Abstract
Over the past years, accumulating evidence has indicated that d-serine is the endogenous ligand for the glycine-modulatory binding site on the NR1 subunit of N-methyl-d-aspartate receptors in various brain areas. d-Serine is synthesized in glial cells and neurons by the pyridoxal-5′ phosphate-dependent enzyme serine racemase, and it is released upon activation of glutamate receptors. The cellular concentration of this novel messenger is regulated by both serine racemase isomerization and elimination reactions, as well as by its selective degradation catalyzed by the flavin adenine dinucleotide-containing flavoenzyme d-amino acid oxidase. Here, we present an overview of the current knowledge of the metabolism of d-serine in human brain at the molecular and cellular levels, with a specific emphasis on the brain localization and regulatory pathways of d-serine, serine racemase, and d-amino acid oxidase. Furthermore, we discuss how d-serine is involved with specific pathological conditions related to N-methyl-d-aspartate receptors over- or down-regulation.
Keywords: d-Serine, Gliotransmitter, Serine racemase, d-Amino acid oxidase, Neurotransmission, NMDA receptors
d-Serine as neuromodulator
Natural amino acids are utilized by mammalian brain and most of them are present in the l-form. The opposite isomers, the d-amino acids, were considered to be restricted to bacteria (e.g., as components of the cell wall of microorganisms): the discovery of d-serine in the brain at concentrations higher than many other l-amino acids has forced researchers to reconsider the view that only l-amino acids occur in mammals. It is now accepted that higher organisms produce d-amino acids (and in particular d-serine) by the enzyme serine racemase (SR), in both astrocytes and neurons [1, 2]. Notably, the relative roles of glial and neuronal d-serine has not yet been defined, and this in our opinion will require further investigations.
d-Serine is a “new” neuromodulator, playing a major role in the central nervous system (CNS). It has been localized to the protoplasmic astrocytes that wrap nerve terminals in areas rich in heterotetrameric N-methyl-d-aspartate receptors (NMDARs) [3], key receptors for excitatory transmission and cognitive functions [4]. d-serine is likely the main endogenous ligand for the strychnine-insensitive glycine-binding site (also referred to as the GlyB site) of NR1 subunit of NMDARs: activation of NMDARs requires glutamate and a coagonist and d-serine might be an alternative to glycine for NMDAR activation [5, 6]. d-serine release from astrocytes in culture is due to a specific activation of metabotropic and non-NMDA ionotropic glutamate receptors; glutamate is also released from glial cells following stimulation of metabotropic and non-NMDA glutamate receptors (for further details, see [7–10]). d-Serine is not restricted to glial cells: it was identified in neurons of the cerebral cortex, the forebrain, the brainstem, and the olfactory bulb [11–13].
Cellular uptake of extracellular d-serine is due to several low-affinity neutral amino acid transporters located in neurons and astrocytes, which are not only selective for d-serine and are Na+ and/or K+-dependent (the glial Na+-dependent transporter resembles the ASCT2 transporters) [7, 14, 15]. In addition, a faster Na+-independent alanine–serine–cysteine transporter 1 (Asc-1) is present on presynaptic terminals, dendrites, and soma of neurons, showing a high affinity for d-serine [15–18]. Further investigations are required to elucidate the role that this transporter might play in regulating synaptic d-serine level. Once d-serine is taken up into the neurons and astrocytes, the peroxisomal flavoenzyme d-amino acid oxidase (DAAO) degrades the neuromodulator. While this seems to be the case in the brainstem and cerebellum, a different mechanism might be active in the forebrain, as DAAO is virtually absent in this region [2]. In fact, histochemical analysis mapping of d-serine and DAAO localization in adult rat brain shows an intense presence of d-serine in the forebrain but a virtual absence in the brainstem and cerebellum, where, instead, DAAO shows the strongest staining (see below) [2, 19].
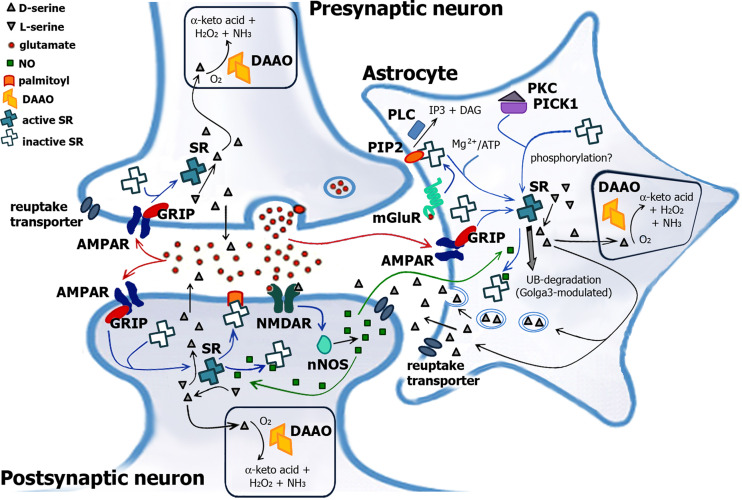
The current view of synaptic NMDAR function regulation by glial-neuronal interaction proposes that glutamate release from neurons stimulates AMPA/kainate receptors on glial cells [7–9], as well as on neurons [12], to release d-serine, which then acts as an endogenous coagonist of NMDAR in the presence of the agonist glutamate [20]. This binding enhances the flux of Ca2+ current through this ligand-gated channel, thus mediating diverse functions (Fig. 1): learning and memory, long-term changes in synaptic plasticity, and neural development [10, 21–23].
Fig. 1.
Model for SR’s and DAAO’s role in d-serine signaling in the brain. Glutamate released from presynaptic neuron acts on the postsynaptic neuron as well as on the surrounding astrocytes (red arrow), activating metabotropic glutamate receptors mGluR5 [and subsequent degradation of PIP2 to inositol triphosphate (IP 3) and diacylglycerol (DAG) by phospholipase C, PLC] and synthesizing d-serine from SR. Activation of glial SR presumably is due to an association with GRIP proteins and/or phosphorylation. Since GRIP-1 is expressed in neurons where the large majority of AMPAR are found, this mechanism might also activate neuronal SR. In the synaptic cleft, d-serine binds to the glycine-binding site on the NMDAR and in conjunction with glutamate results in the opening of the receptor channel. The activity of SR is switched off by different mechanisms: (1) Ca2+ entering the postsynaptic neuron activates neuronal NO synthase, producing NO, which can diffuse into adjacent cells. In astrocytes and neurons, NO gives nitrosylation of SR at Cys113, preventing ATP binding and thus inactivating the enzyme (green arrow). (2) The binding of SR to membrane PIP2 inhibits the enzyme according to a competitive mechanism with ATP. (3) SR is degraded through the ubiquitin-proteasomal system, a process which is modulated by Golga3 (gray arrow). Golga3 interacts with the N-terminal 66 residues of SR, decreasing the ubiquitin/proteasomal degradation of SR, probably interfering with binding of a still unidentified E3-ubiquitin ligase, and increases its steady-state levels (and d-serine synthesis)
The biochemistry of brain serine racemase
Mammalian serine racemase was isolated in 1999 from rodent brain by Snyder’s group [2]. It is a pyridoxal-5′ phosphate (PLP)-containing enzyme that catalyzes the racemization of l-serine to d-serine (Fig. 2a). Subsequently, SR has also been shown to catalyze α,β-elimination of water from l- or d-serine to yield pyruvate and ammonia (Fig. 2b) [24, 25]. Mouse SR (mSR) has a molecular mass of 36.3 kDa (319 amino acids), is a homodimer in solution, shows an activity optimum at pH 8.5 and 37°C, and is highly selective for serine [2]. A few months later, the same group reported on the molecular cloning of mSR cDNA [26]; a sequence comparison with known PLP-containing racemases identified K56 as the lysine residue that forms the Schiff base with PLP during catalysis (Fig. 2a) [26]. Furthermore, the authors demonstrated that SR distribution resembles that of endogenous d-serine, with the highest concentrations in the forebrain and negligible levels in brainstem, and that both SR and d-serine are enriched in astrocytes concentrated in the gray matter [26]. Studies on the recombinant mSR expressed in HEK293 cells [27] demonstrated that divalent cations and ADP/ATP were physiological cofactors of this enzyme [24, 28, 29]. In particular, the racemization and elimination reactions of l-serine as a substrate were activated 5- to tenfold by ≥10 μM MgCl2/CaCl2 and by ≥100 μM ATP. The racemase activity was independently stimulated by both Mg2+ and ATP: in fact, the nucleotide was able to increase SR activity even in the presence of EDTA, and the effect due to divalent ion and ATP was additive, suggesting a role for an Mg-ATP complex in activating SR [28]. It is noteworthy that Mg2+ and ATP modulate SR activity at concentrations well below those found in the cytosol (0.6 and 3–6 mM, respectively). In the presence of 1 mM ATP, the Km for l-serine is decreased ~tenfold with little change in V max [30]. Since ATP and ADP (as well as nonhydrolyzable analogues) are almost equally effective on SR activation, it was argued that its physiological role is not related to an energy requirement for enzyme activity but rather represents a means of lowering the K m for l-serine, reaching a value close to the cytosolic concentration of l-serine. Concerning the elimination activity, EDTA impairs the α,β-elimination reaction (while it does not affect protein stability), which is stimulated by ~100 μM Mg2+ in the absence of exogenous ATP or by 10 μM of divalent ion in the presence of the nucleotide [25, 28]. These results were also confirmed using the recombinant SR purified from bacteria [29]. In this paper, activation of SR by Ca2+ binding was also observed at a concentration two orders of magnitude higher than the resting value within the cell.
Fig. 2.
Reaction mechanism for serine racemization (a) and α,β-elimination (b) by SR [25]. PLP bound to SR through an internal aldimine with Lys56 reacts with l-serine yielding an external aldimine; α-proton abstraction from this intermediate gives a resonance-stabilized carbanion. This intermediate can follow two pathways: for racemization reaction (a), the reprotonation of the intermediate on the opposite face of the planar carbanion generates the external aldimine which releases d-serine via transimination with Lys56; for α,β-elimination reaction (b), the elimination of the β-OH group from the carbanionic intermediate leads to the formation of the aminoacrylate-PLP intermediate; subsequent transimination releases iminopyruvate product, which non-enzymatically hydrolyzes to pyruvate and ammonia
The activity of mSR is inhibited by amino acids containing –SH groups (cysteine and homocysteine) or electron-withdrawing groups on the β-carbon of alanine (β-haloalanine) because they react with the pyridoxal phosphate cofactor to yield thiazolidine derivatives. Most importantly, glycine and l-aspartate are also competitive SR inhibitors (K i is 0.15 and 1.9 mM, respectively), indicating that their in vivo concentration plays a role in d-serine synthesis since glycine is present in astrocytes at relatively high concentrations (3–6 mM) [31]. In contrast, Strísovský et al. [32] reported a different K i value for glycine (=1.6 mM), and also showed that several dicarboxylic acids are strong, competitive inhibitors of SR.
