Abstract
Malignant tumors express multiple factors that have some role in the regulating networks supporting their ectopic growth. Recently, increased interest has been developing in the expression and biological role of the neuropeptides and receptors of the corticotropin releasing factor (CRF) system, the principal neuroendocrine mediator of the stress response, especially in the light of several R&D programs for small molecule antagonists that could present some anticancer therapeutic benefit. In the present article, we review the literature suggesting that the CRF system could be involved in the regulation of human cancer development. Potential implication in growth, metastasis, angiogenesis, or immune parameters via activation of locally expressed receptors could be clinically exploited by presenting targets of new therapeutic approaches.
Keywords: Corticotropin releasing factor, Urocortin, Receptor, Human, Cancer
Introduction
CRF and its family of ligands include the non-mammalian sauvagine and urotensin I, and the mammalian urocortins (Ucns) 1, 2 (stresscopin-related peptide, SRP) and 3 (stresscopin, SCP), which are also found in humans [1–3]. Corticotripin releasing factor (CRF) is a hypothalamic factor that was discovered as an active principal in 1955 [4, 5], whereas ovine CRF was only isolated and sequenced in 1981 [6]. The human CRF is a 41-amino-acid peptide and its gene consists of two exons separated by an intron in its 5′ untranslated region [7]. CRF is the principal coordinator of the hypothalamic–pituitary–adrenal axis mediating behavioral, autonomic, and neuroendocrine responses to stressors [6].
The CRF homologues urocortins (Ucn) are similar sized neuropeptides that exhibit high sequence homology with CRF [8]. Ucn1, Ucn2, and Ucn3 are found in the periphery as well as in the central nervous system [8–11]. Human Ucn1 is expressed in the brain, pituitary, heart, vascular system, reproductive organs, and the gastrointestinal tract [12–16]. Although it is not yet clear whether human tissues express Ucn2 [9, 10], Ucn3 has been reported in the human brain, heart, kidney, reproductive organs, and gastrointestinal tract [17–19].
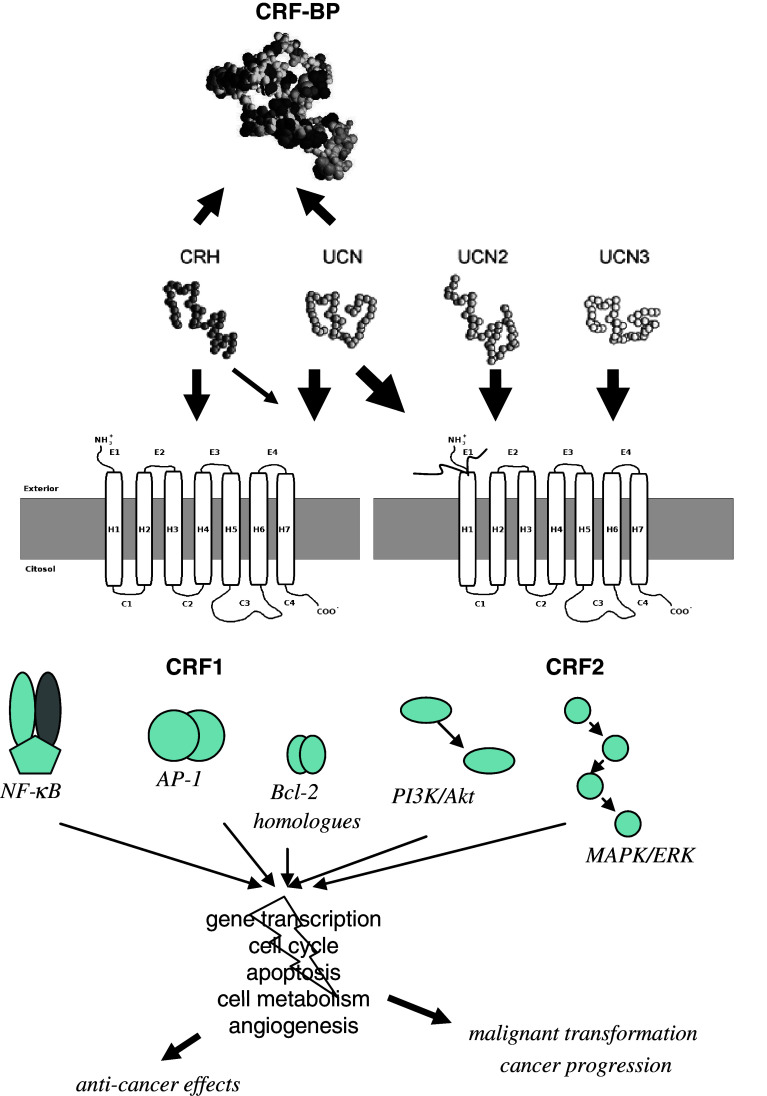
CRF and Ucns mediate stress responses, cardiovascular and immune functions via two CRF receptors, which are found in the CNS, the pituitary, heart, vascular system, and lymphocytes [20, 21]. Both CRF receptors, CRF1 and CRF2, are G protein coupled receptors encoded by two different genes. They share 69% amino acid homology but they have different tissue distributions and pharmacological properties [22, 23]. CRF has tenfold higher affinity for CRF1 than CRF2 receptor. Ucn1 binds to both CRF1 and CRF2 receptors, whereas Ucn2 and Ucn3 are highly selective for the CRF2 receptor with little affinity for the CRF1 receptor [8, 10, 11]. Binding of CRF-related peptides to the CRF receptors stimulates cAMP production and subsequently activates the protein kinase A (PK A) pathway [3, 8]. In addition to CRF receptors, a soluble CRF binding protein (CRF-BP) binds CRF and Ucn1 with different affinities [24] (Fig. 1).
Fig. 1.
The CRF system of peptides and binding sites. Potential signaling pathways related to cancer
Based on studies using experimental models, the CRF family of neuropeptides and their receptors has been suggested to be involved in the development, metastasis, and immune escape of several malignancies. The available information regarding the expression of the CRF system in a wide spectrum of human malignancies, and, moreover, the elucidation of its biological role needed to support a pathophysiological significance of these findings, is so far limited. In this review, we present an overview of recent findings and knowledge about CRF and cancer. In the light of several R&D programs for small molecule antagonists that could present some anticancer therapeutic benefit, a review covering this topic seems to be timely for the critical reading of the literature.
CRF and prostate cancer
In the mid-1980s, the presence of CRF was investigated in formalin-fixed, paraffin-embedded human tissues and was reported in one out of three small-cell prostate carcinomas [25] and in prostate tissue sections from a patient with a small-cell prostate carcinoma with multiple metastases [26]. The CRF-like material could also be demonstrated in an extract of the prostatic tumor by radioimmunoassay, and the material from both plasma and tumor extract eluted at the position of human CRF on gel chromatography. In contrast, CRF immunoreactivity was not found in the prostate tumor of a 57-year-old man with Cushing’s syndrome and a large pituitary prostatic cancer presenting high levels of serum cortizol, ACTH, and CRF [27]. It should be noted, however, that in the earlier studies before the identification of Ucn1 in 1995, CRF-like immunoreactivity detected in prostate tumors could represent cross-reaction of the antibodies used with Ucn rather than CRF, especially when immunoreactivity was not confirmed at the gene expression level. Indeed, expression of Ucn1 in the human benign prostate and prostate cancer has been reported recently. Ucn1 expression was evaluated in three prostatic adenocarcinomas by RT-PCR and immunohistochemistry. Ucn1 mRNA and peptide were demonstrated in all specimens tested, with tumor cells showing moderate to markedly intense cytoplasmic immunoreactivity, and endothelial cells of tumor vessels also being positive. No correlation was found between Ucn1 immunostaining and tumor grade [28].
Reubi et al. [29] evaluated the expression of CRF1 and CRF2 receptors in 11 prostate carcinomas using in vitro autoradiography with subtype-selective CRF analogs and found no CRF receptor expression. In agreement with these data, RT-PCR in lysates of prostate cancer exhibited no expression of CRF2 mRNA in contrast to normal prostate lysates that contained CRF2 mRNA transcripts. At the protein level, CRF2 receptor has been studied by immunofluorescence of 32 cases of prostatic adenocarcinomas in parallel with the corresponding normal tissues. The tumoral neovascular system exhibited no immunopositivity for CRF2 receptor, while in benign tissues, smooth muscle components of the stroma, endothelial cells of blood vessels, and to a lesser extent vascular smooth muscle, were found positive for CRF2. It was concluded that, while Ucn1 expression in prostate cancer is identical to that of non-malignant prostate tissues, expression loss of CRF2 in prostate cancer and its neovascularization may contribute to prostate tumorigenesis, progression, and neoangiogenesis [30].
