Abstract
Sealing of the paracellular cleft by tight junctions is of central importance for epithelia and endothelia to function as efficient barriers between the extracellular space and the inner milieu. Occludin and claudins represent the major tight junction components involved in establishing this barrier function. A special situation emerges at sites where three cells join together. Tricellulin, a recently identified tetraspan protein concentrated at tricellular contacts, was reported to organize tricellular as well as bicellular tight junctions. Here we show that in MDCK cells, the tricellulin C-terminus is important for the basolateral translocation of tricellulin, whereas the N-terminal domain appears to be involved in directing tricellulin to tricellular contacts. In this respect, identification of homomeric tricellulin-tricellulin and of heteromeric tricellulin-occludin complexes extends a previously published model and suggests that tricellulin and occludin are transported together to the edges of elongating bicellular junctions and get separated when tricellular contacts are formed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0313-y) contains supplementary material, which is available to authorized users.
Keywords: Tricellulin, Occludin, Tight junction, Tricellular contacts, Barrier
Introduction
Tight junctions represent intercellular contact sites essential for the maintenance of the polarized morphology of epithelial and endothelial cell layers. Localized at the uppermost part of the lateral cell membrane, tight junctions form barriers sealing the internal space of multicellular organisms from the external environment. In addition, they inhibit diffusion of lipids and proteins between apical and basolateral membrane domains. Ultrastructural analyses by freeze-fracture electron microscopy identified tight junctions as a set of branched intramembranous strands of protein particles bringing the plasma membranes of opposing cells into close contact [1].
The identification of the integral membrane proteins forming the continuous, anastomosing tight junction strands thus was an essential step for a more detailed understanding of the tight junction structure and function [2]. In this respect, occludin and claudins represent the major integral components of bicellular tight junctions (bTJ) [3]. Recently, tricellulin was characterized as a third integral tight junction protein, which predominantly localizes at tricellular junctions [4]. All these tight junction proteins contain four transmembrane domains with N- and C-terminal cytoplasmic tails, two extracelluar loops and a short intracellular loop between transmembrane domains 2 and 3. Furthermore, the Ig superfamily member junctional adhesion molecule (JAM) [5] and coxsackie and adenovirus receptor (CAR) [6] were found associated with tight junction strands. However, JAM is not able to reconstitute tight junction strands as has been reported for occludin and claudins [7].
Occludin was identified as the first integral tight junction protein [8]; nevertheless, its functional role remains unclear. Although occludin is a major component of all tight junctions, the knock-out of the occludin gene in mice did not show a phenotype in respect to tight junction formation and strand morphology as well as barrier function [9, 10]. Meanwhile, 24 different claudins have been identified. Claudins share the tetraspan transmembrane topology with occludin; however, they do not show significant sequence homology and possess much shorter N- and C-terminal cytoplasmic domains. Tissue-specific expression of different combinations of claudins defines tissue-specific barrier characteristics [11]. In this respect, expression of tightening claudins such as claudin-1 [12] and -5 [13, 14] or pore-forming claudins such as claudin-2 [15] and claudin-16 [16–18] affects paracellular permeability for specific cations and/or anions. Particularly the extracellular loops (ECL) of claudins cooperatively determine these properties. The permeability characteristics are preferentially defined by the first ECL, while the second ECL supports trans-interactions between claudins of opposing cell surfaces at tight junctions [19, 20]. Both homophilic and heterophilic cis- and trans-interactions have been observed [11], and peptides directed against the first loop impaired barrier integrity [21].
The identification of tricellulin added a further member to the tight junction tetraspan proteins [4]. Tricellulin is preferentially localized at tricellular contacts where the tight junctional network is extended basolaterally [1]. After strong overexpression, tricellulin also was equally detectable at tri- and bicellular contacts [22]. Similar to occludin, tricellulin reveals extended N- and C-terminal cytoplasmic domains, with the C-terminal tail exhibiting homology to the occludin C-terminus [4]. Despite this homology, occludin and tricellulin are differentially phosphorylated in their C-terminal tails [23]. Knock-down of tricellulin provided the first insight into the function of this protein. The loss of tricellulin severely affected tight junction organization not only at tricellular contacts, but also bTJs were disorganized resulting in disturbed continuity of occludin distribution at bTJ with tear-drop-shaped accumulation of occludin at tricellular tight junctions (tTJ) [4]. Interestingly, when occludin was knocked down in MDCK II cells, tricellulin was redistributed from tTJ to bTJ. Expression of mouse occludin reversed this phenotype [24]. From these observations it was concluded that in occludin knock-out mice, tricellulin may compensate at least for some of the occludin functions. Recent studies analyzing the physiological function of tricellulin revealed that tricellulin in bTJ increases paracellular electrical resistance and decreases permeability to ions and larger solutes. At tricellular contacts tricellulin specifically seals epithelial cell sheets against passage of macromolecules without significantly affecting ion permeation. This property was explained by the fact that tricellular central tubes are wide enough for passage of macromolecules, but too rare to contribute significantly to ion permeability [22]. Furthermore, tricellulin appears to be involved in sealing tricellular corners in the uppermost layer of keratinocytes in culture [25]. In this context, tricellular pores represent an attractive target to enhance delivery of drug molecules across epithelial and endothelial barriers [26]. The important role of tricellulin for epithelial barrier function was further emphasized in studies identifying mutations in the tricellulin C-terminus causing deafness [27–29].
These observations suggest that a crosstalk between tricellulin and occludin has to exist that is involved in formation and organization of tight junction strands. In this respect we hypothesized that an interaction of tricellulin and occludin has to occur. Here we report our biochemical studies investigating tricellulin in respect to its translocation to tight junctions and its ability to form homomeric tricellulin-tricellulin and heteromeric tricellulin-occludin complexes.
Materials and methods
Cell culture and transfection
HEK-293 and MDCK C11 cells were cultured in DMEM high glucose medium and MEM (PAA Laboratories GmbH), respectively, with 10% (v/v) FCS and 100 U/ml penicillin, 100 μg/ml streptomycin in a 5% CO2 atmosphere at 37°C. MDCK II cells were cultured in DMEM low glucose medium with 10% (v/v) FCS and 100 U/ml penicillin, 100 μg/ml streptomycin in a 10% CO2 atmosphere at 37°C. HEK-293 cells were transiently transfected with the calcium phosphate method as described previously [30]. Stable transfection of MDCK C11 [31] cells with empty pCMV14-3xFLAG or the different tricellulin constructs was performed with polyethylenimine (Sigma) as described in [32], and G418-resistant cell clones were established.
