Abstract
It is well known that the co-chaperone p23 regulates Hsp90 chaperone activity in protein folding. In Plasmodium falciparum, a putative p23 (Pfp23) has been identified through genome analysis, but its authenticity has remained unconfirmed since co-immunoprecipitation experiments failed to show its interaction with P. falciparum Hsp90 (PfHsp90). Thus, recombinant Pfp23 and PfHsp90 proteins purified from expressed clones were used in this study. It was clear that Pfp23 exhibited chaperone activity by virtue of its ability to suppress citrate synthase aggregation at 45°C. Pfp23 was also shown to interact with PfHsp90 and to suppress its ATPase activity. Analyses of modeled Pfp23-PfHsp90 protein complex and site-directed mutagenesis further revealed strategically placed amino acid residues, K91, H93, W94 and K96, in Pfp23 to be crucial for binding PfHsp90. Collectively, this study has provided experimental evidence for the inherent chaperone function of Pfp23 and its interaction with PfHsp90, a sequel widely required for client protein activation.
Keywords: Heat shock protein 90, p23, Plasmodiumfalciparum, Chaperone, Site-directed mutagenesis
Introduction
The co-chaperone, p23, is a small protein involved in the cascade of Hsp90 chaperone activity [1, 2]. It is well documented that Hsp90 folds and activates proteins essential in cellular signaling and development (e.g., kinases and transcription factors) by undergoing a cycle of conformational changes driven by the binding and hydrolysis of ATP at its N-terminal domain [3–6]. The interaction between p23 and Hsp90 occurs at the late stage of the chaperone cycle and is ATP-dependent [7–9]. Reportedly, the binding of p23 reduces the rate of ATP hydrolysis and traps Hsp90 in the ATP-bound conformation [10]. It was also proposed that p23 binding prolongs the interaction between Hsp90 and its client proteins, thereby securing their maturation. Subsequently, the hydrolysis of ATP leads to the release of activated client proteins [11]. This latter feature was demonstrated using an in vitro reconstituted Hsp90 chaperone system, whereby the incorporation of p23 significantly increased the competence of mature progesterone and glucocorticoid receptors in binding steroid hormones [12, 13], as such attesting to p23’s importance in driving the Hsp90 functional repertoire.
In the recently resolved crystal structure complex of Hsp82 and SbaI, homologues of Hsp90 and p23 in yeast, respectively, the two proteins were shown to interact through their N-terminal domains [14]. Hsp82 was crystallized in the ATP-bound conformation and possessed a closed ‘lid’ segment positioned over the ATP-binding pocket, which would otherwise remain open in the absence of ATP. In the case of SbaI, the N-terminal domain was found to bind over the ATP-binding pocket of Hsp82, with the closed ‘lid’ forming one of the interaction interfaces. The requirement for this ATP-induced closed ‘lid’ conformation in Hsp82 to interact with SbaI is circumstantially supported by experiments showing the preferential binding of p23 to ATP-bound Hsp90 [7–9].
Apart from regulating Hsp90 chaperone functions, p23 is also capable of binding partially folded proteins and suppressing protein aggregation in vitro [15, 16]. This has led to the suggestion that p23 may itself function as a chaperone, independent of Hsp90. When the C-terminal tail of p23 was truncated, its chaperone activity was abolished, suggesting that the acidic amino acid-rich tail may be instrumental for its function [17]. This intrinsic chaperone activity of p23 has been demonstrated to be essential for the recycling of steroid receptors and telomerase [18, 19].
In Plasmodium falciparum, the species that causes the most severe form of human malaria, a putative homologue of p23 (Pfp23) has been reported [20]. Pfp23 was initially identified as a 36-kDa major phosphoprotein associated with the kinase activity of an unknown protein in P. falciparum [21, 22]. Subsequent procurement of its protein sequence revealed its homology to the co-chaperone p23 [20]. However, validation of its role as a co-chaperone of P. falciparum Hsp90 (PfHsp90) has been unsuccessful so far [20, 23]. Co-immunoprecipitation experiments performed using either antibodies specific to Pfp23 or PfHsp90 did not detect complexes containing both the proteins. However, as alluded to earlier, p23 has important roles in the activation of a plethora of Hsp90 client proteins, many of which are crucial in signal transduction and regulating cell growth. Thus, it would be unusual to exclude its role in P. falciparum, as implicated in reports so far. Therefore, in this study, the putative Pfp23 was probed for its intrinsic chaperone activity and interaction with PfHsp90.
Materials and methods
Cloning of Pfp23 and PfHsp90
The DNA sequences of Pfp23 (PF14_0510) and PfHsp90 (PF07_0029) were retrieved from PlasmoDB, and primers were designed to clone these genes (Table 1). The genes were cloned from the mRNA of P. falciparum 3D7. The mRNA extraction and reverse transcription procedures were carried out as previously described [24].
Table 1.
Primers designed for gene cloning and mutations
| Gene constructs | Primersa (5′ to 3′) |
|---|---|
| PfHsp90 |
F: ATCATTggatccATGTCAACGGAAACATTCGC R: CTGGATctcgagGTCAACTTCTTCCATTTTAGAATCGG |
| Pfp23 |
F: ATCGTAggatccATGCCACTCTATCC R: ATGAATgtcgacGGCTACTGGTTCTTGTACTTCTACTGC |
| Pfp23-truncated mutants: | |
| T1 |
F: ggatccATGATAGAAAATGTAAAGATTGACC |
| T2 |
F: ggatccATGATAGAAAATGTAAAGATTGACC |
| T3 |
F: ggatccATGATAATAAAAAAAGAACAAGAAAGATGG |
| T4 |
F: ggatccATGAACTCCTGGGTAGATACCGATG |
| T5 |
F: ggatccATGGGAGGTATGCCAGATATGAGTC |
| Pfp23-(Δ135–275) |
R: gtcgacAAATTGACTCATATCTGGCATACC |
| Pfp23-(Δ220–275) |
R: gtcgacATTCATATTATTCATATTAGGC |
| Pfp23 single mutants b : | |
| K91A |
F: ATAACGATGGA GCG
AAACACTGGGTTAAATG R: ACCCAGTGTTT CGC
TCCATCGTTATTTAAAG |
| K92A |
F: CGATGGAAAG GCA
CACTGGGTTAAATG R: CATTTAACCCAGTG TGC
CTTTCCATCG |
| H93S |
F: ATGGAAAGAAA AGC
TGGGTTAAATGTGAC R: CACATTTAACCCA GCT
TTTCTTTCCATCG |
| W94S |
F: GGAAAGAAACAC AGC
GTTAAATGTGACTG R: GTCACATTTAAC GCT
GTGTTTCTTTCCATCG |
| V95N |
F: GAAACACTGG AAT
AAATGTGAC R: GTCACATTT ATT
CCAGTGTTTC |
| K96A |
F: AAACACTGGGTT GCA
TGTGACTGGAACTCC R: AGTTCCAGTCACA TGC
AACCCAGTGTTTC |
aRestriction enzyme sites are in lower case, F represents forward primer, R represents reverse primer
bCodons mutated in Pfp23 are bold and underlined
Briefly, the PCR-amplified gene products were separated by agarose gel electrophoresis using 0.8% (w/v) agarose gel, and the bands that corresponded to the expected size of the genes were excised and purified using the MinEluteTM Gel Extraction Kit (Qiagen). Subsequently, the purified fragments were ligated into pCR-BluntII-TOPO Vector using the Zero BluntTM TOPOTM PCR Cloning Kit (Invitrogen). The plasmids of the recombinant clones were extracted using the Wizard® Plus SV Minipreps DNA Purification System from Promega. Full sequencing was carried out using the ABI PRISMTM BigDyeTM Terminator Cycle Sequencing Ready Reaction Kit from Perkin Elmer and analyzed using the ABI PRISM 3100 DNA sequencer to identify recombinant clones that contained the genes of interest.
