Abstract
Celiac disease is characterized by the presence of specific autoantibodies targeted against transglutaminase 2 (TG2) in untreated patients’ serum and at their production site in the small-bowel mucosa below the basement membrane and around the blood vessels. As these autoantibodies have biological activity in vitro, such as inhibition of angiogenesis, we studied if they might also modulate the endothelial barrier function. Our results show that celiac disease patient autoantibodies increase endothelial permeability for macromolecules, and enhance the binding of lymphocytes to the endothelium and their transendothelial migration when compared to control antibodies in an endothelial cell-based in vitro model. We also demonstrate that these effects are mediated by increased activities of TG2 and RhoA. Since the small bowel mucosal endothelium serves as a “gatekeeper” in inflammatory processes, the disease-specific autoantibodies targeted against TG2 could thus contribute to the pathogenic cascade of celiac disease by increasing blood vessel permeability.
Keywords: Celiac disease, Disease-specific autoantibodies, Transglutaminase 2, Vascular permeability, RhoA activation
Introduction
Celiac disease is an autoimmune-mediated enteropathy characterized by the presence of gluten-triggered serum autoantibodies against transglutaminase 2 (TG2) which are highly specific for the disease [1, 2]. Although present in the serum of untreated celiac disease patients, the antibodies are produced in the small-intestinal mucosa [3], where they are found deposited below the epithelial basement membrane as well as around mucosal blood vessels [4, 5]. Interestingly, at the sites where they are sequestered, these autoantibodies can also bind recombinant TG2 in situ and can thus be considered biologically functional [5]. Even though celiac disease has classically been regarded as a T-cell-mediated inflammatory disorder, new evidence is emerging to indicate that the celiac-specific autoantibodies might also play a role in the disease pathogenesis. It has been shown that these autoantibodies inhibit the differentiation [6] and increase the proliferation of epithelial cells [7], reduce the barrier function of epithelium, and activate monocytes [8], thus possibly contributing to the small bowel mucosal pathology. Furthermore, the disease-specific autoantibodies have been reported to induce neuronal cell apoptosis [9] and induce ataxia-like symptoms when injected into the central nervous system of mouse [10]. Interestingly, it has also been published that patients suffering from gluten ataxia, a neurological manifestation of celiac disease, have TG2-targeted autoantibody deposits both in the small intestinal mucosa and also in the brain around blood vessels [11]. Therefore, the celiac-specific autoantibodies may also participate in the development of neurological manifestations in celiac disease and maybe even do so to other extraintestinal manifestations often occurring in conjunction with celiac disease.
We have recently shown that celiac-specific autoantibodies inhibit angiogenesis [12], which might possibly contribute to the altered small bowel mucosal vasculature in untreated celiac disease patients [13, 14]. As abnormal angiogenesis is often associated with increased vascular permeability [15] and further because the functionally active TG2-targeted autoantibodies in celiac disease are deposited around small-bowel mucosal blood vessels, we hypothesized that the disease-specific autoantibodies might modulate vascular permeability. To test this hypothesis, we investigated whether celiac-specific autoantibodies increase endothelial permeability in vitro and, if so, whether this increase is due to altered enzymatic activity of TG2. Moreover, since TG2 is known to enhance RhoA activity [16], implicated in vascular hyperpermeability [17], we studied whether RhoA activation is involved in the endothelial permeability response exerted by celiac autoantibodies targeted against TG2.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs; Clonetics, San Diego, CA) were cultured in EGM-2 medium (Clonetics) supplemented with 20% fetal bovine serum (FBS; Gibco Invitrogen, Paisley, Scotland), 2 mM glutamine (Gibco Invitrogen), 100 U penicillin, 100 μg/ml streptomycin (Gibco Invitrogen) and 25 μg/ml endothelial cell growth supplement (Clonetics).
The human Burkitt’s lymphoma Namalwa cells (CRL-1432; LGC Promochem, Borås, Sweden) were cultured in suspension in a humidified 37°C incubator with a 5% CO2 atmosphere in RPMI-1640 medium containing 7.5% heat-inactivated FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, 4 mM l-glutamine, and 10 mM HEPES buffer (all from Gibco Invitrogen).
For permeability assays, HUVECs were cultured to confluence on a semipermeable Transwell culture insert (Costar, Cambridge, MA) coated with collagen I, prepared as previously described [18].
Purification of serum IgA and IgG autoantibodies
Serum samples from ten IgA-competent celiac patients on a gluten-containing diet and ten non-celiac controls were used in the study. All celiac sera were positive for anti-TG2 and endomysial antibodies whereas all control sera were negative. Total IgA fractions were purified as previously described [12]. Affinity purification of TG2-specific IgA class autoantibodies was not performed because of the high content of oligosaccharide side chains in IgA molecules, which leads to technical difficulties. In order to show that the effects of celiac disease patient IgA are mediated by antibodies targeting TG2, we affinity purified TG2-specific IgG class autoantibodies from IgA-deficient celiac disease patients on a gluten-containing diet for comparison as previously described [12]. As control for celiac-patient-derived anti-TG2-specific IgG, non-celiac total IgG fraction was used. All purified immunoglobulins, both IgA and IgG, were used in the experiments at a concentration of 1 μg/ml and incubated for 24 h unless otherwise stated.
Total IgA fraction from 3 celiac patients and a further 20 celiac serum samples were used in enzyme-linked immunoassay (ELISA) studies.
The study protocol was approved by the Ethics Committees of Tampere University Hospital, Tampere, Finland, and Heim Pál Children’s Hospital, Budapest, Hungary. All individuals involved gave their written informed consent.
Antibodies and inhibitors
Commercially available function-blocking monoclonal anti-TG2 antibody, CUB7402 [19] (NeoMarkers, Fremont, CA), was used in the experiments at a concentration of 60 ng/ml. As negative control, isotype-matched mouse IgG1 (Dako, Copenhagen, Denmark) was applied at the same concentration.
A cell-impermeable TG2 active site inhibitor, R281 [20, 21] was used in experiments in a final concentration of 200 μM. Inhibitor was added 1 h prior to the purified serum immunoglobulins.
The cell permeable Rho inhibitor C3 transferase (Cytoskeletonc, Denver, CO) was added to a final concentration of 1.0 μg/ml and administered 1 h before addition of immunoglobulins.
