Abstract
Activation of peroxisome proliferator-activated receptor (PPAR) δ by GW501516, a specific PPARδ ligand, significantly inhibited interleukin (IL)-1β-induced proliferation and migration of vascular smooth muscle cells (VSMCs). This effect of GW501516 was dependent on transforming growth factor-β, and was mediated through the up-regulation of IL-1 receptor antagonist. The inhibitory effect of GW501516 on VSMC proliferation was associated with cell cycle arrest at the G1 to S phase transition, which was accompanied by the induction of p21 and p53 along with decreased cyclin-dependent kinase 4 expression. Inhibition of cell migration by GW501516 was associated with the down-regulation of matrix metalloproteinase (MMP)-2 and MMP-9 in IL-1β-treated VSMCs. Inhibition of extracellular signal-regulated kinase significantly reduced the GW501516-mediated inhibition of IL-1β-stimulated VSMC proliferation. These results suggest that PPARδ plays an important role in the pathophysiology of diseases associated with the proliferation and migration of VSMCs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0328-4) contains supplementary material, which is available to authorized users.
Keywords: Cell proliferation, IL-1β, PPARδ, TGF-β1, Vascular smooth muscle cells
Introduction
Peroxisome proliferator-activated receptors (PPARs) are a family of ligand-activated nuclear transcriptional factors that have been implicated in many pathophysiological processes, including vascular disorders [1–4]. The three major PPAR isoforms, α (NR1C1), δ (NR1C2), and γ (NR1C3), are encoded by distinct genes. PPARs form transcriptionally active heterodimeric complexes with the retinoid × receptor and regulate gene expression by binding to a specific recognition sequence, termed the PPAR response element (PPRE) [5]. PPARs exhibit different patterns of tissue expression and biological activities, which suggests that they have distinct functions in different cell types [6]. Activation of PPAR α and γ has been demonstrated to interfere atherosclerotic processes by inhibiting proliferation of vessel cells induced by chemokines, angiotensin II, and injury [7–9]. Although PPARδ has been reported to be abundantly expressed in vascular smooth muscle cells (VSMCs), its role in vascular pathophysiology is not clearly understood. Previous studies from different investigators reported somewhat contradictory effects of PPARδ on the proliferation of VSMCs. An earlier study by Zhang et al. [10] reported that PPARδ is up-regulated in neointima during vascular lesion formation and promotes the post-confluent proliferation of VSMCs in vitro. In contrast, recent report indicates that activation of PPARδ inhibits the proliferation of the VSMCs via suppression of platelet-derived growth factor-stimulated cell cycle progression [11]. Consistent with this inhibitory effect of PPARδ on VSMC proliferation, several lines of evidence also suggested that overexpression of prostacyclin synthase, which produces the endogenous PPARδ ligand prostacyclin I2, inhibits VSMC proliferation [12–14]. However, each of these studies were conducted under different physiological environments and/or stimulation. Thus, results of one biological condition may not be extrapolated into different conditions to draw the same conclusions.
VSMCs are the major cell type in blood vessel walls, and their proliferation and migration play important roles in the development of advanced lesions associated with atherosclerosis and restenosis [15, 16]. The maintenance of vascular integrity is one of the major functions of VSMCs, and within the normal vessel wall, the regulation of VSMC proliferation and migration is tightly controlled by organized balance among several humoral mediators, including cell cycle regulators and cytokines [17, 18]. In particular, one of the biologically active mediators synthesized and secreted by VSMCs is interleukin (IL)-1β. IL-1β was reported to act on the VSMCs through both paracrine and autocrine mechanisms to bring about their proliferation and inflammation, which may contribute to intimal hyperplasia and lesion progression in atherosclerosis [19]. Another biological factor involved in the regulation of vascular homeostasis is a naturally occurring antagonist called interleukin-1 receptor antagonist (IL-1Ra). Thus, the relationship between IL-1β and IL-1Ra may play an important role(s) in the regulation of vascular homeostasis including VSMC proliferation [20, 21]. For example, the balance between IL-1β and IL-1Ra has been reported to influence atherosclerosis development in human atherosclerotic arteries [22, 23]. In addition, reduced levels of IL-1Ra led to aggravation of murine atherosclerosis [23], and patients with polymorphism in the IL-1Ra intron 2, which is known to increase IL-1Ra levels and possibly the IL-1Ra:IL-1β ratio, were associated with reduced coronary atherosclerosis [24]. Although it has been well documented that IL-1β and IL-1Ra play a central role in vascular pathogenesis through modulation of pro-atherogenic action including VSMC proliferation [25], the underlying molecular mechanisms that regulate the balance between the actions of IL-1Ra and IL-1β in atherosclerosis are not clearly understood.
Previous studies demonstrated that activation of PPARα and γ potentiates IL-1Ra production in cells such as synovial fibroblasts, chondrocytes, and THP-1 that were stimulated by cytokine or phorbol ester [26–28]. In IL-1β-treated chondrocytes, this action was mediated via PPRE, which is located in the IL-1Ra promoter region. Several lines of evidence indicate that PPARδ may play an important role in inflammatory responses in vascular disorders that involve proliferation and migration of vascular cells [29–32]. Therefore, we examined the effects of ligand-activated PPARδ on IL-1β-stimulated VSMC proliferation and migration. In this study, we show that activation of PPARδ by GW501516 inhibits proliferation and migration of VSMCs induced by IL-1β, and that it exerts its inhibitory effects by regulating IL-1β-induced expression of IL-1Ra and cell cycle regulatory proteins in a process that requires transforming growth factor (TGF)-β1.
Materials and methods
Materials
2-[2-Methyl-4-([4-methyl-2-[4-(trifluoromethyl)phenyl)-1,3-thiazol-5-yl]methyl sulfanyl]phenoxy-acetic acid (GW501516), TβR-I inhibitor, PD98059 (2′-Amino-3′-methoxyflavone), SP600125 [Anthra (1,9-cd)pyrazol-6(2H)-one], and SB203580 [4-(4-Fluorophenyl)-2-(4-methylsulfonylphenyl)-5-(4-pyridyl) imidazole] were obtained from Calbiochem (La Jolla, CA). Mouse monoclonal anti-matrix metalloproteinase (MMP)-1, goat polyclonal anti-MMP-2, rabbit polyclonal anti-MMP-9, donkey anti-goat IgG-HRP, goat anti-rabbit IgG-HRP, and goat anti-mouse IgG-HRP antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal anti-TGF-β antibody and IL-1β were purchased from R&D systems (Minneapolis, MN). Polyclonal rabbit antibodies specific for p53, p21, CDK2, cyclin D1, extracellular signal-regulated kinase (ERK), phospho-ERK, c-Jun NH2-terminal kinase (JNK), phospho-JNK, p38, and phospho-p38, and mouse monoclonal antibodies specific for CDK4 and cyclin E, were obtained from Cell Signaling (Beverly, MA). Other reagents were of the highest grade available.
