Abstract
CD24 is expressed on mammary stem cells and is used as a marker for their isolation, yet its function in the mammary gland still needs to be examined. Here we show that CD24 is expressed throughout the luminal epithelial cell layer, but only weakly in myoepithelial cells. During lactation, CD24 expression was suppressed within alveoli, but upregulated post-lactation, returning to a pre-pregnant spatial distribution. CD24-deficient mice exhibited an accelerated mammary gland ductal extension during puberty and an enhanced branching morphogenesis, resulting in increased furcation in the ductal structure. CD24−/− mammary epithelial cells were able to completely repopulate cleared mammary fat pads and to give rise to fully functional mammary glands. Together, these data suggest that while CD24 is expressed in mammary epithelium compartments thought to contain stem cells, CD24 is not a major regulator of mammary stem/progenitor cell function, but rather plays a role in governing branching morphogenesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0342-6) contains supplementary material, which is available to authorized users.
Keywords: CD24, Mammary gland, Knockout mice, Branching morphogenesis, Lactation, CD52
Introduction
At birth, the mammary gland contains an immature glandular anlage that occupies only a portion of the mammary fat pad in the region of the nipple (reviewed in [1]). During puberty, the ducts grow and fully invade the fat pad. This outgrowth is driven by specialized club-like structures called terminal end buds (TEBs) located at the end of each duct. TEBs are composed of layers of luminal epithelial cells that overlie cap cells, which are basally located at the growing tip of the duct. The cap cell population is thought to contain stem cells that give rise to the myoepithelial and luminal epithelial layers as the mammary ducts invade the fat pad during puberty [2]. Myoepithelial cells form the basal layer of mammary ducts and are closely apposed to the basement membrane. They contain myofilaments within the cytoplasm and are readily recognized after immunostaining with anti-α-smooth muscle actin antibodies [3]. Luminal epithelial cells overlie the myoepithelial layer and can be identified because of the expression of specific marker proteins such as cytokeratin 18 [4].
In the adult, further phases of ductal growth occur during pregnancy and lactation, where lateral buds form along the ducts developing into alveoli that secrete milk. After weaning, the mammary glands involute in a process typified by both apoptosis and tissue remodeling. A multiplicity of hormones and growth factors controls these processes (reviewed in [5]).
The existence of ductal stem cells that can give rise to the different mammary epithelial cell types was first suggested by the observation that bud-free ducts can regenerate the entire mammary tree [6]. The localization of stem cells within the mammary gland remains to be fully delineated. Mammary stem cells in the mouse are thought to have a basal location (reviewed in [7]). One of the markers used to define mammary stem and progenitor cells is CD24 [7–11]. It has been shown that a single CD24+Lin−CD29hi or CD24+Lin−CD49fhi mammary stem cell is capable of generating a functional mammary gland [8, 9]. However, it remains to be demonstrated whether CD24 expression merely acts as a marker for mammary stem cells or has a functional significance.
CD24 comprises a heavily glycosylated mucin-like GPI-linked cell surface protein also known as heat stable antigen (HSA) in the mouse (reviewed in [12]). Glycosylation is cell type dependent and highly variable. A single functional CD24 gene exists in mice and humans, although CD52 is a related protein that is similarly GPI-linked and heavily glycosylated [13]. Besides its expression in the mammary gland, CD24 is also found on stem cells or progenitors of other organs including brain [14], prostate [15] and kidney [16]. Several studies suggest that it plays roles in the development and function of certain hematopoietic cells [17, 18] and in neurite outgrowth and neurogenesis [19–22]. Recently, a role for CD24 in regulating synaptic transmission of myofibers [23] and in suppressing tissue damage-induced immune responses [24] has been shown. Pathologically, CD24 is upregulated or de novo expressed in a variety of tumor types [12, 25].
In this study we have examined the normal postnatal expression pattern and regulation of CD24 in the mammary gland before and after puberty, and during pregnancy, lactation and involution. We show that CD24 is expressed in the mammary epithelium at all stages of mammary gland development and function analyzed, albeit at different intensities both spatially and temporally. Data obtained using mice deficient for CD24 expression suggest that CD24 regulates ductal branching density during pubertal mammary gland development.
Materials and methods
Mice
C57BL/6JolaHsd mice and mice bearing genomic deletion of CD24 on a C57BL/6JolaHsd background [17] used for these experiments were maintained under SPF conditions in accordance with institutional and international guidelines. All of the analyses in this paper were performed using no. 4 mammary glands.
Transplantation of mammary epithelium into cleared mammary fat pads of mice
Mammary fat pads were cleared as previously described [26]. In brief, inguinal fat pads of 3-week-old female nude Balb/c mice (Charles Rivers) were cleared by surgically removing the developing mammary epithelium. Small pieces of epithelium-containing mammary tissue from either 16-week-old CD24−/− or corresponding B6 wt donor mice were implanted. Nine weeks after surgery, the outgrowth of transplants was analyzed by performing whole mounts. In order to check the capacity for functional differentiation (alveoli formation), some mice were mated and transplants taken and analyzed in the last third of pregnancy (E13–E16). The origin of outgrown mammary epithelium was confirmed by performing CD24 staining on frozen sections as described.
RNA preparation and Northern blots
Freshly dissected no. 4 mammary glands were snap-frozen with prior removal of the lymph nodes. Tissue was ground into a powder under liquid nitrogen, and total RNA was isolated using peqGold (Peqlab, Erlangen) according to the manufacturer’s instructions. Northern blots using 1% agarose-formaldehyde gels and 10 μg total RNA were performed as previously described [27]. After crosslinking with UV light, blots were stained with methylene blue solution to ensure equal loading of RNA, photographed and probed using radiolabeled cDNA-probes. A 320-bp CD24 probe was generated by NheI/XhoI digest of a pMSG-CD24 vector. The mouse CD52 and the mouse cytokeratin 18 (mCK18) probes were generated by PCR using forward primer 5′ GCC CAG GAA GAT TTC AGG ATG AA 3′ and reverse primer 5′ GCT GCG CCT TCA CCT CAG CTG A 3′ for CD52 (mouse splenic cDNA as template) and forward primer 5′ GCT ACC TAG ACA AGG TGA AGA GC 3′ and reverse primer 5′ GCC AGC TCT GAC TCC AGA TGC 3′ for mCK18 (mouse mammary gland cDNA as template). The ß-casein probe was prepared as described previously [28].
