Abstract
Akt (PKB) is a critical kinase in cell-survival pathways. Its activity depends on the phosphorylation of Thr308 and Ser473, by PDK1 and mTORC2, respectively. We found that Akt can be further stimulated through phosphorylation of Ser129 by another kinase, CK2. Here we show that phosphorylation of Akt at Ser129 also facilitates its association with Hsp90 chaperone, thus preventing Thr308 dephosphorylation. This is supported by the following observations: (1) phospho-Thr308 decreases when Ser129 is mutated to alanine, (2) this decrease is abolished by cell treatment with okadaic acid (to inactivate PP2A) or geldanamycin (to inactivate Hsp90), (3) phosphorylation of Ser129 neither enhances the activity of PDK1 nor hampers the in vitro activity of PP2A on Thr308, but increases the Hsp90 association to Akt. These data support the view that the antiapoptotic potential of CK2 is at least in part mediated by its ability to maintain Akt in its active form.
Keywords: CK2, CKII, Akt, PKB, Casein kinase 2
Introduction
Akt (also known as protein kinase B, PKB) is a crucial kinase regulating multiple cellular processes, such as cell death, proliferation, growth, and metabolism (for reviews, see [1–5]). Its dysregulated activity has often been related to pathological conditions characterized by a high level of cell proliferation and survival, such as cancer. Three isoforms of Akt exist, Akt 1, 2, and 3 (or PKB α, β, γ, respectively), with a different tissue distribution [6], more ubiquitous for Akt 1 and 2. The three isoforms share a high sequence homology and the same domain structure: an N-terminal PH (pleckstrin homology) domain, connected by a short linker segment to a central kinase domain, followed by a C-terminal regulatory domain. The Akt activation mechanism is quite complex; a number of partner proteins have been reported to modulate Akt activity, such as the carboxyl-terminal modulator protein, CTMP [7], Tcl1 [8, 9], Hsp90/cdc37 [10], and Hsp27 [11]. The basic mechanism leading to Akt activation, however, starts with the up-regulation of phosphatidylinositol 3-kinase (PI3K) in response to external signals; this promotes a rise in the concentration of phosphatidylinositol 3,4,5 trisphosphate (PIP3), which recruits Akt to the plasma membrane by means of its PH domain. At this location, Akt is phosphorylated at two key residues: Thr308 (numbering according to the Akt1 sequence), in the catalytic domain, is the target of the phosphoinositide-dependent kinase 1 (PDK1), while Ser473, in the C-terminal region, is phosphorylated by the complex mTORC2 [12] or by the DNA-dependent protein kinase in response to DNA damage [13]. Only the combined phosphorylation of these two crucial residues confers catalytical competence to Akt.
In addition, we have recently demonstrated [14] that the phosphorylation of Ser129, catalyzed by the protein kinase CK2, promotes a further activation of Akt.
CK2 [15] is another survival kinase, usually present in the cells as a tetrameric holoenzyme, composed of two catalytic subunits (α and/or α′) and two regulatory subunits (β), playing a major role as a docking element for substrates and interacting proteins [16, 17]. CK2 is constitutively active and ubiquitous, but particularly elevated in tumors as compared to normal tissues; for this reason, it has recently been considered as a possible therapeutic target [18–20]. The antiapoptotic role of CK2 is supported by many findings (for reviews, see [17, 18, 21, 22]), and the mechanism seems to be extremely multifaceted, as expected considering the huge number of CK2 substrates [23]. Besides the many targets of CK2 involved in cell life/death, a general cooperation has emerged between CK2 and other survival pathways. In particular, several connections have already been reported between CK2 and the PI3K/PDK1/Akt pathway. In fact, CK2 phosphorylates the lipid phosphatase PTEN (phosphatase and tensin homolog deleted on chromosome 10) and regulates its activity [24] and turnover [25], thus contributing to maintaining a high level of phosphatidylinositols, essential for Akt activation. Moreover, CK2, which can also physically associate to Akt [26], directly phosphorylates its residue Ser129 [14]; this phosphorylation, added to those of Ser473 and Thr308, contributes to a hyperactivated state of Akt.
Here we show that CK2 has an additional role in controlling Akt activity, since it contributes to maintaining a high Thr308 phosphorylation level, thus taking part in the major mechanism of Akt regulation.
Materials and methods
Antibodies
Total Akt1/2/3, phospho-Akt (S473), and Hsp90 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-Akt (T308) and phospho-PDK1 (S241) antibodies were from Cell Signaling Technology (Danvers, MA, USA). HA antibodies were from Sigma (St. Louis, MO, USA), and phospho-Akt (S129) antibodies were produced as described elsewhere [14].
Plasmids
The expression vectors for Akt were the murine HA-tagged Akt1 (pCMV6-HAAkt1) and HA-tagged myrAkt1 (pCMV6-HAmyrAkt1, a constitutively active Akt containing a consensus myristoylation site). S129A Akt mutants were obtained as already described [14]. CK2α cDNA (pcDNA3.1/myc-His) was produced as previously described [27].
Proteins
Inactive ΔPH Akt1 (dephosphorylated) and active PDK1 were kindly provided by Dr. H. McLauchlan and Dr. J. Hastie (Dundee). Active full-length Akt1 (phosphorylated at Thr308 and Ser473) was purchased from Upstate-Millipore (Temecula, CA, USA). Recombinant CK2α was expressed, purified [28], and kindly donated by Dr. S. Sarno (Padova). PP2A (protein phosphatase 2A) purified from rabbit skeletal muscle was kindly provided by Dr. P. Agostinis (Leuven). Purified recombinant Hsp90 was kindly provided by Dr. P. Csermely and Dr. C. Soti (Budapest).
Cell culture, transfection, and treatment
HEK 293T cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma), supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin, in an atmosphere containing 5% CO2.
Cells were plated onto 60-mm-diameter dishes at about 80% confluence and transiently transfected with 4 μg of cDNA by standard calcium-phosphate procedure. All Akt transfections were performed with HA-tagged myrAkt1, except transfections for experiments of IGF-1 and OA (okadaic acid) treatments, where HA-tagged Akt1 plasmid was used. The transfection mixture was removed after 16 h, and cells were lysed 24 h after transfection.
