Abstract
Crustaceans have long been used for peptide research. For example, the process of neurosecretion was first formally demonstrated in the crustacean X-organ–sinus gland system, and the first fully characterized invertebrate neuropeptide was from a shrimp. Moreover, the crustacean stomatogastric and cardiac nervous systems have long served as models for understanding the general principles governing neural circuit functioning, including modulation by peptides. Here, we review the basic biology of crustacean neuropeptides, discuss methodologies currently driving their discovery, provide an overview of the known families, and summarize recent data on their control of physiology and behavior.
Keywords: Transcriptomics, Mass spectrometry, Peptidergic neuromodulation, Neurotransmitter, Neurohormone, Peptidomics, Stomatogastric ganglion, Cardiac ganglion
General biology of neuropeptides
The largest class of signaling molecules used by nervous systems are peptides, short strings of α-amino acids linked by amide bonds. Like all peptides, neuropeptides are encoded within genomes as larger precursor proteins, known as pre/prepro-hormones (Fig. 1). After transcription and translation, a neuropeptide-containing precursor protein is directed into the secretory pathway via a signal sequence present at its amino (N)-terminus. Within this pathway, post-translational processing takes place, and the peptides within the precursor are packaged into secretory vesicles. In some cases, a single peptide is contained within a precursor protein (here termed a pre-hormone), liberated by signal peptidase cleavage of the signal sequence. More commonly, multiple peptides are encoded within a prepro-hormone, each surrounded by sites for cleavage by enzymes such as prohormone convertase (Fig. 1). Following cleavage from the pro-hormone (the precursor protein minus its signal sequence), many peptides undergo extensive post-translational processing, which can result in modifications including, but not limited to, carboxy (C)-terminal amidation, cyclization of N-terminal glutamine/glutamic acid residues, disulfide bridging between cysteines, and sulfation of tyrosines (Fig. 1). The presence of these post-translational modifications is often responsible for a peptide assuming its bioactive conformation.
Fig. 1.
Nucleotide and deduced amino acid sequences of Homarus americanus (Homam) prepro-sulfakinin. A1 Nucleotide sequence of Homam-prepro-sulfakinin cDNA (accession no. EF418605). The open reading frame of the cDNA, including the stop codon, is shown in black font, with two 3′ polyadenylation signal sequences indicated by underline in black. A2 Deduced amino acid sequence of Homam-prepro-sulfakinin. The signal peptide is shown in grey, with prohormone convertase cleavage loci shown in black. The two encoded sulfakinin isoforms are shown in red, with additional precursor-related peptides shown in blue. The asterisk indicates the position of the stop codon. B Putative processing scheme resulting in the production of the two isoforms of Homam-sulfakinin from its precursor protein. The mature conformations of the two sulfakinin isoforms (Homam-SK I and II) are colored red. Figure modified from Dickinson et al. [158]
Once packaged and processed to its mature conformation, a peptide is released from the neuron synthesizing it to exert its effects on a target. These targets can be the neuron releasing the peptide itself (autocrine functioning), tissues in direct apposition/close proximity to the point of release (paracrine actions), or tissues distantly located from the locus of release, where the peptide is delivered via the circulatory system (hormonal delivery). In crustaceans, a single peptide frequently serves both autocrine/paracrine and hormonal roles within a nervous system [1]. Moreover, crustacean neurons often synthesize and release multiple peptides [2], either concurrently or differentially. In fact, the multiplicity of the co-transmitters produced by and released from crustacean neurons has been postulated to allow the generation of complex behavioral output from the “simple,” “hard-wired” neural networks that control behavior in this group of animals.
Unlike classical neurotransmitters, where release is generally limited to the synapse, it is believed that neuropeptides can be secreted at essentially any point along the length of a neuron, and, unlike the synapse, there are no morphological correlates that can be used a priori to define a putative peptide release site. In spite of this, peptides tend to be sequestered within varicose-like terminals located in central and peripheral regions of the nervous system, and, in crustaceans, these regions are generally recognized as areas of release (Fig. 2).
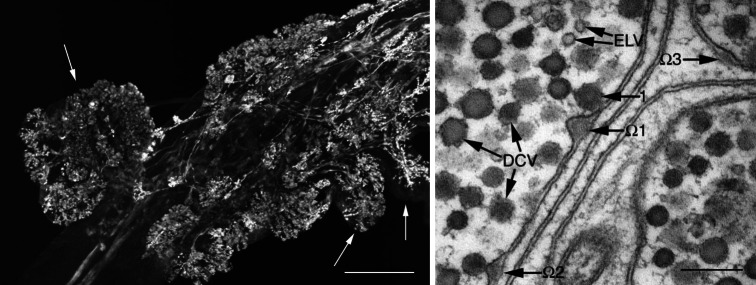
Fig. 2.
General organization of a crustacean neuroendocrine organ; the anterior cardiac plexus (ACP) of Cancer productus is used as an example. (Left panel) The Cancer productus ACP is composed of nerve terminals contained within blister-like protuberances of the anterior cardiac nerve sheath; these are in direct contact with the circulatory system. The confocal image shown illustrates the presence of FMRFamide-like immunoreactivity within these protuberances, several prominent ones indicated by arrows. Scale bar 100 µm. (Right panel) Transmission electron micrograph illustrating morphological correlates of hormone secretion in the Cancer productus ACP. Both dense-core vesicles (DCV), which are likely peptidergic, and electron-lucent vesicles (ELV) are present in these terminals. In this image, one DCV (1) is docked to the plasma membrane, while several others have fused with the membrane and are in the process of exocytosing their contents, creating characteristic ultrastuctural features, i.e., omega (Ω)-figures, on the plasma membrane (Ω2, Ω3). The docked DCV and the three Ω-figures visible in this micrograph create a pseudo-time course of peptide hormone secretion. A DCV first docks to the plasma membrane (1), then fuses with it, releasing its dense-core and forming an Ω-figure (Ω1). The membrane of the DCV is rapidly incorporated into the plasma membrane of the terminal, and the Ω-figure subsides (Ω2 and Ω3). Scale bar 200 nm. Figure modified from Christie et al. [8]
A number of factors determine the sphere of influence of a peptide once it is released from a neuron. Clearly, the presence/absence and relative distribution/concentration of receptors for a given peptide play a critical role in determining whether or not it exerts modulatory activity on a target, as does the quantity of peptide released from the neuron. Moreover, for both locally acting and circulating peptides, the local concentrations of peptidases that can act on a given peptide and the distance that the peptide must diffuse to a target can regulate the concentration of peptide reaching its receptors. Finally, physical barriers within a local release area, or impenetrable ensheathment of a tissue in the case of a hormonally delivered substance, can limit access of a peptide to its receptors on a potential target.
Neural sources of crustacean peptides
The crustacean central nervous system (CNS), like that of all arthropods, is a distributed one, generally consisting of a chain of ganglia interconnected by a longitudinal nerve cord. In decapods, the neural circuits responsible for mediating many behavioral outputs are located within the ganglia of the CNS. For example, the ventilatory rhythms of the gills are generated by a neural network located in the thoracic nervous system [3], while the swimmeret system of the tail is controlled largely by neural circuitry present in the abdominal ganglia [4]. In addition, several offshoots of the CNS are ganglionated, e.g., the stomatogastric nervous system (STNS) and the cardiac ganglion (CG), with the resident somata synapsing within the local neuropil to form the circuitry controlling the rhythmic movements of the foregut and the neurogenic heart, respectively [3, 5, 6]. Peptides released locally within the neuropil of these and other ganglia, in some cases from the circuit elements themselves, are capable of reconfiguring the resident neural circuits, thereby modulating their outputs.
In addition to regions of synaptic interactions, the crustacean nervous system gives rise to a number of neuroendocrine structures, defined as regions of the nervous system in which secretory nerve terminals have direct access to the hemolymph. In decapods, these neuroendocrine sites vary from loosely associated clusters of release terminals located along the ventral nerve cord or in peripheral nerves to highly organized neuroendocrine organs. Two neuroendocrine organs appear to be ubiquitously conserved in decapods [7]: the X-organ-sinus gland (XO-SG) system, typically located in the eyestalk, and the pericardial organ (PO), situated along the lateral walls of the pericardial chamber surrounding the heart. Others, such as the post-commissural organ [7], the anterior cardiac plexus [8] (Fig. 2), and the anterior commissural organ [9], all located within or near the STNS, may be more limited in their phylogenetic conservation. Like locally released modulators, peptide hormones released from these neuroendocrine sites act as powerful modulators of physiology and behavior.
Recent advancements in the methodology for crustacean neuropeptide discovery
The first invertebrate neuropeptide to be fully characterized was red pigment concentrating hormone (RPCH) from the shrimp Pandalus borealis [10]. This peptide was isolated chromatographically/biochemically from a large pool of starting tissue [11] and, upon its bioassay-directed purification, the structure of the peptide was determined by a combination of proteolytic cleavage, Edman analysis, and mass spectrometry [10]. This strategy was commonly employed for peptide discovery in crustaceans for the next quarter century [12–17].
While a number of techniques, including ones similar to those used for the isolation and characterization of RPCH, continue to be employed for peptide discovery [18–20], the emphasis has shifted from the purification and structural elucidation of targeted neuropeptides to an emphasis on peptidomics, the qualitative and quantitative characterization of the complement of endogenous bioactive peptides present in a species. When genomic/transcriptomic information is available, it is possible to use bioinformatics techniques to identify neuropeptide genes and apply processing models to predict neuropeptide sequences. Peptide profiling and de novo sequencing via mass spectrometry (MS) have also played an important role in these efforts. For crustaceans, no genome information is available for public use; thus, the application of genomic analysis is not yet possible. In contrast, many data are available for in silico transcriptome mining and biological mass spectrometry, and these methods are now at the forefront of crustacean peptidomics.
Transcriptome mining
With the advent of new molecular and sequencing technologies, it has become possible to produce expressed sequence tags (ESTs) for mRNA/cDNA libraries from neural and other tissues, or, in some cases, from whole organisms. For an ever-growing number of crustacean species, extensive collections of ESTs have been generated and deposited in publicly accessible databases. These data provide a rich resource for mining transcripts encoding proteins of interest, including neuropeptide precursors. Moreover, on-line software programs available to translate and predict the post-translational processing of the deduced pre/prepro-hormones make transcriptome mining a rapid and readily accessible approach for neuropeptide discovery (Table 1).
Table 1.
Major crustacean peptide families and their modes of identification
| Family (subfamily) | Example | Identification |
|---|---|---|
| A-AST | AGPYSFGLamide | B, T |
| B-AST | GNWNKFQGSWamide | B, T |
| C-AST | pQIRYHQCYFNPISCF | B, T |
| Bursicon α | DECSLRPVIHILSYPGCTSKPIPSFACQGRCTSYVQVSGSKLWQTERSCMCCQESGEREAAITLNCPKPRPGEPKEKKVLTRAPIDCMCRPCTDVEEGTVLAQKIANFIQDSPMDSVPFLK | T |
| Bursicon β | RSYGVECETLPSTIHISKEEYDDTGRLVRVCEEDVAVNKCEGACVSKVQPSVNTPSGFLKDCRCCREVHLRARDITLTHCYDGDGARLSGAKATQHVKLREPADCQCFKCGDSTR | T |
| Corazonin | pQTFQYSRGWTNamide | B, T |
| CCAP | PFCNAFTGCamide | B, T |
| CHH | pQIYDTSCKGVYDRALFNDLEHVCDDCYNLYRTSYVASACRSNCYSNLVFRQCMDDLLMMDEFDQYARKVQMVamide | B, T |
| CPRP | RSTQGYGRMDRILAALKTSPMEPSAALAVQHGTTHPLE | B, T |
| DH (calcitonin-like) | GLDLGLGRGFSGSQAAKHLMGLAAANFAGGPamide | T |
| ETH | DPSPEPFNPNYNRFRQKIPRIamide | T |
| EH | AVAANRKVSICIKNCGQCKKMYTDYFNGGLCGDFCLQTEGRFIPDCNRPDILIPFFLQRLE | T |
| Enkephalin | YGGFM | B |
| FLP (myosuppressin) | pQDLDHVFLRFamide | B, T |
| FLP (NPF) | KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide | T |
| FLP (sNPF) | APALRLRFamide | B, T |
| FLP (sulfakinin) | pQFDEY(SO3H)GHMRFamide | B, T |
| FLP (–FLRFamide) | TNRNFLRFamide | B, T |
| FLP (–YLRFamide) | AYSNLNYLRFamide | B, T |
| FLP (–FVRFamide) | GYSNKNFVRFamide | B |
| Insect kinin | DFSAWAamide | B |
| Neuroparsin | APRCDRHDEEAPKNCKYGTTQDWCKNGVCAKGPGETCGGYRWSEGKCGEGTFCSCGICGGCSPFDGKCGPTSIC | T |
| Orcokinin | NFDEIDRSGFGFN | B, T |
| Orcomyotropin | FDAFTTGFamide | B, T |
| PDH | NSGMINSILGIPRVMTEAamide | B, T |
| Proctolin | RYLPT | B, T |
| Pyrokinin | DFAFSPRLamide | B |
| RPCH | pELNFSPGWamide | B, T |
| RYamide | pEGFYSQRYamide | B |
| SIFamide | GYRKPPFNGSIFamide | B, T |
| TRP | APSGFLGMRamide | B, T |
A-AST A-type allatostatin, B-AST B-type allatostatin, C-AST C-type allatostatin, CCAP crustacean cardioactive peptide, CHH crustacean hyperglycemic hormone, CPRP CHH precursor-related peptide, DH diuretic hormone, ETH ecdysis triggering hormone, EH eclosion hormone, FLP FMRFamide-like peptide, PDH pigment dispersing hormone, RPCH red pigment concentrating hormone, TRP tachykinin-related peptide, NPF neuropeptide F, sNPF short NPF, B sequenced biochemically or via mass spectrometry, T predicted via molecular cloning or transcriptome mining
In crustaceans, a common strategy has been used for transcriptome mining [21–25]. Specifically, known pre/prepro-hormone sequences are used as queries to search the public database for ESTs that encode putatively orthologous proteins. The BLAST program used for these analyses, tblastn, which searches the translated nucleotide database using a protein query, can be searched for transcripts in a general sense or restricted to a desired subset of animals. Positive hits are translated and checked for sequence identity/similarity to the target query. If the hit seems likely to represent a viable transcript, post-translational processing of the deduced protein is subsequently predicted (Fig. 1). Using this protocol, the extant publicly accessible data have recently been used for several taxon-wide surveys of crustacean peptide-encoding ESTs [24, 26], as well as for targeted searches from individual crustacean species [22, 23, 25]. As ESTs are continuously being added to the public database, periodic mining of this resource will certainly reveal additional peptide-encoding transcripts, thereby continuing to expand our knowledge of crustacean peptidergic signaling.
Transcriptome mining offers both pros and cons with respect to other methods of peptide discovery currently in vogue. First, many peptides are present in nervous systems in very small quantities, and thus for many isolation/characterization regimes, large pools of tissue are needed to obtain sequence data. Given this need, standard biochemical/mass spectral sequencing is often not practical, particularly for minute species, such as planktonic crustaceans. Similarly, the rarity of an organism and/or its geographic range can hinder peptide discovery using techniques that require large quantities of tissue. In contrast, transcriptomics is not hampered by a need for large pools of tissue. Moreover, once deposited into the public database, EST sequences provide a stable resource for mining proteins from a given species. In addition, the data obtained from transcriptome mining allow for the unambiguous determination of all amino acids, whereas in other methods, such as some mass spectral platforms, ambiguity occurs for amino acids that are isobaric, e.g., leucine and isoleucine.
Counterbalancing the pros of transcriptomics are a number of limitations. Firstly, for all crustaceans with extant ESTs in the public domain, the sequences thus far deposited represent only a small portion of a transcriptome for any given species. Also, because most ESTs are single pass sequences, miss/uncalled nucleotides can lead to errors in the sequence of a deduced protein. Additionally, predictions of the post-translational processing of the resultant proteins are just that, predictions, which may or may not represent the actual biological processing of a precursor. Moreover, by their very nature, transcriptomes represent only a snapshot of the genes being transcribed in an animal, and thus may be biased by age, sex, physiological state, etc. Therefore, although it is a powerful tool, transcriptomics alone is unlikely to provide a complete peptidome for any species.
Biological mass spectrometry
MS-based techniques have revolutionized the field of neuropeptide discovery [27–30], providing the means to probe complex biological samples and generate detailed structural information with extraordinary sensitivity. In this section, we present a brief overview of the strengths and limitations of the mass spectrometric instruments that have been applied to crustacean neuropeptide identification and summarize how MS instruments and MS-based strategies have been used for the analysis of crustacean tissues and hemolymph.
MS instrumentation for crustacean neuropeptide identification
Three basic elements define the capabilities of all instruments used for MS-based peptidomics: (1) ionization, the production of ions from the biological sample, (2) the measurement of the mass-to-charge ratio (m/z) characteristic of sample components (MS for direct peptide profiling), and (3) the measurement of m/z following the activation and dissociation of isolated ions (MS/MS or MSn for peptide sequencing). Coupled with the resolution of prior chromatographic separations, the attributes of each step define the capabilities and limitations of MS-derived information. The attributes for instruments directed at crustacean neuropeptide characterization are summarized below. The reader is directed to other, more comprehensive, recent reviews of mass spectrometric techniques [31–35] for more detailed information.
Ionization and mass analysis for peptide profiling
Two ionization techniques, matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI), are used extensively for the analysis of peptides in crustacean tissues and tissue extracts [27–30]. Following ionization, many studies have based neuropeptide identification upon the measurement of the m/z ratio via direct peptide profiling. For these measurements, the ability to make mass measurements with both high mass resolution and high mass accuracy is critical. Mass spectrometric instruments used for crustacean neuropeptide identification based upon m/z measurements include instruments that couple MALDI with time-of-flight (TOF) or Fourier transform (FT) mass analyzers. For instruments using ESI, quadrupole (Q)-TOF hybrid mass spectrometers have been the most common.
TOF mass analyzers are used to determine m/z values by accelerating ions down a field-free flight tube and measuring their flight times. This time-based mass analyzer is compatible with the pulsed laser-desorption mode of ion production used for MALDI, and many early applications of mass spectrometry to the analysis of crustacean tissues [36–40] made use of MALDI-TOF instruments. More recently, MALDI-TOF/TOF instruments have been applied to the analysis of crustacean samples [41]. Unique to the field of crustacean neuropeptide analysis, MALDI-FTMS instruments have been applied extensively to the analysis of tissues and tissue and fluid extracts [42–44]. MALDI-FTMS instruments offer ultra-high mass resolution coupled with unique methods of mass calibration, a combination that significantly increases the reliability of m/z-based neuropeptide identifications. However, the ions produced using vacuum-UV MALDI-FTMS are often unstable when trapped for the long times (~10 s) required for FTMS detection, which results in the detection of abundant metastable decay products in the MALDI-FT mass spectra [43]. Later MALDI-FTMS work by Li and co-workers [45] was carried out using a higher pressure MALDI source, which minimizes the problem of metastable decay. Figure 3 shows two representative MALDI-FT mass spectra, drawn from work by Cape et al. [46]. In this study, where direct tissue analysis was one tool used to assess developmental differences in neuropeptides profiles from the lobster Homarus americanus, a single embryonic stomatogastric ganglion (STG) yielded high-quality MALDI spectra (Fig. 3a), which could be compared with the profile generated from an adult STG (Fig. 3b).
Fig. 3.
Direct tissue MALDI-FTMS used for neuropeptide profiling. In this example, a single stomatogastric ganglion (STG) from an a embryonic or b adult Homarus americanus was analyzed. The STG, which is small enough to be analyzed as a whole tissue, was freshly dissected, rinsed in acidified methanol, desalted, and co-crystallized with 2,5-dihydroxybenzoic acid as the MALDI matrix. Figure modified from Cape et al. [46]; used with permission
For instruments relying upon ESI, most work on crustacean peptidomics has made use of hybrid instruments, specifically hybrid Q-TOF mass spectrometers. Q-TOF instruments permit the continuously produced ions generated by the ESI source to be introduced orthogonally into the pulsed TOF mass analyzer. Q-TOF instruments, which can achieve higher resolution and mass accuracy compared with non-hybrid TOF instruments, are extensively used to sequence peptides using MS/MS measurements (described below).
MS/MS for peptide sequencing
To structurally characterize novel neuropeptides, and to confirm the assignment of previously established neuropeptide sequences, tandem mass spectrometry or MS/MS is used to isolate a precursor ion from a sample, dissociate the precursor and generate product ions that, upon mass analysis, can be used for structural characterization. The MS/MS spectrum can be used for the de novo determination of amino acid sequences or, in combination with bioinformatics data, can confirm a proposed neuropeptide amino acid sequence. Tandem mass spectrometry can also be used for the identification of post-translational modifications, such as the sulfation of tyrosine residues. In early work, when MALDI-TOF instruments were most common, MS/MS was carried out using the technique of post-source decay (PSD). With instrument evolution, MALDI-TOF/TOF instruments are now used to isolate precursor peptide ions with higher resolution and to dissociate the precursor using high-energy collision-induced dissociation (CID), which produces more peptide backbone cleavages, as well as amino acid informative fragment ions that can be used to distinguish leucine and isoleucine. For example, Fig. 4a shows a representative MALDI-TOF/TOF MS/MS spectrum for the orcokinin family peptide NFDEIDRSGFGFA ([Ala13]-orcokinin) [47]. The MS/MS spectrum yields almost complete sequence information, and the detection of low mass immonium ions provides information helpful for distinguishing isobaric leucine and isoleucine.
Fig. 4.
a MS/MS spectrum of [Ala13]-orcokinin from the brain of Cancer borealis, measured using a MALDI-TOF/TOF instrument. MS/MS was carried out using air as the collision gas and a 2-kV collision energy. b MS/MS spectrum of an [Ala13]-orcokinin standard, measured using a MALDI-FTMS instrument. MS/MS was carried out using SORI-CID with argon as the collision gas and a Vp amplitude of 6.5 V. The lower energy SORI-CID conditions yield spectra dominated by Asp-Xxx cleavages (cleavages C-terminal to aspartate residues) and fewer product ions that can be used for peptide sequencing. Figure 4a modified from Chen et al. [47]; used with permission
MALDI-FTMS instruments have also been used for MS/MS peptide fragmentation because of their ability to isolate a precursor at high resolution and accurately determine the m/z values of product ions at high resolution. However, the low energy CID process used on most FTMS instruments (sustained off-resonance irradiation, or SORI) and the fact that the MALDI-produced ions are singly charged result in spectra that are often dominated by small neutral losses, little sequential fragmentation and, consequently, fewer structurally useful sequence ions. For example, the MS/MS spectrum of [Ala13]-orcokinin, measured using a MALDI-FTMS instrument (Fig. 4b), shows a spectrum dominated by two y-type ions and fewer fragments that would permit sequencing. With this limitation, confirmation of peptide sequence has often relied upon comparing the MS/MS spectrum of the novel peptide with that of a synthetic reference peptide.
For tissue and hemolymph extracts, ESI-Q-TOF instruments have provided the most powerful tool for peptide sequencing. While MS/MS occurs under lower energy CID conditions, the more highly charged ions produced by ESI yield more detectable fragments that can be used for sequencing. The higher charges, coupled with the rapid, sensitive TOF mass analyzer, have made this technique an effective means for sequencing and identifying large numbers of novel neuropeptides from complex samples.
