Abstract
The number of circulating mesenchymal stem cells (MSC), analyzed after acute myocardial infarction (AMI), was lower in AMI patients who developed heart failure (HF) in the follow-up. Conversely, the circulating levels of tumor necrosis factor (TNF)-α, and osteoprotegerin (OPG) were higher in AMI patients who developed HF with respect to the patients who did not develop HF. In vitro exposure to TNF-α enhanced the migration of MSC in response to TNF-related apoptosis-inducing ligand (TRAIL) and significantly increased the release of OPG by endothelial cells. On the contrary, OPG dose-dependently neutralized the in vitro pro-migratory activity of TRAIL. Thus, TNF-α exhibits opposite effects on MSC migration driven by TRAIL: it is capable of potentiating MSC migration as well as of inhibiting MSC migration as an indirect consequence of OPG induction, which might result in a suboptimal recruitment of circulating MSC after AMI in those patients who develop HF in the follow-up.
Keywords: TNF-α, OPG, TRAIL, Mesenchymal stem cells, Heart failure, Inflammation
Introduction
Several studies have demonstrated that the circulating levels of tumor necrosis factor (TNF)-α are elevated in patients with coronary artery disease (CAD) and, in particular, in patients with acute myocardial infarction (AMI) [1–4]. While most initial studies underlined a negative prognostic role of elevated levels of circulating TNF-α in the physiopathology of heart failure (HF) in AMI patients, other more recent studies have suggested that TNF-α might exert a beneficial role by increasing the recruitment of mesenchymal stem cells (MSC) [5, 6]. Interestingly, one of the major transcriptional targets of TNF-α is osteoprotegerin (OPG), a soluble member of the TNF receptor super-family [7, 8]. The levels of OPG have been found elevated in the serum/plasma of patients affected by CAD, and increased amounts of OPG in these patients represent a risk factor for cardiovascular disease progression [9–20]. On the other hand, it has been recently shown that the circulating levels of one of the two major ligands of OPG, TNF-related apoptosis-inducing ligand (TRAIL) [8], are decreased in CAD patients. Importantly, low levels of TRAIL have a negative prognostic significance for the development of HF and vascular mortality [21, 22]. In line with a protective role of TRAIL against CAD, a subsequent study has demonstrated that elevated levels of soluble TRAIL protect against restenosis in CAD patients [23].
On these bases, we have analyzed the circulating levels of TNF-α, OPG, and TRAIL in a cohort of patients with AMI in relationship with the levels of circulating MSC, which are thought to play a key role in regeneration after AMI [24], and with the development of HF in the follow-up. In parallel, since we have recently demonstrated that TRAIL promotes the in vitro migration of human bone marrow (BM)-derived MSC [25], we have evaluated the potential interplay of the TNF-α/OPG/TRAIL system in in vitro BM-MSC and endothelial culture systems.
Materials and methods
Blood sampling and processing
Blood samples were collected from a total of 80 AMI patients and 40 healthy control subjects, described in Table 1. For the measurement of circulating TNF-α, OPG, and TRAIL, serum samples were obtained from all individuals. Aliquots of serum samples were stored at −80°C and thawed only once before the assays. Assessment of circulating MSC was carried out on freshly collected blood samples, which were kept at room temperature until processing, and analyzed within a few hours. These analyses were possible for a subset (n = 50) of AMI patients and (n = 20) control subjects, following procedures in accordance with the Declaration of Helsinki and approved by the institutional review board (University-Hospital of Ferrara and Udine). All participant subjects gave written informed consent.
Table 1.
Characteristics of the study population
| Variables | Healthy control subjects (n = 40) | AMI patients | ||
|---|---|---|---|---|
| All (n = 80) | With HF (n = 21) | Without HF (n = 59) | ||
| Clinical and biomedical | ||||
| Age (years) | 57.1 ± 9.4 | 62.1 ± 10.8 | 66.6 ± 10.9 | 60.3 ± 11.9 |
| Men (%) | 66.1 | 78.5 | 66.7 | 83.7 |
| BMI (kg/m2) | 23.9 ± 4.2 | 28.0 ± 4.6 | 29.0 ± 4.8 | 27.7 ± 4.8 |
| CK (U/L) | – | 2,149.3 ± 2,022.3 | 3,029.7 ± 2,580.8 | 1,924.6 ± 1,819.4 |
| Peak CK-MB (ng/ml) | – | 205.2 ± 162.1 | 226.3 ± 169.4 | 193.3 ± 157.9 |
| Troponin I at peak (ng/ml) | – | 74.9 ± 61.1 | 89.2 ± 86.4 | 52.5 ± 49.8 |
| BNP (pg/ml) | – | 111.6 ± 87.3 | 124.1 ± 95.5 | 108.4 ± 85.9 |
| CRP (mg/dl) | 0.6 ± 0.4 | 3.7 ± 6.4 | 7.8 ± 12.1 | 2.6 ± 2.9 |
| Cytokines | ||||
| TNF-α (pg/ml) | 17.8 ± 7.5 | 28.3 ± 11.7 | 36.9 ± 16.4 | 25.4 ± 7.8 |
| OPG (pg/ml) | 99.7 ± 36.3 | 168.4 ± 80.1 | 195.6 ± 100 | 158.4 ± 70.6 |
| TRAIL (pg/ml) | 75.4 ± 17.9 | 50.4 ± 20.6 | 32.7 ± 18.5 | 56.7 ± 17.6 |
| OPG/TRAIL ratio | 1.6 ± 0.8 | 4.5 ± 4.2 | 8.3 ± 6.1 | 3.2 ± 2.0 |
Values given as percentage or as mean ± SD
BMI Body mass index, BNP B-type natriuretic peptide, CK creatine kinase, CK-MB creatine kinase-MB fraction, CRP C-reactive protein
