Abstract
We previously demonstrated the antiproliferative and antiangiogenic effects of 3-methylcholanthrene (3MC), an aryl-hydrocarbon receptor (AhR) agonist, in human umbilical vascular endothelial cells (HUVECs). Herein, we unraveled its molecular mechanisms in inhibiting HUVEC motility. 3MC down-regulated FAK, but up-regulated RhoA, which was rescued by AhR knockdown. It led us to identify novel AhR binding sites in the FAK/RhoA promoters. Additionally, 3MC increased RhoA activity via suppression of a negative feedback pathway of FAK/p190RhoGAP. With an increase in membrane-bound RhoA, subsequent stress fiber and focal adhesion complex formation was observed in 3MC-treated cells, and this was reversed by a RhoA inhibitor and AhR antagonists. Notably, these compounds significantly reversed 3MC-mediated anti-migration in a transwell assay. The in vitro findings were further confirmed using an animal model of Matrigel formation in Balb/c mice. Collectively, AhR’s genomic regulation of FAK/RhoA, together with RhoA activation, is ascribable to the anti-migration effect of 3MC in HUVECs.
Keywords: 3-Methylcholanthrene, Aryl-hydrocarbon receptor, Angiogenesis, Cell motility, Adhesion, Focal adhesion kinase (FAK), Resveratrol, Alpha-naphthoflavon (alpha-NF)
Introduction
Angiogenesis, the formation of new blood vessels from preexisting vessels, is a highly coordinated process required for normal development and wound healing. Key components of angiogenesis are the tightly regulated proliferation of endothelial cells and cell motility. Major steps in cell migration include the following: the internal cellular cytoskeleton pushes forward a part of the cell (a “pseudo-pod” or a false leg); adhesion molecules on the cell surface bind to extracellular matrix (ECM) ligands and form links to the cytoskeleton; cells contract from the edges towards the nucleus; and adhesion receptors are released from the cytoskeleton, thus detaching the cell from its previous attachment sites [1].
Cell migration is largely regulated by the formation or disassembly of focal adhesions, which is related to the organization of the actin-containing cytoskeleton. Focal adhesions promote cell adherence to the ECM via specific integrin molecules at the attaching surface of cultured cells which promote cell motility. One major mechanism of signal transduction utilized by integrins involves tyrosine phosphorylation of focal adhesion-associated proteins [2, 3]. Focal adhesion kinase (FAK), a non-receptor protein tyrosine kinase, is associated with the cytoskeleton and with integrin-signaling complexes by binding to Src kinase and paxillin [4, 5]. FAK is rapidly tyrosine-phosphorylated upon cell adhesion, creating a high-affinity binding site for Src and thereby increasing phospholipase C (PLC)γ enzymatic activity [6]. Genetic ablation of FAK results in early embryonic lethality in mice, and cells derived from these knockout embryos demonstrate severe migration and survival defects [7]. Conversely, enhanced FAK signaling up-regulates cell motility and promotes cell survival in an anchorage-independent manner. Additionally, it was revealed that the N-terminal domain of FAK interacts with both STAP-2 and CbI. These interactions may allow STAP-2 to recruit Cbl to FAK, thereby leading to enhancement of the ubiquitin-dependent degradation of FAK [8]. Furthermore, the phosphatase and tensin homolog (PTEN), a key molecule implicated in integrin signaling pathways, interacts with FAK, and inactivates FAK by dephosphorylation [9].
The cytoskeletal reorganization of actin microfilaments is triggered by regulating members of the Rho family of GTPase, such as Rho, Rac, and Cdc42 [10]. At the leading edge of the migrating cell, Rac and Cdc42, respectively regulate lamellipodia and filopodia formation. Rho is required for the formation and maintenance of focal adhesions [11]. Rho GTPases exist in either an inactive GDP-bound or an active GTP-bound form. Two main regulatory proteins interact with Rho GTPases to facilitate an active or inactive conformation: guanine nucleotide exchange factors (GEFs) stimulate the GDP–GTP exchange reaction, and GTPase-activating proteins (GAPs) promote hydrolysis of GTP to GDP [12]. It was demonstrated that RhoA/Rho-dependent kinase (ROCK, a downstream effector of RhoA)-coupled signaling, is essential for controlling cell apoptosis, migration, and angiogenesis [13]. ROCK activates myosin light chain (MLC) phosphorylation through activation of MLC kinase or inactivation of MLC phosphatase. The activation of MLC results in an increase in actomyosin-based contractility, which is important for cell migration [14]. Bartolome et al. [15] showed that activated G(alpha)13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity.
However, the roles of RhoA and ROCK in cell migration are controversial. For instance, it was shown that FAK ablation causes a RhoA-mediated decrease in focal adhesion turnover and consequently eliminates cell migration in FAK−/− primary mouse embryo fibroblasts [7], which is reversed in FAK+/+ cells [16]. Additionally, the pathological consequence of RhoA activation was shown to cause a thrombin-induced increase in endothelial permeability [12] and to increase resistance of aqueous humor outflow in endothelial-like trabecular cells [17].
We previously reported that 3MC not only decreases cell proliferation of human umbilical vascular endothelial cells (HUVECs) by p21 and p27 induction [18] but also eliminates cell adhesion signaling by inhibiting FAK phosphorylation [19]. Herein, we attempted to illustrate the molecular mechanism of 3MC-mediated FAK/RhoA alteration and its downstream target molecules involved in the anti-motility of vascular endothelial cells both in vitro and in vivo.
Materials and methods
Endothelial cell culture
HUVECs were harvested from human umbilical veins by enzymatic dissociation as previously described [20]. Cells were grown in medium 199 (M199; Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS), endothelial cell growth supplement (ECGS, 0.03 mg/ml; Biomedical Technologies, Stoughton, MA, USA), heparin (50 U/ml; Sigma), HEPES (10 mM; Sigma), and kanamycin (0.1 mg/ml; Bioshop, Burlington, ON, Canada) in gelatin-coated plates, and incubated at 37°C in 5% CO2 in air. Cells from passages 5–9 were used.
Analysis of gene expression by reverse-transcriptase polymerase chain reaction (RT-PCR) and Western blot analysis
Total cellular RNA was extracted from cultured cells using Trizol reagent (Sigma). The concentration of total RNA was measured using spectrophotometry. RNA (2 μg) was converted to complementary (c)DNA by a SuperScript™ III Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. cDNA (2 μl) was used in a total 30-μl reaction volume as a template for amplification in the PCR. The PCR was performed under standard conditions in 30 μl (20 mM Tris–HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTP, 200 pM of each primer, and 1 U Taq DNA polymerase). The PCR conditions were 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, 55°C for 45 s, and 72°C for 45 s. The PCR was carried out with specific primers (5′-CGTGAAGCCTTTTCAAGGAG-3′ and 5′-TCCATCCTCATCCGTTCTTC-3′ for FAK, 5′-AAGGACCAGTTCCCAGAGGT-3′ and 5′-TTCTGGGGTCCACTTTTCTG-3′ for RhoA, and 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for GAPDH). Antibodies for pFAK (Biosource, Camarillo, CA, USA), FAK, P190RhoGAP (BD BioSciences PharMingen, San Diego, CA, USA), pMLC, MLC, RhoA, pTyrosine (Santa Cruz Biotechnology, Santa Cruz, CA, USA), pSRC, SRC (Millipore, Burlington, MA, USA), AhR (Biomol Research Laboratories, Plymouth Meeting, PA, USA), ve-cadherin, vinculin (Sigma-Aldrich, St. Louis, MO, USA), pSerine (Chemicon, Temecula, CA, USA), and rhodamine-phalloidin (Molecular Probes, Eugene, OR, USA.) were included in the assay. The cell lysate (30 μg) was electrophoresed on an 8–10% sodium dodecylsulfate (SDS)-polyacrylamide gel and then transblotted onto a Hybond-P membrane (GE Healthcare, Hong Kong). The subsequent procedures were described elsewhere [18, 19].