Immunohistochemistry
Number 4 mammary glands from female mice were dissected and embedded in OCT (Labonord, Mönchengladbach; Germany). The 12-μm frozen sections were cut, dried over night and fixed in ice-cold 100% acetone for 10–15 min. Sections were blocked for 10 min with PBS/10% goat serum/1% rabbit serum followed by an avidin-biotin blocking step (Avidin-Biotin Blocking kit, DAKO, Hamburg, Germany). Sections were incubated with rat anti-mouse CD24 (clone M1/69; RDI, Flanders, NJ) at a concentration of 0.75 μg/ml in PBS for 1 h at room temperature. Unbound antibody was washed off with PBS/0.05% Triton X-100, and sections were incubated with secondary antibody (anti-rat biotin, DAKO) at a dilution of 1:600 in PBS for a further 30 min at room temperature. After washing, slides were incubated for an additional 30 min with alkaline-phosphatase complex (Vectastain ABC-AP kit, Vector Laboratories). After further washing, sections were exposed to a chromogen substrate (New Fuchsin, DAKO) containing Levamisole (DAKO) and counterstained with hematoxilin. For immunofluorescence analysis, the staining procedure was essentially the same except using the CD24 antibody at a concentration of 5 μg/ml. Anti-smooth muscle actin antibody (Sigma, clone 1A4, ascites fluid) was employed at a dilution of 1:500 for 1 h at room temperature. Appropriate secondary Alexa-coupled antibodies (Invitrogen) were employed together with DAPI (1:10,000) for 30 min at room temperature at a dilution of 1:1,000.
H&E staining
Prior to embedding in paraffin, mammary gland specimens were fixed in formalin. For histological analysis, 6-μm sections were cut and stained with hematoxylin and eosin.
Whole mount staining of mammary glands
Number 4 mammary glands were freshly dissected and fixed on a glass slide in methanol:chloroform:acetic acid (6:3:1). After washing in 70% ethanol, glands were stained in carmine solution [0.2% carmine (w/v), 0.5% potassium aluminium sulphate (w/v) and 0.01% thymol (w/v)] and destained with 70% ethanol followed by 95% and 100% ethanol. For long-term storage and photographic documentation, glands were immersed in methyl salicylate (Oil of Wintergreen, Sigma). Whole mounts were photographed using a Leica Stereomicroscope and a AxioCam camera from Zeiss. Analysis of branching was performed by counting the number of ductal endings/endpoints of the whole gland as an indirect means of quantifying bifurcation events. To quantify ductal elongation into the mammary fat pad, the area occupied by extended mammary ducts was measured using the contour tool of Axiovision software Rel 4.7. Statistical significance was calculated using an unpaired two-sided Student’s t test.
Results
CD24 is expressed in postnatal mammary glands and is restricted to the epithelial compartment
CD24 is used as a marker for the isolation of mammary stem and progenitor cells, although the limited expression data published to date suggest that CD24 is expressed more broadly in the mammary gland [8, 9, 29]. To begin to understand the role of CD24 expression on mammary stem and progenitor cells, we set out first to elucidate the temporal and spatial expression of CD24 in the postnatal murine mammary gland. To this end, we used the anti-CD24 antibody M1/69 to stain frozen sections from the mammary glands of pre- and post-pubertal mice in order to determine CD24 expression and localization at the cellular level. Preliminary experiments in which chromogen-based and immunofluorescence-based staining methods for CD24 were compared indicated differences between these techniques with respect to signal linearity, sensitivity and saturation. We therefore used both methods to allow an accurate indication of the CD24 protein expression pattern in the mammary glands to be obtained. Importantly, although there were differences in the relative staining intensities, the same general staining pattern in the different stages was observed with both methods.
CD24 staining was observed in the epithelial ducts of all mice examined (Fig. 1 and Supplementary Figure 1). No notable difference in staining intensity was observed when 4-week-old mice, 6-week-old mice (Fig. 1), 3-month-old mice and 5-month-old mice (data not shown) were compared. Further stainings of mammary tissue at additional time points during puberty from mice 3, 4, 5, 6, 8 and 10 weeks of age are presented in Supplementary Figure 1. Specificity of staining was demonstrated by using the anti-CD24 antibody M1/69 to stain mammary gland sections from CD24 deficient mice (Supplementary Figure 2a), which showed that mammary epithelial cells were not stained in these sections. We conclude that CD24 is expressed in both pre- and postpubertal mammary epithelium at consistently equivalent levels.
Fig. 1.
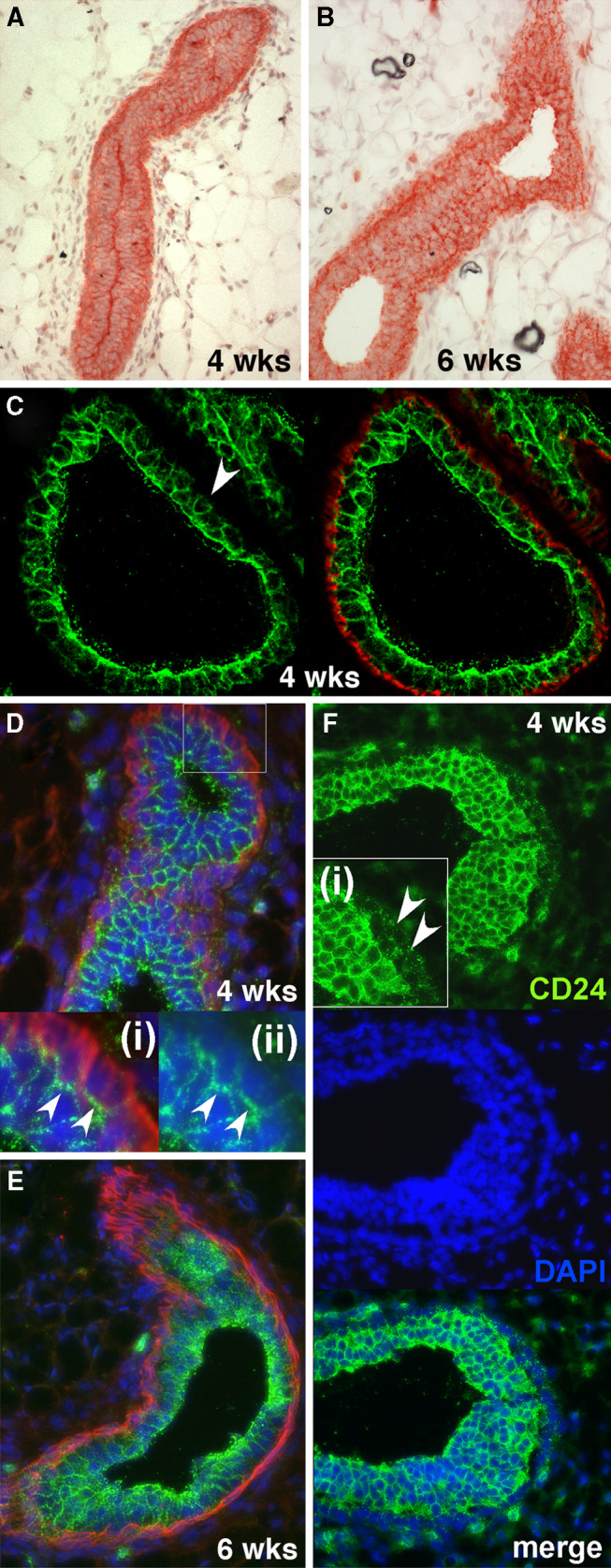
