Abstract
The paradoxical effects of ovarian hormones in both the promotion and prevention of breast cancer have been debated for over 30 years. Genetic studies have demonstrated that ovarian hormones act through NF-κB to stimulate proliferation and ductal elongation, whereas the p53 tumor suppressor protein plays a central role in rendering the mammary epithelium resistant to tumorigenesis. Transcriptional profiles now suggest that ovarian hormones stimulate a constellation of genes that interact with NF-κB and p53 to arbitrate the competing demands for proliferation and surveillance. Genes that participate in chromatin remodeling are among the acute transcriptional responses to estrogens and progestins. These genes are proposed to initiate epigenetic programs that influence the balance between proliferation and surveillance, and render the breast epithelium resistant to tumors.
Keywords: P53, Breast cancer, Estrogen receptor alpha, Estrogen receptor beta, Parity, Risk, Epigenetics
The paradoxical effects of ovarian hormones
Hormonal exposures are prominent among the factors determining risk of breast cancer. Associations between early menarche and late menopause as well as hormone replacement therapies suggest that lifetime exposures to ovarian steroids (estrogens and progestins) are associated with increases in the relative risk of breast cancer of 1.2–1.5 [1]. Elevated levels of circulating estrogens are also associated with increasing risk in a dose-dependent fashion among pre-menopausal women [2]. While estrogens can have direct genotoxic effects by alkylating DNA [3], excessive signaling through the estrogen receptor appears to be the primary mechanism for breast carcinogenesis as modest increases in expression of estrogen receptor alpha (ERα) in transgenic mice resulted in mammary hyperplasias [4]. Levels of estrogen and progesterone receptors vary among strains of mice, influencing mammary susceptibility [5, 6], and polymorphisms in the gene encoding ERα in humans (designated ESR1) have been linked to increased breast cancer risk [7–9]. Therefore, excessive or inappropriate signaling through estrogen and progesterone receptors are key factors determining the risk of breast cancer.
However, ovarian hormones pose a paradox as estrogens and progestins also mediate the protection from breast cancer afforded by parity. A full-term pregnancy early in reproductive life reduces breast cancer incidence by up to 50% [10, 11]. Estrogen and progesterone are sufficient to mimic the effect of pregnancy in reducing the incidence of carcinogen-induced mammary tumors [12–14]. Thus, estrogens alone as well as in combination with progestins are able to engage pathways that are potent inhibitors of breast cancer.
Activity of the p53 tumor suppressor and breast cancer risk
Tumor suppressor genes underlying heritable breast cancers identify pathways that may mediate the protection afforded by parity. The p53 pathway is a prominent candidate as heritable mutations in TP53 are associated with Li-Fraumeni syndrome, and breast cancer is the most common tumor type among women carrying heterozygous mutations [15, 16]. Mutation of TP53 is common in sporadic breast cancers [17] and appears to be a necessary collaborating alteration in breast cancers associated with heritable mutations in BRCA1 [18, 19]. CHK2 and HDM2 (the human ortholog of Mdm2 in mice) are regulators of p53 activity, and polymorphisms in these genes were identified as breast cancer risk alleles [20, 21]. Together, these genetic studies suggested that p53 activity in the mammary epithelium may be limiting and that increased activity of p53 could confer resistance to tumors. Indeed, the apoptotic activity of p53 is reduced in the mammary epithelium of BALB/c-Trp53+/− mice, predisposing them to spontaneous tumors. Conversely, the activity of p53 was increased in parous mice and delayed the onset of mammary tumors in BALB/c-Trp53+/− mice [22], while in the absence of p53 parity failed to reduce mammary tumors [23, 24]. Thus, sufficiency of p53 activity represents a vulnerable link in the barriers to tumorigenesis in the breast epithelium.