Paracellular macromolecular permeability assays
Macromolecule transport across endothelial monolayers grown on semipermeable inserts was assessed by measuring the flux of both 4-kD and 70-kD FITC-labeled dextran (500 μg/ml; Fluka Chemicals, Dorset, UK). Different serum immunoglobulins were administered to the apical chamber of confluent endothelial cells 12 h prior to the addition of the endocytosis blocking agent nocodazole (40 ng/ml; Sigma-Aldrich), whereafter the incubation was continued for a further 12 h. Nocodazole was used to specifically study paracellular permeability. Subsequently, FITC-labelled dextran was added apically and, after 3 h, the fluorescence of the transported FITC-dextran was measured from the bottom chambers with a fluorometer (Victor 2 Counter Plate Reader; Perkin-Elmer Wallac, Turku, Finland) using 485 and 535 nm as the excitation and emission wavelengths, respectively.
Lymphocyte adhesion assays and E-selectin expression
To investigate lymphocyte adhesion to confluent endothelial monolayers, study compounds were added apically as described above. Namalwa lymphocytes were labeled by incubating them with 15 μg/ml biscarboxyethyl carboxyfluorescein acetoxymethyl ester (BCECF; Lambda Fluoreszenztechnologie, Graz, Austria) for 30 min at 37°C in RPMI 1640 and 5% FBS and carefully washed to remove non-bound dye. Human B-lymphocyte chemoattractant (50 ng/ml; Sigma-Aldrich) was added to each basal well and apically administered BCECF-labelled lymphocytes (1 × 106 cells) were incubated with HUVEC monolayers for 60 min at 37°C under orbital rotation. After intensive washing, the adherent lymphocytes were detached using 5 mmol/l EDTA, harvested, and quantified with a fluorometer (Victor 2 Counter Plate Reader) using the same excitation and emission wavelengths as above.
For flow cytometric analysis of E-selectin protein expression, 4 × 105 HUVECs were seeded onto 12-well plates (Nunc, Roskilde, Denmark), grown to 90% confluence, and treated with substances under study for 24 h. The cells were washed with Hanks Balanced Salt Solution (HBSS; Sigma-Aldrich) and trypsinized. Thereafter, collected cells were washed with HBSS stained with monoclonal anti-human E-selectin antibody (20 μg/ml, BBIG-E4-5D11; R&D Systems, Abingdon, UK) for 1 h. The cells were then washed and incubated with Alexa Fluor 488-conjugated anti-mouse secondary antibody (1:1000; Molecular Probes, Eugene, OR) for 30 min. Finally, they were washed and analyzed using a fluorescence-activated cell sorter (Coulter EPICS XL-MCL; Beckman Coulter, High Wycombe, UK). For each experiment, duplicates of 1 × 104 cells were counted and the experiment was repeated three times.
For immunofluorescent staining of the E-selectin, HUVEC cells were plated on type I collagen-coated four chamber polystyrene vessel culture slides (BD Biosciences). Confluent cell layers were fixed in 4% paraformaldehyde, whereafter unspecific binding of antibodies was blocked with 0.5% BSA followed by 60 min incubation with the anti-human E-selectin antibody (1:200; R&D Systems) at room temperature. Subsequently, the cells were incubated with Alexa 568-conjugated secondary antibody (1:1,000; Molecular Probes) for 60 min and washed prior to mounting with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Samples were viewed with an Axiovision 3.0 program (Carl Zeiss Vision, Munchen-Halbergmoos, Germany) with a 100× objective.
Lymphocyte transmigration assays
The lymphocyte transmigration assay was performed similarly to the lymphocyte adhesion assay (see above) except that the incubation time was extended to 3.5 h, whereafter the amount of fluorescent lymphocytes was measured in the basal chamber.
Determination of extracellular TG2 activity
The extracellular TG2 transamidating activity was determined by live cell ELISA. HUVEC cells were plated onto collagen I pre-coated 96 well plates (Becton Dickinson Labware, MA) at a density of 2 × 105 cells per well and grown to confluency. Subsequently, the cells were treated with IgA for 24 h. The active site-directed TG2 inhibitor, R281, was administered 1 h prior to adding antibodies at a concentration of 200 μM.
After incubation with study compounds, TG2 substrate monodancylcadaverine (5 mM; Sigma-Aldrich) was added to each well and incubated at 37°C for 2 h. Thereafter, the wells were washed and fixed with 4% paraformaldehyde. Following fixation and washings, the amount of incorporated monodansylcadaverine was detected with rabbit anti-dancyl antibody (1:200; Gibco Invitrogen). Since the cells were not permeabilized, the assay only detected extracellularly incorporated monodansylcadaverine and thus extracellular TG2 activity. As the secondary antibody, horse radish peroxidise-conjugated polyclonal swine anti-rabbit immunoglobulins (1:1,000; Dako, Denmark) were administered to each well and incubated for 30 min at 37°C. Finally, peroxidase substrate, 3,3′,5,5′-tetramethylbenzidine (Slow Kinetic Form, for ELISA; Sigma-Aldrich) was added and the reaction was stopped by 2.5 M H2SO4. The absorbance at 450 nm was measured by spectrophotometer (Multiskan Ascent; Thermo Labsystems, Vantaa, Finland). To control the confluence of the monolayers, cells were stained with crystal violet. To test whether celiac disease IgA has intrinsic activity, the IgA was bound to the bottom of the wells without HUVECs and the assay procedure was otherwise performed as described above.
Western blotting analysis
Western blotting was performed using the Bio-Rad Mini-Protein Tetra Cell system (Biorad Laboratories, Espoo, Finland). Cells were lysed in Laemmli buffer and 10 μg aliquots per lane were loaded on 10% SDS-polyacrylamide electrophoresis gels. Proteins were transferred to nitrocellulose filters (Hybond C-extra; Amersham Biosciences, Little Chalfont, UK) and non-specific binding was blocked with 5% non-fat powdered milk for 1 h at room temperature. Subsequently, the filters were incubated overnight in the presence of mouse anti-TG2 antibody, CUB7402 (1:2,000) at 4°C. Monoclonal antibody against γ-tubulin (1:8,000; Sigma Aldrich) was chosen as internal control to detect equal loading. Blots were then washed, where after secondary horse radish peroxidise-conjugated anti-mouse antibody (1:2,000; Dako) was applied for 1 h at room temperature, washed, developed with enhanced chemiluminescence (Amersham Life Sciences, GE Healthcare, Little Chalfont, UK), exposed to autoradiography film (Kodak, New Haven, CT) and scanned for densitometry analysis with Kodak 1D image analysis software (Kodak). The TG2 expression was normalized to γ-tubulin. The calculated values are from three independent experiments performed in duplicate with IgA derived from three different celiac disease patients and controls subjects.
