Abstract
ATHB-8, -9, -14, -15, and IFL1/REV are members of a small homeodomain-leucine zipper family whose genes are characterized by expression in the vascular tissue. ATHB-8, a gene positively regulated by auxin (Baima et al., 1995), is considered an early marker of the procambial cells and of the cambium during vascular regeneration after wounding. Here, we demonstrate that although the formation of the vascular system is not affected in athb8 mutants, ectopic expression of ATHB-8 in Arabidopsis plants increased the production of xylem tissue. In particular, a careful anatomical analysis of the transgenic plants indicated that the overexpression of ATHB-8 promotes vascular cell differentiation. First, the procambial cells differentiated precociously into primary xylem. In addition, interfascicular cells also differentiated precociously into fibers. Finally, the transition to secondary growth, mainly producing xylem, was anticipated in transgenic inflorescence stems compared with controls. The stimulation of primary and secondary vascular cell differentiation resulted in complex modifications of the growth and development of the ATHB-8 transgenic plants. Taken together, these results are consistent with the hypothesis that ATHB-8 is a positive regulator of proliferation and differentiation, and participates in a positive feedback loop in which auxin signaling induces the expression of ATHB-8, which in turn positively modulates the activity of procambial and cambial cells to differentiate.
The vascular system of the plant forms a complex but orderly network which connects the leaves and other parts of the shoot with the root. The vascular tissues are composed of two types of conducting tissues: the xylem, which is the conduit for water and mineral salts, and the phloem, through which organic compounds are transported. Xylem is a complex tissue, consisting of tracheary (conducting) elements, fibers, and xylary parenchyma. Like xylem, phloem also is composed of different kinds of cells: sieve elements, companion cells, phloem parenchyma, and fibers (Esau, 1965). Vascular development involves the formation of provascular cells that give rise to the procambium and, after specific events of cyto-differentiation, to both conducting tissues (Steeves and Sussex, 1989). The procambial cells are dense, narrow, and elongated parallel with the longitudinal axis of the organ. In the young, terminal regions of the stem, depending on the characteristics of the future vascular system, the procambium may be a solid cylinder, a hollow cylinder, or a system of discrete cellular strands. In the roots in general, the procambium is a core of tissue from which the entire vascular cylinder originates (Esau, 1965). The vascular tissues can also be formed in the older part of the plant by the activity of the vascular cambium, a secondary meristem. Different from the procambium, the cambial cells are highly vacuolated, and are separated into two cell populations with different cytological features and division plane capabilities: the fusiform and the ray initials forming the axial and the ray system of the secondary vascular structure, respectively (Esau, 1965). Herbaceous plants may lack secondary meristems entirely, or these meristems may be poorly developed. In Arabidopsis, vascular cambium activity (leading to structural changes known as secondary thickening or secondary growth) has been observed both in the mature root and hypocotyl (Dolan et al., 1993; Gendreau et al., 1997) and in the inflorescence stem (Altamura et al., 2001).
Despite a great variety of patterns in vascular systems and organs, it is likely that a common mechanism underlies spatial regulation of vascular tissue formation. Several phytohormones have been implicated in the regulation of vascular tissue formation. However, considerable evidence indicates that auxin is the major signal involved in several aspects of the ontogeny of the vascular system (Aloni, 1987; Sachs, 2000). One of the main peculiarities of auxin is that, of all the known plant hormones, it is the only one that exhibits polar transport (Lomax et al., 1995). In the “canalization hypothesis,” Sachs proposed that the diffusion of this hormone from an auxin source induces the formation of a polar auxin transport system along a narrow file of procambial cells; the polar transport of auxin should result in the formation of vascular strands (Sachs, 1981; Sachs, 1991). However, this model alone cannot account for all aspect of vascular pattern formation. Other mechanisms, such as diffusion-reaction systems and long-distance signaling by hypothetical diffusible substances, may play a role in the process (Dengler and Kang, 2001).
In Arabidopsis, several mutants that interfere with various aspects of vascular development have been isolated (Dengler and Kang, 2001). Some of these mutants have been described with auxin transport or auxin signaling defects, and loss of tissue continuity within the vascular system (Hardtke and Berleth, 1998; Berleth and Sachs, 2001). A recessive mutation in the WOODEN LEG (WOL) gene results in reduced proliferation of procambial cells, altered xylem organization, and absence of phloem cells within the root vascular tissue (Scheres et al., 1995, Mahonen et al., 2000). It is interesting that the WOL gene encodes a putative two-component His kinase with a receptor domain, suggesting that it functions as a signal transducer (Mahonen et al., 2000).
Studies in many cell types have shown that proliferation and differentiation are inversely correlated processes likely involving the activity of distinct transcription factors. REVOLUTA (REV), also known as INTERFASCICULAR FIBERLESS1 (IFL1; Zhong and Ye, 1999; Ratcliffe et al., 2000), recently has been implicated in both processes. A careful analysis of several alleles indicated that IFL1/REV is necessary for lateral meristem initiation and normal organ development (Talbert et al., 1995; Otsuga et al., 2001) as well as proper differentiation of vascular cells of the stem (Zhong et al., 1997). IFL1/REV, together with ATHB-8, -9, -14, and -15, is a member of the HD-ZIP III family (Sessa et al., 1994; Sessa et al., 1998; Baima et al., 2000). The five HD-ZIP III genes encode highly related transcription factors characterized by the presence of the homeodomain-Leu zipper domain associated with steroidogenic acute regulatory protein-related lipid transfer, a putative lipid-binding domain (Ponting and Aravind, 1999).
The restricted expression of ATHB-8 in the provascular cells, its auxin inducibility, and its polar expression in the region of revascularization of wounded stems, suggested that ATHB-8 might play a regulatory role in the development of the vascular system (Baima et al., 1995).
In this study, we used two complementary approaches to investigate ATHB-8 function. We have screened for athb8 insertional mutants and expressed the ATHB-8 coding sequence, in sense and antisense orientation, under the control of the cauliflower mosaic virus (CaMV) 35S promoter in Arabidopsis. Our analysis suggests that although ATHB-8 is not essential for vascular tissue differentiation, its increased expression significantly accelerates and stimulates the formation of the vascular tissue, indicating a role for this gene in the regulation of the activity of the vascular meristems.
RESULTS
Identification of athb8 Mutants
In an attempt to understand ATHB-8 function during vascular development, a collection of 8,000 Arabidopsis plants carrying, on average, six independent insertions of the maize (Zea mays) transposable element En-1 was used for reverse genetic analyses to identify knock-out mutant alleles of ATHB-8. Insertions were identified by using a three-dimensional, PCR-based screening strategy (Baumann et al., 1998) and were confirmed in individual progeny of the selected plant by sequencing.