Cell culture
Rat aortic VSMCs were isolated from free-floating explants of aorta, as described previously [30]. Briefly, the thoracic aorta was dissected from adult male Sprague–Dawley rats, cut longitudinally, and then the endothelial cells were removed. The isolated medial membrane was cut into small pieces and incubated for 1 day. After supplementation with fresh medium, the tissue was incubated for an additional 2–3 days. VSMCs were removed by trypsinization and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 100 U/ml penicillin and 100 μg/ml streptomycin, supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C in an atmosphere of 95% air and 5% CO2.
Small interfering RNA-mediated gene silencing
Cells were seeded into 100-mm culture dishes 18–24 h prior to transfection. Cells were transfected with a control small interfering (si)RNA, rat PPARδ siRNA, or IL-1Ra siRNA (Ambion, Austin, TX) in serum free medium using Welfect-Q (WelGENE, Daegu, Korea). Following incubation for 6 h, the transfection medium was replaced with fresh medium, and then the cells were allowed to incubate for an additional 24–38 h, at which point they were treated for indicated time with reagents. The effect of gene silencing was determined by immunoblot and northern blot analysis, and by MTT and cell migration assays.
Cell proliferation assay
VSMCs transfected with specific siRNAs were seeded into 24-well plates and then synchronized to quiescence by serum starvation for 24 h. Following pretreatment with GW501516 for 24 h in fresh media containing 5% FBS, the cells were treated with IL-1β and then incubated for an additional 24 h. Throughout all the experiments, GW501516 was pretreated for 24 h to elucidate fully the transcriptional activity of PPARδ as shown in our previous reports [30, 32]. For the MTT assay, MTT solution (final 0.1 mg/ml) was added to the culture medium, and then cells were incubated for 4 h. After removal of the medium, formazan crystals, formed after the reduction of MTT by mitochondrial dehydrogenases, were solubilized in acidified isopropanol and quantified by spectroscopy at 570 nm. For analysis of cell proliferation by [3H]-thymidine incorporation, VSMCs were treated with the indicated reagents, as described above, except that they were pulsed with 1.0 μCi/mL of [3H]-thymidine for the last 6 h of incubation. After washing with ice-cold phosphate-buffered saline (PBS), cellular DNA was precipitated with cold 10% trichloroacetic acid and then solubilized in a mixture of 0.5 N NaOH and 0.5% SDS. Thymidine incorporation was determined using a scintillation counter (Beckman Instruments, CA).
Cell migration assay
Cells (monolayers) were grown to confluence on 6-mm tissue culture plates and then pretreated with GW501516 for 24 h. The monolayers were scraped with a sterile single-edged razor blade and then the cells were incubated in fresh medium containing IL-1β for an additional 96 h. Cells were fixed and stained with trypan blue. The number of cells that migrated across the wound edge was counted under a microscope.
Immunoblot analysis
Cells treated with the indicated reagents were washed in ice-cold PBS and then lysed in PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seoul, Korea). An aliquot of the cell lysate was subjected to SDS-polyacrylamide gel electrophoresis and then proteins were transferred onto a Hybond-P+ polyvinylidene difluoride membrane (Amersham Biosciences UK, UK). The membrane was probed with the indicated antibodies, as described previously [32].
Cell cycle analysis
VSMCs seeded onto 100-mm culture dishes were incubated for 24 h and then synchronized to quiescence by serum starvation for 24 h. Fresh media containing GW501516 was added to the cells, followed by incubation for 24 h. Finally, IL-1β was added to the medium and the cells were incubated for an additional 24 h. The cells were trypsinized and collected by centrifugation at 1,000g for 5 min. Cell pellets were fixed in 70% (v/v) ethanol at −70°C for 1 h, then washed twice with ice-cold PBS and resuspended in propidium iodide staining solution [5 μg/ml propidium iodide, 0.7 μg/ml ribonuclease A, 10 mM Tris (pH 7.0), 1 mM NaCl, and 0.1% NP-40]. Following incubation in the dark for 30 min at room temperature, cellular DNA content was measured based on the level of propidium iodide incorporation, and cell cycle profiles were determined using a FACSCalibur™ system (Becton–Dickinson Biosciences, Franklin Lakes, NJ) and CellQuest Pro™ software.
Northern blot analysis
Aliquots of total RNA were heat-denatured at 65°C for 15 min in gel-running buffer (40 mM MOPS, 10 mM sodium acetate, and 1 mM EDTA, pH 7.0) containing 50% formamide, and then subjected to electrophoresis on a 1% agarose gel containing 2.2 M formaldehyde. Size-fractionated RNA was transferred onto a Hybond-N+ nylon membrane (Amersham Biosciences UK) overnight by capillary action, and then hybridized with a 32P-labeled IL-1Ra probe at 68°C in QuikHyb solution (Stratagene, La Jolla, CA). The membrane was washed and then analyzed using a Fuji BAS-2500 Bioimaging Analyzer (Tokyo, Japan). The blots were stripped and rehybridized with a 32P-labeled GAPDH cDNA probe. The cDNA probe for IL-1Ra was generated by PCR using primers that were specific for nucleotides 5–541 of IL-1Ra.
Statistical analysis
Data are expressed as means ± SE. Statistical significance was determined by ANOVA with post hoc Bonferroni test. A value of P < 0.05 was considered statistically significant.
