem Physiol. 1999;41:89–98. [Google Scholar]
- 76.Schlattner U, Vafopoulo X, Steel CGH, Hormann RE, Lezzi M. Non-genomic ecdysone effects and the invertebrate nuclear steroid hormone receptorEcR–new role for an “old” receptor? Mol Cell Endocrinol. 2006;247:64–72. doi: 10.1016/j.mce.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 77.Elmogy M, Iwami M, Sakurai S. Presence of membrane ecdysone receptor in the anterior silk gland of the silkworm Bombyx mori . Eur J Biochem. 2004;271:3171–3179. doi: 10.1111/j.1432-1033.2004.04249.x. [DOI] [PubMed] [Google Scholar]
- 78.Elmogy M, Terashima J, Iga M, Iwami M, Sakurai S. A rapid increase in cAMP in response to 20-hydroxyecdysone in the anterior silk glands of the silkworm, Bombyx mori . Zool Sci. 2006;23:715–719. doi: 10.2108/zsj.23.715. [DOI] [PubMed] [Google Scholar]
- 79.Evans PD, Srivastava DP, Reale V. Rapid, nongenomic tresponses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. In: Smagghe G, editor. Ecdysteroids, structures and functions. Berlin: Springer; 2009. pp. 425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flatt T, Heyland A, Rus F, Porpiglia E, Sherlock C, Yamamoto R, Garbuzov A, Palli SR, Tatar M, Silverman N. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster . J Exp Biol. 2008;211:2712–2724. doi: 10.1242/jeb.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1998;59:1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- 82.Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insect. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 83.Orme MH, Leevers SJ (2005) Flies on steroids: The interplay between ecdysone and insulin signalling. Cell Metab 277–78. doi:10.11016/j.cmet.2005.10.005 [DOI] [PubMed]
- 84.Mirth CK, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster . Curr Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 85.McBrayer Z, Ono H, Shimell M-J, Parvy JP, Beckstead RB, Warren JT, Thummel CS, Dauphin-Villemant C, Gilbert LI, O`Connor MB. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila . Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Truman JW, Hiruma K, Allee JP, MacWhinnie SBG, Champlin D, Riddiford L. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science. 2006;310:667–670. doi: 10.1126/science.1123652. [DOI] [PubMed] [Google Scholar]
- 87.Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP (2008) A nucleostemin family GTPase, NS3, acts in serotinergic neurons to regulate insulin signalling and con troll body size. Genes Dev. doi:10.1101/gad.1670508 [DOI] [PMC free article] [PubMed]
- 88.Hatt PJ, Liebon C, Morinière M, Porcheron P. Roles for insulin and ecdysteroids in differentiation on an insect cell line of epidermal origin. In Vitro Cell Dev Biol Anim. 1994;30A:717–720. doi: 10.1007/BF02631276. [DOI] [PubMed] [Google Scholar]
- 89.Vander Griend DJ, Litvinov IV, Isaacs JT. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle. 2007;15:647–651. doi: 10.4161/cc.6.6.4028. [DOI] [PubMed] [Google Scholar]
- 90.Maruvada P, Baumann CT, Hager GL, Yen PM. Cell cycle-dependent expression of thyroid hormone receptor-beta is a mechanism for variable hormone sensitivity. Mol Biol Cell. 2003;15:1895–1903. doi: 10.1091/mbc.E03-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narayanan R, Edwards DP, Weigel NL. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol. 2005;25:2885–2898. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petre-Draviam CE, Cook SL, Burd CJ, Marshall TW, Wetherill YB, Knudsen KE. Specificity of cyclin D1 for androgen receptor regulation. Cancer Res. 2003;63:4903–4913. [PubMed] [Google Scholar]
- 94.Parthasarathy R, Palli SR. Proliferation and differentiation of intestinal stem cells during metamorphosis of the red flour beetle, Tribolium castaneum . Dev Dyn. 2008;237:893–908. doi: 10.1002/dvdy.21475. [DOI] [PubMed] [Google Scholar]
- 95.Champlin DT, Truman JW. Ecdysteroid control of cell proliferation during optic lobe neurogenesis in the moth Manduca sexta . Development. 1998;125:269–277. doi: 10.1242/dev.125.2.269. [DOI] [PubMed] [Google Scholar]