A balancing act
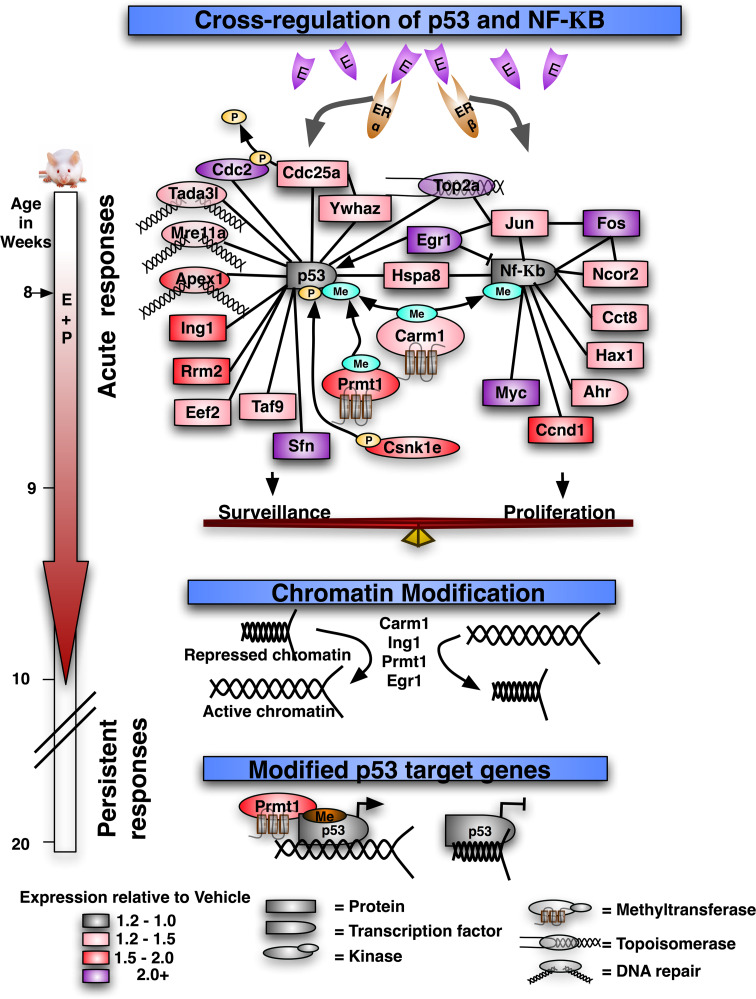
While it is clear that stimulation of mammary tissues with estrogen and progesterone promotes proliferation, p53-mediated apoptosis is also increased [25]. How do these hormones induce opposing actions? Transcriptional profiles of mouse mammary tissues after treatment with estrogen and progesterone provide clues to the basis for the apparent paradox [26]. Using protein–protein interaction databases, we found that interactions with p53 and c-Rel were significantly greater than expect by chance (Fig. 1), suggesting that these are major targets regulated by the hormone treatments. The identification of c-Rel, a subunit of NF-κB, is satisfying because NF-κB is an essential mediator for mammary gland development [27, 28]. Deletion of IKKα (required for activation of NF-κB) in mice phenocopies ERα knockouts [29, 30]. Therefore, NF-κB is presumed to mediate the proliferative effects of estrogens. Although estrogen and progesterone did not alter p53 expression or its basal activity [25], these hormones induced a constellation of genes that potentiates the responsiveness of p53 to genotoxic stimuli [31, 32]. Thus, it appears that the proliferative effects of estrogen and progesterone are coordinated with an increase in genome surveillance.
Fig. 1.
Balancing the responses to estrogens and progestins. Genes differentially expressed after acute exposure to 17β-estradiol and progesterone (4 days) were identified previously [26]. Among these genes, first-neighbor interactions with p53 and NF-kB were significantly over-represented. Chromatin remodeling enzymes together with p53 may target epigenetic alterations responsible for the persistent increase in p53 activity in parous mammary epithelium. The colors indicate the relative levels of mRNA expression in the E + P-treated compared to the vehicle-treated controls (see legend). The interaction database was obtained from NCBI, and the final model is adapted from the visualization using Cytoscape
A subset of the gene products induced by estrogens and progestins interacts with both p53 and NF-κB, and thus is ideally situated to arbitrate the decision between proliferative responses mediated by NF-κB and the surveillance activity of p53 (Fig. 1). Of these, EGR1 is especially intriguing as it acts to both temper transactivation mediated by NF-κB as well as promote the transcriptional activity of p53 [26, 33, 34]. In addition to being increased by estrogen and progesterone, DNA damage activates CArG elements in the EGR1 promoter, resulting in further increases in expression. Proper function of EGR1 is essential for p53-dependent apoptosis in response to irradiation in mouse embryo fibroblasts (MEFs) [35]. In addition to interactions with p53, transcriptional studies of EGR1-deficient MEFs demonstrated interactions with TGFβ signaling. Furthermore, estrogen and progesterone increase active TGFβ1 in the mammary epithelium [36, 37], which collaborates with p53 to restrain proliferation and potentiate radiation-induced apoptosis [25, 37, 38].