As a general rule, PLP-dependent enzymes have the ability to catalyze more than one reaction type with a single substrate, with one reaction being more effective than others. This has also been observed for mSR: while the elimination reaction starting from l-serine shows a higher kinetic efficiency than the racemization reaction (20 and 12 mM−1 min−1, respectively), the V max/K m ratio for racemization of d-serine into l-isomer is threefold higher than for elimination to pyruvate (Table 1) [24, 32]. The α,β-elimination activity was selectively impaired by introducing the Q155D mutation in mSR [25]. This work also demonstrated that the elimination reaction competes with the isomerization reaction mechanism for regulating intracellular d-serine levels, especially in forebrain areas which lack DAAO activity [25].
Table 1.
Comparison of kinetic properties of human and mouse SR (see reaction reported in Fig. 2)
| mSR | hSR | |||||
|---|---|---|---|---|---|---|
| Vmax (min−1) | Km (mM) | Vmax/Km (mM−1 min−1) | Vmax (min−1) | Km (mM) | Vmax/Km (mM−1 min−1) | |
| Racemization reaction | ||||||
| l-Serine | 45.5 ± 0.5 | 3.8 ± 0.1 | 12.0 ± 0.4 | 41.5 ± 1.5 | 4.1 ± 0.2 | 10.0 ± 0.8 |
| d-Serine | 113 ± 3 | 14.5 ± 1.1 | 7.8 ± 0.6 | 84.9 ± 4.2 | 10.8 ± 0.8 | 7.9 ± 0.9 |
| α,β-Elimination reaction | ||||||
| l-Serine | 81.3 ± 0.8 | 4.0 ± 0.5 | 20.3 ± 2.6 | 100 ± 5.1 | 4.7 ± 0.4 | 21.2 ± 2.7 |
| d-Serine | 8.8 ± 0.3 | 3.2 ± 0.3 | 2.7 ± 0.4 | 7.7 ± 0.3 | 9.5 ± 0.6 | 0.81 ± 0.09 |
| l-Serine-o-sulfate | 967 ± 17 | 0.49 ± 0.05 | 1,973 ± 205 | 807 ± 49 | 0.43 ± 0.05 | 1,879 ± 352 |
The cloning and expression of human SR (hSR) was reported in 2000: the gene encompasses seven exons and localizes to chromosome 17q13.3 [33]. The human enzyme (340 amino acids) is ~88% identical to the mSR. The hSR mRNA (2.7 kb) is mainly localized in brain areas containing high levels of endogenous d-serine (i.e., hippocampus and corpus callosum). Subsequently, Northern-blot analysis identified hSR mRNA in brain, heart, skeletal muscle, kidney, and liver tissues: at least three different transcripts were identified [34, 35]. Indeed, multiple protein isoforms of hSR were apparent in Western-blot analysis: a single band at 39 kDa was observed in HEK293-transfected cells, a single band at 42 kDa in human brain extracts, and a band at 62 kDa in kidney and heart, where an additional band at 80 kDa is also apparent [34]: these results require further substantiation by using an additional specific antibody. The most prominent difference in kinetic properties between human and mouse SRs is represented by the threefold higher K m for d-serine in elimination for the human enzyme (Table 1) [36]. Changes are also evident considering classical competitive inhibitors (i.e., malonate or glycine): K i values are two- to fivefold lower for human than for mouse SR [36].
Sequence analysis indicates that SR belongs to the type II-fold group of PLP-dependent enzymes, together with many other racemases and dehydratases. The structure of mammalian SR has not been resolved to date; however, that of SR from Saccharomyces pombe (PDB code 1WTC), which was used as a template for building a 3D model of hSR (Fig. 3a), is known [37]. As a result of this analysis, a model could be proposed for mammalian SR structure and in particular of the active site (the microenvironment surrounding PLP is highly conserved), the putative Ca2+-binding domain and ATP-binding site, the mode of monomer–monomer contact, and interaction with PDZ6 domains (see below). This model suggests that the binding sites for the various effectors are spatially distinct and may modulate enzyme function through different mechanisms [38].
Fig. 3.
a Model of the dimeric structure of hSR as proposed by [37, 38] (reproduced with permission). PLP is shown in blue, Ca2+ in green, and ATP analogue phosphomethylphosphonic acid (ACP) in red. b Dimeric structure of hDAAO in complex with imino-serine (PDB code: 2e49) [52]. FAD cofactor is represented in yellow, ligand (imino serine, SRI) in pink
The biochemistry of human d-amino acid oxidase
d-Amino acid oxidase, a flavin adenine dinucleotide (FAD)-dependent enzyme, catalyzes with a strict stereospecificity the oxidative deamination of d-amino acids to give α-keto acids and ammonia with the concomitant two-electron reduction of the FAD cofactor, which then reoxidizes on dioxygen (Fig. 4) [39, 40]. This flavooxidase was discovered 75 years ago by Krebs after he observed that amino acids belonging to the “d-series were deaminated much more rapidly than the natural isomerides” by fresh pig kidney and liver homogenates [41]. DAAO is almost ubiquitous in eukaryotic organisms, from the simplest ones, such as fungi, to fishes and mammals, where its presence was first detected [40, 41].
Fig. 4.
Reaction catalyzed by DAAO [39, 40]. The hydride-transfer of the α-proton of d-serine to the oxidized N(5) FAD position yields imino pyruvic acid and reduced (anionic) flavin. The imino pyruvic acid is then non-enzymatically hydrolyzed to pyruvate and ammonia; the reduced flavin is re-oxidized by dioxygen yielding hydrogen peroxide
Since 1935 DAAO has been the focus of an overwhelming body of research and has become a model for the oxidase class of flavoenzymes: the chemical aspects of enzyme reactivity have previously been described in detail using the protein purified from pig kidney (pkDAAO) and the recombinant enzymes from microbial sources, in particular the yeast proteins (for a review see [39, 40]). The combination of results gathered from determining the high-resolution structure of DAAO from different sources with the results of linear free-energy correlations and site-directed mutagenesis studies provided strong support for a classical hydride transfer mechanism [40]. The “dark side” of this very well studied enzyme was actually its physiological role, considering the atypical stereochemistry of its substrates. This problem persisted until 1992, when high concentrations of d-serine in the brain were demonstrated, a discovery representing a milestone in our understanding of DAAO function in mammals [5, 42–45]. In humans, the gene encoding for this flavoenzyme is present as a single copy in the genome on chromosome 12 (region 12q23-24), spans 20 kb, and comprises 11 exons and 10 introns [46]. The identification of an internal promoter-like region in the first intron suggested a differential regulation for DAAO gene expression. Up to now, however, no experimental evidence of different mRNA species in human tissues has been reported.
Although the cDNA encoding the human flavoprotein was isolated in 1988 [47], only little was undertaken in the following years to biochemically characterize hDAAO. The main reason for this lies in the difficulty encountered in expressing this human protein in a heterologous system such as E. coli. A robust and reliable process at the lab bioreactor scale was recently established reaching ~100 mg of hDAAO/liter culture [48]. The recombinant hDAAO has been isolated as a holoenzyme, showing the typical absorbance spectrum of the FAD-containing flavoenzymes [49]. The enzyme contains one molecule of non-covalently bound FAD per protein monomer and tightly binds the classical DAAO ligands, such as benzoate, anthranilate and sulfite (K d values for the various ligands are reported in Table 2). The oxidized form of the flavin cofactor is fully converted to the reduced species following the addition of substrate under anaerobic conditions. Intriguingly, the amount of the anionic semiquinone form of FAD produced upon anaerobic photoreduction depends largely on the experimental conditions, ranging from ≤5% (in Tris–HCl buffer, pH 8.0) to ~77% (in bicine buffer, pH 8.3). The addition of 1 mM benzoate caused a 34-mV negative shift of the midpoint redox potential for the two-electron transfer reaction (Table 2) [49].
Table 2.
Properties of human DAAO
| Spectral and binding properties | Values | References | ||
|---|---|---|---|---|
| ε454 nm (mM−1 cm−1) | 12.2 | [49, 69] | ||
| pKa, N(3)-H | 10.3 ± 0.15 | |||
| Kd, FAD (μM) | ||||
| Free form | 8 ± 2 | |||
| Benzoate complex | 0.3 ± 0.1 | |||
| Kd, benzoate (μM) | 7 ± 2 | |||
| Kd, anthranilate (μM) | 40 ± 10 | |||
| Kd, sulfite (μM) | 64 ± 9 | |||
| Em (mV), pH 8.0 | ||||
| Free form | −100 ± 11 | |||
| Benzoate complex | −134 ± 8 |
| Substrate specificity | Vmax,app (s−1) | Km,app (mM) | Vmax,app/Km,app | References |
|---|---|---|---|---|
| d-Serine | 3.0 | 7.5 | 0.4 | [49, 51, 69] |
| d-Alanine | 5.2 | 1.3 | 4 | |
| d-Proline | 10.2 | 8.5 | 1.3 | |
| d-Aspartate | 6.7 | 2,000 | 0.003 | |
| Glycine | 0.9 | 180 | 0.005 | |
| d-Phenylalanine | 15.5 | 1.1 | 14.1 | |
| d-Tyrosine | 14.8 | 1.5 | 9.9 | |
| d-DOPA | 21.7 | 1.5 | 14.5 |
| Temperature-induced unfolding (T m, °C) | Holoenzyme | Apoprotein | References | |
|---|---|---|---|---|
| Far-UV CD (220 nm) | 57.0 ± 1.0 | 51.0 ± 1.0 | [50] | |
| Trp fluorescence (335 nm) | 57.7 ± 0.3 | 48.6 ± 1.0 |
The V max,app and K m,app values are the kinetic parameters determined for the listed substrates at air oxygen saturation (0.25 mM)
The temperature-induced unfolding of holo- and apoprotein forms of hDAAO were determined by temperature ramp experiments following the signals associated to secondary (decrease of CD signal at 220 nm) and tertiary (increase in Trp fluorescence at 335 nm) structure [50]
The human DAAO binds the flavin cofactor weakly (it can be easily isolated from the apoprotein by dialysis in 1 M KBr): the estimated K d for FAD (=8 × 10−6 M) is significantly higher than the one measured for any other known DAAO, which is typically in the 10−7–10−8 M range [40, 49]. For this reason, hDAAO exists as an equilibrium of holo- and apoprotein forms in solution. The very low affinity for the cofactor raised the focal question of how much active hDAAO is possibly present in vivo (and in particular in brain tissues). It is noteworthy that a 20-fold tighter interaction between the FAD and the apoprotein moiety is observed in the presence of a ligand (substrate analogue) in the active site, such as the inhibitor benzoate (K d,FAD = 3 × 10−7 M, a value comparable to that determined for pkDAAO) [49].
