Abstract
Sphingosine 1-phosphate (S1P) is a bioactive lipid that acts through a family of G-protein-coupled receptors. Herein, we report evidence of a novel redox-based cross-talk between S1P and insulin signaling pathways. In skeletal muscle cells S1P, through engagement of its S1P2 receptor, is found to produce a transient burst of reactive oxygen species through a calcium-dependent activation of the small GTPase Rac1. S1P-induced redox-signaling is sensed by protein tyrosine phosphatase-1B, the main negative regulator of insulin receptor phosphorylation, which undergoes oxidation and enzymatic inhibition. This redox-based inhibition of the phosphatase provokes a ligand-independent trans-phosphorylation of insulin receptor and a strong increase in glucose uptake. Our results propose a new role of S1P, recognizing the lipid as an insulin-mimetic cue and pointing at reactive oxygen species as critical regulators of the cross-talk between S1P and insulin pathways. Any possible implication of S1P-directed insulin signaling in diabetes and insulin resistance remains to be established.
Keywords: Sphingosine 1-phosphate, Insulin, Insulin receptor, Protein-tyrosine phosphatase, Reactive oxygen species, Glucose uptake
Introduction
Sphingosine 1-phosphate (S1P), initially regarded as a catabolic product of sphingomyelin metabolism, is presently considered one of the most versatile lipid signaling molecules. Indeed, this lysophospholipid influences a diverse range of cellular processes, including proliferation, differentiation, motility, and survival, largely through ligation to a panel of five distinct G-protein-coupled receptors (GPCRs), named S1P receptors (S1PRs) [1, 2]. Intriguingly, the biological response elicited by S1P in a given cell type appears to be critically dependent on the expression pattern of S1PRs since they are differentially coupled to heterotrimeric G-proteins and downstream signaling pathways. S1P is a physiological component of serum, where it is mainly associated with HDL particles [3], therefore capable of acting on numerous different tissues. In addition, it is becoming increasingly clear that S1P can be autonomously produced by multiple cell types, where it acts in autocrine or paracrine fashion. Many studies have shown that cellular S1P metabolism is quite complex: its biosynthesis is catalyzed by two distinct isoenzymes, named sphingosine kinase (SK)1 and SK2, that play different roles, with SK1 involved in cellular proliferation and motility and SK2 implicated in apoptosis [2]. The catabolism of S1P is also subjected to distinct enzymes; specific S1P phosphatases produce sphingosine, which can be addressed to the sphingolipid salvage pathway, while S1P lyase cleaves it into phospho-ethanolamine and hexadecenal [2].
Of note, the SK/S1P signaling pathway is implicated in a complex cross-talk with growth factors, cytokines, and hormones. Indeed, S1P was initially discovered as a cellular messenger generated in response to platelet-derived growth factor (PDGF), participating in its mitogenic response [4]. Thereafter, it was demonstrated that several extracellular cues exhibit biological activities similar to S1P and that S1PR can be transactivated by ligand-dependent stimulation of various tyrosine kinase receptors [5], serine/threonine kinase receptors [6], death domain receptors [7], as well as GPCRs [8]. Moreover, it appears that the S1P interplay with growth factor and cytokine receptors is bidirectional. In this respect, S1P is capable of transactivating receptor tyrosine kinases (RTKs), such as vascular endothelial growth factor (VEGF) receptor Flk1/KDR [9], PDGFβR [10, 11], and epidermal growth factor receptor (EGFR) [10], as well as transforming growth factor β (TGFβ) signaling pathway [12] in favor of a key role of SK/S1P in regulating the intensity of signals generated by growth factors and cytokines. S1P appears capable of transactivating RTK by either ligand-dependent or ligand-independent mechanisms. So, in injured hepatocytes, it induces PDGF-BB mRNA expression via S1P2 [13], whereas it rapidly enhances tyrosine phosphorylation of EGFR and PDGFβR in vascular smooth cells [10].
Insulin receptor (Ins-R) is an RTK that is critically involved in the hormone-dependent removal of excess glucose from the bloodstream after a meal. In skeletal muscle, its expression contributes to 80% of whole-body insulin-stimulated glucose disposal. In this respect, modulation of Ins-R sensitivity represents a key issue in the struggle against glucose intolerance and type-2 diabetes [14]. It is well-known that Ins-R tyrosine phosphorylation is regulated by different PTPs, among which protein tyrosine phosphatase-1B (PTP1B) plays a predominant role, as recently demonstrated by Fiaschi et al. [15].
Previous studies performed in C2C12 myoblasts have established that S1P regulates multiple signaling pathways and, acting via S1P2, stimulates myogenesis and inhibits migratory response [16, 17].
In this study we provide the first evidence that in these cells S1P induces a novel signaling pathway, which by enhancing reactive oxygen species and thus inhibiting PTP1B, transactivates Ins-R in a ligand-independent fashion. Importantly, these molecular events account for the enhancement of glucose uptake elicited by the bioactive sphingolipid, thereby opening new avenues for the pharmacological control of glycaemic parameters.
Materials and methods
Materials
C2C12 cells were obtained from the European Collection of Cell Cultures (Salisbury, UK). Dulbecco’s Modified Eagle’s Medium (DMEM) was from Cambrex (East Rutherford, NJ, USA), and fetal calf serum (FCS) was from Euroclone (Pero, Italy). Unless specified, all reagents included protease inhibitor cocktail. Bovine serum albumin (BSA), phorbol 12-myristate 13-acetate (PMA), nordihydroguaiaretic acid (NDGA), rotenone, wortmannin, N-acetyl-l-cysteine (NAC), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM), and diphenyleneiodonium chloride (DPI) were purchased from Sigma (Sigma-Aldrich, Milan, Italy). D-erythro-sphingosine 1-phosphate (S1P) was from Calbiochem (San Diego, CA, USA). 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) was from Molecular Probes (the Netherlands). JTE013, LY294002, and Gö6976 were from Tocris Bioscience (Bristol, UK). FTY720-P was a kind gift of Prof. Kleuser (Free University of Berlin, Berlin, Germany). Coomassie Blue reagent was from Bio-Rad (Hercules, CA, USA). Enhanced chemiluminescence reagents and polyvinylidene difluoride (PVDF) membrane were from Amersham Pharmacia Biotech (Uppsala, Sweden). Anti-Ins-R, anti-phospho-Ins-R (Tyr1162/1163), anti-protein-tyrosine phosphatase 1B (PTP1B), anti-Akt, anti-phospho-Akt, anti-mouse, anti-rabbit immunoglobulin G conjugated to horseradish peroxidase, and Blotto (non-fat dry milk) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-Rac1 was from BD Transduction Laboratories (Lexington, KY, USA). Recombinant PTP1B (50 kDa) was a gift of Dr. Paolo Paoli (University of Firenze, Italy).
