Abstract
Melatonin has been proposed as regulating the immune system by affecting cytokine production in immunocompetent cells, enhancing the production of several T helper (Th)1 cytokines. To further investigate the melatonin’s role in IL-2/IL-2R system, we established an inducible T-REx expression system in Jurkat cells in which the protein levels of HIOMT enzyme or MT1 receptor were significantly down-regulated upon tetracycline incubation. We found that T-REx Jurkat cells with lower levels of HIOMT activity, and consequently lower content of endogenous melatonin, showed IL-2 production decrease after activation with lectin. Likewise, tetracycline-inducible stable cell line expressing MT1 antisense produced decreased amounts of IL-2 (mRNA and protein levels) after stimulation. Moreover, in T-Rex-MT1 cells incubated with tetracycline, a sub-optimal PHA dose failed to induce the early activation marker CD25 on the cell surface. The results shown here support the relevance of endogenous melatonin and its signaling in T cell activation.
Keywords: T-REx cells, IL-2, HIOMT, Melatonin, MT1
Introduction
Melatonin (MLT), a widespread substance in the animal kingdom, is the product of a multistep metabolic pathway that starts with the hydroxylation of l-tryptophan by the tryptophan hydroxylase (TPH, EC 1.14.16.4). Decarboxylation of hydroxytryptophan by aromatic amino acid decarboxylase (AAD, EC 4.1.1.28) generates serotonin, which can act as a neurotransmitter, besides its actions as a regulator of vascular tone, an immunomodulator, a growth factor, and a precursor for MLT. In this metabolic pathway, acetylation of serotonin catalyzed by arylalkylamine N-acetyltransferase (AANAT, EC 2.3.1.87) generates N-acetylserotonin (NAS), which is further methylated by hydroxyindole-O methyltransferase (HIOMT, EC 2.1.1.4) [1]. It should be noted that serotonin can be acetylated by other enzymes than just AANAT, such as arylamine N-acetyltransferases (NAT) [2], and in vivo AANAT activity does not always correlate with MLT production [3]. Therefore, the recent evidence demonstrates that HIOMT but not AANAT is the rate-limiting enzyme in MLT biosynthesis [2, 4].
Melatonin is produced by several organs and tissues besides the pineal gland. In mammals, this extrapineal MLT synthesis has been identified in the brain, retina, Harderian gland, ciliary body, lens, thymus, airway epithelium, bone marrow, gonads, placenta, GI tract, skin, and immune cells [1, 5–8]. It can act through membrane bound receptors MT1 and MT2 and nuclear orphan receptors from the RORα/RZRα family [9–11].
Jurkat T cells have also been described as a source of physiologically active MLT, which takes part in IL-2 production through an action mechanism mediated by both membrane and nuclear receptors [12, 13]. Although the MLT/IL-2 connection suggests a relevant role of MLT in immune control, signaling and regulatory mechanisms are still poorly understood. Some in vitro studies to test the immunomodulatory role of MLT have been controversial due to contradictory results which disagree with those obtained in in vivo models in which MLT behaved as a positive modulator of lymphocyte proliferation and cytokine production [14]. This discrepancy could be explained, at least in part, by endogenous MLT production by cultured cells [6, 15] which would be masking the effect of exogenous MLT. Taking account of this hypothesis, we consider that a model lacking of MLT would be useful to study the role of this indolamine in the T cell response. Therefore, we designed two stable inducible cell lines with regulated expression of human HIOMT enzyme and MT1 membrane melatonin receptor and evaluated the influence of endogenous MLT on lymphocyte activation, focusing on the IL-2/IL-2R system. To make it possible, we have applied the tetracycline-regulated technology, T-REx [16], in order to express an antisense sequence that blocks translation of the interest proteins in Jurkat cells. The efficiency of these models was analyzed as well as the physiological effects of such inhibition showing that the alteration in MLT signaling could affect critical functions in T cell biology and, consequently, in the immune system.
Materials and methods
Cell line and culture conditions
The T-REx-Jurkat cell is a derivative of human leukemic T cell line which stably expresses a tetracycline repressor (Invitrogen, Carlsbad, CA, USA). Cells were grown and maintained in RPMI 1640 culture medium supplemented with 25 mM HEPES, 10% FBS, 2 mM l-glutamine, and 10 μg/ml blasticidin. Transfected T-Rex-Jurkat cells were grown in the same medium with 100 μg/ml zeocin.
PCR and cloning
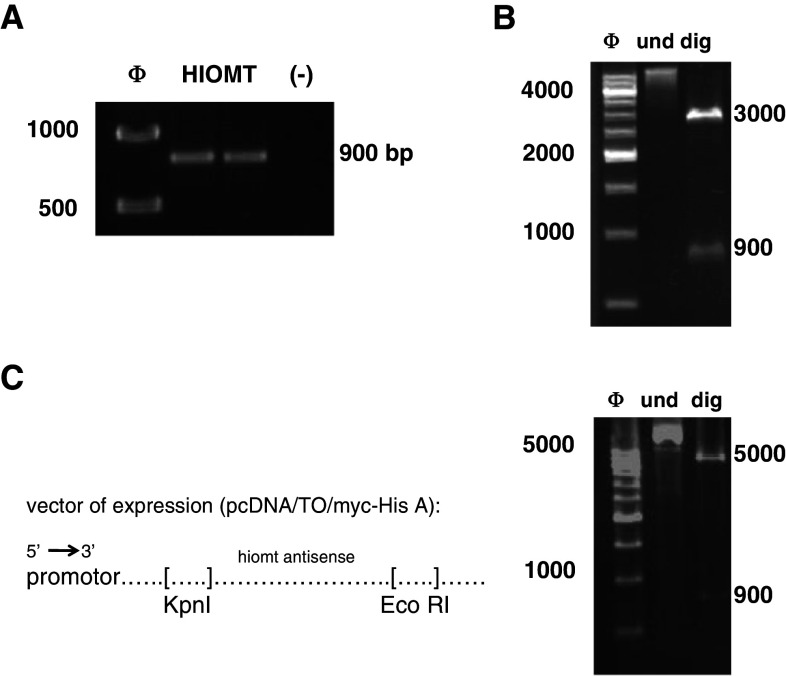
The coding sequence of Hiomt and MT 1 receptor genes were amplified by nested PCR from the human leukaemia lymphoblastic marathon-ready cDNA (BD Biosciences, Franklin Lakes, NJ, USA) using the Advantage 2 PCR enzyme system (Clontech, Palo Alto, CA, USA). The amplification conditions were: 94°C for 30 s for an initial incubation followed by 94°C for 10 s, 74°C for 30 s, and 68°C for 2 min, for five cycles; 94°C for 10 s, 72°C for 30 s, 68°C for 2 min, for 10 cycles; 94°C for 10 s, 70°C for 30 s, 68°C for 2 min for 15 cycles, and a final extension at 68°C for 10 min in a standard buffer. Two-step nested primers for each amplification were designed using Hiomt and MT1-specific sequence information generated by GenBank. Primers used are listed in Table 1, and were designed to place EcoRI and KpnI restriction sites at the 5′ and 3′ends of the amplified sequence. After extraction gel with a QIAquick gel purification kit (Qiagen, Charsworth, CA, USA), the PCR products (865 bp for the Hiomt and 808 bp for the MT1 receptor) were cloned into pGEM-T vector (Promega, Madison, WI, USA) and transformed into JM109 competent cells (Promega). The constructs were confirmed by restriction digestion with EcoRI and KpnI and DNA sequencing (Neocodex, Sevilla, Spain). After inserts liberation from pGEMT-T vector, the HIOMT and the MT1 receptor sequences were inversely cloned into the KpnI and EcoRI restriction sites of the pcDNA4/TO vector (Invitrogen) containing the zeocin-resistance gene and two tetracycline operator sites within the human cytomegalovirus immediate-early promoter to allow for tetracycline-regulated expression of the antisense sequence in transfected cells. The constructs pcDNA4/TO-HIOMT expressing antisense HIOMT and pcDNA4/TO-MT1 expressing antisense MT1 receptor were confirmed by restriction digestion with EcoRI and KpnI and DNA sequencing (Neocodex) (Fig. 1).
