Abstract
A novel homologue of insect defensin designated lucifensin (Lucilia defensin) was purified from the extracts of various tissues (gut, salivary glands, fat body, haemolymph) of green bottle fly (Lucilia sericata) larvae and from their excretions/secretions. The primary sequence of this peptide of 40 residues and three intramolecular disulfide bridges was determined by ESI-QTOF mass spectrometry and Edman degradation and is very similar to that of sapecin and other dipteran defensins. We assume that lucifensin is the key antimicrobial component that protects the maggots when they are exposed to the highly infectious environment of a wound during the medicinal process known as maggot therapy. We also believe that lucifensin is that long-sought larger molecular weight antimicrobial factor of the Lucilia sericata excretions/secretions believed to be effective against pathogenic elements of the wound microbial flora.
Keywords: Antimicrobial peptide, Insect defensin, Maggot therapy, Lucilia sericata
Introduction
Larvae of the green bottle fly (Lucilia sericata Meigen, 1826) are increasingly used as a fast and effective treatment of necrotic wounds where conventional treatments have failed [1]. The application of sterile larvae to an infected non-healing wound results in the removal of necrotic tissue (debridement), disinfection, rapid elimination of infecting microorganisms including methicillin-resistant Staphylococcus aureus (MRSA) and enhancement of the healing process [1, 2]. It has been suggested that the antimicrobial action results from both larval ingestion of wound bacteria [3], which are killed as they pass through the larvae digestive tracts [4], and by antimicrobial activity of larvae components, including salivary gland secretions and faecal waste products [5]. Not surprisingly, many attempts have been made to isolate and identify in the excretion/secretion (ES) antimicrobial agents responsible for suppressing MRSA and other bacteria in infected wounds. In the last decade, several reports have described the presence of three categories of antibacterial factors in the ES of maggots, one category with a molecular mass <0.5 kDa and the other two with molecular masses of 0.5–10 and >10 kDa, respectively [5–8]. The exposure of maggots to the infectious environment of a wound activates their innate defence system, thus resulting in the synthesis of antibacterial peptides and their release into the haemolymph [9]. An important class of these compounds are medium-sized cationic peptides composed of 36–40 amino acid residues that belong to a large group of insect defensins [10]. Defensins of the dipteran species, such as sapecin isolated from the culture medium of the embryonic cell line of the flesh fly Sarcophaga peregrina [11], and two so-called insect defensins isolated from the haemolymph of immunised larvae of the blowfly Phormia terraenovae, are more effective against Gram-positive bacteria than against Gram-negative bacteria [12, 13]. They all are positively charged peptides consisting of 40 amino acid residues, containing three disulphide bridges, and differing from one another by only one amino acid residue [12]. The results of previous investigations indicate that one of the antimicrobial factors detected in the ES of L. sericata larvae possesses several characteristics consistent with the aforementioned dipteran defensins [1].
In this study, we report the sequence determination of a novel defensin, named lucifensin, which we first isolated from the gut of L. sericata larvae. This antimicrobial peptide differs from sapecin and the P. terraenovae defensins by five amino acid residues. In addition, we confirmed the presence of lucifensin in other larval organs, namely the salivary glands, fat body and haemolymph. We also detected its presence in the ES of non-immunised larvae and in washes of maggots removed from the wound of a diabetic patient. Our finding strongly supports the idea that lucifensin is one of the antimicrobial factors from L. sericata ES that have recently been the subject of several attempts at chemical characterisation by other researchers [7].
Materials and methods
Breeding of L. sericata larvae
Larvae of the green bottle fly (L. sericata) hatched from non-sterile eggs were reared in batches of approximately 500 specimens on beef liver in small, open disposable packets made from aluminium foil. At 24 ± 1°C the larvae reach the third (final) instar 3 days after hatching, feed for another 2 days, and then spontaneously leave the packets with food and pupariate in dry sawdust after two more days. Larvae in the middle of the third instar (approximately 4 days after hatching) that were feeding and that had crops still full of ingested food were used for all dissections, as well as haemolymph and ES collections. The culture of L. sericata was initiated with larvae obtained from a hospital after removal from a patient’s wound and was maintained in the laboratory for 2 years.
Dissections
Before dissections of the gut (midgut and hindgut), salivary glands and fat body, the larvae were thoroughly washed in water and placed on ice for >10 min. The tip of the body with the mouth hooks of chilled immobile larvae was cut off with scissors and the rest of the body squeezed between the fingers to expel all internal organs into insect phosphate buffered saline (PBS; Sigma-Aldrich). Individual tissues or organs were then separated, washed clean in PBS and transferred to an ice cold acetonitrile–water (1:1) mixture containing 0.5% trifluoroacetic acid (TFA) for further processing.
Collection of larval haemolymph
Chilled larvae (ca. 80 pieces) were punctured with a fine non-sterile pin in the front segment and gently squeezed to allow the haemolymph to leak out. Individual drops of clean haemolymph were first collected on a parafilm sheet placed on ice to make sure that no visible fragments of other tissue (e.g. fat body) were present. Then the haemolymph was transferred with a pipette to a vial with an equal volume of a cold acetonitrile–water (1:1) mixture containing 0.5% TFA (1.5 mL). The mixture was centrifuged at 15,000g for 15 min to remove the blood cells. The supernatant containing the plasma was used for further processing.
