Abstract
Chromogranin A (CHGA) is ubiquitously expressed in secretory cells of the endocrine, neuroendocrine, and neuronal tissues. Although this protein has long been known as a marker for neuroendocrine tumors, its role in cardiovascular disease states including essential hypertension (EH) has only recently been recognized. It acts as a prohormone giving rise to bioactive peptides such as vasostatin-I (human CHGA1–76) and catestatin (human CHGA352–372) that exhibit several cardiovascular regulatory functions. CHGA is over-expressed but catestatin is diminished in EH. Moreover, genetic variants in the promoter, catestatin, and 3′-untranslated regions of the human CHGA gene alter autonomic activity and blood pressure. Consistent with these findings, targeted ablation of this gene causes severe arterial hypertension and ventricular hypertrophy in mice. Transgenic expression of the human CHGA gene or exogenous administration of catestatin restores blood pressure in these mice. Thus, the accumulated evidence establishes CHGA as a novel susceptibility gene for EH.
Keywords: Chromogranin A, Catestatin, Vasostatin, Cardiovascular, Blood pressure, Anti-hypertensive, Genetics, Hypertension
Introduction
Essential hypertension (EH) is a major public health problem due to its high prevalence (~30–45%) in urban populations [1–3]. It is an asymptotic, chronic disorder that contributes to serious health complications including myocardial infarction, congestive heart failure, stroke, end-stage renal disease (ESRD), and retinal damage. EH is regarded as a “complex genetic trait” caused by multiple susceptibility genes and unhealthy lifestyles involving gene–gene as well as gene–environment interactions that determine the onset and severity of the disease [2, 4, 5].
Various approaches such as candidate-gene approach, genome-wide scanning, “intermediate phenotype” approach, and comparative genomics in animal models are utilized to identify the genes underlying pathogenesis of EH. The major susceptibility genes for EH known thus far belong to several molecular pathways/systems including the renin-angiotensin-aldosterone system that influences vascular volume homeostasis and vascular tone, the sympathetic nervous system that modulates heart rate, cardiac contraction, and vascular tone, and the kallikrein–kinin system that affects renal salt handling and vascular tone, as well as factors involved in endothelial function and vasoactivity such as endothelin and nitric oxide [2, 5–8]. However, only a partial understanding of the underlying mechanisms has been achieved. Indeed, it is not yet clear how many genes are involved in the pathogenesis of EH and how they interact among themselves and other genes. Therefore, the search for the genetic basis of EH is ongoing in order to identify and characterize all susceptibility genes and molecular variants that modulate blood pressure [9–11].
Chromogranin A (CHGA; molecular weight 48 kDa), a well-studied member of the granin family of proteins, is co-stored and co-released with catecholamines from secretory vesicles in adrenal medulla and postganglionic sympathetic axons [12–18]. It binds with calcium and catecholamines [19, 20], interacts with several proteins in regulated secretory pathways [21], and plays a crucial role in the biogenesis of the catecholamine secretory chromaffin vesicles [22–25]. Moreover, CHGA also acts as a prohormone giving rise to several biologically active peptides by proteolytic cleavage such as vasostatin-I (human CHGA1–76, a vasodilator), chromacin (human CHGA176–197, an anti-microbial agent), pancreastatin (human CHGA250–301, a dsyglycemic hormone), and catestatin (human CHGA352–372, a potent inhibitor of catecholamine release from chromaffin cells and adrenergic neurons, and an anti-microbial agent) [26–31]. Notably, plasma CHGA levels are elevated in chronic heart failure patients and associated with clinical severity and prognosis [32]. More recently, circulating CHGA levels have been shown to be an independent prognostic indicator in patients with complicated myocardial infarction [33] as well as in acute coronary syndromes [34].
In view of these intracellular and extracellular functions as well as association with cardiovascular disease states, we hypothesize that CHGA is a susceptibility gene for hypertension. Indeed, systemic ablation of Chga gene caused elevation of blood pressure in mice, and hypertension in these mice was rescued by transgenic expression of the human ortholog or by exogenous administration of catestatin [25]. Moreover, in humans, DNA variants in the promoter region, in the catestatin peptide region, and the 3′-untranslated region (UTR) alter autonomic activity and blood pressure [35–37]. These and other experimental data from cellular, molecular, physiological, and clinical studies providing phenotypic links between CHGA and EH are discussed in this review.
Gene structure and tissue-specific expression of CHGA
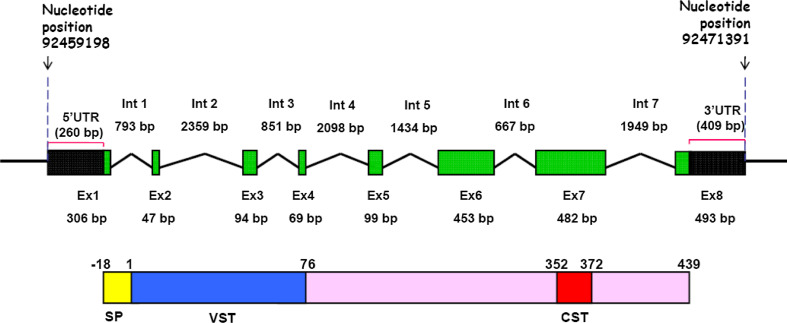
Human CHGA gene (MIM 118910; Accession number NM_001275) spans 12,194 bp in the locus 14q32.12 and consists of 8 exons giving rise to a 2,043-nucleotide transcript, of which 1,374 nucleotides are processed for translation into a 457 residue protein (having an 18 residue signal peptide) while 260 and 409 bp remain as 5′- and 3′- UTRs, respectively (Fig. 1; [38, 39]). The mouse chomogranin A gene (Accession number NM_007693) located in chromosome 12 has similar genomic organization and it translates to a 463 residue polypeptide also having an 18 residue signal peptide [40]. Although the human and mouse CHGA differ in primary sequence as well as length, both have a high content of acidic amino acid residues (implicated for the involvement of this protein in the sorting and packaging of hormones and neuropeptides into secretory granules [12]) and a high degree of conservation at the N- and C-terminal domains (suggested to be important for prohormone-like properties of the protein [41]).
Fig. 1.
Schematic organization of the human CHGA gene and its protein product. Top panel The human CHGA gene spanning 12,194 bp in chromosome 14 (from base position 92459198 to 92471391) consists of 8 exons separated by seven introns (UCSC Genome Browser refGene NM_001275). UTR Untranslated region, Ex exon, Int intron. Bottom panel The translated protein product consists of 457 amino acids, of which the first 18 residues serve as the signal peptide (SP). The mature protein undergoes proteolytic cleavage to generate several bioactive peptides. Localizations of the vasoactive peptide vasostatin-I (1–76 amino acids; VST) and catecholamine release inhibitory peptide catestatin (352–372 amino acids; CST) are indicated. The schemes are not drawn to scale
CHGA is ubiquitously expressed in secretory cells of the endocrine, neuroendocrine, and neuronal tissues [12]. In normal humans, adrenal medulla was reported to be the major tissue source of CHGA while other neuroendocrine tissues possessed only 0.04–25% of the immunoreactivity found in the adrenal medulla, the rank order of concentration (μg/g wet wt) being adrenal medulla > pituitary > pancreas > stomach > small intestine (jejunoileum) > brain (frontal cortex) > parathyroid > thyroid [42]. A similar tissue-specific pattern of CHGA expression was observed in a “humanized” mouse model expressing the human CHGA gene instead of the mouse ortholog [25]. Consistent with these findings, transgenic mice expressing mouse chga promoter–firefly luciferase reporter also displayed neuroendocrine-tissue specific expression of the transgene [43]. Very recently, CHGA has been detected in rat bulbospinal neurons in the rostral ventrolateral medulla, a key cardiovascular nucleus of the brain stem [44].