- 96.Spindler-Barth M, Junger E, Spindler K-D. Vesicle formation and ecdysteroid induced cellular differentiation in the epithelial cell line from Chironomus tentans . Tissue Cell. 1992;24:919–934. [Google Scholar]
- 97.Swiderski RE, O’Connor JD. Modulation of novel-length DOPA decarboxylase transcripts by 20-OH-ecdysone in a Drosophila melanogaster Kc cell subline. Mol Cell Biol. 1986;6:4433–4439. doi: 10.1128/mcb.6.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mottier V, Siaussat D, Bozzolan F, Auzoux-Bordenave S, Porcheron P, Debernard S. The 20-hydroxyecdysone-induced cellular arrest in G2 phase is preceded by an inhibition of cyclin expression. Insect Biochem Mol Biol. 2004;34:51–60. doi: 10.1016/j.ibmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Gerenday A, Fallon AM. Ecdysone-induced accumulation of mosquito cells in the G1 phase of the cell cycle. J Insect Physiol. 2004;50:831–838. doi: 10.1016/j.jinsphys.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Siaussat D, Bozzolan F, Queguiner I, Porcheron P, Debernard S. Cell cycle profiles of EcR, USP, HR3 and B cyclin mRNAs associated to 20E-induced G2 arrest of Plodia interpunctella imaginal wing cells. Insect Mol Biol. 2005;14:151–161. doi: 10.1111/j.1365-2583.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 101.Siaussat D, Bozzolan F, Porcheron P, Debernard S. The 20-hydroxyecdysone-induced signalling pathway in G2/M arrest of Plodia interpunctella imaginal wing cells. Insect Biochem Mol Biol. 2008;38:529–539. doi: 10.1016/j.ibmb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 102.Betanska K, Czogalla S, Spindler-Barth M, Spindler KD (2009) Cell cycle-dependent ecdysteroid localization and concentration in chinese hamster ovary cells. Arch Insect Biochem Physiol (in press) [DOI] [PubMed]
- 103.Siaussat D, Porcheron P, Debernard S (2009) In: Smagghe G (ed) Ecdysteroids, structures and functions. Springer, Berlin, pp 185–204
- 104.Parthasarathy R, Palli SR. Stage- and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti . J Insect Physiol. 2007;53:216–229. doi: 10.1016/j.jinsphys.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Parthasarathy R, Palli SR. Developmental and hormonal regulation of midgut remodeling in a lepidopteran insect, Heliothis virescens . Mech Dev. 2007;124:23–34. doi: 10.1016/j.mod.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 106.Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech Dev. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum . Mech Dev. 2008;125:299–313. doi: 10.1016/j.mod.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parthasarathy R, Tan A, Palli SR. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval–pupal metamorphosis. Mech Dev. 2008;125:601–616. doi: 10.1016/j.mod.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berger EM, Dubrovsky EB. Juvenile hormone molecular actions and interactions during development of Drosophila melanogaster . Vitam Horm. 2005;73:175–215. doi: 10.1016/S0083-6729(05)73006-5. [DOI] [PubMed] [Google Scholar]
- 110.Barchuk AR, Figueiredo VL, Simões ZL. Downregulation of ultraspiracle gene expression delays pupal development in honeybees. J Insect Physiol. 2008;54:1035–1040. doi: 10.1016/j.jinsphys.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 111.Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum . Proc Natl Acad Sci USA. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Konopova B, Jindra M. Broad-complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development. 2008;135:559–568. doi: 10.1242/dev.016097. [DOI] [PubMed] [Google Scholar]
- 113.Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem. 2007;282:37605–37617. doi: 10.1074/jbc.M704595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maki A, Sawatsubashi S, Ito S, Shirode Y, Suzuki E, Zhao Y, Yamagata K, Kouzmenko A, Takeyama K, Kato S. Juvenile hormones antagonize ecdysone actions through co-repressor recruitment to EcR/USP heterodimers. Biochem Biophys Res Commun. 2004;320:262–267. doi: 10.1016/j.bbrc.2004.05.156. [DOI] [PubMed] [Google Scholar]