Expression of CD24 in the murine mammary gland during puberty. a, b CD24 expression detected by Fuchsin chromogen staining in mammary epithelial ducts from mice 4 weeks old (a) and 6 weeks old (b). c–e Expression of CD24 (green fluorescence) and smooth muscle actin (red fluorescence) in mammary glands from 4-week-old mice (c, d) and 6-week-old mice (e). DAPI staining of nuclei (blue fluorescence) was omitted from c for the sake of clarity. The arrowhead in c indicates an example of a luminal cell with only weak CD24 staining on its basally-facing surface. The insets (i) and (ii) in d show larger magnifications of the boxed area in the main picture. In inset (ii) the smooth muscle actin staining has been omitted to allow better visualization of the strong CD24 staining at the interface between luminal and basal cells (indicated by arrowheads) and the weak CD24 staining elsewhere on the basal cells. f CD24 staining (green fluorescence) and DAPI staining of nuclei (blue fluorescence) of a TEB from mice of 4 weeks of age. The arrowheads in inset (i) indicate weak CD24 staining in the cap cells of the TEB
Although CD24 was expressed in all ducts, the staining intensity differed in the different epithelial layers. A rim of strong CD24 staining was often observed on the luminal surface. This was particularly pronounced when the lumen was closed, where strong CD24 staining was usually observed at the interface between the two luminal surfaces (Fig. 1a). With chromogen staining we also often observed a more pronounced staining at the interface between the myoepithelial and luminal epithelial cells (Fig. 1a, b). Co-staining mammary sections with anti-CD24 and anti-smooth muscle actin antibodies revealed two patterns of CD24 expression at the interface between the luminal and myoepithelial layers. Some luminal cells exhibited virtually no CD24 staining at their interface with the myoepithelial cells, despite being strongly CD24 positive on their other surfaces (Fig. 1c, see arrowhead), while others showed a pronounced CD24 staining at their interface with the myoepithelial cells (Fig. 1d). Aside from the interface with luminal cells, CD24 expression was weak or non-detectable in the myoepithelial layer (Fig. 1c–e). In TEBs, cap cells stained weakly positive for CD24 (Fig. 1f).
Our data demonstrate that in contrast to other reports [9], CD24 expression is not expressed on adipocytes or endothelial cells, which may be due to strain-specific differences. However, occasional extra-ductal CD24-positive cells were observed in mammary glands from wild-type mice, and were found to be CD45 positive, indicating a hemapoietic origin (Supplementary Figure 2). Occasionally some periglandular structures stained positive with the antibody in sections of mammary gland from CD24-deficient mice that were not stained in secondary antibody alone controls (Supplementary Figure 2a, b), indicating that the M1/69 antibody can weakly stain non-CD24 epitopes.
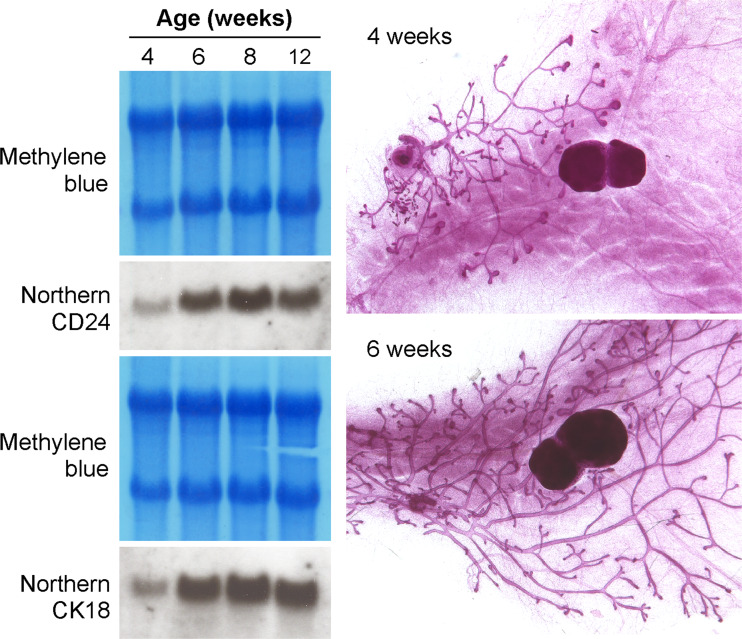
We also performed Northern blots using RNA derived from pre- and post-pubertal mice. Using this method, little CD24 transcription could be detected in the mammary glands of 4-week-old pre-pubertal mice, but after 6 weeks of age, robust and ubiquitously high expression of CD24 was observed (Fig. 2). However, very little mammary epithelium is present in mice of 4 weeks of age in comparison to older mice (as illustrated by the whole mounts in Fig. 2) and thus most RNA will be derived from the non-epithelialized fat pad, diluting out any CD24 transcripts from the epithelial cells. This changing ratio of cell-type specific RNAs that contribute to the total RNA during development of the gland is reflected by an increasing signal for the luminal epithelium-specific marker cytokeratin 18 (Fig. 2).
Fig. 2.
Northern blot of RNA from mammary glands of mice of different ages. The blots were stained with methylene blue to ensure equal RNA loading, then hybridized with a CD24 probe or with a probe for the luminal epithelial marker cytokeratin 18 (CK18). Whole mounts of mammary glands from female mice 4 and 6 weeks of age demonstrate the differences in the extent of mammary duct growth through the mammary fat pad before and during puberty
Expression of CD24 protein is downregulated in lactating alveoli
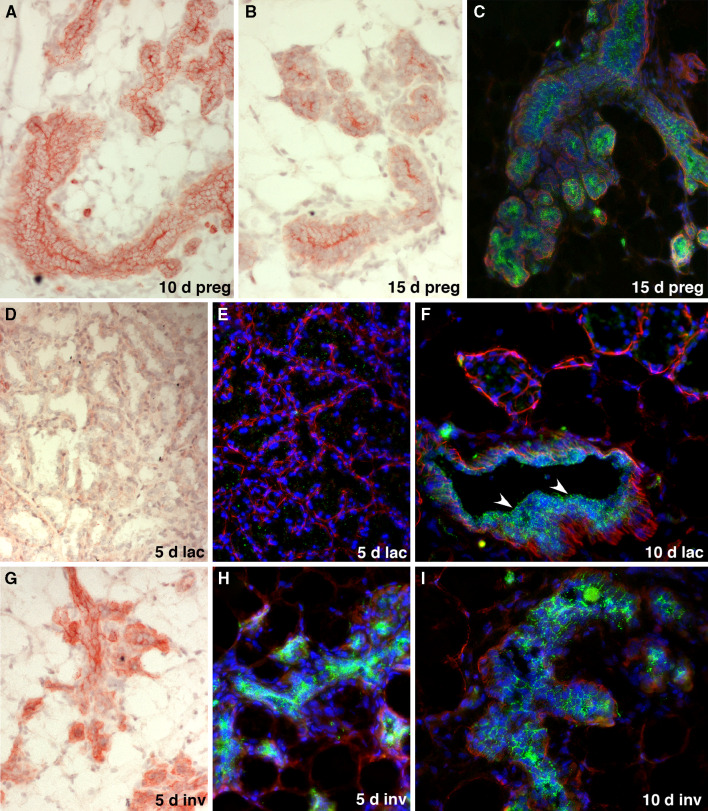
Having shown that CD24 is ubiquitously expressed in the ductal epithelium of virgin mice, we then set out to determine whether expression of CD24 is regulated during pregnancy, lactation and involution. During pregnancy, we observed pronounced staining on the surface of the alveolar cells abutting the closed alveolar lumen (Fig. 3a–c). This strong staining of the alveolar luminal surface was transiently maintained once the alveoli began to open close to partuition (Supplementary Figure 1). During lactation, CD24 staining was virtually undetectable in milk-producing alveoli (Fig. 3d–f). However, collecting ducts retained a robust CD24 expression throughout pregnancy and lactation (Fig. 3f, arrowheads). Strong CD24 expression was re-established in the mammary epithelium soon after involution began subsequent to the cessation of lactation (Fig. 3g–i) and the expression pattern of CD24 returned to that observed in postpubertal non-pregnant mice.