Cell culture and transfection
C2C12 mouse myoblasts were grown in DMEM, supplemented with 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2 humidified atmosphere, and used below the 25th passage. For all the experiments, C2C12 cells were used at about 60% confluence. For transient transfections: pBABE-GFP-RacN17 plasmid was purified using QIAfilter Plasmid Midi kit (Qiagen) and transfected using GeneJammer Transfection Reagent (Stratagene) according to the manufacturer’s instructions. To decrease intracellular Ca2+ concentration, cells were kept in Krebs–Ringer modified buffer (KRB) (125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM Na2HPO4, 5.5 mM glucose, 2 mM l-glutamine, and 20 mM Hepes, 40 μM CaCl2, 0.1% BSA, pH 7.4) containing 40 μM CaCl2.
Intracellular H2O2 assay
Levels of intracellular hydrogen peroxide were measured spectrofluorometrically by the oxidation of H2DCF-DA to DCF-DA as previously described [15]. Cells were serum-deprived for 24 h and then challenged with 1 μM S1P or treated with different inhibitors 45 min before the addition of S1P. Three minutes before the end of stimulation, 10 μM H2DCF-DA was added. Cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 100 mM sodium fluoride, 1% Triton X-100, 2 mM EGTA, 1 mM sodium orthovanadate, 1 mM phenyl-methanesulphonyl-fluoride) with protease inhibitor cocktail (1.04 mM AEBSF, 0.08 μM aprotinin, 0.02 mM leupeptin, 0.04 mM bestatin, 15 μM pepstatin A, 14 μM E-64), and fluorescence was immediately analyzed using a Perkin Elmer Fluorescence Spectrophotometer (excitation wavelength 488 nm, emission wavelength 510 nm). Obtained values were normalized on protein content.
Determination of Rac1 activity
After stimulation, cells were washed twice with ice-cold PBS, lysed in RIPA buffer, and then clarified by centrifugation at 10,000×g for 15 min at 4°C. Briefly, 100 μg/sample of lysates were incubated with 10 μg of GST-PAK-1 fusion protein absorbed on glutathione-Sepharose beads for 2 h at 4° C. Immunoreactive Rac1 linked to GST-PAK-1 was then quantified by anti-Rac1 Western blot analysis [15].
Immunoprecipitation and Western blot analysis
Following stimulation, cells were lysed for 30 min on ice in 500 μl of complete RIPA lysis buffer [containing 0.1% sodium dodecylsulfate-(SDS), 0.5% deoxycholate]. Lysates were clarified by centrifugation (15 min at 10,000×g at 4°C), and 500 μg/sample were immunoprecipitated for 2 h at 4°C with 2 μg of anti-Ins-rβ. Immune complexes were collected on protein A-Sepharose, resuspended in Laemmli’s SDS sample buffer, separated by SDS/PAGE, and transferred onto PVDF, as previously described [18]. For Akt and phospho-Akt analysis, 30 μg/sample of clarified lysates was immunoblotted. Bound antibodies were detected using ECL reagents and analyzed with a Biorad ChemiDoc Imaging System for dedicated chemiluminescent image acquisition.
In-gel phosphatase assay
For detection of protein-tyrosine phosphatase (PTP) activity, a 10% SDS-polyacrylamide gel containing 105 cpm/ml of [32P]-labeled substrate was prepared as described previously [19]. In the modified version of the assay, to detect oxidized PTPs [20], 10 mM iodoacetic acid was added to the samples after degassing the buffer. Samples were subjected to immunoprecipitation with specific antibodies and then analyzed by in-gel assay. After electrophoresis, gels were sequentially washed for the indicated times at room temperature with the following buffers: buffer 1 (overnight)—50 mM Tris pH 8 and 20% isopropanol; buffer 2 (twice, for 30 min)—50 mM Tris pH 8 and 0.3% β-mercaptoethanol; buffer 3 (90 min)—50 mM Tris pH 8, 0.3% β-mercaptoethanol, 6 M guanidine hydrochloride, and 1 mM EDTA; buffer 4 (three washes for 1 h each)—50 mM Tris pH 8, 0.3% β-mercaptoethanol, 1 mM EDTA, and 0.04% Tween 20; buffer 5 (overnight)—50 mM Tris pH 8, 0.3% β-mercaptoethanol, 1 mM EDTA, 0.04% Tween 20, and 4 mM DTT. Gels were then stained with Coomassie brillant blue, de-stained in 40% methanol and 10% acetic acid, dried, and analyzed using a Cyclone system (PerkinElmer).
Glucose uptake assay
To determine glucose uptake, C2C12 cells were seeded in six-well plates, serum-starved for 18 h, and then stimulated with 1 μM S1P or 10 nM insulin for 90 min, or pretreated with inhibitors 45 min before S1P or insulin addition. Glucose transport was assayed essentially as previously described [21]. Briefly, following S1P or insulin challenge, 1 μCi 2-deoxy-D-2-3H-glucose (1 mCi/0.1 mmol) was added to each well and glucose uptake was allowed at 37°C for 10 min. Cells were subsequently washed with cold PBS and lysed in 0.1 M NaOH. Aliquots (550 μl) of the lysates were counted with a β scintillation counter.
Statistical analysis
Statistical analysis was performed using Student’s t test, and P < 0.05 was considered significant. Data are reported as means ± SEM of at least three experiments with similar results. Densitometric analysis of Western blot bands was performed using imaging and analysis software by Bio-Rad (Quantity One).
