Abstract
A costimulatory signal from an inducible costimulator (ICOS) of T cells plays a critical role in immunological homeostasis. This study shows that the interaction of ICOSIg and its ligand (ICOSL) on mouse bone marrow-derived dendritic cells (DCs) induces a p38-MAPK dependent elevation of interleukin 6 (IL-6). It also enhances phagocytosis and the antigen-presentation function of DCs in vitro, further favoring cell-mediated immunity in vivo. As seen for other types of costimulator molecules expressed in the T cells in the CD28 family, it is shown here for the first time that ICOS can also deliver reverse signals through its ligand to ICOSL-expressing cells. These reverse signals in turn transfer positive immunogenic information to bone marrow-derived DCs. Our work therefore provides new recognition of an ICOSL/ICOS signal pathway in immunity and also supplies more evidence that this ICOSL/ICOS signal pathway is a reasonable target for therapeutic drugs.
Keywords: Inducible costimulator, Reverse signal, Dendritic cells, Interleukin-6, p38-MAPK, Immunological homeostasis
Introduction
The coordination of immunological homeostasis through simultaneous forward and reverse signals, as well as through receptors and ligands, has been found in a number of costimulators. Among the most important and best understood of these receptor-ligand pairs are CD28/cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and B7. CD28 is a predominant immunostimulating costimulator that participates in activation, proliferation and differentiation of T cells. CTLA-4, in contrast, provides an intense inhibitory signal. Previous work has proposed that CTLA-4 decreases T cell responses by competing for B7 ligand access to CD28 and/or by delivering a signal to T cells that antagonizes or aborts a CD28 signal, generating a signal that intercepts a TCR-delivered signal [1, 2]. In recent years, Grohmann et al. found that one means of CTLA-4 function was through IFN-γ-dependent modulation of tryptophan catabolism. CTLA-4 also acted as a ligand for B7 receptor molecules that transduced reverse intracellular signals [3–5]. Two years later, the same group demonstrated that CD28 delivered immunostimulatory signals by enhancing dendritic cells (DCs), which produce more IL-6 than IFN-γ through the same B7 receptors [6]. There is also emerging evidence for ‘reverse signaling’ through PD-L proteins into DCs [7–9]. In DC in vitro cultures, soluble PD-1 decreases the expression of DC maturation markers (CD40, CD80, and CD86) and increases IL-10 production by DCs, resulting in a suppressive DC phenotype. However, the functioning and survival of DCs, the expression of genes capable of enhancing the migration of DCs to lymph nodes, and the activation of T cells are all stimulated by a unique PD-L2-specific IgM [7]. This IgM also provides CpG-maturing DCs with a different activation phenotype, generating cells that have both the strong antigen-presenting functions of mature DCs and the antigen-acquiring functions of immature DCs [8, 9]. Researchers have also found reverse signaling through GITRL after the engagement by soluble GITR, which initiates the immunoregulatory pathway of tryptophan catabolism in mouse plasmacytoid DCs [10]. Thus, bidirectional signals among costimulator receptors and ligands appear to be a universal phenomenon and also to play an important, but complicated, role in the interactions of T cells and DCs.
In 1999, it was first reported that ICOS was upregulated on activated CD4+ T and CD8+ T, and was present on effector and memory T cells. Although both Th1 and Th2 cells express ICOS during T cell differentiation, ICOS persists at higher levels in Th2 cells [11–13]. ICOS is also upregulated in activated NK cells, enhancing NK cell function [14]. ICOSL has also been detected on the surface of DCs, B-cells, macrophages, a subset of CD3+ T cells, and other cell types including endothelial cells and some epithelial cells [15]. The most recent research on the ICOS/ICOSL signal pathway has focused mainly on the impact of forward signaling on T cell function [15]. However, few studies have examined whether ICOS can also deliver reverse signals via ICOSL to ICOSL-expressing cells such as DCs, and if so, what effect this may have on function. In the present study, we made use of a soluble fusion protein of human ICOS and IgG1 Fc (ICOSIg) to explore the potential of ICOS as an agonist for the ICOSL expressed on DCs. For the first time, we were also able to provide biological proof that human ICOS can bind specifically to the ICOSL present in mouse DCs. More significantly, we discovered that ICOS can promote a p38-MAPK-dependent expression of IL-6 both in vitro and in vivo. This ability is recruited to deliver an immunostimulatory signal to bone marrow-derived DCs.
Materials and methods
Mice
Five to ten-week-old female BALB/c (H-2d) and C57BL/6 (H-2b) mice were purchased from Joint Ventures Sipper BK Experimental Animal. OVA323–339-specific TCR-transgenic mice (DO11.10), first generated by the Jackson Laboratory, were a gift from professor Xuetao Cao of the Institute of Immunology, SMMU, Shanghai, China, and were bred in specific pathogen-free conditions. The icosl gene knockout mice (ICOSL−/−, C57BL/6, H-2b) were bought from the Jackson Laboratory, while the TLR4−/− mice (C57BL/6, H-2b) were from Model Animal Research Center of China. All the DCs used in in vitro experiments were obtained from wild C57BL/6 mice unless demonstrated, and the in vivo experiments were performed in BALB/c mice. In this paper, all studies were carried out in compliance with National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University, Shanghai.
Generation of ICOSIg and ICOS-anchored CHO cells
The extracellular part of a DNA sequence encoding human ICOS was amplified via PCR from activated splenic mononuclear cells. For ICOSIg (forward primer: 5′-GCG GCC CAG CCG GCC GAA ATC AAT GGT TCT GCC-3′, reverse primer: 5′-GCT CTA GAC TTC AGC TGG CAA AG-3′, designed and synthesized by Sangon Corporation), the extracellular part of the DNA sequence was cloned into a reconstituted expression vector pSecTag2, in which the human IgG1 constant region (Fc), containing hinge regions CH1 and CH2, were pre-inserted in the laboratory. The plasmids were transfected into Chinese hamster ovary (CHO) cells, and the acquired stable ICOSIg-expressing CHO cell lines were then expanded.
The ICOSIg fusion molecule produced was purified from the supernatant using a protein A column. Similar to the natural monovalent dimer, ICOSIg appeared to be a dimeric protein about 100 kDa in molecular size, as evaluated through gel electrophoresis analysis in reducing versus nonreducing conditions (data not shown). As well, SDS-PAGE analysis demonstrated a purity of >90%. To remove endotoxin, Ca2+ was added at a final concentration of 100 μM and the protein solution was passed through an EndoTrap Blue Column (LONZA) according to the manufacturer’s directions. Human IgG1 (Sigma-Aldrich) was also passed through an EndoTrap Blue column for the same target and used as a control in all corresponding experiments. LPS levels in our protein solution were less than 0.06 EU/ml as determined by Tachypleus Amebocyte Lysate assay (KANGBO, China).
For constructing ICOS-anchored CHO cells, the entire sequence of ICOS was cloned with PCR as above, using the forward primer: 5′-GTT GGA TCC GCC ACC ATG AAG TCA GGC CTC-3′ and the reverse primer: 5′-GAG A AT TCT TAT AGG GTC ACA TCT G-3′ (designed and synthesized by Sangon Corporation), then ligated into the retrovirus vector PMSCV-puro. The recombined retrovirus vector was then packed into PT-67 cells to produce recombined retrovirus. The high-titer recombined retroviruses were then collected and used to infect CHO cells. Twenty-four hours later, Puromycin was added to the cultures at a final concentration of 600 μg/ml. After being cloned and subcloned, a CHO cell clone highly expressing ICOS (CHO-ICOS) on the surface was obtained. Control CHO cells transformed with empty PMSCV-puro retrovirus vector (CHO-CON) were also constructed and selected. Before coculturing with DCs, these CHO cells were first treated with 80 μg/ml mitomycin C for 30 min in order to eliminate their further proliferation during their incubation with DCs. The mitomycin C treatment had no obvious effects on the ICOS expression of CHO-ICOS cells (data not shown).
Recombinant mouse-derived ICOSIg (R&D systems; 168-CS, with endotoxin level <1.0 EU per 1 μg) were also used for directly bioactivity comparison of mouse- or human-derived ICOSIg.
