Abstract
This review explores various aspects of the interaction between microtubule targeting agents and tubulin, including binding site, affinity, and drug resistance. Starting with the basics of tubulin polymerization and microtubule targeting agent binding, we then highlight how the three-dimensional structures of drug–tubulin complexes obtained on stabilized tubulin are seeded by precise biological and biophysical data. New avenues opened by thermodynamics analysis, high throughput screening, and proteomics for the molecular pharmacology of these drugs are presented. The amount of data generated by biophysical, proteomic and cellular techniques shed more light onto the microtubule–tubulin equilibrium and tubulin–drug interaction. Combining these approaches provides new insight into the mechanism of action of known microtubule interacting agents and rapid in-depth characterization of next generation molecules targeting the interaction between microtubules and associated modulators of their dynamics. This will facilitate the design of improved and/or alternative chemotherapies targeting the microtubule cytoskeleton.
Keywords: Tubulin, Thermodynamics, Taxanes, Vinca alkaloids, Proteomics, Microtubule, Microtubule targeting agents
Introduction
Microtubules (MTs) are cytoskeletal hollow fibers common to most eukaryotic cells [1]. These dynamic structures result from the interaction of α/β tubulin polymers with microtubule-associated proteins (MAPs) [2, 3]. MTs control cell shape and processes such as motility, mitosis, intracellular vesicle transport, organization, and positioning of membranous organelles [4–7]. The microtubule cytoskeleton is one of the major targets in cancer chemotherapy. MTs exhibit a highly dynamic behavior, undergoing transitions from growing to shrinking phases and resulting in a dynamic exchange of tubulin dimers at microtubule ends [8]. Although this dynamic behavior occurs without general modification of microtubule stability, some key events in the cell, such as transition from nonmigratory to migratory status, or between interphase and mitosis, require alterations in dynamics and overall remodeling of the microtubule network. These processes are driven by MAPs, which either destabilize or stabilize MTs; some of these MAPs participate in the local fine tuning of microtubule end dynamics [2]. Microtubule targeting agents (MTAs) also modify the formation and function of MTs [9]. These compounds, known to interact with tubulin, lead to cell death by altering microtubule dynamics at low, clinically relevant concentrations. At higher doses, they can either stabilize or destabilize MTs thereby changing the cellular unpolymerized to polymerized tubulin ratio and inducing cell death. Natural MTAs such as paclitaxel, vinblastine, or vincristine [10–13] have been used in the clinic for years. Although MTAs are potent antitumoral drugs, their clinical use is limited by hematological and neurological toxicities as well as by tumor resistance that leads to tumor resurgence and death [14]. The attempts to generate better MTAs require a detailed understanding of the drug–tubulin interaction at the molecular and cellular levels. After a rapid description of microtubule polymerization and the latest techniques in the field (Table 1), we will focus on the biophysical characterization of the tubulin binding site of antimicrotubule agents. To understand how the therapeutic window of MTAs could be improved, we will then focus on three topics: first, binding affinity of MTAs for tubulin; second, bioavailability at the tumor site using vectorization and cancer cell targeting; and third, in-depth characterization of the microtubule proteome in cancer cells using mass spectrometry. The quest for the best antimicrotubule agent may involve a combination of high throughput techniques to define the optimal binding parameters of the drug for tubulin in the tumor.
Table 1.
Biophysical approaches for the study of MTAs binding to tubulin
| Analytical ultracentrifugation | Characterization of biomolecular complexes interactions, and macromolecular conformational changes, by sedimentation velocity and sedimentation equilibrium | [11], [62], [72], [74], [75], [77] [147] |
| Differential scanning calorimetry (DSC) | Thermal denaturation of samples to determine macromolecular and domain organization of proteins | [73], [77], [151] |
| Electron diffraction crystallography | Study of matter by firing electrons and observing the resulting interference pattern, three-dimensional structure of proteins at low resolution | [16], [17], [38] |
| Electron microscopy | A beam of electrons create an image of the sample. Magnification is 250 higher than optical microscopy; 5–10 nM objects can be imaged | [5], [11], [22], [23], [34], [62], [74], [86], [125] |
| Fluorescence anisotropy | Characterization of the interaction between two molecules one being fused to a fluorophore. The fluorophore is excited by polarized light but also emit. Upon binding of the partner molecule, the complex will increase the polarization of the emitted light | [43], [55] |
| Fluorescence correlation spectroscopy (FCS) | Characterization of the dynamics of fluorescent species, a common tool for studying molecular dynamics in living cells | [34] |
| Fluorescence resonance energy transfer (FRET) | Distance-dependent excited state interaction in which emission of one fluorophore is coupled to the excitation of another | [48] |
| Isothermal titration calorimetry (ITC) | Measure of heat exchange to probe biomolecular interactions. Simultaneous determination of all binding parameters (n, K, ∆H, and ΔS) | [73], [147], [151] |
| Mass spectrometry–AQUA® peptide technology | Use of a synthetic internal isotopically labeled peptide for absolute quantitation by mass spectrometry | [127] |
| Mass spectrometry–hydrogen deuterium exchange (HDX) | Measure of deuterium incorporation in proteins to probe conformational changes induced by ligands | [49], [50] |
| Mass spectrometry–liquid chromatographic–tandem mass spectrometric analyses | Separation of complex mixture by liquid chromatography coupled to mass spectrometry sequencing | [108], [109] |
| Mass spectrometry–MALDI-TOF | Mass spectrometric method combining laser desorption and time of flight separation of ions to measure the mass of molecules | [40] |
| Mass spectrometry–mass spectrometry imaging | Visualization of the spatial distribution of metabolites, peptides or proteins by their molecular masses in a tissue slice | [130], [131], [132], [133], [134], [135] |
| Mass spectrometry–peptide mass fingerprint (PMF) | Identification of proteins by enzymatic digestion, mass spectrometric analysis, and database searching | [126] |
| Mass spectrometry–electrospray | Soft ionization method to measure the mass of molecules | |
| Optical tweezers | Manipulation and detection of sub-nanometer displacements for sub-micrometer dielectric particles. Used to study single molecules | [35] |
| Photoaffinity labeling | A photochemically reactive molecular entity, specifically associated with a biomolecule, is photo-excited in order to covalently attach a label to the biomolecule. Identification of the resulting photo-crosslinked product provides structural information on the protein’s binding site | [44], [45], [46], [54] [60], [61], [65], [66], [67], [68], [69], [70], [71] |
| Solid state rotational-echo double-resonance (REDOR) | Use of magic angle spinning and cross-polarization to measure the distance between two select labeled heteronuclei | [47] |
| X-ray crystallography | Characterization of three-dimensional structure of a macromolecule by diffraction in a crystal lattice | [42], [63], [143] |
A brief reminder on tubulin
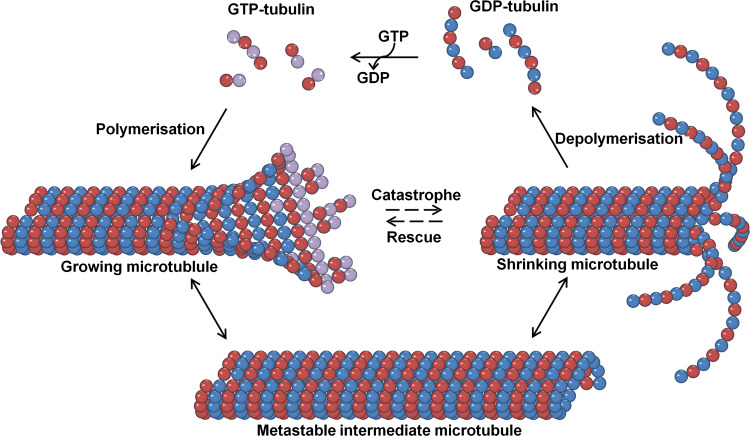
Soluble tubulin is found as a 110-kDa heterodimer of α and β tubulin subunits that assembles itself head to tail into protofilaments associating laterally to build a hollow tube called the microtubule. MTs are polar and dynamic structures formed by 13 protofilaments [15]. Each monomer, namely α or β tubulin, binds a guanosine tri phosphate (GTP) molecule and can roughly be divided in three domains: The N-terminal domain (residues 1–206) containing the nucleotide binding site, the central domain (207–384) involved in lateral/longitudinal contacts, and the C-terminal domain which is mainly involved in the binding of MAPs. The C-terminus (the last 20 amino acids) is disordered and forms a cloud of negative charge around the microtubule [16]. The nucleotide in the α-subunit is buried at the intradimer interface, explaining the nonexchangeability of the GTP in this site while the nucleotide in the β-subunit is partially exposed [17]. GTP bound to β-tubulin is hydrolyzed to GDP after incorporation of tubulin into the microtubule lattice. When a GDP-containing dimer is released upon microtubule disassembly, its assembly competence is regenerated by exchange of GDP for GTP. MTs are dynamic structures constantly switching between polymerization and depolymerization phases via rescues and catastrophes, respectively, that are regulated by GTP and MAPs (Fig. 1). Microtubules longitudinally elongate by addition of dimers or oligomers. They contain GTP-tubulin at the microtubule plus-end which maintains the microtubule stability and protofilaments association [3, 18–20]. The stationary state or pause is a third phase where polymerization and depolymerization are either inhibited or instantaneously compensated to maintain the length of the microtubule constant. Treadmilling is a particular case of this process in which there is a continuous addition of tubulin subunits at one end and disassembly at the other. In that case, microtubules seem to stay of constant length but individual subunits move along the length of the microtubule.
Fig. 1.
Microtubule polymerization phases. Microtubules grow by addition of GTP-tubulin subunits. After their incorporation, subunits are hydrolyzed to become GDP-tubulin. The straight tubulin conformation within the microtubule lattice is stabilized by a ‘cap’ of tubulin-GTP subunits. Closure of the terminal sheet structure generates a metastable, blunt-ended microtubule intermediate, which might pause, undergo further growth, or switch to the depolymerization phase possibly by loss of the GTP-tubulin at the end. GDP-tubulin is free to splay out and the microtubule rapidly shrinks. Microtubules can transition from growth to shrinking phases called catastrophes. Protection of microtubules from shrinking by adding GTP-tubulin at the tip of microtubule is referred to as rescue
Other members of the tubulin family, such as γ-tubulin [21], have a key role in microtubule nucleation [22–24], or like δ-, ε-, η- and ζ-tubulin are constituents of specialized organelles [25–27]. Multiple genes encode for α- and β-tubulin which, especially in vertebrates, generate complex expression profiles. In humans, eight α-isotypes (α1A, α1B, α1C, α3C, α3E, α4A, α8, and αlike3) and seven β-isotypes (βI, βII, βIII, βIVa, βIVb, βV, and βVI) have so far been discovered [28]. In addition, these tubulin isotypes may undergo multiple posttranslational modifications such as polyglutamylation, polyglycylation, acetylation, phosphorylation, tyrosination, and palmitoylation [29]. Questions relative to the functional significance of this tubulin diversity have been the subject of extensive genomic and proteomic studies for more than 20 years [30, 31]. The sequence divergence between isotypes and sites for posttranslational modifications are concentrated on the C-termini of tubulins which are also the sites of the interaction of MAPs with MTs [16]. However, hotspots of sequence divergence between isotypes in the rest of the structure may differentially modulate their respective molecular dynamics [18].