In addition to the acute changes, the mammary epithelium retains a “memory” of hormonal exposures that potentiates p53-dependent apoptosis even after the hormones are withdrawn [22]. Chromatin remodeling enzymes are prominent among the genes induced by estrogen and progesterone, suggesting that epigenetic mechanisms may participate. CARM1 and PRMT1 are histone methylases that bind to p53 and enhance transcriptional activation of p53 target genes [39]. Egr1 also participates directly in methylation of target genes [40] and regulates epigenetic patterning of social behaviors [41]. TGFβ signaling is a target of Egr1 [35] and is among the pathways that have been shown to be increased persistently in parous mammary tissues compared to nulliparous [42]. TGFβ signaling may be critical as p53 collaborates with Smads to form complexes with mSin3A that contribute to gene silencing [43]. While p53 and TGFβ signaling can silence genes, ING1 binds specifically with tri-methylated histone 3 (H3K4me3) on active chromatin [44] and recruit p53 to enhance transcriptional activation of target genes [45]. Therefore, prolonged exposure to estrogen and progesterone during pregnancy stimulates chromatin remodeling proteins that together with p53 orchestrate remodeling of the epigenetic landscape in mammary epithelia to favor a range of protective measures that include p53-mediated apoptosis.
A tale of two receptors
Although circulating levels of estrogen and progesterone are increased during pregnancy, 17β-estradiol appears to be more potent in rendering the mammary epithelium resistant to tumors. Estrogenic compounds have anti-tumor activities in humans [46, 47], and estrogen alone was sufficient to prevent mammary tumors in rodents [48]. Furthermore, the effect of 17β-estradiol was ~threefold more potent than progesterone in potentiating p53-dependent responses in mice [25]. Two estrogen receptors (ERα and ERβ) mediate the actions of estrogens and are encoded by separate genes designated ESR1 and ESR2, respectively. Both ERα and ERβ share a common structure with major divergence localized to the N-terminal transactivation domain. While ERα and ERβ have similar binding affinities for 17β-estradiol (K d of 0.1 and 0.4 Nm, respectively), ERβ showed a greater affinity for phytoestrogens such as genestein [49]. Both estrogen receptors transcriptionally activate consensus estrogen-responsive elements in response to physiologic estrogens and phytoestrogens [50]. In cells that express both receptors, heterodimers also efficiently transactivate reporter genes [51]. However, ERα and ERβ also bind AP1 sites, but yield opposing actions with ERα mediating transcriptional activation, whereas ERβ is inhibitory [52]. Therefore, the effects of ERα and ERβ on gene expression depend on the context of promoter elements.
The luminal epithelia of the mammary gland express both ERα and ERβ, and thus heterodimers may predominate. In contrast, the basal epithelial cells express solely ERβ. Expression of the two estrogen receptors also differs between nulliparous and parous rodents. During pregnancy, the levels of ERβ are increased among multiparous rats compared to nulliparous. This increase is associated with a 50% decrease in BrdU incorporation in the mammary epithelium of parous mice compared to the nulliparous mice [53], suggesting opposing effects of ERα and ERβ.