Results
TGF-β-dependent inhibition of IL-1β-induced proliferation of VSMCs by PPARδ ligand
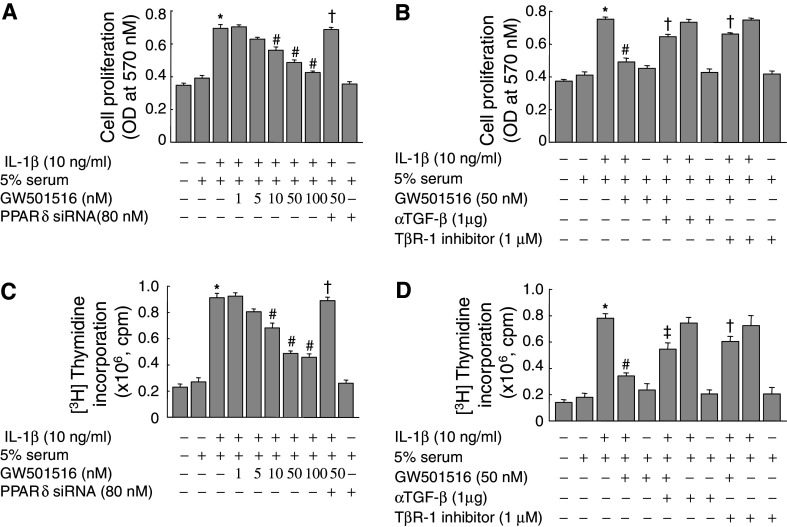
Exposure of VSMCs to IL-1β for 24 h resulted in a significant increase in cell proliferation, as assessed by MTT (Fig. 1a, b) and [3H]-thymidine incorporation assays (Fig. 1c, d). Pretreatment with GW501516, a specific ligand of PPARδ [33], significantly suppressed the IL-1β-induced proliferation of VSMCs in a dose-dependent manner. Similar results were also observed in the VSMCs when L-165041, another specific ligand for PPARδ [34], was used (data not shown). To investigate the role of PPARδ in the suppression of IL-1β-induced proliferation, cells were treated with an siRNA against PPARδ (Supplemental Fig. 1), and then the effect of GW501516 was examined. siRNA-mediated down-regulation of PPARδ nearly abolished the GW501516-mediated suppression of cell proliferation (Fig. 1a, c). The GW501516-induced decrease in VSMC proliferation was also significantly reduced in the presence of a neutralizing antibody against TGF-β or a TGF-β receptor-1 (TβR-1) inhibitor (Fig. 1b, d). These results suggested that the PPARδ-mediated suppression of VSMC proliferation is mediated by TGF-β.
Fig. 1.
Effect of GW501516 on the IL-1β-stimulated proliferation of VSMCs. Cells transfected with a PPARδ siRNA for 38 h were pretreated with GW501516 for 24 h in the presence or absence of a neutralizing anti-TGF-β antibody or a TβR-1 inhibitor and then incubated with IL-1β for 24 h in DMEM containing 5% FBS. VSMC proliferation was determined by MTT (a,b) and [3H]-thymidine incorporation (c,d) assays. Data represents mean ± SE (n = 4). *P < 0.01 versus 5% serum-treated group; # P < 0.01 versus IL-1β-treated group; † P < 0.01, ‡ P < 0.05 versus IL-1β + 50 nM GW501516-treated group
Activation of PPARδ inhibits IL-1β-induced G1–S phase cell cycle progression in a TGF-β-dependent manner
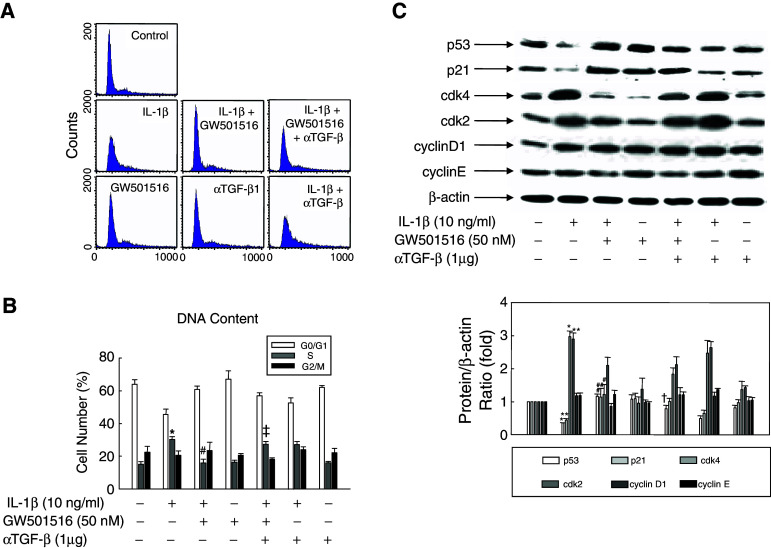
To evaluate the functional role of PPARδ in IL-1β-induced cell proliferation, we examined the effect of GW501516 on cell cycle progression in the presence of IL-1β. VSMCs were synchronized by serum starvation for 24 h, and then pretreated with GW501516, or DMSO as a control, followed by incubation with IL-1β. The number of cells that accumulated in the G0/G1 phase was significantly decreased by treatment with IL-1β alone, and pre-treatment with GW501516 significantly increased the G0/G1 cell population (Fig. 2a, b). In contrast, the proportion of cells entering S phase was increased by IL-1β and significantly declined upon pre-treatment with GW501516. This decrease in cells entering S phase induced by GW501516 was almost completely abolished by treatment with an anti-TGF-β antibody. These results indicated that the activation of PPARδ by GW501516 induces cell cycle arrest in VSMCs, and that this effect is mediated by TGF-β.
Fig. 2.
Effect of GW501516 on cell cycle progression of VSMCs. Cells pretreated with GW501516 and/or a neutralizing anti-TGF-β antibody for 24 h were incubated in DMEM containing 5% FBS and IL-1β for 24 h, and then stained with propidium iodide. DNA content was analyzed by flow cytometry and is presented as relative fluorescence. Representative histogram (a) and plots of cell cycle distribution (b) are shown as the mean ± SE (n = 4). Cells were treated as described for (a) and (b), and the expression of cell cycle-related proteins was analyzed by immunoblot using the indicated specific antibodies (c). Representative blots and densitometric measurements are shown as the mean ± SE (n = 4). *P < 0.01, **P < 0.05 versus 5% serum-treated group; # P < 0.01, ## P < 0.05 versus IL-1β-treated group; † P < 0.01 versus IL-1β + GW501516-treated group
Cell cycle progression is tightly regulated through a complex network of regulatory molecules, including cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors [35]. To determine whether any of these molecules played a role in GW501516-induced cell cycle arrest of IL-1β-treated VSMCs, we examined the expression levels of key cell cycle regulatory proteins using immunoblot analysis. As shown in Fig. 2c, IL-1β treatment caused a marked decrease in the levels of p53 and p21, which are key regulators of progression through the G1–S transition checkpoint [36, 37]. Treatment with GW501516 markedly inhibited this effect of IL-1β on the expression levels of both proteins. We observed a similar effect of GW501516 on the expression of CDK 2 and 4. The effect of GW501516 on the expression of cell cycle regulatory proteins was nearly abolished by treatment with an anti-TGF-β antibody. These results provided evidence that the GW501516-induced activation of PPARδ regulates the expression of cell cycle regulatory proteins in a TGF-β-dependent manner.