A further peculiar feature of hDAAO was made evident through gel permeation chromatography experiments. The native protein is a dimeric holoenzyme in the 1- to 24-mg protein per concentration range [49], an oligomerization state different from that of the closely related porcine DAAO, whose oligomeric state depends on the protein concentration [39]. Moreover, the dimeric state is retained also by apoprotein form of the human enzyme whereas the apoproteins of the other known DAAOs are stable 40-kDa monomers [49]. Differences in protein conformation are apparent between the holo- and apoprotein forms of hDAAO: altered tryptophan fluorescence, higher exposure of hydrophobic surfaces, and higher sensitivity to proteolytic cleavage of the apoprotein form with respect to the holoenzyme [50]. In any case, the far-UV circular dichroism spectrum of the hDAAO apoprotein is similar to that of the holoenzyme, which suggests that a large part of the dimerization interface is conserved in the apoprotein.
Binding of the flavin cofactor only increases the structural stability of hDAAO towards chemical denaturation slightly. In contrast, a most significant effect is evident for the thermal unfolding: the T m value for the unfolding of the apoprotein is 6–9°C lower than that of the holoenzyme (Table 2) [50]. The urea-induced unfolding process of both the hDAAO holo- and apoprotein forms can be considered as a two-step (three-state) process. It is noteworthy that the denaturant-induced dissociation of dimeric hDAAO into monomers produces “adhesive” protein conformers, which are prone to aggregation. The reconstitution process of hDAAO holoenzyme consists of the rapid binding of FAD to the apoprotein, related to attaining enzymatic activity, followed by a slower secondary conformational change [50]. From a physiological point of view, the weak interaction between the apoprotein moiety and its cofactor may represent a means of evolving an enzyme that is largely present in the holoenzyme (active) form only in the presence of an active site ligand, i.e., the substrate d-serine or a still unknown physiological ligand. Considering the overall physiological concentration of d-serine and FAD in human brain tissues, in vivo hDAAO should be largely present in the inactive apoprotein form. It is tempting to speculate that the low affinity of hDAAO for the cofactor has been selected to control d-serine concentration in brain, avoiding an excessive degradation of the neuromodulator. Indeed, this hypothesis also correlates with the long half-life of d-serine in brain (~16 h) [23].
The apparent kinetic parameters indicate that hDAAO possesses a low catalytic efficiency and substrate affinity on the putative physiological substrate d-serine (a higher activity was determined on d-alanine and d-proline, Table 2) [49]. Interestingly, the enzyme can even degrade glycine and d-aspartate (both present at relevant concentrations in the brain); although the relative affinity for these amino acids is very low (see Table 2). hDAAO was also reported to catalyze the oxidation of d-3,4-dihydroxyphenylalanine (d-DOPA) into dihydrophenylpyruvic acid [51]. At low concentration, d-DOPA is oxidized by the human flavoenzyme more efficiently than any previously identified substrate (Table 2) but a substrate inhibition effect is evident at high concentrations (as also observed using d-tyrosine and d-phenylalanine as substrate).
In 2006, we investigated the kinetic mechanism of hDAAO both by the enzyme monitored turnover method (steady-state kinetics) and rapid measurements (pre-steady-state kinetics), using d-alanine and d-serine as substrates [49]. The rate of the main phase of flavin reduction is significantly higher than the turnover number, while the rate for the product release from the reduced enzyme is too low to belong to the catalytic cycle (~0.6–0.9 s−1). The step of reoxidation of reduced flavin is consistent with a second-order reaction in dioxygen (a reoxidation constant of 1.7 ± 0.6 × 104 M−1 s−1 and 1.25 ± 0.4 × 105 M−1 s−1 was calculated for the free and imino acid-complexed reduced enzyme, respectively). Taken together, these kinetic measurements indicated that hDAAO follows a sequential kinetic mechanism in which the rate of product release from the reoxidized enzyme form represents the rate-limiting step in catalysis, analogously to what was observed for pkDAAO [49].
The hDAAO structure contains 11 α-helices and 14 β-strands, which fold into two domains, the FAD-binding domain and the interface domain (Fig. 3b) [52]. The C-terminus of hDAAO (residues 341–347, containing the peroxisomal targeting signal) was not visible on the electron-density map, indicating the flexibility of this region. The overall dimeric structure was identical to the “head-to-head” structure as observed for porcine DAAO but the frequency of substitution at the dimer interface was higher than the overall substitution frequency (33 vs. 15%, respectively). Furthermore, the electrostatic surface potential at the dimer interface of hDAAO is negatively charged while that of the porcine enzyme is positively charged [53, 54]: these changes account for the different stability of the dimeric state of human and pig DAAOs. Both these DAAOs show a loop (residues 217–227), referred to as “the active site lid”, which movement allows substrate binding and product release. When complexed with the substrate-analogue benzoate, the conformation of the active site lid is in a closed state, with the inhibitor sandwiched between the flavin ring and the aromatic ring of Tyr224 [52]. The structure of hDAAO in complex with imino-DOPA showed additional H-bonds between the ligand and Gln53 and His217 [51], thus suggesting that these residues could be involved in determining the substrate specificity. As expected from the 85% sequence identity, the active site geometry was fully conserved between the human and the porcine DAAO at the re-face of the flavin ring [52–54]. However, at the si-face a difference was observed: the conformation of the hydrophobic stretch (VAAGL, residues 47–51) covering the si-face of the flavin ring differs significantly in hDAAO where it is shifted away from FAD, resulting in the loss of the H-bond between the flavin N(5) and the backbone N atom of Ala49. Since no further remarkable differences were observed within 6 Å from FAD, the authors argued that the VAAGL stretch plays an important role in determining the affinity for the cofactor [52].
Regulation of SR and DAAO
Nitrosylation
The work of Suzuki’s group on the human glioblastoma cell line U87 by using NOR-3 as NO donor recently demonstrated that SR activity is inversely regulated by d-serine and nitric oxide. SR activity was enhanced up to fivefold and in a dose-dependent manner by d-serine and was inhibited by NO [55]: indeed, d-serine induces the denitrosylation of SR. Interestingly, DAAO activity was altered by NO in a dose-dependent manner as well but in this case nitrosylation resulted in an enhancement of the flavooxidase activity [56]. The authors proposed that NMDAR-mediated Ca2+ influx at postsynaptic neurons involves Ca2+/calmodulin-dependent activation of neuronal NO synthase (without affecting endothelial NO synthase) with production of NO. This NO diffuses into adjacent astrocytes or neurons to give nitrosylation and inhibition of SR and activation of DAAO (Fig. 1) [55, 56]. This model of SR activation was subsequently confirmed [57]: treatment of mixed cortical neuron-glial cultures with S-nitroso-glutathione (as NO donor) augmented SR S-nitrosylation. Furthermore, Cys113 was identified as the target residue of nitrosylation, using both the recombinant enzyme and HEK293 transfected cells [57]. Cys113 is in close proximity to the putative ATP-binding region of the enzyme and thus nitrosylation might displace ATP from its binding site and inactivate SR: ATP and NO reciprocally activate and inhibit the enzyme by acting at the same protein site [57]. NO produced in neurons may represent a inhibitory feedback mechanism to finely modulate the metabolism of d-serine in astrocytes, in particular to prevent overproduction.
SR-interacting proteins: GRIP, PICK1, and Golga3
The yeast two-hybrid screening technique against a rat hippocampus and cortex cDNA library using SR as bait identified the hepta-PDZ protein GRIP, glutamate receptor interacting protein (usually coupled to the Glu2/3 subunits of the α-amino-3-hydroxy-5-methylisooxazole-4-propionic acid–AMPA–Ca2+ channel) as a binding partner [58]. GRIP proteins play a major role in the proper trafficking and assembly of the glutamate receptors of the AMPA/kainate type (as well as of accessory proteins). In particular, the C-terminal end of mouse and human SR contains a ValSerCys sequence, a motif resembling the type II consensus sequence for binding to PSD95/disc large/ZO-1 (PDZ) domains [59]. SR binds specifically to the PDZ6 domain of GRIP by means of its C-terminal PDZ-binding motif, and it is activated by this interaction. It has been proposed that GRIP proteins were released from AMPA receptors (AMPAR) following their stimulation and phosphorylation, which would allow GRIPs to interact with SR in the cytosol and result in an increase in SR activity (and consequently in d-serine concentration, Fig. 1) [58]. This sequence of events was confirmed by [37], although only a limited (1.5-fold) activation of SR by full-length GRIP was observed. These authors reported that binding to PDZ6 alone was not sufficient for activation: the remaining part of the C-terminal region of GRIP (the PDZ7 module and a linking segment between PDZ6 and PDZ7) was required for full activation of SR, both in vitro and in vivo. GRIP adenovirus-infected P8 glial cerebellum mice cells showed a twofold increased d-serine concentration and granule cell migration by activating NMDAR [58]. This activation process is switched off by releasing GRIP and SR after the GluR2 subunit of AMPAR is phosphorylated, thus preventing calcium from activating SR [38]. The putative phosphorylation of SR following GRIP interaction remains to be established, likewise the ability of GRIP to bring SR in proximity of the calcium channel. Anyway, and in agreement with the results of [58], a modulation of d-serine synthesis by Ca2+ concentration can be postulated, as also suggested by the trimeric GluR2–GRIP–SR complex recently observed using a mouse brain lysate [38].
A further yeast two-hybrid screening analysis using a human hippocampal cDNA library identified the protein interacting with C kinase 1 (PICK1), a different PDZ domain-containing protein, as interactor of SR [60]. PICK1 is a scaffolding protein that has been proposed to regulate subcellular localization and membrane expression of various binding partners. The PDZ domain of PICK1 can interact with lipid membranes, an essential step for the clustering of AMPAR and synaptic plasticity [61]. Although protein kinase C may phosphorylate SR brought into proximity by PICK1 binding (Fig. 1), no data are available regarding the effect of the binding of PICK1 on SR activity. Anyway, an increase in d-serine concentration was observed in HEK293 cells transfected with SR or PICK1 (or both) [62]. By using siRNA to PICK1 to decrease PICK1 expression or by using SR or PICK1 variants that cannot interact with each other, this concentration increase could be prevented. Furthermore, d-serine levels were reduced (~30%) in the forebrain of neonatal PICK1 knockout mice.
In another important study from Wolosker’s laboratory [63], SR was demonstrated to be degraded through the ubiquitin–proteasomal system, a process which is modulated by the Golgi-localized protein, Golgin subfamily A member 3 (Golga3). In fact, the yeast two-hybrid screening identified Golga3 as an interactor of SR [63]. Golga3 interacts with the N-terminal 66 residues of SR to decrease its ubiquitin/proteasomal degradation, probably interfering with binding of a still unidentified E3-ubiquitin ligase (an almost threefold increase in average half-life was observed), and thus increasing SR cellular steady-state levels and d-serine synthesis. Golga3 and SR colocalized in the cytosol perinuclear Golgi region and in neuronal and glia processes in primary cultures [63]. It was proposed that Golga3 mediated binding of SR to the Golgi membrane and thus acts by constituting a pool of available SR to be exported/targeted to different cell compartments.