Reagents, enzymes and antibodies
Polyclonal antibodies against ZO-1, ZO-2 and occludin were obtained from Zymed (Invitrogen). The polyclonal anti-tricellulin antibody was generated as described in [22]. Mouse monoclonal anti-FLAG-M2 and anti-β-actin (AC15) antibodies were purchased from Sigma, and monoclonal mouse anti-HA (6E2) antibody was from Cell Signaling. HRPO-labeled second antibodies were purchased from Dianova and Alexa Fluor™488, and Alexa Fluor™594-labeled antibodies were from Molecular Probes (Invitrogen). Enzymes for molecular biology reagents were obtained from Roche, Fermentas and New England Biolabs.
Plasmid construction
The full-length cDNA of the human tricellulin-a splice variant [22] was used as a template to generate N- and C-terminal deletion constructs. For amplification of ΔN-tricellulin (ΔNTric) (amino acid 176–558) and tricellulin-ΔC (TricΔC) (amino acids 1–356), the following oligonucleotide pairs were used: 5′-GCG GGT ACC GGA TCC GCC GCC ATG GTG GAG GAG TAT AAC CTG AG-3′, 5′-GCG GGT ACC GGA TCC AGA ATA ACC TTG TAC ATC CC-3′ and 5′-GCG GGT ACC GGA TCC GCC GCC ATG TCA AAT GAT GGA AGA TCC-3′, 5′-GCG GGA TCC AGC TGC CTC ATG CCT CCA- 3′. After BamHI digestion, the PCR products were ligated into the BamHI site of p3x-FLAG-CMV14 (Sigma-Aldrich). To generate expression vectors for HA-tagged tricellulin or occludin, we first inserted the sequence for the HA-tag into the pcDNA6-HA-V5/HisB plasmid by ligation of the annealed oligonucleotides 5′-CTA GCC ACC ATG ACC AGC TAC CCA TAC GAT GTT CCA GAT TAC GCT A-3′ and 5′-AGC TTA GCG TAA TCT GGA ACA TCG TAT GGG TAG CTG GCT ATG GTG G-3′ into NheI/HindIII digested pcDNA6-V5/HisB (Invitrogen). The sequence was confirmed by cycle sequencing. The full-length tricellulin, TricΔC or occludin cDNAs were subsequently ligated into the BamHI-site of this vector. For the expression of a ΔNTricΔC-myc6 construct the corresponding cDNA was amplified by PCR using the oligonucleotides described above and ligated into the BamHI site of pCS2+myc6.
Full-length occludin cDNA was used as a template to generate occludin N- and C-terminal deletion constructs [33]. The endogenous BamHI sites in the occludin cDNA were mutated using the Change-IT™ Multiple Mutation Site Directed Mutagenesis Kit (USB). Briefly, the silent mutations were introduced using the primers 5′-CCG CAG AAC CTC TTG TTG GAC CCT GAT ACT GCT GTA TTA AAA-3′ (forward) and 5′-TTT TAA TAC AGC AGT ATC AGG GTC CAA CAA GAG GTT CTG CGG-3′ (reverse). The ΔN and ΔC occludin cDNA was amplified by PCR using the primers 5′-GCG GGA TCC GCC GCC ATG ACC TCT CCT CCA GGA GTG ATT C-3′ (forward) and 5′-CGC GGA TCC CTA TGT TTT CTG TCT ATC ATA GTC-3′ (reverse) or 5′-CGC GGA TCC GCC GCC ATG TCA TCC AGG CCT CTT GAA-3′ (forward) and 5′-GCG GGA TCC TTA CTT TCT TCG AGT TTT CAC AGC-3′ (reverse), respectively, and subcloned into BamHI sites of p3xFLAG CMV10.
Vectors encoding tricellulin fused to an N- or C-terminal cyan/yellow fluorescent protein (CFP/YFP) were generated using primer pairs 5′-GTA CAA AAG CTT GGG CGG AGG CGG GGG CAT GTC AAA TGA TGG AAG ATC C-3′, 5′-GGA TCC CTC GAG TTA AGA ATA ACC TTG TAC ATC-3′ and 5′-GGA TCC AAG CTT GCC GCC ATG TCA AAT GAT GGA AGA TCC-3′, 5′-CGA TCG CTC GAG CCC CCG CCT CCG CCA GAA TAA CCT TGT ACA TCC C-3′ to amplify full-length cDNA. The PCR products subsequently were digested with HindIII and XhoI, and ligated into pcDNA3-NYFP or pcDNA3-NCFP and pcDNA3-CYFP or pcDNA3-CCFP, respectively. Sequences of all plasmid constructs were verified by resequencing.
Co-immunoprecipitation assays
Twenty-four hours before calcium-phosphate transfection with 2 μg of the respective expression plasmids, HEK-293 cells were seeded into six-well plates in a density of 1 × 106 cells/well. The cells were lysed by ultrasonic treatment in RIPA-buffer [25 mM Tris pH 6.8, 150 mM NaCl, 5 mM EDTA, 1% (w/v) sodium-deoxycholate, 1% (v/v) NP40, 0.1% (w/v) SDS and Complete + EDTA protease inhibitor mixture (Roche)] 48 h after transfection. To remove viscous DNA, benzonase was added. Cellular debris was pelleted by centrifugation, and the supernatant was incubated for 1 h at 4°C with Protein-A-Sepharose (GE Healthcare), which had been preincubated with anti-FLAG-M2 antibody (2 and 4 μg for analysis of homomeric and heteromeric complex formation, respectively). Subsequently, beads were washed 3× with RIPA buffer, and the pellet was resuspended in 2× SDS-sample buffer, boiled for 5 min and analyzed by SDS-PAGE and Western blotting. For co-immunoprecipitation experiments from MDCK C11 cells stably expressing the FLAG-tagged tricellulin constructs, 1 × 106 cells/well were seeded into six-well plates and 24 h later were lysed in RIPA-buffer. After benzonase digestion and removal of cell fragments by centrifugation, lysates were incubate with 2 μg of polyclonal anti-occludin antibody (Zymed, Invitrogen) or as a control with rabbit-IgG (Santa Cruz Biotechnology) for 1 h at 4°C. After centrifugation (20,800×g, 10 min, 4°C), the supernatant was incubated with Protein-A-Sepharose beads for 45 min at 4°C. Subsequently, the beads were washed 3× in RIPA buffer, and bound protein complexes were eluted with 2× SDS sample buffer and analyzed by Western blotting.