Generation of bone marrow-derived DCs
DCs were generated from 5- to 8-week-old mice essentially as previously described, with minor modifications [16]. In brief, bone marrow mononuclear cells from mouse femur and tibia were prepared by the depletion of red cells with ammonium chloride Tris-buffer, then cultured in six-well plates in RPMI 1640 medium (Hyclone) supplemented with 10% FCS (PAA), 10 ng/ml of recombinant mouse granulocyte-monocyte, colony-stimulating factor (GM-CSF, R&D) and 1 ng/ml of recombinant mouse IL-4 (PeproTech). Nonadherent cells were gently washed out on day 2 of culture. The remaining loosely adherent clusters were cultured for 3 more days in fresh medium containing the same concentrations of GM-CSF and IL-4. At day 5, DC cells were positively selected with CD11c magnetic microbeads (Miltenyi Biotec; clone: N418).The recovered cells consisted of more than 95% CD11c+ cells. Before further treatment, all the isolated DCs were incubated for 20 min at 4°C with CD16/CD32 antibody for blockage of Fc receptors. For detecting cytokines and surface molecules of DCs following different treatments, the sorted cells were seeded at a density of either 2 × 105 cells/200 μl or 6 × 105 cells/500 μl, in flat-bottomed 96- or 24-well plates, respectively. For T cell co-culture experiments, DCs were first treated with ICOS or its control for 24 h, then washed and cocultured with CD4+ T cells in U-bottomed plates.
Analysis of cell surface marker expression
Fluorescein-conjugated monoclonal antibodies recognizing CD11c (N418), CD80 (16-10A1), CD83 (Michel 17), CD86 (PO3.1), I-Ab (AE6-120.1), ICOSL (HK5.3), CD8 (53-6.7), CD4 (GK1.5), CD25 (PC61), CD69 (H1.2F3), and their respective isotype controls were purchased from eBioscience or Biolegend. Purified antibody to CD16/CD32 (rat IgG2b; clone 2.4G2) was also obtained from Biolegend. Before fluorescent antibody staining, all cells were incubated for 20 min at 4°C with antibody CD16/CD32 for blockage of Fc receptors prior to further manipulation. Fluorescent antibodies and the respective isotype controls were then added according to the supplier’s directions, and cells were incubated for a further 30 min at 4°C. The cells were then washed once with ice-cold PBS pH 7.2 containing 0.1% NaN3 and 0.5% BSA, then resuspended in 300 μl PBS. Flow cytometry (FCM) was performed with a FACSCalibur flow cytometer (Becton Dickson) and data were analyzed using CellQuest software (Becton Dickinson).
ELISA
The concentration of total cytokines in the culture supernatant of DCs alone, or in the T-mixed cultivation system, was measured using a corresponding ELISA kit (eBiosciences, or Bender), according to the protocol provided by the manufacturer.
Quantitative real time PCR
Total RNA was extracted from DCs using TRIzol (Invitrogen Life Technologies) and reverse transcribed to cDNA using a RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). One microgram of the cDNA was used as template for quantitative PCR (SYBR Green, TOYOBO), which was performed using the Lightcycler Detection System (ROCHE) with the ΔΔCt method, according to the manufacturer’s instructions. Primers for quantitative RT-PCR of mouse β-actin, ICOSL, IL-1β, TGF-β1, PGE2, CCL3, CCL4, IL-10, CCL19 CCR7, and IL-10R were designed by TAKARA Corporation according to the corresponding whole mRNA sequences and were synthesized by Sangon Corporation. The primer sequences were as follows:
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β-actin | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
| ICOSL | AGCTCCATGTTTCTAGCGGGTTC | ACCATTGCACCGACTTCAGTCTC |
| TGF-β1 | GTGTGGAGCAACATGTGGAACTCTA | TTGGTTCAGCCACTGCCGTA |
| PGE2 | CTCATCAGCAAGCGCCTCAA | GGTCTTTACCCACGGCTGTCA |
| CCL3 | TGAAACCAGCAGCCTTTGCTC | AGGCATTCAGTTCCAGGTCAGTG |
| CCL4 | CCATGAAGCTCTGCGTGTCTG | GGCTTGGAGCAAAGACTGCTG |
| CCL19 | CGCATCATCCGAAGACTGAAGA | GGCACAGACTTGGCTGGGTTA |
| IL-10R | GTGAACGGACAGGCAATG | TCCCAGAACGGAAGGTAT |
| CCR7 | CTGGTCAGTGCCCAAGTGGA | CTCAAAGTTGCGTGCCTGGA |
| IL-10 | TTTCAAACAAAGGACCAG | GGATCATTTCCGATAAGG |
| IL-1β | TCCAGGATGAGGACATGAGCAC | GAACGTCACACACCAGCAGGTTA |
Western blotting analysis
The activation of p38 was determined by western blotting with antibodies specific for phosphorylated or total p38 kinases. Cells were maintained for different times in a medium supplemented with 1% FCS, treated as indicated, then lyzed in 100 μl of M-PER® Mammalian Protein Extraction Reagent (PIERCE) containing 3% Halt Phosphatase Inhibitor Cocktail (PIERCE). All samples were normalized according to protein concentration, separated on 12% SDS-PAGE gel, then transferred to polyvinylidene difluoride membranes (PVDF; Gelman-Pall, USA) using a wet transfer blotting system (Bio-Rad, USA). After blocking in Tris-buffered saline (TBS 120 mmol/l NaCl, 50 mmol/l Tris-HCl, pH 7.4) containing 0.05% Tween 20 and 1% BSA, the blot was probed with suitable antibodies and incubated overnight at 4°C. The monoclonal antibodies (1:2,000) for phospho-specific p38 MAPK (Cell Signaling, USA) were used to detect phospho-p38 MAPK. The membranes were washed extensively and incubated with horseradish peroxidase conjugated secondary antibodies (1:2,000; Cell Signaling, USA) for 1.5 h at room temperature. After three washings with TBS containing 0.05% Tween 20, the phospho-p38 MAPK was detected by enhanced chemiluminescent detection (ECL; Pierce Rockford, USA). The PVDF membrane was then thoroughly washed with Restore™ Western Blot Stripping Buffer (PIERCE) for stripping primary and secondary antibodies from blots and to enable reprobing of total p38 on the same membrane. β-actin was used as inner reference. For semi-quantitative analysis of the expression of phospho-p38 MAPK, Smartview Software was used to obtain gray scale values of each band. The relative expression levels of phospho-p38 MAPK in each group were calculated as ODp-p38/ODβ-actin.
T cell stimulation assay
The F1 generation of DO11.10 and C57BL/6 was used as a resource for OVA-specific CD4+ T cells in an antigen-presenting experiment. T cells from BALB/c mice were used in a mixed lymphocyte reaction (MLR). Splenocyte populations were harvested and CD4+ T cells were positively selected with CD4-antibody labeled with magnetic microbeads (Miltenyi Biotec). Responder cells (5 × 105) were stimulated in vitro for 3 days with different ratios of DCs derived from triplicates of C57BL/6 in U-bottom 96-wells, together with OVA323–339 polypeptides synthesized by Sangon Corporation (China). The plated cells were pulsed with 3H thymidine 18 h before harvest and counted with a Packard cell harvester and counter (Micro-beta, Wallac). When CFSE dye was used for evaluation of T cell proliferation, the purified CD4+ T cells were first co-incubated with 5 mM CFSE for 15 min, then thoroughly washed and cocultured with DCs for 4 days as described. Cells were then collected and analyzed with FCM.
Analysis of DCs phagocytic ability
Following treatment with soluble proteins, the purified immature or mature DCs were incubated at 37°C for 4 h with FITC-conjugated OVA protein (Molecular Probes) at a final concentration of 100 μg/ml in RPMI 1640 medium containing 10% FCS. After washing twice with ice-cold PBS, pH 7.2, containing 0.1% NaN3 and 0.5% BSA, cells were again suspended in chilled PBS for immediate flow cytometry. Cells incubated with OVA-FITC at 0°C were used as a negative control.