Very recently, Wolosker’s group demonstrated that NMDAR activation promotes translocation of cytosolic SR to the plasma membrane and dendrites in primary neuronal cultures of rat brain, a process that dramatically decreases the enzymatic activity of the PLP-dependent enzyme (Fig. 1) [64]. Binding of SR to membrane was proposed to be due to palmitoylation with a serine or threonine residue and to require phosphorylation at Thr227. It was suggested that SR inhibition by translocation is a fail-safe mechanism to prevent NMDAR overactivation in vicinal cells or synapses. Binding SR to phosphatidylinositol(4,5)biphosphate (PIP2) represents another mechanism for sequestering inactive enzyme to the membrane in glial cells, a process that inhibits the enzyme according to a competitive mechanism with ATP. By activating metabotropic glutamate receptors mGluR5, phospholipase C can degrade PIP2 and thus recover the active SR form (Fig. 1) [65]. Site-directed mutagenesis of residues important for lipid-binding (i.e., K96A, K137A, and L168A SR variants) produced enzyme forms totally resistant to inhibition by PIP2.
In conclusion, brain SR shares numerous properties with other members of the type II-fold PLP-dependent racemase/dehydratase family; however, it is distinct because of specific regulation in the brain through interaction with specific glial or neuronal proteins.
DAAO and pLG72 interaction and significance of a “odd couple”
An analysis of single-nucleotide polymorphisms (SNPs) and haplotypes found an association between schizophrenia and the newly identified primate specific gene G72, encoding for the small protein pLG72 (18 kDa) [66]. The yeast two-hybrid system identified DAAO as a putative pLG72-interacting partner; subsequently, DAAO independently was associated with schizophrenia [67]: both genes are located in a chromosomal region showing evidence for linkage with the disease (see below). Chumakov et al. [66] reported that mixing pkDAAO with a large molar excess of pLG72 enhances basal levels of activity by threefold. Therefore, the authors proposed a model whereby an enhanced expression of pLG72 produces an increase in DAAO activity and a concomitant d-serine depletion, which would result in hypofunction of glutamatergic synapses and thus increase susceptibility of individuals to schizophrenia (see “d-serine and human diseases” paragraph). As suggested by [19], the genetic association is intriguing but the proposed biochemical mechanism does not fit with other observations (see below). In order to investigate the effect of pLG72 on the structural and functional properties of hDAAO, both human proteins were produced as recombinant protein in E. coli [49, 68]. Their use confirmed the in vitro “physical” interaction between hDAAO and pLG72, but failed to reproduce the effect of pLG72 binding to the pig enzyme previously reported by [66]: specifically, pLG72 did not activate mammalian DAAOs [69]. By using a variety of experimental methods (namely, far Western-blot analysis, size exclusion chromatography, and surface plasmon resonance analysis), we demonstrated that pLG72 interacts with both the holo- and the apoprotein forms of hDAAO to yield an ~200-kDa complex, constituted by two hDAAO homodimers (2 × 80 kDa) and two pLG72 molecules (2 × 20 kDa). The K d for complex formation is ~8 × 10−6 M [69]. Binding of pLG72 to hDAAO did not significantly affect the catalytic efficiency and the kinetic parameters of the reaction catalyzed by the enzyme on d-serine, nor was the affinity for the coenzyme or the rate constant of its binding to the apoprotein affected. The main effect of binding is a faster time course for enzyme inactivation (i.e., a decrease in hDAAO stability) when an excess of pLG72 is present (Fig. 5a) [69]. We have attributed this effect to a decrease in the amount of the active form of holoenzyme in solution (Fig. 5a). This inactivation of hDAAO is not related to the depletion of FAD in solution (although pLG72 possesses a binding site for molecules with a large hydrophobic moiety, such as FAD) as the same result was obtained when an excess of free FAD was present in the assay mixture. Visible absorbance and near-UV CD spectroscopy analyses showed that pLG72 binding altered the tertiary structure of hDAAO, suggesting that the modification destabilizes the holoenzyme form [69].
Fig. 5.
Effect of pLG72 on hDAAO activity in vitro (a) and in U87 glioblastoma-transfected cells (b) and on d-serine cellular concentration [69]. a Effect of pLG72 binding on hDAAO activity: black bars activity measured without preincubation or gray bars after 30 min of pre-incubation of a fixed amount of hDAAO (0.1 nmol/ml) with increasing amounts of pLG72; (white bars) effect of pLG72 on the reactivity of the hDAAO-bound FAD. In this latter case, the oxidized hDAAO was incubated with a stoichiometric amount of free FAD and increasing amounts of pLG72 and subsequently was anaerobically reduced by adding 1 mM d-serine: the bars report the percentage of reduced flavin. b (Black bars) Summary histogram of the d-/l-serine ratio (percentage) in U87 control cells (CTRL) and in the same cells transfected with hDAAO or pLG72: the change was significant for hDAAO (p = 0.004) and not significant for pLG72. (Gray bars) Summary histogram of the hDAAO activity (arbitrary units) in the same U87 cells: the change in activity with respect to the control was statistically significant for hDAAO (p = 0.012) and not significant for pLG72. The data are reported as mean ± SEM
Human U87 glioblastoma cells transfected with expression vectors encoding for EGFP-hDAAO or EGFP-pLG72 fused proteins showed significantly lower d-/l-serine ratios (as determined by HPLC analysis) when hDAAO was overexpressed (~10%) with respect to nontransfected cells or EGFP-pLG72 or in cotransfected cells (~13.0%, Fig. 5b). The measured DAAO activity values increased in EGFP-hDAAO-transfected cells but were practically negligible in cells transfected with the EGFP-pLG72 expression plasmid and in cotransfected cells (Fig. 5b) [69]. This result indicated that d-serine levels decrease upon transient transfection of hDAAO in U87 cells, according to the function of this flavoenzyme in the catabolism of the gliotransmitter, and further supported the proposal that in vivo pLG72 interacts with hDAAO, functioning as an endogenous negative modulator of the flavoenzyme.
Furthermore, hDAAO and pLG72 were identified in the same astrocytes of the human cerebral cortex by immunofluorescence experiments on tissue slices [69]; the coimmunoprecipitation of the two proteins further supported this interaction in vivo. Concerning the subcellular localization of DAAO and pLG72 in human cultured astrocytes, only 5% of the cells display overlapping signals, with a mean average of 8.1% colocalization [69]. This suggests that pLG72 interaction with hDAAO is probably driven by specific spatiotemporal stimuli, the features of which remain elusive.
Despite the aforementioned observations, pLG72 function is still controversial. It was recently reported that pLG72 is a mitochondrial protein that promotes robust fragmentation in mammalian cell lines, including human and rat primary neurons [70]. Even more recently, “humanized” BAC transgenic mice (G72Tg) expressing alternatively spliced G72 and G30 transcripts and pLG72 were generated [71]: the G72 gene expression is prominent in granular cells of the cerebellum, the hippocampus, the cortex, and the olfactory bulb. The G72Tg mice exhibited enhanced locomotor responses to PCP, a noncompetitive antagonist of NMDA receptors, which suggests a higher sensitivity of the transgenic animals to drugs affecting the glutamatergic system. The authors concluded that the expression of the human G72/G30 gene locus in mice produces behavioral phenotypes that are relevant to psychiatric disorders (see below).
SR and DAAO distribution in the CNS
Serine racemase
The gene for SR was identified in mice, rats, and humans [26, 33, 72]. It is noteworthy that, differently from other peripheral tissues (see above), a single transcript of 2.6 kb corresponding to SR was identified in brain. The distribution of the PLP-dependent enzyme is very similar to that reported for endogenous d-serine, with the highest expression in the forebrain. The highest levels are found in the hippocampus and corpus callosum, with intermediate levels in substantia nigra and caudate, and very low levels in the amygdala, thalamus, and subthalamic nuclei [26, 33, 34, 72]. In the CNS, SR was initially reported as being expressed mostly by glial fibrillar acidic protein (GFAP)-positive astrocytes [13, 26, 34], with some expression detected in quiescent and activated microglia [13, 73]. However, neither SR nor d-serine is a strict marker of glia: in fact, they both have been subsequently detected in some neurons of the cerebral cortex [12, 13] and in hindbrain glutamatergic neurons [13]. Different studies showed that cultured neurons contained both SR mRNA and protein and that these cells catalyzed d-serine synthesis to levels comparable to those observed within astrocytes [12, 74, 75]. SR neuronal expression was also confirmed by in situ hybridization, which revealed the presence of high levels of SR mRNA in neurons of the brain [75] and in neuronal ganglion cells of the retina [76] where, however, the enzyme was also detected in Müller cells (i.e., retinal glial cells) and astrocytes [77]. By using the same method, a neuronal-like distribution of SR mRNA was demonstrated in the hippocampus, with little or no SR message evident in astrocytes at the corpus callosum [78].
Subsequently, Miya et al. [79] examined SR distribution in the brain during postnatal development and also in cultured cells by using novel SR knockout mice as negative control. Based on results from Northern-blot, Western-blot, and immunohistochemistry analyses, the authors concluded that SR is localized in glutamatergic neurons in the cerebral cortex and in the glutamatergic pyramidal neurons of the hippocampus, where the enzyme is mainly present at postsynaptic sites. In the adult cerebellum, SR expression levels are lower than those reported for the telencephalic regions. They also detected significant, though weak, expression of SR in GABAergic Purkinje cells [79]. Taken together, these findings suggest that the d-serine-producing enzyme is expressed in principal neurons of given neuronal regions, irrespective of the excitatory or inhibitory signature. Furthermore, SR was unambiguously identified by means of mass spectrometrical methods in samples from the perireticular nucleus, a transient structure of developing brain in humans. This observation may indicate the importance of SR function for developing correctly formed corticothalamic and thalamocortical connections, by stimulating d-serine-dependent NMDA receptor activity [80].
d-Amino acid oxidase
This flavoprotein oxidase was observed in mammalian nervous tissues already in 1966, even in humans [81, 82]. Earlier papers reported on DAAO distribution in rat brain by using different cytochemistry methods to assay activity [83–86]: a regional heterogeneity of DAAO activity distribution in rat CNS was observed. Specifically, DAAO appears to be most abundant in the cerebellum and the brainstem with respect to the forebrain. DAAO activity has also been observed in the gray matter of the medulla and thoracic spinal cord, except for discrete areas, including those involved in autonomic function [86]. Different groups provided evidence that the DAAO activity is localized in astrocytes but not in other glial cell types or neurons [83, 84]. A complete map of DAAO distribution in rat brain was subsequently obtained by using an immunocytochemical assay: this work reported the presence of the flavoenzyme in both neuronal and glial cells, albeit at different concentrations [87]. In detail, the richest cell types in DAAO were astrocytes and radial glia cells; ependymal cells also contained moderate amounts of the enzyme, whereas other cells were apparently immunonegative. Regional differences have also been detected: astroglial cells of the caudal brainstem and of the cerebellum are generally more immunoreactive than those located in the forebrain. The neuronal immunoreactivity is generally stronger in the hindbrain than in the forebrain although numerous neuronal subtypes in this region display high staining intensities [87]. DAAO gene expression was also investigated by RT-PCR in cultured rat astrocytes: higher levels were detected in type-1 than in type-2 cells [88]. In addition, and consistent with histochemical studies, DAAO expression levels were higher in cerebellum astrocyte cultures than in cerebral cortex ones [89].