Examples of MS-based approaches for crustacean neuropeptide identification
In this section, we provide representative examples of MS-based strategies that have been used for crustacean neuropeptides analysis.
Characterizing the crustacean neuropeptidome
The vast majority of currently identified crustacean neuropeptides have been determined through large-scale studies directed at the neuropeptidome of particular species. Most studies have focused on the analysis of pooled tissue extracts from a large number of animals, which have then been subjected to off- and on-line chromatographic separations. This peptidomic approach was first applied to tissues from the crab Cancer borealis, where ESI-Q-TOF and MALDI-TOF measurements were used to characterize neuropeptides extracted from the brain and thoracic ganglion [48]. A large number of studies, initially relying heavily upon nanoESI-Q-TOF mass spectrometric analysis [23, 46, 49–51], have followed. In all of these studies, the acquisition of MS/MS data and the use of complementary instrumentation, such as MALDI-FTMS with nanoESI-Q-TOF MS, have played a critical role in supporting peptide identifications. More recent studies have further developed this multipronged approach, using a variety of MS ionization and mass analysis techniques (MALDI-FTMS, MALDI-TOF/TOF, nanoESI-Q-TOF) in combination to enhance the number of neuropeptides that can be detected and sequenced [52]. These studies, which have recently included a bioinformatics component [23, 25], have greatly enhanced our understanding of the range of neuropeptides in the crabs Cancer borealis [52], Carcinus maenas [23], the lobster, Homarus americanus [46, 51], and the shrimp, Litopenaeus vannamei [25]. Li and co-workers [53] have taken a novel approach to analyze the wealth of data provided by information-rich high-resolution mass spectrometric analyses by applying bioinformatics approaches to analyze MALDI-FTMS data for the comparison of the peptidome of five crustacean species.
Targeted neuropeptide identification/analysis
In contrast with peptidomic approaches, mass spectrometry has been used in studies focused on the detailed identification and characterization of specific novel neuropeptides. For example, MS sequencing of the native neuropeptides, coupled with confirmation using neuropeptide standards, has been used to identify a number of peptides, including the SIFamide VYRKPPFNGSIFamide [54], the tachykinin-related peptide (TRP) TPSGFLGMRamide [55], and the pyrokinins SGGFAFSPRLamide and TNFAFSPRLamide [56] (see “Crustacean neuropeptide families” for descriptions of these peptide families).
Bioinformatics-aided MS peptide identification
Early work by Yasuda-Kamatani and Yasuda showed that mass spectrometry, in combination with molecular cloning techniques, provided an efficient strategy for peptide identification [36, 57, 58]. More recently, in silico database searches for putative peptide precursors or molecular cloning approaches, coupled with predictions of peptide processing, have led to the MS confirmation of the structures of novel neuropeptides, including members of the C-type allatostatin (AST) [59–61], orcokinin [62], pigment dispersing hormone (PDH) [63], and SIFamide [64] families (see “Crustacean neuropeptide families” for descriptions of these peptide groups). As mentioned above, bioinformatics-aided MS techniques are also playing an important role in large-scale neuropeptidome studies [23, 25].
Analysis of larger neuropeptides
The ability to characterize larger neuropeptides presents challenges for MS/MS-based sequencing, as illustrated by studies of crustacean hyperglycemic hormone (CHH)- and CHH precursor-related peptide (CPRP)-like peptides. Early attempts to sequence CPRP peptides (30+ amino acid residues), which were extracted from the SG of the crayfish Orconectes limosus, yielded only 65–76% sequence coverage following nanoLC-Q-TOF MS/MS analysis [65]. For the larger (roughly 70 amino acids) CHH-like peptide, extracted from the PO and SG of Carcinus maenas, proteolytic digestion followed by MS/MS sequencing on a Q-TOF instrument still required Edman peptide degradations to determine the complete amino acid sequences [66]. In more recent work, Li and co-workers were able to use a Q-TOF instrument to fully characterize CPRPs from Cancer productus [67], as well as from Cancer borealis and Homarus americanus [49], taking advantage of truncated versions of the full length peptides that were present in the pooled tissue extracts. Most recently, Li and co-workers were able to de novo sequence a full length CHH peptide from the SG of Cancer borealis, making use of both “bottom-up” (tryptic digestion followed by tryptic peptide sequencing) and “top-down” (dissociation of the full-length peptide) characterization strategies [68]. Key to complete sequence characterization was the top-down strategy, implemented using electrospray ionization and high field-strength FTMS instruments. Cleavage of the peptide disulfide bonds was critical for establishing the amino acid sequences.
Novel methodologies for crustacean neuropeptide identification and characterization
Imaging mass spectrometry is an emerging technique that offers advantages over immunohistochemical imaging for localizing neuropeptides within tissue samples because labeling is not required and specific information about small variations in peptide structure (post-translational modifications or sequence variations) is available. MALDI-TOF/TOF MS has been applied by Li’s group to the two- [41] and three-dimensional [47] mapping of neuropeptides in the PO and brain of Cancer borealis. In these studies, MS peptide profiling provided detailed information about peptide localization, high-energy MS/MS experiments were used to confirm neuropeptide identity, and m/z intensity maps provided three-dimensional distributions of selected neuropeptides in brain slices.
Other developments in the area of sample preparation and analysis have involved work by Li and co-workers to improve the production of sequence-specific product ions in MS/MS experiments using peptide derivatization (reductive methylation [69] and methyl esterification [45]). Capillary electrophoresis techniques coupled with MALDI-FTMS analysis have been applied to crustacean tissue extracts [70, 71]. Li and co-workers have developed immunoaffinity-based enrichment techniques (immunoprecipitation and immunodot blot screening), coupled with MALDI-FTMS and nanoLC-ESI-Q-TOF MS/MS, for the targeted analysis of FMRFamide-related peptides in the PO of Cancer borealis [72].
Finally, quantitative peptidomic strategies are emerging as MS-based techniques that can be used to provide insights into neuropeptide function. MS-based quantitative approaches assess the up- or downregulation of peptide concentrations in response to a physiological change, such as an environmental stress or food deprivation [73]. In recent work, Li and co-workers have applied quantitative peptidomic techniques to assess changes in peptide expression in the brain and PO of fed and unfed Cancer borealis [74]. Variations in neuropeptide expression were quantified using stable isotopic labeling of extracted neuropeptides with H2- or D2-formaldehyde. In combination with imaging measurements, two potential feeding centers in the brain (the boundary of the olfactory lobe and the median protocerebrum) were identified.
Crustacean neuropeptide families
While a number of recent review articles have focused on specific families of neuropeptides in crustaceans, there has been no comprehensive review of the extant families of crustacean neuropeptides since that of Keller [75]. Here, we provide a brief overview of each of the neuropeptide families that are currently recognized as existing in crustaceans (Table 1), briefly describing their general structures, and, where possible, their putative modes of action and bioactivities.
A-type allatostatins
The A-type ASTs, first identified in insects, are typified by the C-terminal motif –YXFGLamide, where X represents a variable amino acid. In crustaceans, the existence of A-ASTs was first suggested by immunohistochemical labeling in the STNS in Cancer borealis [76]. The identification of native A-ASTs from Carcinus maenas, e.g., AGPYSFGLamide, followed shortly thereafter [16], with additional isoforms subsequently identified from a number of other decapod species [17, 25, 46, 48, 50–52, 77–79]. A-ASTs have also been identified by transcriptomics from several lower crustaceans, specifically the copepod Calanus finmarchicus [80] and the cladoceran Daphnia pulex [22]; in Calanus, the predicted isoforms exhibit variant –YXFGI/Vamide C-termini, e.g., APYGFGIamide and pQ/QPYNFGVamide [80].
A-ASTs are broadly distributed within the nervous systems of crustaceans, including regions of synaptic neuropil [22, 76, 80, 81] and neuroendocrine organs [1, 22, 80, 81], suggesting that they function as both locally released autocrines/paracrines and circulating hormones.
In crustaceans, the A-ASTs are well-documented inhibitory neuro/myomodulators. The stomatogastric and cardiac neuromuscular systems are two targets of A-type peptides. In the stomatogastric system, A-ASTs decrease the activity of the pyloric neural circuit, which produces the pyloric motor pattern [17, 76], and elicit a decrease in neuromuscular transmission in a number of pyloric and gastric mill muscles [82]. In the CG, whose rhythmic activity drives the heartbeat, A-ASTs decrease cycle frequency, as well as the number and frequency of spikes per burst in cardiac motor neurons [83]. A-type peptides have also been shown to decrease skeletal muscle performance, acting through both pre- and post-synaptic mechanisms [84], and have been implicated in the regulation of methyl farnesoate production by the mandibular organ [85].
B-type allatostatins
Members of the B-type AST family are characterized by the C-terminal motif –WX 6Wamide, where X 6 represents six variable amino acids. Although originally described from insects, B-type peptides, e.g., GNWNKFQGSWamide, have also been identified/predicted from a number of decapod species [21, 23, 25, 50–52], as well as from Daphnia pulex [22].
Mass spectral/molecular studies suggest that B-ASTs are broadly distributed within the nervous systems of at least the decapods, functioning both as locally released autocrines/paracrines and as circulating hormones.
At present, investigations into the physiological roles played by crustacean B-ASTs are limited to a single study on the Cancer borealis STNS [86], here eliciting a decrease in the activity of the ongoing pyloric motor pattern [86].
C-type allatostatins
Authentic C-type peptide
C-type ASTs are characterized by the presence of a pyroglutamine blocked N-terminus, the C-terminal motif –PISCF, and a disulfide bridge between the Cys residues located at positions 7 and 14. While members of this peptide family were long believed to exist only in holometabolous insects, an authentic C-AST, pQIRYHQCYFNPISCF (disulfide bridging between Cys7 and Cys14), was recently shown via transcriptomics and mass spectrometry to be broadly conserved within the Decapoda, being predicted/detected in 29 species representing seven infraorders [25, 60, 61].
Mass spectrometry and immunohistochemistry suggest that pQIRYHQCYFNPISCF likely serves as both a circulating hormone (Dickinson and Christie, unpublished) and a locally released autocrine/paracrine in crustaceans [60, 61, 87]. Additionally, it was detected in the midgut epithelium of Cancer borealis and Homarus americanus, suggesting gut-derived endocrine/paracrine functioning as well [60].
Physiologically, pQIRYHQCYFNPISCF appears to serve as an inhibitory modulator of both the stomatogastric neural circuit, where it decreases the frequency of the pyloric motor pattern [61], and the cardiac neuromuscular system, in which it decreases heart rate (Dickinson and Christie, unpublished).
C-type-like peptide
In addition to the authentic C-AST just described, a C-AST-like peptide, SYWKQCAFNAVSCFamide (disulfide bridging between Cys6 and Cys13), also appears to be broadly conserved within the Crustacea. This peptide was first identified via transcriptome analysis from Daphnia pulex [22], with subsequent transcriptomic/mass spectral detection in a second cladoceran, Daphnia carinata [24], and 25 decapod species [59, 61], including members of five infraorders.
As with the authentic C-AST, mass spectral and immunohistochemical data suggest that SYWKQCAFNAVSCFamide serves as both a locally released autocrine/paracrine [59, 61] and a circulating hormone (Dickinson and Christie, unpublished), at least in decapods.
One target of SYWKQCAFNAVSCFamide is the cardiac neuromuscular system, where application of the peptide modulates the frequency and amplitude of heart contractions [59]. Interestingly, its effects on frequency were mixed, increasing the heart rate in some preparations and decreasing it in others [59]. In addition, the peptide modulates the output of the pyloric motor pattern, eliciting a decrease in cycle frequency [59, 61].
Bursicon
In insects, melanization and sclerotisation of the cuticle following ecdysis are controlled by bursicon, a heterodimeric cysteine knot protein comprised of bursicon α and bursicon β subunit peptides. In crustaceans, the first isoform of each subunit was identified from Carcinus maenas [23, 88], i.e., DECSLRPVIHILSYPGCTSKPIPSFACQGRCTSYVQVSGSKLWQTERSCMCCQESGEREAAITLNCPKPRPGEPKEKKVLTRAPIDCMCRPCTDVEEGTVLAQKIANFIQDSPMDSVPFLK (bursicon α [88]) and RSYGVECETLPSTIHISKEEYDDTGRLVRVCEEDVAVNKCEGACVSKVQPSVNTPSGFLKDCRCCREVHLRARDITLTHCYDGDGARLSGAKATQHVKLREPADCQCFKCGDSTR (bursicon β [88]). Isoforms of the α and/or β subunit peptides have subsequently been identified in several other decapods [24, 25, 60, 61], as well as in Daphnia pulex [22, 88] and the euphausid Euphausia superba [24].
In Carcinus maenas, in situ hybridization studies show that bursicon α- and β-producing neurons are limited to the suboesophageal, thoracic, and abdominal ganglia, with all cells that produce one subunit also producing the other [88]. Interestingly, the bursicon-expressing somata also appear to produce crustacean cardioactive peptide (CCAP; see “Crustacean cardioactive peptide”) [88]. Based on the known projection patterns of the CCAP cells, the bursicon-containing somata likely project to and innervate the PO, suggesting a hormonal mode of delivery for the peptide [88]. The physiological roles played by bursicon in crustaceans remain unknown.
Corazonin
pQTFQYSRGWTNamide (Arg7-corazonin) is a well-known insect neuropeptide. In crustaceans, it was first sequenced via mass spectrometry from Cancer borealis [38]. Subsequent mass spectral investigations have identified it in several other decapods [23, 25, 51]. Prepro-hormones encoding the peptide have also been identified via transcriptome analysis from Litopenaeus vannamei [25] and Daphnia carinata [24].
In decapod species, immunohistochemical and/or mass spectral studies suggest that Arg7-corazonin likely functions as both a circulating hormone and a locally released autocrine/paracrine [23, 25, 51]. Additionally, the Litopenaeus vannamei ESTs that encode Arg7-corazonin were derived from the lymphoid organ [25], suggesting that it may also be produced by non-neural tissues.
The functional roles served by corazonin in crustaceans are currently limited to a single study [89], which suggests an involvement in the control of pigment migration in chromatophores.
Crustacean cardioactive peptide
CCAP
A peptide with the structure PFCNAFTGCamide (disulfide bridging between Cys3 and Cys9) was originally identified from Carcinus maenas [90]; in this species the peptide was cardioactive and thus was named crustacean cardioactive peptide [90]. Following its original description, authentic CCAP was identified via molecular/mass spectral studies in a number of other decapod species [38, 50, 52, 91, 92], with a variant isoform, PFCNAFAGCamide (Ala7-CCAP), predicted via transcriptome mining from Daphnia pulex and Daphnia carinata [22, 24].
In decapods, CCAP is present in both neuroendocrine organs [1, 23, 38, 50–52, 81, 91–95] and regions of the central neuropil [23, 25, 51, 52, 81, 91, 95–97], suggesting it functions as both a circulating hormone and a locally released autocrine/paracrine.
CCAP has been implicated in the control of many physiological processes in decapods. Although the peptide was named for its cardioexcitatory properties [83, 90, 93, 98], it also modulates the stomatogastric neuromuscular system [99–103], induces pigment dispersion in chromatophores [104, 105], induces changes in the light sensitivity of the retina [106], and is implicated in the control of ecdysis [88, 107, 108].
CCAP precursor-related peptides
The known crustacean CCAP-encoding prepro-hormones are predicted to liberate several peptides in addition to CCAP itself [22, 24, 25, 91]. For example, in the lobster Homarus gammarus, four peptides, GPVA, DIGDLLEGKD, SDPSMEGLASSSELDALAKHVLAEAKLWEQLQSKMEMMRSYASRMENHPVY, and STPHTQPRQHLTSTPQQKVETEKQ, are predicted to be produced along with authentic CCAP [91]. DIGDLLEGKD was recently sequenced from the brain and thoracic/abdominal ganglia of Homarus americanus using mass spectrometry [23, 25, 51]. Similarly, DIADLLDGKD, which was predicted from a Litopenaeus vannamei prepro-CCAP, was sequenced via mass spectrometry from the brain and thoracic/abdominal ganglia of this species [25]. The functional roles played by crustacean CCAP precursor-related peptides are largely unknown, although DIGDLLEGKD is cardioactive in Homarus americanus, increasing both the frequency and amplitude of the heartbeat (Wiwatpanit and Dickinson, unpublished data).
Crustacean hyperglycemic hormone superfamily
CHH superfamily
The CHH superfamily is a group of large, 70+ amino acid peptides whose members were originally isolated and characterized from the XO-SG systems of decapods [109–116]; the first CHH to be fully characterized was from Carcinus maenas, i.e., pQIYDTSCKGVYDRALFNDLEHVCDDCYNLYRTSYVASACRSNCYSNLVFRQCMDDLLMMDEFDQYARKVQMVamide [117]. Members of the CHH superfamily can be divided into two subgroups, the CHH subfamily and the molt-inhibiting hormone (MIH)/gonad-inhibiting hormone (GIH)/vitellogenesis-inhibiting hormone (VIH)/mandibular organ-inhibiting hormone (MOIH) subfamily (hereafter termed the MIH subfamily), based on their structures and/or the structures of their precursor proteins. Specifically, members of the CHH subgroup are typically 70–72 amino acids in length, possess six identically placed internal Cys residues (which allow for the formation of three stereotypic disulfide bridges), and the prepro-hormones from which they are cleaved include a second, 30+ amino acid peptide (commonly referred to as CHH precursor-related peptide or CPRP) between the CHH isoform and the signal sequence. In contrast, members of the MIH subgroup are typically larger, 77–78 amino acids long, and possess a similar, though not identical arrangement of Cys resides; their precursors lack the presence of a CPRP. Members of both the CHH and MIH subfamilies have been characterized from a large number of decapod species [109–116], as well as from members of several lower crustacean taxa, e.g., the isopod Armadillidium vulgare [118, 119] and Daphnia pulex [22].
The XO-SG system is a common source of members of the CHH superfamily [120–122]. In addition, isoforms of CHH distinct from those present in the XO-SG have been isolated and characterized from the PO [109–116]. In some species, CHH superfamily members have been identified immunologically in regions of synaptic neuropil, for example, MOIH-like labeling is present throughout the STNSs of several Cancer species [123]. Thus, while originally thought of as endocrine signaling agents, at least some members of the CHH superfamily appear likely to serve as locally released autocrines/paracrines. Moreover, CHH has also been found in epithelial endocrine cells of the fore- and hindguts of Carcinus maenas, implicating members of this peptide family in gut paracrine/endocrine signaling [124, 125].
Members of the CHH superfamily are highly pleiotropic [109–116]. As their names imply, this group of peptides has been implicated in the control of carbohydrate metabolism, ion transport and water uptake, molting, and reproduction [109–116]. The recent immunohistochemical identification of MOIH in the STG of Cancer crabs [123] suggests local paracrine modulation of the neural circuitry involved in the ingestion, chewing, and filtering of food within the foregut as well.
One feature of the CHH superfamily that currently appears to be unique is the existence of chiral variants (L and D) of some family members; in some cases, these variants have been shown to be differentially distributed within the nervous system and to serve distinct functions [109–116].
CPRP
As stated in the “CHH superfamily,” the precursors from which CHHs are derived contain a second peptide, CHH precursor-related peptide or CPRP, between the signal sequence and the CHH isoform. In decapods, CPRPs show considerable sequence identity to one another within members of a given infraorder, e.g., RSTQGYGRMDRILAALKTSPMEPSAALAVQHGTTHPLE and RSAQGMGKMERLLASYRGALEPSTPLGDLSGSLGHPVE in the crabs Carcinus maenas [14] and Cancer pagurus [121], respectively; more variation is seen between the CPRPs of different infraorders, particularly in their C-termini [14]. Although CPRPs are detectable in the hemolymph [126], where they can persist for a considerable period of time [126], nothing is currently known about the functional roles served by them in any species.
Diuretic hormone
In insects, peptides with structural similarity to vertebrate calcitonins have been identified and implicated in diuresis. Transcriptome mining has recently identified homologs of calcitonin-like diuretic hormone (CLDH) in several crustaceans including Daphnia pulex [22], the copepod Caligus clemensi [24], and Homarus americanus [127], e.g., GLDLGLGRGFSGSQAAKHLMGLAAANFAGGPamide from the latter species [127].
In Homarus americanus, RT-PCR tissue profiling shows that the native CLDH is produced by both neuroendocrine somata and somata likely to contribute to modulation in regions of synaptic neuropil, suggesting that CLDH functions as both a circulating hormone and a locally released autocrine/paracrine [127]. Surprisingly, the CG was one portion of the nervous system in which the CLDH-encoding transcript was identified, making it the first intrinsic peptide identified in the crustacean cardiac neuromuscular system [127]; CLDH is cardioactive in Homarus [127].
Ecdysis-triggering hormone
In insects, a group of structurally related peptides possessing –FFXKXXKXVPRXamide (where the Xs represent variable residues) C-termini have been shown to play a critical role in triggering ecdysis. Recently, the first crustacean ecdysis-triggering hormones (ETHs) were predicted via transcriptome mining from Daphnia pulex, i.e., DPSPEPFNPNYNRFRQKIPRIamide and GEGIIAEY(SO3H)MNSESFPHEGSLSNFFLKASKAVPRLamide [22]. The cellular distribution and functions of these peptides remain unknown.
Eclosion hormone
In insects, eclosion hormones (EHs) play critical roles in adult ecdysis. The known insect isoforms of eclosion hormone possess considerable amino acid identity, including six internal Cys residues that allow for the formation of three disulfide bridges. Via transcriptome mining, EHs have recently been identified from the crab Callinectes sapidus [24, 128], the shrimp Marsupenaeus japonicus and Penaeus monodon [24], and the tadpole shrimp Triops cancriformis [24], a branchiopod. The crustacean isoforms, e.g., the Calinectes peptide AVAANRKVSICIKNCGQCKKMYTDYFNGGLCGDFCLQTEGRFIPDCNRPDILIPFFLQRLE [24, 128], show significant sequence similarity to the known insect EHs, and like their insect counterparts, possess 6 Cys residues. At present nothing is known about the cellular distributions or physiological roles played by EHs in crustaceans.
Enkephalin
The peptides YGGFM and YGGFL were isolated and characterized from the thoracic ganglia of Carcinus maenas [129]; these peptides are identical in structure to the vertebrate opioid peptides Met-enkephalin and Leu-enkephalin, respectively. While Carcinus maenas is the only crustacean from which enkephalins have been fully characterized, biochemical/immunohistochemical data suggest they are broadly conserved in the taxon [129–136]; these data also suggest that the enkephalins function both as locally released autocrines/paracrines and circulating hormones in crustaceans.
Enkephalins appear to play a conserved role in the regulation of carbohydrate metabolism; the actions of the enkephalins on this process appear to be species-specific, inducing hypoglycemia in some species and hyperglycemia in others [137–143]. Several lines of evidence suggest that the modulatory activity of enkephalin on carbohydrate metabolism results from their involvement in the regulation of CHH release from the SG. For example, both δ- and β-opioid receptors have been identified in the eyestalk ganglia of crustaceans [144], opioid-binding sites have been localized to CHH-containing terminals of the SG [132], the hypo-/hyperglycemic actions of enkephalins are absent in eyestalk-ablated animals [139–143], and the peptides have been shown/implicated in the inhibition of CHH release in animals exhibiting hypoglycemic responses [135, 138]. Additionally, the enkephalins appear to play roles in the control of pigment granule migration in chromatophores, likely mediated via their regulation of release of other peptide hormones, e.g., PDH or RPCH [145–147], and they have been implicated in the modulatory control of both locomotion [146] and ovarian development [146, 148–153].