Immunofluorescence microscopy
HEK-293 cells were seeded on chamber slides coated with poly-L lysin (Sigma) in a density of 7 × 105 cells per well. After 24 h cells were transfected as described above, and 24 h later cells were washed with PBS/Mg2+/Ca2+, fixed with methanol (10 min at −20°C) and washed again with PBS/Mg2+/Ca2+. MDCK C11 cells stably transfected with empty vector (mock, p3XFLAG-CMV14), Tric-FLAG3, ΔNTric-FLAG3 or TricΔC-FLAG3 were seeded on chamber slides coated with collagen A (Biochrom AG) in a density of 1 × 106 cells/well and fixed after 24 h as described. Non-specific sites were blocked with 5% (v/v) goat serum (PAA) in PBS/Mg2+/Ca2+ for 1 h before the primary antibodies anti-FLAG-M2, anti-HA, anti-myc (9E10) or anti-ZO-1 were added for 1 h at room temperature. After 5× washing with PBS/Mg2+/Ca2+ the Alexa-Fluor488- or Alexa-Fluor594-labeled secondary antibodies and DAPI were added for 30 min at room temperature. Cells were washed again with PBS/Mg2+/Ca2+, and coverslides were mounted using ProTaqs Mount Fluor (Biocyc GmbH&Co. KG). Analysis and photography were performed on a LSM 510 META confocal laser scanning microscope (Zeiss) with a Plan Apochromat Plan Neofluor objective (63×/1.25 oil) at excitation wavelengths of 488, 543 or 405 nm (DAPI). The figures were prepared with Adobe Illustrator without further adjustments.
Fluorescence resonance energy transfer
MDCK II cells were cotransfected with plasmids encoding: claudin-5-CFP (Cld-5-CFP)/claudin-5-YFP (Cld-5-YFP), CFP-tricellulin (CFP-Tric)/YFP-tricellulin (YFP-Tric), tricellulin-CFP (Tric-CFP)/YFP-Tric, Tric-CFP/corticotropin releasing factor receptor-1 (CRFR-1)-YFP (CRFR-1 kindly provided by O. Krätke, Berlin, Germany) or CFP-Tric/CRFR-1-YFP [34]. The position of CFP and YFP before or after the protein name describes N- or C-terminal position, respectively. After 3 days, cells were transferred into HBSS (Hanks’ balanced salt solution) pH 7.5 and analyzed by confocal microscopy (LSM 510 Meta, Carl Zeiss, Jena, Germany). For fluorescence resonance energy transfer (FRET), acceptor photobleaching was applied [34]. The pairs tricellulin-CFP/CRFR-1-YFP and CFP-tricellulin/CRFR-1-YFP were chosen as negative controls, as these proteins represent transmembrane proteins not expected to interact with each other. Cld-5-CFP/Cld-5-YFP was used as positive control, known to interact with each other [34]. Trypan blue staining was used to visualize the plasma membrane [20].
Statistics
Data represent mean values ± SEM. The Kruskal-Wallis test was used to identify significant differences within experiments, and Dunn’s multiple comparison post-test was used to assess the significance of differences between several experimental groups with P < 0.05 considered as significant. The computer package GraphPad Prism version 3.0 (Statcon, San Diego, CA) was employed.
Results
The role of the tricellulin C- and N-terminal tails in basolateral targeting and tricellular localization
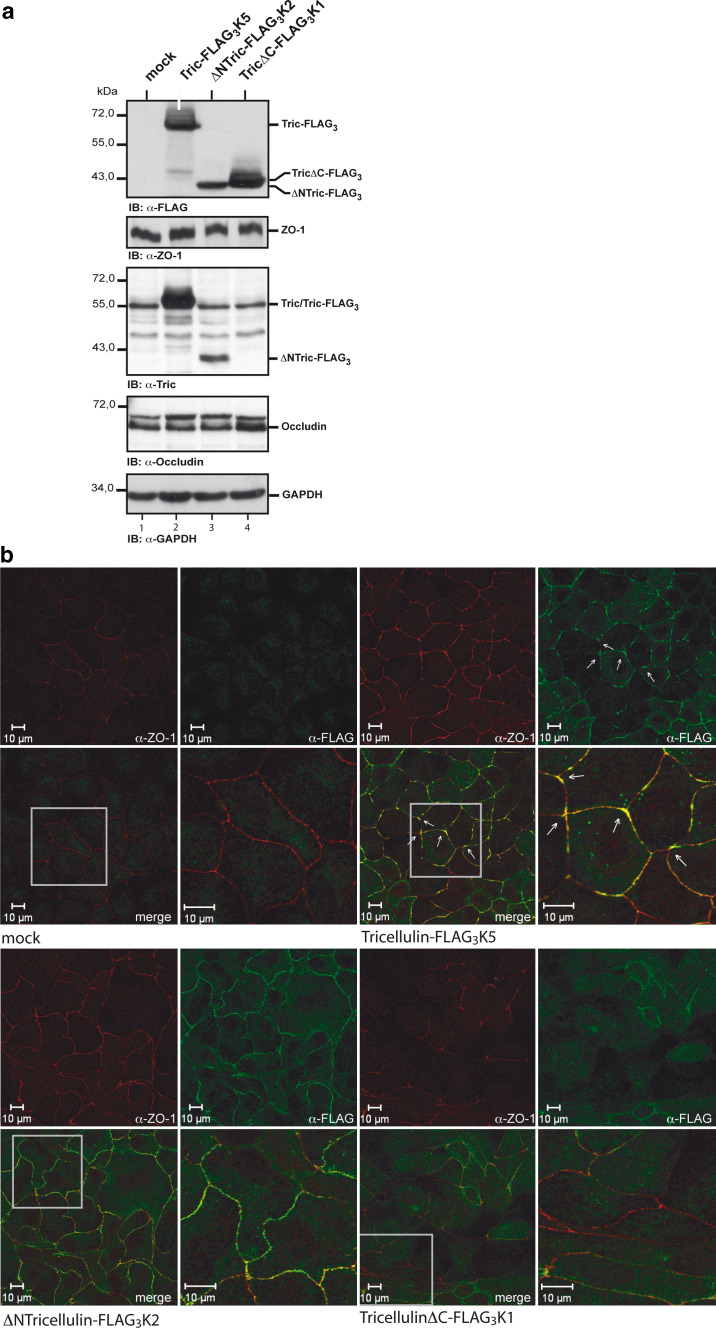
Previous studies have demonstrated that tricellulin is predominantly located at tTJ; however, little is known about the mechanisms responsible for this tricellular localization and how tricellulin affects overall tight junction structure. In this respect, we first wanted to know which domain in tricellulin drives its localization to tricellular contact sites. To address this question, MDCK C11 cells were stably transfected either with full-length tricellulin, ΔNTric or TricΔC, each fused to a C-terminal FLAG3-tag. Expression of the transfected tricellulin constructs was analyzed by Western blotting with an anti-FLAG monoclonal antibody (Fig. 1a, Suppl. Fig. 1A). Analysis of endogenous ZO-1, occludin and tricellulin levels did not show significant differences when vector-transfected cells were compared with cells transfected with tricellulin constructs. GAPDH was used as a loading control.