Most recently, different groups reported on regional and cellular expression of DAAO in human brain. In 2007, Verral et al. [90] focused on areas relevant to schizophrenia and where hDAAO was reputed to be abundant or important. By Western-blot analysis they observed a strong immunospecific signal in cerebellum and spinal cord. On the other hand, in human dorsolateral prefrontal cortex (DPFC) extracts DAAO detection was extremely variable and sometimes absent [90]. In DPFC gray matter sections, hDAAO immunostaining was predominantly neuronal and visible throughout the cytoplasm of pyramidal neuron cell bodies, sometimes granular, and surrounding unstained nuclei. Granular immunoreactivity may reflect the peroxisomal localization of hDAAO staining, as was observed in rat [83, 87]. The authors observed punctuate spots of immunoreactivity throughout the neuropil. Furthermore, small round cells, which were presumed to be glial cells based on their size morphology and location, were stained. In situ hybridization experiments revealed that hDAAO mRNA signals clustered over pyramidal neurons, whereas in the white matter the same signals were marginal [90]. The difficulties in obtaining a reliable signal corresponding to hDAAO in this brain region brought the authors to assume that there is a regional difference in the antigenicity of the enzyme, possibly reflecting differences in post-translational modification or conformation, which differentially affected Western-blot and immunohistochemical detection systems. Subsequently, our group confirmed the presence of DAAO in human brain cortex by Western-blot analysis, immunoprecipitation experiments, and immunofluorescence localization both in tissue slices and secondary cultured astrocytes [69]. So far, the inferred isoforms of hDAAO have not been identified.
Moreover, in the cerebellum hDAAO immunoreactivity localized predominantly to the Purkinje cell layer and therein to small round cells which were recognized as putative Bergmann glia, whereas Purkinje cells themselves were not labeled [90]. The molecular layer and the granule cell layer also demonstrated occasional immunolabeling of small round cells, although in the latter Golgi cells and granule cells were not labeled. In situ hybridization revealed that hDAAO mRNA signals primarily clustered over putatively Bergmann glia cells in the Purkinje cell layer. In midbrain sections, hDAAO staining was, however, strong in neurons containing melanin pigment within the substantia nigra pars compacta (putatively dopaminergic neurons) [90]. This observation is consistent with that reported in rats [87] and it is clinically significant, given that hDAAO can oxidize d-DOPA in an alternative pathway for dopamine synthesis [51].
Most recently, Ono et al. [91] analyzed in parallel the distribution of DAAO mRNA and protein in rat and human brain. Concerning DAAO expression in rat brain, the flavoprotein was detected in the prefrontal cortex and thalamus and its presence was also confirmed in the choroid plexus (CP) and hippocampus. The results of the immunohistochemistry experiments on human brain tissues were in agreement with those obtained with rat samples: the staining corresponding to hDAAO was observed in glial cells of the rhomboencephalon and in the CP. hDAAO-immunopositive cells were detected in the molecular and Purkinje cell layers in the cerebellum, as previously reported [90], as well as in the CP, pons, and medulla oblongata sections, whereas only modest levels of immunoreactivity were observed within the cerebral cortex and in the spinal cord (both in the white and gray matter) [91]. It was suggested that the expression of hDAAO in CP epithelial cells, whose main function is to produce cerebrospinal fluid (CSF), is related to a specific regulatory mechanism governing d-serine levels in the CSF. They assumed that hDAAO in CP modulates the amount of d-serine entering the CSF from blood, and that the levels of this neuromodulator in the CSF influence the composition of the brain interstitial fluid and neuronal tissue function.
Taken together, these findings raise two issues worthy of mention. The first is that in human brain cortex DAAO immunoreactivity was observed both in glial cells [69] and in neurons [90], whereas it is largely—if not exclusively—glial in the cerebellum [90, 91]. The basis and significance for this still remain obscure. The fact that in DPFC hDAAO staining is largely neuronal implies that, to a large extent and at least in the cortex, d-serine catabolism is neuronal rather than glial, consistent with recent observations that the major d-serine transporter Asc-1 is also predominantly neuronal [92]. The second question concerns the reason why hDAAO expression in the cortex is robust but cortically detected activity practically negligible [3, 85]. Two possible responses have been previously proposed [90]. The first is that cortical hDAAO is de facto inactive because it is only a residue from an earlier developmental function, although its significant and prolonged expression argued against this assumption. Otherwise, in the cortex the flavoenzyme might be active on a substrate other than d-serine and catalyze a reaction not detected by the commonly used functional assays; however, this is unlikely and raises the alternative question of how d-serine is degraded in this brain region. Our hypothesis is that in the cortex some kind of negative effector is present along with hDAAO: a small ligand or an interacting protein that is able to down-regulate its activity. This fits nicely with the difficulties encountered in detecting the enzyme activity in tissue slices and it would also explain why the levels of d-serine in the forebrain, and particularly in cortex, are relatively unchanged in adult DAAO-deficient mice while a significant increase in d-serine levels is observed in regions typically displaying low concentrations of neuromodulator in wild-type animals (mainly the cerebellum and the brainstem). Finally, it was argued that in the forebrain areas d-serine concentration is tuned by other mechanisms [93], suggesting that SR would offer an alternative pathway to regulate the intracellular content of the neuromodulator; however, this is yet to be proven.
d-Serine and human diseases
Glial cells play a fundamental role in governing NMDAR function and because of this they may be implicated in pathological events related to neuronal degeneration and altered neurotransmission. Over- or down-stimulation of NMDARs is implicated in psychiatric disorders (such as schizophrenia) and in many acute and chronic degenerative disorders, including stroke, epilepsy, peripheral neuropathies, and Parkinson’s and Alzheimer’s disease (AD).
d-Serine, together with glutamate, is supposed to represent a link between neuroinflammation and excitotoxicity: this effect has been related to neuronal death in AD and other neurodegenerative diseases [73, 94]. Excitotoxicity depends on NMDAR activation, which requires the action of a coagonist at a glutamate-binding site and a coagonist at the glycine-binding site. Neurotoxicity induced by amyloid β-peptide (Aβ) is exacerbated by the release of NMDAR co-agonists, such as d-serine. In fact, neurotoxicity induced on primary hippocampal neurons cells by a primary microglia-conditioned medium treated with 15 μM Aβ (containing elevated levels of d-serine) was rescued when the medium was treated with DAAO, the d-serine degrading enzyme, as well as by addition of 5,7-dichlorokynurenic acid (DKCA), an antagonist of the glycine modulatory site of NMDAR [73]. Furthermore, amyloid precursor protein increased the steady-state level of SR on microglia cells, probably acting at the transcriptional level [94]. A further evidence that d-serine plays an essential role in Aβ-induced neurotoxicity originates from the observation that SR knockout mice showed a 90% decrease in forebrain d-serine content as well as a reduced neurotoxicity induced by NMDA and Aβ peptide injections in forebrain [95].
Concerning amyotrophic sclerosis, glutamate toxicity enhanced by d-serine overproduced in glia has been proposed as a mechanism for motoneuronal death: increased levels of glial d-serine and SR were observed with disease progression [96]. Similarly, endogenous supply of d-serine (as a glycine site agonist) was demonstrated to be important for neuronal injury involving NMDAR overactivation in the cerebral cortex: in fact, DAAO markedly inhibited neuronal damage [97]. Subsequently, ischemic cell death induced by oxygen/glucose deprivation was reported to be not affected by the exogenous addition of glycine or d-serine, although it did depend on NMDAR activation (e.g., by MK-801 administration) [98]. This observation was explained by the extracellular levels of glycine site agonist that are elevated under ischemic conditions, thus saturating the glycine site.
Schizophrenia is a severely debilitating psychiatric disorder that affects ~1% of the population worldwide. It is characterized by the psychotic features of delusions, hallucinations, and disorganized thought as well as by profound cognitive deficits (including impairments in attention, learning, and memory) and by negative symptoms that involve social withdrawal and affective flattening. Schizophrenia is known to be influenced by heritable factors, and genetic studies have identified a number of susceptibility genes that modulate NMDAR function [99]. This indicates that deficient glutamatergic neurotransmission mediated by these receptors may be involved in the pathophysiology of schizophrenia, although monoaminergic theories of the treatment of schizophrenia have dominated research and drug development for many years. Non-competitive antagonists of the NMDAR, such as phencyclidine, elicit schizophrenic-like symptoms in healthy individuals and exacerbate such symptoms in patients [100]. An increasing body of evidence indicates that an abnormality in available d-serine may be involved in the pathogenesis of schizophrenia. This neuromodulator is enriched in brain regions implicated in the pathogenesis of schizophrenia, such as prefrontal and parietal cortex. Moreover, the original association between SNPs and haplotypes in G72 and schizophrenia [66] was supported by a number of subsequent studies: these results imply that genes encoding for proteins involved in d-serine metabolism (SR, DAAO and G72) might potentially contribute to the pathogenesis of schizophrenia (see below). Mice with mutations in the SR gene, which gave a complete loss of enzymatic activity and dramatically reduced d-serine levels, displayed behaviors relevant for schizophrenia (impairment in prepulse inhibition, sociability, and spatial discrimination) [101]. These deficits were augmented by using an NMDAR antagonist and improved by using clozapine (an atypical antipsychotic) or d-serine. Decreased d-serine levels and altered glutamatergic neurotransmission and behavioral abnormalities that reflect hyperactivity and impaired spatial memory have been also observed by [102] using independently generated SR knockout mice.
In 2003, serum levels of d-serine in patients with schizophrenia were demonstrated to be significantly lower than those of healthy controls (1.86 ± 0.53 vs. 2.28 ± 0.59 μM, respectively) [103]. The levels of d-serine measured in cerebrospinal fluid were decreased but unchanged in parietal cortex; SR protein levels and hippocampal SR/DAAO ratio protein levels were significantly decreased in frontal cortex and hippocampus, while the amount of hippocampal hDAAO was 77% higher in patients who have been treated for over 20 years [104]. The decreased level of hippocampal SR in schizophrenic patients disagrees with the slightly increased level identified in the same region by [105] and in dorsolateral prefrontal cortex by [90]. The previous authors, using human postmortem brain tissues, detected a significant increase in SR expression in hippocampus of schizophrenics whereas no changes were observed in any of the cortical areas, suggesting that the alteration in SR levels is region specific [105]. SR Immunoreactivity was also increased in the DPFC but not in the cerebellum in schizophrenia, a change not accompanied by elevated levels of SR mRNA [90]. This last observation suggests regulation at the translational or post-translational level (see above). One possibility is that protein degradation is decreased as SR protein levels are regulated by ubiquitin-dependent proteasomal degradation, a process modulated by the interaction with Golga3 (see above and Fig. 1) [63]: whether Golga3 is altered in schizophrenia is not known. A 37% increase in cerebellar hDAAO activity in schizophrenia as compared to controls, as well as an increase with duration of the illness was also reported [106]. Similarly, a twofold increase in hDAAO activity in postmortem cortex samples from schizophrenia patients as compared to controls was found although no correlation with other factors (duration of disease, gender, age on onset, cumulative lifetime antipsychotic, etc.) was evident [107]. It is also noteworthy that a similar pattern of hDAAO distribution in human brain was apparent between schizophrenic patients and controls [91].