Peptide purification from the larval guts
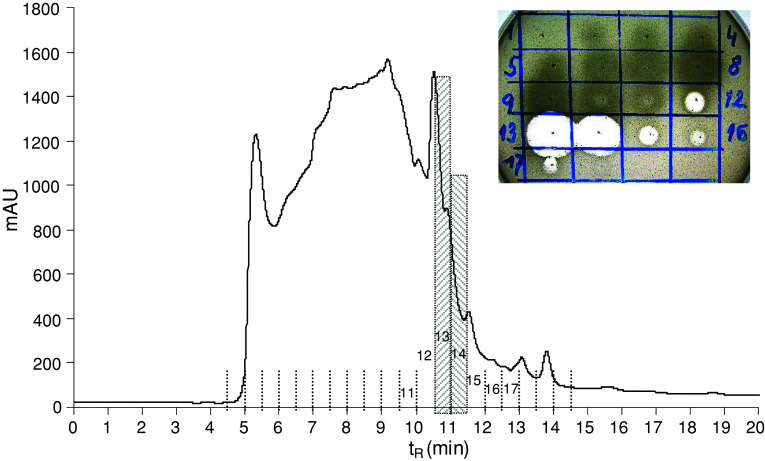
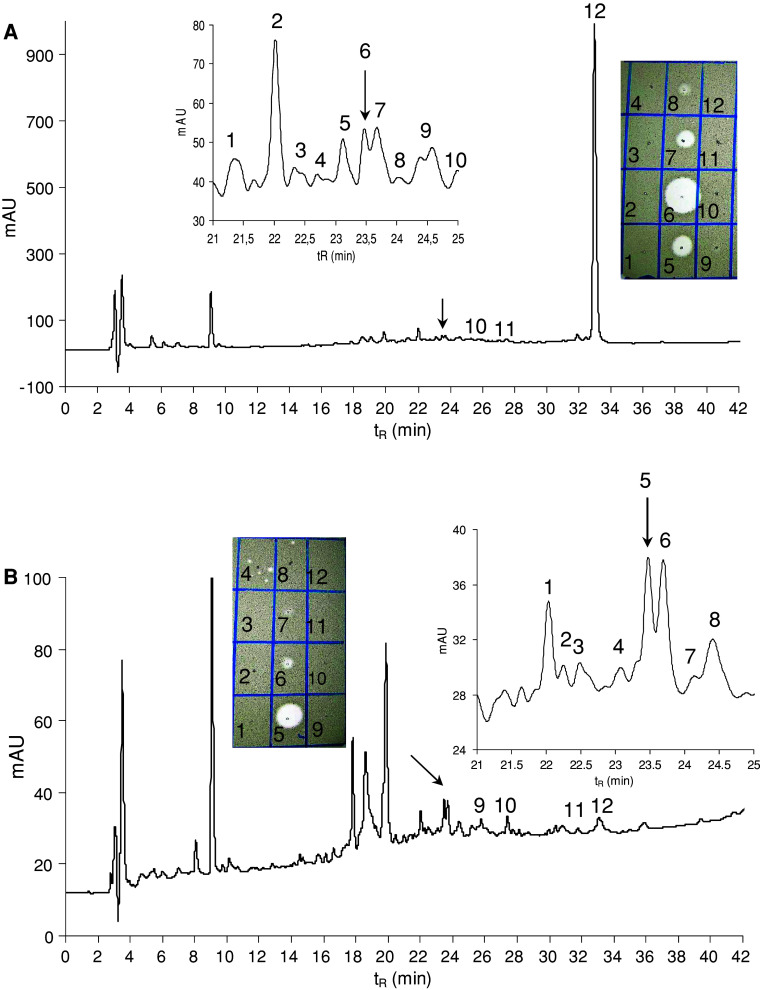
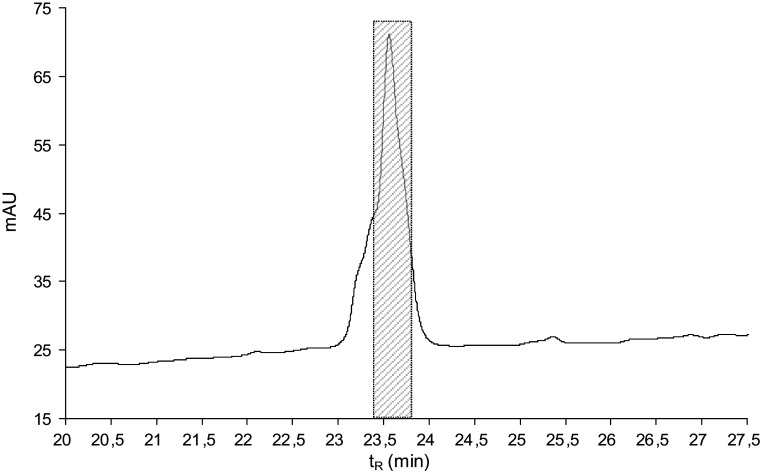
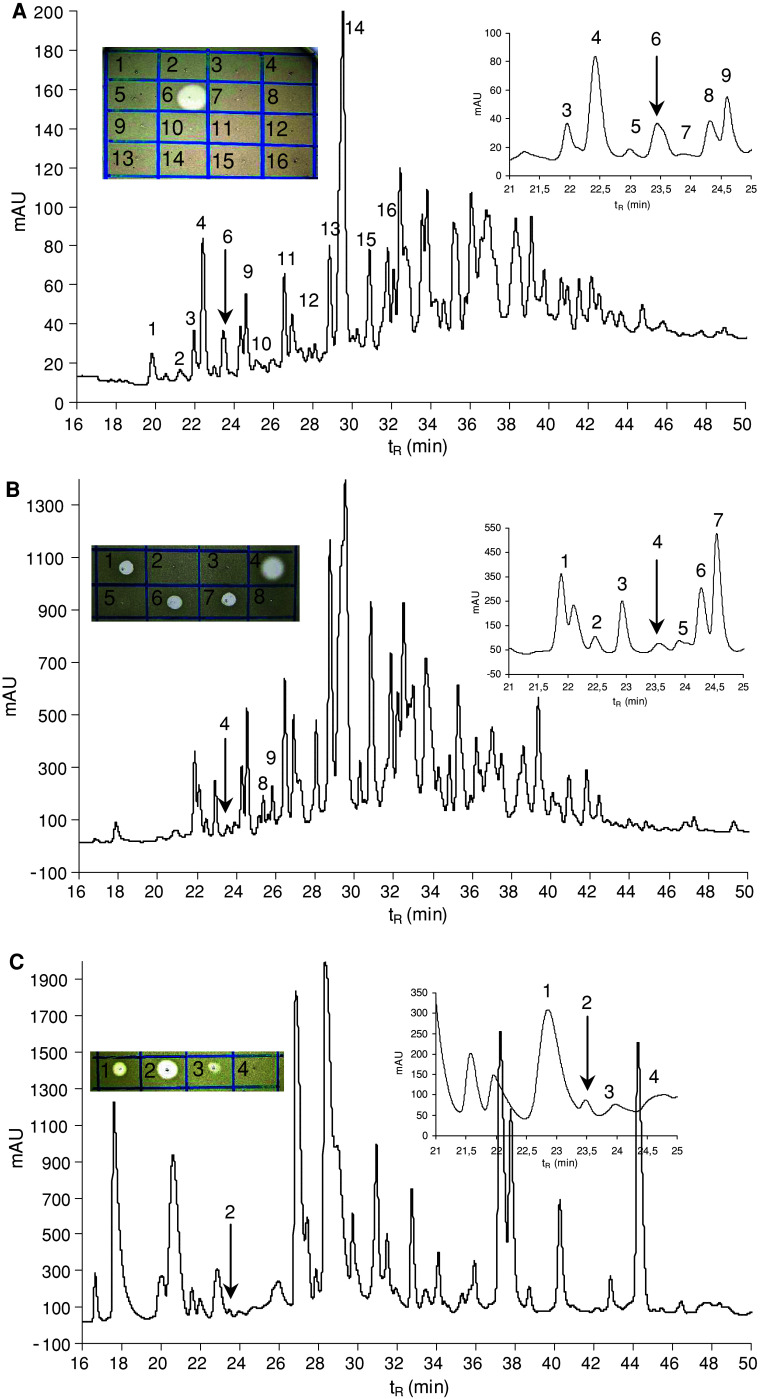
The guts (ca. 200 pieces) were extracted with 1 mL of acetonitrile–water (1:1) mixture containing 0.5% TFA, the extract was centrifuged, and the supernatant was diluted with 0.1% TFA to a 5 mL total volume. The solvent was fourfold ultrafiltered using an Amicon Ultra-15 centrifugal filter device with a 10 kDa molecular weight cut-off membrane at 5°C. The retenate was lyophilised, resulting in 6.2 mg of white lyophilisate exhibiting antimicrobial activity against Micrococcus luteus. The material was dissolved in 100 μL of 0.1 M ammonium acetate buffer, pH 6.2, and fractioned in two consecutive runs by size exclusion high-performance liquid chromatography (HPLC) on a Bio-Sil SEC-125, 300 × 7.8 mm, 5 μm column, (Bio-Rad, Hercules, CA, USA) at a flow rate of 1 mL/min using 0.1 M ammonium acetate buffer, pH 6.2, as a mobile phase (Fig. 1). The fractions were collected and evaporated in a Speed-Vac. The anti-M. luteus activity was detected in fractions 12–17 with the maxima in fractions 13 and 14 (Fig. 1). Fractions 13 and 14 were separately subjected to reversed phase HPLC (RP-HPLC) on a Vydac C-18, 250 × 4.6 mm, 5 μm column (Grace Vydac, Hesperia, CA, USA) at 1 mL/min flow rate using a solvent gradient ranging from 5 to 70% acetonitrile/water/0.1% TFA over 60 min (Fig. 2). The material of all peaks detected at 220 nm was collected, evaporated in the Speed-Vac and tested for the presence of antimicrobial activity. The majority of the activity was detected in the peaks of t R = 23.5 min. The active materials corresponding to these peaks were combined and re-purified by RP-HPLC under the same conditions (Fig. 3). The material in the mid-section of the peak (Fig. 3) was subjected to Edman degradation and analysed by ESI-QTOF mass spectrometry (Fig. 4a).
