Abstract
Burn injury causes an immunosuppression associated with suppressed adaptive immune function. Dendritic cells (DCs) are APCs for which signaling via their Toll-like receptors (TLRs) induces their maturation and activation, which is essential for the adaptive immune response. In this study, we examined if burn injury alters the TLR activity of splenic DCs. After injury, we noticed that DC functions were impaired, characterized by a suppressed capacity to prime naive T cells when triggering the TLR4 signaling cascade using specific ligands (LPS or rHSP60). The observed perturbations on LPS-primed DCs isolated from burned mice exhibited significantly diminished IL-12p40 production and enhanced IL-10 secretion-associated impairment in mitogen-activated protein kinase activation. Interestingly, we observed a decrease of TLR4/MD-2 expression on the CD8α+ DC subset that persisted following LPS stimulation. The altered TLR4 expression on LPS-stimulated CD8α+ DCs was associated with reduced capacity to produce IL-12 after stimulation. Our results suggested that TLR4 reactivity on DCs, especially CD8α+ DCs, is disturbed after burn injury.
Keywords: Toll-like receptor, TLR4, CD8α+ DC subset, Lipopolysaccharides, HSP60, MAP kinase, Interleukine-12, Interleukine-10
Introduction
Dendritic cells (DCs) are unique in their capacity to prime naive T cells and initiate immune responses [1]. This property correlates with their ability to enhance antigen peptides/MHC, co-stimulatory molecules expression, and their ability to produce cytokines when they are stimulated [2]. However, DCs do not display a constitutive co-stimulatory function in situ and are immature immunologically. DCs undergo maturation upon pathogen entry, tissue damage, or danger signals [3]. Maturation is characterized by an enhanced expression of both MHC-class II and co-stimulatory molecules, a production/secretion of stimulatory cytokines, and their responsiveness to chemokines leading them to the T-cell-rich areas of lymphoid organs [2, 4]. In mice, three classes of lymphoid organ-resident conventional DC subsets coexist in spleen (CD8α+CD4−, CD8α−CD4+ and CD8α−CD4−) which selectively and differently regulate the development of Th1 versus Th2 response depending of local environment [5]. IL-12 is produced mainly by CD8α+ DCs that are associated with the Th1 response [6]. In contrast, CD8α− DCs can also produce IL-12 but trigger a Th2 response in the presence of IL-10 producing cells in their microenvironment [6].
Maturation and functions of DCs are influenced by endogenous and exogenous signals like cytokines and pathogen-associated molecular pattern (PAMPs) which bind to pattern recognition receptors (PRRs) [7, 8]. The most common PRRs expressed on DCs are the Toll-like receptors (TLRs) which discriminate between different types of pathogens. Conserved PAMPs such as bacterial lipopolysaccharides (LPS), peptidoglycan, or CpG DNA motif bind to specific TLRs [8]. Moreover, putative endogenous ligands such as endogenous fibrinogen and heat shock protein can also interact with TLRs [9]. With regard to DC maturation, many ligands have been identified such as LPS for TLR4/MD-2 or lipoproteins for TLR2 [10]. Ligand binding to TLRs leads to dimerization and conformational changes that are required for the recruitment of downstream signaling molecules. The signaling pathway from MyD88 leads to the activation of mitogen-activated protein kinase (MAPK), including p38, ERK1/2, and JNK/SAPK, and to the downstream activation of transcription factors such as NF-κB and AP-1 which control the inflammatory response [10, 11]. The LPS-activated DCs produce different types of cytokines depending on the DC subsets and can direct the activation and differentiation of naive CD4+ T cells into Th1, Th2, Th17, or regulatory T cells [12].
Severe burn injury induces an immunosuppressive state which is responsible for the development of opportunistic infections that can lead to sepsis and increase mortality [13–17]. It is well established that burn injury induces a systemic inflammatory response syndrome (SIRS) [18]. For the majority of burned patients, SIRS persists for several days and is often followed by the compensatory anti-inflammatory response syndrome (CARS). The latter syndrome causes a decrease in cell-mediated immune response, which may explain the frequency of septicemia following burn injuries [19]. Our group and others have noted that the immune depression observed after burn injuries is attributed to CD4+ T cell impairment causing suppressed Th1 function [15, 17, 20–22]. Antigen presentation is the first step in the initiation of CD4+ T cell-mediated responses suggesting that the impaired T cell response following injury may be related to APC function. Cell types that have the ability to present antigen to CD4+ T cells are DCs, macrophages, and B cells [2]. It has been demonstrated that defects in the T cell-mediated adaptive immune response can be exacerbated by perturbations of macrophages [23–27]. However, the contribution of DCs in T cell dysfunction following burn injury is poorly documented. After burn injury, it was reported that Fms-like tyrosine kinase 3 ligand (Flt3L) treatments resulted in both stimulation of DC production and enhancement of DC functions restoring their ability to support Th1 cytokine production [28–30]. Previous research from our laboratory using endotoxin mice strain (C3H/HeJ), which has a mutation in the TLR4 receptor, demonstrated that LPS unresponsiveness induced a more pronounced immunosuppression state and an intensive oxidative stress in burn mice [31]. This suggests a role of TLR4 signaling in the control of immune response after burn injury. Burn injury is associated with disrupt normal homeostasis and increase in bacterial translocation, which results in predisposition to infection with a variety of potential pathogens, such as Pseudomonas aeruginosa, Escherichia coli, and all Gram-negative bacteria. McManus and colleagues found that 10% of all burned patients admitted to burn units developed P. aeruginosa bacteremia and 80% of those died [32]. Endotoxin (LPS) is a major component of the outer membrane of Gram-negative bacteria and a critical actor in the pathogenesis of Gram-negative sepsis. This multistep recognition process is initiated by the binding of LPS to the LPS-binding protein (LBP) that conveys LPS to a cell surface receptor complex composed of MD-2 and TLR4 [33]. Given its central role in the pathogenesis of Gram-negative sepsis following burn injury, we assumed that TLR4 is one of the main receptors involved in the immune response following burn injury. More recently, it was established that burn injury primes DC TLR-mediated responses and contributes to heighten the SIRS [34]. An over-stimulation of DCs in the early state after burn injury could induce a desensitized phenotype of those cells against further pathogens. Long-term consequence of severe burn injury on DC differentiation has not yet been established. This is particularly important since an alteration in DC subtypes can influence T cell response and the immune homeostasis after burn injury.
The aim of our work was to determine the consequence of burn injury on DC subpopulations and their functionality in CARS. We demonstrated severe alterations in splenic DC functions at 10 days post-injury. These perturbations were characterized by a decrease in their capacity to prime T cells via a dysregulation of TLR4 expression, maturation induction, and cytokine secretion. We concluded that DCs from burn-injured mice are no longer capable of responding adequately to TLR stimulation, thus leading to an endotoxin tolerance-like state following microbial infections.
Materials and methods
Mouse thermal injury model
All experiments were performed on 8-week-old male C57BL/6 mice (Charles River Laboratories, St-Constant, QC, Canada). Prior to the initiation of any procedure, the mice were acclimatized for 1 week to our central animal facility with strictly controlled temperature, relative humidity, and a 12-h light/dark cycle. Each cage contained five mice, which were fed standard chow (Richmond standard diet; Lab Diet, Richmond, IN, USA) and water ad libitum. The Institutional Animal Care Committee reviewed and approved all procedures performed in accordance with the Canadian Council on Animal Care guidelines. Mice were subjected to a well established thermal injury model that was previously described [17, 31]. Briefly, mice were anesthetized with pentobarbitural (CDMV, St-Hyacinthe, QC, Canada) and the dorsums were shaved. Mice were placed in a custom-insulated mold exposing 20% of their total body surface area (TBSA) and were immersed for 7 s in either room temperature water (22°C) for sham burn or in 90°C water to produce a full-thickness burn. This treatment has been shown to cause full-thickness burn with low mortality (<5%). All animals received by i.p. a mixture of ml 0.9% saline and 0.1 mg/kg of buprenorphine (Reckitt and Coleman Pharmaceuticals, Richmond, VA, USA) for fluid resuscitation while a topical antibacterial agent (silver sulfadiazine cream, Smith and Nephew, QC, Canada) was applied on the burned areas.