CRF and endometrial carcinoma
The first report of CRF expression in human endometrium was in 1995 and showed the presence of CRF mRNA in the human endometrial adenocarcinoma cell line Ishikawa by northern blot hybridization. Immunoreactive CRF was also detectable. Gel filtration chromatography of Ishikawa cell extracts and their culture media showed the presence of the authentic CRF peptide and probably the presence of CRF precursor molecules, whereas immunofluorescence staining of CRF revealed a cytoplasm rich in granules positive for immunoreactive CRF [31]. The possible antiproliferative effect of CRF on the Ishikawa adenocarcinoma cell line was later investigated. CRF induced time- and concentration-dependent inhibition of Ishikawa cell growth. A decrease in telomerase activity, which paralleled tumor growth inhibition, was also observed in CRF-treated samples. The antiproliferative effect involved the CRF1 receptor [32]. In the same cell line, CRF counteracted the increase in cell proliferation caused by estradiol [33]. In addition, 21% of tumor tissues from 19 untreated patients with the diagnosis of primary endometrial cancer expressed the CRF1 receptor gene as shown by RNase protection assay. Ucn1 mRNA and peptide expression were decreased in endometrial adenocarcinoma. The levels of Ucn1 mRNA in 9 endometrial adenocarinoma, both well and poorly differentiated, were shown to be significantly lower than in 13 healthy postmenopausal women used as controls, as evaluated by quantitative RT-PCR. Immunoreactive Ucn1 was found in healthy luminal and grandular epithelial cells but not in neoplastic samples [34].
Finally, in a most recent study, the expression and intracellular localization of CRF and its two receptor subtypes in surgical specimens from 51 untreated endometrial cancer patients as well as normal surrounding tissues were investigated by immunohistochemistry. A diffuse cytoplasmic staining was found in 100% (51 from 51), 92% (34 from 37), and 61% (31 from 51) of tumor specimens for CRF, CRF1 and CRF2 receptor, respectively. The surrounding normal endometrial glands showed a typical paranuclear/apical pattern for CRF and stained for CRF2 receptor at the nuclear level, whereas CRF1 receptor staining was similar to that observed in tumors. CRF2 cytoplasmic pattern was associated with more advanced FIGO stage disease [34, 35]. In contrast, positive correlation was found between CRF1 and progesterone receptor expression suggesting a potential role of this receptor in the characterization of less aggressive tumors. Indeed, progesterone has been found to induce the transcription of the CRF gene in human endometrial stroma [109]. These findings point to a differential involvement of the two receptors in the pathophysiology of endometrial cancer.
CRF and breast cancer
Breast cancer tumors are known to express multiple neuropeptides and their receptors. The presence of immunoreactive CRF, as well as GnRH, GHRH, and somatostatin was firstly studied by immunohistochemistry in 40 pre- and postmenopausal patients with operable breast cancer. CRF was found in 14 out of 40 breast cancers (35%), in the cytoplasm or in the nuclei of the tumor cells, and there was no relationship to the clinical stage of the disease. Positive immunostaining for CRF was present in colloid, lobular, and infiltrating ductal carcinomas [36]. However, these studies need to be confirmed using peptide-specific antibodies that discriminate between CRF and the homologue urocortins. Subsequently, the in vivo and in vitro antineoplastic potential of human CRF was tested in W256 rat mammary carcinoma. CRF treatment significantly inhibited the growth and vascular permeability of the W256 tumors and also exhibited antiproliferative and differentiation-inducing effects in W256 cell growth in vitro. These effects were proved to be CRF receptor mediated, based on the presence of relatively high levels of CRF1 receptor mRNA in W256 cells and by the fact that the effects of human CRF on the tumor were abolished by the CRF receptor antagonist α-helical CRF (9–41) [37].
The sequencing analysis of the 5′ region and the genomic structure of the human CRF1 receptor gene was presented in 2004, using the human neuronal-like teratocarcinoma cells NT2, the human neuroblastoma cells SH-5YSY, and the human breast cancer cells MCF7. The full genomic organization of the human gene for the CRF1 receptor was reported with complete mapping of exons 1–14. CRF and Ucn1 markedly increased promoter activity during transient CRF1 receptor expression studies. Similarly, CRF and Ucn1 up-regulated the endogenous CRF1 receptor at the mRNA level in NT2 and MCF7 cells [38].
In order to unfold the biological role of the CRF1 receptor in breast cancer, the effect of CRF on MCF7 proliferation was tested. CRF significantly inhibited cell growth induced by estradiol, and this effect was not associated with the induction of apoptosis. This CRF inhibition of cell proliferation was counteracted by the nonselective CRF receptor antagonist, astressin, as well as by a CRF1 selective receptor antagonist, antalarmin. It was shown by RNase protection assay that MCF7 cells express constitutively the CRF1 receptor subtype transcript under basal conditions. Finally, the endogenous source of CRF was shown to be the same cells, as MCF7 cells were found to express CRF mRNA under basal conditions and to secrete sizable amounts of immunoreactive CRF. The existence of a paracrine–autocrine inhibitory mechanism operated by CRF in breast cancer cells was suggested [39]. In agreement with these findings, Androulidaki et al. showed by RT-PCR that MCF7 contain high levels of CRF1a mRNA and very low levels of CRF2c mRNA. The other CRF receptor subtypes were not detected. Interestingly, while CRF transiently inhibited apoptosis, it also promoted cell motility and invasiveness most probably via induction of focal adhesion kinase phosphorylation, actin filament reorganization, and production of prostaglandins via Cox 1 [40]. These findings support a role of the CRF system (CRF/CRF1) in breast cancer cell growth, homeostasis, motility, and metastatic potential.
CRF and ovarian cancer
The presence of immunoreactive CRF was firstly reported in ovarian carcinomas in 1986 [41]. CRF is also produced in normal human cycling ovaries [15, 42] being significantly higher in the premenopausal than the postmenopausal ovaries, suggesting that ovarian CRH is related to normal ovarian function during the reproductive lifespan. Recently, Minas et al. examined immunohistochemically the expression of CRF, CRF1 and CRF2 receptors in 47 human ovarian cancer cases. They revealed that the tumor cells produced all the investigated peptides in situ in 68.1, 70.2, and 63.8% of the cases, respectively. Tumor advancement, as assessed by increasing tumor stage, was associated with significantly increased immunohistochemical positivity for CRF. Furthermore, RT-PCR performed in total mRNA extracted from the ovarian cancer cell lines OvCa3 and A2780 revealed the expression of CRF and CRF1 receptor. CRF2 receptor was only expressed by A2780. Immunofluorescence confirmed these results. CRF increased the expression of FasL in OvCa3 and A2780 cells through CRF1, thereby potentiated their ability to induce apoptosis of activated peripheral blood lymphocytes. It was concluded that CRF produced by human ovarian cancer might favor survival and progression of the tumor by promoting its immune privilege [43].
CRF and thyroid cancer
In nonmammalian vertebrates, CRF is considered to be a potent thyrotropin (TSH)-releasing factor in parallel with ACTH [44]. The first report of a CRF relevance to thyroid cancer was in 1976, describing a 45-year-old woman with medullary thyroid carcinoma that showed a large arterio-venous increase in plasma CRF-like activity across the thyroid gland. The tumor tissue also contained CRF-like activity [45]. Subsequently, several studies reported expression of CRF in patients with thyroid cancer. Immunoreactive CRF was detected in one medullary thyroid carcinoma by radioimmunoassay [46] and in two of ten medullary thyroid carcinomas by immunohistochemisty [25]. In addition, in a 58-year-old man with medullary carcinoma of the thyroid, 10–30% of the tumor cells were positive for one out of three CRF antisera tested by immunocytology and CRF was detectable in the tumor extract by radioimmunoassay [47]. Scopa et al. examined immunohistochemically four follicular and eight papillary carcinomas, four Hurtle cell tumors, one medullary cancer, and one insular thyroid carcinoma. Immunoreactive CRF was detected in the cytoplasm of neoplastic follicular cells in 100% of Hurtle cell tumors, in 25% of follicular carcinomas, and in 50% of papillary carcinomas. The medullary and the insular carcinoma were also positive for immunoreactive CRF [48]. A case of Cushing’s syndrome caused by a medullary thyroid carcinoma was found to be CRF positive by immunohistochemistry, as well as material from the liver mass biopsy of a 45-year-old male with medullary thyroid carcinoma with liver metastasis presenting Cushing’s syndrome [49, 50]. Finally, a 38-year-old woman with multiple endocrine neoplasia type II accompanied by thyroid medullary carcinoma was described to express CRF, Ucn1, and Ucn3 by the thyroid carcinoma and the pheochromocytoma, whereas CRF1 and CRF2 receptor immunoreactivity was absent in the thyroid tissue [51]. It was also reported as unpublished data that Ucn1 and Ucn3 were found in the human normal thyroid. It was postulated by the authors that the CRF neuropeptides act on other tissues in an endocrine manner. This is the only study using neuropeptide selective antibodies that confirms the previous findings of CRF expression by the thyroid tumors. There are no other reports on CRF receptor expression in the thyroid gland, whether of a biological role of the system in this organ or in tumors raising there.
CRF and melanoma
The CRF system is expressed in the normal skin. CRF neuropeptide production is regulated by ultraviolet radiation, glucocorticoids, and the phase of the hair cycle. CRF1 is the major receptor in human skin, found in epidermal and dermal compartments, whereas CRF2 is located predominantly in dermal structures. Different CRF biological effects have been shown depending on skin cell type and nutritional status, including modulation of differentiation, proliferation, viability, and immune activity [52].