Attempts to understand the functions of these receptors using cell-based assays have yielded contradictory results with respect to their actions on p53. Using MCF7 cells, exogenous ERα bound the C-terminus of p53 and inhibited transactivation of a PCNA-luciferase reporter [54], while experiments with HeLa cells demonstrated that exogenous ERα bound the N-terminus of p53, sparing it from degradation by HDM2 and enhanced transactivation of a Cdkn1a/p21-luciferase reporter [55]. With respect to regulation of endogenous ESR1, p53 was shown to both inhibit [56] as well as maintain ERα levels in MCF7 cells [57]. In contrast to these cancer-derived cell lines, introducing exogenous ERα into MCF10A cells resulted in transcriptional activation of a reporter gene, but failed to induce proliferation [58]. Similarly, in normal breast epithelial cells immortalized with telomerase (76N-Tert), treatment with estrogen and progesterone inhibited cell proliferation and was associated with an increase in transactivation by p53 [59]. The inconsistency of the results among experiments may reflect variations in the complement of co-activators among cell lines, which strongly influence the activities of ERα and p53 [60, 61].
A more consistent picture emerges from experiments varying ERβ levels. Transient transfection of ERβ enhanced radiation-induced expression of p21 [62]. Similarly, expression of ERβ inhibited proliferation of MCF7 cells and blocked tumor formation as xenografts [63, 64]. Expression of ERβ also inhibited tumor growth in T47D cells, which express a missense mutant of p53 [65, 66]. Thus, ERβ may have p53-independent anti-proliferative activities. Conditional expression of ERβ in MCF7 cells increased expression of pro-apoptotic target genes [67]. Although ERα and ERβ show similar potency in transactivation of reporter genes and appear to bind to similar target sequences identified by chromatin immunoprecipitation [68, 69], co-expression of ERβ along with ERα resulted in dramatic alterations in transcriptional profiles in breast cancer cell lines [68, 70–73], supporting distinct actions and targets for these receptors. It is notable that EGR1 was increased by threefold in MCF7 cells when ERβ was present [70], which is similar to the induction noted in mouse mammary gland after stimulation with E + P [26]. Therefore, the opposing activities of ERα and ERβ appear to be in an equilibrium that determines the cellular response.
Future implications
Although the mechanisms by which ovarian hormones act to both promote and prevent breast cancer remain incomplete, NF-κB and p53 are likely to be major targets. A division of labor between estrogen receptor subtypes is also suggested, with ERα being essential for proliferation, while ERβ favors genome surveillance via p53. EGR1 offers an attractive target for arbitrating the acute response to genotoxic stress. While the acute responses to estrogen and progesterone remain significant, the effects on epigenetic programs are likely to be more profound in determining the balance among responses and the long-term reduction in the risk of breast cancer. Consequently, rather than simply blocking the actions of estrogen and progesterone, one can envision therapies that harness the effects of these hormones to alter the epigenetic profile so as to shift the equilibrium to favor surveillance and a durable resistance to carcinogenesis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-CA095164, R01-CA105452 and R01-ES015739 DJJ).
References
- 1.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237:474–482. doi: 10.1097/00000658-200304000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 3.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA, Flaws JA, Furth PA. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65:681–685. [PMC free article] [PubMed] [Google Scholar]
- 5.Montero GG, Vanzulli SI, Cerliani JP, Bottino MC, Bolado J, Vela J, Becu-Villalobos D, Benavides F, Gutkind S, Patel V, Molinolo A, Lanari C. Association of estrogen receptor-alpha and progesterone receptor A expression with hormonal mammary carcinogenesis: role of the host microenvironment. Breast Cancer Res. 2007;9:R22. doi: 10.1186/bcr1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aupperlee MD, Drolet AA, Durairaj S, Wang W, Schwartz RC, Haslam SZ (2008) Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development. Endocrinology [DOI] [PMC free article] [PubMed]