Enzyme-linked immunoassay with inhibitor-treated TG2
Full-length human recombinant TG2 was expressed in E. coli and purified as described [22]. Maxisorp™ microtiter plates (Nunc) were coated for 60 min at room temperature with 0.3 μg human fibronectin (Sigma-Aldrich) diluted in bicarbonate buffer pH 9.6. The plates were washed three times with tris-buffered saline, pH 7.4, containing 0.1% (v/v) Tween 20 (TBS), and incubated with 0.8 μg human recombinant TG2 in TBS containing 5 mM CaCl2. After washings, the plates were treated with 200 μM R281 in TBS for 20 min and celiac antibodies were added to TG2 for 60 min in the presence of R281. Antibody dilutions were established by previous measurements and were 1:50–1:3,600. Bound antibodies were detected with horse radish peroxidase-conjugated rabbit anti-human IgA (1:5,000; Dako) and 100 μl 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich). The absorbance was read at 450 nm after stopping the reaction with 50 μl 1 M H2SO4. Wells prepared without R281 were used as controls.
RhoA activation assay
To determine RhoA activity, 4 × 105 HUVECs were seeded on 12-well plates (Nunc) and grown to approximately 80%, confluence after which they were exposed to study substances. RhoA activation measurements were made using the absorbance-based G-LISA RhoA Activation Assay Biochem Kit (Cytoskeleton) according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using the Kruskall–Wallis test. The data are presented as mean ± standard error of mean (SEM). A P value < .05 was considered statistically significant.
Results
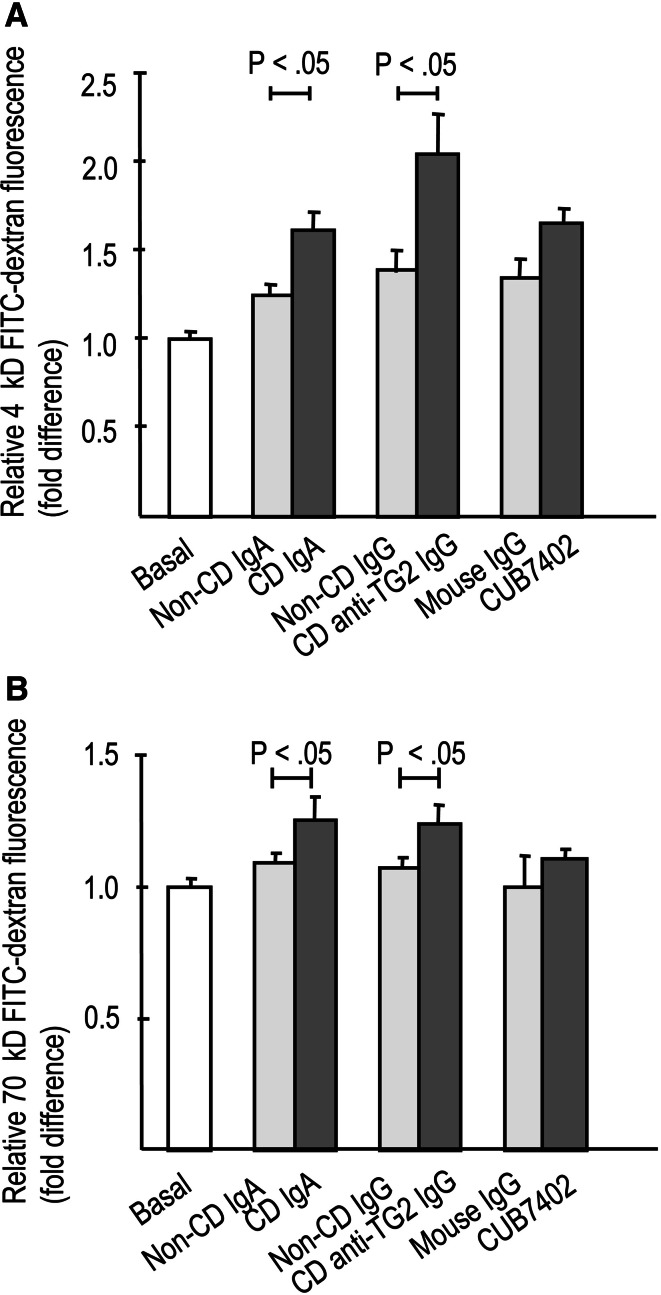
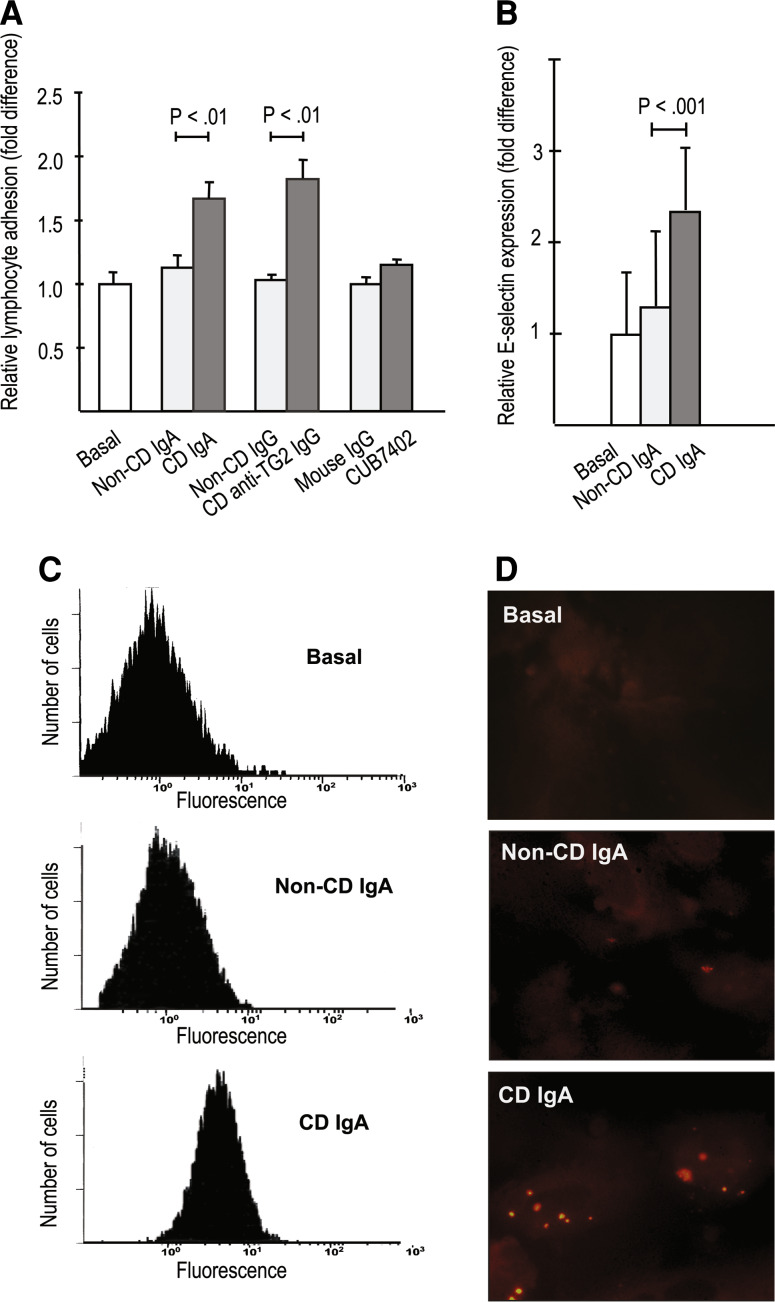
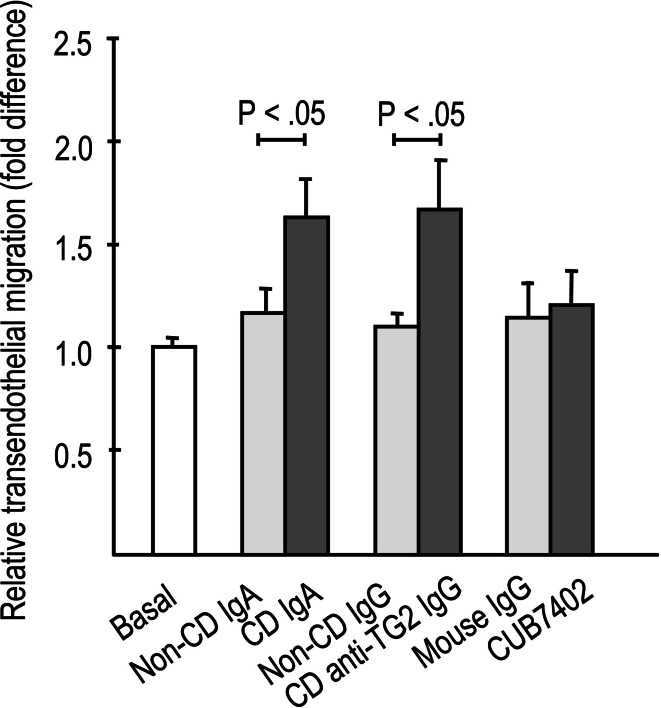
The presence of both celiac disease patient total IgA and affinity-purified IgA-deficient patient anti-TG2-specific IgG antibodies significantly increased the paracellular passage of both 4-kD and 70-kD macromolecules through the endothelial monolayer when compared to non-celiac antibodies (Fig. 1a, b). In parallel, IgA and affinity-purified patient TG2-specific IgG antibodies derived from celiac patients, but not those from control subjects, significantly enhanced lymphocyte adhesion to endothelial cells (Fig. 2a). This enhanced lymphocyte adhesion was accompanied by