Abstract
Melanocytes and Schwann cells are derived from the multipotent population of neural crest cells. Although both cell types were thought to be generated through completely distinct pathways and molecular processes, a recent study has revealed that these different cell types are intimately interconnected far beyond previously postulated limits in that they share a common post-neural crest progenitor, i.e. the Schwann cell precursor. This finding raises interesting questions about the lineage relationships of hitherto unrelated cell types such as melanocytes and Schwann cells, and may provide clinical insights into mechanisms of pigmentation disorders and for cancer involving Schwann cells and melanocytes.
Keywords: Schwann cell precursor, Development, Melanocyte, Neural crest, Stem cell niche, Multipotency, Cell fate specification, Peripheral nerve
Introduction
During vertebrate development, melanocytes and Schwann cell precursors (SCPs) are derived from a group of highly migratory embryonic cells called the neural crest (NC). Neural crest cells (NCCs) arise from the lateral edges of the neural folds at the time of gastrulation. Subsequently, they delaminate and migrate along specific routes to a number of sites where these cells stop and differentiate into a wide variety of derivatives. These include neurons and glial cells of the peripheral nervous system, melanocytes, smooth muscle cells, connective tissue, and cartilaginous and skeletal elements in the head (reviewed in Ref. [1]).
The identity of a NCC is determined in response to both intrinsic and extrinsic influences that operate differently along the neural axis, migratory paths, and homing sites (reviewed in Refs. [2, 3]). At the trunk level, NCCs escape from the neural tube and migrate following stereotypical pathways. Ventrally, the cells that move furthest end up in the vicinity of the dorsal aorta and generate neurons and glia of the sympathetic ganglia. The remaining ventrally migrating cells arrest more dorsally within the anterior sclerotome, close to the neural tube, and generate sensory neurons and glia of the dorsal root ganglia (DRG). In contrast, the late-migrating NCCs follow a dorsolateral path between the dermamyotome and the overlying ectoderm. These cells give rise to melanocytes.
Previous studies on melanocyte origin and migratory path in trunk NC in avian and mouse species led to the prevailing view that all prospective melanocytes arise from NCCs at the dorsal neural tube, migrate between the superficial ectoderm and the dermamyotome, and then invade the skin to eventually differentiate into pigment cells. A recent study performed in our laboratory has readdressed this issue and provided in vivo evidence that large numbers of melanocytes are produced from nerves innervating the skin during development [4]. This study also discovered that, in addition to contributing primarily to glia, SCPs adjacent to nerves are a cellular source of melanocytes. Hence, this study not only calls into question the origin of melanocytes during development but also addresses the plasticity and developmental potential of SCPs. At the same time, it raises interesting questions about the molecular mechanisms regulating skin pigmentation during development, in health, and in pigmentation disorders. It will also serve to refocus the field to the possibility that a stem cell of glial origin could give rise to melanocytes or skin cancer cells in adulthood or during skin repair. In this review, we attempt to place this recent discovery within the perspective of current dogmas in the field and to highlight important issues to be resolved in the future.
Definition, origin, and commitment of Schwann cell precursors
SCPs derive from the NCCs, which migrate into the nascent peripheral nerves during embryonic development. At this level, NC-derived cells that are found both inside and at the edge of the nerves during embryonic days (E) 9.5–14 are called SCPs and no longer possess some biochemical and morphological characteristics of an NCC (reviewed in Ref. [5]). SCPs, also defined by their high dependence on nerve contact for their survival [6], will then mature over time in a defined stepwise process to give rise to all SCs of adult nerves. At the intermediary state the immature SCs can be distinguished from SCPs primarily by the expression of a different set of molecular markers, a basal lamina, and the fact that they no longer depend on axonal survival signals as they possess an autocrine survival circuit [5, 7].
SC lineage progression is strikingly dependent on survival factors, mitogens, and differentiation signals that emanate from the axons with which SCPs and SCs continuously associate. Numerous molecules have been implicated in the regulation of SC development (reviewed in Ref. [5]). One current view postulates that SCPs are generated in the absence of other cues, and their differentiation program is a default pathway in NC lineage. This view has been inferred mainly because no transcription factors that actively promote gliogenesis have been identified, leading to the proposition that gliogenesis might be enabled when neurogenesis or other alternative fates are suppressed.
Important progress has been made in revealing the molecular mechanisms that control the specification of SCPs from NCCs. These advances have been the focus of recent reviews [5, 8]. Although we wish to provide an update, we will primarily review the progress in the perspective of the specific interplay between the nervous system and glia.
The observation that SCPs, unlike immature SCs, undergo rapid cell death if deprived of axons led to the identification of NRG1 (expressed on the peripheral axons) as their major survival signal [9]. Acting through ErbB2/ErbB3 heterodimer receptors [10, 11], the neuregulin signaling was further shown to exert a critical effect on proliferation and differentiation of SCPs [12–17]. However, as neuregulin signaling is essential for the survival of SCPs, and some early migrating NCCs, the exact function of this signaling pathway in specifying SCP fate has been hindered. Overall, the published data reveals that SCPs do form in the absence of neuregulin signaling. The severe SC phenotype apparent at later stages might develop either because of a defect in migration of SCPs into the peripheral nerves or because of their apparent inability to expand in numbers and/or cell death before SCPs could reach the nerve roots [13, 18–20]. At later stages of SC development, neuregulin signaling instructs immature ensheathing cells to become true myelinating SCs and preserves the quiescent state of mature SCs in the adult [17, 21]. This highlights the importance of the neuregulin-based signaling system for virtually all steps of SC differentiation and maintenance.
Several authors suggest that neuregulin signaling may have an early effect on SCP specification by inhibiting neurogenesis, as Notch signaling would do [8, 22–24]. Such signals may function primarily to promote the loss of neuronal differentiation potential, rather than to activate a glial differentiation program within the early condensed DRGs and their associated nerves. In the case of Notch signaling, it has been shown that even a transient activation of Notch in NC stem cells in vitro might be sufficient to cause rapid and irreversible loss of neurogenic activity accompanied by accelerated glial differentiation. In line with this, Weston and his colleagues observed that Delta1, which encodes a Notch ligand, is expressed in early NC-derived neuronal cells in the nascent DRG and that Notch prevents neuronal differentiation while it permits glial differentiation in vitro [25]. Overall, these data suggest a model in which Notch ligands, expressed by neuroblasts in early DRG, would act positively to instruct a cell-heritable switch to gliogenesis in neighboring uncommitted progenitors [26, 27]. More recently, using selective ablations of Notch signaling in either NCCs or SCPs in mouse, two independent studies have reported that Notch signaling has, in fact, multiple regulatory functions in glial specification and differentiation during peripheral nervous system development and maturation, and after injury. Interfering with Notch signaling severely reduced gliogenesis throughout the DRG, despite the ongoing generation of SCPs in peripheral nerves [28]. In a parallel study, Woodhoo et al. [29] demonstrated that the Notch pathway was essential to drive the transition from SCPs to immature SCs. They also showed that Notch regulates the mechanism that controls the proliferation and numbers of SCs and the transition from immature to myelinating SCs by acting as a negative regulatory component. Although the molecular mediators of Notch function in SC development have not been clearly defined, the authors of this study suggested that one role of Notch in generating SCs in vivo was to maintain high levels of ErbB2 receptor in SCPs, leading to enhanced NRG1 signaling, which is required for glial lineage progression.
As exemplified above, the fate of SCPs is tightly regulated by the combinatorial activity of multiple signals intimately connected to the nervous system. Changes in composition or levels of these signals are believed to affect the specification of SC development at early and more advanced stages. Also, as evidenced above, a single protein, e.g. Notch or NRG1, has the capacity to operate across many if not all developmental processes of SC differentiation. This highlights the need for comprehensive characterization of the different states of SCs during development and of the exact interplay between the different regulatory signals.
The origin and migration of melanoblasts: new vistas
Melanoblasts have long been regarded as originating from the NC from where they immediately migrate dorsolaterally between the dermamyotome and the overlying ectoderm, followed by ventral migration through the developing dermis to their final destination, the basal layer of the epidermis and the hair follicles [30]. This proposed origin implies that NCCs are already committed to a melanocyte fate at the level of the neural tube after NC delamination. This model has been primarily supported by radioautographic or quail chick transplantation experiments, vital dye tracing in chick and mouse, and genetic targeting to express reporter markers under melanoblast-specific and melanocyte-specific protein promoters in mouse [31–38]. However, even with these techniques the origin and migration of the melanocyte lineage was not completely defined, because the eventual migration within the prospective dermis to ventral body regions and limbs was not fully addressed. Moreover, this lack of a complete view of how melanocytes are formed during development can also be emphasized by the observation that in Xenopus embryos most of pigment cells utilize a ventral path to later reach the epidermis [39]. It is only recently that new data obtained in our laboratory came to extend our knowledge in the field by unveiling a completely new cellular origin and migration pathway for melanocytes during development [4]. Results of this work suggest a prominent role for SCPs along peripheral nerves as a cellular source of melanocytes in the skin (Fig. 1).
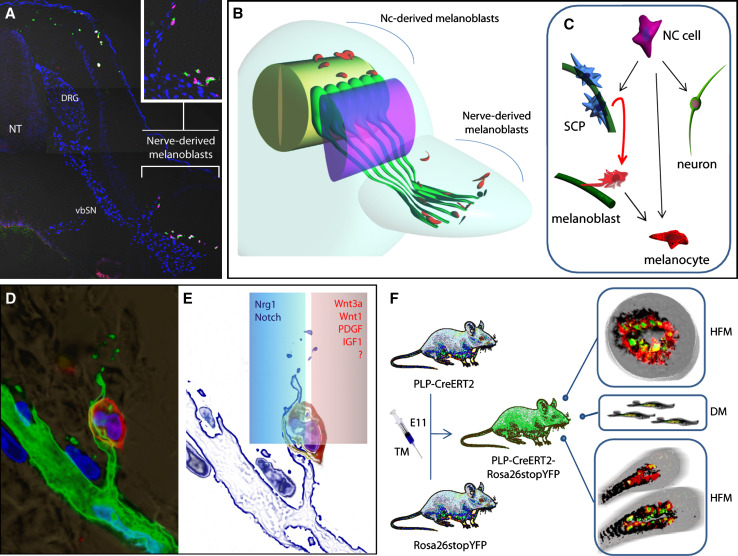
Fig. 1.