Dendritic cell isolation
One or 10 days after burn injury, mice spleens in each group were individually prepared as single-cell suspensions in 10 ml of complete RPMI 1640 (BioMedia, Drummondville, QC, Canada) supplemented with 10% heat-inactivated FBS (BioMedia), 50 μM 2-mercapthethanol (Sigma–Aldrich, Oakville, ONT, Canada) and antibiotics (100U/ml penicillin and 100 μg/ml streptomycin (Biosource, Montreal, QC, Canada)). DC isolation was done as described in [64]. Briefly, spleen cell suspensions were obtained by digestion at 37°C for 30 min with collagenase mix (1 mg/ml Collagenase D and 20 μg/ml of DNase I; Roche Molecular Products, Laval, QC, Canada) in RPMI-1640 medium 2% of FBS. At the end of incubation, splenocyte suspensions were obtained by tearing apart the splenic matrix on a strainer and kept on ice to prevent DC maturation. Thereafter, erythrocytes were removed by hypertonic lysis (Gey’s solution with NH4Cl), viability determined and total cell number counted. Viability of cells was consistently greater than 90%. Spleen cell suspensions (108 total cells) were incubated in 400μL of PBS 0.5% BSA buffer with 200μL heat inactivated normal mouse serum, 100 μg/ml of purified rat anti-mouse CD16/CD32 FcγIII/II Fc-Block Ab (BD Biosciences Pharmingen, Mississauga, ONT, Canada) and 70μL microbead-conjugated hamster anti-mouse CD11c+ (Miltenyi Biotec, Auburn, CA, USA) at 4°C for 15 min. Cells were washed twice and DCs were separated by positive magnetic selection on auto-MACS according to the manufacturer’s instructions. The purity of CD11c + cells was consistently greater than 85% as determined using an R-PE-conjugated hamster anti-mouse CD11c MoAb (BD Biosciences Pharmingen) on a FACScan® flow cytometer (Becton–Dickinson, Mississauga, ON, Canada). WinMdi software was used for analysis.
T cell proliferation assay
To evaluate DC cell capacity to prime T cell proliferation at 10 days, we used T cell hybridomas BO-97.11, the TcRs of which are specific to OVA peptide (323–339) (Peptides International, Louisville, KT, USA) presented by I-Ab cells. First, we pulsed splenic DCs with different OVA concentrations 0, 3, 30, and 300 μg/ml for 1 h. OVA-pulsed splenic DCs were incubated with BO-97.11 T cells at a ratio of 1:4 alone with media or with 1 μg/ml of LPS E. coli (O55:B55; Sigma–Aldrich) for 24 h. The supernatants were harvested for measurement of IL-2 secretion by BO-97.11 T cells, as an indirect marker of cell proliferation, using an ELISA kit [35]. Purified rat anti-mouse IL-2 and biotin rat anti-mouse IL-2 were purchased at BD Biosciences Pharmingen, streptavidin-POD conjugated from Roche, and ABTS substrate (Sigma–Aldrich). All procedures were performed according to the manufacturer’s instruction.
Semi-quantitative RT-PCR
At 10 days, DCs were washed twice in cold PBS and total RNA was extracted with the RNeasy Mini kit from QIAGEN (Mississauga, ON, Canada) according to the manufacturer’s instructions. Random primers (Promega, Madison, WI, USA) and MMLV reverse transcriptase (Invitrogen, Burlington, ON, Canada) were used for cDNA synthesis. PCR amplification of the cDNA was performed in a final volume of 50 μl containing 1.5 mM of MgCl2, 1 unit of GoTaq DNA polymerase (Promega) and 0.5 μM each of TLR4 primers (Invitrogen). Cycling conditions were 94°C for 30 s, 62°C for 30 s, and 72°C for 30 s (Whatman-Biometra T-Gradient thermal cycler; Montreal Biotech, Kirkland, QC, Canada) and the optimum cycle was 41 cycles for TLR4 amplification. The sequence of the specific primers used was for TLR4 forward 5′-CAA GAA CAT AGA TCT GAG CTT CAA CCC-3′ and for TLR4 reverse 5′-GCT GTC CAA TAG GGA AGC TTT CTA GAG-3′. For each sample, mRNA was normalized to the corresponding amount of 18S rRNA using the mouse 18S rRNA Primer set kit (Maxim Biotech, San Francisco, CA, USA). The TLR4 signal for each sample was then divided by its corresponding 18S signal. TLR4 and the 18S-probed membranes were analyzed and quantified using a PhosphoImager apparatus (Molecular Dynamics; Amersham Biosciences).
Analysis of TLR4/MD-2
For the experiments investigating TLR4 modulation and maturation of splenic DCs isolated 10 days post-trauma, we cultured alone with media or with 1 μg/ml of LPS in complete RPMI 1640 media at 37°C for the indicated time. Following incubation, cells were trypsined (0.25% trypsine solution) and washed three times with cold HBSS (Sigma–Aldrich), 0.1% BSA, 0.01% sodium azide (Sigma–Aldrich). TLR4/MD-2 expression on DC subsets CD8α+ and CD8α− was evaluated by flow cytometry using biotin anti-TLR4/MD-2, PE-anti-mouse anti-CD4 Abs (eBioscience, San Diego, CA, USA), FITC-conjugated anti-mouse anti-CD8 Ab (eBioscience), and streptavidine-PECy5 (Cedarlane Laboratories, Hornby, ON, Canada). The isotype control Ab IgG PECy5 (Cedarlane) was used. Following labeling procedures, cells were washed three times with cold HBSS, 0.1% BSA, 0.01% sodium azide and analyzed on FACScan®,while WinMdi software was used for the analysis.
Cytokine ELISA
On day 10 after burn injury, sham and burn DCs (2 × 105 cells/ml) were stimulated with LPS 1 μg/ml in complete RPMI 1640 for 24 h. Serial dilutions of recombinant cytokine standards and culture supernatants were added to individual wells in triplicate, and were subjected to quantification of the cytokine level of IL-12p40 and IL-10 by using specific ELISA kit. The mouse IL-12/IL-23 (total p40) ELISA kit (eBioscience) was used to quantify IL-12p40 and the mouse IL-10 ELISA ready Set-Go (eBioscience) for IL-10.
Western blot of phosphorylated (activated) MAPK
On day 10 following burn injury, splenic DCs (2 × 106 cells) were incubated for 1 h at 37°C in microtubes containing media only after which they were exposed to 1 μg/ml LPS and MAPK activation was evaluated. After 0-, 15-, 30-, and 60-min incubation periods, cells were washed once with PBS 0.5% BSA, lysed in loading buffer and boiled at 95°C for 5 min. Proteins were loaded on a 10% SDS-polyacrylamide gel, separated and transferred onto a nitrocellulose membrane. Membranes were blocked overnight with skim milk and probed for overnight at 4°C with desired antibodies. Specific antibodies for the phosphorylated form of p38 MAPK (BioSource, Camarillo, CA, USA), phospho-p46/p54 SAPK/JNK MAPK (Cell Signaling), or p44/42 ERK1/2 MAPK (Cell Signaling) were used. Membranes were then washed three times in TBST and incubated at room temperature with secondary HRP-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h at room temperature. Visualization of the bands was done by chemiluminescence with the ECL+ system. Thereafter, membranes probed with phospho-specific antibodies were stripped, blocked, and rehybridized with non-phosphorylated anti-p38 (Santa-Cruz Biotechnology), anti-ERK1/2 (Biosource), or anti-JNK Abs (Cell Signaling).
Western blot of endogenous hsp60
Ten days after burn injury, splenocytes were lysed in loading buffer, boiled at 95°C for 5 min, and proteins proceeded to western blot as described above. Membranes were stained overnight at 4°C with mouse anti-hsp60 mAb (Stressgen Biotechnologies, BC, Canada). Following three times washing in TBST, membranes were incubated with secondary HRP-conjugated anti-mouse IgG (Santa Cruz) for 1 h at room temperature. Visualization of the bands was done by chemiluminescence with the ECL+ system. Thereafter, membranes were stripped and reprobed with antibody against mouse actin (A4700; Sigma–Aldrich) to evaluate protein loading in each sample.