FMRFamide-related peptides
Myosuppressin
The myosuppressin subfamily of FMRFamide-like peptides (FLPs) possesses the consensus motif –HVFLRFamide. In decapod crustaceans, a single peptide possessing this C terminus has been identified, pQDLDHVFLRFamide [44]. Mass spectrometry suggests that pQDLDHVFLRFamide is broadly, perhaps ubiquitously, conserved within the Decapoda [44].
Mass spectral tissue profiling suggests that pQDLDHVFLRFamide is broadly distributed within decapod nervous systems [23, 25, 51, 52], likely functioning as both a locally released autocrine/paracrine and a circulating hormone.
Physiologically, pQDLDHVFLRFamide is a powerful modulator of the cardiac neuromuscular system [154].
Neuropeptide F
Members of the neuropeptide F (NPF) subfamily of FLPs are typically 36 amino acids in overall length and possess the C-terminal motif –GRPRFamide, as well as tyrosine residues at positions 10 and 17 from their C-termini. In crustaceans, three NPF-like peptides have recently been predicted via transcriptomics [21, 22], one from Marsupenaeus japonicus, i.e., KPDPSQLANMAEALKYLQELDKYYSQVSRPRFamide, and the others from the cladocerans Daphnia magna and Daphnia pulex, i.e., DGFVMGGGEGGEMTAMADAIKYLQGLDKVYGQAARPRFamide and DGGDVMSGGEGGEMTAMADAIKYLQGLDKVYGQAARPRFamide, respectively. No information is currently available as to the tissue distributions or functional roles played by NPFs in any crustacean.
Short neuropeptide F
A third subfamily of FLPs is the short neuropeptide Fs or sNPFs. Like the NPFs proper, these peptides possess –RXRFamide C-termini, where X is a variable residue; they are shorter in overall length than are the NPFs, typically being ~10 amino acids long. The first crustacean sNPFs, i.e., APALRLRFamide and DRTPALRLRFamide, were identified from the shrimp Macrobrachium rosenbergii [155]. To date, sNPF isoforms have been identified in decapod species encompassing four infraorders [23, 25, 48, 51, 72, 155–157], as well as in the cladoceran Daphnia pulex [22]. Interestingly, a peptide appearing to be an intermediate between the sNPFs and the NPFs proper has recently been predicted from the copepod Lepeoptheirus salmonis, i.e., LSQIKDFY(SO3H)NEAGRPRFamide [24].
Mass spectral tissue profiling suggests that sNPFs are broadly distributed within the decapod CNS [23, 51, 52, 72], serving as both autocrines/paracrines and circulating hormones. At present, the functional roles played by crustacean sNPFs remain unknown.
Sulfakinin
A fourth subfamily of FLPs is the sulfakinins, whose family members are characterized by the C-terminal motif –Y(SO3H)GHM/LRFamide. In crustaceans, sulfakinins have been identified from three decapod species, the first being Penaeus monodon, where two peptides, pQFDEY(SO3H)GHMRFamide and AGGSGGVGGEYDDY(SO3H)GHLRFamide, were biochemically characterized [18]. With the exception of the predicted sulfation state of one tyrosine in the latter peptide, an identical set of peptides was subsequently identified from Litopenaeus vannamei [20]. In Homarus americanus, molecular cloning identified the first crustacean sulfakinin-encoding transcript, with the peptides predicted from it being pEFDEY(SO3H)GHMRFamide and GGGEY(SO3H)DDY(SO3H)GHLRFamide [158].
Immunohistochemistry conducted on the CNS of Penaeus monodon suggests that the sulfakinins have a highly restricted distribution within the nervous system, being detected only in approximately ten neurons in the brain [18]. Moreover, large amounts of tissue were needed as starting material for the isolation and purification of the native isoforms from both Penaeus monodon and Litopenaeus vannamei, suggesting that the sulfakinins are present in low abundance within the CNS [18, 20]. These data are consistent with the sulfakinins serving as locally released modulators rather than hormones in at least penaeid species.
Functionally, the native Homarus americanus isoforms are cardioactive, increasing both the frequency and amplitude of ongoing heart contractions in the lobster [158].
Other FLPs
In addition to the subfamilies just described, a number of other FLPs have been identified from decapod crustaceans. Many of these peptides possess the C-terminal motif –FLRFamide [13, 19, 23, 25, 48, 51, 52, 72, 79, 155, 157, 159–161]. In fact, the first FLPs identified from crustaceans contain this structural element, i.e., TNRNFLRFamide and SDRNFLRFamide from Homarus americanus [13]. Mass spectral tissue surveys suggest that –FLRFamides function as both locally released autocrines/paracrines and circulating hormones [23, 25, 51, 52, 72]. Studies directed at assessing the physiological roles played by extended –FLRFamides suggest that these peptides are powerful modulators of the cardiac and stomatogastric neuromuscular systems, and of exoskeletal muscles in many decapod species [13, 83, 159, 161–169].
Another C-terminal motif seen in multiple crustacean FLPs is –YLRFamide [23, 25, 50, 51, 79, 157, 170], e.g., AYSNLNYLRFamide from Penaeus monodon [157]. Mass spectral tissue profiling suggests that, like most of the other FLP subfamilies, the –YLRFamides are broadly distributed within the nervous system, functioning as both locally release autocrines/paracrines and as circulating hormones [23, 25, 51, 52, 72]. Functionally, –YLRFamides have been shown to modulate the motor outputs of both the cardiac and stomatogastric neuromuscular systems [170].
Recently, two peptides possessing –FVRFamide C-termini were identified from the brain of Litopenaeus vannamei, i.e., GYSNKNFVRFamide and GYSNKDFVRFamide [25]. No information on the functional roles played by these peptides is currently available.
Insect kinin
Members of the insect kinin family possess the consensus motif –FX 1 X 2WGamide, where X 1 and X 2 represent variable amino acids. In crustaceans, the first members of this peptide family were identified from Litopenaeus vannamei, with some isoforms having an Ala for Gly substitution at their C-terminus [171, 172], e.g., DFSAWAamide. A subset of the Litopenaeus vannamei peptides has also been detected via mass spectrometry in Cancer crabs [48, 50].
Data on the distribution of insect kinins in crustaceans are limited to members of the Decapoda [48, 50, 171–173], where they have been found both in regions of central neuropil and in neuroendocrine sites, suggesting autocrine/paracrine and hormonal functioning [48, 173].
Physiologically, application of insect kinins to the STG excited the pyloric rhythm, particularly in preparations with slow ongoing motor patterns [173]. In addition, they consistently enhanced activity in the dorsal gastric (DG) neuron, a member of the gastric mill neural circuit, although the peptide did not elicit or alter the full motor program per se [173, 174]. Insect kinins have also been shown to increase the rate of spontaneous hindgut contractions in crustaceans [171].
Neuroparsin
The neuroparsins are a group of large, structurally related peptides that, in insects, were originally identified as anti-gonadotropic agents, though they have subsequently been shown to be highly pleiotropic. Insect neuroparsins contain 12 cysteine residues, which allow for the formation of 6 disulfide bridges, a hallmark of the family. In crustaceans, neuroparsin-like peptides have recently been predicted via transcriptome mining from several decapods [23–25], as well as from the copepod Caligus rogercresseyi [24]. Like their insect counterparts, these peptides contain Cys residues that are likely to result in a similar set of disulfide bridges, e.g., APRCDRHDEEAPKNCKYGTTQDWCKNGVCAKGPGETCGGYRWSEGKCGEGTFCSCGICGGCSPFDGKCGPTSIC from Carcinus maenas [23, 25]. At present, nothing is known about the tissue distribution or functional roles played by neuroparsins in crustaceans.
Orcokinin
The peptide NFDEIDRSGFGFN was originally isolated from Orconectes limosus [175]; based on its species of origin and myotropic activity on the gut, the peptide was named orcokinin [175]. Since this original description, additional isoforms of orcokinin have been identified from both this and other crustacean species via biochemical, mass spectral and/or molecular analyses [21–25, 36, 37, 39, 40, 43, 48, 50–52, 62, 176, 177]. In most decapods, multiple orcokinin isoforms are present, encoded by a common precursor; for example, 11 orcokinins (seven copies of NFDEIDRSGFGFN, two copies of NFDEIDRSGFGFV and one copy each of NFDEIDRSGFGFA and NFDEIDRTGFGFH) are present in the precursor of the crayfish Procambrarus clarkii [36]. However, in the shrimp Marsupenaeus japonicus, only a single orcokinin appears encoded within its prepro-hormone, i.e., 13 copies of NFDEIDRAGMGFA [21]. Regardless of species, all full-length decapod orcokinins are 13 amino acids long and possess the N-terminal consensus motif NFDEIDR–. Interestingly, in lower crustaceans, i.e., daphnids and copepods, a different situation pertains, namely one or two isoforms per species, with the native peptides being 14 rather than 13 amino acids long, e.g., the Daphnia pulex peptides NLDEIDRSNFGTFA and NLDEIDRSDFGRFV, both of which also exhibit a Leu for Phe substitution at position 2 [22], and NFDEIDRAGFGSFM, NFDEIDRAGFGSLI from the copepod Lernaeocera branchialis [24].
Biochemical, immunohistochemical, and/or mass spectral studies have shown that members of the orcokinin family are broadly distributed within crustacean nervous systems, and are likely to function as both locally released autocrines/paracrines and circulating hormones [23, 39, 40, 46, 51, 52, 62, 94, 178].
Orcokinin bioactivity has been demonstrated for several tissues in decapods. Specifically, in several species, orcokinins increase both the frequency and amplitude of spontaneous hindgut contractions [62, 175]; interestingly, they have little if any modulatory influence on hindgut contractions in others [62]. Orcokinins also modulate the output of the STNS [39, 40].
Orcomyotropin and other orcokinin precursor-related peptides
A peptide with the structure FDAFTTGFamide was originally sequenced from Orconectes limosus [177]. Given its pronounced enhancement of hindgut contractility in this species, the peptide was named orcomyotropin. Orcomyotropin in its authentic form has subsequently been found in a number of other decapod species [37], as have several unamidated, C-terminally extended peptides with significant sequence identity to orcomyotropin, e.g., FDAFTTGFGHN and FDAFTTGFGHS [44, 51]. With the identification of the precursors encoding orcokinin, it became clear that these extended peptides, likely the precursors for orcomyotropin, are encoded (one copy per prepro-hormone) on the same precursor as orcokinins [36, 62].
Mass spectral tissue profiling has shown that orcomyotropin and/or its extended variants are widely distributed within the nervous systems of at least decapods [23, 25, 50–52], suggesting both autocrine/paracrine and hormonal modes of delivery.
At present, investigations into the physiological roles played by crustacean orcomyotropins are limited to a single study where FDAFTTGFamide was shown to be a powerful excitatory modulator of hindgut contractility [177].
In addition to orcomyotropin, several other peptides are encoded on the orcokinin precursor [36, 62]. For example, one copy each of SSEDMDRLGFGFN, GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV, GDY(SO3H)DVYPE, VYGPRDIANLY, and SAE are predicted from the Homarus americanus prepro-hormone [62]. Mass spectrometry confirmed the presence of SSEDMDRLGFGFN and VYGPRDIANLY in the brain, STNS, and SG of Homarus americanus, with desulfated GDYDVYPE detected in the SG [62]. The functional roles served by these and other orcokinin precursor-related peptides remain unknown.
Pigment dispersing hormone
One of the first crustacean neuropeptides to be fully characterized was NSGMINSILGIPRVMTEAamide from the eyestalk ganglia of Pandalus borealis [12]. Given its ability to affect light-adapting pigment movements in the retina, the peptide was named light-adapting distal retinal pigment hormone. The peptide was subsequently found to be a potent pigment granule dispersing agent in chromatophores [179], and hence was dubbed pigment dispersing hormone, the name that is commonly used today. With the subsequent identification of the structurally related peptide NSELINSILGLPKVMNDAamide from the crab Uca pugilator [180], the Pandalus peptide was redesignated α-PDH and the Uca isoform β-PDH. Since their initial descriptions, other PDH isoforms have been identified biochemically, molecularly and/or via mass spectrometry from a wide variety of decapod species (e.g., [49, 50, 63, 181–189]), with those possessing sequence, acidity, and charge similarity to α-PDH forming one subgroup and those with similarity to β-PDH forming a second subfamily [190]. While members of the β-PDH subfamily have been identified in species from a number of decapod infraorders, detection of members of the α-PDH subfamily has thus far been limited to members of the Caridea [191]. In many species, multiple isoforms of PDH are present, e.g., NSELINSILGLPKVMNDAamide and NSELINSLLGISRLMNEAamide in Cancer productus [50, 63]. In addition to decapods, β-PDHs [191] have been identified from Armadillidium vulgare, i.e., NSELINSLLGAPRVLNNAamide [192], and Daphnia pulex, i.e., NSELINSLLGLPRFMKVVamide [22].
Immunohistochemical and/or mass spectral data suggest that PDHs are likely to serve as both autocrines/paracrines and hormones in decapods (e.g., [1, 23, 49–52, 63, 191, 193, 194]). It is important to note that in species with multiple PDH isoforms, one isoform may function primarily as a hormone and the other as an autocrine/paracrine. Which isoform is delivered hormonally versus released locally appears to vary from species to species, even in relatively closely related animals. For example, in Callinectes sapidus, authentic β-PDH has been proposed as the SG hormone, with a second β-PDH isoform proposed as a local transmitter in central neuropil [184], whereas in Cancer productus the modes of delivery for the two PDHs appear flipped [63]. PDH-like peptides have also been detected in neuropilar processes in the central nervous systems of several lower crustaceans, i.e., Calanus finmarchicus [195] and Daphnia pulex [22], suggesting at least a local modulatory functioning in these animals.
Members of the PDH family are classically known for their ability to affect pigment granule translocation, specifically pigment dispersion, within a number of cell types in the eye, as well as in epithelial chromatophores [191]. In addition, the presence of PDH-like immunoreactivity in regions of synaptic neuropil suggests that these peptides are likely to function as locally released neuromodulators; however, to the best of our knowledge, there has been no direct demonstration of this function. In fact, in the STNS, where immunoreactivity is present, β-PDH shows no bioactivity [194]. Anecdotal evidence suggests that the PDH system in crustaceans may also be involved in the generation of circadian rhythmicity [196–198].
Proctolin
A peptide with the structure RYLPT, originally isolated from the cockroach Periplaneta americana and named proctolin, has been identified in authentic form from many decapod crustaceans [23, 25, 38, 50–52, 199–201], the first being the crab Cardisoma carnifex [201]. Recently, the first crustacean proctolin-encoding transcript was identified from Litopenaeus vannamei [25].
The distribution of proctolin in crustacean tissues has been the focus of numerous studies. In decapods, proctolin is widely distributed within the nervous system [23, 38, 50–52, 92, 94, 200, 202–211], and likely serves both as an autocrine/paracrine and as a hormone. In addition, immunohistochemical data indicate that proctolin is present in the central nervous system of members of several lower crustaceans, i.e., the isopod Porcellio scaber [212] and Daphnia pulex [22], suggesting at least a local modulatory role for it in these animals.
Proctolin has widespread modulatory actions in crustaceans. The decapods have received by far the most extensive investigation, and here, proctolin has been shown to modulate exoskeletal muscles/neuromuscular junctions [213–216], the cardiac neuromuscular system [98, 217–222], the stomatogastric neuromuscular system [92, 103, 204, 223–228], the ventilatory system [229], the neural circuitry controlling the swimmerets [230, 231], mechanosensory neurons [232–234], and hindgut contractility [235]. In non-decapods, proctolin has been shown to be a potent myomodulator [236, 237] and to modulate cardiac output [238].
Pyrokinin
Members of the pyrokinin family exhibit the C-terminal motif –FXPRLamide, where X represents a variable residue. In decapods, several pyrokinin isoforms have been identified [23, 25, 51, 52, 56, 239], e.g., DFAFSPRLamide and ADFAFNPRLamide from Litopenaeus vannamei [25, 239].
In decapods, pyrokinins are broadly distributed within the nervous system [23, 25, 51, 52, 56], suggesting both autocrine/paracrine and hormonal functioning.
Assessment of the physiological roles served by pyrokinins in crustaceans is limited to a single study, where their actions on the Cancer borealis stomatogastric neural circuits were examined [56]. Interestingly, and unlike most peptide modulators, pyrokinins had little effect on the pyloric motor pattern, but consistently activated the gastric mill rhythm.
Red pigment concentrating hormone
The first invertebrate neuropeptide to be fully characterized was pELNFSPGWamide, which was isolated from the eyestalk of Pandalus borealis [10]. Based on its ability to affect color change via the aggregation of pigment granules within epithelial erythrophores, this peptide is commonly referred to as red pigment concentrating hormone (RPCH). Since its initial description, authentic RPCH has been identified via a variety of techniques from many other decapod species [92, 240–243]. RPCH was also detected using biochemistry/mass spectrometry in the CNS of Porcellio scaber [244]. While it was long thought that pELNFSPGWamide was the sole RPCH isoform present in crustaceans, recent transcriptome mining has shown that in at least two daphnids, Daphnia magna and Daphnia carinata, a variant isoform, pQVNFSTSWamide, is present [21, 24].
The classic source of RPCH in members of the Decapoda is the XO-SG system [10, 11]. Biochemical, immunohistochemical, and/or mass spectral studies, however, have shown that it is also present in other areas of the decapod nervous system, including other neuroendocrine organs [1, 107] and regions of synaptic neuropil [223, 245–247], suggesting dual endocrine and autocrine/paracrine function. In lower crustaceans, RPCH-like immunoreactivity has been reported in the CNS of Daphnia pulex [22], suggesting at least local modulatory functioning here.
Although RPCH was originally identified based on its ability to affect the concentration of pigment granules in erythrophores, it has subsequently been shown to be highly pleiotropic, modulating the central pattern generating networks present in the STNS [223, 228, 246, 248–252] and CG [83], as well as the motor output of the swimmeret system [247]. Recently, RPCH was implicated in the mobilization of energy stores in Porcellio scaber [244].
RYamide
A family of peptides exhibiting –RYamide C-termini has recently been identified in members of the Decapoda [23, 25, 38, 50, 253], e.g., pEGFYSQRYamide [253].
Mass spectral data suggest that RYamides are present in neuroendocrine stuctures and in regions of central neuropil, serving as both hormones and locally released autocrines/paracrines [23, 25, 38, 50, 52, 253]. Nothing is known about the bioactivity of RYamides in Crustacea.
SIFamide
Members of the SIFamide family of neuropeptides typically exhibit the structure XYRKPPFNGSIFamide, where X represents a variable residue. In most decapod crustaceans, Gly1-SIFamide appears to be the sole isoform present [24, 25, 44, 57, 64, 157]. However, in homarid lobsters, Gly1-SIFamide has been replaced by a Val1 variant [44, 51, 54, 64]. In several mass spectral studies, the peptide PPFNGSIFamide has also been detected, though it is likely a breakdown product of the full-length peptide [64]. In Daphnia pulex, transcriptome mining predicts the SIFamide variant TRKLPFNGSIFamide [254].
Immunohistochemistry and mass spectral tissue profiling suggest that SIFamide is widely distributed within the nervous systems of decapod species [23, 25, 51, 52, 54, 79, 255], but is not present in any neuroendocrine release site. Based on these data it appears that SIFamide functions solely as a locally released autocrine/paracrine. However, SIFamide has also been identified in epithelial endocrine cells of the midgut, suggesting that gut-derived endocrine functioning is possible, as is local autocrine/paracrine modulation of the midgut [256].
While much is known about the identity of the SIFamide isoforms present in decapods [44], comparatively little is known about the physiological roles served by members of this peptide family. In fact, only two direct functional studies currently exist. In Homarus americanus, Val1-SIFamide is a potent modulator of the pyloric neural circuit [54, 64], while in Macrobrachium rosenbergii, injection of the Gly1 isoform increases the level of aggressive behavior in males, and thus appears to play a role in the establishment of dominance hierarchies [257]. Although indirect, anatomical studies have suggested other neuromodulatory roles for the SIFamides in decapods, implicating them in both visual and olfactory control [57, 79, 258].
Tachykinin-related peptide
A family of peptides possessing the C-terminal motif –FX 1GX 2Ramide, where X 1 and X 2 represent variable residues, is broadly conserved in invertebrates. Given their sequence similarity to members of the vertebrate tachykinins, these peptides are commonly referred to as tachykinin-related peptides. The first crustacean TRP identified was APSGFLGMRamide from Cancer borealis [15]. APSGFLGMRamide was subsequently identified in many other decapod species [44] and for some time was believed to be the sole TPR present in members of this crustacean taxon [58]. Recently a second decapod TRP, TPSGFLGMRamide, was identified [55]. Although this peptide is present in a number of decapods [21, 23, 51, 55], it appears to be less broadly conserved than its Ala1 counterpart [44]. Additionally, mass spectral analyses conducted on Litopenaeus vannamei identified several other TRPs, i.e., APAGFLGMRamide, APSGFNGMRamide and APSFGLDMRamide [25], bringing the current number of known decapod isoforms to five. TRPs have also been predicted via transcriptomics from the isopod Eurydice pulchra, i.e., APSGFLGMRamide, VPRRFLGIRamide, APASFLGMRamide, APSAFLGMRamide, and ARSSFLGMRamide [21].
TRPs are widely distributed within the CNSs of decapods, including both synaptic neuropil and neuroendocrine sites [1, 9, 23, 25, 51, 52, 131, 134, 259–262]. In addition, TRPs have been shown to be present in and released from midgut epithelial endocrine cells in several species [55, 256, 263]. Collectively, these data suggest that TRPs function as both locally released autocrines/paracrines and circulating hormones in the Decapoda.
TRP bioactivity has been demonstrated for the decapod stomatogastric and cardiac neuromuscular systems [9, 15, 55, 83, 228, 251, 263–267]; TRP is also implicated in the modulation of photoreceptor sensitivity [268].
Other peptides
CFITNCPPGamide
A peptide with the sequence CFITNCPPGamide was recently predicted from Daphnia pulex [269]; its structure places it within the oxytocin/vasopressin family [269]. The cellular distribution and functional roles played by CFITNCPPGamide remain unknown, and it is unclear how broadly conserved this peptide, or related isoforms, may be in crustaceans.
HIGSLYRamide
HI/LGSI/LYRamide has been identified via mass spectrometry from several decapods [21, 23, 50, 52], the Ile/Leu ambiguity resulting from the isobaric nature of these amino acids. In Carcinus maenas, a partial transcript encoding the peptide has also been identified [21], revealing the structure of the peptide to be HIGSLYRamide [21]. Mass spectral tissue profiling suggests that HIGSLYRamide is widely distributed within decapod nervous systems, serving as both a locally released autocrine/paracrine and a circulating hormone [21, 23, 50, 52]. The functional roles played by HIGSLYRamide are unknown.
Physiological effects of neuropeptides in crustaceans
The most extensively studied effects of crustacean neuropeptides are the modulatory effects they exert on pattern generators in the central nervous system. In addition to effects at the central level, these peptides alter behavior by modulating both sensory receptors and muscle contraction. Moreover, hormonally released neuropeptides control a wide variety of other physiological processes, ranging from metabolism and osmoregulation to the synthesis and release of other hormones. Here, we focus on recent studies of neuropeptides in the crustacean cardiac and the stomatogastric neuromuscular systems, examining the multiple neuropeptides that work together to control them.