Peptides and DTH assays
A skin test was used to measure MHC class I-restricted delayed-type hypersensitivity (DTH) responses of synthetic peptides [3, 6]. These peptides were synthesized by Sangon Corporation, purified by means of reversed-phase HPLC and characterized by amino acid analysis. The sequence of the peptides used is as follows: H-2d-restricted P815AB35–43: LPYLGWLVF; I-Ad-restricted tetanus toxin (tt) 947–967, FNNFTVSFWLRVPKVSASHLE. DCs (5 × 105 per ml) pulsed with P815AB and tt were transferred intravenously into recipients. After 2 weeks, 50 μg P815AB peptide in 30 μl RPMI1640 containing 6% of DMSO was inoculated into the left hind footpads of the mice. As a control, the right hind footpad received the same volume of RPMI1640 containing 6% of DMSO used as a control. The DTH reaction was recorded 24 h later. The animals were sacrificed and their hind feet were removed along the hairline. The results were expressed as the increase in footpad weight of peptide-injected footpads over that of vehicle-injected counterparts. Data shown are the mean ± SD of at least five mice per group.
Statistical analysis
Multiple treatment groups were compared within the individual experiments using one-way ANOVA. All experiments were repeated at least three times except in cases where more replicates were needed.
Results
Soluble ICOSIg binds specifically to ICOSL on DCs
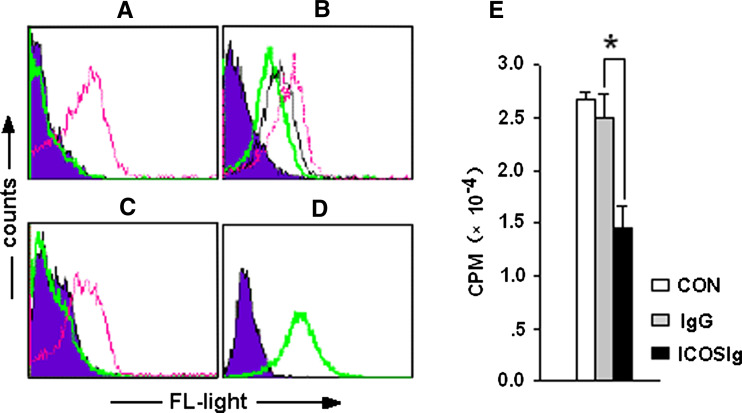
When viewed using a computer-generated modeling program, the ICOS receptor-binding interfaces for mouse and human B7h were similar in appearance [17]. Several previous studies have successfully demonstrated the important roles of the ICOS/ICOSL pathway using human-derived ICOSIg on mouse or rat disease models [18–20]. These data confirmed that human ICOS is able to bind to mouse or rat ICOSL and can function as a native analog. We have obtained abundant human-derived soluble ICOSIg in our previous work. To investigate whether ICOSIg activates a functional response in mouse DCs, we first tested the human fusion protein for its ability to specifically bind ICOSL on mouse bone marrow-derived DCs. Dendritic cells were cocultured with ICOSIg or control human IgG1 for 30 min at 4°C, and detected by flow cytometry (FCM). Treatment with a fluorescent-labeled anti-human IgG1 antibody for another 30 min clearly indicated that ICOSIg could bind to DCs (Fig. 1a). To further investigate the specific binding of ICOS with ICOSL, DCs were treated with anti-ICOSL-PE antibody in the presence of different concentration of ICOSIg. Alternatively, DCs were first cocultured with ICOSIg, then with anti-ICOSL-PE in a competitive binding test. The binding of anti-ICOSL-PE to the ICOSL on DCs was either partially (Fig. 1b) or entirely blocked (Fig. 1c).
Fig. 1.
Specific binding of ICOSIg to ICOSL on DCs inhibits MLR. a–c DCs were treated as described in the text, and the histogram shows the fluorescence labeling-isotype antibodies. a ICOSIg binds specifically to DCs: thin line (red) 60 μg/ml ICOSIg + anti-IgG-PE; bold line (green) 60 μg/ml IgG + anti-IgG-PE. b ICOSIg binds competitively with anti-ICOSL to ICOSL on DCs: dashed line (red) only anti-ICOSL-PE; thin line (black) 30 μg/ml ICOSIg + anti-ICOSL-PE; bold line (green) 60 μg/ml ICOSIg + anti-ICOSL-PE. c Pretreated with ICOSIg completely blocks the specific binding of anti-ICOSL to ICOSL on DCs: thin line (red) 60 μg/ml IgG + anti-ICOSL-PE; bold line (green) 60 μg/ml ICOSIg + anti-ICOSL-PE. d More than 98% of the constructed ICOS transfectants express membrane-bound ICOS as determined by a specific anti-ICOS-PE antibody: histogram: CHO-CON + anti-ICOS-PE; bold line (green) CHO-ICOS + anti-ICOS-PE. e MLR were partially inhibited in the presence of ICOSIg compared to IgG1 (mean ± SD; *P < 0.05)
Previous study has shown that proliferation and activation of T cells were significantly inhibited when the ICOS/ICOSL pathway was abrogated with ICOSIg, which blocks the interaction of ICOS on T cells and of ICOSL on antigen-presenting cells [21]. To determine the bioactivity of soluble ICOSIg, MLR was performed using CD4+ T from BALB/c mice and DCs from C57BL/6 mice in the presence of ICOSIg or control human IgG1. The proliferation of CD4+ T cells was partially inhibited by ICOSIg (Fig. 1e), in agreement with previous findings. These data demonstrate that human-derived soluble ICOSIg can bind specifically to mouse ICOSL on immature DCs and can be used for further functional research in further experiments. We also successfully constructed membrane-anchored ICOS-expressing CHO cells (CHO-ICOS) that express the ICOS molecular structure on the surface in more than 98% of the cells, as determined by PE-labeled anti-ICOS antibody via FCM (Fig. 1d).
ICOSIg enhances expression of IL-6 in DCs
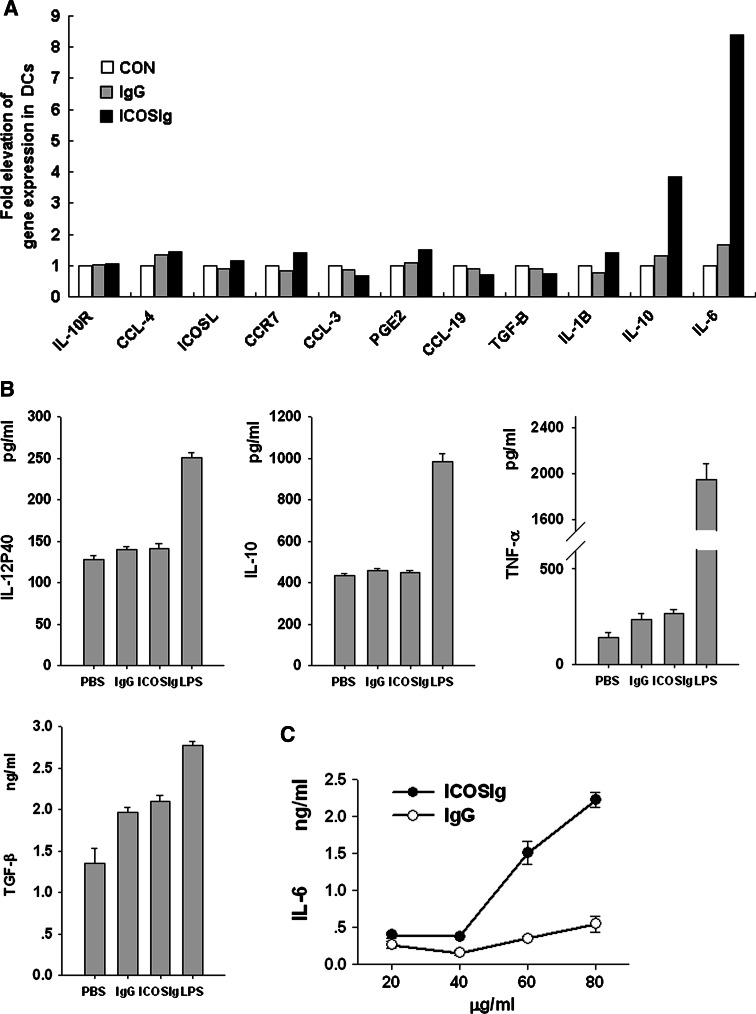
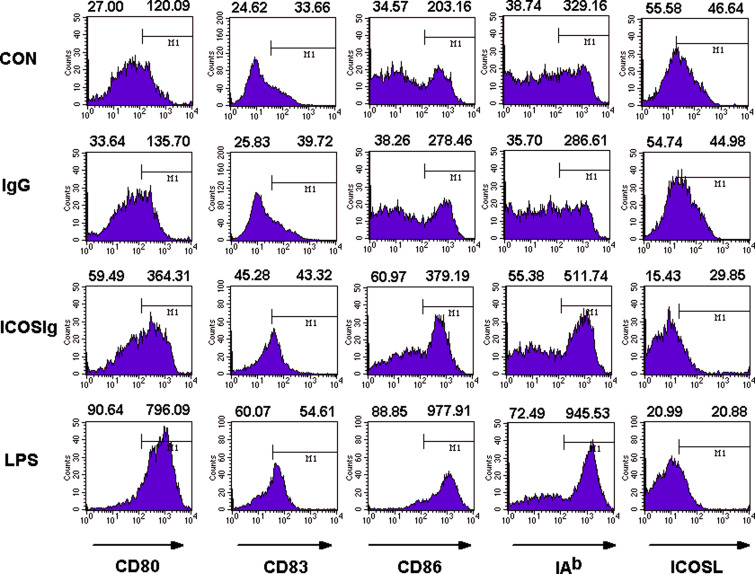