Treatments with okadaic acid (Alexis, Lausen, Switzerland), IGF-1 (Sigma), LY294002 (Sigma), TBB [28], or GA (geldanamycin) (Sigma) were performed during transfection, under conditions indicated in the figure legends and described elsewhere [29].
Cell lysis and Western blot analysis
Cell lysis and Western blot analysis were performed as described [14], in a buffer consisting of 20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, and 0.5% (v/v) Triton X-100. For co-immunoprecipitation experiments, a different lysis buffer was used according to [30], consisting of 20 mM Tris–HCl, pH 7.5, 137 mM NaCl, 1 mM EDTA, 1.5 mM MgCl2, and 0.2% (v/v) Nonidet NP40. Lysis buffers were supplemented with 2 mM dithiothreitol, protease inhibitor cocktail Complete (Roche, Mannheim, Germany), 10 mM NaF, 1 μM okadaic acid, and 1 mM Na vanadate. After Western blot procedure, quantification of the antibodies signal was obtained by chemiluminescence detection on a Kodak Image Station 440CF and analysis with the Kodak 1D Image software.
Hsp90 binding to affinity matrix-purified Akt
Aliquots (100 μg) of total proteins from cells overexpressing HA-tagged Akt [wild type (w.t.) or Ser129Ala mutant] in 10 μl lysis solution were mixed with 250 μl of a buffer containing 20 mM Tris–HCl, pH 7.5, 0.1 M NaCl, 0.1 mM EDTA, and 15 μl of the anti-HA affinity matrix (Roche) previously equilibrated in the same buffer. After 15 min of incubation with gentle rotation at 4°C, the affinity matrix-bound Akt was collected by centrifugation, washed twice, and resuspended in the same buffer. Increasing amounts of recombinant Hsp90 were added (as indicated in the figures), and incubated overnight in a final volume of 25 μl with gentle rotation at 4°C. The pellets, recovered by centrifugation and extensively washed with the same buffer, were analyzed by SDS-PAGE and Western blot.
Immunoprecipitation and co-precipitation experiments
Akt immunoprecipitation was performed starting with 200 μg of proteins from lysate of transfected cells, with anti-HA antibodies, using the protocol described in [28]. The following modifications were introduced, where necessary: immunoprecipitations performed to provide Akt substrate to PP2A were carried out in the presence of 0.8% (v/v) Triton X-100; immunoprecipitations performed to assess the presence of co-precipitating Hsp90 were carried out starting from lysate with a different lysis buffer (see above) and in the presence of 35 μM ATP.
In vitro phosphorylation of Akt
Phosphorylation of recombinant Akt by CK2 was performed as already described [14]. Phosphorylation by PDK1 was achieved by incubating Akt with PDK1 for 10 min at 30°C, in the presence of 50 mM Tris–HCl, pH 7.5, 10 mM MgCl2, 10 μM ATP, [γ-33P]ATP (1,500 cpm/pmol), 0.1 mM EGTA, and 1 mM DTT. Analysis was performed by SDS-PAGE, blotting, electronic autoradiography by Cyclone (Perkin Elmer, Shelton, CT, USA), and Western blot with anti-phospho-Ser129 and anti-phospho-Thr308 antibodies.
In vitro dephosphorylation of Akt by PP2A
Akt (recombinant or immunoprecipitated from Akt-overexpressing cells) was incubated with purified PP2A for 2 h at 30°C, in the presence of 50 mM Tris–HCl, pH 7.5, 5 mM MgCl2, and 5 μM ATP. Dephosphorylation was assessed by Western blot analysis with anti-phospho-Thr308 antibody.
Results
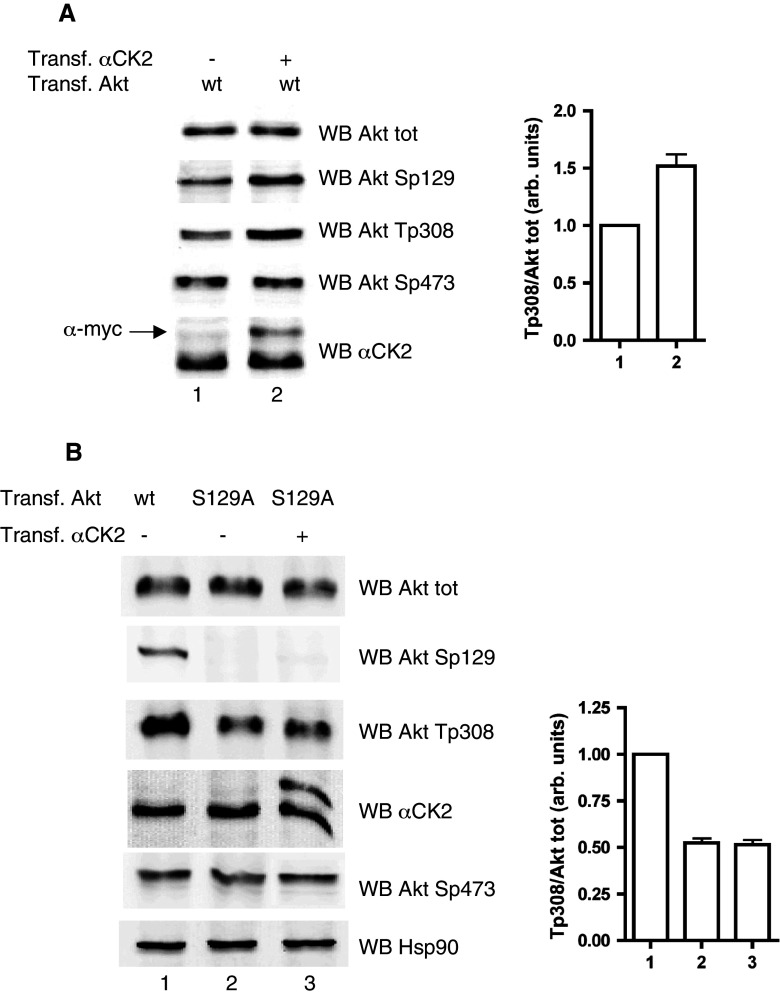
We have previously observed that, upon down-regulation of CK2, the phosphorylation of Akt at Thr308 was significantly reduced, although this residue is not a direct target of CK2 [14]. This correlation has now been confirmed (Fig. 1a) by showing that overexpression of CK2 resulted in a higher level of Thr308 phosphorylation, while Ser473, reported by others [31] to be affected by variations in CK2 activity, was unaffected in our experimental models.