- 7.Zheng W, Long J, Gao YT, Li C, Zheng Y, Xiang YB, Wen W, Levy S, Deming SL, Haines JL, Gu K, Fair AM, Cai Q, Lu W, Shu XO. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009;41:324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchatchou S, Jung A, Hemminki K, Sutter C, Wappenschmidt B, Bugert P, Weber BH, Niederacher D, Arnold N, Varon-Mateeva R, Ditsch N, Meindl A, Schmutzler RK, Bartram CR, Burwinkel B. A variant affecting a putative miRNA target site in estrogen receptor (ESR) 1 is associated with breast cancer risk in premenopausal women. Carcinogenesis. 2009;30:59–64. doi: 10.1093/carcin/bgn253. [DOI] [PubMed] [Google Scholar]
- 9.Maguire P, Margolin S, Skoglund J, Sun XF, Gustafsson JA, Borresen-Dale AL, Lindblom A. Estrogen receptor beta (ESR2) polymorphisms in familial and sporadic breast cancer. Breast Cancer Res Treat. 2005;94:145–152. doi: 10.1007/s10549-005-7697-7. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- 11.Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses’ Health Study. Am J Epidemiol. 1994;139:819–835. doi: 10.1093/oxfordjournals.aje.a117079. [DOI] [PubMed] [Google Scholar]
- 12.Grubbs CJ, Farnell DR, Hill DL, McDonough KC. Chemoprevention of N-nitroso-N-methylurea-induced mammary cancers by pretreatment with 17 beta-estradiol and progesterone. J Natl Cancer Inst. 1985;74:927–931. [PubMed] [Google Scholar]
- 13.Sivaraman L, Stephens LC, Markaverich BM, Clark JA, Krnacik S, Conneely OM, O’Malley BW, Medina D. Hormone-induced refractoriness to mammary carcinogenesis in Wistar–Furth rats. Carcinogenesis. 1998;19:1573–1581. doi: 10.1093/carcin/19.9.1573. [DOI] [PubMed] [Google Scholar]
- 14.Cabanes A, Wang M, Olivo S, deAssis S, Gustafsson JA, Khan G, Hilakivi-Clarke L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–748. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 15.Birch JM, Alston RD, McNally RJ, Evans DG, Kelsey AM, Harris M, Eden OB, Varley JM. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–4628. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 16.Nichols KE, Malkin D, Garber JE, Fraumeni JF, Jr, Li FP. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev. 2001;10:83–87. [PubMed] [Google Scholar]
- 17.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 18.Crook T, Brooks LA, Crossland S, Osin P, Barker KT, Waller J, Philp E, Smith PD, Yulug I, Peto J, Parker G, Allday MJ, Crompton MR, Gusterson BA. p53 mutation with frequent novel condons but not a mutator phenotype in BRCA1- and BRCA2-associated breast tumours. Oncogene. 1998;17:1681–1689. doi: 10.1038/sj.onc.1202106. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Wagner K-U, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng C-X. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumor formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 20.Meijers-Heijboer H, Van Den OA, Klijn J, Wasielewski M, De Snoo A, Oldenburg R, Hollestelle A, Houben M, Crepin E, Veghel-Plandsoen M, Elstrodt F, Van Duijn C, Bartels C, Meijers C, Schutte M, McGuffog L, Thompson D, Easton DF, Sodha N, Seal S, Barfoot R, Mangion J, Chang-Claude J, Eccles D, Eeles R, Evans DG, Houlston R, Murday V, Narod S, Peretz T, Peto J, Phelan C, Zhang HX, Szabo C, Devilee P, Goldgar D, Futreal PA, Nathanson KL, Weber BL, Rahman N, Stratton MR. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–59. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 21.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, Onel K, Yip L, Hwang SJ, Strong LC, Lozano G, Levine AJ. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Dunphy KA, Blackburn AC, Yan H, O’Connell LR, Jerry DJ. Estrogen and progesterone induce persistent increases in p53-dependent apoptosis and suppress mammary tumors in BALB/c-Trp53± mice. Breast Cancer Res. 2008;10:R43. doi: 10.1186/bcr2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickinson ES, Bonilla PJ, Butel JS, Medina D. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–1058. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 24.Medina D, Kittrell FS. p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 25.Becker KA, Lu S, Dickinson ES, Dunphy KA, Mathews L, Schneider SS, Jerry DJ. Estrogen and progesterone regulate radiation-induced p53 activity in mammary epithelium through TGF-beta-dependent pathways. Oncogene. 2005;24:6345–6353. doi: 10.1038/sj.onc.1208787. [DOI] [PubMed] [Google Scholar]