Intracellular cytokine detection
At 10 days after sham or burn injury, DCs were prepared as previously described and 1 × 106 cells were incubated with 1 μg/ml of LPS and 10 μg/ml of brefeldin A (Sigma) for 6 h. Cells were then stained for CD11c, CD8, or CD4 expression using FITC-conjugated anti-CD11c (Myltinei Biotec), FITC-conjugated anti-CD8, or FITC-conjugated anti-CD4 Abs (eBioscience). Cells were washed twice with HBSS 0.1% BSA 0.01% Sodium Azide and then fixed for 20 min with 400 μl of 2% paraformaldehyde in PBS (pH 7.4) at 4°C. Following fixation, cells were washed once then permeabilized in 200 μl of buffer containing 0.1% saponin, 0.1% BSA, and 0.01% sodium azide in PBS. Cytokine staining was done by first pre-treating the fixed and permeabilized cells with 100 μL solution of normal human IgG Ab (Sigma) for 10 min at 4°C, to block nonspecific binding. Fixed and permeabilized cells were incubated with PE-labeled rat anti-mouse IL-12 (p40/p70) (BD Pharmingen) for an additional 1 h at room temperature. Cells were washed twice with permeabilization buffer, and then resuspended in 100 μl of permeabilization buffer. FACScan® flow cytometer was used to detect cytokine expression levels in gated CD11c+, CD4+, or CD8+ DCs subsets and analysis was performed with WinMdi software. PE-labeled isotype control Ab (Cedarlane) was used to judge specific versus nonspecific staining.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Data were analyzed by Mann–Whitney U test, unpaired t test or by one-way ANOVA test and Bonferroni multiple comparison post-test using Graph Pad InStat 3.05 software program (GraphPad Software, San Diego, CA). Differences between groups were considered statistically significant when the probability that they occurred by chance was <5%. Each experiment was performed twice or more with at least five mice per group.
Results
Increase of DCs in the spleen after thermal injury
We have shown previously that burn injury caused an increase of splenocyte number 10 days post-trauma [17]. Since a proportion of spleen cells are not T or B lymphocytes or macrophages, we have determined the percentage of CD11c+ cells following burn injury. Analysis of CD11c+ splenocyte after burn injury showed a decrease in percentage of CD11c+ cells at day 1 followed by a significant increase at day 10 post-injury (Fig. 1a, p < 0.01). Transformation in absolute number of CD11c+ cells showed, at an early time following burn injury (day 1), a decrease of around half in CD11c+ DCs (Fig. 1b; p < 0.05). Later, during the CARS stage (day 10), an increase by 2.5-fold in the number of splenic CD11c+ DCs of burned mice was noted when compared to sham mice (Fig. 1b; p < 0.001). Overall, burn injury causes a modulation in the number of DCs which can play an important role in the immune response post-trauma.
Fig. 1.
Burn injury causes an increase of the number of DCs in spleen 10 days post-injury. C57BL/6 mice were subjected to thermal injury and splenocytes were isolated as described in “Materials and methods” 1 and 10 days post-injury. Dendritic cell percentage (a) and number (b) were evaluated by flow cytometry for both sham and burn mice as described in “Materials and methods”. Data are represented as the mean ± SEM of four independent experiments (n = 5 mice/group; *p < 0.05, **p < 0.01 and ***p < 0.001; Mann–Whitney U test comparing sham vs burn-injured mice)
DC capacity to prime antigen specific T cells after burn injury
Since DCs are the most potent antigen-presenting cells (APC) involved in adaptive immunity, we have examined stimulatory capacity for Ag-specific T cells by evaluating indirect parameter such as IL-2 synthesis. To evaluate MHC class II presentation by TLR4-stimulated splenic DCs incubated, we utilised the BO-97.11 hybridoma, which secretes IL-2 upon recognition of the OVA 323-339 peptide in association with I-Ab [35]. DCs from burned mice isolated at 10 days post-injury more efficiently induced IL-2 production by BO-97.11 T cells when compared to DCs from sham group (Fig. 2a; p < 0.05). Considering that many PAMPs are released after burn injury, we wondered if DCs pulsed in the presence of LPS induced optimal IL-2 production. OVA peptides pulsed DCs in presence of LPS (1 μg/ml) from burned mice were less efficient than sham DCs in their capacity to induce T cell IL-2 production (Fig. 2b; p < 0.05). When compared to the sham group, a decrease of 34.7 and 32.9% in IL-2 production by BO-97.11 T cells were noted for DCs from burned mice in the presence of 30–300 μg/ml of OVA peptide, respectively. Thus, burn injury alone causes an increase in the capacity of DCs to activate T cells. However, a second in vitro LPS stimulation causes a reprogramming phenotype of the DCs towards to decrease APC functions observed by reduce T cells activation, suggesting that injury alters signaling pathway of the TLR4.
Fig. 2.
Burn injury decreases DC capacity to prime naive T cells. T cell hybridomas BO-97.11, the TCR of which is specific to ovabulmine peptide (323–339) presented by I-Ab cells, were used to evaluate DC cell capacity to present antigen and induce T cell proliferation. DCs were pulsed with different OVA concentrations 0, 3, 30, and 300 μg/ml alone with media (a) or with LPS (1μg/ml) (b) or with rHSP60 (5 μg/ml) (d). Then, OVA-pulsed splenic DCs were co-cultured with BO-97.11 T cells at a ratio of 1:4 for 24 h. The supernatants were harvested and IL-2 production by BO-97.11 cells was evaluated by ELISA. Results are presented as mean value ± SEM of three independent experiments (n = 5 mice/group; *p < 0.05; ANOVA test with Bonferroni post test comparing sham vs burn-injured mice of each OVAc concentration). HSP60 expression level in total splenocytes 10 days post-injury (c) . Results are presented as mean value ± SEM of three independent experiments (n = 3 mice/group; **p < 0.01; Mann–Whitney U test)
A common issue of burn injury is the increase of released endogenous activating molecules from necrotic tissues, namely damage-associated molecular pattern molecules which can act as a danger signals. Presence of these endogenous molecules can lead to DC activation as do PAMPs. In humans, burn injury is associated with an increase of the latter type of molecules as shown with extracellular heat-shock proteins [36]. To address this question in our model, we have determined the level of HSP60 in sera from sham and burned mice. HSP60 from burned mice showed a significant decrease for up to 10 days following injury compared to sham mice (data not shown). However, the enhanced protease activity observed in serum from burn injury needs to be considered in these results [37]. Thus, we have determined the HSP60 expression in splenocytes following burn injury. As expected, we noted a significant increase of HSP60 in splenocyte from burned mice 10 days following the burn injury (Fig. 2c; p < 0.01). Since DCs interact with T cells in restricted area and required cognate interaction, which can be influenced by their environment, it is plausible that release of intracellular protein such as HSP60 can influence cells bearing a specific receptor for related molecules. We have sought to determine if endotoxin-free recombinant heat-shock protein 60 (rHSP60), a TLR4 ligand, can modulate DC activity isolated from burn-injured mice. We observed that OVA peptides pulsed DCs in presence of rHSP60 (5 μg/ml) from burned mice were less efficient than sham DCs in their capacity to induce T cell IL-2 production (Fig. 2d; p < 0.05). Taken together, our results showed that in the presence of a second challenge mediated by TLR4 DCs from burn-injured animals less efficiently activate T cells, which can explain the hyporesponsiveness of T cell post-trauma.
Injury decreases LPS-induced TLR4/MD-2 expression on splenic DCs and IL-12 cytokine production
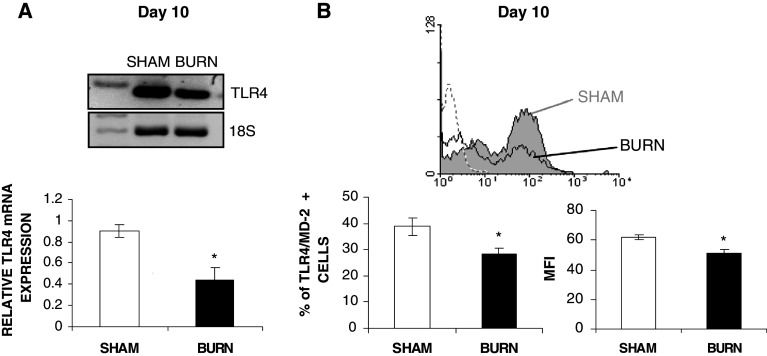
To test whether the inhibitory effect of burn injury on LPS-stimulated DCs were related to decreased TLR4 expression, we have evaluated the TLR4 mRNA by semi-quantitative RT-PCR and TLR4 expression on DCs isolated from both groups. TLR4 mRNA in purified splenic CD11c+DCs 10 days after burn injury was significantly decreased by 2.3 ± 0.5-fold when in comparison to sham group DCs (Fig. 3a; p < 0.05). At the protein level, % CD11c+DCs expressing TLR4/MD-2 was significantly reduced in DCs isolated from burned mice in comparison to sham group. Splenic CD11c+DCs expressing TLR4/MD-2 was significantly decreased 10 days after burn injury with burned mice having 28.4 ± 2.3% CD11c+/TLR4/MD-2 DCs and sham mice 38.7 ± 3.1% CD11c+/TLR4/MD-2 DCs (Fig. 3b; p < 0.05). Moreover, burned splenic CD11c+DCs express lower level of the TLR4/MD-2 receptor compared to sham, as determined by mean fluorescence intensity (MFI) value (Fig. 3b, p < 0.05).