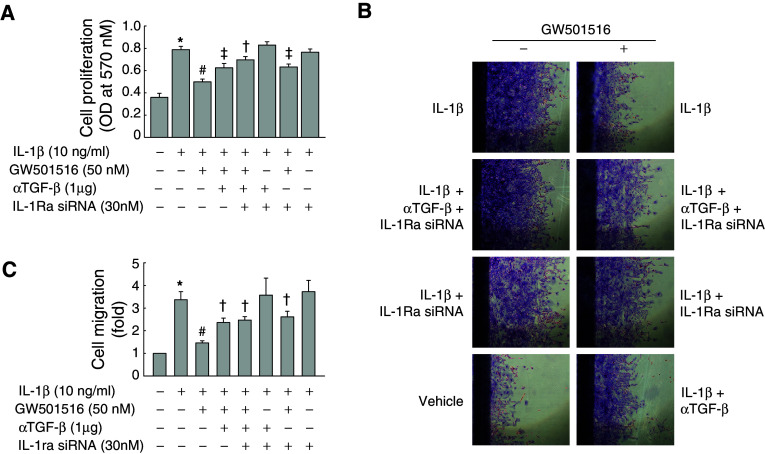
Inhibition of the IL-1β-induced migration of VSMCs by PPARδ is mediated by TGF-β
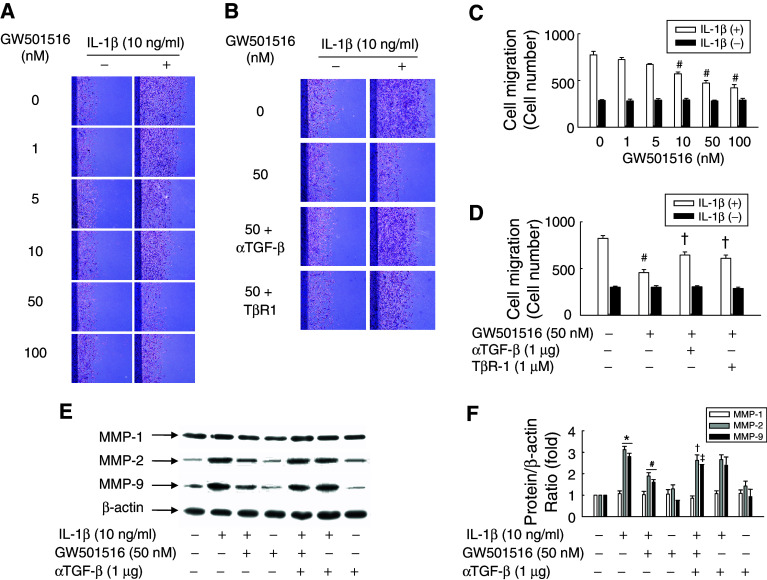
To determine the effect of GW501516 on IL-1β-induced VSMC migration, we carried out an in vitro scrape wounding assay. VSMCs grew actively after plating, typically reaching confluence after 4 days, and the percentage of cells undergoing active proliferation conversely declined to minimum at day 4 when determined by [3H]-thymidine incorporation (Electronic supplementary material, ESM, Fig. 2). Using the proliferation index described above as a reference basis, we found that GW501516 significantly attenuated the IL-1β-induced migration of VSMCs in a dose-dependent manner (Fig. 3a, c). To investigate the role of TGF-β in the PPARδ-mediated inhibition of VSMC migration, cells were treated with an anti-TGF-β antibody or TβR-1 inhibitor, and then IL-1β-induced cell migration was assessed. The activation of PPARδ significantly attenuated IL-1β-induced cell migration, and this effect was significantly decreased in the presence of an anti-TGF-β antibody or TβR-1 inhibitor (Fig. 3b, d). These results clearly indicated that PPARδ is involved in the inhibition of IL-1β-induced cell migration through a TGF-β-dependent mechanism.
Fig. 3.
Effect of GW501516 on the IL-1β-stimulated migration of VSMCs. Cell monolayers pretreated with GW501516 for 24 h in the presence or absence of a neutralizing anti-TGF-β antibody or a TβR-1 inhibitor were scraped with a razor blade, and then incubated with IL-1β in fresh medium containing 5% FBS. After incubation for 96 h, the number of cells that migrated across the wound was counted. Representative photographs (a,b) and cell number (c,d) are shown as the mean ± SE (n = 6). Cells pretreated with GW501516 and/or neutralizing anti-TGF-β antibody for 24 h were incubated with IL-1β for 24 h. The expression levels of MMPs were determined by immunoblot using the indicated antibodies (e) and quantified (f). Representative blots and densitometric measurements are shown as the mean ± SE (n = 4). *P < 0.01 versus 5% serum-treated group; # P < 0.01 versus IL-1β-treated group; † P < 0.01, ‡ P < 0.05 versus IL-1β + GW501516-treated group
Activation of PPARδ down-regulates the expression of MMP-2 and -9, but not MMP-1, in IL-1β-stimulated VSMCs
We next examined the effect of PPARδ activation on the expression of MMPs, which are key regulators of VSMC proliferation and migration [38]. Cells were stimulated with IL-1β in the presence or absence of GW501516. As shown in Fig. 3e and f, GW501516 inhibited the IL-1β-induced expression of MMP-2 and MMP-9, but not MMP-1. Since TGF-β has also been shown to modulate the expression of MMPs [39], we investigated the effect of TGF-β inhibition on the ability of PPARδ to inhibit IL-1β-induced MMP-2 and MMP-9 expression. Treatment of cells with an anti-TGF-β antibody markedly attenuated the effect of GW501516 on MMP-2 and MMP-9 expression in response to IL-1β. These results suggested that MMP-2 and MMP-9 are involved in the TGF-β-dependent inhibition of VSMC migration by PPARδ.