Table 1.
Primers used for amplification of cDNA of HIOMT and MT 1 genes
| Primer | Sequence (5′ → 3′) |
|---|---|
| HIOMT | |
| Forward | CGTCGAATTCTCCCTGAAGCTGCTGAAAGT |
| Reverse | CGACGGTACCGTTCCAGGTCACAAGAAACAGTTAT |
| Nested forward | CGTCGAATTCAAAAGCTTTCTATCGAAACACAGAG |
| Nested reverse | CGACGGTACCGGCATCATAAATGGCTCCTG |
| MT1 | |
| Forward | CGTCGAATTCATCTTCACCATCGTGGTGGA |
| Reverse | CGACGGTACCGGAGACGGTTTCCATTTAACC |
| Nested forward | CGTCGAATTCCTGTCGGTGTATCGGAACAA |
| Nested reverse | CGACGGTACCCTGGCTGTACAGAGCGAGACTAT |
Restriction sites are underlined in the oligonucleotide sequences
Fig. 1.
Cloning of antisense sequences. a Nested-PCR of HIOMT cDNA. Nested PCR using specific primers was performed to place EcoRI and KpnI restriction sites at the 5′ and 3′ends of the amplified sequence. PCR molecular size marker (ϕ) was used, and PCR reaction without cDNA (–) was used as PCR control. b HIOMT cloning in pGEMT vector. After extraction gel, the PCR product was cloned into pGEM-T vector and transformed into JM109 competent cells. The constructs were confirmed by restriction digestion with EcoRI and KpnI and DNA sequencing. c HIOMT Cloning in TREx vector. After inserts liberation from pGEMT-T vector by restriction digestion with EcoRI and KpnI, the HIOMT sequence was inversely cloned into the KpnI and EcoRI restriction sites of the pcDNA4/TO vector (left). The constructs pcDNA4/TO-HIOMT expressing antisense HIOMT were confirmed by restriction digestion with EcoRI and KpnI (right). und: Undigested vector, dig: digested vector. The same procedure was followed to create T-Rex-MT1 cells
Transfection
Before transfection, T-REx-Jurkat cells (1.5 × 106 cells) were grown in the medium described above and, 48 h later, different concentrations of zeocin (from 50 to 1,000 μg/ml) were added to choose the optimal concentration for selection of transfected cells (data not shown). T-REx-Jurkat cells were transfected with pcDNA4/TO-HIOMT or pcDNA4/TO-MT1 by use of DMRIE-C reagent (Invitrogen) in accordance with the manufacturer’s protocol. Aliquot 0.5 ml of Opti-MEM I reduced serum medium (Invitrogen) and 10 μl of DMRIE-C reactive were placed into each well of 6-well sterile plates, then 4 μg of pcDNA4/TO-HIOMT vector or pcDNA4/TO-MT1 vector diluted in 0.5 ml of DMRIE-C was added. After mixing and incubating for 30 min at room temperature, 2–3 × 106 T-Rex-Jurkat cells in 0.5 ml of Opti-MEM I without antibiotics were added to each well, and incubated at 37°C in a CO2 incubator for 4 h. Finally, 2 ml of RPMI medium with 15% serum fetal, 1 μg/ml PMA (phorbol 12-myristate 13-acetate; Sigma), and 50 ng/ml PHA (phytohemagglutinin; Sigma) was added. After 48 h, 100 μg/ml zeocin was added for selection of transfected cells.
Expression studies
Induction of antisense expression in transfected cells was accomplished by exposing cells (1 × 106 cells/ml) to 0.1–1 μg/ml of tetracycline for 24 h. The expression levels of antisense HIOMT were followed by HIOMT activity analysis and MLT determinations. The expression levels of antisense MT1 were tested by western blots and flow cytometry analysis. As control cells were used the same transfected cells without tetracycline addition.
HIOMT activity assay
Transfected cells (1 × 106 cells/ml) were cultured for 24 h with 1 μg/ml of tetracycline and then were centrifuged for 10 min at 600g. The cell precipitate was resuspended in 1 ml of 0.05 M PBS pH 6.8 and disrupted at 4°C using a cell sonicator (Sonics and Materials, Danbury, CT, USA). The cell lysate was centrifuged for 10 min at 3,000g and used for assay of HIOMT activity [17]. Then, 40 μl of this supernatant was mixed with 20 μl 0.05 M PBS, pH 7.9, containing 20 nCi S-[methyl-14C] adenosyl-l-methionine and 3 mM N-acetyl serotonin. The reaction was incubated for 20 min at 37°C and was stopped by the addition of 100 μl 0.2 M sodium borate buffer pH 10 and 1 ml chloroform at 4°C. Synthesized MLT was measured following extraction in 1 ml chloroform and the radioactivity by liquid scintillation spectrometry counted with a beta counter. HIOMT activity was expressed as pmol melatonin/mg protein/h and then normalized as % of control. Protein content was measured following the Bradford protocol [18].