Cell membrane fractionation
Cells treated with 3MC for the indicated times were collected and incubated in 0.1 ml of hypotonic buffer (10 mM Tris (pH 7.5), 0.5 mM EDTA, and 2 mM phenylmethylsulfonyl fluoride) at 4°C for 30 min. After centrifugation, the supernatant (cytosolic fraction) was collected, and the pellet was resuspended in 0.1 ml of radioimmune precipitation assay (RIPA) buffer and incubated at 4°C for 30 min. The resulting fractions were sheared through an insulin syringe with a 29 Gauss needle 100 times. After centrifugation, the supernatant (membrane fraction) was collected for analysis.
Fluorescence microscopy
Cells grown overnight on glass coverslips were treated with or without 100 nM of 3MC for 1 h, then washed once with cold phosphate-buffered saline (PBS) and fixed for 10 min in 4% paraformaldehyde. Cells were then permeabilized by treatment with 0.1% Triton X-100 and 0.05% Tween 20 in PBS. Coverslips were blocked in 10% goat serum at room temperature for 60 min, then stained with rhodamine-phalloidin (ICN Immunobiologicals, Costa Mesa, CA, USA) in a 1:100 dilution or with anti-vinculin at 1:100 (Sigma-Aldrich) overnight at 4°C followed by Texas red-conjugated goat anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 1:100 for 60 min at room temperature. Coverslips were mounted on slides with Vectashield anti-fade (Vector) diluted 1:1 with PBS, and images were obtained with a DMI 6000B CS laser confocal microscope (Leica) using an HCX PL APO l-blue 63x/1.40-0.60 NA oil-immersion objective lens for HUVECs. Images were acquired with a CM350 CCD camera (Applied Precision, Issaquah, WA, USA) using TCS SP5 confocal spectral microscope imaging system software (Leica) and processed with Photoshop 7.0 software (Adobe System, San Diego, CA, USA).
Co-immunoprecipitation (CoIP) for protein interactions among cell motility-related molecules
HUVECs grown in 10-cm dishes with or without 100 nM of 3MC for 1 h were lysed, and pellets were collected to detect interactions among cell motility-related molecules. Briefly, cell lysates were precleared with the addition of protein A-agarose beads for 60 min at 4°C, and then the protein concentrations of the samples were analyzed. Protein (200 μg) was incubated with anti-RhoA or anti-FAK antibodies (2 μg) and protein A plus G agarose beads (20 μg) at 4°C overnight. The precipitates were washed five times with lysis buffer and once with PBS. The pellet was then resuspended in sample buffer (50 mM Tris (pH 6.8), 100 mM bromophenol blue, and 10% glycerol) and incubated at 90°C for 10 min before electrophoresis to release the proteins from the beads. Each sample was then electrophoresed on 10.0% SDS-PAGE gels and transferred to a polyvinylidene difluoride (PVDF) membrane for western blotting with specific antibodies, as described previously [18].
Chromatin immunoprecipitation (ChIP) assay
A ChIP assay was performed according to the instructions of Upstate Biotechnology (Lake Placid, NY, USA) with minor modifications. Briefly, 6 × 105 cells were cultured in 100-mm dishes and treated with or without 3MC for 0–90 min. HUVECs were cross-linked by adding 1% (v/v) formaldehyde, and incubated at room temperature for 10 min, followed by adding glycine to a final concentration of 0.125 M to stop the reaction. Cells were washed twice with ice-cold PBS, re-suspended, and homogenized in 0.5 ml swelling buffer (5 mM PIPES pH 8.0, 0.5% Triton X-100, 0.5 mM PMSF) plus Complete Protease Inhibitor (Roche, Germany). Nuclei pellet was sonicated using Ultrasonics Sonicator at a power setting of 5 for 15 times with 5-s pulse to shear DNA to an average length between 200 and 1,000 bps in 0.5 ml sonication buffer (10 mM EDTA, 50 mM Tris–HCl pH 8.0, 0.5 mM PMSF) containing protease inhibitors. Non-specific binding was reduced by adding 0.1% SDS with 85 mM KCl together. The resulting supernatant was subjected to overnight co-IP using either an anti-AhR antibody, or the same amount of a nonspecific antibody (mouse monoclonal α-tubulin (α-Tu); Sigma Chemical, St. Louis, MO, USA) as a negative control, followed by incubation with a salmon sperm DNA/protein G agarose slurry (Upstate, Charlottesville, VA, USA) to immobilize the DNA–protein–antibody complex. DNA–protein complexes were then eluted with 200 μl of elution buffer (Tris–EDTA buffer containing 1% sodium dodecylsulfate, SDS) for 30 min, and the cross-links were reversed by overnight incubation at 65°C. DNA was purified using a PCR purification kit (Qiagen, Hilden, Germany). CYP1A1, a well-known downstream target of AhR, contains a XRE flanking at −615 to −597 bp of its promoter region by MOTIF Search analysis, as a positive control for cells with 3MC challenge [21]. The DNA filtrates were amplified by PCR with primers flanking the promoter of the FAK, RhoA and CYP1A1 genes containing the putative AhR-binding sites (XREs): FAK forward primer, 5′-TCATTCCTCTGCCTTTTTGC-3′ and FAK reverse primer, 5′-TTTAAACACGGTAGGTACAATGC-3′; and RhoA forward primer, 5′-TGCCCAGGAGACTTAACACC-3′ and RhoA reverse primer, 5′-CAGTCATGGCGGAGTCCT-3′, CYP1A1 forward primer, 5′-TCTGAGTCCTGGCTCTGTCA-3′, and CYP1A1 reverse primer, 5′-CAAGGTGTATCACCCCGAGT-3′. Additionally, the template was replaced with double-distilled (dd)H2O as a negative internal control. The PCR products were electrophoresed on a 2% agarose gel, and PCR products of the expected sizes of 151 and 162 bp were visualized, quantified using an Image analysis system, and eluted from the agarose gel for sequencing to verify the site of amplification.
Electrophoretic mobility shift assay (EMSA)
The EMSA was performed as described previously [22] with minor modifications. To prepare the nuclear protein extracts, HUVECs in 10-cm2 dishes after treatment with 100 nM of 3MC for 1 h were subjected to NE-PER™ nuclear extraction reagents (Pierce, Rockford, IL, USA) with the addition of protease inhibitors. The subsequent procedures for the nuclear protein extraction followed the manufacturer’s instructions. The sequences of the oligonucleotides used were CATGACAATGGCGTAATGAGCCTAA and CATGACAAGGTATTCATGAGCATAA for the putative FAK XRE wild type and mutant, respectively, and TGAAGAGGGTCACGCGCATGTCCTG and TGAAG AGGGGAACTATCATGGACTG for the putative RhoA XRE wild type and mutant, respectively (the conserved and mutated sequences are shown in single- and double-underlines, respectively). The oligonucleotides were end-labeled with biotin according to the manufacturer’s protocol (Pierce Biotechnology, Rockford, IL, USA). Briefly, unlabeled oligonucleotides (1 μM) were incubated in TdT reaction buffer containing biotin-11-dUTP (0.5 μM) and TdT (0.2 U/μl) at 37°C for 30 min, followed by the addition of 2.5 μl EDTA (0.2 M, pH 8.0) to stop each reaction and 50 μl chloroform/isoamyl alcohol to extract the TdT. Extracted nuclear protein (10 μg) was incubated with biotin-labeled (1 pmol) probes at 15°C for 30 min in binding buffer containing 1 μg of poly deoxyinosinedeoxycytidine (dI-dC) (Panomics, Redwood City, CA, USA). For competition with unlabeled oligonucleotides, a 100-fold molar excess of unlabeled oligonucleotides relative to the biotin-labeled probes were added to the binding assay. The mixture were separated on a 6% nondenaturing polyacrylamide gel at 4°C in 1 × TBE (90 mM Tris borate, 2 mM EDTA, pH 8.3) and then transblotted onto a Hybond N+ membrane (Amersham Pharmacia Biotech, Freiburg, Germany). Blots were incubated with blocking buffer, followed by additional streptavidin-horseradish peroxidase (HRP) conjugates. Blots were imaged by means of an enhanced chemiluminescence system.