Two origins of melanocytes in the embryo: NCCs and SCPs from peripheral innervation. a Section through the E4 chick embryo at the forelimb level. MEBL1 and MITF (markers for melanocytes) are labeled in green and red respectively, Sox10 (SCP and melanoblasts marker) is shown in a blue color. Note the NCC-derived melanoblasts labeled with MEBL-1 and MITF at the dorsal skin above the neural tube. Melanoblasts emerging from SCPs are adjacent to the ventral branch of spinal nerve (vbSN) and are spatially separated from the population of NCC-derived melanocytes. DRG, dorsal root ganglion; NT, neural tube. b 3D representation outlining two separate pathways of melanocytes at the forelimb level in avian embryo. Red color represents melanoblasts, green color, nerves; purple, dermamyotome. c Scheme illustrating the relationship between SCPs, melanocytes and neurons as a result of NC differentiation. Conversion of SCPs into melanocytes is shown by the red arrow. In this case SCPs could correspond to the classical glia-melanocyte precursor postulated in earlier work by Elisabeth Dupin and Nicole M. Le Douarin [1, 97]. d Section with melanoblast adjacent to the dorsal ramus of spinal nerve. Nerve fibers are labeled in green, Sox10+ nuclei of SCPs and melanoblasts are shown in blue, and the red stands for melanocyte marker DCT. e Scheme showing the same cell biased by competition between signaling from the nerve (in a blue frame) and other paracrine signals promoting a melanocyte fate (in a red frame). f Identification of SCP-derived melanocytes in hair follicles and in dermis of adult mice by a genetic tracing approach. PLP-CreERT2 mouse strain was crossed with Rosa26stopYFP strain, and pregnant females were injected with tamoxifen (TM) at the post-NC stage (E11). Subsequent analysis identified numerous YFP+ cells among the population of dermal solitude melanocytes (DM) and in melanocytes of the hair follicles (HFM) in PLP-CreERT2—Rosa26stopYFP mice
As presented above, most previous studies on melanocyte development agreed on the conclusion that all melanoblasts arise directly from the dorsal neural tube and follow the so-called dorsolateral pathway to populate the skin. Therefore, the field has focused mainly, if not solely, on the dorsolateral pathway of NCCs migration during early development, disregarding alternative origins. Strikingly, our recent study characterized further the migration of melanoblasts into the skin and provided evidence for a pathway of melanoblast generation, temporally and spatially distinct from the dorsolateral pathway, that is associated with early spinal nerves [4]. This suggested that the NC-derived cells in the ventral pathway might constitute a source of melanoblasts during development. Micromanipulations of chick embryos in ovo confirmed the additional origin of melanoblasts from the ventral pathway. Lineage tracing experiments with PLP-CreERT2/R26YFP transgenic mice identified the SCPs along the peripheral nerves as the main source (within the ventral pathway) of melanoblasts and postnatal mature pigmented melanocytes in the skin.
With the identification of SCPs as a major source of melanocytes during development, we questioned the existence of a similar mechanism operating at the adult stage [4]. Histological analysis of adult Krox20-Cre/R26YFP mice (in which myelinating SCs and their derivatives express YFP) revealed that although Krox-20-positive differentiated SCs most likely could not give rise to melanoblasts during development, these cells remained competent to form pigmented cells if challenged with a new microenvironment resulting from, for instance, a loss of nerve contact [4]. Likewise, cells losing contact with the peripheral nerves during development acquired a melanoblast fate. Together, these data stress the importance of the nerve contact for balancing glial versus melanocyte cell fates. Also because a proportion of newly formed melanocytes in transected Krox20-Cre/R26YFP animals were not YFP-positive, i.e. did not derive from myelinated SCs, it will be interesting to clarify whether unmyelinated SCs can also differentiate into melanocytes.
As presented above, nerve contact is critical for limiting the differentiation of SCPs into melanoblast fate. Therefore, a disruption in specific signals between nerve and SCPs might affect this cell fate transition. In line with this idea, genetic deletion of ErbB3 in mouse embryos led to an increased number of melanoblasts around the distal ends of dorsal spinal nerves at E12, despite an overall reduction in SCPs along the nerves [4], as previously shown [19]. Strikingly, many Sox10-positive cells were still associated with the nerve endings, suggesting that SCPs that survived in mutant embryos were more likely to become melanoblasts. In addition to emphasizing the importance of neuregulin signaling in SCPs development, these data reveal a completely new function for neuregulin as a key player in driving or maintaining SCP cell fate at the expense of melanoblast differentiation.
The effect of NRG1 on melanocyte cell fate was confirmed in vitro, in which addition of NRG1 to cultured early glial cells reduced the number of newly formed melanoblasts [4]. In contrast, IGF1 and PDGF efficiently promoted the differentiation of SCPs into a melanocyte lineage whereas neuregulin signaling reversed this effect. The dependence of SCPs on NRG1 for cell survival lasts only until their transition into immature SCs which produce and respond to the survival factors IGF and PDGF [7]. Hence, it is conceivable that as glial precursors expand in numbers neuregulin signaling becomes limiting, because of competition for nerve contact. In parallel, as increasing amounts of SCPs differentiate into immature SCs and levels of IGF/PDGF increase, the balance of neuregulin and IGF/PDGF signaling could shift in favor of melanocyte differentiation. A similar mechanism might act at the adult stage during nerve damage, leading to increased expression of IGF/PDGF [7] and loss of the nerve-derived neuregulin signaling.
Although these different factors may affect melanocyte differentiation, the complexity of embryonic development raises the question whether they would be capable of triggering the aforementioned responses in vivo. Further, identification of the in vivo mechanisms operating at the distal nerve environment and balancing the production of SCs versus melanocytes will be an important focus for future research.
At first glance, the proposed view of the origin of melanoblasts stands at odds with former descriptions in the field. But, in fact, the nerve-associated production of melanoblasts has been noted in previous investigations. For example, Thomas and Erickson [40] recently described the presence of melanoblasts in the ventral migratory pathway in the proximity to the brachial plexus of HHst25 (E4.5) chick embryos. Likewise, Niwa et al. [41] observed the presence of a population of melanoblasts apparently distinct from the dorsolateral melanocytes and located between the ventral spinal nerves and the dermis in E5 quail embryos. The finding of the nerve-derived pathway of melanocytes may provide an explanation of previous results showing that melanoblast progenitors populate the skin in relatively small defined patches, rarely organized into stripes, as would be assumed if they were derived directly from the NC and traveled as a proliferating mass across the body [42]. It is conceivable that melanocytes arising from SCPs of cutaneous nerves could explain the observed patches of pigmentation when individual labeled NCCs are studied. This phenotype could appear if expansion of melanoblasts largely takes place within and around nerves terminating in specific cutaneous locations.
Finally, zebrafish, a well-known animal model in pigmentation research, has been the subject of many investigations on the possibility of the existence of a melanocyte stem cell population different from the dorsolateral pathway of melanocytes. Below, we will concentrate on discussing the possibility of a connection between the existence of this melanocyte stem cell population and the SCPs at sites of peripheral innervation.
Enigmatic melanocyte stem cells versus Schwann cell precursors in zebrafish
Danio rerio has long been regarded as an ideal model for study of pigmentation patterns. After extensive experiments Yang and Johnson [43] proposed that zebrafish NCCs generate not only specified melanoblasts and pigmented melanocytes but also give rise to a population of quiescent reserve cells which can be recruited by an unknown mechanism during zebrafish development and organ regeneration. The identity of these cells is unknown. However, experiments on zebrafish mutants involving different embryo manipulations provided some clues about what kind of cells these “melanocyte stem cells” could be and revealed the possibility that SCP-melanocyte cell fate transitions could occur in fish during development and regeneration. Experimentally, this proposition is based on the discovery that newly regenerated zebrafish melanocytes originate from an unknown, unpigmented cellular source, and that this process is dependent on ErbB receptor signaling [44].
Historically Goodrich and Nichols [45] first studied pigmentation recovery after fin ablation in zebrafish. Their results demonstrated that melanocytes ranged in age and that any melanocytes positioned in between pigmented regions had to reposition to within striped regions for survival. Three basic models describing the origin of regenerated melanocytes were then proposed in the field (reviewed in Ref. [43]):
simple division and migration of mature pigmented melanocytes;
cell fate change of other cell types with or without subsequent re-establishment of fate-restricted stem cells; and
proliferation and differentiation of totally unknown unpigmented melanocyte stem cells.
In order to address the first hypothesis, researchers incubated fish in the melanin synthesis inhibitor, phenylthiourea (PTU), which does not allow new pigments to form while not affecting previous pigmentation. This enabled melanin to be used as a lineage tracing marker and led to the discovery that preexisting melanin does not get separated into the vast majority of newly formed melanocytes during fin regeneration. Furthermore, after treatment with the melanotoxic molecule MoTP from 30 to 72 hpf (larval pigmentation takes place from 24 to 60 hpf in zebrafish) which led to apoptotic disruption of all DCT+ pigmented melanocytes, researchers noticed the recovery of DCT+ melanoblasts together with slightly pigmented melanocytes. Later the pigmentation pattern regenerated completely [43, 46]. This finding tells us that new larval melanocytes were regenerated from undifferentiated or transdifferentiated cells. Likewise, after laser treatment and PTU incubation, zebrafish could not recover their pigmentation [47]. However, after PTU wash-out, the pigmentation recovered fully. Taken together, these data strongly suggest that regenerating melanocytes are not derived from preexisting pigmented cells but rather from unpigmented undefined precursors [48].
Transdifferentiation of iridiophores and xanthophores into melanophores was first proposed in amphibians [49, 50]. In order to address this idea in zebrafish, Yang and Johnson [43] conducted chemical MoTP-based ablation of pigmented cells in mutant larvae deficient in iridiophores and xanthophores during 14–72 dpf. Shortly after MoTP washout they observed normal regeneration of pigmentation pattern. Nevertheless, these results do not exclude the possibility of cell fate changes from other cell types [43].
Zebrafish mutants are numerous and their phenotypes have enabled identification of many genes and their associated functions, some of them affecting pigmentation patterns: sparse (kit), leopard (cx41.8), jaguar (kir7.1), rose (endothelin receptor b1, ednrb1), picasso (erbb3), nacre (microphthalmia-a, mitfa), panther (colony stimulating factor 1 receptor, csf1r), bonaparte (bnc2), colorless (sox10), and many others (reviewed in Refs. [51–53]). One of the mutants, named picasso, has a defective receptor-like tyrosine kinase gene ErbB3b, which codes part of the heteromeric receptor conveying neuregulin signaling [54]. Similar to mice, zebrafish deficient in ErbB3b have severe loss of glia [19, 55]. Chemical ablation of melanocytes in picasso fish revealed that the normal pattern of pigmentation cannot recover compared with wild type. One suggestion was that the source of melanocytes in adult fish could be represented by a population of NC-derived cells linearly independent of primary larval pigmented melanocytes, and that its generation could be dependent on NRG signaling [44]. This suggests a similarity between the hypothesized “melanocyte stem cells” and SCPs. Therefore, if we assume that zebrafish have a nerve-derived pathway of melanocytes, we could expect that a reduced glial pool will cause depletion in late melanocyte progenitors.