CHGA is also expressed in various cardiac cell types. For example, immunohistochemical studies revealed the presence of CHGA in the secretory granules of nonadrenergic myoendocrine atrial cells of rat heart. Notably, CHGA co-localized with the atrial natriuretic peptide in these myoendocrine granules and appeared to undergo proteolytic processing to a greater extent than in the adrenal medulla [45]. Additionally, immunohistochemical studies in serial sections from atrial and ventricular regions of rat heart demonstrated localization of CHGA along with the calcium channel alpha 1E subunit in Purkinje fibers of both atrium and ventricle [46]. Consistent with these findings, CHGA-derived vasostatin-containing peptides were identified in rat heart extracts, indicating their role in cardiac physiology by an autocrine/paracrine mechanism [47]. More recently, convincing evidences of production and release of CHGA in human ventricular myocardium were provided by Pieroni et al. [48]. They measured >0.5 μg of CHGA/g left ventricular myocardial tissue in patients with dilated and hypertrophic cardiomyopathy by ELISA using four different monoclonal antibodies. It was also shown that CHGA co-localized with the brain nartiuretic peptide (BNP) in ventricular cardiomyocytes and that there was a strong correlation between CHGA and BNP circulating levels. Although CHGA protein was not detectable in the normal myocardial tissue by immunohistochemistry and ELISA, the presence of CHGA mRNA in normal myocardium was confirmed by RT-PCR [48]. The myocardial overproduction of CHGA may contribute to the regulation of heart remodeling in patients with myocardial infarction, cardiomyopathies, and heart failure. It is also noteworthy that CHGA and its proteolytic fragments, including vasostatin-I and catestatin, have been detected in polymorphonuclear neutrophils (PMNs) [30, 49] that are known to accumulate at sites of inflammation. Consistently, over-expression of CHGA and its positive correlation with the inflammation markers procalcitonin and C-reactive protein in serum has recently been reported in patients with systemic inflammatory response syndrome [50]. These observations indicate possible roles for CHGA and/or its peptide-derivatives in inflammatory responses and local modulation of cardiac functions.
Circulating CHGA level is elevated in hypertension and cardiovascular disease states
Since CHGA is ubiquitously distributed in dense-core secretory granules of the endocrine and nervous systems and is co-secreted with catecholamines and neuropeptides into the circulation, it may serve as a valuable indicator of sympathoadrenal activity under both physiological and pathological conditions [12]. Indeed, plasma CHGA concentration correlates with catecholamine release rate [51], indicating that measurement of CHGA levels may yield insights into the pathogenesis of EH. In normal humans, plasma CHGA levels have been reported to range from 0.5 to 5 nM in the literature (recently reviewed in [41]). Such a wide range of concentrations may be due to various differences among the different studies, including the specificity of the antibodies used for the radioimmunoassays. Interestingly, even the time of collection (morning, afternoon, or night) of the blood sample also led to significant differences (up to 40% increase from morning to night) in an Italian population of healthy individuals reflecting circadian rhythms in plasma CHGA [41, 52]. Although this study [52] did not detect a difference in plasma CHGA levels between the two groups, phenotypic links between CHGA and EH have been repeatedly observed in other studies [53–56]. Notably, two studies in Southern California populations showed significant elevations (~4.1 vs ~2.7 nM [54] and ~2.1 vs ~1.3 nM [56]) of plasma CHGA concentrations in EH as compared to the normotensive controls. The releasable sympathoadrenal vesicular stores of CHGA have also been observed to be elevated in EH [56]. Moreover, studies in twin subjects displayed significant heritability in plasma CHGA concentration [37, 56]. Consistent with these findings in humans, expression of this gene was observed to be significantly higher in adrenal glands of rat and mouse models of genetic hypertension [57–59], supporting the phenotypic association between elevated CHGA and EH. However, given that the numbers of individuals investigated in these reports were small, further studies in large populations are required to establish the association between plasma CHGA levels and EH.
The serum CHGA concentration was shown to increase substantially (up to 10–20 nM depending on the severity of the disease) in chronic heart failure in a study comprising 160 Italian subjects [32]. The CHGA level in the circulation was also reported to be elevated in complicated myocardial infarction in 217 patients included in the OPTIMAAL (Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan) trial [33] and in acute coronary syndromes in a Swedish population of 1,268 patients [34]. These studies documented that the circulating CHGA level can serve as an independent prognostic indicator in patients with complicated myocardial infarction [33] as well as an independent predictor of long-term mortality and heart failure hospitalizations in acute coronary syndromes [34]. The plasma CHGA levels were also observed to be significantly higher (~3.0 vs 1.3 nM) in patients with dilated cardiomyopathy and hypertrophic cardiomyopathy than controls in an Italian population [48]. The increased circulating CHGA level in these patients is thought to be significantly contributed by cardiomyocytes, assuming constant release of CHGA from myocardial cells [48] and considering the plasma half-life of CHGA to be 18.4 min [60].
Thus, the elevated CHGA concentration in the circulation is linked to EH, myocardial infarction, and cardiomyopathy, reflecting an important role of this protein as a biomarker for cardiovascular disease states.
Systemic deletion of chga in mice results in alteration of autonomic/cardiovascular physiology
To study the physiological role of CHGA in general and specifically whether it plays a role in regulation of blood pressure, a knockout mouse model was generated by systemic deletion of chga gene using loxP-Cre recombination strategy [25]. Homozygous knockout (chga −/−) mice displayed severe hypertension [up to ~44 mmHg higher systolic blood pressure (SBP) and up to ~26 mmHg higher diastolic blood pressure (DBP)] as compared to wild-type (chga +/+) mice. Heterozygous knockout (chga +/−) mice also displayed hypertension with blood pressure values closer to those for chga −/− mice, indicating that both copies of the gene are essential for maintaining normal blood pressure. Of note, the elevated blood pressure in chga −/− mice was rescued to normalcy by “humanization” of these mice at the CHGA locus (by generation of chga −/− CHGA +/+ mice that contain two copies of human CHGA gene), suggesting that the human gene probably functions identically to the murine ortholog, at least with respect to blood pressure maintenance [25].
In view of the elevated SBP and DBP in chga −/− mice, the left ventricle (LV) morphology in these mice was studied by transthoracic echocardiography. Significant increases in the LV wall thickness (both septal and free wall), LV mass as well as LV internal diameter (cavity size) at both end systole and end diastole were detected in chga −/− mice [25]. Although the mechanism underlying such alteration of cardiac physiology in these knockout mice has not yet been studied, substantially increased afterload due to severe hypertension in these mice may lead to the LV hypertrophy and progression to LV cavity dilation.
Association of naturally occurring human genetic variants of CHGA with blood pressure variation
Re-sequencing the CHGA gene has led to the discovery of several variations in the promoter, 5′-UTR, coding region, 3′-UTR, as well as intronic regions adjacent to the exons [61]. Some of those variants have been found to alter blood pressure and autonomic activity in human populations (listed in Table 1 and described below).
Table 1.