Fig. 3.
Splenic CD11c+DCs have decreased TLR4 mRNA and TLR4/MD-2 protein level after burn injury. RNA was isolated from both sham and burn splenic DCs, and RT-PCR was performed to evaluate TLR4 gene expression (a) in both sham and burned mice. For each TLR4 RT-PCR, 18S ribosomal RNA was evaluated as a housekeeping gene, as described in “Materials and methods”. Results are expressed as relative mRNA TLR4 expression normalized to 18S RNA of two independent experiments (DCs of n = 7 mice/group were pooled). Results are presented as mean value ± SEM of three independent experiments (n = 3 mice/group; *p < 0.05; Mann–Whitney U test). Percentage of splenic DCs expressing TLR4/MD-2 and the MFI of TLR4/MD-2 on splenic DCs (b) were evaluated by flow cytometry using specific Abs as described in “Materials and methods”. TLR4/MD-2 protein level is presented as mean value ± SEM of four independent experiments (n = 5 mice/group, *p < 0.05; Mann–Whitney U test comparing sham vs burn-injured mice). Dotted line isotype control, the filled curve (grey) sham and open curve (white) burn TLR4/MD-2 histogram
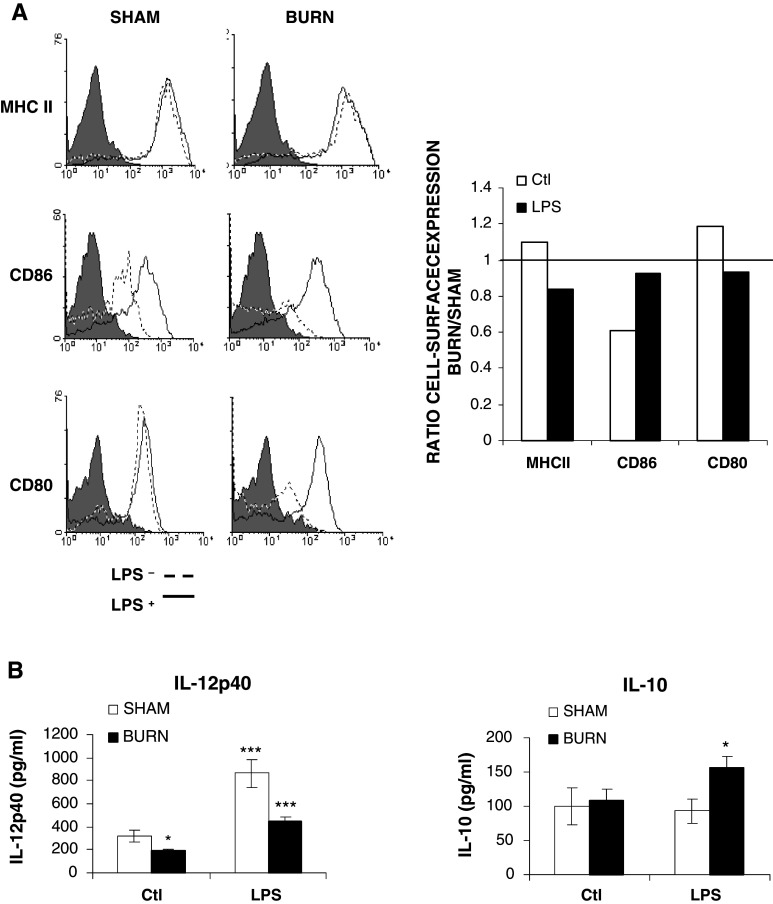
Three signals generated from TLR-activated DCs direct the differentiation and activation of T helper cell Th1 or Th2 subsets, including the dose of antigen presented (signal 1), the type and degree of co-stimulatory molecule expressed (signal 2), and the type of cytokine secreted (signal 3) [2]. Since burn injury promotes antigen-driven Th2-type responses in vivo [26], we have determined if DC perturbations observed after burn injury change TLR4-induced altered co-stimulatory molecule expression and cytokine production. We analyzed the expression of B7.1 (CD80), B7.2 (CD86), and MHC II on LPS-stimulated (1 μg/ml) DCs from both sham and burned animals. At the basal level, CD11c+DCs constitutively expressed CD80, CD86, and MHC II (data not shown). In the spleen, the percentage of CD11c+DCs showed a trend (statistically not significant different) to increasing MHC II and CD80 associated with lower percentage of CD11c+/CD86+ (Fig. 4a). Analysis of the percentage of DCs expressing MHCII and co-stimulatory molecules following LPS-activation revealed no significant difference between the burn and sham groups (Fig. 4a).
Fig. 4.
Burn injury induces a decrease of IL-12p40 associated with an increase of IL-10 production without any changes of CMH II, CD86 and CD80 expression of splenic CD11c+ DCs; a 18 h after incubation with LPS (1 μg/ml) CD11c+DCs were labeled using specific Abs as described in “Materials and methods”. FACS results are representative of one experiment of three independent experiments (DCs of n = 7 mice/group were pooled). The filled curve (grey) isotype control, the dotted line curve unstimulated and the open curve (white) LPS-stimulated histrogram. The culture supernatants were subject to quantification of the proteins levels of: b IL-12p40 and IL-10 by ELISA. Results are presented as mean value ± SEM of three independent experiments (n = 5 mice/group; *p < 0.05, **p < 0.01 and ***p < 0.001; ANOVA test with a Bonferroni post test comparing sham vs burn-injured mice)
The type of cytokine secreted, such as IL-12p40 and IL-10 production, were also determined in LPS-stimulated CD11c+DCs from sham and burn-injured mice. In the resting state, the basal amount of IL-12p40 was significantly decreased in the burn group (Fig. 4b; p < 0.05). After LPS stimulation, a decrease of 50% of IL-12p40 production in the burn group (444.7 ± 25.6 pg/ml) was noted in comparison to the sham group (846.7 ± 58.1 pg/ml) (Fig. 4b; p < 0.001). In order to further assess the cytokine production by DCs, we have determined IL-10 production in supernatant of LPS-stimulated DCs in both groups (Fig. 4b). No change was observed in unstimulated DCs between each group. However, LPS stimulation induced IL-10 production only in DCs from burned animals by 1.6-fold, to reach 173.2 ± 17.9 pg/ml (Fig. 4b; p < 0.05). Our results demonstrate that burn injury causes a decrease in TLR4 expression of DCs associated with a change in the cytokine production towards to an anti-inflammatory cytokine profile.
Burn injury effects on MAP kinase pathway in total splenic DCs
One potential explanation for the impairment of DCs activity after thermal injury could be related to perturbations in TLR signaling, particularly MAPK pathway, which mediate a broad range of physiological processes, including transcription factor activation, maturation, and cytokine production cytokines [38–40]. Therefore, we observed the consequence of burn at 10 days post-injury on splenic DC MAPK activation following TLR4 activation. In order to rule out an over-stimulation of DCs induced by trauma, cells were incubated in the absence of any stimulation for 1 h before stimulation. LPS (1 μg/ml) exposure enhanced p38 MAPK phosphorylation in DCs from both groups with maximum at 30 min. At the basal level, a significant increase of p38 MAPK phosphorylation is observed in DCs obtained from burned mice when compared to DCs from the sham group (Fig. 5a; p < 0.05). As shown in Fig. 5a, CD11c+ CDs stimulated with LPS showed an increase of phospho-p38 level for both sham and burned mice. However, no significant difference was observed in p38 MAPK phosphorylation between DCs of both groups following LPS exposure. Since SAPK/JNK is also activated by stress stimuli and is implicated in inflammatory cytokine production in DCs, we determined its activation. As expected, the pattern of SAPK/JNK activation is similar to that of p38 MAPK and no difference was observed between CD11c+ DCs from sham and burned mice (Fig. 5b). In contrast to the activation of p38 MAPK and SAPK/JNK, ERK1/2 phosphorylation 30 min following LPS-stimulation is significantly less important in DCs from burned mice as compared to those obtained from sham animals (Fig. 5c; p < 0.05). Overall, our results showed that burn injury is not associated with significant altered level of phospho MAPK in total CD11c+ CDs.