All aforementioned studies on the different origins of melanocytes do not provide sufficient evidence proving the reality of nerve-derived melanocyte precursors in zebrafish. Yet they show the possibility of this pathway in fish and inspire future efforts in this direction.
Integrative view on molecular mechanisms of melanocyte formation
An important challenge following the discovery of a nerve-derived pathway of melanocyte formation is how to molecularly define the generation of melanocytes from a specific stem cell population. Indeed, recent results suggest that the mechanisms regulating the transition from NC to melanoblast must be reassessed, because the path from the first to the second cell type seems to be more complex than previously thought [4]. In the past, specification cues—intrinsic or extrinsic—have been successfully adopted to induce/regulate specific differentiation of melanoblasts from NC, including MITF (microphthalmia-associated transcription factor), Sox10, Pax3, FOXD3, and the signaling pathways mediated by Kit, Wnt proteins, and endothelins (EDNs) (reviewed in Ref. [56]). Although the field has advanced substantially during the last 10 years, a more integrated view of melanocyte development has not yet been clearly refined. Below, we will briefly describe our current knowledge in the field and try to integrate, where possible, the new pathway of nerve-derived melanocytes with previous conclusions.
Pax3 is a transcription factor that is expressed by NCCs before they delaminate from the dorsal neural tube [57]. Pax3 is one of the earliest markers of NCCs and mouse embryos lacking Pax3 have multiple NC-related defects [58]. Although Pax3 is clearly necessary for the early development of melanoblasts, promoting the expansion of a pool of restricted progenitor cells [59], it is MITF that is referred to as the master regulator of melanoblast specification (reviewed in Refs. [60, 61]). MITF is expressed in melanoblasts soon after emerging from the neural tube and well before they enter the dorsolateral migratory pathway, coincident with lineage specification [62]. Afterwards, MITF was observed in Sox10-positive cells first inside and later around the ventral spinal nerves [4]. In mouse embryos with severe mutations in the MITF locus, NC-derived cells do not reach the Dct-positive stage [62]. It is clear that MITF is essential at the earliest stages of melanocyte specification, as its down-regulation in cultured melanoblasts turns them into glial cells. Furthermore its misexpression in fibroblasts or in neuroretinal cells causes them to adopt a melanocyte-like fate or to differentiate into melanocytes, respectively [63–65].
During melanocyte lineage, MITF is subjected to a complex array of transcriptional regulation that enables accurate developmental expression of MITF. Genetic analysis concentrated on a melanocyte-specific promoter, that controls melanocyte-restricted expression of MITF, has identified an epistatic relationship between Sox10 and MITF [66, 67], giving a molecular basis for the pigmentary defect in mice devoid of Sox10 activity [12, 68]. Detailed quantification of melanoblast numbers revealed a severe and early loss of melanoblasts in Sox10 mutant animals. Sox10 might also work through pathways other than MITF activation in mouse melanocytes. Using lineage-directed gene transfer, Hou et al. [69] showed, for example, that MITF can rescue survival and partial differentiation but not full pigmentation of Sox10-deficient mouse melanoblasts, unable to lead to the expression of tyrosinase. Sox10 also regulates Dct expression by directly binding to its promoter [70], suggesting that additional melanocytic target genes might require Sox10 activity. Another aspect of Sox10 function during melanocyte lineage is its dose-dependent, differentiation-promoting activity. Indeed, a substantial reduction but not complete absence of melanoblasts was noted in the heterozygous Sox10 mutant mice, accounting for pigmentation defects present in these animals in the adult [12, 70]. However, given the minor defects observed in adult mice, the authors suggested that the loss of melanoblasts was, at least in part, compensated during further development. In fact, the absence of Dct expression in heterozygous Sox10 mutant animals analyzed at E10.5–11.5 is transient, because a large increase in the number of Dct-positive melanoblasts occurs at E13.5 [70]. The melanoblasts populate these animals in a correct spatial manner, but with a mild reduction in number that could be related to the visible absence of melanoblasts at the very dorsal aspect of the mutant embryo compared with wild type. A late wave of SCP-derived melanocytes could account for the appearance of melanoblasts in regions previously lacking Dct-positive cells in heterozygous Sox10 mutant animals. This is consistent with the observation that SCPs along peripheral nerves are maintained in those mutant mice [12]. This could also explain why the very dorsal aspect of the embryos, populated mostly by the dorsolateral pathway of melanocytes, remains devoid of melanocyte at later stages in the mutant animals. Moreover, it is interesting to note that analysis of Kit-positive cells in these animals at E12.5 identified a clear presence of melanoblasts at the exact position where SCP-derived melanoblasts have been observed in regions innervated by the dorsal rami nerves [4, 12]. Taken together, these data suggest a model in which Sox10 could regulate melanocyte formation via both the dorsolateral and ventral pathways of NC migration. According to this model, in the dorsolateral pathway Sox10 expression in melanoblasts may directly upregulate genes or gene cascades that drive melanocyte differentiation. In the ventral pathway, the importance of Sox10 expression in SCP specification is, of course, a prerequisite for any SCP-derived melanocytes to be generated. Indeed, gliogenesis and SC maturation are greatly affected in the absence of Sox10 [12, 23, 71, 72]. However, the unique requirement of specific Sox10 domains for melanocyte formation, with no visible effect on SCP differentiation, indicates a selective function of Sox10 regions on SCP-derived melanoblast differentiation [68]. Additional studies will be required to further delineate the temporal and spatial requirements for Sox10 (domains) in proper melanocyte development and function.
In addition to the aforementioned transcription factors, numerous signaling systems regulate many aspects of melanocyte development. Apart from its role in NC induction and expansion at early stages [3, 73], Wnt signaling has also been directly associated with the early formation of the melanocyte lineage from the NC [74–76]. Wnt1 and Wnt3 double-knockout mice exhibit defects in several NC derivatives, including melanocytes [77]. When the signaling pathway is targeted by beta-catenin ablation in NCCs (beta-catenin is the main effector in the canonical Wnt signaling network [78]), melanocytes and sensory neurons are lost [75]. A change in cell fate specification, rather than a proliferation defect may underlie the loss of melanocytes. These observations might reflect a direct relationship between Wnt signaling and induction of MITF expression, because the MITF promoter harbors a binding site for a beta-catenin-containing transcription factor complex and can be activated by Wnt [79, 80]. The critical function of Wnt signaling in driving melanoblast specification also explains the complete absence of melanocyte lineage in the beta-catenin mutant embryos whereas, in contrast, SCPs form independently of beta-catenin activity in these animals [75]. This also suggests the possibility that beta-catenin-dependent signaling could regulate the transition from SCP to melanocyte at the nerve endings.
Another signaling pathway involving stem cell factor (SCF), which is driven by the activation of Kit receptor tyrosine kinase, is thought to be important for their specification, migration, proliferation, and survival. SCF is expressed in the mesenchyme underlying developing skin in mouse embryos where Kit-expressing melanoblasts migrate to the periphery [81–83]. After melanoblasts colonize the skin, SCF expression is thought to be confined to the dermal papillae of hair follicles, where melanoblasts mature into pigmented melanocytes [84]. A number of in vivo and in vitro approaches have demonstrated that signaling through Kit plays an early role in melanoblast proliferation and survival and a later function in the activation of the gene that encodes for tyrosinase [84–87]. Hence, although Kit signaling does not serve an instructive role in lineage commitment, it is required for the generation of sufficient numbers of melanoblasts and their differentiation into melanocytes. The important role of Kit signaling in melanocyte formation has been particularly emphasized by experiments using transgenic mice expressing SCF in the basal layer of the skin [86]. These mice displayed increased pigmentation. As expected, treatment with anti-Kit monoclonal antibody completely prevented pigmentation of these postnatal transgenic mice. But, unexpectedly, a few days after ceasing treatment, skin pigmentation recovered [86]. Interestingly, this process operated through the generation of new melanocytes differentiated from undefined stem cells at isolated skin spots. This result indicates that there must be a melanocyte stem cell population that can survive independently of Kit. This Kit-independent stem cell population may reside in the hair follicles and migrate outside the follicles in a Kit-dependent manner. The presence of such Kit-independent melanocyte stem cells was previously reported in the hair follicles of normal mice [88, 89]. Alternatively, after ceasing Kit antibodies neutralizing treatment, new emerging melanocytes may derive from a resident pool of SCPs. Although the existence of such a population of SCPs at adulthood is still a matter of debate, it is conceivable that Kit signaling may modulate directly or indirectly the emergence of melanoblasts from SCPs—generating the characteristic spotted pigmentation pattern—and, then, further regulate their proliferation at skin level to eventually expand the pigmented spots to cover the entire skin.
Another signal that has been proved to be involved in melanocyte development is EDN3 and its receptor, endothelin receptor-B (EDNRB). Genetic evidence has indicated that targeted disruption of EDN3 or EDNRB genes produces pigmentary disorders [90, 91]. Based on in vitro data, Reid et al. [92] have further suggested that endothelins may regulate the expansion of the melanocyte progenitor pool and their differentiation into mature melanocytes. The development of melanocytes is a multistep process, and EDNRB is expressed in unspecified NCCs and melanocyte precursors. Exploiting strains of mice in which the expression of the EDNRB gene is regulated, Shin et al. [93] determined that the EDNRB gene must be activated between E11.5 and E13 to obtain a full pigmentation pattern. In a more recent study, Lee et al. [94] showed that in ENDRB mutant mouse, melanoblasts lose their fate in a temporal, rostro-caudal manner along the trunk starting at E11 between the otic vesicle and forelimb, with no increased apoptosis in the dorsolateral pathway. The authors suggested that proliferation and/or migration defects explained the observed phenotype. Alternatively, and based on the fact that both EDN3 and its receptor are expressed in peripheral nerves [95], we also propose the hypothesis that disruption of the END3-ENDRB signaling in SCPs during development may result in the pigmentation defects observed in those mutant animals. It has, in fact, been suggested that endothelins, although promoting the survival of SCPs, have a negative role in SCP–SC fate transition [95]. Moreover, using clonal cultures from trunk NCCs, Le Douarin and her colleagues [96] showed that EDN3 promotes a large increase in survival and proliferation of both melanocyte and glial cells, with a more pronounced effect on melanogenic than on glial precursors. They also revealed that exogenous EDN3 could drive embryonic peripheral nerve cells to express a melanogenic potential [97, 98]. Therefore, EDN3 could be one of the crucial signalings that, together with NRG1, IGF1, PDGF, and Wnts, might compete in vivo at the nerve endings to regulate the balance between SCP and melanocyte fates during development.