Biophysical investigations using spectroscopy, sedimentation velocity, microcalorimetry, and dialysis provided information on the microscopic nature of tubulin polymerization steps. Studies clearly showed that the elongation occurs mainly via the addition of tubulin dimers [32]. Others also showed that XMAP215-mediated stabilization triggered multiple tubulin dimer additions [33]. Very recent electron microscopy and fluorescence correlation spectroscopy (FCS) experiments suggested that microtubule elongation and nucleation involves interactions of short tubulin oligomers rather than dimers [34]. Optical tweezers observing the assembly dynamics of individual microtubules at molecular resolution also showed that MTs can increase their length by amounts exceeding the size of individual dimers (up to three tubulin dimers) [35] (Table 1). It is still uncertain whether these in vitro observations have significance for MTs dynamics in cells where many proteins regulate the equilibrium between the pool of unassembled tubulin and MTs, but these findings help in modeling how a drug might perturb the dynamics of MTs.
The tubulin binding sites of microtubule targeting agents
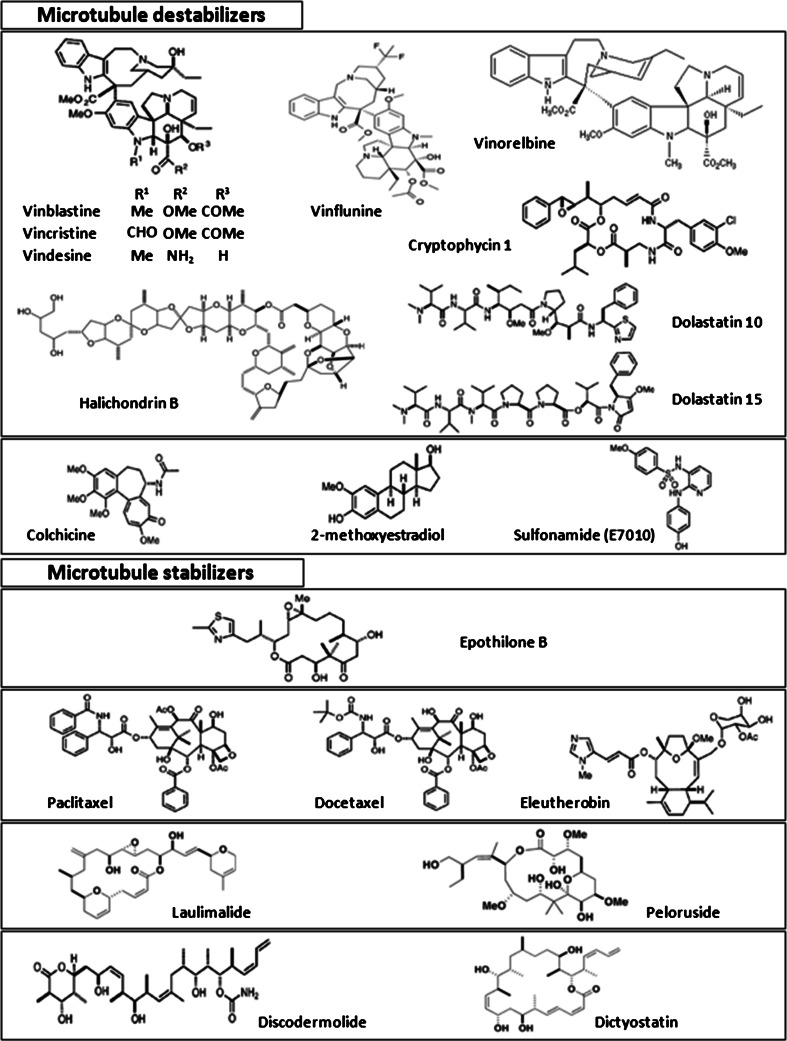
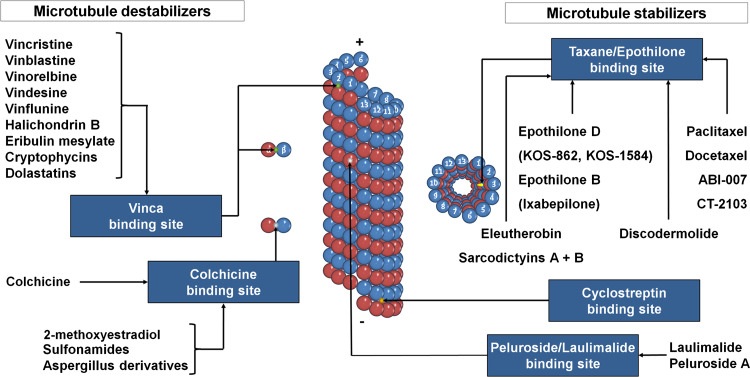
MTAs (for chemical structure, see Fig. 2), known to interact with tubulin, disrupt microtubule dynamics by either stabilizing or destabilizing the polymerized state. This wide class of drugs is composed of more than 30 different molecular backbones interacting with four main tubulin binding sites: the taxane/epothilones site, the laulimalide/peloruside A site, the colchicine site, and the vinca alkaloid site (Fig. 3). Binding to the two first sites stabilizes MTs while binding to the others induces tubulin depolymerization. This old MTA classification as microtubule stabilizers or destabilizers is overly simplistic since drugs that increase or decrease microtubule polymerization at high concentrations, both suppress microtubule dynamics at concentrations 10-100 times lower. At these low concentrations, drugs kinetically stabilize MTs without changing their polymer mass, but can induce mitotic arrest and apoptosis or inhibit cell migration [36, 37]. Figure 3 illustrates one of the fundamental differences between antimicrotubule agents, the binding either on free tubulin or on microtubules. Taxanes preferentially bind the polymeric MT form of tubulin at the lumen of the microtubule pore. Epothilones bind the same site but exploit the tubulin-binding pocket in a unique and independent manner [38]. The stabilizing agent, cyclostreptin, is significantly different since it binds at the outer surface of pores in the microtubule wall. Laulimalide [39] and peloruside A [40] share a binding site distinct from the paclitaxel/epothilone site. Colchicine and molecules binding on the same site induce microtubule depolymerization by inhibiting lateral contacts between protofilaments [41] and have a better affinity for free tubulin heterodimers. Vinca alkaloids’ binding preferentially occurs on free tubulin but also at the end of the microtubule.
Fig. 2.
Structure of microtubule destabilizers and stabilizers
Fig. 3.
Tubulin binding site of MTAs
The flexibility and molecular diversity of tubulin have been major problems for determining its structure and drug binding pockets. Tubulin structure was obtained by electron diffraction of tubulin–zinc sheets stabilized by docetaxel [16], paclitaxel [17], or epothilone A [38]. The three-dimensional model of the tubulin–vinblastine and tubulin–colchicine interactions was obtained by X-ray crystallography in the presence of RB3, a stathmin-like, tubulin-associated protein forming the T2S complex with two tubulin dimers [42] (Table 1).
Drug binding sites are well described although some structural biases are still debated such as the paclitaxel conformation and the unknown tubulin conformation details in the context of the microtubule lattice. Interestingly, the use of alternative approaches can provide very close printouts and almost the same view as X-ray or electron crystallography. The elegant work of Andreu et al. [43] is the perfect plea for thermodynamic analysis and how to determine the binding sites at the molecular level without resolving the structure. These authors investigated the binding of separate parts of paclitaxel, the C-13 side chain and the baccatin III (paclitaxel without the side chain methyl ester), by fluorescence anisotropy (Table 1). The competition isotherms showed that the taxane ring system (C2–C4) of the paclitaxel molecule provides 75% of the free energy change (ΔG). Although not as active as paclitaxel, baccatin III does promote microtubule assembly in the absence of GTP, stabilizes MTs, and competitively inhibits the binding of [3H]-paclitaxel to the microtubule protein. In cells, a large excess of baccatin III completely inhibits microtubule anisotropy of Flutax-2, a fluorescent paclitaxel derivative that retains binding and stabilization properties, which is not the case for the methyl ester side chain. The authors showed that the global conformational change due to baccatin III binding was similar to that of paclitaxel. They also hypothesized a potential hydrogen bond between the 2′OH of the baccatin III and tubulin. This bond would strengthen the interaction without structure modification. Photoaffinity labeling was also extensively used to map the taxane binding site. Paclitaxel analogues with photoreactive substituents at defined positions around the taxane nucleus nicely pinpointed amino acids forming the binding pocket [44–46].
The three-dimensional structure is an amazing printout of these pharmacological data [16]. The binding pocket for paclitaxel is located near the surface of β-tubulin as mapped by electron crystallography. Paclitaxel interacts with the protein by means of three potential hydrogen bonds and multiple hydrophobic contacts. The studies using solid state rotational-echo double-resonance (REDOR), nuclear magnetic resonance (NMR) [47], and fluorescence resonance energy transfer (FRET) [48] (Table 1) demonstrated that the ligand inside the binding site is in a hydrophobically collapsed conformation.
Despite this crucial structure description, there is a need for the visualization of the conformational change in MT dynamics by taxanes. A first step in this direction has been made by the combination of hydrogen exchange (HDX) and mass spectrometry (Table 1). This approach, used first with paclitaxel [49], has been recently applied to the analysis of tubulin regions with altered levels of deuterium incorporation in MTs upon docetaxel, epothilone A, and peloruside A binding [50]. By this method, drug-induced solvent exposure can be translated into changes in molecular entropy. This strategy established the mechanisms by which these drugs stabilize MTs and helped in determining a highly probable site for peloruside A binding. This site is distinct from the taxane/epothilone site, which is consistent with binding competition studies [51]. Interestingly, these works confirmed the computer modeling of tubulin dynamics experimentally [52] and showed the major site of microtubule stabilization lies in the longitudinal tubulin dimer interfaces. Bioactive conformation of peloruside A, laulimalide, and discodermolide have been described by Jiménez-Barbero et al. [53], but a detailed analysis of the binding site for these agents is not yet available.