Melatonin content
Transfected cells were cultured for 24 h (1 × 106 cells/ml) with 1 μg/ml of tetracycline and then were centrifuged for 10 min at 600g.The culture supernatants were collected for MLT determination by specific Melatonin ELISA (Immuno Biological Laboratories, Hamburg, Germany) according to the manufacturer’s instructions. Melatonin from 500 ml of the samples, standards and controls was extracted (90–100% yield recovery) using C18 reversed phase columns (Immuno Biological Laboratories) and methanol elution. The dried extracts (after evaporating methanol) were stored at −20°C for up to 48 h. Melatonin levels were measured in duplicate using 96-well microtiter plates coated with captured antibody goat anti-rabbit Ig. Each microtiter plate was filled either with 50 ml blank reagent, extracted calibrators, extracted samples or extracted standard solutions (containing 0, 3, 10, 30, 100, or 300 pg/ml of melatonin). Then, 50 ml of melatonin biotin and 50 ml of rabbit-antiserum were added into each well, shaken carefully, sealed with adhesive foil and incubated overnight (14–20 h) at 2–8°C. After washing three times with 250 μl diluted assay buffer, 150 μl of anti-biotin conjugate to alkaline phosphatase was added to each well and incubated for 2 h at room temperature. The reaction was developed using p-nitrophenyl phosphate and optical densities were determined at 450 nm in an automatic microplate reader. The sensibility of the MLT assay was 3 pg/ml. Both the intra- and inter-assay coefficients of variation (CV) were <10%.
Western blots
Cells were solubilised for 30 min at 4°C in lysis buffer containing 50 mM HEPES; pH 7,5; 150 mM NaCl; 1,5 mM MgCl2; 1 mM EGTA; 10% glycerol; 1% Triton X-100 with protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA). After centrifugation, protein concentration was determined by the Bradford method. Total proteins [19] were denatured at 85°C for 5 min in Laemmli Buffer 2× (Sigma–Aldrich), subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to PVDF membrane. The membranes were blocked with Tris-buffered saline–0.05% Tween 20 (TBST) containing 5% non-fat dry milk for 1 h at RT. The blots were then incubated with polyclonal anti-human Melatonin Receptor 1A antibody (Abcam, Cambridge, UK) for 2 h, washed in TBST, and further incubated with secondary antibody linked to horseradish peroxidase (Promega). Bound horseradish peroxidase was visualized using a high-sensitive chemiluminescence system (SuperSignal; Pierce, Rockford, IL, USA). The bands obtained in the blots were scanned and analyzed by the PCBAS2.0 program. The amount of protein loaded in each lane was controlled by immunoblot with monoclonal anti-GAPDH antibody (Chemicon, Germany).
Flow cytometry analysis
MT1-transfected cells (1 × 106 cells/ml) were cultured with or without 1 μg/ml of tetracycline. After 24 h, cells were centrifuged, washed in phosphate-buffered saline (PBS) and incubated with polyclonal anti-human MEL-1A-R (N-20) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min. Afterwards, cells were centrifuged, washed with PBS and incubated with FITC-anti goat antibody (BD Biosciences PharmingenTM) for 30 min. Finally, cells were washed and fixed for flow cytometry analysis. To analyze the percentage of cells expressing CD25, after incubation with or without 1 μg/ml tetracycline for 24 h, MT1-transfected cells were cultured in the presence of appropriate stimuli (PHA 0,5 μg/ml; Sigma–Aldrich) for 24 h more to detect the activation marker on the cell surface. PHA, like other carbohydrate-binding proteins, binds to specific configurations of T-lymphocyte glycoprotein’s, including TCR and CD3, and so activates the lymphocytes [20]. At the end of the stimulation time, cells were centrifuged, washed in phosphate-buffered saline (PBS) and resuspended in 100 μl PBS containing ethylenediamine tetraacetic acid (EDTA) (FACS-Flow; BD Biosciences). Finally, cells were incubated with phycoerythrin-conjugated anti-CD25 antibody (BD PharMingen, San Diego,CA) for 20 min, washed and fixed for flow cytometry analysis. We gated Jurkat T cells according to side scatter (SSC) and forward scatter (FSC). A total of 20,000 cells were acquired.
RNA extraction and reverse transcription
Transfected cells were cultured for 24 h with or without 1 μg/ml of tetracycline and then cultured in presence of PHA (8 μg/ml) for 24 h. Cells were collected and stored at −20°C for RNA analysis. Total RNA was extracted from the samples by a modification of Chomczynski and Sacchi’s method using TriPure Isolation Reagent (Roche, Mannheim, Germany) as denaturing solution and appropriate chloroform volume. After cell lysis and RNA extraction, RNA was precipitated with isopropanol, and the pellet was washed in 75% ethanol. The RNA samples were recovered by centrifugation at 14,000g for 5 min and then dried. Each RNA pellet was dissolved in 50 or 100 μl RNase-free water and the quantified spectrophotometrically at 260 nm.
Single-strand cDNA was then synthesized using the following method: 2 μg of RNA was denatured in 19 μl RNase-free water at 85°C for 10 min, and then rapidly chilled on ice. Then, 21 μl of a mixture formed by 1× reverse transcription (RT) buffer, 20 mM dithiothreitol (DTT), 2′-deoxyribonucleoside-5′-triphosphates (0.5 mM of each), 40 U recombinant RNasin ribonuclease inhibitor, 0.5 μg oligo (dT)15 primer and 200 U Moloney murine leukemia virus reverse transcriptase (M-MLV RT) were added to give a final volume of 40 μl and incubated for 60 min at 42° C (all reagents were purchased from Promega). The RT reaction was terminated by placing it on ice after deactivation at 95°C for 5 min.