AhR small interfering (si)RNA preparation and transient transfection
An AhR siRNA (UUACUAUCUUGAAAGAGCCct) duplex was chemically synthesized by Ambion (Austin, TX, USA). HUVECs were seeded in a 6-well plate and transfected with either 100 pmole of AhR siRNA, scrambled control siRNA (#4611, Ambion), or GAPDH siRNA (#4624, Ambion) in a 100-μl volume with siPORT™NeoFX™. The efficiency of siRNA silencing was analyzed by RT-PCR and western blotting after transfection for 24 h, followed by 100 nM of 3MC treatment for 1 h.
Transwell assay
Cell migration assays were performed using a 24-well microchemotaxis chamber (Costar, Cambridge, MA, USA) [23]. Polyvinylpyrrolidone-free polycarbonate filters with a pore size of 8 μm (Nuclepore, North Point, Hong Kong) were coated with 1 mg/ml collagen for 30 min in a 37°C incubator. 3-MC at 1 mM was diluted to appropriate concentrations in M199 supplemented with 10% FBS, and 600 μl of the final dilution was placed in the lower chamber of a microchemotaxis chamber. Confluent cell cultures were washed and trypsinized for the minimum time required to achieve cell detachment. After placing the filter between the lower and upper chambers, 2.5 × 104 cells suspended in 100 μl of M199 containing 2% FBS and indicated concentrations of 3MC were seeded in the upper compartment. The apparatus was then incubated for 16 h at 37°C in a humidified chamber with 5% CO2 to allow cell migration. After the incubation period, the filter was removed, and cells on the upper side of the filter which had not migrated were scraped away with a cotton applicator. The filter was fixed for 30 min at room temperature with 4% paraformaldehyde, washed twice with PBS and stained with crystal violet. The number of cells that had migrated through to the lower surface of each membrane was counted. Each experimental point was repeated six times.
Matrigel angiogenesis assay
The Matrigel angiogenesis assay was performed as described before [24]. Briefly, 500 μl of a liquid mixture of Matrigel (350 μl; BD Biosciences, San Jose, CA, USA), M199 medium (150 μl), heparin (20 U/ml), and VEGF (200 ng/ml) was prepared and injected subcutaneously into 7-week-old Balb/c mice. After Matrigel formed a solid gel plug, mice were divided into four groups: DMSO as the control, 3MC alone, an AhR antagonist (resveratrol), and 3MC combined with resveratrol (n = 8). Mice were intraperitoneally given resveratrol (2 mg/kg) on day 2 or 3MC (2 mg/kg) on day 3 following implantation, and separate chemicals were given every other day for 2 weeks. The plugs allowed VEGF to stimulate angiogenesis. In 2 weeks, mice were sacrificed to remove the Matrigel plugs. Vascularization of plugs was quantified by measuring hemoglobin content using the Drabkin reagent (Sigma-Aldrich) according to the manufacturer’s protocol. All in vivo procedures were approved by the Taipei Medical University Animal Care and Use Committee.
Statistical analysis
Values are expressed as the mean ± SD. The significance of the difference from the control groups was analyzed by Student’s t test or one-way analysis of variance (ANOVA) and Bonferroni’s method as a post hoc test. A value of P < 0.05 was considered statistically significant.
Results
3MC-mediated alteration of FAK/RhoA at both the mRNA and protein levels
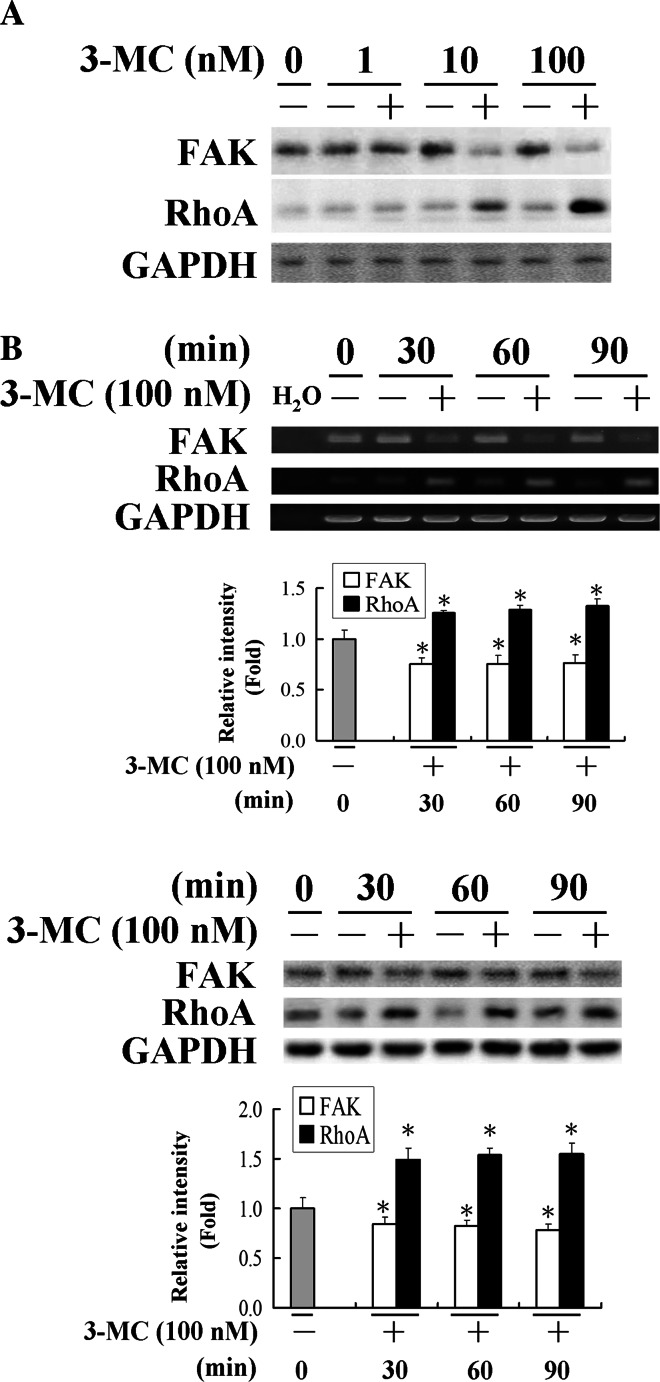
Our group previously showed [19] that inhibition of vascular angiogenesis is involved in the deactivation of FAK. To unravel the molecular mechanisms of its action, we investigated the concentration-dependent effect of 3MC on cell mobility-related molecules including FAK and RhoA by western blot (Fig. 1a) and the time course of their expression pattern at both the mRNA and protein levels (Fig. 1b). Cells were harvested from HUVECs treated with 1, 10 or 100 nM of 3MC for 1 h or with 100 nM of 3MC for 0–90 min, and analyzed by western blotting or RT-PCR analysis. The results in Fig. 1a showed that 3MC concentration-dependently altered FAK and RhoA protein levels that are inverted relative to each other by western blot analysis. Additionally, the results in Fig. 1b demonstrated that challenge of cells with 100 nM of 3MC for 0–90 min significantly suppressed FAK expression levels by approximately 20–30%, but increased the induction of RhoA by approximately 30–60%. Apparently, 3MC decreased FAK, but induced RhoA at both the mRNA and protein levels, which showed a reciprocal correlation (Fig. 1). However, there was no apparent change in other members of the Rho family of small GTPase such as Rac1 or Cdc42 in HUVECs with 3MC challenge (data not shown).
Fig. 1.