Results
S1P challenge induces ROS generation in C2C12 myoblasts
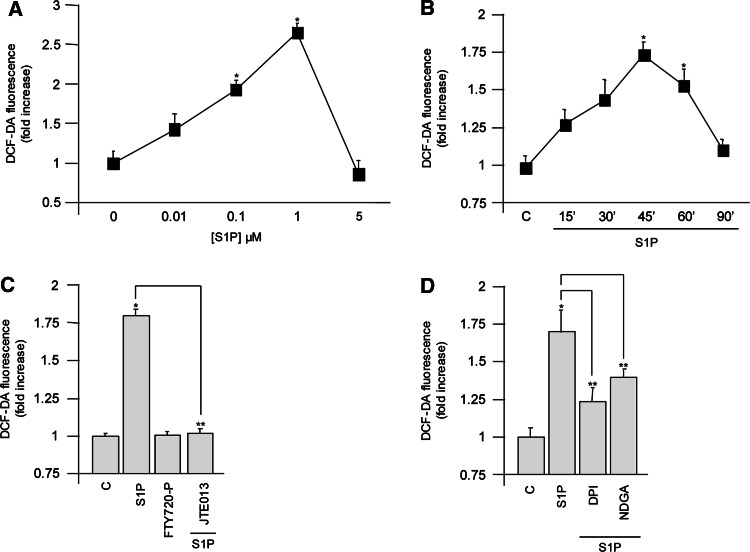
First we investigated whether S1P was able to induce ROS generation in C2C12 myoblasts. To this end, we measured H2O2 production using the fluorescent dye H2DCF-DA as probe. S1P treatment for 30 min dose-dependently increased ROS production with a maximum effect at 1 μM S1P (Fig. 1a). Moreover, 1 μM S1P increased H2O2 in a time-dependent manner, reaching a peak at 45–60 min, while after 90 min from stimulation, ROS levels were very close to control values (Fig. 1b). It is well-known that S1P acts mainly through its binding with specific membrane receptors [1, 2]. Given that C2C12 myoblasts express three receptor subtypes (namely S1P1, S1P2, and S1P3)[22], we analyzed their possible involvement in ROS production. For this purpose, cells were challenged with 10 μM FTY720-P, a S1PR agonist with an affinity comparable to S1P towards all S1PRs except S1P2 [23], or with 1 μM JTE013, a selective antagonist of S1P2, added 45 min before S1P stimulation [24]. Cell treatment with FTY720-P failed to elicit ROS production (Fig. 1c), suggesting that the action of the sphingolipid was independent of S1P1 and S1P3. Conversely, the blockade of S1P2 by JTE013 treatment abrogated the S1P-induced enhancement of ROS, clearly pointing at this receptor subtype as a critical mediator of S1P response (Fig. 1c). To characterize the source of ROS production following S1P treatment, the effect of selective inhibitors of enzymes responsible for intracellular ROS formation was then analyzed. We used 20 μM DPI to inhibit NADPH oxidase [25] and 10 μM NDGA to block 5-lypoxigenase (5-LOX) [26]. Both inhibitors were efficacious in reducing ROS production, with a more marked effect exerted by DPI (Fig. 1d). These data suggest the involvement of NADPH oxidase and 5-LOX in ROS production following S1P challenge. In keeping with several other redox-producing hormones, S1P is also able to activate multiple ROS sources [27].
Fig. 1.
S1P treatment generates transient ROS production in C2C12 myoblasts. Cells were serum-starved before stimulation. C2C12 myoblasts serum starved for 24 h were challenged with different concentrations of S1P (a) or with 1 μM S1P for the indicated time intervals (b). Cells stimulated with 1 μM S1P or 10 μM FTY720-P for 1 h were pretreated or not with 1 μM JTE013 for 45 min (c). Cells challenged with 1 μM S1P for 1 h were pretreated with inhibitors of intracellular ROS sources (20 μM DPI and 10 μM NDGA) for 45 min (d). All data are representative of at least three independent experiments performed in duplicate. *P < 0.05 versus control or **P < 0.05 versus S1P-treated cells
S1P-induced Rac1 activation and Akt phosphorylation are dependent on intracellular calcium release
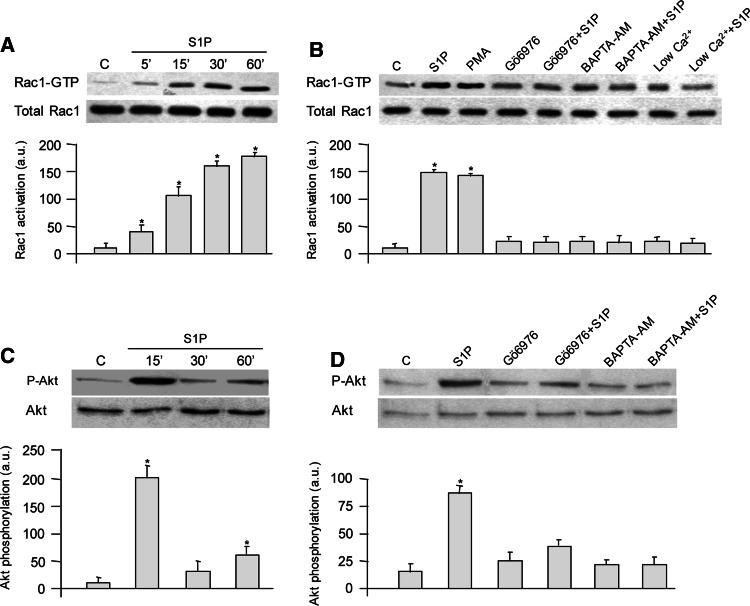
Since the small GTPase Rac1 is a downstream signaling target of several membrane receptors implicated in ROS production [28], C2C12 cells were stimulated with S1P for different time intervals and Rac1 activation was analyzed. As can be observed in Fig. 2a, S1P evoked a rapid and sustained activation of Rac1 already appreciable at 5 min of incubation and maintained up to 60 min, in full agreement with a previous study [16]. Since Ca2+ increase has been found upstream of Rac activation [29] and S1P is capable of elevating cytosolic Ca2+ levels and consequently activating conventional protein kinase C (cPKC) in these cells [22, 30], next we analyzed the molecular mechanism by which Rac1 was activated. To this end C2C12 cells were treated with 500 nM Gö6976, a cPKC inhibitor, or with 15 μM BAPTA-AM, an intracellular calcium chelator, 45 min before S1P addition [31]. As illustrated in Fig. 2b, both treatments completely abolished Rac1 activation, whereas challenge with 300 nM PMA, which irreversibly activates cPKC, induced a significant increase in GTP-Rac1 comparable to that provoked by S1P addition. Moreover, when cytosolic Ca2+ was reduced by 3-h incubation in 40 μM CaCl2/KRB before S1P addition [32], the Rac-GTP increase induced by S1P was abolished (Fig 2b). These data strongly suggest that in C2C12 myoblasts, the activation of small GTPase Rac1 is Ca2+- and cPKC-dependent.
Fig. 2.
S1P challenge induces Rac1 activation in a Ca2+-dependent manner. C2C12 myoblasts were serum-starved for 24 h before stimulation with 1 μM S1P for the specified time intervals (a), or pretreated for 45 min with 500 nM Gö6976, or with 15 μM BAPTA-AM before S1P addition, or maintained in Krebs–Ringer-modified buffer supplemented with 40 μM CaCl2 for 3 h before challenge to reduce intracellular Ca2+ (b). Cells were then lysed and processed as described in “Materials and methods” to quantify active and total Rac1 by Western blot analysis using an antibody anti-Rac1. All data are representative of at least three independent experiments. The bar graphs below the Western blots show the means of the ratio of activated Rac1 to total Rac1. In panels c and d, myoblasts were treated as in a and b, and Akt phosphorylation was evaluated. Total lysates (30 μg/sample) were immunoblotted and revealed with anti-phospho-Akt and anti-Akt antibodies. Blots are representative of at least three independent experiments, and the bar graphs show the means of the ratio of phosphorylated Akt versus Akt. *P < 0.05 versus untreated cells. Densitometric analysis of Western blot bands was performed using imaging and analysis software by Bio-Rad (Quantity One)
Recently, it was demonstrated that S1P is able to trigger a transient Akt phosphorylation in C2C12 myoblasts [17]. In agreement, as shown in Fig. 2c, S1P treatment exerted a biphasic effect on Akt phosphorylation presenting a robust increase at 15 min, and a clear, although less pronounced rise at 60 min. To gain more insight into the mechanism involved in S1P-induced Akt phosphorylation after 60-min challenge, myoblasts were treated with 500 nM Gö6976 or with 15 μM BAPTA-AM before S1P addition. As shown in Fig. 2d, both treatments dramatically reduced Akt phosphorylation supporting the view that Ca2+/cPKC are upstream of Akt activation.
