Abstract
A growing number of publications show that apoptosis induction is often associated with increased autophagy indicating the existence of an interplay between these two important cellular events. The simultaneous activation of both phenomena has been detected not only in experimental settings but also in vivo under physiological and pathological conditions. Despite these studies, the reciprocal influence of the two pathways in vivo has still not been completely understood. It is clear that autophagy and apoptosis are strictly interconnected, as highlighted by the finding that the two pathways share key molecular regulators. Many novel aspects of the crosstalk between apoptosis and autophagy have recently emerged showing how complex is this relationship and how critical is for the overall fate of the cell. In this mini-review we will focus on some key experiments trying to decipher as to whether autophagy contributes to apoptosis modulation in vivo.
Keywords: Ambra1, Beclin1 protein complex, ATG proteins, BH3-only proteins, p53, ER stress, Genotoxic stress
Introduction
It has been suggested that autophagy under specific circumstances, resulting in the total self-digestion of the cell, can act as one of several cell death types (also known as type II cell death [1–3]). Indeed, a type of programmed cell death distinct from apoptosis and necrosis has been reported in vivo in the course of insect metamorphosis, which has been defined as autophagic cell death [3, 4]. However, whether autophagy induction in dying cells actually causes death, or whether it simply occurs as a process alongside it, is still a controversial issue [1]. Many review articles recently appeared in which the authors tried to reconcile the observations concerning the role of autophagy as a survival/death mechanism [1, 2, 5]. It is becoming clear that, in a given cell, autophagy may contribute differently to cell death induction accordingly to the type and degree of environmental changes or stress stimuli. A cell’s response may shift gradually from the elimination of damaged proteins/organelle by autophagy, which leads to its recovery, to the induction of apoptotic pathways determining cell’s demise. In this review, we will focus on selected examples of the in vivo interplay between apoptosis and autophagy under physiological and pathological conditions. For a role of autophagy and other forms of cell death in the control of infections, we refer to Bortoluci and Medzhitov in this issue [6].
The autophagic process
In cell biology, the term autophagy defines the catabolic process regulating the degradation of a cell’s own components through the lysosomal machinery [1, 2]. It is a genetically regulated process that plays an important homeostatic role in cells preserving the balance between the synthesis, degradation, and subsequent recycling of cellular components [7]. Although autophagy was first described in the early 1960s by Christian de Duve [8], the comprehension of its genetic basis remained elusive until mid-1990s when about 30 autophagy-related genes (ATG) were identified in yeast by Oshumi and coworkers [7, 9]. Among them, 18 are indispensable for formation of the autophagosome [9]. The discovery of the basic elements of the autophagy genetic pathways has brought about a renaissance in the studies dealing with this basic cellular process.
It is well defined that autophagy is a complex phenomenon that encompasses at least three distinct autophagic processes. In fact, it may occur via different biochemical and cellular pathways: macroautophagy microautophagy, and chaperone-mediated autophagy (CMA) [1, 2, 5, 10]. Although all three forms of autophagy ultimately end with the degradation of substrates within lysosomes, each one has unique biological features:
Macroautophagy is the most studied mechanism of autophagy involving the formation of a double membraned structure (autophagosome or autophagic vacuole) around a targeted region of the cell, separating the contents from the rest of the cytoplasm [1, 2]. It may involve the sequestration of cytosolic proteins, sugars, lipids, RNA, and organelles, such as mitochondria and perixosomes, into autophagosomes that deliver their contents to the lysosomes [1, 5, 10]. Autophagosomes are formed from the elongation and closure of small membrane structures, known as isolation membranes, probably originating from PI3P-rich microdomains (omegasomes) located on the endoplasmic reticulum [11]. The outer membrane of the autophagosome fuses with a lysosome in the cytoplasm to form an autolysosome or autophagolysosome where its contents are degraded via acidic lysosomal hydrolases [1, 5, 10].