Subsequently, the recombinant clones that contained Pfp23 and PfHsp90 were double-digested with BamHI/SalI and BamHI/XhoI, respectively. Pfp23 was subcloned into the expression vector, pET24a (Novagen), whereas PfHsp90 was ligated to pET24a and pGK [25] expression vectors. PfHsp90 expressed using the pGK vector contained a GST- and a His6 tag and would be used for the GST pull-down assay. However, the more highly expressed PfHsp90 from the recombinant pET24a vector possessed only a His6 tag. This fusion protein would be used for the ATPase assay. The ligated recombinant vectors were transformed into Escherichia coli BL21 (DE3) cells that contained a RIG plasmid [26] for expression of the recombinant fusion proteins. DNA sequencing was performed to authenticate the recombinant clones carrying the genes of interest.
Construction of Pfp23-truncated and single mutants
The recombinant pET24a plasmid that contained Pfp23 was used as a template for the construction of Pfp23-truncated mutants. Pfp23-truncated mutants T1, T2, T3, T4 and T5 were PCR-amplified using appropriate forward primers and a common reverse primer used for Pfp23 wild-type (Pfp23-WT) previously. On the other hand, amplifications of Pfp23-truncated mutants, Pfp23-(Δ135–275) and Pfp23-(Δ220–275) were carried out using appropriate designed reverse primers and a common forward primer from Pfp23-WT (Table 1). Similar cloning procedures described earlier were adopted for the subsequent steps.
The QuikChangeTM Site-directed Mutagenesis Kit from Stratagene was used for the construction of Pfp23 single mutants [27]. Primers were designed to introduce the desired mutations using PCR protocols described previously [28]. DNA sequencing was performed subsequently to verify the changes in the nucleotide sequences prior to transformation into E. coli BL21 (DE3) with the RIG plasmid for recombinant fusion protein expression.
Expression and purification of Pfp23 and PfHsp90
Bacterial cultures carrying the PfHsp90 and Pfp23 recombinant plasmids were grown in 100 ml Luria-Bertani broth with 50 μg/ml kanamycin (Sigma) and 40 μg/ml chloramphenicol (Sigma) for protein expression. The cultures were grown at 37°C with agitation at 250 rpm until an OD600 of 0.8 was reached. Protein expression was induced by the addition of 1 mM isopropyl-β-D-1-thiogalactopyranoside (Sigma) followed by incubation at 16°C with shaking at 250 rpm for 16 h.
Following induction, the cultures were harvested by centrifugation at 6,000 rpm for 5 min at 4°C. The supernatant was discarded, and the pelleted cells were resuspended in cold 50 mM Tris-HCl, pH 7.5 buffer. The cells were lysed via sonication with a 1/8-inch probe at an intensity of 10 μm for eight cycles with 10-s pulse and 10-s rest in each cycle. Cell lysates were subsequently centrifuged at 12,000 rpm for 20 min, and the soluble protein fractions that contained the fusion proteins of interest were subjected to purification.
The PfHsp90 and Pfp23 recombinant proteins, both carrying a C-terminal His6 tag, were purified by affinity chromatography using nickel-nitrilotriacetic acid (Ni-NTA) beads (Qiagen). For each purification, an aliquot of 1 ml of the soluble protein fraction was incubated with 50 μl of Ni-NTA beads for 15 min at 4°C. Unbound proteins were removed by washing thrice with 250 μl of 50 mM Tris-HCl, pH 7.5, 20 mM imidazole buffer. The recombinant proteins were eluted in 50 μl of 50 mM Tris-HCl, pH 7.5, 300 mM imidazole buffer.
To remove co-purified contaminating proteins, the eluted proteins were further purified by gel filtration chromatography on a Superdex 200 column (GE Healthcare). The recombinant proteins were separated at a flow rate of 0.5 ml/min in buffer containing 50 mM Tris-HCl, pH 7.5. The eluted samples were analyzed by SDS-PAGE to determine the fractions that contained the recombinant proteins of interest. These fractions were pooled and concentrated using Amicon Ultra-4 Centrifugal Filter Units with appropriate molecular weight cutoff and stored at 4°C for subsequent assays. The concentrations of the purified fusion proteins were determined by Bradford assay (Biorad) using bovine serum albumin (Sigma) as a standard. Expression and purification of the recombinant proteins were upscaled proportionately when necessary to obtain a sufficient amount of recombinant proteins for the subsequent assays.
Citrate synthase aggregation assay
The intrinsic Hsp90-independent chaperone activity of Pfp23 was assayed based on the aggregation of citrate synthase [29] using a slightly modified protocol. Porcine citrate synthase (Sigma) was dialyzed with 40 mM HEPES, pH 7.5 buffer, overnight and concentrated to an 8.5-μM stock solution. Aggregation was induced by the incubation of citrate synthase (0.15 μM) in 40 mM HEPES, pH 7.5 buffer, at 45°C. The assays were performed in the presence or absence of five-fold excess (0.75 μM) of Pfp23-WT and Pfp23-(Δ220–275). The extent of aggregation was measured by the increase in absorbance due to light scattering and monitored at 334 nm using the Tecan Safire spectrophotometer. The absorbance was recorded every 2.5 min for 20 min.
In vitro interaction between Pfp23 and PfHsp90
The in vitro interaction of Pfp23 and PfHsp90 was investigated using GST pull-down assay. Nickel affinity purified dual-tagged PfHsp90 fusion protein expressed from the pGK vector was used as a bait. For each experiment, 2 μg of PfHsp90 fusion protein was incubated with 50 μl of glutathione Sepharose beads (GE Healthcare) at 4°C for 15 min. The bound fusion proteins were washed thrice with 50 mM of Tris-HCl, pH 7.5, buffer to remove unbound proteins. Subsequently, the beads were reconstituted in 500 μl of binding buffer (50 mM Tris-HCl, pH 7.5, 10 mM KCl, 2 mM dithiothreitol, 0.01% (v/v) Triton X-100). The binding assays were carried out in the presence of 5 mM MgCl2, 5 mM ATP and 10 μg of Pfp23, unless stated otherwise. Samples were incubated at 30°C for 30 min for PfHsp90-Pfp23 complex formation. Thereafter, the samples were washed thrice with 500 μl of binding buffer prior to elution in 150 μl of reduced glutathione buffer. Fifty microliters of the eluted sample was analyzed by SDS-PAGE. Gel image analyses and densitometric measurements were performed using Bio-Rad Molecular Imager Gel Doc XR system equipped with the Quantity One® Software.
ATPase assay
The ATPase activity of PfHsp90 was measured using the pyruvate kinase/lactate dehydrogenase-coupled assay [6, 30]. The assay was carried out in a 200-μl reaction containing 2 μM PfHsp90 incubated in 100 mM Tris-HCl, pH 7.5, 6 mM MgCl2, 20 mM KCl, 0.8 mM ATP, 2 mM phosphoenolpyruvate, 0.2 mM NADH, 50 μg/ml lactate dehydrogenase and 0.2 mg/ml pyruvate kinase at 37°C. The decrease in NADH level, which is in direct stoichiometry to the amount of ATP hydrolysed, was determined spectrophotometrically at 340 nm. The authenticity of PfHsp90 ATPase activity was verified by its sensitivity to inhibition by radicicol (100 μM), a specific Hsp90 ATPase inhibitor. To determine the effect of Pfp23 interaction on PfHsp90 ATPase activity, Pfp23 was added to the assay reactions in increasing concentrations (2–30 μM). In addition, Pfp23 single mutants with differential binding capacity to PfHsp90 were also tested for their influence on PfHsp90 ATPase activity. The Pfp23 single mutants were added in ten-fold molar excess (20 μM), and their effects on the ATPase activity of PfHsp90 were compared to that of Pfp23-WT. All measurements were made using the Tecan Safire spectrophotometer. The ATPase activity reported is expressed as micromole of ATP hydrolysed per micromole of PfHsp90 in 1 min at 37°C.