Fig. 1.
The role of tricellulin N- and C-terminus in its localization. a MDCK C11 cells were stably transfected with tricellulin-FLAG3, ΔNTric-FLAG3 or TricΔC-FLAG3, and expression of the FLAG-tagged proteins was analyzed by Western blotting with the anti-FLAG-M2 antibody. ZO-1, endogenous full-length tricellulin and occludin were detected with anti-ZO-1, anti-occludin and anti-tricellulin polyclonal antibodies, respectively. GAPDH was used as a loading control. b Analysis of the expression and localization of ZO-1 (red) and the FLAG-tagged tricellulin constructs (green) in stably transfected MDCK C11 cells by confocal immunofluorescence microscopy. Nuclei were stained with DAPI. The merged images show co-localization of the tricellulin constructs with ZO-1 at tight junctions. The lower right image for each of the constructs is a magnification of the boxed field in the lower left image
The subcellular distribution of the different tricellulin constructs was examined by confocal immunofluorescence microscopy. Double staining with anti-FLAG and anti-ZO-1 antibodies was used to determine the tight junctional localization of the tricellulin constructs (Fig. 1b). As expected, the vector control did not show FLAG-staining at cell-cell contacts. Full-length tricellulin-FLAG3 showed a clustered distribution at tricellular contacts and in addition discontinuous staining at bTJ. In contrast, ΔNTric-FLAG3 revealed continuous distribution all over the membranes at bicellular and tricellular contact sites without clustering of signals. In particular, accumulation of ΔNTric-FLAG3 at tricellular contacts was not detectable. However, staining of the FLAG-tagged ΔNTric construct was more intense compared to staining of FLAG-tagged full-length tricellulin or TricΔC. Whether this is due to an enhanced half-life of the ΔNTric construct has to be evaluated.
Interestingly, as compared to both full-length tricellulin and ΔNTric-FLAG3, the tricellulin construct lacking the C-terminal cytoplasmic domain (TricΔC-FLAG3) was transported to the cell contact sites less efficiently, although expressed at levels similar to that of full-length tricellulin-FLAG3 and ΔNTric-FLAG3, respectively. These observations suggested that the C-terminal cytoplasmic tail is of importance for efficient transport and targeting of tricellulin to basolateral membranes, while the N-terminal cytoplasmic tail is involved in targeting to tricellular contacts. The experiments described above were also performed with other clones of transfected cells leading to the same results (see Suppl. Fig. 1A and 1B). Further experiments were performed to examine the effect of Ca2+-ion concentrations in the culture medium on the cell surface localization of tricellulin. While lowering Ca2+-ion concentration from 1.8 mM to 5 μM resulted in a disappearance of tricellulin-FLAG3 from the cell surface, this effect could be reversed by switching back to the initial Ca2+-ion concentration of 1.8 mM. No major differences were noted for full-length tricellulin-FLAG3, ΔNTric-FLAG3 and TricΔC-FLAG3 when comparing staining patterns after switching back from low to normal Ca2+ concentrations except a minor delay in reappearance of ΔNTric-FLAG3 at sites of cell–cell contact (not shown).
Tricellulin forms homodimeric complexes
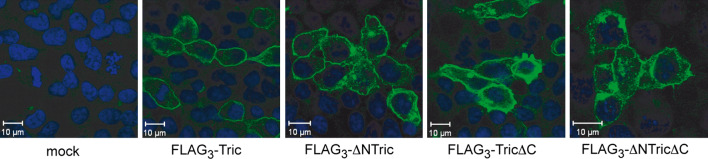
The preferential localization at tricellular cell contacts and especially in basolaterally extended regions where occludin and claudins are excluded suggested that tricellulin molecules form homomeric protein complexes. In order to examine this assumption, co-immunoprecipitation experiments from lysates of HEK-293 cells transiently transfected with FLAG3-tricellulin and HA-tricellulin were performed. HEK-293 cells were chosen for these experiments because they do not express endogenous tight junction complexes and can be easily co-transfected with the different combinations of constructs. Immunofluorescence microscopy of the full-length tricellulin and ΔN-tricellulin constructs with the FLAG-tag fused to the N-terminus in the transiently transfected HEK293 cells showed that the constructs are localized mainly in the cell surface membrane (Fig. 2). In cells expressing high levels of FLAG3-TricΔC, a strong staining all over the cytoplasm was detectable, suggesting that translocation to the membrane is impaired similar to the results obtained in MDCK cells.
Fig. 2.