Microautophagy takes place when lysosomes directly engulf cytoplasmic components by invaginating protrusions of their membrane. It involves the pinocytosis of small quantities of cytosol directly by lysosomes [12].
In Chaperone-mediated autophagy, or CMA, only proteins that have a consensus peptide sequence are recognized by the binding of the HSC70-containing chaperone/co-chaperone complex [12]. In fact, this type of autophagy involves the selective targeting of proteins containing a KFERQ-like peptide motif to lysosomes for degradation The substrate/chaperone complex moves to the lysosomes, where the CMA receptor lysosome-associated membrane protein type-2A (LAMP-2A) recognizes it; the protein is then unfolded and translocated across the lysosome membrane assisted by the lysosomal hsc70 on the other side [12]. CMA differs from macroautophagy and microautophagy since the substrates are transported across the lysosomal membrane on a one-by-one basis, whereas in the macroautophagy and microautophagy, the substrates are engulfed or sequestered in bulk. In addition, CMA is very selective in that it degrades only single proteins and not organelles.
Regulation of macroautophagy in mammals and its interactions with apoptosis
The dissection of the molecular mechanisms regulating the interplay between autophagy and apoptosis in determining the cell fate is at the moment one of the hot topics in cell biology. The original concept that inhibition of apoptosis results in autophagic/necrotic cell death is now being extended, and it is becoming clear that apoptosis and autophagy can act as partners to induce cell death in a coordinated or cooperative fashion [13, 14]. In fact, it has been shown that autophagy proteins can also play a role in cellular events that occur during apoptosis. On the other hand, inhibition of autophagy leads in most cases to an increase susceptibility to apoptotic stimuli, highlighting the pro-survival role of the autophagic process [1, 13, 14]. Moreover, a growing number of proteins that play a negative regulatory role in both events have been identified [15].
Two signalling pathways, the class I and III phosphatidylinositol 3-kinases (PI3K) play important opposite roles in the control of autophagy, with the first one having a repressive effect, with the second being essential for autophagy induction (Fig. 1). The class I PI3K pathway promotes normal cell growth, and its constitutive activation is implicated in tumour development [16, 17]. Other oncoproteins involved in this network, such as Ras and Akt, have been shown to inhibit autophagy. At the same time, class I PI3K negatively regulates apoptosis at different levels, i.e. by Akt-mediated phosphorylation of the Bcl-2 family member Bad [15, 18]. On the other hand, tumor suppressor genes like PTEN, TSC1, and TSC2 play a negative role in class I PI3K signaling networks and are able to stimulate autophagy [1, 2, 15, 19].
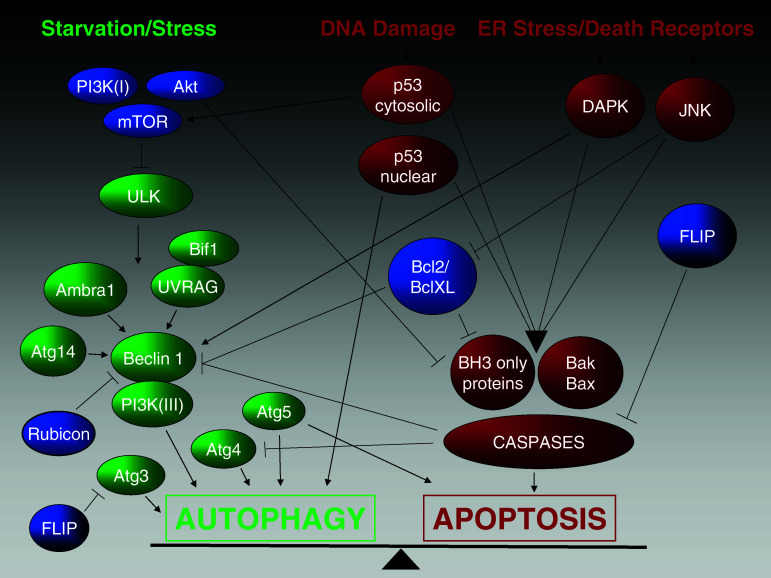
Fig. 1.