Bio-computational analyses of Pfp23 and PfHsp90
Protein sequence alignment was performed using the CLUSTAL W Multiple Alignment Program [31], and the percentage identities were determined by MatGAT 2.02 [32]. Tertiary structures of Pfp23 and PfHsp90 were modeled by the SWISS-MODEL program [33] using the “First Approach Mode”. The Hsp82-SbaI crystal structure complex (PDB accession number: 2cg9) was used as a template for protein modeling. The modeled protein structures were subsequently visualized using the Swiss-PdbViewer (SPdbV) program [34].
Results
Bio-computational analyses of the putative Pfp23
P. falciparum 3D7 genome encodes a putative p23 homologue of 275 amino acid residues. The Pfp23 possesses the characteristic “Hsp20-like chaperone superfamily” domain (residues 2-106) and a C-terminal tail rich in acidic residues. The “Hsp20-like chaperone superfamily” domain, which is also present in the yeast SbaI, is known to be important for Hsp82 interaction [14]. Sequence homology analyses indicated that Pfp23 shared 44% sequence identity with Schizosaccharomyces pombe p23 homologue [20], but exhibited only 19.6 and 23.6% sequence identities in common with the human p23 and Saccharomyces cerevisiae SbaI, respectively. In addition, the putative Pfp23 (275 residues) has a longer protein sequence than the human p23 (160 residues) and S. cerevisiae SbaI (216 residues). Multiple sequence alignment further revealed the difference in protein length of Pfp23 lies in the presence of the unique tandem repeats, GNMGGLx7 (between amino acid residues 135–176) and a longer C-terminal tail (Fig. 1). Similar glycine-rich tandem repeats were also found in p23 of other Plasmodium species, namely P. chabaudi, P. berghei, P. yeolii and P. knowlesi [20]. In comparison, the human p23 only possessed a GGD sequence, whereas the yeast SbaI has two repeats of GGAGGA and a single GGAGGM sequence. So far, the significance of these glycine-rich sequences has yet to be reported and as such its functionality remains enigmatic.
Fig. 1.
Protein sequence alignment of p23 and related sequences. Protein sequences of Homo sapiens p23 (NP_006592.3) and Saccharomyces cerevisiae SbaI (NP_012805.1) were obtained from GenBank, whereas that of Pfp23 (PF14_0510) was retrieved from PlasmoDB. The P. falciparum genome encodes a longer putative p23 homologue that shares only 19.6 and 23.6% amino acid sequence identities with the human p23 and S. cerevisiae SbaI, respectively. The glycine-rich GNMGGLx7 sequence repeats in Pfp23 are bold and underlined. Asterisk identical amino acid residues in all the sequences, colon indicates conserved amino acid substitutions, dot indicates semi-conserved amino acid substitutions
Cloning, expression and purification of Pfp23 and PfHsp90
To clone the putative Pfp23 and PfHsp90, reverse transcription and PCR amplifications were carried out using appropriate primers and mRNA from P. falciparum 3D7. The resultant ORF of Pfp23 was inserted into the pET24a expression vector, whereas that of PfHsp90 was ligated into pET24a and pGK expression vectors. Subsequently, DNA sequencing was performed to verify their authenticities. The recombinant plasmids were transformed into E. coli BL21 (DE3) already harboring the RIG plasmid. The RIG plasmid encodes tRNAs that recognize rare codons present in the AT-rich plasmodial genes [26]. Hence, it was included in anticipation of improving the expression of the recombinant proteins.
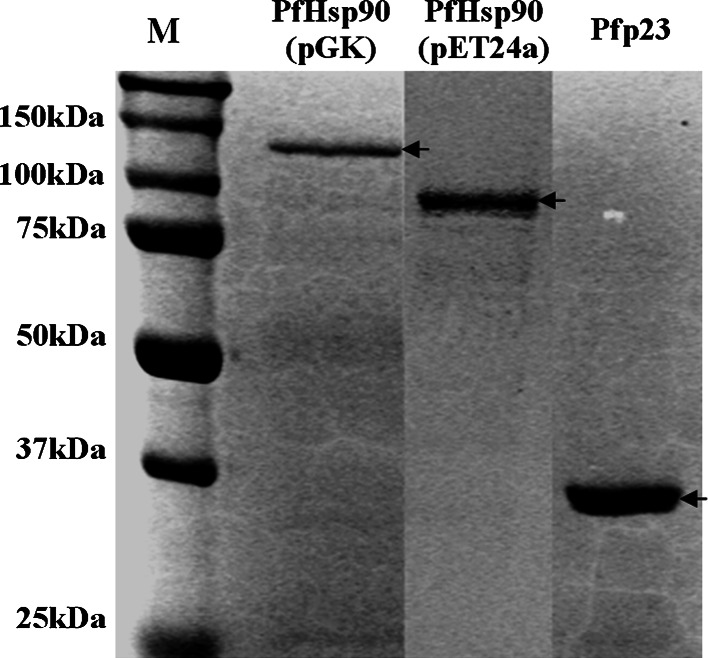
Pfp23 and PfHsp90 were solubly expressed as recombinant proteins. The PfHsp90 fusion protein expressed from the pGK vector contained an N-terminal GST tag and a C-terminal His6 tag. The inclusion of a dual tag allowed an initial purification of PfHsp90 fusion protein using Ni2+ affinity chromatography and gel filtration separation, prior to immobilization on the glutathione Sepharose for the GST pull-down assays. As the subsequent ATPase assay requires large quantities of highly purified proteins, the recombinant PfHsp90 was expressed using the pET24a vector. The recombinant pET24a vector provided significantly higher quantities of PfHsp90 fusion proteins compared to the recombinant pGK vector. Pfp23 was also recombinantly expressed using the pET24a vector, and similar Ni2+ affinity chromatography and gel filtration separation techniques were utilized to purify these fusion proteins. After electrophoretic separation, the recombinant Pfp23 was found to be approximately 36 kDa, whereas PfHsp90 expressed from recombinant pGK and pET24a vectors were 114 kDa and 88 kDa, respectively (Fig. 2).
Fig. 2.