An analysis of SNPs and haplotypes found an association between the gene G72 with schizophrenia [66]. Subsequently, DAAO itself was associated with schizophrenia and combinations of G72/DAAO genotypes had a synergistic effect on disease risk. Both genes are located in chromosomal regions showing evidence for linkage with this disease. Differently from the previous proposal [66], we recently demonstrated that binding of pLG72 to hDAAO decreases the stability of the flavoenzyme (see above) [69]. We proposed that hDAAO hyperactivity due to loss of the negative control exerted by pLG72 interaction decreases d-serine concentration and causes hypofunction of NMDARs, thus predisposing individuals to schizophrenia (see Fig. 6) [69]. Following the original genetic association between G72 gene and schizophrenia [66], significant associations have been reported in various populations, such as French Canadians, Russians, Germans, Palestinian Arabs, South Africans, Ashkenazy Jews, Chinese, Taiwanese, Scots, Koreans, and the Irish (see [108] and references therein). The majority of replication studies of G72 have indicated significant associations of alleles, genotypes, or haplotypes with schizophrenia. For example, one haplotype within the G72 gene was associated to schizophrenia and specifically with one aspect of cognitive performance, semantic fluency [109], or for cognitive variables assessing working memory and attention [110]. However, a few studies have reported that there is no association between G72 gene and schizophrenia [106].
Fig. 6.
Proposed role of the interaction between hDAAO and pLG72 on the d-serine bioavailability at glutamatergic synapses under normal and pathological (schizophrenia susceptibility) conditions [69]. Up to now, pLG72 was identified only in glial cells, therefore we focused on the modulation of hDAAO activity in astrocytes. According to our hypothesis, pLG72 modulates the amount of active hDAAO acting on the stability of the flavoenzyme (left panel). An abnormal, low expression of pLG72 under pathological conditions could result in hyperactivation of hDAAO and decrease d-serine concentration (right panel). Importantly, the decrease in d-serine concentration results in a lower amount of activated NMDAR and, thus, in a hypofunction of the glutamatergic neurotransmission (see also Fig. 1 for details about the overall mechanisms of d-serine regulation at synapsis)
The results from genetic association analyses in SR or DAAO genes and schizophrenia are also quite contrasting. A recent genetic analysis on a Japanese population identified a two and a three SNP-based haplotype of DAAO gene associated with schizophrenia [111]. In contrast, a different association analysis on a Japanese population [35] and Caucasian samples were demonstrated to be not significant [110]: minor ethnic heterogeneity or reduced power may affect the genetic association data, which are at borderline significance. In fact, when this analysis was carried out using a very large sample size, nominal evidence was found for an association of a number of allele and haplotypes with schizophrenia: these associations were no longer positive after correction for multiple testing [108]. Furthermore, the work from [35] did not identify a correlation between d-serine concentration and the SR or DAAO genotypes either. On the other hand, variants in the promoter and 5′-terminus of SR gene demonstrated a significant association with schizophrenia [101, 112, 113], contrary to polymorphisms in the central and 3′-terminus. For example, a significant excess of a SNP of the 5′ region of the SR gene (the IVS1a + 465G → C allele) was found, especially in the paranoid subtype: this allele showed a 60% lower promoter activity [113].
Finally, the level of SR expression was reported to be modulated under different pathological and therapeutic conditions. A significant increase in the level of mRNA and protein expression of SR was observed in all rat brain regions following repeated administration of morphine for 30 days, paralleled by a slight increase in d-serine concentration in cortex, striatum, and hippocampus, while no significant changes in DAAO levels were observed [114]. These observations suggest that chronic treatment with morphine elevates d-serine levels that at least in part are involved in the activation of NMDAR. On the other hand, both mRNA and protein level of SR and d-serine concentrations were decreased in the hippocampus (but not in the cerebral cortex or cerebellum) of Wistar rats, whereas DAAO expression was unchanged [22, 115], thus indicating that the SR-dependent pathway is a main objective of hippocampus-dependent cognitive deficits related to aging.
Conclusions
d-Serine plays an essential role in the mammalian brain, in particular by modulating NMDAR function. It is involved in the mechanism by which astrocytes control synaptic transmission and plasticity. However, a number of important questions remain to be addressed. For example, does a reciprocal control exist between SR and DAAO expression and/or activity levels (in addition to nitrosylation)? Considering its physiological function in human brain, DAAO activity might be finely modulated by interacting proteins or still unknown effectors. More specifically from a pathological point of view: to what extent is chronic potentiation of a hypofunctional or normal NMDAR beneficial? Will excitotoxicity possibly persist over a long period of elevated d-serine levels near the NMDAR? Over- and down-stimulation of NMDARs are crucial initial pathological events in a number of psychiatric and degenerative disorders. In fact, a number of findings from preclinical and clinical investigations demonstrated beneficial effects of NMDAR activators acting at the glycine binding site in the treatment of schizophrenia. A more effective strategy could use molecules acting on proteins involved in d-serine metabolism: these novel therapies will substantially improve treatment, with fewer side-effects than the antipsychotics currently being used. Similarly, and since d-serine has been demonstrated to be deleterious for neurons in ischemia, inflammation, and excitotoxic insults, molecules that affect hDAAO and/or SR activity would presumably be neuroprotective.
Acknowledgments
This work was supported by grants from Fondo di Ateneo per la Ricerca to L. Pollegioni and S. Sacchi, and from Fondazione CARIPLO to L. Pollegioni. We are grateful for the support of Consorzio Interuniversitario per le Biotecnologie and the Centro di Ricerca in Biotecnologie per la Salute Umana (Università degli studi dell’Insubria). The authors are grateful to all members of their laboratory and particularly to Mirella Pilone, Gianluca Molla (even for help in preparing Fig. 3), Laura Caldinelli, and Pamela Cappelletti for helpful discussions.
Abbreviations
- Aβ
Amyloid β-peptide
- AD
Alzheimer’s disease
- AMPA
α-Amino-3-hydroxy-5-methylisooxazole-4-propionic acid
- CNS
Central nervous system
- CP
Choroid plexus
- CSF
Cerebrospinal fluid
- DAAO
d-Amino acid oxidase (EC 1.4.3.3)
- d-DOPA
d-3,4-Dihydroxyphenylalanine
- DPFC
Dorsolateral prefrontal cortex
- FAD
Flavin adenine dinucleotide
- Golga3
Golgin subfamily A member
- GRIP
Glutamate receptor interacting protein
- PDZ
PSD95/disc large/ZO-1
- PICK1
Protein interacting with C kinase 1
- PIP2
Phosphatidylinositol(4,5)biphosphate
- PLP
Pyridoxal-5′ phosphate
- NMDAR
N-methyl-d-aspartate receptor
- NO
Nitric oxide
- SR
Serine racemase (EC 5.1.1.18)
References
- 1.Hashimoto A, Oka T, Nishikawa T. Extracellular concentration of endogenous free d-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- 2.Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator d-serine. Proc Natl Acad Sci USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schell MJ, Molliver ME, Snyder SH. d-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danysz W, Parsons CG. Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- 5.Hashimoto A, Nishikawa T, Oka T, Takahashi K. Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J Neurochem. 1993;60:783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 6.Schell MJ, Brady RO, Jr, Molliver ME, Snyder SH. d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro CS, Reis M, Panizzutti R, de Miranda J, Wolosker H. Glial transport of the neuromodulator d-serine. Brain Res. 2002;929:202–209. doi: 10.1016/s0006-8993(01)03390-x. [DOI] [PubMed] [Google Scholar]
- 8.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martineau M, Galli T, Baux G, Mothet JP. Confocal imaging and tracking of the exocytotic routes for d-serine-mediated gliotransmission. Glia. 2008;56:1271–1284. doi: 10.1002/glia.20696. [DOI] [PubMed] [Google Scholar]
- 10.Oliet SH, Mothet JP. Regulation of N-methyl-d-aspartate receptors by astrocytic d-serine. Neuroscience. 2009;158:275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda E, Ma N, Semba R. Immunohistochemical demonstration of l-serine distribution in the rat brain. Neuroreport. 2001;12:1027–1030. doi: 10.1097/00001756-200104170-00032. [DOI] [PubMed] [Google Scholar]
- 12.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- 13.Williams SM, Diaz CM, Macnab LT, Sullivan RK, Pow DV. Immunocytochemical analysis of d-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia. 2006;53:401–411. doi: 10.1002/glia.20300. [DOI] [PubMed] [Google Scholar]
- 14.Dun Y, Mysona B, Itagaki S, Martin-Studdard A, Ganapathy V, Smith SB. Functional and molecular analysis of d-serine transport in retinal Müller cells. Exp Eye Res. 2007;84:191–199. doi: 10.1016/j.exer.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao Z, Kamboj A, Anderson CM. Functional and immunocytochemical characterization of d-serine transporters in cortical neuron and astrocyte cultures. J Neurosci Res. 2009;87:2520–2530. doi: 10.1002/jnr.22086. [DOI] [PubMed] [Google Scholar]
- 16.Helboe L, Egebjerg J, Møller M, Thomsen C. Distribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur J Neurosci. 2003;18:2227–2238. doi: 10.1046/j.1460-9568.2003.02966.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo H, Kanai Y, Tokunaga M, Nakata T, Chairoungdua A, Ishimine H, Tsukada S, Ooigawa H, Nawashiro H, Kobayashi Y, Fukuda J, Endou H. High affinity d- and l-serine transporter Asc-1: cloning and dendritic localization in the rat cerebral and cerebellar cortices. Neurosci Lett. 2004;358:123–126. doi: 10.1016/j.neulet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Xie X, Dumas T, Tang L, Brennan T, Reeder T, Thomas W, Klein RD, Flores J, O’Hara BF, Heller HC, Franken P. Lack of the alanine–serine–cysteine transporter 1 causes tremors, seizures, and early postnatal death in mice. Brain Res. 2005;1052:212–221. doi: 10.1016/j.brainres.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Schell MJ. The N-methyl d-aspartate receptor glycine site and d-serine metabolism: an evolutionary perspective. Philos Trans R Soc Lond B Biol Sci. 2004;359:943–964. doi: 10.1098/rstb.2003.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martineau M, Baux G, Mothet JP. d-Serine signalling in the brain: friend and foe. Trends Neurosci. 2006;29:481–491. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc Natl Acad Sci USA. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junjaud G, Rouaud E, Turpin F, Mothet JP, Billard JM. Age-related effects of the neuromodulator d-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. J Neurochem. 2006;98:1159–1166. doi: 10.1111/j.1471-4159.2006.03944.x. [DOI] [PubMed] [Google Scholar]