Localization of the indicated FLAG-tagged tricellulin constructs (green) in transiently transfected HEK-293 cells as analyzed by confocal immunofluorescence microscopy. Nuclei were stained with DAPI
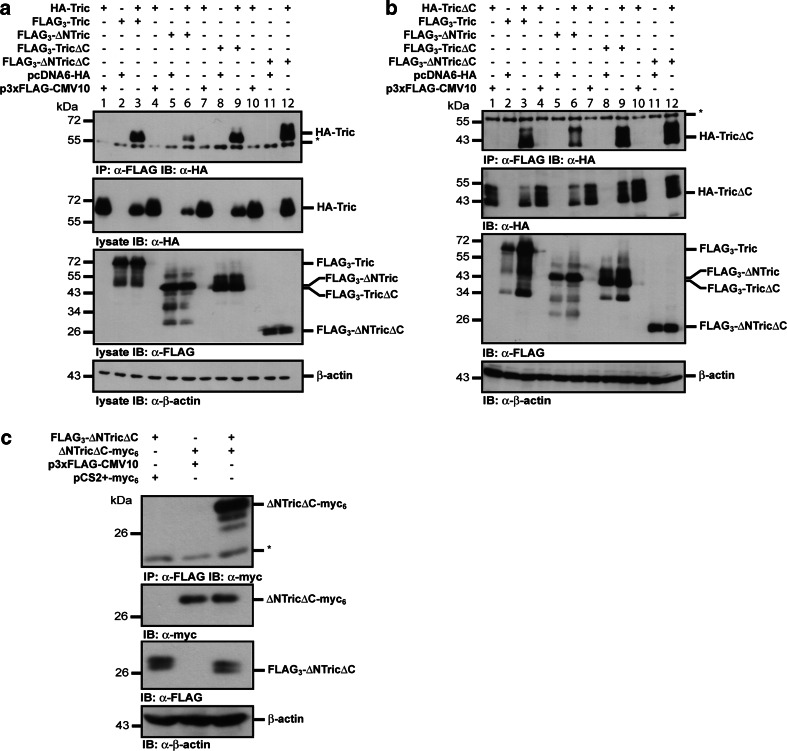
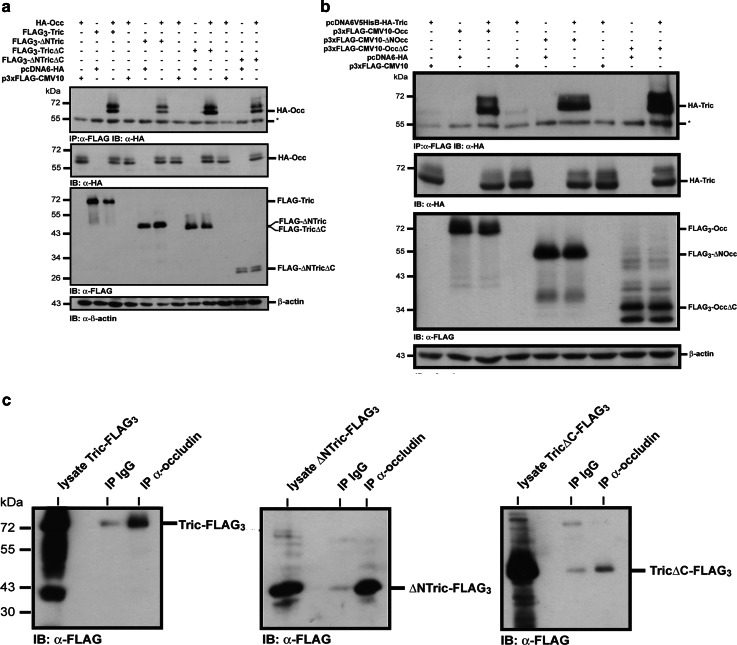
Expression levels of the transfected constructs were analyzed by Western blotting of equal amounts of cell lysate. β-Actin was used as a loading control. Western blotting with anti-FLAG antibody showed that each of the FLAG-tagged tricellulin constructs was expressed at similar levels. Co-expression of FLAG-tagged tricellulin constructs with the exception of FLAG3-ΔNTricΔC with HA-tricellulin lead to a reduced expression of HA-tricellulin. At the moment we cannot explain this observation. In co-immunoprecipitation experiments using the anti-FLAG-M2 monoclonal antibody, HA-tricellulin was found to associate with FLAG-tagged tricellulin. HA-tricellulin could only be co-immunoprecipitated when both the FLAG- and the HA-tagged constructs were co-expressed (Fig. 3a, lanes 1–3).
Fig. 3.
Tricellulin forms homomeric complexes. a Co-immunoprecipitation of HA-tagged full-length tricellulin with the indicated FLAG-tagged tricellulin constructs after transient transfection into HEK-293 cells. Complex formation is only detectable when HA-tricellulin is co-transfected with the FLAG-tagged tricellulin constructs (lanes 3, 6, 9, 12). No interaction was detectable when one of the constructs was transfected alone (lanes 1 and 2, 4 and 5, 7 and 8, 10 and 11). Western blotting of the lysates shows expression of the indicated constructs. β-Actin was used as a loading control. b HA-TricΔC also interacts with the FLAG-tagged tricellulin constructs. Experiments were performed as above. c FLAG-tagged tricellulin with a deleted N- and C-terminus co-immunoprecipitates with a myc6-tagged tricellulin construct without N- and C-terminal domain. The presented immunoprecipitations are representatives of at least three independent experiments. Asterisk heavy chain of the precipitating antibody
Previously, it was shown that the C-terminal domain of occludin mediates occludin dimerization [35, 36]. In this context, we next wanted to know whether similar to occludin the C-terminal domain of tricellulin is involved in complex formation. To address this, FLAG3-ΔNTric or FLAG3-TricΔC was co-transfected with HA-tricellulin, and co-immunoprecipitations were performed as described above. Surprisingly, both N- and C-terminally deleted tricellulin associated with full-length HA-tricellulin protein (Fig. 3a, lanes 4–9), suggesting that either one of the cytoplasmic domains is sufficient to mediate an interaction, or none of the cytoplasmic domains of tricellulin is necessary for dimerization. This assumption was confirmed by the finding that HA-tricellulin also associated with a FLAG3-ΔNTricΔC construct, where both the N- and the C-terminal cytoplasmic domains were deleted (Fig. 3a, lanes 10–12). Moreover, complex formation could also be shown for HA-TricΔC and each of the FLAG-tagged tricellulin constructs (Fig. 3b). To rule out that complex formation is influenced by the type of the tag or by the position of the tag within the tricellulin molecule, co-immunoprecipitation experiments were performed for FLAG-tagged ΔNTricΔC and ΔNTricΔC-myc6 (Fig. 3c). An association of both tricellulins was found, confirming that the interaction of tricellulin molecules occurrs in a way independent of the tag and of its N- or C-terminal position. Moreover, the experiments clearly show that complex formation does not involve the N- and the C-terminal cytoplasmic tail of tricellulin. Since FLAG-ΔNTricΔC is not routed to the cell surface (Fig. 2), the finding that HA-tricellulin also associated with a FLAG3-ΔNTricΔC construct suggests that tricellulin–tricellulin interactions already occur during transport to the cell surface.
