Abstract
Osteoarthritis is one of the most common forms of musculoskeletal disease and the most prominent type of arthritis encountered in all countries. Although great efforts have been made to investigate cartilage biology and osteoarthritis pathology, the treatment has lagged behind that of other arthritides, as there is a lack of effective disease-modifying therapies. Numerous approaches for dealing with cartilage degradation have been tried, but enjoyed very little success to develop approved OA treatments with not only symptomatic improvement but also structure-modifying effect. In this review we discuss the most recent findings regarding the regulation of cartilage biology and pathology and highlight their potential therapeutic values.
Keywords: Osteoarthritis, Cartilage, Cartilage repair, Disease modifying drugs
Introduction
Osteoarthritis (OA) is a progressive joint disease that is characterized by the proceeding destruction of articular cartilage by uncontrolled proteolysis of extracellular matrix and typically leads to a remodeling of affected joints. As a consequence, OA is associated with severe pain and impairment and results in a significant reduction of the quality of life. In addition to the individual burden, the overall impact of OA for Western societies is enormous, because OA is the most common form of arthritis and is associated with high costs, which burdens the health systems worldwide, particularly governments and health insurance companies. The National Arthritis Data Workgroup estimated the prevalence of osteoarthritis in the United States to be 26.9 million individuals in 2005, which constitutes an increase of nearly 30% as compared to the previous 10 years [1, 2]. Importantly, the individual suffering from OA and the socioeconomic impact of this disease are in sharp contrast to our therapeutic options, particularly with respect to early treatment and inhibition of disease progression. Frustratingly, no significant progress has been made in the drug treatment of OA over the last 20 years, with symptomatic pain relief and anti-inflammatory treatment of flares being the major therapeutic principles. At the end stage of OA, the replacement of joints by endoprotheses is mostly the last possible curative approach [3]. Despite major advances in understanding cartilage biology, including extracellular matrix and the metabolism of chondrocytes, key issues of the pathogenesis of OA are still unclear. It has become obvious that OA most likely is not a uniform disease, but a network of mechanisms by which the cartilage responds to stress [4, 5]. Also, inflammation, which has been considered a secondary phenomenon, has been shown to contribute to the perpetuation of disease [6–8]. However, research over the last few years has not only identified key risk factors including genetic predisposition as well as being overweight, metabolic factors, and mechanical stress, all of which accelerate the processes leading to the degenerative loss of cartilage [9, 10], it has also provided us with important insights into pathways, that in some patients mediate a rapid disease progression, while result in stable homeostatic cartilage balance in others [11].
This review will summarize the important aspects of our current knowledge on cartilage physiology and pathology with specific emphasis on cartilage degeneration as observed during OA.
Cartilage biology
Different types of cartilage tissue are present throughout the body at various sites. They are classified histologically into hyaline, elastic, and fibrocartilaginous cartilage depending on their molecular composition [12]. Hyaline cartilage is the predominant form of cartilage and is commonly associated with the skeletal system. Articular cartilage belongs in this group and can be divided in into four zones: the superficial zone, the transitional zone, the radial zone, and the calcified cartilage zone, where the cartilage interfaces with the bone [13]. These zones are characterized by a distinct organization of the collagen network, as well as by differences in the amounts and types of proteoglycans. Type II collagen is the principal molecular component in healthy articular cartilage, but collagens III, VI, IX, X, XI, XII, and XIV all contribute in smaller amounts to the mature matrix [14–16]. The main proteoglycan present in cartilage is aggrecan, which is a large chondroitin sulfate proteoglycan. Other proteoglycans found in cartilage include the syndecans and glypican, the decorin, biglycan, fibromodulin, lumican, epiphycan, and perlecan [17]. The chondrocytes are arranged in a zonal stratification and embedded in the arcade-like network of collagen fibrils intermingled with proteoglycans [16]. This specific organization of articular cartilage and the embedded chondrocytes results from complex developmental processes in which the joints are formed during embryogenesis [18]. This process is called endochondral ossification, and can be divided into four steps [19]: chondrogenesis from early mesenchymal condensations, chondrocyte differentiation and hypertrophy, mineralization of the matrix and invasion of bone cells, and finally, the definitive formation of bone. Chondrogenesis is the first step in endochondral ossification and is based on strongly regulated events that comprise condensation of mesenchymal chondroprogenitor cells, differentiation into chondrocytes, and the patterning of chondrofying tissues into skeletal structures. The composition of ECM changes during the differentiation of mesenchymal cells into chondrocytes. While the expression of collagen I decreases, chondrocytes start producing collagen II, IX, and XI as well as aggrecan, link protein, and Gla protein [19]. This composition of cartilage is largely retained in adult articular cartilage, which can be considered a remnant of initial cartilage formation. In those parts of the embryonic cartilage in which cartilage is replaced by bone, chondrocytes differentiate further. They become hypertrophic and as part of their hypertrophic differentiation express collagen X. With beginning bone formation, cartilage undergoes vascularization by invasion of blood vessels from the perichondrium [18, 19]. The extracellular matrix gets mineralized in part by hypertrophic chondrocytes, and later—during the formation of the definitive bone matrix—through the coordinated action of mineralizing osteoblasts and bone resorbing osteoclasts that migrate into the remodeling cartilage.
In long bones, the process of endochondral ossification is initiated separately in the mesenchymal interzone at each prospective joint site [20, 21]. The interzone is composed of a dense intermediate cell layer and two outer cell layers, each facing the epiphyseal end of adjacent long bone anlagen [21]. Bland et al. [22] have shown that the development of articular chondrocytes derived from the interzone layers. In this process, the outer interzone layers mediate the initial lengthening of long bone anlagen by appositional growth. In contrast, the articular chondrocytes are mainly derived from the intermediate layer [23]. Further studies also indicating different developmental origins of articular, shaft, and growth plate cartilage are given by Rountree and Koyama [24, 25]. However, the underlying mechanisms and the specific developmental roles remain poorly understood [26]. Understanding the specific mechanisms of chondrocyte differentiation during endochondral ossification is crucial for studying cartilage degeneration because several lines of evidence indicate that in the pathogenesis of OA, similar mechanisms are involved in the initiation and progression of disease.
Pathophysiology of cartilage in OA
OA is widely considered a degenerative disease that is characterized by progressive structural changes in joint tissues, principally in articular cartilage, but also in subchondral bone, the synovial membrane, and the synovial fluid. Articular cartilage fibrillation and erosions accompanied by chondrocyte proliferation and loss of matrix proteoglycans, subchondral bone thickening, deformation of the articular surface, osteophyte formation, synovial intima cell hyperplasia, and synovial fibrosis are some key features associated with OA. These changes result from an incompletely understood but large series of functional events [27]. Different factors, including cytokines, growth factors and Wnts, have been identified to induce changes in chondrocyte metabolism or mechanisms leading to matrix degradation.
Influence of cytokines on OA
The induction of the aforementioned phenomenon is partly mediated by pro-inflammatory cytokines, which promote changes of chondrocyte metabolism and phenotype and lead to alterations of cartilage matrix structure. Among the cytokines believed to play a role in the progression of OA, IL-1β and TNF-α are known to be the predominant mediators of inflammation [6, 28, 29], even though recent attempts to block the action of either IL-1β or TNF-α have failed to improve the disease progress. In OA cartilage, they are expressed by chondrocytes and stimulate in an autocrine and paracrine manner their own production [30, 31]. Furthermore, both cytokines affect the physiological chondrocyte metabolism and favor an imbalance between the catabolic and anabolic equilibrium via activation of catabolic pathways [6, 8, 32]. IL-1β and TNF-α are capable of inducing MMPs, aggrecanases, inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), and reducing synthesis of tissue inhibitor of metalloproteinases (TIMP) [10, 33–39]. Moreover, the action of IL-1β and TNF-α is to maintain and to promote the inflammatory process via stimulation of IL-8, IL-6, nitric oxide, and prostaglandin E2 (PGE2) production [29, 40–42]. Cytokines in this place not only mediate inflammation but also contribute to changes in chondrocyte phenotype and cartilage matrix structure as discussed in the section “Changes in chondrocyte metabolism”.
In addition to these inflammatory cytokines, cytokines like growth factors have an important role in regulating cartilage homoeostasis. Thus, it was shown that enhanced levels of anabolic factors, including transforming growth factor-β (TGF-β), fibroblast growth factors (FGFs), and bone morphogenetic proteins (BMPs) trigger the matrix production in different ways [43–45]. On the one hand, it was shown that TGF-β 1, 2, and 3 trigger the matrix production by stimulating the synthesis of type II collagen and proteoglycan in cultured primary chondrocytes and in cartilage explants [43]. On the other hand, various BMPs stimulate the differentiation of mesenchymal precursors into chondrocytes and promote the differentiation of hypertrophic chondrocytes and are thereby capable of increasing the synthesis of type II collagen and aggrecan by articular chondrocytes in vitro [46–48].