The multipotent Schwann cell precursor
Several studies have implied that SCPs may represent a transitory bipotent population of post-migratory NC stem cells that retain the capacity to differentiate into both melanocytes and SCs [99–102]. The authors proposed a cell fate change to explain the transition from glial cells into melanocytes. This idea was further supported by in-vitro culture experiments that illustrate the reversion of melanocytes and peripheral glial cells to a bipotent glial-melanocyte progenitor, which would lie upstream in the NC lineage hierarchy [97, 103–105]. In addition, the same group showed that SCs in culture can be de-differentiated to generate myofibroblasts [106]. They also noted that mature pigmented melanocytes can be reprogrammed in culture to give rise to glial cells, myofibroblasts, and multipotent-like NC progenitors capable of self-renewing [107]. However, such plasticity of glial and melanocyte phenotypes was argued to reflect the flexibility of NCC when challenged in vitro with a new environment rather than a phenotypic differentiation occurring during embryonic development. This issue was resolved by new fate-mapping experiments which conclusively demonstrated that a large number of melanocytes in vivo are linearly related to SCPs [4]. The finding that nerve-associated SCPs undergo multilineage differentiation indicates that nerve development is more complex than previously thought. Among other functions, the growing nerves might then be regarded as stem/progenitor cell niches from which diverse cell types could be generated via inductive recruitment. Melanocytes and SCs have been identified as such cell types [4], but the NC contributes with numerous cell types to a large number of tissues during embryogenesis. Hence, in line with this idea, it will be interesting to verify whether, for instance, endoneurial fibroblasts could be regarded in vivo as SCP-derived. Indeed, both SCs and endoneurial fibroblasts arise from a common desert hedgehog (Dhh)-expressing progenitor population within the nerve environment [108, 109], suggesting that SCPs could give rise to endoneurial fibroblasts in vivo.
With regard to the SCP multipotency, it is particularly interesting to note that the expression of the forkhead box transcriptional repressor FOXD3 [110] is present in the precursors of all NC lineages in the mouse. FOXD3 gradually switches off in those precursor cells as they commit to a specific cell fate and persists in glial precursors at the DRG level and along the peripheral nerves until E14, when SCPs differentiate into SCs [111, 112]. Functionally, FOXD3 has been implicated in the control of differentiation in multiple systems, mostly preventing appropriate maturation [40, 112–115]. Interestingly, accumulated data suggest that this mode of action, through cell fate restriction, is a central hallmark of stem cells in general [116–119]. As such, it has been shown that ectopic expression of FOXD3 in the neural tube promotes a NC-like phenotype and continued expression of FOXD3 in migrating NCCs interferes with their subsequent differentiation in NC derivatives [112, 113], suggesting that FOXD3 may play a critical role in establishing or maintaining proliferating and self-renewing progenitor cell populations. Therefore, retention of FOXD3 expression in SCPs during NC lineage specification strengthens the idea that SCPs are indeed multipotent stem cells. It is also worth noting that SCPs are generated and positioned along the nerves as early as E9.5, and provide trophic support to the nerves. They then differentiate into SCs only from E14 onwards, when peripheral nerves have already grown towards their peripheral targets. Thus, one might ask logically what SCPs do during these 4–5 days? It may be simply assumed that SCPs are deployed along the nerves provisionally before they differentiate into SCs. Instead we propose that they represent dormant stem cell elements deployed along highways and easily recruited locally by environmental signals. As such, SCPs could be regarded as NC stem cell-like cells positioned along the nerves and forced to occupy this spatial niche. During that time, the pool of SCPs must proliferate and respond accurately to secreted signals to differentiate into multiple cell types. It is then crucial that these precursor cells maintain their multipotency throughout these critical stages and expand their cell numbers appropriately. From this point of view, FOXD3 would be one of the factors essential for postponing cell fate decision in the SCP population. Thus, the identification of key factors regulating FOXD3 and expressed at critical locations along the peripheral nerves pathway during development might be an important step towards understanding the cell fate transition from SCPs into the wide range of potential derivatives.
Human pathologies potentially associated with Schwann cell–melanocyte cell fate transition
Numerous pathologies in humans are characterized by pigmentation disorders associated with abnormalities in the peripheral nervous system and are commonly referred to as neurocutaneous diseases. Although for some there are good prognoses they can also be high-risk indicators of melanoma cancer and therefore represent serious medical conditions.
Until recently, there has been no clear link between nervous system disorders and the associated pigmentation in most neurocutaneous diseases. Novel data regarding the role of the nervous system in producing pigmentation and the SCP origin of melanocytes may provide a new perspective towards understanding these diseases. Although the existence of a glial cell–melanocyte fate transition and of SCPs themselves has not yet been established under physiological condition in adulthood, this speculative section is intended to provide examples of diseases in which this transition might occur with pathological outcomes.
Vitiligo vulgaris is a well-known pigmentation disorder caused by obliteration of pigmented melanocytes in the patches of affected skin. Although the physiological mechanisms accounting for this disease are still under question, a few observations suggest involvement of the nervous system [120]. One type of vitiligo, called segmental vitiligo, was found to occur with functional disruption of the cholinergic sympathetic nerves [121]. Moreover, two vitiligo-negative patients who underwent thoracoscopic sympathectomy developed depigmentation areas resembling vitiligo manifestation in the regions with disrupted sympathetic innervation [122]. Another neurocutaneous disease, acquired bilateral nevus of Ota, is manifested by symmetrical hyperpigmentation of the head skin along the branches of the trigeminal nerve [123, 124]. The bilateral symmetry of such hyperpigmentation, and its clear position relative to innervation pattern, makes it possible to hypothesize an SC origin of the excess melanocytes, potentially recruited from the trigeminal nerve. Moreover, nevus of Ota has been associated with segmented vitiligo and melanoma cancer in different human patients [124, 125].
The association between pigmentation disorders and the nervous system has also been noted in some cases of extraepidermal melanoma tumors appearing only within the nerve sheaths and ganglia, without any pigmentation abnormalities at the skin level [126]. For instance, pigmented paragangliomas have been observed along the vagal trunk, in the adrenal gland, and in the uterus, spine, retroperitoneum, bladder, mediastinum, and orbit [127]. Moreover, melanotic Schwannoma is characterized by the presence of SCs together with pigmented melanocytes in the same tumor. Such deadly aggregates can be found deep in the body inside the nerve sheaths and hence are often called nerve sheath tumors [128]. It is not known how these tumorigenic melanocytes appear within the nerve sheaths and other extraepidermal locations. Recent data on the SCP origin of melanocytes and the SC–melanocyte cell fate change in adults under specific conditions [4] could lead to new directions in investigation of the origin of these extracutaneous melanomas, which are the worst kind, and almost 5% of all melanoma tumors.
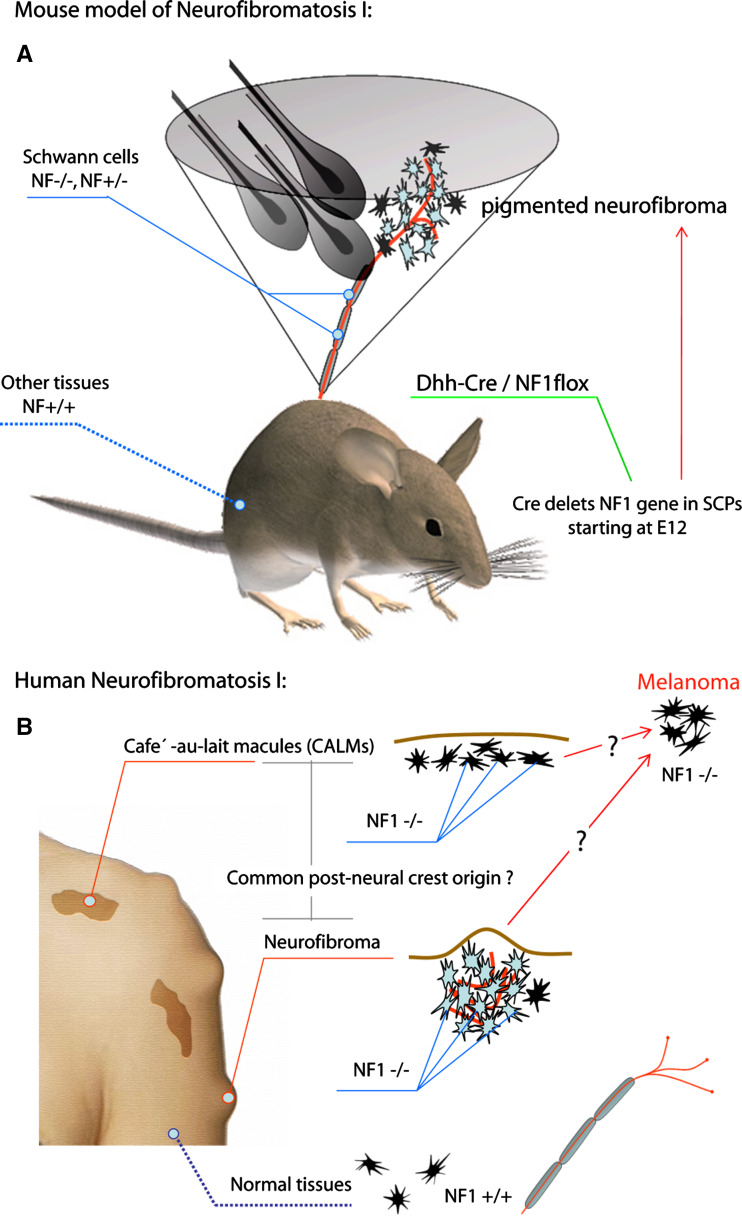
The data on the SCP origin of melanocytes may also help to explain the association between abnormal pigmentation and nervous system disorders observed in patients with neurofibromatosis type 1 (NF1) disease, in which the identification of a common progenitor for the primary tumorigenic cells, identified as early glial cells, and the associated pigmented cells has been substantially supported by experimental data (Fig. 2). NF1 disease is a well studied example of a pigmented body tumor affecting 1 in 3,500 individuals worldwide. Neurofibroma cancer usually consists of axons, SCs, fibroblasts, perineurial cells, mast cells, and melanocytes. Neurofibromas are usually classified into dermal and plexiform neurofibromas. Plexiform neurofibromas can grow from nerves in the skin or from more internal nerve bundles and extend through multiple layers of tissue, a profile that emphasizes its high invasiveness. In addition, approximately 10% of plexiform neurofibromas undergo malignant transformation [129]. Other primary features are the associated abnormal pigmentary defects and the higher incidence of simultaneous occurrence of malignant melanoma [130–132]. Genetic studies on mosaic inactivation of the NF1 gene (neurofibromin) in several patients with NF1 disease identified SC as the major tumorigenic cell type. At the same time, they identified a biallelic inactivation of NF1 in tumorigenic SCs together with associated malignant melanoma cells and NF1-related café-au-lait macules, supporting the idea that melanocytes in neurofibroma tumors could originate from early SC lineage in humans [132, 133]. However, it is only recently that, using different transgenic mice, a post-NC origin of the tumorigenic cell type has been revealed in neurofibroma tumors. More specifically, crossing an NF1 conditional knockout mouse with a battery of deleter strains (Dhh-Cre, Krox20-Cre, PLP-CreERT, and P0-CreERT) driving Cre expression in early glial lineage resulted in successful induction of plexiform neurofibromas associated with hyperpigmentation [134–136]. Conversely, inactivation of NF1 in mature SCs or uncommitted NCCs did not induce tumorigenic properties in the lineage and subsequent NC-derived tissues [136, 137]. These genetic studies provide compelling evidence that plexiform neurofibromas arise most efficiently as a result of NF1 deletion from fetal stem/progenitor cells in developing peripheral nerves. Considering the specific time-dependent requirement of NF1 gene inactivation in SC lineage, one may propose an SCP and/or immature SC origin of melanocytes in NF1 tumors and the associated pigmentation abnormalities and melanoma.