Fig. 5.
Burn injury causes an increase in MAPK phosphorylation in LPS-stimulation CD11c+DCs. On day 10, splenic CD11c+DCs were incubated with 1 μg/ml LPS for the indicated times and p38 (a), SAPK/JNK (b), and ERK1/2 MAPK (c) phosphorylation were measured by western blot as mentioned in “Materials and methods”. Results are presented of three independent experiments. MAPK phophorylation level ratio is presented as mean value ± SEM of three independent experiments (DCs of n = 7 mice/group were pooled, *p < 0.05; unpaired t test comparing sham vs burn-injured mice)
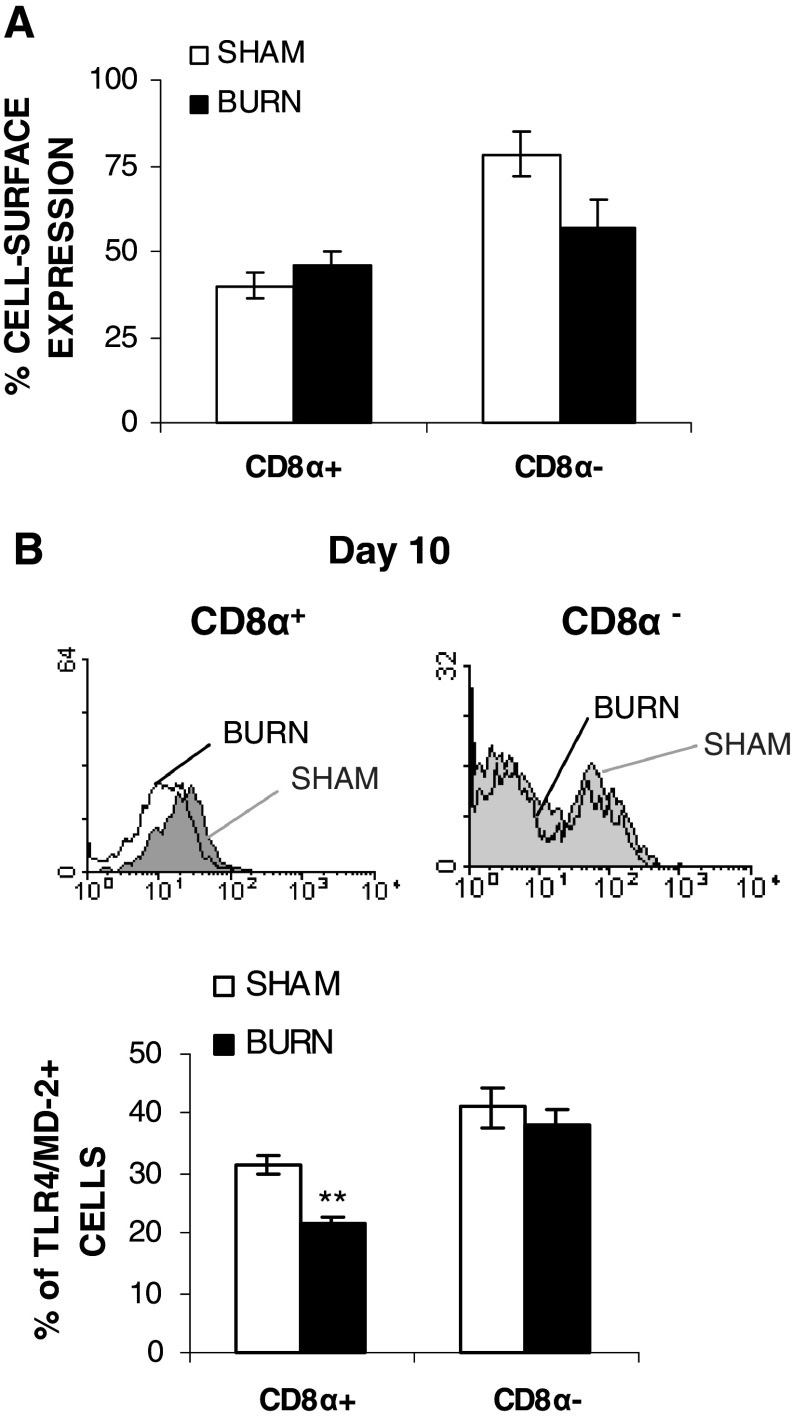
Burn injury affected selectively CD8α+CD11c+DCs subsets
The decreased TLR4 expression and the effect on the MAPK pathway in splenic DCs from burned mice prompted us to determine if more subtle changes can be detected within the DC subsets. At day 10 following burn injury, no significant change was noted in percentage of either the CD8α+CD11c+DC or the CD8α−CD11c+DC sub-populations (Fig. 6a). However, analysis of the TLR4 expression on these subsets revealed that burn injury significantly down-regulated TLR4/MD-2 expression on the CD8α+CD11c+DC sub-population (31.3 ± 1.6 to 21.4% ± 1.3% for sham vs burned mice, **p < 0.01) (Fig. 6b). In contrast, no difference was noted on CD8α−CD11c+DCs (Fig. 6b). These results demonstrated a selective impact of burn injury on TLR4/MD-2 expression on splenic CD8α+CD11c+DC subsets.
Fig. 6.
Effect of burn injury on TLR4/MD2 expression on CD8α+CD11c+DCs and CD8α −CD11c+ sub-populations at day 10. Percentage of splenic CD11c+DC subsets, termed as CD8α+(CD4−)CD11c+ and CD8α−(CD4+)CD11c+ from both sham or burned mice was evaluated at day 10 post-injury (a). Ten days following burn injury, TLR4/MD-2 expression level on both CD8α+(CD4−)CD11c+ and CD8α−(CD4+)CD11c+ from both sham or burned mice was measured by flow cytometry using specific Abs as described in “Materials and methods”. b Results are represented as mean value ± SEM of three independent experiments (n = 7 mice/group; **p < 0.01; Mann–Whitney U test comparing sham vs burn-injured mice). The filled curve (grey) sham and open curve (white) burn TLR4/MD-2 histogram
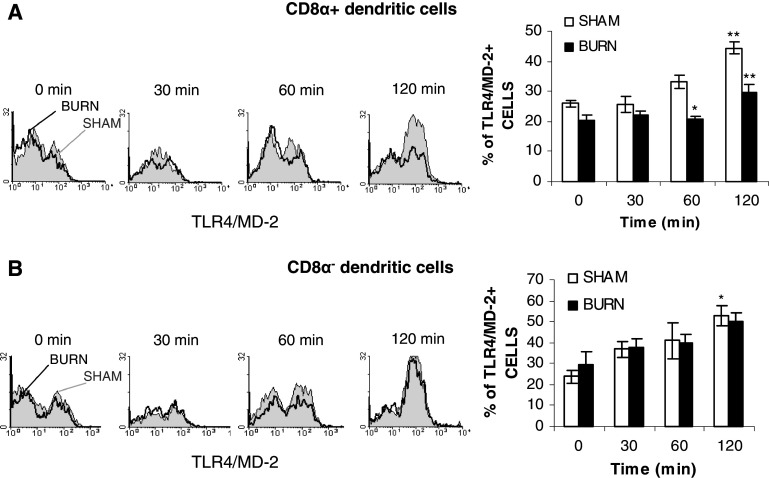
An et al. [10] has reported that TLR4 expression is associated with functional DC states. If burn injury causes impairment of TLR4 modulation on CD11c+DCs and their subsets, this could lead to a DC dysfunctional state. To verify this purpose, DCs isolated from both sham and burned mouse spleen 10 days post-injury were stimulated with the optimal stimulatory dose of LPS (1 μg/ml) for various times. TLR4/MD-2 expression and modulation were analyzed on each DCs subset. LPS stimulation of splenic DCs induced a time-related increase of TLR4/MD-2 expression in total splenic DCs from both groups and no significant change occurs after burn injury (data not shown). This TLR4/MD-2 increase was transient and a normal level of expression was observed at 18 h after LPS stimulation (data not shown). Analysis of the DCs subsets showed that CD8α+CD11c+DCs from burned mice failed to up-regulate TLR4/MD-2 expression in response to LPS (Fig. 7a). After 60 and 120 min of LPS stimulation, significant decrease of TLR4/MD-2 expression on CD8α+CD11c+DCs in burned mice compared to sham mice was observed (Fig. 7a; *p < 0.05 and **p < 0.01). Otherwise, on CD8α−CD11c+DCs, no change was noted in TLR4/MD-2 expression between each group (Fig. 7b). Our results established that burn injury causes a perturbation in up-regulation of TLR4/MD-2 expression on CD8α+CD11c+DCs following LPS-stimulation without a modification in percentage of CD8α+CD11c+DCs.