Displacement titration with labeled ligand is a method of choice to investigate drug competition or synergism in vitro and therefore is useful for determining whether drugs have affinity for common or different binding sites. For laulimalide and peloruside, their site of tubulin binding was investigated using a combination of displacement tests using flutax-2 and a new mass spectrometric-based approach in which peloruside was displaced and detected by matrix-assisted LASER desorption/ionization (MALDI) mass spectrometry [40] (Table 1). Laulimalide and peloruside bind a site on tubulin that is not completely characterized, but is distinct from the common site for paclitaxel, epothilone, and discodermolide. This strategy was also applied to discodermolide binding to tubulin. Xia et al. [54] described the analysis of three photoaffinity-labeled discodermolide analogues which promote microtubule polymerization in the absence of GTP. C19-[3H]BPC-discodermolide analogue is not displaced by paclitaxel, but can displace it since its affinity is three orders of magnitude higher than paclitaxel. Discodermolide and paclitaxel may occupy overlapping binding pockets since competitive behavior exists between the two drugs [55]. This was further suggested by photolabeling with a discodermolide analogue, enzymatic digestion of tubulin, and analysis of resulting peptides by mass spectrometry. Cyclostreptin binding site on microtubules was discovered using mass spectrometry strategies and the binding mechanism was probed with Flutax-2, displacement of radiolabeled taxanes, epothilones, and discodermolide [56]. These studies clearly showed that cyclostreptin binds to the outer surface of a pore in the microtubule wall but also explained how paclitaxel reached its kinetically unfavorable luminal site. The diversity of binding sites for MTs stabilizers on tubulin opens the possibility of synergistic drug action as observed for peloruside A and paclitaxel [57, 58].
Contrary to stabilizing agents that bind only polymerized MTs, destabilizing agents, such as the vinca alkaloids, also bind to soluble tubulin. At the lowest effective concentrations, vinca alkaloids have been shown to affect both dynamic instability and treadmilling of microtubules. At higher concentrations, they depolymerize MTs and at super stoichiometric concentrations they induce the formation of one or two protofilaments spirals or large paracrystals made of these spirals. Before precise X-ray characterization of the vinca alkaloid binding domain [42], key contacts between these drugs and tubulin were investigated using two vinca alkaloid moieties: velbanamine (indole) and vindoline (dihydroindole) [59]. Catharanthine, the closest analogue mimicking velbanamine, was shown to induce the self-association of tubulin into indefinite linear polymers whereas the binding of vindoline was barely detectable. However, this study shows that the covalent link between velbanamine and vindoline is necessary to obtain a strong binding affinity. In the case of vinblastine or vincristine, the binding energy of the two separate halves of these molecules were larger than the binding energy of the intact structure, indicating loss of conformational freedom of the drugs inside the binding site. This study showed that chemical modifications of velbanamine, on the D′ ring, are by far the most important determinants for vinca alkaloid affinity. As an example, vinorelbine or 7′ noranhydrovinblastine modified on the velbanamine part of the molecule has reduced affinity for tubulin. X-ray crystallography shows that vinblastine is in contact with two tubulin heterodimers at the interface between the α-subunit of one and the β-subunit of the other, preventing elongation of the microtubule. Vinblastine binds to residues located toward the inner lumen of the protofilaments and involves longitudinal contacts. Both the velbanamine and the vindoline moieties of vinblastine interact with the protein. This mode of binding is consistent with previous studies using affinity and photoaffinity labeling vinblastine analogs [60, 61].
Several short unconventional natural peptides have also generated hope as potential anticancer drugs. This is the case of cryptophycin depsipeptides, competitors of vinca alkaloids isolated from cyanobacteria. Cryptophycin 1 induces the formation of small ring-shaped oligomers of tubulin [62], and the tubulin binding site of the cryptophycins partially overlaps the vinblastine binding site. Unfortunately, this drug was stopped in phase II clinical trials because of toxicity. Other drugs that partially overlap the vinca alkaloid binding site such as phomopsin and dolastatins, a macrocyclic hexapeptide and pentapeptide, respectively, have been used to clarify the mechanism of tubulin nucleotide exchange and elucidate the ways by which vinca alkaloid ligands prevent microtubule assembly [63]. Some other recently discovered MTAs have the destabilizing effects of the vinca alkaloids without sharing the same site. Halichondrin B [64] and its derivative eribulin are macrolide lactone polyethers that inhibit vinca alkaloid binding through an allosteric mechanism. The localization of the colchicine binding site was obtained using affinity or photoaffinity labeling derivatives. Various studies showed that the binding site would be in the α-subunit [65, 66], the β-subunit [67], on both subunits [68, 69], or at the dimer interface [70, 71]. Finally, the crystallographic structure at 3.5 Å of tubulin bound to colchicine in the T2S complex confirmed the location of colchicine at the intradimer interface. Last but not least, a very recent study, based on mass spectrometry, hypothesized a new binding site on tubulin for caulerpenyne, a cytotoxic sesquiterpene isolated from the seaweed Caulerpa taxifolia [72]. Therefore, we can affirm that the diverse compounds that can bind to different sites on tubulin reflect the complexity of the protein molecular dynamics and raise the possibility that other binding pockets could be targeted by both natural and synthetic products.
Is affinity improvement a real benefit?
Structure–activity relationship studies of microtubule stabilizing agents require the knowledge of four main parameters: n, the stoichiometry; K a, the binding affinity of the compound to MTs; Cr, the critical tubulin concentration for polymerization; and IC50, the concentration of drug inducing 50% cell growth inhibition. For determination of accurate binding parameters, quantification of the free and bound species in solution is sometimes a challenging prerequisite to fulfill. This can be performed by direct measurements such as dialysis, co-sedimentation using radiolabeled, fluorescently tagged ligand, analytical ultracentrifugation, differential scanning calorimetry (DSC), or nuclear magnetic resonance (NMR). Nondirect measurements directly proportional to ligand binding are generated by spectroscopic techniques based on fluorescence or absorption, or by isothermal titration calorimetry (Table 1). The values refine the binding model with conformational change information. One of the great interests of thermodynamic result analysis is that the difference in the binding energy between two drugs congeners can be rationalized in terms of enthalpy and entropy changes which provide crucial structural information for drug improvement depending on the hydrophilic or apolar nature of key interactions [73]. The goal of a MTA is to efficiently kill malignant cells, therefore a simple question emerges: can we correlate IC50 values with tubulin binding affinity?
Most of these parameters have been determined for the MT stabilizing agents as reported in Table 2. Authors have shown that a correlation exists between binding parameters and cytotoxicity for most of the existing taxane/epothilone analogues [74]. Lobert et al. also provide the thermodynamic direction for drug improvement since they assume that compounds with a favorable entropic contribution to binding are less effective in killing cells than those with a favorable enthalpic contribution. Using this approach, they were able to increase paclitaxel affinity for tubulin by three orders of magnitude but with only a tenfold increase in cytotoxicity [75]. Such a lack of correlation is also found for discodermolide [76] (Table 2). In the case of this agent, this could reflect the difference in the binding affinity of discodermolide for the bovine brain tubulin used in binding experiments from tubulin in cancer cells or to difficulties in crossing the cell membrane [76]. However, cytotoxicity can be limited because, to stop the cell cycle, a minimal percentage of MTA is necessary. This theory also explains why peloruside A, a weaker microtubule stabilizing agent than paclitaxel and laulimalide, is still cytotoxic at nanomolar concentrations [40].
Table 2.
Affinity and cytotoxicity of the different microtubule targeting agents
| MTAs | Ka (M−1) (K1K2 values (M−2)) | IC50 (nM) | IC50/K d |
|---|---|---|---|
| Paclitaxel | 1.07 × 107 | 1.4 ± 0.2a | 0.01498 |
| Docetaxel | 3.09 × 107 | 3.0 ± 0.3a | 0.0927 |
| Epothilone A | 3 × 107 | 7.5 ± 1.4a | 0.225 |
| Epothilone B | 60 × 107 | 0.8 ± 0.1a | 4.8 |
| Discodermolide | 526 × 107 | 6.6 ± 0.8a | 34.716 |
| Peloruside A | ND | 16 ± 2b | ND |
| Laulimalide | ND | 3.9 ± 0.4c | ND |
| Vinorelbine | 1.3 × 105 ± 0.2 (1.4 × 1011) | 28d | 0.00364 |
| Vinflunine | 8.8 × 104 ± 2.0 (2.6 × 1010) | 97d | 0.008536 |
| Vincristine | 1.4 × 105 ± 0.4 (2.4 × 1012) | 16d | 0.00224 |
| Vinblastine | 1.2 × 105 ± 0.2 (6.1 × 1011) | 16d | 0.00192 |
| Colchicine | 3.2 × 106 | 16.86 ± 0.63e | 0.053952 |
| Cyclostreptin | ND | 37 ± 8a | ND |
The vinca alkaloids can bind to tubulin according to both the ligand-induced model, where binding precedes elongation, and the ligand-facilitated model, where elongation precedes binding [77, 78]. Sedimentation velocity was one of the main tools used for studying these vinca alkaloid-induced tubulin polymers. According to the Wyman linkage theory, the spiraling potential is described as the product of equilibrium constants, K 1 K 2; K 1 being the drug binding to the tubulin heterodimer, and K 2 the interaction of liganted-heterodimers to make an indefinite spiral polymer. K 3, the drug binding affinity for oligomers, is also a critical equilibrium parameter. For the various vinca alkaloids, the K 1 values (around 105 M−1 in the presence of GTP) are almost identical (Table 2); the difference is more obvious on the K 2 value and therefore on the spiraling potential (K 1 K 2). The link between K 2 and cytotoxicity has been extensively explored [75]. Vincristine has the highest spiraling potential with a K 1 K 2 value around 5 × 1012 M−1 in presence of GTP. Modification of the velbanamine part has been shown to be critical for binding affinity whereas the vindoline part mostly anchors the drug. Vinorelbine having a lower affinity for tubulin has decreased spiraling potential of about one order of magnitude (K 1 K 2 = 2 × 1011 M−1). Structure activity relationship conducted on vinca alkaloids synthetic analogues modified on the velbanamine part of the molecule showed an excellent correlation between decrease of the spiraling potential and IC50 of the drug [74]. For the latest vinca alkaloid in clinical development, vinflunine (20′,20′-difluoro-3′,4′-dihydrovinorelbine) obtained by super acid chemistry [79], the binding process is thermodynamically different from other vinca alkaloids. It is driven by entropy while binding of vinblastine and vincristine to tubulin is enthalpy-driven. The spiraling potential of vinflunine is two orders of magnitude lower than vincristine. IC50 values are around 10 nM for vinblastine or vincristine and two- or tenfold higher for vinorelbine or vinflunine, respectively [75]. Vinflunine is described as an excellent compromise between cytotoxicity and a decreased neurotoxicity.