Fig. 1.
Effect of CK2 on Akt Thr308 phosphorylation. a HEK 293T cells were transfected with w.t. Akt plus myc-tagged αCK2, where indicated (+). b HEK 293T cells were transfected with w.t. Akt or Ser129Ala Akt mutant, with or without myc-tagged CK2α, as indicated. Proteins (10 μg) from total lysates were analyzed by Western blot (WB) with the indicated antibodies. The results are also shown as a bar graph for Thr308 signal normalized to total Akt. Bar labels correspond to lane numbers
To check if Thr308 phosphorylation is influenced by the phosphorylation state of Ser129, the only known CK2 target in Akt, we have mutated Ser129 to Ala, and compared the Thr308 phosphorylation of this mutant, expressed in HEK 293T cells, with that of w.t. Akt. The results (Fig. 1b) show that the Ser129Ala mutant was phosphorylated at Thr308 significantly less than w.t. Akt was. Moreover, in the case of the mutant, overexpression of CK2 did not induce any significant increase in Thr308 phosphorylation (Fig. 1b).
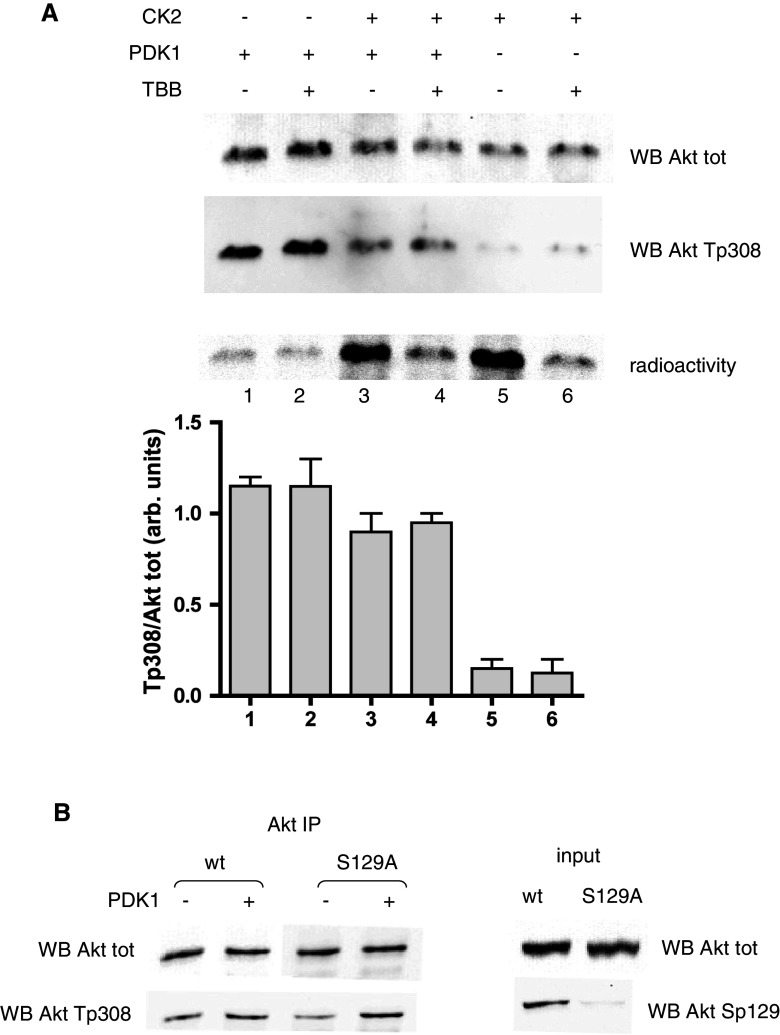
These findings could suggest an effect mediated by protein kinase PDK1, responsible for the Thr308 phosphorylation [32], assuming that Akt phosphorylated at Ser129 might be a better substrate for PDK1. To check this hypothesis, we performed in vitro experiments with recombinant proteins (Fig. 2a). We measured the activity of PDK1 toward Akt that had been phosphorylated by CK2 compared to Akt that had not been pre-incubated with CK2 or that had been incubated in the presence of the CK2-inhibitor TBB (4,5,6,7-tetrabromobenzotriazole) to prevent CK2-dependent phosphorylation. PDK1 efficiency was detected by means of Western blot with the Thr308 phospho-specific antibodies. The results clearly demonstrated that CK2 did not increase the PDK1-dependent phosphorylation of Akt, which was rather decreased, for a reason at present unknown. However, the Akt recombinant protein available for these experiments was a deletion mutant devoid of the PH domain, so we wanted to exclude the possibility that our results were influenced by this deletion. We therefore performed similar experiments by immunoprecipitating Akt from cells expressing w.t. Akt or Ser129Ala mutant, exploiting the fact that only a portion of Thr308 phosphorylation was preserved, so that unphospo-Thr308 was still available for in vitro reaction. Using these immunoprecipitation pellets as substrates for PDK1 (Fig. 2b), we found that, again, PDK1 did not discriminate between the two forms of Akt, either phosphorylated or not by CK2 (distinguishable using anti-phosphoSer129 antibody, see Fig. 2b, right panel). Altogether these experiments disprove the hypothesis that Ser129 phosphorylation facilitates Thr308 phosphorylation by PDK1.
Fig. 2.