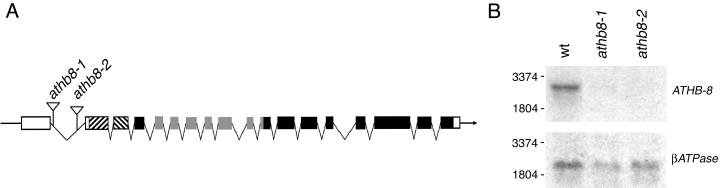
We isolated two independent insertions in ATHB-8 named 6H75 (athb8-1) and 6AAR36 (athb8-2). Both insertions are in the first intron, 383 and 701 bp downstream of the transcription start site in athb8-1 and athb8-2, respectively, and oriented with the 5′ end of the En-1 element toward the 5′ end of ATHB-8 (Fig. 1A). Northern-blot analyses has shown that the ATHB-8 mRNA is below the level of detection in the two tagged mutants (Fig. 1B). Nonetheless, the athb8 knockout plants did not show any phenotypic alteration with respect to overall size and morphology under our growth conditions. Leaf shape and vein pattern were comparable with those of the wild type, and normal, fully fertile flowers were formed. Moreover, root growth and lateral root formation were unaffected in athb8 plants grown on synthetic medium. Finally, the histological analysis of athb8 stem and root revealed a normal vascular system with no morphological alterations (data not shown). The lack of evident phenotypes in athb8 mutants might be explained by a functional redundancy within the HD-ZIP III family because some of the genes are expressed in the vasculature (Zhong and Ye, 1999; Otsuga et al., 2001; C. Steindler, M. Possenti, G. Mozelli, and I. Ruberti, unpublished data).
Figure 1.
Structure of the ATHB-8 gene, En-1 transposon insertion sites, and ATHB-8 expression in athb-8 mutants. A, Schematic drawing of the ATHB-8 gene. The gene has 17 introns, with the first intron located in the 5′ untranslated region just before the start codon. Introns are shown as lines and exons as boxes. White boxes, 5′ and 3′ untranslated region; sketch boxes, HD-Zip domain; gray boxes, START domain. The positions of the two independent En-1 insertions are indicated by white arrowheads. B, Northern-blot analysis of ATHB-8 expression. A DNA fragment corresponding to the 3′ region of the ATHB-8 cDNA (a 272-bp EcoRI DNA fragment; Baima et al., 1995) was used to probe 10 μg of total RNA isolated from 2-week-old Arabidopsis plants. The same blot was probed with a cDNA for the β-subunit of N. plumbaginifolia mitochondrial ATPase (βATPase).
Phenotype of Plants Overexpressing ATHB-8
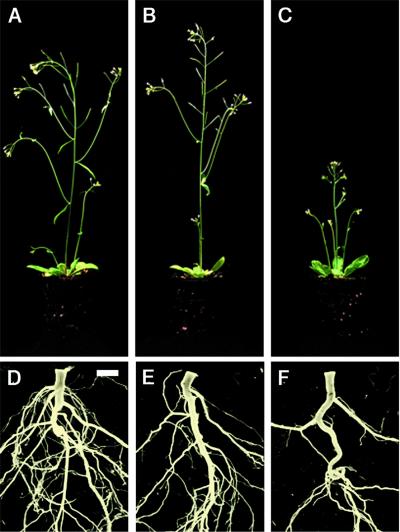
To gain more insight into the role of ATHB-8 in procambial cell differentiation, we attempted to overcome the functional redundancy studying the effects of constitutive ATHB-8 expression. Therefore, we fused the ATHB-8 coding region to the 35S CaMV promoter and introduced this chimeric gene into Arabidopsis plants. Two of the 10 sense ATHB-8 transgenic lines examined, At5/2 and At10/1, had an upward curling of leaf margins, whereas seven had only a slight curling of leaf margin. At bolting, the line At5/2 had a normal elongated, but a more rigid inflorescence stem and a root system slightly less complex compared with the wild-type control (Fig. 2, A, B, D, and E). At10/1, the second line with rolled-up leaves, showed a strong reduction of inflorescence stem elongation (Fig. 2, A and C). The lateral branches of the main stem and the stems of the side shoots were produced normally, although they grew shorter in At10/1 plants than in wild-type controls (Fig. 2, A and C). The At10/1 line also showed a reduced root system (Fig. 2F). The other seven transgenic lines showed a normal inflorescence stem and root system (data not shown).
Figure 2.
Morphology of soil-grown ATHB-8 transgenic plants. A through C, Side view of 4-week-old wild-type (A), At5/2 (B), and At10/1 (C) plants. D through F, Root architecture of 6-week-old wild-type (D), At5/2 (E), and At10/1 (F) plants. Scale bar = 3 mm.
RNA gel-blot analysis was used to examine the ATHB-8 transcript levels in the transgenic plants. The steady state level of ATHB-8 transcripts was found enriched about 3- and 7-fold in the At5/2 and At10/1 transgenic plants, respectively. The ATHB-8 expression level was found slightly increased (between 1.5- and 2-fold) in the other seven transgenic lines compared with the wild type. The At5/2 and At10/1 lines were chosen for further characterization.
To analyze the effect of altered ATHB-8 levels on the root system, we measured the length of the primary root and the formation of lateral roots of 2-week-old seedlings grown on synthetic media. Under this growth conditions the same number of leaves (four) was formed in wild type and transgenic seedlings. The leaves of the transgenic plants were of normal size but clearly rolled up and iponastic (data not shown). The total length of the At10/1 primary root was about the same as that of the wild type (11.34 ± 0.21 and 11.38 ± 0.15 cm, respectively). In contrast, the number of lateral roots formed in the At10/1 transgenic plants was significantly lower than that of the wild type (37.35 ± 1.36 and 48.9 ± 1.49, respectively, P < 0.01). Moreover, At10/1 transgenic plants produced fewer higher order roots, resulting in a root system less complex than that of wild-type plants, as observed in plants grown on soil (Fig. 2F). No significant reduction of lateral root formation was observed in the At5/2 line when grown on synthetic media.
The ATHB-8 coding sequence was put under the control of the 35S promoter also in antisense orientation. None of the Arabidopsis plants expressing the antisense ATHB-8 construct showed any evident phenotypic alterations, although fluorimetric detection of β-glucuronidase activity driven by the bidirectional 35S promoter of the pMON721 vector indicated that the 35S promoter was active in the antisense lines (data not shown).
Altered Levels of ATHB-8 Affect Secondary Growth in the Inflorescence Stem and Root
To investigate in more detail the alterations observed in the inflorescence stem and root of the transgenic lines, a histological analysis of these organs was undertaken.