3-Methylcholanthrene (3MC)-mediated alteration in focal adhesion kinase (FAK)/RhoA in human umbilical vascular endothelial cells (HUVECs). a Concentration-dependent alteration of FAK/RhoA proteins by 3MC. Cells were treated with the indicated concentrations of 3MC for 1 h, and the expressions of FAK/RhoA were determined using GAPDH as an internal control. b Time-course alteration of FAK/RhoA by 3MC. HUVECs released from quiescence by incubation in culture media supplemented with 2% FBS were treated with 100 nM of 3MC or DMSO as the internal control and harvested at the indicated time points. FAK and RhoA levels were assayed by RT-PCR and western blotting, and GAPDH was used as an internal control to verify equivalent loading. A representative experiment is shown. Bar charts in the lower panel show the band intensity of FAK and RhoA by densitometry. Data were derived from three independent experiments and are presented as the mean ± SD. *Significantly different (P < 0.05 vs the control)
Alteration of the activities of FAK and RhoA in HUVECs challenged with 3MC
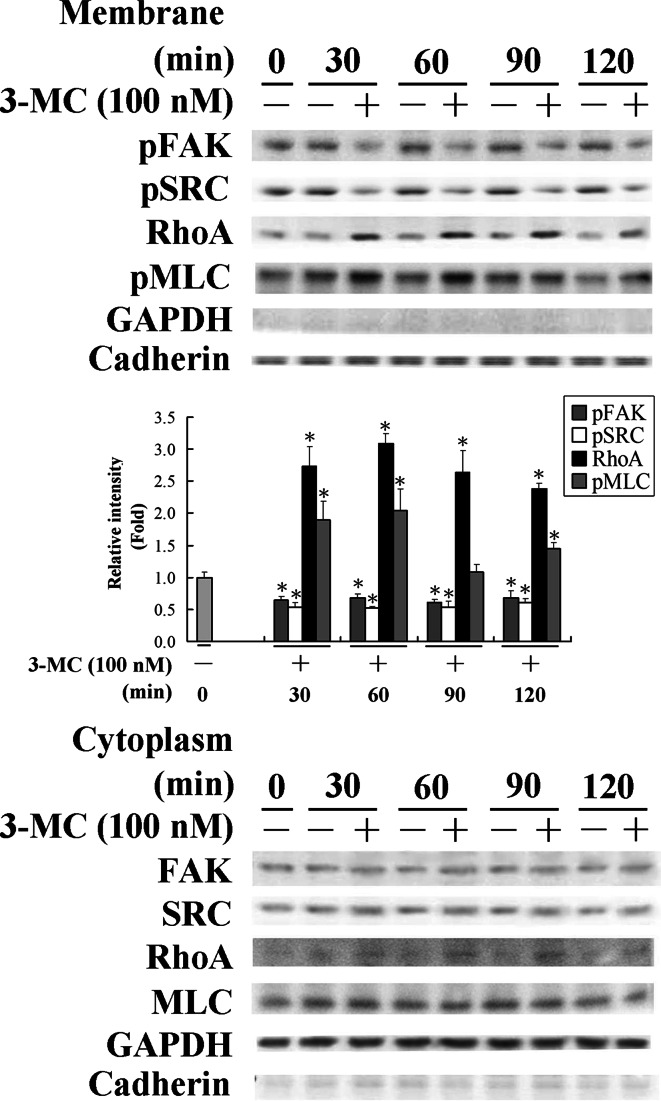
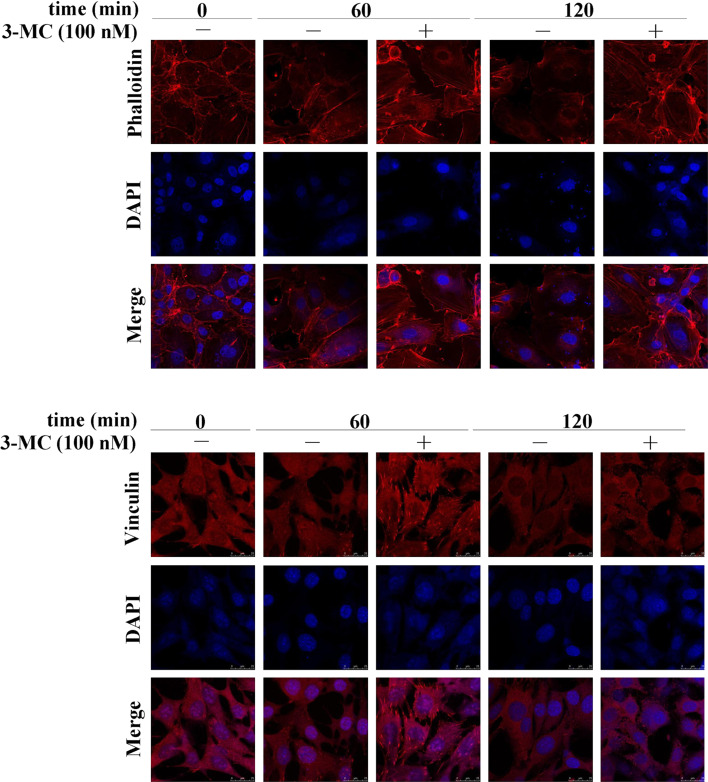
The results in Fig. 1 show that 3MC mediated the alteration in the mRNA and protein levels of FAK and RhoA. To further investigate their activities upon 3MC administration, cell lysates were partitioned into cytosol and membrane fractions. Notably, the activated form of FAK in membranes decreased by approximately 40%, and conversely, the activated form of RhoA anchored to membranes increased by approximately 2.5-fold in HUVECs treated with 3MC (Fig. 2). The decrease in FAK phosphorylation was accompanied by inhibition of SRC phosphorylation by approximately 50%, which is a docking protein for FAK autophosphorylation and in turn SRC phosphorylation. Additionally, the activation of RhoA by 3MC treatment was concomitant with the increase in phosphorylation of the MLC, a downstream effector of RhoA, by approximately 1- to 2-fold at the indicated time points. To further examine the consequence of RhoA activation by 3MC on the morphology of HUVECs, cells were stained with rhodamine-phalloidin for F-actin or incubated with an anti-vinculin antibody followed by a secondary antibody conjugated with Texas red for focal adhesion formation. 3MC-mediated RhoA activation obviously increased stress-fiber formation and the amount of focal adhesion complexes upon treatment for 60 min followed by cell round-up at 120 min of treatment, as examined by fluorescent confocal microscope (Fig. 3).
Fig. 2.
Altered activities of focal adhesion kinase (FAK)/RhoA in human umbilical vascular endothelial cells (HUVECs) challenged with 3-methylcholanthrene (3MC). Cells were treated with 3MC for 0–120 min, and cell lysates were partitioned into cytosolic and membranous fractions as described in “Materials and methods”. An anti-cadherin antibody was used to verify equivalent loading in the membrane fraction. Results are presented as the mean ± SD. of four independent experiments. *Significantly different (P < 0.05 vs the control)
Fig. 3.