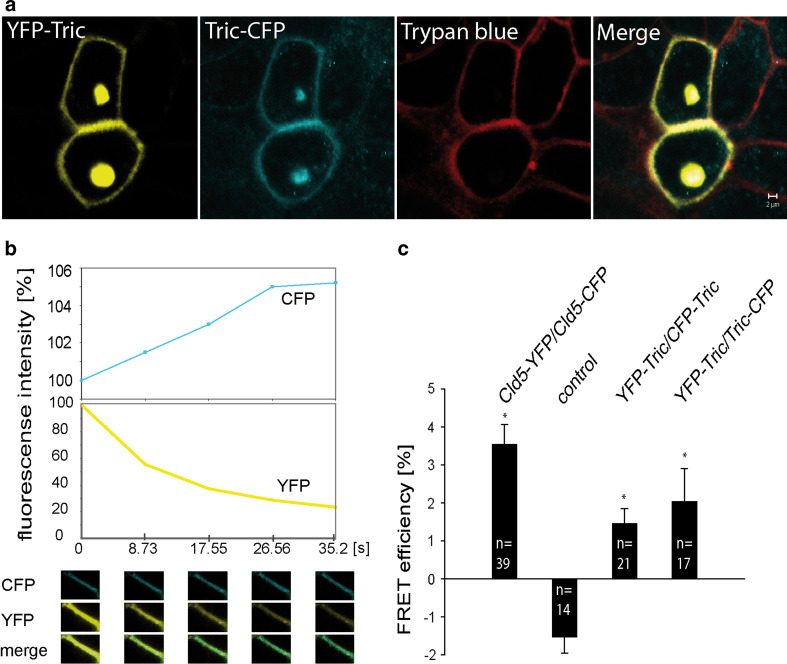
Oligomerization of tricellulin was further verified by FRET assays conducted in living MDCK II cells that endogenously contain tight junction proteins. Confluent MDCK II cells were co-transfected with YFP-Tric/CFP-Tric or YFP-Tric/Tric-CFP. In Fig. 4a the localization of the fusion proteins in the plasma membrane is visualized and compared to the cell membrane marker trypan blue (red) [20]. YFP-Tric (yellow) and Tric-CFP (blue) co-localized all over the cell surface membrane at cellular contact sites (merge). For FRET analyses, contact areas between two transfected cells were marked (Fig. 4a). The FRET efficiency was determined by comparing the CFP fluorescence at cell-cell contacts before and after acceptor (YFP) photo-bleaching (Fig. 4b). As a positive control we used MDCK II cells transfected with Cld-5-YFP/Cld-5-CFP as self-association of claudin-5 is well documented [20, 34]. The FRET efficiency was 3.6 ± 0.5%. As negative controls, MDCK II cells co-transfected with either CRFR-1-YFP/CFP-Tric or CRFR-1-YFP/Tric-CFP were used. The two protein combinations taken together were considered as control. These protein pairs, not expected to interact, showed no considerable signal (–1.5 ± 0.4%). The negative FRET efficiency was caused by a cross photo-bleaching of the CFP by the YFP excitation. MDCK II cells expressing CFP-Tric/YFP-Tric (1.5 ± 0.4%) or Tric-CFP/YFP-Tric (2.0 ± 0.9%) showed a significantly higher FRET efficiency as compared to the negative control (P > 0.05) (Fig. 4b).
Fig. 4.
Localization and homophilic interaction of transfected tricellulin in MDCK II cells. a Fluorescence image of living MDCK II cells co-transfected with YFP-Tric (yellow) and Tric-CFP (blue). The plasma membrane of MDCK II cells was visualized with trypan blue (red). The fusion proteins show plasma membrane localization and co-localization (merge). Bar 2 μm. b FRET (fluorescence resonance energy transfer) analyses using acceptor photo-bleaching. In the area of cell-cell contacts the CFP- and YFP-fluorescence was determined before, during and after four cycles of photo-bleaching of YFP. A representative time series is given with images (lower part) and fluorescence intensities (upper part). c The FRET efficiencies calculated from MDCK-II cells expressing Cld-5-YFP/Cld-5-CFP as positive control; YFP-Tric/CFP-Tric and YFP-Tric/Tric-CFP were significantly higher than for the negative control. These results indicate the specific self-association of tricellulin along one plasma membrane (cis-interaction [20]). Data are mean values ± SEM; *P < 0.05 compared to the control. YFP/CFP yellow/cyan fluorescence protein, Tric tricellulin, Cld-5 claudin-5
Tricellulin forms heteromeric complexes with occludin
It was reported that knock-down of tricellulin affects overall tight junction organization and occludin localization. Vice versa, loss of occludin impairs localization of tricellulin to tricellular contacts [4, 24]. Based on the reported homology of tricellulin and occludin, these findings suggest that tricellulin is also able to form heteromeric complexes with occludin. Co-immunoprecipitation experiments revealed that, indeed, in transiently transfected HEK-293 cells, HA-occludin associated with FLAG-tagged full-length tricellulin and all N- and C-terminal deletion constructs (Fig. 5a). Likewise, when we performed immunoprecipitations with an anti-occludin antibody, endogenous occludin was found to associate with the FLAG-tagged tricellulin constructs in stably transfected MDCK C11 cells (Fig. 5c). Finally, association of full-length tricellulin with both an N- and C-terminally deleted occludin construct was detectable (Fig. 5b).
Fig. 5.
Tricellulin forms heteromeric complexes with occludin. a HEK-293 cells were transiently transfected with the indicated combinations of HA-tagged full-length occludin and FLAG-tagged tricellulin constructs. Heteromeric complexes only formed when both constructs were co-transfected. Lysates were analyzed for expression of the constructs by Western blotting. β-Actin was used as a loading control. b Full-length tricellulin associates with FLAG-tagged ΔNOcc and OccΔC in transiently transfected HEK-293 cells. Asterisk heavy chain of the precipitating antibody. c Immunoprecipitation of endogenous occludin from lysates of MDCK C11 cells stably transfected with the indicated FLAG-tagged tricellulin constructs. Endogenous occludin associates with the full-length tricellulin, ΔNTric and TricΔC. In controls using IgG only, negligible co-precipitation of the tricellulin constructs was detectable
Taken together, these observations indicate that homomeric and heteromeric tricellulin-tricellulin and tricellulin-occludin complex formation occurs and does this in a way independent of the N- and the C-terminal cytoplasmic tail of both occludin and tricellulin. In view of the reported role of occludin and tricellulin in tight junction organization [4, 24], our findings indicate that the association of occludin and tricellulin is of relevance for tricellulin incorporation into tight junction strands and its tricellular localization.