Real-time PCR
Real-time quantitative PCR was performed by the MiniOpticon cycler system (BioRad®). Reactions were done in a 25-μl volume containing 5 μl of RT product as template DNA [21], 2X FastStart SYBR Green Master (Roche Molecular Biochemicals) and 200 nM for each primer set. The following experimental protocol was used: denaturation program (95°C for 10 min), amplification and quantification program repeated 45 times (95°C for 10 s, 60°C for 20 s, 72°C for 30 s with fluorescent data acquisition after extension step), melting curve program (60–95°C with a heating rate of 0.5°C/s with continuous fluorescence measurement), and finally a cooling step at 30°C. The following oligonucleotides (5′ to 3′) (Roche) were used as primers for RT–PCR: human IL-2, ATG TAC AGG ATG CAA CTC CTG TCT T (exon 1) and GTC AGT GTT GAG ATG ATG CTT TGA C; and human β-actin, TCCCTGGAGAAGAGCTACGA (exon 4) and AGCACTGTGTTGGCGTACAG (exon 5).
The cycle number at which the fluorescent signal of a given reaction well crossed the threshold value was denoted as the CT. CT data for multiplex targets were normalized to the internal standard, β-actin CT, by use of the formula: ΔCT = target CT − internal standard CT. Calculations for relative quantitation were performed as outline in User Bulletin #2: ABI Prism 7700 Sequence Detection System (PE Applied Biosystems). The relative expression of each mRNA was calculated with the comparative ΔΔCT method since the amplification efficiencies of the target and the endogenous reference are approximately equal. The calculation of ΔΔCT involves subtraction by the ΔCT calibrator value. This is subtraction of an arbitrary constant, so the standard deviation of ΔΔCT is the same as the standard deviation of the ΔCT value. The relative amount of each mRNA was determined by evaluating the expression of 2−ΔΔCT and then represented as a % of its respective control (cells without tetracycline).
IL-2 determination
Transfected cells were cultured for 24 h with or without 1 μg/ml of tetracycline and then cultured in presence of PHA (8 μg/ml) for 24 h. IL-2 concentrations in the cell free supernatants were determined by using a specific ELISA kit (Pharmingen, San Diego, CA, USA). Mouse anti-human IL-2 in 0.2 M sodium phosphate pH 9.0 was coated overnight to high-binding microtiter plates. The plates were washed twice with PBS/0.05% Tween 20, incubated with 1% BSA in PBS/Tween 20 for 1 h as a blocking step, and washed again. Samples and standards (recombinant human IL-2) were diluted in 1% BSA PBS/Tween 20 and incubated overnight. After being washed three times, biotinylated mouse anti-human IL-2 mAb was added, and bound IL-2 detected using streptavidin-horseradish peroxidase conjugate, and 3,3′, 5,5′tetramethylbenzidine and hydrogen peroxide as the substrate of the enzyme.
Statistical analysis
Differences between two groups were evaluated with Student’s t test. A probability value (P) <0.05 was considered statistically significant. Statistical analyses were performed using SPS SYSTAT 10 program (Systat Software, Richmond, CA, USA).
Results
Suppression of HIOMT activity and MLT production by tetracycline-regulated HIOMT antisense expression
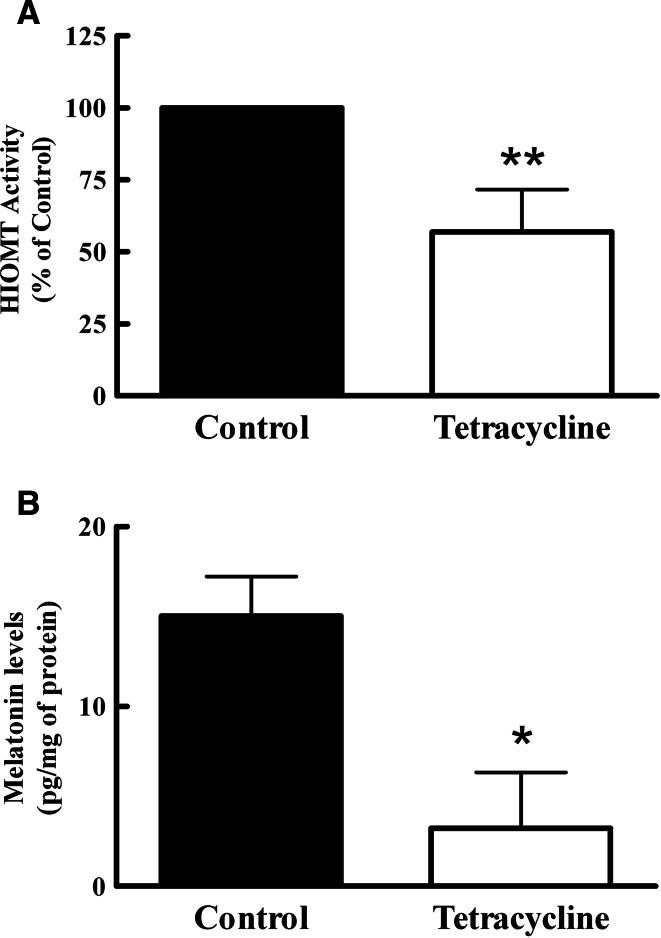
To interfere with the synthesis of endogenous MLT in Jurkat cells, T-Rex-HIOMT cells were cultured with tetracycline (1 μg/ml) for 24 h. Unfortunately, we were not able to analyze the expression of HIOMT protein using western blot analysis because anti-human HIOMT antibody generated in our laboratory failed to recognize native antigen in this assay. For this reason, the efficiency of HIOMT blocking was determined by measuring HIOMT activity, which was reduced by 50% in T-Rex-HIOMT cells treated with tetracycline when they were compared to untreated cells (Fig. 2a). The effect of the antisense expression was also analyzed by the quantification of MLT levels. As we expected, the translational blockade of the Hiomt gene induced by tetracycline leaded to a decrease in MLT content by Jurkat cells (Fig. 2b).
Fig. 2.