Activation of PPARδ potentiates the IL-1β-induced expression of IL-1Ra
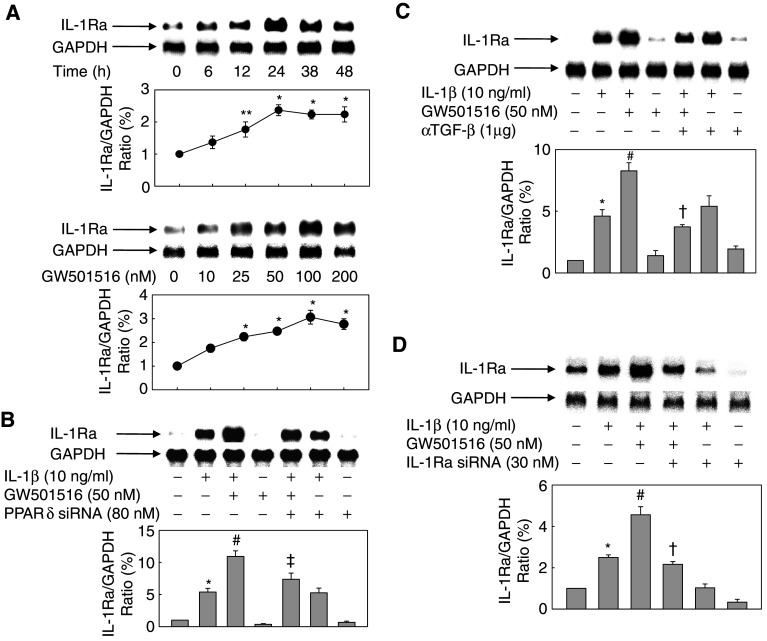
To elucidate the mechanism of inhibition of IL-1β-induced cell proliferation and migration by PPARδ, we examined the effect of GW501516 on the expression of IL-1Ra, a well-known natural antagonist of IL-1β [40]. Treatment of VSMCs with GW501516 in the presence of IL-1β resulted in a significant increase in the levels of IL-1Ra mRNA transcripts in a concentration- and time-dependent manner (Fig. 4a). However, GW501516 did not affect the expression level of IL-β1, a well-known activator of IL-1Ra expression (data not shown). To verify the role of PPARδ in the potentiation of IL-1Ra expression, we examined the effect of GW501516 on cells that were treated with an siRNA against PPARδ. siRNA-mediated down-regulation of PPARδ suppressed the effect of GW501516 on the expression of IL-1Ra (Fig. 4b). The mRNA expression levels of IL-1Ra were also reduced in the presence of a neutralizing antibody against TGF-β (Fig. 4c). In addition, treatment of cells with an siRNA against IL-1Ra almost completely abolished the IL-1β- and/or GW501516-induced expression of IL-1Ra mRNA (Fig. 4d). These results indicated that PPARδ potentiates the effect of IL-1β on IL-1Ra expression, and that this potentiation effect is mediated by TGF-β.
Fig. 4.
Effect of GW501516 on the IL-1β-induced expression of IL-1Ra in VSMCs. a Cells were incubated with 100 nM GW501516 for the indicated periods of time (upper panels) or for 24 h with the indicated concentrations of GW501516 (lower panels). b Cells transfected with a PPARδ siRNA for 38 h were pretreated with GW501516 for 24 h and then incubated with IL-1β for 24 h. c Cells pretreated with GW501516 and/or a neutralizing anti-TGF-β antibody for 24 h were incubated with IL-1β for 24 h. d Cells transfected with an IL-1Ra siRNA for 24 h were pretreated with GW501516 for 24 h and then incubated with IL-1β for 24 h. Northern blot analysis was performed using an IL-1Ra cDNA probe. Representative blots and densitometric measurements are shown as the mean ± SE (n = 4). *P < 0.01, **P < 0.05 versus 5% serum-treated group; # P < 0.05 versus IL-1β-treated group; † P < 0.01, ‡ P < 0.05 versus IL-1β + GW501516-treated group
PPARδ-induced upregulation of IL-1Ra mediates the inhibitory actions of GW501516 on IL-1β-induced VSMC proliferation and migration
To examine the role of IL-1Ra in the inhibition of IL-1β-induced proliferation and migration by GW501516, cells were treated with an siRNA against IL-1Ra, and then the effect of GW501516 on cell proliferation and migration was examined (Fig. 5). siRNA-mediated down-regulation of IL-1Ra inhibited the ability of GW501516 to suppress VSMC proliferation and migration (Fig. 5). The decrease in proliferation and migration of VSMCs induced by GW501516 was partially reversed in the presence of a neutralizing antibody against TGF-β. The effect of combined treatment with IL-1Ra siRNA and anti-TGF-β antibody on VSMC proliferation and migration was similar to that of siRNA or antibody alone. We performed the same experiments using human aortic vascular smooth muscle cells, and observed similar results (ESM, Fig. 3). These results suggested that both TGF-β and IL-1Ra independently mediate the action of PPARδ on the proliferation and migration of VSMCs.
Fig. 5.
Effect of IL-1Ra upregulation by GW501516 on the proliferation and migration of VSMCs. a Cells transfected with an IL-1Ra siRNA for 24 h were pretreated with GW501516 and/or a neutralizing anti-TGF-β antibody for 24 h and then incubated with IL-1β for 24 h in DMEM containing 5% FBS. VSMC proliferation was determined by MTT assay (n = 4). b,c Serum-starved cells were transfected with an IL-1Ra siRNA for 24 h, and then pretreated with GW501516 and/or a neutralizing anti-TGF-β antibody for 24 h. Cell monolayers were scraped with a razor blade, and then the cells were incubated with IL-1β in fresh medium containing 5% FBS. After incubation for 96 h, the number of cells that migrated across the wound was counted. Representative photographs (b) and cell number (c) are shown as the mean ± SE (n = 4). *P < 0.01 versus 5% serum-treated group; # P < 0.01 versus IL-1β-treated group; † P < 0.01, ‡ P < 0.01 versus IL-1β + GW501516-treated group
ERK- and JNK-mediated signaling cascades participate in the PPARδ-mediated regulation of cell proliferation
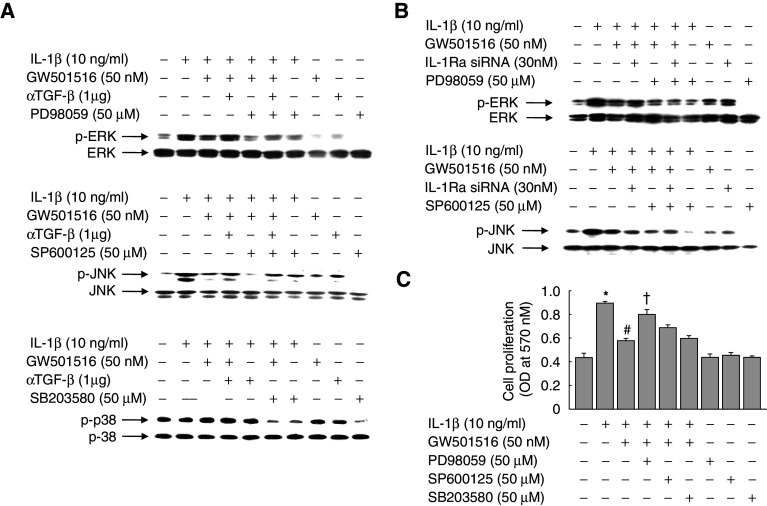
To identify the intracellular signaling cascades that are activated by IL-1β, we examined the effect of IL-1β on the three intracellular mitogen-activated protein kinase (MAPK) cascades, ERK, JNK, and p38, using antibodies that specifically and selectively recognize the activated, phosphorylated forms of ERK, JNK, and p38. VSMCs were treated with IL-1β for different periods of time, and then cell lysates were examined by immunoblot analysis using phospho-specific antibodies. In parallel, total enzyme levels were analyzed using kinase-specific antibodies. IL-1β alone activated the ERK and JNK cascades following a period of delay after treatment, but had no effect on the p38 pathway (ESM, Fig. 4). The IL-1β-induced activation of ERK and JNK was markedly inhibited in the presence of GW501516, and treatment with an anti-TGF-β antibody reversed the effects of GW501516 (Fig. 6a). However, the addition of siRNA against IL-1Ra markedly reversed this GW501516-mediated inhibition of ERK, but not JNK, indicating that the GW501516-induced activation of JNK signaling pathway is not directly related to the anti-proliferative effects of GW501516 via upregulation of IL-1Ra in VSMCs (Fig. 6b).