CHGA SNPs and haplotypes associated with variation in blood pressure
| SNP or haplotype (location, domain) | Study population(s) | Associated phenotype(s) | References |
|---|---|---|---|
| T/C (at −1,014 bp in promoter) | Twins (mostly normotensive) of European ancestry | The C allele predicted lower post-cold stress DBP (lower risk of developing hypertension) | [36] |
| T/G (at −988 bp in promoter) | Twins (mostly normotensive) of European ancestry | The G allele was associated with lower post-cold stress DBP (lower risk of developing hypertension) | [36] |
| G/A (at −462 bp in promoter) |
Twins (mostly normotensive) of European ancestry Hypertensive versus normotensive (~90% European and ~10% Mexican ancestries) |
The A allele predicted lower post-cold stress DBP (lower risk of developing hypertension) G/A genotype (heterozygous) predicted higher SBP and DBP over G/G and A/A genotypes |
[36] |
| C/T (at +11,825 bp with respect to the cap site, i.e. at +87 bp in 3′-UTR) |
Twins (mostly normotensive) of European ancestry Hypertensive versus hypotensive people of European ancestry |
C allele was associated with higher SBP after cold-stress (higher magnitude in men than women) C/C genotype predicted higher basal SBP and DBP than C/T or T/T genotypes, especially in men |
[37] |
| G/A (at +9,559 bp in exon 7; amino acid variation Gly364Ser) | Hypertensive versus normotensive and hypotensive people of European ancestry |
Gly/Ser heterozygotes displayed lower post-cold stress BP than Gly/Gly homozygotes; Significantly lower resting BP values in Gly/Ser heterozygote men than Gly/Gly homozygotes (Ser as protective allele in men) |
[62] |
| Haplotype CGATA (involved SNPs T-1014C, T-988G, G-462A, T-415C and C-89A in promoter) | Twins (mostly normotensive) of European ancestry | Blunted blood pressure response to cold-stress (lower risk of developing hypertension) | [36] |
| Haplotype GGCC (involved SNPs −462 G in promoter, 246Glu/G [at +8,540 bp] in Exon 6, Arg381/C [at +9,610 bp] in exon 7, +87C in 3′-UTR) | Hypertensive versus hypotensive people of European ancestry | Dose-dependently (based on copy number) predicted higher DBP in men | [37] |
| Haplotype ATC (involved SNPs G-462A,T-415C and C-89A in promoter) | Hypertensive-ESRD patients versus controls of African ancestry | ~2.6-fold higher risk of hypertension | [97] |
| Haplotype TC (involved SNPs C+87T in 3′-UTR and G12602C in 3′-end) | Hypertensive-ESRD patients versus controls of African ancestry | ~2.7-fold higher risk of hypertension | [97] |
The base positions are numbered (+/−) with respect to the cap (transcription initiation) site
BP Blood pressure, DBP diastolic blood pressure, SBP systolic blood pressure, ESRD end stage renal disease
The Gly364Ser variant of human catestatin occurring at ~3% allele frequency caused profound changes in cardiac activity in two Southern California populations [62]. Carriers of the 364Ser allele displayed increased baroreceptor sensitivity, increased cardiac parasympathetic activity, and decreased cardiac sympathetic activity. Consistent with the enhanced baroreceptor sensitivity, the pressor response was significantly less in Gly/Ser heterozygotes when compared with Gly/Gly homozygotes following cold stress [62]. The Gly364Ser variant was also associated with significant alterations in resting blood pressure. For example, Gly/Gly homozygotes displayed ~5–6 mmHg higher DBP and ~13 mmHg higher SBP than Gly/Ser heterozygotes [62]. Intriguingly, this lower blood pressure effect (and hence a reduced risk of developing hypertension) for the 364Ser genotype was confined to men [62]. These observations may be explained by an action of this peptide at the central nicotinic–cholinergic synapses in the nucleus of the tractus solitarius in brain stem, although the exact molecular mechanism underlying such alterations in cardiovascular functions associated with the uncommon Gly364Ser variant of catestatin remains to be elucidated.
More recently, two studies in Southern California populations were also carried out to explore association of any common genetic variation at the CHGA locus with regulation of blood pressure.
One case–control study focused on CHGA promoter variants in 919 subjects: 204 hypertensives and 715 normotensives [36]. It was observed that the G-462A variant predicted resting/basal BP in the population with the G allele displaying higher SBP and DBP [36]. Intriguingly, the G/A genotype (heterozygosity) was associated with higher SBP and DBP than G/G and A/A genotypes [36], suggesting molecular heterosis [63]. Of note, in a cohort of predominantly normotensive twin pairs, the most common promoter haplotype TTGTC (designated as “Haplotype A” that contains the G-462 allele, indicated in bold type; allele frequency ~57%) displayed higher post-cold stress DBP as compared to another common haplotype CGATA (designated as “Haplotype B”; allele frequency ~23%), suggesting the promoter Haplotype A as a risk factor genotype for EH [36].
How might be these CHGA promoter variants play a role in the control of BP? Earlier reports documented that both hypertensive and still-normotensive subjects with family history of hypertension (hence at genetic risk) may display altered autonomic function and CHGA expression [54, 64]. Indeed, the CHGA Haplotype A (containing the G-462 allele)-luciferase reporter plasmid showed higher expression of the reporter gene than the Haplotype B-luciferase reporter plasmid in PC12 adrenal chromaffin cells and AtT20 pituitary corticotrophe cells [36]. Consistent with this observation, the CHGA promoter bearing the G-462 allele displayed ~25% higher activity than the −462A allele on the background of Haplotype A [36], clearly showing an alteration of gene expression by these common promoter polymorphisms. Such transcriptional control of CHGA by promoter variants may be an early control point in the development of cardiovascular risk in view of the crucial role of CHGA in the formation of catecholamine storage vesicles as well as in the regulation of transmitter release to the circulation [22, 24, 25].
Another study investigated individuals with extreme blood pressures: 189 and 175 hypertensive men and women versus 281 and 383 hypotensive men and women [37]. These subjects were selected on the basis of their DBP: the inclusion criteria being DBP values of ≥96 and ≤61 mm for men while ≥92 and ≤59 mm for women, thus high and low blood pressure groups differed by more than 33 mmHg DBP. It was found that a common genetic variant in the CHGA UTR (C+87T) was strongly associated with EH, especially in men. Subjects with C/C genotype displayed up to ~12 and ~9 mmHg higher SBP and DBP, respectively, than those with T/T genotype. The most common haplotype GGCC (consisting of the promoter G-462A, coding exon6 Glu[G]246Asp, coding exon7 Arg[C]381Trp and 3′-UTR C+87T SNPs; occurring at ~55% frequency) in this population dose-dependently (from 0 → 1 copy → 2 copies) predicted higher DBP in men [37], thus showing progressing elevation of DBP by the +87C allele in the context of the GGCC haplotype. Notably, the +87C allele was also associated with significantly higher plasma concentration of CHGA [37], an observation consistent with the over-expression of CHGA in EH as reported in earlier studies [54, 56, 64]. Moreover, the C+87T variant also predicted blood pressure response to cold stress in normotensive twin pairs: the C allele that was associated with higher basal blood pressure in the population displayed increased systolic blood pressure response to stress by ~12 mmHg [37], suggesting it as a risk factor allele for EH.
Although the detailed mechanism by which this 3′-UTR SNP alters the blood pressure is not known, similar to the case of the promoter “Haplotype A” (discussed above), this may be mediated by alteration of CHGA expression because CHGA is a key regulator of sympathochromaffin activities [22, 24, 25]. Indeed, in cultured chromaffin cells transfection of CHGA 3′-UTR/luciferase reporter, expression plasmids showed ~30% higher reporter activity in the case of the C allele (that was associated with higher blood pressure) in comparison with the T allele [37]. The altered CHGA expression in turn is expected to modulate catecholamine storage and release processes [24, 25]. Consistent with this suggestion, reduction of endogenous CHGA expression by siRNA in chromaffin cells caused up to 75% depletion of catecholamine storage vesicles [37].
Thus, the C-allele (occurring at ~73% frequency) of the common 3′-UTR variant C+87T emerged as a risk factor for hypertension in males (although not in females) of European ancestry [37]. The mechanism of this sex-dependent effect associated with this genetic variant remains to be elucidated. Nevertheless, influence of this variant on environmental stress-evoked change (as noted above) in BP indicates an early pathogenetic role.
Cardiovascular activities of the CHGA peptides
Consistent with elevated blood pressure and LV hypertrophy of chga −/− mice [25], a number of studies have reported that some CHGA-derived peptides modulate cardiovascular processes and may play important roles in regulation of blood pressure. In this section, we will provide an up-to-date overview of the relevant literature on the two peptides vasostatin-I, and catestatin (Fig. 1) that have been relatively well studied.
Vasostatin-I
This 76-residue, N-terminal peptide (that contains three amphipathic domains: CHGA1–16, CHGA17–38, and CHGA47–66 [35]; Fig. 1) is highly conserved across vertebrate species, from zebrafish to mammals, displaying ~80% sequence homology [65]. It has been reported to exert several modulatory effects of cardiovascular relevance [66]. Studies on isolated segments of human blood vessels (internal thoracic artery and saphenous vein) showed that vasostatin-I inhibited endothelin-1 (ET-1)-induced vasoconstriction and that the inhibitory effect was independent of endothelium and extracellular calcium [26, 67, 68]. Vasostatin-I also displayed inhibitory effect in pressure-activated bovine coronary and adrenal resistance arteries [69, 70]. The mechanism of the vasodilatory effect of vasostatin-1 remains unclear, partly because any cell surface receptor has not yet been identified, although similar sized vasostatin-I binding proteins have been detected in calf aorta smooth muscle cells [68] and bovine parathyroid cells [71]. Nevertheless, involvement of a Gαi/0 subunit has been suggested since pertussis toxin diminished the vasodilatory effect in coronary artery [72].