A schematic model illustrating the crosstalk between apoptosis (red) and autophagy (green) in mammals showing the major players of each pathway that have been shown to also directly influence the other one. Some proteins negatively controlling one or both pathways are also included (blue)
The principal link between PI3K/Akt and the suppression of autophagy is the induction of the target of rapamycin (mTOR) signaling. mTOR is a conserved Ser/Thr kinase that controls cell growth by activating an array of anabolic processes including protein synthesis, transcription, and ribosome biogenesis, and by inhibiting catabolic processes such as mRNA degradation and autophagy [1, 2, 20]. On the other hand, mTOR activity is negatively regulated by multiple stress signals including hypoxia and lack of nutrients, which are inducers of autophagy [20].
At molecular levels, mTOR activity is regulated by the interaction with Rheb, a small GTP-binding protein that activates mTOR in its GTP-bound form [21]. The GTP hydrolysis, promoted by the TSC1/TSC2 dimer formation, inactivates Rheb, thus negatively regulating mTOR kinase activity. Growth factors induce phosphorylation and inactivation TSC1/TSC2 via the class I PI3K/AKT pathway, thus allowing mTOR activation by Rheb and consequent inhibition of autophagy [21, 22]. Conversely, AMPK, which is activated by the high AMP/ATP ratios present when nutrients are limited, induces autophagy through a phosphorylation event that stimulates TSC1/TSC2 activity [23].
The regulation of autophagy operated by mTOR occurs primarily at the initiation step, and involves the phosphorylation and inhibition of ULK kinases (ULK1 and ULK2, the orthologs of the yeast Atg1 kinase) and their associated protein ATG13 [23, 24]. The activation of ULK kinases is an early regulatory step that is essential for the induction of many downstream autophagic events [23, 24]. ULKs form a complex with Atg13 and FIP200, a putative mammalian counterpart of yeast Atg17. The binding of Atg13 stabilizes and activates ULK facilitating the phosphorylation of FIP200 by ULKs [23, 24]. However, the full comprehension of ULK kinases function requires the complete identification of their autophagy-specific protein substrates.
Following the ULK kinases induction, the Beclin1-Class III PI3K complex is activated to produce PI3-P-enriched membranes domains (omegasomes) which act as platforms able to recruit factors required for autophagosomes assembly, namely Atg5-12-16 complex, LC3, and Atg9 [9, 24, 25]. The activity of the Beclin 1-Class III PI3K complex is regulated by the interaction of a series of co-factors, either evolutionarily conserved, such as UVRAG and Atg14/Barkor [26, 27], or with no homologs so far identified in invertebrates, such as Ambra1 and Rubicon [28, 29]. Atg14 and Ambra1 have been shown to be important for Class III PI3K activity required for autophagosome formation, while UVRAG and Rubicon seem to be more directly involved in Class III PI3K-regulated endosomal trafficking; however, the precise role of UVRAG in autophagy induction remains controversial [29]. An important role in the formation of the autophagosome is played by Bif-1 [30]. Bif-1 is a BAR domain-containing protein belonging to the endophilin family, which, by binding membranes, causes their curvature. By interacting with UVRAG, Bif-1 is recruited on the Beclin 1-Class III PI3K complex, indicating that, besides its role in lipid phosphorylation, this complex could also engage factors required for autophagosome expansion [30].