The cardiac neuromuscular system
Peptidergic modulation of the neurogenic heartbeat of crustaceans involves multiple mechanisms acting at multiple sites
The crustacean heart is neurogenic, with contractions driven by the rhythmic output of a central pattern generator located in the CG, which lies within the single-chambered heart (reviewed in [5]). To alter hemolymph flow, neuropeptides can thus exert modulatory effects on the pattern generator (i.e., the CG itself), on the cardiac muscle and/or neuromuscular junction, and/or on the vessels that carry hemolymph from the heart to the tissues. Because all parts of the circulatory system are constantly bathed with the full array of neuropeptides being used as circulating hormones, neuropeptides, which play a major role in controlling circulation, can exert their effects at all of these sites.
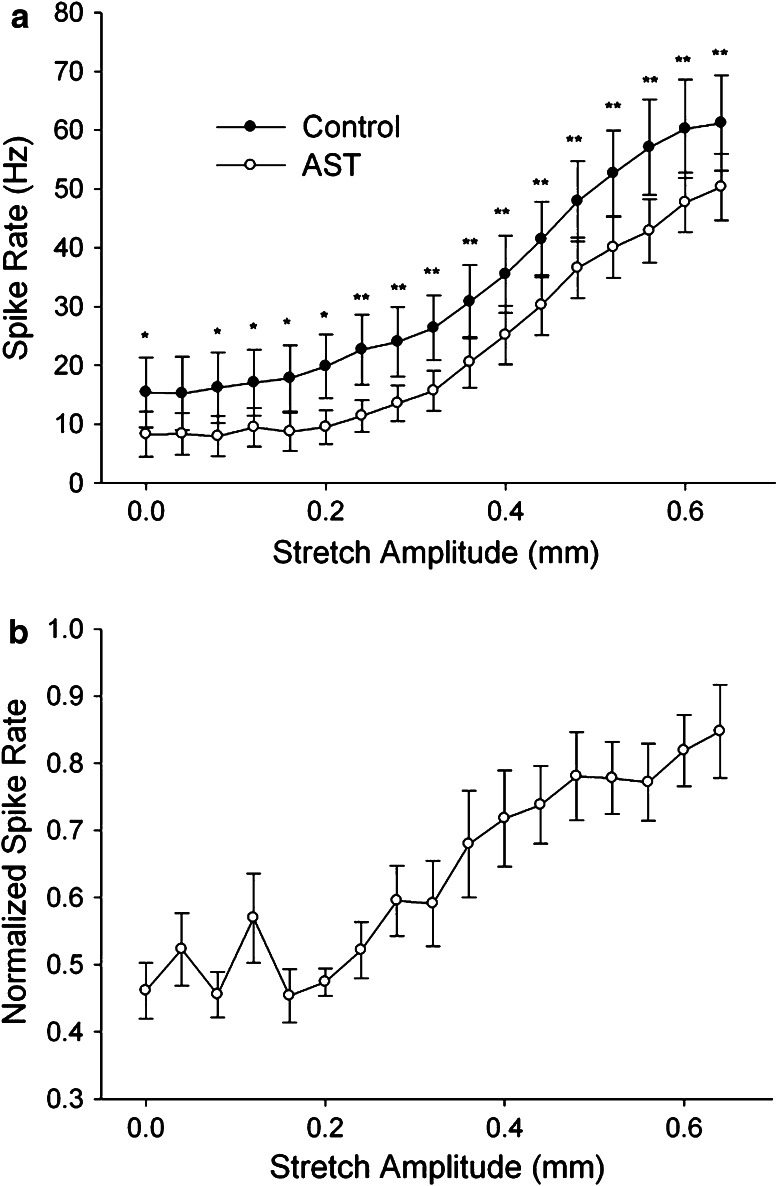
When they are perfused through the isolated whole heart, most neuropeptides that have been examined cause increases in contraction amplitude and frequency (e.g. [93, 127, 158, 159, 162, 164, 169, 221, 270–272]). Only a few peptides examined in Homarus americanus, i.e., myosuppressin [154], the AST-C-like peptide SYWKQCAFNAVSCFamide [59], and the A-AST ASPYAFGLamide (Powers and Dickinson, unpublished), have been shown to result in decreases in either frequency or amplitude.
Modulation of the cardiac central pattern generator
The pattern generator that drives cardiac contractions consists of nine neurons in most crustaceans that have been studied. Four small cells, which have strong endogenous oscillatory properties, drive bursting in the five large motor neurons. In Cancer borealis, Cruz-Bermudez and Marder [83, 170] examined the effects of ten neuropeptides on the rhythmic output of the CG. Of these, seven had excitatory effects, one inhibited the rhythmic output of the ganglion, and two had little or no effect. The only peptide that exhibited inhibitory effects in this study was GGSLYSFGLamide, an insect A-type AST, which also inhibits the pattern generators of the stomatogastric system (see below).
Interestingly, although the FLPs and CCAP generally increased cycle frequency, spike frequency within bursts, and duty cycle in Cancer borealis [83, 170], studies of these peptides in other species have found more complex effects. In Callinectes sapidus, for example, the effects of CCAP on the isolated CG were primarily excitatory, increasing burst duration, duty cycle, and number of spikes/burst [93]; however, cycle frequency did not change. Several FLPs, including TNRNFLRFamide and SDRNFLRFamide and the native Callinectes FLP, GYNRSFLRFamide, exerted similar effects in this species [162]. In the isolated CG of Homarus americanus, several of the identified FLPs have effects similar to those recorded in crabs. TNRNFLRFamide, for example, causes increased spike frequency within bursts and increased burst duration when bath applied to the isolated CG [273].
In contrast, myosuppressin caused a large decrease in cycle frequency in the isolated CG in both Procambarus clarkii [270] and Homarus americanus [154]. At the same time, burst duration increased by at least 50%, and the amplitude of the driver potential (slow wave) that underlies the bursts of action potentials in the motor neurons increased.
Nearly all of the neuropeptides that have been shown to modulate the crustacean heart, including those described above, are released from neuroendocrine organs, but are not present in the CG. Recently, however, the mRNA that encodes a CLDH was localized to the large motor neurons of Homarus americanus CG itself [127]. This peptide profoundly increases both the frequency and amplitude of heart contractions, indicating that it modulates the cardiac neuromuscular system at one or more sites, likely including the CG, which determines the frequency of the heartbeat. This is the first peptide to be found in the CG [127], suggesting the possibility that it may act as an intrinsic neuromodulator of this system.
Effects on cardiac muscle
The motor behavior that results from activity in any central pattern generator is determined not only by the motor output of that CPG, but also by the way that the output is translated into movement by the muscles, i.e., the neuromuscular transform [274–276]. Thus, effects of neuropeptides on the cardiac muscle will likewise alter the behavioral changes that these peptides provoke.
Effects of neuropeptides on cardiac muscle contraction have been examined for relatively few of the many neuropeptides that influence cardiac activity. All but one of the neuropeptides whose modulatory effects on the periphery have been examined are FLPs; all result in increased muscle contraction. For example, TNRNFLRFamide [273] and myosuppressin [154] cause contraction amplitude to increase in response to controlled trains of stimuli in Homarus americanus. Fort et al. [162] found that GYNRSFLRFamide caused an increase in the amplitude of both contractions and excitatory junctional potential amplitudes in response to controlled trains of stimuli in Callinectes sapidus. The FLP in these studies could have exerted its effects on either the muscle itself or the neuromuscular junction. In Homarus americanus, Wilkens et al. [221] demonstrated that both proctolin and SDRNFLRFamide act directly on the muscle, where they enhance contractions caused by direct stimulation of the cardiac muscles. In addition, Wilkens et al. [221] measured increases in intracellular Ca++ concentration when the peptide was present at low concentrations (~10−10M); the results of these experiments and experiments using specific channel blockers suggest that the peptides modulate voltage-gated L-type Ca++ channels at threshold concentrations and activate sarcolemmal Ca++ transporters at higher concentrations.
In addition to effects mediated by changes in heart contractions themselves, hemolymph flow can be altered by changes in the properties of the outflow vessels, particularly changes in the resistance to flow in the arteries. Recordings from a variety of locations within the circulatory system have shown that a number of peptides, including proctolin, TNRNFLRFamide, and CCAP, can increase vascular resistance in both Homarus americanus and the achelatan lobster Jasus edwardsii [98, 222]. In the dorsal abdominal artery, at least part of this increased resistance is due to changes in the valves that lead from this artery into each of the pairs of lateral arteries within the abdomen [98]. Increasing resistance in these valves increases the general resistance in the abdomen and tends to force more hemolymph into the other parts of the lobster. Neuropeptides can also cause an increase in resistance in other parts of the circulatory system, such as the anterior arteries. Although the majority of circulatory vessels in the lobsters are not muscular, a recent study [220] showed that Homarus americanus arteries contain actin, myosin, and tropomyosin. The dorsal abdominal artery contains striated muscle cells and responds to electrical stimulation. Other arteries respond to proctolin with slow circumferential, but not longitudinal, contractions. The magnitude of these contractions appears sufficient to account for the measured increases in vascular resistance [220].
Effects of neuropeptides on the integrated output of the crustacean heart neuromuscular system
Because the CG sits within the heart, and because most neuropeptides are delivered to the heart hormonally, the CG and heart muscles are virtually always exposed to these neuropeptides in concert. The peptides thus exert their effects simultaneously at multiple levels within these pattern generator-effector systems [93, 154, 162, 277]. Because of the presence of a number of feedback loops (e.g., stretch receptors [5, 278, 279] and nitric oxide [280]), the global effects of any given peptide on the integrated system may or may not be predictable from the simple effects on the CPG and/or the isolated muscle. For example, although cycle frequency does not increase in response to any of the FLPs in the isolated Callinectes sapidus CG, frequency increases by as much as 300% when the same peptides are perfused through the whole heart [162]. Similarly, TNRNFLRFamide causes cycle frequency to increase in the whole heart of Homarus americanus, but to decrease in the isolated ganglion [273] (Fig. 5). This difference is likely due to indirect effects of the peptides via the feedback loops. The role of both passive stretch and active contraction of the heart muscle has been examined in the CG of the isopod Ligia pallasii [279]; here stretch or contraction can phase-advance the next burst, resulting in an increase in cycle frequency.
Fig. 5.
Modulation of the heartbeat in Homarus americanus by TNRNFLRFamide involves complex effects at multiple sites. a, b Schematic diagrams of the preparations used to record muscle contraction and activity of the cardiac ganglion. a Contraction of an isolated whole heart was monitored with a force transducer; motor output of the cardiac ganglion was recorded simultaneously with a suction electrode on a motor nerve. b In other preparations, the cardiac ganglion was removed from the heart, and motor output was recorded on the motor nerves in the isolated cardiac ganglion. c Perfusion of TNRNFLRFamide through the heart increases both amplitude and frequency of heart contractions. C1 and C2 are higher speed recordings from the regions shown in c, illustrating the increases in both amplitude and frequency recorded during TNRNFLRFamide perfusion (yellow bar in c; C2). d The effect of TNRNFLRFamide on contraction amplitude in the whole heart is dose-dependent, with significant effects at concentrations as low as 10−10 M. e Cycle frequency of the motor bursts recorded in the whole heart (blue bars) increases in response to perfusion with TNRNFLRFamide, with a threshold of ~10−10 M. In contrast, superfusion of the same peptide over the isolated CG causes a decrease in cycle frequency (green bars). Moreover, effects of this peptide on the isolated CG are seen only at much higher concentrations (e.g., 10−8 M) than those on the whole heart. Scale bars 1.0 g, 100 s in c, 2.5 s in C1 and C2. Figure modified from Stevens et al. [273]
In contrast, central and peripheral mechanisms appear to act largely in concert, and to reinforce one another’s effects, in the response of the whole heart to myosuppressin [154]. Responses in the whole heart are remarkably similar to those recorded in the isolated CG. However, myosuppressin caused a much larger decrease in frequency in the whole heart than in the isolated CG, which drives the contractions. This difference cannot readily be explained by the expected changes in stretch feedback in the whole heart; increased contraction amplitude is predicted to activate stretch receptors and thereby increase, rather than decrease, the heart rate. Thus, in addition to modulating the CG and the muscle/neuromuscular junction, myosuppressin may directly modulate one of the feedback pathways.
Although studies examining the effects of neuropeptides at multiple sites in this integrated neuromuscular system are limited at present, they suggest that the interactions between the central nervous system and the periphery are complex, and are themselves likely to be modulated by neuropeptides. Additional studies examining the effects of the many neuropeptides that act simultaneously at multiple sites are likely to follow and to further enhance our understanding of these important interactions.
The stomatogastric neuromuscular system
Organization of the stomatogastric neuromuscular system
The STNS, a relatively small extension of the central nervous system, consists of four ganglia, the paired commissural ganglia (CoGs), and the unpaired esophageal (OG) and stomatogastric (STG) ganglia, their inter-connecting nerves, motor nerves, and a number of integrated sensory organs. The STNS generates the rhythmic motor patterns that control the four major regions of the crustacean foregut: (1) the esophagus, (2) the cardiac sac, which serves largely for food storage, (3) the gastric mill, consisting of three teeth that shred ingested food, and (4) the pylorus, a set of filters responsible for sorting digested or partially digested food particles. Both the gastric mill and the pyloric patterns are generated in the STG, which contains between 25 and 35 neurons, depending on the species.
The core pyloric motor pattern is triphasic, consisting of alternating bursts of action potentials (period ~1–2 s) in the pyloric dilator (PD)/anterior burster (AB) neurons, followed by bursts in two types of constrictor neurons, the lateral pyloric (LP) and then the pyloric (PY) neurons. In the intact animal and in vitro, this pattern is usually constitutively active as long as the inputs from the anterior CoGs and OG are intact. The PD and AB neurons, which are electrically coupled, function as the pacemaker for the pattern, since the AB is an endogenous burster. Additional neurons include the inferior cardiac (IC) neuron, which usually fires in phase with the LP neuron, and the ventricular dilator (VD) neuron, which fires with the PY neurons.
In contrast to the pyloric pattern, there is no single pacemaker neuron for the gastric mill pattern; instead, this pattern results from the interactions of neurons within the network. The resulting pattern is more or less biphasic, with the neurons that control the power and return strokes for each of the two tooth types (medial tooth; paired lateral teeth) firing in alternation due to reciprocal inhibitory connections. Thus, as initially described for the achelatan lobster Panulirus interruptus (see [281]), the dorsal gastric (DG) and gastric mill (GM) neurons alternate, and the lateral gastric (LG)/medial gastric (MG) neurons fire in alternation with the lateral posterior gastric (LPG neurons). The gastric mill pattern is considerably slower than the pyloric pattern, with a period of 5–20 s, and is active only intermittently in the intact animal. It is considerably less stereotyped than the pyloric pattern; at least part of that variability is related to the extensive interactions of the pattern generating neurons in the STG with modulatory neurons and other inputs from the CoGs. This has been studied most extensively in Cancer borealis, in which numerous projection neurons have been identified and the patterns that result from their activation have been characterized (e.g., [264, 265, 267, 282–287]).
Modulation of the pyloric and gastric motor patterns by neuropeptides: shared mechanisms
The frequency and phasing of the gastric mill and pyloric patterns rely on both the intrinsic membrane properties of the neurons that generate them and the synaptic interactions between those neurons. Thus, alterations of either parameter are likely to modulate the outputs of the pattern generators.
Among the membrane properties that play major roles in the generation of the rhythmic gastric and pyloric patterns are endogenous bursting, plateau potentials (i.e., bi-stability in neuronal membrane potential) and post-inhibitory rebound. These are all modulated by at least one of the neuropeptides present in the STNS. Post-inhibitory rebound in the LP neuron of Panulirus interruptus, for example, is enhanced in the presence of RPCH [248]. Weimann et al. [168] showed that SDRNFLRFamide and TNRNFLFRamide enhance the ability of the DG neuron to produce plateau potentials, sometimes even causing it to fire in endogenous bursts. This alteration is accompanied by an activation of the gastric pattern. The endogenous bursting of the AB neuron is modulated by neuropeptides, as was first shown by Hooper and Marder [227], in experiments in which proctolin enhanced AB bursting.
Because the modulation of intrinsic properties is ultimately the result of changes in specific ionic currents, recent studies have focused on the modulation of membrane currents. Golowasch and Marder [288] initially demonstrated that the peptide proctolin enhances a voltage-dependent inward current that is also sensitive to external Ca++ concentrations [288]. Subsequently, Swensen and Marder [289] determined that six different modulators, including five neuropeptides (proctolin, TNRNFLRFamide, APSGFLGMRamide, CCAP, and RPCH), all converge to modulate this same current. Interestingly, application of these five peptides results in five different patterns because different suites of neurons have receptors for different peptides [290]. In fact, by experimentally adding (via the dynamic clamp) the “proctolin current” to the appropriate suite of neurons in the presence of one peptide, Swensen and Marder [289] were able to elicit the pattern typical of a different peptide.
Like intrinsic properties, the strength of chemical synapses can be modulated by neuropeptides. Few examples of peptidergic synaptic modulation at central synapses have been recorded, and their functional significance varies considerably, suggesting that synaptic modulation may play a number of different roles in pattern generating networks.
First, in Panulirus interruptus, RPCH increases the amplitude of the post-synaptic potentials from the inferior ventricular (IV) neurons onto a large number of its post-synaptic targets, including neurons in both the gastric [250] and pyloric [248] networks. At the same time, RPCH activates the IV neurons to fire in a bursting pattern that drives the cardiac sac pattern. Thus, synaptic modulation is largely responsible for the complete fusion of the cardiac sac and gastric mill motor patterns; it is also among the mechanisms by which RPCH elicits a pyloric pattern that includes complex temporal variation, also tied to the cardiac sac pattern, of the normally stereotyped pyloric pattern [248].
In Cancer borealis, proctolin enhances the strength of the synapse between a pyloric (IC) and a gastric (GM) neuron. Although the functional effects of this change were not examined, the extent to which the gastric and pyloric networks interact is highly variable in this species [291], presenting the possibility that this synaptic enhancement may likewise play a role in modulating the coordination of two networks.
Finally, Thirumalai and Marder [251] showed that RPCH enhances a synapse within the pyloric network in Homarus americanus. The inhibitory synapse from the LP to the PD neuron, which provides the major source of feedback from the constrictor neurons (LP/PY) to the pacemaker group (PD/AB), is strongly potentiated by RPCH; bursting in the pacemakers is simultaneously enhanced. Surprisingly, because of its timing, this synaptic potentiation does not alter the pyloric cycle frequency, but may instead serve to stabilize the pattern as dilator bursting is strengthened [252].
Modulation of the stomatogastric motor patterns by neuropeptides: the role of multiple modulators and co-transmission
Most of the modulatory neurons whose transmitter complement has been identified in the STNS contain more than one transmitter, including neuropeptides (reviewed in [286]). Like modulatory neurons, neuroendocrine organs contain many different neurotransmitters, including neuropeptides (e.g., [1, 23, 49–52]). Although the patterns of hormonal co-release of different peptides remain unknown, varicosities within these organs frequently show co-localized peptides [1]. Given the large number of peptides localized to these organs, it seems inevitable that multiple peptides will, at times, be released more or less together, so that neurons of the STG will be simultaneously exposed to multiple neuropeptides.
This raises several questions, including (1) whether and how modulatory neurons using some of the same co-localized transmitters are able to exert different effects on their target networks and (2) whether the effects of different peptides are simply additive or interact in more complex ways. The first question has been studied extensively in the Cancer borealis STNS, in which three different neurons, all of which modulate the gastric and/or pyloric patterns, contain proctolin as a co-transmitter [2]. The motor patterns that result from the activation of these neurons are distinct, with different patterns being triggered by activity in each of the neurons (e.g., [2]). The mechanisms by which co-transmission in the crab is regulated and by which modulatory neurons containing different complements of co-transmitters exert their effects have been extensively reviewed (e.g., [285, 286, 292–294]). Nonetheless, it is worth noting that not only are co-transmitters likely to activate distinct receptors, but also that the patterns of firing, and therefore of transmitter release, of neurons containing different co-transmitter complements are likely to differ. Both factors may play a role in determining the differential effects of these modulatory neurons. Moreover, even neurons that respond to a given co-transmitter when it is bath-applied may not respond to simulation of the neuron containing that peptide. Possible explanations include the distribution of synapses and the segregation of transmitters within a modulatory neuron.
The second question, regarding the ways in which different peptides interact within a given circuit, may involve the same issues discussed above. However, an early study showed that the influence of proctolin on the cardiac sac network differs dramatically when it was applied alone and when it was applied either shortly after RPCH or in the presence of low (sub-threshold for overt effects) levels of RPCH. Alone, proctolin had no obvious effect; with RPCH, it, like higher concentrations of RPCH alone, activated the cardiac sac pattern in Panulirus interruptus [295]. Although no mechanism was proposed, the subsequent demonstration [289] that RPCH and proctolin converge to activate the same inward current in many STG neurons suggests a possible site for their interactions.
Modulation of sensory feedback within the stomatogastric system
Both the anterior gastric receptor (AGR) and the gastro-pyloric receptor (GPR) neurons monitor stretch in stomach muscles, providing feedback to the stomatogastric pattern generators. Neuropeptides, including TNRNFLRFamide and SDRNFLRFamide, excite these receptors. These effects are more complex than a simple increase in firing frequency, presenting possible mechanisms for the coding of more information. The dendrites of the AGRs in Homarus gammarus fire tonically in the absence of stretch and increase their firing frequency in response to stretch [296]. Exogenously applied TNRNFLRFamide causes them to reversibly switch from a tonic-firing mode to a bursting mode of firing in the absence of stretch. When stretched, the bursting pattern is altered, so that stretch in the presence of the peptide is encoded by three parameters: increased burst frequency, decreased burst duration, and increased spike frequency within bursts [296].
Although TNRNFLRFamide does not cause the firing mode of the GPRs to switch from tonic to bursting in Cancer borealis, these receptors can spontaneously fire in a bursting mode. When they do so, TNRNFLRFamide increases the burst rate, even in the absence of a stretch stimulus [297]. When these receptors are not in bursting mode, TNRNFLRFamide increases the tonic firing rate, as well as the response to stretch.
Although most neuropeptides known to modulate sensory organs in crustaceans result in increases in sensory responses to the same stimulus, one peptide, an A-AST, causes an average decrease in tonic spike frequency as well as in the response to stretch in the GRP [297]. These modulatory effects are both amplitude and history-dependent. Specifically, the relative effects of the peptide are greater when spike frequency in the receptor is low. Thus, the peptide decreases the response to smaller stretches more than that to large stretches (Fig. 6). Moreover, because these receptors adapt to repeated stimulation, response amplitude decreases with repeated stimulation. Consequently, the effects of the peptide tend to be more pronounced in a preparation that has been subject to repeated stimulation.
Fig. 6.
A-type allatostatin (A-AST) decreases the responses of the gastro-pyloric stretch receptor (GPR) in the Cancer borealis stomatogastric system. a Spike frequency in response to stretch increases as a function of stretch amplitude, but is lower at all amplitudes in the presence of AST. b Spike frequency in A-AST is shown normalized to the response in control saline at each stretch amplitude, showing that the effects of A-AST are greater at larger stretch amplitudes. Figure modified from Birmingham et al. [297]; used with permission
In addition to decreasing spike frequency, the A-ASTs have more subtle effects on the timing of spikes in the GPRs [298]. The precision of spike timing increases; that is, there is less jitter in the times at which the spikes occur during a changing stimulus. Thus, if a neuron getting post-synaptic input from the GPRs has a short time constant and is integrating information from multiple sources, the change in jitter could, like the change in average firing frequency, play an important role in information transfer.