Fig. 1.
Size exclusion HPLC profile of lyophilised (3.1 mg) retenate obtained by ultrafiltration of larval gut crude extract at 220 nm on a Bio-Sil (300 × 7.8 mm, 5 μm) column. Isocratic elution at a flow rate of 1 mL/min and 0.1 M ammonium acetate buffer, pH 6.2, as a mobile phase were used. Inset Anti-M. luteus activity (clear zones in the drop diffusion test) of individual fractions delineated in the profile
Fig. 2.
RP-HPLC profiles of the anti-Micrococcus luteus active materials obtained from the previous purification step (size exclusion HPLC) at 220 nm. a Fraction 13, b fraction 14 of Fig. 1. An elution gradient of solvents from 5 to 70% acetonitrile/water/0.1% TFA was applied for 60 min at a flow rate of 1 mL/min. Arrows indicate the anti-M. luteus active peaks containing lucifensin. MS analysis (molecular mass 13,824 Da) and the partial N-terminal Edman degradation of the material isolated as the most prominent peak (peak 12) eluted at 33 min indicate that this may be the insect lysozyme. However, this material was inactive against M. luteus under the tested conditions. Inset Anti-M. luteus activity (clear zones in the drop diffusion test) of selected peaks delineated in the profile
Fig. 3.
RP-HPLC re-purification of the combined anti-Micrococcus luteus active fractions obtained from the previous purification step (RP-HPLC, Fig. 2, arrows). An elution gradient of solvents from 5 to 70% acetonitrile/water/0.1% TFA was applied for 60 min at a flow rate of 1 mL/min. Detection at 220 nm
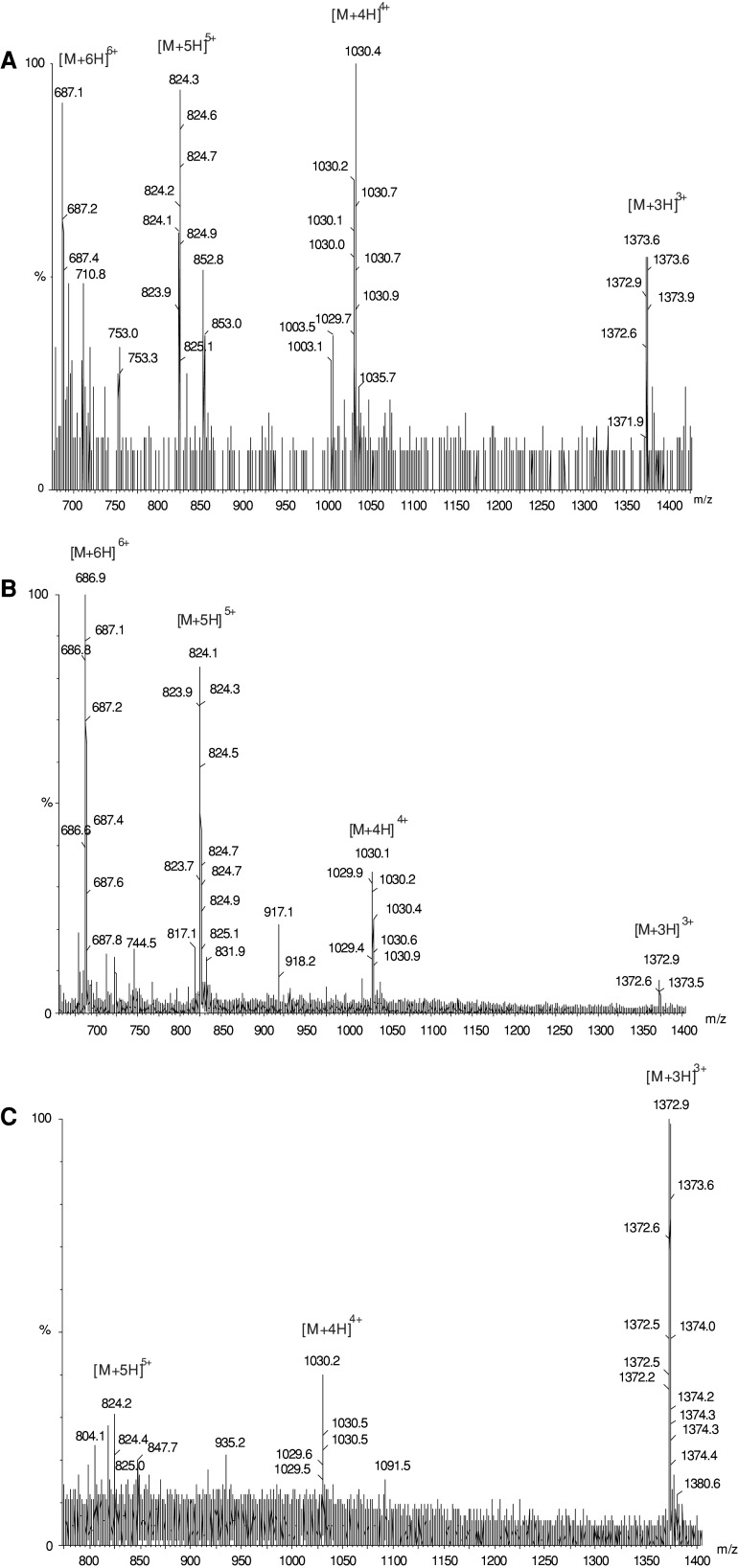
Fig. 4.