It is important to note that Beclin 1-mediated induction of autophagy is inhibited by the antiapoptotic Bcl-2 family members; interestingly, this interaction involves the binding of Bcl-2 to a BH3 domain present in Beclin 1 [31]. Therefore, the protein–protein interaction network dependent on BH3 domains present in many pro- and anti-apoptotic proteins does not seem to be exclusive for the regulation of the apoptotic process but is also involved in the induction of autophagy [31]. In line with this observation, the BH3-only proteins BAD, BNIP3, and BNIP3-like (BNIP3L), also known as NIX, have been shown to play a role both in cell death and autophagy [32, 33]. The molecular mechanisms by which BNIP3 induces both processes are not well understood. BNIP is a weak inducer of cell death and its activity is primarily determined by its transmembrane domain which facilitates the opening of the mitochondrial permeability transition pores. At variance with typical BH3-only proteins causing apoptotic cell death, BNIP3 induces a necrotic type of cell death. On the other hand, BNIP3 and NIX are important for the regulation of the mitochondrial homeostasis by regulating mitophagy, the selective degradation of mitochondria by autophagy [32]. Thus, BNIP3 exhibits a dual nature; it induces cell death by necrosis, and participates in the induction of autophagy, possibly by affecting the binding of Beclin 1 to the Bcl-2 family members [32]. It has recently been shown that the p53-inducible BH3-only protein PUMA, a key mediator DNA damage-dependent apoptosis, is also able to induce mitophagy [34].
Other key effectors regulating the crosstalk between apoptosis and autophagy are the JNK and DAP kinases, which, by catalyzing the phosphorylation of several substrates, plays a major role in apoptosis regulation [34–36]. JNK is able to trigger autophagy by targeting Bcl2/BclXL proteins and abrogating their binding to Beclin 1 [36]. Recently, Beclin 1 has been shown to be among the substrates of DAP kinase, and its phosphorylation reduces its binding to the Bcl-2 family members, thus suggesting a possible mechanism by which DAPk may also induce autophagy [37].
LC3, the mammalian homologue of yeast Atg8, and Atg5/Atg12 play an essential role in the expansion of the autophagosomes [1, 2]. Two conjugation systems are essential for the localization of these proteins on the pre-autophagosomal membrane. In the first step, Atg5 is covalently conjugated to Atg12, a ubiquitin-like protein, a reaction facilitated by Atg7 [5, 7, 9]. The Atg12-Atg5 dimer then binds Atg16L, forming a large protein complex, which is recruited to the forming autophagosome’s isolation membrane through Atg16L [5, 7, 9]. In the second conjugation system, Atg7 and Atg3 mediate the conjugation of Atg8 (mammalian LC3) to the phosphatidylethanolamine (PE). Lipidation of Atg8, which occurs only as a consequence of autophagy induction, converts Atg8 from its soluble, cytoplasmic form (LC3-I) to the membrane-bound, autophagosome-associated form (LC3-II) [9].
Atg5, in addition to its essential role in autophagy, can also act as a positive/negative effector of apoptotic pathways. The effect of Atg5 deficiency on cell death regulation varies accordingly to the apoptotic stimulus. In fact, Atg5-deficient mouse embryo fibroblasts (Atg5−/− MEFs) are more sensitive to cell death triggered by death receptor or starvation than WT MEFs. By contrast, Atg5−/− MEFs are more resistant to cell death induced by genotoxic insults (UV) and stress (menadione and H2O2) [38]. Of note, ERK1/2 is markedly activated in Atg5−/− MEFs, and its inhibition by a MEK1 inhibitor or dominant negative ERK2 enhanced the susceptibility of Atg5−/− MEFs to cell H2O2-induced cell death [38]. Another apoptotic pathway showing a strong crosstalk with autophagy is the one induced by ER stress. It has recently demonstrated that, in cancer cells, autophagy is upstream to apoptosis in ER stress-induced death. Interestingly, the ablation of Ambra1 and ATG5, which inhibit autophagy, reduces apoptosis in glioma cells treated with cannabinoids [39]. DAPk has recently been identified as an important component in the ER stress-induced cell death pathway. In fact, DAPk−/− mice are protected from ER stress induction [40]. Both caspase activation and autophagy induction, events that are activated by ER stress and precede cell death, are significantly attenuated in the DAPk null cells. Notably, in this system, autophagy acts as a parallel cell-killing mechanism in concert with apoptosis, as the depletion of Atg5 or Beclin 1 from fibroblasts significantly protected cells from ER stress-induced death when combined with caspase-3 depletion [40].