Functional assessment of 3-methylcholanthrene (3MC)-mediated RhoA activation by (upper) stress fiber formation and (lower) focal adhesion complex formation in human umbilical vascular endothelial cells (HUVECs). Cells treated with or without 100 nM of 3MC for 0–120 min were detected by rhodamine-phalloidin for stress fiber formation and using an anti-vinculin antibody, followed by a Texas red-conjugated secondary antibody for focal adhesion complex formation. Cell morphology was photographed using a fluorescent confocal microscope, where red-colored filaments represent stress fiber formation (upper) and red-spotted patches indicate focal adhesion complex formation (lower). Identical fields stained for the formations of stress fibers and focal adhesion complexes were also stained using DAPI to reveal the positions of cell nuclei. Magnification ×630
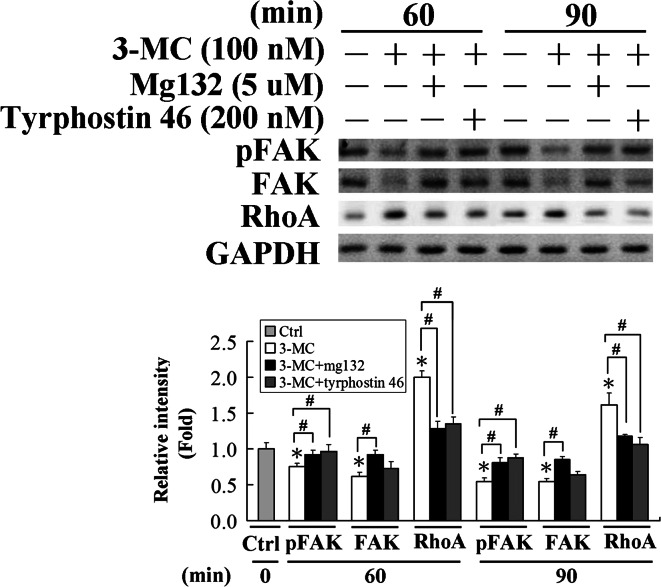
The causal relationship of FAK and RhoA in HUVECs with 3MC administration
Furthermore, the causal relationship of FAK and RhoA were examined using a S26 proteasome inhibitor, MG132, and a phosphatase inhibitor, tyrphostin 46, prior to cell exposure to 3MC for 0–90 min. As demonstrated in Fig. 4, FAK protein level was significantly increased in cells with addition of MG132 treatment, which in turn decreased the 3MC-mediated RhoA induction, implying that FAK negatively regulated the RhoA protein level. Likewise, the increase in FAK phosphorylation achieved by adding the phosphatase inhibitor, tyrphostin 46, obviously reversed 3MC-mediated RhoA induction, suggesting that, in addition to the FAK protein level, FAK activation is also involved in this event.
Fig. 4.
The interplay between focal adhesion kinase (FAK) and RhoA in human umbilical vascular endothelial cells (HUVECs) challenged with 3-methylcholanthrene (3MC). HUVECs were pretreated with S26 proteasome and phosphatase inhibitors, MG132 (5 μM) and tyrphostin 46 (200 nM), respectively for 1 h, prior to 3MC challenge for 0–90 min. pFAK, FAK, and RhoA of total cell lysates were analyzed by western blotting. Membranes were probed with an anti-GAPDH antibody to verify equivalent loading. Bar charts in the lower panel show the band intensities of FAK and RhoA by densitometry. Data were derived from three independent experiments and are presented as the mean ± SD. *Significantly different (P < 0.05 vs the control), #significantly different (P < 0.05 vs 3MC alone)
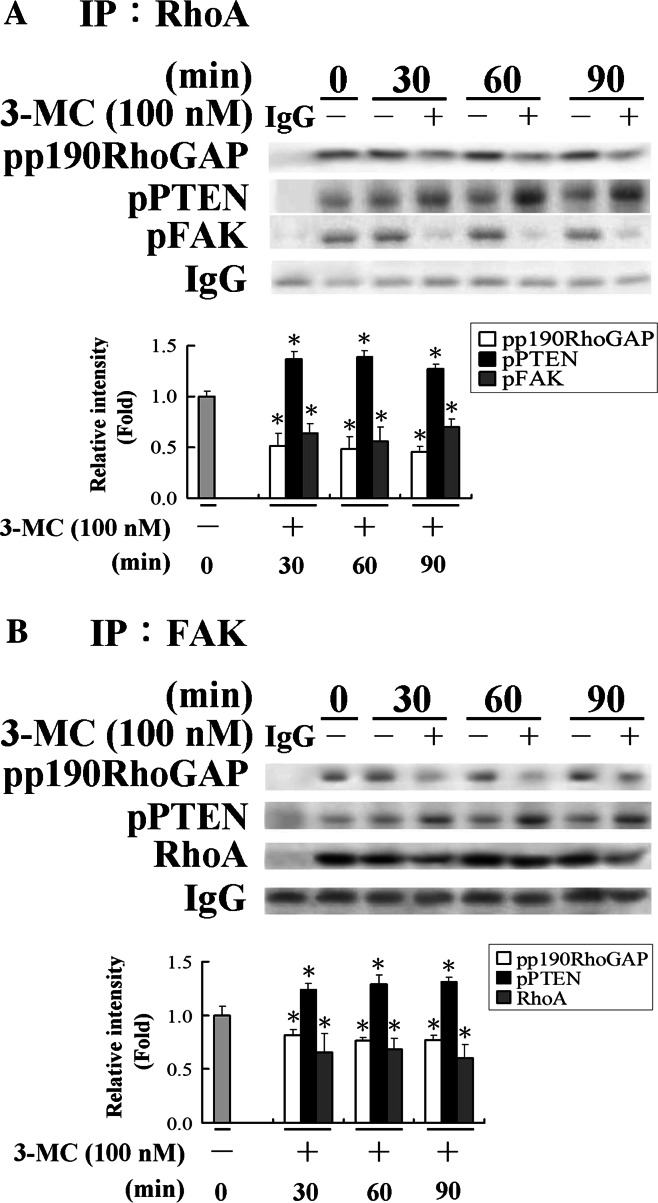
Alteration of the protein interaction pattern among angiogenic-related molecules in HUVECs challenged with 3MC
A growing body of evidence indicates protein interactions among FAK, PTEN (a phosphatase), and p190RhoGAP are involved in the activity regulation [25–28]. For instance, dephosphorylation of FAK by PTEN leads to FAK inactivation. Conversely, phosphorylation of FAK activates p190RhoGAP, which in turn decreases RhoA activity. However, whether FAK interacts with RhoA has not been elucidated. We investigated protein interactions by immunoprecipitation (CoIP) with an anti-RhoA or anti-FAK antibody. Thus, 3MC-mediated complex formation of the aforementioned molecules was assayed by CoIP as described in “Materials and methods”. As demonstrated in Fig. 5a, RhoA interacted with a number of proteins including pFAK, p190RhoGAP, and PTEN. In this complex, PTEN slightly increased, whereas pFAK and p190RhoGAP decreased, in cells exposed to 3MC. Similar to the pattern of using an anti-RhoA antibody in CoIP assay, the results in Fig. 5b using an anti-FAK antibody demonstrate that except for PTEN, the other counterparts associated with FAK decreased upon 3MC administration, suggesting that FAK is inactivated by the increasing phosphorylation of PTEN, thus suppressing the negative feedback of FAK/p190RhoGAP to increase RhoA activity. Analysis of this mechanism of functional regulation demonstrated that RhoA and FAK were associated with each other as RhoA was co-immunoprecipitated with FAK, and vice versa. At the same time, the western blot analysis also used GAPDH as a negative control to exclude non-specific binding, and as expected, no bands were shown in the blot. An IgG light chain was used as an internal control to normalize the quantitated data from immunoblotting.
Fig. 5.