Cytokines and chemokines play an important role in the effect or function of DCs. Therefore, we first assessed the production of cytokines in the supernatants using an ELISA assay, including: IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α, IFN-γ, and TGF-β1. We also determined expression of PGE2, IL-6, IL-10, IL-10 receptor(IL-10R), chemokines CCL3, CCL4, CCL19, and chemokine receptor CCR7 in DCs treated with ICOSIg or control IgG1, with quantitative real-time PCR. The levels of IL-2, IL-4, and IFN-γ in the supernatants were below the limits of the corresponding ELISA kits. There were no obvious differences in most of the other detected compounds (Fig. 2a, b), but intriguingly, we found a notable dose-dependent effect in IL-6 production in response to ICOSIg, both in the supernatant and at the mRNA level, compared to IgG1 control (Fig. 2a, c). Although CD28-Ig induced no substantial changes in the surface expression of MHC-II, CD80, CD83, and CD86 molecules on spleen DCs [6], a slight but definite augmentation of these surface molecules was found on bone marrow-derived DCs treated with ICOSIg (Fig. 3). In contrast, surface expression of ICOSL on DCs decreased after treatment with ICOSIg. Given that the actual level of ICOSL might be disguised by ICOSIg, owing to its competitive binding with PE-anti-ICOSL antibody (as shown in Fig. 1), the mRNA levels of ICOSL in DCs were investigated using quantitative RT-PCR. Consequently, in this case, no difference was seen (Fig. 2a). At the same time, ICOSIg treatment did not induce any obvious apoptosis, death, or proliferation of DCs, as determined by Annexin V/PI counterstain and CCK-8 detection (data not shown).
Fig. 2.
ICOSIg induces IL-6 in DCs. a DCs were treated with ICOSIg or control IgG1 for 8 h after blocking Fc receptors with antibody to CD16/CD32, then collected and detected for mRNA levels of different targets as shown using quantitative RT-PCR(one representative data of two independent experiments shown). b Cytokines shown in the culture supernatant were determined with ELISA after DCs were incubated with 60 μg/ml soluble proteins for 24 h; DCs treated with 200 ng/ml LPS were used as positive control. c IL-6 levels in the culture supernatant were detected using ELISA after DCs were treated with different concentration of proteins for 24 h (in groups of 60 and 80 μg/ml, P < 0.01)
Fig. 3.
ICOSIg improves surface molecules on immature DCs. Immature DCs were administered with 60 μg/ml ICOS-Ig or IgG1 and then treated with different fluorescence-labeling antibody and detected by FCM. Left-hand numbers above each histogram represent the percent of positive-staining cells while the right-hand numbers represent mean fluorescent intensity (MFI) of all the cells gated (data are one representative of six)
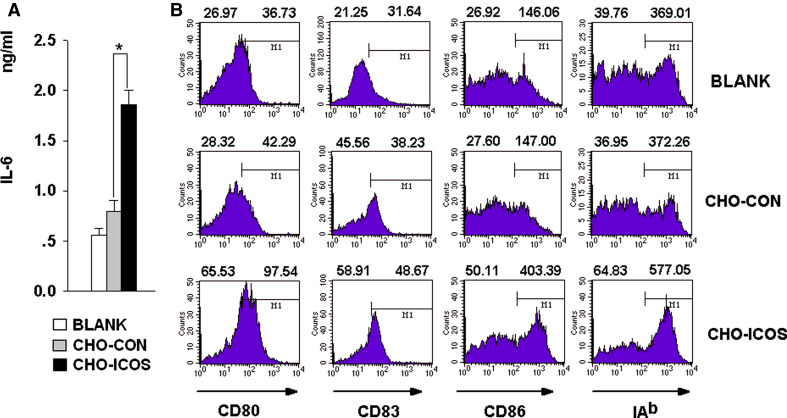
To confirm the reverse signal by soluble ICOSIg, DCs were next co-incubated with CHO-ICOS pretreated with mitomycin C, to mimic the in vivo cell-to-cell interaction. CHO-ICOS cells could also promote DCs to express more IL-6 in the culture supernatants and to express higher surface costimulating molecules compared to control CHO (CHO-CON) cells (Fig. 4a, b). These results suggest that both soluble and membrane-anchored ICOS induce an elevated IL-6 cytokine response and that ICOSIg could be used for further investigation of the interaction between ICOS and ICOSL as an alternative to membrane ICOS.
Fig. 4.
Membrane-anchored ICOS induces expression of IL-6 by DCs. DCs were left untreated, or cocultured with CHO-ICOS or CHO-CON which were pre-treated by mitomycin for 24 h. a The supernatant were collected and detected for IL-6 with ELISA kit (*P < 0.05). b Cells were then collected and washed, treated with different fluorescence-labeling antibody and detected by FCM; CD11c+ cells were gated and the data were analyzed by Cellquest Software. Left-hand numbers above each histogram represent the percent of positive-staining cells while the right-hand numbers represent mean fluorescent intensity (MFI) of all the cells gated (data are one representative of three)
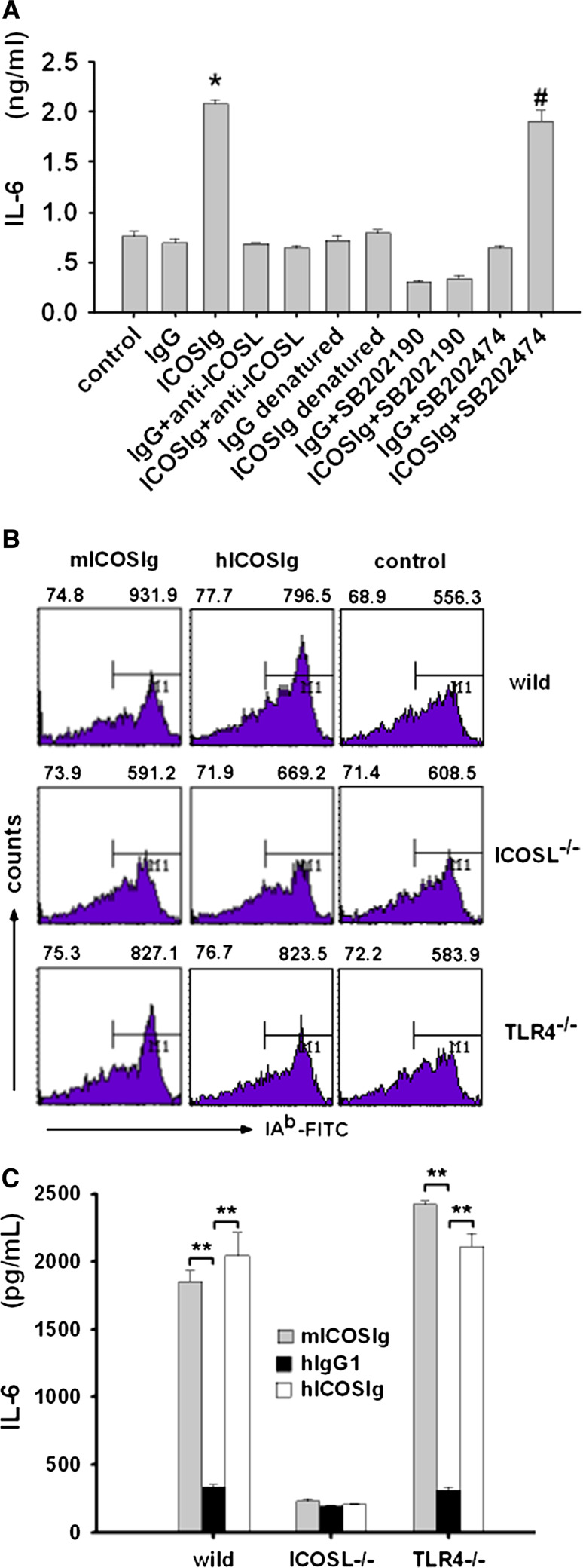
Expression of IL-6 by DCs induced by ICOSIg is ICOSL and p38-MAPK dependence
To confirm that the augmentation of IL-6 in DCs was caused by ICOSIg, we used anti-ICOSL antibody to block the interaction of ICOSIg with ICOSL on DCs. The use of anti-ICOSL abolished the differential expression of IL-6 between the experiment and control groups (Fig. 5a), which indicated that IL-6 was expressed in an ICOSL-dependent fashion. In our experiments, both ICOSIg and IgG1 were treated with an EndoTrap Blue column for removing endotoxin, according to the manipulation procedure. To assess any possible contribution of toll-like receptor (TLR) signaling by remnant endotoxin contamination, the ICOSIg and human IgG1 proteins were denatured in boiling water to abrogate their biological activity and retain the possible residual biological effect of endotoxin, since the latter needs harsher conditions to be destroyed. The denatured ICOSIg lost its ability to promote generation of IL-6 by DCs (Fig. 5a). Thus, ICOSIg induces an ICOSL-dependent elevation of IL-6 expression by immature DCs.