ROS generation depends on multiple signaling pathways
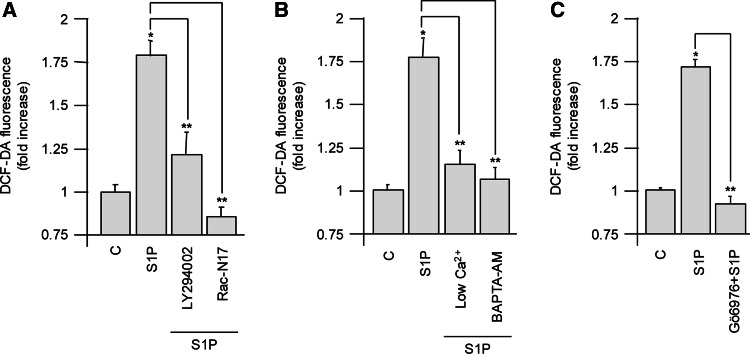
Since the small GTPase Rac1 has been shown to be involved in the regulation of both NADPH oxidase and 5-LOX activities [33], we also investigated the role of Rac1 in ROS production in C2C12 cells by transiently overexpressing the dominant negative mutant of the protein (Rac N17). The functional inhibition of Rac1 abrogated the ROS peak following S1P treatment (Fig. 3a), suggesting that this small GTPase plays a key role in S1P redox signaling. Given that Rac1 activation is regulated by Ca2+ and cPKC [29, 30], we examined if ROS increase upon S1P challenge was influenced by these signaling pathways. Decreasing intracellular Ca2+ concentration by either keeping the cells in 40 μM CaCl2/KRB or chelating the cation by treating myoblasts with BAPTA-AM abolished ROS formation (Fig. 3b). Similarly, inhibition of cPKC by Gö6976 prevented S1P-induced ROS increase (Fig. 3c). Given that the phosphatidylinositol 3-kinase (PI3 K)/Akt pathway plays a pivotal role in modulating ROS production in different cell types [34, 35], to further examine the molecular mechanisms responsible for the observed ROS production by S1P, its role was also investigated. As shown in Fig. 3a, when cells were treated for 45 min with the PI3 K inhibitor LY294002 (10 μM) before the addition of S1P, ROS production was not statistically different from control, demonstrating that PI3 K also plays a role in regulating this process.
Fig. 3.
ROS generation is Ca2+-, cPKC-, PI3 K-, and Rac1-dependent. C2C12 myoblasts were kept in serum-free culturing medium for 24 h, and then 1 μM S1P was added 1 h before ROS measurements. C2C12 myoblasts overexpressing the dominant negative mutant Rac N17 were challenged with 1 μM S1P (a). Cells were pretreated with 15 μM BAPTA-AM for 45 min or kept 3 h in 40 μM CaCl2/KRB (b), with 500 nM Gö6976 for 45 min (c), or with 10 μM LY294002 for 45 min (a), before S1P addition. All data are representative of at least five independent experiments. *P < 0.05 versus control or **P < 0.05 versus S1P treated cells
S1P gives rise to an ROS-dependent, insulin-independent phosphorylation of insulin receptor
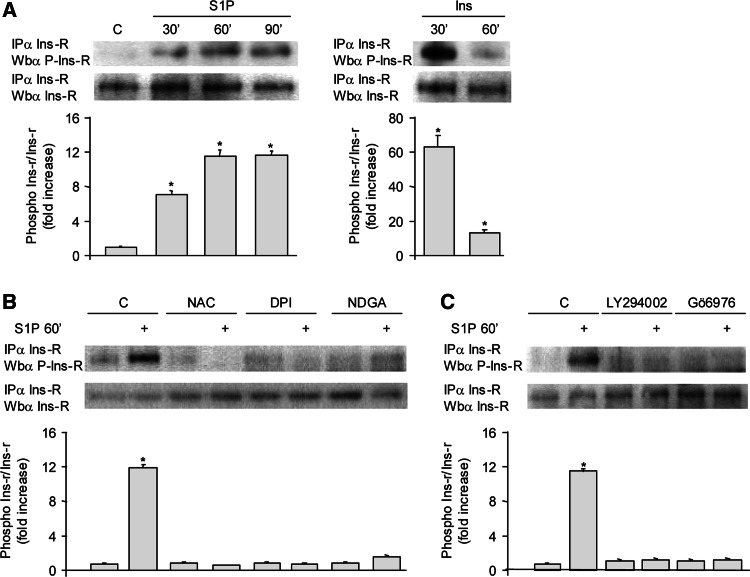
Agonist-induced ROS production represents a common mechanism for the redox-dependent regulation of phosphorylation of a wide variety of TRKs. Interestingly in a recent paper, Fiaschi and colleagues demonstrated that in liver cells adiponectin induced a ligand-independent trans-phosphorylation of Ins-R, mediated by ROS enhancement [15]. In this vein, we investigated whether S1P redox signaling was able to affect the Ins-R phosphorylation state in C2C12 myoblasts. Data presented in Fig. 4a show that 30 min of incubation with 1 μM S1P elicited a trans-phosphorylation of Ins-R, reaching a maximum after 60 min, in agreement with the observed time course of ROS levels in response to the bioactive sphingolipid. To verify that S1P-evoked ROS formation was functionally linked to trans-activation of Ins-R, ROS production was inhibited by incubating cells with 20 μM DPI, 10 μM NDGA, or 20 μM NAC 45 min before S1P challenge. Thereafter, Ins-R was immunoprecipitated from cell lysates. As shown in Fig. 4b, NAC, DPI, and NDGA strongly interfered with S1P-dependent trans-phosphorylation of Ins-R (Fig. 4b). Similar results were obtained when PI3 K or cPKC signaling pathway was inhibited using LY294002 or Gö6976, respectively (Fig. 4c).
Fig. 4.