Effect of CK2 on PDK1 activity towards Thr308. a Recombinant ΔPH-Akt (240 ng) was pre-incubated for 40 min with a radioactive phosphorylation mixture in the presence or in the absence of CK2α (200 ng). Where indicated, TBB (10 μM) was added at the beginning of the radioactive reaction. After pre-incubation, a new nonradioactive mixture was added, together with PDK1 (20 ng) where indicated. Incubations were prolonged for an additional 10 min. Analysis was performed by SDS-PAGE followed by Western blot with the indicated antibodies; since incubation in the presence of CK2 turned out to reduce the amount of total Akt, the results are also shown as as a bar graph for Thr308 signal normalized to total Akt. Radioactivity was detected by electronic autoradiography, then radioactive bands were cut and counted by liquid scintillation; the CK2-dependent phosphorylation degree (lanes 3 and 5) was estimated at 18%. Three determinations were performed for each experiment; mean values ± SEM are shown. b HEK 293T cells were transfected with w.t. Akt or Ser129Ala Akt mutant, Akt was immunoprecipitated from cell lysates, and the washed pellets were incubated with a phosphorylation mixture, in the presence or in the absence of PDK1 (80 ng). Samples were analyzed by Western blot with the indicated antibodies; on the right (input), the Western blot analysis of 10 μg of total lysate proteins is shown
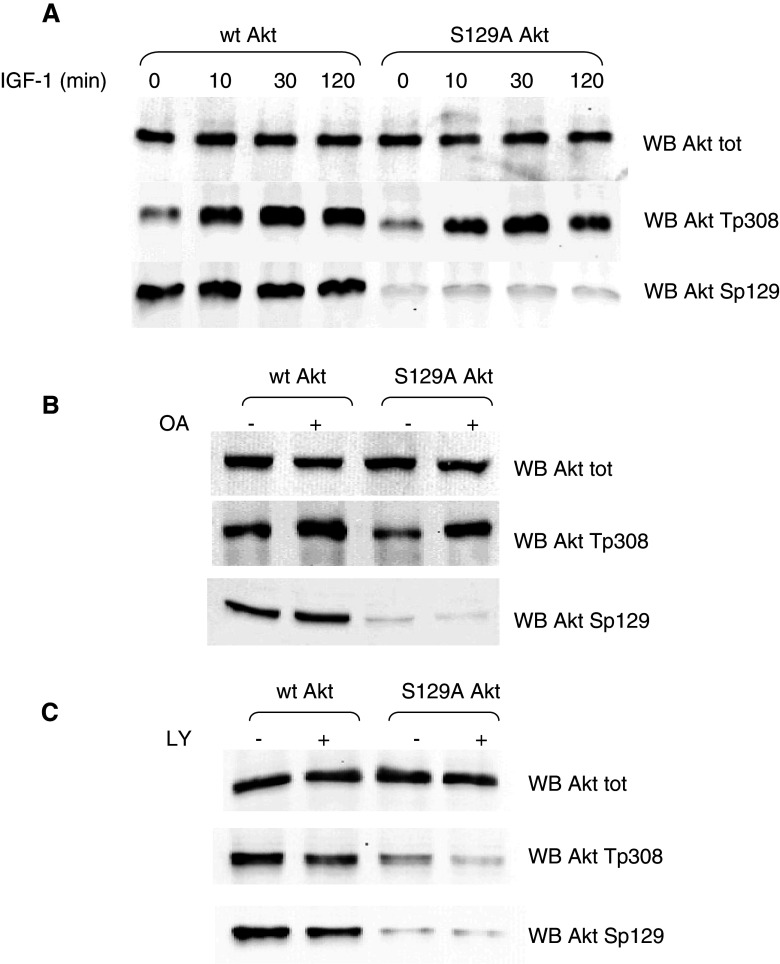
Next, we wanted to analyze the effect of Ser129Ala mutation on Thr308 phosphorylation in response to different cellular conditions variably impinging on Thr308 phosphorylation by PDK1. Starting from starving conditions induced by serum deprivation, we used IGF-1 (insulin-like growth factor-I) to stimulate cells overexpressing w.t. and mutated Akt, and analyzed the Thr308 phosphorylation level (Fig. 3a). While the early phosphorylation in response to the stimulus did not differ significantly, at longer IGF-1 treatment times, a decline in phospho-Thr308 was observed in Ser129Ala Akt mutant, much more evident than in w.t. Akt. This suggested to us that the Thr308 dephosphorylation process, rather than its phosphorylation by PDK1, might be affected by the Ser129 mutation. Consistent with this hypothesis, treatment of cells with okadaic acid, an inhibitor of protein phosphatases (in particular PP2A and PP1 [33]), nearly abrogated the differences between the Ser129Ala mutant and the w.t. Akt (Fig. 3b), as far as Thr308 phosphorylation is concerned. Moreover, in cells treated with LY294002 [34], which blocks the upstream activation of Akt without affecting its dephosphorylation, the higher level of phospho-Thr308 in w.t. compared to mutant Akt was maintained or even increased (Fig. 3c).
Fig. 3.
Effect of Ser129Ala mutation on the Thr308 phosphorylation in response to different cellular conditions. HEK 293T cells were transfected with w.t. Akt or Ser129Ala Akt mutant for 24 h and treated as indicated; 10 μg of total proteins from cell lysates were analyzed by Western blot. a After transfection, cells were serum-deprived for 12 h, then treated with 100 ng/ml IGF-1 for the indicated times. b Cells were treated with 0.5 μM okadaic acid (OA) where indicated for the last 1 h of transfection. c Cells were treated with 25 μM LY294002 (LY) where indicated for the last 2 h of transfection
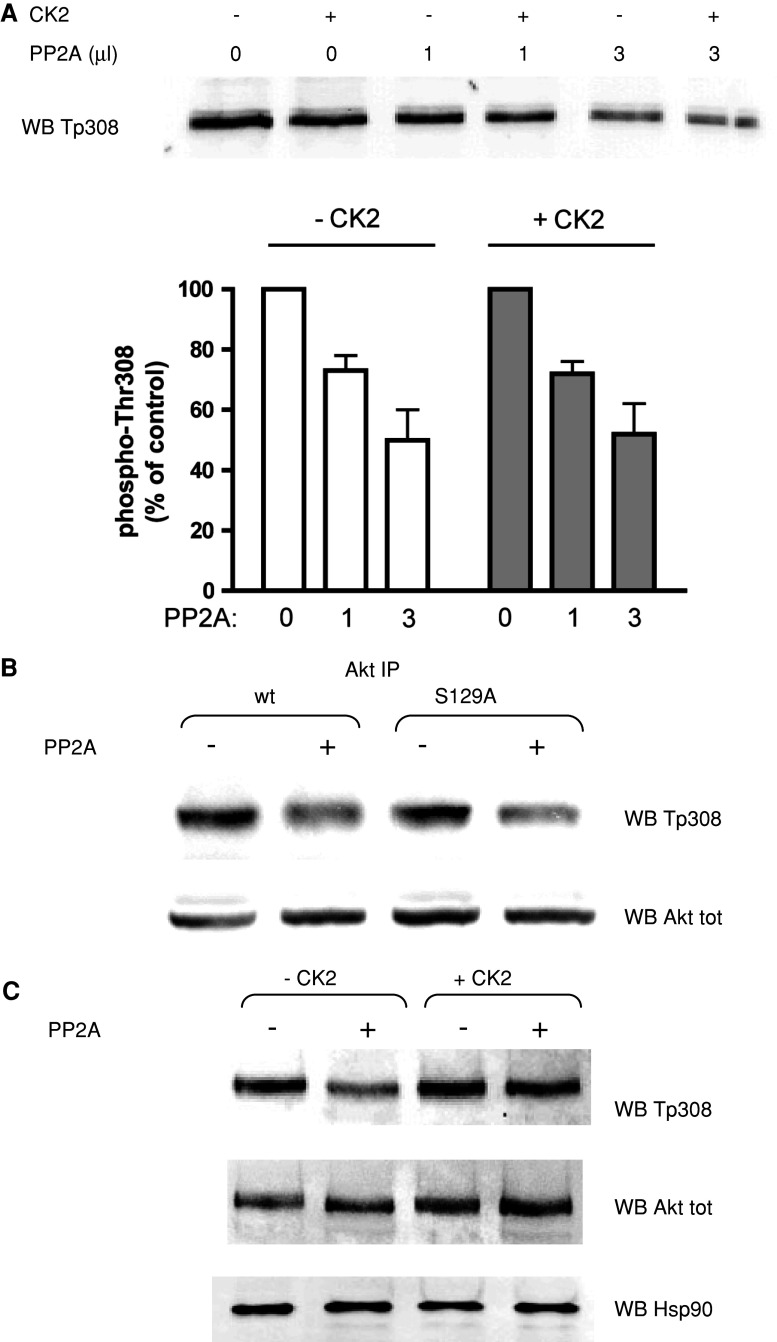