Fig. 5.
ICOSIg induces p38-dependent IL-6 expression via ICOSL on DCs. a Wild DCs (C57BL/6) were treated with sufficient anti-ICOSL antibody or SB202190 and SB202474 for 1 h before being incubated with ICOS-Ig or IgG for another 24 h. In part of the experiments, soluble proteins were denatured with boiling water. IL-6 in the supernatants were then measured by ELISA (*P < 0.05 compared to other groups except ICOS + SB202474 group; # P < 0.05 compared with ICOSIg + SB202190). b,c DCs from different mice (wild, ICOSL−/− and TLR−/−, all these mice were C57BL/6 derived) were blocked with CD16/CD32 antibody and incubated with 60 μg/ml mouse ICOS-Ig, human ICOSIg or human IgG1 for 36 h (1 × 105 cells in 100 μl condition media per duplicate 96-cell flat cells) before being collected and detected for IAb with FCM or IL-6 in the supernatants with ELISA (**P < 0.01, data are one representative of three)
To further identify the important role of ICOSL in the ICOSIg-induced biological-function changes in DCs, we repeated these experiments using DCs obtained from ICOSL−/− and TLR4−/− mice. In this case, mouse-derived ICOSIg was also used for a directly comparison of bioactivity of mouse- or human-derived ICOS on mouse DCs. The slight enhancement of IAb expression on DCs and definite elevation of IL-6 in the supernatant caused by ICOSIg were almost completely abrogated after the deletion of icosl, which, however, were not affected by the deletion of TLR4, the generally accepted receptor of LPS (Fig. 5b, c). These experiments also provided further evidences that ICOSIg, either from mouse or human beings, could function properly by binding specifically to ICOSL on mouse DCs (Fig. 5b, c).
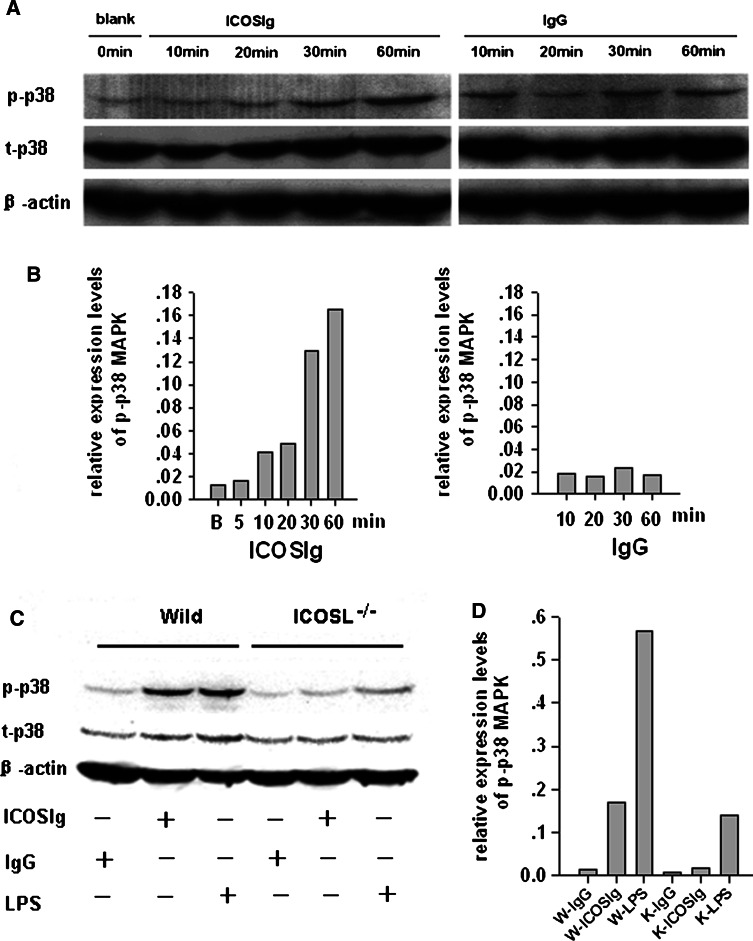
The p38-MAPK signal pathway has been presumed necessary for the production of IL-6 by DCs in response to inflammatory stimuli, and p38-MAPK also plays a critical role in CD28 induction of IL-6 in splenic DCs [6]. To further explore whether activation of the p38-MAPK signal pathway was also included during the generation of IL-6 by DCs treated with ICOSIg, the production of IL-6 was examined in immature bone marrow-derived DCs treated with ICOSIg, in either the presence or absence of SB202190, an inhibitor of p38-MAPK signal pathway, or its inactive analog SB202474. Blockade of p38 activity resulted in an obvious suppression of IL-6 released by DCs and also an abolition of the discrimination in both groups (Fig. 5a), showing a direct association of IL-6 secretion with the activation of p38 signal pathway.
To further dissect the mechanism of elevated IL-6, we next examined whether enhanced activation of p38 protein was induced in DCs after its interaction with ICOSIg. To this end, the phosphorylation levels of p38 protein were evaluated using western blotting analysis. We found a basic phosphorylation of p38 in untreated wild DCs, and the phosphorylation levels were augmented in response to ICOSIg in a time-dependent manner, while no difference could be found in DCs treated with control IgG protein at the observed time points (Fig. 6a, b). Furthermore, ICOSIg induced little change in p38 phosphorylation in ICOSL−/−-derived DCs as compared with those from wild mice (Fig. 6c, d). Total levels of p38 showed no obvious difference in each group (Fig. 6a, c and data not shown). These data suggest that soluble ICOSIg, but not IgG, specifically affects activation of p38 MAPK in DCs.
Fig. 6.