upregulated expression of the endothelial adhesion molecule E-selectin in response to celiac patient IgA (Fig. 2b–d). Similarly, transendothelial migration of lymphocytes was also significantly increased in experiments supplemented with both types of celiac disease patient autoantibodies, total IgA, and affinity-purified patient TG2-specific IgG antibodies (Fig. 3). In contrast, the commercial function-blocking transglutaminase 2 antibody CUB7402 had no significant effect on macromolecular permeability, lymphocyte adhesion, or their transendothelial migration (Figs. 1a, b, 2a, 3).
Fig. 1.
Endothelial paracellular permeability to macromolecules, a 4-kD and b 70-kD FITC-dextran, in the presence of celiac disease (CD) and control patient (non-CD)-derived antibodies as well as commercially available anti-transglutaminase 2 (TG2) antibody, CUB7402. Both types of celiac-patient derived antibodies, CD total IgA and CD anti-TG2-specific IgG, significantly increased macromolecular permeability (a, b) when compared to relevant control antibodies, while CUB7402 had no significant effect. Experiments were performed after 24 h incubation with the antibodies and the measurements were carried out 3 h after administration of the macromolecules. Experiments were performed in duplicate and repeated at least three times. Bars represent mean values and error bars standard error of means. Only significant differences (P < .05) between the relevant antibody groups are depicted
Fig. 2.
Lymphocyte adhesion on endothelial cells. a Lymphocytes adhered more prominently to endothelial monolayer treated with celiac disease (CD) patient-derived antibodies [CD total IgA and CD anti-transglutaminase 2 (TG2)-specific IgG] when compared to control (non-CD) antibodies. Commercially available anti-TG2 antibody CUB7402 had no effect. b CD IgA increased the expression of the endothelial adhesion molecule E-selectin significantly when compared to non-CD IgA (b) analysed by flow cytometry (b, c) and by immunofluorescent labelling (d, in red). Experiments were performed in duplicate and repeated at least three times. Bars represent mean values and error bars standard error of means. Only significant differences (P < .05) between the relevant antibody groups are depicted
Fig. 3.