Fig. 6.
Role of MAPKs on the IL-1β-stimulated proliferation of VSMCs. a Cells were pretreated with a neutralizing anti-TGF-β antibody and/or GW501516 for 24 h and then incubated in DMEM containing 5% FBS in the presence or absence of the indicated reagents for 24 h. b Cells transfected with a IL-1Ra siRNA for 24 h were pretreated with GW501516 for 24 h, and then incubated in DMEM containing 5% FBS in the presence or absence of the indicated reagents for 24 h. Proteins were subjected to immunoblot analysis using phospho-specific antibodies; total kinase levels were analyzed in parallel immunoblots. c Cells pretreated with GW501516 for 24 h were incubated in DMEM containing 5% FBS in the presence or absence of the indicated reagents for 24 h, and proliferation was determined by MTT assay. Data represents the mean ± SE (n = 4). *P < 0.01 versus 5% serum-treated group; # P < 0.01 versus IL-1β-treated group; † P < 0.01 versus IL-1β + GW501516-treated group
To clarify which signaling pathways were involved in the PPARδ-mediated inhibition of IL-1β-induced cell proliferation, we examined the effects of specific inhibitors of the three MAPK cascades. As shown in Fig. 6c, the GW501516-mediated inhibition of IL-1β-induced cell proliferation was significantly attenuated by PD98059, but not SP600125 and SB203580; further evidence that the ERK-mediated signaling pathway is involved in the GW501516-mediated inhibition of cell proliferation. The viability of cells treated with PD98059 (50 μM), SP600125 (50 μM), or SB203580 (50 μM) was >90%, as determined by MTT assay (data not shown).
The role of TGF-β1 in the suppressive effect of PPARδ on IL-1β-induced VSMC proliferation and migration was further confirmed by the exogeneous treatment of TGF-β1 in cultured VSMCs. The addition of TGF-β1 significantly inhibited IL-1β-induced proliferation and migration of VSMCs, and this effect was reduced in the presence of either anti-TGF-β antibody or SB431542, a specific ALK5 receptor antagonist (ESM, Fig. 5). These results strongly suggest functional involvement of TGF-β1 in PPARδ-mediated signaling pathway. However, the effects of TGF-β1 treatment on the suppression of IL-1β-induced VSMC proliferation and migration were not as potent as those of GW501516 treatment. And, together with the fact that GW501516-mediated inhibition was not fully recovered by neutralization of TGF-β, we suggest a hypothesis that activation of PPARδ induces not only the TGF-β signaling pathway but also other cellular transduction pathways that contribute to the inhibition of IL-1β-induced proliferation and migration of VSMCs.
Discussion
The PPAR nuclear receptors play important roles in the regulation of gene expression during vascular homeostasis [10, 41–44]. While PPARα and PPARγ have been implicated in vascular disorders, little is known about the role of PPARδ in VSMCs. Here, this study provides evidence that activation of PPARδ by a specific ligand, GW501516, attenuates the stimulatory effects of IL-1β on proliferation and migration of VSMCs through the TGF-β- and IL-1Ra-mediated processes. PPARδ activation also resulted in the inactivation of both ERK and JNK, but not p38, and this inactivation of the kinases was dependent on TGF-β. The order of sequential events that leads to the regulation of signaling cascades is not currently evident, but on the basis of a transcriptional regulatory function of PPARδ, the ligand-bound nuclear receptor may modulates expression of its target genes which then cause activation of TGF-β signaling pathways to inactivate ERK and JNK pathways. However, the present study suggests that PPARδ-mediated inhibition of IL-1β-induced VSMC proliferation may be mediated through regulation of ERK signaling pathway rather than JNK pathway, because inhibition of ERK significantly reduced the effects of GW510156 on IL-1β-induced VSMC proliferation, whereas the effect of JNK had minimal impact. As opposed to previous studies demonstrating the involvement of p38 pathway in the IL-1β-induced up-regulation of vascular endothelial growth factor [45], the p38 pathway seems to be dispensable for the GW501516-mediated inhibition of IL-1β-induced VSMC proliferation. Accordingly, the member of the MAP kinases in signaling cascades mediating the vascular functions may differ depending upon the target molecules and cell types.
Activation of PPARδ significantly inhibited IL-1β-induced proliferation of VSMCs in a process mediated by TGF-β. Although the beneficial effect of therapeutic inhibition of VSMC proliferation in atherosclerosis is unclear, recent observations suggest the therapeutic potential of antiproliferative therapy for vasculoproliferative disorders [46]. In fact, recent report demonstrated that activation of PPARδ by L-165041 moderately reduces the neointima formation after balloon injury and attenuates expression of proliferation cell nuclear antigen, indicating a proliferation-inhibitory effect in the region of arterial injury [11]. In this respect, PPARδ-dependent inhibition of VSMC proliferation would elicit beneficial effects in atherosclerotic progression. On the other hand, PPARδ is induced in response to PDGF and overexpression of PPARδ in VSMCs causes promotion of the post-confluent cell proliferation [10], emphasizing the complexity of this system in vivo. Further studies are necessary to clarify the exact roles of VSMC proliferation in the atherosclerotic lesion development and progression.
Of particular note is that activated PPARδ appears to play a role in the regulation of cell cycle progression. GW501516 almost completely abolished the accumulation of VSMCs in S phase in response to IL-1β. The altered cell cycle distribution and induction of cell cycle arrest in the G0/G1 phase, with correspondingly fewer cells entering S phase, in GW501516-treated cells suggests that PPARδ is involved in cell cycle inhibition in VSMCs. This finding is in line with a previous study in which a significant inhibition in cell proliferation via suppression of cell cycle progression was demonstrated in VSMCs treated with PDGF [11]. To date, a number of studies have shown that PPARδ promotes the proliferation of VSMCs and endothelial cells [10, 47], and, more frequently, cancer cells [48, 49]. However, the general effects of PPARδ on cellular proliferation are somewhat controversial, as PPARδ also inhibits cellular proliferation in other types of cells, including keratinocytes [50, 51]. Furthermore, PPARδ suppresses inflammatory responses, which can eventually result in cellular proliferation [42]. Although it is difficult to explain these discrepancies and their underlying mechanisms, it is possible that PPARδ regulates the proliferation of a variety of cells under diverse physiological or pathological conditions.