SDS-PAGE analyses of purified Pfp23 and PfHsp90 recombinant proteins. PfHsp90 was expressed from recombinant pGK and pET24a vectors separately. PfHsp90 expressed from the pGK vector contained an N-terminal GST tag and a C-terminal His6 tag, whereas PfHsp90 from the pET24a vector possessed only the C-terminal His6 tag. Similarly, Pfp23 expressed from the recombinant pET24a vector harbored a C-terminal His6 tag. The molecular weights of the recombinant proteins are approximately 114 kDa (PfHsp90 from pGK), 88 kDa (PfHsp90 from pET24a) and 36 kDa (Pfp23). M protein marker (Biorad)
The recombinant Pfp23 possesses chaperone activity
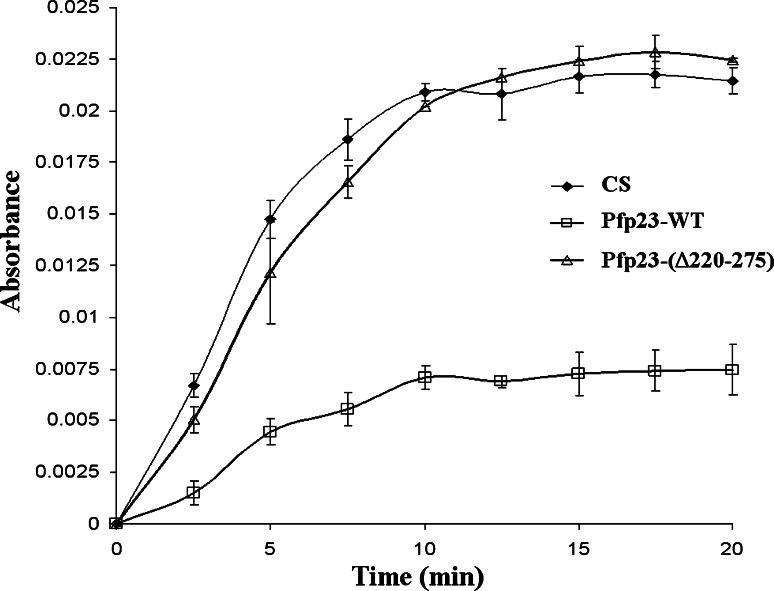
Prior to this study, the functionality of the putative Pfp23 had not been demonstrated. In view of this, Pfp23 was examined for its intrinsic chaperone activity in preventing the aggregation of citrate synthase [29]. Pfp23 was added to citrate synthase in a 5:1 M ratio and incubated at 45°C. Aggregation of citrate synthase was detected by the increase in absorbance at 334 nm over a 20-min interaction time.
In the absence of Pfp23, citrate synthase aggregated spontaneously at 45°C (Fig. 3). The addition of Pfp23 suppressed the aggregation of citrate synthase, as reflected by the decrease in absorbance reading. Similar anti-aggregation activity was observed in human p23, but was abolished when its acidic C-terminal tail was truncated by 30 amino acid residues [17]. To investigate whether the C-terminal tail of Pfp23 also contributes to anti-aggregation activity, a mutant clone of Pfp23 that lacks the C-terminal acidic residues was constructed. Deletion of the C-terminal tail in Pfp23-(Δ220–275) resulted in a total loss of the anti-aggregation activity observed in Pfp23-WT, implying the requirement of the C-terminal tail for its full chaperone activity. No self-aggregation of Pfp23-WT and Pfp23-(Δ220–275) was observed at 45°C in the control experiments performed without citrate synthase (data not shown).
Fig. 3.
The Pfp23 recombinant protein exhibited intrinsic chaperone activity. The intrinsic chaperone activity of Pfp23 was determined by citrate synthase aggregation assay. Spontaneous aggregation of citrate synthase was monitored continuously over 20 min at 45°C by the increase in absorbance. Addition of Pfp23-WT suppressed the aggregation of citrate synthase. The anti-aggregation activity was abolished when Pfp23-WT was substituted with Pfp23-(Δ220–275), which has a truncated C-terminal tail. Results shown are representative of three independent experiments. CS citrate synthase
Pfp23 interaction with PfHsp90 is dependent on MgCl2 and ATP
The role of Pfp23 as a co-chaperone of PfHsp90 in P. falciparum has remained debatable as previous attempts to identify their interaction via co-immunoprecipitation techniques were unsuccessful [20, 23]. As such, their interaction was probed in this study using recombinant proteins and GST pull-down assays. Purified Pfp23 recombinant protein was added to PfHsp90 immobilized on glutathione Sepharose in a 5:1 ratio and incubated either in the presence or absence of 5 mM MgCl2, 5 mM ADP or 5 mM ATP. Under the different conditions tested, the binding of Pfp23 to PfHsp90 was only observed in the presence of MgCl2 and ATP (Fig. 4). Recombinant GST-His6 fusion protein without PfHsp90 was used as a control to ensure that the recombinant tags do not contribute to the interaction observed. Pfp23 binding was absent in the control experiments performed under similar conditions (data not shown).
Fig. 4.
SDS–PAGE analyses of Pfp23 interaction with PfHsp90. The interaction between Pfp23 and PfHsp90 was tested in the presence or absence of 5 mM MgCl2, 5 mM ADP and 5 mM ATP as indicated. Pfp23 was bound to PfHsp90 only in the presence of MgCl2 and ATP. Results shown are representative of three independent experiments
Sequential N-terminal truncation of Pfp23 affects interaction with PfHsp90
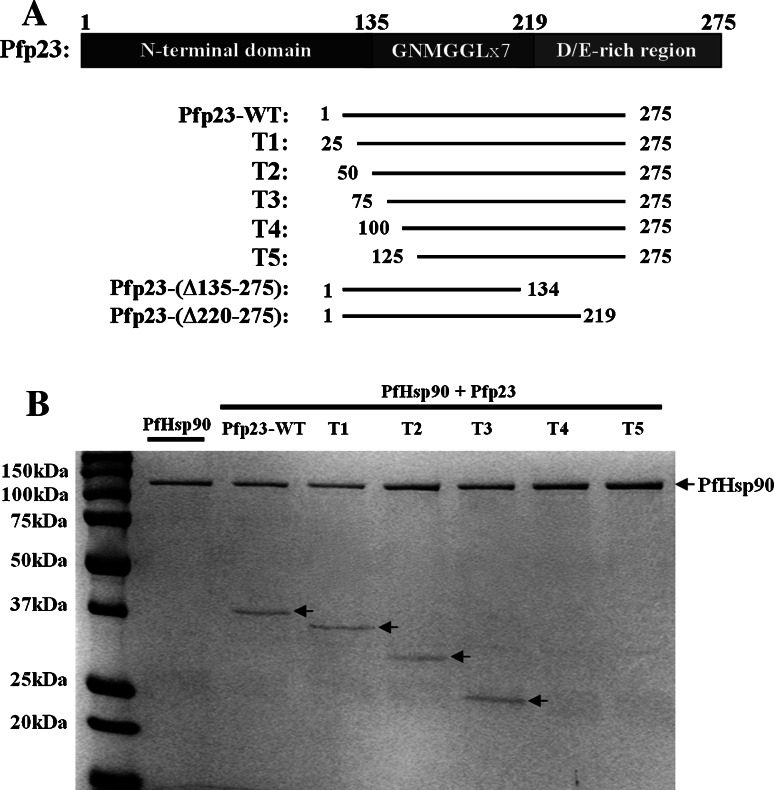
As Pfp23 shares low amino acid sequence identities with the human p23 and yeast SbaI, its interaction with PfHsp90 may potentially differ from that of their homologues. Hence, the interaction between Pfp23 and PfHsp90 was further analyzed. Pfp23 was sequentially truncated by 25 amino acid residues from the N-terminal end to identify regions that are essential for binding PfHsp90 (Fig. 5a).
Fig. 5.