The AMI patients and the control group did not significantly differ for age, sex, and body mass index (BMI), as evaluated by Student’s t test (for age and BMI) or chi-square test (for percentage of men). Criteria adopted for AMI definition finalized to patient enrolling were those of the ESC/ACC Joint Committee [26]. Most of the patients presented with ST-segment elevation myocardial infarction (95%), anterior in location (59%), without signs of HF on admission (92%) and with a mean left ventricular ejection fraction (LVEF %) of 48 ± 11. Primary percutaneous coronary intervention (PCI) reperfusion treatment was carried out according to current guidelines in 60% of the patients, while 21% of the patients received trombolytics. A total of 80% of AMI patients received medication with statins. Exclusion criteria were: myocardial infarction precipitated or confounded by other co-morbidities or secondary to coronary revascularization procedures, the presence or history of any cardiomyopathy or of valvular heart disease more than mild in severity, mechanical complications, cardiogenic shock, malignant disease, inflammatory disease, and severe kidney disease. The AMI patients underwent outpatient visits every 6 months. The main outcome of interest was represented by occurrence of new-onset HF, in accordance with previously proposed criteria [27]. Serial blood samples were collected from the AMI patients. In particular, serial samples were collected between the entry (≤12 h after symptom onset) and the discharge (approximately 7 days), which are collectively referred to as “acute phase” in "Results", and subsequently at 6 months follow-up after AMI.
Flow cytometry
Unfractioned blood samples were stained with directly conjugated monoclonal antibodies: PERCP-CD45, FITC-CD34, and PE-CD90 (BD Biosciences, Franklin Lakes, NJ, USA). Before being acquired by CyAn (Dako, Glostrup, Denmark) or FACScan (BD Biosciences), samples were lysed by FACS Lysing Solution (BD Biosciences) and 200,000–400,000 cells/sample were collected. Every experiment included negative controls. For multi-color staining, single-color stained controls were included in order to create a compensation matrix (Summit Software, Dako). Analyses were performed utilizing SummitSoftware (Dako) and CellQuest (BD Biosciences).
Enzyme-linked immunosorbent assays (ELISA)
Measurement of circulating TNF-α, OPG, and TRAIL were performed in duplicate by using specific, commercially available ELISA kit (Alexis Biochemicals, Lausen, Switzerland, for OPG, and R&D Systems, Minneapolis, MN, for TRAIL and TNF-α) in accordance with the manufacturer’s instructions, and analyzed with an ELISA reader at 450 nm. The same ELISA kits were also used for the measurement of OPG and TRAIL in the supernatants of endothelial cell cultures. Sensitivity of the TNF-α assay was 1.6 pg/ml, and the intra- and inter-assay coefficients of variation (CV) were 4.7 and 5.8%, respectively, and the upper limit of detection (ULOD) was 1,000 pg/ml. Sensitivity of the OPG assay was 2.8 pg/ml, the intra- and inter-assay CV were 9 and <10%, respectively, and the ULOD was 600 pg/ml. Sensitivity of the TRAIL assay was 2.9 pg/ml, and the intra- and inter-assay CV were 3.9 and 6%, respectively, and the ULOD was 1,000 pg/ml. Selected serum samples were run in each ELISA plates, as internal controls, confirming the reproducibility of the determinations over time.
Cell cultures and migration assay
Human bone marrow (BM)-derived MSC and human umbilical vein endothelial cells (HUVEC) were purchased from Lonza (Walkersville, MD). BM-MSC were routinely cultured in MSC-Growth Medium (MSC-GM; Lonza) and were used at the second to sixth passage in all experiments. Migration assays were performed in transwell plates (Corning Costar, Cambridge, MA, USA) 6.5 mm in diameter, with 8-μm pore filters, as described [28]. Briefly, the upper side of the transwell filter was coated for 1 h with collagen IV, and serum-starved MSC (3 × 105), either left unstimulated or pretreated with TNF-α for 20 h, were added to the upper chamber. Recombinant human TRAIL, produced as described [29], was added to the lower chamber in 600 μl of serum-free medium. For the experiments carried out in presence of OPG, 50 pg/ml of TRAIL were pre-incubated for 30 min with or without scalar doses (15–50–150–300 pg/ml) of recombinant full-length OPG (R&D Systems), before adding to the lower chamber in 600 μl of serum-free medium. In some experiments, pre-incubation of TRAIL with recombinant OPG (300 pg/ml) was carried out in the presence of 1 μg of a neutralizing anti-OPG antibody (Ab, mouse IgG1; R&D Systems). Migration observed in the presence of 30% FCS, served as positive control for each experimental round. After 16 h of incubation at 37°C in 5% CO2, the upper side of the filters was carefully washed with PBS, and cells remaining on the upper face were removed with a cotton wool swab. Transwell filters were then fixed and stained using May Grünwald Giemsa. Cells that had migrated were counted using light microscopy at high-power magnification. The average number of migrating cells per field was assessed by counting at least four random fields per filter. Each experiment was done in duplicate.