- 23.Panatier A, Theodosis DT, Mothet J-P, Touquet B, Pollegioni L, Poulain DA, Oliet SHR. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Strísovský K, Jirásková J, Barinka C, Majer P, Rojas C, Slusher BS, Konvalinka J. Mouse brain serine racemase catalyzes specific elimination of l-serine to pyruvate. FEBS Lett. 2003;535:44–48. doi: 10.1016/s0014-5793(02)03855-3. [DOI] [PubMed] [Google Scholar]
- 25.Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, Li P, Toney MD, Kartvelishvily E, Wolosker H. Serine racemase modulates intracellular d-serine levels through an alpha, beta-elimination activity. J BiolChem. 2005;280:1754–1763. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- 26.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panizzutti R, De Miranda J, Ribeiro CS, Engelender S, Wolosker H. A new strategy to decrease N-methyl-d-aspartate (NMDA) receptor coactivation: inhibition of d-serine synthesis by converting serine racemase into an eliminase. Proc Natl Acad Sci USA. 2001;98:5294–5299. doi: 10.1073/pnas.091002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Miranda J, Panizzutti R, Foltyn VN, Wolosker H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-d-aspartate (NMDA) receptor coagonist d-serine. Proc Natl Acad Sci USA. 2002;99:14542–14547. doi: 10.1073/pnas.222421299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook SP, Galve-Roperh I, Martínez del Pozo A, Rodríguez-Crespo I. Direct calcium binding results in activation of brain serine racemase. J Biol Chem. 2002;277:27782–27792. doi: 10.1074/jbc.M111814200. [DOI] [PubMed] [Google Scholar]
- 30.Neidle A, Dunlop DS. Allosteric regulation of mouse brain serine racemase. Neurochem Res. 2002;27:1719–1724. doi: 10.1023/a:1021607715824. [DOI] [PubMed] [Google Scholar]
- 31.Dunlop DS, Neidle A. Regulation of serine racemase activity by amino acids. Brain Res Mol Brain Res. 2005;133:208–214. doi: 10.1016/j.molbrainres.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Strísovský K, Jirásková J, Mikulová A, Rulísek L, Konvalinka J. Dual substrate and reaction specificity in mouse serine racemase: identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the beta-eliminase activity. Biochemistry. 2005;44:13091–13100. doi: 10.1021/bi051201o. [DOI] [PubMed] [Google Scholar]
- 33.De Miranda J, Santoro A, Engelender S, Wolosker H. Human serine racemase: molecular cloning, genomic organization and functional analysis. Gene. 2000;256:183–188. doi: 10.1016/s0378-1119(00)00356-5. [DOI] [PubMed] [Google Scholar]
- 34.Xia M, Liu Y, Figueroa DJ, Chiu CS, Wei N, Lawlor AM, Lu P, Sur C, Koblan KS, Connolly TM. Characterization and localization of a human serine racemase. Brain Res Mol Brain Res. 2004;125:96–104. doi: 10.1016/j.molbrainres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, Nakamura K, Shimizu E, Itokawa M, Mori N, Iyo M, Yoshikawa T. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and d-serine levels. Biol Psychiatry. 2005;57:1493–1503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman HE, Jirásková J, Ingr M, Zvelebil M, Konvalinka J. Recombinant human serine racemase: enzymologic characterization and comparison with its mouse ortholog. Protein Expr Purif. 2009;63:62–67. doi: 10.1016/j.pep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Baumgart F, Mancheño JM, Rodríguez-Crespo I. Insights into the activation of brain serine racemase by the multi-PDZ domain glutamate receptor interacting protein, divalent cations and ATP. FEBS J. 2007;274:4561–4571. doi: 10.1111/j.1742-4658.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 38.Baumgart F, Rodríguez-Crespo I. d-amino acids in the brain: the biochemistry of brain serine racemase. FEBS J. 2008;275:3538–3545. doi: 10.1111/j.1742-4658.2008.06517.x. [DOI] [PubMed] [Google Scholar]
- 39.Pilone MS. d-Amino acid oxidase: new findings. Cell Mol Life Sci. 2000;57:1732–1747. doi: 10.1007/PL00000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G. Physiological functions of d-amino acid oxidases: from yeast to humans. Cell Mol Life Sci. 2007;64:1373–1394. doi: 10.1007/s00018-007-6558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs HA. Metabolism of amino-acids: deamination of amino-acids. Biochem J. 1935;29:1620–1644. doi: 10.1042/bj0291620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. The presence of free d-serine in rat brain. FEBS Lett. 1992;296:33–36. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K. Free d-serine, d-aspartate and d-alanine in central nervous system and serum in mutant mice lacking d-amino acid oxidase. Neurosci Lett. 1993;152:33–36. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- 44.Nagata Y, Yamamoto K, Shimojo T, Konno R, Yasumura Y, Akino T. The presence of free d-alanine, d-proline and d-serine in mice. Biochim Biophys Acta. 1992;1115:208–211. doi: 10.1016/0304-4165(92)90055-y. [DOI] [PubMed] [Google Scholar]
- 45.Nagata Y, Horiike K, Maeda T. Distribution of free d-serine in vertebrate brains. Brain Res. 1994;634:291–295. doi: 10.1016/0006-8993(94)91932-1. [DOI] [PubMed] [Google Scholar]
- 46.Konno R. Assignment of d-amino-acid oxidase gene to a human and a mouse chromosome. Amino Acids. 2001;20:401–408. doi: 10.1007/s007260170036. [DOI] [PubMed] [Google Scholar]
- 47.Momoi K, Fukui K, Watanabe F, Miyake Y. Molecular cloning and sequence analysis of cDNA encoding human kidney d-amino acid oxidase. FEBS Lett. 1988;238:180–184. doi: 10.1016/0014-5793(88)80252-7. [DOI] [PubMed] [Google Scholar]
- 48.Romano D, Molla G, Pollegioni L, Marinelli F. Optimization of human d-amino acid oxidase expression in Escherichia coli . Protein Expr Purif. 2009;68:72–78. doi: 10.1016/j.pep.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Molla G, Sacchi S, Bernasconi M, Pilone MS, Fukui K, Pollegioni L. Characterization of human d-amino acid oxidase. FEBS Lett. 2006;580:2358–2364. doi: 10.1016/j.febslet.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 50.Caldinelli L, Molla G, Sacchi S, Pilone MS, Pollegioni L. Relevance of weak flavin binding in human d-amino acid oxidase. Protein Sci. 2009;18:801–810. doi: 10.1002/pro.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawazoe T, Tsuge H, Imagawa T, Aki K, Kuramitsu S, Fukui K. Structural basis of d-DOPA oxidation by d-amino acid oxidase: alternative pathway for dopamine biosynthesis. Biochem Biophys Res Commun. 2007;355:385–391. doi: 10.1016/j.bbrc.2007.01.181. [DOI] [PubMed] [Google Scholar]
- 52.Kawazoe T, Tsuge H, Pilone MS, Fukui K. Crystal structure of human d-amino acid oxidase: context-dependent variability of the backbone conformation of the VAAGL hydrophobic stretch located at the si-face of the flavin ring. Protein Sci. 2006;15:2708–2717. doi: 10.1110/ps.062421606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattevi A, Vanoni MA, Todone F, Rizzi M, Teplyakov A, Coda A, Bolognesi M, Curti B. Crystal structure of d-amino acid oxidase: a case of active site mirror-image convergent evolution with flavocytochrome b2. Proc Natl Acad Sci USA. 1996;93:7496–7501. doi: 10.1073/pnas.93.15.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizutani H, Miyahara I, Hirotsu K, Nishina Y, Shiga K, Setoyama C, Miura R. Three-dimensional structure of porcine kidney d-amino acid oxidase at 3.0 Å resolution. J Biochem. 1996;120:14–17. doi: 10.1093/oxfordjournals.jbchem.a021376. [DOI] [PubMed] [Google Scholar]
- 55.Shoji K, Mariotto S, Ciampa AR, Suzuki H. Regulation of serine racemase activity by d-serine and nitric oxide in human glioblastoma cells. Neurosci Lett. 2006;392:75–78. doi: 10.1016/j.neulet.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 56.Shoji K, Mariotto S, Ciampa AR, Suzuki H. Mutual regulation between serine and nitric oxide metabolism in human glioblastoma cells. Neurosci Lett. 2006;394:163–167. doi: 10.1016/j.neulet.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 57.Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of d-serine formation. Proc Natl Acad Sci USA. 2007;104:2950–2955. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA. 2005;102:2105–2110. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002;277:5699–5702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- 60.Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J, Yamada T, Ozeki Y, Kawahara R, Okawa M, Huganir RL, Ujike H, Snyder SH, Sawa A. Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry. 2006;11:150–157. doi: 10.1038/sj.mp.4001776. [DOI] [PubMed] [Google Scholar]
- 61.Pan L, Wu H, Shen C, Shi Y, Jin W, Xia J, Zhang M. Clustering and synaptic targeting of PICK1 requires direct interaction between the PDZ domain and lipid membranes. EMBO J. 2007;26:4576–4587. doi: 10.1038/sj.emboj.7601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hikida T, Mustafa AK, Maeda K, Fujii K, Barrow RK, Saleh M, Huganir RL, Snyder SH, Hashimoto K, Sawa A. Modulation of d-serine levels in brains of mice lacking PICK1. Biol Psychiatry. 2008;63:997–1000. doi: 10.1016/j.biopsych.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dumin E, Bendikov I, Foltyn VN, Misumi Y, Ikehara Y, Kartvelishvily E, Wolosker H. Modulation of d-serine levels via ubiquitin-dependent proteasomal degradation of serine racemase. J Biol Chem. 2006;281:20291–20302. doi: 10.1074/jbc.M601971200. [DOI] [PubMed] [Google Scholar]
- 64.Balan L, Foltyn VN, Zehl M, Dumin E, Dikopoltsev E, Knoh D, Ohno Y, Kihara A, Jensen ON, Radzishevsky IS, Wolosker H. Feedback inactivation of d-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc NatlAcad Sci USA. 2009;106:7589–7594. doi: 10.1073/pnas.0809442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci USA. 2009;106:2921–2926. doi: 10.1073/pnas.0813105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D. Genetic and physiological data implicating the new human gene G72 and the gene for d-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, He G, Wang X, Chen Q, Qian X, Lin W, Li D, Gu N, Feng G, He L. Association of DAAO with schizophrenia in the Chinese population. Neurosci Lett. 2004;369:228–233. doi: 10.1016/j.neulet.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 68.Molla G, Bernasconi M, Sacchi S, Pilone MS, Pollegioni L. Expression in Escherichia coli and in vitro refolding of the human protein pLG72. Protein Expr Purif. 2006;46:150–155. doi: 10.1016/j.pep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Sacchi S, Bernasconi M, Martineau M, Mothet JP, Ruzzene M, Pilone MS, Pollegioni L, Molla G. pLG72 modulates intracellular d-serine levels through its interaction with d-amino acid oxidase: effect on schizophrenia susceptibility. J Biol Chem. 2008;283:22244–22256. doi: 10.1074/jbc.M709153200. [DOI] [PubMed] [Google Scholar]