Fig. 3.
Expression of CD24 in the murine mammary gland during pregnancy, lactation and involution. a–c Expression of CD24 in the mammary epithelium of mice pregnant for 10 days (a) and 15 days (b, c) detected by red Fuchsin chromogen staining (a, b) or as green fluorescence (c). Staining for smooth muscle actin (red fluorescence) and DAPI staining of nuclei (blue fluorescence) was also performed in c. Note the reduced CD24 staining in the alveolar epithelial cells, with the exception of the cell surfaces forming the closed lumen of the alveoli. d–f Expression of CD24 in the mammary epithelium of mice that had been lactating for 5 days (d, e) and 10 days (f), detected by red Fuchsin chromogen staining (d) or green fluorescence, together with smooth muscle actin (red fluorescence) and blue DAPI staining of nuclei (e, f). Note the virtual absence of CD24 expression in the alveoli. The arrowheads in f indicate retention of robust CD24 staining in milk collecting ducts. g–i Expression of CD24 in the mammary epithelium of post-lactating mice, 5 days (g, h) and 10 days (i) after the weaning of their pups. CD24 expression was detected by red Fuchsin chromogen staining (g) or by green immunofluorescence, together with smooth muscle actin (red fluorescence) and blue DAPI staining of nuclei (h, i). Note the increased CD24 expression in the remodeled epithelium that rapidly returns to a CD24 expression pattern typical of pre-pregnant ducts
Targeted deletion of CD24 increases ductal branch density
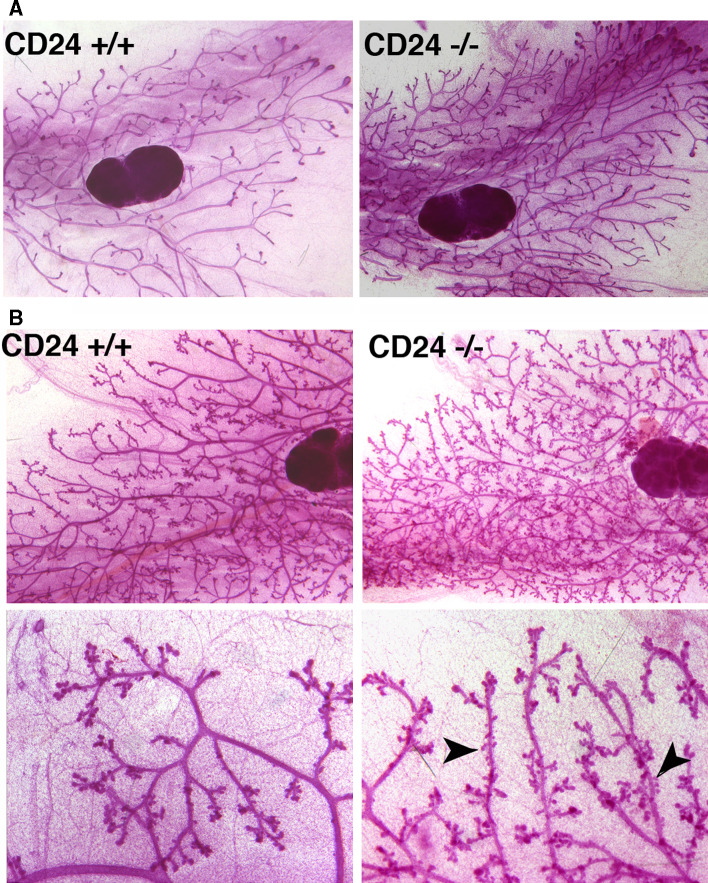
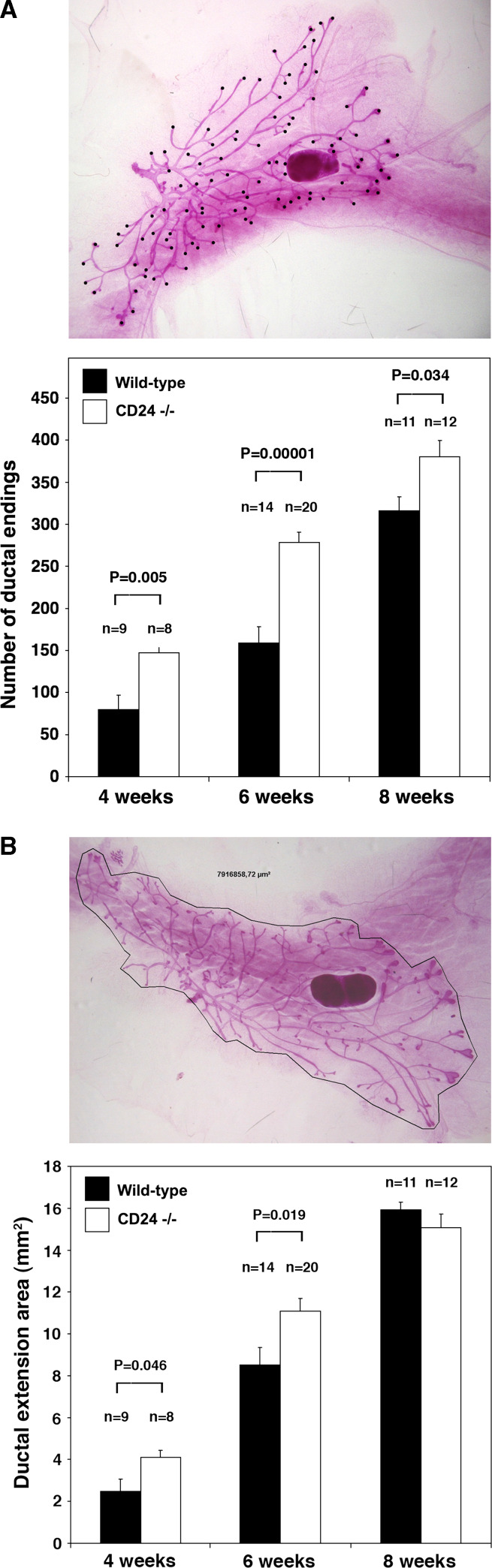
To determine what role CD24 expression has in the development and function of the mammary gland, we analyzed the mammary glands of mice bearing homozygous targeted disruption of the CD24 gene. Whole mounts were prepared from pre- and post-pubertal as well as pregnant, lactating and involuting mammary glands. Wild-type and knockout glands were compared (Fig. 4). We quantified ductal end structures as a measure of numbers of side branches and bifurcations, as well as the total area of ductal extension into the mammary fat pad (Fig. 5). We observed a striking and statistically significant increase in mammary ductal branching in the knockout mammary glands during both puberty (4 and 6 weeks of age), as well as in 8-week-old adult mice (Fig. 5a), resulting in an increased density of mammary ducts. We could observe no significant difference at 3 weeks of age. The ductal extension area was also significantly larger at 4 and 6 weeks of age, but not at 8 weeks (Fig. 5b). We performed Ki-67 staining to examine proliferation of mammary epithelial cells in wild-type and CD24 knockout mice. While there was a trend towards increased proliferation in the mammary epithelial cells of 6-week-old CD24−/− mice compared with wild-type mice, this difference was not significant (Supplementary Figure 3). Using immunostaining for activated caspase 3, we could find no evidence for a difference in apoptosis rates in these cells (data not shown). Together, the data suggest that CD24 loss results in accelerated branching morphogenesis and consequently an increased density of mammary ducts. This is reflected in a more rapid extension of the growing ductal tree during puberty, possibly supported by increased proliferation of the epithelial cells.
Fig. 4.