- 26.Lu S, Becker KA, Hagen MJ, Yan H, Roberts AL, Mathews LA, Schneider SS, Siegelmann HT, Macbeth KJ, Tirrell SM, Blanchard JL, Jerry DJ. Transcriptional responses to estrogen and progesterone in mammary gland identify networks regulating p53 activity. Endocrinology. 2008;149:4809–4820. doi: 10.1210/en.2008-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/S0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 28.Demicco EG, Kavanagh KT, Romieu-Mourez R, Wang X, Shin SR, Landesman-Bollag E, Seldin DC, Sonenshein GE. RelB/p52 NF-kappaB complexes rescue an early delay in mammary gland development in transgenic mice with targeted superrepressor IkappaB-alpha expression and promote carcinogenesis of the mammary gland. Mol Cell Biol. 2005;25:10136–10147. doi: 10.1128/MCB.25.22.10136-10147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minter LM, Dickinson ES, Naber SP, Jerry DJ. Epithelial cell cycling predicts p53 responsiveness to gamma-irradiation during post-natal mammary gland development. Development. 2002;129:2997–3008. doi: 10.1242/dev.129.12.2997. [DOI] [PubMed] [Google Scholar]
- 32.Sivaraman L, Conneely OM, Medina D, O’Malley BW. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman NR, Perkins ND. Inhibition of the RelA(p65) NF-kappaB subunit by Egr-1. J Biol Chem. 2000;275:4719–4725. doi: 10.1074/jbc.275.7.4719. [DOI] [PubMed] [Google Scholar]
- 34.Krones-Herzig A, Adamson E, Mercola D. Early growth response 1 protein, an upstream gatekeeper of the p53 tumor suppressor, controls replicative senescence. Proc Natl Acad Sci USA. 2003;100:3233–3238. doi: 10.1073/pnas.2628034100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, Soni T, Adamson ED, Mercola D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005;65:5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. [DOI] [PubMed] [Google Scholar]
- 36.Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ewan KB, Oketch-Rabah HA, Ravani SA, Shyamala G, Moses HL, Barcellos-Hoff MH. Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice. Am J Pathol. 2005;167:409–417. doi: 10.1016/s0002-9440(10)62985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, Akhurst RJ, Barcellos-Hoff MH. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res. 2002;62:5627–5631. [PubMed] [Google Scholar]
- 39.An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. doi: 10.1016/j.cell.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Cruz CM, Moody SE, Master SR, Hartman JL, Keiper EA, Imielinski MB, Cox JD, Wang JY, Ha SI, Keister BA, Chodosh LA. Persistent parity-induced changes in growth factors, TGF-beta3, and differentiation in the rodent mammary gland. Mol Endocrinol. 2002;16:2034–2051. doi: 10.1210/me.2002-0073. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson DS, Tsai WW, Schumacher MA, Barton MC. Chromatin-bound p53 anchors activated Smads and the mSin3A corepressor to confer transforming-growth-factor-beta-mediated transcription repression. Mol Cell Biol. 2008;28:1988–1998. doi: 10.1128/MCB.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pena PV, Hom RA, Hung T, Lin H, Kuo AJ, Wong RP, Subach OM, Champagne KS, Zhao R, Verkhusha VV, Li G, Gozani O, Kutateladze TG. Histone H3K4me3 binding is required for the DNA repair and apoptotic activities of ING1 tumor suppressor. J Mol Biol. 2008;380:303–312. doi: 10.1016/j.jmb.2008.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ythier D, Larrieu D, Brambilla C, Brambilla E, Pedeux R. The new tumor suppressor genes ING: genomic structure and status in cancer. Int J Cancer. 2008;123:1483–1490. doi: 10.1002/ijc.23790. [DOI] [PubMed] [Google Scholar]
- 46.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel BA. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, Mella O, Howell A. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–116. doi: 10.1023/A:1010619225209. [DOI] [PubMed] [Google Scholar]
- 48.Rajkumar L, Guzman RC, Yang J, Thordarson G, Talamantes F, Nandi S. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98:11755–11759. doi: 10.1073/pnas.201393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/en.138.3.863. [DOI] [PubMed] [Google Scholar]