S1P causes trans-phosphorylation of Ins-R in an ROS-dependent manner. C2C12 myoblasts were serum-starved for 24 h and then stimulated with 1 μM S1P or 10 nM insulin for the indicated time intervals (a). In panels b and c, cells treated as in panel a were pretreated with 20 mM NAC, 20 μM DPI, and 10 μM NDGA (b), or with 10 μM LY294002 and 500 nM Gö6976 (c), 45 min before S1P challenge. Ins-R phosphorylation level was assessed by Western blotting with anti-phospho-Ins-R (Tyr1162/1163) following immunoprecipitation against Ins-R. Data are from one representative experiment, which was repeated three times with analogous results. *P < 0.05 versus control
S1P treatment causes a redox modulation of protein-tyrosine phosphatase 1B activity
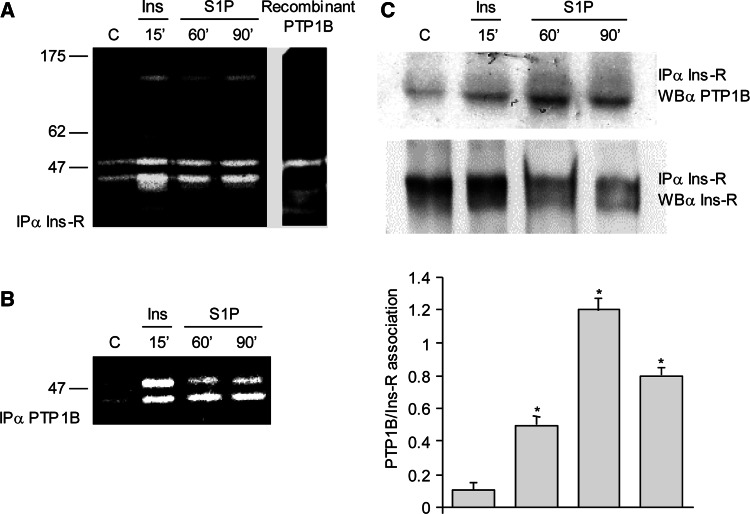
To verify whether PTP1B was implicated in the redox-dependent signaling triggered by S1P, the redox state of PTPs during S1P stimulation was analyzed by a modified PTP in-gel assay [20]. Ins-R was immunoprecipitated from lysates and subjected to SDS-PAGE in a gel containing a radioactive PTP substrate. Gels were then kept in a solution containing a reducing agent to allow full rescue of oxidized PTPs only. Their activity was visualized by appearance of a white area around the band corresponding to the PTP in the gel. We observed that a PTP of a relative molecular mass of 50 kDa increased its oxidation levels upon S1P or insulin stimulation (Fig. 5a). On the basis of the molecular size, the results suggested that the ~50 kDa PTP might be PTP1B. To demonstrate the identity of the ~50 kDa PTP, cell lysates were immunoprecipitated against PTP1B following S1P or insulin challenge. As shown in Fig. 5b, PTP1B oxidation was indeed enhanced by S1P challenge. The presence of two different bands in PTP1B immunoprecipitates is not surprising considering that the occurrence of multiple fragments of PTP1B in in-gel assay has already been reported, probably due to different post-translational modifications or different oxidation states [20].
Fig. 5.
S1P stimulates PTP1B oxidation. Serum-starved C2C12 myoblasts were exposed to 1 μM S1P or 10 nM insulin for the indicated times. Lysates were prepared as described in “Materials and methods,” immunoprecipitated against Ins-R, and then subjected to modified in-gel PTP assay. Recombinant PTP1B was run as control (a). In b, C2C12 myoblasts were treated as described in panel a, lysates were immunoprecipitated with anti-PTP1B antibodies and then subjected to modified in-gel PTP assay. c C2C12 myoblasts were stimulated as in a, and an immunoprecipitation anti-Ins receptor was performed. The amount of PTP1B associated with Ins receptor was evaluated by immunoblotting against PTP1B, and an anti-Ins-R was performed for normalization. All data are representative of at least three independent experiments. The bar graphs show the association level between Ins-R and PTP1B. *P < 0.05 versus untreated cells
By immunoblot analysis, we finally verified that the protein associated with Ins-R in immunoprecipitates was indeed PTP1B (Fig. 5c). Hence these findings show that Ins-R undergoes a ligand-independent activation following a redox-induced inhibition of its dephosphorylation mediated by PTP1B.
S1P enhances cell glucose uptake
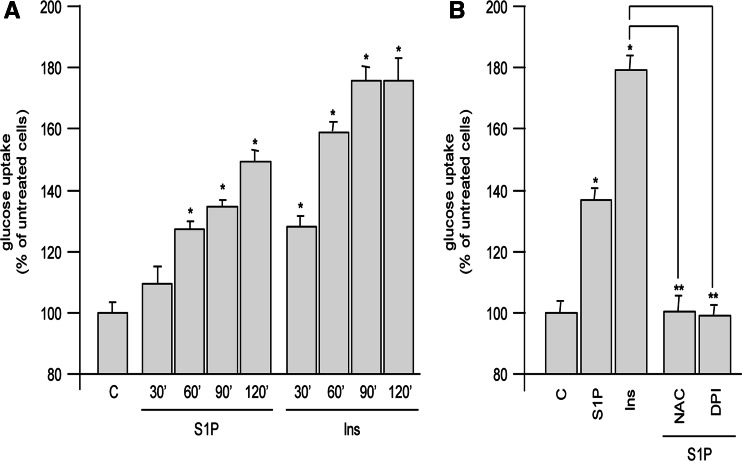
Ins-R phosphorylation is required for the biochemical events that mediate the insulin-induced cellular responses, including the enhancement of cell glucose uptake. Since S1P was able to trans-activate Ins-R, we investigated whether the bioactive lipid could also influence this biochemical parameter. As shown in Fig. 6a, S1P was able to increase glucose uptake by C2C12 myoblasts in a time-dependent manner, which was compatible with the time course of ROS production, as well as of Ins-R trans-phosphorylation. S1P induced a significant glucose uptake 60 min after the bioactive lipid addition with a maximum at 2 h. When insulin was used as positive control, a statistically significant glucose uptake rise was observed already at 30 min, reaching a plateau at 90 min. To confirm that the S1P-stimulated glucose uptake was downstream of ROS production, C2C12 myoblasts were treated with the ROS scavenger NAC or with the NADPH oxidase inhibitor DPI. As shown in Fig. 6b, S1P-dependent glucose uptake was significantly reduced when ROS production was prevented. In keeping with these findings, the stimulatory effect of S1P was also diminished when Ca2+ increase was prevented by cell loading with BAPTA-AM or PI3 K/Akt, or cPKC pathways were blocked by LY294002 or Gö6976 (data not shown).
Fig. 6.