Another important cardiovascular regulatory function associated with vasostatin-I is protection of the integrity of endothelial barrier. It has been shown that vasostatin-I inhibits TNF-α-induced gap formation in arterial endothelial cells of bovine pulmonary and coronary origin, suggesting its effect on endothelial barrier dysfunction in venous as well as arterial vascular beds [73]. Vasostatin-I also partially inhibits thrombin- and VEGF-induced permeability of HUVEC cells [74]. Although the receptors or molecular targets on endothelial cells underlying these effects of vasostatin-I is unclear, involvement of p38MAP kinase signaling cascade via a pertussis toxin (PTX)-sensitive mechanism has been hypothesized (Fig. 2; [41]). However, since endothelial dysfunction is associated with pathophysiology of cardiovascular disease states including EH [75–77], vasostatin-I may have modulatory role in EH by virtue of its endothelial barrier-protective function.
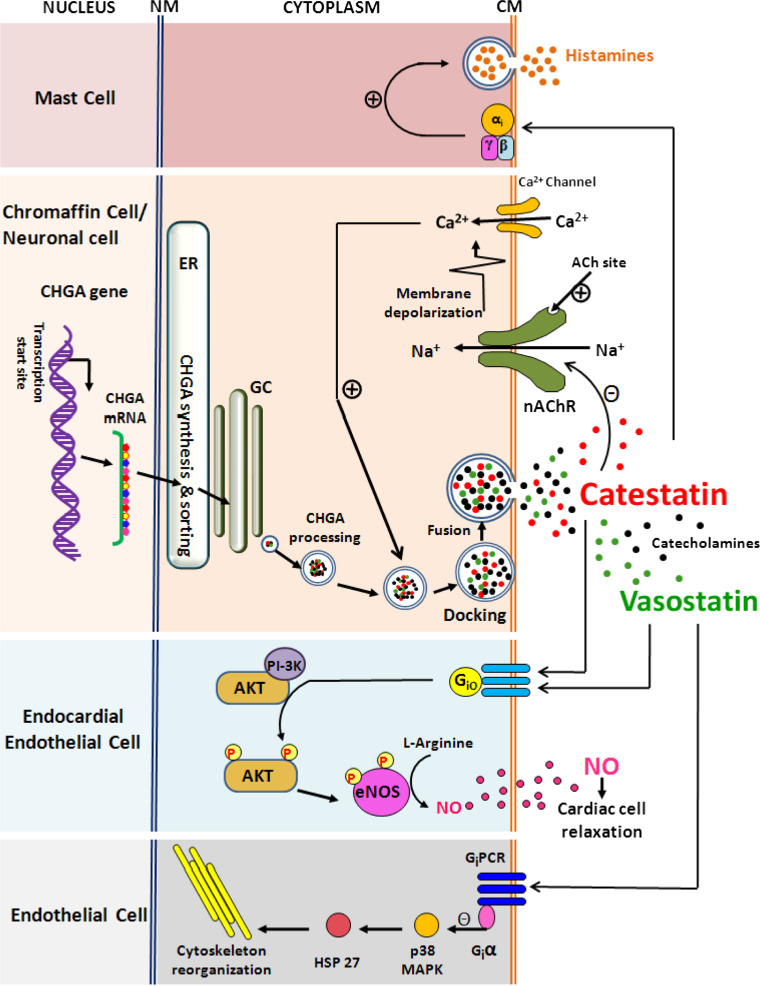
Fig. 2.
Plausible molecular mechanisms of action of the CHGA-derived peptides vasostatin-I and catestatin in various cell types. A schematic of the major signaling pathways by which these peptides may exert their cardiovascular activities is presented. Catestatin acts on (1) mast cells to stimulate release of the vasodilator histamine in a receptor-independent manner via inhibitory heterotrimeric G-proteins [41], (2) chromaffin cells/neurons to inhibit exocytotic release (that involves intracellular free Ca2+-triggered docking of catecholamine storage vesicles on the cytoplasmic membrane) of catecholamines via non-competitive blocking of nAChR [29], (3) endocardial endothelial cells to activate release of the vasorelaxant NO via Protein kinase B signaling to eNOS [95]. Vasostatin-I acts on (1) endothelial endocardial cells to modulate NO release via the PTX-sensitive Gi/0-Akt-eNOS signaling similar to catestatin [82] and (2) endothelial cells to inhibit endothelial dysfunction via down-regulation of p38 MAP kinase phosphorylation involving protection of Giα subunit of Gi proteins, which tonically inhibit downstream signaling through Hsp 27 and the contractile apparatus [41]. ER Endoplasmic reticulum, GC Golgi complex, NM nuclear membrane, CM cytoplasmic membrane, CHGA chormogranin A, Ach Acetylcholine, nAChR nicotinic acetyl choline receptor, NO nitric oxide, Akt Protein kinase B, eNOS endothelial nitric oxide synthase, PI-3K phosphoinositide 3-kinase, MAPK mitogen activated protein kinase, HSP heat shock protein
Several recent studies have reported that vasostatin-I also exerts negative myocardial inotropy (i.e., inhibition of myocardial contraction) both under basal and beta-adrenergic-stimulated conditions [78–81]. In isolated and perfused frog heart, vasostatin-I inhibited myocardial inotropy and counteracted the positive inotropism evoked by the beta-adrenergic agonist isoproterenol [78, 80]. Likewise, in Langendorff-perfused rat heart, the native rat CHGA1–64 peptide corresponding to the human N-terminal vasostatin-I (rCHGA1–64, which contains the disulphide bridge between Cys17 and Cys38 residues) induced negative inotropism and lusitropism (i.e., inhibition of myocardial relaxation) under basal conditions [82]. The rat CHGA1–64 peptide also resulted in coronary dilation [82], consistent with vasostatin-induced vasodilation in human thoracic artery, saphenous vein [26], and bovine coronary arteries [69]. It is, however, noteworthy that the human recombinant vasostatin-I (in contrast with the rCHGA1–64) did not alter the coronary pressure although it displayed similar effects on myocardial contraction and relaxation parameters in the rat heart under unstimulated conditions [83], indicating distinct species-specific vasomotor sensitivities against homologous versus heterologous vasostatin peptides. The rCHGA1–64 also counteracted isoproterenol- and ET-1-induced positive inotropic effects and ET-1-dependent coronary constriction in the rat heart [82]. This is consistent with the abolition of isoproterenol-induced positive inotropism in the rat heart by the human recombinant vasostatin-I [83]. Notably, ET-1 plays important physiological roles in the regulation of normal cardiovascular function, and excessive generation of ET-1 has been linked to major cardiovascular pathologies, including hypertension and heart failure [84]. Therefore, the demonstration of the potent vasodilatory action of the rCHGA1–64 peptide in ET-1 pre-constricted coronaries [82] is of crucial pathophysiological relevance. It is conceivable that, in addition to its anti-adrenergic activity, rCHGA1–64 may function as a cardiac counter-regulatory modulator during exaggerated cardiac hyperactivity, such as neuroendocrine cardiomyopathy and myocardial necrosis [82]. The rCHGA1–64 peptide also reduced papillary muscle contractility under both basal conditions and with beta-adrenergic stimulation [82]. On the other hand, the human recombinant vasostatin-I had no effect on basal contractility of rat papillary muscle although the peptide significantly reduced the effect of isoproterenol-stimulation [85], suggesting a more specific action of the rCHGA1–64 peptide in the rat heart. However, both rCHGA1–64 and human recombinant vasostatin-I peptides promote NO release from endocardial endothelial cells in a calcium-independent/phosphatidylinositol 3-kinase (PI3K)-dependent manner [82, 85]. Additionally, preconditioning with the human vasostatin-I displayed a marked protective effect against the cardiac ischemia-evoked left ventricular infarction [86].
Taken together, cardiodepressive and cardioprotective effects of vasostatin-I appear to involve the PTX-sensitive G-proteins -NO -cyclic GMP -PKG and adenosine A1 receptor -PKC signaling pathways (Fig. 2; [82, 83, 86]). Thus, although a direct function of vasostatin-I in blood pressure regulation has not yet been reported, endogenous vasostatin-I may play important roles under pathophysiological conditions particularly in the presence of intense adrenergic stimuli (e.g., myocardial stress) providing beneficial effects for the stressed heart via vasodilation, protection of endothelial structural integrity, and counteractivity of cardiac contraction and relaxation. Therefore, this multifunctional peptide acting as a modulator of cardiovascular elements may indirectly contribute to the regulation of systemic blood pressure.