Lymphocyte transendothelial migration was significantly increased in the presence of celiac disease (CD)-derived antibodies [CD total IgA and CD anti-transglutaminase 2 (TG2)-specific IgG] when compared to relevant control (non-CD) antibodies. Commercially available anti-TG2 antibody CUB7402 had no effect. Experiments were performed in duplicate and repeated at least three times. Bars represent mean values and error bars standard error of means. Only significant differences (P < .05) between the relevant antibody groups are depicted in figures
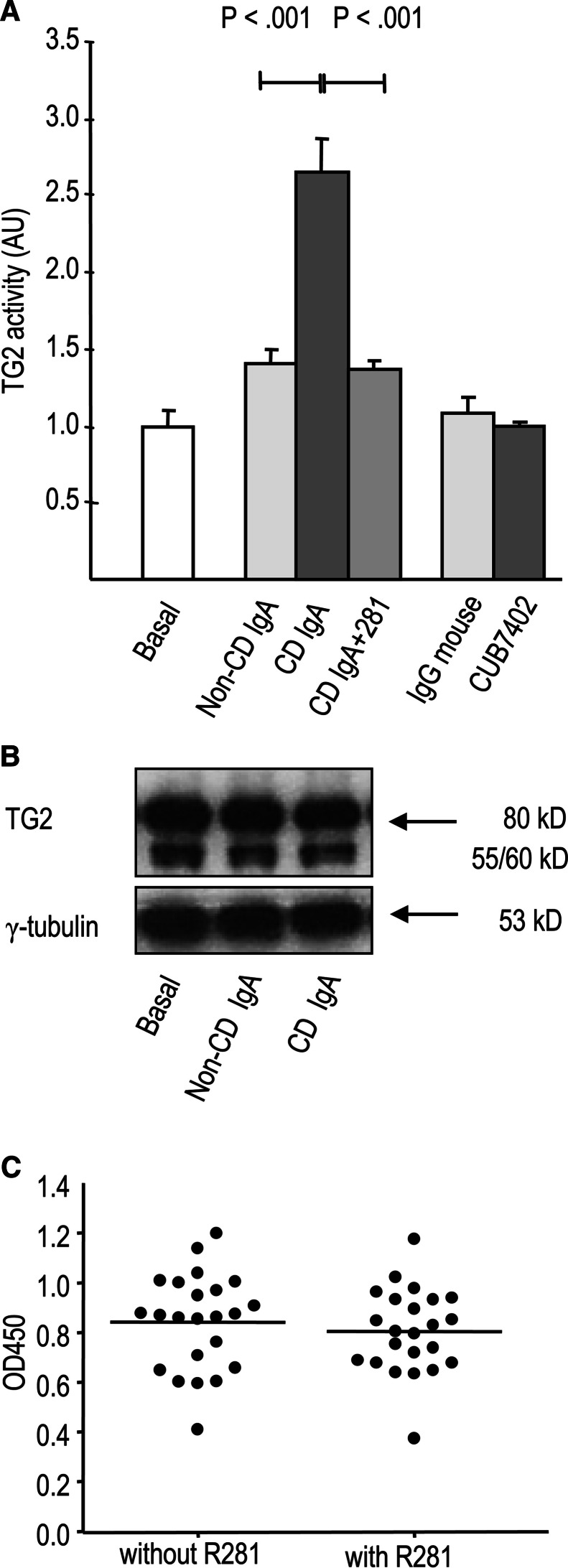
Live cell ELISA for determining the extracellular transamidating activity of TG2 showed that celiac patient IgA, but not IgA derived from control subjects or commercial anti-TG2 antibody CUB7402, increased extracellular activity of TG2 in endothelial cells (Fig. 4a). Celiac patient IgA did not have intrinsic TG2 activity (data not shown) as also shown previously [23]. The celiac patient IgA-induced activation of TG2 could be blocked by the active site-directed TG2 inhibitor R281. The expression level of TG2 protein was unaffected by celiac patient IgA, and the active site-directed TG2 inhibitor 281 had no effect on the binding of celiac IgA to purified recombinant TG2 (Fig. 4b, c).
Fig. 4.
a Celiac disease patient IgA (CD IgA) significantly increased the extracellular enzymatic activity of TG2 in endothelial cells when compared to control patient antibodies (non-CD IgA) whereas commercial transglutaminase 2 (TG2) antibody, CUB7402 had no effect. The co-administration of site-directed TG2 inhibitor, R281 prevented the enhancement of the TG2 activity. Bars represent mean TG2 activity in arbitrary units (AU). b Western blot analysis of TG2 expression in endothelial cells without any treatment (basal) and after administration of non-CD and CD IgA. Data are representative of three independent experiments. c Celiac disease (CD) patient IgA (n = 3) and CD patient serum samples (n = 20) bind to purified TG2 both in the presence and absence of R281 in enzyme-linked immunoassay
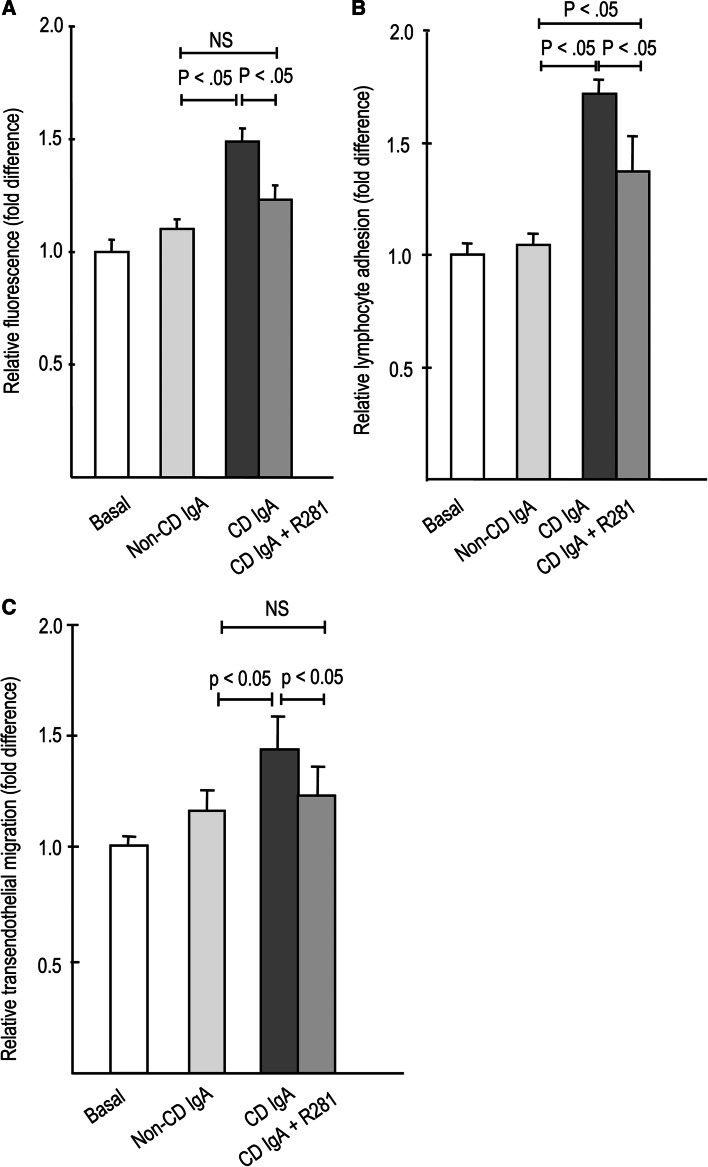
The pretreatment of endothelial cultures with TG2 inhibitor R281 was able to inhibit the increased macromolecular permeability as well as enhance lymphocyte adhesion and transendothelial migration exerted by celiac patient IgA (Fig. 5a–c). Co-administration of R281 with celiac patient IgA rescued the macromolecule permeability and lymphocyte transendothelial migration to the level of control subject IgA (Fig. 5a, c), whereas lymphocyte adhesion to the endothelial cells was only partially rescued (Fig. 5b).