Tetracycline decreased HIOMT activity and melatonin production. T-Rex-HIOMT Jurkat cells were cultured in presence or absence of tetracycline (1 μg/ml) for 24 h. a After treatment, HIOMT activity of those cells was assayed. Data were first expressed in pmol and then averaged from five experiments performed in duplicate. Control value of HIOMT activity: 56.27 ± 17 pmol/mg protein/h. b Melatonin content in cells was measured by ELISA. Statistically significant differences were observed between cells treated with tetracycline and the group without the antibiotic. *P < 0.05, **P < 0.005, Student t test
Suppression of MT1 levels by tetracycline-regulated MT1 antisense expression
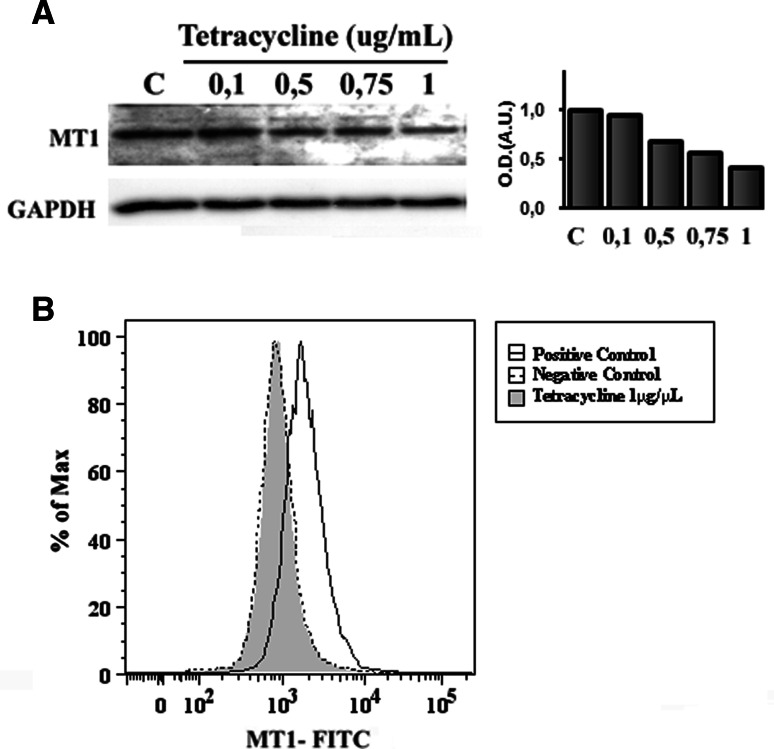
To further assess the efficiency of the tetR-mediated MT1 translation repression, western blot analysis using anti-MT1 antibody was performed. As shown in Fig. 3a, after 24 h, MT1 receptor expression in T-Rex-MT1 cells was decreased in a tetracycline-concentration-dependent manner. As the maximum effect was observed with 1ug/ml of the antibiotic, we chose this concentration as the optimum for the subsequent experiments. Equal loading was controlled by re-probing the blot with an anti-GAPDH antibody.
Fig. 3.
Dose-dependent regulation of MT1 receptor expression in T-Rex-MT1 Jurkat cells by tetracycline. a T-Rex-MT1 Jurkat cells were cultured with different concentrations of tetracycline for 24 h. After treatment, the MT1 receptor protein levels were analyzed by western blot. A representative picture of MT1 from three experiments with similar results is shown (left) as well as a graph representing quantification of the bands by densitometry after normalization against GAPDH (right). Anti-GAPDH immunoblot was employed to check the general amount of protein in the blots. b The expression level of MT1 receptor was measured by flow cytometry. T-Rex-MT1 Jurkat cells were cultured with or without tetracycline (1 μg/ml) for 24 h. After treatment, they were incubated with anti-MT1 primary antibody and stained with FITC-conjugated secondary antibody. Total cells were analysed by a fluorescence activated sorter (FACS) Calibur. Viable cells were gated by side-forward-scatter characteristics. Representative histograms from three independent experiments are shown
To further confirm the inhibition of MT1 receptor levels, flow cytometry was performed in the absence or presence of tetracycline. T-Rex-MT1 cells treated with tetracycline (Fig. 3b) showed a significant fall in FITC fluorescence in comparison to untreated cells (Fig. 3b, positive control), and this expression was comparable to the negative control (Fig. 3b, negative control).
Endogenous MLT is necessary for IL-2 production in Jurkat cells
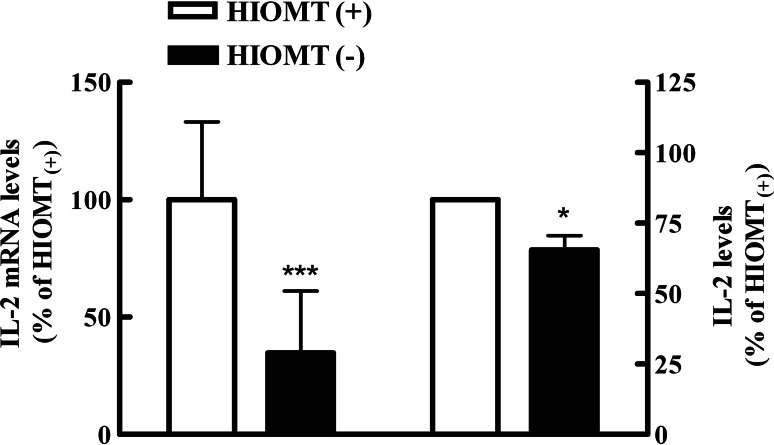
To determine the role of endogenous MLT in the regulation of IL-2 production by lymphocytes, the IL-2 levels in T-Rex-HIOMT cells treated with tetracycline were assessed by RT–PCR and ELISA. After 24 h of tetracycline incubation, PHA-activated cells showed a significant decrease in IL-2 mRNA expression almost reaching 70%. In accordance with this result, the IL-2 protein levels in the corresponding supernatants from these experiments also significantly diminished in presence of tetracycline (Fig. 4).
Fig. 4.