In skin cancer, expression of CRF mRNA was firstly demonstrated in a human squamous carcinoma (C4–1) and a melanoma (SK-MEL188) cell line, using northern blot hybridization, whereas CRF peptide was identified in the same cells by reverse-phase HPLC separation. CRF peptide production was stimulated and inhibited by forskolin and dexamethasone, respectively. Moreover, stimulation of melanogenesis down-regulated CRF1 receptor mRNA expression, without affecting CRF mRNA production [53]. CRF receptor signal transduction pathways were studied in the human melanoma cell line SK-MEL 188 and the hamster melanoma cell line AbC1. CRF induced a rapid and dose-dependent increase in the intracellular Ca2+ in these cells, whereas other peptides of the CRF superfamily, such as sauvagine and urocortin, also induced cytoplasmic calcium increases but at higher concentrations than CRF [54]. These results confirmed the existence of functional CRF receptors in the melanoma cell lines.
CRF and its receptor mRNA expression were also found by RT-PCR in cultured human melanoma cells, nevus cells, and normal melanocytes, with higher levels in melanoma cells. Immunohistochemistry revealed that CRF as well as POMC were strongly expressed in advanced melanomas, such as vertically growing lesions of acral lentiginous, nodular, and metastatic melanomas, in contrast to negative nevus cells. These results indicate that skin tumor progression accentuates CRF, CRF receptor, and POMC expression by melanoma cells [55]. Elevated CRF levels might stimulate expression of POMC peptides, thus indirectly endowing melanoma cells with enhanced growth and metastatic abilities through a α-melanocyte stimulating hormone (MSH) mechanism, forming a local organization structure similar to that found in the hypothalamo–pituitary axis. To support this, CRF and POMC expression coincided in about 50% of advanced melanoma cells [55]. In order to clarify whether high expression of POMC correlates with CRF and the possible role of CRF as a melanoma growth factor, 25 cases of primary malignant melanoma and 20 cases of metastatic melanoma were immunohistochemically analyzed in parallel with five metastatic melanoma cell lines. Thirty-six percent of primary melanoma as well as 67% of metastatic melanoma showed positive staining for CRF. None of the CRF positive specimens were negative for POMC. In 7 out of 9 CRF positive melanomas and in 7 out of 12 CRF positive metastatic melanomas, co-localization of CRF and POMC peptides was shown. In addition, all metastatic melanoma cell lines expressed POMC mRNA that was stimulated in vitro by CRF and suppressed by CRF antagonists [56]. To further support a POMC-related CRF involvement in the development of skin malignancy, examination of the expression patterns of the CRF–POMC axis-related hormones revealed that CRF, ACTH, and α-MSH were strongly expressed in malignant skin tumor cell lines such as G-361 and DX-3, whereas normal and haematological malignant cell lines did not express the CRF–POMC axis-related hormones. Immunohistochemical analysis of skin tumors showed that eight out of ten malignant melanomas, seven out of ten squamous cell carcinomas, and one out of ten basal cell carcinomas had strong immunoreactivity for CRF [57].
CRF affected the migration of melanoma cells studied in the spontaneous murine melanoma cell line B16F0 and its metastatic clone, B16F10. CRF treatment increased the level of B16F10 cell migration in a dose- and time-dependent manner. Pretreatment with an inhibitor of the extracellular signal-regulated protein kinase 1/2 (ERK1/2) blocked this effect, whereas CRF induced its phosphorylation, suggesting that CRF regulates the migration of melanoma cells in the skin during stress through the ERK1/2 signaling pathway [58].
In contrast to these data indicating a tumor promoting role of the CRF system in melanoma, CRF and six analogues, including Ucn1 and sauvagine, were shown to exert antiproliferative effects when tested on Cloudman melanoma cell proliferation and B16 melanoma tumor growth in C57B1/6 mice. CRF and all the six analogues inhibited proliferation of Cloudman cells in culture and also inhibited B16 tumor growth rate in vivo, most likely by activation of endogenous CRF1 receptors and subsequent altered intracellular Ca2+ signaling. This was suggested to hold a therapeutic potential [59].
CRF and lung cancer
Immunoreactive CRF was initially measured by radioimmunoassay in one small-cell lung carcinoma [46] and in 2 of 40 small-cell lung carcinomas [60]. Immunohistochemistry showed peptide expression in 1 of 30 small-cell lung carcinomas, in a poorly differentiated adenocarcinoma [25, 61] and in a patient with Cushing’s syndrome and metastatic small-cell lung cancer. Radioimmunoassay of the patient’s plasma revealed persistently elevated CRF concentrations [62].
In accordance with these data, at the gene expression level, significant amounts of long- and authentic-size CRF mRNAs were detected in one of six patients with pulmonary small-cell carcinoma by northern blot analysis, and authentic size CRF mRNA was detected in two of six patients [63], confirming expression of the CRF gene rather than the homologue urocortins.
CRF, ACTH, or beta-endorphin levels in the bronchoalveolar lavage of 25 patients with lung cancer (17 squamous carcinomas, 4 adenocarcinomas, 2 small-cell carcinomas- and 2 unclassified) were compared to 18 controls measured by radioimmunoassay. CRF and ACTH levels were not significantly different between the two groups. Moreover, histological tumor type was not associated with expression levels of any of the peptides measured [64]. In order to evaluate CRF as a possible tumor marker, plasma CRF levels were determined in a sequence of 103 randomly selected patients with lung cancer without Cushing’s syndrome and in 72 age- and sex-matched controls. Plasma CRF levels of cancer patients were similar to those of controls [65].
To evaluate receptor expression, the radioligand binding, second messenger, and mRNA characteristics of CRF receptors were studied in a variety of small-cell lung carcinoma lines and compared to CRF receptors in the mouse pituitary tumor At-20 cells. Results demonstrated the presence of CRF receptors in the small-cell lung carcinoma cell lines with kinetic, pharmacological, second messenger, and mRNA characteristics comparable to those in pituitary and brain, indicating a possible role for CRF as a regulatory peptide in human small-cell lung carcinoma [66]. Functional lung cancer receptors were shown, since CRF increased the cAMP levels on human lung cancer cell lines NCI-H345, NCI-H720, and NCI-H1299 in a dose-dependent manner. The CRF analogue sauvagine also elevated the cAMP levels. CRF had no effect on cytosolic calcium but stimulated [3H] arachidonic acid release from NCI-H1299 and the clonal growth of NCI-H345 and NCI-H720 cells. All these effects were reversed by the CRF antagonist α-helical CRF (9–41) showing that they are specific receptor-mediated actions [67]. However, CRF1 and CRF2 receptor expression was not detected in 11 non-small-cell lung carcinomas tested by autoradiography, reducing the pathophysiological relevance of the former findings, since CRF receptor expression seems to be a characteristic of the small-cell lung carcinoma lines but not of the actual tumors [29].
Finally, in a mouse cachexia model, administration of a CRF2 agonist (PG-873637) resulted in beneficial effects on muscle weight loss in mice with implanted fast-growing Lewis lung carcinoma. Moreover, the agonist significantly reduced both the number of metastases and their mass. These data suggested a potentially beneficial use of CRF agonists for the treatment of muscle wasting associated with cancer [68].
CRF and gastrointestinal cancers
It is well known that stress affects the function of the gastrointestinal system (GI), under basal and pathological conditions, and the role of the CRF system is well established by a plethora of evidence [69]. Multiple reports show that members of the CRF system, i.e. the receptors as well as their neuropeptide ligands, are expressed throughout the gastrointestinal tract in humans and rats, and may contribute significantly in the regulation of GI motility and response to noxious stimuli. It appears that their distribution differs along the GI lumen, resulting in distinctive physiological effects, a notion that is also supported by pharmacological data. Indeed, various physiological stressors in the colon stimulate its propulsive activity, affecting motility, transit, and defecation, whereas in upper GI tissues they inhibit contractility and delay gastric emptying.
Expression of immunoreactive CRF was first detected by radioimmunoassay in various tumors of the GI in 1985 by Wakabayashi et al., including one adenocarcinoma of the stomach, one adenocarcinoma of the pancreas, one adenocarcinoma of the sigmoid colon, and one adenocarcinoma of the rectum [46]. However, these studies need to be confirmed using peptide-specific antibodies that discriminate between CRF and the homologue urocortins. In a later study, a 28-year-old woman, described with upper gastrointestinal bleeding caused by an active ulcer, a pancreatic head mass, and multiple liver metastases, was found to have a CRF-positive liver biopsy by immunohistochemical analysis [70].