ESI-QTOF mass spectra of various Lucilia sericata defensin (lucifensin) samples obtained by purifications from the larval gut extract (a), haemolymph (b) and from the larval ES (c)
Detection of lucifensin in the salivary glands, fat body and haemolymph
The salivary glands (ca. 180 pieces), fat body (ca. 180 pieces) and plasma of the haemolymph (ca. 1.5 mL) were extracted and the extract ultrafiltered as described above. The anti-M. luteus active retenates obtained as white lyophilisates (0.9, 1.2 and 1.4 mg from the salivary glands, fat body and haemolymph respectively) were fractioned by single RP-HPLC under the conditions described above (Fig. 5). In all three instances, anti-M. luteus activity was detected in the peaks of t R = 23.5 min. In the case of fat body, the presence of antimicrobial activity was detected also in the adjacent fractions. In addition to the antimicrobial activity detection, the presence of lucifensin in the peaks of t R = 23.5 min was verified by mass spectrometry (MS). The same procedure was applied for the lucifensin detection in haemolymph (Fig. 5c). In this case, the material corresponding to the peak of t R = 23.5 min was re-purified by RP-HPLC and then analysed by MS (Fig. 4b).
Fig. 5.
RP-HPLC profiles of the lyophilised retenates obtained by ultrafiltration of crude extracts from three different larval tissues at 220 nm. a Salivary glands, b fat body, c haemolymph. An elution gradient of solvents from 5 to 70% acetonitrile/water/0.1% TFA was applied for 60 min at a flow rate of 1 mL/min. Arrows indicate the anti-Micrococcus luteus active peaks containing lucifensin. Inset Anti-M. luteus activity (clear zones in the drop diffusion test) of the fractions containing lucifensin and other surrounding fractions delineated in the profile. The other peaks not numbered in the chart were collected as larger fractions at consecutive time intervals. Those fractions did not show any anti-M. luteus activity
Detection of lucifensin in the larval ES
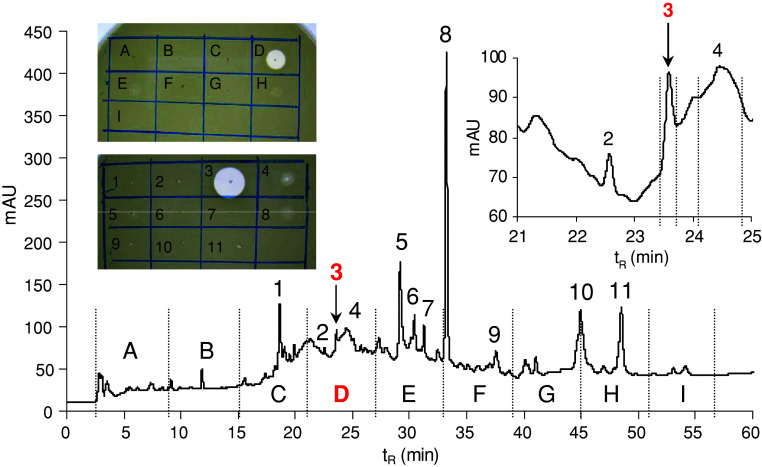
Approximately 300 pieces of feeding larvae of the mid-third instar were removed from liver, thoroughly washed three times with tap water and once with distilled water, then placed into a 250 mL Erlenmeyer flask with 5 mL of distilled water adjusted to pH 3 with acetic acid. Care was taken that the larvae remained immersed in the liquid at the bottom of the flask. After 20 min the liquid was decanted and replaced with a fresh one for another 20 min interval. Both washes of the larval ES were pooled and centrifuged at 15,000g for 5 min to remove all the debris before further processing. The supernatant was fourfold ultrafiltered (10 kDa cut-off membrane) and the retenate lyophilised. This anti-M. luteus active lyophilisate (3.3 mg) was repeatedly (six runs) fractionated by RP-HPLC under the same conditions as above. Figure 6 shows a typical HPLC profile indicating the presence of antimicrobial activity only in the peak of t R = 23.5 min among the other HPLC dominant peaks. The presence of lucifensin in combined anti-M. luteus active material of the peaks t R = 23.5 was verified by MS (Fig. 4c).
Fig. 6.