Fig. 2.
Common post-NC origin of melanocytes and tumorigenic SCs in neurofibromatosis type 1 disease. a Mouse model of neurofibromatosis based on the Dhh-Cre/NF1floxed mouse strain. Dhh promoter activates Cre expression in SCPs starting at E12–E12.5 causing deletion of the floxed NF1 gene. Later these mice develop pigmented neurofibroma tumors. b Neurofibromatosis type 1 in humans. Melanocytes in café-au-lait macules (CALMs) are deficient in NF1 gene on the background of normal melanocytes in the rest of the body. Patients with both neurofibromatosis type 1 and melanoma show biallelic deletion of NF1 in tumorigenic melanocytes. These data inspired us to propose a hypothesis implying common post-NC origin of melanocytes in pigmentation abnormalities together with melanocytes in melanoma tumors and mosaic tumorigenic SCs of neurofibromatosis type 1 patients
The existence of a stem cell of glial lineage residing in adult peripheral nerves is still controversial. If true, such quiescent stem cells could be involved in the development of pigmented and non-pigmented tumors throughout the body. Further advances in our knowledge of the connection between SCPs, SCs, and melanocytes in some pathological conditions, and our understanding of the signaling pathways involved in cell fate changes may help us to treat extracutaneous melanomas, melanocytosis, and other diseases. Further, more complete knowledge of mechanisms controlling production of melanocytes from nerves may assist in drug development toward preventing concomitant melanoma in neurofibromatosis type 1 disease and skin cancer in regions subjected to high solar activity.
Dorsolateral and nerve-derived melanocytes in evolution: a speculative view
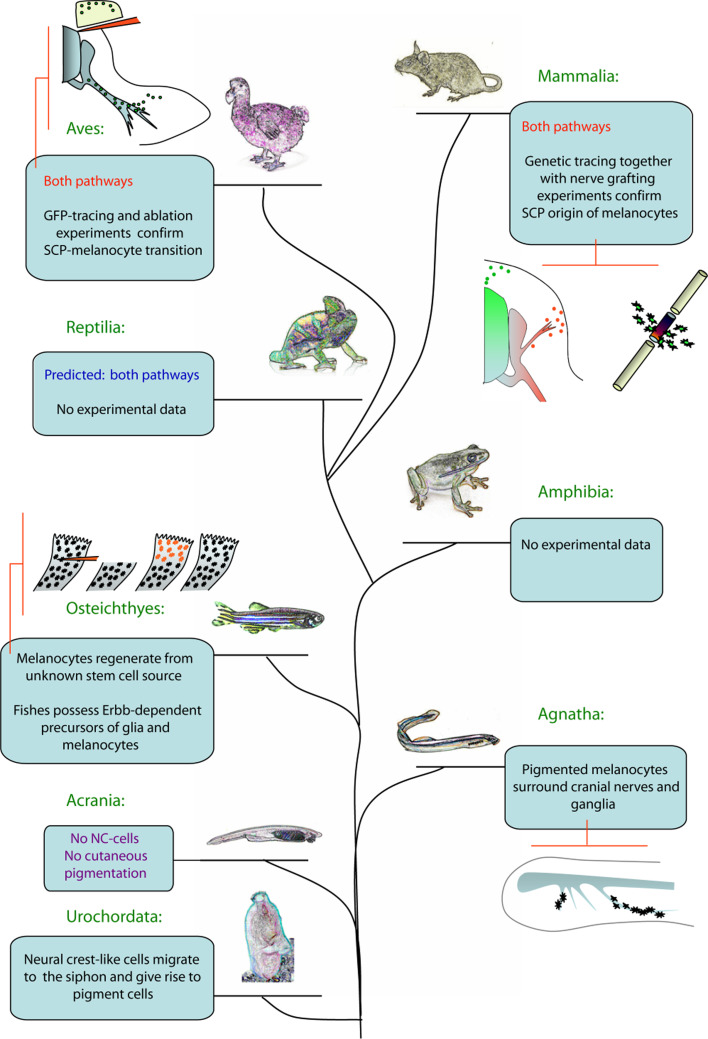
NC-like cells have been described already in Tunicata (Urochordata) in a course of vital dye labeling experiments. The labeled cells were born at the top of the larval neural tube and later migrated to the body wall and siphon primordia where they became pigmented cells. During their migration these cells expressed the typical NC markers HNK-1 and Zic. Those migrating cells could be the only NCCs-like cells in Tunicata, because no other fates and destinations have been identified for them [138–140]. On this basis it has been hypothesized that evolution of the NC lineage started with melanocyte-like migratory cells born in the CNS to generate adult pigmentation in the common ancestor of Tunicata and vertebrates [139]. Millions of years later these cells might have acquired multipotency and hence additional functional properties, eventually spreading their repertoire of fates to glia, sensory neurons, cartilage, and other NC-derived cell types. Alternatively, NCCs have been proposed as originally being derived from primary sensory neurons in antecedent invertebrates [141, 142].
Unlike Tunicata, Amphioxus, a member of Acrania, does not have NC stem cells and cutaneous pigmentation. These animals have very few glia-like supporting cells of uncertain origin in the peripheral nervous system and only extracutaneous ocellus melanocytes [143]. The latest hypothesis in the field of evolution and systematics suggests that currently living members of Tunicata and Acrania represent the side branches on a stem of the evolutionary tree leading to the higher vertebrate taxons [144]. Therefore, these organisms belong to their own crown groups leaving the last common ancestors with vertebrates far away in the past. This particular detail significantly biases our interpretation of how organs and tissues (including NC and pigmentation) evolved in line of early Chordata animals, especially taking into account the passive lifestyle and degeneration of many structures of currently living members of these ancient groups.
Quite modern NC stem cells are found in Agnatha (jawless) fish, and obtain pigmentation and only non-myelinating glial cells. Agnathan fish preceded the emergence of the Gnathostomata group, which includes the real jaw fish, amphibians, reptiles, birds, and mammals. Ultrastructural research on developing larvae of Japanese lamprey (Lampetra japonica) showed the clear presence of mature pigmented melanocytes along the vagal nerve and in contact with other parts of the peripheral nervous system. In particular, the position of their melanocytes strikingly overlapped with the morphology of the cranial nerves and ganglion anlage at specific developmental stages (st28–st29) [145]. This finding is important, because it indicates that some Agnatha potentially have already possessed both mechanisms of making melanocytes: from NC and from SCPs situated along peripheral nerves. An alternative model proposes only migration of pigmented melanocytes along the nerves from NC to cutaneous destinations, thus assigning only navigational function to the innervation.
These studies on lamprey larvae raise questions about the time of origin and intermediate steps in the development of the nerve-derived pathway of melanocytes (Fig. 3). Did this pathway appear simultaneously with the NC-derived pathway of melanocytes or did it appear later as a progressive novelty? Was it evolving through the stage of simple migration and navigation of NC-derived melanoblasts along the nerves in primitive Chordata animals or was the recruitment of glial stem elements from the nerve required in the very beginning?
Fig. 3.
Emergence of the nerve-derived pathway of melanocytes in evolution. This evolutionary tree holds landmarks showing different phenomena putatively related to the conversion of SCPs into melanocytes. Pigmentation of cranial nerves in cyclostome larva and the presence of enigmatic ErbB-dependent nonpigmented melanocyte precursors in zebrafish are good examples of preliminary data showing that nerve-derived melanocytes could potentially appear at one of those levels of organization. Pictograms outline the crucial experiments and observations corroborating the aforementioned idea. Mammalia: on the left pictogram, green symbolizes the NCC-derived melanoblasts with origin in the neural tube (gradient green), and red outlines are melanoblasts derived from SCPs positioned along the nerve (gradient red). The right pictogram depicts the nerve transection experiment (grafted piece is labeled with dark color surrounded by newly formed melanocytes). The Aves pictogram depicts the experiment with surgical ablation of the NC-derived pathway of melanoblasts combined with GFP tracing of NC derivatives. GFP melanoblasts (small circles) are found later in the limb of the embryos with previously deleted dorsal skin and dermamyotome. Osteichtyes; red symbolizes newly formed melanocytes after fin ablation in zebrafish. Melanin does not redistribute in those cells in the presence of PTU, confirming their alternative origin. Agnatha: the nervous system (cranial nerves) of the cyclostome larva is marked by grey color, pigmented melanocytes are black
Apparently, birds and mammals share the presence of both melanocyte-producing pathways suggesting that these pathways did not appear independently as a result of parallel evolution, but were likely to have been present in a common reptilian ancestor. In chick embryo “hot spots” of SCP–melanocyte transitions starting at E4 include dorsal rami of spinal nerves and brachial and lumbosacral plexuses. Later during development melanoblasts appear in proximity to most peripheral nerves, including branches innervating limbs and ventral parts of the developing embryo. In mouse embryo detachment of melanoblasts occurs at dorsal rami of spinal nerves starting from E12, as observed at brachial and thoracic levels [4]. The tracing studies were not sufficient to cover all necessary developmental stages and body regions to confirm that nerves do not contribute with melanocytes in later mouse development. One possible explanation for those differences between chick and mouse includes the evolutionary change in expression pattern of paracrine regulators controlling the process of SCP–melanocyte cell fate transition at the sites close to innervation.