- 50.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- 51.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/me.11.10.1486. [DOI] [PubMed] [Google Scholar]
- 52.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 53.Kass L, Durando M, Ramos JG, Varayoud J, Powell CE, Luque EH, Munoz-de-Toro M. Association of increased estrogen receptor beta2 expression with parity-induced alterations in the rat mammary gland. J Steroid Biochem Mol Biol. 2004;91:29–39. doi: 10.1016/j.jsbmb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Liu W, Konduri SD, Bansal S, Nayak BK, Rajasekaran SA, Karuppayil SM, Rajasekaran AK, Das GM. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem. 2006;281:9837–9840. doi: 10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- 55.Liu G, Schwartz JA, Brooks SC. Estrogen receptor protects p53 from deactivation by human double minute-2. Cancer Res. 2000;60:1810–1814. [PubMed] [Google Scholar]
- 56.Akaogi K, Nakajima Y, Ito I, Kawasaki S, Oie SH, Murayama A, Kimura K, Yanagisawa J. KLF4 suppresses estrogen-dependent breast cancer growth by inhibiting the transcriptional activity of ERalpha. Oncogene. 2009;28:2894–2902. doi: 10.1038/onc.2009.151. [DOI] [PubMed] [Google Scholar]
- 57.Shirley SH, Rundhaug JE, Tian J, Cullinan-Ammann N, Lambertz I, Conti CJ, Fuchs-Young R. Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res. 2009;69:3405–3414. doi: 10.1158/0008-5472.CAN-08-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pilat MJ, Christman JK, Brooks SC. Characterization of the estrogen receptor transfected MCF10A breast cell line 139B6. Breast Cancer Res Treat. 1996;37:253–266. doi: 10.1007/BF01806507. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Tu Y, Smith-Schneider S. Activation of p53, inhibition of telomerase activity and induction of estrogen receptor beta are associated with the anti-growth effects of combination of ovarian hormones and retinoids in immortalized human mammary epithelial cells. Cancer Cell Int. 2005;5:6. doi: 10.1186/1475-2867-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 61.Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 62.Lewandowski SA, Thiery J, Jalil A, Leclercq G, Szczylik C, Chouaib S. Opposite effects of estrogen receptors alpha and beta on MCF-7 sensitivity to the cytotoxic action of TNF and p53 activity. Oncogene. 2005;24:4789–4798. doi: 10.1038/sj.onc.1208595. [DOI] [PubMed] [Google Scholar]
- 63.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.CAN-03-2446. [DOI] [PubMed] [Google Scholar]
- 64.Behrens D, Gill JH, Fichtner I. Loss of tumourigenicity of stably ERbeta-transfected MCF-7 breast cancer cells. Mol Cell Endocrinol. 2007;274:19–29. doi: 10.1016/j.mce.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson JA. Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006;66:11207–11213. doi: 10.1158/0008-5472.CAN-06-0017. [DOI] [PubMed] [Google Scholar]
- 66.Sotoca AM, van den BH, Vervoort J, van der SP, Strom A, Gustafsson JA, Rietjens I, Murk AJ. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008;105:303–311. doi: 10.1093/toxsci/kfn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodges-Gallagher L, Valentine CD, El BS, Kushner PJ. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat. 2008;109:241–250. doi: 10.1007/s10549-007-9640-6. [DOI] [PubMed] [Google Scholar]
- 68.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol. 2008;22:1032–1043. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K. The genome landscape of ERalpha- and ERbeta-binding DNA regions. Proc Natl Acad Sci USA. 2008;105:2604–2609. doi: 10.1073/pnas.0712085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 71.Lin CY, Strom A, Li KS, Kietz S, Thomsen JS, Tee JB, Vega VB, Miller LD, Smeds J, Bergh J, Gustafsson JA, Liu ET. Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 2007;9:R25. doi: 10.1186/bcr1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Secreto FJ, Monroe DG, Dutta S, Ingle JN, Spelsberg TC. Estrogen receptor alpha/beta isoforms, but not betacx, modulate unique patterns of gene expression and cell proliferation in Hs578T cells. J Cell Biochem. 2007;101:1125–1147. doi: 10.1002/jcb.21205. [DOI] [PubMed] [Google Scholar]
- 73.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–1032. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]