RP-HPLC profile of the lyophilised retenate obtained by ultrafiltration of the larval ES at 220 nm. An elution gradient of solvents from 5 to 70% acetonitrile/water/0.1% TFA was applied for 60 min at a flow rate of 1 mL/min. Arrows indicate the anti-Micrococcus luteus active peak containing lucifensin. Inset Anti-M. luteus activity (clear zones in the drop diffusion test) of individual fractions and selected peaks delineated in the profile
Detection of lucifensin in maggots removed from a wound
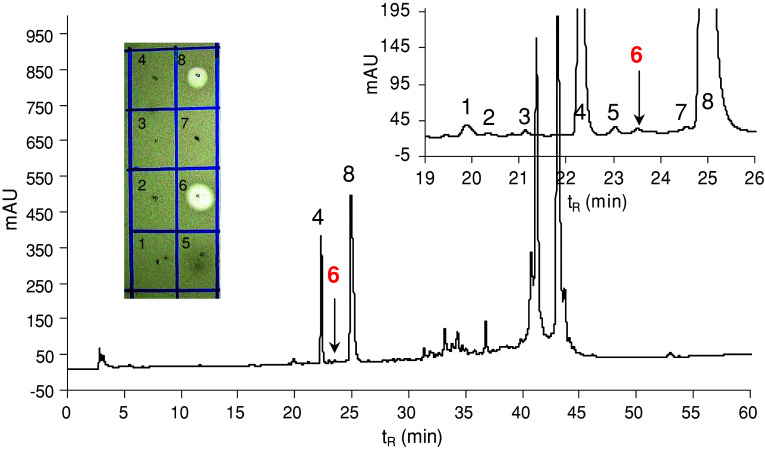
Maggots (45 larvae) removed from the neuroischaemic foot ulcer (Fig. 7) of a female diabetic patient were immediately placed in 5 mL of an acetonitrile–water (1:1) mixture containing 0.5% TFA. After 1 h, the solvent was decanted, centrifuged, and the supernatant fourfold ultrafiltered (10 kDa cut-off membrane) as described above. The retenate was lyophilised (5.4 mg), dissolved in 0.1% TFA, then ultrafiltered once again using an Amicon Ultra-15 centrifugal filter device with a 30 kDa molecular weight cut-off membrane in several consecutive runs. The filtrate was lyophilised (0.4 mg) and fractioned by RP-HPLC as described above (Fig. 8). The antimicrobial activity was detected in the peaks labelled as 6 (t R = 23.5) and 8 (t R = 24.9 min).
Fig. 7.
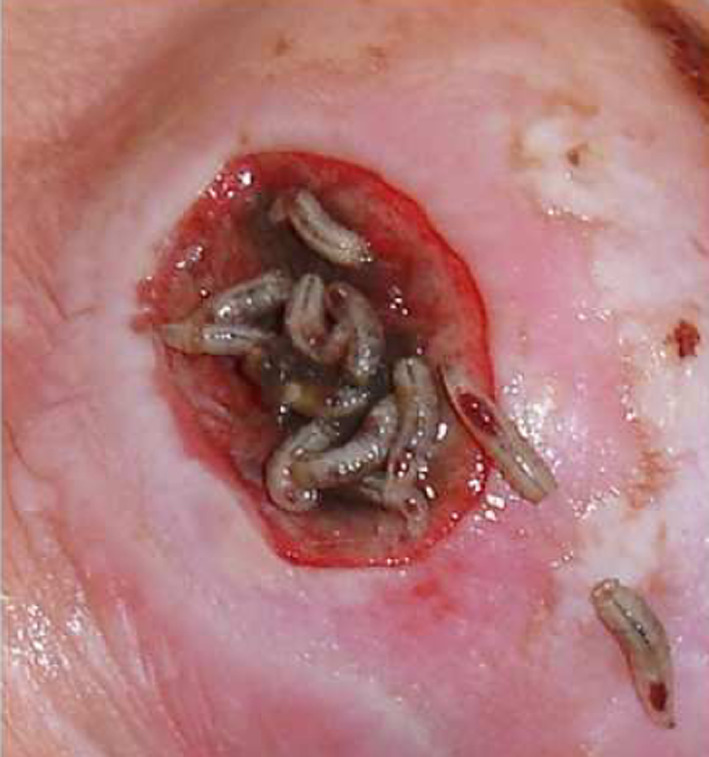
Left toe neuroischaemic foot ulcer of female diabetic patient at the time of larvae removal
Fig. 8.
RP-HPLC profile (at 220 nm) of the lyophilised filtrate (0.4 mg) obtained by ultrafiltration through a 30 kDa cut-off membrane (after the ultrafiltration through a 10 kDa cut-off membrane) of the wash of larvae after removal from the wound. An elution gradient of solvents from 5 to 70% acetonitrile/water/0.1% TFA was applied for 60 min at a flow rate of 1 mL/min. Arrows indicate the anti-Micrococcus luteus active peaks containing lucifensin (peak 6) and mixture of two human α-defensins HNP1 and HNP2 (peak 8). Inset Anti-M. luteus activity (clear zones in the drop diffusion test) of selected peaks delineated in the profile
High-performance liquid chromatography
Size exclusion chromatography and RP-HPLC were carried out on an Agilent Technologies 1200 Series module equipped with a diode-array detector. The elution was monitored by absorption at 220, 254 and 280 nm. The instrument was controlled and UV spectra evaluated using ChemStation Software.
Peptide sequencing by Edman degradation
The N-terminal amino acid sequence was determined on the Procise Protein Sequencing System (491 Protein Sequencer; PE Applied Biosystems, Foster City, CA, USA) using the manufacturer’s pulse-liquid Edman degradation chemistry cycles.
Mass spectrometry
Mass spectra of the peptide were recorded using a Micromass Q-Tof micro mass spectrometer (Waters) equipped with an electrospray ion source. A mixture of acetonitrile and water (1:1) containing 0.1% formic acid was continuously delivered to the ion source at a flow rate of 20 μL/min. Samples dissolved in the mobile phase were introduced using a 2-μL loop. Capillary voltage, cone voltage, desolvation temperature and source temperature were 3.5 kV, 20 V, 150 and 90°C, respectively.
Determination of antimicrobial activity
A simple qualitative estimate of antimicrobial properties was undertaken using the drop diffusion test on Petri dishes by the double-layer technique. The Petri dishes (90 mm in diameter) contained 20 mL of Luria–Bertani (LB) agar (Sigma). Over the surface we poured melted “soft” agar (2 mL), prepared from LB broth (Sigma) and 0.5% agar (Difco) and then mixed with M. luteus (ca. 107 colony forming units). Fresh bacterial cultures were always prepared in the LB broth and added when the melted soft agar cooled to about 45°C. Antimicrobial activity-containing materials (evaporated HPLC fractions) were diluted in water (10 μL) and dropped (2 μL) onto the surface of the solidified upper layer. The plates were incubated at 37°C. Clear zones of inhibition appeared within a few hours and remained clear for days. The potency was semi-quantitatively estimated by the diameter and clarity of the zones of inhibition.