For colchicine, the binding mechanism is simpler than for vinca alkaloids: the drug first forms a reversible pre-equilibrium complex with tubulin dimer, which induces conformational changes in tubulin [80]. Colchicine effectively inhibits cell growth, mitosis [81], secretion, cell elongation, and cell motility. This MTA has been used to treat gout for more than 2,000 years, and pseudo-gout and Familial Mediterranean Fever (FMF) for several decades [82, 83], but its high toxicity at doses necessary for antitumor effects have impaired any anticancer therapy development. Substoichiometric concentrations of colchicine promote inhibition of microtubule assembly. Binding of colchicine to tubulin is a slow, quasi-irreversible and temperature-dependent process (Table 2). Colchicine was considered lost for chemotherapic treatments due to its high toxicity, possibly a consequence of its quasi-irreversible binding to tubulin. However, studies have shown that modifying colchicines substituents drastically alter binding affinity and therefore inhibition of tubulin polymerization and cytotoxicity [84]. In spite of the severe toxicity associated with colchicine, several colchicine site-binding agents that bind reversibly to tubulin were developed, such as combretastatin A4 [85]. Derivatives of estradiol, such as 2-methoxyestradiol, bind reversibly at the colchicine site and are used as an antiangiogenic agents [86, 87]. Curacin A, a thiazoline-containing lipid from marine cyanobacteria, has poor structural similarity with colchicine, but strongly inhibits its binding in a quasi-irreversible way [88]. Despite its potential toxicity, this natural substance has been the subject of intense combinatorial chemistry development to improve its solubility and stability [89]. Studies on the curacin biosynthetic pathway led to the discovery of enzymes with unprecedented catalytic activities that may afford the synthesis of biologically active metabolites [90]. Although not strictly proportional, the relationship between affinity to tubulin and cytotoxicity in cancer cell lines holds up for most of the MTAs. At the higher end of the affinity range, we find some synthetic analogues of the taxane family like the C2-modified 10-deacetyl-7-propionyl cephalomannine derivatives [91] and discodermolide which are the most cytotoxic. However, cytotoxicity is not as strong as expected. As remarkably explained by Matesanz et al., due to tubulin quantity in cell, the minimal amount needed to kill these cells imposes a limit on the lowest dose that can be used. At the lower end, sarcodyctins are of lower binding efficiency and therefore lower cytotoxicity. For vinca alkaloids, there is also an excellent correlation between the spiraling potential and cytotoxicity. However, if we consider the vinflunine case, lower spiraling potential seems to offer therapeutic benefit. At the opposite extreme, dolastatin 10, with a high affinity for tubulin and strong accumulation in cells compared to vinca alkaloids [92], has been found clinically inefficient probably because of low bioavailability. In fact, the ratio amount of tubulin versus concentration of available active drug species within the cell is rarely established but would bridge molecular and cellular pharmacology of MTAs.
Reaching the target
Attempts to increase MTA affinity should be accompanied by efforts to improve bioavailability. Thus, novel formulations are engineered to control active drug concentration within the cell. Paclitaxel, due to its poor solubility, is formulated in the cremorphor EL (CrEL) solvent system, a polyoxyethylated castor oil vehicle responsible for side effects such as acute hypersensitivity reactions and peripheral neuropathy [93]. Covalently linking the natural fatty acid DHA to paclitaxel to form the docosahexaenoic acid (DHA)-paclitaxel (Taxoprexin®) [94] or the 7-methylthiomethyl ether substitution (BMS-184476) were designed to expose melanoma cells to higher doses of paclitaxel for a longer time. A better transport is also ensured by the formulation of paclitaxel encapsulated in positively charged lipid-based complexes (EndoTAG-1) [95]. Colloidal suspensions of nanoparticle albumin-bound paclitaxel or Nab-paclitaxel (ABI-007; AbraxaneTM) [96] may also enhance antitumor activity via targeting of SPARC, a protein up-regulated in the poor prognostic factor head and neck cancer [97]. Carbon nanotubes or nanodiamonds can also be used to deliver active agents to the interior of the cells [98]. The nanoparticles are coupled to the drug through supramolecular bonding. The drug-coupled and functionalized nanoparticles may also be targeted to specific cells through modification of the hydrophilic polymer by an antibody directed to a cell surface marker. Nanoformulations based on superparamagnetic iron oxide nanoparticles coated with biocompatible polymers are also of great interest in the controlled delivery of antitumoral agents. With the drive of a magnetic field, the drug can be concentrated at a target site, far away from the reticular endothelial system [99].
Bioavailability is also linked to drug efflux by ABC proteins responsible for multidrug resistance (MDR), including P-glycoprotein (P-gp). The C-4 methyl carbonate paclitaxel (BMS-275183) [100] and the 14-beta-hydroxydeacetylbaccatin (Ortataxel) analogues [101] are active against paclitaxel-resistant tumors including those harboring tubulin mutations or overexpressing P-gp. Other stabilizing agents such as epothilones are not susceptible to the MDR-mediated drug resistance. Laulimalide [102], discodermolide [51], and dictyostatin [103] are poor P-gp substrates and therefore could be effective against paclitaxel-resistant cells presumably because of a weaker binding affinity for P-gp. Another example is given by the loss of epoxy ring in the isolaulimalide structure which induces a decrease of affinity for both tubulin and P-gp when compared to laulimalide [104]. In the case of vinflunine, modification of the velbanamine moiety is likely to induce a decrease of the binding affinity for P-gp and therefore limit drug efflux [105]. The nanomolar affinity of taxanes to P-gp is independent of tubulin binding [91]. Metabolism of MTAs is beyond the scope of this paper and has been extensively reviewed. Briefly, liquid chromatography coupled to electrospray ionization mass spectrometry is the method of choice to study the stability of MTAs [106]. Structural alterations that increase drug stability can provide clinical benefit. For example, in the epothilone family, Ixabepilone (BMS-247550) [107], a drug recently approved for the treatment of aggressive metastatic or locally advanced breast cancer that fails to respond to currently available chemotherapies, bears a lactame group instead of a lactone moiety which provides protection from degradation. Mooberry et al. [102] synthesized five analogues of laulimalide which also enhanced stability. In a few instances, unstable drugs can generate metabolites more active than parent compounds [108]. For example, the metabolism of dolastatine and dictyostatin is also critical for their activity. The well-characterized high phosphatase activity in the tumor microenvironment led to the design of combretastatin A4-phosphate and estramustine phosphate prodrugs that are specially metabolized to their active form at the desired site of action [109, 110].
Quantitative proteomics is perfect to identify proteins involved in drug resistance. For this purpose, a quantitative separation technique is associated with mass spectrometry. The two-dimensional gel electrophoresis within gel dyes (2D-DIGE) [111] allows the labeling of up to two extracts and a standard that co-migrates on the same gel. For example, using quantitative proteomics, Yang et al. [112] have undertaken the comparison of the protein profile between the vincristine-resistant human gastric cancer cell line, SGC7901/VCR, and its parental cell line, SGC7901, by a subcellular proteomics approach. They found that there are 30 differentially expressed proteins which are associated with drug resistance. Quantitative proteomics can also been done using peptides labeled with stable isotopes. They can be incorporated chemically (ICAT, [113]; iTraq, [114]) or metabolically from cell culture (SILAC, [115]). These stable isotopes have a known mass difference which allows the comparison of the mass spectrometry signal of the same peptide under different conditions and, by extension, the estimation of the relative protein abundance between two proteomes. Last but not least, the constant increase in data treatment capabilities allow ion current comparison and label-free quantitative proteomics [116]. Proteins identified as playing a role in drug efficacy belong to a wide variety of families as diverse as cell cycle and checkpoint proteins, calcium binding proteins, chaperones, cytoskeletal [117], redox and metabolism proteins, and cell cycle polypeptides.
The role of tubulin isotype composition in MTA efficacy
Preclinical studies have shown high levels of expression of class III β-tubulin are associated with paclitaxel resistance in human cancer cell lines (lung cancer [118], ovarian cancer [119], prostate cancer [120], breast cancer [118], and pancreatic cancer [121]) and with docetaxel resistance in human pancreatic cancer cell lines [121, 122]. Vinca alkaloids response is also dependant on the isotypic distribution of tubulin. Kavallaris et al. [123] showed that class III β-tubulin is downregulated in acute lymphoblastic leukemia cells lines made resistant to vincristine and vinblastine; however, some studies have shown that class III β-tubulin could be overexpressed in the case of vinblastine-resistant erythroleukemic cell line [124]. The reasons why class III β-tubulin is involved in drug resistance is unclear; some suggest thermodynamic reasons since βIII-tubulin requires the highest critical concentration of tubulin for assembly, others suggest that amino acid residue changes in the drug binding site are the cause. The presence of βIII-tubulin on mitochondria, its interaction with voltage-dependent anion channel (VDAC) [125] and the presence of proteins involved in adaptation to oxidative stress and glucose deprivation in the βIII-tubulin interactome is also of interest for further investigations [126]. The validation of βIII-tubulin as a biomarker in oncology is yet to be established quantitatively because it is not clear if the apparent increase of its expression in many aggressive solid tumors is specific to this isotype or reflect a global increase in tubulin expression. The problem represented by precise, unbiased quantitation of βIII-tubulin in tumor biopsies maybe solved by recent developments in quantitative mass spectrometry-based approaches. Absolute quantification is achievable with the AQUA® peptide technology. In the case of βIII-tubulin, tryptic AQUA® peptides specific to this isotype harbor one stable isotope-labeled amino acid. This allows easy separation by mass spectrometry from sample-generated βIII-tubulin peptides. The AQUA® peptides are spiked into the tumor cell lysate and subsequently digested with trypsin. Tryptic peptides are separated by HPLC, and the synthetic AQUA® peptides serve as internal standards for absolute quantification based on m/z peaks ratio in MS spectra [127] (Table 1).
New MTAs could specially target tubulin isotypes and more precisely to the βIII-tubulin isotype. Freedman and co-workers believe in the in silico design of tubulin isotype-specific MTAs [128]. For this, they propose to dock large chemical databases on tubulin structures to obtain lead compounds. High throughput screening (HTS) methods, usually based on fluorescence assays, verify the binding or the polymerization properties of molecule to tubulin [129]. Microcalorimetry is also of interest to test binding parameters since new high throughput systems are able to run up to 75 samples a day with only 100 μg of protein per experiment. The robotized systems can run up to 384 samples unattended. The taxane, epothilone, vinca alkaloid, and colchicine binding sites also harbor specific amino acid substitution among β-tubulin isotypes (Table 3).
Table 3.