Mammary glands from CD24-deficient mice exhibit accelerated ductal branching morphogenesis during puberty. a Whole mounts of no. 4 mammary glands from 6-week-old wild-type (CD24+/+) and CD24-deficient (CD24−/−) mice. b Example of whole mounts of no. 4 mammary glands from wild-type (CD24+/+) and CD24-deficient (CD24−/−) mice that had been pregnant for 10 days. Arrowheads in the higher magnification pictures of the mammary glands from CD24-deficient mice indicate the precocious development of alveoli in comparison to wild-type mice
Fig. 5.
Quantification of no. 4 mammary gland branching morphogenesis and ductal area extension in wild-type and CD24-deficient mice during puberty. a Upper panel: Representative picture showing how ductal endings (marked with black dots) were quantified. Lower panel: Quantification of numbers of ductal endings as a measure of increased side branching and bifurcations. The age of the mice in weeks that were analyzed is indicated, as is the number (n) of animals analyzed in each group. P values were calculated using an unpaired two-sided t test. Error bars The standard error of the mean. b Upper panel: Representative picture showing how ductal area extension (circumscribed with a black line) was quantified. Lower panel Quantification of ductal extension area. The age of the mice in weeks that were analyzed is indicated, as is the number (n) of animals analyzed in each group. P values were calculated using an unpaired two-sided t test. Error bars Indicate the standard error of the mean
In mammary glands from pregnant CD24 knockout mice, we also observed a trend towards increased lateral bud density and subsequent alveolar development in comparison to wild-type mice, although statistical significance could not be demonstrated (Fig. 4b). Analysis of glands from lactating mice did not reveal any morphological differences between CD24−/− and wild-type animals (data not shown). Furthermore, the ability of mice to successfully lactate and nourish their offspring was equivalent when wild-type and CD24-deficent mice were compared. Thus, CD24 deficiency does not observably affect the ability of mammary glands to properly develop and function.
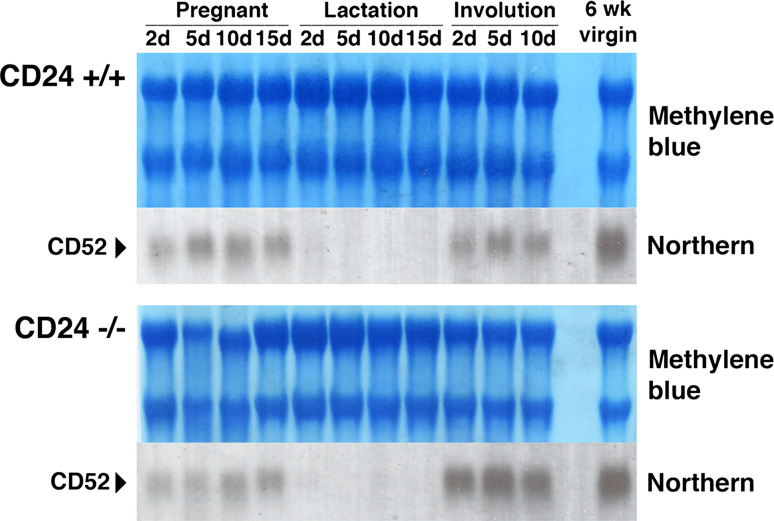
To examine the possibility that loss of CD24 is masked in CD24-deficient mice due to compensatory expression of a protein that takes over the function of CD24, we analyzed the expression of CD52, the only CD24 paralog that has been detected in the mammalian genome. However, no substantial differences in CD52 expression levels could be detected during pregnancy, lactation and involution when mammary glands from wild-type and knockout mice were compared (Fig. 6). Furthermore, as expected, no CD24 expression could be detected immunohistologically in tissues from CD24 knockout mice (Fig. 7c, i, Supplementary Figure 2), ruling out the possibility that the pattern of CD24 expression observed in wild-type mice is contributed to by a cross-reactivity of the anti-CD24 antibody with CD52.
Fig. 6.
The CD24 paralog CD52 is not upregulated in the mammary glands of CD24-deficient mice. RNA was prepared from the mammary glands of wild-type (CD24+/+) and CD24-deficient (CD24−/−) mice. Mammary glands were prepared from mice in pregnancy, lactation and undergoing post-lactational involution, as well as from 6-week-old virgin mice. The number of days (d) through each process is indicated. Northern blots prepared with the RNA were stained with methylene blue to ensure equal RNA loading, then hybridized with a CD52 probe
Fig. 7.