HUVEC were grown on 0.2% gelatin-coated tissue culture plates in M199 endothelial growth medium supplemented with 20% FBS, 10 mg/ml heparin, and 50 mg/ml ECGF (all from Lonza). In all experiments, endothelial cells were used between the 3rd and 5th passage in vitro. When indicated, endothelial cells and BM-MSC were treated with human recombinant TNF-α purchased from R&D Systems.
Statistical analyses
Data are calculated and shown as mean ± SD or as median and Interquartile Range (IQR), according to the distribution. Differences in mean values across study phases were analyzed using analysis of variance (ANOVA) for repeated measures. Comparisons between groups were performed with Student’s t test and with chi-square test. A two-sided P value <0.05 has been chosen as statistically significant.
Results
The number of circulating MSC and the serum levels of TNF-α are acutely increase after AMI
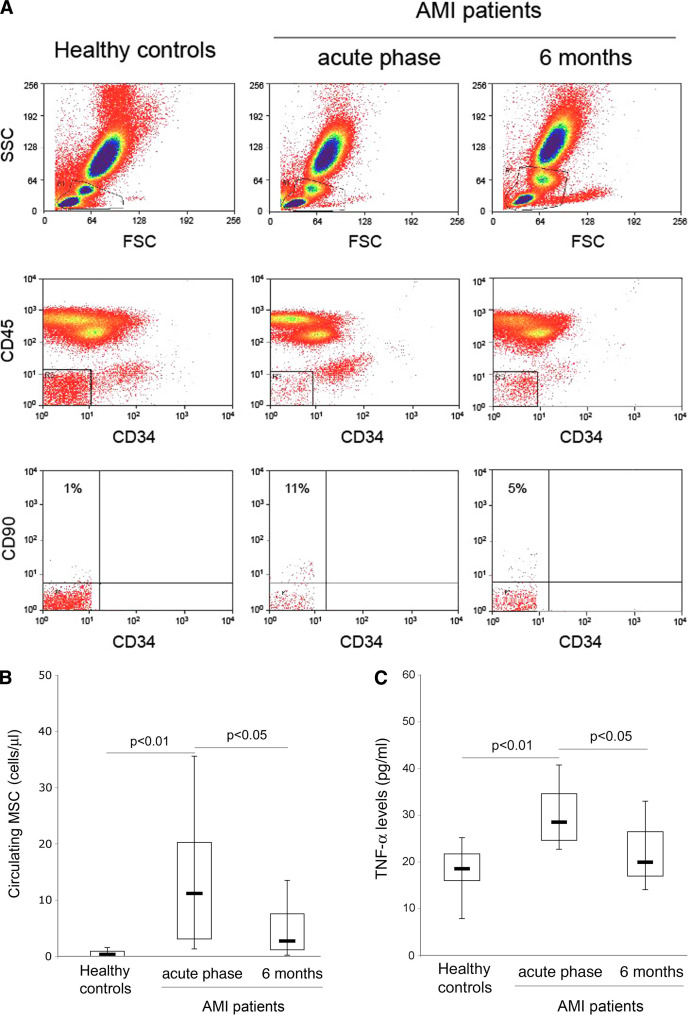
MSC have been implicated in heart regeneration [24]. Therefore, in the first group of experiments, the presence of putative (CD34−/CD45−/CD90+) circulating MSC was analyzed in AMI patients in acute phase and 6 months after AMI in comparison with healthy control donors by multicolor flow cytometry (Fig. 1a). In the acute phase after AMI, MSC resulted to be significantly (P < 0.01) recruited into the bloodstream of the patients (Fig. 1b). Indeed, while these cells were virtually undetectable in healthy control subjects, the median number of circulating MSC measured acutely after AMI was of 12.5 cells/μl (15.6 ± 16.3, mean ± SD), decreasing 6 months after AMI. Since it has been shown that the migratory response of MSC to chemotactic stimuli is modulated by TNF-α [5, 6, 30], we have also analyzed the serum levels of TNF-α in the AMI patients in comparison with normal control subjects. As shown in Fig. 1c, TNF-α levels were significantly elevated in the acute phase after AMI and decreased at later time.
Fig. 1.