Results
Purification of the lucifensin for primary structure determination
The physicochemical properties of insect defensins (medium sized, cationic, disulfide bridges containing peptides) influenced us in the selection of effective purification methods. The strongly acidic acetonitrile/water/0.5% TFA mixture as an extraction solvent provided good solubility of a cationic peptide while protecting its stability against enzymatic digestion and disulfide bridge reshuffling. Successive ultrafiltration of crude extracts through 10 kDa molecular weight cut-off membrane resulted in massive removal of sticky low molecular weight junk material, yielding the retenate as a mixture of high molecular weight compounds exhibiting antimicrobial activity against Gram-positive bacteria. Surprisingly, 4 kDa molecular weight lucifensin and even some other peptides of lower molecular masses were always retained in the retenate. For the primary sequence determination, lucifensin was purified from the larval gut using size exclusion HPLC chromatography on a Bio-Sil column as the second purification step. This facilitated substantial removal of high molecular weight compounds, as shown in Fig. 1. Most of the antimicrobial activity was detected in two relatively narrow fractions (hatch-marked area in Fig. 1), which were further purified by RP-HPLC (Fig. 2) with monitoring at 220 nm. The maximum anti-M. luteus activity in both fractions was surprisingly detected in very tiny peaks at t R = 23.5 min. Combining the material from two consecutive RP-HPLC runs gave a peptide, which after another RP-HPLC re-purification (Fig. 3), was subjected to Edman degradation.
Primary structure determination
When the sample was sequenced by Edman degradation using 40 cycles, it yielded the following N-terminal sequence: Ala-Thr-X-Asp-Leu-Leu-Ser-Gly-Thr-Gly-Val-Lys-His-Ser-Ala-X-Ala-Ala-His-X-Leu-Leu-Arg-Gly-Asn-Arg-Gly-Gly-Tyr-X-Asn-Gly-Arg-Ala-Ile-X-Val-X-Arg-Asn, assuming that all six undetermined amino acid residues (X) were cysteine. The molecular mass of lucifensin measured by ESI-QTOF MS was manually calculated from the m/z values of multiply (3×, 4×, 5× and 6×) charged molecular ions found in the mass spectra (Fig. 4a), resulting in a monoisotopic molecular mass of 4,113.6. This is in good agreement with the calculated value of 4,113.89, based on the sequence determined by Edman degradation and assuming that the six cysteine residues form three disulfide bridges. Our results show that lucifensin differs from Phormia terranovae defensins A and B and from Sarcophaga peregrina sapecin by five amino acid residues (Val11, Lys12, Arg33, Ala34, and ILe35). The sequences of some other dipteran defensins with their molecular masses (monoisotopic) are shown in Scheme 1.
Scheme 1.
Sequence comparison of the lucifensin (Lucilia sericata defensin) with defensins from other cyclorrhaphous dipteran species. [a] calculated monoisotopic molecular masses of defensins
Detection of lucifensin in the salivary glands, fat body and haemolymph
In a search for the occurrence of lucifensin in other larval tissues, the purification procedure was simplified by omitting the size exclusion chromatography step. Based on the knowledge of lucifensin HPLC retention time, we could use relatively small numbers of larval organs in order to obtain an anti-M. luteus HPLC active fraction that eluted at t R = 23.5 min and confirm the presence of lucifensin in it by mass spectrometry. The quantity of about 1 mg of retenates obtained by the ultrafiltration of crude tissue extract was loaded on the RP-HPLC column. Figure 5 shows the RP-HPLC profiles of the ultrafiltered samples (retenates) of two larval tissue extracts and the plasma of the haemolymph indicating the presence of lucifensin in the peak of t R = 23.5 min (arrow). In each case, the presence of lucifensin in that peak was confirmed by the detection of multiply charged molecular ions in the mass spectra derived from its molecular mass. Figure 4b shows as an example the mass spectrum of the re-purified peptide isolated from the haemolymph in the peak t R = 23.5 min (Fig. 5c).
Detection of lucifensin in larval ES
Acidified ES obtained from approximately 300 larvae was ultrafiltered and the lyophilised retenate (3.3 mg) was repeatedly fractioned by RP-HPLC. In the first HPLC run (Fig. 6), nine 6 mL volume fractions were collected for antimicrobial activity determination. Almost all anti-M. luteus activity was contained in fraction D with retention time 21–27 min. In the second run the material of only selected peaks was collected for the activity test, which clearly showed that anti-M. luteus activity is concentrated in the third peak at t R = 23.5 min (Fig. 6). Materials contained in the 23.5 min peaks from six HPLC runs were combined, and the presence of lucifensin therein was proved by MS (Fig. 4c). The results of our experiments indicate that lucifensin (Lucilia defensin) is that antimicrobial factor of ES whose presence was already detected by other researchers as a factor with molecular weight 0.5–10 kDa [1, 7]. Until now, however, this factor has not been further characterised.
Detection of lucifensin in the maggots removed from the wound
The larvae used for this experiment were obtained from a neuroischaemic diabetic foot ulcer (Fig. 7) at the end of a 4-day maggot therapy. We expected that the washes of larvae contained lucifensin derived from their surface and probably from their ES released into the solvent during the transport from the hospital to the lab. RP-HPLC profile (not shown) of the high molecular weight fraction (retenate) obtained after ultrafiltration through a 10 kDa cut-off membrane showed mainly high molecular weight compounds originating from the wound exudate. The appearance of a very tiny peak at t R = 23.5 min could indicate the presence of a negligible amount of lucifensin. We assumed that another successive ultrafiltration of the lucifensin-containing material through a 30 kDa cut-off membrane would result in substantial enrichment of lucifensin in the filtrate and in the reduction of compounds originating from the wound exudate. However, the RP-HPLC profile of the filtrate (Fig. 8) showed that some undesired high molecular compounds were not removed by ultrafiltration through the 30 kDa cut-off membrane. Nevertheless, the presence of lucifensin in the very tiny peak number 6 (t R = 23.5 min) (Fig. 8) was proved by its remarkably selective anti-M. luteus activity among surrounding peaks (Fig. 8). On the other hand, the HPLC analysis (not shown) still showed the presence of lucifensin in the retenate (30 kDa cut-off membrane) when the peak t R = 23.5 min was tested for antimicrobial activity. As shown in Fig. 8, the anti-M. luteus activity was also detected in the large peak number 8. The MS and the partial N-terminal automatic Edman degradation analyses of peak 8 revealed the presence of two human α-defensins [14] HNP1 (3,439.2 Da found, 3,439.51 Da calc.) and HNP2 (3,368.1 Da found, 3,368.47 Da calc.) in that material. These two host defence peptides were apparently produced and released by the components of the host immune system, including some blood cells (neutrophils) (Fig. 7) as the innate immune response against infection. Even though for the antimicrobial test the material of peak 8 was diluted four times against the material of peak 6, the anti-M. luteus activity of the very tiny peak 6 was more intense than the activity of peak 8 (Fig. 8).