Fig. 5.
Effects of site-directed transglutaminase 2 inhibitor R281 on a 4-kD macromolecule permeability, b lymphocyte adhesion, and c lymphocyte transendothelial migration in the presence of celiac (CD)- and control patient (non-CD)-derived total IgA. TG2 inhibition blocked the effects of CD IgA on macromolecular permeability (a) and lymphocyte transendothelial migration (c), and attenuated their effect on lymphocyte adhesion (b). Bars represent mean values of experiments performed in duplicate and repeated at least three times. Error bars indicate standard error of means. P < .05 was considered significant
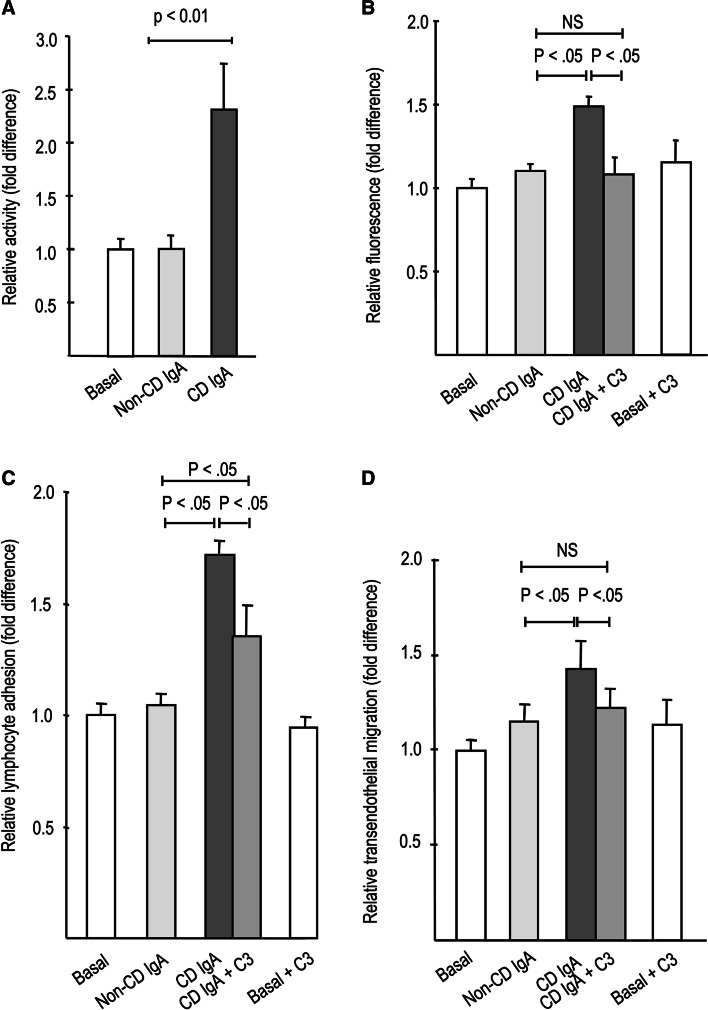
As shown in Fig. 6a, supplementation of celiac disease patient IgA to endothelial cells increased the activity of RhoA, a well-characterized signaling molecule contributing to vascular hyperpermeability [17]. The activation of RhoA by celiac autoantibodies was constitutive, since the activity was elevated at all time-points measured (data not shown). Inhibition of Rho activity by C3 endotoxin abolished the effects of celiac IgA on macromolecular permeability and lymphocyte transendothelial migration and attenuated the effects on lymphocyte adhesion to endothelial cells (Fig. 6b–d). C3 did not inhibit basal responses in the permeability, lymphocyte adhesion, or lymphocyte transmigration (Fig. 6b–d).
Fig. 6.
Role of Rho activity in response to celiac disease (CD) patient IgA. a RhoA activity in endothelial cells is significantly increased after 24 h administration with CD but not with control (non-CD) patient IgA. Co-administration of the Rho inhibitor C3 endotoxin together with CD IgA abolished the effect of celiac antibodies on the paracellular permeability of 4 kD FITC-dextran (b) and lymphocyte transendothelial migration (d), and diminished lymphocyte adhesion to endothelial cells (c). Bars represent mean values of experiments performed in duplicate and repeated at least three times. Error bars indicate standard error of means. P < .05 was considered significant
Discussion
The endothelium serves as a “gatekeeper” in the inflammatory process, and the regulation of vascular permeability is thus critical in this context, but to date the vascular barrier function in celiac disease has not been thoroughly investigated. The focus of this study was on whether celiac disease-specific autoantibodies against TG2, which are deposited around mucosal blood vessels in the small intestine of untreated celiac disease patients [4, 5, 14], modulate blood vessel permeability in an endothelial cell-based in vitro model. We demonstrated that celiac disease autoantibodies indeed increase endothelial permeability to macromolecules and enhance lymphocyte adhesion and their transendothelial migration. Since IgA-deficient celiac patient-derived affinity-purified anti-TG2-specific IgG class antibodies exerted effects similar to patient total IgA, it would appear that modulation of the vascular function is mediated specifically by the TG2-targeted autoantibody population.
As regards the enhanced lymphocyte adhesion onto endothelial cells in response to celiac patient IgA, we observed that it is accompanied by upregulated expression of the lymphocyte adhesion molecule, E-selectin. Interestingly, there are reports that in untreated celiac disease, the expression of lymphocyte adhesion molecules such as intracellular adhesion molecule-1, E-selectin, and mucosal addressin cell adhesion molecule 1 are upregulated in the intestinal mucosa [24–26]. One could thus hypothesize that celiac patient IgA sequestered on small-bowel mucosal blood vessels might enhance the expression of these lymphocyte-recruiting molecules and thereby contribute to the migration of lymphocytes across the endothelial barrier into the underlying lamina propria.