Alteration of the protein interaction pattern among angiogenic-related proteins in human umbilical vascular endothelial cells (HUVECs) challenged with 3-methylcholanthrene (3MC) using an anti-RhoA (a) or anti-FAK (b) antibody. 3MC-mediated complex formation of angiogenic-related proteins in HUVECs. Cells treated with 3MC or DMSO for 0–90 min were immunoprecipitated by an anti-RhoA or anti-FAK antibody (2 μg) and protein A plus G agarose beads (20 μl). The pulled-down complex was detected with anti-pPTEN, pP190RhoGAP, FAK, or RhoA by western blotting. The blot also used GAPDH as a negative control to exclude non-specific binding, and as expected, no bands were shown in the blot. An IgG light chain was used as an internal control to normalize the Western blot data. Bar charts in the lower panel show the band intensities of FAK and RhoA by densitometry. Data were derived from three independent experiments and are presented as the mean ± SD. *Significantly different (P < 0.05 vs the control)
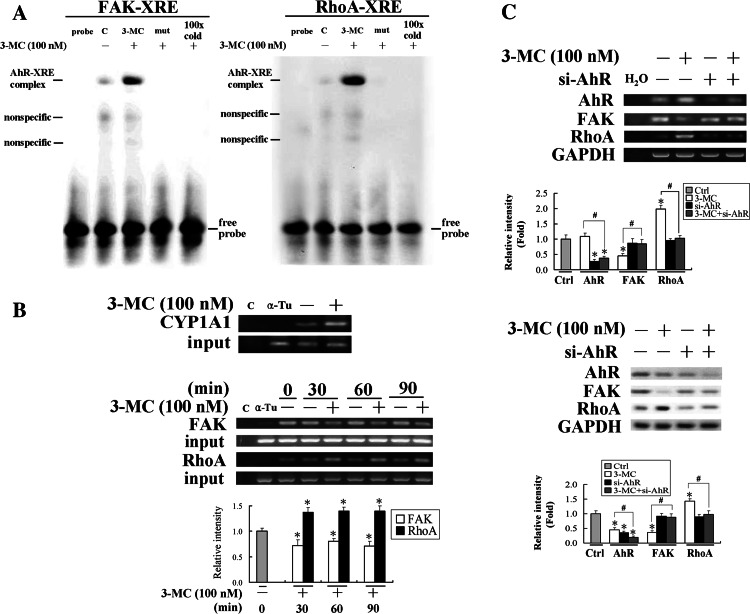
AhR genomic regulation of the alteration in FAK and RhoA protein levels by ChIP and siRNA assays
We further investigated the genomic regulation of FAK and RhoA mRNA levels by 3MC. Importantly, based on the silico analysis using MatInspector Professor software, there are putative XREs in the FAK/RhoA promoter regions at the positions of −160 to −136 bp for FAK and −455 to −431 bp for RhoA. To prove that AhR is capable of binding to those putative XREs, EMSA was carried out. The results of EMSA assay in Fig. 6a using putative XREs derived from FAK/RhoA promoter regions showed that 3MC increased the DNA binding activities of AhR to XREs upon 3MC treatment, of which the binding activities were abolished by their respective mutants and the competition of a 100-fold molar excess of unlabeled oligonucleotides relative to the radiolabeled probe. Furthermore, the effects of 3MC on the association of AhR to XREs in FAK/RhoA promoters were examined by ChIP assay using CYP1A1 as a positive control. We caused the 3MC-induced association of AhR with the XREs by pulling down the XRE fragments of the FAK/RhoA gene promoters using an anti-AhR antibody. The immunoprecipitated XRE fragments were amplified by PCR to examine the association of the AhR to the fragments using primers derived from FAK/RhoA promoters flanking XREs. The association of AhR to XRE in the RhoA promoter region increased by approximately 50%, but conversely, that in the FAK fraction decreased by approximately 40% upon 3MC administration, as shown in Fig. 6b. We further confirmed that the AhR was indeed involved in the transcriptional regulation of FAK and RhoA using an AhR siRNA technique. As shown in Fig. 6b, cells with AhR knockdown rescued 3MC-mediated alterations in both mRNA and protein levels of FAK and RhoA.
Fig. 6.
The aryl-hydrocarbon receptor (AhR)-dependent mechanism in the alteration of focal adhesion kinase (FAK)/RhoA in human umbilical vascular endothelial cells (HUVECs) challenged with 3-methylcholanthrene (3MC). a EMSA analysis of DNA binding activity of AhR to the putative XREs in FAK/RhoA promoters upon 3MC administration in HUVECs. The putative XREs of FAK/RhoA promoters predicted by MatInspector Professional software and their respective mutants were used as probes in the EMSA assay, as described in “Materials and methods”. Representative results of three separate experiments are shown. b Cells were harvested and subjected to a chromatin immunoprecipitation (ChIP) assay. The DNA associated with the AhR was immunoprecipitated with an anti-AhR antibody, and PCR amplification was used to determine the extent of AhR association with the functional AhR-binding sites (XREs) in the FAK/RhoA promoter fragments of 151 and 162 bp, respectively. CYP1A1 was used as a positive control for the binding of AhR to XRE by 3MC challenge and distilled water (C) and anti-α-tubulin (α-Tu) were respectively used as negative controls for the PCR and ChIP assays. c Cells knocked down with the AhR rescued 3MC-mediated alteration in FAK/RhoA levels. Cells transfected with the siAhR duplex as described in “Materials and methods” were treated with 100 nM 3MC for 1 h, followed by assessment of the expressions of FAK and RhoA at both the mRNA and protein levels. Representative results of three separate experiments are shown and data are presented as the mean ± SD. *Significantly different (P < 0.05 vs the control), #significantly different (P < 0.05 vs 3MC alone)
Functional assessments of 3MC-mediated changes in the properties of HUVECs and implications of AhR antagonists and a RhoA inhibitor
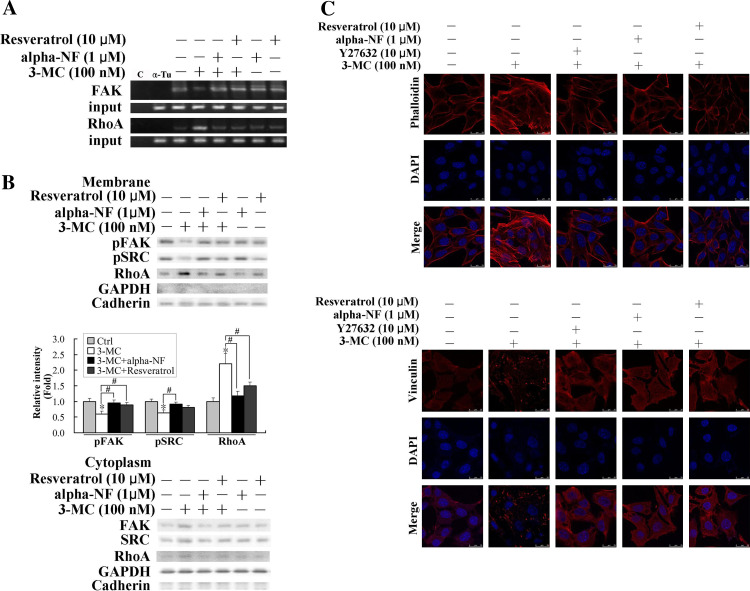
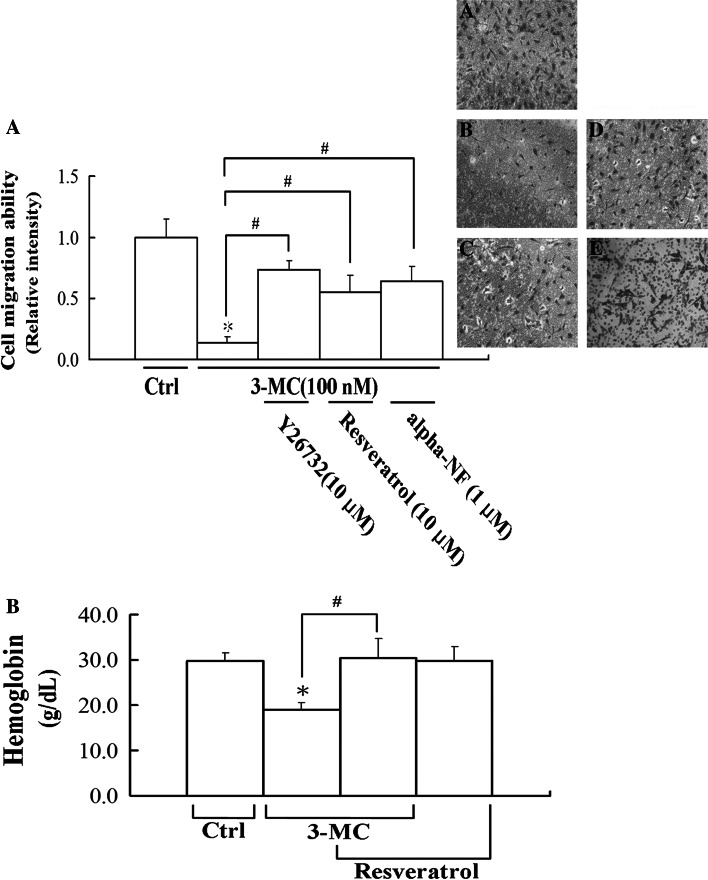
Given that the AhR is essential for the alteration of FAK/RhoA, the AhR antagonists, alpha-NF and resveratrol, were employed to examine their therapeutic intervention on the action of 3MC. Therefore, the effects of AhR antagonists on the results of the ChIP analyses were examined. We demonstrated that AhR antagonists, alpha-NF and resveratrol, reversed 3MC-mediated alteration of the association of AhR to XREs using the ChIP assay, as shown in Fig. 7a. Additionally, results of cytosol-membrane partitioning of RhoA in Fig. 7b show that alpha-NF and resveratrol partially rescued 3MC-mediated alterations in the activation of FAK, SRC, and RhoA. The effects of RhoA-mediated increases in stress fiber and focal adhesion complex formation were also confirmed using an inhibitor of ROCK (the RhoA effector), Y27632, and the AhR antagonists, alpha-NF and resveratrol. Data presented in Fig. 7c demonstrate that the 3MC-mediated effects were reversed by Y27632 and AhR antagonists, suggesting the essential roles of RhoA and the AhR in these effects. Moreover, the consequence of RhoA-mediated effects on HUVECs was evaluated in a microchemotaxis chamber. Importantly, the data presented in Fig. 8a demonstrate that 3MC-mediated inhibition of chemotactic migration by approximately 85% was significantly rescued by Y27632, resveratrol, and alpha-NF, which also imply the correlation of the increases in stress fiber and focal adhesion complex formation with the inhibition in cell motility. Our previous publication [19] showed that 3MC (5–500 nM) concentration-dependently inhibits capillary-like tube formation of HUVECs in Matrigel, which is partially reversed by the addition of alpha-NF (0.5–1 μM). The anti-migration effect of 3MC in vitro was further confirmed in vivo using Matrigel formation. Mice were subcutaneously implanted with Matrigel mixed with VEGF as a chemotactic force in the thigh to stimulate neoangiogenesis followed by 3MC challenge every 2 days for 2 weeks. The effect of 3MC on VEGF-mediated neoangiogenesis was assessed by the amount of hemoglobin in the removed Matrigel. Interestingly, mice with 3MC insults showed a significantly lesser extent of neoangiogenesis by approximately 60% relative to those given DMSO, which was significantly rescued by the AhR antagonist, resveratrol (Fig. 8b).