Discussion
It is generally accepted that the larval ES plays an important role in wound disinfection during the maggot therapy process. Already long ago it was shown that the larval secretions of L. sericata contain a variety of alkaline compounds including ammonium carbonate, calcium, allantoin and urea that inhibit bacterial growth [2]. More recently, other bacteriostatic low molecular weight organic compounds were identified in the ES of maggots [7, 8]. It also has been proposed that the larvae of L. sericata release antimicrobial substances into the wound as part of their innate response to the infection [15], and a number of septic injury-inducible genes were identified in medicinal maggots [16]. Such antimicrobial components have been isolated in vitro from larval ES by several research groups. They report that these components have features in common with insect antimicrobial peptides belonging to the defensin group [1, 15] for which the generic term insect defensins was proposed some time ago [12]. It is surprising that structural characterisation of Lucilia defensin has not yet been reported, since the first insect defensins isolated from related dipteran species were entirely characterised two decades ago [11, 12, 17–20]. In this paper, we report for the first time the primary sequence of L. sericata defensin, for which we coined the name lucifensin. Our results suggest that lucifensin could be the long-sought larger molecular weight bactericide [1, 7] and the main component of effective antimicrobial activity contained in ES of L. sericata larvae that is apparently involved in controlling various populations of pathogenic bacteria (such as MRSA) during the larval therapy of refractory wounds [21]. The presence of lucifensin in the washes of larvae picked out from a treated wound supports this contention. In this case, when comparing the ratio of amounts of lucifensin to HNP1 and HNP2 with their antimicrobial activity ratio, the activity of lucifensin against Gram-positive M. luteus was seemingly higher than that of the mixture of the two human α-defensins (Fig. 8). To validate this observation, a higher quantity of lucifensin will be prepared synthetically for detailed antimicrobial testing in vitro using a larger set of pathogenic bacteria.
One of the reasons we succeeded in the isolation and complete identification of lucifensin’s primary structure from a relatively small amount of starting material (200–300 larvae) was the use of very sensitive M. luteus as the bioindicator in the antimicrobial drop diffusion assay. In our initial experiments that used B. subtilis as the antimicrobial activity indicator, the clear zones of the drop diffusion assay were several times less intense, which resulted in frequent omissions of lucifensin in HPLC fractions. Interestingly, the zone inhibition assay had not been effective in demonstrating the antimicrobial activity of L. sericata ES in another lab [7] when using S. aureus or E. coli. Even though the presence of lucifensin in tissue extracts or in ES was manifested in the HPLC profiles by the appearance of negligible peaks (Figs. 2, 5, 6, 8), we were able to determine its primary sequence completely starting with purification from the extract of just 200 gut pieces. By comparison, the haemolymph from 3,000 larvae was used to estimate the sequence of Phormia terranovae defensin [12].
The fact that the present study revealed the presence of lucifensin not only in the larval ES but also in the extracts of various tissues as well as in haemolymph indicates that the peptide is constitutively expressed as a part of the systemic immune mechanism and synthesized mainly in the fat body, a functional homologue of vertebrate liver. As shown in Fig. 5b, the presence of other compounds with antimicrobial activity other than lucifensin was detected in the fat body. Since no other antimicrobial compounds besides lucifensin were detectable in ES or other tissue extracts, we may speculate that these additional antimicrobial compounds are intrinsic metabolites of the fat body. The larva releases lucifensin as a part of its innate response to bacterial challenge, a mechanism already proposed for other insect defensins [10]. The question as to how lucifensin is excreted from the body (orally, anally or both ways) was not addressed by our study. However, the presence of the peptide in the salivary gland material does not rule out its presence in the regurgitated liquid for extracorporeal digestion.
It is generally presumed that the haemolymph and probably ES of sterile larvae would contain considerably less antimicrobial potency than those larvae challenged by microbes. In some laboratories, therefore, the larvae of dipteran species were immunised by bacteria in advance of the antimicrobial peptides isolation procedure [12, 22]. Larvae used in our experiments were not kept in a sterile environment. Exposure to the microbes in their food—the decaying beef liver—simulates to a certain extent the microenvironment of an infected wound, although the microbial populations in both situations can markedly differ. Moreover, in addition to being a potential health threat to larvae, the microbes are also a source of food for the larvae as well as competitors for available nutrients in natural situations. Therefore, the release of defensive molecules of the innate immune system can be also considered as an evolutionary adaptation of carnivorous fly larvae to life in discrete, highly competitive growth environments; using the external antibiotics, the larvae control the decaying process caused by their microbial food competitors. A common experience of fly keepers is that a decaying piece of meat or liver infested with maggots stinks far less than a similar piece of flesh left alone to microbial degradation. This serves as circumstantial evidence for this antimicrobial interaction of the blowfly larvae.
Insect lysozymes are proteins, approximately 14 kDa in size, produced mainly by the fat body and pericardial cells and secreted to the haemolymph in response to bacterial or fungal infection. To our surprise, the fraction containing the dominant peak of lysozyme in the HPLC profile of the gut extract showed no anti-M. luteus activity (Fig. 2A) whereas hen egg-white lysozyme was active in the parallel test. This observation is in accordance with the literature showing that fly lysozyme has an acidic pH optimum of lytic activity towards M. luteus and no enzymatic activity above pH 7 [23].
Practical considerations
Wound infections and sepsis cause an increasing risk of serious medical complications, which may result in considerable morbidity. Such wounds include ulcers caused by peripheral vascular disease, chronic infection, pressure sores and complications due to diabetes. In particular, diabetic foot syndrome and infection result in foot amputation in 15–25% of diabetic patients with foot ulcers [24]. In such cases where available topical antimicrobial therapy is of limited use, maggot therapy is a salvage option.
Bacterial resistance to conventional antibiotics is a major concern and the main reason for the research aimed at developing new therapeutics. The discovery of a new insect antimicrobial defensin—lucifensin—from the body tissues and ES of L. sericata larvae may have potential as an agent for new topical therapeutic applications in the treatment of serious surface wound infections. A promising aspect in the treatment of non-healing wounds could be the combination of conventional antibiotics with lucifensin and/or use of this antimicrobial peptide as a supportive means to bolster the healing effect of maggot therapy. In order to support this proposal, the production of a higher quantity of lucifensin through total chemical synthesis will be necessary to provide the material for further research. Such a synthesis via solid-phase peptide synthesis protocol is currently in progress at our laboratory.