- 115.Henrich VC, Burns E, Yelverton DP, Christensen E, Weinberger C. Juvenile hormone potentiates ecdysone receptor-dependent transcription in a mammalian cell culture system. Insect Biochem Mol Biol. 2003;33:1239–1247. doi: 10.1016/j.ibmb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Planelló R, Martínez-Guitarte JL, Morcillo G. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius . Chemosphere. 2008;71:1870–1876. doi: 10.1016/j.chemosphere.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 117.Lye CM, Bentley MG, Galloway T. Effects of 4-nonylphenol on the endocrine system of the shore crab, Carcinus maenas . Environ Toxicol. 2008;23:309–318. doi: 10.1002/tox.20344. [DOI] [PubMed] [Google Scholar]
- 118.Le Goff G, Hilliou F, Siegfried BD, Boundy S, Wajnberg E, Sofer L, Audant P, ffrench-Constant RH, Feyereisen R. Xenobiotic response in Drosophila melanogaster: sex dependence of P450 and GST gene induction. Insect Biochem Mol Biol. 2006;36:674–682. doi: 10.1016/j.ibmb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 119.King-Jones K, Horner MA, Lam G, Thummel CS. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila . Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Bathori M, Toth N, Hunyadi A, Marki A, Zador E. Phytoecdysteroids and anabolic-androgenic steroids–structure and effects in humans. Curr Med Chem. 2008;15:75–91. doi: 10.2174/092986708783330674. [DOI] [PubMed] [Google Scholar]
- 121.Lafont R, Dinan L. Innovative and future applications for ecdysteroids. In: Smagghe G, editor. Ecdysteroids, structures and functions. Berlin: Springer; 2009. pp. 551–578. [Google Scholar]
- 122.Dhadialla TS, Carlson GR, Lee DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- 123.Mommaerts V, Sterk G, Smagghe G. Bumblebees can be used in combination with juvenile hormone analogues and ecdysone agonists. Ecotoxicology. 2006;15:513–521. doi: 10.1007/s10646-006-0087-z. [DOI] [PubMed] [Google Scholar]
- 124.Harmatha J, Dinan L. Biological activity of natural and synthetic ecdysteroids in the BII bioassay. Arch Insect Biochem Physiol. 1997;35:219–225. doi: 10.1002/(SICI)1520-6327(1997)35:1/2<219::AID-ARCH20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 125.Li J, Qi H, Qi LW, Yi L, Li P. Simultaneous determination of main phytoecdysones and triterpenoids in radix achyranthis bidentatae by high-performance liquid chromatography with diode array-evaporative light scattering detectors and mass spectrometry. Anal Chim Acta. 2007;596:264–272. doi: 10.1016/j.aca.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Carmichael JA, Lawrence MC, Graham LD, Pilling PA, Epa VC, Noyce L, Lovrecz G, Windler DA, Pawlak-Skrezecz A, Eaton RE, Hannan GN, Hill RJ. The X-ray structure of a hemipteran ecdysone receptor ligand-binding domain: comparison with a lepidopteran ecdysone receptor ligand-binding domain and implications for insecticide design. J Biol Chem. 2005;280:22258–22269. doi: 10.1074/jbc.M500661200. [DOI] [PubMed] [Google Scholar]
- 127.Lapenna S, Friz J, Barlow A, Palli SR, Dinan L, Hormann RE. Ecdysteroid ligand–receptor selectivity—exploring trends to design orthogonal gene switches. FEBS J. 2008;275:5785–5809. doi: 10.1111/j.1742-4658.2008.06687.x. [DOI] [PubMed] [Google Scholar]
- 128.Fujita T, Nakagawa Y. QSAR and mode of action studies of insecticidal ecdysone agonists. SAR QSAR Environ Res. 2007;18:77–88. doi: 10.1080/10629360601053943. [DOI] [PubMed] [Google Scholar]
- 129.Bordas B, Belai I, Lopata A, Szanto Z. Interpretation of scoring functions using 3D molecular fields. Mapping the diacyl-hydrazine-binding pocket of an insect ecdysone receptor. J Chem Inf Model. 2007;47:176–185. doi: 10.1021/ci600317v. [DOI] [PubMed] [Google Scholar]
- 130.Holmwood G, Schindler M. Protein structure based rational design of ecdysone agonists. Bioorg Med Chem. 2009;17:4046–4070. doi: 10.1016/j.bmc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 131.Dhadialla TS, Le D, Palli SR, Raikhel A, Carlson GR. A photoaffinity, non-steroidal, ecdysone agonist, RH-131039: characterization of binding and functional activity. Insect Biochem Mol Biol. 2007;37:865–875. doi: 10.1016/j.ibmb.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Gu JB, Sun YT, Peng HJ. Construction of a yeast model for screening Aedes albopictus ecdysone agonist pesticides. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2007;25:376–380. [PubMed] [Google Scholar]