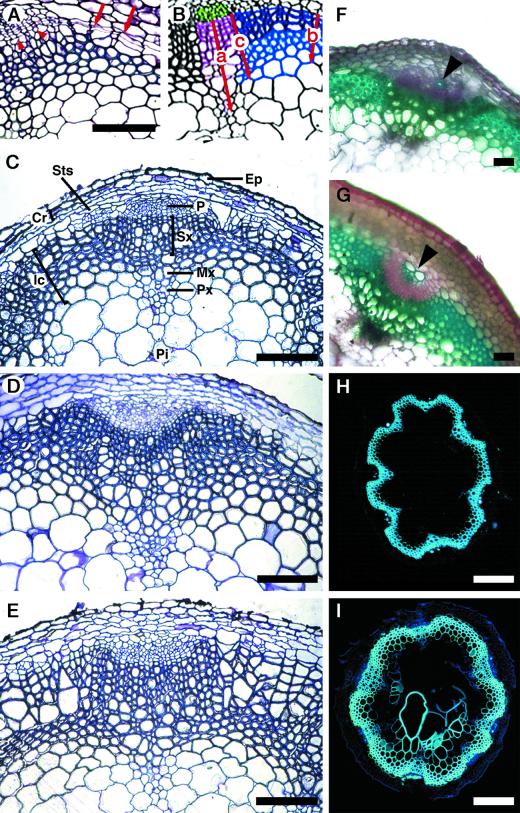
Wild-type Arabidopsis inflorescence stem has a vascular pattern similar to that found in most dicots (Altamura et al., 2001). Two weeks after bolting (5-week-old plants), in the basal end of the wild-type stem a vascular cambium producing secondary vascular tissues was clearly present (Fig. 3A). The interfascicular cambium was located outside the sclerified arcs between the primary vascular bundles (arrows, Fig. 3A) and was connected to the fascicular cambium forming a continuous ring of meristematic cells (arrowheads, Fig. 3A) producing mainly sclerified xylary tissue. A detailed description of the secondary growth in the Arabidopsis inflorescence stem will be published elsewhere (Altamura et al., 2001).
Figure 3.
Histological analysis of the secondary vascular structure in the inflorescence stem of ATHB-8 transgenic plants. A, Transverse section of the basal end of the stem showing the onset of the activity of the interfascicular (arrows) and fascicular (arrowheads) cambium in 5-week-old wild-type plants. B, Schematic diagram showing the organization of the Arabidopsis vascular system in stem undergoing secondary growth: phloem (green), fascicular xylem (purple), interfascicular xylem (dark blue), interfascicular fiber cells (light blue); the arrows indicate the radius of the: (a) fascicular xylem, (b) middle part of the interfascicular arc, and (c) interfascicular arc flanking the bundle. The average measurements are indicated in Table I. C–E, Transverse sections of the basal end of stem showing the secondary structure of the vascular system in 6-week-old stem. C, Wild type. D, At5/2. E, At10/1. F through I, Transverse sections of the basal part of the stem were taken from 6-week-old (F and G) or 8-week-old (H and I) plants showing other secondary growth events peculiar to ATHB-8 transgenic plants. G, Significant production of phloem fiber sclereids (arrowhead); I, lignification of a wide part of the pith was observed in the stem of At10/1 (G and I) and not in the wild type (F and H). Sections were stained with toluidine blue (A–E) and carmin-iodine mixture (F and G). H and I, Autofluorescence of lignified cell walls. Scale bar: 100 μm (A–E, H, and I) and 20 μm (F and G). Ep, Epidermis; Cr, cortex; Ic, interfascicular cells; Mx, metaxylem; P, phloem; Pi, pith; Px, protoxylem; Sts, starch sheath; Sx, secondary xylem.
The comparative analysis between wild-type and transgenic plants was done at the stage of green siliques, about 3 weeks after bolting, when the inflorescence stem is fully developed. At this stage, sections taken from the basal part of the stem of At5/2 and At10/1 transgenic plants showed an anatomical structure similar to that of wild type with a higher production of lignified tissue (Fig. 3, C–E), in agreement with the observation that the inflorescence stem of the two transgenic lines appeared more rigid compared with the wild-type stem (see Fig. 2, A–C). To quantify the production of lignified tissue in wild-type and transgenic plants we measured the radius of the fascicular xylem (indicated as a, Fig. 3B), the middle part of the interfascicular arc (b, Fig. 3B), and the region flanking the bundle (c, Fig. 3B). The results of this analysis are shown in Table I. The major contribution to the production of lignified tissue in both the transgenic lines was derived from an increased activity of both the fascicular and interfascicular cambium (mainly producing secondary xylem) compared with the wild type. At the end of the life cycle, the bundles and the interfascicular regions of the transgenic plants consistently continued to show a conspicuous increase in xylem formation compared with the wild type (data not shown). Other events occurring during the secondary growth, and peculiar to transgenic plants, were an increase in the production of phloem fiber sclereids (Fig. 3, F and G) and the lignification of a wide part of the pith (Fig. 3, H and I).
Table I.
Histological analysis of stem and root
| WT | At5/2 | At10/1 | |
|---|---|---|---|
| Fascicular xylem (a) | 153.4 ± 4.1 | 188.7 ± 4.5a | 190.8 ± 6b |
| Interfascicular arc (middle part) (b) | 93.9 ± 4.7 | 149.6 ± 7.4a | 166.5 ± 4.4b |
| Interfascicular arc (flanking the bundle) (c) | 118.2 ± 5.4 | 162.4 ± 4.7a | 170.8 ± 4.4b |
| Root vascular region (d) | 184.2 ± 5.9 | 183.7 ± 7.8 | 250.4 ± 6.9b |
| Root periderm (e) | 56.2 ± 4.9 | 60.5 ± 10.3 | 53.3 ± 6.1 |
| Root (f) | 240.4 ± 9.5 | 244.2 ± 17.7 | 303.7 ± 12.6b |
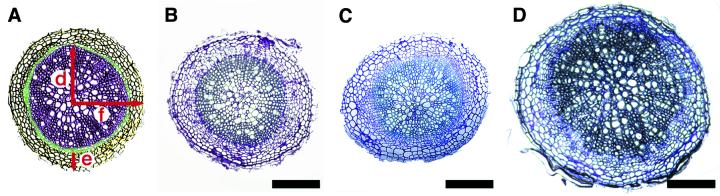
A similar analysis was also performed in the root, another organ undergoing secondary growth (Dolan et al., 1993). At 3 weeks after bolting, a normal organization of the different cell types composing the root was detected, although the final diameter of the At10/1 transgenic root was quite large compared with that of the At5/2 and wild type (compare root radii, Table I). This difference was due mainly to an excess of secondary xylem formation (Fig. 4, A–D).
Figure 4.