Other factors determining the outcome of peptidergic modulation: state dependence, life history, and evolutionary considerations
One of the earliest principles to come from studies of peptidergic modulation of the STNS is the concept of state-dependent modulation [299, 300]. Initially, it was shown that the effects of excitatory peptides or the activity of modulatory neurons was stronger when the starting pattern was weak than when it was strong. It has subsequently become clear that the same is true for inhibition: the inhibitory ASTs are likewise more effective on an initially weak pattern [61, 76, 86]. While the “state” is clearly a complex function of ion channels present in many neurons within the system, the factors determining channel distribution are considerably less clear. This question becomes particularly interesting when one considers the large number of peptides present in the STNS, and then asks how and whether all of them are effective modulators of the system. While it is clear that many of the modulatory peptides located in the STG do alter the patterns produced there, it is equally clear that, at least under some conditions, a number of peptides within the ganglion have little or no effect on the activity of the pyloric and gastric mill patterns. Thus, for example, the recently identified Homarus americanus CLDH, which alters the output of the CG, is found in the STG, but appears to have little or no effect on the patterns generated there (Dickinson, unpublished). Likewise, pEGFYSQRYamide is found within the STG, but does not appear to alter the patterns produced there (Dickinson, unpublished data).
In the short term, the presence of other neuromodulators is a major factor in determining the level of activity and thus the state of the system. The experiments on Panulirus interruptus described above, in which proctolin activates the cardiac sac pattern in the presence of low concentrations of RPCH or just after RPCH application, but not in its absence [295], illustrate one such example. This is also seen in decentralization, when inputs to the STG are removed; in most species, this leads to a cessation of the gastric pattern and either a decrease or cessation of the pyloric pattern. In Homarus americanus, the PD neurons continue to burst strongly, but at a decreased frequency [301]. If, however, the preparations are maintained in this state, deprived of all neuromodulatory inputs, for hours to days, activity returns to something resembling normal in many species, e.g., Jasus lalandii [302, 303], Cancer borealis [304, 305], and Homarus gammarus [301]. The mechanisms underlying this change, and the role played by neuropeptides in it, have recently been examined in Cancer borealis [306, 307]. These studies showed that the alterations in ionic currents that underlie recovery after decentralization, including decreased co-regulation of specific currents, are controlled not only by activity in the pattern generator, but also by neuromodulators, notably proctolin. Interestingly, the currents whose expressions were altered by decentralization and whose coordinated expression was regulated over the time (hours to days) involved in the recovery from decentralization were not the currents that are acutely modulated by proctolin, suggesting the possibility that neuropeptides have longer term, more subtle effects than have previously been studied.
It is also worth considering and speculating on the determinants of the extent to which peptides alter activity on longer time scales: developmentally, over the course of seasons and years, and evolutionarily.
A number of studies in Homarus americanus [101, 228, 266, 308, 309] and Homarus gammarus [310, 311] have examined the roles of neuropeptides in the development of the pattern generators of the STNS, at least partly by assessing the effects of the peptides at different developmental stages (for review see [308, 312, 313]). Interestingly, the full complement of STG neurons and modulatory projection neurons is present very early in development. However, modulatory transmitters are acquired only gradually. Thus, some peptides, including FLRF-like peptides, proctolin and RPCH, are all present by the mid-embryonic stage, whereas A-ASTs and TRPs are acquired only near or after the end of the embryonic stage (for review see [312, 313]). Moreover, although all of the STG neurons are present, the motor patterns that are generated in the embryonic and larval stages differ dramatically from those generated in the adult. Specifically, the embryonic STG produces only a single rhythm, in which all of the STG neurons participate, whereas clearly distinct pyloric and gastric mill patterns are generated by the adult STG [310, 311]. A number of experiments suggest that the differential organization is at least partly a function of modulation, including peptidergic effects. Thus, blocking all modulation eliminates rhythmic activity in the embryonic nervous system and eliminates most rhythmic activity in the adult nervous system. Adding back a modulator (oxotremorine in the published experiments) restores activity, but results in the generation of two patterns with different frequencies in both the embryo and the adult, suggesting that the specific pattern of modulators released in the embryo is responsible for maintaining the single embryonic pattern. In spite of this, a number of peptides have different effects on the embryonic and adult motor patterns, while the effects of others are remarkably similar at these different life stages. For example, in recordings from neurons that show pyloric activity in the adult, CCAP activates a pyloric-like triphasic pattern, albeit one with a relatively low cycle frequency, in the embryonic STNS [101]. RPCH likewise produces similar patterns in the adult and the embryo [228] (Fig. 7). In contrast, SIFamide activates different classes of neurons in the embryo and the adult [228] (Fig. 7). Thus, while peptides may play an important role in sculpting the pattern generators and the resultant stomatogastric patterns during development, it is clear that developmental stage partly determines the responses of the STG pattern generators to peptides.
Fig. 7.
Peptides modulate the same system differently in adults (a, b, c) and embryos (d, e, f) of Homarus americanus. (a) When inputs from the anterior ganglia are blocked in the adult, the only rhythmically active neurons in the STG are the PD neurons, which fire in regular bursts at a low cycle frequency. b Superfusion of the isolated STG with SIFamide activates bursting in the PD neurons, but has little effect on the other two neurons shown, the LP neuron of the pyloric network, and the DG neuron from the gastric mill network. c RPCH causes an increase in activity in all three neurons shown. d In the embryo, recordings are from muscles innervated by the same neurons whose activity is shown in the adult neuronal recordings. In control saline, when modulatory inputs are blocked, none of the neurons are rhythmically active. e Superfusion with SIFamide activates all three neurons, with the PD and DG innervated muscles (pdm, lpm) firing in bursts that are more or less synchronous, while the LP-innervated muscle (dgm) is activated more or less tonically. This contrasts with the adult, in which only one of the neurons (i.e., PD) is activated. f RPCH activates activity in all three muscles; however, in contrast to what is seen in the adult, they all fire with the same cycle frequency. c and f are from different preparations than a/b and d/e, respectively; note also that they are on slightly different time scales. Scale bars 5 mV, 5 s. Figure modified from Rehm et al. [228]; used with permission
After metamorphosing to their adult forms, crustaceans continue to undergo periodic changes in their internal as well as external environments. Perhaps the most physiologically demanding change is the periodic molt cycle. As the cuticle is removed in ecdysis, crustaceans swallow water to increase their size, which might be expected to alter functioning of the foregut, although only one study has examined molting. Clemens et al. [314] found that the pyloric rhythm continued relatively unchanged except for a brief time during ecdysis itself. They likewise found that the depression of the pattern that occurred during this time could be largely accounted for by a hypoxia induced by molting. Nonetheless, it is known that peptide levels, for example CCAP, change significantly during the molt cycle [108], suggesting the possibility that the effects of peptides may change as a function of the molt cycle. For example, given that CCAP strongly activates the pyloric pattern between molts, how is it that this pattern remains relatively constant as CCAP levels increase with the approaching molt? The extent to which, and the mechanism by which, the molt cycle influences peptide effects on the STNS as well as on other systems (e.g., the heart) are areas of investigation that are likely to provide insight into fundamental aspects of the function of peptides in physiological control.
On an evolutionary time scale, the structure and the required function of the stomatogastric system as a whole have undergone significant changes. These are reflected in the structure of the foregut and of the STNS. Nonetheless, the pattern generators of the STNS remain remarkably similar between species, with changes most evident in the strengths of the synaptic connections (both electrical and chemical) between neurons within the system (for review see [313]). However, peptidergic modulation appears to be less conserved, and homologous neurons often contain different peptide complements (for review see [313]). Additionally, even when the same modulatory peptides are present, the ability of the pattern-generating neurons of the STNS to respond to them may change over evolutionary time. For example, virtually the same complement of peptides is present in the STNS and neuroendocrine organs of Cancer crabs and the kelp crab Pugettia producta, which has a much more restricted diet than Cancer species [92]. However, the Pugettia STNS appears to be much less sensitive to many of these peptides, including both CCAP and the TRP APSGFLGMRamide [92].
Conclusions and future directions
Much has changed in the 15 years since the last comprehensive review of crustacean neuropeptides [75] was undertaken. The tools currently being used for peptide discovery have changed substantially; consequently, in contrast to the fewer than two dozen neuropeptides characterized from the entirety of the crustacean taxon, more than two dozen peptide families have now been characterized from many individual species. For some peptide families, over two dozen isoforms have been characterized from a single animal. The functional roles played by peptides in members of the Crustacea continue to expand; most, if not all, peptides now appear to be highly pleiotropic within this taxon. Clearly much work remains to be done before we will understand fully the roles served by the diverse set of crustacean neuropeptides, and undoubtedly many more peptides remain to be discovered.
Acknowledgments
This work was supported through institutional funds provided by Mount Desert Island Biological Laboratory.
References
- 1.Christie AE, Skiebe P, Marder E. Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis . J Exp Biol. 1995;198:2431–2439. doi: 10.1242/jeb.198.12.2431. [DOI] [PubMed] [Google Scholar]
- 2.Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci. 1999;19:6774–6783. doi: 10.1523/JNEUROSCI.19-16-06774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- 4.Mulloney B, Skinner FK, Namba H, Hall WM. Intersegmental coordination of swimmeret movements: mathematical models and neural circuits. Ann NY Acad Sci. 1998;860:266–280. doi: 10.1111/j.1749-6632.1998.tb09055.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooke IM. Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol Bull. 2002;202:108–136. doi: 10.2307/1543649. [DOI] [PubMed] [Google Scholar]
- 6.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 7.Cooke I, Sullivan R. Hormones and neurosecretion. In: Bliss D, Atwood H, Sandeman D, editors. The biology of crustacea. New York: Academic Press; 1982. pp. 205–290. [Google Scholar]
- 8.Christie AE, Cain SD, Edwards JM, Clason TA, Cherny E, Lin MH, Manhas AS, Sellereit KL, Cowan NG, Nold KA, Strassburg HP, Graubard K. The anterior cardiac plexus: an intrinsic neurosecretory site within the stomatogastric nervous system of the crab Cancer productus . J Exp Biol. 2004;207:1163–1182. doi: 10.1242/jeb.00856. [DOI] [PubMed] [Google Scholar]
- 9.Messinger DI, Kutz KK, Le T, Verley DR, Hsu YW, Ngo CT, Cain SD, Birmingham JT, Li L, Christie AE. Identification and characterization of a tachykinin-containing neuroendocrine organ in the commissural ganglion of the crab Cancer productus . J Exp Biol. 2005;208:3303–3319. doi: 10.1242/jeb.01787. [DOI] [PubMed] [Google Scholar]
- 10.Fernlund P, Josefsson L. Crustacean color-change hormone: amino acid sequence and chemical synthesis. Science. 1972;177:173–175. doi: 10.1126/science.177.4044.173. [DOI] [PubMed] [Google Scholar]
- 11.Fernlund P, Josefsson L. Chromactivating hormones of Pandalus Borealis. Isolation and purification of the ‘red-pigment-concentrating hormone’. Biochim Biophys Acta. 1968;158:262–273. doi: 10.1016/0304-4165(68)90139-6. [DOI] [PubMed] [Google Scholar]
- 12.Fernlund P. Structure of a light-adapting hormone from the shrimp, Pandalus borealis . Biochim Biophys Acta. 1976;439:17–25. doi: 10.1016/0005-2795(76)90155-0. [DOI] [PubMed] [Google Scholar]
- 13.Trimmer BA, Kobierski LA, Kravitz EA. Purification and characterization of FMRFamidelike immunoreactive substances from the lobster nervous system: isolation and sequence analysis of two closely related peptides. J Comp Neurol. 1987;266:16–26. doi: 10.1002/cne.902660103. [DOI] [PubMed] [Google Scholar]
- 14.Tensen CP, Verhoeven AHM, Gaus G, Janssen KPC, Keller R, Vanherp F. Isolation and amino acid sequence of crustacean hyperglycemic hormone precursor-related peptides. Peptides. 1991;12:673–681. doi: 10.1016/0196-9781(91)90119-a. [DOI] [PubMed] [Google Scholar]
- 15.Christie AE, Lundquist CT, Nassel DR, Nusbaum MP. Two novel tachykinin-related peptides from the nervous system of the crab Cancer borealis . J Exp Biol. 1997;200:2279–2294. doi: 10.1242/jeb.200.17.2279. [DOI] [PubMed] [Google Scholar]
- 16.Duve H, Johnsen AH, Maestro JL, Scott AG, Jaros PP, Thorpe A. Isolation and identification of multiple neuropeptides of the allatostatin superfamily in the shore crab Carcinus maenas . Eur J Biochem. 1997;250:727–734. doi: 10.1111/j.1432-1033.1997.00727.x. [DOI] [PubMed] [Google Scholar]
- 17.Dircksen H, Skiebe P, Abel B, Agricola H, Buchner K, Muren JE, Nassel DR. Structure, distribution, and biological activity of novel members of the allatostatin family in the crayfish Orconectes limosus . Peptides. 1999;20:695–712. doi: 10.1016/s0196-9781(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 18.Johnsen AH, Duve H, Davey M, Hall M, Thorpe A. Sulfakinin neuropeptides in a crustacean. Isolation, identification and tissue localization in the tiger prawn Penaeus monodon . Eur J Biochem. 2000;267:1153–1160. doi: 10.1046/j.1432-1327.2000.01113.x. [DOI] [PubMed] [Google Scholar]
- 19.Sithigorngul P, Saraithongkum W, Longyant S, Panchan N, Sithigorngul W, Petsom A. Three more novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant freshwater prawn Macrobrachium rosenbergii . Peptides. 2001;22:191–197. doi: 10.1016/s0196-9781(00)00382-x. [DOI] [PubMed] [Google Scholar]
- 20.Torfs P, Baggerman G, Meeusen T, Nieto J, Nachman RJ, Calderon J, De Loof A, Schoofs L. Isolation, identification, and synthesis of a disulfated sulfakinin from the central nervous system of an arthropod, the white shrimp Litopenaeus vannamei . Biochem Biophys Res Commun. 2002;299:312–320. doi: 10.1016/s0006-291x(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 21.Christie AE, Cashman CR, Brennan HR, Ma MM, Sousa GL, Li L, Stemmler EA, Dickinson PS. Identification of putative crustacean neuropeptides using in silico analyses of publicly accessible expressed sequence tags. Gen Comp Endocrinol. 2008;156:246–264. doi: 10.1016/j.ygcen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Gard AL, Lenz PH, Shaw JR, Christie AE. Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea; Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen Comp Endocrinol. 2009;160:271–287. doi: 10.1016/j.ygcen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Ma MM, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li L. Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen Comp Endocrinol. 2009;161:320–334. doi: 10.1016/j.ygcen.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christie AE, Durkin CS, Hartline N, Ohno P, Lenz PH. Bioinformatic analyses of the publicly accessible crustacean expressed sequence tags (ESTs) reveal numerous novel neuropeptide-encoding precursor proteins, including ones from members of several little studied taxa. Gen Comp Endocrinol. 2010;167:164–178. doi: 10.1016/j.ygcen.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Ma M, Gard AL, Xiang F, Wang J, Davoodian N, Lenz PH, Malecha SR, Christie AE, Li L. Combining in silico transcriptome mining and biological mass spectrometry for neuropeptide discovery in the Pacific white shrimp Litopenaeus vannamei . Peptides. 2010;31:27–43. doi: 10.1016/j.peptides.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie AE. Neuropeptide discovery in Ixodoidea: an in silico investigation using publicly accessible expressed sequence tags. Gen Comp Endocrinol. 2008;157:174–185. doi: 10.1016/j.ygcen.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Boonen K, Landuyt B, Baggerman G, Husson SJ, Huybrechts J, Schoofs L. Peptidomics: the integrated approach of MS, hyphenated techniques and bioinformatics for neuropeptide analysis. J Sep Sci. 2008;31:427–445. doi: 10.1002/jssc.200700450. [DOI] [PubMed] [Google Scholar]
- 28.Hummon AB, Amare A, Sweedler JV. Discovering new invertebrate neuropeptides using mass spectrometry. Mass Spectrom Rev. 2006;25:77–98. doi: 10.1002/mas.20055. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Sweedler JV. Peptides in the brain: mass spectrometry-based measurement approaches and challenges. Annu Rev Anal Chem. 2008;1:451–483. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- 30.DeKeyser SS, Li L. Mass spectrometric charting of neuropeptides in arthropod neurons. Anal Bioanal Chem. 2007;387:29–35. doi: 10.1007/s00216-006-0596-x. [DOI] [PubMed] [Google Scholar]
- 31.Westman-Brinkmalm A, Brinkmalm G. Mass spectrometry instrumentation. In: Silberring J, Ekman R, editors. Mass spectrometry and hyphenated techniques in neuropeptide research. New York: Wiley; 2002. pp. 47–105. [Google Scholar]
- 32.Chen C-H. Review of a current role of mass spectrometry for proteome research. Anal Chim Acta. 2008;624:16–36. doi: 10.1016/j.aca.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed FE. Utility of mass spectrometry for proteome analysis: Part I. Conceptual and experimental approaches. Exp Rev Proteom. 2008;5:841–864. doi: 10.1586/14789450.5.6.841. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed FE. Utility of mass spectrometry for proteome analysis: Part II. Ion-activation methods, statistics, bioinformatics and annotation. Exp Rev Proteom. 2009;6:171–197. doi: 10.1586/epr.09.4. [DOI] [PubMed] [Google Scholar]
- 35.El-Aneed A, Cohen A, Banoub J. Mass spectrometry, review of the basics: electrospray, MALDI, and commonly used mass analyzers. Appl Spectrosc Rev. 2009;44:210–230. [Google Scholar]
- 36.Yasuda-Kamatani Y, Yasuda A. Identification of orcokinin gene-related peptides in the brain of the crayfish Procambarus clarkii by the combination of MALDI-TOF and on-line capillary HPLC/Q-Tof mass spectrometries and molecular cloning. Gen Comp Endocrinol. 2000;118:161–172. doi: 10.1006/gcen.1999.7453. [DOI] [PubMed] [Google Scholar]
- 37.Skiebe P, Dreger M, Borner J, Meseke M, Weckwerth W. Immunocytochemical and molecular data guide peptide identification by mass spectrometry: orcokinin and orcomyotropin-related peptides in the stomatogastric nervous system of several crustacean species. Cell Mol Biol. 2003;49:851–871. [PubMed] [Google Scholar]
- 38.Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis . J Neurochem. 2003;87:642–656. doi: 10.1046/j.1471-4159.2003.02031.x. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E. Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J Comp Neurol. 2002;444:227–244. doi: 10.1002/cne.10139. [DOI] [PubMed] [Google Scholar]
- 40.Skiebe P, Dreger M, Meseke M, Evers JF, Hucho F. Identification of orcokinins in single neurons in the stomatogastric nervous system of the crayfish, Cherax destructor . J Comp Neurol. 2002;444:245–259. doi: 10.1002/cne.10145. [DOI] [PubMed] [Google Scholar]
- 41.DeKeyser SS, Kutz-Naber KK, Schmidt JJ, Barrett-Wilt GA, Li L. Imaging mass spectrometry of neuropeptides in decapod crustacean neuronal tissues. J Proteome Res. 2007;6:1782–1791. doi: 10.1021/pr060603v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutz Kimberly K, Schmidt Joshua J, Li L. In situ tissue analysis of neuropeptides by MALDI FTMS in-cell accumulation. Anal Chem. 2004;76:5630–5640. doi: 10.1021/ac049255b. [DOI] [PubMed] [Google Scholar]
- 43.Stemmler EA, Provencher HL, Guiney ME, Gardner NP, Dickinson PS. Matrix-assisted laser desorption/ionization Fourier transform mass spectrometry for the identification of orcokinin neuropeptides in crustaceans using metastable decay and sustained off-resonance irradiation. Anal Chem. 2005;77:3594–3606. doi: 10.1021/ac0502347. [DOI] [PubMed] [Google Scholar]
- 44.Stemmler EA, Cashman CR, Messinger DI, Gardner NP, Dickinson PS, Christie AE. High-mass-resolution direct-tissue MALDI-FTMS reveals broad conservation of three neuropeptides (APSGFLGMRamide, GYRKPPFNGSIFamide and pQDLDHVFLRFamide) across members of seven decapod crustaean infraorders. Peptides. 2007;28:2104–2115. doi: 10.1016/j.peptides.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Ma MM, Kutz-Naber KK, Li L. Methyl esterification assisted MALDI FTMS characterization of the orcokinin neuropeptide family. Anal Chem. 2007;79:673–681. doi: 10.1021/ac061536r. [DOI] [PubMed] [Google Scholar]