We have recently reported the expression of the CRF system in the human liver, using RT-PCR and immunohistochemistry. Both mRNA and immunoreactivity of Ucn1 were found in all the human liver biopsies examined. Ucn1 was localized in hepatocytes. CRF1 and CRF2α receptor gene expression was also found, and receptor protein had a similar distribution to Ucn 1. Finally, Ucn 1 and CRF receptor expression was demonstrated in hepatic biopsies from a variety of liver pathologies, including primary or metastatic liver carcinoma and cirrhosis. We concluded that the CRF system is expressed by human liver under normal and pathological conditions, Ucn 1 being the major ligand [71] that may act in an autocrine manner through activation of the local CRF receptors. In order to unfold a functional biological role of these effectors in liver physiology and pathogenesis, we tested the effects of the CRF system in the hepatocellular apoptotic process, using a rat experimental model of common bile duct surgical ligation, leading to obstructive jaundice, cholestasis, and apoptosis induction in the hepatic parenchyma. Administration of selective and non-selective CRF antagonists showed that the endogenous CRF system promotes the cholestasis-induced apoptotis via CRF1 activation. In contrast, CRF2 seems to mediate an early and a late apoptosis-preventing phenomena, i.e. elevated gene transcript levels of the anti-apoptotic bcl-2 at the first postoperative day and increased rat serum hepatocyte growth factor (HGF) levels at the third postoperative day, acting opposed to CRF1. No effect was observed under basal conditions. These data support a CRF-based apoptosis-regulating mechanism in the liver that may contribute to carcinogenesis. This notion is further supported by data showing a role of hepatic CRF receptors on tumor growth and angiogenesis. Both in vivo and in vitro effects of Ucn1 were evaluated in human hepatome cell lines SMMC-7721 and HepG2, human umbilical vein endothelial cell (HUVEC), and human hepatocellular carcinoma tissues. Ucn1 inhibited the growth of hepatocellular carcinoma and reduced tumor microvessel density in nude mice. Ucn1 administered in tumor-bearing mice inhibited the growth of established tumors in vivo. In addition, in vitro three-dimensional culture assay showed that Ucn1 inhibited angiogenesis via CRF2 activation. Finally, Ucn1 inhibited the proliferation, promoted the apoptosis of endothelial cells, and down-regulated VEGF expression in vivo via CRF2 [72].
Both CRF receptors are expressed in normal intestine [14, 73] in proximity to their ligands, indicating the formation of autocrine–paracrine regulatory loops. CRF immunoreactivity and mRNA have been revealed in the human colonic mucosa [74]. Ucn1 is also evident in epithelial and lamina propria cells of the colonic mucosa [14, 75]. Furthermore, mRNA from the two newer members of the CRF peptide family, Ucn2 and 3, was detected in tissues of the lower GI tract [9, 11]. Although the biological role of the peripherally expressed CRF ligands in the intestinal tissue is not clear, it has been postulated that they may participate in several aspects of immune-humoral mechanisms within the gut and this could be related to carcinogenesis. Indeed, it is well established that stress is implicated in the development of inflammatory bowel disease (IBD), a high risk pre-condition for the development of colon cancer, via initial nervous disturbance and subsequent immune dysfunction through brain-gut interactions. The CRF system is involved in the inflammatory process within the gastrointestinal tract, via vagal and peripheral pathways, as implied by multiple reports reviewed recently by our group [69]. No expression of CRF1 and CRF2 receptors was detected in ten colon carcinomas by in vitro autoradiography with subtype-selective CRF analogs [29] indicating that receptor loss may contribute to malignant transformation and/or tumor progression either as a causal or as a resulting effect.
The presence of the CRF receptors and ligands has also been reported in the normal gastric mucosa [76–78]. The level of immunoreactive Ucn1 was higher in gastric biopsies from patients with active Helicobacter pylori (HP) gastritis than in normal controls. Ucn1 was localized by immunohistochemistry in gastric epithelial cells and in inflammatory elements of the surrounding negative for Ucn1 gastric stroma. After eradication of HP infection, Ucn1 levels increased dramatically compared with pretreatment values whereas nonresponders did not show any significant change. It was concluded that Ucn1 expression is related to HP infection and its progression in gastric mucosa, and this may imply a role in HP-related carcinogenesis. Finally, using the gastric cancer cell line AGS transiently transfected to express functional CRF2, we showed that activation of this receptor reduced the degree of apoptosis and had no effect on the proliferation rate and PGE2 release, indicating a regulating mechanism of an important parameter of gastric cell regeneration and malignant transformation [78].
CRF signaling pathways related to cancer
Multiple data contribute to the description of the possible molecular pathways responsible for the CRF system involvement in the mechanisms of tumorigenesis and/or antitumor processes in cancer cells, through regulation of oncogenes or tumor suppressor or other genes.
CRF is a regulator of the activity of nuclear transcription factor B (NF-κB). NF-κB is a regulator of genes that control cell proliferation and cell survival. Aberrant activation of NF-κB is frequently observed in many cancers. Active NF-κB turns on the expression of genes that keep the cell proliferating and protect the cell from conditions that would otherwise cause death via apoptosis. It has been shown that NF-κB is expressed in the brain and that its DNA binding activity is inhibited by CRF in hippocampal neurons as well as in a pituitary corticotroph cell line, the AtT20 cells, under normal or oxidative stress-induced conditions [79]. This finding has been associated with the neuroprotective effects of CRF during hypoxia. The regulation of corticotroph NF-κB activity by CRF is also correlated with the activation of the pituitary POMC gene as shown in AtT20 cells transiently transfected with a POMC–luciferase construct mutated at an NF-κB binding site [80]. Inhibition of NF-κB DNA binding activity may represent an anticancer action of CRF. In contrast, other findings showed that in leucocytes CRF enhanced the antigen-specific antibody response through the CRF1 receptor by elevation of NF-κB activity [81]. Also, in mouse thymocytes, CRF has been shown to induce the NF-κB DNA-binding activity in a time- and dose-dependent manner, with parallel degradation of its inhibitor protein inhibitor [82] through the protein kinase A and protein kinase C signaling pathways. Similarly, in neonatal rat cardiomyocytes, Ucn activates NF-κB, ERK, and p38 MAP kinases and their inhibitors block Ucn-induced IL-6 release, suggesting that these molecules participate in this regulatory mechanism [83].These varying results indicate cell- or receptor-specific actions.
The early-response transcription factor activator protein 1 (AP-1), a dimer of Jun and Fos, has also been implicated in the transactivation of the CRF gene. c-fos induction has been widely used as a marker for neuronal activation, and it is often co-localized in CRF expressing neuronal circuits (for review see [84]), whereas it represents a cellular proto-oncogene implicated in the malignant transformation and cancer progression. c-fos is induced by stressors in CRF neurons in vivo and the PKC pathway can activate CRF gene transcription in vitro, whereas the 5′ flanking region of vertebrate CRF genes contains several AP-1 binding sites [85]. It could therefore be related to CRF involvement in carcinogenesis.
Other evidence suggests that the activation of MAP kinases by CRF involves tissue-specific intracellular proteins and signaling pathways. CRF receptors mediate induction of ERK1/2 phosphorylation in a cAMP-independent way [86]. Similarly, Ucn induced ERK1/2 activation in human pregnant myometrium via CRF1, an effect inhibited by protein kinase A activation [87]. A potent cardioprotective effect against hypoxic insults through activation of both Akt and ERK1/2 has been proposed for Ucn2 and Ucn3 that bind exclusively CRF2 [88–90]. The phosphatidylinositol 3-kinase (PI3 K)/Akt pathway is implicated in a great spectrum of tissue responses and cellular processes. Akt regulates cellular survival and metabolism by binding and regulating many downstream effectors, e.g. NF-κB, the Bcl-2 family of proteins, and murine double minute 2 (MDM2). It is therefore a key modulator of cell survival, cell cycle, metabolism, and angiogenesis, the main processes involved in carcinogenesis. Recently, the PI3 K pathway has been suggested to play a critical role in CRF1-mediated effects, more specifically those of the subtype CRF1a [91]. Moreover, in the human monocytic THP-1 cells, CRF activated the phosphatidylinositol 3-kinase (PI3 K)/Akt and ERK1/2 pathways via CRF2, leading to cell survival activation through stimulation of the antiapoptotic factor Bcl-2 [92]. These findings imply that CRF receptor signaling is implicated in carcinogenesis-related pathways which could therefore be regulated by CRF ligands.
The human Y-79 retinablastoma cell line expresses functional CRF receptors [93] and presents a suitable model for the study of homologous desensitization and signal transduction pathways [94–98]. In this cell line, cytoprotective effects of CRF, involving suppression of pro-apoptotic pathways at a site upstream of activation of procaspase-3 and the involvement of PK A in the mediation of the anti-apoptotic effect of CRF, have been shown [99].
Interestingly, in order to identify some of the intracellular substrates mediating the actions of small-molecule CRF1 antagonist NBI30775, that is currently being clinically tested as an antidepressant, Post et al. studied its effects after acute administration in a stress-independent animal model [100]. NBI30775 induced the nuclear translocation of glucocorticoid receptors and BAG-1, a Bcl2-associated athanogene that enhances its anti-apoptotic effect. It also suppressed the DNA-binding activity of AP-1, identifying these molecules as some of the drug’s intracellular targets, which could be related to anti-cancer therapy. Drug treatment resulted in a modest, insignificant reduction of DNA-NF-κB binding activity.