Histological analysis of the secondary vascular structure in the root of ATHB-8 plants. A, Schematic diagram showing secondary growth in the Arabidopsis root: xylem (dark purple), cambium and phloem (green), periderm (yellow); the arrows indicate the radius of the (d) vascular region, (e) periderm, and (f) entire root. B through D, Transverse sections showing the degree of secondary vascular growth in wild-type (B), At5/2 (C), and At10/1 (D) root. The transverse sections were taken from the region immediately below the hypocotyl-root junction of 6-week-old plants grown on soil. Sections were stained with toluidine blue. Scale bar: 100 μm.
Altered Levels of ATHB-8 Affect Primary Vascular Development, and the Onset of Secondary Growth in the Inflorescence Stem
A careful analysis of the flowering time and, subsequently, of the growth rate of the stem revealed that there were no significant differences between wild-type, At5/2, and At10/1 transgenic plants until the stems were about 5 cm long (4–5 d after bolting in our growth conditions). Starting from this point, the At10/1 transgenic stem displayed a continuous growth at a slower elongation rate resulting, at the end of the life cycle, in a stem about 17 cm long, compared with the mean length of 45 cm for the wild-type and At5/2 stems. To investigate in more detail if the alteration in stem height observed in the At10/1 line correlated with an alteration of vascular development, a histological analysis was undertaken before the plants showed a difference in the height of the stem. To obtain comparable samples from morphologically divergent plants, sections were taken from the apical and basal part of inflorescence stems about 1 and 5 cm in length.
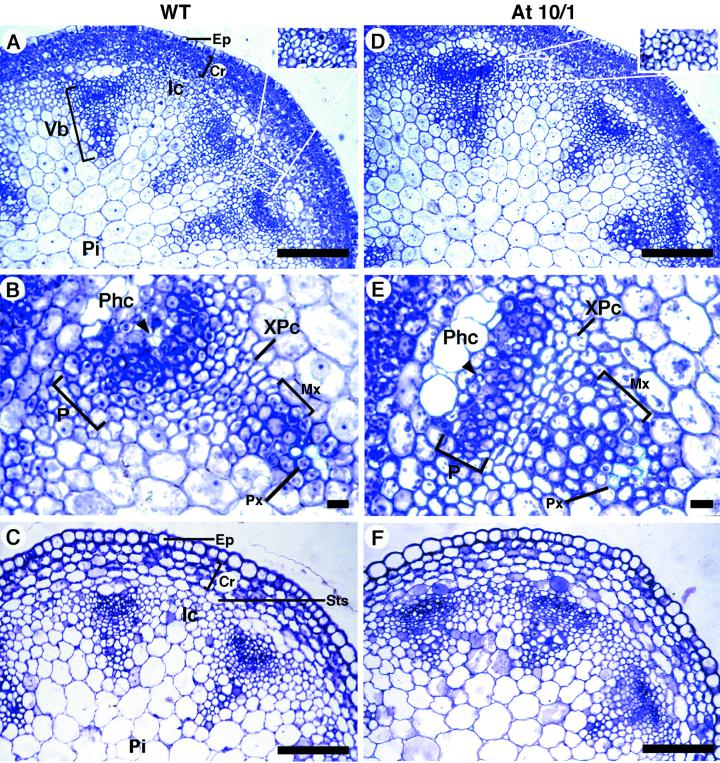
In the apical part of 1-cm-tall Arabidopsis stems, the eustelic bundles showed the characteristic collateral arrangement, with the phloem located outside the xylem (Fig. 5A). At this stage, groups of meristematic cells were still present in the interfascicular regions (see inset, Fig. 5A). Within the bundle, the primary phloem had a typical round shape mainly composed of densely cytoplasmic cells surmounted by a few enlarged phloem cap cells and a few differentiated protophloem cells (Fig. 5B). The primary xylem consisted of immature metaxylem cells with a radial alignment, and few differentiated protoxylem cells (Fig. 5B). A small group of xylary procambial cells arranged in a radial series was located between phloem and xylem (Fig. 5B). Overall, most of the bundles assumed the shape of an isosceles triangle. The analysis of At10/1 transgenic plants revealed that the bundles were wider compared with those of the wild type at the same stem level and assumed the shape of an equilateral triangle (Fig. 5, D and E). The different shape of the transgenic bundles is due to a flattening of the phloem and an increased number of files of radially aligned xylary procambial cells differentiating into metaxylem (Fig. 5, B and E). The different organization of the xylary procambium resulted in a higher tangential extension of this tissue compared with the controls. Furthermore, the immature metaxylem cells did not show any pattern of radial seriation (Fig. 5E) and the interfascicular regions were differentiated (parenchymatic; see inset in Fig. 5D). The different shape of the phloem was evidenced also by the number and spatial arrangement of the phloem cap cells surmounting each bundle. In the basal part of 1-cm-long stems, the presence of flattened phloem in the transgenic plants was even more evident (Fig. 5, C and F). At this developmental stage most of the wild-type bundles had lost the radial alignment of the immature metaxylem cells (Fig. 5C).
Figure 5.
Histological analysis of the primary vascular structure in 1-cm-long inflorescence stem of ATHB-8 transgenic plants. A through C, Wild type. D through F, At10/1. A and D, Transverse sections showing representative bundles of the apical part of 1-cm-long stem (eustele); the insets show magnifications of the interfascicular region indicating a lower meristematic activity of the transgenic plant compared with the control. B and E, Magnification of typical vascular bundles. Note the absence of radially aligned immature xylem cells and the flattened phloem in the transgenic primary bundle. The arrowheads show protophloem cells. C and F, Transverse sections showing representative bundles of the basal part of 1-cm-long stem. Sections were stained with toluidine blue. Scale bar: 100 μm (A, C, D, and F) and 10 μm (B and E). Ep, Epidermis; Cr, cortex; Ic, interfascicular cells; Mx, metaxylem; P, phloem; Phc, phloem cap cells; Pi, pith; Px, protoxylem; Sts, starch sheath; Vb, vascular bundle; XPc, xylary procambium.