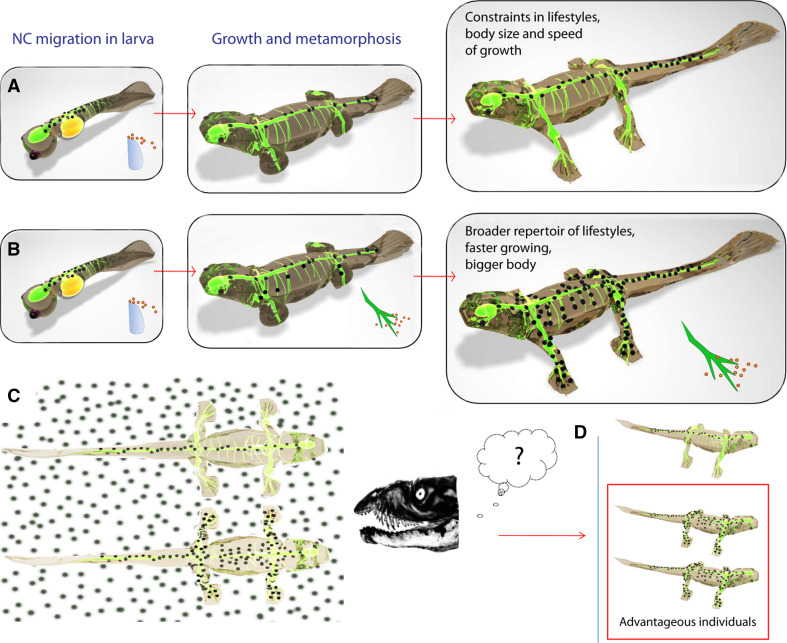
Advantages associated with the nerve-derived pathway of melanocytes potentially include facilitated delivery of stem elements to the remote destinations inside the fast-growing embryonic body (Fig. 4), simplification of intrinsic navigational programs in melanocyte precursors, and cutback of multifarious pathfinding cues spread in developing embryo. The nervous system starts to develop early and reaches all peripheral parts of the embryo very quickly, following complex navigational cues on the way to target tissues including skin. Navigation of a single cell to a specific destination is an elaborate process as the embryonic body is overflowing with pathfinding cues intended for dozens of different cell types. Using nerves as railways for navigation and as a source of stem cell elements seems to be a reliable strategy for populating the skin and extracutaneous locations with melanocytes. The combination of likely conversion of one cell type into another in NC-lineage and the abundance of a pervasive innervation makes implementation of this strategy highly reasonable and expected in evolution. As such, melanocytes could be produced everywhere in proximity to nerve fibers and within migratory distance of cutaneous sites even in large embryos, provided that extrinsic master regulators are appropriately expressed. For example, recent results show that NC-derived melanocytes are not able to populate efficiently all parts of the embryonic skin in the chick. Indeed, after surgical ablation of the nerve-derived pathway, melanocytes originating directly from the NC stay restricted to the dorso-lateral area without increasing their numbers over time. These primary cells do not repopulate the rest of the skin during later days of embryonic development [4]. Although the mature pigmentation pattern in those animals was not analyzed, it seems likely that chicks require SCP-derived melanocytes in order to develop sufficient pigmentation covering all parts of the body.
Fig. 4.
Advantage of a hypothetical vertebrate possessing the nerve-derived pathway of melanocytes compared with a fellow creature with only NC-derived melanocytes. a Development and pigmentation of imaginary vertebrate with NC-derived melanocytes only. b Development and pigmentation of imaginary vertebrate with both pathways generating primary NC-derived melanocytes and later nerve-derived melanocytes. c Insufficient pigmentation is disadvantageous to such individuals (shown in a) faced with active predators. d Presence of both mechanisms producing melanocyte progenitors ensures better camouflage and survival. a–d Green color outlines the nervous system of an animal. Developmental strategies of living creatures always meet environmental criteria and constraints for better fitting and survival. Even tiny growing animals, larvae, should be safe during their growth and maturation. For this reason the speed of their development and their body size should be dependent on their ability to become fully pigmented at the proper time. Furthermore, the number of melanocyte progenitors generated at such early stages must be sufficient to provide a pigmentation pattern of an adult animal later. Insufficiently pigmented individuals are easily eliminated by predators, and, solely because of this, they must hide their bodies, acquire benthic way of life, their activity becomes restricted to the dark part of the day, they are forced to adapt to very deep or permanently dark habitats, for example caves. However, acquisition of mechanisms providing the animal with nerve-derived melanocytes could solve these tricky constraints, giving more developmental flexibility to the system. Animals possessing both ways of making melanocytes can gain faster growth rates and bigger bodies, solely because the speed of larval growth no longer depends on the migration of initial melanocyte precursors. Instead, new precursors can be recruited locally, providing the animal with specific pigmentation pattern. Hence, the lifestyle of the animal could potentially reflect the contribution of each pathway of melanocytes. We could also expect that the nerve-derived pathway of melanocytes appeared in evolution earlier than vertebrate embryos acquired protecting shells
In conclusion, there is no substantial data that convincingly indicate the level of organization at which nerve-derived melanocytes first appear. Therefore, we cannot discard an evolutionary scenario depicting the emergence of a nerve-derived pathway of melanocytes as an advantageous novelty later in evolution (Fig. 4). Like insect wings that degenerated and reappeared many times [146], this new acquisition of a nerve-derived pathway could have followed a similar generation–degeneration profile during evolution. Conversely, it is conceivable that a nerve-derived pathway of melanocytes could have been implemented concomitantly with the NC-derived pathway. In line with this, instead of trying to depict differences in melanocyte pathways across taxa, dissimilarities would be rather more noticeable between species (even within one systematic group) displaying different lifestyles, body proportions and speed of embryonic and postnatal development. Therefore, it will be interesting to compare the relative contribution of the two identified melanocyte pathways in regard to the particular morphological, developmental, and lifestyle characteristics of different animals.
Concluding remarks
The realization that diverse cell types, including melanocytes, can arise from a common pool of precursors located along the peripheral nerves raises numerous questions. An important issue concerns the precise lineage relationship between SCPs-derived subpopulations. In particular, the plasticity of the restrictions towards definable SCP-derived subpopulations has to be elucidated. In this respect, although after nerve injury SCs seem capable of cell fate transition into melanocytes [4, 147], it remains to be addressed whether the reverse is also possible and if such a remarkable reprogramming is valid for other SCP-derived subpopulations. It will be also interesting to see to what extent such fate changes are part of normal physiology. Furthermore, it remains unclear what dictates the decision of SCPs to adopt one fate versus another. NRG1 and IGF/PDGF may play negative and positive roles, respectively, in specification of melanocyte cell fates. How these and other signals impinge on the transcriptional machinery that directs SC, melanocytes, and endoneurial fibroblast cell fates and other yet undefined SCP derivatives remains to be determined. The relative contributions of various SCP-derived subpopulations to the diverse organs or tissues they populate also remain to be resolved.
Another issue is the possible heterogeneity of the SCP pool during development and in different anatomical regions. Evidence has been provided that endoneurial fibroblasts are linearly derived from a Dhh-positive SCP population [109]. Analysis of Dhh expression in NC lineage revealed its presence in SCPs starting from E12.5 onwards [108]. In parallel, results demonstrated that this particular Dhh-positive precursor population does not give rise to melanocytes in the hair follicle [148] whereas, in contrast, earlier PLP-positive SCPs do [4]. Hence, these results unveil an apparent heterogeneity in SCP population with distinct preferences for the range of cell types generated. Such a behavior might arise from changing extrinsic signals along the SCP pathway. Following this notion, specific factors would mediate cell fate biases of SCP subpopulations. A switch in competence or cell fate bias consequently should be reflected by a shift in the molecular profile of a particular SCP subpopulation, as exemplified, for instance, by Dhh expression in late SCPs [108, 109]. It could also simply be assumed that cell autonomous mechanisms might operate over time in mediating changes in the intrinsic responsiveness of SCPs to particular extracellular signals. In line with this, SCPs would switch between different competent states during the successive stages of SC lineage development. Such cell-intrinsic mechanisms could potentially complement the control of cell fate commitment via extrinsic signaling.
Limited knowledge exists today regarding the existence of SCPs subpopulations and their derivatives and their relationships to one another or the molecular networks that regulate their self-renewal or lineage differentiation states. Further analysis of embryonic and adult SCPs (if those cells truly exist) with regard to their derivation promises to enhance our understanding of NCC development and related diseases and may serve as a platform for developing therapies to treat diseases related to dysfunctions in organs, tissues, or cell types to which SCPs contribute.
Acknowledgments
We would like to thank Patrik Ernfors and Ruani Fernando for critical reading of the manuscript. We also thank Anastasia Ermolaeva and Maria Golubeva for helping us with design of the figures. I. Adameyko was supported by the Swedish Medical Research Council. F. Lallemend was supported by the Swedish Medical Research Council and the European Union.
Contributor Information
Igor Adameyko, Phone: +46-85-2487620, FAX: +46-8-341960, Email: igor.adameyko@ki.se.
Francois Lallemend, Phone: +46-85-2487620, FAX: +46-8-341960, Email: francois.lallemend@ki.se.