S1P enhances glucose uptake in C2C12 myoblasts. Cells were serum-starved for 18 h and then stimulated with 1 μM S1P or 10 nM insulin for different periods of time. Subsequently, 1 μCi 2-deoxy-D-2-3H-glucose (1 mCi/0.1 mmol) was added to each well, and glucose uptake was allowed at 37°C for 10 min (a). Otherwise, cells were pretreated with the indicated inhibitors 45 min before S1P or insulin addition (b). Aliquots (500 μl) of the lysates were counted in liquid scintillation counter. All data are representative of at least five independent experiments. *P < 0.05 versus untreated cells or **P < 0.05 versus S1P-treated cells
Discussion
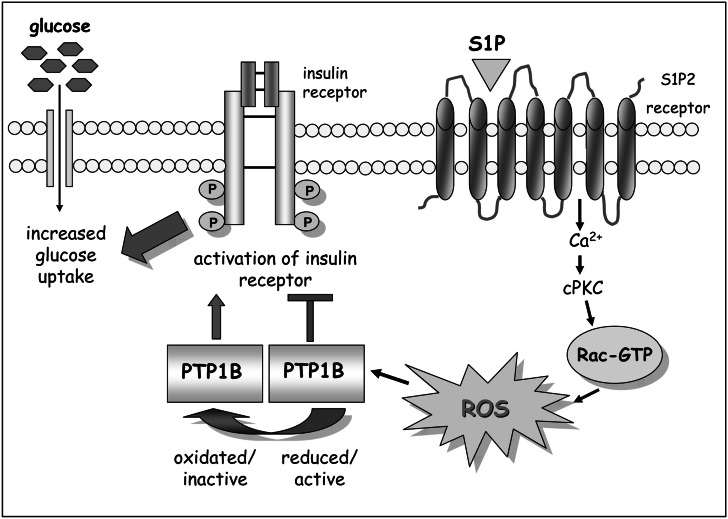
The data reported herein describe a novel signaling pathway triggered by S1P in myoblasts involving a cross-talk with Ins-R. Our findings lead to three major conclusions: (1) ROS have a major role in the signaling cascade triggered by S1P, (2) these oxidants trigger oxidation and inactivation of PTP1B, thereby enhancing the tyrosine phosphorylation of Ins-R in the absence of the natural ligand, (3) this trans-phosphorylation of Ins-R gives rise to an enhancement of glucose uptake in response to S1P (Fig. 7).
Fig. 7.
Proposed mechanism of action of S1P in modulating glucose uptake through trans-activation of Ins-R. S1P signaling induces a Ca2+-dependent ROS burst that triggers oxidation and inactivation of PTP1B. This event causes a ligand-independent activation of Ins-R leading to an increased glucose uptake following SP1 stimulation
Studies over the past years have demonstrated that H2O2 actively participates in several biological processes including cell growth, induction and maintenance of the transformed state, programmed cell death, and cellular senescence [36]. Several studies revealed that a redox-dependent signaling is engaged by several extracellular stimuli, including EGF [37], PDGF [38], insulin [39, 40], VEGF [36], and integrin receptors [38]. H2O2 is a mild oxidant and may oxidize cysteine residues in proteins to cysteine sulfenic acid or disulfide, both of which are readily re-reduced to cysteine by various cellular reductants. H2O2-susceptible proteins include several transcription factors, the p21/Ras family of proto-oncogenes, as well as PTPs and protein tyrosine kinases [38].
In keeping with another recent report [41], we now include the bioactive lipid S1P among factors engaging a redox-based signaling. Interestingly, by employing a selective pharmacological agonist of S1PR and a specific S1P2 antagonist, we demonstrated that S1P-mediated ROS generation is dependent on S1P2. This receptor subtype, although co-expressed in C2C12 cells together with S1P1 and S1P3, appears to be a dominant transducer of S1P, being involved in transmitting the pro-myogenic effect of S1P as well as its anti-migratory action [16, 17]. The ability of S1P to increase ROS has also been reported for other cell types [41–43], although the implicated S1PR subtypes have not been characterized except for the indirect implication of S1P1 in NIH-3T3 fibroblasts, on the basis of the sensitivity to pertussis toxin of the S1P response [41]. The identification of S1P2 as majorly responsible for ROS generation in myoblasts in response to S1P treatment reinforces the notion that S1PR-mediated signaling is highly cell-specific.
The functional relevance of ROS for several growth factor signaling processes has been demonstrated by blocking their accumulation using many antioxidant drugs [39, 44]. Accordingly, we observed that the NADPH oxidase inhibitor DPI as well as the 5-LOX inhibitor NDGA greatly reduces the amount of ROS generated in response to S1P, thereby suggesting an involvement of both the NADPH oxidase and 5-LOX in the S1P-dependent redox signaling. As expected, S1P activates the small GTPase Rac1 concomitantly with ROS generation and the transfection of the dominant negative Rac1 completely inhibits the generation of ROS in response to S1P, thereby confirming the involvement of the small GTPase as an upstream regulator of ROS source [28].
A second notable finding is that the generation of ROS in response to S1P2 activation is strongly reliant on Ca2+-dependent signaling. Indeed, both Rac activation and the consequent redox signaling are potently inhibited by both Ca2+ depletors and cPKC inhibitor. The ability of S1P to signal through mobilization of Ca2+ intracellular stores [30] and to activate known Ca2+-dependent signaling pathways such as cPKC [45] in these cells has been already reported, in particular S1P2 and S1P3 have been shown to be implicated in the S1P-induced Ca2+-transient. The finding that ROS generation upon S1P challenge mainly involves S1P2 is in substantial agreement with the previous report and suggests that the Ca2+ increase elicited by the two receptor subtypes is upstream of distinct biological events, probably due to a different spatial localization within the cell. The novelty of our findings is that the S1P2-induced Ca2+-dependent pathway is hierarchically positioned upstream of Rac1 activation and the consequent redox signaling. In keeping with our findings, Ca2+ and redox signaling have been described as strictly linked. For example, direct or PKC-mediated activation of several NADPH oxidase isoforms by intracellular Ca2+ signals has been previously evidenced [46–48]. In addition, calcium-mediated generation of ROS has been reported for PDGF signaling in both embryoid bodies and during vasculogenesis of embryonic stem cells [49].
H2O2-susceptible proteins include PTPs, which are strongly but transiently inhibited through oxidation of their catalytic cysteine [38]. Redox signaling in response to several growth factors and cytokines has been correlated with the transient negative regulation of PTPs, which represents a strategy adopted by cells to promote RTK signaling by simply avoiding its prompt inactivation by PTPs [38]. Such reversible oxidation has been reported for the activation of many RTKs by different PTPs, both in response to their engagement by their natural ligands or in a ligand-independent manner. Known PTPs involved in RTK redox signaling are PTP1B for Ins-R [39, 44] and EGFR [50], as well as low molecular weight-PTP, PTEN, or SHP2 for PDGFR [40, 51].
The data presented herein suggest that, besides their function as downstream modulators of RTK signaling upon ligand binding, ROS act as upstream key molecules in RTK trans-activation, leading to a ligand-independent signal transduction. Here we report that Ins-R activation is achieved through a ligand-independent mechanism, involving transient oxidation and inactivation of PTP1B. This phosphatase is one of the main regulators of insulin signaling, and its redox regulation has been already reported during both ligand-dependent [44] and ligand-independent activation of Ins-R. Previously Meng et al. reported that, although the phosphatase is constitutively associated with Ins-R, in response to ligand activation of the kinase, a NADPH-driven generation of ROS produces a transient inactivation of the receptor-associated PTP1B, thereby shifting the equilibrium towards tyrosine phosphorylation of the receptor [40].