- 70.Kvajo M, Dhilla A, Swor DE, Karayiorgou M, Gogos JA. Evidence implicating the candidate schizophrenia/bipolar disorder susceptibility gene G72 in mitochondrial function. Mol Psychiatry. 2008;13:685–696. doi: 10.1038/sj.mp.4002052. [DOI] [PubMed] [Google Scholar]
- 71.Otte DM, Bilkei-Gorzó A, Filiou MD, Turck CW, Yilmaz O, Holst MI, Schilling K, Abou-Jamra R, Schumacher J, Benzel I, Kunz WS, Beck H, Zimmer A. Behavioral changes in G72/G30 transgenic mice. Eur Neuropsychopharmacol. 2009;19:339–348. doi: 10.1016/j.euroneuro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 72.Konno R. Rat cerebral serine racemase: amino acid deletion and truncation at carboxy terminus. Neurosci Lett. 2003;349:111–114. doi: 10.1016/s0304-3940(03)00801-2. [DOI] [PubMed] [Google Scholar]
- 73.Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and d-serine release from microglia by amyloid beta-peptide. J Neuroinflammation. 2004;1:2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshikawa M, Nakajima K, Takayasu N, Noda S, Sato Y, Kawaguchi M, Oka T, Kobayashi H, Hashimoto A. Expression of the mRNA and protein of serine racemase in primary cultures of rat neurons. Eur J Pharmacol. 2006;548:74–76. doi: 10.1016/j.ejphar.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 75.Yoshikawa M, Takayasu N, Hashimoto A, Sato Y, Tamaki R, Tsukamoto H, Kobayashi H, Noda S. The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch Histol Cytol. 2007;70:127–134. doi: 10.1679/aohc.70.127. [DOI] [PubMed] [Google Scholar]
- 76.Dun Y, Duplantier J, Roon P, Martin PM, Ganapathy V, Smith SB. Serine racemase expression and d-serine content are developmentally regulated in neuronal ganglion cells of the retina. J Neurochem. 2008;104:970–978. doi: 10.1111/j.1471-4159.2007.05015.x. [DOI] [PubMed] [Google Scholar]
- 77.Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. d-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolosker H, Dumin E, Balan L, Foltyn VN. d-amino acids in the brain: d-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 79.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 80.Hepner F, Pollak A, Ulfig N, Yae-Kyung M, Lubec G. Mass spectrometrical analysis of human serine racemase in foetal brain. J Neural Transm. 2005;112:805–811. doi: 10.1007/s00702-005-0304-6. [DOI] [PubMed] [Google Scholar]
- 81.Neims AH, Zieverink WD, Smilack JD. Distribution of d-amino acid oxidase in bovine and human nervous tissues. J Neurochem. 1966;13:163–168. doi: 10.1111/j.1471-4159.1966.tb07508.x. [DOI] [PubMed] [Google Scholar]
- 82.Goldstein DB. d-amino acid oxidase in brain: distribution in several species and inhibition by pentobarbitone. J Neurochem. 1966;13:1011–1016. doi: 10.1111/j.1471-4159.1966.tb10299.x. [DOI] [PubMed] [Google Scholar]
- 83.Arnold G, Liscum L, Holtzman E. Ultrastructural localization of d-amino acid oxidase in microperoxisomes of the rat nervous system. J Histochem Cytochem. 1979;27:735–745. doi: 10.1177/27.3.39097. [DOI] [PubMed] [Google Scholar]
- 84.Horiike K, Tojo H, Arai R, Yamano T, Nozaki M, Maeda T. Localization of d-amino acid oxidase in Bergmann glial cells and astrocytes of rat cerebellum. Brain Res Bull. 1987;19:587–596. doi: 10.1016/0361-9230(87)90076-1. [DOI] [PubMed] [Google Scholar]
- 85.Horiike K, Tojo H, Arai R, Nozaki M, Maeda T. d-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res. 1994;652:297–303. doi: 10.1016/0006-8993(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 86.Kappor R, Kapoor V. Distribution of d-amino acid oxidase (DAO) activity in the medulla and thoracic spinal cord of the rat: implications for a role for d-serine in autonomic function. Brain Res. 1997;771:351–355. doi: 10.1016/s0006-8993(97)00886-x. [DOI] [PubMed] [Google Scholar]
- 87.Moreno S, Nardacci R, Cimini A, Cerù MP. Immunocytochemical localization of d-amino acid oxidase in rat brain. J Neurocytol. 1999;28:169–185. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- 88.Urai Y, Jinnouchi O, Kwak KT, Suzue A, Nagahiro S, Fukui K. Gene expression of d-amino acid oxidase in cultured rat astrocytes: regional and cell type specific expression. Neurosci Lett. 2002;324:101–104. doi: 10.1016/s0304-3940(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 89.Cristiano L, Bernardo A, Cerù MP. Peroxisome proliferator-activated receptors (PPARs) and peroxisomes in rat cortical and cerebellar astrocytes. J Neurocytol. 2001;30:671–683. doi: 10.1023/a:1016525716209. [DOI] [PubMed] [Google Scholar]
- 90.Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ono K, Shishido Y, Park HK, Kawazoe T, Iwana S, Chung SP, Abou El-Magd RM, Yorita K, Okano M, Watanabe T, Sano N, Bando Y, Arima K, Sakai T, Fukui K. Potential pathophysiological role of d-amino acid oxidase in schizophrenia: immunohistochemical and in situ hybridization study of the expression in human and rat brain. J Neural Transm. 2009;116:1335–1347. doi: 10.1007/s00702-009-0289-7. [DOI] [PubMed] [Google Scholar]
- 92.Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, Patel S. Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating d-serine reuptake in the mouse CNS. Eur J Neurosci. 2007;25:1757–1766. doi: 10.1111/j.1460-9568.2007.05446.x. [DOI] [PubMed] [Google Scholar]
- 93.Morikawa A, Hamase K, Inoue T, Konno R, Niwa A, Zaitsu K. Determination of free d-aspartic acid, d-serine and d-alanine in the brain of mutant mice lacking d-amino acid oxidase activity. J Chromatogr B Biomed Sci Appl. 2001;757:119–125. doi: 10.1016/s0378-4347(01)00131-1. [DOI] [PubMed] [Google Scholar]
- 94.Wu S, Basile AS, Barger SW. Induction of serine racemase expression and d-serine release from microglia by secreted amyloid precursor protein (sAPP) Curr Alzheimer Res. 2007;4:243–251. doi: 10.2174/156720507781077241. [DOI] [PubMed] [Google Scholar]
- 95.Inoue R, Hashimoto K, Harai T, Mori H. NMDA- and beta-amyloid1–42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci. 2008;28:14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasabe J, Chiba T, Yamada M, Okamoto K, Nishimoto I, Matsuoka M, Aiso S. d-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 2007;26:4149–4159. doi: 10.1038/sj.emboj.7601840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Katsuki H, Nonaka M, Shirakawa H, Kume T, Akaike A. Endogenous d-serine is involved in induction of neuronal death by N-methyl-d-aspartate and simulated ischemia in rat cerebrocortical slices. J Pharmacol Exp Ther. 2004;311:836–844. doi: 10.1124/jpet.104.070912. [DOI] [PubMed] [Google Scholar]
- 98.Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A. Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci. 2007;81:740–749. doi: 10.1016/j.lfs.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 100.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 101.Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH, Roder JC. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of d-serine in patients with schizophrenia: evidence in support of the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- 104.Bendikov I, Nadir S, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Steffek AE, Haroutunian V, Meador-Woodruff JH. Serine racemase protein expression in cortex and hippocampus in schizophrenia. Neuroreport. 2006;17:1181–1185. doi: 10.1097/01.wnr.0000230512.01339.72. [DOI] [PubMed] [Google Scholar]
- 106.Burnet PW, Eastwood SL, Bristow GC, Godlewska BR, Sikka P, Walker M, Harrison PJ. d-Amino acid oxidase activity and expression are increased in schizophrenia. Mol Psychiatry. 2008;13:658–660. doi: 10.1038/mp.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain d-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Ohi K, Hashimoto R, Yasuda Y, Yoshida T, Takahashi H, Iike N, Fukumoto M, Takamura H, Iwase M, Kamino K, Ishii R, Kazui H, Sekiyama R, Kitamura Y, Azechi M, Ikezawa K, Kurimoto R, Kamagata E, Tanimukai H, Tagami S, Morihara T, Ogasawara M, Okochi M, Tokunaga H, Numata S, Ikeda M, Ohnuma T, Ueno S, Fukunaga T, Tanaka T, Kudo T, Arai H, Ohmori T, Iwata N, Ozaki N, Takeda M. Association study of the G72 gene with schizophrenia in a Japanese population: a multicenter study. Schizophr Res. 2009;109:80–85. doi: 10.1016/j.schres.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 109.Opgen-Rhein C, Lencz T, Burdick KE, Neuhaus AH, DeRosse P, Goldberg TE, Malhotra AK. Genetic variation in the DAOA gene complex: impact on susceptibility for schizophrenia and on cognitive performance. Schizophr Res. 2008;103:169–177. doi: 10.1016/j.schres.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, Coppola R, Egan MF, Weinberger DR. The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology. 2006;31:2022–2032. doi: 10.1038/sj.npp.1301049. [DOI] [PubMed] [Google Scholar]
- 111.Ohnuma T, Shibata N, Maeshima H, Baba H, Hatano T, Hanzawa R, Arai H. Association analysis of glycine- and serine-related genes in a Japanese population of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:511–518. doi: 10.1016/j.pnpbp.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Goltsov AY, Loseva JG, Andreeva TV, Grigorenko AP, Abramova LI, Kaleda VG, Orlova VA, Moliaka YK, Rogaev EI. Polymorphism in the 5′-promoter region of serine racemase gene in schizophrenia. Mol Psychiatry. 2006;11:325–326. doi: 10.1038/sj.mp.4001801. [DOI] [PubMed] [Google Scholar]
- 113.Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, Hamase K, Zaitsu K, Kuroda S. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry. 2007;61:1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 114.Yoshikawa M, Shinomiya T, Takayasu N, Tsukamoto H, Kawaguchi M, Kobayashi H, Oka T, Hashimoto A. Long-term treatment with morphine increases the d-serine content in the rat brain by regulating the mRNA and protein expressions of serine racemase and d-amino acid oxidase. J Pharmacol Sci. 2008;107:270–276. doi: 10.1254/jphs.08030fp. [DOI] [PubMed] [Google Scholar]
- 115.Turpin FR, Potier B, Dulong JR, Sinet PM, Alliot J, Oliet SH, Dutar P, Epelbaum J, Mothet JP, Billard JM (2009) Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2009.09.001. Epub ahead of print [DOI] [PubMed]