Conclusion
In the present review, we summarize data from the existing literature describing expression and pathophysiological significance of the members of the CRF system of neuropeptides and receptors in human cancers (Table 1). CRF has been found in small percentages of prostate, thyroid, lung, breast, and GI tumors and higher percentages of ovarian, endometrium, and skin malignancies. However, it should be mentioned that until the late 1990s when the homologues Ucns were characterized, the specificity of the antibodies used for CRF detecting assays should be re-evaluated and the reported results reconfirmed. Receptor expression is also reported in ovarian, endometrial, breast, skin, and liver, where they may mediate growth and apoptotic, immune, and metastatic parameters. It is clear that the two receptor types exhibit different distributions and hold distinct roles in cancer cells, which could even be counteracting. On the other hand, receptor loss may contribute to malignant transformation and tumor growth in prostate, colon, and lung cancer. No value as a tumor marker has been found for CRF and CRF receptors in lung and breast cancer, respectively, whereas in endometrial cancer, CRF1 correlated with less aggressive tumors, whereas CRF2 did so to advanced stage tumors. In the skin, a CRF/POMC mechanism may contribute to MSH driven-carcinogenesis, and finally, in the stomach, Ucn1 may be involved in HP-related cancer development. Multiple cancer cell lines from different origins express CRF peptides and receptors (Table 2) and present suitable models for the clarification of the role of the CRF system in human malignancy. In addition, synthetic peptide and non-peptide CRF ligands with differing antagonistic intrinsic activities have been synthesized for research utility, whereas over 100 patent claims have been made during the last 15 years for low molecular weight, non-peptide, selective CRF1 receptor antagonists [101], which are currently being tested for their therapeutic potential against depression and other stress-related disorders [102, 103]. In addition, Ucn1 and Ucn2 are being clinically evaluated in the treatment of human heart failure [104, 105]. Direct and indirect evidence [106] supporting a role of the CRF receptors in the growth of certain human cancers, present them as novel targets for anticancer therapy, using these compounds which are readily available for clinical use. However, further studies are needed in order to firmly support these speculations.
Table 1.
Expression of CRF family of neuroeptides and receptors in various human cancers
| Positive/total tissues studied | Molecule | Technique | Reference |
|---|---|---|---|
| Prostate Cancer | |||
| 1/3 | CRF | IHC | [25] |
| 1/1 | CRF | RIA | [26] |
| 3/3 | Ucn1 | RT–PCR, IHC | [28] |
| 0/11 | CRF1 and CRF2 | Autoradiography | [29] |
| 0/32 | CRF2 | RT–PCR, IF | [27] |
| 0/1 | CRF | IHC | [30] |
| Thyroid | |||
| 1/1 medullary | 1 for CRF | RIA | [45] |
| 1/1 medullary | 1 for CRF | RIA | [46] |
| 2/10 medullary | 2 for CRF | IHC | [25] |
| 1/1 medullary | 1 for CRF | RIA | [47] |
| 1/4 follicular | CRF | IHC | [48] |
| 4/8 papillary | CRF | ||
| 4/4 Hurtle cell | CRF | ||
| 1/1 medullary | CRF | ||
| 1/1 insular | CRF | ||
| 1/1 medullary | CRF | IHC | [49] |
| 1/1 liver metastasis from medullary | CRF | IHC | [50] |
| 1/1 medullary | CRF, Ucn1, Ucn3 | IHC | [51] |
| Ovarian cancer | |||
| 32/47 | CRF | IHC | [43] |
| 33/47 | CRF1 | ||
| 30/47 | CRF2 | ||
| Endometrial cancer | |||
| 4/19 | CRF1 | RNase protection assay | [33] |
| 9/9 | Ucn1 mRNA | Quantitative RT-PCR | [34] |
| 51/51 | CRF | IHC | [35] |
| 34/37 | CRF1 | ||
| 31/51 | CRF2 | ||
| Melanoma | |||
| Human melanoma cells | CRF, CRFRs | RT-PCR, IHC | [55] |
| 9/25 primary | CRF | IHC | [56] |
| 12/18 metastatic | CRF | ||
| 8/10 malignant | CRF | IHC | [57] |
| 7/10 squamous cell carcinomas | CRF | ||
| 1/10 basal cell carcinomas | CRF | ||
| Lung cancer | |||
| 1/1 SCLC | CRF | RIA | [46] |
| 1/30 SCLC | CRF | IHC | [25] |
| 1/1 adenocarcinoma | CRF | IHC | [61] |
| 2/40 SCLC | CRF | RIA | [60] |
| 3/6 SCLC | CRFmRNA | Northern blot hybridization | [63] |
| 1/1 metastatic SCLC | CRF | RIA | [62] |
| 0/11 non-SCLC | CRF1 and CRF2 | Autoradiography | [29] |
| Gastrointestinal cancer | |||
| Adenocarcinomas 1/1 stomach | CRF | RIA | [46] |
| 1/1 pancreas | CRF | ||
| 1/1 sigmoid colon | CRF | ||
| 1/1 rectum | CRF | ||
| 0/10 colon cancer | CRF1 and CRF2 | Autoradiography | [29] |
| Liver metastasis from 1 malignant gastrinoma | CRF | IHC | [70] |
|
3/3 primary hepatocellular cancer 1/1 cholangiocarcinoma 3 metastatic hepatic cancer |
Ucn1, CRF1 and CRF2 | IHC | [71] |
| Breast cancer | |||
| 14/40 | CRF | IHC | [36] |
| Other malignant tumors | |||
| 1/1 pituitary cancer | CRF |
IHC, Gel filtration Northern blot analysis |
[107] |
| 1/1 Ewing’s sarcoma | CRF | IHC | [108] |
IHC Immunohistochemistry, IF immunofluorescence, RT-PCR reverse-transcription polymerase chain reaction, RIA radioimmunoassay, SCLC small-cell lung cancer
Table 2.
Expression of CRF neuropeptides and receptors in cancer cell lines
| Cell line | Molecule | Technique | Reference |
|---|---|---|---|
|
OvCa3 human ovarian cancer A2780 human ovarian cancer |
CRF, CRF1 CRF, CRF1, CRF2 |
RT-PCR, IF RT-PCR, IF |
[43] |
| Ishikawa human endometrial adenocarcinoma |
CRFmRNA CRF peptide CRF |
Northern blot hybridization Gel filtration chromatography Immunofluorescence |
[31] |
|
C4–1 human squamous carcinoma SK-MELL 188 human melanoma |
CRFmRNA CRF peptide |
Northern blot hybridization, chromatography | [33] |
|
G-361 human melanoma DX-3 |
CRF, POMC, ACTH, αMSH | [57] | |
| MCF7 human breast cancer |
CRF1 CRFmRNA |
RNase protection assay RT-PCR |
[38] |
| MCF7 human breast cancer |
CRF1a mRNA CRF2c mRNA |
RT-PCR RT-PCR |
[40] |
| W256 rat mammary carcinoma | CRF1 | RT-PCR | [37] |
| NCI-H345, NCI-H720, NCI-H1299 human SCLC | CRF receptors | cAMP induction | [67] |
| NCI-H69, H82, H146, H209, H345, H446, and H510A, SCLC | CRF receptors | Radiologand binding assay | [66] |
| NT2 human neuronal-like teratocarcinoma | CRF1 | RT-PCR | [38] |
| SH-5YSY human neuroblastoma | CRF1 | RT-PCR | [38] |
| Y-79 human retinoblastoma | CRFRs | RT-PCR | [93] |
IHC Immunohistochemistry, IF immunofluorescence, RT-PCR reverse-transcription polymerase chain reaction, SCLC small-cell lung cancer
References
- 1.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pohl S, Darlison MG, Clarke WC, Lederis K, Richter D. Cloning and functional pharmacology of two corticotropin-releasing factor receptors from a teleost fish. Eur J Pharmacol. 2001;430:193–202. doi: 10.1016/s0014-2999(01)01391-7. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW. Cloning and characterization of human urocortin published erratum appears in endocrinology 1996. Endocrinology. 1996;137(9):3896–70. doi: 10.1210/endo.137.9.8756563. [DOI] [PubMed] [Google Scholar]
- 4.Guillemin R, Rosenberg B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955;57:599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- 5.Saffran M, Schally AV. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955;33:408–415. [PubMed] [Google Scholar]
- 6.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Hara E, Suzuki H, Sasano H, Shibahara S. Expression of heme oxygenase isozyme mRNAs in the human brain and induction of heme oxygenase-1 by nitric oxide donors. J Neurochem. 1996;67:482–489. doi: 10.1046/j.1471-4159.1996.67020482.x. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor see comments. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 9.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 10.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iino K, Sasano H, Oki Y, Andoh N, Shin RW, Kitamoto T, Totsune K, Takahashi K, Suzuki H, Nagura H, Yoshimi T. Urocortin expression in human pituitary gland and pituitary adenoma. J Clin Endocrinol Metab. 1997;82:3842–3850. doi: 10.1210/jcem.82.11.4371. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Totsune K, Sone M, Murakami O, Satoh F, Arihara Z, Sasano H, Iino K, Mouri T. Regional distribution of urocortin-like immunoreactivity and expression of urocortin mRNA in the human brain. Peptides. 1998;19:643–647. doi: 10.1016/s0196-9781(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 15.Muramatsu Y, Sugino N, Suzuki T, Totsune K, Takahashi K, Tashiro A, Hongo M, Oki Y, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in normal cycling human ovaries. J Clin Endocrinol Metab. 2001;86:1362–1369. doi: 10.1210/jcem.86.3.7299. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab. 2002;87:340–346. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Totsune K, Murakami O, Saruta M, Nakabayashi M, Suzuki T, Sasano H, Shibahara S. Expression of urocortin III/stresscopin in human heart and kidney. J Clin Endocrinol Metab. 2004;89:1897–1903. doi: 10.1210/jc.2003-031663. [DOI] [PubMed] [Google Scholar]