Comparison of the circulating MSC and TNF-α levels between healthy control subjects and AMI patients. Circulating levels of putative MSC were quantified in blood of AMI patients and of healthy donors by multi-parametric flow-cytometry, utilizing the CD34/CD45/CD90 antibody combination (a, b). a Representative FACS plots of controls and AMI patients, examined during the acute phase and at 6 months after AMI, show the gating strategy adopted to identify circulating MSC. Mononuclear cells were chosen on the basis of their physical properties (FSC/SSC; top panels). CD45 −/CD34 − cells (middle panels) were gated and this subpopulation was further characterized for the expression of CD90; percentages of CD90 +/CD34 − are reported in the top left quadrants (bottom panels). b Circulating cell concentration is expressed as number of cells/μl of blood. c Serum levels of TNF-α were evaluated in AMI patients and in healthy control subjects by ELISA. In b and c, horizontal bars at median, upper, and lower edges of box are 75th and 25th percentiles, lines extending from box are 10th and 90th percentiles
Patients who developed HF in the follow-up exhibit lower levels of circulating MSC and higher levels of TNF-α in the acute phase after AMI
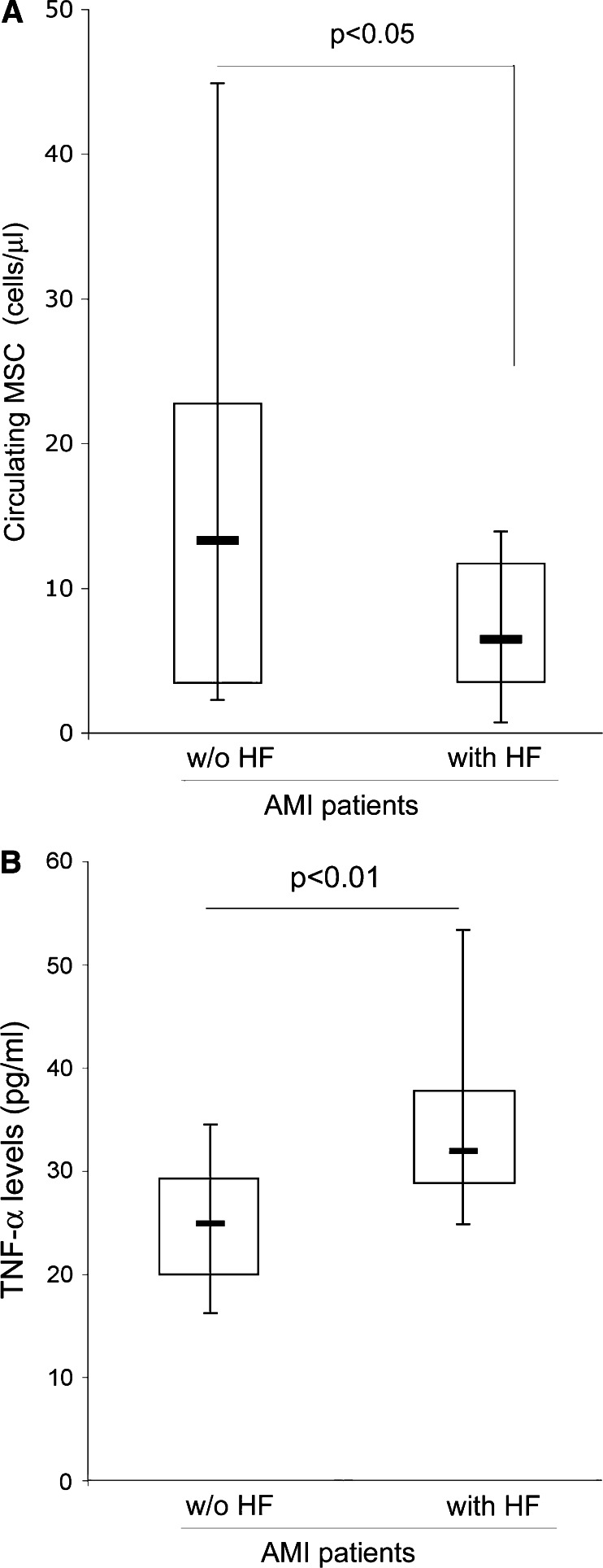
In order to investigate the potential relationship between MSC mobilization during the acute phase after AMI and a major prognostic outcome, such as the development of heart failure (HF), we have analyzed the number of MSC in the AMI patients subdivided in two groups based on the occurrence of HF in the follow-up (Table 1). We observed that the number of circulating MSC in the acute phase after AMI was significantly (P < 0.05) lower in patients who developed HF (6.4 cells/μl median; 10.4 ± 10.2, mean ± SD) with respect to patients who did not develop HF (13.3 cells/μl median; 16.9 ± 17.6, mean ± SD) (Fig. 2a). In contrast, the circulating levels of TNF-α (Fig. 2b), as well as OPG, were significantly (P < 0.05) higher in AMI patients who developed HF (Table 1). It is also noteworthy that among the AMI patients only 28.8% of patients who developed HF were under medical treatment with statins, while 71.2% of AMI patients without HF in the follow-up received treatment with statins.
Fig. 2.