Recently it has been reported that an insulin-mimetic adipokine, adiponectin, similarly affects activation of Ins-R in a ligand-independent manner as well [15]. Indeed, stimulation of the GPCR-like AdipoR1 or AdipoR2 receptors gives rise to oxidation of PTP1B, successively culminating in enhancement of Ins-R phosphorylation, although in the absence of the ligand. The present findings indicate that S1P is similarly capable of affecting the Ins-R activation state, although the two cues appear to be active in different tissues. Indeed, adiponectin elicits a ligand-independent insulin activation exclusively in liver, being completely inefficient in eliciting a similar response in myoblasts [15]. In this regard it will be important to ascertain whether S1P2, which is implicated in liver regeneration [13], also participates in a similar insulin-mimetic effect in hepatocytes. Notably, the S1P-dependent redox regulation of PTP1B identified here was found to be responsible for the ligand-independent phosphorylation of Ins-R. In accord with previous data [18, 44], PTP1B is constitutively associated with Ins-R, although we observed an increase in the amount of receptor-recruited phosphatase in response to S1P treatment. This event is concomitant and likely causally correlated with oxidation of the phosphatase. In keeping with the present finding that implicates PTP1B, it has been reported for several phosphatases that the oxidation of their catalytic cysteines converts their behavior from active enzymes to dominant negative mutants, thereby increasing their binding of substrates [44, 51–53]. In this view PTP1B, while oxidized by S1P redox signaling, increases its binding to its substrate Ins-R, thereby enhancing its tyrosine phosphorylation.
S1P has already been involved in several cross-talk mechanisms, including bidirectional communications between RTK and its receptors. First the ability of RTKs, including PDGFR, VEGFR and EGFR, to activate S1P signaling was shown, mainly through de novo synthesis of bioactive lipid owing to activation of SK [9–11]. A second mechanism, described for S1P-PDGF cross-talk in vascular smooth cells, involves the occurrence of a PDGFR-S1P1 complex responsible for a specific integrative signal [11]. A third mechanism, again described for S1P-PDGF cross-talk, implicates a specific ligand-independent activation of PDGFR, owing to activation of the kinase Src elicited by S1P signaling [41]. The herein proposed redox-based cross-talk between S1P and Ins-R not only represents an additional new mechanism exerted by S1P to regulate RTKs, but introduces ROS as key regulators of this communication. It is conceivable that the multiplicity of connections between S1P and RTKs is reminiscent of the pleiotropic action of this bioactive lipid. S1P is a widely recognized muscle active factor. Indeed, the lipid has been reported to enhance differentiation in myoblasts, as well as to regulate the proliferation and survival of both resident and nonresident muscle stem cells [17, 54, 55]. Now we show that the action of S1P in myoblasts involves the regulation of a new metabolic process as well. Indeed, S1P-mediated trans-activation of Ins-R leads to a sustained increase in glucose uptake, suggesting a shift of muscle metabolism towards anabolic targets.
This insulin-mimetic effect is in keeping with several other observations. First, S1P shares with insulin the ability to elicit differentiation of myoblasts and maturation of myofibers [56]. Second, it has already been shown that in skeletal muscle cells S1P metabolism participates in insulin signaling, since pharmacological inhibition of SK1 reduced insulin-stimulated glucose uptake, while its overexpression mimicked insulin action in vivo [57]. However, in that previous study no clue was put forward with regard to the molecular mechanism by which SK1 acts. The present demonstration that S1P can activate signaling downstream of insulin in a ligand-independent manner provides the mechanistic explanation of the insulin-mimetic action of SK1 overexpression, thereby highlighting the existence of a positive feedback mechanism responsible for a sustained activation of Ins-R. However, it remains to be investigated if insulin transactivation plays a role in myoblast differentiation upon S1P challenge. Overall, our results contribute a new explanation about the role of S1P in skeletal muscle cells, identifying the bioactive lipid as an insulin-mimetic cue, strongly suggesting that its altered production might be related to the pathophysiology of insulin resistance and diabetes.
Acknowledgements
This research was supported by grants from the University of Florence (ex 60%) and Ente Cassa di Risparmio di Pistoia e Pescia to P.B. and by the Italian Association for Cancer Research, the Interuniversity Biotechnology Consortium, the Ente Cassa di Risparmio di Firenze, and the Tuscany Regional Project TRESOR to P.C.
Footnotes
E. Rapizzi and M. L. Taddei contributed equally to this work.
References
- 1.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 3.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 4.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 5.Pyne NJ, Waters C, Moughal NA, Sambi BS, Pyne S. Receptor tyrosine kinase-GPCR signal complexes. Biochem Soc Trans. 2003;31:1220–1225. doi: 10.1042/BST0311220. [DOI] [PubMed] [Google Scholar]
- 6.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 7.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 8.Mulders AC, Hendriks-Balk MC, Mathy MJ, Michel MC, Alewijnse AE, Peters SL. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:2043–2048. doi: 10.1161/01.ATV.0000237569.95046.b9. [DOI] [PubMed] [Google Scholar]
- 9.Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS) J Biol Chem. 2002;277:42997–43001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- 10.Tanimoto T, Lungu AO, Berk BC. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res. 2004;94:1050–1058. doi: 10.1161/01.RES.0000126404.41421.BE. [DOI] [PubMed] [Google Scholar]
- 11.Waters C, Sambi B, Kong KC, Thompson D, Pitson SM, Pyne S, Pyne NJ. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J Biol Chem. 2003;278:6282–6290. doi: 10.1074/jbc.M208560200. [DOI] [PubMed] [Google Scholar]
- 12.Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, Schafer-Korting M, Pfeilschifter J, Huwiler A. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- 13.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, Tran-Van-Nhieu J, Manin S, Poelstra K, Chun J, Carpentier S, Levade T, Mallat A, Lotersztajn S. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 14.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/S0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 15.Fiaschi T, Buricchi F, Cozzi G, Matthias S, Parri M, Raugei G, Ramponi G, Chiarugi P. Redox-dependent and ligand-independent trans-activation of insulin receptor by globular adiponectin. Hepatology. 2007;46:130–139. doi: 10.1002/hep.21643. [DOI] [PubMed] [Google Scholar]