A proposed molecular mechanism connecting autophagy and apoptosis, which seems to be independent from the cell death stimulus, is the cleavage of Atg5 by calpain [13]. Apoptosis induction results in the translocation of its newly generated N-terminal product to the mitochondria, where it interacts with Bcl-XL and promotes cytochrome C release and caspase activation [13]. By this mechanism, apoptosis may limit the pro-survival function of autophagy enhancing its killing power.
Alternatively, it has been recently shown that, in T cells, the induction of autophagy may result in the formation of a DISC-like complex including Atg5–12/Atg16L, FADD, caspase 8, and RIPK1. The assembly of this protein complex counteracts the hyperactive autophagic induction consequent to T cell activation that, if uncontrolled, results in “necroptotic” cell death [41]. Altogether, these findings point out Atg5 as a key molecule connecting the autophagic with the apoptotic pathways.
It is interesting to note that ATG5 is not the only autophagy protein to be cleaved during apoptosis induction. Recently, both Beclin 1 and ATG4d have been shown to be targets of caspases [42, 43]. Cleavage of Beclin 1 results in the inhibition of its autophagic activity, a proposed mechanism to prevent the prosurvival function of autophagy once apoptosis has started. Instead, caspase-cleaved ATG4d unmasks a potential BH3 domain of the protein, causing its translocation to mitochondria where it acts, similarly to ATG5, as a proapoptotic factor.
The final delivery of mature autophagosomes to the lysosomes is achieved by a machinery shared with other vesicle trafficking pathways. In this context, it is relevant to note the function carried out by UVRAG as a partner of the Class C Vps/HOPS proteins involved in the fusion machinery [44]. It is further interesting to note that the crosstalk with apoptosis also takes place in these late stages of autophagy. In fact, it has been shown that the proapoptotic protein p53 controls the expression of DRAM, a lysosomal protein, critical for its ability to induce autophagy [45].
Lessons from knockout mice
The most reliable clues about the importance of autophagy for cell death induction in mammals derive from knockout studies in mice deprived of key regulatory autophagy genes. Interestingly, not all the knockout mice display lethal effects showing quite distinct phenotypes, thus suggesting that some of these genes may have additional functions whose impairment causes embryonic mouse defects. For example, Ambra1, Beclin 1, and FIP200 knockout mice die during development showing an increased numbers of apoptotic cells [28, 46, 47], thus indicating that the crosstalk between autophagy and apoptosis already takes place during embryogenesis. Notably, Ambra1 and Beclin 1 have been shown to control cell growth, in addition to regulating the formation of the phagophore [28, 46]. By contrast, mice deficient for either Atg5 or Atg7 appear normal at birth but die within the first day after delivery [48, 49]. These deficient neonates exhibit reduced amino acid concentrations in plasma and tissues, and display defects in the maintenance of energy homeostasis that is essential for survival during neonatal starvation [46, 48].
The majority of information about the interplay between autophagy and apoptosis derives from the tissue-specific ablation of Atg5 and Atg7 genes. Mice lacking either Atg5 or Atg7 in the central nervous system show behavioral and movement defects, and die within 28 weeks after birth [46, 48]. These deficiencies cause massive neuronal death in the cortex and cerebellum indicating that autophagy is essential for the survival of neural cells, and that its impairment is implicated in the pathogenesis of neurodegenerative disorders. Similarly, neural-specific deletion of FIP200, the ortholog of yeast autophagy protein Atg17, results in cerebellar degeneration accompanied by progressive neuronal death and neurite degeneration [47].
Apoptosis plays a fundamental role in the homeostasis of the immune system by shaping the immunological repertoire in the embryo as well as in adult tissues. Notably, in Atg5-deficient mice, although lymphocytes underwent full differentiation, the numbers of thymocytes and peripheral T and B lymphocytes were reduced [50]. Atg5−/−CD8 + T lymphocytes display a drastic increase in cell death, and both Atg5−/− CD4+ and CD8+ T cells failed to undergo efficient proliferation after TCR stimulation [50]. These results indicate a critical role for autophagy in multiple aspects of lymphocyte function and suggest that its activation is essential for lymphocyte survival and proliferation.