Catestatin
A growing body of evidence shows that this 21 residue, cationic and hydrophobic peptide (Fig. 1) plays important roles in the regulation of blood pressure and cardiac function [87]. The plasma catestatin concentration was reported to inversely correlate with hypertension. For example, the plasma catestatin level was diminished in not only established hypertensives but also in their still normotensive offspring. This is in contrast with the observation that the plasma concentration of the parent molecule CHGA is elevated in hypertensive individuals, indicating deficiency in the processing of this prohormone in hypertension. Notably, family history was observed to have a strong influence: despite having similar blood pressures, normotensive subjects with a positive family history displayed significantly lower catestatin levels than those having a negative family history [88]. Thus, the decline in plasma catestatin may be a very early event (even pre-hypertensive) in the course of development of hypertension rather than a late response to the disease state, suggesting a pathophysiologic role of catestatin.
What might be the mechanism of catestatin-mediated blood pressure regulation? Studies in adrenal chromaffin cells [29, 89, 90], in voltage-clamped Xenopus laevis oocytes [91] as well as in mice [43] documented that this peptide acts as a potent antagonist of nicotinic cholinergic receptor, the physiological trigger to efferent autonomic outflow. Therefore, diminished catestatin in circulation may result in augmented catecholamine secretion. Indeed, in addition to lower plasma catestatin levels, individuals with a positive family history of hypertension displayed ~2-fold elevation in urinary epinephrine excretion than those with a negative family history [88]. Corroboratively, the resting arterial plasma norepinephrine concentration was reported to be ~1.7-fold higher in these subjects when compared with those having no family history of EH [92]. Consistent with such apparent tonic sympathoinhibitory effect of catestatin, subjects with lower plasma catestatin displayed higher blood pressure elevations to cold stress [88]. The enhanced pressor response to a sympathoadrenal stressor in catestatin-deficient subjects suggests an adrenergic mechanism whereby diminished circulatory catestatin may lead to the development of hypertension in later years of life [88]. Thus, the diminished plasma catestatin level emerges as an “intermediate phenotype” for EH.
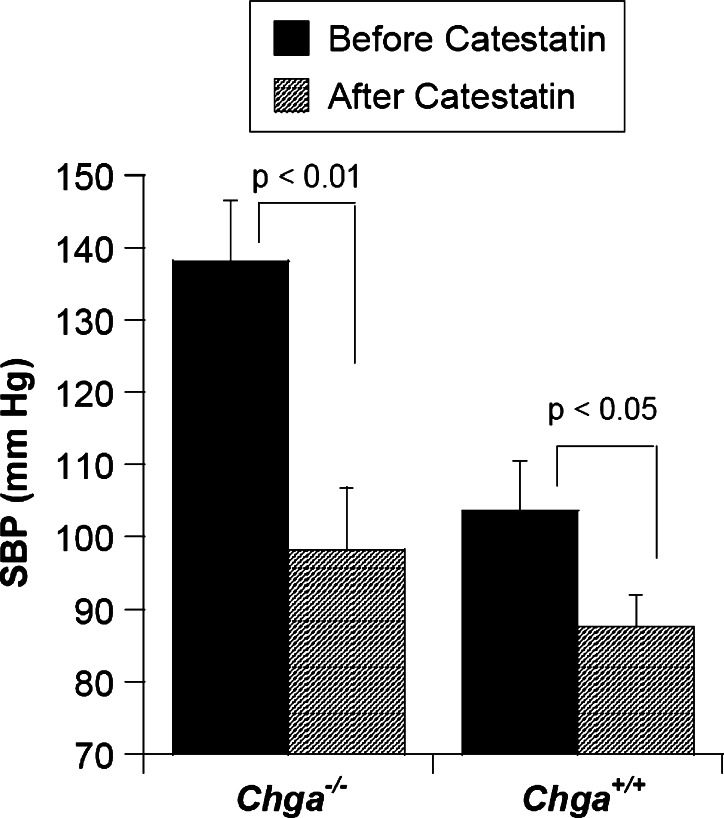
Consistent with these observations in humans, infusion of exogenous human catestatin resulted in a substantial reduction (from 138 to 98 mmHg) of elevated SBP in chga −/− mice. The SBP of wild-type (chga +/+) mice was also found to decrease (from 104 to 88 mmHg), after catestatin injection (Fig. 3; [25]. Although the exact mechanism of the depression of blood pressure after catestatin infusion is not yet known, the antagonistic activity of this peptide to nicotinic cholinergic receptor may play an important role by acting as a ‘physiological brake’ on transmitter release from the sympathochromaffin system (Fig. 2). In corroboration with this explanation, plasma catecholamine levels in chga −/− mice (that lack catestatin because of the absence of the parent molecule) were ~2-fold higher than those in chga +/+ mice [25]. Of note, in an experimental rat model, catestatin displayed vasodepression activity by augmentation of histamine release into the circulation [93], possibly via stimulation of mast cells in a receptor-independent manner involving PTX-sensitive inhibitory GTP-binding proteins (Fig. 2; [41]). However, it is not yet known whether the hypotensive effect of catestatin in mice is contributed by the vasodilatory action of histamine released from mast cells.
Fig. 3.
Catestatin infusion results in lowering of blood pressure in mice. Systolic blood pressure (SBP) was measured before and 120 min after injection of catestatin (20 nmol/25 g body weight, intraperitoneally; expected to result in a concentration of ~4 μM in the extracellular space) to four chga knock out (chga −/−) and four wild-type littermate (chga +/+) by telemetry (chga −/−). The data for generating this plot were taken from [25]. The data were evaluated by unpaired Student’s t test with the InStat 3 program (GraphPad Software). An exaggerated lowering of SBP was observed in the case of chga −/− mice displaying anti-hypertensive effect of the peptide
More recently, it has been reported that (similar to vasostatin-I) catestatin also acts as a cardio-suppressive agent exhibiting reduction of stroke volume and stroke work, inhibition of the positive inotropic effect evoked by isoproterenol/ET-1, in isolated avascular frog heart [94]. The underlying mechanism involves eNOS, cGMP, and ET-1 receptor since pre-treatment with inhibitors of these signaling molecules abolishes this catestatin effect [94]. Catestain has also been reported to exhibit negative inotropism and negative lusitropism under basal conditions, and to inhibit/counteract isoproterenol- or ET-1-induced positive inotropism and coronary constriction in Langendorff-perfused rat heart model [95], similar to vasostatin-I. Angelone et al. [95] also tested the myocardial effects of two naturally occurring human variants of catestatin (viz. Gly364Ser and Pro370Leu) along with the wild-type peptide. In contrast to the wild-type catestatin, the Gly364Ser variant did not affect basal cardiac performance but abolished isoproterenol-induced positive inotropism and lusitropism, while Pro370Leu variant decreased the RPP (an index of cardiac work) and inhibited/reduced isoproterenol-induced positive inotropism and lusitropism [95]. The inotropic and lusitropic effects of catestatin are abolished by inhibition of beta2-adrenergic receptors, Gi/o protein, nitric oxide, or cGMP, indicating involvement of beta2-adrenergic receptors-Gi/o protein-nitric oxide-cGMP signaling mechanisms (Fig. 2; [95]). These cardio-protective effects of catestatin reveal a new cardiovascular role for this anti-hypertensive peptide, particularly under harmful conditions of abnormal systemic/intra-cardiac excitatory stimuli (e.g., as catecholamines and ET-1) targeting the heart as seen in hypertensive cardiomyopathy.
Thus, both vasostatin and catestatin exert cardiosuppressive effects in experimental animal models. Although detailed molecular mechanisms are not yet established, both peptides appear to rely on the release of NO from endocardial endothelium cells (Fig. 2). It remains to be explored whether these peptides act in an additive or competitive manner in ex-vivo (Langendorff model) as well as in vivo conditions.
These two CHGA-derived peptides may also exert beneficial effects during cardiovascular dysfunction provoked by inflammation. For example, chromofungin (CHGA47–66, that corresponds to the antifungal domain of vasostatin-I) and catestatin have recently been shown to stimulate exocytosis from PMNs (the central effectors in innate immune response to inflammatory stimuli) by inducing calcium influx [96]. Both these peptides penetrate into the PMNs, bind with calmodulin, and activate store-operated calcium channels via calcium-independent phospholipase A2. This study clearly depicts immunomodulatory function of the vasostatin-I fragment and catestatin, suggesting important roles for these CHGA-derived peptides as mediators in the cross-talk between the neuroendocrine and immune systems, which may be relevant in several cardiovascular pathological conditions.