Antisense HIOMT expression inhibits IL-2 production from PHA activated Jurkat cells. T-Rex-HIOMT Jurkat cells were cultured with or without tetracycline (1 μg/ml) and then incubated for 24 h with PHA (8 μg/ml). Left Real-time PCR was performed and results of IL-2 mRNA expression were calculated using the method of 2−ΔΔCT in relation to stimulated cells without tetracycline after normalization against β-actin. Then, they were expressed as a percentage and the values in HIOMT(+) cells were considered as 100%. Data were obtained from three experiments performed in triplicate. ΔCtHIOMT(+) ± SD: 3.906 ± 1.0531; ΔCtHIOMT(−) ± SD: 5.433 ± 1.687. Right IL-2 content was determined by ELISA in culture supernatants. Data are expressed as percentage of mean values ± SD of 5 experiments performed in triplicate. Control value of IL-2 production: 304.73 ± 90.55 pg/ml. HIOMT(+) Cells without tetracycline, HIOMT(−) cells with tetracycline. Statistically significant differences were observed between cells treated with tetracycline and the group without the antibiotic. *P < 0.05, ***P < 0.0001, Student t test
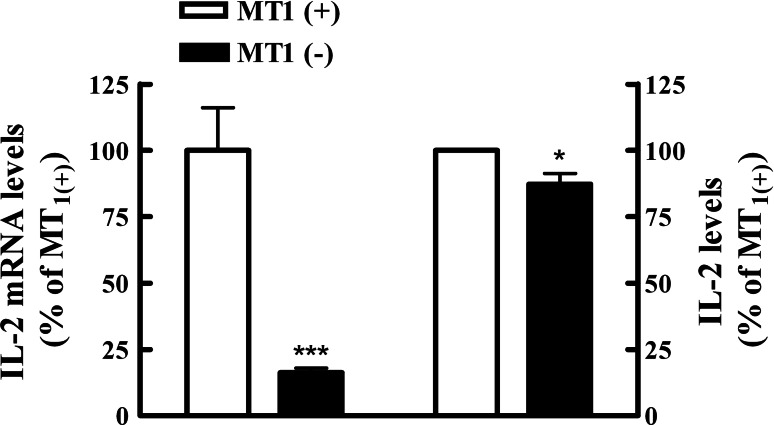
To study the involvement of membrane MLT signalling, Jurkat cells transfected with the tetracycline-controlled MT1 antisense expression system were cultured with tetracycline for 24 h and then stimulated with PHA 8 μg/ml. Then, 24 h later, IL-2 mRNA levels decreased by 80% in MT1 down-regulated cells (Fig. 5). Accordingly, IL-2 levels in the supernatant of cultures were also significantly lower as compared with untreated cells.
Fig. 5.
Antisense MT1 receptor expression inhibits PHA activated Jurkat cell IL-2 mRNA transcripts and supernatant concentration. T-Rex-MT1 Jurkat cells were cultured with or without tetracycline (1 μg/ml) and then incubated for 24 h with PHA (8 μg/ml). Left Real-time PCR was performed and results of IL-2 mRNA expression were calculated using the method of 2−ΔΔCT in relation to stimulated cells without tetracycline after normalization against β-actin. Then, they were expressed as percentage and the values in MT1(+) cells were considered as a 100%. Data were obtained from three experiments performed in triplicate. ΔCtMT1(+) ± SD: 0.73 ± 0.549; ΔCtMT1(−) ± SD: 3.355 ± 0.382. Right IL-2 content was determined by ELISA in culture supernatants. Data are expressed as percentage of mean values ± SD of 5 experiments performed in triplicate. Control value (100%) of IL-2 production: 543.55 ± 131.85 pg/ml. MT1(+) Cells without tetracycline; MT1(−) cells with tetracycline. Statistically significant differences were observed between cells treated with tetracycline and the group without the antibiotic. *P < 0.05, ***P < 0.0001, Student t test
To rule out the possibility that the effect observed in IL-2 levels was due to the inhibition of protein translation promoted by tetracycline, untransfected T-REx-Jurkat cells were also treated with tetracycline and assayed for proliferation. Data did not show any significant effect of tetracycline on cell proliferation or IL-2 production (data not shown). However, normal Jurkat cells treated with tetracycline showed a decrease in IL-2 production after PHA stimulation (data not shown) which shows that T-Rex-Jurkat cells are prepared to be cultured with tetracycline without suffering any toxic effect.
MT1 melatonin receptors are necessary for CD25 expression
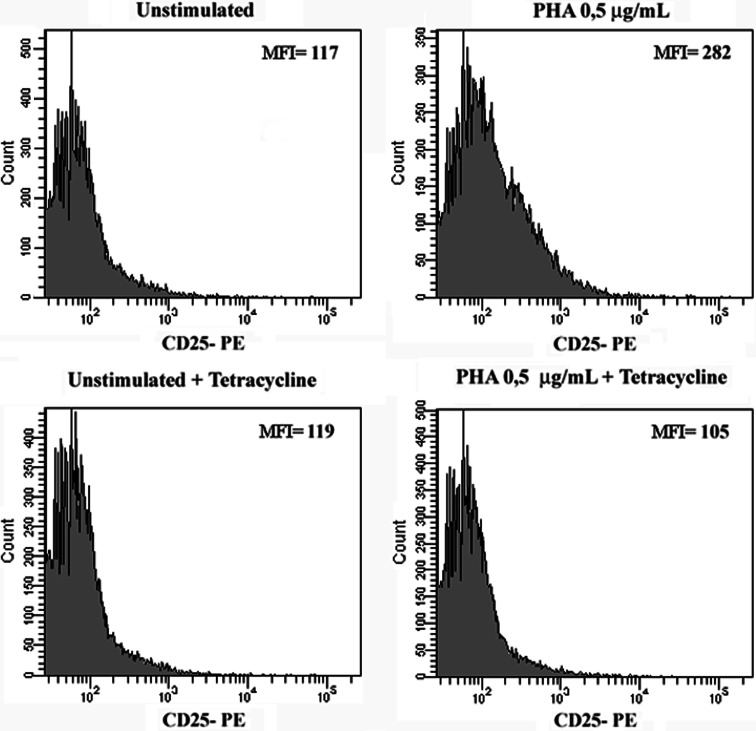
We have previously reported that endogenous MLT is involved in the regulation of the IL-2/IL-2R expression through an action mechanism involving both membrane and nuclear receptors [15]. It is well known that, under T cell activation through its antigen receptor, one of the most rapid consequences is the de novo synthesis of IL-2 which is quickly followed by the expression of the α-chain (CD25) on the cell surface [22]. In the present study, T-Rex-MT1 cells were incubated with or without tetracycline and then cultured with PHA 0.5 μg/ml to induce the early activation marker CD25 on the cell surface. As shown in the representative histogram in Fig. 6, CD25 expression was up-regulated by PHA activation (b) and this increased expression was inhibited by pre-incubation with tetracycline.
Fig. 6.