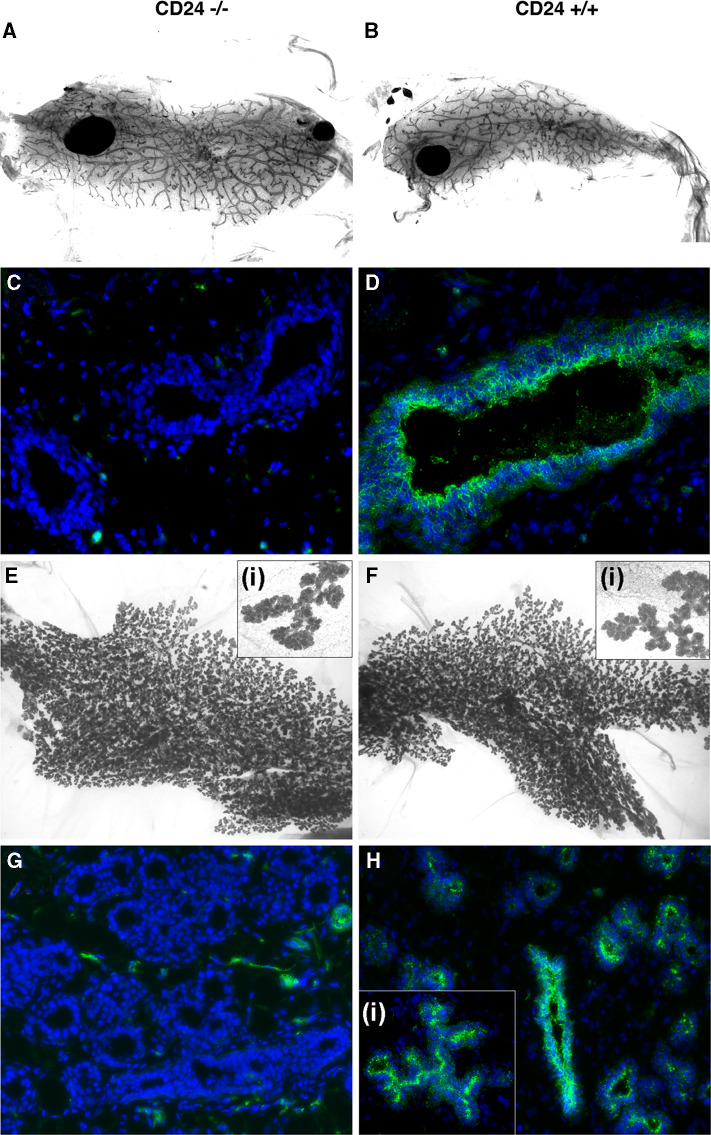
CD24−/− mammary transplants reconstitute the mammary gland as efficiently as CD24+/+ transplants and are able to functionally differentiate. a Representative whole mount of outgrown transplanted CD24−/− mammary epithelium 9 weeks after transplantation into a cleared mammary fat pad. b Corresponding representative whole mount of outgrown transplanted CD24+/+ mammary epithelium. Note that in both a and b, the ducts grew out into every part of the cleared fat pad. c, d Frozen sections of transplants from virgin animals stained with CD24 antibody (green fluorescence) and DAPI staining of nuclei (blue fluorescence) to confirm the origin of the transplanted CD24-deficient epithelium (c) and transplanted CD24+/+ wild-type epithelium (d). e, f and insets: Whole mount analysis of transplants during late pregnancy (around E15.5) taken from animals transplanted with CD24−/− (e) or wild-type (f) mammary epithelium. g, h and inset: Corresponding frozen sections of pregnant transplants stained with anti-CD24 antibody (green fluorescence) and DAPI staining of nuclei (blue fluorescence). CD24-deficient transplanted epithelium (g) does not stain with the CD24 antibodies as expected, whereas wild-type epithelium (h) shows a specific CD24 staining pattern [see also inset (i)], as previously shown in Fig. 2. The occasional CD24-positive cells in G are derived from the wild-type stroma of the recipient and are most probably cells of the immune system
CD24−/− mammary epithelial cells can fully reconstitute functional mammary glands
The lack of major developmental or functional defects in mammary glands from CD24 knockout mice implies that CD24 has no critical or major role in the mammary stem cell compartment. Nevertheless, CD24−/− mammary stem/progenitor cells might have a reduced potential or capacity to reconstitute a gland in a cleared fat pad. To determine whether this is the case, we performed transplantation assays where small pieces of mammary duct-containing tissue from CD24−/− mice or the corresponding wild-type control tissue were transplanted into cleared fat pads of recipient mice. Outgrowth was analyzed 9 weeks after transplantation. In 6/6 experimental animals analyzed, we observed that both wild-type as well as CD24−/− transplants completely repopulated the cleared mammary fat pads, giving rise to mammary ducts that completely occupied the fat pad (Fig. 7a, b, representative pair of whole mounts). To confirm that the epithelial ducts were of wild-type or CD24−/− origin, frozen sections were stained with CD24 antibody. As shown in Fig. 7c and d, wild-type transplants stained positively for CD24 as expected, whereas knockout epithelium was clearly negative.
To investigate if the transplants were able to differentiate functionally and undergo alveologenesis, recipients were mated and analyzed in late pregnancy (around E15.5). Whole mounts as well as frozen sections were performed. In all pregnant mice analyzed (3/3 animals), we found full and complete alveologenesis, and no morphological differences were observed between glands reconstituted with wild-type and CD24−/− cells (Fig. 7e–h), indicating a normal hormonal responsiveness of the outgrown ducts. Again, outgrowths derived from fragments of wild-type mammary glands stained positively for CD24, while transplants derived from CD24−/− mice did not (Fig. 7g, h). These results indicate that CD24−/− mammary ductal stem/progenitor cells have the same capacity to reconstitute cleared mammary fat pads as those from CD24+/+ mice.
Discussion
There is increasing interest in CD24 as a marker of mammary stem cells. Here we report the first comprehensive survey of CD24 expression and function in the murine mammary gland. We found that the expression levels of CD24 remained relatively constant during postnatal life, except during lactation where it was virtually absent from alveoli. Expression of CD24 returned to normal levels during involution following the cessation of lactation.
Genomic deletion of CD24 promoted mammary gland branching morphogenesis, resulting in the enhanced development of branch bifurcations and secondary branching during puberty and an increased ductal density in adult mice. During pregnancy we also observed a tendency towards increased lateral bud density and subsequent alveolar development in CD24-deficient mice, which presumably also reflects altered regulation of branching morphogenesis. We conclude that CD24 expression is a negative regulator of branching morphogenesis. While the regulation of ductal branching morphogenesis remains to be fully understood, it is clear that it involves a complex interplay between systemic hormones (for example, estrogen and progesterone), local growth factor signaling (for example via EGFR) and the surrounding stroma (reviewed in [30]). It would seem unlikely that dysregulated estrogen levels or altered ovarian function is involved in the effects on branching morphogenesis in CD24-deficient mice, as CD24 is not normally expressed in the ovary (Supplementary Figure 2c, see also [25]), and we could also not detect differences in circulating estrogen levels between wild-type and CD24-deficient mice (data not shown). Negative regulators of branching morphogenesis have been described, including TGF-β1 [31–33] and Sprouty2, a negative regulator of FGFR2 [34]. CD24 may negatively regulate morphogenesis by influencing the activity of these or other cell surface proteins. For example, it has been shown to regulate the activity of receptors such as CXCR4 [35] and the neural recognition molecule L1, in which context it inhibits neurite outgrowth [20] and neurogenesis [21]. We and others have also shown that CD24 can activate β1-containing integrins [36, 37]. However, it is unlikely that this activity plays any role in the CD24-mediated negative regulation of branching morphogenesis, as β1-containing integrin activation is required for mammary gland morphogenesis [38, 39] and for the maintenance of a functional stem cell population [40].