- 18.Saruta M, Takahashi K, Suzuki T, Fukuda T, Torii A, Sasano H. Urocortin 3/stresscopin in human colon: possible modulators of gastrointestinal function during stressful conditions. Peptides. 2005;26:1196–1206. doi: 10.1016/j.peptides.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Florio P, Torres PB, Torricelli M, Toti P, Vale W, Petraglia F. Human endometrium expresses urocortin II and III messenger RNA and peptides. Fertil Steril. 2006;86:1766–1770. doi: 10.1016/j.fertnstert.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology. 1999;140:5651–5658. doi: 10.1210/endo.140.12.7223. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Kageyama K, Sakihara S, Nigawara T. Physiological roles of urocortins, human homologues of fish urotensin I, and their receptors. Peptides. 2004;25:1689–1701. doi: 10.1016/j.peptides.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin- releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 23.Vale WW, Vaughn J, Perrin M. Corticotropin-releasing factor (CRF) family of ligands and their receptors. Endocrinologist. 1997;7:3S–9S. [Google Scholar]
- 24.Jahn O, Radulovic J, Stiedl O, Tezval H, Eckart K, Spiess J. Corticotropin-releasing factor binding protein–a ligand trap? Mini Rev Med Chem. 2005;5:953–960. doi: 10.2174/138955705774329500. [DOI] [PubMed] [Google Scholar]
- 25.Asa SL, Kovacs K, Vale W, Petrusz P, Vecsei P. Immunohistologic localization of corticotrophin-releasing hormone in human tumors. Am J Clin Pathol. 1987;87:327–333. doi: 10.1093/ajcp/87.3.327. [DOI] [PubMed] [Google Scholar]
- 26.Fjellestad-Paulsen A, Abrahamsson PA, Bjartell A, Grino M, Grimelius L, Hedeland H, Falkmer S. Carcinoma of the prostate with Cushing’s syndrome. A case report with histochemical and chemical demonstration of immunoreactive corticotropin-releasing hormone in plasma and tumoral tissue. Acta Endocrinol (Copenhagen) 1988;119:506–516. [PubMed] [Google Scholar]
- 27.Yamada Y, Ohashi A, Inoue T, Sakaguchi K, Tsujimura T, Okamoto D, Itatani H, Fujimoto N, Kusaka K, Fushimi H. Cushing’s syndrome with a large pituitary adenoma producing both corticotropin-releasing hormone (CRH) and adrenocorticotropin (ACTH) Int Med. 2002;41:549–554. doi: 10.2169/internalmedicine.41.549. [DOI] [PubMed] [Google Scholar]
- 28.Arcuri F, Cintorino M, Florio P, Floccari F, Pergola L, Romagnoli R, Petraglia F, Tosi P, Teresa Del Vecchio M. Expression of urocortin mRNA and peptide in the human prostate and in prostatic adenocarcinoma. Prostate. 2002;52:167–172. doi: 10.1002/pros.10094. [DOI] [PubMed] [Google Scholar]
- 29.Reubi JC, Waser B, Vale W, Rivier J. Expression of CRF1 and CRF2 receptors in human cancers. J Clin Endocrinol Metab. 2003;88:3312–3320. doi: 10.1210/jc.2002-021853. [DOI] [PubMed] [Google Scholar]
- 30.Tezval H, Jurk S, Atschekzei F, Serth J, Kuczyk MA, Merseburger AS. The involvement of altered corticotropin releasing factor receptor 2 expression in prostate cancer due to alteration of anti-angiogenic signaling pathways. Prostate. 2009;69:443–448. doi: 10.1002/pros.20892. [DOI] [PubMed] [Google Scholar]
- 31.Makrigiannakis A, Zoumakis E, Margioris AN, Theodoropoulos P, Stournaras C, Gravanis A. The corticotropin-releasing hormone (CRH) in normal and tumoral epithelial cells of human endometrium. J Clin Endocrinol Metab. 1995;80:185–189. doi: 10.1210/jcem.80.1.7829610. [DOI] [PubMed] [Google Scholar]
- 32.Graziani G, Tentori L, Portarena I, Barbarino M, Tringali G, Pozzoli G, Navarra P. CRH inhibits cell growth of human endometrial adenocarcinoma cells via CRH-receptor 1-mediated activation of cAMP-PKA pathway. Endocrinology. 2002;143:807–813. doi: 10.1210/endo.143.3.8694. [DOI] [PubMed] [Google Scholar]
- 33.Graziani G, Ferrandina G, Pozzoli G, Vergati M, Muzi A, Legge F, Tentori L, Scambia G, Navarra P. Corticotropin-releasing hormone receptor-1 in human endometrial cancer. Oncol Rep. 2006;15:375–379. [PubMed] [Google Scholar]
- 34.Florio P, De Falco G, Leucci E, Torricelli M, Torres PB, Toti P, Dell’Anna A, Tiso E, Santopietro R, Leoncini L, Petraglia F. Urocortin expression is downregulated in human endometrial carcinoma. J Endocrinol. 2006;190:99–105. doi: 10.1677/joe.1.06726. [DOI] [PubMed] [Google Scholar]
- 35.Miceli F, Ranelletti FO, Martinelli E, Petrillo M, Scambia G, Navarra P, Ferrandina G. Expression and subcellular localization of CRH and its receptors in human endometrial cancer. Mol Cell Endocrinol. 2009;305:6–11. doi: 10.1016/j.mce.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Ciocca DR, Puy LA, Fasoli LC, Tello O, Aznar JC, Gago FE, Papa SI, Sonego R. Corticotropin-releasing hormone, luteinizing hormone-releasing hormone, growth hormone-releasing hormone, and somatostatin-like immunoreactivities in biopsies from breast cancer patients. Breast Cancer Res Treat. 1990;15:175–184. doi: 10.1007/BF01806354. [DOI] [PubMed] [Google Scholar]
- 37.Tjuvajev J, Kolesnikov Y, Joshi R, Sherinski J, Koutcher L, Zhou Y, Matei C, Koutcher J, Kreek MJ, Blasberg R. Anti-neoplastic properties of human corticotropin releasing factor: involvement of the nitric oxide pathway. In Vivo. 1998;12:1–10. [PubMed] [Google Scholar]
- 38.Parham KL, Zervou S, Karteris E, Catalano RD, Old RW, Hillhouse EW. Promoter analysis of human corticotropin-releasing factor (CRF) type 1 receptor and regulation by CRF and urocortin. Endocrinology. 2004;145:3971–3983. doi: 10.1210/en.2004-0194. [DOI] [PubMed] [Google Scholar]
- 39.Graziani G, Tentori L, Muzi A, Vergati M, Tringali G, Pozzoli G, Navarra P. Evidence that corticotropin-releasing hormone inhibits cell growth of human breast cancer cells via the activation of CRH-R1 receptor subtype. Mol Cell Endocrinol. 2007;264:44–49. doi: 10.1016/j.mce.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Androulidaki A, Dermitzaki E, Venihaki M, Karagianni E, Rassouli O, Andreakou E, Stournaras C, Margioris AN, Tsatsanis C. Corticotropin releasing factor promotes breast cancer cell motility and invasiveness. Mol Cancer. 2009;8:30. doi: 10.1186/1476-4598-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda T, Tomori N, Yajima F, Odagiri E, Demura H, Shizume K. Characterization of immunoreactive corticotropin and corticotropin: releasing factor in human adrenal and ovarian tumours. Acta Endocrinol (Copenhagen) 1986;111:546–552. doi: 10.1530/acta.0.1110546. [DOI] [PubMed] [Google Scholar]
- 42.Zoumakis E, Chatzaki E, Charalampopoulos I, Margioris AN, Angelakis E, Koumantakis E, Gravanis A. Cycle and age-related changes in corticotropin-releasing hormone levels in human endometrium and ovaries. Gynecol Endocrinol. 2001;15:98–102. [PubMed] [Google Scholar]