Fig. 7.
Restoration of 3-methylcholanthrene (3MC)-mediated alteration in properties of human umbilical vascular endothelial cells (HUVECs) by the RhoA inhibitor and AhR antagonists. Elimination of 3MC-mediated alteration in focal adhesion kinase (FAK)/RhoA by aryl-hydrocarbon receptor (AhR) antagonists including resveratrol and alpha-NF in ChIP assay (a) and western blot analysis of cell fractionation (b). In a, cells were with similar treatments as Fig. 6a, except that some samples were pretreated with AhR antagonists, alpha-NF and resveratrol, for 1 h prior to 3MC administration for 1 h. Distilled water (c) and anti-α-tubulin (α-Tu) were respectively used as negative controls for the PCR and the ChIP assays. In b, cells with the indicated treatment were partitioned into cytosol and membrane fractions, both of which were probed with anti-FAK, anti-SRC, and anti-RhoA antibodies to assess their activity. Bar charts in the lower panel show the band intensity of pFAK, pSRC, and RhoA with additional 100 nM 3MC treatment with respect to that of each individual pretreatment and values represent the mean ± SD. *Significantly different (P < 0.05 vs the control), #significantly different (P < 0.05 vs 3MC alone). b Reversal of 3MC-mediated formation of stress fibers and the focal adhesion complex by Y27632 and AhR antagonists, alpha-NF and resveratrol, in HUVECs. Cells grown on coverslips were pretreated with 10 μM of Y27632 or resveratrol, or 1 μM of alpha-NF for 1 h prior to 3MC challenge for 1 h. The methods for staining the formation of the stress fiber and focal adhesion complexes are described in “Materials and methods”. Magnification ×630
Fig. 8.
Restoration of antiangiogenic activity of 3-methylcholanthrene (3MC) in vivo by resveratrol or alpha-NF using Transwell and Matrigel plug assays. a Elimination of 3MC-mediated inhibition in cell motility by Y27632 and aryl-hydrocarbon receptor (AhR) antagonists. The migration of human umbilical vascular endothelial cells (HUVECs) was evaluated in microchemotaxis chambers. Cells grown in the 24-well microchemotaxis chamber were treated with the indicated chemicals and assayed at 16 h of the treatment. Numbers of cells migrating through to the lower surface of each membrane were counted, as described in “Materials and methods”. Cells migrating in the transwells were photographed and A–E represent the control, 3MC alone, 3MC + Y27632, 3MC + resveratrol, and 3MC + alpha-NF groups, respectively. Magnification ×100. b Angiogenesis was assessed using a Matrigel angiogenesis assay. The mean hemoglobin concentration of plugs excised from animals with various treatments was quantified as described in “Materials and methods”. Six samples were analyzed in each group, and values represent the mean ± SD. *Significantly different (P < 0.05 vs the control), #significantly different (P < 0.05 vs 3MC alone)
Discussion
In the present study, we examined the molecular mechanisms of an aryl hydrocarbon receptor agonist, 3MC, on cell migration and its dependence on the AhR using a human endothelial cell system. We demonstrated that 3MC treatment caused a reciprocal alteration in protein levels and activities of FAK and RhoA; the increase in RhoA activity was further proven through suppressed negative feedback of FAK/p190RhoGAP. Moreover, cell migration was inhibited by 3MC, but reversed by the AhR antagonists, resveratrol and alpha-NF, and a Rock inhibitor, Y27632, suggesting the essential roles of AhR and RhoA activation in this process.
The novel finding of this study is that we identified functional AhR-binding sites (XREs) in the FAK/RhoA promoter regions which regulate FAK/RhoA expression levels in response to 3MC challenge. This was done using EMSA to provide direct evidence of the binding of AhR to XRE in the FAK/RhoA promoter region, which can be abolished by their respective mutants and competition of a 100-fold molar excess of unlabeled oligonucleotides relative to the radiolabeled probes (Fig. 6a). In contrast to the EMSA result of FAK shown in Fig. 6a, Fig. 6b demonstrated that 3MC decreased the association of AhR to the XRE fragment of the FAK promoter in the ChIP assay, which might be due to steric hindrance of other co-repressors or transcription factors involved in this complex formation upon 3MC challenge. However, elucidating the underlying molecular mechanism requires further investigation. Additionally, the alteration in FAK/RhoA levels by 3MC was rescued in HUVECs with AhR knockdown (Fig. 6c) and additional treatments of AhR antagonists (Fig. 7a), suggesting the genomic regulation of the AhR in HUVECs.
Interestingly, the mRNA and protein levels of FAK decreased upon 3MC challenge across all the time points examined (Fig. 1). The protein and activity of FAK were rescued by Mg132 and tyrphostin 46, a S26 proteasome and phosphatase inhibitor, respectively, with a concomitant recovery of 3MC-mediated increase in RhoA activity (Fig. 4). This suggests that in addition to the upregulation of RhoA by the AhR, FAK also plays an important role in the negative regulation of RhoA activity. Nevertheless, determining the mechanisms of the AhR-mediated decrease in the stability of the FAK protein and mRNA remains for further investigation.