In recent studies in which mammary epithelial cells were isolated and separated according to their CD24 expression, it was found that the CD24negative subpopulation corresponded to non-epithelial cells, CD24low to myoepithelial/basal cells and CD24high to luminal epithelial cells [11, 41]. Our findings are in agreement with these studies. Of these populations, only the CD24low subpopulation was able to repopulate cleared mammary fat pads efficiently with the complete spectrum of mammary epithelial subtypes, indicating that the CD24low subpopulation contains mammary stem cells. Furthermore, isolated mammary stem cells are CD24 positive [8]. However, our findings that genomic deletion of CD24 does not have a pronounced effect on mammary gland development and function, and that CD24−/− mammary epithelial cells are able to functionally reconstitute cleared mammary fat pads speak against a major functional role for CD24 in regulating the biology of mammary progenitor and/or stem cells. In this regard it is important to note that we found no evidence for compensatory upregulation of CD52, the only paralog of CD24. While CD24 has recently been suggested to control the proliferation/differentiation balance between transit-amplifying and committed differentiated cells in the context of skin and the brain [42], we found no evidence for a similar role for CD24 in the mammary gland.
CD24 is upregulated in a wide variety of tumors and correlates with tumor progression [12]. Significantly increased CD24 expression is observed in breast tumors, both in comparison to normal tissues and during tumor progression, and this expression is prognostically relevant [43]. We have shown that CD24 promotes tumor growth and metastasis in animal models of breast cancer, increases their adhesion to ECM components and promotes their motility in vitro [37]. CD24 expressed on the surface of breast cancer cells promotes rolling on P-selectin-coated surfaces and affects CXCR4 function, probably also of relevance to metastasis [35, 44, 45]. The studies presented here suggest that CD24 does not contribute decisively to mammary progenitor and/or stem cell function, and confirm that it is expressed much more widely than just in the mammary stem cell/progenitor compartment. Thus, the stem cell marker function of CD24 probably simply reflects its expression in an epithelial compartment that contains mammary stem cells, which in combination with other markers renders it useful for isolating stem cells. On the other hand, we did observe a clear effect of loss of CD24 on branching morphogenesis. During branching morphogenesis the ductal epithelium invades the surrounding fat pad to form an organized mammary tree. Many of the molecular mechanisms that control this process are subverted by breast cancer cells during tumor progression (reviewed in [46]). TGF-β, for example, acts both as a negative regulator of branching morphogenesis and promotes invasion and metastasis [46–48]. We therefore suggest that the molecular functions of CD24 in the breast cancer context may be related to the role of CD24 in regulating branching morphogenesis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1. CD24 staining at various time points of mammary gland development. The ages of the mice from which the glands were taken are indicated. Immunostaining of CD24 (green fluorescence), smooth muscle actin (red fluorescence) and DAPI staining of nuclei (blue fluorescence) is shown (TIFF 23271 kb)
Supplementary Figure 2. A. CD24 -/- gland stained with anti-CD24 M1/69 antibody (green) and anti-SMA antibody (red). Nuclei are stained with DAPI (blue). The arrows indicate staining of periductal structures in the stroma, indicating that the M1/69 antibody can weakly stain non-CD24 epitopes. B. Virgin CD24 +/+ gland stained with an isotype control for the CD24 M1/69 antibody. C. Section of murine ovary immunostained for CD24 (green fluorescence), smooth muscle actin (red fluorescence) and DAPI staining of nuclei (blue fluorescence). These data show an absence of CD24 expression in ovarian tissue. D-F. Virgin CD24 +/+ gland double stained with CD24 (green fluorescence) and CD45 (red fluorescence), indicating that CD24 signals in stromal cells can be due to CD45-positive cells from the hemapoetic system (TIFF 15451 kb)
Supplementary Figure 3. Proliferation rates in mammary epithelial cells from 6-week-old CD24 +/+ and CD24 -/- mice. A. Representative section of mammary gland stained with Ki-67 antibody (brown nuclear stain). The sections were counterstained with hematoxylin. B. Quantification of Ki-67 staining. The number of animals analyzed (n) is indicated. For quantification of proliferation, 8–10 independent fields from each histological section were photographed (Axiovision microscope, Zeiss; 20× objective). A minimum of 2,600 mammary epithelial cells were evaluated per animal. The number of Ki-67-positive mammary epithelial nuclei versus the total number of mammary epithelial nuclei was counted and expressed as %. The mean and standard error are presented. Statistical significance was calculated using an unpaired two-sided Student’s t-test (TIFF 6197 kb)
Acknowledgments
We thank Norma Howells and Selma Huber for excellent technical assistance with the animal experiments, Diana Plaumann and Stefanie Dukowic-Schulze for their outstanding practical help, and Marina Glukhova for her support and advice. This work was supported by grants from the European Union (FP6 STREP project BRECOSM, contract no. LSHC-CT-2004-503224) and the German National Genome Research Network (NGFN2). M.A.D is “chargée de recherche” at the “Institut de la Santé et de la Recherche Médicale”.