Circulating levels of MSC and TNF-α in AMI patients in relationship to the occurrence of HF after AMI. Circulating levels of MSC (a) and TNF-α (b), determined in the samples of AMI patients in the acute phase, were analyzed in relationship to the occurrence of HF during the follow-up after AMI. Horizontal bars at median, upper, and lower edges of box are 75th and 25th percentiles, lines extending from box are 10th and 90th percentiles
TNF-α modulates the in vitro migratory response of BM-MSC to TRAIL
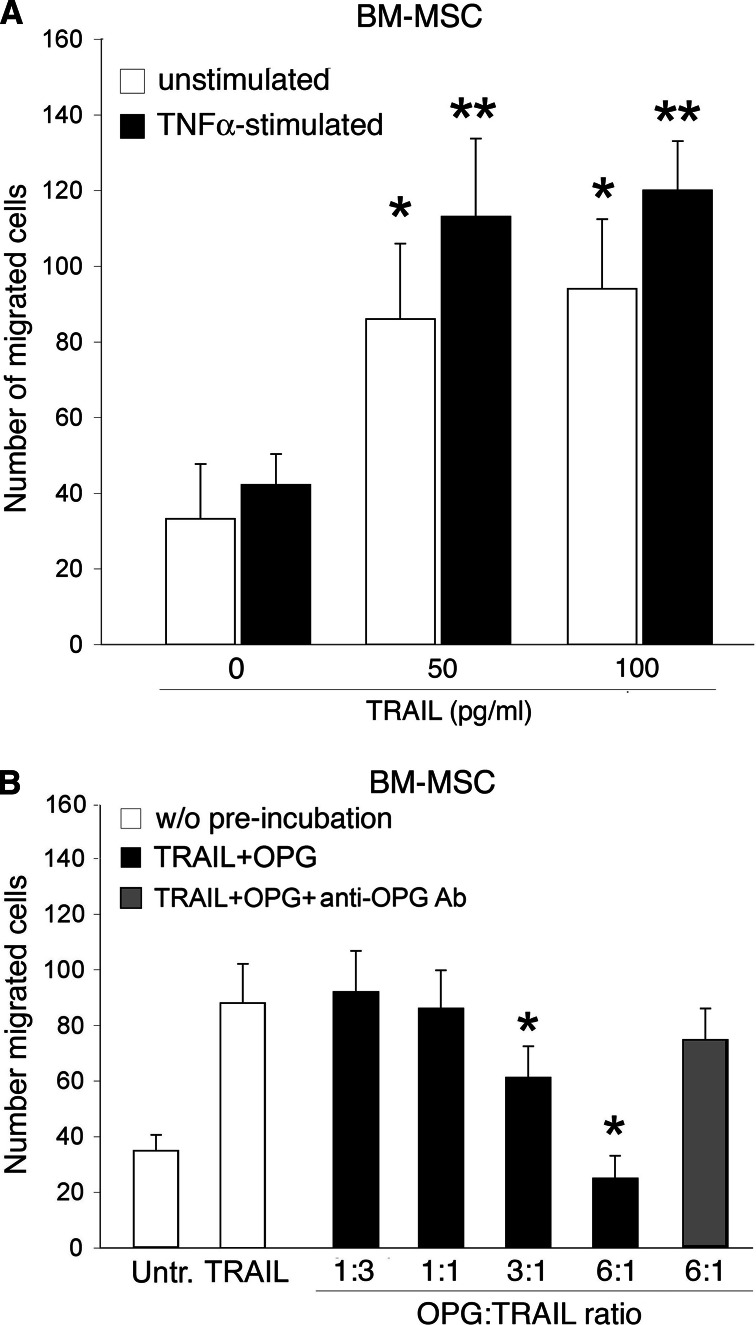
Based on the results obtained analyzing the AMI patients, we have next explored in vitro the potential interplay among TNF-α, OPG and TRAIL on the migration of BM-derived MSC. Since recombinant soluble TRAIL (50–100 pg/ml) significantly (P < 0.05) induced the migration of BM-derived MSC (Fig. 3a, white columns), we sought to investigate whether pre-exposure to TNF-α (100 pg/ml) affects the in vitro migratory response of BM-MSC to TRAIL. It is important to point out that in these in vitro experiments both TNF-α and TRAIL were used at concentrations comparable to those found in human serum/plasma. On the other hand, previous studies reporting the ability of TNF-α to promote MSC migration used higher concentrations of TNF-α (1–10 ng/ml) [30], which are not in the physiological range. Overnight pre-stimulation of MSC with TNF-α alone marginally affected BM-MSC migration, while it induced a reproducible (P < 0.05) increase in the migratory response of MSC to TRAIL (Fig. 3a, black columns). Although OPG has been shown to neutralize TRAIL cytotoxicity in apoptotic assays mostly carried out in tumor cells [31], its ability to interfere with the pro-migratory activity of TRAIL on MSC has never been tested. For this purpose, in the next experiments the effect of OPG on TRAIL-driven MSC migration was assessed by pre-incubating TRAIL with scalar doses of recombinant human OPG. When used at an OPG:TRAIL ratio up to 1:1, similar to that found in the blood of healthy donors, OPG did not affect the MSC migration in response to TRAIL (Fig. 3b). Of note, at OPG:TRAIL ratios of 3:1 and 6:1, similar to those found in acute AMI patients, in particular in patients who developed HF (Table 1), OPG significantly (P < 0.05) decreased (at a ratio of 3:1) or abrogated (at a ratio of 6:1) the TRAIL-mediated induction of MSC migration (Fig. 3b). When recombinant OPG (300 pg/ml) was pre-incubated with TRAIL in the presence of anti-OPG neutralizing Ab (1 μg/ml), the pro-migratory activity of TRAIL was restored (Fig. 3b).
Fig. 3.