Mechanisms of matrix degradation
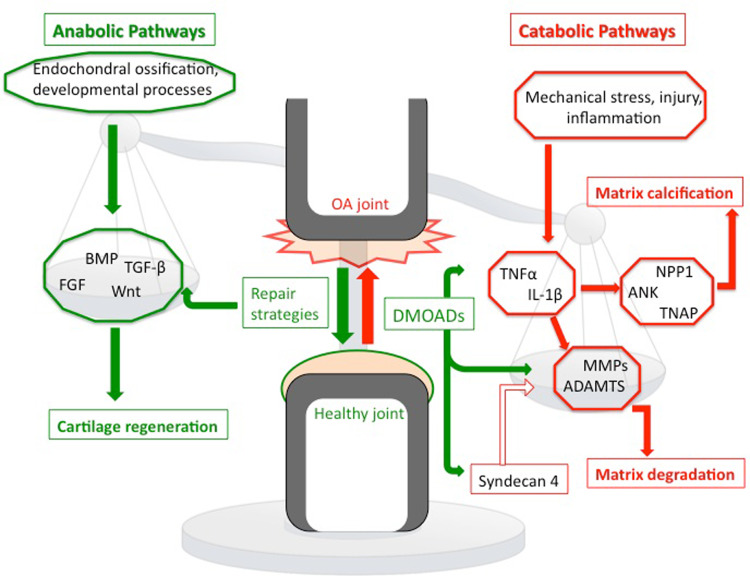
The precise mechanisms of cartilage degradation are still unclear, but a complex interplay of environmental, metabolic, and biochemical factors has been proposed to be responsible for the progressive loss of cartilage matrix in OA. Abnormal mechanical loading and synovial inflammation are only two factors contributing to dysregulation of chondrocyte function (Fig. 1). They favor an imbalance between anabolic and catabolic processes in chondrocytes [30]. In early stages of OA, matrix-degrading enzymes are overexpressed, resulting in a loss of matrix, in particular at the cartilage surface. Subsequently, there is an increase of water content in the matrix and a decrease of proteoglycans and cleavage of collagen type II. Due to damages in the structure of the collagen network, there is also a loss of tensile strength in the cartilage and, thereby, altered biomechanical properties of cartilage with a reduced stiffness [5, 16, 49]. Chondrocytes try to compensate for these effects by enhanced proliferation and synthesize of both collagen type II (COL2AI) and proteoglycans [4, 30]. Notably, there appear to be significant differences between the individual zones of cartilage. In the upper zones of OA articular cartilage, expression of COL2AI is significantly suppressed while collagen type III and fibronectin is upregulated during the progression of matrix destruction [50]. Thus, chondrocytes yield a different kind of matrix structure, which is more susceptible to erosion and to loss of proteoglycans [50, 51]. In contrast, the middle and deeper zones exhibit a normal metabolism, producing predominantly cartilaginous ECM molecules [30]. Though the repair attempts by enhanced collagen type II and proteoglycan production can stop progression of disease for several years, particularly chondrocytes of the superficial zone are unable to compensate fully for proteoglycan loss. Finally, repair attempts are outmatched by degradation due to the enhanced expression and activation of matrix degrading enzymes, and complete loss of cartilage tissue is the consequence [5].
Fig. 1.
Key factors balancing the anabolic and catabolic pathways of the OA joint. A dysregulation of chondrocyte function leads to an imbalance of anabolic and catabolic pathways with enhanced matrix degradation and calcification, as well as reduced matrix production. Anabolic mechanisms are labeled with green color and catabolic pathways are marked with red color
The predominant proteinases responsible for the characteristic matrix degradation in OA are matrix metalloproteinases (MMPs) and aggrecanases [52–54]. MMPs belong to a family of zinc-dependent proteases that are structurally and functionally related. It is known that the activation level of MMPs correlates with the incident of OA in human. Under physiological conditions, the activity of MMPs is tightly regulated at several levels, including transcriptional and post-transcriptional levels, in order to maintain proper balance between anabolism and catabolism. However, it has been shown that under the specific conditions of early OA and during OA progression there is an imbalance of MMP regulation towards enhanced activity. Pro-inflammatory cytokines such as IL-1-β and TNF-α stimulate the production of MMP-1, MMP-3, and MMP-13 [28, 35, 55]. In addition, cyclic compressive loading further increases MMP-2 and MMP-9 expression and activity [56] and mechanical injury results in elevated MMP-3 mRNA levels [57]. Once MMPs are released, tissue inhibitors of matrix metalloproteinases (TIMPs) regulate their proteolytic and biologic activity. TIMPs are the major endogenous metalloproteinase inhibitors synthesized by the same cells that produce MMPs. In non-arthritic human cartilage, there is a small excess of TIMP activity over MMP activity favoring a balance of ECM homeostasis. However, in OA cartilage, the MMP-TIMP balance is shifted towards MMP and, thereby, to an excess of activated MMPs leading to cartilage degradation [58]. With respect to their expression profile in OA and their functional properties, the groups of collagenases (MMP-1, -8, and -13), stomelysins (MMP-3, -10, -11) and gelatinases (MMP-2, -9) are considered as those with the highest impact on OA cartilage breakdown [55, 59–61]. Members of the collagenase family, mainly MMP-1, -8, and -13, contribute to the pathological cleavage of collagen fibrils in OA, since they have been described to cleave native fibrillar collagen [38, 54, 55]. Although all these MMPs are active on collagen fibrils, they are biochemically distinct. MMP-13 is able to cause the distinctive collagen II breakdown in OA [62], as it preferentially hydrolyses this type of collagen and is about ten times more active on this molecule than MMP-1 and MMP-8. Of note, it has been demonstrated that MMP-13 also degrades aggrecan, giving it a dual role in OA matrix degradation [63]. MMP-1 has a higher specificity for type III collagen, whereas MMP-8 is particularly active on type I collagen [64]. Hence, as the fibrillar network in mammalian cartilage is made up of primarily type II collagen [65], MMP-13 activity is discussed as pivotal for the collagen cleavage in OA. In immunochemical analyses of human knee samples, collagenases have been shown to be elevated in arthritic cartilage, whereas no MMPs were identified in full-thickness normal cartilage specimens [66]. Further evidence for an association of MMP-1, -8, and -13 action and enhanced ECM cleavage in OA is given by the observation that these MMPs are predominantly observed in the superficial zone of cartilage. As already mentioned, the superficial zone of OA cartilage is distinct for significant degenerative matrix changes. The expression of the other MMPs degrading non-collagen matrix compounds is also elevated in human OA cartilage. Although levels of both gelatinases (MMP-2 and -9) are enhanced in human OA cartilage, their expression levels are below that of collagenases [33]. They are capable of cleaving denatured collagen, gelatin, and type IV and V collagen [33]. However, mouse studies demonstrate that inhibition of MMPs, long thought as effectors of cartilage degradation in OA, also provide negative effects on cartilage homeostasis. This finding was achieved using MMP-2 knockout mice in an antibody-induced arthritis model, where even more severe clinical and histological arthritis was observed than in wild-type mice [65]. Using the MMP-9 knockout, it was shown that these mice are also even more susceptible to OA compared to wild-type mice [23]. The stromelysins have a substrate preference for proteoglycans, elastin, laminin, and fibronectin. Of the three members, MMP-3 is reported to be increased in human OA cartilage [67] and it is of importance that in addition to cleave cartilage molecules, MMP-3 is one major enzyme associated with the activation of other MMPs [33].