- 43.Minas V, Rolaki A, Kalantaridou SN, Sidiropoulos J, Mitrou S, Petsas G, Jeschke U, Paraskevaidis EA, Fountzilas G, Chrousos GP, Pavlidis N, Makrigiannakis A. Intratumoral CRH modulates immuno-escape of ovarian cancer cells through FasL regulation. Br J Cancer. 2007;97:637–645. doi: 10.1038/sj.bjc.6603918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Groef B, Van der Geyten S, Darras VM, Kuhn ER. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates. Gen Comp Endocrinol. 2006;146:62–68. doi: 10.1016/j.ygcen.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Birkenhager JC, Upton GV, Seldenrath HJ, Krieger DT, Tashjian AH., Jr Medullary thyroid carcinoma: ectopic production of peptides with ACTH- like, corticotrophin releasing factor-like and prolactin production- stimulating activities. Acta Endocrinol (Copenhagen) 1976;83:280–292. doi: 10.1530/acta.0.0830280. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi I, Ihara T, Hattori M, Tonegawa Y, Shibasaki T, Hashimoto K. Presence of corticotropin-releasing factor-like immunoreactivity in human tumors. Cancer. 1985;55:995–1000. doi: 10.1002/1097-0142(19850301)55:5<995::aid-cncr2820550513>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Delgrange E, Maiter D, Donckier J, Tourniaire J. Influence of age on the clinical presentation of prolactinomas in male patients. Gerontology. 1999;45:160–164. doi: 10.1159/000022079. [DOI] [PubMed] [Google Scholar]
- 48.Scopa CD, Mastorakos G, Friedman TC, Melachrinou M, Merino MJ, Chrousos GP. Presence of immunoreactive corticotropin releasing hormone in thyroid lesions. Am J Pathol. 1994;145:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 49.Tagliabue M, Pagani A, Palestini N, Manieri C, Martina V. Multiple endocrine neoplasia (MEN IIB) with Cushing’s syndrome due to medullary thyroid carcinoma producing corticotropin-releasing hormone. Panminerva Med. 1996;38:41–44. [PubMed] [Google Scholar]
- 50.Chrisoulidou A, Pazaitou-Panayiotou K, Georgiou E, Boudina M, Kontogeorgos G, Iakovou I, Efstratiou I, Patakiouta F, Vainas I. Ectopic Cushing’s syndrome due to CRH secreting liver metastasis in a patient with medullary thyroid carcinoma. Hormones (Athens) 2008;7:259–262. doi: 10.1007/BF03401514. [DOI] [PubMed] [Google Scholar]
- 51.Kageyama K, Sakihara S, Yamashita M, Takahashi K, Kawashima S, Tanabe J, Tsutaya S, Yasujima M, Suda T. A case of multiple endocrine neoplasia type II accompanied by thyroid medullary carcinoma and pheochromocytomas expressing corticotropin-releasing factor and urocortins. Am J Med Sci. 2008;335:398–402. doi: 10.1097/MAJ.0b013e31815200f8. [DOI] [PubMed] [Google Scholar]
- 52.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 54.Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Lett. 1998;435:187–190. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- 55.Funasaka Y, Sato H, Ichihashi M. Expression of corticotropin releasing hormone in malignant melanoma. Ann N Y Acad Sci. 1999;885:391–393. doi: 10.1111/j.1749-6632.1999.tb08696.x. [DOI] [PubMed] [Google Scholar]
- 56.Sato H, Nagashima Y, Chrousos GP, Ichihashi M, Funasak Y. The expression of corticotropin-releasing hormone in melanoma. Pigment Cell Res. 2002;15:98–103. doi: 10.1034/j.1600-0749.2002.1o063.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim MH, Cho D, Kim HJ, Chong SJ, Lee KH, Yu DS, Park CJ, Lee JY, Cho BK, Park HJ. Investigation of the corticotropin-releasing hormone-proopiomelanocortin axis in various skin tumours. Br J Dermatol. 2006;155:910–915. doi: 10.1111/j.1365-2133.2006.07442.x. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Park H, Yang Y, Kim TS, Bang SI, Cho D. Enhancement of cell migration by corticotropin-releasing hormone through ERK1/2 pathway in murine melanoma cell line, B16F10. Exp Dermatol. 2007;16:22–27. doi: 10.1111/j.1600-0625.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 59.Carlson KW, Nawy SS, Wei ET, Sadee W, Filov VA, Rezsova VV, Slominski A, Quillan JM. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21:1173–1179. [PubMed] [Google Scholar]
- 60.Tsuchihashi T, Yamaguchi K, Abe K, Yanaihara N, Saito S. Production of immunoreactive corticotropin-releasing hormone in various neuroendocrine tumors. Jpn J Clin Oncol. 1992;22:232–237. [PubMed] [Google Scholar]
- 61.Bahro M, Pfeifer U, Dammrich J. Involvement of autophagic degradation in ACTH-induced skeletal muscle atrophy. Clin Neuropathol. 1992;11:64–70. [PubMed] [Google Scholar]
- 62.Auchus RJ, Mastorakos G, Friedman TC, Chrousos GP. Corticotropin-releasing hormone production by a small cell carcinoma in a patient with ACTH-dependent Cushing’s syndrome. J Endocrinol Invest. 1994;17:447–452. doi: 10.1007/BF03347737. [DOI] [PubMed] [Google Scholar]
- 63.Suda T, Tozawa F, Dobashi I, Horiba N, Ohmori N, Yamakado M, Yamada M, Demura H. Corticotropin-releasing hormone, proopiomelanocortin, and glucocorticoid receptor gene expression in adrenocorticotropin- producing tumors in vitro. J Clin Invest. 1993;92:2790–2795. doi: 10.1172/JCI116898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calogero AE, Polosa R, Neville E, D’Agata R. Measurements of hormonal peptides in the bronchoalveolar fluid as tumor markers of lung cancer. J Endocrinol Invest. 1995;18:354–358. doi: 10.1007/BF03347837. [DOI] [PubMed] [Google Scholar]
- 65.Calogero AE, Minacapilli G, Nicolosi AM, Moncada ML, Mistretta A, Latteri SF, Polosa P, D’Agata R. Limited clinical usefulness of plasma corticotropin-releasing hormone, adrenocorticotropin and beta-endorphin measurements as markers of lung cancer. J Endocrinol Invest. 1992;15:581–586. doi: 10.1007/BF03344929. [DOI] [PubMed] [Google Scholar]
- 66.Dieterich KD, Lehnert H. Decreased expression of corticotropin-releasing factor-binding protein mRNA in ACTH-secreting pituitary adenomas. Exp Clin Endocrinol Diabetes. 2000;108:59–62. doi: 10.1055/s-0032-1329217. [DOI] [PubMed] [Google Scholar]
- 67.Moody TW, Zia F, Venugopal R, Korman LY, Goldstein AL, Fagarasan M. Corticotropin-releasing factor stimulates cyclic AMP, arachidonic acid release, and growth of lung cancer cells. Peptides. 1994;15:281–285. doi: 10.1016/0196-9781(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 68.Argiles JM, Figueras M, Ametller E, Fuster G, Olivan M, de Oliveira CC, Lopez-Soriano FJ, Isfort RJ, Busquets S. Effects of CRF2R agonist on tumor growth and cachexia in mice implanted with Lewis lung carcinoma cells. Muscle Nerve. 2008;37:190–195. doi: 10.1002/mus.20899. [DOI] [PubMed] [Google Scholar]
- 69.Paschos KA, Charsou C, Constantinidis TC, Anagnostoulis S, Lambropoulou M, Papachristou F, Simopoulos K, Chatzaki E (2010) Corticotropin releasing hormone receptors mediate opposing effects in cholestasis-induced liver cell apoptosis. Endocrinology (in press) [DOI] [PubMed]
- 70.Park SY, Rhee Y, Youn JC, Park YN, Lee S, Kim DM, Song SY, Lim SK. Ectopic Cushing’s syndrome due to concurrent corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secreted by malignant gastrinoma. Exp Clin Endocrinol Diabetes. 2007;115:13–16. doi: 10.1055/s-2007-948212. [DOI] [PubMed] [Google Scholar]
- 71.Simopoulos C, Christodoulou E, Lambropoulou M, Tsaroucha AK, Kakolyris S, Polychronidis A, Karayiannakis AJ, Chatzaki E. Neuropeptide urocortin 1 and its receptors expressed human liver. Neuroendocrinology. 2009;89:315–326. doi: 10.1159/000187136. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Xu Y, Xu Y, Zhu H, Zhang R, Zhang G, Li S. Urocortin’s inhibition of tumor growth and angiogenesis in hepatocellular carcinoma via corticotrophin-releasing factor receptor 2. Cancer Invest. 2008;26:359–368. doi: 10.1080/07357900701788106. [DOI] [PubMed] [Google Scholar]
- 73.Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 74.Kohno M, Kawahito Y, Tsubouchi Y, Hashiramoto A, Yamada R, Inoue KI, Kusaka Y, Kubo T, Elenkov IJ, Chrousos GP, Kondo M, Sano H. Urocortin expression in synovium of patients with rheumatoid arthritis and osteoarthritis: relation to inflammatory activity. J Clin Endocrinol Metab. 2001;86:4344–4352. doi: 10.1210/jcem.86.9.7827. [DOI] [PubMed] [Google Scholar]
- 75.Kozicz T, Arimura A. Distribution of urocortin in the rat’s gastrointestinal tract and its colocalization with tyrosine hydroxylase. Peptides. 2002;23:515–521. doi: 10.1016/s0196-9781(01)00639-8. [DOI] [PubMed] [Google Scholar]
- 76.Chatzaki E, Charalampopoulos I, Leontidis C, Mouzas IA, Tzardi M, Tsatsanis C, Margioris AN, Gravanis A. Urocortin in human gastric mucosa: relationship to inflammatory activity. J Clin Endocrinol Metab. 2003;88:478–483. doi: 10.1210/jc.2002-020853. [DOI] [PubMed] [Google Scholar]