Effect of TNF-α on migratory response of BM-MSC to TRAIL. a Migration capacity of unstimulated and TNF-α-stimulated BM-MSC toward recombinant TRAIL, used at the indicated concentrations, was evaluated in Transwell plates as described in the Methods. *P < 0.05 with respect to untreated cultures (without cytokines); **P < 0.05 with respect to the corresponding unstimulated culture exposed to the same TRAIL concentration. b Effects of recombinant OPG on BM-MSC migration driven by TRAIL. After plating BM-MSC in the upper compartment of Transwell plates, soluble recombinant TRAIL (100 pg/ml) was used either alone (w/o pre-incubation) or after pre-incubation with OPG (TRAIL + OPG), used at the indicated OPG:TRAIL ratios, in the absence or presence of neutralizing anti-OPG Ab. *P < 0.05 with respect to cells treated with TRAIL in the absence of OPG. In a and b, data are the mean ± SD of results from at least three independent experiments, each performed in duplicate
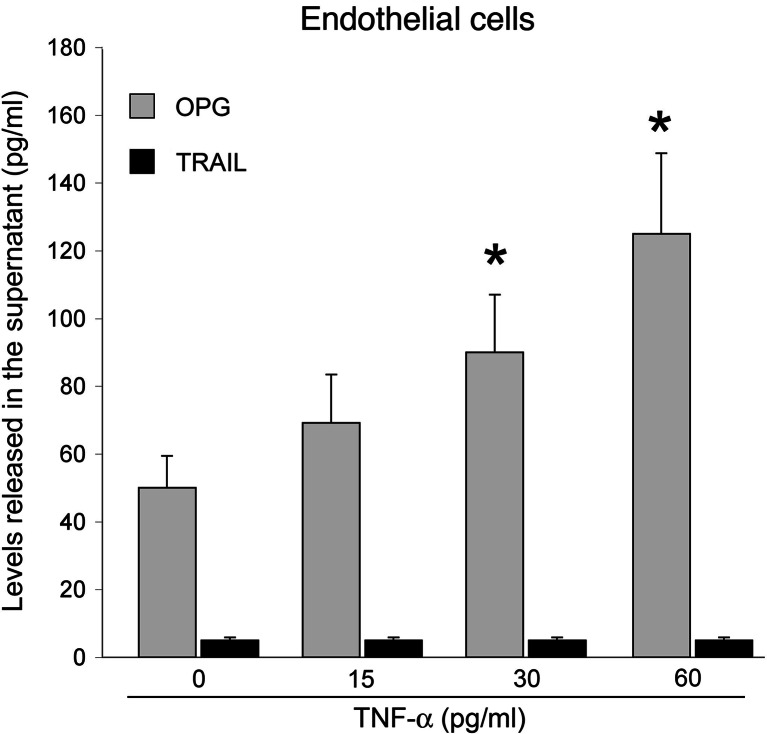
Since endothelial cells represent a major source of circulating OPG [8], in parallel experiments, we have investigated whether TNF-α is able to modulate the expression and release of OPG and/or TRAIL. As shown in Fig. 4 (gray columns), TNF-α significantly (P < 0.05) up-regulated, dose- and time-dependently, the release of OPG in HUVEC culture medium. On the other hand, HUVEC did not release appreciable levels of TRAIL either in basal culture conditions or after stimulation with TNF-α (Fig. 4, black columns).
Fig. 4.
Effect of TNF-α on the release of OPG and TRAIL by endothelial cells. OPG and TRAIL levels were assessed by ELISA in culture supernatants of endothelial cells stimulated for 20 h with the indicated concentrations of TNF-α. Data are the mean ± SD of results from four independent experiments, each performed in duplicate. *P < 0.05 with respect to unstimulated cells
Discussion
Mobilization of both CD34+ hematopoietic stem/progenitor cells [32–34] and MSC [24] from bone marrow has been described in the acute phase after AMI. Although the relative contribution of these stem cell populations to heart regeneration after AMI is incompletely understood, we have previously demonstrated that CD34+ hematopoietic cells do not express TRAIL receptors [8]. On the other hand, MSC express functional levels of surface TRAIL-R2 [25]. Although various chemotactic factors and cytokines have been described as enhancing MSC migration [30, 35, 36], in this study we have focused our attention on the potential effects of TNF-α on TRAIL-induced migration of MSC. Since TNF-α is increased in damaged and inflamed myocardial tissues [5, 6], it has been speculated that the release of TNF-α at the site of injury might mobilize MSC from bone marrow, thereby initiating the process of MSC recruitment [37]. However, the data on the potential physiopathological role of circulating TNF-α in the pathogenesis of CAD are conflicting [1–6]. In this respect, we found that: (1) in the acute phase after AMI, both circulating levels of MSC and TNF-α are more elevated in AMI patients with respect to normal healthy control subjects, and significantly decrease at later time points after AMI; and (2) in the acute phase after AMI, patients who developed HF in the follow-up show higher levels of TNF-α and OPG, but lower levels of circulating MSC, with respect to AMI patients who did not develop HF. On the basis of these in vivo data, we have analyzed the ability of TNF-α to modulate the migratory activity of MSC in the absence or presence of TRAIL and OPG when used in the range of concentrations found in human plasma/serum of normal individuals and AMI patients. While recombinant TNF-α and OPG alone marginally affected MSC migration, soluble TRAIL promoted the migration of BM-derived MSC. Of interest, pre-exposure of BM-MSC to TNF-α induced a significant increase of the migratory response to TRAIL, in keeping with previous data suggesting that TNF-α potentiates the migration response of MSC to chemokines [30]. In the same in vitro context, an additional important finding of our study was that the migratory response of BM-MSC to TRAIL was dose-dependently inhibited by pre-incubation with soluble OPG. In particular, the pro-migratory activity of TRAIL was completely abrogated when OPG and TRAIL were used at an OPG/TRAIL ratio (6:1) similar to that found in AMI patients who developed HF. Moreover, when endothelial cells, which represent a major source of circulating OPG [8], were exposed to TNF-α, the release of OPG (but not of TRAIL) was significantly enhanced. Although we are aware that we have not directly linked the ability of TNF-α to increase the in vitro production of OPG by endothelial cells to the inhibitory activity of OPG on MSC migration in response to TRAIL, the ability of recombinant OPG to dose-dependently suppress the migratory response of MSC to TRAIL offers a potential physiopathological mechanism for previous data, which have clearly established a solid link between elevated levels of circulating OPG and risk of cardiovascular events [9–20]. It is also noteworthy that a higher percentage of patients receiving statins before AMI showed lower levels of circulating TNF-α and OPG and a better prognosis. Consistently with our present findings, it has been previously shown that statins are able to down-regulate the TNF-α-induced secretion of OPG by vascular cells [38].