The activation of PPARδ potentiated the induction of expression of IL-1Ra by IL-1β, with corresponding inhibitory effects on VSMC proliferation and migration. The findings that IL-1Ra, which is a naturally occurring antagonist of IL-1β signaling, mediated the action of GW501516 and that the effects of GW501516 were significantly decreased by treatment of cells with an IL-1Ra siRNA suggesting that PPARδ regulates the proliferation and migration of VSMCs through the potentiation of IL-1β-stimulated IL-1Ra expression. Recently, it was reported that the activation of PPARδ stimulates production of the secreted IL-1Ra in IL-1β-stimulated skin fibroblasts [52]. In agreement with this previous report, GW501516 potentiated the expression of IL-1Ra in VSMCs that were exposed to IL-1β, whereas GW501516 alone had no effect on IL-1Ra expression. These results indicate that IL-1Ra expression is not induced at the transcriptional level by GW501516. While we cannot clearly determine the mechanism of PPARδ-mediated potentiation of IL-1Ra expression, it is possible that PPARδ modulates the stability or degradation of IL-1Ra mRNA in IL-1β-stimulated VSMCs. Further studies are needed to clarify the mechanism of IL-1Ra up-regulation by PPARδ.
GW501516 inhibited the IL-1β-induced migration of VSMCs in a TGF-β-dependent manner. This inhibitory effect of PPARδ was associated with the regulation of expression of MMP-2 and -9, which play critical roles in the migration of VSMCs through their ability to breakdown the extracellular matrix [53]. The inhibition of MMP-2 and MMP-9 by tissue inhibitor of metalloproteinases (TIMPs) overexpression [54] or synthetic peptide inhibitor [38] inhibits the migration of VSMCs in vitro, and neointima formation in vivo. Thus, the regulation of MMP activity is an important mechanism of regulation of VSMC migration. The association of TGF-β1 with cell migration has been documented in several studies. In human meningeal cells and keratinocytes, the levels of MMP-9 increase after stimulation with TGF-β1 through MAPK and Smad3 signaling pathways [55, 56]. In contrast, TGF-β suppresses tumor necrosis factor-induced MMP-9 expression through a prostaglandin E2- and cAMP-dependent mechanism in monocytes [57]. Our results showed that GW501516 markedly suppresses IL-1β-induced MMP-2 and -9 expression in a TGF-β-dependent manner. To our knowledge, this is the first demonstration that TGF-β is involved in the inhibition of IL-1β-induced migration of VSMCs by PPARδ. Although additional experiments are needed to clarify the details of the PPARδ/TGF-β interaction in the inhibition of cell migration, the current study supports the therapeutic potential of PPARδ ligands in the treatment of pathologic cardiovascular conditions, such as restenosis and atherosclerosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare (A080433), Republic of Korea.
Footnotes
H. J. Kim and M. Y. Kim contributed equally to this work.
References
- 1.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–939. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/er.20.5.649. [DOI] [PubMed] [Google Scholar]
- 4.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 5.Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/en.137.1.354. [DOI] [PubMed] [Google Scholar]
- 7.Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B. The role of PPARs in atherosclerosis. Trends Mol Med. 2002;8:422–430. doi: 10.1016/S1471-4914(02)02385-7. [DOI] [PubMed] [Google Scholar]
- 8.Graf K, Xi XP, Hsueh WA, Law RE. Troglitazone inhibits angiotensin II-induced DNA synthesis and migration in vascular smooth muscle cells. FEBS Lett. 1997;400:119–121. doi: 10.1016/S0014-5793(96)01371-3. [DOI] [PubMed] [Google Scholar]
- 9.Law RE, Meehan WP, Xi XP, Graf K, Wuthrich DA, Coats W, Faxon D, Hsueh WA. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–1905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Fu M, Zhu X, Xiao Y, Mou Y, Zheng H, Akinbami MA, Wang Q, Chen YE. Peroxisome proliferator-activated receptor delta is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J Biol Chem. 2002;277:11505–11512. doi: 10.1074/jbc.M110580200. [DOI] [PubMed] [Google Scholar]
- 11.Lim HJ, Lee S, Park JH, Lee KS, Choi HE, Chung KS, Lee HH, Park HY. PPAR delta agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2009;202:446–454. doi: 10.1016/j.atherosclerosis.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Hara S, Morishita R, Tone Y, Yokoyama C, Inoue H, Kaneda Y, Ogihara T, Tanabe T. Overexpression of prostacyclin synthase inhibits growth of vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;216:862–867. doi: 10.1006/bbrc.1995.2701. [DOI] [PubMed] [Google Scholar]
- 13.Imai H, Numaguchi Y, Ishii M, Kubota R, Yokouchi K, Ogawa Y, Kondo T, Okumura K, Murohara T. Prostacyclin synthase gene transfer inhibits neointimal formation by suppressing PPAR delta expression. Atherosclerosis. 2007;195:322–332. doi: 10.1016/j.atherosclerosis.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Yamakawa T, Tanaka S, Yamakawa Y, Kamei J, Numaguchi K, Motley ED, Inagami T, Eguchi S. Lysophosphatidylcholine activates extracellular signal-regulated kinases 1/2 through reactive oxygen species in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:752–758. doi: 10.1161/01.ATV.0000015903.02749.71. [DOI] [PubMed] [Google Scholar]
- 15.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;129:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz RS, Murphy JG, Edwards WD, Camrud AR, Vliestra RE, Holmes DR. Restenosis after balloon angioplasty. A practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
- 17.Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Rakesh K, Agrawal DK. Cytokines and growth factors involved in apoptosis and proliferation of vascular smooth muscle cells. Int Immunopharmacol. 2005;5:1487–1506. doi: 10.1016/j.intimp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 20.Bonin PD, Fici GJ, Singh JP. Interleukin-1 promotes proliferation of vascular smooth muscle cells in coordination with PDGF or a monocyte derived growth factor. Exp Cell Res. 1989;181:475–482. doi: 10.1016/0014-4827(89)90104-3. [DOI] [PubMed] [Google Scholar]
- 21.French JF, Schroeder KK, Akeson AL, Dage RC, Bowlin TL. Identification of a specific receptor for interleukin-1 in vascular smooth muscle cells: regulation by interleukin-1 and interleukin-6. Eur J Pharmacol. 1993;233:109–112. doi: 10.1016/0014-2999(93)90355-L. [DOI] [PubMed] [Google Scholar]
- 22.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y, Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 24.Olofsson PS, Sheikine Y, Jatta K, Ghaderi M, Samnegård A, Eriksson P, Sirsjö A. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1beta:interleukin-1 receptor antagonist balance in atherosclerosis. Circ J. 2009;73:1531–1536. doi: 10.1253/circj.CJ-08-1150. [DOI] [PubMed] [Google Scholar]