- 77.Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, Crowe PD, Petroski R, Tache Y, Grigoriadis DE. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J Neurochem. 2004;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- 78.Chatzaki E, Lambropoulou M, Constantinidis TC, Papadopoulos N, Tache Y, Minopoulos G, Grigoriadis DE. Corticotropin-releasing factor (CRF) receptor type 2 in the human stomach: Protective biological role by inhibition of apoptosis. J Cell Physiol. 2006;209:905–911. doi: 10.1002/jcp.20792. [DOI] [PubMed] [Google Scholar]
- 79.Lezoualc’h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non- amyloidogenic amyloid beta precursor protein and with the suppression of nuclear factor-kappaB. Mol Endocrinol. 2000;14:147–159. doi: 10.1210/mend.14.1.0403. [DOI] [PubMed] [Google Scholar]
- 80.Karalis KP, Venihaki M, Zhao J, van Vlerken LE, Chandras C. NF-kappaB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem. 2004;279:10837–10840. doi: 10.1074/jbc.M313063200. [DOI] [PubMed] [Google Scholar]
- 81.Smith EM, Gregg M, Hashemi F, Schott L, Hughes TK. Corticotropin Releasing Factor (CRF) activation of NF-kappaB-directed transcription in leukocytes. Cell Mol Neurobiol. 2006;26:1021–1036. doi: 10.1007/s10571-006-9040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao J, Karalis KP. Regulation of nuclear factor-kappaB by corticotropin-releasing hormone in mouse thymocytes. Mol Endocrinol. 2002;16:2561–2570. doi: 10.1210/me.2001-0334. [DOI] [PubMed] [Google Scholar]
- 83.Huang M, Kempuraj D, Papadopoulou N, Kourelis T, Donelan J, Manola A, Theoharides TC. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappa B activation. J Mol Endocrinol. 2009;42:397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- 84.Yao M, Denver RJ. Regulation of vertebrate corticotropin-releasing factor genes. Gen Comp Endocrinol. 2007;153:200–216. doi: 10.1016/j.ygcen.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 85.Vamvakopoulos NC, Sioutopoulou TO. Human corticotropin-releasing hormone receptor gene (CRHR) is located on the long arm of chromosome 17 (17q12-qter) Chromosome Res. 1994;2:471–473. doi: 10.1007/BF01552870. [DOI] [PubMed] [Google Scholar]
- 86.Rossant CJ, Pinnock RD, Hughes J, Hall MD, McNulty S. Corticotropin-releasing factor type 1 and type 2alpha receptors regulate phosphorylation of calcium/cyclic adenosine 3′, 5′-monophosphate response element-binding protein and activation of p42/p44 mitogen-activated protein kinase. Endocrinology. 1999;140:1525–1536. doi: 10.1210/endo.140.4.6656. [DOI] [PubMed] [Google Scholar]
- 87.Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol Endocrinol. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- 88.Brar BK, Railson J, Stephanou A, Knight RA, Latchman DS. Urocortin increases the expression of heat shock protein 90 in rat cardiac myocytes in a MEK1/2-dependent manner. J Endocrinol. 2002;172:283–293. doi: 10.1677/joe.0.1720283. [DOI] [PubMed] [Google Scholar]
- 89.Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, Yellon DM, Latchman DS. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- 90.Chanalaris A, Lawrence KM, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS. Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. J Mol Cell Cardiol. 2003;35:1295–1305. doi: 10.1016/s0022-2828(03)00244-x. [DOI] [PubMed] [Google Scholar]
- 91.Punn A, Levine MA, Grammatopoulos DK. Identification of signaling molecules mediating corticotropin-releasing hormone-R1alpha-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol Endocrinol. 2006;20:3179–3195. doi: 10.1210/me.2006-0255. [DOI] [PubMed] [Google Scholar]
- 92.Chandras C, Koutmani Y, Kokkotou E, Pothoulakis C, Karalis KP. Activation of phosphatidylinositol 3-kinase/protein kinase B by corticotropin-releasing factor in human monocytes. Endocrinology. 2009;150:4606–4614. doi: 10.1210/en.2008-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olianas MC, Lampis G, Onali P. Human Y-79 retinoblastoma cells express functional corticotropin- releasing hormone receptors. Brain Res. 1992;593:304–306. doi: 10.1016/0006-8993(92)91324-8. [DOI] [PubMed] [Google Scholar]
- 94.Olianas MC, Lampis G, Onali P. Human Y-79 retinoblastoma cells exhibit specific corticotropin-releasing hormone binding sites. J Neurochem. 1995;64:402–407. doi: 10.1046/j.1471-4159.1995.64010402.x. [DOI] [PubMed] [Google Scholar]
- 95.Hauger RL, Dautzenberg FM, Flaccus A, Liepold T, Spiess J. Regulation of corticotropin-releasing factor receptor function in human Y-79 retinoblastoma cells: rapid and reversible homologous desensitization but prolonged recovery. J Neurochem. 1997;68:2308–2316. doi: 10.1046/j.1471-4159.1997.68062308.x. [DOI] [PubMed] [Google Scholar]
- 96.Gutknecht E, Hauger RL, Van der Linden I, Vauquelin G, Dautzenberg FM. Expression, binding, and signaling properties of CRF2(a) receptors endogenously expressed in human retinoblastoma Y79 cells: passage-dependent regulation of functional receptors. J Neurochem. 2008;104:926–936. doi: 10.1111/j.1471-4159.2007.05052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, Dautzenberg FM. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Mol Pharmacol. 2009;75:648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- 98.Dautzenberg FM, Hauger RL. G-protein-coupled receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology. 2001;40:394–407. doi: 10.1016/s0028-3908(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 99.Radulovic M, Hippel C, Spiess J. Corticotropin-releasing factor (CRF) rapidly suppresses apoptosis by acting upstream of the activation of caspases. J Neurochem. 2003;84:1074–1085. doi: 10.1046/j.1471-4159.2003.01594.x. [DOI] [PubMed] [Google Scholar]
- 100.Post A, Ohl F, Almeida OF, Binder EB, Rucker M, Welt S, Binder E, Holsboer F, Sillaber I. Identification of molecules potentially involved in mediating the in vivo actions of the corticotropin-releasing hormone receptor 1 antagonist, NBI30775 (R121919) Psychopharmacology (Berl) 2005;180:150–158. doi: 10.1007/s00213-004-2134-x. [DOI] [PubMed] [Google Scholar]
- 101.Halasz I, Rittenhouse PA, Zorrilla EP, Redei E. Sexually dimorphic effects of maternal adrenalectomy on hypothalamic corticotrophin-releasing factor, glucocorticoid receptor and anterior pituitary POMC mRNA levels in rat neonates. Brain Res Dev Brain Res. 1997;100:198–204. doi: 10.1016/s0165-3806(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 102.Chatzaki E, Minas V, Zoumakis E, Makrigiannakis A. CRF receptor antagonists: utility in research and clinical practice. Curr Med Chem. 2006;13:2751–2760. doi: 10.2174/092986706778521977. [DOI] [PubMed] [Google Scholar]
- 103.Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 104.Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, Rademaker MT, Richards M. Urocortin 2 infusion in human heart failure. Eur Heart J. 2007;28:2589–2597. doi: 10.1093/eurheartj/ehm340. [DOI] [PubMed] [Google Scholar]
- 105.Davis ME, Pemberton CJ, Yandle TG, Lainchbury JG, Rademaker MT, Nicholls MG, Frampton CM, Richards AM. Effect of urocortin 1 infusion in humans with stable congestive cardiac failure. Clin Sci (Lond) 2005;109:381–388. doi: 10.1042/CS20050079. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Li S. Corticotropin-releasing factor family and its receptors: tumor therapeutic targets? Biochem Biophys Res Commun. 2007;362:785–788. doi: 10.1016/j.bbrc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 107.Nishi Y, Takayanagi R, Yanase T, Haji M, Hasegawa Y, Nawata H. Inhibin-like immunoreactivity produced by the adrenal gland is circulating in vivo. Fukuoka Igaku Zasshi. 2000;91:8–20. [PubMed] [Google Scholar]
- 108.Preeyasombat C, Sirikulchayanonta V, Mahachokelertwattana P, Sriphrapradang A, Boonpucknavig S. Cushing’s syndrome caused by Ewing’s sarcoma secreting corticotropin releasing factor-like peptide. Am J Dis Child. 1992;146:1103–1105. doi: 10.1001/archpedi.1992.02160210105034. [DOI] [PubMed] [Google Scholar]
- 109.Makrigiannakis A, Margioris AN, Chatzaki E, Zoumakis E, Chrousos GP, Gravanis A. The decidualizing effect of progesterone may involve direct transcriptional activation of corticotrophin-releasing hormone from human endometrial stromal cells. Mol Hum Reprod. 1999;5:789–796. doi: 10.1093/molehr/5.9.789. [DOI] [PubMed] [Google Scholar]