In addition to MMPs, aggrecanases are of particular importance in cartilage turnover [68]. In contrast to collagenases, their main activity is against the proteoglycan aggrecan, which is the main proteoglycan of articular cartilage. Aggrecan is a large aggregating proteoglycan that contains chondroitin sulphate and keratan sulphate attached to a multidomain protein core. It aggregates by binding to hyaluronan and this is further associated with a separate globular link protein to form stable large-molecular-weight molecules. Healthy cartilage requires a high concentration of aggrecan to provide effective weight-bearing properties. These properties are mainly due to the high osmotic pressure of aggrecan molecules, because aggrecan has a high density of negative-charged glycosaminoglycan chains [69, 70]. During OA, the loss of aggrecan is an early event in the degradation of articular cartilage and results in decrement of functional and structural ECM integrity followed by an irreversible loss of collagens [71]. The (a disintegrin and metalloproteinase with thrombospondin motifs) ADAMTS family of zinc metalloproteinases comprises 14 currently known members. All ADAMTS protein consist of an N-terminal prodomain, a catalytic domain with a zinc-binding motif, a disintegrin domain, and on the C-terminus, a cysteine-rich domain, at least one thrompospondin motif and spacer domains [72]. Of the ADAMTSs, ADAMTS-4 and ADAMTS-5 have gained special attention as it has been demonstrated that recombinant human ADAMTS-4 and ADAMTS-5 are substantially more active against aggrecan than the other members of the ADAMTS family [73]. ADAMTS-4 expression is stimulated by proinflammatory cytokines IL-1β and TNF-α, whereas ADAMTS-5 is constitutively expressed in chondrocytes [36, 74, 75]. In line with the former results, a TNF-α blocker and an IL-1β neutralizing antibody inhibited ADAMTS-4 expression by human synoviocytes significantly without any alterations of ADAMTS-5 expression, either alone or in combination [76]. Interestingly, human recombinant ADAMTS4 has higher rates of aggrecan cleavage than ADAMTS-5 [77]. This observation in combination with the expression profile indicates that ADAMTS-4 might be more detrimental for the health of cartilage. However, in mouse cartilage explants, ADAMTS-5 is strongly stimulated by IL-1 with no effect on ADAMTS-4 expression [74]. Further evidence emphasizing ADAMTS-5 to be the major aggrecanase in cartilage is given by studies with ADAMTS-4- and ADAMTS-5-deficient mice. ADAMTS-5 knockout prevents mice from cartilage degradation in both inflammatory and in joint-instability model of arthritis [78, 79]. Not only ADAMTS-5 knockout mice but also ADAMTS-5-resistant aggrecan knock-in mouse, show protection from OA development [78]. Thus, mainly ADAMTS-5 contributes to cartilage destruction in mice; while it has to be determined whether ADAMTS-5 is also the major aggrecanase in human OA cartilage or whether ADAMTS-4 equally contributes to the pathogenesis of human OA. In vitro studies with human cartilage show that both ADAMTS-4 and ADAMTS-5 are expressed in human OA cartilage and contribute to ECM breakdown during disease progression. Suppression of ADAMTS-4 and ADAMTS-5, individually or in combination, with siRNA attenuate the degradation of cartilage in cytokine-stimulated human cartilage explants [52]. Hence, this study does not support a dominant role for either of these enzymes in OA pathogenesis.
Changes in chondrocyte metabolism
Another, though far less well understood hallmark of OA is the hypertrophic differentiation of articular chondrocytes and subsequent functional events associated with this differentiation. These include the perichondrocytic degradation of the cartilage matrix by aforementioned proteases but also cartilage mineralization. Cartilage mineralization is of interest because recent data suggest that in human osteoarthritis (OA), mineralization of cartilage is re-initiated as part of the pathogenic changes associated with the terminal, hypertrophic differentiation of diseased chondrocytes [80, 81]. While in healthy articular cartilage most chondrocytes maintain a stable resting phenotype and resist proliferation and differentiation, articular chondrocytes from osteoarthritic joints form clusters by proliferating more strongly and eventually develop hypertrophy, typically close to areas of mineralized cartilage matrix and near sites of surface lesions [40, 82]. Recent evidence suggests that hypertrophic chondrocytes in the growth plate and in osteoarthritic cartilage share certain similarities, and that the calcification of hyaline cartilage is a regular event in human OA [81, 83]. One key finding linking chondrocyte hypertrophy to similar mechanisms involved in endochondral ossification is the expression of HIF-2 alpha during both processes. It has been that HIF-2 alpha is the most potent inducer of Col10A1 transcription activity, thereby inducing hypertrophic differentiation [82]. It has also been shown that HIF-2 alpha is capable of the induction of different matrix degrading enzymes like MMP-1, -3, -9, -12, and -13 as well as the aggrecanase ADAMTS-4, which leads to an imbalance of anabolic and catabolic factors favoring the catabolic side [84]. Another phenomenon linking osteoarthritis with endochondral ossification is the occurence of calcification. However, it has to be mentioned that the calcification of matrix during OA is of a different nosologic entity than calcification in crystal-induced arthropathies such as chondrocalcinosis [85, 86]. The mineralization of the matrix during OA surrounding late hypertrophic chondrocytes is due to the deposition of basic calcium phosphate (BCP) [82, 87]. Hypertrophic chondrocytes release membrane-bound matrix vesicles containing a specific combination of proteins, annexins, phosphate transporters, and phosphatases which provide the nucleation site for mineralization [81, 88]. Matrix mineralization of cartilage and vesicles is dependent on alkaline phosphatase activity, which hydrolyses organic phospho compounds. Interestingly, type X collagen, as a marker for hypertrophic chondrocytes, is highly expressed in mineralizing growth plate cartilage and several studies have suggested that the expression of type X collagen is associated with mineral growth [82, 89, 90]. It is one key observation of the recent year that similar processes are obviously initiated in human OA [81, 82]. The mechanisms that are involved in pathological cartilage calcification during OA, and particularly the pathways that link chondrocyte differentiation to the calcification of the surrounding matrix, are not completely understood. However, changes in the synthesis and transport of inorganic pyrophosphate (PPi) as well as altered extracellular PPi (ePPi) metabolism have been associated strongly with this process [81, 91]. High extracellular levels of Pi lead to an increase in BCP generation and prevent generation of calcium pyrophosphate dihydrate (CPPD) crystals. Under physiological conditions, PPi potently suppresses the deposition and propagation of BCP, and the maintenance of relatively high extracellular PPi concentrations by chondrocytes is a vital physiologic mechanism to prevent articular cartilages from calcifying [83, 92]. Based on the observation that extracellular PPi is increased in articular cartilage in direct association with OA, it has been suggested that supersaturation of the extracellular matrix with PPi along with alterations of the structure and composition of the damaged OA matrix alters the solubility product of PPi and Ca2+ and ultimately leads to cartilage calcification [83, 93, 94]. Although the underlying mechanisms are not fully clear, three molecules have been identified as central regulators of PPi metabolism: the tissue nonspecific alkaline phosphatase (TNAP), which hydrolyzes PPi, the multiple-pass transmembrane protein ANK, which mediates intracellular to extracellular channeling of PPi, and the nucleotide pyrophosphatase phosphodiesterase (NPP1), which is encoded by the enpp1 gene and generates PPi from nucleoside triphosphates. Interestingly, the deletion of ANK in mice results in extensive joints, menisci, and ligament mineralization, suggesting that extracellular pyrophosphate acts as an inhibitor of mineralization [95]. This finding is supported by the finding of severe calcifications of cartilaginous tissue and aorta in mice and humans carrying a mutation in the NPP1 gene [92, 96, 97].
In OA cartilage, IL-1 triggers the loss of a differentiated chondrocyte phenotype by suppressing the expression of a number of genes associated with differentiated phenotype, including the ones for type II collagen and proteoglycans [8, 98]. Additionally, in vitro treatment of chondrocytes with IL-1 facilitates the production of BCP crystals and calcification of the surrounding matrix, presumably by downregulation of NPP1 expression [29]. This finding is consistent with reports suggesting a contribution of inflammation to the production of minerals like BCP and CPPD during OA [93]. As previously noted, calcification with BCP of articular matrix is an indissociable process of OA and correlates with the expression of collagen X, a hypertrophic chondrocyte marker [94]. Different crystals, like MSU, BCP, and CPPD, are capable of mediating cartilage degradation indirectly by inducing pro-inflammatory cytokines (IL-1, TNF-α), nitric oxide production, MMP production, and activation and COX-1, COX-2, and PGE2 production [82, 99, 100]. However, it is not clear whether the crystals themselves induce these processes, or the crystals are coated with proteins inducing these effects. There is accumulating evidence that for example MSU crystals have a high affinity of binding proteins and that the coating of these crystals regulates the cellular response [37, 101].
Mechanisms and mediators of cartilage regeneration
As noted in the guidelines of the American College of Rheumatology, current medical therapies for OA largely focus on symptom relief, in particular of pain [102]. Even though they provide symptomatic benefit in a number of patients, there is no treatment option available for slowing down or even stopping the progression of cartilage degeneration [103]. Thus, there is a dire need to improve the options to treat localized cartilage lesions and degradation of cartilage in OA.