Based on these clues, we tested the in vitro activity of the PP2A, i.e., the phosphatase acting on phospho-Thr308 [35], using purified Akt as substrate, without observing, however, any significant difference in PP2A activity on Akt that had been previously phosphorylated by CK2 on Ser129 compared to control Akt, pre-incubated under the same conditions, but in the absence of CK2 (Fig. 4a). In this in vitro experiment, the phosphorylation degree induced by CK2 was about 47%. Although we do not know the exact extent of in vivo phosphorylation, we can assume that it is similar to that observed in vitro, since the ratio between the signal in WB with anti-phosphoSer129 Akt and anti-total Akt was similar in in vitro and in vivo experiments [14]. Therefore, the in vitro results reasonably reflect the in vivo situation. Moreover, we also performed experiments in which PP2A was assayed on Thr308 of Akt immunoprecipitated from cells; the phosphatase activity was very similar towards the w.t. Akt and the Ser129Ala mutant (Fig. 4b). These data tend to rule out the possibility that phosphorylation of Ser129 directly influences susceptibility of Thr308 to dephosphorylation by PP2A.
Fig. 4.
Effect of CK2-dependent phosphorylation on PP2A activity towards phospho-Thr308. a Recombinant active Akt (200 ng) was preincubated for 30 min with a phosphorylation mixture in the absence or in the presence of CK2α (100 ng). The degree of Akt phosphorylation induced by CK2 under the conditions used for this experiment was about 47%. Incubations were further prolonged for 2.5 h, with the addition of purified PP2A as indicated. Thr308 phosphorylation state was measured by means of Western blot (WB) with the phospho-specific antibody; the quantification of the signal is shown in the bottom panel, expressed as percentage of phosphorylation compared to the control (no PP2A addition). Three different determinations were performed; means ± SEM are shown. b HEK 293T cells were transfected with w.t. Akt or Ser129Ala Akt mutant, Akt was immunoprecipitated from cell lysates, and the washed pellets were incubated with 3 μl of purified PP2A. Thr308 phosphorylation state and total Akt amount were assessed by means of WB with the indicated antibodies. c Recombinant active Akt (200 ng) was preincubated for 30 min in a phosphorylation mixture with or without CK2α (100 ng). Then recombinant Hsp90 (150 ng) was added, followed by purified PP2A (3 μl), where indicated, and incubations were prolonged for a further 2.5 h. Analysis was performed by means of WB with the indicated antibodies
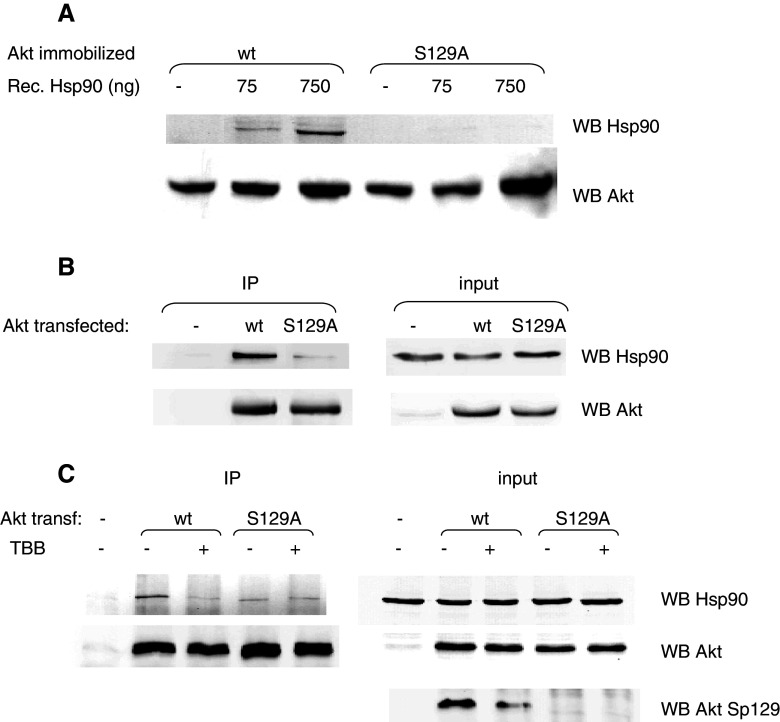
Since it has been reported that the formation of an Akt/Hsp90 complex protects phospho-Thr308 from PP2A-mediated dephosphorylation [30], we wondered if the effect of CK2 on Thr308 dephosphorylation might be mediated by the chaperone protein Hsp90. To check this point, we added recombinant Hsp90 to our in vitro dephosporylation assay, and we observed a protective effect much more evident when Akt was previously phosphorylated by CK2 (Fig. 4c). We therefore exploited two different approaches to analyze the effect of CK2 on the Akt/Hsp90 binding. Firstly, we performed in vitro experiments with recombinant Hsp90 protein, whose ability to bind to Akt immobilized on resin was measured, either under conditions where Akt was phosphorylated by CK2 (Akt from cells expressing w.t. protein) or not (Akt from cells expressing the Ser129Ala mutant). The results (Fig. 5a) showed that the Ser129Ala mutant displays a lower affinity for Hsp90 compared to w.t. Akt. Secondly, to evaluate the in vivo binding of w.t. and mutant Akt to Hsp90, we performed