The potential role of MSC in heart regeneration has been underscored in previous studies demonstrating that MSC can be mobilized from BM and migrate into the infarcted myocardium, where they are thought to exert their regenerative activity mainly by releasing pro-angiogenic cytokines and also by exerting their anti-inflammatory activity [39, 40]. Since the molecular mediators involved in MSC migration are poorly understood, our current data suggest that the reciprocal fluctuations of the levels of inflammatory cytokines, such as TNF-α and OPG, and of other cytokines, such as TRAIL, in the bloodstream of AMI patients might play an important role in recruiting BM-derived MSC into the circulation. TNF-α seems to exhibit two independent and apparently contrasting effects on MSC migration: on the one hand, it contributes to enhance the migratory response of MSC toward TRAIL, but, on the other hand, it might indirectly suppress the biological activity of TRAIL by increasing the release of its soluble neutralizing receptor OPG (as summarized and depicted in Fig. 5). It is likely that the net effect of the interplay among these cytokines will be determined by their concentrations and/or ratio. Since TRAIL exerts anti-inflammatory [41, 42] and anti-atherosclerotic [43] effects, a TNF-α-dependent elevation of OPG might have pathogenic implications in the outcome of AMI by neutralizing the beneficial activity of TRAIL in the vascular system, including the mobilization of BM-derived MSC.
Fig. 5.
Schematic representation of the potential interplay among TNF-α, OPG, and TRAIL in AMI
Acknowledgment
This work was supported by a grant from “Programma di Ricerca Regione-Università 2007/2009” (Regione Emilia Romagna) to R.F. and P.S.
References
- 1.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 2.Feldman AM, Combes A, Wagner D, Kadokami T, Kubota T, Li YY, McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–544. doi: 10.1016/S0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 4.Akasaka Y, Morimoto N, Ishikawa Y, Fujita K, Ito K, Kimura-Matsumoto M, Ishiguro S, Morita H, Kobayashi Y, Ishii T. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Mod Pathol. 2006;19:588–598. doi: 10.1038/modpathol.3800568. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Park HJ, Hong MH, Kang PM, Morgan JP, Jeong MH, Cho JG, Park JC, Ahn Y. TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front Biosci. 2009;14:2845–2856. doi: 10.2741/3417. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Li SH, Liu SM, Wu J, Weisel RD, Zhuo YF, Yau TM, Li RK, Fazel SS. Improvement in cardiac function after bone marrow cell therapy is associated with an increase in myocardial inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H43–H50. doi: 10.1152/ajpheart.00613.2008. [DOI] [PubMed] [Google Scholar]
- 7.Simonet WS, Lacey DL, Dunstan C, Kelly M, Chang MS, Luthy R, Nhuyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliot R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 8.Zauli G, Secchiero P. The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Fact Rev. 2006;17:245–257. doi: 10.1016/j.cytogfr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. doi: 10.1161/01.CIR.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 10.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 11.Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, Squire IB, Gullestad L, Bollerslev J, Dickstein K, Aukrust P. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–1976. doi: 10.1016/j.jacc.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 12.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 13.Ueland T, Yndestad A, Øie E, Florholmen G, Halvorsen B, Frøland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 14.Avignon A, Sultan A, Piot C, Elaerts S, Cristol JP, Dupuy AM. Osteoprotegerin is associated with silent coronary artery disease in high-risk but asymptomatic type 2 diabetic patients. Diabetes Care. 2005;28:2176–2180. doi: 10.2337/diacare.28.9.2176. [DOI] [PubMed] [Google Scholar]
- 15.Shin JY, Shin YG, Chun CH. Elevated serum osteoprotegerin levels are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care. 2006;29:1664–1666. doi: 10.2337/dc06-0631. [DOI] [PubMed] [Google Scholar]
- 16.Secchiero P, Corallini F, Pandolfi A, Consoli A, Candido R, Fabris B, Celeghini C, Capitani S, Zauli G. An increased osteoprotegerin (OPG) serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am J Pathol. 2006;169:2236–2244. doi: 10.2353/ajpath.2006.060398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abedin M, Omland T, Ueland T, Khera A, Aukrust P, Murphy SA, Jain T, Gruntmanis U, McGuire DK, de Lemos JA. Relation of osteoprotegerin to coronary calcium and aortic plaque. Am J Cardiol. 2007;99:513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 18.Helske S, Kovanen PT, Lindstedt KA, Salmela K, Lommi J, Turto H, Werkkala K, Kupari M. Increased circulating concentrations and augmented myocardial extraction of osteoprotegerin in heart failure due to left ventricular pressure overload. Eur J Heart Fail. 2007;28:1894–1903. doi: 10.1016/j.ejheart.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Omland T, Ueland T, Jansson AM, Persson A, Karlsson T, Smith C, Herlitz J, Aukrust P, Hartford M, Caidahl K. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51:627–633. doi: 10.1016/j.jacc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 20.Nybo M, Rasmussen L. The capability of plasma osteoprotegerin as a predictor of cardiovascular disease. A systematic literature review. Eur J Endocrinol. 2008;159:603–608. doi: 10.1530/EJE-08-0554. [DOI] [PubMed] [Google Scholar]