Mechanisms of cartilage regeneration
A great deal of attention has been paid to the restoration and repair of distinct cartilage lesions and defects, not only as a healing attempt but also for a better understanding of cartilage-regenerating approaches in OA. Such approaches include the delivery of an efficient combination of bioactive compounds to the site of injury to trigger, enhance, or even substitute the intrinsic repair and formation of hyaline-like cartilage. As cartilaginous defects constitute one major risk factor for OA [104], such strategies would ideally also slow down and prevent the development of OA from such lesions. Currently, there are surgical approaches to treat cartilage defects, such as microfracturing of the subchondral bone, mosaicplasty, and the transplantation of autologous osteochondral grafts [104], with a good track record after 2–9 years [105]. Nevertheless, further improvement is necessary as these procedures largely lead to the formation of fibrous tissue, chondrocyte death, and further cartilage degeneration [106–108].
The observation of such fibrous cartilage repair, both in men [105, 109] and in animal models [110, 111], along with the demonstration of progenitor-like chondrocytes and stem cells in the synovial membrane and other joint tissues [112, 113], indicate the presence of intrinsic repair mechanisms in articular cartilage. However, the fibrocartilaginous tissue that results from these regenerative attempts often does not restore the physiological structure of normal articular cartilage showing that hyaline cartilage has restricted capacity to heal [114, 115]. It appears that there are some obstacles that prevent the restoration of cartilage damage to functional hyaline cartilage, depending on the injury model used, the animal model, and the intra-individual differences. To understand these, a number of animal studies have been performed. Prominent examples include histological analyses of full-thickness defect healing in rabbits [107, 108, 116].
In these studies, full-thickness defects of articular cartilage are drilled. They span the entire depth of articular cartilage and continue into the subchondral bone and bone marrow space. The occurring local bleeding then serves as the basis for spontaneous repair, as they facilitate the invasion of different stem cell types from the bone-marrow space, from adipose and vascular tissue and from bone [107, 108, 112, 117]. In defect voids between 1 and 2 mm, a blood clot fills the defect immediately, which consists of fibrin molecules and various types of blood cells, in particular platelets [112]. Thereby, large quantities of growth factors reach the site of injury [107, 108, 117]. Notably, these fibrin molecules adhere to the surface edges in the bony compartment, but only rarely to those of articular cartilage. Later, the fibrin network serves to orient mesenchymal cell ingrowth along its axis, setting in after a few days. Their occurrence results in resorption of the fibrin clot and development of a vascularized, scar-like tissue [107, 108, 112]. Between the days 10 and 14, these mesenchymal cells start to pass the stages of endochondral ossification with progressive differentiation of cells to chondroblasts, chondrocytes, and osteoblasts, laying down cartilage and bone matrices in their appropriate location within the repair tissue. However, instead of hyaline cartilage with its arcade-like organization of its fibers and the zonal stratification of its chondrocytes, the collagenous tissue generated during spontaneous repair is rich in flattened fibrocartilaginous cells, generating a fibrous type of cartilage with inferior mechanical competence [107, 108, 112]. Also, the newly generated cartilage does not firmly adhere to and integrate into the native cartilage adjacent to it [112, 117]. This is partly caused by the failure of collagenous fibrils to project and to intermingle with the surrounding fibril network. Considerably, there early traces of degeneration in the newly synthesized cartilage have been described which occur after 20–48 weeks and advance over time [107, 108, 112]. Furthermore, the native cartilage adjacent to the defect becomes necrotic over time. The functional incompetence of the repair tissue, together with the lack of integration into the surrounding cartilage, may be one explanation for the poor outcomes of repair processes. Nevertheless, it has been recognized that in a number of studies there have been animals that showed good to excellent histological repair in the absence of any specific treatment [112] and these individual successes are supported by data from some clinical studies in humans [109, 112]. These findings suggest that inter-individual variations of the response to cartilage defects contribute crucially to efficacy or failure of repair processes. These most likely include a diverse pattern of cells, growth factors, and cytokines, as well as the activation of distinct signaling pathways.
Recent studies indicate that particularly morphogenetic pathways are reactivated during the signaling response to injury of adult human cartilage, which include BMPs, FGFs, and Wnt [110]. Additionally, factors regulating chondrocyte action in the growth plate remain important mediators that regulate the metabolism and homeostasis of mature articular cartilage [118, 119]. These observations form a rationale behind the idea that the same growth factor families that play a role in embryonic cartilage development are also pivotal for the activation of repair and morphogenesis of repair tissue. Consequently, a great deal of interest has focused on these families of growth and differentiation factors.
The TGF- and BMP- family
The TGF-β family consists of over 35 members and includes TGF-β proteins and bone morphogenetic proteins (BMPs). They play important roles in development and homeostasis of various tissues regulating cell proliferation, differentiation, apoptosis, migration, and extra cellular matrix metabolism [120]. Activated members of the TGF-β family interact with type I and type II receptors [121, 122]. Upon receptor phosphorylation, members of the Smad family are phosphorylated, and the activated Smads associate with Co-Smad and translocate to the nucleus where they can act as transcription factors [122, 123]. Members of the TGF-β family are considered potent stimulators of chondrocyte proliferation and matrix homeostasis exerting a beneficial anabolic or “repair” response on articular cartilage. Application of TGF-β1 and -2 increases aggrecan and collagen gene expression and prevent the loss of proteoglycan in articular cartilage during experimental OA [123, 124], while blocking of TGF-β2 receptor leads to enhanced proteoglycan loss and reduces the articular thickness [125]. Interestingly, it has been reported that members of the TGF-β family promote the differentiation of MSCs to chondrocytes [126] and that TGF-β signaling enhances repair of full-thickness cartilage defects by triggering chondrocyte differentiation, improving integration between repair tissue and adjacent native tissue and inhibiting the activity of matrix metalloproteinases [127].
Despite such promising data, therapeutic studies with TGF-β1 revealed major adverse effects. As shown by van Beuningen et al. and Bakker et al., injection of TGF-β1 and adenovirus-mediated delivery of TGF-β1 into a normal murine knee joint resulted in joint fibrosis and osteophyte formation [128] (Bakker 2001, #289).
Similar effects as for TGF-β have been observed for several BMP molecules. Different BMPs have been shown to stimulate chondrogenic differentiation of MSCs [129, 130], enhance the synthesis of type II collagen, and aggrecan by chondrocytes in vitro [131]. It has also been demonstrated that BMPs boost the healing response to full-thickness defects of cartilage when combined with microfracture [132] or when delivered locally by transfected muscle-derived stem cells [133]. In the first clinical trials with BMP as the therapeutic agent, the weekly intra-articular injection of BMP-7 was capable of inhibiting osteoarthritis progression in rabbits [134]. However, because of the findings in clinical trials with TGF-β, which belongs to the same protein family, it seems likely that BMPs may also exhibit various side-effects when injected into the joints.
The FGF-family
The human FGF family comprises 22 secreted glycoproteins, which exert their action trough four transmembrane tyrosine kinases (FGFR1-4) [134, 135]. Cell surface heparan-sulfate proteoglycans (HSPGs) are capable of binding the liberated FGF, thus facilitating signaling in a paracrine manner. HSPGs are also required for the interaction of FGFs with FGFRs and contribute to the development of a stable complex between FGF and FGFR. When two FGFR molecules are recruited into the complex of FGF-HSPG, they dimerize with autophosphorylation by intrinsic tyrosine kinase. Upon activation, FGFRs interact with multiple single-transduction molecules, such as the ras-mitogen-activated protein kinases, the phosphoinositide-3-OH kinases (PI3K) and phospholipase C (PLC)γ. Due to the variety of potential downstream targets and the individual unique signaling pattern of different FGF–FGFR complexes, FGF signaling is complex and the effects differ considerably between different cell tissues and cell types [136]. However, it is widely assumed that FGFs display diverse functions in development, homeostasis, injury repair, and regeneration [135]. Thus, FGF–FGFR interaction affects chondrogenesis and cartilage homeostasis by regulating chondrocyte proliferation during the embryonic and postnatal growth [44, 137]. Defining the specific FGF–FGFR interaction responsible for chondrogenesis has been difficult, as expression levels of ligands and receptors overlap [138]. In growth-plates, FGFR1 is expressed primarily by terminally differentiated chondrocytes and osteoblasts whereas proliferating chondrocytes express specifically FGFR3 [138]. As for the ligands, FGF-2 and FGF-18 are discussed as prominent regulators of cartilage homeostasis in adult tissue [138]. FGF-18 is shown to exert anabolic effects in cartilage via activation of FGFR3. This interaction leads to ECM production and cell differentiation, while cell proliferation is inhibited. The role of FGF2 is controversial, as it is associated with both catabolic and anabolic effects on adult matrix homeostasis. FGF2 action is likely to be mediated by FGFR1, which leads to an enhanced activity of matrix-degrading enzymes, inhibition of matrix production, and increased cell proliferation. Noteworthy, both FGFs are demonstrated to facilitate repair of damaged cartilage in vivo. FGF2 released from a carrier of fibrin sealant promotes healing of full-thickness defects of the articular cartilage in rabbits [45]. Intra-articular injections of FGF18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced OA [139]. Nonetheless, a cautionary note is merited regarding the use of FGFs in cartilage repair strategies, as there are contradictory observations in different in vivo and in vitro studies, and variable effects of FGFs when comparing different tissues of different joints [45, 140]. The complexity of FGF signaling emphasizes the need for a better understanding of FGF signaling, before FGFs are to be used for clinical approaches.