References
- 1.Smalley M, Ashworth A. Stem cells and breast cancer: a field in transit. Nat Rev Cancer. 2003;3:832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 2.Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 3.Radnor CJ. Myoepithelial cell differentiation in rat mammary glands. J Anat. 1972;111:381–398. [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor-Papadimitriou J, Lane EB. Keratin expression in the mammary gland. In: Neville MC, Daniels C, editors. The mammary gland: development, regulation and function. New York: Plenum Publishing Co.; 1987. pp. 181–215. [Google Scholar]
- 5.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 6.Daniel CW, De Ome KB, Young JT, Blair PB, Faulkin LJ., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci USA. 1968;61:53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 9.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 10.Deugnier MA, Faraldo MM, Teuliere J, Thiery JP, Medina D, Glukhova MA. Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dbeta cell line. Dev Biol. 2006;293:414–425. doi: 10.1016/j.ydbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/B:HIJO.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- 13.Tone M, Nolan KF, Walsh LA, Tone Y, Thompson SA, Waldmann H. Structure and chromosomal location of mouse and human CD52 genes. Biochim Biophys Acta. 1999;1446:334–340. doi: 10.1016/s0167-4781(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 14.Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 15.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen PJ, Lorenz B, Muller AM, Wenger RH, Brombacher F, Simon M, von der Weid T, Langhorne WJ, Mossmann H, Kohler G. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood. 1997;89:1058–1067. [PubMed] [Google Scholar]
- 18.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shewan D, Calaora V, Nielsen P, Cohen J, Rougon G, Moreau H. mCD24, a glycoprotein transiently expressed by neurons, is an inhibitor of neurite outgrowth. J Neurosci. 1996;16:2624–2634. doi: 10.1523/JNEUROSCI.16-08-02624.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleene R, Yang H, Kutsche M, Schachner M. The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem. 2001;276:21656–21663. doi: 10.1074/jbc.M101790200. [DOI] [PubMed] [Google Scholar]
- 21.Belvindrah R, Rougon G, Chazal G. Increased neurogenesis in adult mCD24-deficient mice. J Neurosci. 2002;22:3594–3607. doi: 10.1523/JNEUROSCI.22-09-03594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieoullon V, Belvindrah R, Rougon G, Chazal G. mCD24 regulates proliferation of neuronal committed precursors in the subventricular zone. Mol Cell Neurosci. 2005;28:462–474. doi: 10.1016/j.mcn.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Jevsek M, Jaworski A, Polo-Parada L, Kim N, Fan J, Landmesser LT, Burden SJ. CD24 is expressed by myofiber synaptic nuclei and regulates synaptic transmission. Proc Natl Acad Sci USA. 2006;103:6374–6379. doi: 10.1073/pnas.0601468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristiansen G, Denkert C, Schluns K, Dahl E, Pilarsky C, Hauptmann S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161:1215–1221. doi: 10.1016/S0002-9440(10)64398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young LJT (2000) The cleared mammary fat pad and the transplantation of mammary gland morphological structures and cells. In: Ip MM and Ash BB (eds) Methods in mammary gland biology and breast cancer research. Kluwer Academic/Plenum Publishers, New York, pp 67–74
- 27.Hofmann M, Fieber C, Assmann V, Gottlicher M, Sleeman J, Plug R, Howells N, von Stein O, Ponta H, Herrlich P. Identification of IHABP, a 95 kDa intracellular hyaluronate binding protein. J Cell Sci. 1998;111:1673–1684. doi: 10.1242/jcs.111.12.1673. [DOI] [PubMed] [Google Scholar]
- 28.Hebbard L, Steffen A, Zawadzki V, Fieber C, Howells N, Moll J, Ponta H, Hofmann M, Sleeman J. CD44 expression and regulation during mammary gland development and function. J Cell Sci. 2000;113:2619–2630. doi: 10.1242/jcs.113.14.2619. [DOI] [PubMed] [Google Scholar]
- 29.Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, Dexter T, Davies S, Bulmer K, Ford E, Parry S, Budroni M, Palmieri G, Neville AM, O’Hare MJ, Lakhani SR. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64:3037–3045. doi: 10.1158/0008-5472.CAN-03-2028. [DOI] [PubMed] [Google Scholar]
- 30.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel CW, Robinson S, Silberstein GB. The role of TGF-beta in patterning and growth of the mammary ductal tree. J Mammary Gland Biol Neoplasia. 1996;1:331–341. doi: 10.1007/BF02017389. [DOI] [PubMed] [Google Scholar]
- 32.Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu P, Ewald A, Martin G, Werb Z. Essential function of FGF signalling pathway during branching morphogenesis of the mammary gland. Dev Biol. 2005;283:680–681. [Google Scholar]
- 35.Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119:314–325. doi: 10.1242/jcs.02741. [DOI] [PubMed] [Google Scholar]
- 36.Hahne M, Wenger RH, Vestweber D, Nielsen PJ. The heat-stable antigen can alter very late antigen 4-mediated adhesion. J Exp Med. 1994;179:1391–1395. doi: 10.1084/jem.179.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- 38.Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- 39.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieoullon V, Belvindrah R, Rougon G, Chazal G. Mouse CD24 is required for homeostatic cell renewal. Cell Tissue Res. 2007;329:457–467. doi: 10.1007/s00441-007-0395-5. [DOI] [PubMed] [Google Scholar]
- 43.Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schluns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H, Dietel M. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9:4906–4913. [PubMed] [Google Scholar]
- 44.Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. Faseb J. 1998;12:1241–1251. doi: 10.1096/fasebj.12.12.1241. [DOI] [PubMed] [Google Scholar]
- 45.Friederichs J, Zeller Y, Hafezi-Moghadam A, Grone HJ, Ley K, Altevogt P. The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res. 2000;60:6714–6722. [PubMed] [Google Scholar]
- 46.Lanigan F, O’Connor D, Martin F, Gallagher WM. Molecular links between mammary gland development and breast cancer. Cell Mol Life Sci. 2007;64:3159–3184. doi: 10.1007/s00018-007-7386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 48.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. CD24 staining at various time points of mammary gland development. The ages of the mice from which the glands were taken are indicated. Immunostaining of CD24 (green fluorescence), smooth muscle actin (red fluorescence) and DAPI staining of nuclei (blue fluorescence) is shown (TIFF 23271 kb)
Supplementary Figure 2. A. CD24 -/- gland stained with anti-CD24 M1/69 antibody (green) and anti-SMA antibody (red). Nuclei are stained with DAPI (blue). The arrows indicate staining of periductal structures in the stroma, indicating that the M1/69 antibody can weakly stain non-CD24 epitopes. B. Virgin CD24 +/+ gland stained with an isotype control for the CD24 M1/69 antibody. C. Section of murine ovary immunostained for CD24 (green fluorescence), smooth muscle actin (red fluorescence) and DAPI staining of nuclei (blue fluorescence). These data show an absence of CD24 expression in ovarian tissue. D-F. Virgin CD24 +/+ gland double stained with CD24 (green fluorescence) and CD45 (red fluorescence), indicating that CD24 signals in stromal cells can be due to CD45-positive cells from the hemapoetic system (TIFF 15451 kb)
Supplementary Figure 3. Proliferation rates in mammary epithelial cells from 6-week-old CD24 +/+ and CD24 -/- mice. A. Representative section of mammary gland stained with Ki-67 antibody (brown nuclear stain). The sections were counterstained with hematoxylin. B. Quantification of Ki-67 staining. The number of animals analyzed (n) is indicated. For quantification of proliferation, 8–10 independent fields from each histological section were photographed (Axiovision microscope, Zeiss; 20× objective). A minimum of 2,600 mammary epithelial cells were evaluated per animal. The number of Ki-67-positive mammary epithelial nuclei versus the total number of mammary epithelial nuclei was counted and expressed as %. The mean and standard error are presented. Statistical significance was calculated using an unpaired two-sided Student’s t-test (TIFF 6197 kb)