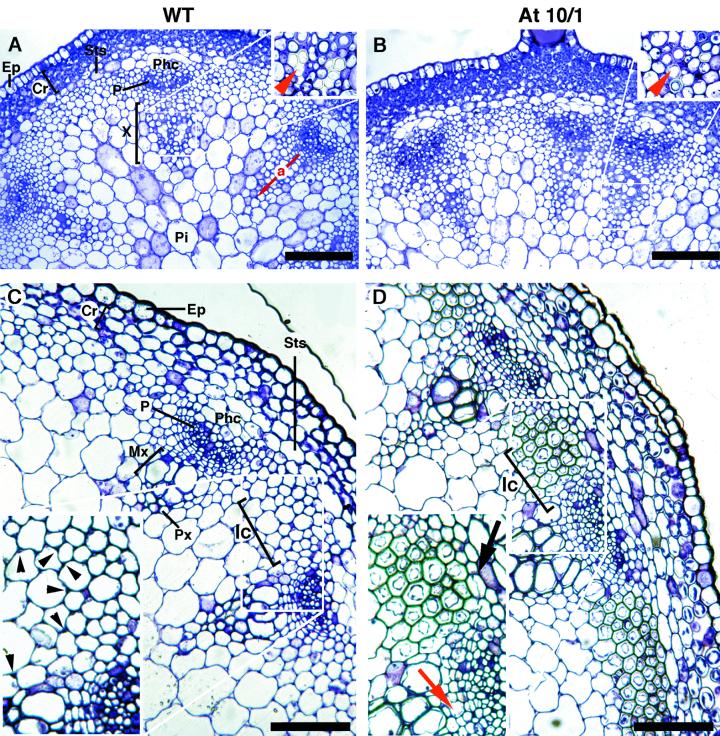
In the apical region of 5-cm-tall wild-type stems, vascular bundles contained few layers of procambium between primary phloem and xylem (Fig. 6A); in the latter, protoxylem and some metaxylem cells showed well lignified walls (see inset in Fig. 6A). In At10/1 stems at the same stage the radial extension of the primary xylem was similar to the controls (23.9 ± 1.3 and 20.6 ± 2.7 μm, respectively; Fig. 6, A and B); however, the thickness of the protoxylem walls was significantly (P < 0.01) higher in the transgenic bundles than in the controls (1.4 ± 0.02 and 1.1 ± 0.03 μm, respectively; see inset in Fig. 6, A and B).
Figure 6.
Histological analysis of the vascular structure in 5-cm-long inflorescence stem of ATHB-8 transgenic plants. A and C, Wild type. B and D, At10/1. A and B, Transverse sections showing representative bundles of the apical part of 5-cm-long stem (eustele). The red arrow (a) indicates the radius of the primary xylem considered for statistical analysis. A magnification of the protoxylem cells (indicated by red arrowhead in the insets) shows a higher secondary wall deposition in the transgenic plant compared with the control. C and D, Transverse sections showing representative bundles of the basal end of 5-cm-long stem. Note the deposition of secondary wall thickenings (black arrowheads) at the angles of the interfascicular cells in C, compared with the lignified interfascicular cells in D. The onset of the interfascicular cambium (black arrow) and a secondary xylem cell (red arrow) are also shown in D. Sections were stained with toluidine blue. Scale bar: 100 μm (A–D). Ep, Epidermis; Cr, cortex; Ic, interfascicular cells; Mx, metaxylem; P, phloem; Phc, phloem cap cells; Pi, pith; Px, protoxylem; Sts, starch sheath; Sx, secondary xylem; X, xylem.
The main difference between the transgenic and wild-type plants was observed in the basal region of the 5-cm stem (Fig. 6, C and D). In fact, the early events leading to secondary growth in the vascular system were observed only in At10/1 plants and not in the controls. In particular, the interfascicular regions of the transgenic stem showed cells with evenly lignified secondary walls (Fig. 6, C and D) and periclinal divisions at their flanks in contiguity with the cambial cells of the bundles (onset of interfascicular cambium; black arrow, Fig. 6D). Within the bundles, series of new cells, produced by periclinal divisions in the cambium, were also observed (onset of the production of secondary xylem by the fascicular cambium; red arrow, Fig. 6D). In the controls, instead the typical eustele was still visible, with the interfascicular cells showing only the deposition of secondary wall thickenings at the angles (arrowheads, Fig. 6C).
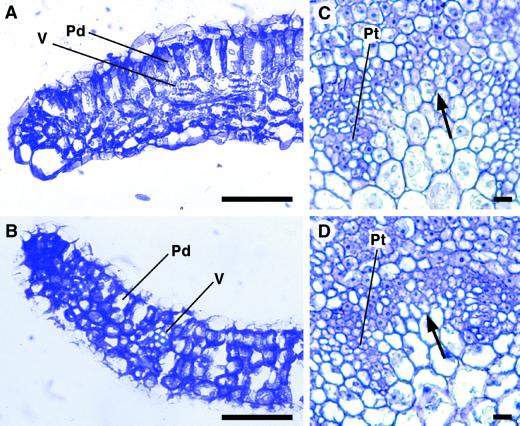
Overexpression of ATHB-8 also affected primary vascular development in the Arabidopsis leaf and pedicel. The number of leaves and the timing of leaf initiation and bolting were essentially the same in wild-type and At10/1 transgenic plants. We analyzed the structure of the seventh leaf (the last leaf of the rosette) at bolting time. The histological analysis revealed that the wild-type leaf was fully differentiated (Fig. 7A), whereas the transgenic At10/1 leaf still exhibited meristematic features. In particular, at the margins, the palisade parenchyma was not yet fully differentiated (Fig. 7B). Compared with the wild type, the transgenic leaves also showed a reduced presence both of the intercellular spaces of the spongy parenchyma and the sub-stomatal chambers underneath the adaxial and abaxial epidermis (Fig. 7, A and B).
Figure 7.
Histological analysis of the primary vascular structure in leaf and pedicel of ATHB-8 transgenic plants. A and C, Wild type. B and D, At10/1. A and B, Transverse sections showing a lateral portion of the lamina. Sections were taken at the middle of the seventh leaf of 3-week-old plants. Note the presence of meristematic tissue at the margins of the transgenic leaf in B. C and D, Transverse sections showing differentiating procambial traces in flower pedicels. In the interfascicular region (arrow) the meristematic component is higher in the transgenic plants than in the control. Sections were stained with toluidine blue. Scale bar: 50 μm (A and B and 10 μm (C and D). Pd, Palisade cells; Pt, procambial traces; V, vein.
The analysis of the vasculature in the median region of the seventh leaf revealed that the radial extension of the xylem in the midrib was significantly higher (P < 0.01) in At10/1 than in wild type (i.e. 23.3 ± 1.1 and 18.8 ± 1 μm, respectively). The number of vascular bundles (veins) in the median region of the leaf conversely did not change significantly, being by average 15.3 ± 0.8 in At10/1 plants and 14.5 ± 0.5 in wild-type controls.
At the receptacle base of the pedicel, in transection, the procambium assumes the outline of a ring; thus, the procambial traces are connected in the interfascicular regions by arcs of meristematic cells from which the external part of the medullary rays differentiate. The meristematic component of these interfascicular regions was higher in At 10/1 transgenic plants than in controls (Fig. 7, C and D). In the procambial traces some protophloem and protoxylem elements were present both in wild-type and transgenic plants.