In this study, we demonstrated for the first time that the alteration of FAK/RhoA levels is AhR-dependent, and also that the increased activity of RhoA is subject to suppression by negative feedback of FAK and p190RhoGAP that was inactivated by 3MC. Additionally, a tetra-complex formation of the above-mentioned molecules was identified by coimmunoprecipitation assay in our study (Fig. 4). Moreover, PTEN was shown to directly interact with FAK, thereby inactivating FAK by dephosphorylation [9]. Interestingly, the association of PTEN and FAK increased with 3MC treatment according to co-IP immunoprecipitation using an anti-FAK antibody, which might be ascribable for FAK inactivation. RhoA activation increased the formation of the stress fiber and focal adhesion complex, and MLC phosphorylation, which might be attributed to the inhibition in cell migration, since inhibition of ROCK, a downstream target of RhoA, by Y27632 reversed this phenomenon. Therefore, our results also correlated stress fiber formation and the focal adhesion complex by RhoA activation with the anti-migration effect of 3MC in HUVECs. This result agrees with another study showing that FAK knockout in mice caused constitutive RhoA activation, with subsequent inhibition of focal adhesion turnover, thereby inhibiting cell migration and embryonic survival [16]. The finding of an increase in focal adhesion complex formation in this study and a decrease in focal adhesion turnover by RhoA activation might also account for our previous finding that 3MC decreases cell reattachment after subculturing [19]. In addition, the pathological influences of RhoA activation were shown in various systems. For instance, increased RhoA activity is responsible for a thrombin-induced increase in endothelial permeability due to increased contraction, which can be rescued by increasing p190RhoGAP phosphorylation by FAK [12]. Moreover, activation of RhoA increases resistance to aqueous humor outflow by influencing actomyosin assembly, cell adhesive interactions, and the expression of ECM proteins and cytokines in endothelial-like trabecular cells [17].
However, the roles of RhoA and Rock in migration and angiogenesis are equivocally discussed. In contrast to our finding, inhibition of RhoA activity using a dominant negative RhoA (DNRho) or Y27632 was shown to inhibit chemotaxis [29]. Likewise, inhibition of RhoA/Rock activation by trapidil, a platelet aggregation inhibitor and vasodilator agent, was ascribed to its inhibitory effect on migration [30]. Determining whether the opposite effects of RhoA in chemotaxis is cell type- or stimulator-dependent requires further investigation. Moreover, we surmise that the outcome of the combined alteration of FAK/RhoA is different to the increasing RhoA activity alone. However, this is a speculation and remains to be further examined.
The pathologic significance of this finding is that an AhR agonist, 3MC, a component of environmental pollutants, eliminated migration of endothelial cells by suppressing the FAK signaling pathway through an AhR-dependent mechanism, which could be reversed by resveratrol or alpha-NF (AhR antagonists) both in vitro and in vivo. This implies their potential benefits in preventing AhR agonist-mediated cardiovascular toxicities, which further confirmed our previous findings that AhR antagonists rescue 3MC-mediated inhibition of neoangiogenesis using capillary-like tube formation and HUVEC proliferation [18, 19], and that 3MC decreased HUVEC proliferation and suppressed cell adhesion signaling by inhibiting FAK phosphorylation are essential for anti-angiogenesis. Here, we illustrated the molecular mechanisms of 3MC-mediated FAK alteration and its downstream target molecules involved in anti-migration processes. Despite AhR-mediated p21/p27 induction causing the cell cycle arrest of HUVECs in our previous report [18], determining whether the cytosolic parts of p21 and p27 are involved in the anti-migration effect of 3MC warrant further investigation.
In conclusion, results of the present study demonstrate that 3MC is a potent anti-migration and antiangiogenic molecule in HUVECs. Notably, we demonstrated that the alteration of FAK/RhoA levels is subject to the AhR’s genomic regulation, and increasing activity of RhoA is due to the suppression of negative feedback regulation of FAK and p190RhoGAP by 3MC. These findings may contribute to a better understanding of the potency of AhR agonists in inducing vasculotoxicity and the possible therapeutic intervention of using AhR antagonists including alpha-NF and resveratrol.
Acknowledgment
This study was supported by a grant [NSC97-2320-B-038-017-MY3(1-3)] from the National Science Council, Taiwan.
References
- 1.Manes S, Mira E, Gomez-Mouton C, Lacalle RA, Martinez C. Cells on the move: a dialogue between polarization and motility. IUBMB Life. 2000;49:89–96. doi: 10.1080/15216540050022386. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa S, Li C, Ni CZ, Takayama S, Reed JC, Ely KR. Structural analysis of Siah1 and its interactions with Siah-interacting protein (SIP) J Biol Chem. 2003;278:1837–1840. doi: 10.1074/jbc.M210263200. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Chattopadhyay A, Ji QS, Owen JD, Ruest PJ, Carpenter G, Hanks SK. Focal adhesion kinase promotes phospholipase C-gamma1 activity. Proc Natl Acad Sci USA. 1999;96:9021–9026. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 8.Sekine Y, Tsuji S, Ikeda O, Sugiyma K, Oritani K, Shimoda K, Muromoto R, Ohbayashi N, Yoshimura A, Matsuda T. Signal-transducing adaptor protein-2 regulates integrin-mediated T cell adhesion through protein degradation of focal adhesion kinase. J Immunol. 2007;179:2397–2407. doi: 10.4049/jimmunol.179.4.2397. [DOI] [PubMed] [Google Scholar]
- 9.Gunther W, Skaftnesmo KO, Arnold H, Terzis AJ. Molecular approaches to brain tumour invasion. Acta Neurochir. 2003;145:1029–1036. doi: 10.1007/s00701-003-0099-x. [DOI] [PubMed] [Google Scholar]
- 10.Chan AY, Bailly M, Zebda N, Segall JE, Condeelis JS. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J Cell Biol. 2000;148:531–542. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 13.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 14.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartolome RA, Wright N, Molina-Ortiz I, Sanchez-Luque FJ, Teixido J. Activated G(alpha)13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity. Cancer Res. 2008;68:8221–8230. doi: 10.1158/0008-5472.CAN-08-0561. [DOI] [PubMed] [Google Scholar]
- 16.Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008;295:C1057–C1070. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang PH, Lin YH, Lee YH, Hou HH, Hsu SP, Juan SH. Molecular mechanisms of p21 and p27 induction by 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, involved in antiproliferation of human umbilical vascular endothelial cells. J Cell Physiol. 2008;215:161–171. doi: 10.1002/jcp.21299. [DOI] [PubMed] [Google Scholar]
- 19.Juan SH, Lee JL, Ho PY, Lee YH, Lee WS. Antiproliferative and antiangiogenic effects of 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, in human umbilical vascular endothelial cells. Eur J Pharmacol. 2006;530:1–8. doi: 10.1016/j.ejphar.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 2004;32:4512–4523. doi: 10.1093/nar/gkh782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih CM, Lin H, Liang YC, Lee WS, Bi WF, Juan SH. Concentration-dependent differential effects of quercetin on rat aortic smooth muscle cells. Eur J Pharmacol. 2004;496:41–48. doi: 10.1016/j.ejphar.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Falk W, Goodwin RH, Jr, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 24.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest J Tech Meth Pathol. 1992;67:519–528. [PubMed] [Google Scholar]
- 25.Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai J, Lin H, Nie Z, Wu J, Canete-Soler R, Schlaepfer WW, Schlaepfer DD. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278:24865–24873. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 27.Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 28.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanda S, Miyata Y, Kanetake H. Role of focal adhesion formation in migration and morphogenesis of endothelial cells. Cell Signal. 2004;16:1273–1281. doi: 10.1016/j.cellsig.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Ishikura K, Fujita H, Hida M, Awazu M. Trapidil inhibits platelet-derived growth factor-induced migration via protein kinase A and RhoA/Rho-associated kinase in rat vascular smooth muscle cells. Eur J Pharmacol. 2005;515:28–33. doi: 10.1016/j.ejphar.2005.04.013. [DOI] [PubMed] [Google Scholar]