References
- 1.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 2.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 3.Raible DW. Development of the neural crest: achieving specificity in regulatory pathways. Curr Opin Cell Biol. 2006;18:698–703. doi: 10.1016/j.ceb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, Fritz N, Beljajeva A, Mochii M, Liste I, Usoskin D, Suter U, Birchmeier C, Ernfors P. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 6.Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 7.Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 9.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 10.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syroid DE, Maycox PR, Burrola PG, Liu N, Wen D, Lee KF, Lemke G, Kilpatrick TJ. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc Natl Acad Sci USA. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the Schwann cell precursor pool. J Cell Biol. 2000;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi AD, Bunge RP, Lofgren JA, Meima L, Hefti F, Nikolics K, Sliwkowski MX. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995;15:1329–1340. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/S0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 19.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 20.Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 22.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 23.Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- 24.Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 25.Wakamatsu Y, Maynard TM, Weston JA. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- 26.Kubu CJ, Orimoto K, Morrison SJ, Weinmaster G, Anderson DJ, Verdi JM. Developmental changes in Notch1 and numb expression mediated by local cell-cell interactions underlie progressively increasing delta sensitivity in neural crest stem cells. Dev Biol. 2002;244:199–214. doi: 10.1006/dbio.2002.0568. [DOI] [PubMed] [Google Scholar]
- 27.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/S0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MK, Yeager K, Morrison SJ. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development. 2007;134:2435–2447. doi: 10.1242/dev.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson CA. From the crest to the periphery: control of pigment cell migration and lineage segregation. Pigment Cell Res. 1993;6:336–347. doi: 10.1111/j.1600-0749.1993.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 31.Bronner ME, Cohen AM. Migratory patterns of cloned neural crest melanocytes injected into host chicken embryos. Proc Natl Acad Sci USA. 1979;76:1843–1847. doi: 10.1073/pnas.76.4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston MC. A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat Rec. 1966;156:143–155. doi: 10.1002/ar.1091560204. [DOI] [PubMed] [Google Scholar]
- 33.Le Douarin N. A biological cell labeling technique and its use in experimental embryology. Dev Biol. 1973;30:217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 35.Rawles ME. Some observations on the developmental properties of the presumptive hind-limb area of the chick. Anat Rec. 1947;99:648. [PubMed] [Google Scholar]
- 36.Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- 37.Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- 38.Weston JA. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev Biol. 1963;6:279–310. doi: 10.1016/0012-1606(63)90016-2. [DOI] [PubMed] [Google Scholar]
- 39.Collazo A, Bronner-Fraser M, Fraser SE. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development. 1993;118:363–376. doi: 10.1242/dev.118.2.363. [DOI] [PubMed] [Google Scholar]
- 40.Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–1858. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niwa T, Mochii M, Nakamura A, Shiojiri N. Plumage pigmentation and expression of its regulatory genes during quail development—histochemical analysis using Bh (black at hatch) mutants. Mech Dev. 2002;118:139–146. doi: 10.1016/S0925-4773(02)00256-3. [DOI] [PubMed] [Google Scholar]
- 42.Wilkie AL, Jordan SA, Jackson IJ. Neural crest progenitors of the melanocyte lineage: coat colour patterns revisited. Development. 2002;129:3349–3357. doi: 10.1242/dev.129.14.3349. [DOI] [PubMed] [Google Scholar]
- 43.Yang CT, Johnson SL. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development. 2006;133:3563–3573. doi: 10.1242/dev.02533. [DOI] [PubMed] [Google Scholar]
- 44.Hultman KA, Budi EH, Teasley DC, Gottlieb AY, Parichy DM, Johnson SL. Defects in ErbB-dependent establishment of adult melanocyte stem cells reveal independent origins for embryonic and regeneration melanocytes. PLoS Genet. 2009;5:e1000544. doi: 10.1371/journal.pgen.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodrich H, Nichols R. The development and the regeneration of the color pattern in Brachydanio rerio . J Morphol. 1931;52:513–523. doi: 10.1002/jmor.1050520207. [DOI] [Google Scholar]
- 46.O’Reilly-Pol T, Johnson SL. Melanocyte regeneration reveals mechanisms of adult stem cell regulation. Semin Cell Dev Biol. 2009;20:117–124. doi: 10.1016/j.semcdb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang CT, Sengelmann RD, Johnson SL. Larval melanocyte regeneration following laser ablation in zebrafish. J Invest Dermatol. 2004;123:924–929. doi: 10.1111/j.0022-202X.2004.23475.x. [DOI] [PubMed] [Google Scholar]
- 48.Rawls JF, Johnson SL. Zebrafish kit mutation reveals primary and secondary regulation of melanocyte development during fin stripe regeneration. Development. 2000;127:3715–3724. doi: 10.1242/dev.127.17.3715. [DOI] [PubMed] [Google Scholar]
- 49.Ide H, Akira E. Differentiation and transdifferentiation of amphibian chromatophores. Prog Clin Biol Res. 1988;256:35–48. [PubMed] [Google Scholar]
- 50.Thibaudeau G, Holder S. Cellular plasticity among axolotl neural crest-derived pigment cell lineages. Pigment Cell Res. 1998;11:38–44. doi: 10.1111/j.1600-0749.1998.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 51.Pickart MA, Sivasubbu S, Nielsen AL, Shriram S, King RA, Ekker SC. Functional genomics tools for the analysis of zebrafish pigment. Pigment Cell Res. 2004;17:461–470. doi: 10.1111/j.1600-0749.2004.00189.x. [DOI] [PubMed] [Google Scholar]
- 52.Rawls JF, Mellgren EM, Johnson SL. How the zebrafish gets its stripes. Dev Biol. 2001;240:301–314. doi: 10.1006/dbio.2001.0418. [DOI] [PubMed] [Google Scholar]
- 53.Lang MR, Patterson LB, Gordon TN, Johnson SL, Parichy DM. Basonuclin-2 requirements for zebrafish adult pigment pattern development and female fertility. PLoS Genet. 2009;5:e1000744. doi: 10.1371/journal.pgen.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008;135:2603–2614. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS. erbb3 and erbb2 are essential for Schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–524. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 56.Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 57.Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay P, Kessel M, Gruss P. A transgenic neuroanatomical marker identifies cranial neural crest deficiencies associated with the Pax3 mutant Splotch. Dev Biol. 1995;171:317–329. doi: 10.1006/dbio.1995.1284. [DOI] [PubMed] [Google Scholar]
- 59.Hornyak TJ, Hayes DJ, Chiu LY, Ziff EB. Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech Dev. 2001;101:47–59. doi: 10.1016/S0925-4773(00)00569-4. [DOI] [PubMed] [Google Scholar]
- 60.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 62.Opdecamp K, Nakayama A, Nguyen MT, Hodgkinson CA, Pavan WJ, Arnheiter H. Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop–helix-zipper transcription factor. Development. 1997;124:2377–2386. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- 63.Planque N, Raposo G, Leconte L, Anezo O, Martin P, Saule S. Microphthalmia transcription factor induces both retinal pigmented epithelium and neural crest melanocytes from neuroretina cells. J Biol Chem. 2004;279:41911–41917. doi: 10.1074/jbc.M404964200. [DOI] [PubMed] [Google Scholar]
- 64.Planque N, Turque N, Opdecamp K, Bailly M, Martin P, Saule S. Expression of the microphthalmia-associated basic helix-loop–helix leucine zipper transcription factor Mi in avian neuroretina cells induces a pigmented phenotype. Cell Growth Differ. 1999;10:525–536. [PubMed] [Google Scholar]
- 65.Tachibana M, Takeda K, Nobukuni Y, Urabe K, Long JE, Meyers KA, Aaronson SA, Miki T. Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet. 1996;14:50–54. doi: 10.1038/ng0996-50. [DOI] [PubMed] [Google Scholar]
- 66.Lee M, Goodall J, Verastegui C, Ballotti R, Goding CR. Direct regulation of the Microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J Biol Chem. 2000;275:37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- 67.Verastegui C, Bille K, Ortonne JP, Ballotti R. Regulation of the microphthalmia-associated transcription factor gene by the Waardenburg syndrome type 4 gene, SOX10. J Biol Chem. 2000;275:30757–30760. doi: 10.1074/jbc.C000445200. [DOI] [PubMed] [Google Scholar]
- 68.Schreiner S, Cossais F, Fischer K, Scholz S, Bosl MR, Holtmann B, Sendtner M, Wegner M. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development. 2007;134:3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- 69.Hou L, Arnheiter H, Pavan WJ. Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc Natl Acad Sci USA. 2006;103:9081–9085. doi: 10.1073/pnas.0603114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potterf SB, Mollaaghababa R, Hou L, Southard-Smith EM, Hornyak TJ, Arnheiter H, Pavan WJ. Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev Biol. 2001;237:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/S0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 72.Peirano RI, Goerich DE, Riethmacher D, Wegner M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol. 2000;20:3198–3209. doi: 10.1128/MCB.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu J, Saint-Jeannet JP, Klein PS. Wnt-frizzled signaling in neural crest formation. Trends Neurosci. 2003;26:40–45. doi: 10.1016/S0166-2236(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 74.Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 75.Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin EJ, Erickson CA, Takada S, Burrus LW. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev Biol. 2001;233:22–37. doi: 10.1006/dbio.2001.0222. [DOI] [PubMed] [Google Scholar]
- 77.Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 78.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 79.Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14:158–162. [PMC free article] [PubMed] [Google Scholar]
- 80.Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275:14013–14016. doi: 10.1074/jbc.C000113200. [DOI] [PubMed] [Google Scholar]
- 81.Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122:3023–3033. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- 82.Kluppel M, Nagle DL, Bucan M, Bernstein A. Long-range genomic rearrangements upstream of Kit dysregulate the developmental pattern of Kit expression in W57 and Wbanded mice and interfere with distinct steps in melanocyte development. Development. 1997;124:65–77. doi: 10.1242/dev.124.1.65. [DOI] [PubMed] [Google Scholar]
- 83.Manova K, Bachvarova RF. Expression of c-kit encoded at the W locus of mice in developing embryonic germ cells and presumptive melanoblasts. Dev Biol. 1991;146:312–324. doi: 10.1016/0012-1606(91)90233-S. [DOI] [PubMed] [Google Scholar]
- 84.Yoshida H, Kunisada T, Kusakabe M, Nishikawa S, Nishikawa SI. Distinct stages of melanocyte differentiation revealed by analysis of nonuniform pigmentation patterns. Development. 1996;122:1207–1214. doi: 10.1242/dev.122.4.1207. [DOI] [PubMed] [Google Scholar]