Interaction between Pfp23-WT and truncated mutants with PfHsp90. a Schematic representation of the Pfp23-truncated mutants constructed in this study. A 25-amino acid serial truncation was performed from the N-terminal end of Pfp23 (T1 to T5) to identify the region that is essential for binding PfHsp90. Pfp23-(Δ220–275) was constructed to investigate the intrinsic chaperone activity of Pfp23. On the other hand, Pfp23-(Δ135–275) was generated to determine whether the GNMGGLx7 sequence repeats are essential for PfHsp90 interaction. b Purified Pfp23-WT and the respective truncated mutants were incubated with PfHsp90 in the presence of 5 mM MgCl2 and 5 mM ATP. Truncated mutants T4 and T5 were unable to bind PfHsp90, as shown in the SDS-PAGE analysis. Results presented are representative of three independent experiments
A total of five Pfp23-truncated mutants (T1 to T5) were constructed, cloned, expressed and purified under similar conditions to those described for Pfp23-WT. All the truncated mutants were solubly expressed in E. coli BL21 (DE3) (data not shown). Pfp23-truncated mutants T1, T2 and T3 retained their binding to PfHsp90, whereas further truncations in T4 and T5 abolished interaction with PfHsp90 (Fig. 5b). The results indicated that certain amino acid residues located between the 75th to 100th positions in Pfp23 are crucial for binding PfHsp90. To identify these amino acid residues, the corresponding region in yeast SbaI was determined by primary sequence alignment and examined for the presence of Hsp82-interacting residues. Inspection of the region using the yeast Hsp82-SbaI crystal structure complex revealed an interface in SbaI (K113, Y114, P115, Y116, I117 and K118) that interacts with the closed ‘lid’ segment of Hsp82 (Fig. 6a) [14]. This interface corresponded to the amino acid residues K91, K92, H93, W94, V95 and K96 in Pfp23 and was observed to be in proximity to PfHsp90 in the modeled complex (Fig. 6b). Hence, these residues may potentially be essential for PfHsp90 interaction, such that when deleted (in Pfp23-truncated mutants T4), Pfp23’s competency in binding PfHsp90 is undermined.
Fig. 6.

Analyses of the modeled structures of Pfp23 and PfHsp90. a PfHsp90 (orange) and Pfp23 (green) were modeled against the yeast Hsp82 (gold)-SbaI (brown) crystal structure complex (PDB accession number: 2cg9). The modeled Pfp23 shares similar structural features with SbaI and possesses an extended loop (highlighted in grey) that is projected towards the closed ‘lid’ segment of PfHsp90. Only the N-terminal domains of PfHsp90 and Hsp82 were shown in the graphical presentation. b Amino acid residues K91, K92, H93, W94, V95 and K96, located at the extended loop of Pfp23, were observed to be in proximity to the closed ‘lid’ segment of PfHsp90. Pfp23 residue H93 was observed to be in close contact with the hydrophobic residues F104 and I114 in PfHsp90 (top right). Similarly, the residue W94 in Pfp23 is directed towards residues I108 and M105 in PfHsp90 (bottom right). AMP-PNP a non-hydrolysable analogue of ATP
Residues K91, H93, W94 and K96 in Pfp23 are essential for PfHsp90 interaction
To investigate the importance of the amino acid residues K91, K92, H93, W94, V95 and K96 in Pfp23 for PfHsp90 interaction, single mutants were generated. Firstly, Pfp23 residues K91, K92 and K96 were each mutated to an alanine to examine the importance of the lysine side chains for binding PfHsp90. As for residues H93 and W94, mutation to a polar serine residue was proposed to disrupt any hydrophobic interaction formed between Pfp23 and PfHsp90. Similarly, the hydrophobic amino acid residue, V95, was mutated to a polar asparagine residue as a previous study showed that substitution at the corresponding position in SbaI (I117N) abolished interaction with Hsp82 [35]. All the Pfp23 mutant proteins were solubly expressed, purified and tested for PfHsp90 interaction under similar binding conditions to those described for Pfp23-WT. Pfp23 mutants K91A, H93S, W94S and K96A displayed significant reduction in their binding to PfHsp90, retaining less than 25% of Pfp23-WT’s binding (Fig. 7). However, Pfp23 mutants K92A and V95 N retained more than 85% of Pfp23-WT’s binding to PfHsp90. Recombinant GST-His6 fusion protein without PfHsp90 was used as a control, and no interaction with Pfp23 mutants was observed when tested under similar conditions (data not shown).
Fig. 7.
Effects of Pfp23 mutations on PfHsp90 interaction. a Pfp23-WT and mutants were incubated with PfHsp90 in the presence of 5 mM MgCl2 and 5 mM ATP. The complexes formed were analyzed by SDS-PAGE. b The binding of Pfp23 mutants to PfHsp90 was analyzed by densitometry. The percentage of PfHsp90 bound was calculated with respect to the PfHsp90 control (single asterisk), whereas Pfp23 mutants binding to PfHsp90 was determined with respect to Pfp23-WT (double asterisk). Pfp23 single mutants K91A, H93S, W94S and K96A displayed drastically reduced binding to PfHsp90, whereas single mutants K92A and V95 N retained binding comparable to Pfp23-WT to PfHsp90. Results shown are representative of three independent experiments
The GNMGGLx7 sequence repeats in Pfp23 are not essential for PfHsp90 interaction
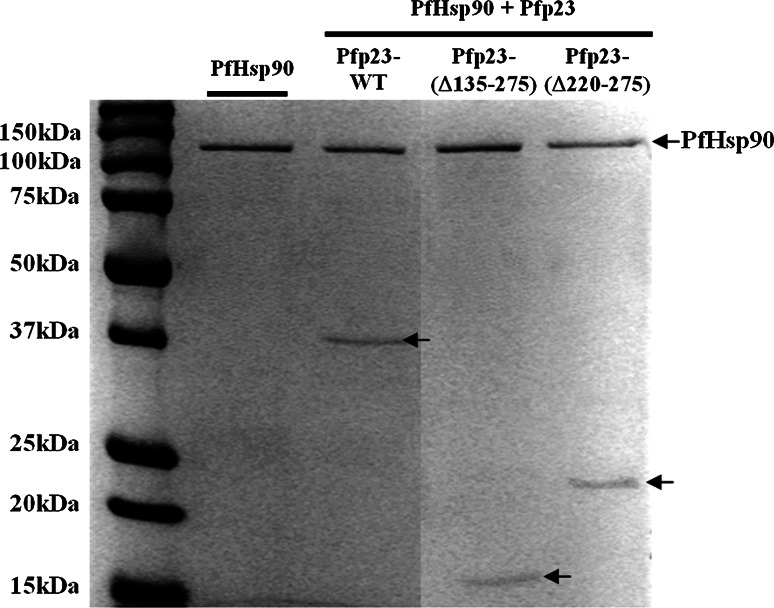
The protein sequence of Pfp23 contains GNMGGLx7 sequence repeats, which have yet to be functionally characterized. To determine if the sequence repeats are important for PfHsp90 interaction, two additional truncated mutants, Pfp23-(Δ220–275) and Pfp23-(Δ135–275), were constructed. The Pfp23-(Δ220–275) mutant contained the GNMGGLx7 sequence repeats but lacked the C-terminal tail, whereas Pfp23-(Δ135–275) had both the sequence repeats and the C-terminal tail deleted (Fig. 8). SDS-PAGE analysis showed that Pfp23-(Δ220–275) retained interaction with PfHsp90. Similarly, further removal of the GNMGGLx7 sequence repeats in Pfp23-(Δ135–275) did not affect the mutant’s competency in binding PfHsp90. Hence, the results indicated that the GNMGGLx7 sequence repeats are not essential for PfHsp90 interaction.
Fig. 8.
SDS-PAGE analyses of Pfp23-(Δ220–275) and Pfp23-(Δ135–275) interactions with PfHsp90. To investigate the importance of the GNMGGLx7 sequence repeats in Pfp23 for PfHsp90 interaction, Pfp23-(Δ135–275) was constructed. Using Pfp23-(Δ220–275) mutant, the C-terminal tail of Pfp23 was shown to be dispensable for PfHsp90 interaction. Further deletion of the GNMGGLx7 sequence repeats in Pfp23-(Δ135-275) did not affect its binding to PfHsp90, indicating that the sequence repeats are not essential for PfHsp90 interaction
Pfp23 inhibits the ATPase activity of PfHsp90
The binding and hydrolysis of ATP are known to induce conformational changes in Hsp90, essential for the chaperoning of its client proteins [3–5]. To determine whether the binding of Pfp23 affects the hydrolysis of ATP in PfHsp90, and henceforth, the regulation of its chaperone function, ATPase assay was carried out.