DISCUSSION
The experiments described here, together with the analysis of tobacco thin cell layer explants from ATHB-8 transgenic plants (Baima et al., 2000), provide evidence that the product of the Arabidopsis ATHB-8 gene is involved in the regulation of vascular development. In particular, the histological analysis of transgenic plants suggests that ATHB-8 is likely to act as a differentiation-promoting transcription factor regulating the activity of procambial and cambial cells. ATHB-8 belongs to HD-ZIP III, a small family of five highly related HD-Zip proteins (Sessa et al., 1994; Sessa et al., 1998; Zhong and Ye, 1999; Baima et al., 2000). Expression analysis of IFL1/REV (Zhong and Ye, 1999), ATHB-8 (Baima et al., 1995), and ATHB-9 and ATHB-14 (C. Steindler, M. Possenti, G. Mozelli, and I. Ruberti, unpublished data) indicated that four members of the HD-ZIP III family are expressed in the vascular system. The expression pattern of the fifth gene (ATHB-15, the most related to ATHB-8) has not been determined yet. The presence in the vasculature of HD-Zip proteins highly related to ATHB-8 suggests some redundancy within the HD-ZIP III family and might explain the lack of evident phenotypes in athb8 mutants and in 35S::αATHB-8 antisense plants. Some alterations observed in transgenic plants ectopically expressing ATHB-8 might be arisen by activation of target genes regulated by other HD-ZIP III proteins in wild-type plants.
Primary and Secondary Growth Are Anticipated in the ATHB-8 Transgenic Stems
A careful analysis of the wild-type stem indicated that the Arabidopsis cambium produced much more xylem than phloem (Altamura et al., 2001). Therefore, the increased production of xylem found in the ATHB-8 transgenic lines could be attributed to a stimulation of cambial cell proliferation and differentiation mainly producing xylem parenchyma cells. We also found that a relatively high expression of ATHB-8 in transgenic plants affects the formation of the primary vascular tissue. For instance, a higher production of xylem was found in the primary vein of the transgenic leaf, and an increased procambial activity was observed in pedicels. Moreover, significant morphological changes were found in the bundles of transgenic plants at early stages of vascular development compared to the controls. Among them, a higher number of files of radially aligned procambial cells engaged in xylem formation were observed (see Fig. 3). In addition, the differentiation of the interfascicular (extraxylary) fiber cells was accelerated in the transgenic plants. The above results are in good agreement with previous observations suggesting that both vascular strands and fibers are induced along the paths of auxin flow (Aloni, 1987). An alteration of auxin signaling might explain the lack of differentiated fiber cells in most of the inflorescence stem of the ifl1/rev mutant. The finding of sclerified fibers around the vascular bundles in the upper region led to the hypothesis that the lateral diffusion of auxin from the vascular bundles might be responsible for the differentiation of the cells (Zhong et al., 1997). In a similar manner, it has been shown that the block of auxin flow within the bundles in the Atpin1 mutant determines the proliferation and differentiation of cambial cells in regions of the inflorescence stem adjacent to the auxin-synthesizing cauline leaf (Gälweiler et al., 1998).
The expression of ATHB-8 is modulated by auxin, and strongly and precociously induced in revascularization processes caused by wounding, in neo-formed cambial cells and parenchima cells which differentiate without undergoing cell division (Baima et al., 1995). Therefore, in the presence of an auxin source, a positive feedback loop might be activated in provascular cells stimulating the expression of the ATHB-8 gene and, subsequently, cell division and cyto-differentiation toward the formation of the vascular tissue. In the transgenic plants, the threshold for the activation of the procambial and cambial cell seems to be lowered by the increased levels of ATHB-8. In agreement with this hypothesis is the observation that elevated levels of ATHB-8 enhance xylogenesis in tobacco thin cell layer explants grown in hormone-free medium. Furthermore, a medium containing auxin strongly enhanced the formation and amount of organized vascular strands in the transgenic explants, suggesting an increased sensitivity to auxin and, eventually, a higher capacity of ordered growth of the cambium (Baima et al., 2000).
Pleiotropic Phenotypes May Be a Consequence of Precocious Vascular Cell Differentiation
In addition to the changes in the development of the vasculature, ATHB-8 transgenic plants have some other noticeable phenotypes, including reduced internode elongation and reduced number of lateral roots which may have arisen secondarily from a precocious formation of the vascular system.
Flowering Stem
In most plants, primary growth is essentially equivalent to grow in length. It is thought that the activity of lateral meristems, which are involved in radial growth, can develop in regions of the plant that have stopped elongating. Plants exposed to environmental signals such as wind or touch are generally shorter and stockier, and often have altered flexibility. These changes in development in response to mechano-stimulation are collectively known as thigmomorphogenesis (Mitchell, 1996; Ennos, 1997). Arabidopsis responds to wind with a decrease in the inflorescence elongation rate that eventually results in shorter mature primary inflorescence stems (Johnson et al., 1998). A similar, although more dramatic effect, has been observed in the severe ATHB-8 transgenic line. The decreased elongation of the transgenic stem might be a consequence of the accelerated differentiation of the primary vasculature, which in turn provokes the anticipated transition to secondary growth. It is noteworthy that the ifl1-1 mutant, which lacks almost completely interfascicular fiber differentiation, produces long stems (Zhong et al., 1997).
Root System
The enhanced vascularization might also be responsible for the much less complex root system in the transgenic plants than in the controls. In roots undergoing secondary growth, part of the vascular cambium is formed from pericycle cells (Esau, 1965; Dolan et al., 1993). As lateral roots are also formed by dedifferentiation and proliferation of mature pericycle cells, the decreased formation of secondary roots might be due to a precocious recruitment of the pericycle cells for the process of secondary vascular growth. The lower production of lateral roots alternatively might be the result of an alteration of auxin distribution or signaling. A complete phenotypic analysis of the Arabidopsis hy5 mutant showed that this mutant, besides other alterations, has a reduced secondary vascular growth of the hypocotyl and root and an enhanced production of lateral roots compared with the wild type (Oyama et al., 1997). These phenotypes are opposite to those of ATHB-8 transgenic plants. It has been suggested that HY5 may be involved in signaling pathway(s) mediated by auxin and in this respect HY5 might act in the process of secondary thickening as a positive regulator of ATHB-8 and other members of the HD-ZIP III family possibly involved in secondary vascular growth.
CONCLUSIONS
In this study, we analyzed transposon-tagged and transgenic athb8 mutants. The phenotypic data fit nicely with the expression pattern of ATHB-8 (Baima et al., 1995) and point to a role for ATHB-8 in controlling the activity of vascular meristems. On the other hand, the study of the IFL1/REV gene function, through the phenotypic analysis of several alleles and the definition of its expression pattern in wild-type plants, showed that IFL1/REV is implicated in different aspects of plant development: initiation of lateral meristems, normal organ development, and proper development of the vascular tissue (Talbert et al., 1995; Zhong and Ye, 1999; Otsuga et al., 2001). Recent studies indicated that the HD-ZIP III genes have distinct but overlapping patterns of expression. For instance, both ATHB-9 and -14 are expressed in procambial cells, and ATHB-14 is also expressed in the shoot apical meristem and leaf primordia (C. Steindler, M. Possenti, G. Mozelli, and I. Ruberti, unpublished data). All HD-ZIP III proteins share nearly identical Leu zipper domains (Sessa et al., 1998). Thus, depending on the relative concentration of each HD-ZIP III protein in a given cell type, different combinations of homo- and hetero-dimeric complexes might form. Although it is likely that all HD-ZIP III proteins bind the same signaling molecule(s) in distinct cell types through the START domain (Ponting and Aravind, 1999), the different homo- and hetero-dimeric complexes might have opposite functions by acting as positive or negative regulators of common target genes. To unravel the interplay of these proteins in the control of meristem activity, a complex biochemical and genetic analysis of multiple conventional and transgenic mutants will be required.