Conclusions and perspectives
A number of phenotypic studies (for example, over-expression of the CHGA mRNA in genetically hypertensive rodents [58, 59], higher plasma concentration of the CHGA protein in hypertensive individuals [54, 56], and lower concentration of plasma catestatin in established hypertensives, as well as their still normotensive offspring [88]) over the past several years led to the development of a hypothesis that the CHGA gene may be linked to EH. Consistent with this hypothesis, ablation of the gene in mice was observed to result in major alterations of autonomic/cardiovascular physiology including severe hypertension and left ventricular hypertrophy [25], providing a direct association of this gene with EH. Interestingly, the hypertension in chga −/− mice was rescued by “humanization” of these mice at the CHGA locus or by exogenous administration of catestatin [25], implying a hypotensive effect of the CHGA-derived catestatin peptide. However, because CHGA also gives rise to the vasoactive peptide vasostatin-I in vivo, the rescue of hypertension by expression of human CHGA might also have resulted in or been contributed by vasostatin-I. Therefore, generation and functional characterization of mouse models that specifically cannot produce catestatin and vasostatin-I peptides may provide better insight into the individual role of these peptides in the pathogenesis of hypertension.
Vasostatin-I may also function as a cardiocirculatory homeostatic stabilizer because it is a potent inhibitor of cardiac contraction and relaxation, a non-competitive counter-regulator of beta-adrenergic stimulation and a protecting agent in ischemic preconditioning [81], although this peptide (unlike catestatin) has not directly been associated with pathogenesis of hypertension. Nonetheless, the observation that the native rat vasostatin peptide rCHGA1–64 functions as a potent vasodilator on ET-1 pre-constricted coronaries [82] is of crucial pathophysiological relevance in view of significant roles of ET-1 in hypertension and heart failure [84]. Moreover, functional interactions among vasostatins, catecholamines, and neuropeptides are likely to be important for maintaining vascular homeostasis, particularly under intense cardio-excitatory stimuli [81, 95].
Complementary to observations in mice and rats implicating a pathogenetic role for CHGA in hypertension, the human Gly364Ser variant of catestatin was strongly associated with blood pressure variation and prediction of risk for EH (Gly as the risk factor allele), especially in men [62]. It is noteworthy that no naturally occurring variant of the vasostatin-I peptide has so far been reported. Furthermore, it is also not known whether the plasma concentration of vasostatin-I is diminished in patients with established hypertension and those with a genetic risk for this disorder, as observed in the case of catestatin. This investigation may not only provide a direct association of this peptide with EH, it would also be crucial towards understanding whether the proteolytic processing of CHGA to these active peptides of cardiovascular relevance takes place in a uniform manner or a particular peptide is processed preferentially over others in EH. More recently, common variations in CHGA promoter region (viz. G at the −462 position instead of A and TTGTC haplotype containing the G-462 allele as risk factor genotypes) have been associated with alteration of blood pressure in humans [36]. Additionally, a common UTR variant (C+87T) and the most common haplotype, GGCC, containing the C+87 allele was strongly associated with EH [37] in men. The underlying mechanism of blood pressure regulation by promoter/UTR variants is likely to be alteration of CHGA expression that would change the autonomic tone causing sustained alterations in blood pressure in the later period of life. Taken together, common genetic variants at the CHGA locus influence blood pressure and risk for EH, at least in people of European ancestry. Of note, two CHGA haplotypes have recently been shown to increase the risk for hypertension in ESRD-patients of African ancestry [97].
Thus, the accumulated experimental evidence establishes CHGA as a novel susceptibility gene for EH. Replication studies in subjects with different ancestries will permit assessment of the role of CHGA in EH in the general population that may ultimately lead to the possible clinical application of both the genotypic data (certain genetic variants as risk factor/protective alleles) and phenotypic data (circulating levels of CHGA and its proteolytically-cleaved peptides as biomarkers) for management of cardiovascular diseases.
Acknowledgements
We are grateful to all the researchers who participated in the studies on characterization of chromogranin A and made valuable contributions in this field. We are thankful to the Center for Industrial Consultancy and Sponsored Research at IIT Madras, Department of Biotechnology and Council for Scientific and Industrial Research, Govt. of India for financial support.
Conflict of interest
None
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, Subcommittee Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Gong M, Hubner N. Molecular genetics of human hypertension. Clin Sci (Lond) 2006;110:315–326. doi: 10.1042/CS20050208. [DOI] [PubMed] [Google Scholar]
- 3.Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, Gupta R, Joshi P, Kerkar P, Thanikachalam S, Haridas KK, Jaison TM, Naik S, Maity AK, Yusuf S. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy KM. The genetics of essential hypertension. Br J Clin Pharmacol. 2001;51:5–11. doi: 10.1046/j.1365-2125.2001.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/S0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 6.Kohara K, Tabara Y, Nakura J, Imai Y, Ohkubo T, Hata A, Soma M, Nakayama T, Umemura S, Hirawa N, Ueshima H, Kita Y, Ogihara T, Katsuya T, Takahashi N, Tokunaga K, Miki T. Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens Res. 2008;31:203–212. doi: 10.1291/hypres.31.203. [DOI] [PubMed] [Google Scholar]
- 7.Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 8.Pravenec M, Kurtz TW. Molecular genetics of experimental hypertension and the metabolic syndrome: from gene pathways to new therapies. Hypertension. 2007;49:941–952. doi: 10.1161/HYPERTENSIONAHA.107.086900. [DOI] [PubMed] [Google Scholar]
- 9.Yagil Y, Yagil C. The search for the genetic basis of hypertension. Curr Opin Nephrol Hypertens. 2005;14:141–147. doi: 10.1097/00041552-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Puddu P, Puddu GM, Cravero E, Ferrari E, Muscari A. The genetic basis of essential hypertension. Acta Cardiol. 2007;62:281–293. doi: 10.2143/AC.62.3.2020818. [DOI] [PubMed] [Google Scholar]
- 11.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taupenot L, Harper KL, O’Connor DT. The chromogranin–secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 13.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helle KB. Some chemical and physical properties of the soluble protein fraction of bovine adrenal chromaffin granules. Mol Pharmacol. 1966;2:298–310. [PubMed] [Google Scholar]
- 15.Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967;215:58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- 16.Iacangelo AL, Eiden LE. Chromogranin A: current status as a precursor for bioactive peptides and a granulogenic/sorting factor in the regulated secretory pathway. Regul Pept. 1995;58:65–88. doi: 10.1016/0167-0115(95)00069-N. [DOI] [PubMed] [Google Scholar]
- 17.Aunis D, Metz-Boutigue MH. Chromogranins: current concepts. Structural and functional aspects. Adv Exp Med Biol. 2000;482:21–38. doi: 10.1007/0-306-46837-9_2. [DOI] [PubMed] [Google Scholar]
- 18.Huttner WB, Gerdes HH, Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991;16:27–30. doi: 10.1016/0968-0004(91)90012-K. [DOI] [PubMed] [Google Scholar]