Sequence variations in the drug binding sites of beta tubulin isotypes
| Binding sites | Isotypes | Inter-isotypic mutations |
|---|---|---|
| Paclitaxel binding site | III | Ser275-Ala |
| VI | Val23-Met, Ser25-Gly, Asp26-Glu, Ser275-Ala, Arg276-Gln | |
| Colchicine binding site | III | Cys239-Ser, Ala315-Thr, Thr351-Val |
| V | Cys239-Ser, Ala315-Thr, Thr351-Val | |
| VI | Val236-Ile, Cys239-Ser, Ala315-Thr, Thr351-Val | |
| Vinblastine binding site | III | Thr218-Ala |
Substitutions observed between βI and the other human β-tubulin isotypes in paclitaxel, colchicine, and vinblastine binding sites
Localizing microtubule isotypes and binding agents in the cell by mass spectrometry
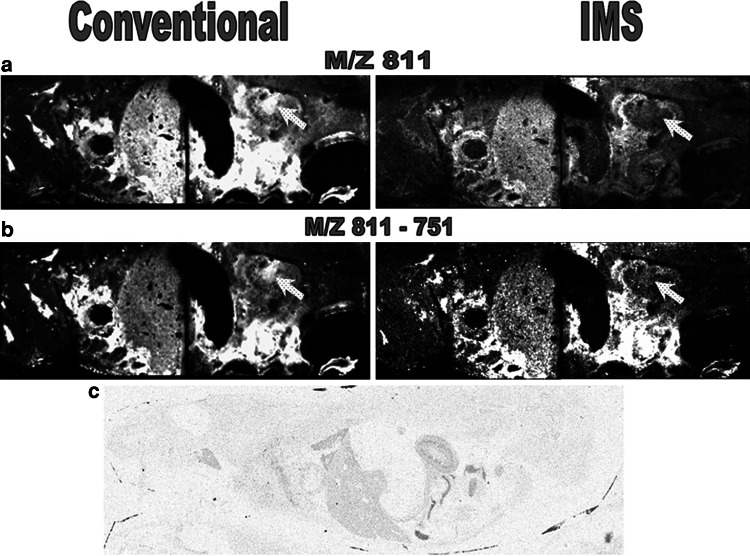
Colocalization and imaging of drugs with tubulin or tubulin isotypes within the cell is also a critical goal. Molecular mass spectrometry imaging is announced as a promising tool to fulfill this challenge (Table 1). This technique complementary to classical imaging techniques not described in this review has already been used to locate curacine A and other metabolites in its native cyanobacteria [130], and vinblastine and its metabolites in rat tissues [131], without labeling the drug (Fig. 4). However, although promising, this technique still has a spatial resolution based on the size of the laser beam (100–200 μM) that is too low to go down to the cellular scale. The use of the alternative mass microscope approach could decrease resolution to the μm scale [132]. Powerful mass spectrometers using secondary ion mass spectrometry coupled to new ion sources equipped with C60 guns open new avenues in the field of mass spectrometry imaging [133]. With this technique, imaging of small molecules in the cell nucleus becomes possible. The biological step size of 40–60 nm is not far from being compatible with mass spectrometry imaging. Identifying specific microtubule isotypes or a drug will require different processes. Vinblastine and its metabolites can be localized within a tissue slice using ion mobility, which enables separation of species with the same mass (isobaric) using the conformational properties of the ions [131] (Fig. 4). In order to assess the heterogeneity of tubulin composition in a tumor, enzymatic digestion could be performed in situ prior to determining the distribution of tubulin isotype-specific peptides using sequencing by mass spectrometry (MS/MS). Another way is to use the “Tag-Mass” concept. This strategy allows the indirect detection by MALDI imaging of different classes of probes such as DNA, cDNA, single stranded cRNA, or antibody probes chemically attached with a reporter group or “Tag-Mass” indicator through a photocleavable linker [134]. Lemaire et al. [135] used this technique to image overexpressed Reg-alpha, a member of the proteasome activator 11S, in carcinoma. The “Tag-Mass” concept could be used to characterize the composition of tubulin isotypes in different tumors using highly specific antibodies.
Fig. 4.
MALDI-MS/MS and MALDI-IMS-MS/MS whole body imaging of rats dosed at 6 mg/kg IV with vinblastine. Distribution of the precursor ion m/z 811.4 (vinblastine) and production m/z 751 (vinblastine dissociation product) is shown. Comparison of a m/z 811 and b m/z 811-751 images obtained using MALDI-IMS-MS/MS and conventional MALDI-MS/MS clearly demonstrate the advantages of ion mobility separation within MALDI xenobiotic imaging. The main difference observed is indicated; the distribution of the ions of interest within the renal pelvis. White contrast indicates vinblastine concentration. c Whole body autoradiograph showing the distribution of 3H in a 1-h post-dose rat dosed with 6 mg/kg IV 3H vinblastine, confirming the distribution observed with MALDI-IMS-MS/MS. Reprinted with the authorization of the American Chemical Society
Other targets of microtubule dynamics
In addition to their direct effects on tubulin and/or MTs, MTAs can modify the activity of MAPs through activation/inactivation of posttranslational modification pathways, formation of ternary complexes with tubulin, or degradation. It is necessary to understand the fine mechanism of action of these secondary MAP-mediated effects of MTAs. Such effects of MTAs have been shown on MAPs including stathmin, tau, and MAP-4 [136–142]. Will MAPs one day be used as a prognosis factor or even as a therapeutic target? Promising results have been obtained concerning stathmin since the characterization of its structure in complex with tubulin [143]. Stathmin is known to promote microtubule depolymerization at MTs ends by increasing the catastrophe rate by sequestering free tubulin, thus lowering the pool of “assembly competent” tubulin [144]. Structurally, the correlation between a high level of expression of stathmin and a higher sensitivity to vinblastine [136–138, 140, 145] could be explained by a simultaneous binding of stathmin and vinblastine on tubulin [146]. This hypothesis was recently confirmed by microcalorimetry providing the first direct evidence of a functional synergy between stathmin and vinblastine [147]. Using isothermal titration calorimetry (ITC) and analytical ultracentrifugation, it was indeed demonstrated that vinblastine increased the stathmin binding constant for tubulin 50-fold and that stathmin had the same effect on the vinblastine binding constant for tubulin. Stathmin can thus be seen as a novel mediator of cell sensitivity to vinblastine, thereby underscoring its potential as a promising target for cancer therapeutics.
This is of high clinical significance since high stathmin expression predicted an unfavorable prognosis in patients with ovarian cancer who received paclitaxel and platinum chemotherapy [148].
Other studies have shown that other MAPs, including tau or even MAP4, might also be used as predictive factors to identify patients most at risk of recurrence and those most likely to benefit from taxane treatment [149]. This importance of MAPs in tumor biology, which has been mostly described with stathmin and tau, could be extended to other MAPs regulated via phosphorylation such as EB1 [150]. Since binding of MAPs to MTs is tightly regulated by phosphorylation, the study of the phosphoproteome is another new direction to understand MTAs action on the microtubule network. Finally, MAPs are not the only proteins that can be targeted to indirectly affect MT dynamics. Vinca alkaloids can bind to other proteins such as calmodulin, a calcium binding protein; this binding modulates the interaction with the stable tubule-only polypeptide (STOP) known to regulate microtubule dynamics [151]. These kinds of multidisciplinary investigations may broaden the MTAs arsenal from the diverse class of tubulin interacting agents to active molecules altering direct and indirect regulators of MTs dynamics.
References
- 1.Bryan J, Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci USA. 1971;68:1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 3.Nogales E, Wang HW. Structural intermediates in microtubule assembly and disassembly: how and why? Curr Opin Cell Biol. 2006;18:179–184. doi: 10.1016/j.ceb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Ballestrem C, Magid N, Zonis J, Shtutman M, Bershadsky A. Interplay between the actin cytoskeleton, focal adhesions, and microtubules. In: Ridley A, Peckham M, Clark P, editors. Cell motility. From molecules to organisms. Chichester: Wiley; 2004. pp. 75–99. [Google Scholar]
- 5.Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970;24:625–640. [PubMed] [Google Scholar]
- 6.Kline-Smith SL, Walczak CE. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell. 2004;15:317–327. doi: 10.1016/j.molcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Wittmann T, Hyman A, Desai A. The spindle: a dynamic assembly of microtubules and motors. Nat Cell Biol. 2001;3:E28–E34. doi: 10.1038/35050669. [DOI] [PubMed] [Google Scholar]
- 8.Hill TL, Chen Y. Phase changes at the end of a microtubule with a GTP cap. Proc Natl Acad Sci USA. 1984;81:5772–5776. doi: 10.1073/pnas.81.18.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson L, Panda D, Jordan MA. Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators. Cell Struct Funct. 1999;24:329–335. doi: 10.1247/csf.24.329. [DOI] [PubMed] [Google Scholar]
- 10.Rowinsky EK, Calvo E. Novel agents that target tublin and related elements. Semin Oncol. 2006;33:421–435. doi: 10.1053/j.seminoncol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981;91:479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derry WB, Wilson L, Jordan MA. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- 13.Dhamodharan R, Jordan MA, Thrower D, Wilson L, Wadsworth P. Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol Biol Cell. 1995;6:1215–1229. doi: 10.1091/mbc.6.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973;59:267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 17.Lowe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 18.Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci USA. 1994;91:11358–11362. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billger MA, Bhattacharjee G, Williams RC., Jr Dynamic instability of microtubules assembled from microtubule-associated protein-free tubulin: neither variability of growth and shortening rates nor “rescue” requires microtubule-associated proteins. Biochemistry. 1996;35:13656–13663. doi: 10.1021/bi9616965. [DOI] [PubMed] [Google Scholar]
- 20.Joshi HC. Microtubule dynamics in living cells. Curr Opin Cell Biol. 1998;10:35–44. doi: 10.1016/s0955-0674(98)80084-7. [DOI] [PubMed] [Google Scholar]
- 21.Oakley CE, Oakley BR. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans . Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- 22.Moritz M, Braunfeld MB, Guenebaut V, Heuser J, Agard DA. Structure of the gamma-tubulin ring complex: a template for microtubule nucleation. Nat Cell Biol. 2000;2:365–370. doi: 10.1038/35014058. [DOI] [PubMed] [Google Scholar]