immunoprecipitation experiments, under conditions respectful of multiprotein complexes. Figure 5b shows that, when w.t. Akt was immunoprecipitated, the amount of co-precipitating Hsp90 was much higher than in the case of the Ser129Ala Akt mutant. Then we tried to mimic constitutive phosphorylation of Ser129 by mutating Ser129 to an aspartic acid, which sometimes can substitute for the effect of a phosphorylated residue. However, the results obtained with this mutant (not shown) were very similar to those observed with the Ser129Ala mutant, in terms of phospho-Thr308 level and Hsp90 association. These results seem to indicate that the introduction of a negatively charged side chain at position 129 is not sufficient to mimic phosphorylation of Ser129, but they do not allow us to completely exclude that the mutation of Ser129Ala is accompanied by a structural defect not related to the phosphorylation state. To confirm that the reduced association of mutant Akt with Hsp90 was due to the lack of phosphorylation, we treated cells overexpressing w.t. Akt with the CK2-inhibitor TBB [28]. After having assessed that the level of Ser129 phoshorylation was actually decreased by the treatment (Fig. 5c, right panel), we measured the amount of Hsp90 co-precipitating with Akt, and we found that CK2 inhibition produced an effect similar to the Ser129Ala mutation (Fig. 5c, left panel). As expected, no effect was observed on Hsp90 association when TBB treatment was applied to cells expressing the Ser129Ala mutant instead of the w.t. Akt.
Fig. 5.
Association of Hsp90 protein to w.t. and Ser129Ala Akt. a HA-tagged Akt (w.t. or Ser129Ala mutant) was expressed in HEK 293T cells, immobilized on resin (anti-HA Affinity Matrix, Roche), and incubated with increasing amounts of purified recombinant Hsp90. After washing, the resin-bound proteins Hsp90 and Akt were detected by Western blot (WB) with the specific antibodies. b HEK 293T cells were transfected with empty vector (−), HA-tagged w.t. Akt (w.t.), or HA-tagged Ser129Ala Akt (S129A). Lysate proteins (200 μg) were used for immunoprecipitation with anti-HA antibodies. The left panel [immunoprecipitation (IP)] shows the WB analysis of the pellets for the amount of precipitated Akt and co-precipitated Hsp90. The right panel (input) shows WB with the same antibodies of 10 μg of proteins from total lysates. c HEK 293T cells were transfected with HA-tagged w.t. Akt (w.t.) or HA-tagged Ser129Ala Akt (S129A), and treated, where indicated, with 20 μM TBB for 3 h. Then, 200 μg of lysate proteins was used for immunoprecipitation with anti-HA antibodies. Left panel (IP) shows the WB analysis of the pellets for the amount of precipitated Akt and co-precipitated Hsp90. Right panel (input) shows WB with the indicated antibodies of 10 μg of proteins from total lysates
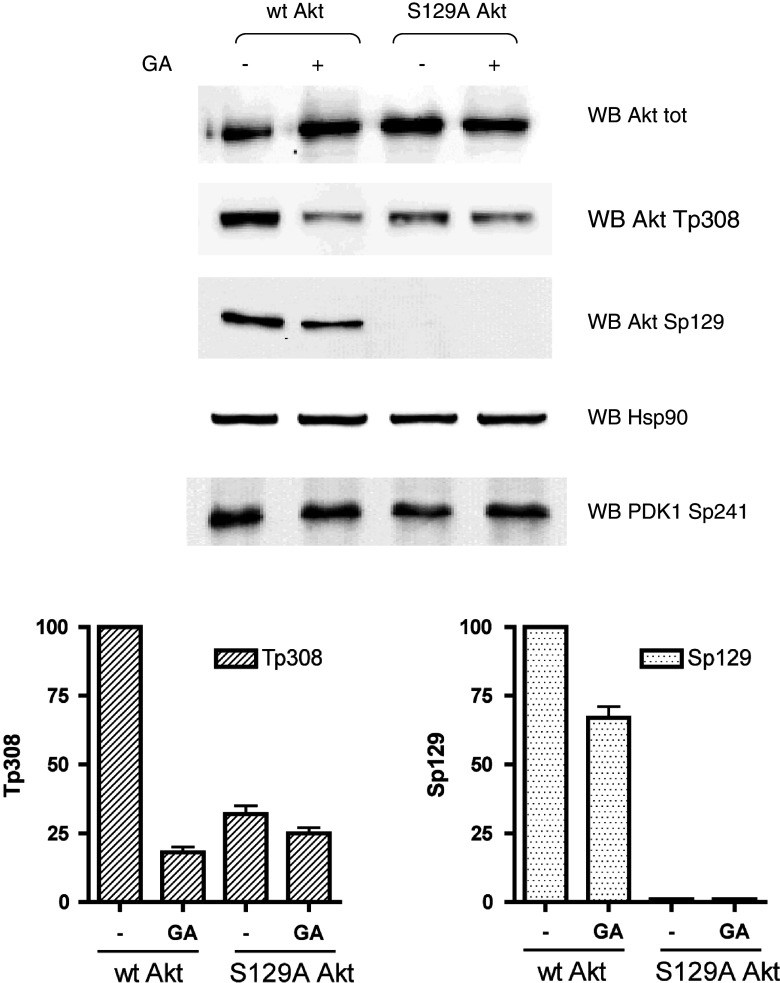
Next, we reasoned that, if a stronger association to Hsp90 is responsible for a higher phospho-Thr308 level in w.t. than in Ser129A Akt, the abrogation of Hsp90 function in the cells would abolish these differences. We therefore treated the cells with the Hsp90 inhibitor GA (geldanamycin) [36], and we found (Fig. 6) that, without any effect on PDK1 activation, the Thr308 phosphorylation of w.t. Akt was dramatically reduced by GA, reaching a level very similar to that of the Ser129Ala mutant, in which phospho-Thr308 was unaffected by the GA treatment. Also the level of Ser129 phosphorylation was slightly reduced by GA, by a mechanism that is presently unclear.