- 85.Hou L, Panthier JJ, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development. 2000;127:5379–5389. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 86.Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, Shultz LD, Nishikawa S, Hayashi S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- 87.Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- 88.Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, Era T, Sakakura T. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okura M, Maeda H, Nishikawa S, Mizoguchi M. Effects of monoclonal anti-c-kit antibody (ACK2) on melanocytes in newborn mice. J Invest Dermatol. 1995;105:322–328. doi: 10.1111/1523-1747.ep12319939. [DOI] [PubMed] [Google Scholar]
- 90.Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 91.Puffenberger EG, Hosoda K, Washington SS, Nakao K, deWit D, Yanagisawa M, Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 92.Reid K, Turnley AM, Maxwell GD, Kurihara Y, Kurihara H, Bartlett PF, Murphy M. Multiple roles for endothelin in melanocyte development: regulation of progenitor number and stimulation of differentiation. Development. 1996;122:3911–3919. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- 93.Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402:496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- 94.Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol. 2003;259:162–175. doi: 10.1016/S0012-1606(03)00160-X. [DOI] [PubMed] [Google Scholar]
- 95.Brennan A, Dean CH, Zhang AL, Cass DT, Mirsky R, Jessen KR. Endothelins control the timing of Schwann cell generation in vitro and in vivo. Dev Biol. 2000;227:545–557. doi: 10.1006/dbio.2000.9887. [DOI] [PubMed] [Google Scholar]
- 96.Lahav R, Dupin E, Lecoin L, Glavieux C, Champeval D, Ziller C, Le Douarin NM. Endothelin 3 selectively promotes survival and proliferation of neural crest-derived glial and melanocytic precursors in vitro. Proc Natl Acad Sci USA. 1998;95:14214–14219. doi: 10.1073/pnas.95.24.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dupin E, Real C, Glavieux-Pardanaud C, Vaigot P, Le Douarin NM. Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proc Natl Acad Sci USA. 2003;100:5229–5233. doi: 10.1073/pnas.0831229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nataf V, Le Douarin NM. Induction of melanogenesis by tetradecanoylphorbol-13 acetate and endothelin 3 in embryonic avian peripheral nerve cultures. Pigment Cell Res. 2000;13:172–178. doi: 10.1034/j.1600-0749.2000.130309.x. [DOI] [PubMed] [Google Scholar]
- 99.Ciment G, Glimelius B, Nelson DM, Weston JA. Reversal of a developmental restriction in neural crest-derived cells of avian embryos by a phorbol ester drug. Dev Biol. 1986;118:392–398. doi: 10.1016/0012-1606(86)90009-6. [DOI] [PubMed] [Google Scholar]
- 100.Nichols DH, Kaplan RA, Weston JA. Melanogenesis in cultures of peripheral nervous tissue. II. Environmental factors determining the fate of pigment-forming cells. Dev Biol. 1977;60:226–237. doi: 10.1016/0012-1606(77)90121-X. [DOI] [PubMed] [Google Scholar]
- 101.Nichols DH, Weston JA. Melanogenesis in cultures of peripheral nervous tissue. I. The origin and prospective fate of cells giving rise to melanocytes. Dev Biol. 1977;60:217–225. doi: 10.1016/0012-1606(77)90120-8. [DOI] [PubMed] [Google Scholar]
- 102.Sherman L, Stocker KM, Morrison R, Ciment G. Basic fibroblast growth factor (bFGF) acts intracellularly to cause the transdifferentiation of avian neural crest-derived Schwann cell precursors into melanocytes. Development. 1993;118:1313–1326. doi: 10.1242/dev.118.4.1313. [DOI] [PubMed] [Google Scholar]
- 103.Dupin E, Glavieux C, Vaigot P, Le Douarin NM. Endothelin 3 induces the reversion of melanocytes to glia through a neural crest-derived glial-melanocytic progenitor. Proc Natl Acad Sci USA. 2000;97:7882–7887. doi: 10.1073/pnas.97.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–3023. doi: 10.1038/sj.onc.1206460. [DOI] [PubMed] [Google Scholar]
- 105.Trentin A, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA. 2004;101:4495–4500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Real C, Glavieux-Pardanaud C, Vaigot P, Le-Douarin N, Dupin E. The instability of the neural crest phenotypes: Schwann cells can differentiate into myofibroblasts. Int J Dev Biol. 2005;49:151–159. doi: 10.1387/ijdb.041940cr. [DOI] [PubMed] [Google Scholar]
- 107.Real C, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Clonally cultured differentiated pigment cells can dedifferentiate and generate multipotent progenitors with self-renewing potential. Dev Biol. 2006;300:656–669. doi: 10.1016/j.ydbio.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 108.Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, Levavasseur F, Raghoenath S, Grosveld F, Meijer D. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17:1380–1391. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 111.Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- 112.Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- 113.Curran K, Raible DW, Lister JA. Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev Biol. 2009;332:408–417. doi: 10.1016/j.ydbio.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu D, Yoder M, Sutton J, Hromas R. Forced expression of Genesis, a winged helix transcriptional repressor isolated from embryonic stem cells, blocks granulocytic differentiation of 32D myeloid cells. Leukemia. 1998;12:207–212. doi: 10.1038/sj.leu.2400948. [DOI] [PubMed] [Google Scholar]
- 116.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dejosez M, Krumenacker JS, Zitur LJ, Passeri M, Chu LF, Songyang Z, Thomson JA, Zwaka TP. Ronin is essential for embryogenesis and the pluripotency of mouse embryonic stem cells. Cell. 2008;133:1162–1174. doi: 10.1016/j.cell.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, Hermanson O, Rosenfeld MG. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 119.Pietersen AM, van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol. 2008;20:201–207. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 120.Yu HS. Melanocyte destruction and repigmentation in vitiligo: a model for nerve cell damage and regrowth. J Biomed Sci. 2002;9:564–573. doi: 10.1007/BF02254984. [DOI] [PubMed] [Google Scholar]
- 121.Koga M. Vitiligo: a new classification and therapy. Br J Dermatol. 1977;97:255–261. doi: 10.1111/j.1365-2133.1977.tb15180.x. [DOI] [PubMed] [Google Scholar]
- 122.Westphal FL, de Campos JR, Ribas J, de Lima LC, Lima Netto JC, da Silva MS, Westphal DC. Skin depigmentation: could it be a complication caused by thoracic sympathectomy? Ann Thorac Surg. 2009;88:e42–43. doi: 10.1016/j.athoracsur.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 123.Long TF, Liu L, He L, Shen LD, Gu H, Yang Z, Tu Y, Ruan RH, Liu Y. Androgen, estrogen and progesterone receptors in acquired bilateral nevus of Ota-like macules. Pigment Cell Melanoma Res. 2010;23:144–146. doi: 10.1111/j.1755-148X.2009.00644.x. [DOI] [PubMed] [Google Scholar]
- 124.Patterson CR, Acland K, Khooshabeh R. Cutaneous malignant melanoma arising in an acquired naevus of Ota. Australas J Dermatol. 2009;50:294–296. doi: 10.1111/j.1440-0960.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 125.Luo XY, Xu AE, Li JC, Guan CP. Naevus of Ota associated with segmental vitiligo: a case report. J Eur Acad Dermatol Venereol. 2009;24:611–612. doi: 10.1111/j.1468-3083.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 126.Hussein MR. Extracutaneous malignant melanomas. Cancer Invest. 2008;26:516–534. doi: 10.1080/07357900701781762. [DOI] [PubMed] [Google Scholar]
- 127.Reddy CE, Panda NK, Vaiphei K, Powari M. Pigmented vagal paraganglioma. J Laryngol Otol. 2003;117:584–587. doi: 10.1258/002221503322113102. [DOI] [PubMed] [Google Scholar]
- 128.Kaehler KC, Russo PA, Katenkamp D, Kreusch T, Neuber K, Schwarz T, Hauschild A. Melanocytic schwannoma of the cutaneous and subcutaneous tissues: three cases and a review of the literature. Melanoma Res. 2008;18:438–442. doi: 10.1097/CMR.0b013e32831270d7. [DOI] [PubMed] [Google Scholar]
- 129.Mautner VF, Friedrich RE, von Deimling A, Hagel C, Korf B, Knofel MT, Wenzel R, Funsterer C. Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma. Neuroradiology. 2003;45:618–625. doi: 10.1007/s00234-003-0964-6. [DOI] [PubMed] [Google Scholar]
- 130.De Schepper S, Boucneau J, Lambert J, Messiaen L, Naeyaert JM. Pigment cell-related manifestations in neurofibromatosis type 1: an overview. Pigment Cell Res. 2005;18:13–24. doi: 10.1111/j.1600-0749.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 131.Guillot B, Dalac S, Delaunay M, Baccard M, Chevrant-Breton J, Dereure O, Machet L, Sassolas B, Zeller J, Bernard P, Bedane C, Wolkenstein P. Cutaneous malignant melanoma and neurofibromatosis type 1. Melanoma Res. 2004;14:159–163. doi: 10.1097/00008390-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 132.Rubben A, Bausch B, Nikkels A. Somatic deletion of the NF1 gene in a neurofibromatosis type 1-associated malignant melanoma demonstrated by digital PCR. Mol Cancer. 2006;5:36. doi: 10.1186/1476-4598-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maertens O, De Schepper S, Vandesompele J, Brems H, Heyns I, Janssens S, Speleman F, Legius E, Messiaen L. Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81:243–251. doi: 10.1086/519562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng H, Chang L, Patel N, Yang J, Lowe L, Burns DK, Zhu Y. Induction of abnormal proliferation by nonmyelinating Schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 137.Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M, Conway SJ, Parada LF, Zhu Y, Morrison SJ. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 2008;13:129–140. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jeffery WR. Ascidian neural crest-like cells: phylogenetic distribution, relationship to larval complexity, and pigment cell fate. J Exp Zool B Mol Dev Evol. 2006;306:470–480. doi: 10.1002/jez.b.21109. [DOI] [PubMed] [Google Scholar]
- 139.Jeffery WR. Chordate ancestry of the neural crest: new insights from ascidians. Semin Cell Dev Biol. 2007;18:481–491. doi: 10.1016/j.semcdb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 140.Jeffery WR, Strickler AG, Yamamoto Y. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature. 2004;431:696–699. doi: 10.1038/nature02975. [DOI] [PubMed] [Google Scholar]
- 141.Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- 142.Donoghue PC, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30:530–541. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wicht H, Lacalli T. The nervous system of amphioxus: structure, development, and evolutionary significance. Can J Zool. 2005;83:122–150. doi: 10.1139/z04-163. [DOI] [Google Scholar]
- 144.Stach T. Chordate phylogeny and evolution: a not so simple three-taxon problem. J Zool. 2008;276:117–141. doi: 10.1111/j.1469-7998.2008.00497.x. [DOI] [Google Scholar]
- 145.Kuratani S, Ueki T, Aizawa S, Hirano S. Peripheral development of cranial nerves in a cyclostome, Lampetra japonica: morphological distribution of nerve branches and the vertebrate body plan. J Comp Neurol. 1997;384:483–500. doi: 10.1002/(SICI)1096-9861(19970811)384:4<483::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 146.Whiting MF, Bradler S, Maxwell T. Loss and recovery of wings in stick insects. Nature. 2003;421:264–267. doi: 10.1038/nature01313. [DOI] [PubMed] [Google Scholar]
- 147.Rizvi TA, Huang Y, Sidani A, Atit R, Largaespada DA, Boissy RE, Ratner N. A novel cytokine pathway suppresses glial cell melanogenesis after injury to adult nerve. J Neurosci. 2002;22:9831–9840. doi: 10.1523/JNEUROSCI.22-22-09831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D, Beermann F, Barrandon Y, Sommer L. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]