Fig. 7.
Inhibition of TLR4 up-regulation following LPS stimulation on the CD8α+CD11c+DC sub-population isolated from burned mice 10 days post-injury. Splenic DCs were cultured with 1 μg/ml of LPS at 37°C in a 5% CO2 atmosphere for the indicated times. TLR4/MD-2 expression was measured by flow cytometry on CD8α+(CD4−)CD11c+ DCs (a) and CD8α−(CD4+)CD11c+DCs (b) in total splenic DCs as described in “Materials and methods”. Results are presented as mean value ± SEM of three independent experiments (n = 7 mice/group; *p < 0.05 and **p < 0.01; ANOVA test with a Bonferroni post test comparing sham vs burn-injured mice). The filled curve (grey) sham and open curve (white) burn TLR4/MD-2 histogram
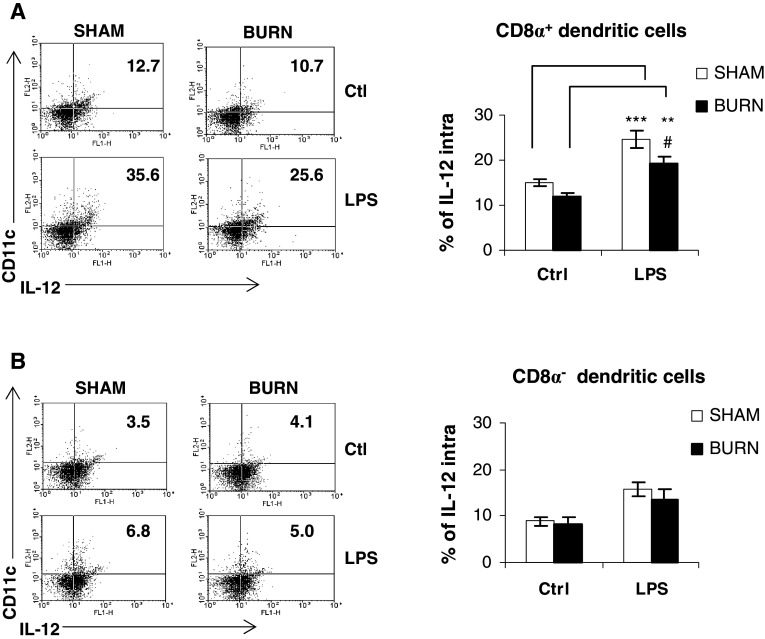
Burn injury suppresses IL-12 production by LPS-stimulated CD8α+CD11c+DCs subsets
The CD8α+ subset of DCs is one of major producers of IL-12 which plays a central role in Th1 response [6]. CD8α−DCs can also produce IL-12 but mediate a Th2 response in the presence of IL-10-producing cells in their microenvironment [6]. It is well documented that burn injury is associated with an increase of the Th2 response on the late phase. Thus, we have determined the capacity of both DC subpopulations to produce IL-12 after LPS-stimulation. For this purpose, the intracellular levels of IL-12p40/p70 in both DC subsets were determined. Following LPS stimulation, a significant increase by 1.6-fold in percentage CD8α+CD11c+DCs positive for intracellular IL-12p40/p70 was noted in the sham group (Fig. 8a; 15.0 ± 0.90 to 24.57 ± 1.93% for unstimulated vs LPS-stimulated, ***p < 0.001). Resting CD8α+CD11c+DCs purified from burned mice have reduced percentage expression of intracellular IL-12p40/p70 when compared to those isolated from sham group. Their stimulation with LPS led to an increase of IL-12p40/p70 synthesis (Fig. 8a; **p < 0.01), but to a lower extent than observed in CD8α+CD11c+DCs from sham animals (Fig. 8a; 24.57 ± 1.93 to 19.23 ± 1.38% for sham vs burned mice, #p < 0.05). The latter significant change represents a 20% decrease in the CD8α+CD11c+DCs response of burned mice (Fig. 8a). In contrast, sham CD8α−CD11c+DCs, either in resting state or after LPS stimulation, have no significant difference, even though a slight increase of IL-12p40/p70 was detected (Fig. 8b). With regards to burn CD8α−CD11c+DCs, no difference was detected between the sham and burn groups (Fig. 8b).
Fig. 8.
Decreased production of IL-12 pro-inflammatory cytokine from LPS-stimulated CD8α+CD11c+DCs isolated 10 days after burn injury. Intracellular IL-12p40/p70 was evaluated on DC subsets CD8α+ (a) and CD8α− (b) after LPS (1 μg/ml) stimulation on both sham and burned mice. Representative FACS plots showing intracellular IL-12p40/p70 on gated CD8α+CD11c+ DCs and CD8α−CD11c+DCs; the numbers in the upper-right quadrants represent the percent-positive staining cells. Results are presented as mean value ± SEM of three independent experiments (n = 4 mice/group, #p < 0.05, **p < 0.01 and ***p < 0.001; ANOVA test with a Bonferroni post test comparing sham vs burn-injured mice)
Discussion
Several studies have established that burn injuries are associated with major complications including immunosuppression which can contribute to the development of septicemia [13, 14, 18]. The contributive factors of the inability of injured patients to resist potential fatal infections remain elusive. Many studies have focused on monocyte/macrophage functions after burn injury but little is known about DC’s involvement in immune regulation in the post-injury period [38]. In an experimental burn model, Kataranovski et al. [41] have noted an increase of DCs in wound-draining lymph nodes at 24–72 h after injury. More recently, Lederer’s group showed that burn injury-primed macrophages and dendritic cells for enhanced TLR2 and TLR4-induced IL-1β, IL-6, and TNF-α production in the spleen [34]. At this time, the consequence of burn injury effect on the innate immune system remained elusive since it was demonstrated that these immune perturbations can be protective in an infection model [42]. In a burn injury model, DC functions in adaptive immune responses have never been determined. In trauma patients, including burned patients, it has been suggested that impaired T cell responses after trauma may be related to a decreased promotion of T cell activation by DCs, subsequent to impairment in DC differentiation from monocyte precursors [43].
The present study was undertaken to evaluate the major role of DCs in the initiation and regulation of immune response to antigen. Analysis of DC number showed a biphasic modulation characterized by a depletion the day following burn injury and a marked increase at day 10. Depletion at day 1 can be due to the stress response involving the high level of glucocorticoid at this time point [44]. It was observed that DCs, particularly those that are CD8+ lymphoid-derived, appear to be lost in the spleens of septic patients and mice 24 h post-trauma [45, 46]. This observed depletion of splenic DCs was caused primarily by the loss of immature DCs rather than mature DCs. They found that this depletion of immature DCs was neither LPS nor FasL dependent [45]. The observation that the depletion of DCs was not FasL sensitive is in keeping with the observation of several groups. Interestingly, polymicrobial sepsis seemed to cause divergent effects on splenic DCs and peritoneal DCs. The peritoneal DCs showed a more active response to septic challenge because the capacity of peritoneal DCs isolated from septic mice to trigger T cell proliferation was enhanced [45]. Therefore, sepsis affected DCs isolated from different organs differently as they are exposed to PAMPs in a different way. In burn sepsis mice, it was observed an impairment of DC development from progenitors, which could also explain the decrease of DCs [47]. However, the exact mechanisms for DC decrease are still unknown. Increase of DCs at 10 days of burn injury is not restricted to DCs, as seen with lymphocytes and myeloid cells [17, 48]. This observed modulation in DC numbers suggested that DCs are perturbed by the stress and tissue damage associated with burn injury in mice. As DCs are important cells in effective T cell response, we reasoned that DC number alterations or APC function might explain, in part, the altered T cell-mediated immunity that occurs after injury.