The Wnt family of molecules
Members of the Wnt family regulate a variety of biological processes, including embryonic development, body patterning, tissue morphogenesis, and tumorigenesis [120]. During the last decade, studies reveal that Wnt signaling also has an important role in many aspects of cartilage biology. To date, 19 Wnt genes have been identified in the human genome. Mostly Wnts act on target cells by binding to Frizzeld (FZD) receptor proteins and LRP-5/6 co-receptor proteins, which activate several signal transduction pathways. The canonical Wnt pathway affects cellular functions by regulation of β-catenin levels. In the absence of Wnt activating the canonical pathway, β-catenin is targeted for proteasome-mediated degradation by a multi-protein complex comprising glycogen synthase kinase 3β (GSK-3β) [141]. The presence of appropriated Wnt ligands leads to inhibition of GSK-3β and, thereby, to β-catenin accumulation and translocation into the nucleus. Within the nucleus, it acts in concert with Tcf/Lef transcription factors [141]. The β-catenin-Tcf/Lef transcriptional activity regulates expression of a various target genes such as cyclin D1, c-jun, c-myc, E-cadherin, and MMP-7, and MMP-26 [142]. Non-canonical Wnt signaling is mostly a β-catenin-independent mechanism like the Wnt/calcium and Wnt/JNK pathways in vertebrates and the Wnt/planar cell polarity pathway (PCP) in flies [143]. There are several antagonists for Wnt signaling. One of the best characterized families is the Dickkopf (Dkk) family binding to LRP-5/6 and antagonizing canonical pathway. Another antagonist is the secreted frizzled-related protein (sFRP) family binding directly to Wnt ligands and inhibiting both canonical and non-canonical Wnt pathways. Distinct components of Wnt signaling have been shown to regulate chondrogenesis at the stage of mesenchymal condensation and/or cartilage nodule formation. It is described that Wnt4, which is expressed in developing joints and cartilage, accelerates hypertrophy, whereas Wnt5a which is expressed in perichondrium, prolongates hypertrophy [144, 145]. A very recent report by Diarra et al. stresses the role of Wnt signaling in the remodeling of adult joint tissue, as they show that inhibition of the Wnt-inhibitor Dickkopf-1 reverse a bone erosive RA pattern into the bone-forming pattern of osteoarthritis [146]. The work of Loughlin et al., which suggests that mutations in the secreted frizzled-related protein 3 are linked to female hip OA, also gives evidence for a role of Wnt signaling in osteoarthritis and joint remodeling [147]. Another work also shows that the knockout of Frzb in mice is associated with increased cartilage loss in OA, due to increased Wnt signaling and MMP-3 expression and activity [148]. Both studies indicate a potential role of Wnt signaling in cartilage biology and repair.
Further evidence for a role of Wnt signaling is given by microarray analysis of injured cartilage showing a striking up-regulation of Wnt-16, nuclear accumulation of β-catenin, and up-regulation of several Wnt target genes after acute injury of cartilage or in OA [118]. Several Wnt members have been implicated in both early and late skeletal development by promoting chondrocyte proliferation and hypertrophic maturation [149, 150]. However, there are also Wnt members that inhibit chondrocyte differentiation and maturation, such as Wnt-1 and Wnt-4. Additionally, it has been shown that in particular, members activating the canonical Wnt signaling induce ossification and suppression of chondrogenesis [150, 151]. Similar to these contradictory observations, β-catenin is required for both osteogenesis and chondrogenesis in adult tissues, but is dispensable for chondrogenesis in embryonic development [152, 153]. Collectively, the Wnt/β–catenin network is suggested to have a dual role depending on several conditions. Indeed, during embryonic development, the system balances negative and positive influences on chondrogenesis, while it is implicated as a positive regulator during postnatal development. Later in life, it also seems to contribute to pathogenesis of joint conditions, such as OA [150]. Overall, these results highlight the importance of Wnt signaling for chondrogenesis but also emphasize the need for a better understanding.
Disease-modifying approaches in osteoarthritis
Due to the small number of leukocytes in the synovial fluid and the absence of systemic inflammation, OA is considered a primarily degenerative arthropathy. However, the disease is frequently accompanied by inflammatory flares [154, 155], and a variety of findings suggest that this inflammatory process contributes at least to the progression of OA [29]. Also, in different studies analyzing the effect of mechanical injury, chondrocytes have been shown to exert an increased expression of inflammatory mediators and stress response factors [49]. Moreover, it is described that a number of cartilage macromolecules in the synovial fluid released after cartilage destruction have significant inflammatory and immunogenic properties [29]. Therefore, interfering with inflammatory mediators and pathways has been one approach when designing novel strategies for the treatment of OA.
Inhibition of inflammatory mediators
The impact of inflammation on disease progression suggests that modulation of inflammation through its major mediators, IL-1 and TNF-α, might have a value by reducing the catabolic imbalance in OA cartilage and preventing degradation and alterations of articular cartilage matrix. In treatment of RA, TNF-α and IL-1 antagonists are successfully proven to be effective disease modifiers and are already applied in the clinic [156, 157].
Initial in vitro studies with OA cartilage explants demonstrated that the inhibition of IL-1 via IL-1-receptor antagonist (IL-1RA) attenuated the production of MMPs and other inflammatory mediators and reduced matrix degradation, while significantly increasing synthesis of type II collagen and aggrecan [28]. According to the in vitro data, the intra-articular application of IL-1RA as well as the in vivo transfer of IL-1RA gene into OA knee joints has proved disease-modifying efficacy in dfferent animal models as both have led to a reduction of cartilage destruction [158–161]. Despite this favorable evidence from in vitro and in animal models, current studies on the inhibition of IL-1R in human OA indicate limited effectiveness of IL-1RA as DMOAD and are still scarce. Although intra-articular injection of IL-1RA has improved both pain and clinical outcome in an open-label, 12-week safety study [163], it does not appear to have lasting beneficial effects on the signs and symptoms of knee OA. In two different multicenter, placebo-controlled, double-blind, clinical trials in 2005 and in 2009, it was demonstrated that intra-articular injection of IL-1RA is not associated with improvement in OA symptoms compared with placebo [164, 165]. Rather, there is at least some evidence that IL-1 is required for normal cartilage remodeling and homeostasis and that, therefore, inhibition of IL-1 itself may do more harm than good [166, 167].
In contrast to rheumatoid arthritis, where several anti-tumor necrosis factor alpha (TNF-α) have been approved as disease-modifying drugs [157], only a few experimental trials have assessed the efficacy of blocking this cytokine for the treatment of OA. In a small, open-label study concerning the effect of TNF-α antibodies in the treatment of erosive hand OA describe only a modest, not significant symptom improvement and just individual patients had some benefit [168].
Inhibitors of cartilage degeneration
Based on the above data, it has been assumed that the action of MMPs causes the loss of both collagens and proteoglycans in the progression of OA cartilage degradation. Thus, it is no surprise that modulation of MMPs as cartilage-degrading enzymes has been a key strategy to halt matrix degradation and, thereby, to gain disease modification. As a consequence, a number of small-molecular-weight compounds have been developed that can act as selective inhibitors of proteolytic MMP activity. However, biologic systems are sometimes much more complex than predicted. A clinical report from a 1-year dose–response study of the efficacy and safety of an oral MMP inhibitor in patients with mild to moderate knee arthritis showed increased incidence of musculoskeletal adverse effects [169]. Also, there was no structure modification after 1 year of treatment, as no change in joint space width could be detected. MMP-inhibitors have also failed in clinical trials investigating their efficacy in other fields such as cardiology and oncology [170–174]. These disappointing results lead to the termination of most MMP inhibitor trails.