- 23.Keating TJ, Borisy GG. Immunostructural evidence for the template mechanism of microtubule nucleation. Nat Cell Biol. 2000;2:352–357. doi: 10.1038/35014045. [DOI] [PubMed] [Google Scholar]
- 24.Job D, Valiron O, Oakley B. Microtubule nucleation. Curr Opin Cell Biol. 2003;15:111–117. doi: 10.1016/s0955-0674(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Oakley BR. An abundance of tubulins. Trends Cell Biol. 2000;10:537–542. doi: 10.1016/s0962-8924(00)01857-2. [DOI] [PubMed] [Google Scholar]
- 26.Dutcher SK. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol. 2001;13:49–54. doi: 10.1016/s0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 27.McKean PG, Vaughan S, Gull K. The extended tubulin superfamily. J Cell Sci. 2001;114:2723–2733. doi: 10.1242/jcs.114.15.2723. [DOI] [PubMed] [Google Scholar]
- 28.Luduena RF. Are tubulin isotypes functionally significant. Mol Biol Cell. 1993;4:445–457. doi: 10.1091/mbc.4.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 30.Verdier-Pinard P, Pasquier E, Xiao H, Burd B, Villard C, Lafitte D, Miller LM, Angeletti RH, Horwitz SB, Braguer D. Tubulin proteomics: towards breaking the code. Anal Biochem. 2009;384:197–206. doi: 10.1016/j.ab.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verdier-Pinard P, Wang F, Burd B, Angeletti RH, Horwitz SB, Orr GA. Direct analysis of tubulin expression in cancer cell lines by electrospray ionization mass spectrometry. Biochemistry. 2003;42:12019–12027. doi: 10.1021/bi0350147. [DOI] [PubMed] [Google Scholar]
- 32.Al-Bassam J, van Breugel M, Harrison SC, Hyman A. Stu2p binds tubulin and undergoes an open-to-closed conformational change. J Cell Biol. 2006;172:1009–1022. doi: 10.1083/jcb.200511010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozziconacci J, Sandblad L, Wachsmuth M, Brunner D, Karsenti E. Tubulin dimers oligomerize before their incorporation into microtubules. PLoS One. 2008;3:e3821. doi: 10.1371/journal.pone.0003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerssemakers JW, Munteanu EL, Laan L, Noetzel TL, Janson ME, Dogterom M. Assembly dynamics of microtubules at molecular resolution. Nature. 2006;442:709–712. doi: 10.1038/nature04928. [DOI] [PubMed] [Google Scholar]
- 36.Braguer D, Barret JM, McDaid H, Kruczynski A. Antitumor activity of vinflunine: effector pathways and potential for synergies. Semin Oncol. 2008;35:S13–S21. doi: 10.1053/j.seminoncol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Pourroy B, Honoré S, Pasquier E, Bourgarel-Rey V, Kruczynski A, Briand C, Braguer D. Antiangiogenic concentrations of vinflunine increase the interphase microtubule dynamics and decrease the motility of endothelial cells. Cancer Res. 2006;66:3256–3263. doi: 10.1158/0008-5472.CAN-05-3885. [DOI] [PubMed] [Google Scholar]
- 38.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone A on alpha, beta-tubulin by electron crystallography. Science. 2004;305:866–869. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 39.Pryor DE, O’Brate A, Bilcer G, Diaz JF, Wang Y, Kabaki M, Jung MK, Andreu JM, Ghosh AK, Giannakakou P, Hamel E. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry. 2002;41:9109–9115. doi: 10.1021/bi020211b. [DOI] [PubMed] [Google Scholar]
- 40.Gaitanos TN, Buey RM, Diaz JF, Northcote PT, Teesdale-Spittle P, Andreu JM, Miller JH. Peloruside A does not bind to the taxoid site on beta-tubulin and retains its activity in multidrug-resistant cell lines. Cancer Res. 2004;64:5063–5067. doi: 10.1158/0008-5472.CAN-04-0771. [DOI] [PubMed] [Google Scholar]
- 41.Terkeltaub R. Pathogenesis and treatment of crystal-induced inflammation. In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions. 15. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 2357–2372. [Google Scholar]
- 42.Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435:519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- 43.Andreu JM, Barasoain I. The interaction of baccatin III with the taxol binding site of microtubules determined by a homogeneous assay with fluorescent taxoid. Biochemistry. 2001;40:11975–11984. doi: 10.1021/bi010869+. [DOI] [PubMed] [Google Scholar]
- 44.Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Characterization of the Taxol binding site on the microtubule. Identification of Arg(282) in beta-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 45.Rao S, Horwitz SB, Ringel I. Direct photoaffinity labeling of tubulin with taxol. J Natl Cancer Inst. 1992;84:785–788. doi: 10.1093/jnci/84.10.785. [DOI] [PubMed] [Google Scholar]
- 46.Carboni JM, Farina V, Rao S, Hauck SI, Horwitz SB, Ringel I. Synthesis of a photoaffinity analog of taxol as an approach to identify the taxol binding site on microtubules. J Med Chem. 1993;36:513–515. doi: 10.1021/jm00056a013. [DOI] [PubMed] [Google Scholar]
- 47.Paik Y, Yang C, Metaferia B, Tang S, Bane S, Ravindra R, Shanker N, Alcaraz AA, Johnson SA, Schaefer J, O’Connor RD, Cegelski L, Snyder JP, Kingston DG. Rotational-echo double-resonance NMR distance measurements for the tubulin-bound Paclitaxel conformation. J Am Chem Soc. 2007;129:361–370. doi: 10.1021/ja0656604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y, Malak H, Chaudhary AG, Chordia MD, Kingston DG, Bane S. Distances between the paclitaxel, colchicine, and exchangeable GTP binding sites on tubulin. Biochemistry. 1998;37:6636–6644. doi: 10.1021/bi9719760. [DOI] [PubMed] [Google Scholar]
- 49.Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the mechanism of microtubule stabilization by Taxol. Proc Natl Acad Sci USA. 2006;103:10166–10173. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huzil JT, Chik JK, Slysz GW, Freedman H, Tuszynski J, Taylor RE, Sackett DL, Schriemer DC. A unique mode of microtubule stabilization induced by peloruside A. J Mol Biol. 2008;378:1016–1030. doi: 10.1016/j.jmb.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowalski RJ, Giannakakou P, Gunasekera SP, Longley RE, Day BW, Hamel E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol Pharmacol. 1997;52:613–622. [PubMed] [Google Scholar]
- 52.Keskin O, Durell SR, Bahar I, Jernigan RL, Covell DG. Relating molecular flexibility to function: a case study of tubulin. Biophys J. 2002;83:663–680. doi: 10.1016/S0006-3495(02)75199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiménez-Barbero J, Amat-Guerri F, Snyder JP. The solid state, solution and tubulin-bound conformations of agents that promote microtubule stabilization. Curr Med Chem Anticancer Agents. 2002;2:91–122. doi: 10.2174/1568011023354416. [DOI] [PubMed] [Google Scholar]
- 54.Xia S, Kenesky CS, Rucker PV, Smith AB, 3rd, Orr GA, Horwitz SB. A photoaffinity analogue of discodermolide specifically labels a peptide in beta-tubulin. Biochemistry. 2006;45:11762–11775. doi: 10.1021/bi060497a. [DOI] [PubMed] [Google Scholar]
- 55.Madiraju C, Edler MC, Hamel E, Raccor BS, Balachandran R, Zhu G, Giuliano KA, Vogt A, Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP, Day BW. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry. 2005;44:15053–15063. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]
- 56.Buey RM, Calvo E, Barasoain I, Pineda O, Edler MC, Matesanz R, Cerezo G, Vanderwal CD, Day BW, Sorensen EJ, López JA, Andreu JM, Hamel E, Díaz JF. Cyclostreptin binds covalently to microtubule pores and lumenal taxoid binding sites. Nat Chem Biol. 2007;3:117–125. doi: 10.1038/nchembio853. [DOI] [PubMed] [Google Scholar]
- 57.Hamel E, Day BW, Miller JH, Jung MK, Northcote PT, Ghosh AK, Curran DP, Cushman M, Nicolaou KC, Paterson I, Sorensen EJ. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol Pharmacol. 2006;70:1555–1564. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 58.Wilmes A, Bargh K, Kelly C, Northcote PT, Miller JH. Peloruside A synergizes with other microtubule stabilizing agents in cultured cancer cell lines. Mol Pharm. 2007;4:269–280. doi: 10.1021/mp060101p. [DOI] [PubMed] [Google Scholar]
- 59.Prakash V, Timasheff SN. Mechanism of interaction of vinca alkaloids with tubulin: catharanthine and vindoline. Biochemistry. 1991;30:873–880. doi: 10.1021/bi00217a042. [DOI] [PubMed] [Google Scholar]
- 60.Safa AR, Hamel E, Felsted RL. Photoaffinity labeling of tubulin subunits with a photoactive analogue of vinblastine. Biochemistry. 1987;26:97–102. doi: 10.1021/bi00375a014. [DOI] [PubMed] [Google Scholar]
- 61.Rai SS, Wolff J. Localization of the vinblastine-binding site on beta-tubulin. J Biol Chem. 1996;271:14707–14711. doi: 10.1074/jbc.271.25.14707. [DOI] [PubMed] [Google Scholar]
- 62.Barbier P, Gregoire C, Devred F, Sarrazin M, Peyrot V. In vitro effect of cryptophycin 52 on microtubule assembly and tubulin: molecular modeling of the mechanism of action of a new antimitotic drug. Biochemistry. 2001;40:13510–13519. doi: 10.1021/bi010926z. [DOI] [PubMed] [Google Scholar]
- 63.Cormier A, Marchand M, Ravelli RB, Knossow M, Gigant B. Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep. 2008;9:1101–1106. doi: 10.1038/embor.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem. 1991;266:15882–15889. [PubMed] [Google Scholar]
- 65.Schmitt H, Atlas D. Specific affinity labelling of tubulin with bromocolchicine. J Mol Biol. 1976;102:743–758. doi: 10.1016/0022-2836(76)90289-8. [DOI] [PubMed] [Google Scholar]
- 66.Williams RF, Mumford CL, Williams GA, Floyd LJ, Aivaliotis MJ, Martinez RA, Robinson AK, Barnes LD. A photoaffinity derivative of colchicine: 6′-(4′-azido-2′-nitrophenylamino)hexanoyldeacetylcolchicine. Photolabeling and location of the colchicine-binding site on the alpha-subunit of tubulin. J Biol Chem. 1985;260:13794–13802. [PubMed] [Google Scholar]