- 16.Becciolini L, Meacci E, Donati C, Cencetti F, Rapizzi E, Bruni P. Sphingosine 1-phosphate inhibits cell migration in C2C12 myoblasts. Biochim Biophys Acta. 2006;1761:43–51. doi: 10.1016/j.bbalip.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Donati C, Meacci E, Nuti F, Becciolini L, Farnararo M, Bruni P. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 2005;19:449–451. doi: 10.1096/fj.04-1780fje. [DOI] [PubMed] [Google Scholar]
- 18.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 19.Burridge K, Nelson A. An in-gel assay for protein tyrosine phosphatase activity: detection of widespread distribution in cells and tissues. Anal Biochem. 1995;232:56–64. doi: 10.1006/abio.1995.9961. [DOI] [PubMed] [Google Scholar]
- 20.Markova B, Gulati P, Herrlich PA, Bohmer FD. Investigation of protein-tyrosine phosphatases by in-gel assays. Methods. 2005;35:22–27. doi: 10.1016/j.ymeth.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 22.Meacci E, Vasta V, Donati C, Farnararo M, Bruni P. Receptor-mediated activation of phospholipase D by sphingosine 1-phosphate in skeletal muscle C2C12 cells. A role for protein kinase C. FEBS Lett. 1999;457:184–188. doi: 10.1016/S0014-5793(99)01033-9. [DOI] [PubMed] [Google Scholar]
- 23.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda H, Satoh H, Yanase M, Inoue Y, Tomiya T, Arai M, Tejima K, Nagashima K, Maekawa H, Yahagi N, Yatomi Y, Sakurada S, Takuwa Y, Ogata I, Kimura S, Fujiwara K. Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology. 2003;124:459–469. doi: 10.1053/gast.2003.50049. [DOI] [PubMed] [Google Scholar]
- 25.Reis K, Halldin J, Fernaeus S, Pettersson C, Land T. NADPH oxidase inhibitor diphenyliodonium abolishes lipopolysaccharide-induced down-regulation of transferrin receptor expression in N2a and BV-2 cells. J Neurosci Res. 2006;84:1047–1052. doi: 10.1002/jnr.21005. [DOI] [PubMed] [Google Scholar]
- 26.Ding XZ, Kuszynski CA, El Metwally TH, Adrian TE. Lipoxygenase inhibition induced apoptosis, morphological changes, and carbonic anhydrase expression in human pancreatic cancer cells. Biochem Biophys Res Commun. 1999;266:392–399. doi: 10.1006/bbrc.1999.1824. [DOI] [PubMed] [Google Scholar]
- 27.Luchtefeld M, Drexler H, Schieffer B. 5-Lipoxygenase is involved in the angiotensin II-induced NAD(P)H-oxidase activation. Biochem Biophys Res Commun. 2003;308:668–672. doi: 10.1016/S0006-291X(03)01456-6. [DOI] [PubMed] [Google Scholar]
- 28.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/S0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 29.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 30.Meacci E, Cencetti F, Formigli L, Squecco R, Donati C, Tiribilli B, Quercioli F, Zecchi OS, Francini F, Bruni P. Sphingosine 1-phosphate evokes calcium signals in C2C12 myoblasts via Edg3 and Edg5 receptors. Biochem J. 2002;362:349–357. doi: 10.1042/0264-6021:3620349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Formigli L, Meacci E, Vassalli M, Nosi D, Quercioli F, Tiribilli B, Tani A, Squecco R, Francini F, Bruni P, Zecchi OS. Sphingosine 1-phosphate induces cell contraction via calcium-independent/Rho-dependent pathways in undifferentiated skeletal muscle cells. J Cell Physiol. 2004;198:1–11. doi: 10.1002/jcp.10366. [DOI] [PubMed] [Google Scholar]
- 32.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelletier S, Duhamel F, Coulombe P, Popoff MR, Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol Cell Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, Salgia R, Podar K, Griffin JD, Sattler M. Activation of the PI3 K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 36.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 37.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 38.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 39.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 40.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/S1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 41.Catarzi S, Giannoni E, Favilli F, Meacci E, Iantomasi T, Vincenzini MT. Sphingosine 1-phosphate stimulation of NADPH oxidase activity: relationship with platelet-derived growth factor receptor and c-Src kinase. Biochim Biophys Acta. 2007;1770:872–883. doi: 10.1016/j.bbagen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Lee M, Han SS. Choline phosphate potentiates sphingosine-1-phosphate-induced Raf-1 kinase activation dependent of Ras–phosphatidylinositol-3-kinase pathway. Cell Signal. 2002;14:373–379. doi: 10.1016/S0898-6568(01)00263-7. [DOI] [PubMed] [Google Scholar]
- 43.Okajima F, Tomura H, Sho K, Kimura T, Sato K, Im DS, Akbar M, Kondo Y. Sphingosine 1-phosphate stimulates hydrogen peroxide generation through activation of phospholipase C–Ca2+ system in FRTL-5 thyroid cells: possible involvement of guanosine triphosphate-binding proteins in the lipid signaling. Endocrinology. 1997;138:220–229. doi: 10.1210/en.138.1.220. [DOI] [PubMed] [Google Scholar]
- 44.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 45.Meacci E, Donati C, Cencetti F, Oka T, Komuro I, Farnararo M, Bruni P. Dual regulation of sphingosine 1-phosphate-induced phospholipase D activity through RhoA and protein kinase C-alpha in C2C12 myoblasts. Cell Signal. 2001;13:593–598. doi: 10.1016/S0898-6568(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 46.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 47.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5. Calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 49.Lange S, Heger J, Euler G, Wartenberg M, Piper HM, Sauer H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc Res. 2009;81:159–168. doi: 10.1093/cvr/cvn258. [DOI] [PubMed] [Google Scholar]
- 50.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. doi: 10.1074/jbc.272.51.32071. [DOI] [PubMed] [Google Scholar]
- 51.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 52.Gross S, Knebel A, Tenev T, Neininger A, Gaestel M, Herrlich P, Bohmer FD. Inactivation of protein-tyrosine phosphatases as mechanism of UV-induced signal transduction. J Biol Chem. 1999;274:26378–26386. doi: 10.1074/jbc.274.37.26378. [DOI] [PubMed] [Google Scholar]
- 53.Ostman A, Bohmer FD. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 2001;11:258–266. doi: 10.1016/S0962-8924(01)01990-0. [DOI] [PubMed] [Google Scholar]
- 54.Donati C, Cencetti F, Nincheri P, Bernacchioni C, Brunelli S, Clementi E, Cossu G, Bruni P. Sphingosine 1-phosphate mediates proliferation and survival of mesoangioblasts. Stem Cells. 2007;25:1713–1719. doi: 10.1634/stemcells.2006-0725. [DOI] [PubMed] [Google Scholar]
- 55.Nagata Y, Partridge TA, Matsuda R, Zammit PS. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol. 2006;174:245–253. doi: 10.1083/jcb.200605028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conejo R, Valverde AM, Benito M, Lorenzo M. Insulin produces myogenesis in C2C12 myoblasts by induction of NF-kappaB and downregulation of AP-1 activities. J Cell Physiol. 2001;186:82–94. doi: 10.1002/1097-4652(200101)186:1<82::AID-JCP1001>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 57.Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li JF, Yi J, Yuan YJ, Zhang QW, Mi J, Wang LS, Duan HF, Wu CT. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia. 2007;50:891–900. doi: 10.1007/s00125-006-0589-5. [DOI] [PubMed] [Google Scholar]