- 133.Beatty J, Smagghe G, Ogura T, Nakagawa Y, Spindler-Barth M, Henrich VC. Ecdysteroid receptor properties of two species from different insect orders compared in a heterologous cell culture system. FEBS J. 2009;276:3087–3098. doi: 10.1111/j.1742-4658.2009.07026.x. [DOI] [PubMed] [Google Scholar]
- 134.Spindler-Barth M, Spindler K-D. Ecdysteroid resistant subclones of the epithelial cell line from Chironomus tentans (Insecta, Diptera). I. Selection and characterization of resistant clones. In Vitro Cell Dev Biol Anim. 1998;34:116–122. doi: 10.1007/s11626-998-0093-y. [DOI] [PubMed] [Google Scholar]
- 135.Kayser H, Winkler T, Spindler-Barth M. 26-hydroxylation of ecdysteroids is catalyzed by a typical cytochrome P-450-dependent oxidase and related to ecdysteroid resistance in an insect cell line. Eur J Biochem. 1997;248:707–716. doi: 10.1111/j.1432-1033.1997.00707.x. [DOI] [PubMed] [Google Scholar]
- 136.Swevers L, Soin T, Mosallanejad H, Iatrou K, Smagghe G. Ecdysteroid signaling in ecdysteroid-resistant cell lines from the polyphagous noctuid pest Spodoptera exigua . Insect Biochem Mol Biol. 2008;38:825–833. doi: 10.1016/j.ibmb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 137.Retnakaran A, Gelbic I, Sundaram M, Tomkins W, Ladd T, Primavera M, Feng Q, Arif B, Palli R, Krell P. Mode of action of the ecdysone agonist tebufenozide (RH-5992), and an exclusion mechanism to explain resistance to it. Pest Manag Sci. 2001;57:951–957. doi: 10.1002/ps.377. [DOI] [PubMed] [Google Scholar]
- 138.Retnakaran A, Krell P, Feng Q, Arif B. Ecdysone agonists: mechanism and importance in controlling insect pests of agriculture and forestry. Arch Insect Biochem Physiol. 2003;54:187–199. doi: 10.1002/arch.10116. [DOI] [PubMed] [Google Scholar]
- 139.Xie J, Nair A, Hermiston TW. A comparative study examining the cytotoxicity of inducible gene expression system ligands in different cell types. Toxicol In Vitro. 2008;22:261–266. doi: 10.1016/j.tiv.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 140.Panguluri SK, Kumar P, Palli SR. Functional characterization of ecdysone receptor gene switches in mammalian cells. FEBS J. 2006;273:5550–5563. doi: 10.1111/j.1742-4658.2006.05545.x. [DOI] [PubMed] [Google Scholar]
- 141.Magnenat L, Schwimmer LJ, Barbas CF. Drug-inducible and simultaneous regulation of endogenous genes by single-chain nuclear receptor-based zinc-finger transcription factor gene switches. Gene Ther. 2008;15:1223–1232. doi: 10.1038/gt.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lessard J, Aicha SB, Fournier A, Calvo E, Lavergne E, Pelletier M, Labrie C. Characterization of the RSL1-dependent conditional expression system in LNCap prostate cancer cells and development of a single vector format. Prostate. 2007;67:808–819. doi: 10.1002/pros.20559. [DOI] [PubMed] [Google Scholar]
- 143.Tavva VS, Dinkins RD, Palli SR, Collins GB. Development of a methoxyfenozide-responsive gene switch for applications in plants. Plant J. 2006;45:457–469. doi: 10.1111/j.1365-313X.2005.02628.x. [DOI] [PubMed] [Google Scholar]
- 144.Tavva VS, Dinkins RD, Palli SR, Collins GB. Development of a tightly regulated and highly inducible ecdysone receptor gene switch for plants through the use of retinoid X receptor chimeras. Transgenic Res. 2007;16:599–612. doi: 10.1007/s11248-006-9054-y. [DOI] [PubMed] [Google Scholar]
- 145.Tavva VS, Palli SR, Dinkins RD, Collins GB. Improvement of a monopartite ecdysone receptor gene switch and demonstration of its utility in regulation of transgene expression in plants. FEBS J. 2008;275:2161–2176. doi: 10.1111/j.1742-4658.2008.06370.x. [DOI] [PubMed] [Google Scholar]
- 146.Kimbrell DA, Beutler B. The evolution and genetics of innate immunity. Nat Rev Genet. 2001;2:256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]