Formation of homomeric and heteromeric complexes of tricellulin and occludin could occur either laterally within the same membrane (“cis-complexes”) or at contact sites between opposing cells (“trans-complexes”). In order to examine whether the observed complexes represent cis- or trans-complexes, MDCK C11 cells stably expressing either tricellulin-FLAG3 or HA-tricellulin were mixed in a 1:1 ratio and allowed to form cell-cell contacts. Cells were then lyzed, and co-immunoprecipitation experiments were performed as described above. When cells expressing either FLAG-tricellulin or HA-tricellulin were mixed, no complex formation between FLAG- and HA-tagged tricellulin was detectable (not shown). Likewise, no complex formation was observable when cells expressing either FLAG-tagged occludin or HA-tagged tricellulin were mixed. These results support the conclusion that the tricellulin-tricellulin and tricellulin-occludin complexes observed in our experiments represent cis-complexes formed within the same membrane by lateral cis-interactions of the proteins.
Discussion
In this study we reported biochemical analyses on tricellulin translocation to tight junctions where it forms homomeric tricellulin-tricellulin complexes and heteromeric tricellulin-occludin complexes. By overexpressing tagged tricellulin constructs in MDCK C11 cells, the specific role of the different domains within tricellulin was addressed. Full-length FLAG-tagged tricellulin was localized at tricellular contacts and in addition depending on the expression level in discontinuous patches in bTJ as reported previously by Ikenouchi et al. [4]. In contrast, transport to the cell surface of tricellulin with a deleted C-terminal domain was impaired, whereas more intracellular aggregates were detectable. In contrast, N-terminally deleted tricellulin was efficiently translocated to the cell surface and was evenly distributed in bTJ with no accumulation at tTJ. These observations differ from data reported by Ikenouchi et al. [24] who showed that both N- and C-terminally deleted tricellulins colocalize with claudin-1 at the cell surface. This difference may be due to the fact that Ikenouchi et al. used L cells, which do not express endogenous tight junction proteins and required claudin-1 to concentrate tricellulin at sites of cell contact. MDCK C11 cells possess a complete set of all major tight junction proteins, exhibit a polarized morphology and more adequately represent a native epithelium. Consistent with the findings by Ikenouchi et al. [24], a tricellulin construct with both the N- and C-terminal cytoplasmic tails deleted did not translocate to the cell surface and mainly accumulated in intracellular aggregates. This suggests that the tricellulin C-terminus is important for the transport of tricellulin to the basolateral cell surface, whereas the N-terminus appears to play a role in the distribution of tricellulin between bTJ and tTJ. However, it cannot be excluded that overexpression may affect targeting of constructs, e.g., in competing for a limited number of factors regulating the transport of tricellulin. Further studies are required to define the molecular mechanisms involved in more detail.
Previous studies have shown self-association of occludin in bTJ [34]. Based on its homology with tricellulin and on the preferential localization of tricellulin at tTJ, we assumed that tricellulin may form homomeric complexes at tricellular contacts. This assumption was clearly supported by co-immunoprecipitation experiments showing homomeric tricellulin-tricellulin complexes and by FRET analyses that further confirmed tricellulin dimerization. Occludin was reported to dimerize via a coiled-coil motif in its C-terminal cytoplasmic tail [35, 36]. Thus, it was expected that deletion of the tricellulin C-terminus might affect self-association. Surprisingly, TricΔC still formed complexes with full-length tricellulin. In line with this observation, we also could not detect an association of recombinant GST-TricC and MBP-TricC fusion proteins containing the complete cytoplasmic C-terminal tail of tricellulin in pull-down experiments (not shown). In addition, ΔNTric and even ΔNTricΔC associated with full-length tricellulin as well as with HA-TricΔC (Fig. 3), suggesting that self-association involves the transmembrane domains and/or the extracellular and/or the intracellular loops. This assumption is further supported by the observation that even FLAG3-ΔNTricΔC and ΔNTricΔC-myc6 formed complexes (Fig. 3c). Interestingly, although most of the ΔNTricΔC protein does not translocate to the cell surface and resides in the ER [24], complex formation was detectable. This suggests that homomeric interaction already can occur in the ER and Golgi apparatus. The minor amounts of ΔNTricΔC detectable at the membrane probably have been transported to the cell surface in association with endogenous full-length proteins. From these observations, we assume that homomeric interactions of native full-length tricellulin proteins already can form within the ER and/or Golgi-apparatus during their biosynthetic transport to the cell surface. However, this has to be analyzed in more detail in future studies.
Furthermore, it also had to be considered that the complexes detected in our experiments represented trans-interactions between the tricellulin constructs. When we co-cultured cells expressing either FLAG-tagged or HA-tagged tricellulin constructs, subsequent co-immunoprecipitation experiments did not reveal the formation of homomeric or heteromeric complexes, and from these observations we conclude that tricellulin homodimerization occurs by cis-interactions within the same membrane and not by trans-interactions of opposing cells. The results of our FRET experiments also indicate cis-interaction because FRET from the CFP-tag of one tricellulin to the YFP-tag of another one is only possible within one plasma membrane, but not between those of two opposing cells because the distance is larger than necessary for FRET (≤6 nm [34]).
In addition, we could detect heteromeric tricellulin-occludin complexes. As observed for the homomeric tricellulin interaction, deletion of neither the N- nor C-terminus or of both termini of tricellulin affected binding to full-length occludin. Vice versa, occludin constructs with deleted N- and C-terminal domains also associated with full-length tricellulin. Ikenouchi et al. [24] suggested a mechanism for the tricellular localization of tricellulin, where tricellulin is targeted to the edges of elongating bTJs. Our results now extend this mechanism in postulating that tricellulin and occludin share a common transport pathway directing both proteins to these elongating edges. On their way to the cell surface and in initial phases when they arrive at tight junctions, occludin and tricellulin form complexes that are separated when tricellular contacts are established. This requires tight regulation of the tricellulin-occludin interactions, for example, by phosphorylation.
Taken together, we conclude that tricellulin cis-interaction is mediated by the tricellulin transmembrane and/or extracellular domains. Lateral dimerization of transmembrane domains of cell surface proteins such as E-cadherin [37], APP [38] or the erythropoietin receptor [39] have been previously reported to occur and to be of functional importance. However, the structural basis for the homomeric tricellulin interaction has to be further analyzed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1: (A) Western blot analysis of the expression of the FLAG-tagged tricellulin constructs in stably transfected MDCK C11 cell clones. Please note that clone K5 expresses higher levels of the full-length tricellulin construct compared to clone K13. (B) Localization of the tricellulin constructs in the different clones by confocal immunofluorescence microscopy after PFA fixation. In clone K5 localization of Tric-FLAG3 is not restricted to tricellular contacts but extends into the bicellular tight junctions as previously reported [4]. (TIFF 17745 kb)
Suppl. Fig. 2: Confocal immunofluorescence images (XYZ scans) reveals co-localization of the tricellulin constructs with ZO-1 at tight junctions. Please note that the TricDC-FLAG3 construct is less efficiently translocated to the tight junctions. For the image presented here, a cell that shows relatively strong membrane staining of TricDC-FLAG3 was chosen. (TIFF 26130 kb)
Acknowledgments
This work was supported by the DFG Research Group FOR 721 and the Sonnenfeld-Stiftung. We thank Dr. Michael Schaefer for providing pcDNA3-NYFP, pcDNA3-NCFP, pcDNA3-CYFP and pcDNA3-CCFP vectors and Luise Kosel for technical assistance.