In the presence of released endogenous danger signals following burn injury—released by tissues undergoing stress, damage, or abnormal death—DCs are primed [3]. We established that ex vivo purified DCs have an increased capacity to stimulate T cells after burn injury. In the model used, the modulation of T cell activation in presence of DCs cannot be due to their capacity to capture and process OVA protein, since we have used an OVA peptide which did not require processing. T cell hybridomas used in this study are constantly more primed in presence of OVA peptide pulsed-differentiated DC than undifferentiated DC or fixed DC, although co-stimulation is not absolutely required [49]. The increased capacity to stimulate T cells suggested to us that burn injury may prime DCs in vivo into a more mature phenotype associated with a release of inflammatory cytokines. However, our results showed neither a significant increase of the maturation state (data no shown) nor an increase of IL-12p40 production of CD11c+ DCs of burned mice that could explain the increased capacity to stimulate T cells. Although DCs respond to primary encounter with PAMPs following burn injury exposure to subsequent pathogens, such as LPS, might render DCs temporarily hyporesponsive to LPS. This phenomenon has been referred to as endotoxin desensitization or LPS tolerance [50]. Interestingly, co-incubation of DCs with LPS decreased their capacity to activate T cells. Importantly, DCs requirement for T cell hybridoma activation was less stringent then those of naïve T cells, suggesting that observed effect of decreasing are underestimated. In future studies, it will be useful to use purified OTII TCR transgenic CD4+ T cells, as they are more sensitive to the altered DC antigen-presentating capacity than the T cell hybridomas. Nevertheless, this result can be related to a desensitized state of DCs. We showed after burn injury a decrease of TLR4 in both mRNA and protein expression on total splenic DCs. LPS-activated DCs from burned mice have decreased release of IL-12 and an increased of IL-10 production when compared to DCs isolated from sham mice. Enhanced IL-10 production by TLR4-primed DCs has been demonstrated after TLR restimulation for DCs [51]. Creus et al. [52] have reported on liver DCs that TLR4 down-regulation resulted in a decreased response to specific ligands, inducing a reduced or altered activation of hepatic adaptive immune responses. This phenomenon of LPS tolerance was also shown on macrophages or monocytes that were no longer capable of responding to LPS stimulation [53]. This suggests that injury may have desensitized DCs to TLR4 stimulation after injury in a manner similar to what has been reported for LPS tolerance. For this study, we have used commercially available LPS that could be contaminated with TLR2 bioactive compounds [54]. Since then, we have used physiological concentrations enabling us to assume that TLR4 is the main receptor involved in our observations. However, a recent study has demonstrated that TLR4 is the main receptor involved in the Gram-negative sepsis, as anti-TLR4 antibodies inhibited lethal endotoxin shock and E. coli sepsis [55]. They have shown that TLR2 was not a key player in the pathogenesis of Gram-negative sepsis even though some Gram-negative endotoxin species are also sensed by TLR2. Indeed, unlike the TLR4−/− mice, the TLR2−/− mice produced an abundant amount of cytokines during E. coli sepsis and had a rapidly fatal clinical course identical to that of wild-type mice.
Down-regulation of TLR4 seemed to be associated with CARS since Paterson et al. [34] showed no marked injury-induced changes in TLR4 expression levels at 1 day after injury corresponding to SIRS. One day after burn injury, we showed no marked difference in TLR4/MD-2 expression on DCs (data not shown), but a decrease of DC number in spleen was noted. Lyons et al. [56] have demonstrated in injured patients (including major burns) increased IL-10 production which correlated with subsequent septic events. This decreased resistance to subsequent infection may be related to a dysregulation of TLR4 signaling on DCs, since it was observed that IL-10 production may suppress IL-12 production by TLR3 and TLR4 signaling in human DCs [57]. Lederer and co-workers reported a slightly increase of intracellular TLR4/MD-2 expression on F4/80+ macrophages at 1 day following burn injury [58]. However, by 7 days after burn injury, the TLR4 mRNA and intracellular TLR4/MD-2 expression on F4/80+ macrophages was clearly increased [58]. They have demonstrated that burn injury primes mouse splenic macrophages and DCs to produce significantly higher levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 in response to in vitro LPS stimulation within 1 day after injury, with a more pronounced increase in response by 7 days after injury [34]. Moreover, it was demonstrated by a higher mortality in burned, but not sham, mice given purified LPS at 7 days after injury, and by negligible mortality when injured mice were challenged on day 1 with the same dose of LPS [59]. Based on these observations, the hyperactivity of macrophages was more important in the late phase of the injury. Macrophage’s hyperactivity was noted by an increase of MAPK activation and an increased production of cytokines [25]. We showed an increase of p38 activation in freshly isolated DCs at 10 days post-injury suggesting that splenic DCs might have been primed by endogenous ligands present after burn injury. LPS-induced TLR4 DCs stimulation showed a trend to increase MAPK (p38 and SAPK/JNK) activation in burned mice compared to sham. In contrast, LPS stimulation induced decreased ERK1/2 MAPK activation in DCs of burned mice. It is surprising that in this study we did not observe the opposite in the profile of MAPK activation since p38 and SAPK/JNK MAPK are implicated in IL-12p40 production and ERK1/2 in IL-10 of DCs. Discrepancy of MAPK activation can be related to maturation/activation of DCs. Indeed, it was demonstrated that SB203580, an inhibitor of p38 MAPK pathway, can inhibit or increase TLR4-mediated IL-12p40 production in function of DC activation/maturation [51]. DCs or macrophages increase of IL-10 production mediated by TLR4 stimulation was associated with ERK1/2 MAPK enhancement and p38 MAPK activity [51, 52]. Another possibility to explain this observation in MAPK pathway in our model is the heterogeneity of the DC preparation used. Indeed, by the methods used, we isolated all splenic DC subsets, and it is likely that only one of three DC populations found in spleen can be affected by burn injury.
The three conventional DC subsets present in mice spleen are CD8α+CD11c+, CD8α−CD11c+, and double negative. All subsets vary in their cytokine profile and, possibly, in their functions [1]. Our analysis of CD8α−CD11c+ at day 10 after burn injury demonstrated no major perturbations of TLR4 expression and IL-12 production at basal level and in their response to LPS. In contrast, we noted for CD8α+CD11c+ a decrease of TLR4/MD-2 expression in freshly isolated cells and an absence of up-regulation expression of this receptor after LPS-induced maturation. Moreover, intracellular IL-12 was decreased after LPS-induced activation in CD8α+CD11c+DCs obtained from burned mice. CD8α+DCs are mainly found in splenic T cell zone [60] and play a dominant role in cross-priming and cross-tolerance, and preferentially induce a Th1-biased response [61]. The Th2-type response to soluble Ags is due to CD8α−DCs [5, 6, 62]. Maldonado-Lopez et al. [6] have demonstrated that CD8α+DCs, in contrast to CD8α−DCs, can produce IL-12 as early as 1 h after in vivo stimulation and in the apparent absence of any priming. Our results indicated that impairment of TLR4 signaling led to decreased IL-12 production of CD8α+CD11c+DCs which could explain the decrease of T cell activation and Th1 pro-inflammatory response following thermal injury. After burn injury, adaptive immune response is characterized by Th2-type response [21, 26]. O’Sullivan et al. [21] have also reported similar findings that major injury leads to a diminished IL-12 production and an increased production of IL-4 and IL-10. This imbalance between Th1 versus Th2 cytokine response has been associated with decreased resistance to infection after burn injury. Presently, experiments are being conducted in our laboratory to determine if post-maturation DCs are capable of inducing differentiation of both Th1 and Th2 cells. The lower number of each DC subsets represents a major limitation and required an important number of mice. For this reason, we are also working to develop an in vitro model to study the endotoxin tolerance or the post-maturation reprogramming of each DC subsets.
Overall, our study showed a dysfunction of the DC subset involved in adaptive immune response after 10 days of burn injury. A better understanding of which DC subset is affected and their impaired functions can lead to development of new therapeutic avenue to reduce the immunosuppressive state in CARS period. Until today, a tolerance state was defined as a deletion of cell functional activity. However, the term “cellular reprogramming’’, previously proposed by Zhang and Morisson [63] to characterize endotoxin tolerance, appears to be the most appropriate one to define the events occurring among circulating leukocytes during critical illness. Our study has shown that burn injury caused a state of reprogramming DC subsets that are no longer capable of responding adequately to any subsequent challenge. Since various populations of DCs are central to the orchestration microenvironment and are involved in the generation of regulatory T cells to preserve their integrity and maintain local homeostasis, a better characterization of the effect of trauma on these populations can result in a better management of secondary response of immune system to injury.