Fig. 6.
Effect of geldanamycin (GA) on the Thr308 phosphorylation of w.t. and Ser129Ala mutant Akt. HEK 293T cells were transfected with w.t. Akt or Ser129Ala Akt mutant for 24 h and treated with 10 μM GA for the last 1 h of transfection, where indicated; 10 μg of total proteins from cell lysates were analyzed by Western blot with the indicated antibodies. In the lower panel, quantification of phospho-Thr308 and phospho-Ser129 of three different experiments (means ± SEM) are shown
Discussion
The work described in this paper discloses a new mechanism by which CK2 up-regulates Akt, showing that phosphorylation of Akt at Ser129—in addition to conferring higher activity per se [14]—also ensures a higher phosphorylation level at Thr308. While data obtained by altering the activity of cellular CK2 (by CK2 inhibition or overexpression) could be explained assuming an indirect effect of CK2, mediated by one or more of its many substrates involved in PI3K/PDK1/Akt pathways [such as PTEN [24, 25], Hsp90 [37], or cdc37 (cell division cycle 37) [38]], the effects observed by mutating Ser129 to a non-phosphorylatable residue allow us to conclude that Ser129 phosphorylation is directly involved in determining a higher level of phospho-Thr308. In principle, this could be due either to an increased phosphorylation by the Thr308 kinase (PDK1) or to a decreased dephosphorylation by the Thr308 phosphatase (PP2A). Our results show that Ser129 phosphorylation does not affect Thr308 phosphorylation by PDK1 (Fig. 2); somewhat surprisingly, PP2A also displays similar activity towards immunoprecipitated Akt either phosphorylated or not at Ser129 (Fig. 4). We observed, however, that if lysis and immunoprecipitation were performed under conditions respectful of multiprotein complexes, the chaperone protein Hsp90, as expected [30], co-precipitated with Akt, and its amount was significantly reduced when Ser129 was not phosphorylated (Fig. 5). Since Hsp90 hampers the accessibility of phospho-Thr308 to the phosphatase PP2A [30], it is expected that a more stable complex will result in a slower in vivo dephosphorylation process. Consistently, when Hsp90 function was inhibited by GA, phospho-Thr308 level of w.t. Akt decreased to the level observed with the Ser129Ala mutant (Fig. 6). In Fig. 5, it is also noteworthy that the effect of cell treatment with the CK2 inhibitor TBB was even more dramatic than the mutation of Ser129 in terms of reduced Hsp90 association; this is not completely unexpected considering that TBB blocks the action of CK2 also on other substrates related to the Akt pathway. For example, Hsp90 itself is a CK2 substrate, as is another protein, the co-chaperone cdc37, which has been reported to participate in the Akt/Hsp90 complex [38]. For this latter, however, we have no evidence for a possible role in mediating the effect of Ser129 on Thr308 phosphorylation (not shown).
The mechanism by which the presence of a phosphate group on Ser129 increases the affinity of Akt for Hsp90 is still a matter of conjecture; data obtained in far western (overlay) experiments tend to rule out a direct binding of Hsp90 to the region of Akt containing (phospho)Ser129. In fact, when Ser129 peptide or phospho-peptide was incubated over a membrane exposing Hsp90, no association was observed, as judged from the WB with anti-Ser129 antibodies (not shown). Negative results were also obtained the other way around, i.e., immobilizing Ser129 peptide on the membrane and overlaying with Hsp90 (not shown). Our results are consistent with previously reported data [30] showing that the Akt region involved in the interaction with Hsp90 encompasses residues 229–309, thus not including Ser129. We have to assume, therefore, that the phosphorylation of Ser129 confers to Akt a conformation more suitable for Hsp90 binding. A crystal structure of this region would greatly help in understanding this mechanism; unfortunately, Ser129 is located in the Akt linker region, connecting the PH and the catalytic domains, and, so far, no structure of this portion is available, but only models that are considered highly speculative [39]. For the time being, we can only speculate that this linker region, endowed with a high flexibility, can variably interact with remote domains of Akt in a phosphorylation-dependent manner.
Given the constitutive activity of CK2, it is possible that Akt phosphorylation at Ser129 also occurs under resting conditions, preceding phosphorylation at Thr308 by PDK1 in response to cellular signals. It seems more likely, however, that CK2 intervention occurs when phosphate is already present on Thr308 and is aimed at maintaining this activation state. This possibility is supported by preliminary results suggesting that active Akt is a better substrate for CK2 than unphosphorylated Akt (unpublished data; also compare the degree of CK2-dependent phosphorylation obtained in experiments of Fig. 2, i.e., 18% phosphorylation of inactive Akt, with that of Fig. 4, 47% phosphorylation of active Akt). This would be consistent with a situation in which CK2 plays its role after PDK1, preferentially phosphorylating those molecules displaying an active conformation owing to the presence in them of phospho-Thr308 (and possibly phospho-Ser473). Whatever the order of Akt phosphorylation might be, our results suggest that Ser129 dephosphorylation precedes Thr308 dephosphorylation and, in a way, is a prerequisite. Interestingly, Ser129 phosphorylation is not affected by the okadaic acid treatment (Fig. 3), ruling out the implication of the okadaic-sensitive phosphatases, PP2A and PP1 [33]. Further work will be necessary to elucidate these aspects, but implication of different enzymes mediating Ser129 and Thr308 dephosphorylation would be consistent with the hierarchical concept of one phospho-site preventing the dephosphorylation of the other. We also observed a slight reduction in Ser129 phosphorylation in cells treated with the PI3K inhibitor LY294002 (Fig. 3); although this compound has been reported as a CK2 inhibitor as well [40], we do not have any evidence of cellular CK2 inhibition at the concentration used [14]. Rather, considering that LY294002 treatment reduces the Akt phospho-Thr308, the preference of CK2 for phosphorylating Akt already in the active conformation can be a possible explanation of this observation.