Using a sensitive enzyme-coupled assay, PfHsp90 was shown to have an ATPase activity of 0.504 ± 0.044 μmolATP/min/μmolPfHsp90 at 37°C. The activity measured was sensitive to inhibition by radicicol, a specific Hsp90 ATPase inhibitor, and thus ensured that the activity detected was not attributed to other co-purified ATPase contaminants. The activity observed is approximately ten times more than the human Hsp90 ATPase activity (0.034–0.089 μmolATP/min/μmolHsp90) [10, 36], but comparable to that reported for yeast Hsp82 (0.40–1.05 μmolATP/min/μmolHsp82) [5, 6, 37, 38].
The effect of Pfp23 interaction on PfHsp90 ATPase activity was subsequently investigated by the addition of Pfp23 in increasing concentrations to the assay reactions (Fig. 9a). Introduction of an equimolar concentration of Pfp23 had a negligible effect on the ATPase activity of PfHsp90. However, when added in ten-fold molar excess, Pfp23 reduced the ATPase activity of PfHsp90 to approximately 20% of its original activity.
Fig. 9.
Effects of Pfp23-WT and mutants on PfHsp90 ATPase activity. a Pfp23 was added in increasing concentrations to the ATPase assay reactions containing a fixed amount of PfHsp90 (2 μM). The ATPase activity of PfHsp90 was inhibited by Pfp23 in a concentration-dependent manner. At ten-fold molar excess, Pfp23 inhibited the ATPase activity of PfHsp90 to approximately 20% of its original activity. b Pfp23 single mutants were tested for their ability to inhibit the ATPase activity of PfHsp90. Pfp23 single mutants known to have reduced binding to PfHsp90 (K91A, H93S, W94S and K96A) displayed a negligible inhibitory effect on its ATPase activity. However, Pfp23 single mutants (K92A and V95 N) that exhibited comparable binding to PfHsp90 as Pfp23-WT inhibited PfHsp90 ATPase activity to approximately 35% of its original activity. Pfp23-WT and mutants were added in ten-fold molar excess to PfHsp90 in all the assay reactions. Results shown are representative of two independent experiments
To further verify whether the ATPase inhibitory effect was due to the interaction of Pfp23 with PfHsp90, Pfp23 single mutants with differential PfHsp90 binding levels were used. As expected, Pfp23 single mutants (K91A, H93S, W94S and K96A), which displayed drastically reduced PfHsp90 binding, had little inhibitory effect on the ATPase activity of PfHsp90 (Fig. 9b). PfHsp90 retained approximately 90% of its ATPase activity in the presence of Pfp23 single mutants K91A, H93S and W94S, respectively. Nevertheless, Pfp23 single mutant K96A was able to suppress the ATPase activity of PfHsp90 to approximately 65% of its original activity. On the contrary, Pfp23 single mutants (K92A and V95 N) that have a binding capacity to PfHsp90 similar to Pfp23-WT significantly inhibited the ATPase activity of PfHsp90 to approximately 35% of its original activity. The results clearly demonstrated the inhibitory effect that Pfp23 binding has on the ATPase activity of PfHsp90, and this may have significance in the regulation of PfHsp90 chaperone function in vivo.
Discussion
Treatment of malaria is increasingly challenging with the emergence of new strains that are resistant to conventional anti-malaria drugs such as chloroquine and pyrimethamine-sulfadoxine [39, 40]. Coupled with the lack of efficacious vaccines, there is a need to search for new anti-malarials and drug targets. Recent studies have recognized the potential of targeting PfHsp90 for malaria therapy as its inhibition effectively arrests the intra-erythrocytic development of P. falciparum [23, 41, 42]. Nevertheless, there are some considerations on the use of Hsp90-specific inhibitors as anti-malarials. How selective inhibition of PfHsp90 can be achieved in humans remains a concern since the Hsp90s in the two organisms are fairly conserved, with 63% amino acid identity [43]. Furthermore, the paucity of knowledge on the Hsp90 chaperone system in P. falciparum hinders the understanding of its function in the malaria parasite. Hence, identifying factors that regulate the function of PfHsp90 and its affinity for the inhibitors is imperative to facilitate the assessment of its relevance as a drug target.
One of the factors that influences Hsp90 activity and sensitivity to the inhibitors is the binding of co-chaperones [44–48]. However, thus far, PfHsp90 has only been demonstrated to form complexes with P. falciparum Hsp70, PP5, PKBP35 and α-tubulin [23, 49, 50]. It is surprising that homologues of co-chaperones (e.g., p23, Hop), which are known to be essential in regulating the chaperone function of Hsp90 in human and yeast [2], were not detected in those PfHsp90 complexes isolated. Inevitably, this has raised doubts about the functional conservation of these putative co-chaperones in P. falciparum. As the interaction between p23 and Hsp90 is essential for client protein activation, and recently has been shown to influence the sensitivity of yeast to Hsp90-specfic inhibitors [44], the interaction status of their homologues in P. falciparum may have implications on the modus operandi of PfHsp90 and its affinity for the drugs. Hence, in this study, the putative co-chaperone Pfp23 was investigated for its functionality and interaction with PfHsp90.
The purified Pfp23 recombinant protein was shown to possess intrinsic chaperone activity by virtue of its ability to suppress the aggregation of citrate synthase at 45°C. However, removal of the C-terminal tail of Pfp23 resulted in the loss of this anti-aggregation activity and attests to the need to have a full-fledged Pfp23 protein to elicit the chaperone activity. Pfp23 was subsequently probed for its interaction with PfHsp90 to assess its potential role as a co-chaperone in P. falciparum. Using GST pull-down assays, Pfp23 was observed to bind PfHsp90 in the presence of MgCl2 and ATP. The conditions conducive for binding were similar to that of the human and yeast homologues [7, 8], where the Mg2+ ion is essential as a cofactor for ATP binding in Hsp90 [51]. Truncation experiments indicated that the unique GNMGGLx7 sequence repeats in Pfp23 are dispensable for PfHsp90 interaction. Instead, when coupled to computational analyses, the results from the truncation study suggested that the 91st to 96th amino acid residues in Pfp23 may form an essential interface for binding PfHsp90. This interface (consisting of amino acid residues K91, K92, H93, W94, V95 and K96) is located at an extended loop of Pfp23 and was observed to be in proximity to the closed ‘lid’ segment of PfHsp90 (Fig. 6a). Site-directed mutagenesis experiments affirmed the importance of residues K91, H93, W94 and K96 for PfHsp90 interaction. Their respective single amino acid substitutions, K91A, H93S, W94S and K96A, resulted in drastically reduced binding to PfHsp90. However, Pfp23 mutants with K92A or V95 N substitution displayed PfHsp90 binding levels comparable to Pfp23-WT. The effects of these mutations were analyzed by computationally modeled structures to further comprehend the molecular forces that are involved in their interaction.
With the aid of modeled tertiary structures, the side chains of K91, H93, W94 and K96 in Pfp23 were observed to be projected towards the closed ‘lid’ segment of PfHsp90 (Fig. 6b). Under the in vitro binding conditions used (at pH 7.5), the side chain of the histidine residue (H93) would be uncharged, suggesting that the main force of interaction is hydrophobic in nature. This was supported by the observation that its side chain is located close to the hydrophobic residues F104 and I114, of PfHsp90 (Fig. 6b). Similar hydrophobic interaction would be expected of Pfp23 residue W94, which was visualized to be in proximity to I108 and the aliphatic side chain of M105 in PfHsp90. Conceivably, substitutions of H93 and W94 to a polar serine residue, respectively, would disrupt the hydrophobic interaction and result in the diminished binding to PfHsp90, as observed in this study.