ICOSIg activates p38-MAPK signal pathway in DCs. The 5-day immature DCs derived from wild or ICOSL−/− mice were resuspended in 100 μl 1640 culture medium containing 1% FCS, with 5 × 106 total cell numbers in each group. After being incubated for different times (a) or 60 min (c) with 200 μg/ml ICOSIg or IgG (a,b), or with 100 ng/ml LPS (c), DCs were collected and treated with cell lysis solution; the lysate were then used for detecting phosphorylated and total p38 protein using western blot. The relative gray scale values of each p-p38 MAPK band were also listed (b vs a and d vs c), which were obtained by Smartview Software and calibrated with those of each β-actin, respectively. p-p38 Phosphorylation p38 protein, t-p38 total p38 protein, β-actin inner reference protein, W- wild-derived DCs, K- ICOSL−/−-derived DCs (data are one representative of three)
ICOSIg promotes immunostimulating function of DCs including: phagocytosis, antigen-presenting and initiating mixed lymphocyte reaction
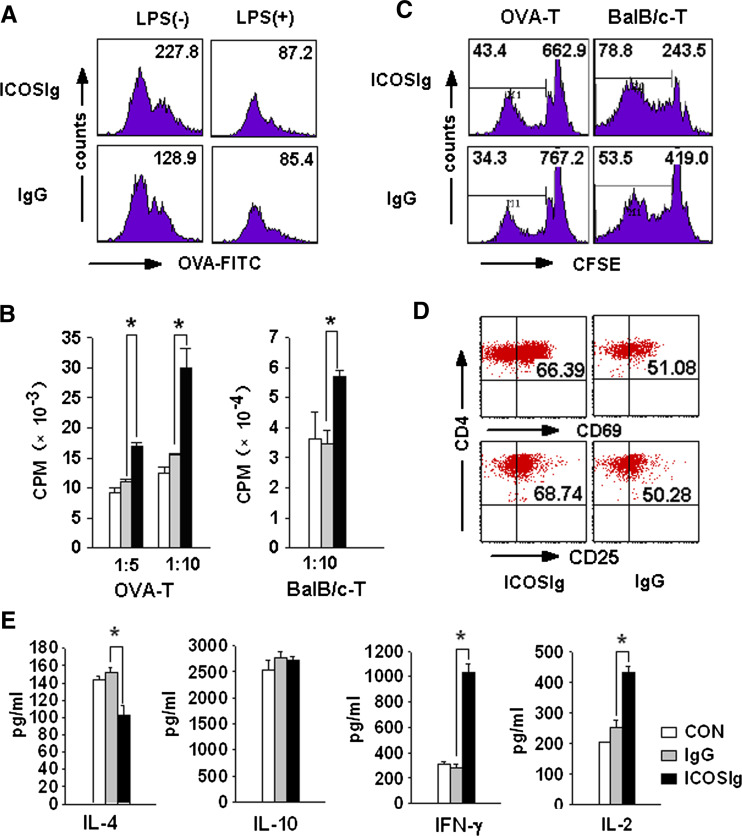
IL-6 has long been recognized as a proinflammatory cytokine [6, 25]. Therefore, the augmented expression of IL-6 might provide 5-day bone marrow-derived DCs with an immunostimulatory phenotype. To confirm this assumption, DCs were incubated with FITC-conjugated chicken OVA (FITC-OVA) after being treated with either ICOSIg or control IgG1. The amount of fluorescence incorporated into DCs was used as an indicator of antigen uptake. As shown in (Fig. 7a, left), DCs treated with ICOSIg glowed brighter than did the DCs treated with the control IgG1, considering the enhanced antigen uptake. A PD-L2-specific IgM has been reported to provide CpG-maturing DCs with a different activation phenotype, with the cells having the strong antigen-presenting functions of mature DCs and the antigen-acquiring functions of immature DCs [8]. We then tried to investigate whether ICOSIg could also promote LPS-inducing mature DCs to acquire antigen. The 5-day DCs were first treated with 100 ng/ml LPS for 24 h and then with 60 μg/ml ICOSIg for another 24 h before being pulsed by FITC-OVA. No positive results were found by FCM (Fig. 7a, right).
Fig. 7.
ICOSIg induces immunogenic DCs in vitro. a Immature DCs were treated with ICOSIg or IgG1 in the absence (left) or presence (right) of 100 ng/ml LPS for 24 h, then washed thoroughly and cocultured with 100 μg/ml FITC labeling OVA protein and detected by FCM. Numbers shown represent MFI of all the cells gated. b–e Immature DCs were pretreated by ICOSIg. b Cocultured with OVA-specific CD4+ T in the presence of OVA323–339 peptides (left) or with CD4+ T obtained from spleen of BALB/c mouse (right) for another 72 h and H3-thymine was added before the last 18 h. X-axis gives the ratio of DC: T(*P < 0.05). c Cocultured with CD4+ T prestained by CFSE agent for 5 days and analyzed by FCM. Left-hand numbers represent the percent of offspring T cells after more than three times of cell division, right-hand numbers represent the MFI of all the cells gated. d Cocultured with OVA-specific CD4+ T as in (b). T cells were then washed and evaluated the expression of CD25 and CD69. e Cocultured with OVA-specific CD4+ T as in (d). The supernatant of the coculture system were then collected and assayed for different cytokines with corresponding ELISA Kit (*P < 0.05)
The ability of DCs coactivated with ICOSIg to present antigens to T lymphocytes was next investigated. OVA323–339-specific CD4+ T cells were cocultured with DCs pretreated with ICOSIg or control IgG1. 200 nM OVA323–339 peptides were added, and incorporation of [3H] thymidine was used to evaluate T cell activation. Treatment of 5-day DCs with ICOSIg partially upregulated the cells’ ability to activate antigen-specific T cells compared with the control groups (Fig. 7b), whereas no obvious activation could be found when DCs were cocultured with T cells without OVA323–339-specific peptides (data not shown). When DCs from C57 mice were co-incubated with CD4+ T cells from BALB/c mice, to initiate a MLR, higher T cell proliferation could also be obtained in a group of DCs treated with ICOSIg (Fig. 7b).
Because 3H incorporation can only capture the T cell proliferation during the last 18 h of coincubation, we repeated the assay with CFSE-labeled antigen-specific or allogeneic CD4+ T cells, which permits visualization of the entire division history during the coculture. The division profile of CFSE-labeled T cells cocultured with DCs pretreated by ICOSIg was correlated with the results of 3H incorporation. The ability of antigen presentation and stimulating proliferation of allogeneic T cells was augmented (Fig. 7c). Collectively, these data suggest that ICOSIg promotes the immunostimulatory ability of immature DCs in vitro.
ICOSIg pulsed DCs induce activation and Th1 differentiation bias of CD4+ T cell
To determine the biological sequelae of ICOSIg-treated DCs in primary CD4+ T cell differentiation, OVA-specific CD4+ T cells were cultured with ICOSIg-loaded DC for 72 h. Increased IL-2 and IFN-γ levels were found in the supernatant of T cells cocultured with ICOSIg-pretreated DCs, but no difference existed in expression of IL-10 (Fig. 7e). This finding suggests that the ICOSIg-pulsed DCs might promote a Th1 differentiation bias from CD4+ T cells. Primary experiments showed no difference in IL-17 expression in both groups (data not shown). CD25 and CD69, the main activation markers of T cells, were also elevated in OVA-specific CD4+ T cells co-incubated with DCs pretreated by ICOSIg (Fig. 7d).