We have also tried to evaluate the relevance of Ser129 phosphorylation to Akt functionality in cells. We have already shown [14] that the Akt Ser129 mutant displays reduced activity towards GSK3β or a specific peptide substrate. New preliminary results would indicate that w.t. Akt ensures a higher level of cell survival in response to an apoptotic stimulus, compared to the mutant not phosphorylatable by CK2, expressed at a similar level. However, in our experiments only a minority of cells were transfected, thus preventing any reliable analysis about the effects of the mutation on transfected cell survival; future experiments exploiting stable transfection or cell sorting will be necessary to clarify this point. Although most of the experiments were performed on cells ectopically expressing Akt, it is important to stress, in this respect, that the implication of CK2 in the direct phosphorylation of Akt Ser129 and in increasing the level of Thr308 phosphorylation was previously demonstrated to occur physiologically in nontransfected Jurkat cells [14]. Therefore, we can assume that all the findings reported here reflect biologically relevant events.
In summary, our results outline a new mechanism by which CK2 controls Akt activity. By ensuring high levels of phospho-Thr308, the key residue for Akt activation, CK2 can significantly potentiate the Akt-mediated catalysis. Consequently, cells where CK2 expression/activity are abnormally high (as frequently observed, e.g., in tumors) may also undergo unwanted hyperactivation of Akt. As the Akt signaling is one of the most critical pathways in promoting cell survival, this can in turn result in marked apoptosis resistance and enhancement of the tumor phenotype, contributing to that vicious circle where CK2 plays a major role as a key suppressor of apoptosis [21] and a cancer driver, although no genetic alterations of CK2 are known to be responsible for specific kinds of tumors [41].
Acknowledgements
We thank the protein production team of the Division of Signal Transduction Therapy at the University of Dundee, Scotland (coordinated by Hilary McLauchlan and James Hastie), for providing inactive Akt and active PDK1; Dr. S. Sarno (Padova, Italy) for providing recombinant CK2; Dr. P. Csermely and Dr. C. Soti (Budapest, Hungary) for providing recombinant Hsp90; Dr. P. Agostinis (Leuven, Belgium) for providing PP2A; and Dr. O. Marin (Padova, Italy) for the synthesis of Ser129 peptide and phospho-peptide. This work was supported by grants from the University of Padova (Progetto Ateneo 2005 to M.R.) and the Italian Ministry of University and Research (PRIN-2007 to M.R.) and from AIRC and EC (PRO-KINASERESEARCH 503467 to L.A.P.).
Footnotes
Giovanni Di Maira and Francesca Brustolon contributed equally to this work.
References
- 1.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 2.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sale EM, Sale GJ. Protein kinase B: signalling roles and therapeutic targeting. Cell Mol Life Sci. 2008;65:113–127. doi: 10.1007/s00018-007-7274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 7.Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, Hemmings BA. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 2001;294:374–380. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 8.Laine J, Künstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell. 2000;6:395–407. doi: 10.1016/S1097-2765(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 9.Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, Russo G, Tsichlis P, Croce CM. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA. 2000;97:3028–3033. doi: 10.1073/pnas.040557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 11.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/S0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 12.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 13.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBα/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes cell survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 15.Pinna LA. Protein kinase CK2: a challenge to canons. J Cell Sci. 2002;115:3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 16.Guerra B, Boldyreff B, Sarno S, Cesaro L, Issinger OG, Pinna LA. CK2: a protein kinase in need of control. Pharmacol Ther. 1999;82:303–313. doi: 10.1016/S0163-7258(98)00064-3. [DOI] [PubMed] [Google Scholar]
- 17.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: implications for cancer therapy. Curr Cancer Drug Targets. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2—a key suppressor of apoptosis. Adv Enzyme Regul. 2008;48:179–187. doi: 10.1016/j.advenzreg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagano MA, Cesaro L, Meggio F, Pinna LA. Protein kinase CK2: a newcomer in the ‘druggable kinome’. Biochem Soc Trans. 2006;34:1303–1306. doi: 10.1042/BST0341303. [DOI] [PubMed] [Google Scholar]
- 23.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 24.Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:145–153. doi: 10.1016/S0014-5793(02)03274-X. [DOI] [PubMed] [Google Scholar]
- 25.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 26.Guerra B. Protein kinase CK2 subunits are positive regulators of AKT kinase. Int J Oncol. 2006;28:685–693. [PubMed] [Google Scholar]
- 27.Salvi M, Sarno S, Marin O, Meggio F, Itarte E, Pinna LA. Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 2006;580:3948–3952. doi: 10.1016/j.febslet.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’) FEBS Lett. 2001;496:44–48. doi: 10.1016/S0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 29.Di Maira G, Brustolon F, Bertacchini J, Tosoni K, Marmiroli S, Pinna LA, Ruzzene M. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007;26:6915–6926. doi: 10.1038/sj.onc.1210495. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao CC, Ma YL, Lee EH. Protein kinase CK2 impairs spatial memory formation through differential cross talk with PI-3 kinase signaling: activation of Akt and inactivation of SGK1. J Neurosci. 2007;27:6243–6248. doi: 10.1523/JNEUROSCI.1531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 33.Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-E. [DOI] [PubMed] [Google Scholar]
- 34.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 35.Andjelković M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uehara Y. Natural product origins of Hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:325–330. doi: 10.2174/1568009033481796. [DOI] [PubMed] [Google Scholar]
- 37.Miyata Y, Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- 38.Miyata Y, Nishida E. CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol Cell Biol. 2004;24:4065–4074. doi: 10.1128/MCB.24.9.4065-4074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar CC, Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 40.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarno S, Pinna LA. Protein kinase CK2 as a druggable target. Mol Biosyst. 2008;4:889–894. doi: 10.1039/b805534c. [DOI] [PubMed] [Google Scholar]