- 67.Grover S, Boye O, Getahun Z, Brossi A, Hamel E. Chloroacetates of 2- and 3-demethylthiocolchicine: specific covalent interactions with tubulin with preferential labeling of the beta-subunit. Biochem Biophys Res Commun. 1992;187:1350–1358. doi: 10.1016/0006-291x(92)90451-p. [DOI] [PubMed] [Google Scholar]
- 68.Lin CM, Ho HH, Pettit GR, Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry. 1989;28:6984–6991. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 69.Floyd LJ, Barnes LD, Williams RF. Photoaffinity labeling of tubulin with (2-nitro-4-azidophenyl)deacetylcolchicine: direct evidence for two colchicine binding sites. Biochemistry. 1989;28:8515–8525. doi: 10.1021/bi00447a037. [DOI] [PubMed] [Google Scholar]
- 70.Wolff J, Knipling L, Cahnmann HJ, Palumbo G. Direct photoaffinity labeling of tubulin with colchicine. Proc Natl Acad Sci USA. 1991;88:2820–2824. doi: 10.1073/pnas.88.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uppuluri S, Knipling L, Sackett DL, Wolff J. Localization of the colchicine-binding site of tubulin. Proc Natl Acad Sci USA. 1993;90:11598–11602. doi: 10.1073/pnas.90.24.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourdron J, Barbier P, Allegro D, Villard C, Lafitte D, Commeiras L, Parrain JL, Peyrot V. Caulerpenyne binding to tubulin: structural modifications by a non conventional pharmacological agent. Med Chem. 2009;5:182–190. doi: 10.2174/157340609787582891. [DOI] [PubMed] [Google Scholar]
- 73.Lafitte D, Lamour V, Tsvetkov PO, Makarov AA, Klich M, Deprez P, Moras D, Briand C, Gilli R. DNA gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5′-methyl group of the noviose. Biochemistry. 2002;41:7217–7223. doi: 10.1021/bi0159837. [DOI] [PubMed] [Google Scholar]
- 74.Verdier-Pinard P, Gares M, Wright M. Differential in vitro association of vinca alkaloid-induced tubulin spiral filaments into aggregated spirals. Biochem Pharmacol. 1999;58:959–971. doi: 10.1016/s0006-2952(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 75.Lobert S, Fahy J, Hill BT, Duflos A, Etievant C, Correia JJ. Vinca alkaloid-induced tubulin spiral formation correlates with cytotoxicity in the leukemic L1210 cell line. Biochemistry. 2000;39:12053–12062. doi: 10.1021/bi001038r. [DOI] [PubMed] [Google Scholar]
- 76.Buey RM, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, Mooberry S, Andreu JM, Díaz JF. Microtubule interactions with chemically diverse stabilizing agents: thermodynamics of binding to the paclitaxel site predicts cytotoxicity. Chem Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Diaz JF, Menendez M, Andreu JM. Thermodynamics of ligand-induced assembly of tubulin. Biochemistry. 1993;32:10067–10077. doi: 10.1021/bi00089a023. [DOI] [PubMed] [Google Scholar]
- 78.Na GC, Timasheff SN. Interaction of vinblastine with calf brain tubulin: multiple equilibria. Biochemistry. 1986;25:6214–6222. doi: 10.1021/bi00368a057. [DOI] [PubMed] [Google Scholar]
- 79.Fahy J, Duflos A, Ribet JP, Jacquesy JC, Berrier C, Jouannetaud MP, Zunino F. Vinca alkaloids in superacidic media: a method for creating a new family of antitumor derivatives. J Am Chem Soc. 1997;119:8576–8577. [Google Scholar]
- 80.Bhattacharyya B, Wolff J. Promotion of fluorescence upon binding of colchicine to tubulin. Proc Natl Acad Sci USA. 1974;71:2627–2631. doi: 10.1073/pnas.71.7.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dustin P. Microtubules. Berlin: Springer; 1978. [Google Scholar]
- 82.Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;18:CD006190. doi: 10.1002/14651858.CD006190. [DOI] [PubMed] [Google Scholar]
- 83.Lidar M, Livneh A. Familial Mediterranean fever: clinical, molecular and management advancements. Neth J Med. 2007;65:318–324. [PubMed] [Google Scholar]
- 84.ter Haar E, Rosenkranz HS, Hamel E, Day BW. Computational and molecular modeling evaluation of the structural basis for tubulin polymerization inhibition by colchicine site agents. Bioorg Med Chem. 1996;4:1659–1671. doi: 10.1016/0968-0896(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 85.Sun L, Vasilevich NI, Fuselier JA, Coy DH. Abilities of 3, 4-diarylfuran-2-one analogs of combretastatin A-4 to inhibit both proliferation of tumor cell lines and growth of relevant tumors in nude mice. Anticancer Res. 2004;24:179–186. [PubMed] [Google Scholar]
- 86.Hamel E, Lin CM, Flynn E, D’Amato RJ. Interactions of 2-methoxyestradiol, an endogenous mammalian metabolite, with unpolymerized tubulin and with tubulin polymers. Biochemistry. 1996;35:1304–1310. doi: 10.1021/bi951559s. [DOI] [PubMed] [Google Scholar]
- 87.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 88.Verdier-Pinard P, Sitachitta N, Rossi JV, Sackett DL, Gerwick WH, Hamel E. Biosynthesis of radiolabeled curacin A and its rapid and apparently irreversible binding to the colchicine site of tubulin. Arch Biochem Biophys. 1999;370:51–58. doi: 10.1006/abbi.1999.1363. [DOI] [PubMed] [Google Scholar]
- 89.Wipf P, Reeves JT, Day BW. Chemistry and biology of curacin A. Curr Pharm Des. 2004;10:1417–1437. doi: 10.2174/1381612043384853. [DOI] [PubMed] [Google Scholar]
- 90.Gu L, Wang B, Kulkarni A, Geders TW, Grindberg RV, Gerwick L, Håkansson K, Wipf P, Smith JL, Gerwick WH, Sherman DH. Metamorphic enzyme assembly in polyketide diversification. Nature. 2009;459:731–735. doi: 10.1038/nature07870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang CG, Barasoain I, Li X, Matesanz R, Liu R, Sharom FJ, Yin DL, Díaz JF, Fang WS. Overcoming tumor drug resistance with high-affinity taxanes: a SAR study of C2-modified 7-acyl-10-deacetyl cephalomannines. ChemMedChem. 2007;2:691–701. doi: 10.1002/cmdc.200700002. [DOI] [PubMed] [Google Scholar]
- 92.Verdier-Pinard P, Kepler JA, Pettit GR, Hamel E. Sustained intracellular retention of dolastatin 10 causes its potent antimitotic activity. Mol Pharmacol. 2000;57:180–187. [PubMed] [Google Scholar]
- 93.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17:735–749. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 94.Bradley MO, Swindell CS, Anthony FH, Witman PA, Devanesan P, Webb NL, Baker SD, Wolff AC, Donehower RC. Tumor targeting by conjugation of DHA to paclitaxel. J Control Release. 2001;74:233–236. doi: 10.1016/s0168-3659(01)00321-2. [DOI] [PubMed] [Google Scholar]
- 95.Schuch G. EndoTAG-1. MediGene. Curr Opin Investig Drugs. 2005;6:1259–1265. [PubMed] [Google Scholar]
- 96.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–1044. [PubMed] [Google Scholar]
- 97.Trieu V, Hwang J, Desai N (2007) Nanoparticle albumin-bound (nab) technology may enhance antitumor activity via targeting of SPARC protein. New targets and delivery system for cancer diagnosis and treatment (SKCC). San Diego, Abstract 53
- 98.Faklaris O, Garrot D, Joshi V, Druon F, Boudou JP, Sauvage T, Georges P, Curmi PA, Treussart F. Detection of single photoluminescent diamond nanoparticles in cells and study of the internalization pathway. Small. 2008;4:2236–2239. doi: 10.1002/smll.200800655. [DOI] [PubMed] [Google Scholar]
- 99.Ciofani G, Riggio C, Raffa V, Menciassi A, Cuschieri A. A bi-modal approach against cancer: magnetic alginate nanoparticles for combined chemotherapy and hyperthermia. Med Hypotheses. 2009;73:80–82. doi: 10.1016/j.mehy.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 100.Rose WC, Long BH, Fairchild CR, Lee FY, Kadow JF. Preclinical pharmacology of BMS-275183, an orally active taxane. Clin Cancer Res. 2001;7:2016–2021. [PubMed] [Google Scholar]
- 101.Distefano M, Scambia G, Ferlini C, Gaggini C, De Vincenzo R, Riva A, Bombardelli E, Ojima I, Fattorossi A, Panici PB, Mancuso S. Anti-proliferative activity of a new class of taxanes (14beta-hydroxy-10-deacetylbaccatin III derivatives) on multidrug-resistance-positive human cancer cells. Int J Cancer. 1997;72:844–850. doi: 10.1002/(sici)1097-0215(19970904)72:5<844::aid-ijc22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 102.Mooberry SL, Randall-Hlubek DA, Leal RM, Hegde SG, Hubbard RD, Zhang L, Wender PA. Microtubule-stabilizing agents based on designed laulimalide analogues. Proc Natl Acad Sci USA. 2004;101:8803–8808. doi: 10.1073/pnas.0402759101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin Y, Fournier JH, Balachandran R, Madiraju C, Raccor BS, Zhu G, Edler MC, Hamel E, Day BW, Curran DP. Synthesis and biological evaluation of (−)-16-normethyldictyostatin: a potent analogue of (−)-dictyostatin. Org Lett. 2005;7:2873–2876. doi: 10.1021/ol050808u. [DOI] [PubMed] [Google Scholar]
- 104.Mooberry SL, Tien G, Hernandez AH, Plubrukarn A, Davidson BS. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999;59:653–660. [PubMed] [Google Scholar]
- 105.Hill BT. Vinflunine, a second generation novel Vinca Alkaloid with a distinctive pharmacological profile, now in clinical development and prospects for future mitotic blockers. Curr Pharm Des. 2001;7:1199–1212. doi: 10.2174/1381612013397456. [DOI] [PubMed] [Google Scholar]
- 106.Wen B, Fitch WL. Analytical strategies for the screening and evaluation of chemically reactive drug metabolites. Expert Opin Drug Metab Toxicol. 2009;5:39–55. doi: 10.1517/17425250802665706. [DOI] [PubMed] [Google Scholar]
- 107.Vahdat L. Ixabepilone: a novel antineoplastic agent with low susceptibility to multiple tumor resistance mechanisms. Oncologist. 2008;13:214–221. doi: 10.1634/theoncologist.2007-0167. [DOI] [PubMed] [Google Scholar]
- 108.Zorza G, Van Heugen JC, De Graeve J, Puozzo C. Development of a sensitive liquid chromatography method coupled with a tandem mass spectrometric detection for the clinical analysis of vinflunine and 4-O-deacetyl vinflunine in blood, urine and faeces. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:294–302. doi: 10.1016/j.jchromb.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 109.Kirwan IG, Loadman PM, Swaine DJ, Anthoney DA, Pettit GR, Lippert JW, 3rd, Shnyder SD, Cooper PA, Bibby MC. Comparative preclinical pharmacokinetic and metabolic studies of the combretastatin prodrugs combretastatin A4 phosphate and A1 phosphate. Clin Cancer Res. 2004;10:1446–1453. doi: 10.1158/1078-0432.ccr-0518-03. [DOI] [PubMed] [Google Scholar]