It is important to note that a commercially available anti-TG2 antibody, CUB7402, did not exert effects on endothelial permeability similar to those of celiac patient-derived total IgA and affinity-purified anti-TG2-specific antibodies. This discrepancy might be explained by the different modes of action of the distinct antibodies. According to our results, celiac patient-derived IgA increased the transamidating activity of TG2 while CUB7402 did not. CUB7402 is known to block the enzymatic activity of TG2 [19], but in our assay we did not see such an inactivation when comparing to baseline activity of TG2, likely because our assay consisted of measuring TG2 activity with a live cell assay performed with confluent cell cultures, where TG2 has been reported in the literature to be catalytically inactive [27]. There are divergent results published on the modulation of TG2 activity by the celiac disease patient TG2-targeted autoantibodies. Most of the articles report diminished TG2 enzymatic activity in response to celiac patient anti-TG2-specific autoantibodies [7, 28–30], whereas Kiraly and coworkers report enhanced enzymatic activity of TG2 in the presence of celiac patient autoantibodies [23]. Although our results are in line with those published by Kiraly and coworkers, it is fairly difficult to explain all the conflicting results above otherwise than by methodological differences. Since we were able to prevent the celiac IgA-induced enhancement of TG2 activity by active-site directed TG2 inhibitor R281, and because celiac IgA did not upregulate TG2 protein expression in endothelial cells, our results indeed suggest that celiac patient IgA increases the extracellular transamidating activity of TG2 at least in endothelial cells. Whether this finding can be generalized to other cell types and tissues remains to be solved.
It has been earlier suggested that celiac patient autoantibodies targeted against TG2 would bind to the catalytic triad [31], making it thus possible that in our TG2 activity assay the inhibitor R281, when bound to the active site, would inhibit the binding of the celiac IgA. Since R281 is a site-directed irreversible inhibitor requiring Ca2+ for its activity [21], it is likely to trap the enzyme in its open extended conformation, as has been shown with comparable inhibitor compounds [32]. However, our binding studies with fibronectin-bound Ca2+-activated TG2 that mimic accessibility of extracellular TG2 epitopes demonstrate that the binding of this inhibitor to TG2 does not render the celiac patient antibodies unable to recognize the inactivated enzyme. Although our ELISA results do not tell whether TG2 adopted the open conformation during the assay or not, the results directly prove that the celiac epitopes are still accessible to patient antibodies while the active site is occupied by the inhibitor, thus the epitope of the celiac patient antibodies are distinct from the active site of TG2.
We next sought to study whether an active site TG2 inhibitor, R281, could counteract the endothelial barrier-disrupting properties of celiac IgA. This seemed indeed to be the case, as pretreatment of endothelial cultures with R281 abolished the effects of celiac disease autoantibodies on macromolecular permeability and lymphocyte transendothelial migration and attenuated the effect on lymphocyte adhesion to endothelial cells. To our knowledge, there are only few reports on TG2 or transglutaminases in general in the context of vascular permeability. However, according to the literature, at least two members of the transglutaminase family, transglutaminase 1 and Factor XIII, would actually appear to preserve the endothelial barrier [20, 33]. Our findings on the interplay between celiac disease autoantibodies and TG2 thus open a new scenario in the modulation of vascular biology in celiac disease.
As RhoA has been shown to play a key role in signaling to increase endothelial permeability [34, 35] and because TG2 has also been reported to be able to activate RhoA [16, 36], we sought to establish whether celiac patient autoantibodies would also activate RhoA. Our findings indeed show that celiac disease patient IgA activates RhoA constitutively in endothelial cells. We were also able to demonstrate that the inactivation of RhoA by C3 endotoxin reduced the celiac patient IgA-enhanced endothelial barrier dysfunction to the control level in the case of macromolecular permeability and lymphocyte transendothelial migration, and also significantly although not to the control level in lymphocyte adhesion to endothelial cells suggesting that patient autoantibodies exert their function through the RhoA pathway.
Since both the enzymatic inactivation of TG2 and RhoA reduced macromolecular permeability and lymphocyte transendothelial migration to the control level, it would seem that the activation of TG2 and RhoA are operating in the same pathway activated by celiac patient autoantibodies and that this pathway is the major, if not sole, pathway activated. Instead, the process of celiac autoantibody-induced lymphocyte adhesion to endothelial cells seems to be a more complex process involving more than one signaling pathway as the enhanced lymphocyte adhesion could only partially be blocked by inhibition of TG2 and RhoA.
It is interesting to note that celiac patient autoantibodies increase vascular permeability, as shown in this paper, but also inhibit angiogenesis [12]. This suggests they operate differently from the classic angiogenic factor family of vascular endothelial growth factors (VEGF) which also induce vascular permeability but, instead of inhibiting, actually induce angiogenesis [37]. The effects exerted by VEGF are mediated by the same VEGF receptors and the different cellular responses use overlapping signaling pathways [38]. Hence, the pathways triggered by celiac patient autoantibodies appear to be distinct from those activated by VEGF and could involve the modulation of a complex interplay of endothelial cell TG2 with extracellular matrix and the integrin network [39]. However, the precise mechanism by which the celiac patient autoantibodies targeted against TG2 exert their function remains to be investigated in future studies.
We conclude that celiac patient autoantibodies targeting TG2 increase the transamidating activity of TG2 and activate RhoA and that these changes lead to increased blood vessel permeability. Consequently, celiac autoantibodies acting in concert with proinflammatory cytokines to increase blood vessel permeability could potentiate the small bowel mucosal inflammatory response, eventually leading to extensive mucosal remodeling in celiac disease and also contributing to the development of extraintestinal manifestations.
Acknowledgements
The authors wish to thank Mr. Jorma Kulmala for technical assistance. The Celiac Disease Study Group has been financially supported by the Research Council for Health, the Academy of Finland, the Juselius Foundation, the Paediatric Research Foundation, the Competitive Research Funding of the Pirkanmaa Hospital District, the Research Fund of the Finnish Celiac Society, the Hungarian Scientific Research Fund (OTKA K61868), and the European Commission (contract number MRTN-CT-2006-036032).