Effect of antisense MT1 expression on CD25 expression in Jurkat cells. T-Rex-MT1 Jurkat cells were cultured with or without tetracycline (1 μg/ml) and then incubated for 24 h with a suboptimal concentration of PHA (0.5 μg/ml). The expression level of CD25 was measured by flow cytometry with a fluorescence activated sorter (FACS)Calibur. Viable cells were gated by side-forward-scatter characteristics. One-color immunofluorescence analysis was performed with anti-CD25-PE (x-axis). Basal levels of fluorescence in un-activated cells (with or without tetracycline) are also shown. MFI Medium fluorescence intensity. Representative histograms from three independent experiments are shown
Discussion
Our previous studies have demonstrated that human lymphocytes in culture not only synthesize MLT [6] but this endogenous melatonin is also biologically active because it is involved in the regulation of the IL-2/IL-2R expression through a mechanism of action in which both membrane and nuclear receptors are implicated [12, 15]. However, many questions about the MLT effects on the immune system are difficult to answer because there are not good models of immune cells where MLT production had been abolished. The ability to suppress gene expression in vitro and in vivo is a valuable tool for studying biological processes. Through these models, it is possible to regulate the expression of specific proteins and study these effects on downstream signaling processes.
Several inducible gene expression systems have been developed and the inhibition of a specific protein expression is now possible [23]. The tetracycline-regulated technology, T-REx, employs the use of host cell lines that have been stably transfected with a constitutively expressed tetR protein and a plasmid containing the gene of interest, under the control of the cytomegalovirus (CMV) promoter upstream of a dimeric tetracycline operator (tetO2) [24]. In the absence of tetracycline, four tetR molecules binds to tetO2 thereby repressing activation, while the addition of tetracycline in the culture medium causes the tetR molecules to be released from tetO2 resulting in the activation of transcription [25].
In the present study, we created two stable inducible cell lines with regulated expression of human HIOMT enzyme and MT1 membrane melatonin receptor as a starter material to evaluate the influence of endogenous MLT and the interaction with its receptor on lymphocyte activation.
For the first model, considering that MLT is enzymatically formed, the only way to avoid its biosynthesis was blocking one of the two last enzymes. Thus, we decided to create a cell line with regulated expression of the last enzyme HIOMT using T-Rex system. The efficiency of HIOMT blocking was about 50%, and it was monitored measuring HIOMT activity and melatonin production. Both parameters showed a perfect correlation as many authors have described [26, 27].
With the second cellular model, we wanted to block one of the MLT action mechanisms, and we focused on the inhibition of MT1 receptor translation since no specific antagonist for this receptor subtype has been reported. The choice of MT1 and not MT2 was based in the fact that the majority of MLT effects described for lymphocytes seem to be mediated through this receptor subtype [28]. However, some evidence shows that MLT-induced enhancement of immune function is also mediated via MT2 receptors, as Drazen et al. have shown about splenocyte proliferation in mice [29, 30].
The inhibition in MT1 receptor protein levels by tetracycline was evaluated with double confirmation through western blot and also by flow cytometry analysis. The difference between the results obtained with both techniques could be due to a different specificity of antibodies, since the antibody used in the cytometer was not the same as the one we used in western blot. To avoid permeabilization of cells, a polyclonal antibody raised against extracellular N-terminus extreme of the receptor was used in flow cytometer.
Once the efficiency of the models was evaluated, we analyzed the physiological effect of MLT decreasing and the down-regulation of its membrane receptor in terms of IL-2 production. Assessment of cytokine expression during an immune response is a critical requirement for studies of basic immune mechanisms and the pathogenesis of disease. As an alternative to measuring cytokine protein levels, analysis of cytokine mRNA may also be appropriate, because production of many cytokines is primarily transcriptionally regulated. Thus, those cells where the expression of antisense against HIOMT was induced with tetracycline showed less IL-2 levels after stimulation with an optimum dose of PHA, especially at transcriptional level. The same effect was found in the cells with less expression of MT1 receptor, which would suggest that endogenous MLT action in lymphocyte activation, reflected as IL-2 production, is mediated, at least in part, through its MT1 receptor. Actually, we have previously described a significant interaction between membrane and nuclear melatonin receptors, since activated cells with less expression of MT1 receptor also showed decreased expression of ROR/RZR mRNA [13]. These data support the hypothesis that considers MLT endogenously synthesized in lymphocytes as an autocrine, intracrine and paracrine factor involved in the regulation of IL-2 production.
Over recent years, several specific melatonin membrane and nuclear receptor agonists and antagonists have been described, which have been extensively used in the study of melatonin effects in several systems, including the immune system. In fact, previous papers published by our group [12, 13, 15] have shown a decrease on IL-2 production in lymphocytes after the incubation with luzindole and CGP 55644. However, the use of molecular approaches for melatonin action’s inhibition is now considered more correct and easier to control than pharmacological ones.
The expression of IL-2 ends up a CD25 expression in membrane. IL-2Rα expression is undetectable on resting T cells but it can be induced by exposure to antigen or mitogen [31] after the up-regulation of IL-2 gene happens. In this line, Carrillo-Vico et al. showed that peripheral blood mononuclear cells (PBMCs) cultured with PHA and melatonin receptor antagonists had a deficient expression of CD25 protein and it would be overcome adding exogenous melatonin, which supported the role of the indolamine in the process [28]. In our conditions, it was reasonable to think that, if the decrease in melatonin-MT1 receptor interaction induced less expression of IL-2 production, it may also affect CD25 expression as an indirect effect. Confirming our hypothesis, pre-incubation of T-Rex-MT1 cells with tetracycline prevented its activation induced by PHA reaching CD25 levels close to control values (unstimulated cells). These results show us the permissive role of melatonin-MT1 receptor system in T lymphocyte activation after mitogenic response.
IL-2, or T-cell growth factor (TCGF), is produced mainly by T helper (CD4+) lymphocytes, stimulates cell-mediated immune responses, controls growth and differentiation of B lymphocytes, and intensifies proliferation and activity of all cytotoxic cell clones [32]. The finding of endogenous MLT production by human lymphocytes is related to enhanced release of IL-2 and up-regulation of IL-2R suggests that MLT may be involved in the clonal expansion of antigen-stimulated human T lymphocytes. The results shown here confirm MLT as an intrinsic factor involved in the natural activation of cells in the immune response, and they are consistent with those previously observed in PBMCs using MT1, MT2, and RORa pharmacological blockers [15].