Apart from that, in silico analyses of the Pfp23 residues, K91 and K96, did not reveal any electrostatic interaction between the charged group in the lysine side chains with PfHsp90 residues. This is consistent with the observation that the interaction between Pfp23 and PfHsp90 was stable at high salt concentrations of up to 300 mM KCl (data not shown), suggesting that electrostatic force plays a minor role in their interaction. Instead, results from the mutation study suggest that the long aliphatic side chains of the lysine residues may be important in contributing to the hydrophobic interaction between the Pfp23 residues, H93 and W94, with PfHsp90. Substitution to an alanine with a much shorter side chain would greatly diminish the hydrophobicity and proximity required for binding PfHsp90, and this may account for the lack of PfHsp90 interaction as observed.
On the contrary, the Pfp23 mutant K92A retained a similar PfHsp90 binding capacity to that of the Pfp23-WT. This implies that the residue K92 is not essential for PfHsp90 interaction. Likewise, replacing V95 with an asparagine resulted in a mutant that retained comparable binding to PfHsp90 as the Pfp23-WT. This result is surprising as the corresponding mutation in yeast SbaI (I117N) had rendered it defective in binding Hsp82 [35]. However, using the modeled structures, the side chain of V95 was observed to be projected away from PfHsp90. In view of this, Pfp23 mutant V95N's ability to retain its binding to PfHsp90 is likely to be attributed to the lack of interaction between the amino acid residue V95 and PfHsp90.
The results of the truncation study have also highlighted that a large segment of Pfp23’s N-terminal domain (1st to 74th amino acid residues) can be deleted without affecting its interaction with PfHsp90. This segment corresponds to the region in SbaI (1st to 93rd amino acid residues) where three other Hsp82-interacting interfaces (amino acid residues 13–16, 31–37, 85–91) were found [14]. It is difficult to postulate whether the deletion of these interfaces in SbaI (similar to that of Pfp23-truncated mutants T4) would affect Hsp82 interaction, as only a few amino acid residues located at these interfaces were conserved at the corresponding positions in Pfp23.
Probing the significance of Pfp23 binding on PfHsp90 function, results from the ATPase assays revealed that Pfp23 is capable of suppressing the hydrolysis of ATP by PfHsp90. The ATPase inhibitory effect of Pfp23 is positively correlated with its binding capacity to PfHsp90. Pfp23 single mutants (K91A, H93S, W94S and K96A) that were defective in binding PfHsp90 were unable to inhibit PfHsp90 ATPase activity as effectively as Pfp23-WT. The observation is consistent with that reported for the human and yeast homologues [10, 11, 52], and it was suggested that the binding of p23 traps Hsp90 in the ATP-bound state and prolongs its interaction with the client proteins. Nevertheless, it is noteworthy that the ATPase inhibitory effect was only observed when Pfp23 was added in large molar excess. While this may indicate limitations of the in vitro environment for stable binding of Pfp23 to PfHsp90, it may also highlight the transient interaction between the two proteins. Coupled with semi-quantitative mass spectrometry data that suggested that Pfp23 is present at a low physiological concentration in P. falciparum [53, 54], these observations could explain why previous studies were unsuccessful in identifying the PfHsp90-Pfp23 complex through co-immunoprecipitation techniques [20, 23].
With the recognition of PfHsp90 as a potential drug target against malaria, there is increasing interest in the development of assays for testing Hsp90-specific inhibitors as anti-malarials. In a recent study, PfHsp90 was shown to be capable of supporting the survival of a yeast strain with both Hsp90 genes (Hsc82 and Hsp82) deleted, which is otherwise lethal [42]. This has led to the proposal of exploiting the viable yeast strain complemented with PfHsp90 to screen for inhibitors that are selective against the parasite Hsp90. While this method offers the advantage of an in vivo setting for drug screening, the slower growth profile reported may reflect non-optimal heterologous interaction between PfHsp90 and the yeast co-chaperones and client proteins. Since the binding of co-chaperones has a major influence on the affinity of Hsp90 for the inhibitors [44–48], it alerts us to the need to assess the sensitivity of PfHsp90 to the inhibitors in the presence of native P. falciparum co-chaperones, using a priori an in vitro assay system. Providing an experimentally endorsed PfHsp90 chaperone complex is important to set the stage correctly for testing inhibitors against this protein consortium in P. falciparum. To this end, it is thus essential to determine and validate experimentally the interaction between PfHsp90 and its putative co-chaperones, particularly that of p23 with its underscored regulatory function and influence on Hsp90’s affinity for the inhibitors [44, 55].
Taken together, this study demonstrated that the P. falciparum genome encodes a functional p23 homologue and has provided valuable evidence concerning its in vitro interaction with PfHsp90. The inhibitory effect that Pfp23 exerts on the ATPase activity of PfHsp90 further highlighted its potential regulatory role on the chaperone function of PfHsp90 in vivo. In addition, truncation experiments and site-directed mutagenesis demonstrated significant roles played by certain amino acid residues in Pfp23, i.e., K91, H93, W94 and K96 in PfHsp90 interaction. Analyses of the modeled structures further revealed these residues to be strategically located at an extended loop of Pfp23 that presumably interacts with the closed ‘lid’ segment of PfHsp90 via hydrophobic forces. As the interaction between Pfp23 and PfHsp90 was not observed in previous studies [20, 23], this may underline the technical difficulties in using co-immunoprecipitation to identify PfHsp90 complexes. In view of this, a similar GST pull-down approach would be useful and may be adopted to investigate the interactions between PfHsp90 and other putative co-chaperones (e.g., Hop), which were also not detected in previous studies.