ICOSIg favors DCs induction of cell-mediated immunity
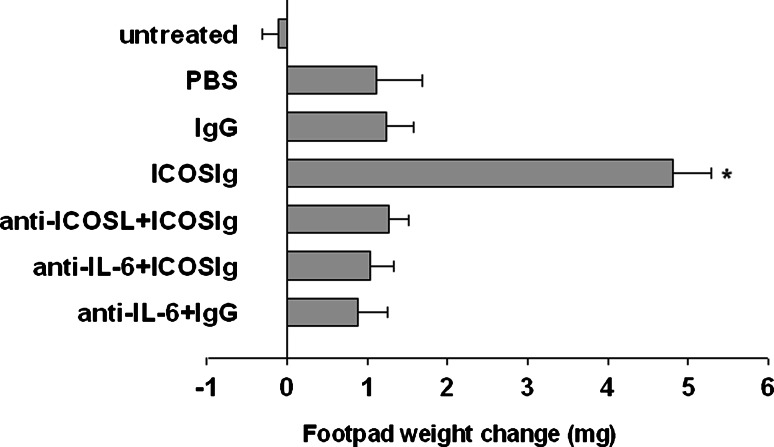
Mouse splenic DCs can present peptide antigens in an immunogenic or tolerogenic way, with the distinction depending on either the occurrence of specialized DC subsets or the maturation or activation state of the DCs [22]. Environmental factors are crucial in conditioning the outcome of DC presentation of the synthetic nonapeptide P815AB, which is related to a poorly immunogenic antigen of mouse mastocytoma P1.HTR31 [23, 24]. Because the nonapeptide P815AB was H-2d restricted, we had to perform this experiment in BALB/c (H-2d) mice. After transferring peptide-pulsed DCs into recipient mice, the induction of immunity versus tolerance can be monitored by a skin test assay. This can be done through intrafootpad challenge with the eliciting peptide, and this DTH model has been successfully used to reveal numbers of reverse signals delivered in members of costimulator families [3, 6, 10]. Five-day immature bone marrow-derived DCs in vitro were pulsed with P815AB and tt, and injected into recipient hosts that we assayed for 2 weeks for the development of MHC class I-restricted skin test reactivity. We either left the DCs untreated or treated them with ICOSIg in the presence or absence of anti-IL-6 and anti-ICOSL. Although previous results have shown that unfractionated splenic DCs presented poorly immunogenic peptides [25, 26], a slight but definite reactivity was found after the transfer of conditioned 5-day bone marrow-derived CD11c+ DCs (Fig. 8). DCs conditioned by ICOSIg elicited stronger footpad reactivity, and this effect was negated by the neutralization of IL-6 or by use of anti-ICOSL (Fig. 8). Thus, ICOSIg favors immunostimulatory function of bone marrow-derived immature DCs, through the induction of IL-6 in an ICOSL-dependent manner, both in vitro and in vivo.
Fig. 8.
DCs pretreated by ICOSIg induces DTH. Immature DCs derived from BALB/c mice were pulsed with P815AB and tt peptides after being treated with IgG1 or ICOS-Ig (60 μg/ml) in vitro, with or without anti-IL-6 treatment. In one group, DCs were first blocked with anti-ICOSL for 1 h before being treated as described above. The priming ability of the conditioned, peptide-pulsed DCs was examined by transfer into homogenic recipient mice, which were assayed at 2 weeks for skin test reactivity to the eliciting peptide. Reactivity was measured as the increase in footpad weight of peptide-injected footpads over that of vehicle-injected counterparts. Untreated Normal mouse with no treatment. Each of the results (mean ± SD) is from one experiment representative of three, with at least five mice per group per experiment (*P < 0.01)
Discussion
Although there is a controversy as to whether ICOS mainly regulates only Th2 or Th1 cells or both, recent work has shown that the ICOS/ICOSL signal pathway plays an important role, in either a CD28-independent or -dependent fashion, in augmenting T cell differentiation and cytokine production. In addition, costimulatory signals through ICOS provide critical T cell help to B cells in B cell differentiation, immunoglobulin class switching, germinal center formation, and memory B cell development [13, 15, 26–32]. Signals of ICOS/ICOSL have also been found to play critical, but to some degree complicated, roles in several model systems of different diseases, including autoimmunity, asthma and allergy, infectious disease, transplantation, and cardiovascular system diseases. This makes the ICOS/ICOSL pathway a new and attractive potential therapeutic target [15, 28, 33–42]; however, more details need to be further investigated in order to thoroughly elucidate this signal pathway before clinical application. Among these is the need to determine whether ICOS can also deliver a reverse signal via ICOSL, as do the other co-stimulators mentioned in the "Introduction".
In this article, we used soluble ICOSIg to bind ICOSL on DCs to investigate whether ICOS could deliver reverse signals of some type to DCs and mediate an immune response. Our data showed that ICOSIg binding to ICOSL on immature DCs induced a dose-dependent augmentation of cytokine IL-6, although no other cytokines or chemokines were detected whose release required early activation of p38 MAPK. Moreover, there is an increase in the expression of surface molecules, as determined by FCM, including CD80, CD83, CD86, and IAb on these modified DCs, but ICOSL showed no difference in both groups of DCs, as determined by quantitave RT-PCR.
These primary data also suggested that the elevated IL-6 and enhanced expression of surface molecules were not due to the change of maturation states of immature DCs, since mature DCs cause a wide increase of multiple cytokines and surface molecules as shown in our later experiments. Membrane-anchored ICOS expressed on the surface of CHO cells confirmed the reverse signal delivered by ICOSIg, and soluble ICOSIg was thus used for convenience in the subsequent experiments to discover the property of the reverse signal, rather than membrane-expressed ICOS.
In our experiment, icosl gene knockout mice were also used as a resource for in vitro producing immature DCs and for confirming the newly uncovered reverse signal through ICOSL on DCs by ICOS. Bone marrow cells from ICOSL−/− mice, however, gave lower yields of DCs when using the same protocol shown in “Methods” as compared to wild mice or TLR4−/− (data not shown). Meanwhile, DCs from ICOSL−/− mice displayed a lower ability of producing IL-6 and lower basic phosphorylation levels of p38 (Figs. 5c, 6c). More surprisingly, LPS also induced lower p38 phosphorylation in the ICOSL−/−-derived DCs compared with that in wild-derived DCs (Fig. 6c, d). Further work is needed to disclose the precise mechanisms. These phenomena, together with the reverse signal found in our article, may suggest that signals from ICOSL play important roles in the development and biological function of DCs. In the following experiments, therefore, we used DCs from wild but not ICOSL−/− mice for further investigation of the direct potential mechanism under the ICOSL/ICOS reverse signal, avoiding bias caused by the deletion of icosl itself. In our experiments, tlr4 gene deletion did not abrogate the increase of IL-6 induced by ICOS which further excluded the possible function of contaminated LPS. Although we cannot completely rule out other non-specific stimulators that might contribute to the IL-6 expression, e.g., CPG, ICOSL−/− mice used here provided efficient evidence of the specific enhancement effects on IL-6 production and p38 phosphorylation.
It has been reported that mouse splenic DCs treated with soluble CD28 predominantly expressed interleukin 6 and then enhanced T cell-mediated immunity to tumor and self peptides in vivo [6]. Therefore, ICOSIg pre-treated DCs producing cytokine IL-6 might also have an elevated immunostimulatory function. Further investigation showed that the ability for phagocytosis and antigen-presenting of DCs conditioned by ICOSIg were markedly enhanced compared to that of the controls. The OVA-specific CD4+ T cells co-incubated with DC conditioned by ICOSIg produced more IFN-γ, but less IL-4, revealing the internal association of augmented Th1 response with the increased immunostimulatory function observed. In vivo study confirmed the increased immunostimulatory function of DCs pre-treated by ICOSIg, displaying an IL-6 and ICOSL-dependent manner, to promote cell-mediated and MHC class I-restricted skin test reactivity. The elevated base level of DC-mediated DTH observed in our experiment, compared to those in previous reports [3, 6], might be due to the different sources and thus different functional states of the DCs.
This study reports for the first time, to our knowledge, the use of in vitro induced bone marrow-derived immature DCs in the classic mouse MHC class I-restricted DTH model to evaluate function of the conditioned DCs [3, 6, 10]. Therefore, ICOS can deliver an immunogenic signal to DCs both in vitro and in vivo, although its biological function on DCs seems not as strong as that of CD28, and thus a higher concentration of protein is needed to obtain the observed changes [6].