- 46.Cape SS, Rehm KJ, Ma M, Marder E, Li L. Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic lobster, Homarus americanus . J Neurochem. 2008;105:690–702. doi: 10.1111/j.1471-4159.2007.05154.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen RB, Hui LM, Sturm RM, Li L. Three dimensional mapping of neuropeptides and lipids in crustacean brain by mass spectral imaging. J Am Soc Mass Spectrom. 2009;20:1068–1077. doi: 10.1016/j.jasms.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huybrechts J, Nusbaum MP, Vanden Bosch L, Baggerman G, De Loof A, Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab, Cancer borealis . Biochem Biophys Res Commun. 2003;308:535–544. doi: 10.1016/s0006-291x(03)01426-8. [DOI] [PubMed] [Google Scholar]
- 49.Fu Q, Goy Michael F, Li L. Identification of neuropeptides from the decapod crustacean sinus glands using nanoscale liquid chromatography tandem mass spectrometry. Biochem Biophys Res Commun. 2005;337:765–778. doi: 10.1016/j.bbrc.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 50.Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, De la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- 51.Ma MM, Chen RB, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC, Goy MF, Christie AE, Li L. Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus . Gen Comp Endocrinol. 2008;156:395–409. doi: 10.1016/j.ygcen.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma MM, Wang JH, Chen RB, Li L. Expanding the crustacean neuropeptidome using a multifaceted mass spectrometric approach. J Proteome Res. 2009;8:2426–2437. doi: 10.1021/pr801047v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt JJ, McLlwain S, Page D, Christie AE, Li L. Combining MALDI-FTMS and bioinformatics for rapid peptidomic comparisons. J Proteome Res. 2008;7:887–896. doi: 10.1021/pr070390p. [DOI] [PubMed] [Google Scholar]
- 54.Christie AE, Stemmler EA, Peguero B, Messinger DI, Provencher HL, Scheerlinck P, Hsu YW, Guiney ME, de la Iglesia HO, Dickinson PS. Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val(1)-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus . J Comp Neurol. 2006;496:406–421. doi: 10.1002/cne.20932. [DOI] [PubMed] [Google Scholar]
- 55.Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J Neurochem. 2007;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- 56.Saideman SR, Ma MM, Kutz-Naber KK, Cook A, Torfs P, Schoofs L, Li L, Nusbaum MP. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- 57.Yasuda A, Yasuda-Kamatani Y, Nozaki M, Nakajima T. Identification of GYRKPPFNGSIFamide (crustacean-SIFamide) in the crayfish Procambarus clarkii by topological mass spectrometry analysis. Gen Comp Endocrinol. 2004;135:391–400. doi: 10.1016/j.ygcen.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda-Kamatani Y, Yasuda A. APSGFLGMRamide is a unique tachykinin-related peptide in crustaceans. Eur J Biochem. 2004;271:1546–1556. doi: 10.1111/j.1432-1033.2004.04065.x. [DOI] [PubMed] [Google Scholar]
- 59.Dickinson PS, Wiwatpanit T, Gabranski ER, Ackerman RJ, Stevens JS, Cashman CR, Stemmler EA, Christie AE. Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J Exp Biol. 2009;212:1140–1152. doi: 10.1242/jeb.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stemmler EA, Bruns EA, Cashman CR, Dickinson PS, Christie AE. Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. Gen Comp Endocrinol. 2010;165:1–10. doi: 10.1016/j.ygcen.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma MM, Szabo TM, Jia CX, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickinson PS, Stemmler EA, Barton EE, Cashman CR, Gardner NP, Rus S, Brennan HR, McClintock TS, Christie AE. Molecular, mass spectral, and physiological analyses of orcokinins and orcokinin precursor-related peptides in the lobster Homarus americanus and the crayfish Procambarus clarkii . Peptides. 2009;30:297–317. doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu YW, Stemmler EA, Messinger DI, Dickinson PS, Christie AE, De la Iglesia HO. Cloning and differential expression of two beta-pigment-dispersing hormone (beta-PDH) isoforms in the crab Cancer productus: evidence for authentic beta-PDH as a local neurotransmitter and beta-PDH II as a humoral factor. J Comp Neurol. 2008;508:197–211. doi: 10.1002/cne.21659. [DOI] [PubMed] [Google Scholar]
- 64.Dickinson PS, Stemmler EA, Cashman CR, Brennan HR, Dennison B, Huber KE, Peguero B, Rabacal W, Goiney CC, Smith CM, Towle DW, Christie AE. SIFamide peptides in clawed lobsters and freshwater crayfish (Crustacea, Decapoda, Astacidea): a combined molecular, mass spectrometric and electrophysiological investigation. Gen Comp Endocrinol. 2008;156:347–360. doi: 10.1016/j.ygcen.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Bulau P, Meisen I, Schmitz T, Keller R, Peter-Katalinic J. Identification of neuropeptides from the sinus gland of the crayfish Orconectes limosus using nanoscale on-line liquid chromatography tandem mass spectrometry. Mol Cell Proteom. 2004;3:558–564. doi: 10.1074/mcp.M300076-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Dircksen H, Bocking D, Heyn U, Mandel C, Chung JS, Baggerman G, Verhaert P, Daufeldt S, Plosch T, Jaros PP, Waelkens E, Keller R, Webster SG. Crustacean hyperglycaemic hormone (CHH)-like peptides and CHH-precursor-related peptides from pericardial organ neurosecretory cells in the shore crab, Carcinus maenas, are putatively spliced and modified products of multiple genes. Biochem J. 2001;356:159–170. doi: 10.1042/0264-6021:3560159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu Q, Christie AE, Li L. Mass spectrometric characterization of crustacean hyperglycemic hormone precursor-related peptides (CPRPs) from the sinus gland of the crab, Cancer productus . Peptides. 2005;26:2137–2150. doi: 10.1016/j.peptides.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 68.Ma MM, Chen RB, Ge Y, He H, Marshall AG, Li L. Combining bottom-up and top-down mass spectrometric strategies for de novo sequencing of the crustacean hyperglycemic hormone from Cancer borealis . Anal Chem. 2009;81:240–247. doi: 10.1021/ac801910g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu Q, Li L. De novo sequencing of neuropeptides using reductive isotopic methylation and investigation of ESI QTOF MS/MS fragmentation pattern of neuropeptides with N-terminal dimethylation. Anal Chem. 2005;77:7783–7795. doi: 10.1021/ac051324e. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Ma M, Chen R, Li L. Enhanced neuropeptide profiling via capillary electrophoresis off-line coupled with MALDI FTMS. Anal Chem. 2008;80:6168–6177. doi: 10.1021/ac800382t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang JH, Jiang XY, Sturm RM, Li L. Combining tissue extraction and off-line capillary electrophoresis matrix-assisted laser desorption/ionization Fourier transform mass spectrometry for neuropeptide analysis in individual neuronal organs using 2,5-dihydroxybenzoic acid as a multi-functional agent. J Chromatogr A. 2009;1216:8283–8288. doi: 10.1016/j.chroma.2009.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma MM, Sturm RM, Kutz-Naber KK, Fu Q, Li L. Immunoaffinity-based mass spectrometric characterization of the FMRFamide-related peptide family in the pericardial organ of Cancer borealis . Biochem Biophys Res Commun. 2009;390:325–330. doi: 10.1016/j.bbrc.2009.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fricker LD, Lim JY, Pan H, Che FY. Peptidomics: identification and quantification of endogenous peptides in neuroendocrine tissues. Mass Spectrom Rev. 2006;25:327–344. doi: 10.1002/mas.20079. [DOI] [PubMed] [Google Scholar]
- 74.Chen R, Hui L, Cape SS, Wang J, Li L. Comparative neuropeptidomic analysis of food intake via a multifaceted mass spectrometric approach. ACS Chem Neurosci. 2010;1:204–214. doi: 10.1021/cn900028s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keller R. Crustacean neuropeptides––structures, functions and comparative aspects. Experientia. 1992;48:439–448. doi: 10.1007/BF01928162. [DOI] [PubMed] [Google Scholar]
- 76.Skiebe P, Schneider H. Allatostatin peptides in the crab stomatogastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. J Exp Biol. 1994;194:195–208. doi: 10.1242/jeb.194.1.195. [DOI] [PubMed] [Google Scholar]
- 77.Duve H, Johnsen AH, Scott AG, Thorpe A. Allatostatins of the tiger prawn, Penaeus monodon (Crustacea: Penaeidea) Peptides. 2002;23:1039–1051. doi: 10.1016/s0196-9781(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 78.Yin GL, Yang JS, Cao JX, Yang WJ. Molecular cloning and characterization of FGLamide allatostatin gene from the prawn, Macrobrachium rosenbergii . Peptides. 2006;27:1241–1250. doi: 10.1016/j.peptides.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 79.Yasuda-Kamatani Y, Yasuda A. Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of crayfish Procambarus clarkii . J Comp Neurol. 2006;496:135–147. doi: 10.1002/cne.20903. [DOI] [PubMed] [Google Scholar]
- 80.Christie AE, Sousa GL, Rus S, Smith CM, Towle DW, Hartline DK, Dickinson PS. Identification of A-type allatostatins possessing -YXFGI/Vamide carboxy-termini from the nervous system of the copepod crustacean Calanus finmarchicus . Gen Comp Endocrinol. 2008;155:526–533. doi: 10.1016/j.ygcen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Skiebe P. Allatostatin-like immunoreactivity in the stomatogastric nervous system and the pericardial organs of the crab Cancer pagurus, the lobster Homarus americanus, and the crayfish Cherax destructor and Procambarus clarkii . J Comp Neurol. 1999;403:85–105. [PubMed] [Google Scholar]
- 82.Jorge-Rivera JC, Marder E. Allatostatin decreases stomatogastric neuromuscular transmission in the crab Cancer borealis . J Exp Biol. 1997;200:2937–2946. doi: 10.1242/jeb.200.23.2937. [DOI] [PubMed] [Google Scholar]
- 83.Cruz-Bermudez ND, Marder E. Multiple modulators act on the cardiac ganglion of the crab, Cancer borealis . J Exp Biol. 2007;210:2873–2884. doi: 10.1242/jeb.002949. [DOI] [PubMed] [Google Scholar]
- 84.Kreissl S, Weiss T, Djokaj S, Balezina O, Rathmayer W. Allatostatin modulates skeletal muscle performance in crustaceans through pre- and postsynaptic effects. Eur J Neurosci. 1999;11:2519–2530. doi: 10.1046/j.1460-9568.1999.00674.x. [DOI] [PubMed] [Google Scholar]
- 85.Kwok R, Zhang JR, Tobe SS. Regulation of methyl farnesoate production by mandibular organs in the crayfish, Procambarus clarkii: a possible role for allatostatins. J Insect Physiol. 2005;51:367–378. doi: 10.1016/j.jinsphys.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Fu Q, Tang LS, Marder E, Li L. Mass spectrometric characterization and physiological actions of VPNDWAHFRGSWamide, a novel B type allatostatin in the crab, Cancer borealis . J Neurochem. 2007;101:1099–1107. doi: 10.1111/j.1471-4159.2007.04482.x. [DOI] [PubMed] [Google Scholar]
- 87.Wilson CH, Christie AE. Distribution of allatostatin C (AST-C)-like immunoreactivity in the central nervous system of the copepod Calanus finmarchicus . Gen Comp Endocrinol. 2010;167:252–260. doi: 10.1016/j.ygcen.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilcockson DC, Webster SG. Identification and developmental expression of mRNAs encoding putative insect cuticle hardening hormone, bursicon, in the green shore crab Carcinus maenas . Gen Comp Endocrinol. 2008;156:113–125. doi: 10.1016/j.ygcen.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Porras MG, De Loof A, Breuer M, Arechiga H. Corazonin promotes tegumentary pigment migration in the crayfish Procambarus clarkii . Peptides. 2003;24:1581–1589. doi: 10.1016/j.peptides.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Stangier J, Hilbich C, Beyreuther K, Keller R. Unusual cardioactive peptide (CCAP) from pericardial organs of the shore crab Carcinus maenas . PNAS. 1987;84:575–579. doi: 10.1073/pnas.84.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chung JS, Wilcockson DC, Zmora N, Zohar Y, Dircksen H, Webster SG. Identification and developmental expression of mRNAs encoding crustacean cardioactive peptide (CCAP) in decapod crustaceans. J Exp Biol. 2006;209:3862–3872. doi: 10.1242/jeb.02425. [DOI] [PubMed] [Google Scholar]
- 92.Dickinson PS, Stemmler EA, Christie AE. The pyloric neural circuit of the herbivorous crab Pugettia producta shows limited sensitivity to several neuromodulators that elicit robust effects in more opportunistically feeding decapods. J Exp Biol. 2008;211:1434–1447. doi: 10.1242/jeb.016998. [DOI] [PubMed] [Google Scholar]
- 93.Fort TJ, Garcia-Crescioni K, Agricola HJ, Brezina V, Miller MW. Regulation of the crab heartbeat by crustacean cardioactive peptide (CCAP): central and peripheral actions. J Neurophysiol. 2007;97:3407–3420. doi: 10.1152/jn.00939.2006. [DOI] [PubMed] [Google Scholar]
- 94.Pulver SR, Marder E. Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus . J Comp Neurol. 2002;451:79–90. doi: 10.1002/cne.10331. [DOI] [PubMed] [Google Scholar]
- 95.Stangier J, Hilbich C, Dircksen H, Keller R. Distribution of a novel cardioactive neuropeptide (CCAP) in the nervous system of the shore crab Carcinus maenas . Peptides. 1988;9:795–800. doi: 10.1016/0196-9781(88)90124-6. [DOI] [PubMed] [Google Scholar]
- 96.Mulloney B, Namba H, Agricola HJ, Hall WM. Modulation of force during locomotion: differential action of crustacean cardioactive peptide on power-stroke and return-stroke motor neurons. J Neurosci. 1997;17:6872–6883. doi: 10.1523/JNEUROSCI.17-18-06872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trube A, Audehm U, Dircksen H. Crustacean cardioactive peptide-immunoreactive neurons in the ventral nervous system of crayfish. J Comp Neurol. 1994;348:80–93. doi: 10.1002/cne.903480104. [DOI] [PubMed] [Google Scholar]
- 98.Wilkens JL. Possible mechanisms of control of vascular resistance in the lobster Homarus americanus . J Exp Biol. 1997;200:487–493. doi: 10.1242/jeb.200.3.487. [DOI] [PubMed] [Google Scholar]
- 99.DeLong ND, Kirby MS, Blitz DM, Nusbaum MP. Parallel regulation of a modulator-activated current via distinct dynamics underlies comodulation of motor circuit output. J Neurosci. 2009;29:12355–12367. doi: 10.1523/JNEUROSCI.3079-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirby MS, Nusbaum MP. Peptide hormone modulation of a neuronally modulated motor circuit. J Neurophysiol. 2007;98:3206–3220. doi: 10.1152/jn.00795.2006. [DOI] [PubMed] [Google Scholar]
- 101.Richards KS, Marder E. The actions of crustacean cardioactive peptide on adult and developing stomatogastric ganglion motor patterns. J Neurobiol. 2000;44:31–44. [PubMed] [Google Scholar]
- 102.Weimann JM, Skiebe P, Heinzel HG, Soto C, Kopell N, JorgeRivera JC, Marder E. Modulation of oscillator interactions in the crab stomatogastric ganglion by crustacean cardioactive peptide. J Neurosci. 1997;17:1748–1760. doi: 10.1523/JNEUROSCI.17-05-01748.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jorge-Rivera JC, Sen K, Birmingham JT, Abbott LF, Marder E. Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J Neurophysiol. 1998;80:2559–2570. doi: 10.1152/jn.1998.80.5.2559. [DOI] [PubMed] [Google Scholar]
- 104.Nery LEM, Da Silva MA, Castrucci AMD. Possible role of non-classical chromatophorotropins on the regulation of the crustacean erythrophore. J Exp Zool. 1999;284:711–716. doi: 10.1002/(sici)1097-010x(19991101)284:6<711::aid-jez13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 105.Granato FC, Tironi TS, Maciel FE, Rosa CE, Vargas MA, Nery LEM. Circadian rhythm of pigment migration induced by chromatrophorotropins in melanophores of the crab Chasmagnathus granulata . Comp Biochem Physiol A Mol Integr Physiol. 2004;138:313–319. doi: 10.1016/j.cbpb.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 106.Gaus G, Stieve H. The effect of neuropeptides on the ERG of the crayfish Orconectes limosus . Zeitschrift Fur Naturforschung C. 1992;47:300–303. doi: 10.1515/znc-1992-3-421. [DOI] [PubMed] [Google Scholar]
- 107.Chung JS, Webster SG. Expression and release patterns of neuropeptides during embryonic development and hatching of the green shore crab, Carcinus maenas . Development. 2004;131:4751–4761. doi: 10.1242/dev.01312. [DOI] [PubMed] [Google Scholar]
- 108.Phlippen MK, Webster SG, Chung JS, Dircksen H. Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph. J Exp Biol. 2000;203:521–536. doi: 10.1242/jeb.203.3.521. [DOI] [PubMed] [Google Scholar]
- 109.Böcking D, Dircksen H, Keller R. The crustacean neuropeptides of the CHH/MIH/GIH family: stuctures and biological activities. In: Wiese K, editor. The crustacean nervous system. Heidelberg: Springer; 2002. pp. 84–97. [Google Scholar]
- 110.Chan SM, Gu PL, Chu KH, Tobe SS. Crustacean neuropeptide genes of the CHH/MIH/GIH family: implications from molecular studies. Gen Comp Endocrinol. 2003;134:214–219. doi: 10.1016/s0016-6480(03)00263-6. [DOI] [PubMed] [Google Scholar]
- 111.Chung JS, Zmora N, Katayama H, Tsutsui N. Crustacean hyperglycemic hormone (CHH) neuropeptides family: functions, titer, and binding to target tissues. Gen Comp Endocrinol. 2010;166:447–454. doi: 10.1016/j.ygcen.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 112.Fanjul-Moles ML. Biochemical and functional aspects of crustacean hyperglycemic hormone in decapod crustaceans: review and update. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:390–400. doi: 10.1016/j.cbpc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 113.Lacombe C, Greve P, Martin G. Overview on the sub-grouping of the crustacean hyperglycemic hormone family. Neuropeptides. 1999;33:71–80. doi: 10.1054/npep.1999.0016. [DOI] [PubMed] [Google Scholar]
- 114.Nakatsuji T, Lee CY, Watson RD. Crustacean molt-inhibiting hormone: structure, function, and cellular mode of action. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:139–148. doi: 10.1016/j.cbpa.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 115.Soyez D. Occurrence and diversity of neuropeptides from the crustacean hyperglycemic hormone family in arthropods––a short review. Neuropeptides Dev Aging. 1997;814:319–323. doi: 10.1111/j.1749-6632.1997.tb46174.x. [DOI] [PubMed] [Google Scholar]
- 116.Van Herp F. Molecular, cytological and physiological aspects of the crustacean hyperglycemic hormone family. Soc Exp Biol Sem Ser. 1998;65:53–70. [Google Scholar]
- 117.Kegel G, Reichwein B, Weese S, Gaus G, Peter-Katalinic J, Keller R. Amino acid sequence of the crustacean hyperglycemic hormone (CHH) from the shore crab, Carcinus maenas . FEBS Lett. 1989;255:10–14. doi: 10.1016/0014-5793(89)81051-8. [DOI] [PubMed] [Google Scholar]
- 118.Greve P, Sorokine O, Berges T, Lacombe C, Van Dorsselaer A, Martin G. Isolation and amino acid sequence of a peptide with vitellogenesis inhibiting activity from the terrestrial isopod Armadillidium vulgare (Crustacea) Gen Comp Endocrinol. 1999;115:406–414. doi: 10.1006/gcen.1999.7330. [DOI] [PubMed] [Google Scholar]
- 119.Martin G, Sorokine O, Vandorsselaer A. Isolation and molecular characterization of a hyperglycemic neuropeptide from the sinus gland of the terrestrial isopod Armadillidium vulgare (Crustacea) Eur J Biochem. 1993;211:601–607. doi: 10.1111/j.1432-1033.1993.tb17587.x. [DOI] [PubMed] [Google Scholar]
- 120.Chung JS, Wilkinson MC, Webster SG. Determination of the amino acid sequence of the moult-inhibiting hormone from the edible crab, Cancer pagurus . Neuropeptides. 1996;30:95–101. doi: 10.1016/s0143-4179(96)90061-x. [DOI] [PubMed] [Google Scholar]
- 121.Chung JS, Wilkinson MC, Webster SG. Amino acid sequences of both isoforms of crustacean hyperglycemic hormone (CHH) and corresponding precursor-related peptide in Cancer pagurus . Regul Pept. 1998;77:17–24. doi: 10.1016/s0167-0115(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 122.Wainwright G, Webster SG, Wilkinson MC, Chung JS, Rees HH. Structure and significance of mandibular organ-inhibiting hormone in the crab, Cancer pagurus: involvement in multihormonal regulation of growth and reproduction. J Biol Chem. 1996;271:12749–12754. doi: 10.1074/jbc.271.22.12749. [DOI] [PubMed] [Google Scholar]
- 123.Hsu YW, Messinger DI, Chung JS, Webster SG, de la Iglesia HO, Christie AE. Members of the crustacean hyperglycemic hormone (CHH) peptide family are differentially distributed both between and within the neuroendocrine organs of Cancer crabs: implications for differential release and pleiotropic function. J Exp Biol. 2006;209:3241–3256. doi: 10.1242/jeb.02372. [DOI] [PubMed] [Google Scholar]
- 124.Chung JS, Dircksen H, Webster SG. A remarkable, precisely timed release of hyperglycemic hormone from endocrine cells in the gut is associated with ecdysis in the crab Carcinus maenas . PNAS. 1999;96:13103–13107. doi: 10.1073/pnas.96.23.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Webster SG, Dircksen H, Chung JS. Endocrine cells in the gut of the shore crab Carcinus maenas immunoreactive to crustacean hyperglycaemic hormone and its precursor-related peptide. Cell Tissue Res. 2000;300:193–205. doi: 10.1007/s004410000176. [DOI] [PubMed] [Google Scholar]
- 126.Wilcockson DC, Chung JS, Webster SG. Is crustacean hyperglycaemic hormone precursor-related peptide a circulating neurohormone in crabs? Cell Tissue Res. 2002;307:129–138. doi: 10.1007/s00441-001-0469-8. [DOI] [PubMed] [Google Scholar]
- 127.Christie AE, Stevens JS, Bowers MR, Chapline MC, Jensen DA, Schegg KM, Goldwaser J, Kwiatkowski MA, Pleasant TK, Shoenfeld L, Tempest LK, Williams CR, Wiwatpanit T, Smith CM, Beale KM, Towle DW, Schooley DA, Dickinson PS. Identification of a calcitonin-like diuretic hormone that functions as an intrinsic modulator of the American lobster, Homarus americanus, cardiac neuromuscular system. J Exp Biol. 2010;213:118–127. doi: 10.1242/jeb.037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zitnan D, Kim YJ, Zitnanova I, Roller L, Adams ME. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen Comp Endocrinol. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luschen W, Buck F, Willig A, Jaros PP. Isolation, sequence-analysis, and physiological properties of enkephalins in the nervous tissue of the shore crab Carcinus maenas L. PNAS. 1991;88:8671–8675. doi: 10.1073/pnas.88.19.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dircksen H. Fine structure of the neurohemal sinus gland of the shore crab, Carcinus maenas, and immuno-electron microscopic identification of neurosecretory endings according to their neuropeptide contents. Cell Tissue Res. 1992;269:249–266. doi: 10.1007/BF00319616. [DOI] [PubMed] [Google Scholar]