- 21.Secchiero P, Corallini F, Ceconi C, Parrinello G, Volpato S, Ferrari R, Zauli G. Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS One. 2009;4:e4442. doi: 10.1371/journal.pone.0004442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niessner A, Hohensinner PJ, Rychli K, Neuhold S, Zorn G, Richter B, Hülsmann M, Berger R, Mörtl D, Huber K, Wojta J, Pacher R. Prognostic value of apoptosis markers in advanced heart failure patients. Eur Heart J. 2009;30:789–796. doi: 10.1093/eurheartj/ehp004. [DOI] [PubMed] [Google Scholar]
- 23.Satoh D, Inami N, Shimazu T, Kajiura T, Yamada K, Iwasaka T, Nomura S (2009) Soluble TRAIL prevents RANTES-dependent restenosis after percutaneous coronary intervention in patients with coronary artery disease. J Thromb Thrombolysis doi:10.1007/s11239-009-0364-9 [DOI] [PubMed]
- 24.Li SC, Wang L, Jiang H, Acevedo J, Chang AC, Loudon WG. Stem cell engineering for treatment of heart diseases: potentials and challenges. Cell Biol Int. 2009;33:255–267. doi: 10.1016/j.cellbi.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Secchiero P, Melloni E, Corallini F, Beltrami AP, Alviano F, Milani D, D’Aurizio F, di Iasio MG, Cesselli D, Bagnara GP, Zauli G. Tumor necrosis factor-related apoptosis-inducing ligand promotes migration of human bone marrow multipotent stroma cells. Stem Cells. 2008;26:2955–2963. doi: 10.1634/stemcells.2008-0512. [DOI] [PubMed] [Google Scholar]
- 26.The Joint European Society of Cardiology/American College of CardiologyCommittee Myocardial infarction redefined—a consensus document for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 27.The Task Force on Heart Failure of the European Society of Cardiology Guidelines for the diagnosis of heart failure. Eur Heart J. 1995;16:741–751. [PubMed] [Google Scholar]
- 28.Secchiero P, Zerbinati C, Rimondi E, Corallini F, Milani D, Grill V, Forti G, Capitani S, Zauli G. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci. 2004;61:1965–1974. doi: 10.1007/s00018-004-4197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milani D, Zauli G, Rimondi E, Celeghini C, Marmiroli S, Narducci P, Capitani S, Secchiero P. Tumour necrosis factor-related apoptosis-inducing ligand sequentially activates pro-survival and pro-apoptotic pathways in SK-N-MC neuronal cells. J Neurochem. 2003;86:126–135. doi: 10.1046/j.1471-4159.2003.01805.x. [DOI] [PubMed] [Google Scholar]
- 30.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 31.Zauli G, Melloni E, Capitani S, Secchiero P. Role of full-length osteoprotegerin in tumor cell biology. Cell Mol Life Sci. 2009;66:841–851. doi: 10.1007/s00018-008-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 33.Turan RG, Brehm M, Koestering M, Tobias Z, Bartsch T, Steiner S, Picard F, Ebner P, Schannwell CM, Strauer BE. Factors influencing spontaneous mobilization of CD34+ and CD133+ progenitor cells after myocardial infarction. Eur J Clin Invest. 2007;37:842–851. doi: 10.1111/j.1365-2362.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- 34.Grundmann F, Scheid C, Braun D, Zobel C, Reuter H, Schwinger RH, Müller-Ehmsen J. Differential increase of CD34, KDR/CD34, CD133/CD34 and CD117/CD34 positive cells in peripheral blood of patients with acute myocardial infarction. Clin Res Cardiol. 2007;96:621–627. doi: 10.1007/s00392-007-0543-7. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki Y, Nishimura M, Sekiya K, Suehiro F, Kanawa M, Nikawa H, Hamada T, Kato Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119–129. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- 36.Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- 37.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Tal Cohen E, Hohensinner PJ, Kaun C, Maurer G, Huber K, Wojta J. Statins decrease TNF-alpha-induced osteoprotegerin production by endothelial cells and smooth muscle cells in vitro. Biochem Pharmacol. 2007;73:77–83. doi: 10.1016/j.bcp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 41.Zauli G, Pandolfi A, Gonelli A, Di Pietro R, Guarnieri S, Ciabattoni G, Rana R, Vitale M, Secchiero P. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially up-regulates nitric oxide and prostanoid production in primary human endothelial cells. Circ Res. 2003;92:732–740. doi: 10.1161/01.RES.0000067928.83455.9C. [DOI] [PubMed] [Google Scholar]
- 42.Secchiero P, Corallini F, di Iasio MG, Gonelli A, Barbarotto E, Zauli G. TRAIL counteracts the proadhesive activity of inflammatory cytokines in endothelial cells by down-modulating CCL8 and CXCL10 chemokine expression and release. Blood. 2005;105:3413–3419. doi: 10.1182/blood-2004-10-4111. [DOI] [PubMed] [Google Scholar]
- 43.Secchiero P, Candido R, Corallini F, Zacchigna S, Toffoli B, Rimondi E, Fabris B, Giacca M, Zauli G. Systemic TRAIL delivery shows anti-atherosclerotic activity in apoE-null diabetic mice. Circulation. 2006;114:1522–1530. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]