- 19.Videen JS, Mezger MS, Chang YM, O’Connor DT. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- 20.Mahapatra NR, Mahata M, Hazra PP, McDonough PM, O’Connor DT, Mahata SK. A dynamic pool of calcium in catecholamine storage vesicles. Exploration in living cells by a novel vesicle-targeted chromogranin A-aequorin chimeric photoprotein. J Biol Chem. 2004;279:51107–51121. doi: 10.1074/jbc.M408742200. [DOI] [PubMed] [Google Scholar]
- 21.Mahapatra NR, Taupenot L, Courel M, Mahata SK, O’Connor DT. The trans-Golgi proteins SCLIP and SCG10 interact with chromogranin A to regulate neuroendocrine secretion. Biochemistry. 2008;47:7167–7178. doi: 10.1021/bi7019996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/S0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 23.Courel M, Rodemer C, Nguyen ST, Pance A, Jackson AP, O’Connor DT, Taupenot L. Secretory granule biogenesis in sympathoadrenal cells: identification of a granulogenic determinant in the secretory prohormone chromogranin A. J Biol Chem. 2006;281:38038–38051. doi: 10.1074/jbc.M604037200. [DOI] [PubMed] [Google Scholar]
- 24.Kim T, Zhang CF, Sun Z, Wu H, Loh YP. Chromogranin A deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J Neurosci. 2005;25:6958–6961. doi: 10.1523/JNEUROSCI.1058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 27.Strub JM, Goumon Y, Lugardon K, Capon C, Lopez M, Moniatte M, Van Dorsselaer A, Aunis D, Metz-Boutigue MH. Antibacterial activity of glycosylated and phosphorylated chromogranin A-derived peptide 173–194 from bovine adrenal medullary chromaffin granules. J Biol Chem. 1996;271:28533–28540. doi: 10.1074/jbc.271.45.28533. [DOI] [PubMed] [Google Scholar]
- 28.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- 29.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briolat J, Wu SD, Mahata SK, Gonthier B, Bagnard D, Chasserot-Golaz S, Helle KB, Aunis D, Metz-Boutigue MH. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol Life Sci. 2005;62:377–385. doi: 10.1007/s00018-004-4461-9. [DOI] [PubMed] [Google Scholar]
- 31.Radek KA, Lopez-Garcia B, Hupe M, Niesman IR, Elias PM, Taupenot L, Mahata SK, O’Connor DT, Gallo RL. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008;128:1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 33.Estensen ME, Hognestad A, Syversen U, Squire I, Ng L, Kjekshus J, Dickstein K, Omland T. Prognostic value of plasma chromogranin A levels in patients with complicated myocardial infarction. Am Heart J. 2006;152:927–927. doi: 10.1016/j.ahj.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J. 2009;30:25–32. doi: 10.1093/eurheartj/ehn513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao F, Keiser HR, O’Connor DT. Malignant and benign pheochromocytoma: chromaffin granule transmitters and the response to medical and surgical treatment. Ann N Y Acad Sci. 2002;971:530–532. doi: 10.1111/j.1749-6632.2002.tb04519.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, Salem RM, Shih PA, Das M, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, O’Connor DT. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int. 2008;74:115–125. doi: 10.1038/ki.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, Vaingankar SM, Wen G, Salem RM, Das M, Cockburn MG, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, Taupenot L, O’Connor DT. Naturally occurring human genetic variation in the 3′-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol. 2008;52:1468–1481. doi: 10.1016/j.jacc.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konecki DS, Benedum UM, Gerdes HH, Huttner WB. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987;262:17026–17030. [PubMed] [Google Scholar]
- 39.Mouland AJ, Bevan S, White JH, Hendy GN. Human chromogranin A gene: molecular cloning, structural analysis, and neuroendocrine cell specific expression. J Biol Chem. 1994;269:6918–6926. [PubMed] [Google Scholar]
- 40.Wu HJ, Rozansky DJ, Parmer RJ, Gill BM, O’Connor DT. Structure and function of the chromogranin A gene. Clues to evolution and tissue-specific expression. J Biol Chem. 1991;266:13130–13134. [PubMed] [Google Scholar]
- 41.Helle KB, Corti A, Metz-Boutigue M-H, Tota B. The endocrine role of chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takiyyuddin MA, Cervenka JH, Pandian MR, Stuenkel CA, Neumann HP, O’Connor DT. Neuroendocrine sources of chromogranin-A in normal man: clues from selective stimulation of endocrine glands. J Clin Endocrinol Metab. 1990;71:360–369. doi: 10.1210/jcem-71-2-360. [DOI] [PubMed] [Google Scholar]
- 43.Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, O’Connor DT. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J Biol Chem. 2003;278:32058–32067. doi: 10.1074/jbc.M305545200. [DOI] [PubMed] [Google Scholar]
- 44.Gaede AH, Lung MS Pilowsky PM (2009) Catestatin attenuates the effects of intrathecal nicotine and isoproterenol. Brain Res. doi:10.1016/j.brainres.2009.09.088 [DOI] [PubMed]
- 45.Steiner HJ, Weiler R, Ludescher C, Schmid KW, Winkler H. Chromogranins A and B are co-localized with atrial natriuretic peptides in secretory granules of rat heart. J Histochem Cytochem. 1990;38:845–850. doi: 10.1177/38.6.2139887. [DOI] [PubMed] [Google Scholar]
- 46.Weiergräber M, Pereverzev A, Vajna R, Henry M, Schramm M, Nastainczyk W, Grabsch H, Schneider T. Immunodetection of alpha1E voltage-gated Ca2+ channel in chromogranin-positive muscle cells of rat heart, and in distal tubules of human kidney. J Histochem Cytochem. 2000;48:807–819. doi: 10.1177/002215540004800609. [DOI] [PubMed] [Google Scholar]
- 47.Glattard E, Angelone T, Strub JM, Corti A, Aunis D, Tota B, Metz-Boutigue MH, Goumon Y. Characterization of natural vasostatin-containing peptides in rat heart. FEBS J. 2006;273:3311–3321. doi: 10.1111/j.1742-4658.2006.05334.x. [DOI] [PubMed] [Google Scholar]
- 48.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–1127. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- 49.Lugardon K, Raffner R, Goumon Y, Corti A, Delmas A, Bulet P, Aunis D, Metz-Boutigue MH. Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J Biol Chem. 2000;275:10745–10753. doi: 10.1074/jbc.275.15.10745. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D, Lavaux T, Sapin R, Lavigne T, Castelain V, Aunis D, Metz-Boutigue MH, Schneider F. Serum concentration of chromogranin A at admission: an early biomarker of severity in critically ill patients. Ann Med. 2009;41:38–44. doi: 10.1080/07853890802199791. [DOI] [PubMed] [Google Scholar]
- 51.Dimsdale JE, O’Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci. 1992;51:519–525. doi: 10.1016/0024-3205(92)90029-O. [DOI] [PubMed] [Google Scholar]
- 52.Bernini G, Moretti A, Salvetti A. Chromogranin A in normal subjects, essential hypertensive and adrenalectomized patients. Clin Endocrinol. 2002;57:41–50. doi: 10.1046/j.1365-2265.2002.01557.x. [DOI] [PubMed] [Google Scholar]
- 53.Hsiao RJ, Parmer RJ, Takiyyuddin MA, O’Connor DT. Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine (Baltimore) 1991;70:33–45. [PubMed] [Google Scholar]
- 54.O’Connor DT. Plasma chromogranin A. Initial studies in human hypertension. Hypertension. 1985;7:176–179. doi: 10.1161/01.hyp.7.3_pt_2.i76. [DOI] [PubMed] [Google Scholar]
- 55.Takiyyuddin MA, Cervenka JH, Sullivan PA, Pandian MR, Parmer RJ, Barbosa JA, O’Connor DT. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. doi: 10.1161/01.cir.81.1.185. [DOI] [PubMed] [Google Scholar]
- 56.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA, O’Connor DT. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 57.Schober M, Howe PR, Sperk G, Fischer-Colbrie R, Winkler H. An increased pool of secretory hormones and peptides in adrenal medulla of stroke-prone spontaneously hypertensive rats. Hypertension. 1989;13:469–474. doi: 10.1161/01.hyp.13.5.469. [DOI] [PubMed] [Google Scholar]
- 58.O’Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, Mahata SK, Wu H, Kennedy BP, Ziegler MG, Wright FA, Schlager G, Parmer RJ. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 59.Fries RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, O’Connor DT. Neuroendocrine transcriptome in genetic hypertension: multiple changes in diverse adrenal physiological systems. Hypertension. 2004;43:1301–1311. doi: 10.1161/01.HYP.0000127708.96195.E6. [DOI] [PubMed] [Google Scholar]