- 131.Fingerman M, Hanumante MM, Kulkarni GK, Ikeda R, Vacca LL. Localization of substance P-like, leucine-enkephalin-like, methionine-enkephalin-like, and FMRFamide-like immunoreactivity in the eyestalk of the fiddler crab, Uca pugilator . Cell Tissue Res. 1985;241:473–477. doi: 10.1007/BF00214565. [DOI] [PubMed] [Google Scholar]
- 132.Hanke J, Jaros PP, Willig A. Autoradiographic localization of opioid binding-sites combined with immunogold detection of leu-enkephalin, crustacean hyperglycemic hormone and molt inhibiting hormone at the electron-microscopic level in the sinus gland of the shore crab, Carcinus maenas . Histochemistry. 1993;99:405–410. doi: 10.1007/BF00717053. [DOI] [PubMed] [Google Scholar]
- 133.Leung MK, Kessler H, Whitefield K, Murray M, Martinez EA, Stefano GB. The presence of enkephalin-like substances in the eyestalk and brain of the land crab Gecarcinus lateralis . Cell Mol Neurobiol. 1987;7:91–96. doi: 10.1007/BF00734992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mancillas JR, McGinty JF, Selverston AI, Karten H, Bloom FE. Immunocytochemical localization of enkephalin and substance P in retina and eyestalk neurones of lobster. Nature. 1981;293:576–578. doi: 10.1038/293576a0. [DOI] [PubMed] [Google Scholar]
- 135.Ollivaux C, Dircksen H, Toullec JY, Soyez D. Enkephalinergic control of the secretory activity of neurons producing stereoisomers of crustacean hyperglycemic hormone in the eyestalk of the crayfish Orconectes limosus . J Comp Neurol. 2002;444:1–9. doi: 10.1002/cne.1426. [DOI] [PubMed] [Google Scholar]
- 136.Palmisano A, Marino G, Di Marzo V, Morris HR, Howlett TA, Tomlin S. RIA/chromatographic evidence for novel opioid peptide(s) in Squilla mantis ganglia. Neuropeptides. 1986;7:281–289. doi: 10.1016/0143-4179(86)90022-3. [DOI] [PubMed] [Google Scholar]
- 137.Lorenzon S, Brezovec S, Ferrero EA. Species-specific effects on hemolymph glucose control by serotonin, dopamine, and L-enkephalin and their inhibitors in Squilla mantis and Astacus leptodactylus (Crustacea) J Exp Zool A Comp Exp Biol. 2004;301:727–736. doi: 10.1002/jez.a.59. [DOI] [PubMed] [Google Scholar]
- 138.Nagabhushanam R, Sarojini R, Reddy PS, Devi M, Fingerman M. Opioid peptides in invertebrates: localization, distribution and possible functional roles. Curr Sci. 1995;69:659–671. [Google Scholar]
- 139.Sarojini R, Nagabhushanam R, Fingerman M. Dopaminergic and enkephalinergic involvement in the regulation of blood glucose in the red swamp crayfish, Procambarus clarkii . Gen Comp Endocrinol. 1995;97:160–170. doi: 10.1006/gcen.1995.1015. [DOI] [PubMed] [Google Scholar]
- 140.Kishori B, Premasheela B, Ramamurthi R, Reddy PS. Evidence for a hyperglycemic effect of methionine-enkephalin in the prawns Penaeus indicus and Metapenaeus monocerus . Gen Comp Endocrinol. 2001;123:90–99. doi: 10.1006/gcen.2001.7655. [DOI] [PubMed] [Google Scholar]
- 141.Kishori B, Reddy PS. Role of methionine-enkephalin on the regulation of carbohydrate metabolism in the rice field crab Oziotelphusa senex senex . C R Biol. 2005;328:812–820. doi: 10.1016/j.crvi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 142.Reddy PS, Basha MR. On the mode of action of methionine enkephalin, FK 33–824 and naloxone in regulating the hemolymph glucose level in the fresh water field crab Oziotelphusa senex senex . Z Naturforschung C. 2001;56:629–632. doi: 10.1515/znc-2001-7-824. [DOI] [PubMed] [Google Scholar]
- 143.Reddy PS, Kishori B. Methionine-enkephalin induces hyperglycemia through eyestalk hormones in the estuarine crab Scylla serrata . Biol Bull. 2001;201:17–25. doi: 10.2307/1543521. [DOI] [PubMed] [Google Scholar]
- 144.Hanke J, Willig A, Yinon U, Jaros PP. Delta and kappa opioid receptors in eyestalk ganglia of a crustacean. Brain Res. 1997;744:279–284. doi: 10.1016/S0006-8993(96)01114-6. [DOI] [PubMed] [Google Scholar]
- 145.Kulkarni GK, Fingerman M. Distal retinal pigment of the fiddler crab, Uca pugilator: Release of the dark-adapting hormone by methionine enkephalin and FMRFamide. Pigment Cell Res. 1987;1:51–56. doi: 10.1111/j.1600-0749.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 146.Martinez EA, Murray M, Leung MK, Stefano GB. Evidence for dopaminergic and opioid involvement in the regulation of locomotor activity in the land crab Gecarcinus lateralis . Comp Biochem Physiol C Toxicol Pharmacol. 1988;90:89–93. doi: 10.1016/0742-8413(88)90103-x. [DOI] [PubMed] [Google Scholar]
- 147.Quackenbush LS, Fingerman M. Regulation of neurohormone release in the fiddler crab, Uca pugilator: effects of gamma-aminobutyric acid, octopamine, Met-enkephalin, and beta-endorphin. Comp Biochem Physiol C Toxicol Pharmacol. 1984;79:77–84. doi: 10.1016/0742-8413(84)90166-x. [DOI] [PubMed] [Google Scholar]
- 148.Kishori B, Reddy PS. Influence of leucine-enkephalin on moulting and vitellogenesis in the freshwater crab, Oziotelphusa senex senex (Fabricius, 1791) (Decapoda, Brachyura) Crustaceana. 2003;76:1281–1290. [Google Scholar]
- 149.Sarojini R, Nagabhushanam R, Fingerman M. Evidence for opioid involvement in the regulation of ovarian maturation of the fiddler-crab, Uca pugilator . Comp Biochem Physiol A Physiol. 1995;111:279–282. [Google Scholar]
- 150.Sarojini R, Nagabhushanam R, Fingerman M. In vivo assessment of opioid agonists and antagonists on ovarian maturation in the red swamp crayfish, Procambarus clarkii . Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:149–153. doi: 10.1016/s0742-8413(96)00108-9. [DOI] [PubMed] [Google Scholar]
- 151.Sarojini R, Nagabhushanam R, Fingerman M. An in vitro study of the inhibitory action of methionine enkephalin on ovarian maturation in the red swamp crayfish, Procambarus clarkii . Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;117:207–210. doi: 10.1016/s0742-8413(96)00108-9. [DOI] [PubMed] [Google Scholar]
- 152.Nagabhushanam R, Sarojini R, Fingerman M. In vivo assessment of opioid agonists and antagonists on ovarian maturation in the red swamp crayfish, Procambarus clarkii . Am Zool. 1995;35:26A. doi: 10.1016/s0742-8413(96)00108-9. [DOI] [PubMed] [Google Scholar]
- 153.Reddy PS. Involvement of opioid peptides in the regulation of reproduction in the prawn Penaeus indicus . Naturwissenschaften. 2000;87:535–538. doi: 10.1007/s001140050773. [DOI] [PubMed] [Google Scholar]
- 154.Stevens JS, Cashman CR, Smith CM, Beale KM, Towle DW, Christie AE, Dickinson PS. The peptide hormone pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites. J Exp Biol. 2009;212:3961–3976. doi: 10.1242/jeb.035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sithigorngul P, Saraithongkum W, Jaideechoey S, Longyant S, Sithigorngul W. Novel FMRFamide-like neuropeptides from the eyestalk of the giant freshwater prawn Macrobrachium rosenbergii . Comp Biochem Physiol B Biochem Mol Biol. 1998;120:587–595. [Google Scholar]
- 156.Sithigorngul P, Pupuem H, Krungkolsem C, Longyant S, Panchan N, Chaivisuthangkura P, Sithigorngul W, Petsom A. Four novel PYFs: members of NPY/PP peptide superfamily from the eyestalk of the giant tiger prawn Penaeus monodon . Peptides. 2002;23:1895–1906. doi: 10.1016/s0196-9781(02)00176-6. [DOI] [PubMed] [Google Scholar]
- 157.Sithigorngul P, Pupuem J, Krungkasem C, Longyant S, Chaivisuthangkura P, Sithigorngul W, Petsom A. Seven novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant tiger prawn Penaeus monodon . Comp Biochem Physiol B Biochem Mol Biol. 2002;131:325–337. doi: 10.1016/s1096-4959(01)00499-7. [DOI] [PubMed] [Google Scholar]
- 158.Dickinson PS, Stevens JS, Rus S, Brennan HR, Goiney CC, Smith CM, Li L, Towle DW, Christie AE. Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus . J Exp Biol. 2007;210:2278–2289. doi: 10.1242/jeb.004770. [DOI] [PubMed] [Google Scholar]
- 159.Krajniak KG. The identification and structure-activity relations of a cardioactive FMRFamide-related peptide from the blue crab Callinectes sapidus . Peptides. 1991;12:1295–1302. doi: 10.1016/0196-9781(91)90210-g. [DOI] [PubMed] [Google Scholar]
- 160.Krajniak KG, Price DA, Greenberg MJ. GYNRSFLRFamide, a novel FMRFamide-related peptide from Callinectes sapidus . Am Zool. 1990;30:A29. [Google Scholar]
- 161.Mercier AJ, Orchard I, Tebrugge V, Skerrett M. Isolation of two FMRFamide-related peptides from crayfish pericardial organs. Peptides. 1993;14:137–143. doi: 10.1016/0196-9781(93)90021-8. [DOI] [PubMed] [Google Scholar]
- 162.Fort TJ, Brezina V, Miller MW. Regulation of the crab heartbeat by FMRFamide-like peptides: multiple interacting effects on center and periphery. J Neurophysiol. 2007;98:2887–2902. doi: 10.1152/jn.00558.2007. [DOI] [PubMed] [Google Scholar]
- 163.Jorge-Rivera JC, Marder E. TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis . J Comp Physiol A Sens Neurol Behav Physiol. 1996;179:741–751. doi: 10.1007/BF00207353. [DOI] [PubMed] [Google Scholar]
- 164.McGaw IJ, McMahon BR. The FMRFamide-related peptides F1 and F2 alter hemolymph distribution and cardiac-output in the crab Cancer magister . Biol Bull. 1995;188:186–196. doi: 10.2307/1542084. [DOI] [PubMed] [Google Scholar]
- 165.Mercier AJ, Schiebe M, Atwood HL. Pericardial peptides enhance synaptic transmission and tension in phasic extensor muscles of crayfish. Neurosci Lett. 1990;111:92–98. doi: 10.1016/0304-3940(90)90350-i. [DOI] [PubMed] [Google Scholar]
- 166.Meyrand P, Marder E. Matching neural and muscle oscillators: control by FMRFamide-like peptides. J Neurosci. 1991;11:1150–1161. doi: 10.1523/JNEUROSCI.11-04-01150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verley DR, Doan V, Trieu Q, Messinger DI, Birmingham JT. Characteristic differences in modulation of stomatogastric musculature by a neuropeptide in three species of Cancer crabs. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:879–886. doi: 10.1007/s00359-008-0359-6. [DOI] [PubMed] [Google Scholar]
- 168.Weimann JM, Marder E, Evans B, Calabrese RL. The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis . J Exp Biol. 1993;181:1–26. doi: 10.1242/jeb.181.1.1. [DOI] [PubMed] [Google Scholar]
- 169.Worden MK, Kravitz EA, Goy MF. Peptide F1, an N-terminally extended analog of FMRFamide, enhances contractile activity in multiple target tissues in lobster. J Exp Biol. 1995;198:97–108. doi: 10.1242/jeb.198.1.97. [DOI] [PubMed] [Google Scholar]
- 170.Cruz-Bermudez ND, Fu Q, Kutz-Naber KK, Christie AE, Li L, Marder E. Mass spectrometric characterization and physiological actions of GAHKNYLRFamide, a novel FMRFamide-like peptide from crabs of the genus Cancer . J Neurochem. 2006;97:784–799. doi: 10.1111/j.1471-4159.2006.03747.x. [DOI] [PubMed] [Google Scholar]
- 171.Nieto J, Veelaert D, Derua R, Waelkens E, Cerstiaens A, Coast G, Devreese B, Van Beeumen J, Calderon J, De Loof A, Schoofs L. Identification of one tachykinin- and two kinin-related peptides in the brain of the white shrimp, Penaeus vannamei . Biochem Biophys Res Commun. 1998;248:406–411. doi: 10.1006/bbrc.1998.8964. [DOI] [PubMed] [Google Scholar]
- 172.Nieto J, Veenstra J, Waelkens E, Calderón J, Baggerman G, Veelaert D, De Loof A, Schoofs L. Isolation and identification of one tachykinin and three kinin-related peptides in the central nervous system of Penaeus vannamei. In: Schram FR, von Vaupel Klein JC, editors. Proceedings of the fourth international crustacean congress. Köln: Brill; 1999. pp. 951–960. [Google Scholar]
- 173.Saideman SR, Christie AE, Torfs P, Huybrechts J, Schoofs L, Nusbaum MP. Actions of kinin peptides in the stomatogastric ganglion of the crab Cancer borealis . J Exp Biol. 2006;209:3664–3676. doi: 10.1242/jeb.02415. [DOI] [PubMed] [Google Scholar]
- 174.Blitz DM, Christie AE, Marder E, Nusbaum MP. Distribution and effects of tachykinin-like peptides in the stomatogastric nervous system of the crab, Cancer borealis . J Comp Neurol. 1995;354:282–294. doi: 10.1002/cne.903540209. [DOI] [PubMed] [Google Scholar]
- 175.Stangier J, Hilbich C, Burdzik S, Keller R. Orcokinin: a novel myotropic peptide from the nervous system of the crayfish, Orconectes limosus . Peptides. 1992;13:859–864. doi: 10.1016/0196-9781(92)90041-z. [DOI] [PubMed] [Google Scholar]
- 176.Bungart D, Hilbich C, Dircksen H, Keller R. Occurrence of analogs of the myotropic neuropeptide orcokinin in the shore crab, Carcinus maenas: evidence for a novel neuropeptide family. Peptides. 1995;16:67–72. doi: 10.1016/0196-9781(94)00145-v. [DOI] [PubMed] [Google Scholar]
- 177.Dircksen H, Burdzik S, Sauter A, Keller R. Two orcokinins and the novel octapeptide orcomyotropin in the hindgut of the crayfish Orconectes limosus: identified myostimulatory neuropeptides originating together in neurones of the terminal abdominal ganglion. J Exp Biol. 2000;203:2807–2818. doi: 10.1242/jeb.203.18.2807. [DOI] [PubMed] [Google Scholar]
- 178.Bungart D, Dircksen H, Keller R. Quantitative-determination and distribution of the myotropic neuropeptide orcokinin in the nervous system of Astacidean crustaceans. Peptides. 1994;15:393–400. doi: 10.1016/0196-9781(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 179.Kleinholz LH. Purified hormones from the crustacean eyestalk and their physiological specificity. Nature. 1975;258:256–257. doi: 10.1038/258256a0. [DOI] [PubMed] [Google Scholar]
- 180.Rao KR, Riehm JP, Zahnow CA, Kleinholz LH, Tarr GE, Johnson L, Norton S, Landau M, Semmes OJ, et al. Characterization of a pigment-dispersing hormone in eyestalks of the fiddler crab Uca pugilator . PNAS. 1985;82:5319–5322. doi: 10.1073/pnas.82.16.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.de Kleijn DPV, Linck B, Klein JM, Weidemann WM, Keller R, van Herp F. Structure and localization of mRNA encoding a pigment dispersing hormone (PDH) in the eyestalk of the crayfish Orconectes limosus . FEBS Lett. 1993;321:251–255. doi: 10.1016/0014-5793(93)80119-f. [DOI] [PubMed] [Google Scholar]
- 182.DesmoucellesCarette C, Sellos D, VanWormhoudt A. Molecular cloning of the precursors of pigment dispersing hormone in crustaceans. Biochem Biophys Res Commun. 1996;221:739–743. doi: 10.1006/bbrc.1996.0666. [DOI] [PubMed] [Google Scholar]
- 183.Klein JM, Dekleijn DPV, Keller R, Weidemann WM. Molecular-cloning of crustacean pigment dispersing hormone precursor. Biochem Biophys Res Commun. 1992;189:1509–1514. doi: 10.1016/0006-291x(92)90246-h. [DOI] [PubMed] [Google Scholar]
- 184.Klein JM, Mohrherr CJ, Sleutels F, Riehm JP, Rao KR. Molecular cloning of 2 pigment-dispersing hormone (PDH) precursors in the blue crab Callinectes sapidus reveals a novel member of the PDH neuropeptide family. Biochem Biophys Res Commun. 1994;205:410–416. doi: 10.1006/bbrc.1994.2680. [DOI] [PubMed] [Google Scholar]
- 185.Lohr J, Klein J, Webster SG, Dircksen H. Quantification, immunoaffinity purification and sequence analysis of a pigment-dispersing hormone of the shore crab, Carcinus maenas (L.) Comp Biochem Physiol B Biochem Mol Biol. 1993;104:699–706. doi: 10.1016/0305-0491(93)90200-o. [DOI] [PubMed] [Google Scholar]
- 186.McCallum ML, Rao KR, Riehm JP, Mohrherr CJ, Morgan WT. Primary structure and relative potency of an analog of beta-PDH (pigment-dispersing hormone) from the crayfish Procambarus clarkii . Pigment Cell Res. 1991;4:201–208. doi: 10.1111/j.1600-0749.1991.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 187.Ohira T, Nagasawa H, Aida K. Molecular cloning of cDNAs encoding two pigment-dispersing hormones and two corresponding genes from the kuruma prawn (Penaeus japonicus) Mar Biotechnol. 2002;4:463–470. doi: 10.1007/s10126-002-0042-9. [DOI] [PubMed] [Google Scholar]
- 188.Ohira T, Tsutsui N, Kawazoe I, Wilder MN. Isolation and characterization of two pigment-dispersing hormones from the whiteleg shrimp, Litopenaeus vannamei . Zool Sci. 2006;23:601–606. doi: 10.2108/zsj.23.601. [DOI] [PubMed] [Google Scholar]
- 189.Yang WJ, Aida K, Nagasawa H. Characterization of chromatophorotropic neuropeptides from the kuruma prawn Penaeus japonicus . Gen Comp Endocrinol. 1999;114:415–424. doi: 10.1006/gcen.1999.7266. [DOI] [PubMed] [Google Scholar]
- 190.Rao KR. Crustacean pigmentary-effector hormones: chemistry and functions of RPCH, PDH, and related peptides. Am Zool. 2001;41:364–379. [Google Scholar]
- 191.Rao KR, Riehm JP. Pigment-dispersing hormones. Ann NY Acad Sci. 1993;680:78–88. doi: 10.1111/j.1749-6632.1993.tb19676.x. [DOI] [PubMed] [Google Scholar]
- 192.Knowles AC. Isolation and characterization of a pigment-dispersing hormone from the terrestrial isopod, Armadillidium vulgare. Pensacola, Florida: The University of West Florida; 1992. [Google Scholar]
- 193.Mangerich S, Keller R. Localization of pigment-dispersing hormone (PDH) immunoreactivity in the central nervous system of Carcinus maenas and Orconectes limosus (Crustacea), with reference to FMRFamide immunoreactivity in O. limosus . Cell Tissue Res. 1988;253:199–208. doi: 10.1007/BF00221755. [DOI] [PubMed] [Google Scholar]
- 194.Mortin LI, Marder E. Differential distribution of beta-pigment-dispersing hormone (beta-PDH)-like immunoreactivity in the stomatogastric nervous-system of 5 species of decapod crustaceans. Cell Tissue Res. 1991;265:19–33. doi: 10.1007/BF00318135. [DOI] [PubMed] [Google Scholar]
- 195.Sousa GL, Lenz PH, Hartline DK, Christie AE. Distribution of pigment dispersing hormone- and tachykinin-related peptides in the central nervous system of the copepod crustacean Calanus finmarchicus . Gen Comp Endocrinol. 2008;156:454–459. doi: 10.1016/j.ygcen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 196.Harzsch S, Dircksen H, Beltz B. Development of pigment-dispersing hormone-immunoreactive neurons in the American lobster: homology to the insect circadian pacemaker system? Cell Tissue Res. 2009;335:417–429. doi: 10.1007/s00441-008-0728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sullivan JM, Genco MC, Marlow ED, Benton JL, Beltz BS, Sandeman DC. Brain photoreceptor pathways contributing to circadian rhythmicity in crayfish. Chronobiol Int. 2009;26:1136–1168. doi: 10.3109/07420520903217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Verde MA, Barriga-Montoya C, Fuentes-Pardo B. Pigment dispersing hormone generates a circadian response to light in the crayfish, Procambarus clarkii . Comp Biochem Physiol A Mol Integr Physiol. 2007;147:983–992. doi: 10.1016/j.cbpa.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 199.Bishop CA, Wine JJ, O’Shea M. Neuropeptide proctolin in postural motoneurons of the crayfish. J Neurosci. 1984;4:2001–2009. doi: 10.1523/JNEUROSCI.04-08-02001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schwarz TL, Lee GMH, Siwicki KK, Standaert DG, Kravitz EA. Proctolin in the lobster: the distribution, release, and chemical characterization of a likely neurohormone. J Neurosci. 1984;4:1300–1311. doi: 10.1523/JNEUROSCI.04-05-01300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sullivan RE. A proctolin-like peptide in crab pericardial organs. J Exp Zool. 1979;210:543–552. [Google Scholar]
- 202.Beltz BS, Pontes M, Helluy SM, Kravitz EA. Patterns of appearance of serotonin and proctolin immunoreactivities in the developing nervous system of the American lobster. J Neurobiol. 1990;21:521–542. doi: 10.1002/neu.480210402. [DOI] [PubMed] [Google Scholar]
- 203.Christie AE, Baldwin D, Turrigiano G, Graubard K, Marder E. Immunocytochemical localization of multiple cholecystokinin-like peptides in the stomatogastric nervous system of the crab Cancer borealis . J Exp Biol. 1995;198:263–271. doi: 10.1242/jeb.198.1.263. [DOI] [PubMed] [Google Scholar]
- 204.Marder E, Hooper SL, Siwicki KK. Modulatory action and distribution of the neuropeptide proctolin in the crustacean stomatogastric nervous system. J Comp Neurol. 1986;243:454–467. doi: 10.1002/cne.902430403. [DOI] [PubMed] [Google Scholar]
- 205.Siwicki KK, Beltz BS, Kravitz EA. Proctolin in identified serotonergic, dopaminergic, and cholinergic neurons in the lobster, Homarus americanus . J Neurosci. 1987;7:522–532. doi: 10.1523/JNEUROSCI.07-02-00522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Siwicki KK, Beltz BS, Schwarz TL, Kravitz EA. Proctolin in the lobster nervous system. Peptides. 1985;6:393–402. doi: 10.1016/0196-9781(85)90404-8. [DOI] [PubMed] [Google Scholar]
- 207.Siwicki KK, Bishop CA. Mapping of proctolinlike immunoreactivity in the nervous systems of lobster and crayfish. J Comp Neurol. 1986;243:435–453. doi: 10.1002/cne.902430402. [DOI] [PubMed] [Google Scholar]
- 208.Skiebe P, Dietel C, Schmidt M. Immunocytochemical localization of FLRFamide-, proctolin-, and CCAP-like peptides in the stomatogastric nervous system and neurohemal structures of the crayfish, Cherax destructor . J Comp Neurol. 1999;414:511–532. [PubMed] [Google Scholar]
- 209.Skiebe P, Ganeshina O. Synaptic neuropil in nerves of the crustacean stomatogastric nervous system: an immunocytochemical and electron microscopical study. J Comp Neurol. 2000;420:373–397. [PubMed] [Google Scholar]
- 210.Stangier J, Dircksen H, Keller R. Identification and immunocytochemical localization of proctolin in pericardial organs of the shore crab, Carcinus maenas . Peptides. 1986;7:67–72. doi: 10.1016/0196-9781(86)90063-x. [DOI] [PubMed] [Google Scholar]
- 211.Wood DE, Nishikawa M, Derby CD. Proctolin-like immunoreactivity and identified neurosecretory cells as putative substrates for modulation of courtship display behavior in the blue crab, Callinectes sapidus . J Comp Neurol. 1996;368:153–163. doi: 10.1002/(SICI)1096-9861(19960422)368:1<153::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 212.Herbert ZS, Molnar L, Pollak E, Eckert M. Proctolin-immunoreactive neurons in the central nervous system of Porcellio scaber . Neurobiology. 2001;9:41–42. [PubMed] [Google Scholar]