Immune homeostasis is a delicate balance between the immune defense against foreign pathogens and suppression of the immune system to maintain self-tolerance and prevent autoimmune disease. Maintenance of this balance involves the different interaction of T cells and antigen-presenting cells. Costimulatory molecules play an important role in controlling the differentiation aspect of T cells, by providing positive or negative signals through factors not only on T cells but also on antigen-presenting cells, i.e., CD28/CTLA-4, CD40L, PD-1, and GITR on T cells or CD80/CD86, CD40, PD-L1/PD-L2, and GITRL on DCs, respectively [3–10]. This is also the first time, to our knowledge, that ICOS has been shown to deliver an immunostimulatory signal to DCs by promoting expression of IL-6 via ICOSL. These conditioned DCs show an immunogenic feature and favor the cell-mediated immunity in vivo. Considering the far lower expression of ICOS on T cells and ICOSL on DCs than that shown by CD28/CTLA-4 and CD80/CD86, respectively, the observed inferior biological signal delivered by ICOS via ICOSL might in fact not be as weak as that delivered by CD28/CTLA-4 to CD80/CD86 in a single immune cell, and thus may participate in maintaining the whole immunological homeostasis. These data provide new recognition of the ICOS/ICOSL signal pathway role in immunity. This may lead to a reconsideration of previous confusing experimental data that indicated that the timing of ICOS blockade significantly influences therapeutic effects [15, 33–36]. Finally, these data provide more evidence justifying the reasonable design of therapeutic drugs targeting the ICOSL/ICOS pathway.
Acknowledgments
We would like take the opportunity to thank Professor Xuetao Cao for kindly providing us with OVA-transgene mouse (DO11.10) and Doctor Liwei Dong for SB202190, an inhibitor of p38 MPAK pathway, and its inactive analog, SB202474. The authors declare that there were no financial interest conflicts raised during this study.
References
- 1.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA-4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallarino F, Fields PE, Gajewski TF. B7–1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T-cell activation in the absence of CD28. J Exp Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 4.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by Human CD4+ T-cells triggers indoleamine 2, 3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 6.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan S, Celis E, Pease LR. B7-DC cross-linking restores antigen uptake and augments antigen-presenting cell function by matured dendritic cells. Proc Natl Acad Sci USA. 2005;102:11438–11443. doi: 10.1073/pnas.0501420102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, Chen L, Rodriguez M, Pease LR. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196:1393–1398. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 12.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T-cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/S1074-7613(00)00011-X. [DOI] [PubMed] [Google Scholar]
- 13.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4 + T-cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, Yoshinaga SK, Lanier LL. Inducible costimulator costimulates cytotoxic activity and IFN-γ production in activated murine NK cells. J Immunol. 2002;169:3676–3685. doi: 10.4049/jimmunol.169.7.3676. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 16.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakkour S, Sha WC. Mapping of the ICOS binding surface of murine B7 h using an unbiased, cellular library of B7 h mutants created by cyclical packaging rescue. J Immunol Methods. 2008;332:151–161. doi: 10.1016/j.jim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Fabian D, Gong N, Vogt K, Volk HD, Pleyer U, Ritter T. The influence of inducible costimulator fusion protein (ICOSIg) gene transfer on corneal allograft survival. Graefes Arch Clin Exp Ophthalmol. 2007;245:1515–1521. doi: 10.1007/s00417-007-0629-y. [DOI] [PubMed] [Google Scholar]
- 19.Afek A, Harats D, Roth A, Keren G, George J. A functional role for inducible costimulator (ICOS) in atherosclerosis. Atherosclerosis. 2005;183:57–63. doi: 10.1016/j.atherosclerosis.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Matsui Y, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Tezuka K, Nakayama Y, Morimoto J. Adenovirus-mediated gene transfer of ICOSIg fusion protein ameliorates ongoing experimental autoimmune myocarditis. Hum Gene Ther. 2003;14:521–532. doi: 10.1089/104303403764539314. [DOI] [PubMed] [Google Scholar]
- 21.Aicher A, Hayden LM, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- 22.Shortman K, Heath WR. Immunity or tolerance? That is the question for dendritic cells. Nat Immunol. 2001;2:988–989. doi: 10.1038/ni1101-988. [DOI] [PubMed] [Google Scholar]
- 23.Grohmann U, Fallarino F, Silla S, Bianchi R, Belladonna ML, Vacca C, Micheletti A, Fioretti MC, Puccetti P. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166:277–283. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 24.Grohmann U, Bianchi R, Orabona C, Fallarino F, Vacca C, Micheletti A, Fioretti MC, Puccetti P. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003;171:2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- 25.Grohmann U, Fallarino F, Bianchi R, Belladonna ML, Vacca C, Orabona C, Uyttenhove C, Fioretti MC, Puccetti P. IL-6 inhibits the tolerogenic function of CD8 alpha + dendritic cells expressing indoleamine 2, 3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 27.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 28.Villegas EN, Lieberman LA, Mason N, Blass SL, Zediak VP, Peach R, Horan T, Yoshinaga S, Hunter CA. A role for inducible costimulator protein in the CD28-independent mechanism of resistance to Toxoplasma gondii. J Immunol. 2002;169:937–943. doi: 10.4049/jimmunol.169.2.937. [DOI] [PubMed] [Google Scholar]
- 29.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T-cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald RJ, McAdam AJ, Van der Woude D, Satoskar AR, Sharpe AH. Cutting edge: inducible costimulator protein regulates both Th1 and Th2 responses to cutaneous leishmaniasis. J Immunol. 2002;168:991–995. doi: 10.4049/jimmunol.168.3.991. [DOI] [PubMed] [Google Scholar]
- 31.Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D. The role of ICOS in the development of CD4 T-cell help and the reactivation of memory T-cells. Eur J Immunol. 2007;37:1796–1808. doi: 10.1002/eji.200636661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castelli L, Comi C, Chiocchetti A, Nicola S, Mesturini R, Giordano M, D’Alfonso S, Cerutti E, Galimberti D, Fenoglio C, Tesser F, Yagi J, Rojo JM, Perla F, Leone M, Scarpini E, Monaco F, Dianzani U. ICOS gene haplotypes correlate with IL10 secretion and multiple sclerosis evolution. J Neuroimmunol. 2007;186:193–198. doi: 10.1016/j.jneuroim.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Rottman JB, Smith T, Tonra JR, Ganley K, Bloom T, Silva R, Pierce B, Gutierrez-Ramos JC, Ozkaynak E, Coyle AJ. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2:605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 34.Ansari MJ, Fiorina P, Dada S, Guleria I, Ueno T, Yuan X, Trikudanathan S, Smith RN, Freeman G, Sayegh MH. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clin Immunol. 2008;126:140–147. doi: 10.1016/j.clim.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Katsumata Y, Harigai M, Sugiura T, Kawamoto M, Kawaguchi Y, Matsumoto Y, Kohyama K, Soejima M, Kamatani N, Hara M. Attenuation of experimental autoimmune myositis by blocking ICOS–ICOS ligand interaction. J Immunol. 2007;179:3772–3779. doi: 10.4049/jimmunol.179.6.3772. [DOI] [PubMed] [Google Scholar]
- 36.Sporici RA, Beswick RL, von Allmen C, Rumbley CA, Hayden LM, Ledbetter JA, Perrin PJ. ICOS ligand costimulation is required for T-cell encephalitogenicity. Clin Immunol. 2001;100:277–288. doi: 10.1006/clim.2001.5074. [DOI] [PubMed] [Google Scholar]
- 37.Rutitzky LI, Ozkaynak E, Rottman JB, Stadecker MJ. Disruption of the ICOS-B7RP-1 costimulatory pathway leads to enhanced hepatic immunopathology and increased γ interferon production by CD4 T-cells in murine schistosomiasis. Infect Immun. 2003;71:4040–4044. doi: 10.1128/IAI.71.7.4040-4044.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittrücker HW, Kursar M, Köhler A, Yanagihara D, Yoshinaga SK, Kaufmann SH. Inducible costimulator protein controls the protective T-cell response against Listeria monocytogenes. J Immunol. 2002;169:5813–5817. doi: 10.4049/jimmunol.169.10.5813. [DOI] [PubMed] [Google Scholar]
- 39.Nanji SA, Hancock WW, Anderson CC, Adams AB, Luo B, Pawlick RL, Wang L, Coyle AJ, Larsen CP, Shapiro AM. Multiple combination therapies involving blockade of ICOS/B7RP-1 costimulation facilitate long-term islet allograft survival. Am J Transplant. 2004;4:526–536. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 40.Salama AD, Yuan X, Nayer A, Chandraker A, Inobe M, Uede T, Sayegh MH. Interaction between ICOS-B7RP1 and B7-CD28 costimulatory pathways in alloimmune responses in vivo. Am J Transplant. 2003;3:390–395. doi: 10.1034/j.1600-6143.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 41.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 42.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]