Acknowledgments
This work was supported by Grant No. 203/08/0536 of the Czech Science Foundation and by Research Project No. Z40550506 of the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic. We thank our technical assistants Mrs. Jarmila Titzenthalerová and Mrs. Jitka Čermáková for help with the breeding the files and dissections of larval organs. We also thank Gale A. Kirking at English Editorial Services, s.r.o. for assistance with the English.
References
- 1.Kerridge A, Lappin-Scott H, Stevens JR. Antibacterial properties of larval secretions of the blowfly, Lucilia sericata . Med Vet Entomol. 2005;19:333–337. doi: 10.1111/j.1365-2915.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 2.Parnés A, Lagan KM. Larval therapy in wound management. A review . Int J Clin Pract. 2007;61:488–493. doi: 10.1111/j.1742-1241.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 3.Lerch K, Linde H-J, Lehn N, Grifka J. Bacteria ingestion by blowfly larvae: an in vitro study. Dermatology. 2003;207:362–366. doi: 10.1159/000074115. [DOI] [PubMed] [Google Scholar]
- 4.Mumcuoglu KY, Miller J, Mumcuoglu M, Friger M, Tarshis M. Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae) J Med Entomol. 2001;38:161–166. doi: 10.1603/0022-2585-38.2.161. [DOI] [PubMed] [Google Scholar]
- 5.Bexfield A, Bond AE, Roberts EC, Dudley E, Nigam Y, Thomas S, Newton RP, Ratcliffe NA. The antibacterial activity against MRSA strains and other bacteria of a <500 Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae) Microbes Infect. 2008;10:325–333. doi: 10.1016/j.micinf.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Thomas S, Andrews AM, Hay NP, Bourgoise S. The anti-microbial activity of maggot secretions: results of a preliminary study. J Tissue Viability. 1999;9:127–132. doi: 10.1016/s0965-206x(99)80032-1. [DOI] [PubMed] [Google Scholar]
- 7.Bexfield A, Nigam Y, Thomas S, Ratcliffe NA. Detection and partial characterization of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA) Microbes Infect. 2004;6:1297–1304. doi: 10.1016/j.micinf.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Huberman L, Gollop N, Mumcuoglu KY, Breuer E, Bhusare SR, Shai Y, Galun R. Antimicrobial substances of low molecular weight isolated from the blowfly, Lucilia sericata . Med Vet Entomol. 2007;21:127–131. doi: 10.1111/j.1365-2915.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann JA, Hetru C. Insect defensins: inducible antimicrobial peptides. Immunol Today. 1992;13:411–415. doi: 10.1016/0167-5699(92)90092-L. [DOI] [PubMed] [Google Scholar]
- 11.Matsuyama K, Natori S. Purification of three antibacterial proteins from the culture medium of NIH-Sape-4, an embryonic cell line of Sarcophaga peregrina . J Biol Chem. 1988;263:17112–17116. [PubMed] [Google Scholar]
- 12.Lambert J, Keppi E, Dimarcq J-L, Wicker C, Reichhart J-M, Dunbar B, Lepage P, Van Dorsselaer A, Hoffmann J, Forthergill J, Hoffmann D. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antimicrobial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc Natl Acad Sci USA. 1989;86:262–266. doi: 10.1073/pnas.86.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K, Natori S. Purification, sequence and antimicrobial activity of two novel sapecin homologues from Sarcophaga embryonic cells: similarity of sapecin B to charybdotoxin. Biochem J. 1993;291:275–279. doi: 10.1042/bj2910275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Ericksen B, Tucker K, Lubkowski J, Lu W. Synthesis and characterization of human α-defensins 4–6. J Peptide Res. 2004;64:118–125. doi: 10.1111/j.1399-3011.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 15.Nigam Y, Bexfield A, Thomas S, Ratcliffe NA. Maggot therapy: the science and implication for CAM. Part II-maggots combat infection. eCAM. 2006;3:303–308. doi: 10.1093/ecam/nel022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altincicek B, Vilcinskas A. Septic injury-inducible genes in medicinal maggots of the green blow fly Lucilia sericata . Insect Mol Biol. 2009;18:119–125. doi: 10.1111/j.1365-2583.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 17.Dimarcq J-L, Hoffmann D, Meister M, Bulet P, Lanot R, Reichhart J-M, Hoffmann JA. Characterization and transcriptional profiles of a Drosophila gene encoding an insect defensin. Eur J Biochem. 1994;221:201–209. doi: 10.1111/j.1432-1033.1994.tb18730.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanzawa H, Shimada I, Kuzuhara T, Komano H, Kohda D, Inagaki F, Natori S, Arata Y. 1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin. FEBS Lett. 1990;269:413–420. doi: 10.1016/0014-5793(90)81206-4. [DOI] [PubMed] [Google Scholar]
- 19.Lepage P, Bitsch F, Roecklin D, Keppi E, Dimarcq J-L, Reichhart J-M, Hoffmann JA, Roitsch C, Van Dorsselaer A. Determination of disulfide bridges in natural and recombinant insect defensin A. Eur J Biochem. 1991;196:735–742. doi: 10.1111/j.1432-1033.1991.tb15872.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim JI, Iwai H, Kurata S, Takahashi M, Masuda K, Shimada I, Natori S, Arata Y, Sato K. Synthesis and characterization of sapecin and sapecin B. FEBS Lett. 1994;342:189–192. doi: 10.1016/0014-5793(94)80498-2. [DOI] [PubMed] [Google Scholar]
- 21.Jaklič D, Lapanje A, Zupančič K, Smrke D, Grunde-Cimerman N. Selective antimicrobial activity of maggots against pathogenic bacteria. J Med Microbiol. 2008;57:617–625. doi: 10.1099/jmm.0.47515-0. [DOI] [PubMed] [Google Scholar]
- 22.Lauth X, Nesin A, Briand J-P, Roussel J-P, Hetru C. Isolation, characterization and chemical synthesis of a new insect defensin from Chironomus plumosus (Diptera) Insect Biochem Mol Biol. 1998;28:1059–1066. doi: 10.1016/S0965-1748(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Nakamura M, Hotani T, Imoto T. Insect lysozyme from house fly (Musca domestica) larvae: possible digestive function based on sequence and enzymatic properties. J Biochem. 1995;118:546–551. doi: 10.1093/oxfordjournals.jbchem.a124943. [DOI] [PubMed] [Google Scholar]
- 24.Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446–451. doi: 10.2337/diacare.26.2.446. [DOI] [PubMed] [Google Scholar]