- 25.Sasu S, Beasley D. Essential roles of IkappaB kinases alpha and beta in serum- and IL-1-induced human VSMC proliferation. Am J Physiol Heart Circ Physiol. 2000;278:1823–1831. doi: 10.1152/ajpheart.2000.278.6.H1823. [DOI] [PubMed] [Google Scholar]
- 26.François M, Richette P, Tsagris L, Fitting C, Lemay C, Benallaoua M, Tahiri K, Corvol MT. Activation of the peroxisome proliferator-activated receptor alpha pathway potentiates interleukin-1 receptor antagonist production in cytokine-treated chondrocytes. Arthritis Rheum. 2006;54:1233–1245. doi: 10.1002/art.21728. [DOI] [PubMed] [Google Scholar]
- 27.Moulin D, Bianchi A, Boyault S, Sebillaud S, Koufany M, Francois M, Netter P, Jouzeau JY, Terlain B. Rosiglitazone induces interleukin-1 receptor antagonist in interleukin-1beta-stimulated rat synovial fibroblasts via a peroxisome proliferator-activated receptor beta/delta-dependent mechanism. Arthritis Rheum. 2005;52:759–769. doi: 10.1002/art.20868. [DOI] [PubMed] [Google Scholar]
- 28.Meier CA, Chicheportiche R, Juge-Aubry CE, Dreyer MG, Dayer JM. Regulation of the interleukin-1 receptor antagonist in THP-1 cells by ligands of the peroxisome proliferator-activated receptor gamma. Cytokine. 2002;18:320–328. doi: 10.1006/cyto.2002.1945. [DOI] [PubMed] [Google Scholar]
- 29.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Ham SA, Kim SU, Hwang JY, Kim JH, Chang KC, Yabe-Nishimura C, Kim JH, Seo HG. Transforming growth factor-beta1 is a molecular target for the peroxisome proliferator-activated receptor delta. Circ Res. 2008;102:193–200. doi: 10.1161/CIRCRESAHA.107.158477. [DOI] [PubMed] [Google Scholar]
- 31.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, Hsueh WA, Tangirala RK. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci USA. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ, Kim MY, Jin H, Kim HJ, Kang SS, Kim HJ, Lee JH, Chang KC, Hwang JY, Yabe-Nishimura C, Kim JH, Seo HG. Peroxisome proliferator-activated receptor delta regulates extracellular matrix and apoptosis of vascular smooth muscle cells through the activation of transforming growth factor-{beta}1/Smad3. Circ Res. 2009;105:16–24. doi: 10.1161/CIRCRESAHA.108.189159. [DOI] [PubMed] [Google Scholar]
- 33.Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger J, Leibowitz MD, Doebber TW, Elbrecht A, Zhang B, Zhou G, Biswas C, Cullinan CA, Hayes NS, Li Y, Tanen M, Ventre J, Wu MS, Berger GD, Mosley R, Marquis R, Santini C, Sahoo SP, Tolman RL, Smith RG, Moller DE. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J Biol Chem. 1999;274:6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- 35.Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol. 1999;287:821–828. doi: 10.1006/jmbi.1999.2640. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 37.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 38.Zempo N, Koyama N, Kenagy RD, Lea HJ, Clowes AW. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol. 1996;16:28–33. doi: 10.1161/01.atv.16.1.28. [DOI] [PubMed] [Google Scholar]
- 39.Akool el-S, Doller A, Müller R, Gutwein P, Xin C, Huwiler A, Pfeilschifter J, Eberhardt W. Nitric oxide induces TIMP-1 expression by activating the transforming growth factor beta-Smad signaling pathway. J Biol Chem. 2005;280:39403–39416. doi: 10.1074/jbc.M504140200. [DOI] [PubMed] [Google Scholar]
- 40.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 41.Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;21:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 42.Rival Y, Benéteau N, Taillandier T, Pezet M, Dupont-Passelaigue E, Patoiseau JF, Junquéro D, Colpaert FC, Delhon A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002;435:143–151. doi: 10.1016/S0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa D, Nomiyama T, Nakamachi T, Heywood EB, Stone JF, Berger JP, Law RE, Bruemmer D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ Res. 2006;98:50–59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 44.Lim S, Jin CJ, Kim M, Chung SS, Park HS, Lee IK, Lee CT, Cho YM, Lee HK, Park KS. PPARgamma gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol. 2006;26:808–813. doi: 10.1161/01.ATV.0000204634.26163.a7. [DOI] [PubMed] [Google Scholar]
- 45.Jung YD, Liu W, Reinmuth N, Ahmad SA, Fan F, Gallick GE, Ellis LM. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/A:1012291524723. [DOI] [PubMed] [Google Scholar]
- 46.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 47.Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, Tanabe T, Warner TD, Bishop-Bailey D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 48.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004;10:245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 49.Abdollahi A, Schwager C, Kleeff J, Esposito I, Domhan S, Peschke P, Hauser K, Hahnfeldt P, Hlatky L, Debus J, Peters JM, Friess H, Folkman J, Huber PE. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci USA. 2007;104:12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-beta/delta(PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin HE, Peraza MA, Billin AN, Willson TM, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor beta inhibits colon carcinogenesis. Cancer Res. 2006;66:4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 52.Chong HC, Tan MJ, Philippe V, Tan SH, Tan CK, Ku CW, Goh YY, Wahli W, Michalik L, Tan NS. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol. 2009;184:817–831. doi: 10.1083/jcb.200809028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 54.Forough R, Koyama N, Hasenstab D, Lea H, Clowes M, Nikkari ST, Clowes AW. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res. 1996;79:812–820. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto T, Takahashi S, Nakamura E, Nagaya K, Hayashi T, Fujieda K. Transforming growth factor-beta1 induces matrix metalloproteinase-9 expression in human meningeal cells via ERK and Smad pathways. Biochem Biophys Res Commun. 2009;383:475–479. doi: 10.1016/j.bbrc.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 56.Santibáñez JF, Guerrero J, Quintanilla M, Fabra A, Martínez J. Transforming growth factor-beta1 modulates matrix metalloproteinase-9 production through the Ras/MAPK signaling pathway in transformed keratinocytes. Biochem Biophys Res Commun. 2002;296:267–273. doi: 10.1016/S0006-291X(02)00864-1. [DOI] [PubMed] [Google Scholar]
- 57.Vaday GG, Schor H, Rahat MA, Lahat N, Lider O. Transforming growth factor-beta suppresses tumor necrosis factor alpha-induced matrix metalloproteinase-9 expression in monocytes. J Leukoc Biol. 2001;69:613–621. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.