- 110.Dahllof B, Billstrom A, Cabral F, Hartley-Asp B. Estramustine depolymerizes microtubules by binding to tubulin. Cancer Res. 1993;53:4573–4581. [PubMed] [Google Scholar]
- 111.Marouga R, David S, Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem. 2005;382:669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- 112.Yang YX, Chen ZC, Zhang GY, Yi H, Xiao ZQ. A subcelluar proteomic investigation into vincristine-resistant gastric cancer cell line. J Cell Biochem. 2008;104:1010–1021. doi: 10.1002/jcb.21687. [DOI] [PubMed] [Google Scholar]
- 113.Oda Y, Owa T, Sato T, Boucher B, Daniels S, Yamanaka H, Shinohara Y, Yokoi A, Kuromitsu J, Nagasu T. Quantitative chemical proteomics for identifying candidate drug targets. Anal Chem. 2003;75:2159–2165. doi: 10.1021/ac026196y. [DOI] [PubMed] [Google Scholar]
- 114.Chong PK, Gan CS, Pham TK, Wright PC. Isobaric tags for relative and absolute quantitation (iTRAQ) reproducibility: implication of multiple injections. J Proteome Res. 2006;5:1232–1240. doi: 10.1021/pr060018u. [DOI] [PubMed] [Google Scholar]
- 115.Ong SE, Foster LJ, Mann M. Mass spectrometric-based approaches in quantitative proteomics. Methods. 2003;29:124–130. doi: 10.1016/s1046-2023(02)00303-1. [DOI] [PubMed] [Google Scholar]
- 116.Wang G, Wu WW, Zeng W, Chou CL, Shen RF. Label-free protein quantification using LC-coupled ion trap or FT mass spectrometry: reproducibility, linearity, and application with complex proteomes. J Proteome Res. 2006;5:1214–1223. doi: 10.1021/pr050406g. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J, MacRae TH. Nucleotide dependence and cytoplasmic localization of a 49-kDa microtubule cross-linking protein from the brine shrimp, Artemia . J Biol Chem. 1994;269:3053–3062. [PubMed] [Google Scholar]
- 118.Burkhart CA, Kavallaris M, Band Horwitz S. The role of beta-tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta. 2001;1471:O1–O9. doi: 10.1016/s0304-419x(00)00022-6. [DOI] [PubMed] [Google Scholar]
- 119.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998;77:562–566. doi: 10.1038/bjc.1998.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu B, Staren ED, Iwamura T, Appert HE, Howard JM. Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J Surg Res. 2001;99:179–186. doi: 10.1006/jsre.2001.6126. [DOI] [PubMed] [Google Scholar]
- 122.Liu B, Staren E, Iwamura T, Appert H, Howard J. Taxotere resistance in SUIT Taxotere resistance in pancreatic carcinoma cell line SUIT 2 and its sublines. World J Gastroenterol. 2001;7:855–859. doi: 10.3748/wjg.v7.i6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD, Haber M. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001;61:5803–5809. [PubMed] [Google Scholar]
- 124.Dumontet C, Jaffrezou JP, Tsuchiya E, Duran GE, Chen G, Derry WB, Wilson L, Jordan MA, Sikic BI. Resistance to microtubule-targeted cytotoxins in a K562 leukemia cell variant associated with altered tubulin expression and polymerization. Bull Cancer. 2004;91:E81–E112. [PubMed] [Google Scholar]
- 125.Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- 126.Cicchillitti L, Penci R, Di Michele M, Filippetti F, Rotilio D, Donati MB, Scambia G, Ferlini C. Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol Cancer Ther. 2008;7:2070–2079. doi: 10.1158/1535-7163.MCT-07-2370. [DOI] [PubMed] [Google Scholar]
- 127.Brun V, Masselon C, Garin J, Dupuis A. Isotope dilution strategies for absolute quantitative proteomics. J Proteomics. 2009;72:740–749. doi: 10.1016/j.jprot.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 128.Freedman H, Huzil JT, Luchko T, Luduena RF, Tuszynski JA. Identification and characterization of an intermediate taxol binding site within microtubule nanopores and a mechanism for tubulin isotype binding selectivity. J Chem Inf Model. 2009;49:424–436. doi: 10.1021/ci8003336. [DOI] [PubMed] [Google Scholar]
- 129.Vassal E, Barette C, Fonrose X, Dupont R, Sans-Soleilhac E, Lafanechère L. Miniaturization and validation of a sensitive multiparametric cell-based assay for the concomitant detection of microtubule-destabilizing and microtubule-stabilizing agents. J Biomol Screen. 2006;11:377–389. doi: 10.1177/1087057106286210. [DOI] [PubMed] [Google Scholar]
- 130.Esquenazi E, Coates C, Simmons L, Gonzalez D, Gerwick WH, Dorrestein PC. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol Biosyst. 2008;4:562–570. doi: 10.1039/b720018h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trim PJ, Henson CM, Avery JL, McEwen A, Snel MF, Claude E, Marshall PS, West A, Princivalle AP, Clench MR. Matrix-assisted laser desorption/ionization-ion mobility separation-mass spectrometry imaging of vinblastine in whole body tissue sections. Anal Chem. 2008;80:8628–8634. doi: 10.1021/ac8015467. [DOI] [PubMed] [Google Scholar]
- 132.Luxembourg SL, Mize TH, McDonnell LA, Heeren RM. High-spatial resolution mass spectrometric imaging of peptide and protein distributions on a surface. Anal Chem. 2004;76:5339–5344. doi: 10.1021/ac049692q. [DOI] [PubMed] [Google Scholar]
- 133.Piehowski PD, Carado AJ, Kurczy ME, Ostrowski SG, Heien ML, Winograd N, Ewing AG. MS/MS methodology to improve subcellular mapping of cholesterol using TOF-SIMS. Anal Chem. 2008;80:8662–8667. doi: 10.1021/ac801591r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lemaire R, Stauber J, Wisztorski M, Van Camp C, Desmons A, Deschamps M, Proess G, Rudlof I, Woods AS, Day R, Salzet M, Fournier I. Tag-mass: specific molecular imaging of transcriptome and proteome by mass spectrometry based on photocleavable tag. J Proteome Res. 2007;6:2057–2067. doi: 10.1021/pr0700044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lemaire R, Menguellet SA, Stauber J, Marchaudon V, Lucot JP, Collinet P, Farine MO, Vinatier D, Day R, Ducoroy P, Salzet M, Fournier I. Specific MALDI imaging and profiling for biomarker hunting and validation: fragment of the 11S proteasome activator complex, Reg alpha fragment, is a new potential ovary cancer biomarker. J Proteome Res. 2007;6:4127–4134. doi: 10.1021/pr0702722. [DOI] [PubMed] [Google Scholar]
- 136.Iancu C, Mistry SJ, Arkin S, Wallenstein S, Atweh GF. Effects of stathmin inhibition on the mitotic spindle. J Cell Sci. 2001;114:909–916. doi: 10.1242/jcs.114.5.909. [DOI] [PubMed] [Google Scholar]
- 137.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. [PubMed] [Google Scholar]
- 138.Alli E, Yang JM, Ford JM, Hait WN. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol. 2007;71:1233–1240. doi: 10.1124/mol.106.029702. [DOI] [PubMed] [Google Scholar]
- 139.Mistry SJ, Bank A, Atweh GF. Synergistic antiangiogenic effects of stathmin inhibition and taxol exposure. Mol Cancer Res. 2007;5:773–782. doi: 10.1158/1541-7786.MCR-06-0290. [DOI] [PubMed] [Google Scholar]
- 140.Iancu C, Mistry SJ, Arkin S, Atweh GF. Taxol and anti-stathmin therapy: a synergistic combination that targets the mitotic spindle. Cancer Res. 2000;60:3537–3541. [PubMed] [Google Scholar]
- 141.Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol. 2007;18(Suppl 12):xii15–20. doi: 10.1093/annonc/mdm534. [DOI] [PubMed] [Google Scholar]
- 142.Martello LA, Verdier-Pinard P, Shen HJ, He L, Torres K, Orr GA, Horwitz SB. Elevated levels of microtubule destabilizing factors in a Taxol-resistant/dependent A549 cell line with an alpha-tubulin mutation. Cancer Res. 2003;63:1207–1213. [PubMed] [Google Scholar]
- 143.Gigant B, Curmi PA, Martin-Barbey C, Charbaut E, Lachkar S, Lebeau L, Siavoshian S, Sobel A, Knossow M. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102:809–816. doi: 10.1016/s0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 144.Steinmetz MO. Structure and thermodynamics of the tubulin–stathmin interaction. J Struct Biol. 2007;158:137–147. doi: 10.1016/j.jsb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 145.Alli E, Yang JM, Hait WN. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene. 2007;26:1003–1012. doi: 10.1038/sj.onc.1209864. [DOI] [PubMed] [Google Scholar]
- 146.King MV, DeVries JL, Pepinsky R. An X-ray diffraction determination of the chemical structure of colchicine. Acta Crystallogr B. 1952;5:437. [Google Scholar]
- 147.Devred F, Tsvetkov PO, Barbier P, Allegro D, Horwitz SB, Makarov AA, Peyrot V. Stathmin/Op18 is a novel mediator of vinblastine activity. FEBS Lett. 2008;582:2484–2488. doi: 10.1016/j.febslet.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Su D, Smith SM, Preti M, Schwartz P, Rutherford TJ, Menato G, Danese S, Ma S, Yu H, Katsaros D. Stathmin and tubulin expression and survival of ovarian cancer patients receiving platinum treatment with and without paclitaxel. Cancer. 2009;115:2453–2463. doi: 10.1002/cncr.24282. [DOI] [PubMed] [Google Scholar]
- 149.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 150.Peth A, Boettcher JP, Dubiel W. Ubiquitin-dependent proteolysis of the microtubule end-binding protein 1, EB1, is controlled by the COP9 signalosome: possible consequences for microtubule filament stability. J Mol Biol. 2007;368:550–563. doi: 10.1016/j.jmb.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 151.Makarov AA, Tsvetkov PO, Villard C, Esquieu D, Pourroy B, Fahy J, Braguer D, Peyrot V, Lafitte D. Vinflunine, a novel microtubule inhibitor, suppresses calmodulin interaction with the microtubule-associated protein STOP. Biochemistry. 2007;46:14899–14906. doi: 10.1021/bi701803s. [DOI] [PubMed] [Google Scholar]
- 152.He L, Yang CP, Horwitz SB. Mutations in beta-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol Cancer Ther. 2001;1:3–10. [PubMed] [Google Scholar]