MATERIALS AND METHODS
Plant Growth and Analysis
Arabidopsis seeds were cold treated for 3 to 4 d at 4°C in the dark, then germinated and grown in soil at 21°C in growth chambers with a 16-/8-h light/dark cycle. The analysis of the phenotypic and anatomical characters of Arabidopsis in planta was performed on at least five specimens, randomly chosen within homogeneous populations of plants. Macroscopic images were acquired with the Abel-Color Acquisition Tool 1.1.5 software (AB.EL Electronics, Inc., Rome) using a COHU 2252-1000 camera mounted on a Wild MZ8 microscope (Leica, Wetzlar, Germany). For root growth analysis, seeds were surface sterilized for 10 min in 30% (v/v) commercial bleach and 0.02% (w/v) Triton X-100, rinsed three times with sterile, distilled water, and dried in a laminar flow hood. Sterile seeds were placed on ARA medium (Kemper et al., 1992) and cold treated for 3 to 4 d at 4°C in the dark. Square plates were incubated in vertical position for 4 d at 21°C under a 16-h light/8-h dark cycle light cycle. Seedlings were then transferred to petri dishes (15 cm ℘ [diameter of the plant]) containing the ARA medium, sealed with 3-m tape (Micropore, Borken, Germany) and incubated for 11 d as above. Each dish contained five transgenic and five wild-type seedlings. The total length of the primary root and the number of lateral roots (including not emerged primordia) present on 20 plants of each genotype were estimated under a Wild MZ8 microscope (Leica).
Identification of athb8 Insertional Mutants
A PCR-based screen was performed, according to a three-dimensional grid as described by Baumann et al. (1998), on a population of Arabidopsis Columbia (Col) plants that carries about 50,000 independent insertions of the autonomous maize transposable element En-1 (Wisman et al., 1998). Primers used in PCR reactions were: 8/II, 5′-GGGAAGTACGTGAGGTACACTCCTGA-3′; 8/III, 5′-TTGACCACTCGTCACCAC TGACTCAC -3′; 8/V, 5′-GGGGAAGAAGACCAGCGGCTCTTAGA-3′; and 8/VI, 5′-AGAGGAATGATGCGGAAACCGGAAGG-3′. Each oligonucleotide was used with both En-1 specific primers En205 and En8130. From selected lines, the presence of the transposon in the gene was confirmed by genomic PCR and the insertion site was determined by subsequent sequence analyses. Mutant plants were backcrossed to the parental Col wild type at least four times to eliminate unlinked transposon insertions. The presence of the specific transposon in the ATHB-8 gene was monitored in each generation by PCR and Southern-blot analyses of HindIII-digested genomic DNA. Plants containing only the desired insertion were selfed to obtain homozygous.
Construction of Transgenes and Plant Transformation
The entire ATHB-8 cDNA from the ATG to the TGA codon was amplified with the primers ATHB-8 5′ (5′-GGGCCCTGCAGCTCGAGGGGAGGAGGAAGCAAT-3′) and ATHB-8 3′ (5′-CCCGGGTCGACGGTCATATAAAAGACCAG-3′), digested with PstI and SalI, and cloned into pBluescript KSII vector (Stratagene, Heidelberg). The cloned fragment was checked by sequencing, excised by digestion with XhoI, and cloned into an XhoI-SalI-digested pMON 721 vector derivative containing a bidirectional CaMV35S promoter and the β-glucuronidase encoding sequence (kindly provided by Prof. Nam-Hai Chua, Rockefeller University, New York; Aoyama et al., 1995). Clones with the ATHB-8 sequence in either sense and antisense orientation were recovered and subsequently introduced by standard methods into Agrobacterium tumefaciens strain GV3101 pMP90RK (Koncz and Schell, 1986). Wassilewskija (WS) ecotype was used for A. tumefaciens-mediated transformation by the vacuum infiltration method (Bechtold et al., 1993). Arabidopsis plants were screened for segregation on plates containing 50 μg mE−1 kanamycin, and for the level of transgene expression by either standard β-glucuronidase fluorimetric assay (Jefferson et al., 1987) or northern hybridization. All the detailed morphological analysis of the transgenic plants expressing the sense construct were done on the T5 and T3 generations of the homozygous At10/1 and At5/2 Arabidopsis lines, respectively.
Microscopy
Seedlings, stems, and roots were fixed overnight in 1% (v/v) glutaraldehyde-4% (v/v) formaldehyde in 50 mm sodium phosphate buffer (pH 7.2). After washing for 30 min in the same buffer, the samples were dehydrated through a graded series of ethanol and embedded in Technovit 7100 (Kulzer, Hereaus, Wehrheim, Germany) as indicated by the manufacturer. Sections were made on an HM 330 microtome (Microm, Waldorf, Germany) at 5-μm thickness and stained with 0.1% (w/v) toluidine blue. For autofluorescence analysis the sections were observed with an Axiolab epifluorescence microscope (Zeiss, Jena, Germany ) using a beam splitter (cutoff 395 nm), an excitation filter (353–377 nm), and a barrier filter (397 nm). Leaves were excised from the seventh node of the rosette (counting from the first node above the cotyledonary node). The leaf explants were fixed in 3.7% (v/v) formaldehyde, 5% (v/v) acetic acid, and 50% (v/v) ethanol, dehydrated in the tertiary butyl alcohol series, and paraffin embedded. Serial transverse sections were made with a Microm HM 330 microtome at 7-μm thickness and stained with 0.1% (w/v) toluidine blue.
Hand-cut sections of stems were stained with carmin-iodine green mixture (Altamura et al., 2001).
For the statistic analysis, micrographs were acquired with a DXC-101P camera (Sony, Milano, Italy) mounted on a Zeiss Axiophot microscope. The images were digitized with the Image Grabber 24 1.2 software (Neotech, La Palma, Los Angeles) and analyzed using OptiLab/Pro 2.6.1 software (Graftek, Mirmande, France). The significance of differences between means was evaluated by the Student's t test and those between percentages by the χ2 test.