References
- 1.Korponay-Szabo IR, Laurila K, Szondy Z, Halttunen T, Szalai Z, Dahlbom I, Rantala I, Kovacs JB, Fesus L, Maki M. Missing endomysial and reticulin binding of coeliac antibodies in transglutaminase 2 knockout tissues. Gut. 2003;52:199–204. doi: 10.1136/gut.52.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 3.Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–4176. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 4.Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, Fesus L, Maki M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641–648. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmi TT, Collin P, Korponay-Szabo IR, Laurila K, Partanen J, Huhtala H, Kiraly R, Lorand L, Reunala T, Maki M, Kaukinen K. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut. 2006;55:1746–1753. doi: 10.1136/gut.2005.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halttunen T, Maki M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology. 1999;116:566–572. doi: 10.1016/S0016-5085(99)70178-2. [DOI] [PubMed] [Google Scholar]
- 7.Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C. Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology. 2007;132:1245–1253. doi: 10.1053/j.gastro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R, Puccetti A. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006;3:e358. doi: 10.1371/journal.pmed.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervio E, Volta U, Verri M, Boschi F, Pastoris O, Granito A, Barbara G, Parisi C, Felicani C, Tonini M, De Giorgio R. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology. 2007;133:195–206. doi: 10.1053/j.gastro.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 10.Boscolo S, Sarich A, Lorenzon A, Passoni M, Rui V, Stebel M, Sblattero D, Marzari R, Hadjivassiliou M, Tongiorgi E. Gluten ataxia: passive transfer in a mouse model. Ann N Y Acad Sci. 2007;1107:319–328. doi: 10.1196/annals.1381.034. [DOI] [PubMed] [Google Scholar]
- 11.Hadjivassiliou M, Maki M, Sanders DS, Williamson CA, Grunewald RA, Woodroofe NM, Korponay-Szabo IR. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology. 2006;66:373–377. doi: 10.1212/01.wnl.0000196480.55601.3a. [DOI] [PubMed] [Google Scholar]
- 12.Myrsky E, Kaukinen K, Syrjanen M, Korponay-Szabo IR, Maki M, Lindfors K. Coeliac disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis. Clin Exp Immunol. 2008;152:111–119. doi: 10.1111/j.1365-2249.2008.03600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke WT, Holmes GKT. Coeliac disease. Edinburgh: Churchill Livingstone; 1984. [Google Scholar]
- 14.Myrsky E, Syrjanen M, Korponay-Szabo IR, Maki M, Kaukinen K, Lindfors K. Altered small-bowel mucosal vascular network in untreated coeliac disease. Scand J Gastroenterol. 2009;44:162–167. doi: 10.1080/00365520802400875. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci USA. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janiak A, Zemskov EA, Belkin AM. Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol Biol Cell. 2006;17:1606–1619. doi: 10.1091/mbc.E05-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 18.Halttunen T, Marttinen A, Rantala I, Kainulainen H, Maki M. Fibroblasts and transforming growth factor beta induce organization and differentiation of T84 human epithelial cells. Gastroenterology. 1996;111:1252–1262. doi: 10.1053/gast.1996.v111.pm8898639. [DOI] [PubMed] [Google Scholar]
- 19.Birckbichler PJ, Upchurch HF, Patterson MK, Jr, Conway E. A monoclonal antibody to cellular transglutaminase. Hybridoma. 1985;4:179–186. doi: 10.1089/hyb.1985.4.179. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner W, Golenhofen N, Weth A, Hiiragi T, Saint R, Griffin M, Drenckhahn D. Role of transglutaminase 1 in stabilisation of intercellular junctions of the vascular endothelium. Histochem Cell Biol. 2004;122:17–25. doi: 10.1007/s00418-004-0668-y. [DOI] [PubMed] [Google Scholar]
- 21.Griffin M, Mongeot A, Collighan R, Saint RE, Jones RA, Coutts IG, Rathbone DL. Synthesis of potent water-soluble tissue transglutaminase inhibitors. Bioorg Med Chem Lett. 2008;18:5559–5562. doi: 10.1016/j.bmcl.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Korponay-Szabo IR, Vecsei Z, Kiraly R, Dahlbom I, Chirdo F, Nemes E, Fesus L, Maki M. Deamidated gliadin peptides form epitopes that transglutaminase antibodies recognize. J Pediatr Gastroenterol Nutr. 2008;46:253–261. doi: 10.1097/MPG.0b013e31815ee555. [DOI] [PubMed] [Google Scholar]
- 23.Kiraly R, Vecsei Z, Demenyi T, Korponay-Szabo IR, Fesus L. Coeliac autoantibodies can enhance transamidating and inhibit GTPase activity of tissue transglutaminase: dependence on reaction environment and enzyme fitness. J Autoimmun. 2006;26:278–287. doi: 10.1016/j.jaut.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Ensari A, Ager A, Marsh MN, Morgan S, Moriarty KJ. Time-course of adhesion molecule expression in rectal mucosa of gluten-sensitive subjects after gluten challenge. Clin Exp Immunol. 1993;92:303–307. doi: 10.1111/j.1365-2249.1993.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelinkova L, Tuckova L, Sanchez D, Krupickova S, Pozler O, Nevoral J, Kotalova R, Tlaskalova-Hogenova H. Increased levels of circulating ICAM-1, E-selectin, and IL-2 receptors in celiac disease. Dig Dis Sci. 2000;45:398–402. doi: 10.1023/A:1005489316037. [DOI] [PubMed] [Google Scholar]
- 26.Di Sabatino A, Rovedatti L, Rosado MM, Carsetti R, Corazza GR, MacDonald TT. Increased expression of mucosal addressin cell adhesion molecule 1 in the duodenum of patients with active celiac disease is associated with depletion of integrin alpha4beta7-positive T cells in blood. Hum Pathol. 2009;40:699–704. doi: 10.1016/j.humpath.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 29.Esposito C, Paparo F, Caputo I, Rossi M, Maglio M, Sblattero D, Not T, Porta R, Auricchio S, Marzari R, Troncone R. Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut. 2002;51:177–181. doi: 10.1136/gut.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anjum N, Baker PN, Robinson NJ, Aplin JD. Maternal celiac disease autoantibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod Biol Endocrinol. 2009;7:16. doi: 10.1186/1477-7827-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrne G, Ryan F, Jackson J, Feighery C, Kelly J. Mutagenesis of the catalytic triad of tissue transglutaminase abrogates coeliac disease serum IgA autoantibody binding. Gut. 2007;56:336–341. doi: 10.1136/gut.2006.092908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noll T, Wozniak G, McCarson K, Hajimohammad A, Metzner HJ, Inserte J, Kummer W, Hehrlein FW, Piper HM. Effect of factor XIII on endothelial barrier function. J Exp Med. 1999;189:1373–1382. doi: 10.1084/jem.189.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- 35.Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- 36.Singh US, Kunar MT, Kao YL, Baker KM. Role of transglutaminase II in retinoic acid-induced activation of RhoA-associated kinase-2. EMBO J. 2001;20:2413–2423. doi: 10.1093/emboj/20.10.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breen EC. VEGF in biological control. J Cell Biochem. 2007;102:136–1358. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 39.Bergamini CM, Griffin M, Pansini FS. Transglutaminase and vascular biology: physiopathologic implications and perspectives for therapeutic interventions. Curr Med Chem. 2005;12:2357–2372. doi: 10.2174/0929867054864804. [DOI] [PubMed] [Google Scholar]