Moreover, they support the relevance of a good cellular model lacking of MLT to explore the various signals triggered by the indolamine and then discuss its role into biological function. The results obtained in our models were sufficiently robust in relation to the influence of MLT in IL-2 regulation, even in spite of the depth of inhibition with the antisense was not as high as we expected since other authors have described almost a 90% inhibition in the target protein with the T-REx system [33, 34].
The induction of IL-2 expression following T cell stimulation depends on the activation of several transcription factors [35], so further studies are necessary to investigate if the signaling pathways activated for MLT would be involving the regulation of some of these factors. The respective functions of these molecules, the mode in which they are activated, and the identities and functions of their targets are some of the questions that still need to be answered to more fully describe this complex mechanism.
Acknowledgments
This paper was supported by grants from Ministerio de Sanidad FIS (06/0091) and Red-FIS (RD06/0013/0001) and Grupo Excelencia del Plan Andaluz de Investigacion (P06-CTS-01604).
Footnotes
P. J. Lardone and A. Rubio contributed equally to this work.
References
- 1.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 3.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270:3335–3344. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- 5.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 6.Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, Garcia-Maurino S, Reiter RJ, Guerrero JM. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18:537–539. doi: 10.1096/fj.03-0694fje. [DOI] [PubMed] [Google Scholar]
- 7.Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni JM. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res. 2000;28:193–202. doi: 10.1034/j.1600-079X.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- 8.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/A:1020107915919. [DOI] [PubMed] [Google Scholar]
- 9.Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem. 1994;269:28531–28534. [PubMed] [Google Scholar]
- 10.Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12:143–150. [PubMed] [Google Scholar]
- 11.Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–d1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- 12.Lardone PJ, Carrillo-Vico A, Naranjo MC, De FB, Vallejo A, Karasek M, Guerrero JM. Melatonin synthesized by Jurkat human leukemic T cell line is implicated in IL-2 production. J Cell Physiol. 2006;206:273–279. doi: 10.1002/jcp.20461. [DOI] [PubMed] [Google Scholar]
- 13.Lardone PJ, Carrillo-Vico A, Molinero P, Rubio A, Guerrero JM. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: significance in IL-2 production. Cell Mol Life Sci. 2009;66:516–525. doi: 10.1007/s00018-008-8601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo-Vico A, Lardone PJ, Fernandez-Santos JM, Martin-Lacave I, Calvo JR, Karasek M, Guerrero JM. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J Clin Endocrinol Metab. 2005;90:992–1000. doi: 10.1210/jc.2004-1429. [DOI] [PubMed] [Google Scholar]
- 16.Yao F, Svensjo T, Winkler T, Lu M, Eriksson C, Eriksson E. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther. 1998;9:1939–1950. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- 17.Champney TH, Holtorf AP, Steger RW, Reiter RJ. Concurrent determination of enzymatic activities and substrate concentrations in the melatonin synthetic pathway within the same rat pineal gland. J Neurosci Res. 1984;11:59–66. doi: 10.1002/jnr.490110107. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Pierpaoli W, Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci USA. 1994;91:787–791. doi: 10.1073/pnas.91.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Didier M, Aussel C, Pelassy C, Fehlmann M. IL-1 signaling for IL-2 production in T cells involves a rise in phosphatidylserine synthesis. J Immunol. 1988;141:3078–3080. [PubMed] [Google Scholar]
- 21.Paskaloglu K, Sener G, Ayangolu-Dulger G. Melatonin treatment protects against diabetes-induced functional and biochemical changes in rat aorta and corpus cavernosum. Eur J Pharmacol. 2004;499:345–354. doi: 10.1016/j.ejphar.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 23.Xu ZL, Mizuguchi H, Mayumi T, Hayakawa T. Regulated gene expression from adenovirus vectors: a systematic comparison of various inducible systems. Gene. 2003;309:145–151. doi: 10.1016/S0378-1119(03)00506-7. [DOI] [PubMed] [Google Scholar]
- 24.Jones J, Nivitchanyong T, Giblin C, Ciccarone V, Judd D, Gorfien S, Krag SS, Betenbaugh MJ. Optimization of tetracycline-responsive recombinant protein production and effect on cell growth and ER stress in mammalian cells. Biotechnol Bioeng. 2005;91:722–732. doi: 10.1002/bit.20566. [DOI] [PubMed] [Google Scholar]
- 25.Fussenegger M. The impact of mammalian gene regulation concepts on functional genomic research, metabolic engineering, and advanced gene therapies. Biotechnol Prog. 2001;17:1–51. doi: 10.1021/bp000129c. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Hidalgo M, de la Lastra CA, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, Guerrero JM. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp Gerontol. 2009;44:328–334. doi: 10.1016/j.exger.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes PA, Bothorel B, Clesse D, Monteiro AW, Calgari C, Raison S, Simonneaux V, Markus RP. Local corticosterone infusion enhances nocturnal pineal melatonin production in vivo. J Neuroendocrinol. 2009;21:90–97. doi: 10.1111/j.1365-2826.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- 28.Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernandez-Montesinos R, Guerrero JM, Pozo D. The modulatory role of melatonin on immune responsiveness. Curr Opin Invest Drugs. 2006;7:423–431. [PubMed] [Google Scholar]
- 29.Drazen DL, Nelson RJ. Melatonin receptor subtype MT2 (Mel 1b) and not mt1 (Mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity. Neuroendocrinology. 2001;74:178–184. doi: 10.1159/000054684. [DOI] [PubMed] [Google Scholar]
- 30.Drazen DL, Bilu D, Bilbo SD, Nelson RJ. Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1476–R1482. doi: 10.1152/ajpregu.2001.280.5.R1476. [DOI] [PubMed] [Google Scholar]
- 31.Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14:63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- 32.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Kiiver K, Tagen I, Zusinaite E, Tamberg N, Fazakerley JK, Merits A. Properties of non-structural protein 1 of Semliki forest virus and its interference with virus replication. J Gen Virol. 2008;89:1457–1466. doi: 10.1099/vir.0.2008/000299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guggenberger C, Ilgen D, Adamski J. Functional analysis of cholesterol biosynthesis by RNA interference. J Steroid Biochem Mol Biol. 2007;104:105–109. doi: 10.1016/j.jsbmb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Tsatsanis C, Patriotis C, Tsichlis PN. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-kappaB. Oncogene. 1998;17:2609–2618. doi: 10.1038/sj.onc.1202460. [DOI] [PubMed] [Google Scholar]