References
- 1.Johnson JL, Toft DO. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J Biol Chem. 1994;269:24989–24993. [PubMed] [Google Scholar]
- 2.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 3.Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- 4.Grenert JP, Johnson BD, Toft DO. The importance of ATP binding and hydrolysis by Hsp90 in formation and function of protein heterocomplexes. J Biol Chem. 1999;274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- 5.Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan W, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri ES, Litwack G, Toft D. Nucleotides and two functional states of hsp90. J Biol Chem. 1997;272:8007–8012. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 8.Fang Y, Fliss AE, Rao J, Caplan AJ. SBA1 encodes a yeast Hsp90 cochaperone that is homologous to vertebrate p23 proteins. Mol Cell Biol. 1998;18:3727–3734. doi: 10.1128/mcb.18.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan WP, Owen BA, Toft DO. The influence of ATP and p23 on the conformation of hsp90. J Biol Chem. 2002;277:45942–45948. doi: 10.1074/jbc.M207754200. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, Grossmann JG, Laue ED, Robinson CV, Jackson SE. The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Biol. 2006;356:746–758. doi: 10.1016/j.jmb.2005.11.085. [DOI] [PubMed] [Google Scholar]
- 11.Richter K, Walter S, Buchner J. The co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J Mol Biol. 2004;342:1403–1413. doi: 10.1016/j.jmb.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 12.Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem. 1997;272:21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- 13.Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- 14.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715. [DOI] [PubMed] [Google Scholar]
- 16.Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- 17.Weikl T, Abelmann K, Buchner J. An unstructured C-terminal region of the Hsp90 co-chaperone p23 is important for its chaperone function. J Mol Biol. 1999;293:685–691. doi: 10.1006/jmbi.1999.3172. [DOI] [PubMed] [Google Scholar]
- 18.Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- 19.Toogun OA, Zeiger W, Freeman BC. The p23 molecular chaperone promotes functional telomerase complexes through DNA dissociation. Proc Natl Acad Sci USA. 2007;104:5765–5770. doi: 10.1073/pnas.0701442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiser MF. A Plasmodium homologue of cochaperone p23 and its differential expression during the replicative cycle of the malaria parasite. Parasitol Res. 2003;90:166–170. doi: 10.1007/s00436-003-0929-z. [DOI] [PubMed] [Google Scholar]
- 21.Wiser MF. Proteolysis of a 34 kDa phosphoprotein coincident with a decrease in protein kinase activity during the erythrocytic schizont stage of the malaria parasite. J Eukaryot Microbiol. 1995;42:659–664. doi: 10.1111/j.1550-7408.1995.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 22.Wiser MF, Plitt B. Plasmodium berghei, P. chabaudi, and P. falciparum: similarities in phosphoproteins and protein kinase activities and their stage specific expression. Exp Parasitol. 1987;64:328–335. doi: 10.1016/0014-4894(87)90043-9. [DOI] [PubMed] [Google Scholar]
- 23.Banumathy G, Singh V, Pavithra SR, Tatu U. Heat shock protein 90 function is essential for Plasmodium falciparum growth in human erythrocytes. J Biol Chem. 2003;278:18336–18345. doi: 10.1074/jbc.M211309200. [DOI] [PubMed] [Google Scholar]
- 24.Lye YM, Chan M, Sim TS. Pfnek3: an atypical activator of a MAP kinase in Plasmodium falciparum. FEBS Lett. 2006;580:6083–6092. doi: 10.1016/j.febslet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Loke P, Sim TS. Mutational analysis of tyrosine-191 in the catalysis of Cephalosporium acremonium isopenicillin N synthase. J Biochem. 2000;127:585–589. doi: 10.1093/oxfordjournals.jbchem.a022644. [DOI] [PubMed] [Google Scholar]
- 26.Baca AM, Hol WG. Overcoming codon bias: a method for high-level overexpression of Plasmodium and other AT-rich parasite genes in Escherichia coli . Int J Parasitol. 2000;30:113–118. doi: 10.1016/S0020-7519(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 27.Loke P, Sim TS. A comparison of three site-directed mutagenesis kits. Z Naturforsch C. 2001;56:810–813. doi: 10.1515/znc-2001-9-1021. [DOI] [PubMed] [Google Scholar]
- 28.Chin HS, Goo KS, Sim TS. A complete library of amino acid alterations at N304 in Streptomyces clavuligerus deacetoxycephalosporin C synthase elucidates the basis for enhanced penicillin analogue conversion. Appl Environ Microbiol. 2004;70:607–609. doi: 10.1128/AEM.70.1.607-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchner J, Grallert H, Jakob U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Meth Enzymol. 1998;290:323–338. doi: 10.1016/S0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- 30.Ali JA, Jackson AP, Howells AJ, Maxwell A. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry. 1993;32:2717–2724. doi: 10.1021/bi00061a033. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campanella JJ, Bitincka L, Smalley J. MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics. 2003;4:29. doi: 10.1186/1471-2105-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guex N, Diemand A, Peitsch MC. Protein modelling for all. Trends Biochem Sci. 1999;24:364–367. doi: 10.1016/S0968-0004(99)01427-9. [DOI] [PubMed] [Google Scholar]
- 34.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oxelmark E, Knoblauch R, Arnal S, Su LF, Schapira M, Garabedian MJ. Genetic dissection of p23, an Hsp90 cochaperone, reveals a distinct surface involved in estrogen receptor signaling. J Biol Chem. 2003;278:36547–36555. doi: 10.1074/jbc.M305960200. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human hsp90 by a client protein. J Mol Biol. 2002;315:787–798. doi: 10.1006/jmbi.2001.5245. [DOI] [PubMed] [Google Scholar]
- 37.Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter K, Reinstein J, Buchner J. N-terminal residues regulate the catalytic efficiency of the Hsp90 ATPase cycle. J Biol Chem. 2002;277:44905–44910. doi: 10.1074/jbc.M208457200. [DOI] [PubMed] [Google Scholar]
- 39.Bloland PB, Lackritz EM, Kazembe PN, Were JB, Steketee R, Campbell CC. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. J Infect Dis. 1993;167:932–937. doi: 10.1093/infdis/167.4.932. [DOI] [PubMed] [Google Scholar]
- 40.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF, Winstanley PA, Watkins WM, Nzila AM. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17:582–588. doi: 10.1016/S1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Musiyenko A, Barik S. The heat shock protein 90 of Plasmodium falciparum and antimalarial activity of its inhibitor, geldanamycin. Malar J. 2003;2:30. doi: 10.1186/1475-2875-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wider D, Peli-Gulli MP, Briand PA, Tatu U, Picard D. The complementation of yeast with human or Plasmodium falciparum Hsp90 confers differential inhibitor sensitivities. Mol Biochem Parasitol. 2009;164:147–152. doi: 10.1016/j.molbiopara.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, Pavithra SR, Tatu U. Three-dimensional structure of heat shock protein 90 from Plasmodium falciparum: molecular modelling approach to rational drug design against malaria. J Biosci. 2007;32:531–536. doi: 10.1007/s12038-007-0052-x. [DOI] [PubMed] [Google Scholar]
- 44.Forafonov F, Toogun OA, Grad I, Suslova E, Freeman BC, Picard D. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol. 2008;28:3446–3456. doi: 10.1128/MCB.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes JL, Sharp SY, Hobbs S, Workman P. Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2008;68:1188–1197. doi: 10.1158/0008-5472.CAN-07-3268. [DOI] [PubMed] [Google Scholar]
- 46.Piper PW, Millson SH, Mollapour M, Panaretou B, Siligardi G, Pearl LH, Prodromou C. Sensitivity to Hsp90-targeting drugs can arise with mutation to the Hsp90 chaperone, cochaperones and plasma membrane ATP binding cassette transporters of yeast. Eur J Biochem. 2003;270:4689–4695. doi: 10.1046/j.1432-1033.2003.03866.x. [DOI] [PubMed] [Google Scholar]
- 47.Piper PW, Panaretou B, Millson SH, Trumana A, Mollapour M, Pearl LH, Prodromou C. Yeast is selectively hypersensitised to heat shock protein 90 (Hsp90)-targetting drugs with heterologous expression of the human Hsp90beta, a property that can be exploited in screens for new Hsp90 chaperone inhibitors. Gene. 2003;302:165–170. doi: 10.1016/S0378-1119(02)01102-2. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Masison DC. Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Sti1 (Hop1) J Biol Chem. 2005;280:34178–34185. doi: 10.1074/jbc.M505420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobson S, Kar B, Kumar R, Adams B, Barik S. A novel tetratricopeptide repeat (TPR) containing PP5 serine/threonine protein phosphatase in the malaria parasite, Plasmodium falciparum . BMC Microbiol. 2001;1:31. doi: 10.1186/1471-2180-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar R, Adams B, Musiyenko A, Shulyayeva O, Barik S. The FK506-binding protein of the malaria parasite, Plasmodium falciparum, is a FK506-sensitive chaperone with FK506-independent calcineurin-inhibitory activity. Mol Biochem Parasitol. 2005;141:163–173. doi: 10.1016/j.molbiopara.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 52.Siligardi G, Hu B, Panaretou B, Piper PW, Pearl LH, Prodromou C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J Biol Chem. 2004;279:51989–51998. doi: 10.1074/jbc.M410562200. [DOI] [PubMed] [Google Scholar]
- 53.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 54.Prieto JH, Koncarevic S, Park SK, Yates J, 3rd, Becker K. Large-scale differential proteome analysis in Plasmodium falciparum under drug treatment. PLoS One. 2008;3:e4098. doi: 10.1371/journal.pone.0004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felts SJ, Toft DO. p23, a simple protein with complex activities. Cell Stress Chaperones. 2003;8:108–113. doi: 10.1379/1466-1268(2003)008<0108:PASPWC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]