Northern Analysis
RNA was isolated from whole plants grown for 2 weeks on soil. RNA was isolated as previously described (Carabelli et al., 1993). Ten micrograms of total RNA was separated, blotted to supported nitrocellulose membranes (Hybond C-extra; Amersham, Brauschweigh, Germany), and hybridized according to Baima et al. (1995). The amount of ATHB-8 transcripts was quantitated by scanning the x-ray films with a laser densitometer (Ultroscan XL, LKB, Bromma, Sweden ) and normalized to the amount of total RNA by transcript quantification of the nuclear gene encoding the β-subunit of the mitochondrial ATPase, a gene that is known to be constitutively expressed (Carabelli et al., 1993).
Footnotes
This research was supported in part by the European Union Biotechnology Program (contract no. BIO4–CT960217 to G.M.), by the Consiglio Nazionale delle Ricerche Target Project on Biotechnology (to G.M. and I.R.), and by the Ministero dell Università e della Ricerca Scientifica e Tecnologica-Consiglio Nazionale delle Ricerche Strategic Project on Biotechnology (to G.M. and I.R.). S.B. was the recipient of a European Molecular Biology Organization short-term fellowship.
LITERATURE CITED
- Aloni R. Differentiation of vascular tissues. Annu Rev Plant Physiol. 1987;38:179–204. [Google Scholar]
- Altamura MM, Possenti M, Matteucci A, Baima S, Ruberti I, Morelli G (2001) The development of the vascular system in the Arabidopsis floral stem. New Phytol (in press) [DOI] [PMC free article] [PubMed]
- Aoyama T, Dong C-H, Wu Y, Carabelli M, Sessa G, Ruberti I, Morelli G, Chua N-H. Ectopic expression of the Arabidopsis transcriptional activator ATHB-1 alters leaf cell fate in tobacco. Plant Cell. 1995;7:1773–1785. doi: 10.1105/tpc.7.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G. The expression of the ATHB-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;121:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- Baima S, Tomassi M, Matteucci A, Altamura MM, Ruberti I, Morelli G. Role of the ATHB-8 gene in xylem formation. In: Savidge R, Barnett J, Napier R, editors. Cambium: The Biology of Wood Formation. Oxford: βIOS Scientific Publishers LTD; 2000. pp. 445–455. [Google Scholar]
- Baumann E, Lewald J, Saedler H, Schulz B, Wisman E. Successful PCR-based reverse genetic screens using an En-1 mutagenised Arabidopsis thaliana population generated via single-seed descent. Theor Appl Genet. 1998;97:729–734. [Google Scholar]
- Bechtold N, Ellis JE, Pellettier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris. 1993;316:1194–1199. [Google Scholar]
- Berleth T, Sachs T. Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Sci. 2001;4:57–62. doi: 10.1016/s1369-5266(00)00136-9. [DOI] [PubMed] [Google Scholar]
- Carabelli M, Sessa G, Baima S, Morelli G, Ruberti I. The Arabidopsis ATHB-2 and -4 genes are strongly induced by far-red-rich light. Plant J. 1993;4:469–479. doi: 10.1046/j.1365-313x.1993.04030469.x. [DOI] [PubMed] [Google Scholar]
- Dengler N, Kang J. Vascular patterning and leaf shape. Curr Opin Plant Sci. 2001;4:50–56. doi: 10.1016/s1369-5266(00)00135-7. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Ennos AR. Wind as an ecological factor. Trends Ecol Evol. 1997;12:108–111. doi: 10.1016/s0169-5347(96)10066-5. [DOI] [PubMed] [Google Scholar]
- Esau K. Plant Anatomy. Ed 2. New York: John Wiley & Sons; 1965. [Google Scholar]
- Gälweiler L, Changui G, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Hofte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;2:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Sistrunk ML, Polisensky DH, Braam J. Arabidopsis thaliana responses to mechanical stimulation do not require ETR1 or EIN2. Plant Physiol. 1998;116:643–649. doi: 10.1104/pp.116.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper E, Grevelding C, Schell J, Masterson R. Improved method for the transformation of Arabidopsis thaliana with chimeric dihydrofolate reductase constructs which confer methotrexate resistence. Plant Cell Rep. 1992;11:118–121. doi: 10.1007/BF00232162. [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publisher; 1995. pp. 509–530. [Google Scholar]
- Mahonen AR, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. A novel two-component hybrid molecule regulates vascular morphogenesis of the vascular root. Genes Dev. 2000;14:2938–2943. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CA. Recent advances in plant response to mechanical stress: theory and application. Hortscience. 1996;31:31–35. [PubMed] [Google Scholar]
- Otsuga D, DeGuzman B, Prigge MJ, Drews G, Clark SE. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001;25:223–236. doi: 10.1046/j.1365-313x.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Riechmann JL, Zhang JZ. INTERFASCICULAR FIBERLESS1 is the same gene as REVOLUTA. Plant Cell. 2000;12:315–317. doi: 10.1105/tpc.12.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Adv Bot Res. 1981;9:151–262. [Google Scholar]
- Sachs T. Cell polarity and tissue patterning in plants. Development Suppl. 1991;1:83–93. [Google Scholar]
- Sachs T. Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol. 2000;41:649–656. doi: 10.1093/pcp/41.6.649. [DOI] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Hauser M-T, Janmaat K, Weisbeek P, Benfey PN. Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995;121:53–62. [Google Scholar]
- Sessa G, Carabelli M, Ruberti I, Lucchetti S, Baima S, Morelli G. Identification of distinct families of HD-ZIP proteins in Arabidopsis thaliana. In: Puigdomenech P, Coruzzi G, editors. Molecular-Genetic Analysis of Plant Development and Metabolism. Berlin: Springer; 1994. pp. 411–426. [Google Scholar]
- Sessa G, Steindler C, Morelli G, Ruberti I. The Arabidopsis ATHB-8, -9 and -14 genes are members of a small gene family coding highly related HD-Zip proteins. Plant Mol Biol. 1998;38:609–622. doi: 10.1023/a:1006016319613. [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM. Patterns in Plant Development. Ed 2. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- Talbert PB, Adler HT, Parks DW, Comai L. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development. 1995;121:2723–2735. doi: 10.1242/dev.121.9.2723. [DOI] [PubMed] [Google Scholar]
- Wisman E, Hartman U, Sagasser M, Baumann E, Palme K, Hahlabrock K, Saedler H, Weisshaar B. Knock-out mutants from an En-1 mutagenized Arabidopsis thaliana population generate phenylpropanoid biosynthesis phenotypes. Proc Natl Acad Sci USA. 1998;95:12432–12437. doi: 10.1073/pnas.95.21.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Taylor JJ, Ye ZH. Disruption of interfascicular fiber differentiation in an Arabidopsis mutant. Plant Cell. 1997;9:2159–2170. doi: 10.1105/tpc.9.12.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye ZH. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucin zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]