Despite the unresolved question about the specific importance of ADAMTS-4 and ADAMTS-5 in human OA, a great deal of research has also focused on the development and investigation of ADAMTS-inhibitors. TIMP-3 and α2-makroglobulin have been identified as potential endogenous inhibitors of ADAMTS-4 and -5 [175]. However, their physiological relevance has to be further evaluated. In 2007, the first specific ADAMTS-4 and -5 inhibitor has entered phase I clinical trial, which is about drug safety and potential short-term efficacy. Actually, the contradictory results of pre-clinical studies regarding the importance of both ADAMTS in OA pathogenesis and the early stage of clinical investigations stress the dire need for research to be done on the pre-clinical and clinical site. Only very recently the work of Echtermeyer Bertrand et al. has strengthened the interest in ADAMTS-5 as a potential target for OA [176]. In this study, it was demonstrated not only that syndecan-4, a transmembrane heparan sulfate proteoglycan, is induced specifically in hypertrophic chondrocytes in OA. It was also shown that syndecan-4 is crucial in regulating IL-1 induced expression of MMP-3 and ADAMTS-5 activity by targeting it to the cell surface. Hence, these findings suggest that enhanced expression of syndecan-4 is not only a distinct event during osteoarthritis, but also seems to be pivotal to the disease process as it directly affects the proteolytic activity. Of importance, it was shown that loss of syndecan-4 in genetically modified mice as well as intra-articular injections of specific syndecan-4 antibodies protected mouse cartilage from OA matrix-breakdown by limiting ADAMTS-5 activation.
Footnotes
D. Umlauf and S. Frank contributed equally to this work.
References
- 1.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamel MB, Toth M, Legedza A, Rosen MP. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee—decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168(13):1430–1440. doi: 10.1001/archinte.168.13.1430. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 5.Martinek V. Anatomy and pathophysiology of articular cartilage. Dtsche Z Sportmed. 2003;54(6):166–170. [Google Scholar]
- 6.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–246. [PubMed] [Google Scholar]
- 7.Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40(1):1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 8.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427:S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 9.Loughlin J. The genetic epidemiology of human primary osteoarthritis: current status. Expert Rev Mol Med. 2005;7(9):1–12. doi: 10.1017/S1462399405009257. [DOI] [PubMed] [Google Scholar]
- 10.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling. Clin Orthop Relat Res. 2004;423:7–16. doi: 10.1097/01.blo.0000131638.81519.de. [DOI] [PubMed] [Google Scholar]
- 12.Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, Caplan AI. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem. 2002;50(8):1049–1058. doi: 10.1177/002215540205000807. [DOI] [PubMed] [Google Scholar]
- 13.Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33(1):1–13. doi: 10.1016/S8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 14.Burgeson RE, Hebda PA, Morris NP, Hollister DW. Human cartilage collagens. Comparison of cartilage collagens with human type V collagen. J Biol Chem. 1982;257(13):7852–7856. [PubMed] [Google Scholar]
- 15.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop.Relat Res. 2001;391(Suppl):S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 17.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12(2):69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 18.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 19.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthr Cartil. 2000;8(5):309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 20.Mitrovic D. Development of di-arthrodial joints in rat embryo. Am J Anat. 1978;151(4):475–485. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- 21.Holder N. Experimental investigation into early development of chick elbow joint. J Embryol Exp Morphol. 1977;39:115–127. [PubMed] [Google Scholar]
- 22.Bland YS, Ashhurst DE. Development and ageing of the articular cartilage of the rabbit knee joint: distribution of the fibrillar collagens. Anat Embryol. 1996;194(6):607–619. doi: 10.1007/BF00187473. [DOI] [PubMed] [Google Scholar]
- 23.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169(5):2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 24.Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. Plos Biol. 2004;2(11):1815–1827. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, Kingsley DM, Iwamoto M, Enomoto-Iwamoto M, Pacifici M. A distinct cohort of progenitor cells participates in synovial Joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316(1):62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75(3):237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- 27.Pritzker KP. Animal models for osteoarthritis: processes, problems and prospects. Ann Rheum Dis. 1994;53(6):406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor a in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease—potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis—an introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3(2):107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, Abramson SB, Amin AR. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type IIIL-1 decoy receptor—potential for pharmacological intervention. J Biol Chem. 2000;275(51):40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 32.Aigner T, Soeder S, Haag J. Il-1 beta and BMPS—interactive players of cartilage matrix degradation and regeneration. Eur Cells Mater. 2006;12:49–56. doi: 10.22203/ecm.v012a06. [DOI] [PubMed] [Google Scholar]
- 33.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage—associations with degenerative changes. Arthritis Rheum. 2001;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Vuolteenaho K, Moilanen T, Knowles RG, Moilanen E. The role of nitric oxide in osteoarthritis. Scand J Rheumatol. 2007;36(4):247–242U245. doi: 10.1080/03009740701483014. [DOI] [PubMed] [Google Scholar]
- 35.Richardson DW, Dodge GR. Effects of interleukin-1 beta and tumor necrosis factor-alpha on expression of matrix-related genes by cultured equine articular chondrocytes. Am J Vet Res. 2000;61(6):624–630. doi: 10.2460/ajvr.2000.61.624. [DOI] [PubMed] [Google Scholar]
- 36.Pratta MA, Scherle PA, Yang GJ, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003;48(1):119–133. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- 37.Morgan MP, Whelan LC, Sallis JD, McCarthy CJ, Fitzgerald DJ, McCarthy GM. Basic calcium phosphate crystal-induced prostaglandin E2 production in human fibroblasts: role of cyclooxygenase 1, cyclooxygenase 2, and interleukin-1beta. Arthritis Rheum. 2004;50(5):1642–1649. doi: 10.1002/art.20223. [DOI] [PubMed] [Google Scholar]
- 38.Mort J, Billington C. Articular cartilage and changes in arthritis: matrix degradation. Arthritis Res. 2001;3(6):337–341. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burrage PS, Brinckerhoff CE. Molecular targets in osteoarthritis: metalloproteinases and their inhibitors. Curr Drug Targets. 2007;8(2):293–303. doi: 10.2174/138945007779940098. [DOI] [PubMed] [Google Scholar]
- 40.Hyc A, Osiecka-Iwan A, Jozwiak J, Moskalewski S. The morphology and selected biological properties of articular cartilage. Ortop Traumatol Rehabil. 2001;3(2):151–162. [PubMed] [Google Scholar]
- 41.Benito MJ, Veale DJ, Fitzgerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelletier JP, McCollum R, Cloutier JM, Martel-Pelletier J. Synthesis of metalloproteases and interleukin 6 (IL-6) in human osteoarthritic synovial membrane is an IL-1 mediated process. J Rheumatol Suppl. 1995;43:109–114. [PubMed] [Google Scholar]
- 43.Grimaud E, Heymann D, Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13(3):241–257. doi: 10.1016/S1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 44.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39(Suppl 1):S88–S96. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 45.Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420(1):82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bobacz K, Gruber R, Soleiman A, Graninger WB, Luyten F, Erlacher L. Cartilage-derived morphogenetic protein-1 and -2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis. Osteoarthr Cartil. 2002;10(5):394–401. doi: 10.1053/joca.2002.0522. [DOI] [PubMed] [Google Scholar]
- 47.Bobacz K, Gruber R, Soleiman A, Erlacher L, Smolen JS, Graninger WB. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003;48(9):2501–2508. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 48.Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35(9):1323–1340. doi: 10.1016/S1357-2725(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 49.Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schunke M. Pathomechanisms of cartilage destruction by mechanical injury. Annals of Anatomy Anatomischer Anzeiger. 2005;187(5–6):473–485. doi: 10.1016/j.aanat.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Fukui N, Ikeda Y, Ohnuki T, Tanaka N, Hikita A, Mitomi H, Mori T, Juji T, Katsuragawa Y, Yamamoto S, Sawabe M, Yarnane S, Suzuki R, Sandell LJ, Ochi T. Regional differences in chondrocyte metabolism in osteoarthritis. Arthritis Rheum. 2008;58(1):154–163. doi: 10.1002/art.23175. [DOI] [PubMed] [Google Scholar]
- 51.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54(11):3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 52.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, Fosang AJ. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair (vol 117, pg 1627, 2007) J Clin Investig. 2008;118(11):3812. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fosang AJ, Little CB. Drug insight: aggrecanases as therapeutic targets for osteoarthritis. Nat Clin Pract Rheumatol. 2008;4(8):420–427. doi: 10.1038/ncprheum0841. [DOI] [PubMed] [Google Scholar]