Acknowledgments
This work was supported by a grant from the Fondation des Pompiers du Québec pour les Grands-Brûlés. Michele D'Elia and Julie Patenaude were supported by a research award from the FRSQ-Fondation de la Recherche en Santé du Québec.
References
- 1.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/S0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 4.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–282. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(−) dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 2001;167:4345–4350. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- 7.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 9.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An H, Yu Y, Zhang M, Xu H, Qi R, Yan X, Liu S, Wang W, Guo Z, Guo J, Qin Z, Cao X. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. TAB 2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/S1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 12.Hirata N, Yanagawa Y, Ebihara T, Seya T, Uematsu S, Akira S, Hayashi F, Iwabuchi K, Onoe K. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Mol Immunol. 2008;45:2734–2742. doi: 10.1016/j.molimm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan ST, O’Connor TP. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50:615–623. doi: 10.1016/S0007-1226(97)90507-5. [DOI] [PubMed] [Google Scholar]
- 14.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Barlow Y. T lymphocytes and immunosuppression in the burned patient: a review. Burns. 1994;20:487–490. doi: 10.1016/0305-4179(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 16.Saffle JR, Sullivan JJ, Tuohig GM, Larson CM. Multiple organ failure in patients with thermal injury. Crit Care Med. 1993;21:1673–1683. doi: 10.1097/00003246-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Patenaude J, D’Elia M, Hamelin C, Garrel D, Bernier J. Burn injury induces a change in T cell homeostasis affecting preferentially CD4+ T cells. J Leukoc Biol. 2005;77:141–150. doi: 10.1189/jlb.0703314. [DOI] [PubMed] [Google Scholar]
- 18.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/S1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 19.Efron P, Moldawer LL. Sepsis and the dendritic cell. Shock. 2003;20:386–401. doi: 10.1097/01.SHK.0000092698.10326.6f. [DOI] [PubMed] [Google Scholar]
- 20.O’Suilleabhain CB, Kim S, Rodrick MR, Mannick JA, Lederer JA. Injury induces alterations in T-cell NFkappaB and AP-1 activation. Shock. 2001;15:432–437. doi: 10.1097/00024382-200115060-00004. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan X, Yarmush D, Leeder A, Yarmush ML, Mitchell RN. Burn-induced immunosuppression: attenuated T cell signaling independent of IFN-gamma- and nitric oxide-mediated pathways. J Leukoc Biol. 2008;83:305–313. doi: 10.1189/jlb.0407228. [DOI] [PubMed] [Google Scholar]
- 23.Daniel T, Alexander M, Hubbard WJ, Chaudry IH, Choudhry MA, Schwacha MG. Nitric oxide contributes to the development of a post-injury Th2 T-cell phenotype and immune dysfunction. J Cell Physiol. 2006;208:418–427. doi: 10.1002/jcp.20677. [DOI] [PubMed] [Google Scholar]
- 24.Schwacha MG, Chaudry IH. The cellular basis of post-burn immunosuppression: macrophages and mediators. Int J Mol Med. 2002;10:239–243. [PubMed] [Google Scholar]
- 25.Alexander M, Daniel T, Chaudry IH, Schwacha MG. MAP kinases differentially regulate the expression of macrophage hyperactivity after thermal injury. J Cell Physiol. 2004;201:35–44. doi: 10.1002/jcp.20050. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z, Kavanagh E, Zang Y, Dolan SM, Kriynovich SJ, Mannick JA, Lederer JA. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171:3983–3990. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- 27.Kelly JL, O’Suilleabhain CB, Soberg CC, Mannick JA, Lederer JA. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39–45. doi: 10.1097/00024382-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infect Immun. 2003;71:3058–3067. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J Immunol. 2008;180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 31.Jobin N, Garrel DR, Bernier J. Increased burn-induced immunosuppression in lipopolysaccharide-resistant mice. Cell Immunol. 2000;200:65–75. doi: 10.1006/cimm.2000.1619. [DOI] [PubMed] [Google Scholar]
- 32.McManus AT, Mason AD, McManus WF, Pruitt BA. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4(2):219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 34.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 35.Hugo P, Kappler JW, Godfrey DI, Marrack PC. A cell line that can induce thymocyte positive selection. Nature. 1992;360:679–682. doi: 10.1038/360679a0. [DOI] [PubMed] [Google Scholar]
- 36.Ogura H, Hashiguchi N, Tanaka H, Koh T, Noborio M, Nakamori Y, Nishino M, Kuwagata Y, Shimazu T, Sugimoto H. Long-term enhanced expression of heat shock proteins and decelerated apoptosis in polymorphonuclear leukocytes from major burn patients. J Burn Care Rehabil. 2002;23:103–109. doi: 10.1097/00004630-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Rund TE, Kierulf P, Godal HC, Aune S, Aasen AO. Studies on pathological plasma proteolysis in severely burned patients using chromogenic peptide substrate assays: a preliminary report. Adv Exp Med Biol. 1984;167:449–454. doi: 10.1007/978-1-4615-9355-3_39. [DOI] [PubMed] [Google Scholar]
- 38.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–2182. doi: 10.1182/blood.V98.7.2175. [DOI] [PubMed] [Google Scholar]
- 40.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 41.Kataranovski M, Nikolic T, Velickovic M, Colic M, Pejnovic N, Vojinovic J. Increased activity of lymph node cells in experimental thermal injury: changes in accessory cells in injured area-draining lymph nodes. Burns. 2000;26:525–534. doi: 10.1016/S0305-4179(00)00018-8. [DOI] [PubMed] [Google Scholar]
- 42.Maung AA, Fujimi S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, Stallwood C, Onderdonk AB, Mannick JA, Lederer JA. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J Immunol. 2008;180:2450–2458. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 43.Zhang PZ, Qin FJ, Ma CX, Su H, Yu DN, Li C. Characterization and biological significance of peripheral blood dendritic cell subsets in patients with severe burn. Zhonghua Yi Xue Za Zhi. 2007;87:2275–2277. [PubMed] [Google Scholar]
- 44.D’Elia M, Patenaude J, Hamelin C, Garrel DR, Bernier J. Corticosterone binding globulin regulation and thymus changes after thermal injury in mice. Am J Physiol Endocrinol Metab. 2005;288:E852–E860. doi: 10.1152/ajpendo.00407.2004. [DOI] [PubMed] [Google Scholar]
- 45.Ding Y, Chung C-S, Newton S, Chen Y, Carlton S, Albina J, Ayala A. Polymicrobial sepsis induces different effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–144. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 47.Muthu K, He LK, Melstrom K, Szilagyi A, Gamelli RL, Shankar R. Perturbed bone marrow monocyte development following burn injury and sepsis promote hyporesponsive monocytes. J Burn Care Res. 2008;29:12–21. doi: 10.1097/BCR.0b013e31815fa499. [DOI] [PubMed] [Google Scholar]
- 48.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23:115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 49.Met O, Buus S, Claesson MH. Peptide-loaded dendritic cells prime and activate MHC-class I-restricted T cells more efficiently than protein-loaded cross-presenting DC. Cell Immunol. 2003;222:126–133. doi: 10.1016/S0008-8749(03)00128-X. [DOI] [PubMed] [Google Scholar]
- 50.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanagawa Y, Onoe K. Enhanced IL-10 production by TLR4- and TLR2-primed dendritic cells upon TLR restimulation. J Immunol. 2007;178:6173–6180. doi: 10.4049/jimmunol.178.10.6173. [DOI] [PubMed] [Google Scholar]
- 52.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 53.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 54.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 55.Roger T, Froidevaux C, Le Roy D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci USA. 2009;106:2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226:450–458. doi: 10.1097/00000658-199710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Re F, Strominger JL. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol. 2004;173:7548–7555. doi: 10.4049/jimmunol.173.12.7548. [DOI] [PubMed] [Google Scholar]
- 58.Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 59.Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, Lederer JA. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 60.Neuenhahn M, Busch DH. Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens. Immunol Lett. 2007;114:66–72. doi: 10.1016/j.imlet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065X.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 62.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/S1471-4906(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 64.Robinson SP, Stagg AJ (eds) (2001) Dendritic cell protocols. Methods in molecular medicine, vol 64. Humana Press, New Jersey