The heart is another organ in which autophagy plays an essential homeostatic role, its impairment causing cardiac dysfunction and the development of cardiomyopathy [51]. In fact, cardiac-specific deficiency of Atg5 induces hypertrophy, left ventricular dilatation, and contractile dysfunction, accompanied by increased levels of ubiquitination [51]. This functional deficiency is paralleled by the appearance of a large number of TUNEL-positive cardiomyocytes [52]. It is important to mention that the in embryogenesis heart-specific Atg5-deficiency showed no such cardiac phenotypes. In keeping with this finding, the conditional null mice developed cardiac dysfunction and left ventricular dilatation only 1 week after treatment with pressure overload [52]. These results indicate that autophagy under physiological conditions plays a protective mechanism in the heart, maintaining cardiomyocyte size and global cardiac structure and function.
The complexity of the interplay between autophagy and apoptosis and its importance in embryogenesis has also been highlighted in embryoid bodies derived from cells lacking the autophagy genes, Atg5 or beclin 1 [53]. These embryonal structures, which resemble the blastocysts, in the absence of autophagy are unable to undergo cavitation, one of the earliest event involving apoptosis in mammalian development [53]. It has been suggested that this defect is due to persistence of cell corpses, rather than impairment of apoptosis. In fact, apoptotic cells in autophagy gene null embryoid bodies fail to express the proper “eat-me” signals required for the clearance of apoptotic bodies, and secrete lower levels of the “come-get-me” signal, lysophosphatidylcholine [53], thus suggesting that autophagy contributes to dead-cell clearance, at least during the early stages of during mammalian development.
Another interesting link between the Bcl2 family and autophagy is that derived from the study on NIX (BNIP3L), a Bcl-2-related protein that is upregulated during terminal erythroid differentiation [32]. Analysis of NIX-deficient mice has revealed a defect in erythroid development that is not directly related to the regulation of cell death but to an impairment of mitochondrial clearance during erythrocytes differentiation. Interestingly, NIX does not function through established proapoptotic pathways nor does it mediate the induction of autophagy in erythroid cells. Rather, NIX is required for the selective incorporation of mitochondria into autophagosomes [32].
Autophagy/apoptosis crosstalk in tumors
One of the major clues today is the comprehension of the crosstalk between autophagy and apoptosis in tumor development [10]. In fact, oncogenesis and tumor survival are influenced by perturbations of the molecular machinery that controls both processes. Several tumor suppressor proteins (e.g., beclin 1, Bif-1, BH3-only proteins, DAPk, PTEN, UVRAG, and p19ARF) promote the autophagic pathway [15, 31]. Conversely, numerous oncoproteins, including phosphatidylinositol 3-kinase, Akt1, and anti-apoptotic members of the Bcl-2 family, suppress autophagy. Recently, it has been shown that both cFLIP and vFLIP of Kaposi’s sarcoma-associated herpesvirus, in addition to inhibiting apoptosis mediated by death receptors, suppress autophagy by preventing Atg3 from binding and processing LC3 [54]. These findings identify another negative regulatory step common to autophagy and apoptosis, besides the antiapoptotic Bcl2 family proteins, which can play a fundamental role facilitating the early stages of oncogenesis.
A complex role in the regulation of autophagy is played by p53, one of the most important tumor suppressor proteins [55]. In fact, p53 regulates autophagy both in a positive and negative fashion, depending on its subcellular localization [56].
Irrespective of the status of p53, basal levels of autophagy appear to inhibit tumor development. Autophagy may contribute to the prevention of tumorigenesis by degrading damaged proteins and organelles that might cause genomic instability [57]. Indeed, cells with deletion of Beclin1 or Atg5 display increased DNA damage, gene amplification, and aneuploidy. A major role in this process is played by p62. Inhibition of autophagy leads to an increase of p62 which promotes tumorigenesis by inducing oxidative stress and NFkB activation [58].