- 54.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4(3):128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 55.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 56.Blain EJ, Gilbert SJ, Wardale RJ, Capper SJ, Mason DJ, Duance VC. Up-regulation of matrix metalloproteinase expression and activation following cyclical compressive loading of articular cartilage in vitro. Arch Biochem Biophys. 2001;396(1):49–55. doi: 10.1006/abbi.2001.2575. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52(8):2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 58.Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 60.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 61.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases—structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 62.Knäuper V, L¢pez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 63.Martel-Pelletier J, Pelletier JP. Wanted—the collagenase responsible for the destruction of the collagen network in human cartilage! Br J Rheumatol. 1996;35(9):818–820. doi: 10.1093/rheumatology/35.9.818. [DOI] [PubMed] [Google Scholar]
- 64.Miwa HE, Gerken TA, Hering TM. Effects of covalently attached chondroitin sulfate on aggrecan cleavage by ADAMTS-4 and MMP-13. Matrix Biol. 2006;25(8):534–545. doi: 10.1016/j.matbio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15(5):805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 66.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, VanWart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8(2):367–376. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 68.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267(6):3581–3584. [PubMed] [Google Scholar]
- 69.Kaushal GP, Shah SV. The new kids on the block: ADAMTSs, potentially multifunctional metalloproteinases of the ADAM family. J Clin Invest. 2000;105(10):1335–1337. doi: 10.1172/JCI10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardingham TE, Fosang AJ, Dudhia J. The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage. Eur J Clin Chem Clin Biochem. 1994;32(4):249–257. [PubMed] [Google Scholar]
- 71.Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124(4):687–693. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- 72.Brooks PM. The burden of musculoskeletal disease—a global perspective. Clin Rheumatol. 2006;25(6):778–781. doi: 10.1007/s10067-006-0240-3. [DOI] [PubMed] [Google Scholar]
- 73.Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2(3):322–329. doi: 10.1016/S1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- 74.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21(6):499–511. doi: 10.1016/S0945-053X(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 75.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46(10):2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 76.Tortorella MD, Malfait AM, Deccico C, Arner E, Nagase H. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation (vol 9, pg 539, 2001) Osteoarthr Cartil. 2002;10(1):82. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 77.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE (2006) The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 8(6) [DOI] [PMC free article] [PubMed]
- 78.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 79.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 80.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang ZY, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56(2):575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 81.Fuerst M, Niggemeyer O, Lammers L, Schafer F, Lohmann C, Ruther W. Articular cartilage mineralization in osteoarthritis of the hip. BMC Musculoskelet Disord. 2009;10:166. doi: 10.1186/1471-2474-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, Rutsch F, Schafer FKW, Niggemeyer O, Steinhagen J, Lohmann CH, Pap T, Ruther W. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60(9):2694–2703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 83.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281(1):C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 84.Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, Nakamura K, Tokunaga K, Chung UI, Kawaguchi H. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16(6):678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 85.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, Min BH, Chun JS. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 86.Ea HK, Liote F. Calcium pyrophosphate dihydrate and basic calcium phosphate crystal-induced arthropathies: update on pathogenesis, clinical features, and therapy. Curr Rheumatol Rep. 2004;6(3):221–227. doi: 10.1007/s11926-004-0072-6. [DOI] [PubMed] [Google Scholar]
- 87.Ea HK, Liote F. Advances in understanding calcium-containing crystal disease. Curr Opin Rheumatol. 2009;21(2):150–157. doi: 10.1097/BOR.0b013e3283257ba9. [DOI] [PubMed] [Google Scholar]
- 88.Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137(5):1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang W, Kirsch T. Annexin V/beta5 integrin interactions regulate apoptosis of growth plate chondrocytes. J Biol Chem. 2006;281(41):30848–30856. doi: 10.1074/jbc.M605937200. [DOI] [PubMed] [Google Scholar]
- 90.Arias JL, Nakamura O, Fernandez MS, Wu JJ, Knigge P, Eyre DR, Caplan AI. Role of type X collagen on experimental mineralization of eggshell membranes. Connect Tissue Res. 1997;36(1):21–33. doi: 10.3109/03008209709160211. [DOI] [PubMed] [Google Scholar]
- 91.Fleisch H. Diphosphonates: history and mechanisms of action. Metab Bone Dis Relat Res. 1981;3(4–5):279–287. doi: 10.1016/0221-8747(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 92.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 93.Lotz M, Rosen F, McCabe G, Quach J, Blanco F, Dudler J, Solan J, Goding J, Seegmiller JE, Terkeltaub R. Interleukin 1 beta suppresses transforming growth factor-induced inorganic pyrophosphate (PPi) production and expression of the PPi-generating enzyme PC-1 in human chondrocytes. Proc Natl Acad Sci USA. 1995;92(22):10364–10368. doi: 10.1073/pnas.92.22.10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rutsch F, Terkeltaub R. Parallels between arterial and cartilage calcification: what understanding artery calcification can teach us about chondrocalcinosis. Curr Opin Rheumatol. 2003;15(3):302–310. doi: 10.1097/00002281-200305000-00019. [DOI] [PubMed] [Google Scholar]
- 95.Kirsch T. Determinants of pathological mineralization. Curr Opin Rheumatol. 2006;18(2):174–180. doi: 10.1097/01.bor.0000209431.59226.46. [DOI] [PubMed] [Google Scholar]
- 96.Koshizuka Y, Ikegawa S, Sano M, Nakamura K, Nakamura Y. Isolation of novel mouse genes associated with ectopic ossification by differential display method using ttw, a mouse model for ectopic ossification. Cytogenet Cell Genet. 2001;94(3–4):163–168. doi: 10.1159/000048809. [DOI] [PubMed] [Google Scholar]
- 97.Koshizuka Y, Kawaguchi H, Ogata N, Ikeda T, Mabuchi A, Seichi A, Nakamura Y, Nakamura K, Ikegawa S. Nucleotide pyrophosphatase gene polymorphism associated with ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res. 2002;17(1):138–144. doi: 10.1359/jbmr.2002.17.1.138. [DOI] [PubMed] [Google Scholar]
- 98.Okawa A, Nakamura I, Goto S, Moriya H, Nakamura Y, Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet. 1998;19(3):271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 99.Bai G, Howell DS, Howard GA, Roos BA, Cheung HS. Basic calcium phosphate crystals up-regulate metalloproteinases but down-regulate tissue inhibitor of metalloproteinase-1 and -2 in human fibroblasts. Osteoarthr Cartil. 2001;9(5):416–422. doi: 10.1053/joca.2000.0407. [DOI] [PubMed] [Google Scholar]
- 100.Molloy ES, Morgan MP, Doherty GA, McDonnell B, Hilliard M, O’Byrne J, Fitzgerald DJ, McCarthy GM. Mechanism of basic calcium phosphate crystal-stimulated cyclo-oxygenase-1 up-regulation in osteoarthritic synovial fibroblasts. Rheumatology (Oxford, England) 2008;47(7):965–971. doi: 10.1093/rheumatology/ken144. [DOI] [PubMed] [Google Scholar]
- 101.Bardin T, Varghese Cherian P, Schumacher HR. Immunoglobulins on the surface of monosodium urate crystals: an immunoelectron microscopic study. J Rheumatol. 1984;11(3):339–341. [PubMed] [Google Scholar]
- 102.Terkeltaub R, Tenner AJ, Kozin F, Ginsberg MH. Plasma protein binding by monosodium urate crystals. Analysis by two-dimensional gel electrophoresis. Arthritis Rheum. 1983;26(6):775–783. doi: 10.1002/art.1780260612. [DOI] [PubMed] [Google Scholar]
- 103.Altman RD, Hochberg MC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee—2000 update. Arthritis Rheum. 2000;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 104.Clouet J, Vinatier C, Merceron C, Pot-vaucel M, Maugars Y, Weiss P, Grimandi G, Guicheux J. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov Today. 2009;14(19–20):913–925. doi: 10.1016/j.drudis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Smith GD, Knutsen G, Richardson JB. A clinical review of cartilage repair techniques. J Bone Joint Surg Br. 2005;87B(4):445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 106.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 107.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthr Cartil. 1999;7(1):15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 108.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 109.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee—a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthopaedica Scandinavica. 1996;67(2):165–168. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 110.Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee—a natural history study. J Bone Joint Surg Am. 2003;85A:8–16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 111.Breinan HA, Hsu HP, Spector M. Chondral defects in animal models—effects of selected repair procedures in canines. Clin Orthop Relat Res. 2001;391:S219–S230. doi: 10.1097/00003086-200110001-00021. [DOI] [PubMed] [Google Scholar]
- 112.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular-cartilage. J Bone Joint Surg Am. 1993;75A(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 113.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 114.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJR, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 115.Mankin HJ. Reaction of articular-cartilage to injury and osteoarthritis.1. N Engl J Med. 1974;291(24):1285–1292. doi: 10.1056/NEJM197412122912406. [DOI] [PubMed] [Google Scholar]
- 116.Mankin HJ. The response of articular-cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 117.Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model—a preliminary study. J Bone Joint Surg Am. 2001;83A(1):53–64. doi: 10.2106/00004623-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 118.Dell’Accio F, De Bari C, Eltawil NA, Vanhummelen P, Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening. Arthritis Rheum. 2008;58(5):1410–1421. doi: 10.1002/art.23444. [DOI] [PubMed] [Google Scholar]