- 60.Corti A, Gasparri A, Chen FX, Pelagi M, Brandazza A, Sidoli A, Siccardi AG. Characterisation of circulating chromogranin A in human cancer patients. Br J Cancer. 1996;73:924–932. doi: 10.1038/bjc.1996.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O’Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O’Connor DT. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 63.Comings DE, MacMurray JP. Molecular heterosis: a review. Mol Genet Metab. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- 64.Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O’Connor DT. Chromogranin A. Storage and release in hypertension. Hypertension. 1990;15:237–246. doi: 10.1161/01.hyp.15.3.237. [DOI] [PubMed] [Google Scholar]
- 65.Montero-Hadjadje M, Vaingankar S, Elias S, Tostivint H, Mahata SK, Anouar Y. Chromogranins A and B and secretogranin II: evolutionary and functional aspects. Acta Physiol (Oxf) 2008;192:309–324. doi: 10.1111/j.1748-1716.2007.01806.x. [DOI] [PubMed] [Google Scholar]
- 66.Helle KB (2009) The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res(PMID: 19640932) (in press) [DOI] [PubMed]
- 67.Aardal S, Helle KB. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept. 1992;41:9–18. doi: 10.1016/0167-0115(92)90509-S. [DOI] [PubMed] [Google Scholar]
- 68.Angeletti RH, Aardal S, Serck-Hanssen G, Gee P, Helle KB. Vasoinhibitory activity of the synthetic peptides from the amino terminus of the adrenomedullary chromogranin A. Acta Physiol Scand. 1994;152:11–19. doi: 10.1111/j.1748-1716.1994.tb09780.x. [DOI] [PubMed] [Google Scholar]
- 69.Brekke JF, Osol GJ, Helle KB. N-terminal chromogranin-derived peptides as dilators of bovine coronary resistance arteries. Regul Pept. 2002;105:93–100. doi: 10.1016/S0167-0115(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 70.Brekke JF, Kirkeleit J, Lugardon K, Helle KB. Vasostatins: dilators of bovine resistance arteries. Adv Exp Med Biol. 2000;482:239–246. doi: 10.1007/0-306-46837-9_19. [DOI] [PubMed] [Google Scholar]
- 71.Russell J, Gee P, Liu SM, Angeletti RH. Stimulation of parathyroid hormone secretion by low calcium is inhibited by amino terminal chromogranin peptides. Endocrinology. 1994;135:337–342. doi: 10.1210/en.135.1.337. [DOI] [PubMed] [Google Scholar]
- 72.Helle KB. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol Rev. 2004;79:769–794. doi: 10.1017/S146479310400644X. [DOI] [PubMed] [Google Scholar]
- 73.Blois A, Srebro B, Mandalà M, Corti A, Helle KB, Serck-Hanssen G. The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regul Pept. 2006;135:78–84. doi: 10.1016/j.regpep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Ferrero E, Scabini S, Magni E, Foglieni C, Belloni D, Colombo B, Curnis F, Villa A, Ferrero ME, Corti A. Chromogranin A protects vessels against tumor necrosis factor alpha-induced vascular leakage. FASEB J. 2004;18:554–556. doi: 10.1096/fj.03-0922fje. [DOI] [PubMed] [Google Scholar]
- 75.Watson T, Goon PK, Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 76.Landmesser U, Drexler H. The clinical significance of endothelial dysfunction. Curr Opin Cardiol. 2005;20:547–551. doi: 10.1097/01.hco.0000179821.11071.79. [DOI] [PubMed] [Google Scholar]
- 77.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 78.Tota B, Mazza R, Angelone T, Nullans G, Metz-Boutigue MH, Aunis D, Helle KB. Peptides from the N-terminal domain of chromogranin A (vasostatins) exert negative inotropic effects in the isolated frog heart. Regul Pept. 2003;114:123–130. doi: 10.1016/S0167-0115(03)00112-5. [DOI] [PubMed] [Google Scholar]
- 79.Imbrogno S, Angelone T, Corti A, Adamo C, Helle KB, Tota B. Influence of vasostatins, the chromogranin A-derived peptides, on the working heart of the eel (Anguilla anguilla): negative inotropy and mechanism of action. Gen Comp Endocrinol. 2004;139:20–28. doi: 10.1016/j.ygcen.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Corti A, Mannarino C, Mazza R, Colombo B, Longhi R, Tota B. Vasostatins exert negative inotropism in the working heart of the frog. Ann N Y Acad Sci. 2002;971:362–365. doi: 10.1111/j.1749-6632.2002.tb04497.x. [DOI] [PubMed] [Google Scholar]
- 81.Tota B, Angelone T, Mazza R, Cerra MC. The chromogranin A-derived vasostatins: new players in the endocrine heart. Curr Med Chem. 2008;15:1444–1451. doi: 10.2174/092986708784567662. [DOI] [PubMed] [Google Scholar]
- 82.Cerra MC, Gallo MP, Angelone T, Quintieri AM, Pulerà E, Filice E, Guérold B, Shooshtarizadeh P, Levi R, Ramella R, Brero A, Boero O, Metz-Boutigue MH, Tota B, Alloatti G. The homologous rat chromogranin A1-64 (rCGA1-64) modulates myocardial and coronary function in rat heart to counteract adrenergic stimulation indirectly via endothelium-derived nitric oxide. FASEB J. 2008;22:3992–4004. doi: 10.1096/fj.08-110239. [DOI] [PubMed] [Google Scholar]
- 83.Cerra MC, De Iuri L, Angelone T, Corti A, Tota B. Recombinant N-terminal fragments of chromogranin-A modulate cardiac function of the Langendorff-perfused rat heart. Basic Res Cardiol. 2006;101:43–52. doi: 10.1007/s00395-005-0547-2. [DOI] [PubMed] [Google Scholar]
- 84.Brunner F, Brás-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–531. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Gallo MP, Levi R, Ramella R, Brero A, Boero O, Tota B, Alloatti G. Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2906–H2912. doi: 10.1152/ajpheart.01253.2006. [DOI] [PubMed] [Google Scholar]
- 86.Cappello S, Angelone T, Tota B, Pagliaro P, Penna C, Rastaldo R, Corti A, Losano G, Cerra MC. Human recombinant chromogranin A-derived vasostatin-1 mimics preconditioning via an adenosine/nitric oxide signaling mechanism. Am J Physiol Heart Circ Physiol. 2007;293:H719–H727. doi: 10.1152/ajpheart.01352.2006. [DOI] [PubMed] [Google Scholar]
- 87.Mahapatra NR. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc Res. 2008;80:330–338. doi: 10.1093/cvr/cvn155. [DOI] [PubMed] [Google Scholar]
- 88.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 89.Mahata SK, Mahata M, Wakade AR, O’Connor DT. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A(344–364)): identification of amino acid residues crucial for activity. Mol Endocrinol. 2000;14:1525–1535. doi: 10.1210/me.14.10.1525. [DOI] [PubMed] [Google Scholar]
- 90.Mahapatra NR, Mahata M, Mahata SK, O’Connor DT. The chromogranin A fragment catestatin: specificity, potency and mechanism to inhibit exocytotic secretion of multiple catecholamine storage vesicle co-transmitters. J Hypertens. 2006;24:895–904. doi: 10.1097/01.hjh.0000222760.99852.e0. [DOI] [PubMed] [Google Scholar]
- 91.Herrero CJ, Alés E, Pintado AJ, López MG, García-Palomero E, Mahata SK, O’Connor DT, García AG, Montiel C. Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. J Neurosci. 2002;22:377–388. doi: 10.1523/JNEUROSCI.22-02-00377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrier C, Cox H, Esler M. Elevated total body noradrenaline spillover in normotensive members of hypertensive families. Clin Sci (Lond) 1993;84:225–230. doi: 10.1042/cs0840225. [DOI] [PubMed] [Google Scholar]
- 93.Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/S0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 94.Mazza R, Gattuso A, Mannarino C, Brar BK, Barbieri SF, Tota B, Mahata SK. Catestatin (chromogranin A344–364) is a novel cardiosuppressive agent: inhibition of isoproterenol and endothelin signaling in the frog heart. Am J Physiol Heart Circ Physiol. 2008;295:H113–H122. doi: 10.1152/ajpheart.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang D, Shooshtarizadeh P, Laventie BJ, Colin DA, Chich JF, Vidic J, de Barry J, Chasserot-Golaz S, Delalande F, Van Dorsselaer A, Schneider F, Helle KB, Aunis D, Prévost G, Metz-Boutigue MH. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS One. 2009;4:e4501. doi: 10.1371/journal.pone.0004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salem RM, Cadman PE, Chen Y, Rao F, Wen G, Hamilton BA, Rana BK, Smith DW, Stridsberg M, Ward HJ, Mahata M, Mahata SK, Bowden DW, Hicks PJ, Freedman BI, Schork NJ, O’Connor DT. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol. 2008;19:600–614. doi: 10.1681/ASN.2007070754. [DOI] [PMC free article] [PubMed] [Google Scholar]