On the contrary, chemotherapy- and metabolic stress-induced activation of the autophagic pathway reportedly contribute to the survival of formed tumors, thereby favoring resistance. To address this issue, the role of autophagy in a Myc-induced model of lymphoma generated from cells derived from p53 null mice was examined [57]. Such tumors are resistant to apoptosis due to a lack of nuclear p53. De novo expression of p53 via a tamoxifen-inducible transgene led to tumor regression followed by tumor recurrence. Activation of p53 was associated with the rapid appearance of apoptotic cells and the induction of autophagy in surviving cells. Inhibition of autophagy by Atg5 short hairpin RNA enhanced the ability of either p53 activation or alkylating drug therapy to induce tumor cell death [59]. These studies provide evidence that autophagy serves as a survival pathway in tumor cells treated with apoptosis activators and propose a rationale for the use of autophagy inhibitors in combination with therapies designed to induce apoptosis in human cancers. Autophagy inhibition would therefore represent a major therapeutic target for chemosensitization. Future studies should further clarify the molecular mechanisms at the basis of the interplay between apoptosis and autophagy which will be an important source of molecular targets for clinical applications.
Concluding remarks
Although in the last 10–15 years the question whether autophagy may indeed act as bonafide cell death mechanism has stimulated a great debate in the scientific community, much remains unanswered. Considering that autophagy physiologically ensures the metabolic supply to starving cells by degrading intracellular components, it may contribute to generate metabolites by sacrificing not only some components of a cell but it can also, under long-lasting extreme conditions to sustain viability, kill the whole cell, and the organism may utilize xeno-cannibalism as ultima ratio to survive [60]. Indeed, this type of phenomena has been reported under pathological conditions in eukaryotic cells. Although, in many cases, enhanced autophagy has been associated with cell death, most of these reports failed to demonstrate a causal role for autophagy in initiating a killing pathway. However, in some instances, the inhibition of autophagic proteins leads to a decreased cell death rate, clearly indicating a pro-death effect of these factors. On the other hand, in many physiological settings, autophagy acts, by promoting cell survival, as an antagonist of apoptosis. As outlined in this mini-review, a complex relationship exists between autophagy and the apoptotic cell death pathway, where regulators of apoptosis also function as regulators of autophagic activation. Accordingly, understanding the effects of this complex interplay on pathological settings might unveil novel important strategies for therapeutic approaches.
Acknowledgments
This work was supported by grants from the Ministry of Health of Italy “Ricerca Corrente” and “Ricerca Finalizzata” to G.M.F. and M.P., EU Marie Curie grant “TRACKS”, AIRC, Banca S. Paolo and Fondazione Telethon to M.P. The support of the EU grant “Apo-Sys” to M.P. is also acknowledged.