- 119.Onyekwelu I, Goldring MB, Hidaka C. Chondrogenesis, joint formation, and articular cartilage regeneration. J Cell Biochem. 2009;107(3):383–392. doi: 10.1002/jcb.22149. [DOI] [PubMed] [Google Scholar]
- 120.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27(5):307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, Kim C, Tang Y, Shetty K, Mishra B, Mishra L (2007) TGF-beta signaling in development. Sci Signal 2007(399): cm1 [DOI] [PubMed]
- 122.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15(1):1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 123.Roberts AB. TGF-[beta] signaling from receptors to the nucleus. Microbes Infect. 1999;1(15):1265–1273. doi: 10.1016/S1286-4579(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 124.Critchlow MA, Bland YS, Ashhurst DE. The effect of exogenous transforming growth factor-beta 2 on healing fractures in the rabbit. Bone. 1995;16(5):521–527. doi: 10.1016/8756-3282(95)00085-R. [DOI] [PubMed] [Google Scholar]
- 125.Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Stimulation of articular cartilage repair in established arthritis by local administration of transforming growth factor-beta into murine knee joints. Lab Invest. 1998;78(2):133–142. [PubMed] [Google Scholar]
- 126.Scharstuhl A, Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169(1):507–514. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 127.Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, Johnstone B, Evans CH, Ghivizzani SC. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005;12(2):219–228. doi: 10.1016/j.ymthe.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 128.Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, Richards CD, van den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondrosteophyte formation. Osteoarthr Cartil. 2001;9(2):128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 129.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71(2):279–290. [PubMed] [Google Scholar]
- 130.Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthr Cartil. 2008;16(10):1121–1130. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2,-4, and-6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 132.Grunder T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, Aicher WK. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthr Cartil. 2004;12(7):559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthr Cartil. 2006;14(11):1126–1135. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 134.Hayashi M, Muneta T, Ju YJ, Mochizuki T, Sekiya I (2008) Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther 10(5) [DOI] [PMC free article] [PubMed]
- 135.Krejci P, Prochazkova J, Bryja V, Kozubik A, Wilcox WR. Molecular pathology of the fibroblast growth factor family. Hum Mutat. 2009;30(9):1245–1255. doi: 10.1002/humu.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martin GR. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998;12(11):1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- 137.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 138.Horton WA, Degnin CR. FGFs in endochondral skeletal development. Trends Endocrinol Metab. 2009;20(7):341–348. doi: 10.1016/j.tem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br. 2007;89B(5):693–700. doi: 10.1302/0301-620X.89B5.18450. [DOI] [PubMed] [Google Scholar]
- 140.Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr Cartil. 2005;13(7):623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 141.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 142.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 143.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20(11):1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 144.Montcouquiol M, Crenshaw EB, Kelley III MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 145.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127(14):3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 146.Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ. 1999;41(1):29–40. doi: 10.1046/j.1440-169x.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- 147.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 148.Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, Ferreira A, Ciesielski C, Carson DA, Corr M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. 2004;101(26):9757–9762. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andrade AC, Nilsson O, Barnes KM, Baron J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone. 2007;40(5):1361–1369. doi: 10.1016/j.bone.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yates KE, Shortkroff S, Reish RG. Wnt influence on chondrocyte differentiation and cartilage function. DNA Cell Biol. 2005;24(7):446–457. doi: 10.1089/dna.2005.24.446. [DOI] [PubMed] [Google Scholar]
- 151.Day TF, Guo XZ, Garrett-Beal L, Yang YZ. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 152.Chen M, Zhu M, Awad H, Li TF, Sheu TJ, Boyce BF, Chen D, O’Keefe RJ. Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. J Cell Sci. 2008;121(9):1455–1465. doi: 10.1242/jcs.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow ECY, Lin AC, Alman BA. beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007;282(1):526–533. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 154.Koutroumpas AC, Alexiou IS, Vlychou M, Sakkas LI. Comparison between clinical and ultrasonographic assessment in patients with erosive osteoarthritis of the hands. Clin Rheumatol. 2010;29(5):511–516. doi: 10.1007/s10067-009-1348-z. [DOI] [PubMed] [Google Scholar]
- 155.Vlychou M, Koutroumpas A, Malizos K, Sakkas LI. Ultrasonographic evidence of inflammation is frequent in hands of patients with erosive osteoarthritis. Osteoarthr Cartil. 2009;17(10):1283–1287. doi: 10.1016/j.joca.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 156.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36(6):1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 157.Taylor PC, Feldmann M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):578–582. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- 158.Caron JP, Fernandes JC, MartellPelletier J, Tardif G, Mineau F, Geng CS, Pelletier JP. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis—suppression of collagenase-1 expression. Arthritis Rheum. 1996;39(9):1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 159.Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, Sheppard M, Krishnan BR, Pelletier JP. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints—prevention of osteoarthritis progression. Am J Pathol. 1999;154(4):1159–1169. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9(1):12–20. doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- 161.Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, Fernandes JC, Martel-Pelletier J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40(6):1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 162.Zhang XL, Mao ZB, Yu CL. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004;22(4):742–750. doi: 10.1016/j.orthres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 163.Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32(7):1317–1323. [PubMed] [Google Scholar]
- 164.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum Arthritis Care Res. 2009;61(3):344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 165.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Kiefer P, Zhou L, Bevirt T, Appleton B. Results from a double-blind, placebo-controlled, multicenter trial of a single intra-articular injection of anakinra (kineret (R)) in patients with osteoarthritis of the knee. Arthritis and Rheumatism. 2005;52(9):S507. [Google Scholar]
- 166.Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin-1 beta, interleukin-1 beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48(12):3452–3463. doi: 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- 167.Fan Z, Soder S, Ehler S, Fundel K, Aigner T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007;171(3):938–946. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Magnano MD, Chakravarty EF, Broudy C, Chung L, Kelman A, Hillygus J, Genovese MC. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol. 2007;34(6):1323–1327. [PubMed] [Google Scholar]
- 169.Krzeski P, Buckland-Wright C, Balint G, Cline GA, Stoner K, Lyon R, Beary J, Aronstein WS, Spector TD (2007) Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res Ther 9(5) [DOI] [PMC free article] [PubMed]
- 170.Bissett D, O’Byrne KJ, von Pawel J, Gatzemeier U, Price A, Nicolson M, Mercier R, Mazabel E, Penning C, Zhang MH, Collier MA, Shepherd FA. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J Clin Oncol. 2005;23(4):842–849. doi: 10.1200/JCO.2005.03.170. [DOI] [PubMed] [Google Scholar]
- 171.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48(1):15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 172.King J, Zhao J, Clingan P, Morris D. Randomised double-blind placebo-control study of adjuvant treatment with the metalloproteinase inhibitor, marimastat in patients with inoperable colorectal hepatic metastases: significant survival advantage in patients with musculoskeletal side-effects. Anticancer Res. 2003;23(1B):639–645. [PubMed] [Google Scholar]
- 173.Leff RL, Elias I, Ionescu M, Reiner A, Poole AR. Molecular changes in human osteoarthritic cartilage after 3 weeks of oral administration of BAY 12–9566, a matrix metalloproteinase inhibitor. J Rheumatol. 2003;30(3):544–549. [PubMed] [Google Scholar]
- 174.Miller KD, Saphner TJ, Waterhouse DM, Chen TT, Rush-Taylor A, Sparano JA, Wolff AC, Cobleigh MA, Galbraith S, Sledge GW. A randomized phase II feasibility trial of BMS-275291 in patients with early stage breast cancer. Clin Cancer Res. 2004;10(6):1971–1975. doi: 10.1158/1078-0432.CCR-03-0968. [DOI] [PubMed] [Google Scholar]
- 175.Tortorella MD, Arner EC, Hills R, Easton A, Korte-Sarfaty J, Fok K, Wittwer AJ, Liu RQ, Malfait AM. alpha(2)-Macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279(17):17554–17561. doi: 10.1074/jbc.M313041200. [DOI] [PubMed] [Google Scholar]
- 176.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15(9):1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]