- 213.Bishop CA, Krouse ME, Wine JJ. Peptide cotransmitter potentiates calcium channel activity in crayfish skeletal muscle. J Neurosci. 1991;11:269–276. doi: 10.1523/JNEUROSCI.11-01-00269.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bishop CA, Wine JJ, Nagy F, O’Shea MR. Physiological consequences of a peptide cotransmitter in a crayfish nerve-muscle preparation. J Neurosci. 1987;7:1769–1779. doi: 10.1523/JNEUROSCI.07-06-01769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kravitz EA, Glusman S, Harris-Warrick RM, Livingstone MS, Schwarz T, Goy MF. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioural studies. J Exp Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- 216.Schwarz TL, Harris-Warrick RM, Glusman S, Kravitz EA. A peptide action in a lobster neuromuscular preparation. J Neurobiol. 1980;11:623–628. doi: 10.1002/neu.480110611. [DOI] [PubMed] [Google Scholar]
- 217.Freschi JE. Proctolin activates a slow, voltage-dependent sodium current in motoneurons of the lobster cardiac ganglion. Neurosci Lett. 1989;106:105–111. doi: 10.1016/0304-3940(89)90210-3. [DOI] [PubMed] [Google Scholar]
- 218.Miller MW, Sullivan RE. Some effects of proctolin on the cardiac ganglion of the Maine lobster, Homarus americanus (Milne Edwards) J Neurobiol. 1981;12:629–639. doi: 10.1002/neu.480120611. [DOI] [PubMed] [Google Scholar]
- 219.Sullivan RE, Miller MW. Dual effects of proctolin on the rhythmic burst activity of the cardiac ganglion. J Neurobiol. 1984;15:173–196. doi: 10.1002/neu.480150302. [DOI] [PubMed] [Google Scholar]
- 220.Wilkens JL, Cavey MJ, Shovkivska I, Zhang ML, ter Keurs H. Elasticity, unexpected contractility and the identification of actin and myosin in lobster arteries. J Exp Biol. 2008;211:766–772. doi: 10.1242/jeb.007658. [DOI] [PubMed] [Google Scholar]
- 221.Wilkens JL, Shinozaki T, Yazawa T, ter Keurs H. Sites and modes of action of proctolin and the FLP F-2 on lobster cardiac muscle. J Exp Biol. 2005;208:737–747. doi: 10.1242/jeb.01430. [DOI] [PubMed] [Google Scholar]
- 222.Wilkens JL, Taylor HH. The control of vascular resistance in the southern rock lobster, Jasus edwardsii (Decapoda: Palinuridae) Comp Biochem Physiol A Mol Integr Physiol. 2003;135:369–376. doi: 10.1016/s1095-6433(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 223.Dickinson PS, Marder E. Peptidergic modulation of a multioscillator system in the lobster. I. Activation of the cardiac sac motor pattern by the neuropeptides proctolin and red pigment-concentrating hormone. J Neurophysiol. 1989;61:833–844. doi: 10.1152/jn.1989.61.4.833. [DOI] [PubMed] [Google Scholar]
- 224.Heinzel HG. Gastric mill activity in the lobster. II. Proctolin and octopamine initiate and modulate chewing. J Neurophysiol. 1988;59:551–565. doi: 10.1152/jn.1988.59.2.551. [DOI] [PubMed] [Google Scholar]
- 225.Heinzel HG, Selverston AI. Gastric mill activity in the lobster. III. Effects of proctolin on the isolated central pattern generator. J Neurophysiol. 1988;59:566–585. doi: 10.1152/jn.1988.59.2.566. [DOI] [PubMed] [Google Scholar]
- 226.Hooper SL, Marder E. Modulation of a central pattern generator by two neuropeptides, proctolin and FMRFamide. Brain Res. 1984;305:186–191. doi: 10.1016/0006-8993(84)91138-7. [DOI] [PubMed] [Google Scholar]
- 227.Hooper SL, Marder E. Modulation of the lobster pyloric rhythm by the peptide proctolin. J Neurosci. 1987;7:2097–2112. doi: 10.1523/JNEUROSCI.07-07-02097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rehm KJ, Deeg KE, Marder E. Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus . J Neurosci. 2008;28:9828–9839. doi: 10.1523/JNEUROSCI.2328-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mercier AJ, Wilkens JL. Modulatory effects of proctolin on a crab ventilatory muscle. J Neurobiol. 1985;16:401–408. doi: 10.1002/neu.480160507. [DOI] [PubMed] [Google Scholar]
- 230.Acevedo LD, Hall WM, Mulloney B. Proctolin and excitation of the crayfish swimmeret system. J Comp Neurol. 1994;345:612–627. doi: 10.1002/cne.903450411. [DOI] [PubMed] [Google Scholar]
- 231.Mulloney B, Acevedo LD, Bradbury AG. Modulation of the crayfish swimmeret rhythm by octopamine and the neuropeptide proctolin. J Neurophysiol. 1987;58:584–597. doi: 10.1152/jn.1987.58.3.584. [DOI] [PubMed] [Google Scholar]
- 232.el Manira A, Rossidurand C, Clarac F. Serotonin and proctolin modulate the response of a stretch-receptor in crayfish. Brain Res. 1991;541:157–162. doi: 10.1016/0006-8993(91)91091-e. [DOI] [PubMed] [Google Scholar]
- 233.Pasztor VM, Bush BM. Peripheral modulation of mechano-sensitivity in primary afferent neurons. Nature. 1987;326:793–795. doi: 10.1038/326793a0. [DOI] [PubMed] [Google Scholar]
- 234.Pasztor VM, Bush BM. Primary afferent responses of a crustacean mechanoreceptor are modulated by proctolin, octopamine, and serotonin. J Neurobiol. 1989;20:234–254. doi: 10.1002/neu.480200406. [DOI] [PubMed] [Google Scholar]
- 235.Mercier AJ, Lee J. Differential effects of neuropeptides on circular and longitudinal muscles of the crayfish hindgut. Peptides. 2002;23:1751–1757. doi: 10.1016/s0196-9781(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 236.Erxleben CFJ, Desantis A, Rathmayer W. Effects of proctolin on contractions, membrane resistance, and non-voltage-dependent sarcolemmal ion channels in crustacean muscle fibers. J Neurosci. 1995;15:4356–4369. doi: 10.1523/JNEUROSCI.15-06-04356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hager SR, Bittar EE. Hormones and the barnacle muscle fiber as a preparation. Comp Biochem Physiol C Toxicol Pharmacol. 1985;81:247–252. doi: 10.1016/0742-8413(85)90001-5. [DOI] [PubMed] [Google Scholar]
- 238.Tsukamoto YF, Kuwasawa K. Neurohormonal and glutamatergic neuronal control of the cardioarterial valves in the isopod crustacean Bathynomus doederleini . J Exp Biol. 2003;206:431–443. doi: 10.1242/jeb.00077. [DOI] [PubMed] [Google Scholar]
- 239.Torfs P, Nieto J, Cerstiaens A, Boon D, Baggerman G, Poulos C, Waelkens E, Derua R, Calderon J, De Loof A, Schoofs L. Pyrokinin neuropeptides in a crustacean––isolation and identification in the white shrimp Penaeus vannamei . Eur J Biochem. 2001;268:149–154. doi: 10.1046/j.1432-1327.2001.01858.x. [DOI] [PubMed] [Google Scholar]
- 240.Carlsen J, Christensen M, Josefsson L. Purification and chemical structure of the red pigment-concentrating hormone of the prawn Leander adspersus . Gen Comp Endocrinol. 1976;30:327–331. doi: 10.1016/0016-6480(76)90083-6. [DOI] [PubMed] [Google Scholar]
- 241.Linck B, Klein JM, Mangerich S, Keller R, Weidemann WM. Molecular-cloning of crustacean red pigment concentrating hormone precursor. Biochem Biophys Res Commun. 1993;195:807–813. doi: 10.1006/bbrc.1993.2117. [DOI] [PubMed] [Google Scholar]
- 242.Martinez-Perez F, Zinker S, Aguilar G, Valdes J, Arechiga H. Circadian oscillations of RPCH gene expression in the eyestalk of the crayfish Cherax quadricarinatus . Peptides. 2005;26:2434–2444. doi: 10.1016/j.peptides.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 243.Stemmler EA, Gardner NP, Guiney ME, Bruns EA, Dickinson PS. The detection of red pigment-concentrating hormone (RPCH) in crustacean eyestalk tissues using matrix-assisted laser desorption/ionization-Fourier transform mass spectrometry: [M + Na](+) ion formation in dried droplet tissue preparations. J Mass Spectrom. 2006;41:295–311. doi: 10.1002/jms.989. [DOI] [PubMed] [Google Scholar]
- 244.Zrala J, Kodrík D, Zahradníčková H, Zemek R, Socha R. A novel function of red pigment-concentrating hormone in crustaceans: Porcellio scaber (Isopoda) as a model species. Gen Comp Endocrinol. 2010;166:330–336. doi: 10.1016/j.ygcen.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 245.Fenelon VS, Kilman V, Meyrand P, Marder E. Sequential developmental acquisition of neuromodulatory inputs to a central pattern generating network. J Comp Neurol. 1999;408:335–351. doi: 10.1002/(sici)1096-9861(19990607)408:3<335::aid-cne3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 246.Nusbaum MP, Marder E. A neuronal role for a crustacean red pigment concentrating hormone-like peptide: neuromodulation of the pyloric rhythm in the crab, Cancer borealis . J Exp Biol. 1988;135:165–181. [Google Scholar]
- 247.Sherff CM, Mulloney B. Red pigment concentrating hormone is a modulator of the crayfish swimmeret system. J Exp Biol. 1991;155:21–35. doi: 10.1242/jeb.155.1.21. [DOI] [PubMed] [Google Scholar]
- 248.Dickinson PS, Hauptman J, Hetling J, Mahadevan A. RPCH modulation of a multi-oscillator network: effects on the pyloric network of the spiny lobster. J Neurophysiol. 2001;85:1424–1435. doi: 10.1152/jn.2001.85.4.1424. [DOI] [PubMed] [Google Scholar]
- 249.Dickinson PS, Mecsas C, Hetling J, Terio K. The neuropeptide red pigment concentrating hormone affects rhythmic pattern generation at multiple sites. J Neurophysiol. 1993;69:1475–1483. doi: 10.1152/jn.1993.69.5.1475. [DOI] [PubMed] [Google Scholar]
- 250.Dickinson PS, Mecsas C, Marder E. Neuropeptide fusion of two motor pattern generator circuits. Nature. 1990;344:155–158. doi: 10.1038/344155a0. [DOI] [PubMed] [Google Scholar]
- 251.Thirumalai V, Marder E. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J Neurosci. 2002;22:1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Thirumalai V, Prinz AA, Johnson CD, Marder E. Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol. 2006;95:1762–1770. doi: 10.1152/jn.00764.2005. [DOI] [PubMed] [Google Scholar]
- 253.Stemmler EA, Bruns EA, Gardner NP, Dickinson PS, Christie AE. Mass spectrometric identification of pEGFYSQRYamide: a crustacean peptide hormone possessing a vertebrate neuropeptide Y (NPY)-like carboxy-terminus. Gen Comp Endocrinol. 2007;152:1–7. doi: 10.1016/j.ygcen.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Verleyen P, Huybrechts J, Schoofs L. SIFamide illustrates the rapid evolution in arthropod neuropeptide research. Gen Comp Endocrinol. 2009;162:27–35. doi: 10.1016/j.ygcen.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 255.Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- 256.Christie AE, Kutz-Naber KK, Stemmler EA, Klein A, Messinger DI, Goiney CC, Conterato AJ, Bruns EA, Hsu YW, Dickinson PS. Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly(1)-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus . J Exp Biol. 2007;210:699–714. doi: 10.1242/jeb.02696. [DOI] [PubMed] [Google Scholar]
- 257.Vazquez-Acevedo N, Rivera Nilsa M, Torres-Gonzalez Alejandra M, Rullan-Matheu Y, Ruiz-Rodriguez Eduardo A, Sosa Maria A. GYRKPPFNGSIFamide (Gly-SIFamide) modulates aggression in the freshwater prawn Macrobrachium rosenbergii . Biol Bull. 2009;217:313–326. doi: 10.1086/BBLv217n3p313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Polanska MA, Yasuda A, Harzsch S. Immunolocalisation of crustacean-SIFamide in the median brain and eyestalk neuropils of the marbled crayfish. Cell Tissue Res. 2007;330:331–344. doi: 10.1007/s00441-007-0473-8. [DOI] [PubMed] [Google Scholar]
- 259.Goldberg D, Nusbaum MP, Marder E. Substance P-like immunoreactivity in the stomatogastric nervous systems of the crab Cancer borealis and the lobsters Panulirus interruptus and Homarus americanus . Cell Tissue Res. 1988;252:515–522. doi: 10.1007/BF00216638. [DOI] [PubMed] [Google Scholar]
- 260.Langworthy K, Helluy S, Benton J, Beltz B. Amines and peptides in the brain of the American lobster: immunocytochemical localization patterns and implications for brain function. Cell Tissue Res. 1997;288:191–206. doi: 10.1007/s004410050806. [DOI] [PubMed] [Google Scholar]
- 261.Sandeman RE, Sandeman DC, Watson AHD. Substance-p antibody reveals homologous neurons with axon terminals among somata in the crayfish and crab brain. J Comp Neurol. 1990;294:569–582. doi: 10.1002/cne.902940405. [DOI] [PubMed] [Google Scholar]
- 262.Schmidt M. Distribution of presumptive chemosensory afferents with FMRFamide- or substance P-like immunoreactivity in decapod crustaceans. Brain Res. 1997;746:71–84. doi: 10.1016/s0006-8993(96)01187-0. [DOI] [PubMed] [Google Scholar]
- 263.Christie AE, Cashman CR, Stevens JS, Smith CM, Beale KM, Stemmler EA, Greenwood SJ, Towle DW, Dickinson PS. Identification and cardiotropic actions of brain/gut-derived tachykinin-related peptides (TRPs) from the American lobster Homarus americanus . Peptides. 2008;29:1909–1918. doi: 10.1016/j.peptides.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 264.Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J Neurosci. 2000;20:8943–8953. doi: 10.1523/JNEUROSCI.20-23-08943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol. 2008;211:1000–1011. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rehm KJ, Taylor AL, Pulver SR, Marder E. Spectral analyses reveal the presence of adult-like activity in the embryonic stomatogastric motor patterns of the lobster, Homarus americanus . J Neurophysiol. 2008;99:3104–3122. doi: 10.1152/jn.00042.2008. [DOI] [PubMed] [Google Scholar]
- 267.Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- 268.Glantz RM, Miller CS, Nassel DR. Tachykinin-related peptide and GABA-mediated presynaptic inhibition of crayfish photoreceptors. J Neurosci. 2000;20:1780–1790. doi: 10.1523/JNEUROSCI.20-05-01780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stafflinger E, Hansen KK, Hauser F, Schneider M, Cazzamali G, Williamson M, Grimmelikhuijzen CJP. Cloning and identification of the first oxytocin/vasopressin-like receptor and its ligand from insects. PNAS. 2008;105:3262–3267. doi: 10.1073/pnas.0710897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mercier AJ, Russenes RT. Modulation of crayfish hearts by FMRFamide-related peptides. Biol Bull. 1992;182:333–340. doi: 10.2307/1542253. [DOI] [PubMed] [Google Scholar]
- 271.Saver MA, Wilkens JL. Comparison of the effects of five hormones on intact and open heart cardiac ganglionic output and myocardial contractility in the shore crab Carcinus maenas . Comp Biochem Physiol A Mol Integr Physiol. 1998;120:301–310. [Google Scholar]
- 272.Wilkens JL, Mercier AJ. Peptidergic modulation of cardiac performance in isolated hearts from the shore crab Carcinus maenas . Physiol Zool. 1993;66:237–256. [Google Scholar]
- 273.Stevens J, Christie A, Dickinson P. Modulation of Homarus americanus cardiac activity by the peptide hormone TNRNFLRFamide. MDIBL Bull. 2008;47:82–85. [Google Scholar]
- 274.Brezina V, Orekhova IV, Weiss KR. The neuromuscular transform: the dynamic, nonlinear link between motor neuron firing patterns and muscle contraction in rhythmic behaviors. J Neurophysiol. 2000;83:207–231. doi: 10.1152/jn.2000.83.1.207. [DOI] [PubMed] [Google Scholar]
- 275.Brezina V, Orekhova IV, Weiss KR. Optimization of rhythmic behaviors by modulation of the neuromuscular transform. J Neurophysiol. 2000;83:260–279. doi: 10.1152/jn.2000.83.1.260. [DOI] [PubMed] [Google Scholar]
- 276.Brezina V, Weiss KR. The neuromuscular transform constrains the production of functional rhythmic behaviors. J Neurophysiol. 2000;83:232–259. doi: 10.1152/jn.2000.83.1.232. [DOI] [PubMed] [Google Scholar]
- 277.Fort TJ, Brezina V, Miller MW. Modulation of an integrated central pattern generator-effector system: dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus . J Neurophysiol. 2004;92:3455–3470. doi: 10.1152/jn.00550.2004. [DOI] [PubMed] [Google Scholar]
- 278.Garcia-Crescioni K, Fort Timothy J, Stern E, Brezina V, Miller Mark W. Feedback from peripheral musculature to central pattern generator in the neurogenic heart of the crab Callinectes sapidus: role of mechanosensitive dendrites. J Neurophysiol. 2010;103:83–96. doi: 10.1152/jn.00561.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sakurai A, Wilkens JL. Tension sensitivity of the heart pacemaker neurons in the isopod crustacean Ligia pallasii . J Exp Biol. 2003;206:105–115. doi: 10.1242/jeb.00050. [DOI] [PubMed] [Google Scholar]
- 280.Mahadevan A, Lappe J, Rhyne RT, Cruz-Bermudez ND, Marder E, Goy MF. Nitric oxide inhibits the rate and strength of cardiac contractions in the lobster Homarus americanus by acting on the cardiac ganglion. J Neurosci. 2004;24:2813–2824. doi: 10.1523/JNEUROSCI.3779-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Selverston AI, Russell DF, Miller JP. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol. 1976;7:215–290. doi: 10.1016/0301-0082(76)90008-3. [DOI] [PubMed] [Google Scholar]
- 282.Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Norris BJ, Coleman MJ, Nusbaum MP. Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis . J Neurophysiol. 1994;72:1451–1463. doi: 10.1152/jn.1994.72.4.1451. [DOI] [PubMed] [Google Scholar]
- 284.Norris BJ, Coleman MJ, Nusbaum MP. Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system. J Neurophysiol. 1996;75:97–108. doi: 10.1152/jn.1996.75.1.97. [DOI] [PubMed] [Google Scholar]
- 285.Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 287.Saideman SR, Blitz DM, Nusbaum MP. Convergent motor patterns from divergent circuits. J Neurosci. 2007;27:6664–6674. doi: 10.1523/JNEUROSCI.0315-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Golowasch J, Marder E. Proctolin activates an inward current whose voltage dependence is modified by extracellular Ca2+ . J Neurosci. 1992;12:810–817. doi: 10.1523/JNEUROSCI.12-03-00810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci. 2001;21:4050–4058. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric pyloric neurons in the crab stomatogastric system. J Neurophysiol. 1991;65:111–122. doi: 10.1152/jn.1991.65.1.111. [DOI] [PubMed] [Google Scholar]
- 292.Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 293.Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol. 2002;60:378–387. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- 294.Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- 295.Dickinson PS, Fairfield WP, Hetling JR, Hauptman J. Neurotransmitter interactions in the stomatogastric system of the spiny lobster: one peptide alters the response of a central pattern generator to a second peptide. J Neurophysiol. 1997;77:599–610. doi: 10.1152/jn.1997.77.2.599. [DOI] [PubMed] [Google Scholar]
- 296.Combes D, Simmers J, Moulins M. Conditioned dendritic oscillators in a lobster mechanoreceptor neurone. J Physiol Lond. 1997;499:161–177. doi: 10.1113/jphysiol.1997.sp021918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Birmingham JT, Billimoria CP, DeKlotz TR, Stewart RA, Marder E. Differential and history-dependent modulation of a stretch receptor in the stomatogastric system of the crab, Cancer borealis . J Neurophysiol. 2003;90:3608–3616. doi: 10.1152/jn.00397.2003. [DOI] [PubMed] [Google Scholar]
- 298.Billimoria CP, DiCaprio RA, Birmingham JT, Abbott LF, Marder E. Neuromodulation of spike-timing precision in sensory neurons. J Neurosci. 2006;26:5910–5919. doi: 10.1523/JNEUROSCI.4659-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). II. State-dependent modulation of rhythmic motor activity. J Neurosci. 1989;9:1600–1607. doi: 10.1523/JNEUROSCI.09-05-01600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). I. Identification and characterization. J Neurosci. 1989;9:1591–1599. doi: 10.1523/JNEUROSCI.09-05-01591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mizrahi A, Dickinson PS, Kloppenburg P, Fenelon V, Baro DJ, Harris-Warrick RM, Meyrand P, Simmers J. Long-term maintenance of channel distribution in a central pattern generator neuron by neuromodulatory inputs revealed by decentralization in organ culture. J Neurosci. 2001;21:7331–7339. doi: 10.1523/JNEUROSCI.21-18-07331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J Neurosci. 1998;18:2212–2225. doi: 10.1523/JNEUROSCI.18-06-02212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Thoby-Brisson M, Simmers J. Transition to endogenous bursting after long-term decentralization requires de novo transcription in a critical time window. J Neurophysiol. 2000;84:596–599. doi: 10.1152/jn.2000.84.1.596. [DOI] [PubMed] [Google Scholar]
- 304.Haedo RJ, Golowasch J. Ionic mechanism underlying recovery of rhythmic activity in adult isolated neurons. J Neurophysiol. 2006;96:1860–1876. doi: 10.1152/jn.00385.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Luther JA, Robie AA, Yarotsky J, Reina C, Marder E, Golowasch J. Episodic bouts of activity accompany recovery of rhythmic output by a neuromodulator- and activity-deprived adult neural network. J Neurophysiol. 2003;90:2720–2730. doi: 10.1152/jn.00370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang Y, Khorkova O, Rodriguez R, Golowaschi J. Activity and neuromodulatory input contribute to the recovery of rhythmic output after decentralization in a central pattern generator. J Neurophysiol. 2009;101:372–386. doi: 10.1152/jn.01290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Marder E, Richards KS. Development of the peptidergic modulation of a rhythmic pattern generating network. Brain Res. 1999;848:35–44. doi: 10.1016/s0006-8993(99)02118-6. [DOI] [PubMed] [Google Scholar]
- 309.Richards KS, Simon DJ, Pulver SR, Beltz BS, Marder E. Serotonin in the developing stomatogastric system of the lobster, Homarus americanus . J Neurobiol. 2003;54:380–392. doi: 10.1002/neu.10136. [DOI] [PubMed] [Google Scholar]
- 310.Le Feuvre Y, Fenelon VS, Meyrand P. Central inputs mask multiple adult neural networks within a single embryonic network. Nature. 1999;402:660–664. doi: 10.1038/45238. [DOI] [PubMed] [Google Scholar]
- 311.Casasnovas B, Fenelon VS, Meyrand P. Ontogeny of rhythmic motor networks in the stomatogastric nervous system. J Comp Physiol A Sens Neural Behav Physiol. 1999;185:361–365. [Google Scholar]
- 312.Fenelon V, Le Feuvre Y, Bem T, Meyrand P. Maturation of rhythmic neural network: role of central modulatory inputs. J Physiol Paris. 2003;97:59–68. doi: 10.1016/j.jphysparis.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 313.Fenelon VS, Feuvre Y, Meyrand P. Phylogenetic, ontogenetic and adult adaptive plasticity of rhythmic neural networks: a common neuromodulatory mechanism? J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:691–705. doi: 10.1007/s00359-004-0533-4. [DOI] [PubMed] [Google Scholar]
- 314.Clemens S, Massabuau JC, Meyrand P, Simmers J. Changes in motor network expression related to moulting behaviour in lobster: role of moult-induced deep hypoxia. J Exp Biol. 1999;202:817–827. doi: 10.1242/jeb.202.7.817. [DOI] [PubMed] [Google Scholar]