References
- 1.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 3.Zakeri Z, Bursch W, Tenniswood M, Lockshin RA. Cell death: programmed, apoptosis, necrosis, or other? Cell Death Differ. 1995;2:87–96. [PubMed] [Google Scholar]
- 4.Baehrecke EH. Autophagic programmed cell death in Drosophila. Cell Death Differ. 2003;10:940–945. doi: 10.1038/sj.cdd.4401280. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortoluci KR , Medzhitov R (2010) Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci (in press) [DOI] [PMC free article] [PubMed]
- 7.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 8.Deter RL, Baudhuin P, de Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol. 1967;35:11–16. doi: 10.1083/jcb.35.2.C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae . FEBS Lett. 2007;58:2156–2161. doi: 10.1016/j.febslet.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 10.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Simonsen A, Stenmark H. Self-eating from an ER-associated cup. J Cell Biol. 2008;182:621–622. doi: 10.1083/jcb.200807061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massey AC, Zhang C, Cuervo AM. Chaperone-mediated autophagy in aging and disease. Curr Top Dev Biol. 2006;73:205–235. doi: 10.1016/S0070-2153(05)73007-6. [DOI] [PubMed] [Google Scholar]
- 13.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 14.Ullman E, Fan Y, Stawowczyk M, Chen H, Yue Z, Zong W. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ. 2008;15:422–425. doi: 10.1038/sj.cdd.4402234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 16.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae . J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 19.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 20.Díaz-Troya S, Pérez-Pérez ME, Florencio FJ, Crespo JL. The role of TOR in autophagy regulation from yeast to plants and mammals. Autophagy. 2008;4:851–865. doi: 10.4161/auto.6555. [DOI] [PubMed] [Google Scholar]
- 21.Arsham AM, Neufeld TP. Thinking globally and acting locally with TOR. Curr Opin Cell Biol. 2006;18:589–599. doi: 10.1016/j.ceb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 23.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita M, Suzuki NN, Obara K, Fujioka Y, Ohsumi Y, Inagaki F. Structure of Atg5.Atg16, a complex essential for autophagy. J Biol Chem. 2007;282:6763–6772. doi: 10.1074/jbc.M609876200. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumor suppressor activity of a novel Beclin 1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 2008;105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, Gruss P, Piacentini M, Chowdhury K, Cecconi F. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 29.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, Yoshimori T. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Meyerkord CL, Wang HG. Bif-1/Endophilin B1: a candidate for crescent driving force in autophagy. Cell Death Differ. 2009;16:947–955. doi: 10.1038/cdd.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA-and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135–1145. doi: 10.1038/cdd.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bialik S, Kimchi A. The death-associated protein kinases: structure, function and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Sinha S, Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4:949–951. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A. DAPk-mediated phosphorylation on the BH3 domain of Beclin-1 promotes dissociation of Beclin-1 from Bcl-XL to induce autophagy. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyo JO, Nah J, Kim HJ, Lee HJ, Heo J, Lee H, Jung YK. Compensatory activation of ERK1/2 in Atg5-deficient mouse embryo fibroblasts suppresses oxidative stress-induced cell death. Autophagy. 2008;4:315–321. doi: 10.4161/auto.5525. [DOI] [PubMed] [Google Scholar]
- 39.Salazar M, Carracedo A, Salanueva IJ, Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C, Torres S, García S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, González-Feria L, Iovanna JL, Guzmán M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Sabanay H, Mizushima N, Yoshimori T, Kimchi A. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–1886. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 41.Bell BD, Walsh CM. Coordinate regulation of autophagy and apoptosis in T cells by death effectors: FADD or foundation. Autophagy. 2009;5:238–240. doi: 10.4161/auto.5.2.7512. [DOI] [PubMed] [Google Scholar]
- 42.Betin VM, Lane JD. Caspase cleavage of Atg4D stimulates GABARAP-L1 processing and triggers mitochondrial targeting and apoptosis. J Cell Sci. 2009;122:2554–2566. doi: 10.1242/jcs.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo S, Rubinsztein DC (2009) Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ Aug 28 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 44.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crighton D, Wilkinson S, Ryan KM. DRAM links autophagy to p53 and programmed cell death. Autophagy. 2007;3:72–74. doi: 10.4161/auto.3438. [DOI] [PubMed] [Google Scholar]
- 46.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang CC, Wang C, Peng X, Gan B, Guan JL (2009) Neural specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem. Nov 24. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 48.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 49.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 52.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 53.Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, Gilpin C, Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR, Chang H, Zhou FC, Gao SJ, Liang C, Jung JU. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11:1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green DR, Kroemer G. Cytoplasmic functions of the tumor suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matarrese P, Ciarlo L, Tinari A, Piacentini M, Malorni W. Xeno-cannibalism as an exacerbation of self-cannibalism: a possible fruitful survival strategy for cancer cells. Curr Pharm Des. 2008;14:245–252. doi: 10.2174/138161208783413239. [DOI] [PubMed] [Google Scholar]