References
- 1.Staehelin LA. Further observation on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 2.Schulzke JD, Fromm M. Tight junction: molecular structure meets function. Ann NY Acad Sci. 2009;1165:1–6. doi: 10.1111/j.1749-6632.2009.04925.x. [DOI] [PubMed] [Google Scholar]
- 3.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 8.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Krause G, Winkler L, Mueller SL, Haselhoff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Furuse M, Hata M, Furuse K, Yoshida A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter AH, Schulzke JD, Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 14.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 16.Günzel D, Amasheh S, Pfaffenbach S, Richter JF, Kausalya PJ, Hunziker W, Fromm M. Claudin-16 affects transcellular Cl− secretion in MDCK cells. J Physiol (Lond) 2009;587:3777–3793. doi: 10.1113/jphysiol.2009.173401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of the ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 18.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 19.Krause G, Winkler L, Piehl C, Blasig IE, Piontek J, Müller SL. Structure and function of extracellular claudin domains. Ann NY Acad Sci. 2009;1165:34–43. doi: 10.1111/j.1749-6632.2009.04057.x. [DOI] [PubMed] [Google Scholar]
- 20.Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 21.Mrsny RJ, Brown GT, Gerner-Smidt K, Buret AG, Meddings JB, Quan C, Koval M, Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol. 2008;172:905–915. doi: 10.2353/ajpath.2008.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dörfel MJ, Westphal JK, Huber O. Differential phosphorylation of occludin and tricellulin by CK2 and CK1. Ann NY Acad Sci. 2009;1165:69–73. doi: 10.1111/j.1749-6632.2009.04043.x. [DOI] [PubMed] [Google Scholar]
- 24.Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19:4687–4693. doi: 10.1091/mbc.E08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlüter H, Moll I, Wolburg H, Franke WW. The different structures containing tight junction proteins in epidermal and other stratified epithelial cells, including squamous cell metaplasia. Eur J Cell Biol. 2007;86:645–655. doi: 10.1016/j.ejcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 26.González-Mariscal L, Hernández S, Vega J. Inventions designed to enhance drug delivery across epithelial and endothelial cells through the paracellular pathway. Recent Pat Drug Deliv Formul. 2008;2:145–176. doi: 10.2174/187221108784534117. [DOI] [PubMed] [Google Scholar]
- 27.Chishti MS, Bhatti A, Tamim S, Lee K, McDonald ML, Leal SM, Ahmad W. Splice-site mutations in the TRIC gene underlie autosomal recessive nonsyndromic hearing impairment in Pakistani families. J Hum Genet. 2008;53:101–105. doi: 10.1007/s10038-007-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramzan K, Shaikh RS, Ahmad J, Khan SN, Riazuddin S, Ahmed ZM, Friedman TB, Wilcox ER, Riazuddin S. DFNB48, a new nonsyndromic recessive deafness locus, maps to chromosome 15q23–q25.1. Hum Genet. 2005;116:407–412. doi: 10.1007/s00439-004-1205-8. [DOI] [PubMed] [Google Scholar]
- 29.Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, Belyantseva IA, Forge A, Riazuddin S, Friedman TB. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiske J, Albring KF, Huber O. The tumor suppressor Fhit acts as a repressor of β-catenin transcriptional activity. Proc Natl Acad Sci USA. 2007;104:20344–20349. doi: 10.1073/pnas.0703664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gekle M, Wünsch S, Oberleithner H, Silbernagel S. Characterization of two MDCK-cell subtypes as model system to study principal cell and intercalated cell properties. Pflügers Arch. 1994;428:157–162. doi: 10.1007/BF00374853. [DOI] [PubMed] [Google Scholar]
- 32.Ehrhardt C, Schmolke M, Matzke A, Knoblauch A, Will C, Wixler V, Ludwig S. Polyethylenimine, a cost-effective transfection reagent. Signal Transduct. 2006;6:179–184. doi: 10.1002/sita.200500073. [DOI] [Google Scholar]
- 33.Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- 34.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J. On the self-association of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63:505–514. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Fanning AS, Anderson JM, Lavie A. Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J Mol Biol. 2005;352:151–164. doi: 10.1016/j.jmb.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Müller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G. The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 37.Huber O, Kemler R, Langosch D. Mutations affecting transmembrane segment interactions impair adhesiveness of E-cadherin. J Cell Sci. 1999;112:4415–4423. doi: 10.1242/jcs.112.23.4415. [DOI] [PubMed] [Google Scholar]
- 38.Münter LM, Voigt P, Harmeier A, Kaden D, Gottschalk KE, Weise C, Pipkorn R, Schaefer M, Langosch D, Multhaup G. GxxxG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Abeta42. EMBO J. 2007;26:1702–1712. doi: 10.1038/sj.emboj.7601616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubatzky KF, Ruan W, Gurezka R, Cohen J, Ketteler R, Watowich SS, Neumann D, Langosch D, Klingmüller U. Self assembly of the transmembrane domain promotes signal transduction through the erythropoietin receptor. Curr Biol. 2001;11:110–115. doi: 10.1016/S0960-9822(01)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1: (A) Western blot analysis of the expression of the FLAG-tagged tricellulin constructs in stably transfected MDCK C11 cell clones. Please note that clone K5 expresses higher levels of the full-length tricellulin construct compared to clone K13. (B) Localization of the tricellulin constructs in the different clones by confocal immunofluorescence microscopy after PFA fixation. In clone K5 localization of Tric-FLAG3 is not restricted to tricellular contacts but extends into the bicellular tight junctions as previously reported [4]. (TIFF 17745 kb)
Suppl. Fig. 2: Confocal immunofluorescence images (XYZ scans) reveals co-localization of the tricellulin constructs with ZO-1 at tight junctions. Please note that the TricDC-FLAG3 construct is less efficiently translocated to the tight junctions. For the image presented here, a cell that shows relatively strong membrane staining of TricDC-FLAG3 was chosen. (TIFF 26130 kb)